Abstract
Background:
Breast cancer-related lymphedema impacts 30% to 47% of women who undergo axillary lymph node dissection (ALND). Studies evaluating the effectiveness of prophylactic lymphovenous bypass (LVB) at the time of ALND have had small patient populations and/or short follow-up. The aim of this study is to quantitatively and qualitatively evaluate prophylactic LVB in patients with breast cancer.
Methods:
A retrospective review of patients who underwent ALND from 2018 to 2022 was performed. Patients were divided into cohorts based on whether they underwent prophylactic LVB at the time of ALND. Primary outcomes included 30-day complications and lymphedema. Lymphedema was quantitatively evaluated by bioimpedance analysis, with L-dex scores >7.1 indicating lymphedema.
Results:
One-hundred five patients were identified. Sixty-four patients (61.0%) underwent ALND and 41 patients (39.0%) underwent ALND+LVB. Postoperative complications were similar between the cohorts. At a median follow-up of 13.3 months, lymphedema occurred significantly higher in the ALND only group compared with ALND+LVB group (50.0% vs 12.2%; P < 0.001). ALND without LVB was an independent risk factor for lymphedema development (odds ratio, 4.82; P = 0.003).
Conclusions:
Prophylactic LVB decreases lymphedema and is not associated with increased postoperative complications. A multidisciplinary team approach is imperative to decrease lymphedema development in this patient population.
Keywords: lyMPHA, lymphedema, lymphovenous bypass, microsurgery, super-microsurgery
INTRODUCTION
Lymphedema is a debilitating, chronic condition characterized by progressive swelling of an extremity because of impaired drainage and excessive retention of lymphatic fluid. Patients experience decreased quality of life owing to pain, restricted function, and the appearance of the affected limb.1 Although lymphedema can occur as a primary process due to the abnormal congenital development of lymphatics, it is more commonly acquired secondary to surgical, traumatic, infectious, inflammatory, or neoplastic obstruction of lymphatic fluid drainage.2,3 In the United States, most cases of lymphedema occur in the upper extremities after mastectomy, axillary lymph node dissection (ALND), with or without adjuvant radiation.4–6 It is estimated that 30% to 47% of patients who undergo ALND will develop breast cancer-related lymphedema, and this risk increases in those who receive radiation therapy.7–10
There is no cure for lymphedema and the optimal treatment is unknown.11,12 Conservative therapies, such as compression garments and complete decongestive therapy, have focused on symptomatic relief.13–15 Debulking procedures, such as liposuction or direct excision, are unable to restore the affected limb to its premorbid state.10 Microsurgical treatment of lymphedema such as lymphovenous bypass (LVB) or vascularized lymph node transfer have demonstrated efficacy but require a separate operation.10,16,17 Given the long-lasting morbidity associated with the development of lymphedema, focus has shifted toward the risk-reduction and prevention.
First described by Boccardo et al18 in 2009, lymphatic microsurgical preventive healing approach is a surgical procedure performed at the time of ALND to prevent lymphedema in high-risk patients. It involves the prophylactic anastomosis of one or more lymphatic vessels transected at the time of ALND to nearby veins.10,18 By repairing transected lymphatics at the time of ALND, lymphatic microsurgical preventive healing approach offers a preventative surgical approach to lymphedema in patients at high risk for developing lymphedema. This is in contrast to LVBs performed for patients with established lymphedema, which are usually performed in the distal upper extremity for technical feasibility as distal functional lymphatics are more easily identified compared with proximal lymphatic vessels near the axilla.10 The goal of this study is to compare the postoperative complications and the development of lymphedema in patients undergoing ALND alone versus ALND with immediate prophylactic LVB.
METHODS
Data Collection and Statistical Analysis
Following institutional board approval (STUDY 00004860), a prospectively maintained database of patients with breast cancer who underwent ALND at 2 tertiary care centers from 2018 to 2022 was created. Inclusion criteria consisted of patients aged 18 or older with a diagnosis of breast cancer with either clinically or radiographic positive axillary nodes or positive sentinel lymph nodes who underwent tumor resection and ALND. Patients were excluded if they underwent only sentinel lymph node biopsy or were lost to follow-up. Variables recorded in the database included patient demographics, comorbidities, breast cancer characteristics, oncologic therapies (eg, radiation and chemotherapy), type of breast oncologic resection (eg, mastectomy or lumpectomy), immediate reconstruction, early postoperative complications, circumferential limb measurements, and bioimpedance spectroscopy (SOZO, ImpediMed Ltd, Australia) findings.
Primary outcomes included early postoperative complications (within 30 days of surgery) and lymphedema development. A subanalysis of patients who underwent prophylactic LVB was also performed to identify perioperative factors associated with lymphedema development. Continuous variables are reported as means and SDs, whereas categorical variables are described as frequencies and percentages. Univariate analyses were performed using χ2 or Fisher exact test (n < 7) for categorical variables and Student t test for continuous variables. Potential confounders were controlled for using multivariate regression analyses. Data analysis was performed using STATA version 17.0 (StataCorp, College Station, TX) with statistical significance defined at values of P ≤ 0.05.
Perioperative Management
Following their diagnosis of breast cancer, patients were evaluated for surgery by breast surgeons (M.B., P.W., or I.T.G) and plastic and reconstructive microsurgeons (K.L.F. or L.K.T.). Patients with known or suspected axillary nodal disease requiring ALND were offered prophylactic LVB to be performed at the time of ALND. Per our multidisciplinary team approach and lymphedema surveillance screening program, patients were referred preoperatively to physiatry (E.M.W. or K.P.) for baseline bioimpedance measurements and further education about lymphedema. Unless there was a contraindication, bioimpedance spectroscopy was used as the primary tool for lymphedema monitoring. Based on the data from Stout Gergich et al,19 patients were screened before undergoing ALND and then at 3-month intervals for the first 2 years postoperatively. Thereafter, they were transitioned to screening every 6 months as risk after 2 years progressively decreases. Testing was considered positive if L-dex was >7.1 in patients who did not have preoperative testing completed or an increase in 10 for those patients with pre-op baseline measurements.20 If patients were found to have a positive L-dex, they were provided a prescription for a compression sleeve (20–30 mm Hg) and advised to wear during daytime hours. After 6 weeks of use, they were asked to return for retesting. Patients were instructed to avoid raising their arm >90° (shoulder height) for 2 weeks after surgery. Then they were instructed to start gentle range-of-motion exercises.
Operative Technique
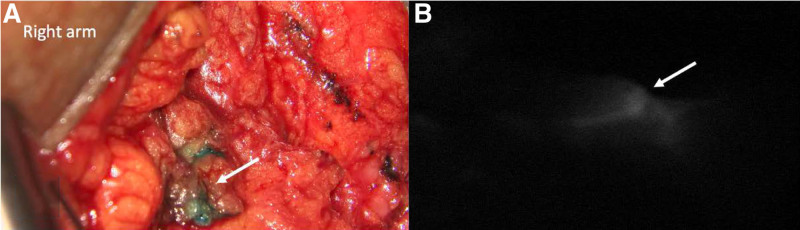
Breast tumor resection (eg, mastectomy or lumpectomy) and ALND were performed by the breast surgical oncologists. Axillary node dissection was performed with the intention of preserving branches of the axillary vein with some length, as able, for possible LVB targets. Following completion of ALND, the reconstructive microsurgeon entered the operating room to perform prophylactic LVB and/or breast reconstruction. Reverse axillary lymphatic mapping was performed using indocyanine green that was injected into the second and fourth webspaces of the hand while the breast surgeon was performing ALND. Using the SPY-PHI near-infrared camera (Stryker, Kalamazoo, MI), afferent lymphatics were evaluated and visible lymphatics were traced out from distal to proximal (Fig. 1). Depending on availability, methylene blue or isosulfan blue was also injected into the skin just distal to the axilla to aid in identification of lymphatic vessels. Using the Mitaka microscope (Mitaka, Denver, CO) at 10× magnification and the near-infrared camera, transected lymphatic channels were identified within the axilla. Recipient veins were chosen based on length, proximity to a valve, and limited back flow. They were dissected to add additional length to reach lymphatic targets. If needed for positioning, an Acland clamp was placed on the vein proximally. The lumen of the vein and lymphatic channels were irrigated. Using a 9-0 nylon suture, a temporary U-stitch was placed to bring the lymphatic ends into the vein. The lymphatic was then brought into the vein in an end-to-end fashion and telescoped to ensure the lymphatic end was within the vein and flowing. Anastomosis was performed using 9-0 nylon interrupted sutures that were placed from the adventitial lymphatic channel to the vein. Multiple lymphatics were brought into the vein, as able, based on the size, location, and reach. Other anastomosis techniques included vein coupler (Synovis Micro Companies, Alliance, Inc., Birmingham, AL) or vein graft, as indicated. The Acland clamp was then removed from the vein, and minimal back-bleeding from the anastomosis was ensured. Under microscopy, a successful immediate LV microanastomosis was ensured by observing dye filling into the recipient vein. Before axillary closure, care was taken to ensure that the surgical drain did not disrupt the LV anastomosis. The number of LVB anastomoses performed was dependent on the availability of lymphatic vessels and nearby vein branches, being mindful of total operative time.
FIGURE 1.
Afferent lymphatics. A, Afferent lymphatic vessel filled with blue dye (arrow) that was transected during axillary lymph node dissection. B, Lymphatic vessel (arrow) is illuminated during reverse axillary lymphatic mapping.
RESULTS
A total of 105 patients were identified during the 3-year study period, of which 64 patients (61.0 %) underwent ALND alone and 41 patients (39.0%) underwent ALND and immediate prophylactic LVB. The majority of the study population was female (n = 103, 98.1%) and average age and body mass index (BMI) were 58.1 years and 30.8 kg/m2, respectively. On average, the ALND+LVB cohort was significantly younger compared with the ALND only group (55.0 vs 60.1 years; P = 0.045). The prevalence of obesity, smoking, diabetes, and breast cancer type was similar between the 2 groups.
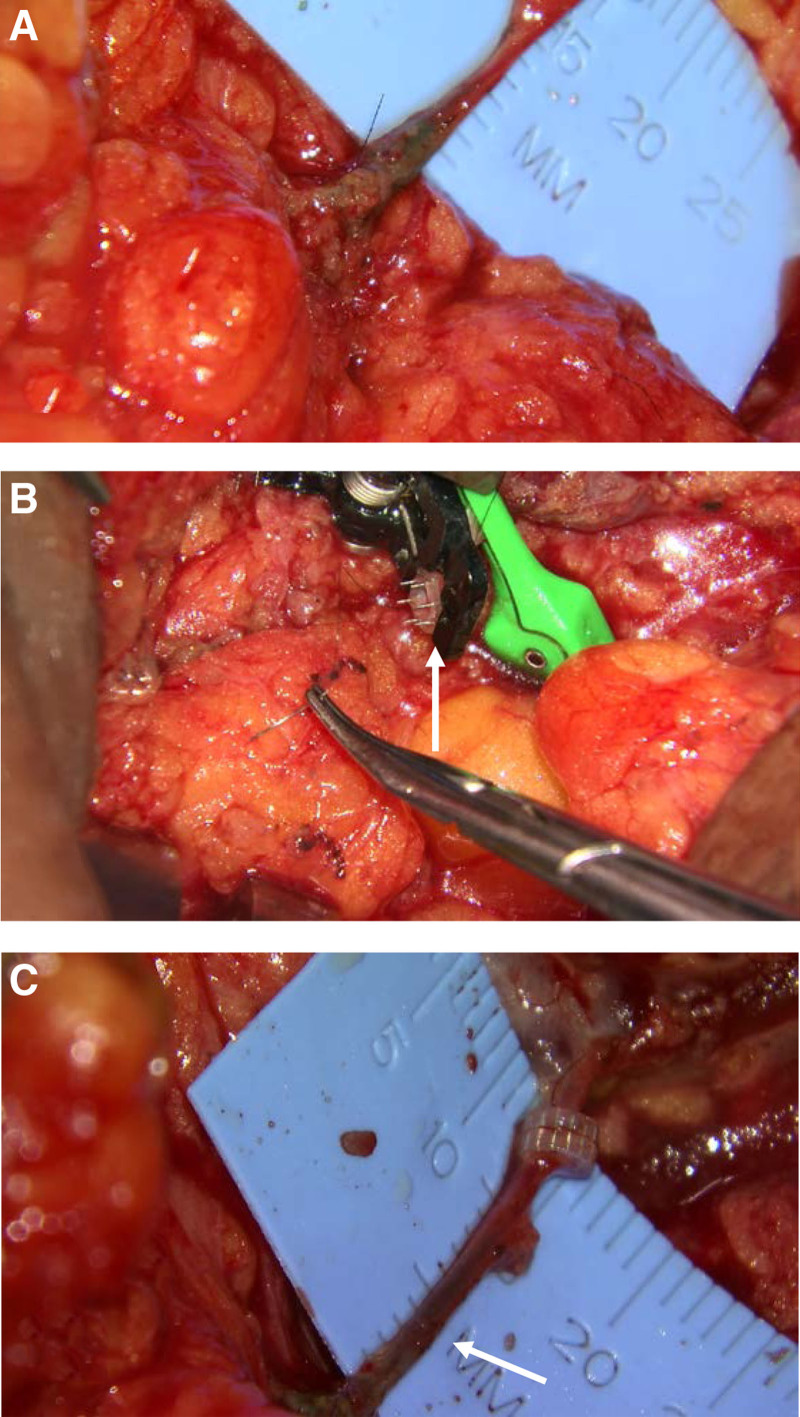
Almost half of the entire study population underwent axillary lymph node biopsy before ALND (n = 49; 46.7%). The proportion of patients who received chemotherapy and/or radiation was similar between cohorts. The average number of lymph nodes removed during ALND was significantly higher in the ALND+LVB group (15.9 vs 13.0, P = 0.007). Oncoplastic reconstruction was performed more commonly in the LVB group (80.5% vs 35.9%; P < 0.001). Operative duration was significantly longer in the LVB group, as well (318.6 vs 236.0 minutes; P < 0.001). Among the patients who underwent prophylactic LVB, the average number of LV anastomoses performed was 1.9 ± 0.9. The majority of microanastomoses were performed using end-to-end suture anastomosis (n = 29; 70.7%), followed by vein coupler (n = 9; 22.0%), vein graft (n = 2; 4.9%), and a combination of anastomotic techniques (n = 1; 2.4%) (Fig. 2). Table 1 describes perioperative and intraoperative factors stratified by cohort.
FIGURE 2.
Types of lymphovenous anastamosis. A. Suture end-to-end anastamosis with multiple lymphatics to single vein with blue dye seen within the vein. B. Coupler-assisted lymphovenous anastomosis. C. Lymphovenous anastomosis using vein graft (arrow).
TABLE 1.
Perioperative and Intraoperative Factors
| Variable | Total (n = 105) | ALND Only (n = 64) | ALND + LVB (n = 41) | P |
|---|---|---|---|---|
| Female | 103 (98.1%) | 63 (98.4%) | 40 (97.6%) | 1.000 |
| Age, y | 58.1 ± 12.6 | 60.1 ± 12.8 | 55.0 ± 11.8 | 0.045 |
| BMI (kg/m2) | 30.8 ± 9.1 | 29.8 ± 9.0 | 32.4 ± 9.1 | 0.179 |
| Obesity (BMI ≥ 30 kg/m2) | 47 (44.8%) | 27 (42.2%) | 20 (48.8%) | 0.507 |
| Race | 0.168 | |||
| Caucasian | 75 (71.4%) | 49 (76.6%) | 26 (63.4%) | |
| Black | 20 (19.0%) | 10 (15.6%) | 10 (24.4%) | |
| Hispanic | 4 (3.8%) | 1 (1.6%) | 3 (7.3%) | |
| Asian | 5 (4.8%) | 4 (6.2%) | 1 (2.4%) | |
| Diabetes mellitus | 21 (20.0%) | 13 (20.3%) | 8 (19.5%) | 0.920 |
| Congestive heart failure | 2 (1.9%) | 1 (1.6%) | 1 (2.4%) | 1.000 |
| Prior venous thromboembolism | 5 (4.8%) | 2 (3.1%) | 3 (7.3%) | 0.376 |
| Current smoker | 7 (6.7%) | 5 (7.8%) | 2 (4.9%) | 0.731 |
| Albumin (g/dL) | 4.0 ± 0.7 | 4.0 ± 0.5 | 4.1 ± 0.9 | 0.088 |
| Breast cancer type | 0.275 | |||
| Ductal carcinoma in situ | 2 (1.9%) | 1 (1.6%) | 1 (2.4%) | |
| Invasive ductal carcinoma | 88 (83.8%) | 55 (85.9%) | 33 (80.5%) | |
| Invasive lobular carcinoma | 9 (8.6%) | 6 (9.4%) | 3 (7.3%) | |
| Papillary carcinoma | 1 (0.9%) | 1 (1.6%) | 0 (0%) | |
| Mammary carcinoma | 3 (2.9%) | 0 (0%) | 3 (7.3%) | |
| Inflammatory carcinoma | 2 (1.9%) | 1 (1.6%) | 1 (2.4%) | |
| Prior node biopsy | 49 (46.7%) | 28 (43.7%) | 21 (51.2%) | 0.454 |
| Chemotherapy | 0.394 | |||
| None | 16 (15.2%) | 12 (18.7%) | 4 (9.8%) | |
| Neoadjuvant | 37 (35.2%) | 19 | 18 (43.9%) | |
| Adjuvant | 24 (22.9%) | 16 (25.0%) | 8 (19.5%) | |
| Both | 28 (26.7%) | 17 (26.6%) | 11 (26.8%) | |
| Radiation | 0.513 | |||
| None | 27 (25.7%) | 19 (29.7%) | 8 (19.5%) | |
| Neoadjuvant | 5 (4.8%) | 4 (6.2%) | 1 (2.4%) | |
| Adjuvant | 69 (65.7%) | 39 (60.9%) | 30 (73.2%) | |
| Both | 4 (3.8%) | 2 (3.1%) | 2 (4.9%) | |
| No. of nodes removed during ALND | 14.1 ± 5.4 | 13.0 ± 4.7 | 15.9 ± 6.0 | 0.007 |
| Cancer surgery | <0.001 | |||
| Lumpectomy | 19 (18.1%) | 4 (6.2%) | 15 (36.6%) | |
| Mastectomy | 86 (81.9%) | 60 (93.7%) | 26 (63.4%) | |
| Oncoplastic reconstruction | 56 (53.3%) | 23 (35.9%) | 33 (80.5%) | <0.001 |
| Operative duration (min) | 268.6 ± 108.5 | 236.0 ± 101.4 | 318.6 ± 100.7 | <0.001 |
| Lymphovenous bypass factors | ||||
| No. anastomoses performed | — | — | 1.9 ± 0.9 | — |
| Anastomotic techniques | — | — | ||
| Suture anastomosis | 29 (70.7%) | |||
| Vein coupler | 9 (22.0%) | |||
| Vein graft | 2 (4.9%) | |||
| Combination | 1 (2.4%) | |||
BMI indicates body mass index.
Bold values indicate statistical significance at the p<0.05 level.
Table 2 summarizes the early postoperative complications and long-term outcomes. The overall complication rate was 13.3% (n = 14), which was similar between the cohorts. At a median follow-up of 13.3 months, lymphedema development occurred significantly more frequently in the ALND only cohort (50.0% vs 12.2%; P < 0.001). Likewise, the median L-dex ratio at the most recent follow-up date was significantly higher in the ALND only group (9.9 vs 1.0; P < 0.001). Breast cancer recurrence and cancer-related mortality were similar between the groups.
TABLE 2.
Postoperative Complications and Long-term Outcomes
| Variable | Total (n = 105) | ALND Only (n = 64) | ALND + LVB (n = 41) | P |
|---|---|---|---|---|
| Early postoperative complications | ||||
| 30-day complications | 14 (13.3%) | 8 (12.5%) | 6 (14.6%) | 0.775 |
| Surgical site infection | 7 (6.7%) | 2 (3.1%) | 5 (12.2%) | 0.107 |
| Dehiscence | 2 (1.9%) | 0 (0%) | 2 (4.9%) | 0.150 |
| Seroma | 9 (8.6%) | 7 (10.9%) | 2 (4.9%) | 0.477 |
| Reoperation | 11 (10.5%) | 6 (9.4%) | 5 (12.2%) | 0.747 |
| Long-term outcomes | ||||
| Lymphedema | 37 (35.2%) | 32 (50.0%) | 5 (12.2%) | <0.001 |
| Median L-dex ratio (IQR) | 4.0 (0.5, 10.2) | 9.9 (6.6, 18.15) | 1.0 (-1.3, 4.0) | <0.001 |
| Bioimpedance only | 4 (3.8%) | 4 (6.2%) | 0 (0%) | |
| Symptoms only | 21 (20.0%) | 18 (28.1%) | 3 (7.3%) | |
| Symptoms + bioimpedance | 11 (10.5%) | 9 (14.1%) | 2 (4.9%) | |
| Cancer recurrence | 8 (7.6%) | 7 (10.9%) | 1 (2.4%) | 0.145 |
| Deceased | 9 (8.6%) | 5 (7.8%) | 4 (9.8%) | 0.839 |
| Median follow-up duration (mo), IQR | 13.3 (7.7, 21.3) | 10.9 (4.0, 17.6) | 15.6 (10.3, 28.3) | 0.064 |
IQR indicates interquartile range.
Bold values indicate statistical significance at the p<0.05 level.
Multivariate logistic regression analysis revealed oncoplastic reconstruction to be an independent predictor of postoperative complications (odds ratio 37.69; 95% confidence interval: 3.16–450.02; P = 0.004). The only independent risk factor for lymphedema development was ALND alone without immediate prophylactic LVB (odds ratio, 4.82; 95% confidence interval: 1.69–13.77; P = 0.003) (Table 3).
TABLE 3.
Multivariate Regression Analysis for Independent Predictors of Lymphedema
| Variable | Odds Ratio (95% CI) | P |
|---|---|---|
| Radiation | 0.88 (0.33–2.36) | 0.798 |
| Chemotherapy | 1.33 (0.40–4.45) | 0.645 |
| Oncoplastic reconstruction | 0.98 (0.37–2.56) | 0.962 |
| ALND without LVB | 4.82 (1.69–13.77) | 0.003 |
| Diabetes | 1.54 (0.50–4.72) | 0.451 |
| Obesity | 0.76 (0.31–1.84) | 0.542 |
CI indicates confidence interval.
Bold values indicate statistical significance at the p<0.05 level.
Subanalysis of only the ALND+LVB group revealed that vein grafts were significantly associated with development of lymphedema (P = 0.034). The prevalence of anastomoses performed using suture or venous couplers were similar among patients who developed lymphedema and those who did not. Number of LVB anastomoses performed, oncologic chemotherapy, and radiation were not significantly associated with the development of lymphedema. The postoperative bioimpedance spectroscopy findings revealed a significantly higher L-dex ratio in patients with lymphedema (11.8 vs −1.1; P < 0.001) (Table 4).
TABLE 4.
Subanalysis of Perioperative Factors in ALND + LVB Patients Only
| Variable | Lymphedema (n = 8) | No. of Lymphedema (n = 33) | P |
|---|---|---|---|
| Type of anastomosis* | |||
| End-to-end suture | 4 (50.0%) | 26 (78.8%) | 0.178 |
| Vein coupler | 2 (25.0%) | 7 (21.2%) | 1.000 |
| Vein graft | 3 (37.5%) | 0 (0%) | 0.034 |
| Number of anastomoses performed | 1.9 ± 0.6 | 1.9 ± 1.0 | 0.958 |
| Oncoplastic reconstruction | 7 (87.5%) | 26 (78.8%) | 1.000 |
| Radiation | |||
| Neoadjuvant | 1 (12.5%) | 2 (6.1%) | 0.488 |
| Adjuvant | 6 (75.0%) | 26 (78.8%) | 1.000 |
| Chemotherapy | |||
| Neoadjuvant | 6 (75.0%) | 23 (69.7%) | 1.000 |
| Adjuvant | 3 (37.5%) | 16 (48.5%) | 0.703 |
| OR duration (min) | 375.9 ± 108.0 | 304.7 ± 95.5 | 0.073 |
| Postoperative L-dex ratio | 11.8 ± 6.6 | −1.1 ± 4.6 | <0.001 |
*One patient underwent a combination of anastomotic techniques.
OR indicates operating room.
Bold values indicate statistical significance at the p<0.05 level.
DISCUSSION
This study demonstrates that in high-risk patients, immediate prophylactic LVB at the time of ALND significantly decreases the rate of lymphedema development (12.2% vs 50.0%), without increasing postoperative complications. Risk factors for lymphedema include axillary node dissection, age older than 65 years, obesity, neoadjuvant chemotherapy, and postmastectomy radiation.21 The findings in this study parallel results reported in earlier studies,11,22 but include patients treated at 2 large medical centers with a longer median follow-up duration (13.3 months), incorporate various modalities to diagnose lymphedema, and highlight the importance of a multidisciplinary team approach in management of this complex patient population. In addition, our results are valuable as our comparative cohorts underwent cancer therapies at the same institutions, had similar comorbidities, and were clinically followed by physiatrists who employed the same protocols to diagnose lymphedema. Because our group screens all patients after ALND, we included patients with early subclinical lymphedema based on the bioimpedance spectroscopy and clinical lymphedema based on the symptoms (even minor symptoms). This may explain why a higher rate of lymphedema was reported in our patients who did not undergo LVB compared with the other studies. This study demonstrated significantly lower median L-dex scores in patients who underwent prophylactic LVB compared with those who did not (9.9 vs 1.0). Although bioimpedance spectroscopy has been shown to be more sensitive for detection of early lymphedema,7 there is a paucity of studies that include this quantitative evaluation for lymphedema diagnosis.
Despite increased efforts toward breast conservation therapy and sentinel node mapping, lymphedema remains a common and devastating complication following ALND.21 Given the irreversible and progressive nature of lymphedema, and improved microsurgery techniques, recent focus has shifted toward primary risk-reduction strategies, including immediate prophylactic LVB at the time of ALND. In a meta-analysis by Johnson et al,8 lymphedema rates were significantly higher in patients who underwent ALND alone versus those who underwent LVB and ALND (14.1% vs 2.1%; P = 0.029). However, a significant amount of heterogeneity regarding the definition of lymphedema existed among the 19 articles included in the meta-analysis. Moreover, only 2 articles utilized bioimpedance spectroscopy to diagnose lymphedema.23,24 In a retrospective study with a median follow-up of 6 months, Feldman et al24 reported a significant reduction of lymphedema rates from 50% to 12.5% in patients who underwent ALND+LVB. This was replicated in the retrospective study by Hahamoff et al,23 which found a reduction in lymphedema development from 40% to 12.5% in patients following introduction of prophylactic LVB.
With greater breast cancer survivorship, it is likely that lymphedema prevalence will also increase. Therefore, it is critical that a multidisciplinary team approach that includes breast surgeons, medical oncologists, plastic and reconstructive microsurgeons, radiation oncologists, physiatrists, and physical therapists be instituted to closely follow this patient population. At our institution, discussions about lymphedema risk and referral to physiatry and plastic surgery have become a routine aspect of interdisciplinary weekly conferences. Close surveillance of high-risk patients by multiple providers is important as prompt detection of lymphedema and early treatment can slow disease progression.25 Furthermore, it may increase patient participation in lymphedema surveillance programs.23
In the ALND+LVB group, the majority of patients underwent end-to-end suture anastomosis, followed by vein coupler, vein graft, or a combination of anastomotic techniques. Subanalysis of this cohort revealed that the patients who had a vein graft as part of their LV anastomosis (n = 3) developed lymphedema. In contrast, there was no significant difference in lymphedema development among those who underwent coupled versus hand-sewn LVB. Despite being statistically significant for lymphedema, we cannot make any conclusions on the utility of vein grafts as 2 of the 3 patients who received vein grafts were morbidly obese and had advanced stage breast cancer; thus, the development of lymphedema may have been due to a more hostile surgical environment for both the breast surgeon and microsurgeon, including difficulty of the axillary dissection and inability to preserve vein recipients as they were involved in the disease process. To our knowledge, this is the first study to examine the impact of LV anastomotic type on development of lymphedema in patients undergoing prophylactic LVB at the time of ALND. Larger, multiinstitutional studies are necessary to further evaluate if one type of microsurgical anastomosis is superior versus another.
This study is not without limitations. Our results may be influenced by a selection bias. Patients were selected for prophylactic LVB with ALND on the basis of referral to the senior author. In 2018, when our team began performing prophylactic LVB with ALND, patients were referred sequentially to 3 different plastic surgeons for reconstruction; the senior author was the only one who performed LVB at that time. Within 18 months, all patients with suspected axillary nodal involvement regardless of reconstruction plans were referred to a plastic surgeon who could offer LVB. This may explain why a higher proportion of the ALND+LVB cohort pursued oncoplastic reconstruction compared with the ALND alone group. Moreover, our mean follow-up duration of 13.3 months is shorter than the previously reported 24-month timeline of lymphedema onset after ALND.11,26 Because of the tertiary referral nature of our practice, many patients come from long distances, thus, long-term follow-up can be difficult to obtain for patients who pursue follow-up closer to their home. A shortcoming of the lymphedema screening program is the number of patients who fail to return to lymphedema clinic after surgery. Reasons cited by patients for not continuing with the screening program include being overwhelmed by multiple doctor’s visits, copay (or other financial) concerns, and not having swelling in their arm. Unfortunately, because they were lost to follow-up, we are unable to quantify who went on to show signs of clinical lymphedema and who remained asymptomatic. Additionally, perhaps univariate analysis to identify intraoperative LVB factors that increased risk of lymphedema may have been more revealing if equal proportions of the various types of LVB anastomotic techniques had been performed. Nevertheless, this study adds to the current, but scarce lymphedema prevention literature and highlights the importance of a multidisciplinary team approach for the management of these complex patients. Larger, multiinstitutional studies are warranted to further evaluate the longevity of prophylactic LVB in this patient population.
CONCLUSIONS
This study demonstrates that immediate prophylactic LVB in high-risk patients undergoing ALND is effective at reducing the rates of lymphedema development at an average follow-up of 13.3 months. This study adds to the scarce literature evaluating simultaneous ALND and prophylactic LVB with use of multiple modalities to measure lymphedema and highlights our multidisciplinary approach to the management of this complex patient population.
Acknowledgments
R.D. did acquisition of data, analysis and interpretation of data, drafting the article, and gave final approval of the version to be published. D.S., N.G., and P.T. did acquisition of data, analysis and interpretation of data, revising the article, and gave final approval of the version to be published. Group Members of MedStar Breast Surgery Division: M.B., P.W., I.T.G., and K.L.F. did acquisition of data, revising the article, and gave final approval of the version to be published. E.M.W. and K.P. did acquisition of data and interpretation of data, revising the article, and gave final approval of the version to be published. L.K.T. conception and design, acquisition of data, interpretation of data, revising the article, and gave final approval of the version to be published.
Footnotes
Published online 2 May 2023
Disclosure: The authors declare that they have nothing to disclose.
REFERENCES
- 1.Fu MR, Kang Y. Psychosocial impact of living with cancer-related lymphedema. Semin Oncol Nurs. 2013;29:50–60. [DOI] [PubMed] [Google Scholar]
- 2.Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77:1009–1020. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Chang DW. Surgical treatment of primary lymphedema. Lymphat Res Biol. 2017;15:220–226. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Verhey EM, Torres-Guzman RA, et al. Outcomes of lymphovenous anastomosis for upper extremity lymphedema: a systematic review. Plast Reconstr Surg Glob Open. 2021;9:e3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrell RM, Halyard MY, Schild SE, et al. Breast cancer-related lymphedema. Mayo Clin Proc. 2005;80:1480–1484. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie TC, Sayegh HE, Brunelle CL, et al. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg. 2018;7:379–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipman K, Luan A, Stone K, et al. Lymphatic microsurgical preventive healing approach (LYMPHA) for lymphedema prevention after axillary lymph node dissection-a single institution experience and feasibility of technique. J Clin Med. 2021;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson AR, Kimball S, Epstein S, et al. Lymphedema incidence after axillary lymph node dissection: quantifying the impact of radiation and the lymphatic microsurgical preventive healing approach. Ann Plast Surg. 2019;82(4S):S234–S241. [DOI] [PubMed] [Google Scholar]
- 9.Shah C, Vicini FA. Breast cancer-related arm lymphedema: incidence rates, diagnostic techniques, optimal management and risk reduction strategies. Int J Radiat Oncol Biol Phys. 2011;81:907–914. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Garza RM, Chang DW. Lymphatic microsurgical preventive healing approach (LYMPHA) for the prevention of secondary lymphedema. Breast J. 2020;26:721–724. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein B, Le NK, Robertson E, et al. Reverse lymphatic mapping and immediate microsurgical lymphatic reconstruction reduces early risk of breast cancer-related lymphedema. Plast Reconstr Surg. 2022;149:1061–1069. [DOI] [PubMed] [Google Scholar]
- 12.Schaverien MV, Aldrich MB. New and emerging treatments for lymphedema. Semin Plast Surg. 2018;32:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezzo J, Manheimer E, McNeely ML, et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Systemat Rev. 2015;5:CD003475. doi:10.1002/14651858.CD003475.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moseley AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol. 2007;18:639–646. [DOI] [PubMed] [Google Scholar]
- 15.Oremus M, Dayes I, Walker K, et al. Systematic review: conservative treatments for secondary lymphedema. BMC Cancer. 2012;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva AK, Chang DW. Vascularized lymph node transfer and lymphovenous bypass: Novel treatment strategies for symptomatic lymphedema. J Surg Oncol. 2016;113:932–939. [DOI] [PubMed] [Google Scholar]
- 17.Chang DW, Masia J, Garza R, 3rd, et al. Lymphedema: surgical and medical therapy. Plast Reconstr Surg. 2016;138(3 Suppl):209S209s–209S218S. [DOI] [PubMed] [Google Scholar]
- 18.Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: a new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol. 2009;16:703–708. [DOI] [PubMed] [Google Scholar]
- 19.Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. [DOI] [PubMed] [Google Scholar]
- 20.Fu MR, Cleland CM, Guth AA, et al. L-dex ratio in detecting breast cancer-related lymphedema: reliability, sensitivity, and specificity. Lymphology. 2013;46:85–96. [PMC free article] [PubMed] [Google Scholar]
- 21.Basta MN, Wu LC, Kanchwala SK, et al. Reliable prediction of postmastectomy lymphedema: the risk assessment tool evaluating lymphedema. Am J Surg. 2017;213:1125–1133.e1. [DOI] [PubMed] [Google Scholar]
- 22.Jonczyk MM, Jean J, Graham R, et al. Trending towards safer breast cancer surgeries? Examining acute complication rates from a 13-year NSQIP analysis. Cancers (Basel). 2019;11:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahamoff M, Gupta N, Munoz D, et al. A lymphedema surveillance program for breast cancer patients reveals the promise of surgical prevention. J Surg Res. 2019;244:604–611. [DOI] [PubMed] [Google Scholar]
- 24.Feldman S, Bansil H, Ascherman J, et al. Single institution experience with lymphatic microsurgical preventive healing approach (LYMPHA) for the primary prevention of lymphedema. Ann Surg Oncol. 2015;22:3296–3301. [DOI] [PubMed] [Google Scholar]
- 25.Torres Lacomba M, Yuste Sánchez MJ, Zapico Goñi A, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ. 2010;340:b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDuff SGR, Mina AI, Brunelle CL, et al. Timing of lymphedema after treatment for breast cancer: when are patients most at risk? Int J Radiat Oncol Biol Phys. 2019;103:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]