Abstract
Objective:
Sleep is a multi-dimensional health behavior associated with elevated risk of substance use. This is the first study to utilize a latent class approach to characterize sleep health across multiple dimensions and across time from late adolescence to emerging adulthood, and to examine associations with alcohol and cannabis use trajectories.
Methods:
The sample included 2995 emerging adults (mean ages = 18 to 24 years across six waves of data collection; 54% female) who provided data on sleep dimensions (quality, duration, and social jetlag) and frequency and consequences of alcohol and cannabis use. Longitudinal latent class analysis (LLCA) models characterized participants according to the three sleep dimensions. Latent growth models examined trajectories of frequency and consequences of alcohol or cannabis use over time among emergent sleep classes, with and without controlling for covariates.
Results:
LLCA models identified four sleep classes: good sleepers (n= 451; 15.2%); untroubled poor sleepers (n= 1024; 34.2%); troubled, moderately good sleepers (n=1056; 35.3%); and suboptimal sleepers (n= 460; 15.4%). Good sleepers reported significantly lower levels of alcohol or cannabis use and consequences, and less of an increase in alcohol consequences as compared to suboptimal sleepers.
Conclusions:
Persistent poor sleep health was associated with higher levels of alcohol and cannabis use and consequences, and greater increases in alcohol-related consequences during the transition from late adolescence to emerging adulthood. Findings have important clinical implications, highlighting that addressing multi-dimensional sleep health may be an important, novel target of intervention to reduce substance use frequency and consequences.
Keywords: sleep, alcohol, marijuana use, longitudinal, adolescents, emerging adulthood
1. INTRODUCTION
Emerging adulthood (typically defined as ages 18–24) is a critical period of development characterized by increased independence from parents, changes in biology and behavior, and development of health behaviors that may influence well-being into adulthood 1,2. For example, substance use increases during this time3,4, and is associated with negative consequences across the lifespan, including risk of substance use disorders, mental and physical health morbidity, and premature mortality5–9. Sleep is also a critical health behavior that undergoes substantial changes during adolescence and emerging adulthood and is consistently associated with multiple indicators of health and functioning, including risk of substance use, depression, suicide, and cardiometabolic dysregulation10–18. Given that emerging adulthood is a time of increased flexibility in schedules, less parental involvement, and removal of early high school start times (a primary constraint to sufficient sleep in adolescent years) 19,20, this represents a critical period for identifying profiles of sleep health and how such profiles may be associated with alcohol and cannabis use trajectories over time.
Evidence suggests that alcohol and cannabis use may negatively affect sleep and bidirectional associations may exist21; however, a greater understanding is needed of how sleep health profiles over time associate with alcohol and cannabis use, as addressing sleep health may serve as a novel target of intervention to reduce substance use and associated consequences. Considerable longitudinal evidence suggests that poor sleep health may be a risk factor for substance use in childhood, adolescence, and adulthood22–31. To date, however, few studies have addressed sleep health and substance use in emerging adulthood. This gap is notable given that sleep undergoes dramatic changes during this period32, and rates of alcohol and cannabis use increase 33,34.
Given that substance use peaks during emerging adulthood, studying this period affords the opportunity to investigate more clinically relevant substance use outcomes, including changes in frequency of use and consequences, whereas in younger samples it is often infeasible to investigate such outcomes due to low base rates of use4. For example, in our prior work with the current sample, we assessed sleep and substance use trajectories when youth were ages 14 to 16 and continuing for 6 annual waves of data (up to age 21); however, these analyses focused on presence or absence of any alcohol or cannabis use in the past month due to low base rates at younger ages. Thus, there remains a need to investigate how longitudinal sleep health associates with other clinically relevant outcomes, including frequency and consequences of use.
Moreover, prior research (including our own28) has focused on sleep or circadian rhythm disturbances, viewed in isolation, in association with substance use, thereby demonstrating that isolated symptoms including short sleep duration, insomnia or poor sleep quality, and “social jetlag” (i.e., discrepancy between weekday and weekend sleep timing) are cross-sectionally and longitudinally associated with substance use35–41. However, specific phenotypes of sleep disturbances (e.g., combination of insomnia and short sleep duration) are more strongly associated with adverse health outcomes than either sleep disturbance examined in isolation42–44. Further, sleep and circadian disturbances often co-occur10,45, and are considered a transdiagnostic process that underlies other health issues, including problematic substance use46. In fact, a growing body of sleep research suggests that a multi-dimensional approach to conceptualizing sleep health may better reflect clinically-relevant sleep disturbances, and may elucidate common mechanisms and novel targets for intervention46–48.
As articulated by Buysse47, “[S]leep health is a multidimensional pattern of sleep-wakefulness, adapted to individual, social, and environmental demands, that promotes physical and mental well-being.” Buysse’s definition simultaneously highlights the potential role of sleep health in contributing to key health outcomes, and reflects awareness that the specific patterns or profiles of sleep-wakefulness and their association with specific health outcomes are influenced (i.e., “adapted”) by multi-level factors, including the developmental stage of the individual.
Guided by this framework and with an eye towards intervention47, the current study conceptualizes sleep health as a dynamic and multi-dimensional construct comprised of different dimensions of sleep and circadian functioning that may be more or less relevant across various stages of development. This approach may be clinically useful as it may help to identify individuals who suffer from a constellation of sleep and circadian disturbances and may facilitate tailored treatment approaches to specific needs of a given developmental stage45,49. This is particularly salient among adolescents and emerging adults, where insufficient sleep duration and erratic sleep-wake patterns are highly prevalent, but not necessarily indicative, in isolation, of clinically relevant symptoms.
The present study is the first to utilize a longitudinal latent class approach to conceptualize a multi-dimensional sleep health construct, comprised of key sleep health dimensions salient among emerging adults (i.e., sleep duration, quality, and social jetlag), in association with longitudinal trajectories of alcohol and cannabis use frequency and consequences, in a large and diverse sample of late adolescents and emerging adults. We selected these dimensions as prior research has demonstrated that short and long sleep duration50, poor sleep quality51, and social jetlag are longitudinally associated with substance use outcomes, undergo considerable changes from late adolescence through emerging adulthood52,53, and can be measured via survey assessments. Other constructs, such as variability in sleep timing are also relevant for these developmental periods and are consistent with the sleep health framework, but are more difficult to measure via surveys47. The current work builds upon and extends prior research and our prior longitudinal work in this cohort28 by 1) examining frequency of use and consequences associated with use, as opposed to presence or absence of use2) considering sleep health as a multidimensional construct, versus a set of individual symptoms, 3) examining these associations into early adulthood, and 4) applying a longitudinal latent class approach, which yields novel information concerning patterning of specific sleep health dimensions over time and their association with substance use outcomes. We hypothesized that those in the poorest sleep health class would have the greatest frequency and consequences of alcohol and cannabis use.
2.0. METHODS
2.1. Participants
This study utilized six waves of data from wave 8 (June 2015 to May 2016) to wave 13 (July 2020 to July 2021) from a large multi-wave study of participants in southern California. The sample included 2,995 participants (mean age 18.3 at Wave 8 and 23.6 at Wave 13) enrolled when participants were in 6th and 7th grades (ages 11 to 13) in 2008 for a one-year substance prevention program, CHOICE. Participants were representative of the 16 middle schools in southern California from which they were recruited54. Participants transitioned to over 200 different high schools and were re-contacted and re-consented to complete annual web-based surveys. Average retention rate across waves 1–13 is 85%, with 82% of the sample completing 4 or more waves between wave 8–13. Participants were paid $50 for completing each survey. The study has a Certificate of Confidentiality, and all procedures were approved by the [BLIND] Human Subjects Protection Committee.
2.2. Measures
2.2.1. Sleep Health Dimensions
The sleep health composite measure was derived at 6 waves (waves 8 to 13) from three dichotomous indicators of sleep, based on the following dimensions: sleep quality, sleep duration, and social jetlag. This approach is consistent with prior research using a sleep health framework48,55 and has clinical utility as it provides meaningful distinctions among groups. We dichotomize each dimension into “good” (1) versus “suboptimal” (0) categories.
Sleep quality was assessed with an item from the 15-item Patient Health Questionnaire (PHQ) Somatic Symptom Severity Scale measure56 asking “during the past 4 weeks, how much have you been bothered by trouble sleeping?”, with responses ranging from “not bothered at all” to “bothered a lot”. A response of “not bothered at all” was categorized as good in the dichotomous indicator for sleep quality, and responses of “bothered a little” and “bothered a lot” were categorized as suboptimal.
Sleep duration was calculated by taking the difference between self-reported bed and wake times for weekdays and weekends separately. Given that this calculated measure does not account for wakefulness during the sleep period, it is actually a measure of “time in bed”. However, to be consistent with the literature, which has used similar methods57 and to facilitate interpretability, we refer to this variable as “sleep duration”. Based on established recommendations for sleep duration in adolescents and young adults which recognize that there may be health consequences associated with both short and long duration 58,59, sleep duration was considered “good” if the time between bed and wake time was between 8 and 10 hours, inclusive; sleep duration less than 8 hours or greater than 10 hours was deemed “suboptimal”.
Social jetlag was calculated as the absolute difference between the mid-point of sleep during weekdays and the mid-point of sleep during weekends. Mid-point of sleep was calculated as the half-way point between self-reported bed and wake times. Larger discrepancies in weekday versus weekend sleep midpoint is indicative of greater circadian misalignment. Based on prior research60,61, we set thresholds for good social jetlag (having < 2 hours of difference between weekday and weekend mid-sleep) and suboptimal social jetlag (>2 hours of difference between weekday and weekend mid-sleep).
2.2.2. Alcohol and Cannabis Use Outcomes
Alcohol and cannabis use were assessed with separate items asking “during the past month (30 days), how many days did you use [at least one drink of alcohol]/[marijuana (pot, weed, grass, hash, bud, sins)]?” At waves 8–10, response options ranged from 1 = 0 days to 7 = 20–30 days. Items were re-coded such that the mid-point was taken for responses with ranges (e.g., 3–5 days re-coded to 4 days) and response values represented actual number of days used (e.g., 0 days coded as 0), resulting in a range of 0–25 days. At waves 11–13, respondents entered a value from 0–30; these responses were re-coded to match the range from the earlier waves (e.g., responses between 3–5 days were re-coded to 4).
Alcohol and cannabis use consequences were assessed (waves 8–13) by asking respondents how often in the past year things happened to them because of drinking alcohol or using marijuana (e.g., missed school or work or other obligations). Response options ranged from “never” to “20 or more times.” Items were dichotomized to indicate that the consequence happened one or more times in the past year versus never; dichotomous indicators were summed to create consequence scores, with ranges of 0–5 (alcohol) and 0–4 (marijuana).
2.2.3. Covariates
Respondents reported age, sex assigned at birth (male or female), race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latinx/o, Asian, and other/multi-racial), and maternal education (dichotomized as associate degree/college or higher vs some college/high school or less). Given strong associations between mental health and sleep and substance use62, mental health was included as a covariate using the validated Mental Health Inventory (MHI-5)63, comprised of five items assessing past month anxiety and depression symptoms. Finally, we controlled for middle school intervention status.
2.3. Analytic Plan
The analytic strategy was comprised of two steps: 1) identify longitudinal latent classes based on multidimensional sleep health measures; and 2) estimate and compare trajectories of alcohol and cannabis frequency and consequences by multi-dimensional sleep classes. First, we conducted a longitudinal latent class analysis (LLCA), a type of mixture model focused on modeling patterns across time rather than scaled change as is typical of growth mixture models. In a traditional growth mixture model, a given number of classes might emerge wherein each class is defined by a distinct intercept and slope. LLCA classifies individuals based on patterns over time and as such do not assume any growth function (e.g., linear or quadratic). Thus, class assignment is based on similar item response patterns. LLCA models were estimated in Mplus version 8.0 64 using the manual three-step approach 65. We included all sleep health measures across waves in the model and estimated a series of LLCAs until converging on an optimal solution. We determined the optimal class solution for the multi-dimensional sleep health measure by evaluating the combination of several fit indices including: negative two log likelihood (−2LL), Akaike Information Criteria (AIC), Bayesian Information Criteria (BIC), the sample size adjusted Bayesian Information Criteria (aBIC), the Vuong-Lo-Mendell-Rubin adjusted likelihood ratio test (VLMR), and the Lo-Mendell-Rubin (LMR) adjusted likelihood ratio test. For all log likelihood measures (−2LL, AIC, BIC, and aBIC), lower values indicate better fit 66. Likelihood ratio testing (VLRM and LMR) provides a significance test evaluating the improvement of an additional class (e.g., 4 versus 5 classes) 66. We also considered the theoretical interpretation and sample size of the emergent classes. Thus, both statistical and theoretical rationale, concerning typical clinical presentation of sleep profiles, guided our final model selection67.
Second, we used logits for the classification probabilities for the most likely latent class membership obtained from the LLCA model and then estimated growth models across latent classes. We repeated this process for each longitudinal use or consequence measure. We estimated intercept, linear (slope), and non-linear (quadratic) growth factors for each model and tested for statistical differences between classes (e.g., do slopes differ between classes?) using a Wald test. For all growth models, we included the set of covariates listed previously. Finally, although analyzing racial/ethnic or gender differences in the longitudinal sleep health classes is beyond the scope of this paper, it is important for descriptive purposes. We therefore provide this information in supplemental Table 1.
3.0. RESULTS
3.1. Sample Description
The sample was on average 18.3 years old (SD = 0.8) at wave 8, 53.7% female, 20.4% non-Hispanic White, 2.2% non-Hispanic Black, 45.9% Hispanic, 19.9% Asian, and 11.5% other/multi-racial (Table 1). Table 2 reports descriptives for each sleep health dimension. The proportion of respondents with good sleep quality steadily decreased over time, from 56% at the first time point to 48% at the last time point. In contrast, the proportion of respondents in good categories for sleep duration (33–44%) and social jetlag (62–74%) increased across time points. Supplemental Table 1 includes information on sociodemographic differences in the longitudinal sleep classes (e.g., Classes 2 (49%) and 4 (53%) had a significantly greater proportion of Hispanic respondents compared to Classes 1 (40%) and 3 (42%); and Class 3 (60%) had a greater proportion of females compared to all other classes (47–54%)).
Table 1.
Sample Descriptives at Wave 8
| Covariates | |
|---|---|
|
| |
| Age (wave 8), mean (SD), years | 18.3 (0.8) |
| Male, n (%) | 1,385 (46.3%) |
| Race/ethnicity, n (%) | |
| Non-Hispanic White | 612 (20.4%) |
| Non-Hispanic Black | 67 (2.2%) |
| Hispanic | 1,374 (45.9%) |
| Non-Hispanic Asian | 596 (19.9%) |
| Non-Hispanic Other/Multi-racial | 345 (11.5%) |
| Maternal education, n (%) | |
| Some college/high school or less | 1,236 (55.1%) |
| Associate degree or bachelor’s degree and higher | 1,007 (44.9%) |
| CHOICE intervention, n (%) | 1,521 (50.9%) |
| MHI-5 (wave 8), mean (SD) | 65.3 (20.4) |
Table 2.
Substance Use and Sleep Health Dimensions (Continuous and Binary Indicators) from Wave 8 to Wave 13
| Wave 8 | Wave 9 | Wave 10 | Wave 11 | Wave 12 | Wave 13 | |
| Alcohol Use, past month, mean (SD) of days | 1.8 (4.1) | 2.3 (4.4) | 3.5 (5.2) | 4.7 (6.2) | 4.5 (6.1) | 4.5 (6.3) |
| Cannabis Use, past month, mean (SD) of days | 2.3 (6.3) | 2.9 (6.9) | 3.5 (7.5) | 3.6 (7.6) | 3.4 (7.6) | 3.8 (7.9) |
| Alcohol Consequences, mean (SD) | 0.8 (1.3) | 1.0 (1.4) | 1.2 (1.5) | 1.3 (1.5) | 1.3 (1.5) | 1.1 (1.4) |
| Cannabis Consequences, mean (SD) | 0.3 (0.7) | 0.3 (0.8) | 0.3 (0.8) | 0.3 (0.8) | 0.3 (0.7) | 0.3 (0.7) |
| Sleep Quality*, mean (SD) | 1.6 (0.7) | 1.6 (0.7) | 1.6 (0.7) | 1.7 (0.7) | 1.7 (0.7) | 1.7 (0.7) |
| Good Sleep Quality, n (%) | 1396 (55.9%) | 1329 (55.1%) | 1209 (49.9%) | 1222 (49.2%) | 1224 (48.5%) | 1155 (48.1%) |
| Sleep Duration (hours), mean (SD) | 8.3 (1.2) | 8.3 (1.2) | 8.1 (1.2) | 8.1 (1.2) | 8.2 (1.2) | 8.3 (1.1) |
| Good Sleep Duration, n (%) | 813 (32.5%) | 881 (36.6%) | 811 (33.7%) | 808 (32.8%) | 908 (36.2%) | 1063 (44.4%) |
| Social Jetlag (hours), mean (SD) | 1.7 (1.1) | 1.6 (1.1) | 1.6 (1.1) | 1.6 (1.1) | 1.6 (1.1) | 1.4 (1.1) |
| Good Social Jetlag, n (%) | 1492 (61.7%) | 1540 (65.4%) | 1499 (64.8%) | 1618 (67.6%) | 1661 (66.0%) | 1771 (74.1%) |
Note.
1 = Not bothered at all by trouble sleeping, 3 = Bothered a lot.
“Good” indicates meeting defined threshold criteria for the given sleep dimension.
3.2. Deriving Longitudinal, Multi-dimensional Sleep Classes
Based on results from likelihood ratio tests (e.g., VLMR) from the LLCAs, in combination with theoretical consideration, a four-class solution best represented longitudinal patterns of average sleep duration, healthy social jetlag, and trouble sleeping. Model fit indices and likelihood ratio tests are presented in Table 3. All information criteria (−2LL, AIC, BIC, aBIC) improved as the number of extracted classes increased; however, the five-class solution was not a statistical improvement over the four-class as indicated by VLMR (p=.1214) and LMR (p=.123) likelihood ratio tests. Item probabilities across all waves and by class are presented in Figure 1. The resulting four classes were: Class 1 (n=454, 15.2%) -- good sleepers (good across all dimensions); Class 2 (n=1024, 34.2%) -- untroubled poor sleepers (good sleep quality, but suboptimal sleep duration and social jetlag). Class 3 (n=1056, 35.3%) - troubled moderately good sleepers (suboptimal sleep quality, but moderately good sleep duration and social jetlag). Class 4 (n=460, 15.4%) -- poor sleepers (suboptimal across all 3 dimensions).
Table 3.
Model Fit Statistics and Likelihood Ratio Tests for Longitudinal Latent Class Analyses
| Class | −2LL | df | AIC | BIC | aBIC | VLMR | LMR |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 57701.058 | 18 | 57737.059 | 57845.137 | 57787.944 | -- | -- |
| 2 | 55237.202 | 37 | 55311.202 | 55533.363 | 55415.800 | 0.0000 | 0.0000 |
| 3 | 54469.358 | 56 | 54581.358 | 54917.603 | 54739.669 | 0.0008 | 0.0009 |
| 4 | 54074.636 | 75 | 54224.636 | 54674.964 | 54436.660 | 0.0001 | 0.0001 |
| 5 | 53872.094 | 94 | 54060.095 | 54624.505 | 54325.831 | 0.1214 | 0.1230 |
Note. AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; aBIC = Sample size-adjusted BIC; VLMR = Vuong-Lo-Mendell-Rubin Likelihood Ratio Test; LMR = Lo-Mendell-Rubin Likelihood Ratio Test. (*) denotes the current class structure (k classes) was a significant improvement over the previous class structure (k-1 classes). (#) denote models with non-convergence perturbations.
Figure 1.
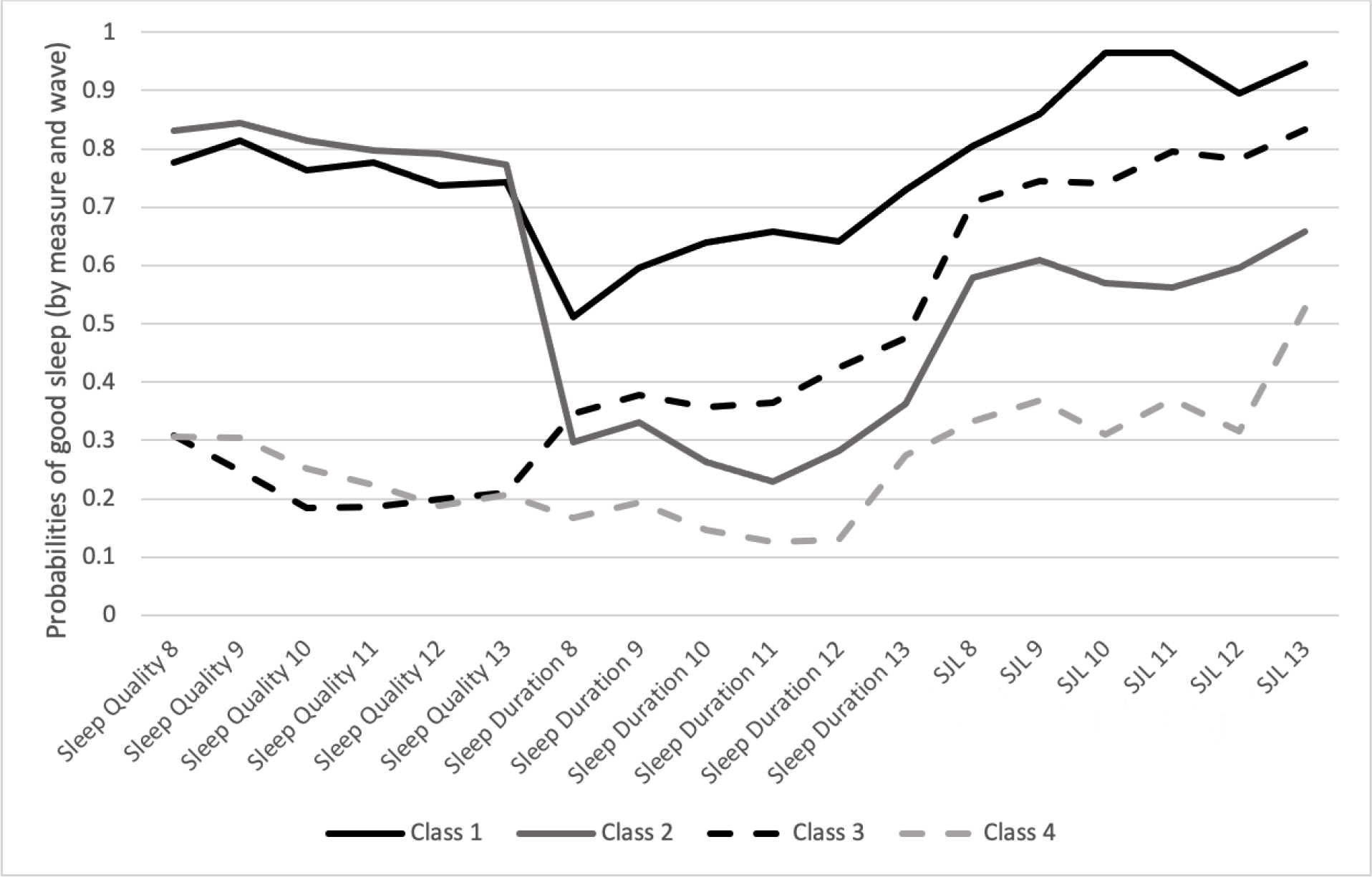
Longitudinal patterns of multidimensional sleep measures.
Note. Class 1: Good sleepers (good across all dimensions); Class 2: Untroubled, poor sleepers (good sleep quality but suboptimal for sleep duration and social jetlag), Class 3: Troubled, moderately good sleepers (suboptimal sleep quality, but moderately good across other 2 dimensions), and Class 4: Poor sleepers (suboptimal across all dimensions).
3.3. LLCA Growth Models
Using class probabilities from the LLCA, we fit growth models for alcohol use, cannabis use, alcohol consequences, and cannabis consequences to explore differences in trajectories by sleep class. Growth parameters (intercept, slope, quadratic) are presented for each longitudinal measure and for each class in Table 4. Trajectory plots by class for each longitudinal measure are presented in Figure 2. For each longitudinal measure, we tested to see if growth parameters differed across sleep health classes.
Table 4.
Growth parameters by class for each longitudinal substance use and consequences measures
| Class 1 | Class 2 | Class 3 | Class 4 | |
|---|---|---|---|---|
| Alcohol Frequency | ||||
| Intercept | 1.491 p<.001 |
1.372 p<.001 |
1.727 p<.001 |
2.079 p<.001 |
| Slope | 1.079 p<.001 |
1.116 p<.001 |
1.291 p<.001 |
1.319 p<.001 |
| Quadratic | −0.101 p<.001 |
−0.105 p<.001 |
−0.123 p<.001 |
−0.147 p<.001 |
| Alcohol Consequences | ||||
| Intercept | 0.664 p<.001 |
0.586 p<.001 |
0.859 p<.001 |
0.900 p<.001 |
| Slope | 0.255 p<.001 |
0.329 p<.001 |
0.371 p<.001 |
0.419 p<.001 |
| Quadratic | −0.042 p<.001 |
−0.05 p<.001 |
−0.061 p<.001 |
−0.065 p<.001 |
| Cannabis Frequency | ||||
| Intercept | 1.947 p<.001 |
2.164 p<.001 |
2.606 p<.001 |
2.545 p<.001 |
| Slope | 0.761 p<.001 |
0.685 p<.001 |
0.639 p<.001 |
0.869 p<.001 |
| Quadratic | −0.109 p<.001 |
−0.082 p=.001 |
−0.064 p=.013 |
−0.107 p=.003 |
| Cannabis Consequences | ||||
| Intercept | 0.231 p<.001 |
0.199 p<.001 |
0.318 p<.001 |
0.316 p<.001 |
| Slope | 0.035 p=.078 |
0.057 p<.001 |
0.064 p<.001 |
0.081 p<.001 |
| Quadratic | −0.008 p=.023 |
−0.01 p<.001 |
−0.013 p<.001 |
−0.015 p<.001 |
Figure 2.
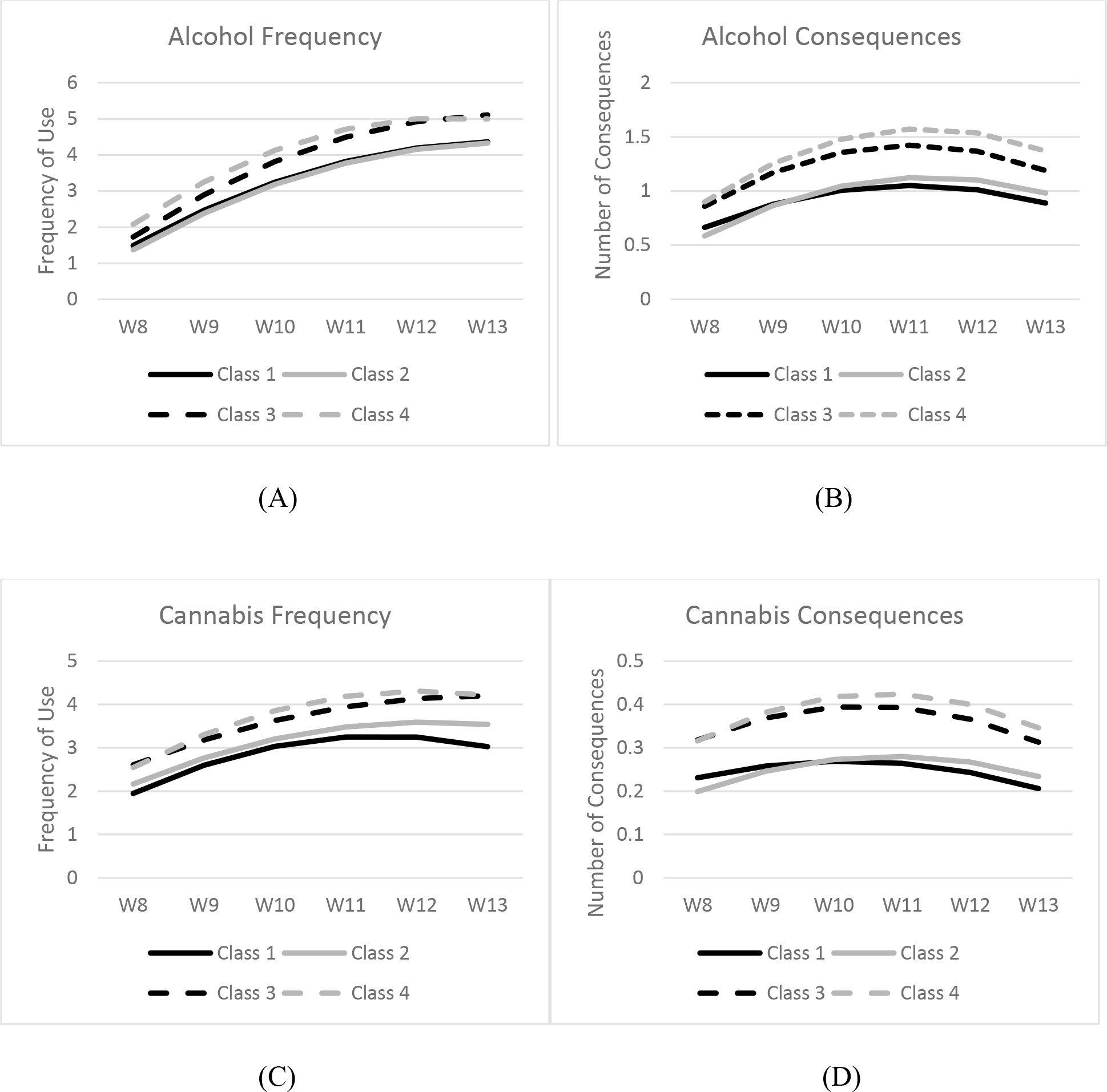
Alcohol and Cannabis use frequency and consequences over time (Wave 8–13) according to sleep health class. Mean ages at each wave were: W8: 18.3, W9: 19.4, W10: 20.7, W11: 21.6, W12: 22.6, W13: 23.6. Panel A shows the alcohol use frequency trajectories by sleep class. Panel B shows the trajectories of alcohol-related consequences by sleep class. Panel C shows the cannabis use frequency trajectories by sleep class. Panel D shows the trajectories of cannabis-related consequences by sleep class. Class 1: Good sleepers (good across all dimensions); Class 2: Untroubled, poor sleepers (good sleep quality but suboptimal for sleep duration and social jetlag), Class 3: Troubled, moderately good sleepers (suboptimal sleep quality, but moderately good across other 2 dimensions), and Class 4: Poor sleepers (suboptimal across all dimensions).
Alcohol Frequency.
Trajectories of alcohol use by sleep class are depicted in panel A of Figure 1. All sleep classes reported some level of initial alcohol use which increased over time, and then stabilized between waves 12 and 13. The poor sleeper class had the highest levels of initial alcohol use, and this was significantly higher than the good sleeper class (χ2=7.01, p=.008) and untroubled poor sleeper class (χ2=12.17, p=.001). Further the troubled, moderately good sleeper class had significantly higher initial alcohol use frequency than the untroubled poor sleeper class (χ2=5.51, p=.019). There were no significant differences across classes in terms of change in alcohol use over time (i.e., slopes and quadratic).
Alcohol Consequences.
Trajectories of alcohol consequences by sleep class are depicted in panel B of Figure 1. All sleep classes reported some initial alcohol consequences, which increased over time, but then started to decline at wave 12. Poor sleepers had greater initial alcohol consequences than both the good sleepers (χ2=11.99, p=.001) and untroubled poor sleepers (χ2=36.36, p<.0001). Similarly, troubled, moderately good sleepers had greater consequences than both good sleepers (χ2=11.78, p=.001) and untroubled poor sleepers (χ2=31.24, p<.0001). Regarding linear change, poor sleepers had a steeper increase in consequences than good sleepers (χ2=10.18, p=.001) and untroubled poor sleepers (χ2=4.14, p=.04). Troubled, moderately good sleepers had a steeper increase in consequences than healthy sleepers (χ2=7.15, p=008). Lastly, regarding the deceleration or downturn (i.e., quadratic term), good sleepers had a significantly less pronounced downturn or decline in consequences than troubled, moderately good sleepers (χ2=4.92, p=.03) and 4 (χ2=5.2, p=.023).
Cannabis Frequency.
Trajectories of cannabis use frequency by sleep class are depicted in panel C of Figure 1. All sleep classes reported some level of initial cannabis use which increased over time, and then either stabilized or began to decelerate between waves 12 and 13. Relative to the good sleeper class, troubled, moderately good sleepers (χ2=6.091, p=.014) and poor sleepers (χ2=3.66, p=.056) reported greater initial cannabis use frequency. There were no significant differences across classes in terms of change in use over time (i.e., slopes and quadratic).
Cannabis Consequences.
Trajectories of cannabis consequences by sleep class are depicted in panel D of Figure 1. All sleep classes reported some initial cannabis consequences, which increased over time (except for troubled, moderately good sleepers), but then started to decline at wave 12. Unhealthy sleepers had greater initial consequences than both the good sleepers (χ2=4.93, p=.026) and untroubled poor sleepers (χ2=13.25, p=.0003). Similarly, troubled, moderately good sleepers had greater consequences than both good sleepers (χ2=7.17, p=.007) and untroubled poor sleepers (χ2=18.44, p<.0001). There were no significant differences across classes in terms of change in consequences over time (i.e., slopes and quadratic).
4.0. DISCUSSION
The current study evaluated longitudinal associations between multiple dimensions of sleep health and alcohol and cannabis use and associated consequences during emerging adulthood. We examined sleep quality, sleep duration, and social jetlag as key components of sleep health in emerging adulthood,32 as these dimensions undergo considerable changes during these developmental periods and are associated with substance use outcomes31,52,53,68,69. Descriptively, our findings showed interesting developmental changes, with the proportion of respondents with good sleep quality (based on our dichotomous definitions) steadily decreasing over time, whereas the proportion of respondents in good categories for sleep duration and social jetlag increased over time. This is consistent with prior evidence demonstrating that developmentally, the peak in circadian phase delay (i.e., evening preference) tends to occur in one’s early 20s69, after which it tends to shift earlier, but at the same time, social jetlag is reduced, as sleep is less constrained by early school start times70.
Importantly, prior research has focused on individual sleep health dimensions using a variable-centered approach71. For example, in our prior work28 in this sample when youth were ages 16–22, we analyzed changes in several sleep health dimensions individually over time and their longitudinal associations with any alcohol or cannabis use. In contrast, in the current study, using a sleep health perspective47 and a person-centered analytic approach71, we analyzed multiple sleep health dimensions simultaneously to identify four longitudinal sleep health classes from late adolescence into emerging adulthood and examined the associations between these sleep health classes and substance use outcomes. Furthermore, given the older age range of the current sample, we were able to examine associations between longitudinal sleep health classes and the frequency and consequences of alcohol or cannabis use, as opposed to a binary outcome of “any” versus “no” use as in our prior paper.
The classes included: good sleepers (good across all dimensions), untroubled poor sleepers (good sleep quality but suboptimal for sleep duration and social jetlag), troubled moderately good sleepers (suboptimal sleep quality, but moderately good across other 2 dimensions), and poor sleepers (suboptimal across all dimensions). The majority of emerging adults were either troubled but had good sleep, or not troubled but had suboptimal sleep. Only 15% were good across all dimensions or suboptimal across all dimensions over time. Findings highlight the considerable heterogeneity in sleep profiles and the clinical relevance of addressing the subjective experience of poor quality sleep, even in the absence of other quantitative indicators of sleep disturbance.
The poor sleeper class reported significantly higher frequency of alcohol use and greater linear increases as compared to good sleepers or untroubled poor sleepers. Further, troubled but otherwise good sleepers reported significantly higher frequency of initial alcohol use as compared to the untroubled, poor sleepers and significantly greater increases in alcohol use than good sleepers. Thus, the presence of insomnia-related symptoms such as poor sleep quality seem to be more strongly associated with frequent alcohol use, consistent with prior evidence demonstrating that insomnia-related symptoms are often more strongly linked with negative health outcomes than quantitative dimensions of sleep (e.g., sleep duration)72–74.
We observed a similar pattern for initial frequency of cannabis use, with good sleepers reporting less frequent use than either troubled, moderately good sleepers, or poor sleepers. Similarly, regarding initial levels of cannabis use consequences, poor sleepers reported more consequences than good sleepers and untroubled poor sleepers, whereas troubled, moderately good sleepers reported greater initial consequences as compared to good sleepers or untroubled, poor sleepers. However, there were no sleep health class differences in trajectories of cannabis use frequency or consequences over time.
Overall, findings underscore key developmental patterns in sleep health profiles and alcohol and cannabis use and consequences over time. Consistent with a sleep health perspective, the majority of this sample of emerging adults experienced problematic sleep health on some dimensions (as opposed to being healthy across all dimensions). Further, those with more optimal sleep profiles reported less alcohol or cannabis use. Results suggest the relative importance of subjective sleep quality as a potential intervention target as the presence of poor sleep quality, alone or in combination with other poor sleep health dimensions, may portend poorer substance use-related outcomes.
Findings have important clinical implications for identifying populations that may be most at-risk, and highlight the need for deploying evidence-based, behavioral interventions that target insomnia (e.g., cognitive behavioral therapy for insomnia)75 and transdiagnostic approaches (e.g., Trans-C76) that also address common circadian disruptions among this age group. Given that our findings cannot rule out bidirectional associations, which have been reported in prior work77, it is also critical for interventions to address motivations for using substances, which may include misconceptions about the soporific effects of cannabis or alcohol78, and to provide psychoeducation concerning the effects of alcohol and cannabis on sleep.
Findings must be considered in light of limitations. First, we utilized self-report, which may have introduced common method variance or self-report bias. Furthermore, we selected sleep health dimensions a priori, based on prior research demonstrating the importance of these sleep dimensions during late adolescence and emerging adulthood and that could be measured with survey items. However, other sleep dimensions (e.g., daytime sleepiness, snoring, variability) as well as other substance use outcomes including amount of the substance, may also be relevant. Relatedly, multi-dimensional sleep health is an evolving construct and our specific approach to operationalizing sleep health with dichotomous indicators was motivated by a clinical perspective. However, an important unanswered question is whether sleep health is more strongly associated with substance use outcomes compared to sleep measures viewed in isolation. Furthermore, there are limitations with regard to the dichotomous approach we used to characterize each dimension. Namely, there are not established guidelines for what constitutes “good” or “suboptimal” social jetlag. However, prior research suggests that social jetlag greater than two hours (as defined herein as “suboptimal”) is associated with greater depression and higher blood cortisol levels79,80. In addition, even for sleep duration where published recommendations exist, there is discrepancy in recommendations, with some establishing an upper limit for sleep duration59,81and others not82. There is also inconsistency in recommendations with regard to age cutoffs for the two developmental periods covered in the current study—adolescents and young adults. Thus, it is possible that findings regarding latent sleep health classes and their associations with substance use outcomes would be different based on different operationalizations of sleep health. Our analyses adjusted for several relevant covariates; however, other factors, such as family or social support, personality characteristics, or other sociodemographic factors may influence findings. Furthermore, our supplemental analyses suggest several sociodemographic differences in longitudinal sleep profiles. Examining how the association between longitudinal sleep health classes and substance use outcomes may differ by sociodemographic characteristics was beyond the scope of this paper but is a critically important opportunity for future research. In addition, we did not examine potential mechanisms underlying associations between sleep health and substance use levels and trajectories; however, prior research suggests that altered neural responses to reward, as well as impacts on affective and behavioral regulation may be candidate mechanisms83,84. Finally, we chose to address how sleep affects trajectories of substance use; future research is needed to examine potential bidirectional associations over time, in order to determine how and when to most effectively intervene.
In sum, findings indicate that emerging adults who face a cluster of sleep and circadian disturbances may be at greatest risk for problematic alcohol and cannabis use, and that those with poor sleep quality were more likely to use substances and report consequences from use. Results highlight the importance of transdiagnostic approaches to treat poor sleep quality as well as circadian rhythm disruptions common to this age group, as a potential strategy to reduce risk of alcohol and cannabis use-related consequences and thereby decrease the likelihood of continued problems into adulthood.
Supplementary Material
Acknowledgments
This research was supported by three grants from the National Institute on Alcohol Abuse and Alcoholism (R01AA016577, R01AA020883, R01AA025848; PI: D’Amico). The authors would like to thank the study participants and districts and schools who participated and supported the CHOICE project. We also thank Jennifer Parker for overseeing the survey administration.
References
- 1.Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000;55(5):469–480. [PubMed] [Google Scholar]
- 2.Sussman S, Arnett JJ. Emerging adulthood: Developmental period facilitative of the addictions. Evaluation & the Health Professions. 2014;37(2):147–155. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico EJ, Tucker JS, Miles JNV, Ewing BA, Shih RA, Pedersen ER. Alcohol and marijuana use trajectories in a diverse longitudinal sample of adolescents: examining use patterns from age 11 to 17 years. Addiction 2016;111(10):1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 1975–2014: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan.;2015. [Google Scholar]
- 5.Schulte MT, Hser Y-I. Substance Use and Associated Health Conditions throughout the Lifespan. Public Health Rev. 2014;35(2): https://web-beta.archive.org/web/20150206061220/http://www.publichealthreviews.eu/upload/pdf_files/20150206061214/20150206061200_Schulte_Hser.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukstein OG, Brent DA, Perper JA, et al. Risk factors for completed suicide among adolescents with a lifetime history of substance abuse: a case-control study. Acta psychiatrica Scandinavica. 1993;88(6):403–408. [DOI] [PubMed] [Google Scholar]
- 7.Mertens JR, Flisher AJ, Fleming MF, Weisner CM. Medical conditions of adolescents in alcohol and drug treatment: comparison with matched controls. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2007;40(2):173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark DB, Martin CS, Cornelius JR. Adolescent-onset substance use disorders predict young adult mortality. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2008;42(6):637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss HB, Chen CM, Yi HY. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 2014;136:51–62. [DOI] [PubMed] [Google Scholar]
- 10.Hasler BP, Pedersen SL. Sleep and circadian risk factors for alcohol problems: a brief overview and proposed mechanisms. Current opinion in psychology. 2019;34:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: A 13-year prospective study. Sleep. 2004;27(4):661–666. [DOI] [PubMed] [Google Scholar]
- 12.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annual review of psychology. 2015;66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. [DOI] [PubMed] [Google Scholar]
- 14.Ivanenko A, Crabtree VM, Gozal D. Sleep and Depression in Children and Adolescents. Sleep Med Rev. 2005;9(2):115–129. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami N, Takatsuka N, Schimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27:282–283. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koren D, Dumin M, Gozal D. Role of sleep quality in the metabolic syndrome. Diabetes, metabolic syndrome and obesity : targets and therapy. 2016;9:281–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris LM, Huang X, Linthicum KP, Bryen CP, Ribeiro JD. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Scientific Reports. 2020;10(1):13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens J Insufficient Sleep in Adolescents and Young Adults: An Update on Causes and Consequences. Pediatrics. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troxel WM, Wolfson AR. The intersection between sleep science and policy: introduction to the special issue on school start times. Sleep health. 2017;3(6):419–422. [DOI] [PubMed] [Google Scholar]
- 21.Pasch KE, Latimer LA, Cance JD, Moe SG, Lytle LA. Longitudinal Bi-directional Relationships Between Sleep and Youth Substance Use. J Youth Adolescence. 2012;41(9):1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasler BP, Bruce S, Scharf D, Ngari W, Clark DB. Circadian misalignment and weekend alcohol use in late adolescent drinkers: preliminary evidence. Chronobiology international. 2019;36(6):796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasler BP, Franzen PL, de Zambotti M, et al. Eveningness and later sleep timing are associated with greater risk for alcohol and marijuana use in adolescence: Initial findings from the National Consortium on Alcohol and Neurodevelopment in Adolescence. Alcoholism: Clinical and Experimental Research. 2017;41(6):1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcoholism, Clinical and Experimental Research. 2004;28(4):578–587. [DOI] [PubMed] [Google Scholar]
- 25.Wong MM, Brower KJ, Zucker RA. Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep medicine. 2009;10(7):787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong MM, Roberson G, Dyson R. Prospective relationship between poor sleep and substance-related problems in a national sample of adolescents. Alcoholism, clinical and experimental research. 2015;39(2):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavernier R, Munroe M, Willoughby T. Perceived morningness–eveningness predicts academic adjustment and substance use across university, but social jetlag is not to blame. Chronobiology international. 2015;32(9):1233–1245. [DOI] [PubMed] [Google Scholar]
- 28.Troxel WM, Rodriguez A, Seelam R, et al. Longitudinal associations of sleep problems with alcohol and cannabis use from adolescence to emerging adulthood. Sleep. 2021;44(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortuna LR, Cook B, Porche MV, Wang Y, Amaris AM, Alegria M. Sleep disturbance as a predictor of time to drug and alcohol use treatment in primary care. Sleep Medicine. 2018;42:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen-Louie TT, Brumback T, Worley MJ, et al. Effects of sleep on substance use in adolescents: a longitudinal perspective. Addiction biology. 2018;23(2):750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho C-Y, Lin S-H, Tsai M-C, Yu T, Strong C. Impact of Cumulative Unhealthy Sleep Practices in Adolescence on Substance Use in Young Adulthood Estimated Using Marginal Structural Modeling. Frontiers in Neuroscience. 2020;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfson AR. Adolescents and Emerging Adults’ Sleep Patterns: New Developments. Journal of Adolescent Health. 2010;46(2):97–99. [DOI] [PubMed] [Google Scholar]
- 33.Tucker JS, Ellickson PL, Orlando M, Martino SC, Klein DJ. Substance use trajectories from early adolescence to emerging adulthood: A comparison of smoking, binge drinking, and marijuana use. Journal of Drug Issues 2005;35:307–332. [Google Scholar]
- 34.Tucker JS, Rodriguez A, Dunbar MS, et al. Cannabis and tobacco use and co-use: Trajectories and correlates from early adolescence to emerging adulthood. Drug Alcohol Depend. 2019;204:107499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fakier N, Wild LG. Associations among sleep problems, learning difficulties and substance use in adolescence. J Adolescence. 2011;34(4):717–726. [DOI] [PubMed] [Google Scholar]
- 36.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11(3):191–199. [DOI] [PubMed] [Google Scholar]
- 37.Gromov I, Gromov D. Sleep and Substance Use and Abuse in Adolescents. Child Adol Psych Cl. 2009;18(4):929–946. [DOI] [PubMed] [Google Scholar]
- 38.Johnson EO, Breslau N. Sleep problems and substance use in adolescence. Drug and Alcohol Dependence. 2001;64(1):1–7. [DOI] [PubMed] [Google Scholar]
- 39.Troxel WM, Ewing BA, D’Amico EJ. Examining Racial/Ethnic Disparities in the Association Between Adolescent Sleep and Alcohol or Marijuana Use. Sleep Health. 2015;1(2):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenney SR, Paves AP, Grimaldi EM, LaBrie JW. Sleep quality and alcohol risk in college students: examining the moderating effects of drinking motives. J Am Coll Health. 2014;62(5):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruce ES, Lunt L, McDonagh JE. Sleep in adolescents and young adults. Clin Med (Lond). 2017;17(5):424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vgontzas AN, Calhoun S, Liao DP, Karataraki M, Pejovic S, Bixler EO. Insomnia with Objective Short Sleep Duration Is Associated With Type 2 Diabetes A population-based study. Diabetes Care. 2009;32(11):1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vgontzas AN, Fernandez-Mendoza J. Insomnia with Short Sleep Duration: Nosological, Diagnostic, and Treatment Implications. Sleep Med Clin. 2013;8(3):309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong L, Martinez AJ, Buysse DJ, Harvey AG. A composite measure of sleep health predicts concurrent mental and physical health outcomes in adolescents prone to eveningness. Sleep Health. 2019;5(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvey AG, Murray G, Chandler RA, Soehner A. Sleep disturbance as transdiagnostic: Consideration of neurobiological mechanisms. Clin Psychol Rev. 2011;31(2):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furihata R, Hall MH, Stone KL, et al. An Aggregate Measure of Sleep Health Is Associated With Prevalent and Incident Clinically Significant Depression Symptoms Among Community-Dwelling Older Women. Sleep. 2017;40(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong L, Dolsen MR, Martinez AJ, Notsu H, Harvey AG. A transdiagnostic sleep and circadian intervention for adolescents: six-month follow-up of a randomized controlled trial. J Child Psychol Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geoffroy PA, Tebeka S, Blanco C, Dubertret C, Le Strat Y. Shorter and longer durations of sleep are associated with an increased twelve-month prevalence of psychiatric and substance use disorders: Findings from a nationally representative survey of US adults (NESARC-III). J Psychiatr Res. 2020;124:34–41. [DOI] [PubMed] [Google Scholar]
- 51.Catrett CD, Gaultney JF. Possible Insomnia Predicts Some Risky Behaviors Among Adolescents When Controlling for Depressive Symptoms. The Journal of genetic psychology. 2009;170(4):287–309. [DOI] [PubMed] [Google Scholar]
- 52.Logan RW, Hasler BP, Forbes EE, et al. Impact of Sleep and Circadian Rhythms on Addiction Vulnerability in Adolescents. Biol Psychiatry. 2018;83(12):987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31(4):276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Amico EJ, Green HDJ, Miles JNV, Zhou AJ, Tucker JA, Shih RA. Voluntary after school alcohol and drug programs: If you build it right, they will come. Journal of Research on Adolescence. 2012;22(3):571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furihata R, Saitoh K, Suzuki M, et al. A composite measure of sleep health is associated with symptoms of depression among Japanese female hospital nurses. Compr Psychiatry. 2020;97:152151. [DOI] [PubMed] [Google Scholar]
- 56.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: Validity of a New Measure for Evaluating the Severity of Somatic Symptoms. Psychosom Med. 2002;64(2):258–266. [DOI] [PubMed] [Google Scholar]
- 57.Kurina LM, McClintock MK, Chen J-H, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all-cause mortality: a critical review of measurement and associations. Annals of epidemiology. 2013;23(6):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. [DOI] [PubMed] [Google Scholar]
- 59.Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2016;12(6):785–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sűdy ÁR, Ella K, Bódizs R, Káldi K. Association of Social Jetlag With Sleep Quality and Autonomic Cardiac Control During Sleep in Young Healthy Men. 2019;13(950). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hena M, Garmy P. Social Jetlag and Its Association With Screen Time and Nighttime Texting Among Adolescents in Sweden: A Cross-Sectional Study. 2020;14(122). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siennick SE, Widdowson AO, Woessner MK, Feinberg ME, Spoth RL. Risk Factors for Substance Misuse and Adolescents’ Symptoms of Depression. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2017;60(1):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berwick DM, Murphy JM, Goldman PA, Ware JE Jr., Barsky AJ, Weinstein MC. Performance of a Five-Item Mental Health Screening Test. Med Care. 1991;29(2):169–176. [DOI] [PubMed] [Google Scholar]
- 64.Muthén LK, Muthén BO. Mplus User’s Guide. Eight Edition. Los Angeles, CA:: Muthén & Muthén.; 2012–2018. [Google Scholar]
- 65.Nylund-Gibson K, Grimm R, Quirk M, Furlong M. A Latent Transition Mixture Model Using the Three-Step Specification. Structural Equation Modeling: A Multidisciplinary Journal. 2014;21(3):439–454. [Google Scholar]
- 66.Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Structural Equation Modeling: A Multidisciplinary Journal. 2007;14(4):535–569. [Google Scholar]
- 67.Lanza ST, Patrick ME, Maggs JL. Latent Transition Analysis: Benefits of a Latent Variable Approach to Modeling Transitions in Substance Use. J Drug Issues. 2010;40(1):93–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McMahon DM, Burch JB, Wirth MD, et al. Persistence of social jetlag and sleep disruption in healthy young adults. Chronobiol Int. 2018;35(3):312–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18(1):80–90. [DOI] [PubMed] [Google Scholar]
- 70.Carskadon MA. Sleep in Adolescents: The Perfect Storm. Pediatric clinics of North America. 2011;58(3):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergman LR, Cairns RB, Nilsson L-G, Nystedt L. Developmental science and the holistic approach. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2000. [Google Scholar]
- 72.Bin YS. Is Sleep Quality More Important Than Sleep Duration for Public Health? Sleep. 2016;39(9):1629–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeSantis AS, Dubowitz T, Ghosh-Dastidar B, et al. A preliminary study of a composite sleep health score: associations with psychological distress, body mass index, and physical functioning in a low-income African American community. Sleep Health: Journal of the National Sleep Foundation. 2019;5(5):514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark AJ, Salo P, Lange T, et al. Onset of Impaired Sleep and Cardiovascular Disease Risk Factors: A Longitudinal Study. Sleep. 2016;39(9):1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline.0(0):jcsm.8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harvey AG, Hein K, Dolsen MR, et al. Modifying the Impact of Eveningness Chronotype (“Night-Owls”) in Youth: A Randomized Controlled Trial. J Am Acad Child Adolesc Psychiatry. 2018;57(10):742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pasch KE, Laska MN, Lytle LA, Moe SG. Adolescent Sleep, Risk Behaviors, and Depressive Symptoms: Are They Linked? Am J Health Behav. 2010;34(2):237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winiger EA, Hitchcock LN, Bryan AD, Cinnamon Bidwell L. Cannabis use and sleep: Expectations, outcomes, and the role of age. Addict Behav. 2021;112:106642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levandovski R, Sasso E, Hidalgo MP. Chronotype: a review of the advances, limits and applicability of the main instruments used in the literature to assess human phenotype. Trends Psychiatry Psychother. 2013;35(1):3–11. [DOI] [PubMed] [Google Scholar]
- 80.Rutters F, Lemmens SG, Adam TC, et al. Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms. 2014;29(5):377–383. [DOI] [PubMed] [Google Scholar]
- 81.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. [DOI] [PubMed] [Google Scholar]
- 82.Panel CC. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcoholism, Clinical and Experimental Research. 2013;37(4):558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research: Neuroimaging. 2013;214(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


