Abstract
Background
In the primary analysis of the PREDICT trial, a higher hemoglobin target (11–13 g/dl) with darbepoetin alfa did not improve renal outcomes compared with a lower hemoglobin target (9–11 g/dl) in advanced chronic kidney disease (CKD) without diabetes. Prespecified secondary analyses were performed to further study the effects of targeting higher hemoglobin levels on renal outcomes.
Methods
Patients with an estimated glomerular filtration rate (eGFR) 8–20 ml/min/1.73 m2 without diabetes were randomly assigned 1:1 to the high- and low-hemoglobin groups. The differences between the groups were evaluated for the following endpoints and cohort sets: eGFR and proteinuria slopes, assessed using a mixed-effects model in the full analysis set and the per-protocol set that excluded patients with off-target hemoglobin levels; the primary endpoint of composite renal outcome, evaluated in the per-protocol set using the Cox model.
Results
In the full analysis set (high hemoglobin, n = 239; low hemoglobin, n = 240), eGFR and proteinuria slopes were not significantly different between the groups. In the per-protocol set (high hemoglobin, n = 136; low hemoglobin, n = 171), the high-hemoglobin group was associated with reduced composite renal outcome (adjusted hazard ratio: 0.64; 95% confidence interval: 0.43–0.96) and an improved eGFR slope (coefficient: + 1.00 ml/min/1.73 m2/year; 95% confidence interval: 0.38–1.63), while the proteinuria slope did not differ between the groups.
Conclusions
In the per-protocol set, the high-hemoglobin group demonstrated better kidney outcomes than the low-hemoglobin group, suggesting a potential benefit of maintaining higher hemoglobin levels in patients with advanced CKD without diabetes.
Clinical trial registration
Clinicaltrials.gov (identifier: NCT01581073).
Supplementary Information
The online version contains supplementary material available at 10.1007/s10157-023-02362-w.
Keywords: Renal anemia, CKD, Kidney, eGFR slope, Proteinuria
Introduction
Chronic kidney disease (CKD) is a globally significant burden that affects society, with an estimated prevalence of 10–13% worldwide. Moreover, the number of patients with CKD requiring renal replacement therapy is estimated to be 4.9–7.0 million [1, 2]. Anemia is a common complication among patients with CKD not requiring dialysis [3, 4]. The prevalence of anemia increases as the stage of CKD progresses, from 8% at stage 1 to 53% at stage 5 [5]. Observational studies have suggested that anemia may be a biomarker independently associated with increased cardiovascular (CV) and kidney events [6–8]. Erythropoiesis-stimulating agents (ESAs) have been widely used to treat renal anemia in patients with CKD on dialysis and those not requiring dialysis. However, interventional studies using ESAs in patients with CKD not requiring dialysis have reported conflicting results [9–14], and the optimal target hemoglobin levels for patients with CKD are unknown [10–13].
A small randomized controlled trial (RCT) conducted by Gouva et al. has demonstrated favorable effects of early intervention using erythropoietin alfa on renal outcomes [9]. Thereafter, three large RCTs failed to show the clinical benefits of targeting higher hemoglobin levels. In the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta (CREATE) trial, using epoetin beta to target high hemoglobin levels (13.0–15.0 g/dl) vs. low hemoglobin levels (10.5–11.5 g/dl) also failed to reduce the incidence of CV events, while the number of patients requiring dialysis therapy significantly increased [11]. In the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial, patients with CKD not requiring dialysis were randomly assigned to target either a high (13.0–13.5 g/dl) or a low hemoglobin level (10.5–11.0 g/dl) using epoetin alfa [12]. However, targeting higher hemoglobin levels was associated with a significantly higher risk of a composite outcome of death and CV events. In the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT), patients with CKD not requiring dialysis who have diabetes were randomly assigned to a placebo and a group receiving darbepoetin alfa to achieve a hemoglobin level of ≥ 13 g/dl [13]. Similarly, darbepoetin alfa failed to reduce the risk of the two primary composite outcomes (CV outcome [death or a CV event] and renal outcome [death or a renal event]). However, it significantly increased the risk of stroke. A meta-analysis report has concluded that targeting higher hemoglobin levels with ESAs reduces the need for blood transfusions but increases the risk of CV and kidney events in patients with CKD [16, 17].
Recently, hypoxia-inducible factor prolyl-hydroxylase inhibitors (HIF-PHIs) have been available. Almost all the studies have successfully demonstrated that the efficiency of HIF-PHIs in treating anemia is comparable with that of ESAs [18–21]. However, no clinical studies have ever shown the clear advantage of HIF-PHIs over ESAs or placebo on the kidney or CV outcomes in patients with CKD not requiring dialysis. Moreover, the optimal target hemoglobin levels using HIF-PHIs for patients with CKD not requiring dialysis have not been previously reported [22].
Tsubakihara et al. conducted an RCT in Japan to investigate the renal protective effects [14, 15]. Although the primary analysis was negative, post-hoc analyses demonstrated that maintaining hemoglobin levels (11–13 g/dl) with darbepoetin alfa improved renal outcomes as compared to maintaining hemoglobin levels (9–11 g/dl) with epoetin alfa in patients at stage 5 CKD not requiring dialysis, particularly those without diabetes. Based on these findings, we conducted an RCT, namely ‘Prevention of end-stage kidney disease (ESKD) by Darbepoetin Alfa in CKD Patients with Non-diabetic Kidney Disease (PREDICT) trial.’ This trial aimed to prove our hypothesis that targeting a higher hemoglobin level (11–13 g/dl) with darbepoetin alfa would prevent ESKD as compared to targeting a lower hemoglobin level (9–11 g/dl) in patients with advanced CKD without diabetes [23]. The primary analysis revealed that targeting a higher hemoglobin level did not significantly improve renal outcomes compared with targeting a lower hemoglobin level [24]. It is noteworthy that in the PREDICT trial, the prognosis of the high-hemoglobin group demonstrated a tendency to improve (hazard ratio [HR] 0.78; 95% confidence interval [CI] 0.60–1.03), whereas the prognosis in the three studies mentioned above [11–13] yielded opposite results.
In this prespecified secondary analysis of the PREDICT trial, we aimed to further clarify the effects of targeting hemoglobin levels using darbepoetin alfa on renal outcomes, including the primary endpoint of composite renal endpoint in the per-protocol set (PPS) and the secondary endpoints of eGFR and proteinuria slopes in the full analysis set (FAS) and PPS, in patients with advanced CKD without diabetes.
Materials and methods
Study design
The PREDICT trial was a multicenter, randomized, open-label, parallel-group study performed in patients with an estimated glomerular filtration rate (eGFR) of 8–20 ml/min/1.73 m2, renal anemia with a hemoglobin level < 10 g/dl and no diabetes. The detailed study design and methods have been previously described [23, 24]. The trial was designed, implemented, and overseen by the PREDICT Executive Committee, along with representatives of the Translational Research Center for Medical Innovation, Kobe, Japan, a third-party organization. The study was registered at Clinicaltrials.gov (identifier: NCT01581073).
Study population and randomization
A total of 491 patients with CKD without diabetes, aged 20–85 years, with an eGFR of 8–20 ml/min/1.73 m2 calculated with the Japanese equation [25] and with a hemoglobin level < 10 g/dl, were randomly assigned to a high- (11–13 g/dl) or a low-hemoglobin (9–11 g/dl) group using darbepoetin alfa in a 1:1 ratio. The registration period was from December 2011 to June 2014, and the observation period lasted two years after the last patient enrollment, up to June 2016.
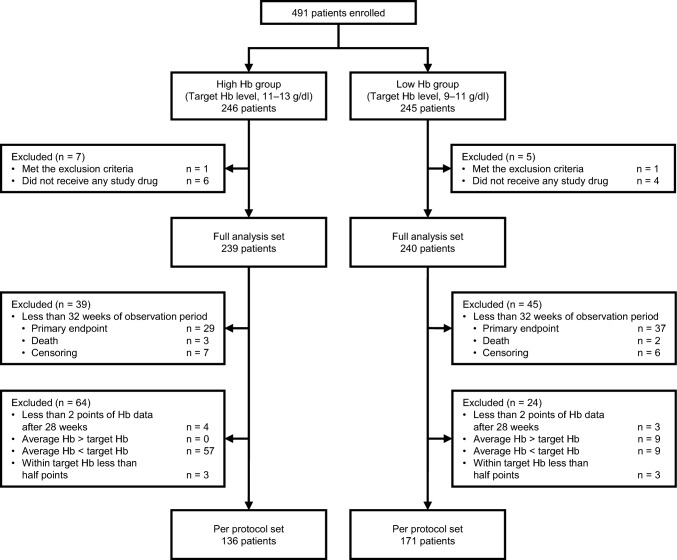
PPS was defined as patients in the FAS whose hemoglobin level had been measured at least twice after 28 weeks (inevitably, the observation period was ≥ 32 weeks), the mean of which was within the target range, and more than half of each measurement was within the target range (Fig. 1).
Fig. 1.
Diagram of patient flow. Hb hemoglobin
Outcomes
The outcomes of the PREDICT trial have also been described previously [23, 24]. The primary endpoint was the onset of a composite renal endpoint, including the initiation of dialysis, kidney transplantation, reaching an eGFR of 6 ml/min/1.73 m2, and ≥ 50% of reduction in eGFR. Among the prespecified secondary endpoint, the initiation of dialysis, ≥ 50% reduction in eGFR, all-cause death, composite CV event, stroke, myocardial infarction, and the development of malignancy have already been reported in our previous report [24]. Here, in addition to the composite renal endpoint in the PPS, the unreported and prespecified secondary endpoints of changes in eGFR and proteinuria in the FAS and PPS were assessed. Serious adverse events including all-cause death, CV events, Malignant neoplasms in the PPS were also assessed.
Statistical analysis
The methods of statistical analysis were described previously [23, 24]. The differences in the primary endpoint between the study groups in the PPS were assessed using the Kaplan–Meier method with a log-rank test. Multivariable Cox regression analysis was also performed to estimate HRs with 95% CIs for the composite renal endpoint in the PPS. The analyses were censored on the date of death and the end of the observation period (2 years from baseline) without a composite renal endpoint. The proportional hazards assumption was checked using Schoenfeld residual, and no violation was recorded. Changes in eGFR and proteinuria were assessed as eGFR slope (ml/min/1.73 m2/year) and proteinuria slope (g/gCr/year), respectively. The eGFR and proteinuria slopes were calculated using a linear mixed effects model with an unstructured variance–covariance matrix and patient-level random slopes and intercepts. The effects of the higher hemoglobin targeting on eGFR and proteinuria slopes were evaluated by adding an interaction term between the high-hemoglobin group and time in the mixed-effects model using the FAS and PPS, respectively. The multivariable models were adjusted for sex, age, baseline eGFR, baseline hemoglobin, systolic blood pressure, and proteinuria. These covariates as well as the interaction terms between each covariate and time were included in the model.
All test statistics used in the analyses were the results of two-sided tests with a significance level of 5%. Data are reported as number (percentage), mean ± standard deviation, median (interquartile range), or estimated value with 95% CI as appropriate. Normality was assessed by inspecting histograms. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata/MP 17.0 (StataCorp., College Station, TX, USA).
Results
Analysis of cohort sets
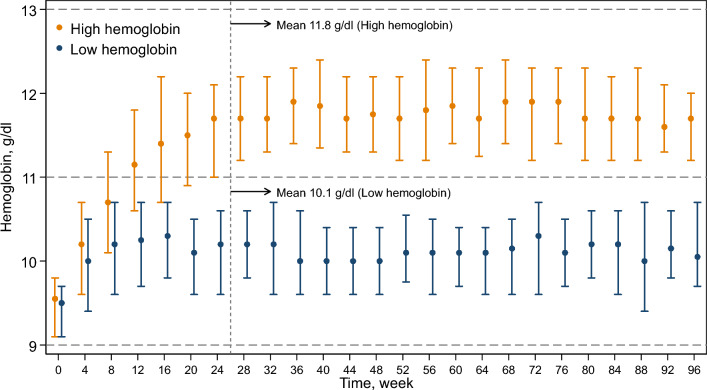
Of the 479 patients in the FAS (high-hemoglobin group, 239; low-hemoglobin group, 240), 307 were included in the PPS (high-hemoglobin group, 136; low-hemoglobin group, 171) (Fig. 1). The patients’ characteristics at baseline are summarized in Table 1. There was no significant difference between the study groups. Figure 2 illustrates the transition of hemoglobin levels by study group in the PPS. The mean hemoglobin levels after 28 weeks were 11.8 g/dl and 10.1 g/dl in the high- and low-hemoglobin groups, respectively. The medians (interquartile ranges) darbepoetin alfa doses in each visit ranged from 67 to 100 µg/4 weeks and 33 to 55 µg/4 weeks in the high- and low-hemoglobin groups (Supplementary Fig. 1).
Table 1.
Baseline characteristics in per-protocol set
| Total | High-hemoglobin group | Low-hemoglobin group | |
|---|---|---|---|
| n = 307 | n = 136 | n = 171 | |
| Male sex | 171 (56) | 73 (54) | 98 (57) |
| Age, years | 71 ± 11 | 71 ± 10 | 70 ± 11 |
| Body mass index, kg/m2 | 22.2 ± 3.1 | 22.0 ± 3.2 | 22.4 ± 3.0 |
| Smoking | |||
| Current/ever | 245 (80) | 109 (80) | 136 (80) |
| Never | 34 (11) | 13 (9.6) | 21 (12) |
| Unknown | 28 (9.1) | 14 (10) | 14 (8.2) |
| Etiology of chronic kidney disease | |||
| Chronic glomerulonephritis | 83 (27) | 30 (22) | 53 (31) |
| Hypertensive nephrosclerosis | 164 (53) | 79 (58) | 85 (50) |
| Polycystic kidney disease | 30 (10) | 17 (12) | 13 ( 8) |
| Others | 30 (10) | 10 ( 7) | 20 (12) |
| History of cardiovascular disease | |||
| Acute myocardial infarction | 12 (3.9) | 5 (3.7) | 7 (4.1) |
| Heart failure | 29 (9.4) | 17 (12) | 12 (7.0) |
| Stroke | 27 (8.8) | 13 (9.6) | 14 (8.2) |
| Peripheral artery disease | 5 (1.7) | 1 (0.7) | 4 (2.4) |
| Erythropoiesis-stimulating agents naive | 214 (70) | 97 (71) | 117 (68) |
| Systolic blood pressure, mm Hg | 132 ± 15 | 131 ± 17 | 132 ± 14 |
| Diastolic blood pressure, mm Hg | 72 ± 12 | 71 ± 12 | 73 ± 11 |
| eGFR, ml/min/1.73 m2 | 14 ± 3 | 14 ± 3 | 14 ± 3 |
| Chronic kidney disease | |||
| Stage 4 | 122 (40) | 59 (43) | 63 (37) |
| Stage 5 | 185 (60) | 77 (57) | 108 (63) |
| Hemoglobin, g/dl | 9.4 ± 0.5 | 9.4 ± 0.5 | 9.3 ± 0.6 |
| Ferritin, ng/ml | 148 (96–233) | 144 (92–222) | 150 (97–241) |
| Transferrin saturation, % | 32 ± 11 | 32 ± 11 | 33 ± 11 |
| Uric acid, mg/dl | 7.2 ± 1.7 | 7.2 ± 1.8 | 7.2 ± 1.6 |
| Albumin, g/dl | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 |
| Proteinuria, mg/dl | 58 (21–116) | 47 (20–113) | 61 (25–117) |
| C-reactive protein, mg/dl | 0.09 (0.03–0.20) | 0.09 (0.03–0.20) | 0.08 (0.03–0.20) |
| Iron supplements | 91 (30) | 42 (31) | 49 (29) |
| Antihypertensive drugs | 278 (91) | 124 (91) | 154 (90) |
| ACE inhibitors | 38 (12) | 17 (12) | 21 (12) |
| Angiotensin II receptor blockers | 187 (61) | 91 (67) | 96 (56) |
| Lipid-lowering drugs | 116 (38) | 55 (40) | 61 (36) |
| Spherical carbonaceous adsorbent | 63 (21) | 27 (20) | 36 (21) |
eGFR estimated glomerular filtration rate, ACE angiotensin-converting enzyme
Continuous variables are presented as mean ± standard deviation if normally distributed, and as median (interquartile range) if non-normally distributed. Categorical variables are presented as n (percent)
Fig. 2.
Transition of hemoglobin levels of the two groups in per-protocol set (n = 307). The plots with capped spikes are medians with interquartile ranges. Values are mean hemoglobin after 28 weeks in each study group. Target hemoglobin levels were 11–13 g/dl in the high-hemoglobin group and 9–11 g/dl in the low-hemoglobin group
Changes in eGFR and proteinuria in FAS
The crude mean ± SD eGFR slopes in the high- and low-hemoglobin groups were − 3.27 ± 3.32 and − 3.77 ± 3.25 ml/min/1.73 m2/year, respectively. In the multivariable analysis, the high-hemoglobin group was not associated with eGFR slope (coefficient: + 0.59; 95% CI − 0.06 to + 1.24; P = 0.075), while male sex, lower age, and higher proteinuria at baseline were significantly associated with faster eGFR decline (Supplementary Fig. 2).
The crude mean ± SD proteinuria slopes in the high- and low-hemoglobin groups were + 0.65 ± 0.063 and + 0.62 ± 0.055 g/gCr/year, respectively, with no significant difference in the multivariable model (Supplementary Table 1).
Composite renal endpoint in PPS
The composite renal endpoint occurred in 40 (29%) and 69 (40%) patients in the high- and low-hemoglobin groups, respectively. The Kaplan–Meier plots of the composite renal endpoint in the PPS are provided in Fig. 3 (log-rank test: P = 0.045). In the multivariable Cox proportional hazard regression analysis, high hemoglobin levels indicated a lower risk of composite renal endpoint (adjusted HR 0.64; 95% CI 0.43–0.96; P = 0.031). Male sex, lower age, lower eGFR, and higher proteinuria were associated with a higher risk of composite renal endpoint (Fig. 4).
Fig. 3.
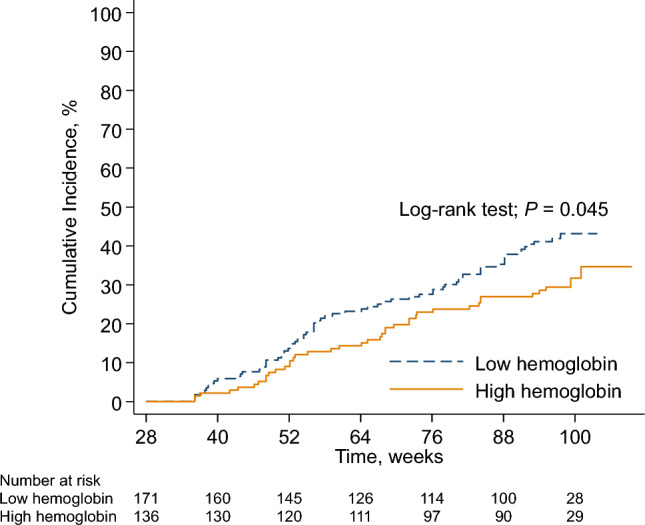
Cumulative incidence of the composite renal endpoint by study group in per-protocol set (n = 307). The composite renal endpoint includes initiation of maintenance dialysis, kidney transplantation, eGFR < 6 ml/min/1.73 m2, and a 50% reduction in eGFR. eGFR estimated glomerular filtration rate
Fig. 4.
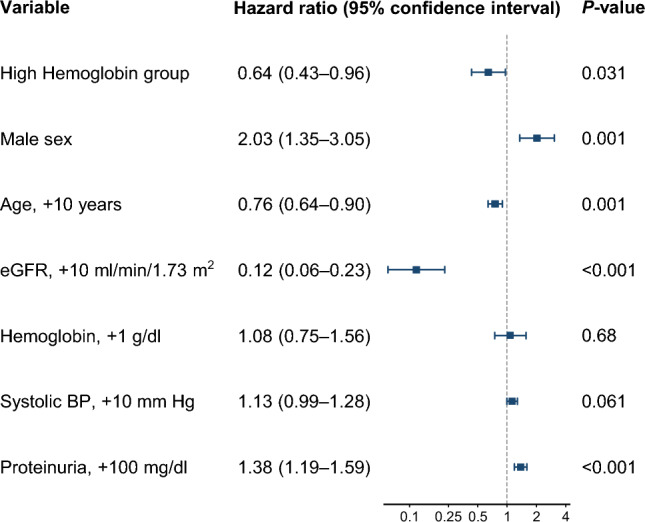
Multivariable Cox proportional hazard regression for the composite renal endpoint in per-protocol set (n = 307). The composite renal endpoint includes initiation of maintenance dialysis, kidney transplantation, eGFR < 6 ml/min/1.73 m2, and a 50% reduction in eGFR. eGFR estimated glomerular filtration rate, BP blood pressure
Changes in eGFR and proteinuria and adverse events in PPS
The changes in eGFR and proteinuria were also evaluated in the PPS. The crude mean ± SD eGFR slopes in the high- and low-hemoglobin groups were − 2.17 ± 3.05 and − 3.19 ± 2.61 ml/min/1.73 m2/year, respectively. In the multivariable analyses, the high-hemoglobin group was associated with improved eGFR slope (coefficient: + 1.00; 95% CI 0.38–1.63; P = 0.002), while lower age and higher proteinuria at baseline were associated with faster eGFR decline (Fig. 5). The crude mean ± SD proteinuria slopes in the high- and low-hemoglobin groups were + 0.48 ± 0.080 and + 0.53 ± 0.068 g/gCr/year, respectively, with no significant difference between the study groups in the multivariable model similar to the results in the FAS (Supplementary Table 1). The frequencies of serious adverse events were not significantly different between the two groups (Supplementary Table 2).
Fig. 5.
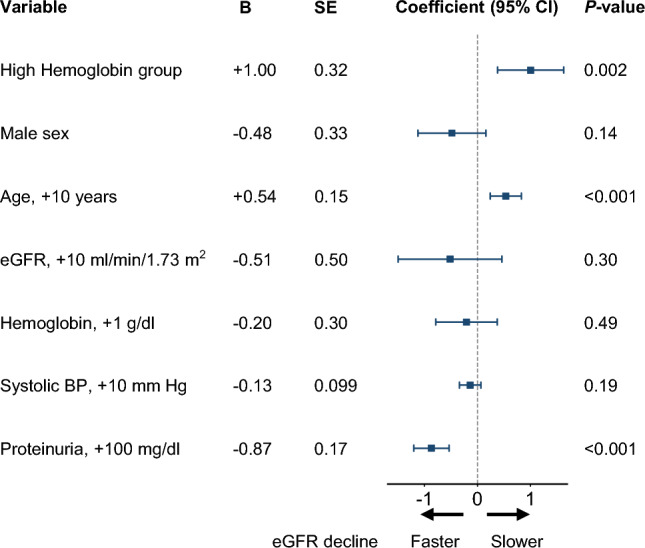
Association between eGFR slope (ml/min/1.73 m2/year) and each covariate in-per protocol set (n = 307). Values are for the interaction term between each variable and time in the mixed-effects model for eGFR. B unstandardized regression coefficient, SE standard error, CI confidence interval, eGFR estimated glomerular filtration rate, BP blood pressure
Discussion
In this prespecified secondary analysis of the PREDICT trial, we found that targeting a higher hemoglobin level at 11–13 g/dl with darbepoetin alfa did not improve eGFR and proteinuria slopes as compared to targeting a lower hemoglobin level at 9–11 g/dl in patients with advanced CKD without diabetes. These were in line with the results of the primary analysis in our study that a higher hemoglobin target did not reduce the composite renal outcome [24]. On the other hand, the PPS analysis that excluded patients with off-target hemoglobin levels demonstrated a 36% reduction in the composite renal endpoint and an improvement in eGFR slope of 1 ml/min/1.73 m2/year in the high-hemoglobin group compared to the low-hemoglobin group. These were in contrast to the results of previous RCTs indicating that higher hemoglobin targeting was linked to a relatively worse prognosis [11–13, 16].
In the FAS analyses of the eGFR and proteinuria slopes, which was the prespecified secondary endpoints, we could not demonstrate the clear benefit of higher hemoglobin targeting using darbepoetin alfa. The composite renal endpoint was reduced by 22% in the high-hemoglobulin group (vs. low-hemoglobin group) as reported previously [24], and the eGFR decline was 0.59 ml/min/1.73 m2/year smaller as presented in this study, although not statistically significant (P = 0.08 and P = 0.075, respectively). Regarding the proteinuria slope analysis, the mean baseline value was only 58 mg/dL. Therefore, it is difficult to draw any conclusion about the anti-proteinuric effects in this study. Our previous report has also revealed that the rates of CV events were not significantly different between the groups. The PPS analyses in this study suggested that high-hemoglobin targeting was associated with better kidney outcomes among patients with advanced CKD without diabetes and who maintained the target hemoglobin levels without violating the protocol. Darbepoetin alfa, when used properly to maintain the target hemoglobin level at 11–13 g/dl, may exert good effects on the kidney as previously described [14, 15].
As aforementioned, a meta-analysis has demonstrated the potential harm of targeting a higher hemoglobin level (> 13 g/dl) using ESAs in patients with CKD not requiring dialysis [16]. One of the main differences was the target level in the high-hemoglobin group; the target in the three major RCTs was > 13 g/dl [11–13], whereas in our study, the target was not normalization but the middle range at 11–13 g/dl. Taken together with our previous report [24], the PREDICT study demonstrates that maintaining hemoglobin levels at 11–13 g/dl compared with 9–11 g/dl did not at least worsen the prognosis of the patients.
However, the results of the PPS analysis need to be interpreted with caution. Hypo-responsiveness to ESAs was reported to be associated with worse kidney outcomes and CV prognosis [25, 26]. Patients unable to achieve the target hemoglobin level may have been hypo-responsive to ESAs. Especially in the high-hemoglobin group, 57 out of 200 patients were excluded because the lower hemoglobin levels than the target. Therefore, it is still difficult to conclude from the PPS analysis that maintaining hemoglobin levels at 11–13 g/dl protects the kidney more than at 9–11 g/dl.
Concerning the mechanisms of ESAs on the CV prognosis and renal outcomes, both beneficial and harmful effects have been postulated. The beneficial effects for organ protection may be independent of anemia correction but can be attributed to the non-hematological effects of rHuEPO that prevent tissue damage [27]. The potential harm of ESAs may include elevated blood pressure, increased viscosity, and increased platelet number and aggregation [28]. The balance of these factors may have led to different results in RCTs, including this study, depending on the setting of the trial, such as target hemoglobin levels, dosage and kinds of ESA reagents, as well as participants’ characteristics with different risks for CV and renal events. Two RCTs involving patients with CKD receiving kidney transplantation have revealed the renoprotective effects of maintaining normal hemoglobin levels with ESAs on renal outcomes [29, 30]. The participants were relatively young and had a lower risk of CV events than those in other RCTs involving patients with CKD in the pre-dialysis stage.
Regarding the other factors, male sex and higher urinary protein levels at baseline were independently associated with worse renal outcomes in line with those of previous studies [31, 32]. Our study also showed that older age was associated with better renal outcomes after adjusting for other clinical factors. This is probably because those who had hypertensive nephrosclerosis with slower CKD progression were relatively old, while those who had glomerulonephritis with rapid CKD progression were relatively young [33].
Recently, HIF-PHIs have been utilized in treating renal anemia in patients with CKD not requiring dialysis. Many preclinical studies have shown the potential benefit of HIF-PHIs for ischemic organ damage, including the heart and kidney [35–36]. To date, clinical studies have not demonstrated the detrimental effects of HIF-PHIs [17–21, 37, 38]. Since the mechanism of HIF-PHIs function is different from that of ESAs [34, 39, 40], the data of hemoglobin targeting studies obtained by ESA treatment cannot be directly applied to understand the potential benefit or harm of HIF-PHIs in the clinical setting. RCTs to analyze the effects of targeting high vs. low hemoglobin levels on the prognosis of patients with CKD not requiring dialysis using HIF-PHIs will provide clinically useful information. Due to the difference in the relatively lower incidence of CV events in patients with CKD in Japan than in Western countries [41], clinical trials focusing on renal outcomes by targeting high hemoglobin levels with HIF-PHIs should be conducted in each region.
Our study had several potential limitations. First, only patients with CKD in Japan were included in the study, and Japan has a much lower incidence of CV events. Therefore, our results may underestimate the potential harm of CV diseases and may not be generalized to all patients with CKD in other places. Nevertheless, this could also be a strength of our study. Since the rates of CV events in our study were low, only 8% in the high- and 7% in the low-hemoglobin groups [24], the net effects of higher hemoglobin levels on renal outcomes were focused on without considering the indirect effects of CV events. Second, the difference in achieved hemoglobin levels was not more significant than expected, leading to insufficient power to detect the group differences in outcomes. The PREDICT trial was planned and conducted based on the data from the previous study performed in Japan [14], where the difference in the hemoglobin levels between high- and low-hemoglobin groups was approximately 2 g/dl. However, the actual difference in this study was only 1.2 g/dl in the FAS and 1.7 g/dl in the PPS. Third, a prespecified method for making up the PPS may not be the best. Patients who could not reach the higher target levels were excluded due to the violation of protocol. As mentioned above, the patients may have been hyporesponsive to ESA therapy and did not necessarily intend to violate protocols. Due to the selection bias from this, the PPS analysis may overestimate the true effects of targeting higher hemoglobin levels.
Conclusions
In summary, in this prespecified secondary analysis of the PREDICT trial, the FAS analysis failed to demonstrate that targeting a high hemoglobin level at 11–13 g/dl with darbepoetin alfa reduced the eGFR decline or proteinuria increase as compared to targeting a low hemoglobin level at 9–11 g/dl in patients with advanced CKD without diabetes. In the PPS that excluded patients whose hemoglobin levels did not match the target, the high-hemoglobin group demonstrated better kidney outcomes than the low-hemoglobin group, suggesting the potential benefit of maintaining higher hemoglobin levels. Further prospective studies are needed to determine the optimal hemoglobin target levels using ESAs or HIF-PHIs in patients with CKD not requiring dialysis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to express our deepest gratitude to the patients, investigators, and staff at the study sites for their contribution to the study. The following investigators participated in the PREDICT trial (the numbers of enrolled patients are in parentheses): Terumasa Hayashi, Osaka General Medical Center (38); Takeyuki Hiramatsu, Konan Kosei Hospital (35); Hirofumi Tamai, Anjo Kosei Hospital (31); Yoshiyasu Iida, Yokkaichi Municipal Hospital (30); Tomohiro Naruse, Kasugai Municipal Hospital (18); Hideto Oishi, Komaki City Hospital (18); Shoichi Maruyama, Nagoya University Hospital (17); Shunya Uchida, Teikyo University School of Medicine (16); Hideaki Shimizu, Chubu Rosai Hospital (15); Kunio Morozumi, Japanese Red Cross Nagoya Daini Hospital (13); Hisashi Kurata, Toyota Kosei Hospital (12); Nobuhito Hirawa, Yokohama City University Medical Center (12); Saori Nishio, Hokkaido University Hospital (12); Yukio Yuzawa, Fujita Health University (11); Ichiei Narita, School of Medicine, Niigata University (10); Makoto Mizutani, Handa City Hospital (9); Isao Aoyama, Chukyo Hospital (9); Hideaki Yoshida, Sapporo Medical University Hospital (9); Kouji Kaneda, Oita Red Cross Hospital (9); Masaomi Nangaku, The University of Tokyo Graduate School of Medicine (8); Hideki Hirakata, Japanese Red Cross Fukuoka Hospital (8); Satoshi Suzuki, Kainan Hospital (8); Hiroki Adachi, Kanazawa Medical University Hospital (7); Eriko Kinugasa, Showa University, Northern Yokohama Hospital (7); Kei Kurata, Tosei General Hospital (7); Hiroshi Morinaga, Okayama University Hospital (6); Yusuke Tsukamoto, Itabashi Chuo Medical Center (6); Kazuhiro Tsuruya, Kyushu University Hospital (5); Ryoichi Ando, Musashino Red Cross Hospital (5); Shizunori Ichida, Japanese Red Cross Nagoya Daiichi Hospital (5); Teiichi Tamura, Yokosuka Kyosai Hospital (5); Takao Masaki, Hiroshima University Hospital (4); Takashi Wada, Kanazawa University Hospital (4); Hirokazu Honda, Showa University Koto Toyosu Hospital (4); Junichiro Yamamoto, Tsushima City Hospital (4); Yoshitaka Isaka, Osaka University Hospital (4); Eri Muso, Tazuke Kofukai Medical Research Institute, Kitano Hospital (4); Yasuhiro Komatsu, St. Luke's International Hospital (4); Norimi Ohashi, Ogaki Municipal Hospital (4); Taiga Hara, Kagawa University Hospital (4); Kiyoshi Ikeda, Ikeda Vascular Access, Dialysis and Internal Medicine Clinic (3); Kazuyoshi Okada, Nihon University School of Medicine (3); Tetsuhiko Yoshida, Hamanomachi Hospital (3); Seiya Okuda, Kurume University Hospital (3); Hiromichi Suzuki, Saitama Medical University (3); Takeshi Nakanishi, Hyogo College of Medicine (3); Harumichi Higashi, St Mary's Hospital (3); Arimasa Shirasaki, Ichinomiya Municipal Hospital (3); Shuichiro Endo, Kyoto University Hospital (2); Yutaka Osawa, Niigata Rinko Hospital (2); Ryuji Aoyagi, Tachikawa General Hospital (2); Yasuhiko Tomino, Juntendo University Hospital (2); Tetsu Akimoto, Jichi Medical University (2); Tsuyoshi Watanabe, Fukushima Medical University (2); Jiro Toyonaga, Iizuka Hospital (2); Motoko Tanaka, Akebono Clinic (2); Yoshitaka Ishibashi, Japanese Red Cross Medical Center (2); Shigehiro Uezono, Miyazaki Prefectural Miyazaki Hospital (2); Masako Sakakibara, Nagoya Memorial Hospital (2); Hajime Yamazaki, Nagaoka Red Cross Hospital (1); Hideki Takano, Tokyo Teishin Hospital (1); Hirofumi Ikeda, Munakata Medical Association Hospital (1); Takuma Takata, Nagaoka Chuo General Hospital (1); Hiroshi Yamashita, Toyota Memorial Hospital (1); Kunihiro Yamagata, University of Tsukuba (1); Toshinobu Sato, Japan Community Health Care Organization, Sendai Hospital (1); Ashio Yoshimura, Showa University Fujigaoka Hospital (1); Keiichi Tamagaki, Kyoto Prefectural University of Medicine (1); Kazuhiro Sonomura, Omihachiman Community Medical Center (1); Akira Iguchi, Ojiya General Hospital (1); Masahito Tamura, Hospital of the University Occupation and Environmental Health (1); Ryota Yasukawa, Sado General Hospital (1); Takanobu Morihiro, Masuko Hospital (1); Manei Oku, Ikeda Hospital (1).
Terumasa Hayashi, Takeyuki Hiramatsu, Hirofumi Tamai, Yoshiyasu Iida, Tomohiro Naruse, Hideto Oishi, Shoichi Maruyama, Shunya Uchida, Hideaki Shimizu, Kunio Morozumi, Hisashi Kurata, Nobuhito Hirawa, Saori Nishio, Yukio Yuzawa, Ichiei Narita, Makoto Mizutani, Isao Aoyama, Hideaki Yoshida, Kouji Kaneda, Masaomi Nangaku, Hideki Hirakata, Satoshi Suzuki, Hiroki Adachi, Eriko Kinugasa, Kei Kurata, Hiroshi Morinaga, Yusuke Tsukamoto, Kazuhiro Tsuruya, Ryoichi Ando, Shizunori Ichida, Teiichi Tamura, Takao Masaki, Takashi Wada, Hirokazu Honda, Junichiro Yamamoto, Yoshitaka Isaka, Eri Muso, Yasuhiro Komatsu, Norimi Ohashi, Taiga Hara, Kiyoshi Ikeda, Kazuyoshi Okada, Tetsuhiko Yoshida, Seiya Okuda, Hiromichi Suzuki, Takeshi Nakanishi, Harumichi Higashi, Arimasa Shirasaki, Shuichiro Endo, Yutaka Osawa, Ryuji Aoyagi, Yasuhiko Tomino, Tetsu Akimoto, Tsuyoshi Watanabe, Jiro Toyonaga, Motoko Tanaka, Yoshitaka Ishibashi, Shigehiro Uezono, Masako Sakakibara, Hajime Yamazaki, Hideki Takano, Hirofumi Ikeda, Takuma Takata, Hiroshi Yamashita, Kunihiro Yamagata, Toshinobu Sato, Ashio Yoshimura, Keiichi Tamagaki, Kazuhiro Sonomura, Akira Iguchi, Masahito Tamura, Ryota Yasukawa, Manei Oku
Funding
The present study was supported by Translational Research Center for Medical Innovation (Kobe, Japan).
Declarations
Conflict of interest
Dr. Maruyama has received honoraria from Kyowa Kirin, Chugai, Astellas, and Mitsubishi Tanabe; grants and/or research funding from Kyowa Kirin, Chugai, Astellas, Mitsubishi Tanabe, and Torii. Dr. Kurasawa has received honoraria from Kyowa Kirin.
Ethical approval
The protocol was approved by the institutional review board of each participating center, in accordance with the principles of the Declaration of Helsinki; Ethical Guidelines on Clinical Studies of the Ministry of Health, Labour, and Welfare of Japan (Tokyo, Japan); and International Conference on Harmonization Good Clinical Practice guidelines.
Informed consent
All participants in the study provided written informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shoichi Maruyama, Email: marus@med.nagoya-u.ac.jp.
PREDICT Investigators:
Takeyuki Hiramatsu, Hirofumi Tamai, Yoshiyasu Iida, Tomohiro Naruse, Hideto Oishi, Shunya Uchida, Hideaki Shimizu, Kunio Morozumi, Hisashi Kurata, Nobuhito Hirawa, Saori Nishio, Yukio Yuzawa, Makoto Mizutani, Isao Aoyama, Hideaki Yoshida, Kouji Kaneda, Satoshi Suzuki, Hiroki Adachi, Eriko Kinugasa, Kei Kurata, Hiroshi Morinaga, Yusuke Tsukamoto, Kazuhiro Tsuruya, Ryoichi Ando, Shizunori Ichida, Teiichi Tamura, Takao Masaki, Takashi Wada, Hirokazu Honda, Junichiro Yamamoto, Yoshitaka Isaka, Eri Muso, Yasuhiro Komatsu, Norimi Ohashi, Taiga Hara, Kiyoshi Ikeda, Kazuyoshi Okada, Tetsuhiko Yoshida, Seiya Okuda, Hiromichi Suzuki, Takeshi Nakanishi, Harumichi Higashi, Arimasa Shirasaki, Shuichiro Endo, Yutaka Osawa, Ryuji Aoyagi, Yasuhiko Tomino, Tetsu Akimoto, Tsuyoshi Watanabe, Jiro Toyonaga, Motoko Tanaka, Yoshitaka Ishibashi, Shigehiro Uezono, Masako Sakakibara, Hajime Yamazaki, Hideki Takano, Hirofumi Ikeda, Takuma Takata, Hiroshi Yamashita, Kunihiro Yamagata, Toshinobu Sato, Ashio Yoshimura, Keiichi Tamagaki, Kazuhiro Sonomura, Akira Iguchi, Masahito Tamura, Ryota Yasukawa, and Manei Oku
References
- 1.Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 2.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS ONE. 2014;9:e84943. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker AM, Schneider G, Yeaw J, Nordstrom B, Robbins S, Pettitt D. Anemia as a predictor of cardiovascular events in patients with elevated serum creatinine. J Am Soc Nephrol. 2006;17:2293–2298. doi: 10.1681/ASN.2005020183. [DOI] [PubMed] [Google Scholar]
- 5.Sofue T, Nakagawa N, Kanda E, Nagasu H, Matsushita K, Nangaku M, et al. Prevalence of anemia in patients with chronic kidney disease in Japan: a nationwide, cross-sectional cohort study using data from the Japan Chronic Kidney Disease Database (J-CKD-DB) PLoS ONE. 2020;15:e0236132. doi: 10.1371/journal.pone.0236132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue JL, St Peter WL, Ebben JP, Everson SE, Collins AJ. Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis. 2002;40:1153–1161. doi: 10.1053/ajkd.2002.36861. [DOI] [PubMed] [Google Scholar]
- 7.Weiner DE, Tighiouart H, Vlagopoulos PT, Griffith JL, Salem DN, Levey AS, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803–1810. doi: 10.1681/ASN.2004070597. [DOI] [PubMed] [Google Scholar]
- 8.Keane WF, Zhang Z, Lyle PA, Cooper ME, de Zeeuw D, Grunfeld JP, et al. Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL study. Clin J Am Soc Nephrol. 2006;1:761–767. doi: 10.2215/CJN.01381005. [DOI] [PubMed] [Google Scholar]
- 9.Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004;66:753–760. doi: 10.1111/j.1523-1755.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 10.Kidney disease: improving global outcomes (KDIGO) CKD work group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 11.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 12.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckard KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 14.Tsubakihara Y, Akizawa T, Iwasaki M, Shimazaki R. High hemoglobin levels maintained by an erythropoiesis-stimulating agent improve renal survival in patients with severe renal impairment. Ther Apher Dial. 2015;19:457–465. doi: 10.1111/1744-9987.12308. [DOI] [PubMed] [Google Scholar]
- 15.Tsubakihara Y, Gejyo F, Nishi S, Iino Y, Watanabe Y, Suzuki M, et al. High target hemoglobin with erythropoiesis-stimulating agents has advantages in the renal function of non-dialysis chronic kidney disease patients. Ther Apher Dial. 2012;16:529–540. doi: 10.1111/j.1744-9987.2012.01082.x. [DOI] [PubMed] [Google Scholar]
- 16.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Grag AX, et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153:23–33. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]
- 17.Palmer SC, Saglimbene V, Craig JC, Navaneethan SD, Strippoli GF. Darbepoetin for the anaemia of chronic kidney disease. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD009297.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akizawa T, Macdougall IC, Berns JS, Bernhardt T, Steadtler G, Taguchi M, et al. Long-term efficacy and safety of molidustat for anemia in chronic kidney disease: DIALOGUE extension studies. Am J Nephrol. 2019;49:271–280. doi: 10.1159/000499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chertow GM, Pergola PE, Farag YMK, Agarwal R, Arnold S, Bako G, et al. Vadadustat in patients with anemia and non–dialysis-dependent CKD. N Engl J Med. 2021;384:1589–1600. doi: 10.1056/NEJMoa2035938. [DOI] [PubMed] [Google Scholar]
- 20.Barratt J, Andric B, Tataradze A, Schömig M, Reusch M, Valluri U, et al. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a Phase 3, randomized, open-label, active-controlled study (DOLOMITES) Nephrol Dial Transplant. 2021;36:1616–1628. doi: 10.1093/ndt/gfab191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh AK, Carroll K, McMurray JJV, Solomon S, Jha V, Johansen K, et al. Daprodustat for the treatment of anemia in patients not undergoing dialysis. N Engl J Med. 2021;385:2313–2324. doi: 10.1056/NEJMoa2113380. [DOI] [PubMed] [Google Scholar]
- 22.Parfrey P. Hypoxia-inducible factor prolyl hydroxylase inhibitors for anemia in CKD. N Engl J Med. 2021;38:2390–2391. doi: 10.1056/NEJMe2117100. [DOI] [PubMed] [Google Scholar]
- 23.Imai E, Maruyama S, Nangaku M, Hirakata H, Hayashi T, Narita I, et al. Rationale and study design of a randomized controlled trial to assess the effects of maintaining hemoglobin levels using darbepoetin alfa on prevention of development of end-stage kidney disease in non-diabetic CKD patients (PREDICT Trial) Clin Exp Nephrol. 2016;20:71–76. doi: 10.1007/s10157-015-1133-z. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Maruyama S, Nangaku M, Narita I, Hirakata H, Tanabe K, et al. Darbepoetin alfa in patients with advanced CKD without diabetes: randomized, controlled trial. Clin J Am Soc Nephrol. 2020;15:608–615. doi: 10.2215/CJN.08900719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Kato H, Nangaku M, Hirakata H, Wada T, Hayashi T, Sato H, et al. Rationale and design of oBservational clinical Research In chronic kidney disease patients with renal anemia: renal proGnosis in patients with Hyporesponsive anemia To Erythropoiesis-stimulating agents, darbepoetiN alfa (BRIGHTEN Trial) Clin Exp Nephrol. 2018;22:78–84. doi: 10.1007/s10157-017-1427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahlmann FH, Fliser D. Erythropoietin and renoprotection. Curr Opin Nephrol Hypertens. 2009;18:15–20. doi: 10.1097/MNH.0b013e32831a9dde. [DOI] [PubMed] [Google Scholar]
- 28.Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007;2:1274–1282. doi: 10.2215/CJN.02380607. [DOI] [PubMed] [Google Scholar]
- 29.Choukroun G, Kamar N, Dussol B, Etienne I, Cassuto-Viguier E, Toupance O, et al. Correction of postkidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol. 2012;23:360–368. doi: 10.1681/ASN.2011060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsujita M, Kosugi T, Goto N, Futamura K, Nishihira M, Okada M, et al. The effect of maintaining high hemoglobin levels on long-term kidney function in kidney transplant recipients: a randomized controlled trial. Nephrol Dial Transplant. 2019;34:1409–1416. doi: 10.1093/ndt/gfy365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996;49:800–805. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa T, Sakamaki K, Koiwa F, Akizawa T, Hishida A, CKD-JAC Study Investigators Clinical prediction models for progression of chronic kidney disease to end-stage kidney failure under pre-dialysis nephrology care: results from the Chronic Kidney Disease Japan Cohort Study. Clin Exp Nephrol. 2019;23:189–198. doi: 10.1007/s10157-018-1621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Eckardt KU. HIF activation against CVD in CKD: novel treatment opportunities. Semin Nephrol. 2018;38:267–276. doi: 10.1016/j.semnephrol.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Shu S, Wang Y, Zheng M, Liu Z, Cai J, Tang C, et al. Hypoxia and Hypoxia and hypoxia-inducible factors in kidney injury and repair. Cells. 2019;8:207. doi: 10.3390/cells8030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreiber T, Salhöfer L, Quinting T, Fandrey J. Things get broken: the hypoxia-inducible factor prolyl hydroxylases in ischemic heart disease. Basic Res Cardiol. 2019;114:16. doi: 10.1007/s00395-019-0725-2. [DOI] [PubMed] [Google Scholar]
- 37.Macdougall IC, Akizawa T, Berns JS, Bernhardt T, Krueger T. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14:28–39. doi: 10.2215/CJN.02510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akizawa T, Nangaku M, Yamaguchi T, Arai M, Koretomo R, Matsui A, et al. A placebo-controlled, randomized trial of enarodustat in patients with chronic kidney disease followed by long-term trial. Am J Nephrol. 2019;49:165–174. doi: 10.1159/000496929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakashita M, Tanaka T, Nangaku M. Hypoxia-inducible factor-prolyl hydroxylase domain inhibitors to treat anemia in chronic kidney disease. Contrib Nephrol. 2019;198:112–123. doi: 10.1159/000496531. [DOI] [PubMed] [Google Scholar]
- 40.Hammond FR, Lewis A, Elks PM. If it's not one thing, HIF's another: immunoregulation by hypoxia inducible factors in disease. FEBS J. 2020;287:3907–3916. doi: 10.1111/febs.15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka K, Watanabe T, Takeuchi A, Ohashi Y, Nitta K, Akizawa T, et al. Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int. 2017;91:227–234. doi: 10.1016/j.kint.2016.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.