Abstract
Background.
Military trainees are at increased risk for Staphylococcus aureus colonization and infection. Disease prevention strategies are needed, but a S. aureus vaccine does not currently exist.
Methods.
We enrolled US Army Infantry trainees (Fort Benning, GA) in a phase 2, randomized, double-blind, placebo-controlled trial of NDV-3A, a vaccine containing a recombinant adhesin/invasion protein of Candida albicans that has structural similarity to the S. aureus protein clumping factor A. Study participants received one intramuscular dose of NDV-3A or placebo (adjuvant alone) within 72 hours of arrival on base. Longitudinal nasal and oral (throat) swabs were collected throughout the 14-week Infantry training cycle. Safety, immunogenicity, and efficacy of NDV-3A against S. aureus nasal / oral acquisition were the endpoints.
Results.
The NDV-3A candidate had minimal reactogenicity and elicited robust antigen-specific B- and T-cell responses. During the 56-day post-vaccination period, there was no difference in the incidence of S. aureus nasal acquisition between those who were randomized to receive NDV-3A vs. placebo (25.6% vs. 29.1%; vaccine efficacy [VE]: 12.1%; p=0.31). In time-to-event analysis, there was no difference between study groups with respect to the S. aureus colonization-free interval (VE: 13%; p=0.29). Similarly, the efficacy of NDV-3A against S. aureus oral acquisition was poor (VE: 2.4%; p=0.52).
Conclusions.
A single dose of NDV-3A did not prevent nasal nor oral acquisition of S. aureus in a population of military trainees at high risk for colonization.
Clinical Trials Registration:
Keywords: Staphylococcus aureus, MRSA, vaccine, colonization, military trainees
INTRODUCTION
Staphylococcus aureus causes an array of clinical syndromes, ranging from relatively mild skin and soft-tissue infections (SSTI) to life-threatening endocarditis and sepsis. An effective S. aureus vaccine has yet to be realized [1], owing in part to an incomplete understanding of the immune correlates of protection against disease. Although S. aureus SSTIs elicit anti-toxin serum antibodies, these responses are neither durable nor protective against disease recurrence [2]. Animal and human data highlight that T-cells play an important role in the defense against Staphylococcal infection [3–5]; however, significant knowledge gaps remain [6].
Strategies in the development of S. aureus vaccines have included the use of non-target antigens [7]. One such candidate, NDV-3A (NovaDigm Therapeutics, Inc.; Grand Forks, ND), contains the N-terminal portion of the agglutinin-like sequence 3 (Als3) protein of Candida albicans [8, 9], which shares both structural and functional homology with Staphylococcal clumping factor A (ClfA) [9]. The NDV-3A candidate has undergone extensive preclinical evaluation [10–13], is safe and immunogenic in humans [14], and was effective against recurrent vulvovaginal candidiasis [15].
Whether immunization with NDV-3A can confer immunity to Staphylococcal infection also has been the subject of ongoing investigation. In murine models, NDV-3 (a precursor to NDV-3A) vaccination reduced the progression and severity of S. aureus SSTIs and was associated with induction of interleukin-17A (IL-17A) and interleukin-22 (IL-22) [16], two components of the Th17 pathway that are known to play a role in the defense of skin and mucocutaneous barriers [17, 18]. The suggestion that this vaccine may protect against S. aureus in humans was derived from the findings of a phase 1 safety and immunogenicity trial of NDV-3 [clinicaltrials.gov identifier NCT01447407]. In a subset of study participants who were persistently nasally colonized with S. aureus, the post-vaccination prevalence of S. aureus nasal colonization was lower among NDV-3 recipients than among controls at day 56 following a single dose (25% vs. 75%; p=0.06; J. P. Hennessey Jr., unpublished data).
While the trial was not designed nor sufficiently powered to evaluate the impact of NDV-3 on S. aureus nasal colonization, these findings were intriguing and encouraging nonetheless, and supported the further evaluation of vaccine efficacy (VE) in a high-risk population. Herein, we describe the results of a phase 2 trial of NDV-3A against S. aureus colonization among US Army Infantry trainees, a population known to be at increased risk for S. aureus colonization and infection [19, 20].
METHODS
We conducted an individually randomized, double-blind, placebo-controlled trial of NDV-3A from January 2018-August 2019 among US Army Infantry trainees at Fort Benning, GA. This study was reviewed and approved by the Uniformed Services University of the Health Sciences Institutional Review Board (IDCRP-104). Vaccine and placebo lots were manufactured using current Good Manufacturing Practices, stored at 2°C–8°C, and monitored for stability. The NDV-3A vaccine candidate is a recombinant form of Als3 that was produced in Saccharomyces cerevisiae, purified (>98% purity), and formulated with aluminum hydroxide [14]. The placebo lot contained all vaccine components except recombinant Als3 protein.
Infantry training cycles are typically 14 weeks long (9 weeks of basic training, followed by 5 weeks of advanced individual training). Infantry companies are composed of ~200 soldiers who train together, reside in the same barracks, and are physically segregated from other training companies for the duration of training. Immediately following their arrival, soldiers undergo a 7–10 day period of in-processing prior to the initiation of the actual training cycle. We targeted four companies (training start: January 2018; June 2018; September 2018; and January 2019) for trial participation. Trainees were briefed on the trial within 24 hours of their arrival. In an auditorium-style setting, a study investigator presented the trial rationale, design, and requirements and gave the trainees an opportunity to ask questions. Military members who were on the investigative team were dressed in civilian attire. An ombudsman was present to ensure that the information provided was adequate and accurate and that trainees’ participation in the trial was strictly voluntary. Officers or drill sergeants in the trainees’ chain of command were not present during trial recruitment.
Inclusion criteria were as follows: 17–42 years of age; assigned to one of the four companies; medically cleared to engage in a military training program; and agreeable to be reached by phone or email 6 months post-vaccination. Because Infantry training was not gender integrated at the time of trial initiation, only males were recruited. Exclusion criteria were receipt of any investigational drug, investigational vaccine, or investigational device in the previous 30 days; a clinically significant SSTI at screening; an inflammatory or other dermatologic condition at screening; self-reported history of allergic response(s), anaphylaxis, or other serious reactions to previous vaccinations; self-reported use of any immunosuppressive drugs in the previous 4 weeks; receipt of any blood products in the previous 3 months; donation of blood/plasma in the previous 28 days; a temperature of ≥100.4°F at screening; or any other medical and/or social reason which, in the opinion of the investigators, would have increased the subject’s risk of an adverse reaction during the study.
The schedule of study visits, designed to minimize the trial’s impact on the Infantry training schedule, is presented in Table 1. Following provision of written informed consent, participants underwent a baseline evaluation to confirm eligibility and to collect demographic information, medical history, and any medications taken in the previous 30 days. Participants completed a written questionnaire on SSTI risk factors, as well as the frequency of personal hygiene practices (e.g., hand washing and use of hand sanitizer). A sample of whole blood (up to 55 mL) and saliva (Oracol S14 Plus; Malvern Medical Devices, United Kingdom) was collected for baseline immune status. Lastly, nasal and throat swabs were collected to assess baseline S. aureus colonization status.
Table 1.
Description of Study Activities and Timeline for a Phase 2 S. aureus Vaccine Trial among US Army Infantry Trainees at Fort Benning, Georgia
| Study Day / Visit Number | ||||||||
|---|---|---|---|---|---|---|---|---|
| −3 | 0 | 14 | 28 | 56 | 90 | 180 (e) | SSTI | |
| 1 | 2 | 3 | 4 | 5 | 6 | N/A | N/A | |
| Study Activity | ||||||||
| Recruitment and enrollment | • | |||||||
| Assessment of subject eligibility | • | |||||||
| Obtain medical history and current medications | • | |||||||
| Perform directed physical examination | • | • | ||||||
| Verification of subject eligibility | • | |||||||
| Obtain vital signs (pre- and post-vaccination) | • | |||||||
| Randomization followed by administration of study vaccine | • | |||||||
| Administer risk factor and personal hygiene survey | • | • | • | • | • | • | ||
| Record medications | • | • | • | • | • | • | • | |
| Collect pre-vaccination blood for safety laboratory assays | • | |||||||
| Collect post-vaccination blood for safety laboratory assays | • | |||||||
| Record solicited adverse events | • | |||||||
| Collect memory aid, if applicable | • | |||||||
| Record unsolicited adverse events | • | • | • | |||||
| Record AESIs and SAEs | • | • | • | • | • | • | ||
| Obtain interval medical history | • | • | • | • | • | • | ||
| Immunology Endpoints | ||||||||
| Collect serum for antibody assays | • | • | • | • | • | • | ||
| Collect whole blood for T-cell assays | • | • | ||||||
| Collect saliva for antibody assays | • | • | • | |||||
| Microbiology Endpoints | ||||||||
| Collect nasal and throat swabs | • | • | • | • | • | • | ||
| Collect wound swab | • | |||||||
SSTI: skin and soft tissue infection; N/A: not applicable; AESI: adverse event of special interest; SAE: serious adverse event
Administration of the study vaccine occurred 48–72 hours after enrollment. Study investigators reviewed and verified subject eligibility criteria and confirmed the subject’s willingness to participate. Pre-vaccination vital signs were documented, and a 7-mL blood sample was obtained for clinical safety laboratory assessment. If the subject had received a tetanus toxoid vaccine on the same day, the study vaccine was administered in the contralateral arm unless contraindicated. Subjects were observed for at least 60 minutes post-vaccination. An assessment of the injection site was conducted, and subjects were asked about the occurrence of any adverse events (AE). Post-vaccination vital signs were documented. To monitor AEs in the post-vaccination period, subjects also received a 14-day memory aid and were instructed on its use.
Approximately 14 days post-vaccination, participants were questioned about any AEs they had experienced. Participants completed a risk factor and hygiene questionnaire and submitted the following specimens: nasal and throat swabs; saliva samples; and whole blood. These procedures were repeated at both the day 28 and day 56 post-vaccination visits, with the exception of saliva collection, which occurred only on the day 56 visit. The final in-person study visit occurred approximately 90 days post-vaccination (~7 days prior to the completion of the training cycle). Nasal and throat swabs and whole blood samples were obtained, and participants were again questioned about AE, risk factors, and personal hygiene practices.
Approximately 180 days post-vaccination, participants were contacted via phone or email to collection information on any AE, as well as any associated medication(s) that the subject may have received since their last study visit.
Participants who developed SSTIs during training were asked to complete a risk factor and personal hygiene questionnaire. If applicable (purulent SSTI only), a study coordinator collected a research culture swab from the infection site. At the conclusion of the trial, study coordinators reviewed the electronic medical records of study participants to capture information on any medically-attended SSTI that were not previously identified.
Safety was described as the proportion (95% confidence interval [CI]) of participants who had any solicited AE. Differences between groups were calculated, including two-sided p values, with Fisher’s exact test.
Nasal and throat swabs were evaluated for the presence of S. aureus by streaking on agar plates. Methicillin-resistant S. aureus (MRSA) was identified by detection of colonies on mannitol salt agar (MSA) CHROMagar™ (CHROMagar, Paris, France) MRSA. Methicillin-sensitive S. aureus was identified by detection of colonies on MSA plates with beta-hemolysis and no colonies on CHROMagar™ MRSA plate.
Plasma and peripheral blood mononuclear cells (PBMCs) were isolated from blood samples, as previously described [14]. The ELISAs for measurement of serum anti-Als3 immunoglobulin G (IgG) and immunoglobulin A1 (IgA1) and ELISpot assays to measure the proportion of PBMCs that showed Als3-specific production of interferon-γ (IFN-γ) and IL-17A were performed as previously described [14].
The Immunogenicity Subgroup for the study was designed to include 10% of the planned number of study participants (in a 2:1 ratio from NDV-3A and placebo groups) and included subjects from all four vaccination cohorts selected evenly and at random from a listing of subjects with samples from all immunogenicity time points available for testing. Serum and saliva samples were analyzed for anti-Als3 IgG and IgA1 [14]. The geometric mean titer (GMT) of antibodies to Als3 prior to and after dosing of NDV-3A or placebo (along with 95% CI) were calculated for each study group. Fold rise in antibody titer at post-vaccination time point(s) relative to baseline were calculated for each individual and seroconversion rate (i.e., proportion of subjects with >4-fold rise) for serum IgG was calculated for each study group. Saliva samples were collected at day 56 but were not processed for this immunogenicity evaluation.
The trial was designed with 80% power to detect a difference in the efficacy of NDV-3A and placebo in preventing incident S. aureus nasal acquisition 56 days post-vaccination, with α (1-sided) of 0.025, assuming: 65% of participants were not nasally colonized with S. aureus at baseline; 50% rate of nasal acquisition in placebo recipients; VE of 50%; and a 10% loss to follow-up. This design called for 210 participants each in the NDV-3A and placebo groups for a total of 420 participants.
The primary efficacy outcome was the incidence of S. aureus nasal colonization by day 56 post-vaccination among participants who were colonization negative at baseline. The proportion of participants in each group was summarized with point estimates and their 95% Clopper Pearson CI. Expressed as a percentage, VE was defined as 1 minus the relative rate of S. aureus acquisition in the NDV-3A group compared with that in the placebo group. Time to S. aureus nasal acquisition was evaluated using interval-censored Kaplan-Meier analysis, and VE was calculated as 1 minus the Kaplan-Meier estimated risk ratio. For the time-to-event analysis, for subjects who dropped out of the study before Visit 5 (day 56±7), the study endpoint was right censored at the subject’s last visit. The VE estimates for oral and nasal/oral acquisition were calculated using the same method. The nasal/oral endpoint was defined as a subject who had a positive S. aureus culture on either the nasal or the oral swab for a given timepoint.
Statistical analyses were conducted with SAS version 9.4 (SAS, Cary, NC).
RESULTS
In total, 809 soldiers from four Infantry training companies were briefed on the trial. Of these, 401 (49.6%) consented to participate (Figure 1). Nineteen (4.7%) trainees were withdrawn prior to vaccination (one had a clinically significant SSTI at the screening visit, and the remaining 18 voluntarily withdrew). A total of 382 trainees were vaccinated, but an administrative error in the handling of two study vials precluded an identification of the study group for two participants. The data from these two participants were excluded from the analysis.
Figure 1.
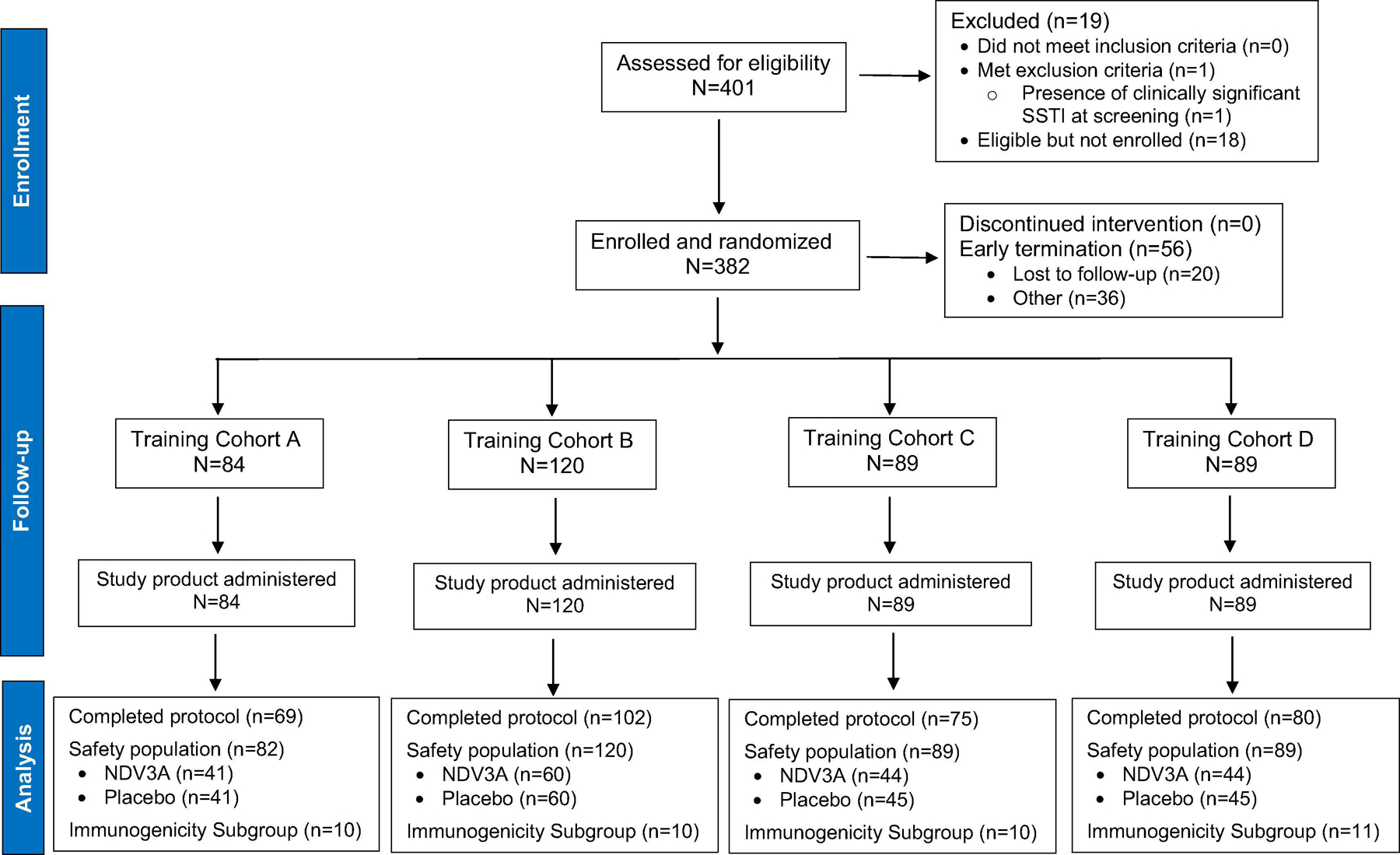
CONSORT Diagram for a S. aureus Vaccine Trial at Fort Benning
Of the 380 participants, 189 were randomized to receive NDV-3A and 191 were randomized to receive placebo (Table 2). By training company, participants were distributed as follows: Company A (n=84 [2 subjects excluded]), Company B (n=120), Company C (n=89), and Company D (n=89). All participants were male with a median (range) age of 20 (17–35) years. Approximately 76% of the study participants were White and 10% were Black. The reported frequency of baseline SSTI risk factors was low. The demographic and epidemiologic characteristics of trial participants did not differ between study groups.
Table 2.
Demographic and Epidemiologic Characteristics of the Study Population (NDV-3A vs. Placebo).
| Subject Characteristic | NDV-3A (N = 189)a | Placebo (N = 191)a | Distribution bv Training Companv | |||
|---|---|---|---|---|---|---|
| Cohort A (N = 82)a | Cohort B (N = 120) | Cohort C (C = 89) | Cohort D (N = 89) | |||
| Median (range) age, years | 20 (17–35) | 20 (17–34) | 20 (17–32) | 20 (18–31) | 19 (18–35) | 20 (18–34) |
| Ethnicity, Number (%) | ||||||
| Not Hispanic or Latino | 152 (80.42) | 146 (76.44) | 64 (78.05) | 93 (77.50) | 70 (78.65) | 71 (79.78) |
| Hispanic or Latino | 37 (19.58) | 45 (23.56) | 18 (21.95) | 27 (22.50) | 19 (21.35) | 18 (20.22) |
| Race, Number (%) | ||||||
| American Indian or Alaska Native | 0 (0) | 1 (0.52) | 0 (0) | 0 (0) | 0 (0) | 1 (1.12) |
| Asian | 4 (2.12) | 3 (1.57) | 0 (0) | 1 (0.83) | 3 (3.37) | 3 (3.37) |
| Native Hawaiian or Other Pacific Islander | 2 (1.06) | 1 (0.52) | 1 (1.22) | 1 (0.83) | 0 (0) | 1 (1.12) |
| Black or African American | 19 (10.05) | 19 (9.95) | 8 (9.76) | 8 (6.67) | 8 (8.99) | 14 (15.73) |
| White | 145 (76.72) | 144 (75.39) | 60 (73.17) | 99 (82.50) | 68 (76.40) | 62 (69.66) |
| Multi-Racial | 8 (4.23) | 8 (4.19) | 5 (6.10) | 4 (3.33) | 3 (3.37) | 4 (4.49) |
| Unknown | 11 (5.82) | 15 (7.85) | 8 (9.76) | 7 (5.83) | 7 (7.87) | 4 (4.49) |
| Known/suspected skin infection or MRSA infection in past 12 monthsb | 1 (0.54) | 1 (0.53) | 0 (0) | 0 (0) | 2 (2.25) | 0 (0) |
| Contact with a person with known /suspected skin infection or MRSA infection in past 3 monthsb | 1 (0.54) | 3 (1.59) | 0 (0) | 1 (0.87) | 3 (3.37) | 0 (0) |
| Antibiotic use in the past 6 monthsb | 7 (3.78) | 8 (4.26) | 4 (4.88) | 7 (6.09) | 2 (2.25) | 2 (2.30) |
Two subjects were excluded from all analyses due to treatment vial mix-up, resulting in that NDV-3A versus Placebo administration could not be determined for the two subjects who were vaccinated on the same date around the same time.
Among those who responded to these particular questions (185 from the NDV-3A group, 188 from the placebo group).
The distribution of subjects experiencing solicited AEs is presented in Supplemental Table 1. Of the solicited systemic symptoms, headache and arthralgia were most commonly reported (5.3% each) in the NDV-3A group, followed by feverishness (4.8%), fatigue and myalgias (4.2% each). Except for arthralgias, which were significantly higher in the NDV-3A (5.3%) than placebo (1.0%) recipients, none of the individually solicited systemic events were significantly different between study groups. The frequency of systemic symptoms among those randomized to receive NDV-3A was low and mild in nature (Supplemental Figure 1). Of those NDV-3A recipients reporting local symptoms, 16.9% and 2.6% reported pain of mild or moderate severity, respectively (Supplemental Figure 2).
The data on the 41 participants (27 NDV-3A, 14 placebo) included in the immunogenicity subgroup analysis are presented in Table 3. The post-vaccination GMT (95% CI) of anti-Als3 serum IgG among NDV-3A recipients was 63,397 (33,686–119,313), with 100% of recipients achieving ≥4-fold rise in antibody concentration. Serum IgG titers among NDV-3A recipients subsequently declined, albeit to levels indicative of a very robust antibody response (Figure 2). At day 90, the GMT was 11,774 (4,890–28,349), with a ≥4-fold rise evident in 89% of NDV-3A recipients.
Table 3.
Immunogenicity of NDV-3A – Baseline and Day 14 IgG and IgA1 GMT, NDV-3A vs. Placebo; Day 14 GMFR, NDV-3A vs. Placebo.
| Time Point and Immunology Endpoint | NDV-3A, n = 27 | Placebo, n = 14 |
|---|---|---|
| Visit 1 (pre-vaccination) | ||
| GMT (95% CI) | ||
| Als3-IgG | 374 (221–633) | 682 (251–1,858) |
| Als3-IgA1 | 3,892 (1,852–8,178) | 19,724 (7,074–54,998) |
| Visit 3 (Day 14) | ||
| GMT (95% CI) | ||
| Als3-IgG | 63,397 (33,686–119,313) | 666 (213–2,084) |
| Als3-IgA1 | 110,541 (60,541–201,833) | 17,044 (5,358–54,223) |
| GMFR (95% CI)a | ||
| Als3-IgG | 169.7 (80.5–357.7) | 1.0 (0.7–1.3) |
| Als3-IgA1 | 28.4 (12.0–67.5) | 0.9 (0.6–1.3) |
| Seroconversion %, ≥4-Fold Rise (95% CI)b Als3-IgG | 100 (87.2–100) | 7.1 (0.2–33.9) |
| Visit 4 (Day 28) | ||
| Als3-IgG GMT (95% CI) | 37,601 (19,423–72,791) | 700 (238–2,060) |
| Als3-IgG GMFR (95% CI)a | 100.6 (44.7–226.4) | 1.0 (0.8–1.4) |
| Als3-IgG Seroconversion %, ≥4-Fold Rise (95% CI)b | 96.3 (81.0–99.9) | 0 (0–23.2) |
| Visit 5 (Day 56) | ||
| Als3-IgG GMT (95% CI) | 28,184 (15,537–51,125) | 766 (256–2,292) |
| Als3-IgG GMFR (95% CI)a | 75.4 (36.1–157.4) | 1.1 (0.7–1.8) |
| Als3-IgG Seroconversion %, ≥4-Fold Rise (95% CI)b | 92.6 (75.7–99.1) | 7.1 (0.2–33.9) |
| Visit 6 (Day 90–115) | ||
| Als3-IgG GMT (95% CI) | 11,774 (4,890–28,349) | 402 (122–1,325) |
| Als3IgG GMFR (95% CI)a | 31.5 (11.1–89.8) | 0.6 (0.2–2.0) |
| Als3IgG Seroconversion %, ≥4-Fold Rise (95% CI)b | 88.9 (70.8–97.6) | 7.1 (0.2–33.9) |
| Peak Titer c | ||
| Als3-IgG GMT (95% CI) | 65,051 (34,474–122,746) | 882 (290–2,685) |
| Als3-IgG GMFR (95% CI)a | 174.1 (82.5–367.2) | 1.3 (0.8–2.1) |
| Als3-IgG Seroconversion %, ≥4-Fold Rise (95% CI)b | 100 (87.2–100) | 14.3 (1.8–42.8) |
CI – confidence interval; GMFR – geometric mean fold rise; GMT – geometric mean titer.
GMFR represents geometric mean fold rise in antibody compared to the pre-vaccination study Visit 1.
Seroconversion % represents the percentage of subjects with at least a 4-Fold Rise in antibody titer compared to pre-vaccination (Visit 1).
Peak titer summarizes the maximum post-baseline result for each subject.
Figure 2.
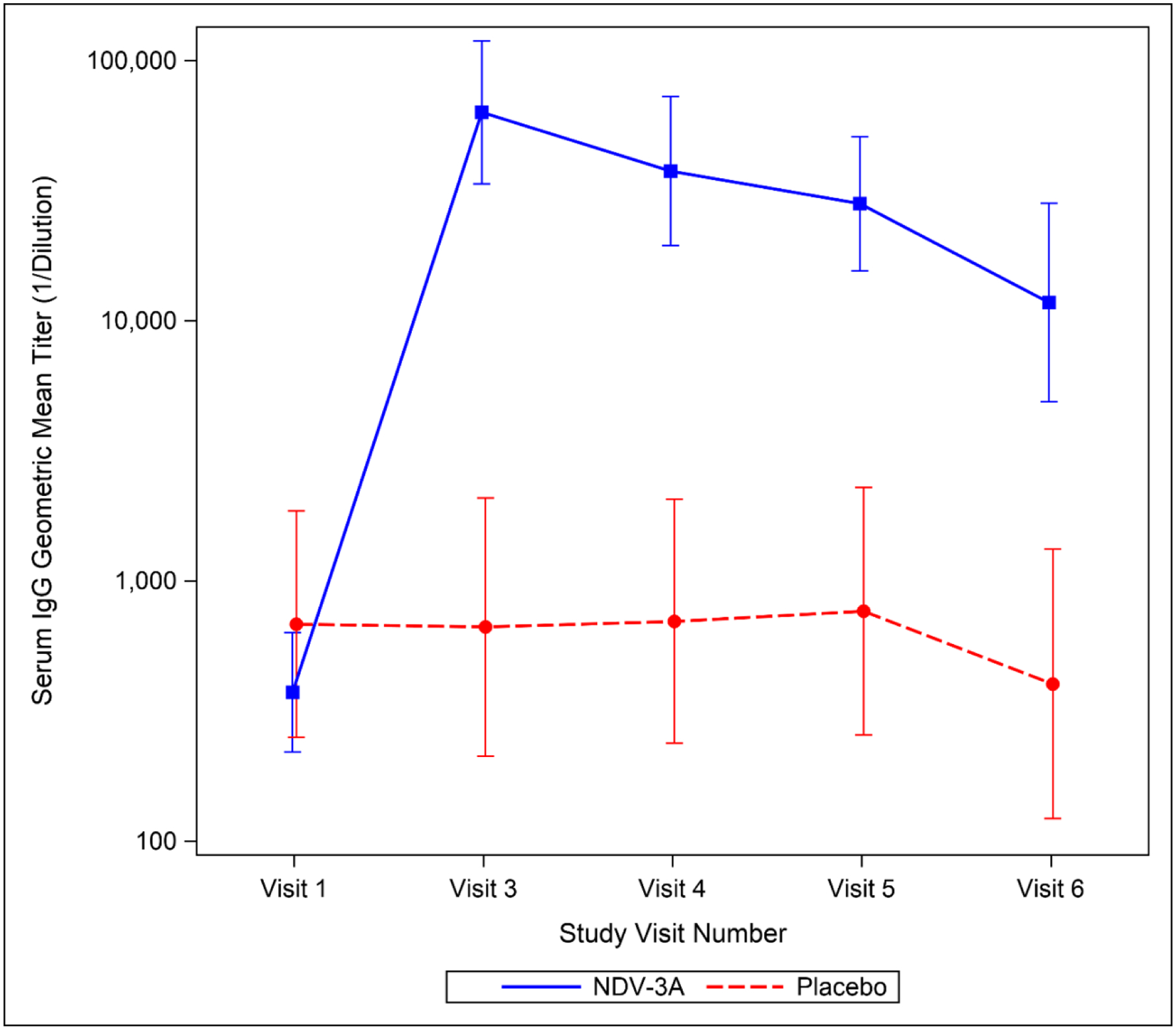
Time Trend Plot of Serum IgG GMT by Time Point and Study Group. Point estimates and 95% CIs are included for the subjects in each group (NDV-3A, n=27; placebo, n=14).
The data on salivary IgG and IgA1 responses are presented in Supplemental Table 2. The post-vaccination GMT (95% CI) of anti-Als3 IgG in saliva was significantly higher among recipients of NDV-3A as compared to controls, 55 (28–108) vs. 9 (5–15), respectively. This represented a geometric mean fold rise (GMFR; 95% CI) of 8.1 (4.0–16.3) in vaccinees as compared to 1.0 (0.7–1.4) in controls. Among NDV-3A recipients, post-vaccination GMTs of anti-Als3-IgA1 in saliva were considerably higher among those who were nasally colonized with S. aureus at the baseline visit vs. those who were not (2,833 vs. 268, respectively; Supplementary Table 2b). The post-vaccination GMT (95% CI) of anti-Als3 IgA1 among vaccinees was 494 (169–1,446) as compared to 291 (49–1,733) among controls, but the between-group differences in GMFR of salivary IgA1 were not significantly different (NDV-3A: 4.6 [1.8–12.3] vs. placebo: 2.9 [0.3–31.8]).Sixty-three percent of vaccinees had a positive IFN-γ response (i.e., difference of 20 spot forming units [SFU] per 106 PMBCs between day 1 and day 14) compared to 21% of controls. The average difference in IFN-γ between day 1 and day 14 was 124.2 and 47.5 SFU per 106 PBMC for the NDV-3A and placebo recipients, respectively. For IL-17A, 44% of NDV-3A recipients had a positive response compared to 14% of controls with an average difference from day 1 to day 14 of 89.7 and 55.3 SFU per 106 PBMC, respectively.
The number and percent of trial participants who were colonized at baseline is presented in Supplemental Table 3. The proportion of NDV-3A vs. placebo recipients who were colonized with S. aureus on the nasal, oral, and nasal/oral sites prior to vaccination did not significantly differ between study groups (nasal: 33.3% vs. 31.9%; oral: 49.2% vs. 47.6%; nasal/oral: 59.8% vs. 58.6%, respectively). These individuals were excluded from the analysis of S. aureus acquisition in the post-vaccination period along with five participants (2 NDV-3A, 3 placebo) who withdrew from military training prior to contributing efficacy data.
Among those individuals not colonized at baseline, the rates of nasal, oral, and nasal/oral acquisition of S. aureus by day 56 are presented in Table 4. Among 125 NDV-3A and 127 placebo recipients, 32 (25.6%) and 37 (29.1%) nasally acquired S. aureus, respectively. Rates of oral (29.2% vs. 29.9%) and nasal/oral (39.5% vs. 42.1%) acquisition of S. aureus did not differ between study groups. Similarly, there were no between-group differences in acquisition rates nor colonization prevalence at the day 28 or day 90 visits (Figure 3). Among participants who were colonized at baseline, the prevalence of S. aureus nasal, oral, and nasal/oral colonization at post-vaccination visits did not differ between NDV-3A vs. placebo recipients (Supplemental Figure 3).
Table 4.
Number and Proportions of Subjects with Positive S. aureus Nasal (a), Oral (b), and Nasal/Oral (c) Cultures by Visit Number and Treatment Arm Among S. aureus Nasal, Oral, and Nasal/Oral Colonization Negative Subjects at Screening
| Study Visit | No. of Incident S. aureus Colonization within Interval (No. at risk)a | Interval Proportion (95% CI)b | Cumulative No. of S. aureus Colonization up to the Study Visit | Cumulative Proportion (95% CI)b | No. of Incident S. aureus Colonization within Interval (No. at risk)a | Interval Proportion (95% CI)b | Cumulative No. of S. aureus Colonization up to the Study Visit | Cumulative Proportion (95% CI)b |
|---|---|---|---|---|---|---|---|---|
| Subjects with Positive S. aureus Nasal Cultures | ||||||||
| NDV-3A Vaccine (N=125) c | Placebo (N=127) c | |||||||
| Visit 3 | 19 (125) | 0.15 (0.09–0.23) |
19 | 0.15 (0.09–0.23) |
17 (125) | 0.14 (0.08–0.21) |
17 | 0.14 (0.08–0.21) |
| Visit 4 | 6 (99) | 0.06 (0.02–0.13) |
25 | 0.20 (0.13–0.28) |
9 (106) | 0.08 (0.04–0.16) |
26 | 0.20 (0.14–0.29) |
| Visit 5 | 7 (93) | 0.08 (0.03–0.15) |
32 | 0.26 (0.18–0.34) |
11 (92) | 0.12 (0.06–0.20) |
37 | 0.29 (0.21–0.38) |
| Visit 6 | 4 (78) | 0.05 (0.01–0.13) |
36 | 0.29 (0.21–0.38) |
4 (77) | 0.05 (0.01–0.13) |
41 | 0.32 (0.24–0.41) |
| Subjects with Positive S. aureus Oral Cultures | ||||||||
| NDV-3A Vaccine (N=96) c | Placebo (N=97) c | |||||||
| Visit 3 | 15 (96) | 0.16 (0.09–0.25) |
15 | 0.16 (0.09–0.25) |
15 (95) | 0.16 (0.09–0.25) |
15 | 0.15 (0.09–0.24) |
| Visit 4 | 11 (77) | 0.14 (0.07–0.24) |
26 | 0.27 (0.19–0.37) |
7 (81) | 0.09 (0.04–0.17) |
22 | 0.23 (0.15–0.32) |
| Visit 5 | 2 (64) | 0.03 (0.004–0.11) |
28 | 0.29 (0.20–0.39) |
7 (72) | 0.10 (0.04–0.19) |
29 | 0.30 (0.21–0.40) |
| Visit 6 | 3 (60) | 0.05 (0.01–0.14) |
31 | 0.32 (0.23–0.43) |
4 (65) | 0.06 (0.02–0.15) |
33 | 0.34 (0.25–0.44) |
| Subjects with Positive S. aureus Nasal/Oral Cultures | ||||||||
| NDV-3A Vaccine (N=76) c | Placebo (N=76) c | |||||||
| Visit 3 | 18 (76) | 0.24 (0.15–0.35) |
18 | 0.24 (0.15–0.35) |
15 (76) | 0.20 (0.12–0.31) |
15 | 0.20 (0.12–0.31) |
| Visit 4 | 9 (55) | 0.16 (0.08–0.29) |
27 | 0.36 (0.25–0.47) |
8 (60) | 0.13 (0.06–0.25) |
23 | 0.30 (0.20–0.42) |
| Visit 5 | 3 (45) | 0.07 (0.01–0.18) |
30 | 0.39 (0.28–0.51) |
9 (51) | 0.18 (0.08–0.31) |
32 | 0.42 (0.31–0.54) |
| Visit 6 | 3 (40) | 0.08 (0.02–0.20) |
33 | 0.43 (0.32–0.55) |
3 (42) | 0.07 (0.02–0.20) |
35 | 0.46 (0.35–0.58) |
Number still at risk (S. aureus negative) at the beginning of the interval (subjects who do not have data available at a given visit are not included in the number at risk for that visit)
Clopper-Pearson 95% confidence interval
Number of subjects in the full analysis population with negative S. aureus nasal colonization at screening
Figure 3.
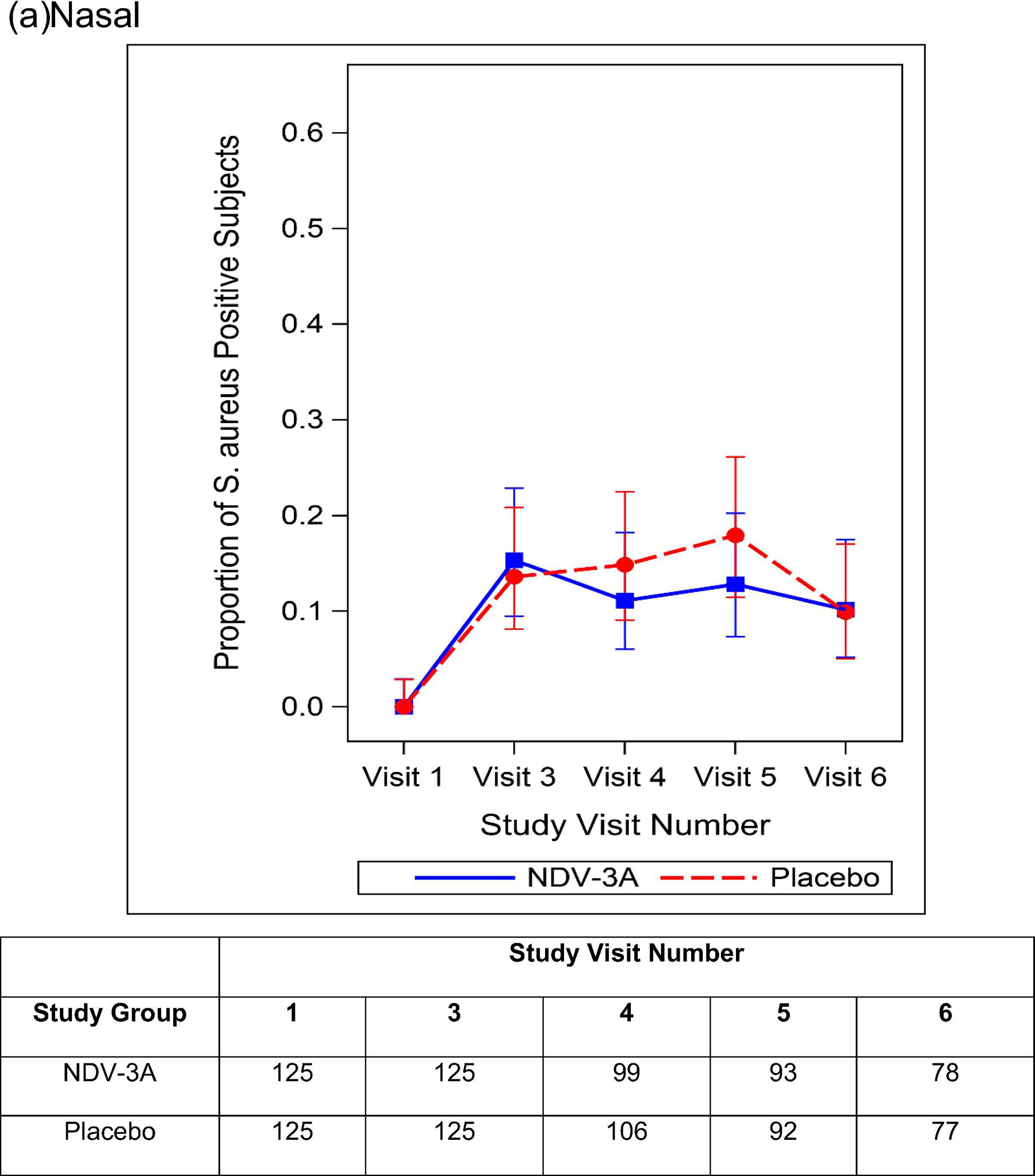
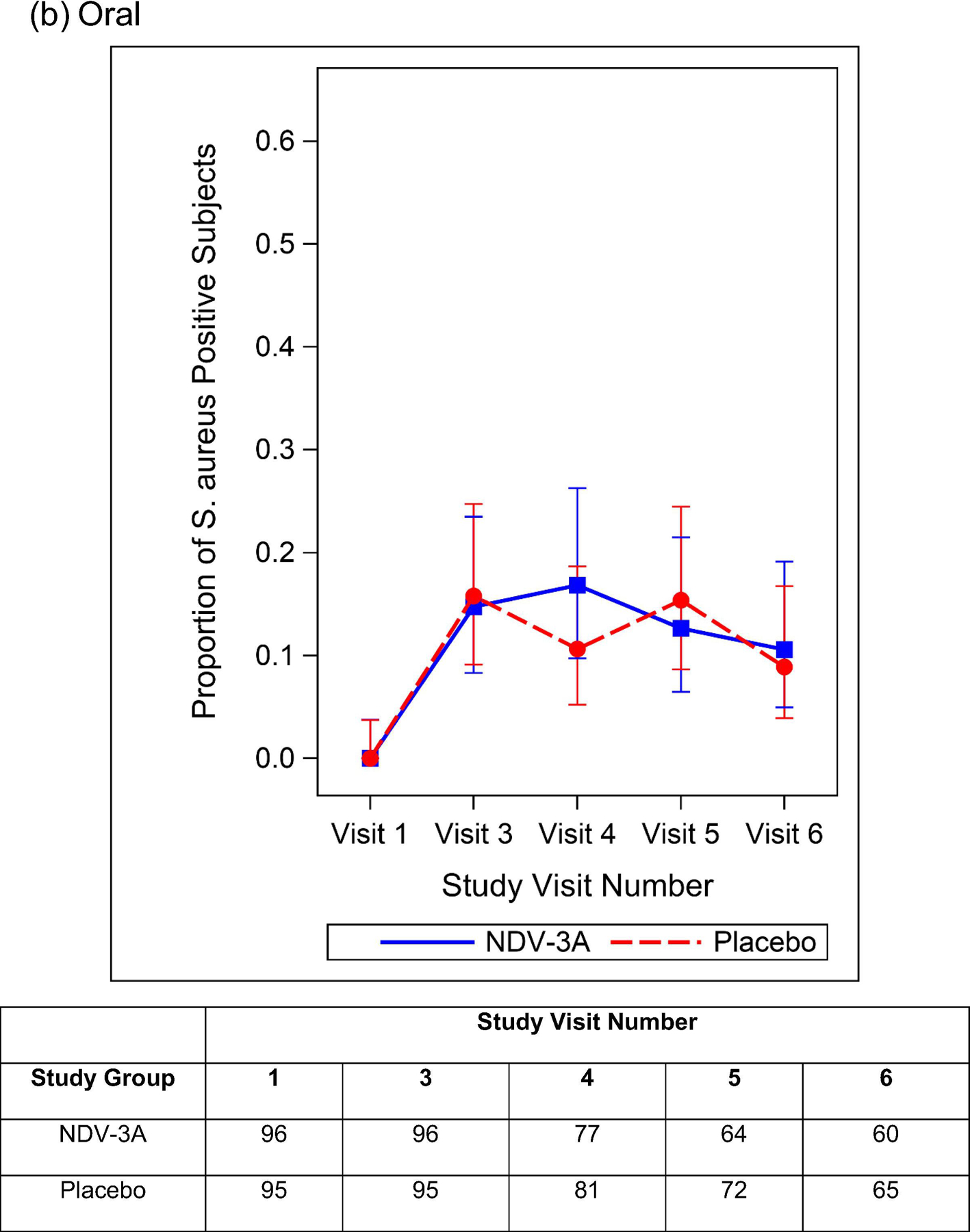
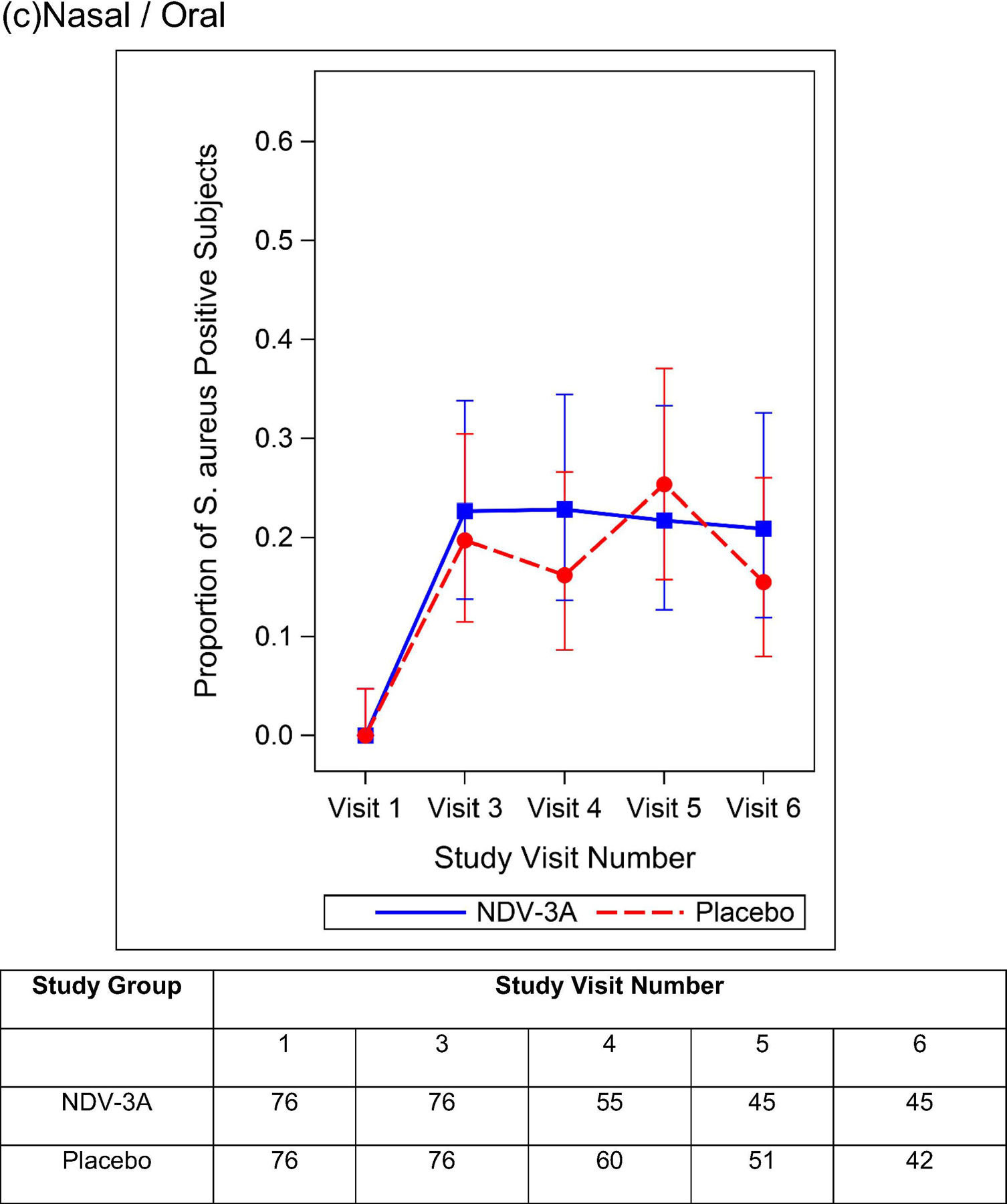
Proportion and 95% Clopper Pearson Confidence Interval of S. aureus Positive Participants By Treatment Group and Study Visit Number -- Baseline S. aureus Nasal (a), Oral (b), and Nasal/Oral (c) Colonization Negative Subjects. The number of participants included in each analysis timepoint is included in the table.
Estimates of NDV-3A VE against nasal, oral, and nasal/oral acquisition of S. aureus are presented in Table 5. In summary, a single dose of NDV-3A was not efficacious for the prevention of nasal, oral, and nasal/oral acquisition of S. aureus (VEnasal [95% CI]: 12.1% [-31.6%, 41.3%], p=0.31; VEoral: 2.4% [-50.9%, 36.9%], p=0.52; VEnasal/oral: 6.2% [-37.6%, 36.1%], p=0.43). In time-to-event analysis, there was no difference between study groups with respect to the S. aureus nasal colonization-free interval (VEnasal: 13.0% [-29.1%, 41.4%], p=0.29; VEoral: 1.1% [-52.0%, 35.7%], p=0. 51; VEnasal/oral: 5.4% [-38.0%, 35.1%], p=0.47).
Table 5.
Vaccine Efficacy Against S. aureus Colonization Detected by Positive Nasal, Oral, and Nasal/Oral Culture by 56 Days Post Vaccination – Baseline S. aureus Nasal/Oral Colonization Negative Subjects
| Endpoint at 56 days post-vaccination | Vaccine Efficacya (95% CI) | p-valueb |
|---|---|---|
| Positive nasal culture | 12.1% (−31.6%, 41.3%) | 0.31 |
| Positive oral culture | 2.4% (−50.9%, 36.9%) | 0.52 |
| Positive nasal/oral culture | 6.2% (−37.6%, 36.1%) | 0.43 |
CI: confidence interval
Vaccine efficacy calculated using unadjusted relative risk ratio (VE: 1 – Relative Risk)
p value from Fisher’s exact 1-tailed test
A total of 13 participants (6 NDV-3A, 7 placebo) developed a SSTI while enrolled in the study. Impetigo (n=6) was the most common diagnosis, followed by folliculitis (n=2), cellulitis (n=2), abscess (n=1), furuncle (n=1), and pilonidal cyst with an abscess (n=1).
DISCUSSION
Prior to this study, no S. aureus vaccine candidate had ever been evaluated in military trainees, a group in whom the increased risk of S. aureus colonization and SSTI has been well-documented [21–23]. We found that a single dose of NDV-3A, administered 7–10 days prior to the initiation of a 14-week military training cycle, showed no efficacy against incident nasal/oral acquisition of S. aureus, no efficacy in delaying the nasal/oral acquisition of S. aureus, and no efficacy in the termination of existing S. aureus nasal/oral colonization. This was observed despite evidence that NDV-3A was highly immunogenic in this population of young adult males, in terms of the anamnestic response to a single dose of vaccine, the high seroconversion rate and the frequency and intensity of T cell responses. These responses were comparable to what was observed in previous trials of NDV-3 [14, 15]. The NDV-3A candidate elicited very high titers of anti-Als3 IgG in both serum and saliva, the latter of particular interest for S. aureus vaccine development because the nasal/oral cavity is a reservoir for S. aureus and nasal colonization is a risk factor for infection. Nevertheless, receipt of NDV-3A did not impact rates of S. aureus acquisition or colonization in this military training population.
NDV-3A was initially developed as vaccine for Candida infection [11], but the structural homology between Als3 and the S. aureus protein ClfA accelerated its development as a vaccine for Staphylococcal infection [9]. The ClfA belongs to a family of bacterial surface proteins known as microbial surface components recognizing adhesive matrix molecules (MSCRAMM) that mediate bacterial adhesion to host cells [24]. Specifically, ClfA binds human fibrinogen in the initial stages of S. aureus infection [25, 26]. The anti-ClfA serum antibodies have been shown to inhibit the binding of S. aureus to human fibrinogen in vitro [27]. However, the ability of ClfA-containing vaccines to prevent S. aureus colonization has yet to be demonstrated in humans; in a phase 1 trial of a three-antigen S. aureus vaccine (SA3Ag) containing recombinant ClfA, no impact on S. aureus nasal / oropharyngeal colonization was observed [28].
The incorporation of a Candida protein in a vaccine to prevent Staphylococcal colonization and infection represents a novel strategy in the field of vaccinology: the principle of ‘convergent immunity’, whereby an epitope from one organism is used to induce protective immunity against another [7]. C. albicans and S. aureus inhabit the same epidermal / mucosal space in the human host and have, thus, evolved similar mechanisms of colonization and pathogenesis. The N-terminal domain of Als3 is structurally similar to that of corresponding regions of S. aureus MSCRAMMs, including ClfA, despite their low sequence similarity (19%) [7]. Serum from individuals vaccinated with recombinant Als3 demonstrated opsonophagocytic activity against S. aureus in vitro [29]. In murine models, vaccination with NDV-3A protected against S. aureus invasive infection, as well as SSTIs [4, 16, 30]. In a subset of participants enrolled in a phase 1 trial of NDV-3, the prevalence of S. aureus nasal colonization was lower among those randomized to receive NDV-3A vs. placebo, but since the trial was not designed nor adequately powered to evaluate the colonization outcome, the difference did not reach statistical significance (J. P. Hennessey Jr., unpublished data).
To systematically evaluate the efficacy of NDV-3A against S. aureus colonization, a military training population was preferentially selected, given the unique epidemiologic characteristics of the training environment (e.g., closed cohorts, shared housing, and highly regimented schedule) and the well-documented, increased risk of S. aureus colonization and infection among military trainees [22, 31]. The reasons that NDV-3A did not prevent the acquisition of S. aureus, despite achieving high titers of anti-Als3 antibody, are not known. The vaccine was administered <72 hours after the arrival of trainees on base, and 7–10 days prior to the initiation of the actual training cycle. It is possible that a single dose of NDV-3A is insufficient for protection against S. aureus colonization and that a second dose would be needed. However, the early and intense exposure of trainees to S. aureus during the first 2–3 weeks of the training cycle poses a considerable challenge to the utility of a two-dose schedule. It is possible that the levels of anti-Als3 IgG in saliva were suboptimal for the prevention of S. aureus acquisition. It is also conceivable that anti-Als3 IgG in saliva do not possess the affinity or the functional characteristics against S. aureus that were observed in vitro. Lastly, it is possible that ClfA has no role in the colonization of the nasal epithelium by S. aureus, and therefore, that anti-Als3/anti-ClfA antibodies, though present, afforded no protection against acquisition of S. aureus.
There are limitations to this study. First, while the trial was conducted in a military training population known to be at increased risk for S. aureus colonization and infection, the design of the study needed strike a balance between what was scientifically sound and what was logistically feasible. For example, obtaining weekly swabs may have yielded more granular data on the dynamics of S. aureus colonization in this population. However, this would have created additional disruptions to the highly regimented military training schedule that may have compromised the training command’s overall support of the trial. Second, with respect to the primary endpoint, we chose culture-based determination of S. aureus colonization instead of a quantitative PCR assay. It is possible that the utilization of molecular-based assays may have revealed important differences between study groups. Third, the relative infrequency of sampling for S. aureus colonization precluded a classification of participants who were intermittently as opposed to persistently colonized.
As efforts toward the development of other S. aureus vaccines continue, lessons from this trial and trials in other high-risk populations must be carefully considered. First, single-antigen vaccines are unlikely to be effective for prevention of S. aureus infection or colonization [32]. Second, a vaccine for the prevention of S. aureus SSTIs will likely differ greatly from a vaccine for the prevention of S. aureus invasive disease [33]. Third, vaccines that prevent S. aureus colonization may, in turn, prevent disease by combatting a major risk factor for infection. To this end, data from a murine model showed that immunization with S. aureus clumping factor B (ClfB), a major determinant in nasal carriage, effectively reduced colonization [34]. Finally, the pursuit of vaccine-based strategies for the prevention of S. aureus infection among military trainees remains critically important, given the disproportionate risk of disease in these populations and the demonstrated ineffectiveness of hygiene-based control measures [21]. All authors attest they meet the ICMJE criteria for authorship
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the US Army Training and Doctrine Command, the 30th Adjutant General Reception Battalion, and the 198th Infantry Battalion at Fort Benning, GA, for their support of this vaccine trial, and most especially, to the US Army Infantrymen who agreed to participate in the study in spite of their demanding training schedule. We are grateful for the IDCRP site managers, clinical research coordinators, and laboratory personnel whose tireless dedication contributed to the successful execution of the trial. We also extend our gratitude to the investigative team members from the Uniformed Services University, Fort Belvoir Community Hospital, Walter Reed Army Institute of Research, and the Benning Martin Army Community Hospital for their assistance in the execution of study activities, and to Leigh Carson for editorial assistance with the manuscript.
FINANCIAL SUPPORT
This work (IDCRP-104) was conducted by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USU) through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. This project was supported by an award from the US Army Medical Materiel Development Activity (USAMMDA; award PMB.16.232/16–0725-ISA to JWB). This project was supported with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-Al-5072 and from the Defense Health Program, U.S. Department of Defense, under award HU0001190002. Funders were not involved in the design or execution of the study; the collection, analysis, or interpretation of data; the writing of the manuscript; or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
T.C., M.T.C., J.P.H., and M.M.S. are employees and shareholders of NovaDigm Therapeutics, Inc. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
T.C., M.T.C., J.P.H., and M.M.S. are employees and shareholders of NovaDigm Therapeutics, Inc.
All other authors report no potential conflicts of interest.
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions or policies of Uniformed Services University of the Health Sciences (USU), Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the National Institute of Health or the Department of Health and Human Services, the Department of Defense (DoD), the Departments of the Army, Navy, or Air Force, or the U.S. Government. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
REFERENCES
- 1.Fowler VG, Jr., Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect 2014;20 Suppl 5:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, et al. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013;56:1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolata JB, Kuhbandner I, Link C, Normann N, Vu CH, Steil L, et al. The Fall of a Dogma? Unexpected High T-Cell Memory Response to Staphylococcus aureus in Humans. J Infect Dis 2015;212:830–8. [DOI] [PubMed] [Google Scholar]
- 4.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 2009;5:e1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 2009;206:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broker BM, Mrochen D, Peton V. The T Cell Response to Staphylococcus aureus. Pathogens 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeaman MR, Filler SG, Schmidt CS, Ibrahim AS, Edwards JE, Jr., Hennessey JP, Jr. Applying Convergent Immunity to Innovative Vaccines Targeting Staphylococcus aureus. Front Immunol 2014;5:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 2007;5:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, et al. Functional and structural diversity in the Als protein family of Candida albicans. J Biol Chem 2004;279:30480–9. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim AS, Luo G, Gebremariam T, Lee H, Schmidt CS, Hennessey JP Jr., et al. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine 2013;31:5549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, et al. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis 2006;194:256–60. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim AS, Spellberg BJ, Avanesian V, Fu Y, Edwards JE, Jr. The anti-Candida vaccine based on the recombinant N-terminal domain of Als1p is broadly active against disseminated candidiasis. Infect Immun 2006;74:3039–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spellberg BJ, Ibrahim AS, Avenissian V, Filler SG, Myers CL, Fu Y, et al. The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect Immun 2005;73:6191–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, et al. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012;30:7594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards JE Jr., Schwartz MM, Schmidt CS, Sobel JD, Nyirjesy P, Schodel F, et al. A Fungal Immunotherapeutic Vaccine (NDV-3A) for Treatment of Recurrent Vulvovaginal Candidiasis-A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis 2018;66:1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeaman MR, Filler SG, Chaili S, Barr K, Wang H, Kupferwasser D, et al. Mechanisms of NDV-3 vaccine efficacy in MRSA skin versus invasive infection. Proc Natl Acad Sci U S A 2014;111:E5555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 2010;120:1762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroder JM. The role of keratinocytes in defense against infection. Curr Opin Infect Dis 2010;23:106–10. [DOI] [PubMed] [Google Scholar]
- 19.Millar EV, Chen WJ, Schlett CD, Cui T, Crawford KB, Lanier JB, et al. Frequent use of chlorhexidine-based body wash associated with a reduction in methicillin-resistant Staphylococcus aureus nasal colonization among military trainees. Antimicrob Agents Chemother 2015;59:943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millar EV, Rice GK, Schlett CD, Elassal EM, Cer RZ, Frey KG, et al. Genomic epidemiology of MRSA infection and colonization isolates among military trainees with skin and soft tissue infection. Infection 2019. [DOI] [PubMed] [Google Scholar]
- 21.Ellis MW, Schlett CD, Millar EV, Wilkins KJ, Crawford KB, Morrison-Rodriguez SM, et al. Hygiene strategies to prevent methicillin-resistant Staphylococcus aureus skin and soft tissue infections: a cluster-randomized controlled trial among high-risk military trainees. Clin Infect Dis 2014;58:1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millar EV, Rice GK, Elassal EM, Schlett CD, Bennett JW, Redden CL, et al. Genomic Characterization of USA300 Methicillin-Resistant Staphylococcus aureus (MRSA) to Evaluate Intraclass Transmission and Recurrence of Skin and Soft Tissue Infection (SSTI) Among High-Risk Military Trainees. Clin Infect Dis 2017;65:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millar EV, Rice GK, Schlett CD, Elassal EM, Cer RZ, Frey KG, et al. Genomic epidemiology of MRSA infection and colonization isolates among military trainees with skin and soft tissue infection. Infection 2019;47:729–37. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien L, Kerrigan SW, Kaw G, Hogan M, Penadés J, Litt D, et al. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol Microbiol 2002;44:1033–44. [DOI] [PubMed] [Google Scholar]
- 25.Geoghegan JA, Ganesh VK, Smeds E, Liang X, Hook M, Foster TJ. Molecular characterization of the interaction of staphylococcal microbial surface components recognizing adhesive matrix molecules (MSCRAMM) ClfA and Fbl with fibrinogen. J Biol Chem 2010;285:6208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Josefsson E, Higgins J, Foster TJ, Tarkowski A. Fibrinogen binding sites P336 and Y338 of clumping factor A are crucial for Staphylococcus aureus virulence. PLoS One 2008;3:e2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Wang X, Thompson CD, Park S, Park WB, Lee JC. Preclinical Efficacy of Clumping Factor A in Prevention of Staphylococcus aureus Infection. MBio 2016;7:e02232–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall HS, Baber J, Richmond P, Nissen M, Shakib S, Kreiswirth BN, et al. S. aureus colonization in healthy Australian adults receiving an investigational S. aureus 3-antigen vaccine. J Infect 2019;79:582–92. [DOI] [PubMed] [Google Scholar]
- 29.Luo G, Hennessey J, Schmidt C, Fu Y, Yeaman M, Filler S. Human vaccination with rAls3p-N produces antibodies that enhance phagocyte-mediated killing of C. albicans and S. aureus and mitigate Als3 functions. 18th Congress of the International Society for Human and Animal Mycology; Berlin2012. [Google Scholar]
- 30.Lin L, Ibrahim AS, Baquir B, Avanesian V, Fu Y, Spellberg B. Immunological surrogate marker of rAls3p-N vaccine-induced protection against Staphylococcus aureus. FEMS Immunol Med Microbiol 2009;55:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis MW, Schlett CD, Millar EV, Crawford KB, Cui T, Lanier JB, et al. Prevalence of nasal colonization and strain concordance in patients with community-associated Staphylococcus aureus skin and soft-tissue infections. Infect Control Hosp Epidemiol 2014;35:1251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A 2006;103:16942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luna BM, Nielsen TB, Cheng B, Pantapalangkoor P, Yan J, Boyle-Vavra S, et al. Vaccines targeting Staphylococcus aureus skin and bloodstream infections require different composition. PLoS One 2019;14:e0217439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, Risley A, et al. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect Immun 2006;74:2145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


