Abstract
The nonstructural NS2 proteins of autonomous parvoviruses are known to act in a host cell-dependent manner and to play a role in viral DNA replication, efficient translation of viral mRNA, and/or encapsidation. Their exact function during the parvovirus life cycle remains, however, still obscure. We report here the characterization of the interaction with the NS2 proteins from the parvovirus minute virus of mice (MVM) and rat as well as mouse homologues of the human CRM1 protein, a member of the importin-beta family recently identified as an essential nuclear export factor. Using the two-hybrid system, we could detect the interaction between the carboxy-terminal region of rat CRM1 and each of the three isoforms of NS2 (P [or major], Y [or minor], and L [or rare]). NS2 proteins were further shown to interact with the full-length CRM1 by coimmunoprecipitation experiments using extracts from both mouse and rat cell lines. Our data show that CRM1 preferentially binds to the nonphosphorylated isoforms of NS2. Moreover, we observed that the treatment of MVM-infected cells with leptomycin B, a drug that specifically inhibits the CRM1-dependent nuclear export pathway, leads to a drastic accumulation of NS2 proteins in the nucleus. Both NS2 interaction with CRM1 and nuclear accumulation upon leptomycin B treatment strongly suggest that these nonstructural viral proteins are actively exported out of the nuclei of infected cells via a CRM1-mediated nuclear export pathway.
Minute virus of mice (MVM) is an autonomously replicating parvovirus that depends on host-cell factors expressed during S phase to complete its life cycle. This requirement results in the restriction of productive MVM infection to proliferating tissues and may contribute to the oncotropism displayed by this virus (16, 51). The genome of MVM consists of a linear, single-stranded, negative-sense DNA molecule of approximately 5,000 nucleotides (nt) with nonidentical palindromic hairpin ends and contains two overlapping transcription units (16). The right-hand part of the genome encodes the capsid proteins VP1 and VP2 under the control of the P38 promoter. The left-hand part of the genome is driven by the P4 promoter and encodes two types of nonstructural proteins, NS1 and NS2, which are implicated in various steps of parvovirus growth. The 83-kDa NS1 phosphoprotein accumulates in the nuclei of infected cells and is involved in viral DNA replication and modulation of viral and cellular promoters (18, 62). NS1 displays various biochemical activities which are required for viral genome amplification, such as ATP binding and ATPase activity, covalent and noncovalent DNA binding, and helicase- and site-specific endonuclease activity (18). NS1 also activates in trans transcription from both its own P4 promoter (22) and the P38 promoter, which drives expression of the capsid genes (23, 40), and may act in the modulation of the cellular environment (2, 47, 62). Indeed, NS1 has been shown to be the major effector of parvovirus-induced cytotoxicity, with NS2 enhancing cell killing in some but not all cell lines tested (7, 11, 37, 41).
Unlike NS1, the role of the nonstructural NS2 proteins during the parvovirus life cycle is not yet clearly understood. NS2 proteins from the murine virus MVM consist of three isoforms that differ at their carboxy termini as a result of alternative splicing events (17). They have a molecular mass in the range of 25 kDa, and all three isoforms share a common amino-terminal domain with NS1, which comprises the first 85 N-terminal amino acids (aa) (16). NS2 polypeptides exist in phosphorylated and nonphosphorylated forms, which are mainly located in the cytoplasm; however, nonphosphorylated NS2 can also be detected in the nuclei of infected cells (13, 17). NS2 are the predominant virus-encoded proteins detected early in S phase of infected cells, but their accumulation rapidly diminishes as the proteins exhibit a relatively short half-life (about 1 h), and the activity of the P4 promoter declines later in infection (14, 17, 54).
Although their mode of action is not known, NS2 proteins from MVM were shown to be absolutely required for productive infection in cells from their natural host species both in tissue cultures and in animals (10, 12, 42, 43). Indeed, NS2 mutants of MVM, which encode truncated or no NS2 polypeptides, have been reported to exhibit multiple defects in DNA replication as well as in the translation and assembly of capsid proteins (12, 15, 42, 43). These defects led to a drastic reduction of the production of progeny NS2 mutant virions in mouse cells, while the production of these viruses was much less affected in nonmurine cells. A similar host species-specific defect in viral DNA replication has also been described for NS2 mutants of the closely related rat parvovirus H-1 (38). In this case, the expression of all viral proteins was strongly reduced, and experiments with reporter gene constructs suggested that a sequence present in the 3′ untranslated region of all viral mRNAs might render them susceptible to translational modulation by NS2 (39). In contrast, the NS2 proteins from canine parvovirus appeared to act in a host-independent manner (66).
Due to the small size of their genomes, which encode only a limited number of proteins, parvoviruses heavily depend on cellular helper functions for their life cycle (16). Furthermore, the viral regulatory products are multifunctional proteins showing a variety of activities. It can then be speculated that the NS proteins need to interact with specific cellular products in order to achieve their functions. Indeed, we previously showed that NS2 proteins from MVM interact with at least two members of the 14-3-3 protein family (9). However, the function of this protein complex remains to be elucidated. In order to better understand the role of NS2 during parvovirus replication, we looked for other interacting partners, by using the two-hybrid system (25). The present study shows that the chromosome region maintenance protein CRM1, a nuclear export factor (29, 30, 44, 48, 57), interacts with all three isoforms of NS2 and is probably involved in an active export of the viral proteins NS2 out of the nuclei of infected cells.
MATERIALS AND METHODS
Bacterial and yeast strains.
The following bacterial strains were used: Escherichia coli Sure (Stratagene) for amplification of the rat cDNA library, HB101 for recovery of the activation domain plasmids, and JM109 for subcloning steps. All bacterial cultures were grown in Luria-Bertani medium supplemented with appropriate antibiotics.
The following Saccharomyces cerevisiae strains were used: HF7c (Mata ura3-52 his3-200 lys2-801 ade2-101 trp1-901 leu2-3,112 gal4-542 gal80-538 LYS2::GAL1-HIS3 URA3::(GAL4 17 mers)3-CYC1-lacZ) and SFY526 (Mata ura3-52 his3-200 lys2-801 ade2-101 trp1-901 leu2-3,112 canr gal4-542 gal80-538 URA3::GAL1-lacZ). Both yeast strains were grown at 30°C in 2% Bacto Tryptone–1% Bacto yeast extract–2% glucose (pH 5.8) (YPD complete medium) or in 0.67% Bacto yeast nitrogen base–2% glucose (pH 5.8) (SD minimal medium) containing the appropriate amino acid supplements.
Viruses and mammalian cell lines.
The FREJ4 cell line (64), a derivative of the established FR3T3 Fisher rat cell line (55) transformed by the c-Ha-ras oncogene, was grown in Dulbecco’s modified Eagle’s medium supplemented with 1 mM sodium pyruvate and 10% donor calf serum. Mouse A9 fibroblasts (59) were grown in Eagle’s minimum essential medium supplemented with 1% nonessential amino acids and 5% fetal bovine serum.
MVM was propagated in A9 cells, titrated, and purified as described by Tattersall and Bratton (59). Mutant NS2-T149A virus was produced in A9 cells upon transfection with the infectious clone DB-D1-4-914 (6), by using the classical CaPO4 coprecipitation technique (63), and further titrated and purified as previously described (59).
Construction of yeast expression vectors.
Vectors pGBT9 (Clontech), which harbors the yeast selectable gene TRP1 as well as the GAL4 DNA-binding domain (BD) (aa 1 to 149) coding sequence, and pGAD424 (Clontech), which contains the yeast selectable gene LEU2 and the sequence encoding the GAL4 activation domain (AD) (aa 768 to 881), were both used for the expression of fusion proteins. Recombinant vectors were constructed and amplified according to standard procedures (52).
(i) pGBT9NS2P.
The NS2 major isoform (NS2P) coding region from MVM was isolated as a NcoI-BamHI fragment from the plasmid pTM1-NS2p (kindly provided by J. Nüesch and P. Tattersall, Yale University, New Haven, Conn.) and inserted, in frame with the GAL4-BD sequence, into pGBT9 with the help of an adapter encompassing an NcoI restriction site and generated through the annealing of oligonucleotides 5′ AATTCACCATGGTTAAC 3′ and 5′ AATTGTTAACCATGGTG 3′.
(ii) pGBT9NS2Y and pGBT9NS2L.
The NS2 minor (NS2Y) and rare (NS2L) isoform coding regions were isolated from the plasmids pTM1-NS2y (a generous gift of J. Nüesch and P. Tattersall, Yale University) and pET-NS2l, respectively, and inserted into the vector pGBT9 as described for the pGBT9NS2P construction.
(iii) pET-NS2l.
The NS2 major isoform (NS2P) coding region from MVM was isolated as a NcoI-BamHI fragment from pTM1-NS2p and subsequently cloned into the plasmid pET-16b (Novagen) in order to generate the pET-NS2p construct. A XhoI-BamHI subfragment, which contains nt 2072 to 2280 and nt 2377 to 2635 from the MVM sequence, was then excised from pET-NS2p and replaced by a PCR-produced XhoI-BamHI fragment, which contains MVM nt 2072 to 2280 and nt 2399 to 2447, to give rise to the construct pET-NS2l. This change allowed the replacement of the MVM sequence encoding the carboxy terminus of the NS2P isoform with the one corresponding to the NS2L isoform. The region encompassing the inserted PCR product was further sequenced to ensure that no PCR-derived mutations were present.
(iv) FREJ4 cDNA library containing pGADNot.
The generation of FREJ4 cDNAs and their insertion, in fusion with the GAL4-AD sequence, into pGADNot, a NotI-containing pGAD424 vector, to create a FREJ4-cDNA plasmid library, was previously described (19).
Yeast two-hybrid screen.
Yeast transformations were performed essentially as previously described (5, 19), with recombinant pGBT9 and pGAD424 shuttle vectors. Both plasmids harbor the yeast 2μm and the bacterial ColE1 origins of replication. The expression of GAL4-BD and GAL4-AD fusion proteins are driven by the constitutively active yeast ADH1 promoter.
The yeast strain HF7c, containing the HIS3 and lacZ reporter genes under the control of two independent promoters, was transformed with the bait plasmid pGBT9NS2P by using a lithium acetate protocol (31). The resulting strain was selected and grown in Trp-deficient SD medium. A 2-liter culture of pGBT9NS2P-expressing HF7c cells in growing phase (optical density at 600 nm of 0.6) was transformed with 20 μg of plasmid DNA from the FREJ4 cDNA library. Double transformants were grown on plates containing SD medium lacking Trp and Leu, to select for the presence of both the bait and the library plasmids, and deprived of His, to select for protein-protein interactions. Positive clones were selected a second time on a Trp− Leu− His− medium, and those that were still able to grow were subsequently assayed for β-galactosidase activity.
β-Galactosidase assay.
The β-galactosidase activity of histidine-positive clones was tested on filters and in vivo with the yeast strains HF7c and SFY526, respectively, which both contain a lacZ reporter gene but under the control of different GAL4 promoters.
β-Galactosidase filter assays were performed according to a standard protocol (8). Briefly, positive clones were streaked onto Whatman filters which were then incubated overnight at 30°C on Trp− Leu− selective plates. Filters, which were rapidly frozen in liquid nitrogen, were laid down onto a second Whatman filter previously wetted in buffer Z (100 mM NaPO4 [pH 7.0], 10 mM KCl, 1 mM MgSO4) supplemented with 5 mM β-mercaptoethanol and 335 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) per ml. Positive clones developed a blue color after 15 to 60 min of incubation.
In vivo β-galactosidase assays were performed as follows. Fresh transformants were streaked onto Trp− Leu− selective dishes, supplemented with a final concentration of 100 mM NaPO4 [pH 7.0] and 40 μg of X-Gal per ml, and grown for 48 h at 30°C. When expressing the lacZ reporter gene, yeast cells developed a blue color.
DNA sequencing.
Sequencing of the crm1 cDNA insert from the plasmid pGADcrm1 was performed on both strands by the 4base lab company (Reutlingen, Germany). Sequences were compared with data bank entries, by using the HUSAR (Heidelberg Unix Sequence Analysis Resources) system (56).
Antibodies.
The polyclonal rabbit antibodies SP8 and SP6 are directed against carboxy-terminal peptides of MVM proteins NS1 (NS1C) and NS2 (NS2C), respectively. The generation of these antibodies was previously described (9, 24). The monoclonal mouse antibody 3D9, directed against the amino acid sequence encoded by nt 1110 to 1638 of MVM DNA (17), is specific for NS1 and was kindly provided by D. Pintel (University of Missouri—Columbia). The antibody anti-hCRM1, directed against the human crm1 gene product (28), was kindly provided by M. Fornerod (European Molecular Biology Laboratory, Heidelberg, Germany).
Cell labeling and protein immunoprecipitation.
The labeling of mock- or MVM-infected cells, protein extraction, and immunoprecipitation were performed as previously described (9). Briefly, subconfluent cell cultures were mock- or MVM-infected at a multiplicity of infection (MOI) of 10 PFU per cell. At 18 h postinfection, cells were metabolically labeled for 2 h with 200 μCi of Tran35S label (1,000 Ci/mmol; ICN Pharmaceuticals) in Met- and Cys-free Eagle’s minimum essential medium supplemented with 5% dialyzed fetal calf serum. Cells were subsequently lysed with either the so-called Raf buffer (20 mM Tris [pH 8.0], 137 mM NaCl, 10% glycerol, 1% Nonidet P-40) or radioimmunoprecipitation assay buffer (10 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]), both containing a mixture of proteinase inhibitors, and proteins were harvested after a clear spin. Equal amounts (107 cpm) of labeled protein extracts were immunoprecipitated with 2 to 5 μl of either polyclonal rabbit antiserum, directed against MVM NS1, NS2, or human CRM1 proteins, and immunocomplexes were analyzed by electrophoresis on SDS-polyacrylamide gels.
Cell synchronization and leptomycin B treatment.
Highly synchronized A9 cells were prepared by an isoleucine-aphidicolin double-block protocol, as previously described (14, 16). Briefly, cells were grown on coverslips in an isoleucine-free medium for 48 h at 37°C, in a 5% CO2 atmosphere, followed by 10 h of incubation in a complete cell culture medium containing aphidicolin at a 12-μg/ml final concentration. Synchronized cells were either mock treated or infected with wild-type MVM at an MOI of 10 PFU/cell in the presence of aphidicolin and subsequently incubated for 10 h in an aphidicolin-containing medium (37°C with 5% CO2). Cells were then released from the aphidicolin treatment and further incubated in a complete medium for either 10 or 12 h. When needed, leptomycin B was added to the cultures at a final concentration of 10 nM for either 3 h (starting from 7 h postrelease) or 12 h (for the duration of release). Leptomycin B was kindly provided to us by B. Wolff-Winiski (Novartis, Vienna, Austria).
Immunofluorescence.
Highly synchronized A9 cells, which were grown and infected on coverslips, were fixed in 1% formaldehyde for 10 min and permeabilized with cold methanol for 5 min, cold acetone for 2 min, and 1% saponin for 15 min. Cells were washed first with 0.2% Tween, then with 2 mM MgCl2, and further preincubated with 1% goat serum. Cells were then successively incubated at room temperature for 1 h each with the polyclonal rabbit serum SP6 (1:700 dilution) or the monoclonal mouse antibody 3D9 (1:20 dilution), as primary antibodies, and fluorescein isothiocyanate-conjugated goat anti-rabbit (1:150 dilution) or rhodamine-conjugated goat anti-mouse (1:200 dilution) secondary antibodies, respectively, followed by three phosphate-buffered saline washes. All solutions and dilutions were prepared in phosphate-buffered saline. Coverslips were then quickly treated with DAPI (4′,6-diamidino-2-phenylindole), dried with ethanol, and mounted on glass slides in the presence of polyvinyl alcohol (Elvanol; Serva, Heidelberg, Germany). Labeled cells were examined with a Leica microscope, at a ×63 magnification with an oil immersion objective, and photographed with ASA400 Ektachrome films.
Nucleotide sequence accession number.
The nucleotide sequence of the cDNA encoding the C-terminal domain of the rat crm1 gene product has been deposited in the GenBank database under accession no. AJ238278.
RESULTS
Identification of a potential interacting partner for NS2P by the two-hybrid system.
To identify new cellular partners of NS2, we performed a two-hybrid screen by using as a bait the major isoform of NS2 (NS2P) fused to the BD of GAL4. We have chosen to screen this bait against a cDNA library that was derived from the ras-transformed rat cell line FREJ4 (64) and that was already successfully used to detect a parvovirus protein interacting partner (19). Yeast transformation with this library allowed the expression of rat cDNA-encoded proteins fused to the AD of GAL4.
Both plasmids were introduced into the HF7c yeast strain, and 3.5 × 106 double transformants were plated on a medium lacking the amino acids tryptophan and leucine, to select for the presence of both bait-expressing and cDNA library-containing plasmids, and lacking histidine to select for interactions between the bait NS2P and rat cDNA library-encoded proteins. From about 2,000 histidine-positive clones, 6 were also positive when assayed for β-galactosidase activity on filters. Plasmid DNA from these positive clones was extracted, amplified after transformation of E. coli HB101, and reintroduced together with the BD-NS2P coding plasmid or the empty pGBT9 vector into HF7c and SFY526 yeast strains. Both strains contain the lacZ reporter gene, however, under the control of different GAL4-responsive promoters. Transformed HF7c and SFY526 yeast cells were then assayed for β-galactosidase activity either on filters or after streaking onto X-Gal-containing plates. After this second selection, five of the six isolated clones were discarded because their encoded prey either did not activate reporter gene expression anymore in the presence of NS2 or induced the reporter gene in an NS2-independent fashion. Only one clone, called X6, was positive in both yeast strains in the presence of the bait and negative when using the empty vector that only expresses the GAL4-BD. Figure 1A shows the activation of the β-galactosidase reporter gene in the SFY526 strain when both NS2P and cDNA-X6 were coexpressed, which led to a blue staining of growing yeast cells. Under conditions in which no NS2-X6 interaction could take place (coexpression of either BD and AD, BD and AD-cDNA-X6, or BD-NS2P and AD) the reporter gene was not induced, yielding white yeast colonies. The latter control indicated that the viral protein NS2P was not able on its own to activate the promoter which drives the expression of the reporter gene, even when NS2 is targeted to this promoter through its fusion with the GAL4-BD. Taken together, these results showed that the isolated cDNA X6 encodes a polypeptide interacting with NS2P, but not with the GAL4-BD alone. It should also be stated that the reconstitution of the GAL4 transactivator activity was demonstrated through its effect on two distinct GAL4-responsive promoters (directing the expression of two different reporter genes in the HF7c yeast strain). This promoter independence further demonstrated that activation was mediated through a protein-protein interaction, in which NS2P was specifically involved.
FIG. 1.
All three NS2 isoforms interact with the C-terminal part of rat CRM1 in the two-hybrid system. The specific interaction of MVM NS2P (A and B), NS2Y (B), or NS2L (B) isoform with the C-terminal domain of the rat crm1 gene product was revealed by means of β-galactosidase assays. SFY526 yeast cells expressing the indicated bait and prey pairs were streaked onto Trp− Leu− selective dishes containing X-Gal and grown for 48 h at 30°C. A blue or a white color indicates a positive or negative interaction, respectively.
The cDNA product interacting with NS2 corresponds to the C-terminal part of the CRM1 protein.
The nucleotide sequence of the FREJ4 cDNA from clone X6, isolated through the two-hybrid screen, was determined. X6 contains a 2.1-kb insert showing a typical polyadenylation signal (AATTAAA) at nt 1982 and a poly(A) tail from nt 2002 (Fig. 2). When translated into an amino acid sequence, the cDNA X6 was predicted to encode a 625-aa polypeptide fused in frame with the GAL4-AD (Fig. 2). Protein data bank searches with the BLAST algorithm from the HUSAR system (56) revealed that the isolated cDNA encodes the C-terminal part of a rat homologue of the human and yeast CRM1 proteins (Fig. 3). A comparison of the predicted rat CRM1 carboxy terminus and the corresponding amino acid sequences of the crm1 gene products from other species showed a strong level of homology (up to 97 and 51% identity with human and yeast CRM1 proteins, respectively) (Fig. 3 and Table 1). This indicates that the crm1 gene products are highly conserved throughout evolution, at least in their carboxy-terminal regions.
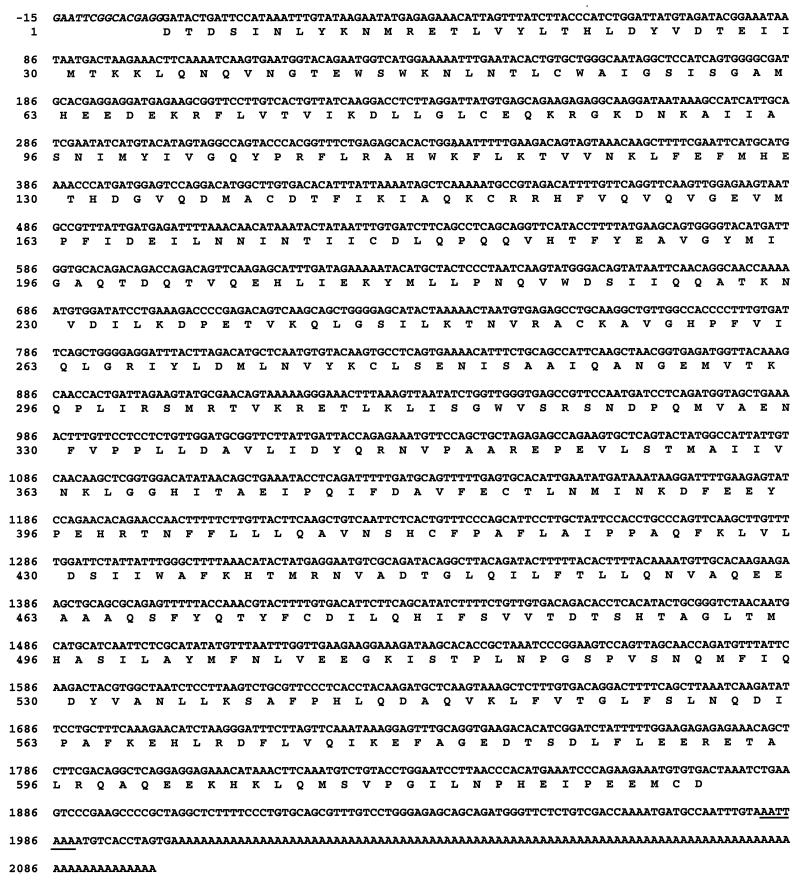
FIG. 2.
Nucleotide sequence of the cDNA encoding the C-terminal domain of the rat crm1 gene product and, below it, the deduced amino acid sequence given in single-letter code. Nucleotides in italics (numbered −15 to −1) indicate the end of the sequence encoding the Gal4-activating domain, at its site of fusion with the isolated cDNA. The polyadenylation site present at the end of the cDNA sequence is underlined. This sequence has received the GenBank accession no. AJ238278.
FIG. 3.
Carboxy-terminal domains of CRM1 proteins are conserved throughout evolution. The alignment of the translated rat CRM1 C-terminal sequence (r) with those from humans (h), fission yeast (f), and budding yeast (b) is shown. White letters represent identical amino acids between the rat sequence and the sequence of at least one other species.
TABLE 1.
Percentage of amino acid homology between the rat CRM1 C-terminal sequence and the CRM1 C-terminal sequences from other various species
| CRM1 | Amino acid homology (%) with rat CRM1
|
|
|---|---|---|
| Identitya | Similarityb | |
| Human | 97 | 98 |
| C. elegansc | 59 | 78 |
| S. pombe | 57 | 73 |
| S. cerevisiae | 51 | 74 |
Percentage of identical amino acids between the rat sequence and CRM1 protein sequences from other species.
Percentage of conserved amino acids between the rat sequence and CRM1 protein sequences from other species.
Caenorhabditis elegans.
The C-terminal part of rat CRM1 interacts with all three NS2 isoforms in the two-hybrid system.
To determine whether, like the major MVM NS2P isoform, the minor NS2Y and rare NS2L isoforms can also interact with rat CRM1 protein, we coexpressed BD fusions with NS2P, NS2Y, and NS2L as baits, together with the AD-cDNA X6 prey (encoding the C-terminal part of rat CRM1) in SFY526 yeast cells. As shown in Fig. 1B, each of these three combinations gave rise to blue colonies on X-Gal-containing plates, whereas the coexpression of each of the BD-NS2 baits with a prey consisting of the sole GAL4-AD failed to activate lacZ reporter gene expression and led to the formation of white colonies. Similar results were obtained with the HF7c yeast strain (data not shown). We concluded from these results that while being unable to activate the reporter gene promoter on their own, all three NS2 isoforms can form a complex with the rat CRM1 carboxy terminus. Since yeast cells turned blue with equivalent color intensities and after similar time intervals, irrespective of the NS2 isoform used as a bait, no gross differences in the relative affinities of the different NS2 isoforms for the CRM1 product were revealed in the present assay.
The full-length CRM1 and NS2 proteins are coimmunoprecipitated from MVM-infected rat cell extracts.
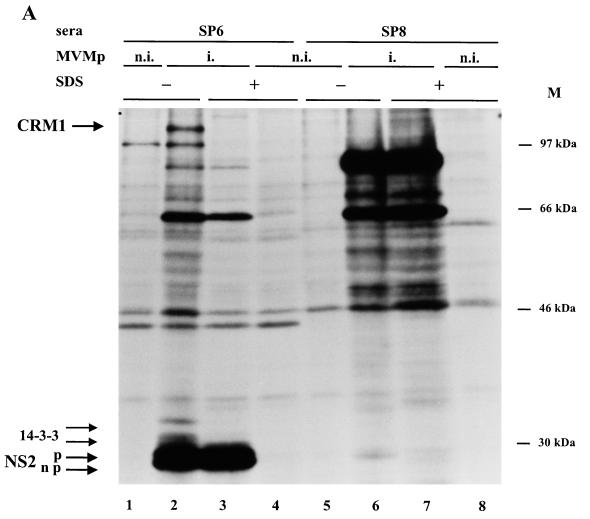
To determine whether NS2 proteins are able to interact with the full-length CRM1 protein in rat cells, coimmunoprecipitation reactions were performed by using lysates of MVM- and mock-infected FREJ4 cells metabolically labeled with [35S]methionine-cysteine and the SP6 and SP8 antibodies directed against the carboxy-terminal domains of NS2 and NS1 proteins, respectively. When cell extracts were prepared in the absence of SDS, the anti-NS2 serum SP6 precipitated not only the viral NS2 and associated cellular 14-3-3 proteins, as previously described (9), but also a product with an apparent molecular mass of about 110 kDa (Fig. 4A and B, lanes 2), a size which is in the molecular mass range reported for CRM1 proteins of various species (1, 28, 60). The precipitation of the 110-kDa protein was specific for conditions allowing the formation of NS2 immunocomplexes, since this product was not observed in cell lysates prepared in the presence of SDS (Fig. 4A, lane 3, and 4B, lane 1). In addition, no 110-kDa product was recovered either when treating noninfected cell extracts with SP6 (Fig. 4A, lanes 1 and 4, and 4B, lane 3) or when using SP8 antibodies that recognize the viral NS1 protein (Fig. 4A, lanes 5 to 8).
FIG. 4.
Specific coimmunoprecipitation of NS2 and full-length CRM1 proteins from MVM-infected FREJ4 cell extracts. Lysates of 35S-labeled FREJ4 cells, infected (i.) or not infected (n.i.) with MVM at an MOI of 10 PFU per cell, were prepared in the presence (+) or absence (−) of SDS and immunoprecipitated with antisera directed against either the NS2 C terminus (SP6) (A and B), the NS1 C terminus (SP8) (A), or the hCRM1 C terminus (anti-hCRM1) (B). The autoradiograms show immunoprecipitated materials after separation by electrophoresis through 10% (A) or bipartite 6 to 10% (B) SDS-polyacrylamide gels. Arrows to the left point at phosphorylated (p) and nonphosphorylated (np) NS2 (25 kDa), 14-3-3 (30 and 32 kDa), NS1 (83 kDa), and CRM1 (110 kDa) proteins. M, molecular sizes (in kilodaltons) of prestained protein standards.
To determine whether the 110-kDa product coprecipitating with NS2 indeed corresponds to CRM1, coimmunoprecipitation experiments were performed with antibodies directed against the human CRM1 protein (hCRM1) (28). As illustrated in Fig. 4B, the anti-hCRM1 antibodies precipitated both the 110-kDa product and a protein comigrating with the faster-migrating form of NS2. The latter protein is likely to correspond to a subpopulation of NS2, since it was not detected when noninfected cell extracts were used (data not shown). From these results and knowing that the 110-kDa and NS2 products were the only detectable infection-specific proteins shared by the anti-NS2 and anti-CRM1 immunoprecipitates, we concluded that the 110-kDa product is able to interact with some but not all forms of NS2 in rat cells. Furthermore, the 110-kDa protein coprecipitating with NS2-specific antibodies (Fig. 4B, lane 2) reacted with anti-hCRM1 antibodies in an immunoprecipitation-Western blot analysis (data not shown). Given the specificity of the anti-CRM1 antibodies used (28) and the reported molecular mass of the CRM1 proteins around 110 kDa (1, 28, 60), these data pointed to full-length rat CRM1 as an interacting partner of NS2. These results, however, do not allow us to determine whether NS2 binds to CRM1 in a direct or indirect manner. It is also worth noting that the members of the 14-3-3 protein family, which were previously shown to interact with NS2 (9) (Fig. 4A and B, lanes 2), were not coimmunoprecipitated with the anti-hCRM1 antibodies (Fig. 4B, lane 4), suggesting that the NS2 molecules interacting with 14-3-3 proteins do not associate with CRM1 at the same time.
Interaction between CRM1 and NS2 proteins also occurs in mouse cells.
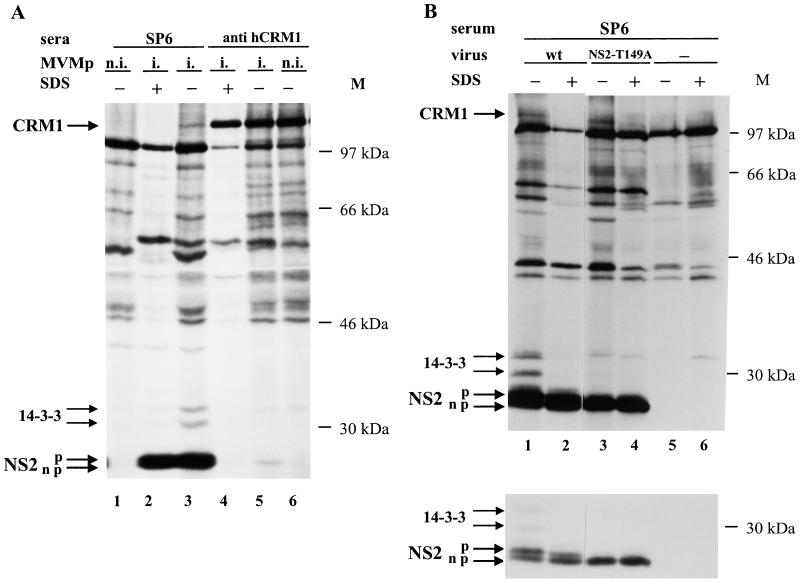
It was previously described that NS2 proteins from MVM are absolutely required for parvovirus replication in cells from the natural host—the mouse—while they are dispensable for a productive virus infection in nonmurine cells (12, 15, 42, 43). If the association of NS2 and CRM1 proteins was relevant for NS2 functioning, such an interaction should also take place in mouse cells. Indeed, the above-mentioned coimmunoprecipitation of NS2 and CRM1 proteins could also be demonstrated in extracts prepared from MVM-infected mouse A9 cells. As illustrated in Fig. 5A, NS2 and CRM1 proteins were both precipitated with either the anti-NS2 serum SP6 (lane 3) or the anti-hCRM1 antibodies (lane 5). The coimmunoprecipitation of NS2 and CRM1 occurred only in infected cell extracts prepared in the absence of SDS. Furthermore, as previously described for infected rat cell extracts, only the fast-migrating forms of NS2 coprecipitated with CRM1 (Fig. 5A, lane 5). Altogether, these data pointed to an association of mouse CRM1 with parvovirus NS2 proteins. The interaction of CRM1 and NS2 in natural host cells would be consistent with a role of this complex in NS2 functioning, although it does not appear to contribute to the host cell dependence of NS2 activity.
FIG. 5.
Association of CRM1 with the nonphosphorylated forms of NS2 in MVM-infected mouse cell extracts. 35S-labeled extracts were prepared from A9 cells that were mock treated (n.i.) or infected (i.) with either wild-type (wt) MVM (A and B) or mutant (NS2-T149A) MVM (B). Immunoprecipitation reactions were performed with SP6 antiserum (A and B) or anti-hCRM1 antibodies (A) in the presence (+) or absence (−) of SDS and analyzed as described in the legend to Fig. 4. Upper and lower panels in panel B correspond to autoradiograms of the same gel exposed for long and short times, respectively. Arrows to the left point at phosphorylated (p) and nonphosphorylated (np) NS2, 14-3-3, and CRM1 proteins. M, molecular sizes (in kilodaltons) of prestained protein standards.
CRM1 preferentially associates with the nonphosphorylated forms of NS2.
MVM NS2 proteins were previously shown to exist in both phosphorylated and unphosphorylated forms (13, 17). To determine whether phosphorylation plays a role in the interaction of NS2 with CRM1, mouse A9 cells were infected with NS2-T149A virus, an MVM mutant producing NS2 proteins whose phosphorylation is drastically impaired. Indeed, when NS2-specific antibodies were used in immunoprecipitation reactions, only fast-migrating forms of NS2-T149A were recovered from 35S-labeled cell extracts (Fig. 5B, lower panel), while no NS2-T149A protein could be detected in 32P-labeled cell extracts (data not shown) (6). As shown in Fig. 5B, the crm1 gene product was coprecipitated with the mutated NS2-T149A protein as efficiently as with wild-type NS2 (lanes 3 and 4 versus lanes 1 and 2). Therefore, NS2 phosphorylation proved to be dispensable for the interaction of the viral product with CRM1. Moreover, as stated above, coimmunoprecipitation reactions performed with hCRM1-recognizing antibodies led to the selective precipitation of the fast-migrating forms of NS2, besides the crm1 gene product (Fig. 5A, lane 5). Since the lower NS2 band has been assigned to the unphosphorylated form of the viral product (13, 17), this fact and above results strongly indicate that the nuclear export factor CRM1 preferentially interacts with nonphosphorylated NS2. This finding is in keeping with previously published data showing that nonphosphorylated NS2 can be detected in both the cytoplasms and nuclei of infected cells, whereas phosphorylated forms of NS2 are confined to the cytoplasm (17).
NS2 proteins accumulate in the nuclei of infected murine cells after treatment with leptomycin B.
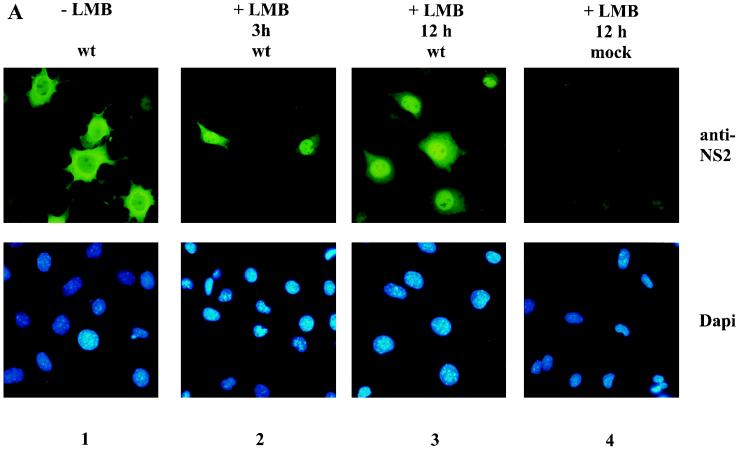
It was previously reported that CRM1 is a member of the importin-β family of transport proteins (28, 32) and is involved in the export of various proteins and RNAs from the nucleus to the cytoplasm (26, 29, 30, 34, 35, 44, 48, 57, 68). Our finding that NS2 interacts with CRM1 led us to hypothesize that nuclear NS2 might be exported to the cytoplasm via a CRM1-dependent pathway. Leptomycin B, a cytotoxin isolated from Streptomyces, was recently reported to specifically bind to CRM1 (36), thereby inhibiting CRM1-dependent nuclear export without directly affecting the other known nuclear transport pathways (29, 49, 67). If the main cytoplasmic localization of NS2 was, at least in part, a consequence of CRM1-mediated export, then leptomycin B would be expected to induce a nuclear accumulation of NS2 in infected cells. We tested this possibility by determining the subcellular localization of the viral NS2 proteins in MVM-infected A9 cells, treated or not with leptomycin B, by using SP6 serum in indirect immunofluorescence assays. Cells were synchronized and infected at the G1-to-S border and subsequently released into S phase in the presence or absence of 10 nM leptomycin B. As illustrated in Fig. 6A (upper panels 1 and 3), a 12-h leptomycin B treatment (starting from the release time point) resulted in a striking nuclear accumulation and cytoplasmic depletion of NS2 proteins, in comparison with untreated cells showing a mainly cytoplasmic localization of the viral product. Similar results were obtained with infected A9 cells treated with leptomycin B for 3 h, starting from 7 h postrelease (Fig. 6A, upper panel 2). The 12-h incubation of infected cells with leptomycin B did not alter the nuclear accumulation of the parvovirus NS1 protein (Fig. 6B), arguing against a general disruption of nucleocytoplasmic transports through this treatment. Although an indirect effect of leptomycin B on the nuclear accumulation of NS2 cannot be ruled out, these results strongly suggest that NS2 proteins are actively exported from the nuclei of MVM-infected mouse cells through a pathway involving the cellular carrier CRM1. This contention is substantiated by our above-mentioned data, showing the ability of NS2 to interact with CRM1.
FIG. 6.
Subcellular distribution of NS2 in MVM-infected mouse cells. A9 cells blocked at the G1-to-S transition were infected with MVM and released into S phase by incubation for either 10 or 12 h in a complete medium containing (+ LMB) or not containing (− LMB) 10 nM leptomycin B (LMB). The drug was given either for the whole duration of release (12 h) or during the last 3 h of a 10-h release period (3 h). Cells were further analyzed by indirect immunofluorescence assay with the NS2-specific SP6 serum (A) or the NS1-specific 3D9 antibody (B). Magnification, ×270. wt, wild type.
Altogether, our data indicate that (i) most of the NS2 proteins synthesized in the cytoplasm are moving into the nucleus and (ii) active nuclear export participates in the predominantly cytoplasmic localization of NS2.
DISCUSSION
CRM1 as a new cellular partner of MVM-NS2 proteins.
NS2 proteins from the parvovirus MVM comprise several isoforms that differ in their carboxy-terminal regions, due to alternate splicing of a precursor mRNA, and are termed major (NS2P), minor (NS2Y), and rare (NS2L), according to their relative abundance in parvovirus-infected cells (13, 17). It is not known so far whether these isoforms have different or redundant functions. The identification of cellular proteins that physically interact with NS2 isoforms constitutes a first step towards improving our understanding of NS2 functions during the parvovirus life cycle. We previously reported that the phosphorylated forms of NS2 specifically interact with two members of the highly conserved 14-3-3 protein family (6, 9). The present study led to the identification of the nuclear export factor CRM1 as a novel NS2-interacting partner. The interaction of NS2 with CRM1 was demonstrated in the yeast two-hybrid system and further confirmed in mammalian cell extracts through coimmunoprecipitation experiments. The association of CRM1 and NS2 proteins was observed both in cells in which NS2 is dispensable for parvovirus replication (FREJ4 rat cells) and in natural host cells in which NS2 is absolutely required for a productive MVM infection (A9 mouse cells), suggesting that CRM1-NS2 complexes may play a role in NS2 functioning and/or regulation but are not involved in the host species dependence of NS2 activity.
Our data also show that CRM1 preferentially associates with the nonphosphorylated forms of NS2. This result is in keeping with the fact that CRM1 is a mainly nuclear protein (1, 28) and that only the nonphosphorylated forms of NS2 are found in the nuclei of infected cells (17). In contrast, 14-3-3 proteins proved to interact only with phosphorylated NS2 (6). CRM1 immunoprecipitates from infected cell extracts contained NS2 but no detectable 14-3-3 proteins, in agreement with the targeting of distinct NS2 subpopulations by 14-3-3 and CRM1 proteins, respectively. It is not known at present whether the phosphorylation pattern confers a distinct function on NS2 or plays a regulatory role. However, that may be possible, and our study points to the CRM1 protein as a new candidate for host participants in the regulation and/or functioning of NS2.
The CRM1 protein was first identified in the fission yeast Schizosaccharomyces pombe, in which mutational inactivation of the corresponding gene causes abnormal chromosome morphology (1). More recently, hCRM1 was identified as a protein interacting with CAN/Nup214, a nucleoprotein associated with nuclear pore complexes (28). Several groups have provided evidence that both the human and yeast CRM1 proteins are importin-β family members (28, 32) that form a complex with leucine-rich nuclear export signal (NES)-containing peptides and function as essential nuclear export factors (3, 29, 30, 44, 48, 57). Several viral or cellular proteins, containing a leucine-rich NES and previously known to be exported from the nucleus or to shuttle through the nuclear membrane, were recently found to interact with CRM1 and/or to exit the nucleus via a CRM1-dependent pathway (34, 35, 48, 68). Others groups reported that leptomycin B, a cytotoxin that abrogates CRM1-mediated transport (29, 36, 67), inhibited the nuclear export of various proteins (33, 49, 58, 61, 65, 67). Altogether, these data led us to postulate that the nonstructural NS2 proteins from MVM are exported out of the nucleus via a CRM1-dependent pathway.
NS2 as a shuttling protein?
In proliferating mouse fibroblasts infected with MVM, the viral NS2 proteins are localized in the cytoplasmic and, to a lesser extent, nuclear compartments (17). It was commonly assumed that due to their small size, the 25-kDa NS2 proteins could enter and exit the nucleus through passive diffusion, since the cutoff between facilitated transport and diffusion across the nuclear pore complex is considered to be about 40 to 50 kDa (20). This hypothesis was supported by the fact that no known nuclear localization signal (NLS) has been detected so far in the NS2 sequence, whereas a bipartite NLS similar to that of nucleoplasmin is present in the mainly nuclear parvovirus protein NS1 (45). The present study is at odds with this assumption by showing that at least the export of NS2 out of the nucleus takes place through an active process. Indeed, MVM NS2 proteins not only were able to interact with the nuclear exportin CRM1 but were also retained in the nuclei of infected cells upon leptomycin B treatment. The fact that most NS2 proteins remained nuclear in the presence of leptomycin B, whereas they accumulated in the cytoplasms of untreated cells, strongly argues for a CRM1-mediated nuclear export of NS2 proteins in murine cells.
The nuclear accumulation of NS2 proteins in the presence of leptomycin B was concomitant with their depletion from the cytoplasm. Therefore, it appears that NS2 proteins normally shuttle through the nuclear envelope, with most of the polypeptides entering the nucleus at some time after their synthesis, followed by their efficient reexport to the cytoplasm through the CRM1-dependent process. Under normal conditions, a steady state is achieved, in which most of the NS2 proteins are cytoplasmic. This heterogeneous intracellular distribution could be assigned to the higher yield of NS2 cytoplasmic production, versus transport, to the greater efficiency of the export, versus import pathway, and/or to the interaction of NS2 with a protein(s) sequestering the viral product outside the nucleus. Indeed, NS2 interaction with the 14-3-3 proteins (9) may conceivably contribute to impeding the reimport or diffusion of NS2 into the nucleus.
Biological relevance of NS2 shuttling between nucleus and cytoplasm.
The biological function of NS2 shuttling is supported by recent data showing a late defect in the cytoplasmic accumulation of NS2 proteins from Aleutian mink disease virus in cells which were not able to sustain a productive parvovirus infection (46). Such a late function in parvovirus infection has also been suggested for the NS2 proteins of MVM (15), although NS2 may also play a role at earlier steps of the virus life cycle (15, 46). The CRM1-NS2 interaction may be important for the transport of NS2 proteins on their own. Thus, due to their relatively small size and the absence of an NLS, NS2 proteins may diffuse into the nucleus and need to be efficiently reexported for proper functioning in the cytoplasm. Furthermore, the active transport of NS2 through the nuclear membrane may point to a potential function of these proteins in the transfer of other macromolecules between the nucleus and cytoplasm. Since MVM NS2 is actively exported from the nucleus, the viral product may act as a carrier of nuclear molecules that need to be transferred to the cytoplasm. Certain MVM mutants, expressing no or truncated NS2 proteins, were reported to be impaired in the translation of viral RNAs, which was speculated to result from a mislocation of the viral transcripts in the cytoplasm (43). It is worth noting in this respect that since its discovery, CRM1 has been shown to bind a number of cellular and viral products and/or to lead to their nuclear export (30, 34, 35, 44, 48, 57, 68). The viral proteins known to shuttle through the nuclear pore complex, via a CRM1-mediated and/or leucine-rich NES-dependent export pathway, include several products that are also involved in the nuclear export of specific viral RNAs, such as human immunodeficiency virus type 1 Rev (27, 67), human T-cell leukemia virus type 1 Rex (4, 34), herpes simplex virus type 1 ICP27 (50, 53), and adenovirus E4-34kDa (21). Altogether these data raise the intriguing question of whether MVM NS2 proteins act in a similar way and play a role in the transport of parvovirus mRNAs, a possibility that remains to be tested experimentally. The further investigation of NS2-CRM1 interaction should shed light on both the mechanisms of NS2 nuclear export and the role(s) of NS2 proteins in the parvovirus life cycle. A potential leucine-rich NES motif, similar to the one present in human immunodeficiency virus Rev (26), has been identified in the region common to all three MVM NS2 isoforms (data not shown), as well as in the amino terminus of Aleutian mink disease virus NS2 proteins (46). Site mutagenesis studies are in progress to disrupt this motif in order to evaluate the relevance of NS2-CRM1 complexes to parvovirus replication.
ACKNOWLEDGMENTS
We acknowledge Maarten Fornerod (EMBL, Heidelberg, Germany) and David Pintel (University of Missouri—Columbia) for providing antibodies directed against human CRM1 and MVM NS1, respectively, Barbara Wolff-Wininsky (Novartis, Vienna, Austria) for supplying leptomycin B, and Jürg Nüesch and Peter Tattersall (Yale University, New Haven, Conn.) for the kind gift of plasmids pTM1-NS2p and pTM1-NS2y. We are indebted to Maarten Fornerod and Jürg Nüesch for helpful discussions and critical reading of the manuscript. The technical assistance of Michèle Klein is gratefully acknowledged.
This work was partly supported by both the German-Israeli Foundation for Scientific Research and Development and La Ligue Nationale Française contre le Cancer. U.B. is a graduate fellow of the Deutsches Krebsforschungszentrum.
REFERENCES
- 1.Adachi Y, Yanagida M. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene, crm1+, which encodes a 115-kDa protein preferentially localized to the nucleus and its periphery. J Cell Biol. 1989;108:1195–1207. doi: 10.1083/jcb.108.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anouja F, Wattiez R, Mousset S, Caillet-Fauquet P. The cytotoxicity of the parvovirus minute virus of mice nonstructural protein NS1 is related to changes in the synthesis and phosphorylation of cell proteins. J Virol. 1997;71:4671–4678. doi: 10.1128/jvi.71.6.4671-4678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askjaer P, Jensen T H, Nilsson J, Englmeier L, Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J Biol Chem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- 4.Ballaun C, Farrington G K, Dobrovnik M, Rusche J, Hauber J, Böhnlein E. Functional analysis of human T-cell leukemia virus type 1 Rex response element: direct RNA binding of Rex protein correlates with in vivo activity. J Virol. 1991;65:4408–4413. doi: 10.1128/jvi.65.8.4408-4413.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel P L, Chien C-T, Sternglanz R, Fields S. Using the two-hybrid system to detect protein-protein interactions. In: Hartley D A, editor. Cellular interactions in development: a practical approach. Oxford, United Kingdom: Oxford University Press; 1993. pp. 153–179. [Google Scholar]
- 6.Bodendorf U, Corbau R, Rommelaere J, Salomé N. Abstracts of the VIIth International Parvovirus Workshop. Heidelberg, Germany: Deutsches Krebsforschungszentrum; 1997. Analysis of 14-3-3 protein interaction with the non-structural products NS2 of minute virus of mice, abstr. P25. [Google Scholar]
- 7.Brandenburger A, Legendre D, Avalosse B, Rommelaere J. NS-1 and NS-2 proteins may act synergistically in the cytopathogenicity of parvovirus MVMp. Virology. 1990;174:576–584. doi: 10.1016/0042-6822(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 8.Breeden L, Nasmyth K. Regulation of the yeast HO gene. Cold Spring Harbor Symp Quant Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- 9.Brockhaus K, Plaza S, Pintel D J, Rommelaere J, Salomé N. Nonstructural proteins NS2 of minute virus of mice associate in vivo with 14-3-3 protein family members. J Virol. 1996;70:7527–7534. doi: 10.1128/jvi.70.11.7527-7534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownstein D G, Smith A L, Johnson E A, Pintel D J, Naeger L K, Tattersall P. The pathogenesis of infection with minute virus of mice depends on expression of the small nonstructural protein NS2 and on the genotype of the allotropic determinants VP1 and VP2. J Virol. 1992;66:3118–3124. doi: 10.1128/jvi.66.5.3118-3124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caillet-Fauquet P, Perros M, Brandenburger A, Spegelaere P, Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990;9:2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cater J E, Pintel D J. The small non-structural protein NS2 of the autonomous parvovirus minute virus of mice is required for virus growth in murine cells. J Gen Virol. 1992;73:1839–1843. doi: 10.1099/0022-1317-73-7-1839. [DOI] [PubMed] [Google Scholar]
- 13.Clemens K E, Cerutis D R, Burger L R, Yang C Q, Pintel D J. Cloning of minute virus of mice cDNAs and preliminary analysis of individual viral proteins expressed in murine cells. J Virol. 1990;64:3967–3973. doi: 10.1128/jvi.64.8.3967-3973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemens K E, Pintel D J. The two transcription units of the autonomous parvovirus minute virus of mice are transcribed in a temporal order. J Virol. 1988;62:1448–1451. doi: 10.1128/jvi.62.4.1448-1451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotmore S F, D’Abramo A M, Jr, Carbonell L F, Bratton J, Tattersall P. The NS2 polypeptide of parvovirus MVM is required for capsid assembly in murine cells. Virology. 1997;231:267–280. doi: 10.1006/viro.1997.8545. [DOI] [PubMed] [Google Scholar]
- 16.Cotmore S F, Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- 17.Cotmore S F, Tattersall P. Alternate splicing in a parvoviral nonstructural gene links a common amino-terminal sequence to downstream domains which confer radically different localization and turnover characteristics. Virology. 1990;177:477–487. doi: 10.1016/0042-6822(90)90512-p. [DOI] [PubMed] [Google Scholar]
- 18.Cotmore S F, Tattersall P. DNA replication in the autonomous parvoviruses. Semin Virol. 1995;6:271–281. [Google Scholar]
- 19.Cziepluch C, Kordes E, Poirey R, Grewenig A, Rommelaere J, Jauniaux J-C. Identification of a novel cellular TPR-containing protein, SGT, that interacts with the nonstructural protein NS1 of parvovirus H-1. J Virol. 1998;72:4149–4156. doi: 10.1128/jvi.72.5.4149-4156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dingwall C, Laskey R A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- 21.Dobbelstein M, Roth J, Kimberly W T, Levine A J, Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a Rev-like signal sequence. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doerig C, Hirt B, Antonietti J-P, Beard P. Nonstructural protein of parvoviruses B19 and minute virus of mice controls transcription. J Virol. 1990;64:387–396. doi: 10.1128/jvi.64.1.387-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doerig C, Hirt B, Beard P, Antonietti J P. Minute virus of mice non-structural protein NS-1 is necessary and sufficient for trans-activation of the viral P39 promoter. J Gen Virol. 1988;69:2563–2573. doi: 10.1099/0022-1317-69-10-2563. [DOI] [PubMed] [Google Scholar]
- 24.Faisst S, Faisst S R, Dupressoir T, Plaza S, Pujol A, Jauniaux J-C, Rhode S L, Rommelaere J. Isolation of a fully infectious variant of parvovirus H-1 supplanting the standard strain in human cells. J Virol. 1995;69:4538–4543. doi: 10.1128/jvi.69.7.4538-4543.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 26.Fischer U, Huber J, Boelens W C, Mattaj I W, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 27.Fischer U, Meyer S, Teufel M, Heckel C, Lührmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti K G, Fransen J, Grosveld G. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 1997;16:807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 31.Gietz R D, Schiestl R H, Willems A R, Woods R A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 32.Görlich D, Dabrowski M, Bischoff F R, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagting A, Karlsson C, Clute P, Jackman M, Pines J. MPF localization is controlled by nuclear export. EMBO J. 1998;17:4127–4138. doi: 10.1093/emboj/17.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hakata Y, Umemoto T, Matsushita S, Shida H. Involvement of human CRM1 (exportin 1) in the export and multimerization of the Rex protein of human T-cell leukemia virus type 1. J Virol. 1998;72:6602–6607. doi: 10.1128/jvi.72.8.6602-6607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kehlenbach R H, Dickmanns A, Gerace L. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT in vitro. J Cell Biol. 1998;14:863–874. doi: 10.1083/jcb.141.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudo N, Wolff B, Sekimoto T, Schreiner E P, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 37.Legrand C, Rommelaere J, Caillet-Fauquet P. MVM(p) NS-2 protein expression is required with NS-1 for maximal cytotoxicity in human transformed cells. Virology. 1993;195:149–155. doi: 10.1006/viro.1993.1355. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Rhode S L., III Nonstructural protein NS2 of parvovirus H-1 is required for efficient viral protein synthesis and virus production in rat cells in vivo and in vitro. Virology. 1991;184:117–130. doi: 10.1016/0042-6822(91)90828-y. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Rhode S L., III The parvovirus H-1 NS2 protein affects viral gene expression through sequences in the 3′ untranslated region. Virology. 1993;194:10–19. doi: 10.1006/viro.1993.1229. [DOI] [PubMed] [Google Scholar]
- 40.Lorson C, Burger L R, Mouw M, Pintel D J. Efficient transactivation of the minute virus of mice P38 promoter requires upstream binding of NS1. J Virol. 1996;70:834–842. doi: 10.1128/jvi.70.2.834-842.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mousset S, Ouadrhiri Y, Caillet-Fauquet P, Rommelaere J. The cytotoxicity of the autonomous parvovirus minute virus of mice nonstructural proteins in FR3T3 rat cells depends on oncogene expression. J Virol. 1994;68:6446–6453. doi: 10.1128/jvi.68.10.6446-6453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naeger L K, Cater J, Pintel D J. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J Virol. 1990;64:6166–6175. doi: 10.1128/jvi.64.12.6166-6175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naeger L K, Salomé N, Pintel D J. NS2 is required for efficient translation of viral mRNA in minute virus of mice-infected murine cells. J Virol. 1993;67:1034–1043. doi: 10.1128/jvi.67.2.1034-1043.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neville M, Stutz F, Lee L, Davis L I, Rosbash M. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr Biol. 1997;7:767–775. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 45.Nüesch J P F, Tattersall P. Nuclear targeting of the parvoviral replicator molecule NS1: evidence for self-association prior to nuclear transport. Virology. 1993;196:637–651. doi: 10.1006/viro.1993.1520. [DOI] [PubMed] [Google Scholar]
- 46.Oleksiewicz M B, Wolfinbarger J B, Bloom M E. A comparison between permissive and restricted infections with Aleutian mink disease parvovirus (ADV): characterization of the viral protein composition at nuclear sites of virus replication. Virus Res. 1998;56:41–51. doi: 10.1016/s0168-1702(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 47.Op De Beeck A, Caillet-Fauquet P. The NS1 protein of the autonomous parvovirus minute virus of mice blocks cellular DNA replication: a consequence of lesions to the chromatin? J Virol. 1997;71:5323–5329. doi: 10.1128/jvi.71.7.5323-5329.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for the role of Crm1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 49.Otero G C, Harris M E, Donello J E, Hope T J. Leptomycin B inhibits equine infectious anemia virus Rev and feline immunodeficiency virus Rev function but not the function of the hepatitis B virus posttranscriptional regulatory element. J Virol. 1998;72:7593–7597. doi: 10.1128/jvi.72.9.7593-7597.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phelan A, Dunlop J, Clements J B. Herpes simplex virus type 1 protein IE63 affects the nuclear export of virus intron-containing transcripts. J Virol. 1996;70:5255–5265. doi: 10.1128/jvi.70.8.5255-5265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rommelaere J, Cornelis J J. Antineoplastic activity of parvoviruses. J Virol Methods. 1991;33:233–251. doi: 10.1016/0166-0934(91)90024-t. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 53.Sandri-Golden R M. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998;12:868–879. doi: 10.1101/gad.12.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoborg R V, Pintel D J. Accumulation of MVM gene products is differentially regulated by transcription initiation, RNA processing and protein stability. Virology. 1991;181:22–34. doi: 10.1016/0042-6822(91)90466-o. [DOI] [PubMed] [Google Scholar]
- 55.Seif R, Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977;24:721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senger M, Glatting K-H, Ritter O, Suhai S. X-HUSAR, an X-based graphical interface for the analysis of genomic sequences. Comput Methods Programs Biomed. 1995;46:131–141. doi: 10.1016/0169-2607(94)01610-r. [DOI] [PubMed] [Google Scholar]
- 57.Stade K, Ford C S, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 58.Taagepera S, McDonald D, Loeb J E, Whitaker L L, McElroy A K, Wang J Y J, Hope T J. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:7457–7462. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tattersall P, Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983;46:944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toda T, Shimanuki M, Saka Y, Yamano H, Adachi Y, Shirakawa M, Kyogoku Y, Yanagida M. Fission yeast pap1-dependent transcription is negatively regulated by an essential nuclear protein, crm1. Mol Cell Biol. 1992;12:5474–5484. doi: 10.1128/mcb.12.12.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanacker J-M, Rommelaere J. Non-structural proteins of autonomous parvoviruses: from cellular effects to molecular mechanisms. Semin Virol. 1995;6:291–297. [Google Scholar]
- 63.van der Eb A J, Graham F L. Assay of transforming activity of tumor virus DNA. In: Grossman L, Moldave K, editors. Methods in enzymology. Vol. 65. New York, N.Y: Academic Press; 1980. pp. 826–839. [DOI] [PubMed] [Google Scholar]
- 64.van Hille B, Duponchel N, Salomé N, Spruyt N, Cotmore S F, Tattersall P, Cornelis J J, Rommelaere J. Limitations to the expression of parvoviral nonstructural proteins may determine the extent of sensitization of EJ-ras-transformed rat cells to minute virus of mice. Virology. 1989;171:89–97. doi: 10.1016/0042-6822(89)90514-x. [DOI] [PubMed] [Google Scholar]
- 65.Wada A, Fukuda M, Mishima M, Nishida E. Nuclear export of actin: a novel mechanism regulating the subcellular localization of a major cytoskeletal protein. EMBO J. 1998;17:1635–1641. doi: 10.1093/emboj/17.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang D, Yuan W, Davis I, Parrish C R. Nonstructural protein-2 and the replication of canine parvovirus. Virology. 1998;240:273–281. doi: 10.1006/viro.1997.8946. [DOI] [PubMed] [Google Scholar]
- 67.Wolff B, Sanglier J J, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Bardes E S G, Moore J D, Brennan J, Powers M A, Kornbluth S. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 1998;12:2131–2143. doi: 10.1101/gad.12.14.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]