Summary
Background
A considerable number of published reviews have addressed the effects of physical exercise on mental health, cognitive function, or attention-deficit hyperactivity (ADHD) symptoms as outcomes in children and adolescents with ADHD. Their findings have often conflicted, therefore, there is an urgent need to synthesise a hierarchy of the evidence and examine the credibility of previous meta-analyses. To establish the robustness of these findings, we conducted an additional meta-analysis on a number of individual studies that were not covered in previous reviews but were suitable for inclusion in our own study.
Methods
Three reviewers independently searched Web of Science, Psych INFO, Embase, Cochrane Library, PubMed, SPORTDiscus, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) for meta-analyses published between database inceptions to December 1, 2022. The individual studies were also screened from 1 January 2015 to 1 December 2022. We included meta-analyses and eligible individual studies that addressed the effects of exercise on at least one outcome of mental health, cognitive function, or ADHD symptoms in children and adolescents with ADHD. We excluded systematic reviews and articles that lacked sufficient data for a meaningful second analysis. The effect estimates (Hedges’ g), 95% confidence interval (95% CI), 95% prediction interval (95% PI), small study effects, and excess significance bias were calculated. Finally, we categorised the meta-analyses based on the credibility of the evidence criteria and their quality using a Measurement Tool to Assess Systematic Reviews 2 checklist. This umbrella review was registered with PROSPERO, CRD42022361331.
Findings
Of 181 listed review articles and 60 individual papers, 10 reviews and 12 individual articles were included in the meta-analyses. This yielded 37 meta-analyses based on 106 study estimates. Evidence was highly suggestive for the effectiveness of exercise (class II) for improving inattention (G = 0.92, 95% CI: 0.44–1.39, 95%), inhibitory control (G = 0.82, 95% CI: 0.52–1.13), and cognitive flexibility (G = 0.52, 95% CI: 0.32–0.72). However, evidence for the effectiveness of exercise on emotional, social, and working memory outcomes was weak, and these results were not significant for hyperactivity and behavioural functioning.
Interpretation
Improvement of cognitive flexibility, inhibitory control, and inattention in children and adolescents with ADHD was highly suggested by exercise interventions. However, results were weak for other outcomes (emotional functioning, social functioning, and working memory). Further high-quality randomised controlled trials are, therefore, warranted to determine the effectiveness of exercise on weak outcomes.
Funding
None.
Keywords: Attention deficit hyperactivity disorder, Executive function, Physical activity, Youth, Special educational needs
Research in context.
Evidence before this study
We searched Web of Science, Psych INFO, Embase, Cochrane Library, PubMed, SPORTDiscus, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases from inception to December 1, 2022, for meta-analyses of randomised controlled trials (RCTs) or non-randomised controlled trials (nRCT) that evaluated at least one of the mental health, cognitive function, and attention-deficit/hyperactivity disorder (ADHD) symptoms variables in children and adolescents with ADHD. We have also added individual studies that did not exist in any of those meta-analyses but were eligible for adding to our umbrella review. Our research identified eight variables concerning mental health (social functioning, emotional functioning, and behavioural functioning), executive function (higher level of cognitive function, e.g., working memory, cognitive flexibility, and inhibitory control), and ADHD symptoms (inattention problems, hyperactive or impulsive problem) in children and adolescents with ADHD. Some results have been inconsistent across studies, and it is unclear whether the claimed associations are prone to biases in the literature, such as excess significance bias and publication bias. The results of some studies have been inconsistent, leading to doubts over the validity of the claimed efficacy of physical exercise on mental health, cognitive function, and ADHD symptoms variables and the potential influence of biases such as publication bias in the literature.
Added value of this study
To address the limitations of previous meta-analyses, we conducted an umbrella review. We performed a variety of bias assessments and used credibility criteria for determining the level of credibility of the efficacy of physical exercise intervention on mental health, cognitive function, and ADHD symptoms. We found and analysed 106 unique study estimates of the effectiveness of physical exercise on mental health, cognitive function, and ADHD symptoms. Among eight identified variables, there was highly suggestive evidence that cognitive flexibility, inattention problem, and inhibitory control were improved by physical exercise. Based on a sensitivity analysis, improving the inhibitory control, and working memory after physical exercise intervention was graded as highly suggestive evidence.
Implications of all the available evidence
Our findings, supported by highly suggestive evidence, propose promising insights for clinicians and physical education teachers in helping patients with ADHD to enhance their cognitive function and symptoms related to ADHD. It should be noted that most of the identified studies were not RCTs, and they usually overestimated the effect of the interventions. There is, therefore, a need for high-quality RCTs to enable the effectiveness of physical activity interventions on mental health, cognitive function, and ADHD symptoms to be accurately assessed.
Introduction
Adolescence is a pivotal phase during which individuals undergo significant changes and face various pressures, such as the development of identity and independence while transitioning to adulthood, alongside forces of maturation, academic expectations, and evolving social roles.1 This stage is compounded by psychosocial adversity experienced in childhood and early adolescence.1 The world's present population is 7.2 billion, of whom almost 3 billion are under the age of 25. Around 1.2 billion young individuals are adolescents aged 10–19.2 A significant proportion (between 3% and 7%) of school-age children and adolescents worldwide suffer from a disorder known as Attention Deficit Hyperactivity Disorder (ADHD).3,4
ADHD is the most prevalent neurodevelopmental disorder worldwide. According to twin and family studies, ADHD is estimated to be 74% heritable.5 Molecular genetic research has shown that a third of heritability is influenced by common genetic variants.6 Besides genetics, a recent umbrella review also found convincing environmental risk factors for ADHD, e.g., maternal pre-pregnancy obesity and acetaminophen consumption.7
Anatomically, neuroimaging studies on children and adolescents with ADHD have confirmed a significant reduction in volume, delayed maturation, delayed degeneration of the bilateral amygdala, accumbens, and hippocampus,8 and lessening in the volume of the right frontal lobes.9 Barkley's theory of ADHD,10 supported by neuropsychological studies, have documented executive dysfunction11 (e.g., inhibitory control,12 working memory deficits13) and non-executive deficits (e.g., perception,14 memory,15 timing,16 and changes in motivational processes) in children and adolescents with ADHD.17
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), ADHD is diagnosed based on the manifestation of two factors: inattention and hyperactivity/impulsivity.18 The debilitating effects of these symptoms are apparent in their significant negative impact on the afflicted patient's social, academic, and occupational functioning.18,19 Given the unique needs and vulnerabilities of children and adolescents with ADHD,20 there is an urgent need for the development of strategies to improve their cognitive functioning, mental well-being, and symptomatology.
Conventional approaches to treating children with ADHD include both pharmacological interventions (e.g., psychostimulant medication) and non-pharmacological therapies (e.g., psychotherapy, and cognitive-behavioural techniques21). These treatments may have unwelcome side effects (including poor medication tolerance, lack of response to treatment, and potential for developing dependence22), or be expensive.23 Physical activity (PA) as a non-pharmacological intervention to improve the cognitive and mental health outcomes of children with ADHD has therefore attracted attention in recent years. PA has been defined as any type of bodily movement that increases energy expenditure above the baseline resting level.24 Similarly, physical exercise has been defined as planned, structured, and repetitive PA performed with deliberate intention.24,25 Previous meta-analyses have indicated that physical exercise participation yields benefits for psychological and social functioning among children and adolescents.25, 26, 27 Lubans et al. (2016) postulated that physical exercise interventions have the potential to improve cognitive function and mental health through three distinct mechanisms.8 These are (1) the neurobiological mechanism (i.e., changes in the structural and functioning composition of the brain such as stimulating brain-derived neurotrophic factor28); (2) the psychosocial mechanism (i.e., satisfying basic psychological needs such as through social interaction29); and (3) the behavioural mechanism (i.e., changes in associated behaviours such as through self-regulation and coping skills and improved sleep quality30). Among the three potential mechanisms, it was concluded that the psychosocial mechanism had the strongest impact on mental health outcomes, whereas the role of neurobiological and behavioural mechanisms in enhancing mental health outcomes was inconclusive due to limited studies.31 In addition to these mechanisms, the intervention components of physical exercise (e.g., type, mode, and intensity) are considered as effect moderators. For example, physical exercise types such as aerobic exercise (AE) and cognitive engaging exercise (CEE) have been used to promote cognitive and mental health outcomes. AE is a structured and repetitive physical exercise (e.g., cycling, running) that utilises oxygen to produce energy grounded in the cardiovascular fitness hypothesis32 while CEE consists of close and open motor skills which require attentional resources and proficiency (e.g. ballgames33). Physical exercise mode consists of acute (e.g., single bout of 5- to 60-min) which are commonly associated with transient improvements, including a positive mood shift, the release of endorphins, and an increase in cerebral blood flow34,35 and chronic (e.g., three sessions per week for 8-week) interventions36 that often produce physiological adaptations. Chronic physical exercise positively affects brain structure (e.g., improved white matter integrity in the genu of the corpus callosum37) and function (e.g., increased activation in brain regions, including the anterior cingulate and superior frontal gyrus38,39), while physical exercise intensity consists of light, moderate, moderate to vigorous, and vigorous levels.40 In general, chronic physical exercise interventions with moderate intensity, through different mechanisms, have been found to enhance neurotransmitter systems, upregulate BDNF, increase neurogenesis, and improve psychological well-being.41
As far as measuring the efficacy of physical exercise interventions on mental health, cognitive function, and ADHD symptoms is concerned, systematic reviews and meta-analyses have been found to be robust tools to support clinical decision-making.31,42 However, as with all experimental studies, reviews are also prone to inconsistency and methodological biases (e.g., excess significant bias and publication bias), so that different studies or meta-analyses on identical patients may reach divergent conclusions.43 Even meta-analyses examining identical variables may produce contradictory results. For instance, Cerrillo-Urbina et al., 201544 and Seiffer et al., 202145 observed a significant effect of physical exercise on the social functioning of ADHD. By contrast, a subsequent meta-analysis by Sun et al., 2022 reported that social functioning could not be altered by physical exercise intervention. Such conflicting findings hinder efforts to derive firm evidence-based conclusions from individual meta-analyses.
In recent years, umbrella reviews have emerged as a useful additional tool for evidence synthesis, because they are not subject to the methodological limitations and biases of conducted meta-analyses.46,47 An umbrella review gathers evidence from a large body of published studies by assessing the credibility of multiple meta-analyses in a consistent, transparent, and reproducible framework.48 An umbrella review, in particular, is intended to guide clinicians to the best available evidence relevant to a specific decision.49,50 Recent umbrella reviews have indicated positive associations between physical activity or physical exercise and mental health outcomes in the healthy human population,51,52 and in children and adolescents with ADHD.53
The aims of this study were therefore: (a) to synthesise a hierarchy of evidence into a single composite score and examine the efficacy of physical exercise as a potential treatment for mental health issues, cognitive dysfunction, and ADHD symptoms in children and adolescents with ADHD primarily; (b) to conduct an algorithmic evaluation of the credibility of the evidence to support the efficacy of different physical exercise interventions through objective, transparent and reproducible criteria; (c) to examine the credibility of identified moderators including physical exercise type, intensity, and mode in published meta-analyses; (d) to analyse the quality, strength, and limitations of the meta-analyses; and (e) to highlight current gaps in the literature and make recommendations for future research.
Methods
To report the findings of the present umbrella review, we strictly adhered to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline (Appendix pp 4–6) and considered the Aromataris and colleagues’ recommendations.24 At least two independent investigators screened, extracted data, and appraised included studies (SD, MHDF, and DSB). This umbrella review was registered with PROSPERO (number CRD42022361331), and the protocol can be accessed online.
Literature search strategy and eligibility criteria
Three independent reviewers (SD, MHDF, and DSB) systematically explored Web of Science, Psych INFO, Embase, Cochrane Library, PubMed, SPORTDiscus, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) for systematic reviews and meta-analysis articles published between database inception and 7 November 2021. Furthermore, two additional searches were conducted on 12 September 2022, and 1 December 2022, to ensure completeness. We selected eligible reviews by sequentially screening the titles, abstracts, and full text (Fig. 1). We also screened all references from eligible studies to maximise the accuracy of the search. Any discrepancies between authors were resolved through debate. In cases where a unanimous decision could not be reached, the corresponding author (CHPS) made the final decision. Appendix (p 7) provides full details of the search strategy, including the terms used.
Fig. 1.
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flow chart of the literature search and screening process.
We included meta-analyses and systematic reviews that provided meta-analyses of prospective studies, e.g., randomised controlled trials (RCT) and non-randomised controlled trials (nRCT), that examined the impact of a physical exercise intervention on any outcomes of mental health, cognitive function, or ADHD symptoms in children and adolescents with ADHD. The language restriction did not apply. The definitions for mental health align with those established by the World Health Organization (WHO), and the understanding of cognitive function is drawn from prior research in psychology. The criteria for ADHD symptoms comply with those included in the Diagnostic and Statistical Manual of Mental Disorders version 5 (DSM-5). Further information can be found in (Appendix p 13). The included studies used categorical ADHD diagnosis criteria in line with those of the Diagnostic and Statistical Manual of Mental Disorders (DSM), the International Classification of Diseases (ICD), or hospital profiles of the patients.
We excluded the reviews that did not evaluate at least one mental health or cognitive function factor or ADHD symptoms. We also excluded articles that did not run meta-analyses and articles that did not provide adequate data for re-analysis (e.g., the primary study estimates or sufficient data to calculate these with appropriate formula). We also excluded animal studies and conference abstracts. To avoid overlaps, if more than one meta-analysis addressed an identical topic with the same primary studies, we selected the one with the higher methodological score base on A Measurement Tool to Assess Systematically Reviews 2 (AMSTAR 2).48 The meta-analyses excluded during the text-screening stage are listed in the Appendix (p 8). First, we preferred the meta-analysis of studies with adjusted estimates over those with crude estimates. Then, we used AMSTAR 254 to rate meta-analyses based on their quality and selected the highest methodological scoring ones (Appendix pp 9–10). In cases where two or more meta-analyses achieved a similar score, we picked the one with more included studies. Whenever a review substantially overlapped with another review but contained a few individual studies that had not been included in other reviews, we only included the new studies, and excluded the overlaps. This approach was consistent with that set out in the Cochrane Library handbook for umbrella reviews.55
Absent studies search
To strengthen the results of this umbrella review, we conducted an additional search for individual studies that were suitable for our purposes but had not been included in any of the meta-analyses previously considered.22 A comprehensive explanation of our search process, including the PRISMA flow chart and the criteria for selecting and excluding absent studies, is set out in the Appendix (p 11).
Data extraction
For each of the included meta-analyses, two investigators (SD and MHDF) separately extracted the first author's surname and year of publication, together with the number of cases and controls with ADHD and the size of the total study population. We also extracted the maximum adjusted individual study estimate and its corresponding 95% confidence interval along with the metrics used in the original analyses, such as the standard mean difference (SMD), mean difference (MD), Cohen's d, and Hedges' g (G). We also extracted the primary study designs of the included meta-analyses (e.g., RCT, nRCT) and their risk of bias assessment (Table 1). We exclusively considered meta-analyses that studied the efficacy of physical exercise on patients with ADHD mental health, cognitive function, and ADHD symptoms (Fig. 2). If other psychological intervention methods were included, we extracted the related data of physical exercise intervention and re-analysed it.
Table 1.
Effect of exercise on mental health and cognitive function of ADHD children and adolescents graded by the classification of the evidence.
| Outcome | Source | Number of ADHD cases; age range | Number of study estimates | Included Study design | Effect metrics | Effect size (95% CI) and random effect P valuea | 95% PI | I2 (%) | Egger Z score and P value | Large heterogeneity, small study effect, excess significant bias | AMSTAR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Convincing (class I) | |||||||||||
| Highly suggestive (class II) | |||||||||||
| Inhibitory control | Liang and colleague56 (2021) | 406; 8–16 | 11 | RCT, nRCT | SMD | 0.76 (0.38 to 1.15); P = 0.001 | −0.44 to 1.97 | 68% | 2.17; P = 0.058 | Large heterogeneity | High |
| Suggestive (III) | |||||||||||
| Executive function | Sun and colleague57 (2022) | 322; 6–15 | 12 | RCT, nRCT | SMD | 1.22 (0.61 to 1.82); P < 0.00001 | −1.12 to 3.6 | 81% | 1.19; P = 0.26 | Large heterogeneity | High |
| Behavioural functioning | Xie_b and colleague58 (2021) | 202; 5–41a | 12 | Pre-Post study | SMD | 0.35 (0.20 to .49); P < 0.001 | 0.20 to 0.5 | 0% | 1.44; P = 0.18 | None | High |
| Emotional functioning | Xie_b and colleague58 (2021) | 233; 5–41a | 14 | Pre-Post study | SMD | 0.42 (0.26 to .58); P < 0.001 | 0.10 to 0.74 | 29% | 1.02; P = 0.33 | None | High |
| Hyperactive or impulsive | Xie_b and colleague58 (2021) | 282; 5–41a | 15 | Pre-Post study | SMD | 0.68 (0.40 to 0.95); P < 0.001 | −0.26 to 1.62 | 66% | 1.57; P = 0.14 | Large heterogeneity | High |
| Inattention | Xie_b and colleague58 (2021) | 296; 5–41a | 16 | Pre-Post study | SMD | 0.61 (0.37 to 0.83); P < 0.001 | −0.072 to 1.29 | 51% | 1.38; P = 0.18 | Large heterogeneity | High |
| Weak (IV) | |||||||||||
| Inattention | Bustamante and colleague59 (2022) | 432; 5–13 | 9 | RCT, nRCT, and Pre-post study | G | 0.41 (0.07 to 0.74); P = 0.016 | −0.41 to 1.23 | 61% | 1.18; P = 0.28 | Large heterogeneity | High |
| Emotional functioning | Cerrillo-Urbina and colleague44 (2015) | 64; 8–16 | 2 | RCT, nRCT | SMD | 0.66 (0.13 to 1.18); P = 0.01 | 0.13 to 1.19 | 0% | NA | Excess significant bias | High |
| Executive function | Cerrillo-Urbina and colleague44 (2015) | 102; 8–16 | 3 | RCT, nRCT | SMD | 0.58 (0.15 to 1); P = 0.008 | 0.04 to 1.12 | 8% | 6.09; P = 0.1 | None | High |
| Hyperactive symptoms | Cerrillo-Urbina and colleague44 (2015) | 58; 8–16 | 2 | RCT, nRCT | SMD | 0.56 (0.04 to 1.08); P = 0.04 | 0.04 to 1.07 | 0% | NA | None | High |
| Impulsivity | Cerrillo-Urbina and colleague44 (2015) | 58; 8–16 | 2 | RCT, nRCT | SMD | 0.56 (0.04 to 1.08); P = 0.04 | 0.04 to 1.07 | 0% | NA | None | High |
| Inattention | Cerrillo-Urbina and colleague44 (2015) | 142; 8–16 | 5 | RCT, nRCT | SMD | 0.84 (0.48 to 1.20); P = 0.000005 | 0.47 to 1.20 | 0% | 3.05; P = 0.055 | None | High |
| Social functioning | Cerrillo-Urbina and colleague44 (2015) | 53; 8–16 | 2 | RCT, nRCT | SMD | 0.59 (0.03 to 1.16); P = 0.04 | 0.03 to 1.15 | 0% | NA | None | High |
| Cognitive flexibility | Liang and colleague56 (2021) | 311; 8–16 | 8 | RCT, nRCT | SMD | 0.78 (0.33 to 1.23); P = 0.003 | −0.58 to 2.22 | 68% | 5.91; P = 0.001 | Large heterogeneity; small study effect | High |
| Working memory | Liang and colleague56 (2021) | 198; 8–16 | 5 | RCT, nRCT | SMD | 0.38 (0.03 to 0.73); P = 0.03 | −0.17 to 0.93 | 63% | 2.3; P = 0.1 | Large heterogeneity | High |
| Social functioning | Seiffer and colleague45 (2021) | 120; 7–16 | 5 | RCT, nRCT, and Pre-post study | G | 0.46 (0.08 to 0.85); P = 0.017 | 0.08 to 0.85 | 0% | 3.5; P = 0.038 | Small study effect | High |
| Cognition | Sibbick and colleague60 (2022) | 2554; 5–18 | 58 | RCT, nRCT, and Pre-post study | SMD | 0.18 (0.12 to 0.25); P < 0.01 | 0.12 to 0.25 | 68% | −1.7; P = 0.1 | Large heterogeneity | Moderate |
| Cognitive flexibility | Sibbick and colleague60 (2022) | 1048; 5–18 | 18 | RCT, nRCT, and Pre-post study | SMD | 0.21 (0.09 to 0.32); P = 0.001 | 0.09 to 0.32 | 42% | −0.65; P = 0.52 | None | Moderate |
| Inattention | Sibbick and colleague60 (2022) | 892; 5–18 | 20 | RCT, nRCT, and Pre-post study | SMD | 0.20 (0.09 to 0.32); P = 0.001 | 0.09 to 0.32 | 56% | −1.21; P = 0.24 | Large heterogeneity | Moderate |
| Inhibitory control | Sibbick and colleague60 (2022) | 453; 5–18 | 15 | RCT, nRCT, and Pre-post study | SMD | 0.18 (0.03, 0.33); P = 0.002 | 0.03 to 0.33 | 46% | −0.91; P = 0.37 | None | Moderate |
| Executive function | Sung and colleague61 (2022) | 431; 5–33a | 14 | RCT, nRCT | G | 0.71 (.0.31 to 1.10); P = 0.0004 | −0.75 to 2.17 | 95% | 1.5; P = 0.15 | Large heterogeneity | High |
| Inhibition on high cognitive demand task | Welch and colleague62 (2021) | 219; 8–15 | 7 | RCT, nRCT | SMD | 0.71 (0.08 to 1.34); P = 0.03 | −0.96 to 2.39 | 80% | 1.38; P = 0.22 | Large heterogeneity | High |
| Cognitive Flexibility | Zhang and colleague63 (2020) | 81; 7–12 | 2 | RCT | SMD | 0.82 (0.12 to 1.5); P = 0.02 | −0.15 to 1.79 | 43% | NA | None | High |
| Inhibitory Control | Zhang and colleague63 (2020) | 191; 7–12 | 5 | RCT | SMD | 1.07 (0.20 to 1.9); P = 0.01 | −0.92 to 3.07 | 87% | 1.31; P = 0.28 | Large heterogeneity | High |
| Not Significant (NS) | |||||||||||
| Hyperactive or impulsive | Bustamante and colleague59 (2022) | 297; 5–13 | 6 | RCT, nRCT, and Pre-post study | G | 0.18 (−0.05 to 0.41); P = 0.12 | −0.1 to 0.46 | 7% | 0.87; P = 0.43 | None | High |
| Working Memory | Sibbick and colleague60 (2021) | 155; 5–18 | 5 | RCT, nRCT, and Pre-post study | SMD | 0.02 (−0.22 to 0.26); P = 0.87 | −0.22 to 0.26 | 0% | 2.83; P = 0.06 | None | Moderate |
| Behavioural functioning | Sun and colleague57 (2022) | 78; 6–15 | 3 | RCT, nRCT | SMD | 0.24 (0.69 to −0.21); P = 0.3 | −0.21 to 0.68 | 0 | −0.47; P = 0.72 | None | High |
| Emotional functioning | Sun and colleague57 (2022) | 98; 6–15 | 4 | RCT, nRCT | SMD | 0.72 (−0.11 to 1.55); P = 0.09 | −1.17 to 2.63 | 0.71 | 0.68; P = 0.57 | Large heterogeneity | High |
| Hyperactive | Sun and colleague57 (2022) | 161; 6–15 | 4 | RCT, nRCT | SMD | −0.06 (−0.37 to 0.26); P = 0.70 | −0.37 to 0.25 | 0 | 0.56; P = 0.63 | None | High |
| Inattention | Sun and colleague57 (2022) | 355; 6–15 | 9 | RCT, nRCT | SMD | 0.60 (0.11 to 1.10); P = 0.02 | −1.3 to 2.63 | 76% | 1.79; P = 0.11 | Large heterogeneity | High |
| Social functioning | Sun and colleague57 (2022) | 113; 6–15 | 4 | RCT, nRCT | SMD | 0.27 (0.09 to 0.64); P = 0.14 | −0.09 to 0.64 | 0% | −0.61; P = 0.6 | None | High |
| Inattention | Welch and colleague62 (2021) | 70; 8–15 | 2 | RCT, nRCT | SMD | 0.76 (−0.41 to 1.93); P = 0.03 | −1.14 to 2.65 | 80% | NA | Large heterogeneity | High |
| Inhibition on low cognitive demand task | Welch and colleague62 (2021) | 26; 8–15 | 2 | RCT, nRCT | SMD | −0.10 (−2.91 to 2.72); P = 0.95 | −4.79 to 4.61 | 90% | NA | Large heterogeneity | High |
| Behavioural functioning | Xie_a and colleague58 (2021) | 212; 5–411 | 6 | RCT, nRCT | SMD | 0.1 (−0.28 to 0.48); P = 0.61 | −0.73 to 0.94 | 68% | 0.37; P = 0.72 | Large heterogeneity | High |
| Emotional functioning | Xie_a and colleague58 (2021) | 207; 5–411 | 6 | RCT, nRCT | SMD | 0.47 (−0.16, 1.09); P = 0.24 | −1.68 to 2.77 | 93% | 1.24; P = 0.28 | Large heterogeneity | High |
| Hyperactive or impulsive | Xie_a and colleague58 (2021) | 202; 5–411 | 6 | RCT, nRCT | SMD | 0.03 (−0.19 to 0.25); P = .58 | −0.5 to 0.69 | 38% | 0.64; P = 0.55 | None | High |
| Inattention | Xie_a and colleague58 (2021) | 253; 5–411 | 8 | RCT, nRCT | SMD | 0.71 (0.11 to 1.33); P = 0.022 | −1.65 to 3.22 | 84% | 1.71; P = 0.14 | Large heterogeneity | High |
| Working memory | Zhang and colleague63 (2020) | 145; 7–12 | 3 | RCT | SMD | 0.43 (−0.005 to 0.86); P = 0.052 | −0.22 to 1.09 | 41% | −2.47; P = 0.24 | None | High |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; AMSTAR 2, A Measurement Tool to Assess Systematic Reviews 2; CI, confidence interval; G, Hedges' g; NA, not available; nRCT, non-randomised controlled trial; PI, prediction interval; RCT randomised control trial; SMD, standard mean difference.
We excluded the individual studies in which the participants aged older than 18 for re-analysing.
Fig. 2.
The outcomes discovered by a systematic search, missing outcomes in the literature.
Absent study data extraction
We used a standardised form to gather information from absent studies, covering each study's reference, design, and location. We carefully recorded three main types of information: participant characteristics, physical exercise intervention details, and findings. We documented the total sample size, sex, age range, and diagnostic method for each study's participants. The physical exercise interventions included the type, mode, frequency, and assessment process. In the Appendix (p 12), we have tabulated the information collected from the absent studies.
Data analysis
We conducted a series of statistical tests to determine the robustness and consistency of the effect of physical exercise on the mental health and cognitive function outcomes of children and adolescents with ADHD following previous umbrella reviews.7,64,65 We used individual study estimates to re-analyse each eligible meta-analysis. All estimates in the original meta-analysis were converted to Hedges’ g (G) to facilitate interpretation.
Whenever necessary, the direction of the effect was switched. Therefore, positive effects systematically reflected improvements, such as symptom reduction or competency improvements. In cases where two different groups were compared (e.g., two experimental groups and one control group), the resulting effect sizes were conservatively assumed to derive from the same patients. We calculated the summary effect estimate and values of the eligible meta-analyses. Statistical significance was set at . We ran Cochran's Q test and calculated the I2 statistic for heterogeneity ( indicates high heterogeneity).66 We calculated the 95% prediction interval (95% PI), which is the range in which we expect the effect of the intervention will be found in 95% of further studies.67 We used Egger et al.‘s asymmetry test to assess the existence of small study effects within regression calculations.68 In the regression asymmetry test with a more conservative effect in the largest study, a evidenced a bias resulting from small-study effects. We calculated the possibility of excess significance bias for statistically significant meta-analyses using a literature bias that compares the expected and observed numbers of statistically significant individual studies ().69
We performed meta-analyses using a random-effect model to consider potential methodological limitations in experimental/quasi-experimental studies that could result in spurious significance.70 A sensitivity analysis was conducted by excluding nRCTs and studies with a high risk of bias identified by the Cochrane Risk of Bias (RoB) Tool. We also performed an overall calculation to present a more comprehensible and recent depiction of the potency of physical exercise interventions. This methodology has been previously endorsed in the guidelines proposed by Aromataris et al.71 We used the R studio software72 (V 4.1.2) and related packages (e.g., metafor V 3.8–1,73 meta V 6.1–0,74 and metaviz V 0.3.175) for the statistical analysis, and two-tailed statistical tests were conducted for Q-test (in heterogeneity and outlier selection), Egger test, and Z-test (in random-effect model estimations).76 For measuring the quality and recency of the included meta-analysis, two independent investigators (SD and MHDF) scored them by means of the AMSTAR 2 checklist.54 Reviews that scored critically low were excluded from the study.
Moderator analyses
Most of the included meta-analyses performed a subgroup analysis to identify potential moderators to explain heterogeneity in study estimates.45,56, 57, 58, 59, 60, 61, 62, 63 We followed their criteria and re-ran the moderator analysis to test the credibility of their subgroup analysis by examining type (aerobic, cognitive engaging exercise), intensity (light, moderate, moderate to vigorous, and vigorous), and mode (acute and chronic). We conducted the subgroup analyses in accordance with the guidelines provided in the Cochrane Library Handbook. The number of included studies in each subgroup was confirmed (K < 10) before the test could run. 1. Following, the between-group test should be significant (p < 0.1 is significant in sub-group analysis). 2. Each category must have contained at least five studies (e.g., five in acute, five in chronic) and have involved at least 1800 participants.55,77
Appraisal of the credibility of the evidence
In accordance with the prior umbrella reviews,7,48,64,78,79 we evaluated the credibility of evidence regarding the efficacy of each physical exercise intervention on each outcome into five categorical classes, using an algorithmic approach. The evidence was either convincing (Class I), highly suggestive (Class II), suggestive (Class III), weak (Class IV), or not significant (Class NS). The results were tabulated into a spreadsheet. This study adopted a criteria set derived from two classification systems commonly used in umbrella reviews concerning identical patients (Table 2).48,80 The credibility of the evidence was re-run four times to examine the robustness of the classes attributed in the primary analysis: (1) independently, for each included meta-analysis without removing overlaps (Table 1); (2) for each included meta-analysis after removing overlaps (Fig. 3, Appendix pp 21 and 22); (3) after calculating the overall results with all included study estimates (Appendix p 23); and (4) after calculating the overall results with RCT designs studies and without a high risk of bias in a sensitivity analysis (Appendix p 23).
Table 2.
Criteria used for the classification of the evidence.
| Class |
|||||
|---|---|---|---|---|---|
| Convincing (Class I) | Highly suggestive (Class II) | Suggestive (Class III) | Weak (Class IV) | Not significant (Class ns) | |
| Criteria | number ADHD cases strictly >500 | number of ADHD cases >350 | number of ADHD cases >200 | – | – |
| p-value was strictly <0.001 | p-value was strictly <0.001 | p-value was strictly <0.001 | p-value was strictly <0.05 | p-value was ≥0.05 | |
| Heterogeneity (Iˆ2) < 50% | largest study found statistically significant effects | – | – | – | |
| 95% PI excluded the null | – | – | – | – | |
| no detection of small study effect | – | – | – | – | |
| low risk of bias for >75% participants | low risk of bias for >50% participants | – | – | – | |
| met all MASTAR-2 critical > methodological criteria (5/5) | met all AMSTAR-2 critical methodological criteria (4/5) | – | – | – | |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; AMSTAR 2, A Measurement Tool to Assess Systematic Reviews 2; CI, confidence interval; PI, prediction interval
Fig. 3.
The forest plot of pooled estimates of each included meta-analysis, the 95% confidence interval (95% CI) that is plotted by black lines, the 95% prediction interval (95% PI) that is plotted by grey lines, and the number of summarised studies and classes of each meta-analysis More details are provided in the Appendix (pp21 and 22).
Ethics
The publicly available desensitised data were used in this study.
Role of the funding source
There was no funding received for this study.
Results
Database
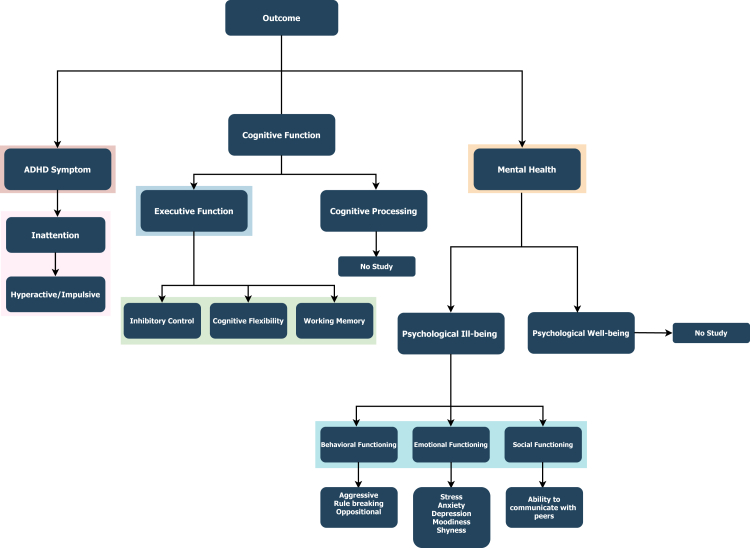
We identified 181 meta-analyses from the seven databases, of which ten were finally eligible (Fig. 1).44,45,56, 57, 58, 59, 60, 61, 62, 63 Due to the absent individual studies searches from the same databases, we identified 12 individual studies (Appendix pp 11 and 12). The 10 eligible meta-analyses (45 included individual studies in total) provided 37 meta-analyses yielded from 106 study estimates based upon the data of 1702 children and adolescents with ADHD. Eight variables were identified for cognitive function, across three main areas: (1) executive function (cognitive flexibility, inhibitory control, and working memory); (2) mental health (social functioning, behavioural functioning, and emotional functioning); and (3) ADHD symptoms (inattention, hyperactivity, or impulsiveness). We illustrated the identified outcomes after a systematic search (Fig. 2), tabulated the questionnaires, and tested each study used to measure the concerned outcome (Appendix pp 14–22). Twenty-nine of those studies were RCT-designed studies, of which 15 had an nRCT design. In total, 23 individual studies considered the effectiveness of physical exercise interventions upon inattention, 20 upon inhibitory control, 11 upon cognitive flexibility and emotional functioning, 10 on working memory and behavioural functioning, and nine upon social functioning. Study estimate metrics were either SMD or G. A total of 18 (48%) physical exercise interventions were statistically significant under the random effect model, of which six (16%) had a , three (8%) had , eight (21%) had a , and 19 (51%) were not significant. Fourteen (38%) physical exercise intervention studies displayed considerable heterogeneity (). Four (11%) had a small study effect, and one study (3%) had excess significance bias. In 11 (30%) studies, the 95% prediction interval excluded the null.
Quality assessment
All included meta-analyses were evaluated using the AMSTAR 2 checklist. Of 10 included meta-analyses, nine were graded high quality (range 10–13), and one was graded medium quality (scored 7) (Table 1). Based on the AMSTAR-2 methodological quality assessment criteria (5 items in AMSTAR-2), three studies scored 5, four studies scored 4, two studies scored 3, and one study scored 2. Table 1 presents a comprehensive overview of the primary attributes of each meta-analysis, including effect size, risk of bias tests, and meta-analyses quality. This table distinguishes between the various outcomes of interest in each meta-analysis before removing overlaps.
Hierarchical level of evidence: mental health, cognitive function, ADHD symptom factors
The overall meta-analysis, based on 106 study estimates, indicates the positive effect of physical exercise graded as the highly-suggestive class (class II) on cognitive flexibility (G = 0.52, 95% CI: 0.32–0.72, 95% PI 0.20–0.85, ; N = 521), and inhibitory control (G = 0.82, 95% CI: 0.52–1.13, 95% PI −0.36 to 2.01, ; N = 801) as executive function factors. Inattention problem (G = 0.92, 95% CI: 0.44–1.39, 95% PI −1.12 to 3.08, ; N = 875), as a factor of ADHD symptoms, was highly suggestive (class II) as well. Outliers were detected in the executive function and ADHD symptoms data. After removing outliers, the effect of physical exercise on cognitive flexibility (G = 0.46, 95% CI: 0.27–0.64, 95% PI 0.27–0.64, ; N = 501) and inattention factors remained in the highly suggestive class (G = 0.7, 95% CI: 0.37–1.04, 95% PI −0.7 to 2.11, ; N = 835). Although the effect size was lower, it was still significant (Appendix p 23).
The evidence weakly (class IV) supported the positive effect of physical exercise upon two mental health factors: emotional functioning (G = 0.81, 95% CI: 0.29–1.34, 95% PI −0.82 to 2.45, ; N = 385) and social functioning (G = 0.90, 95% CI 0.25–1.55, 95% PI −0.97 to 2.77, ; N = 262); and upon one of the executive function factors: working memory (G = 0.61, 95% CI 0.20–1.02, 95% PI −0.57 to 1.79, ; N = 447). After removing the outlier, social functioning (G = 0.61, 95% CI 0.23–0.99, 95% PI −0.21 to 1.43, p = 1e-3; N = 222) continued to exist in weak classification and working memory (G = 0.42, 95% CI 0.21 to 0.64, 95% PI 0.094 to 0.748, ; N = 417) levelled up to highly suggestive evidence. The evidence was not significant for the effectiveness of physical exercise on either behavioural functioning or hyperactive/impulsive behaviors . The sunset funnel plots for visually assessing the publication bias are provided in the Appendix (pp 28–42).
Sensitivity subset analyses
For sensitivity analysis, we only included RCTs (Randomised Controlled Trials) with a low or medium risk of bias. Sixty-five study estimates were selected for the sensitivity of the analysis (Appendix p 23). The effects of physical exercise on inhibitory control (G = 0.84, 95% CI 0.42–1.27, 95% PI −0.58 to 2.27, ; N = 499) and working memory (G = 0.38, 95% CI 0.17–0.6, 95% PI 0.08–0.68, ; N = 389) were graded as highly suggestive evidence, while the effect of physical exercise intervention on cognitive flexibility was graded as suggestive (G = 0.60, 95% CI 0.37–0.82, 95% PI 0.37–0.82, ; N = 333). By contrast, the effect on inattention was graded as weak (G = 0.9, 95% CI 0.16–1.65, 95% PI −1.77 to 3.58, ; N = 574), and was not significant for behavioural functioning , emotional functioning , hyperactive/impulsive , and social problem ().
Subgroup analyses (moderator effects)
Based on the Cochrane library handbook73,81 criteria mentioned in the method, none of the contender variables were identified as moderators. However, the meta-analysis results classified based on type, mode, and intensity are reported in Appendix (pp 24–27). We emphasise that the possibility of comparing different exercise types, modes, and intensities is implausible at this stage.
Discussion
To the best of our knowledge, this umbrella review is the first of its kind to evaluate the efficacy of physical exercise on the mental health, cognitive function, and ADHD symptoms of children and adolescents with ADHD, based on a set of algorithmic criteria. The findings of our umbrella review help to advance our understanding of currently available studies in this line of research. Specifically, our analyses indicated that the ameliorative effect of physical exercise interventions on some of the executive function (cognitive flexibility and inhibitory control) outcomes and one of the ADHD symptoms (inattention) were graded highly suggestive (class II). However, the effect of physical exercise on mental health outcomes, e.g., behavioural, emotional, and social, were graded weak (IV) or not significant (NS). Moreover, as an executive function outcome, working memory was graded as weak (class IV). The effectiveness of physical exercise on cognitive flexibility was not graded as convincing evidence, because of the small study effect. Meanwhile, substantial heterogeneity, including the null in 95% PI, led to highly suggestive (II) inattention and inhibitory control outcomes.
While all results were downgraded after sensitivity analyses, it is striking that the effectiveness of physical exercise on the working memory of patients with ADHD, which was graded as weak, was upgraded to highly suggestive (II). Only a small sample size (N = 389) in the analyses prevented it from achieving the status of convincing evidence (I). The essential requirements for conducting a subgroup meta-analysis are not satisfied, based on Cochrane Library guidelines. Therefore, it appears that we could not confirm earlier published subgroup analysis results in the literature.45,56, 57, 58, 59, 60, 61, 62, 63
The fact that inattention problems, cognitive flexibility, and inhibitory control in children and adolescents with ADHD were positively affected by physical exercise was proven by highly suggestive evidence.
Numerous neurobiological rationales have attempted to account for the psychological differences observed between children and adolescents with ADHD and their typically developing peers. Based upon magnetic resonance imaging (MRI) findings, prefrontal cortex (PFC) development is delayed in children with ADHD.82,83 The PFC is centrally responsible for cognitive functions, and dopamine in the PFC regulates inattention, inefficient executive function, or difficulty in modulating motor activity.82 Specifically, the ‘dynamic developmental behavioural’ theory of ADHD states that an impaired function of the dopaminergic paths/signals results in improper modulation of non-dopaminergic pathways, e.g., glutamic and γ-aminobutyric acid (GABA) pathways, resulting in lower inhibition or complete disinhibition of responses.84
Several pharmacological and non-pharmacological interventions have been recognised for managing patients with ADHD.85 Among the non-pharmacological modalities, physical exercise has been widely acknowledged as a prominent treatment approach.86 The mechanism of the physical exercise effect in humans with ADHD has yet to be determined. In animal studies, however, physical exercise prevented dopaminergic neuronal death by blocking microglial activation and enhancing dopamine levels, resulting in an improvement in the symptoms caused by Parkinson's disease and stress.40,41 In a rat model of ADHD, treadmill physical exercise enhanced TH gene expression, which plays an indirect but essential role in producing catecholamine, e.g., dopamine87; while swimming lessened ADHD symptoms by boosting the expression of dopamine and restricting the expression of the dopamine D2 receptor.88
In human literature, we identified two mechanisms for the effectiveness of physical exercise in improving executive function and mental health. First, the neurobiological mechanism: studies have shown that participation in physical exercise boosted arousal levels, cerebral blood circulation, and neurotransmitter secretion, and could promote cognitive function and raise the parietal P3 amplitude of the event-related potential (ERP).45, 46, 47, 48, 49, 50, 51, 52, 53, 71, 54, 55, 64, 65, 66, 67, 68, 69, 70, 72, 73, 74, 75, 76, 63, 59, 61, 58, 60, 56, 57, 62, 77, 78, 79, 80, 82, 83, 84, 85, 86, 81, 89, 90, 87, 88, 91, 92, 93, 94 Second, learning or development mechanism or physical exercise -induced executive function improvements. Participating in physical exercise interventions facilitates cognitive development by providing a learning experience.95 Physical skills require attention, instruction, remembering, and inhibition of irrelevant environmental stimulations.72 For example, participating in cognitive engagement exercise skill physical exercise such as basketball or football requires decision-making, anticipating, and inhibitory control to accomplish specific tasks.73 There are more opportunities for improvement for those with the poorest baseline performance (e.g., children and adolescents with ADHD), whereas those with high baseline performance have fewer opportunities to improve.96 There may be inherent reinforcement of executive function in these learning processes. However, more research is needed to test this claim.
Although this is the first quantitative umbrella review to systematically assess the robustness of physical exercise intervention on mental health, cognitive flexibility, and ADHD symptoms, our study has the following limitations. First, despite applying the most stringent criteria to assess the credibility of physical exercise intervention on patients with ADHD in line with previous umbrella reviews, we cannot be certain that all biases inherent to individual studies were excluded. It is worth noting that biases might have been caused by the study design and ADHD diagnosis. Second, due to the lack of evidence, we could not re-analyse data based on ADHD subcategories, namely, impulsive type or hyperactive type, or their co-occurrence. Third, we could not identify potential moderator effects including types of physical exercise (e.g., acute vs. chronic) and age group (e.g., children vs. adolescents) because the number of studies necessary to do so was insufficient. Fourth, due to the lack of low RoB in RCT studies, we had to include RCTs with medium to low RoB in the sensitivity of analysis, which may have resulted in an overestimation of the actual effect of physical exercise on some factors. Fifth, few prospective studies have investigated cognitive processing domains, specifically reaction time, which is an etiologically characteristic of ADHD.97 As a result, our umbrella review was necessarily limited to the factors previously considered by existing meta-analyses. Sixth, our study focused on evaluating the effectiveness of physical exercise interventions for children and adolescents with ADHD. As a result, we did not compare the outcomes with other non-pharmacological treatments such as cognitive behavioural therapy. We recommend that future systematic reviews and meta-analyses discuss the reasons for existing bias and state the source of funding, which are two items in the AMSTAR-2 checklist that impact the review's quality scoring. We also recommend that authors should avoid double counting in their meta-analyses. Each study estimate in a meta-analysis must be independent; as a result, two tests from one study in a meta-analysis could not be included.
There is currently insufficient evidence regarding some factors of psychological well-being (e.g., quality of life, resilience, self-esteem) and ill-being (e.g., anxiety, depression, aggression) to determine whether physical exercise has a practical impact on ADHD children's mental health; therefore, we suggest that authors conduct high-quality RCT studies on mental health concerning patients. The current research gap is shown in Fig. 2.
At present, we do not sufficiently understand the underlying mechanisms for the effectiveness of physical exercise in improving mental health and cognitive function in patients with ADHD. Accordingly, we recommend that authors examine the neurobiological, psychological, and behavioural mechanisms underlying the efficacy of physical exercise in this respect.
Finally, based on the methodological analysis of the included reviews, we recommend that future studies report whether assessors were blinded during data collection. A failure to blind can result in substantial methodological bias and lower grades in the RoB tool.
In summary, our umbrella review examined the hierarchy of evidence regarding the efficacy of physical exercise interventions on various outcomes in children and adolescents with ADHD, including mental health, executive function, and ADHD symptoms. We utilised an algorithmic approach to accomplish this mapping. Among the 106 study estimates covering eight different outcomes for children and adolescents with ADHD, our findings suggested that physical exercise interventions have a notable impact on enhancing cognitive flexibility, inhibitory control, inattention, and working memory. However, the evidence for the efficacy of physical exercise interventions in improving emotional and social functioning was comparatively weak. As a result, we cannot conclusively affirm that physical exercise would yield meaningful effects on social and emotional functioning as mental health outcomes. Considering the uncertainties emphasised in our discussion, conducting further RCTs with a meticulous assessment of potential biases is imperative. These studies should specifically investigate the impact of physical exercise on psychological well-being such as resilience and quality of life, as well as psychological ill-being such as stress, anxiety, and depression in children and adolescents with ADHD.
Contributors
SD and CHPS designed the study. SD, DSB, and MHDF performed a literature review search and screened its results. CHPS, SD, and MHDF accessed and verified the underlying data, and produced the figures and tables. SD and CHPS interpreted the data. Any discrepancies were resolved via discussion between CHPS, SD, and MHDF. SD wrote the first draft of the manuscript and then critically revised by JCSY, CCYT, SHSW, and CHPS. The authors approved the final version. MHDF and DSB contributed equally. CHPS supervised the entire study. All authors approved the manuscript before publication.
Data sharing statement
The data used in this review was derived from publicly available systematic reviews and meta-analyses.
Declaration of interests
We declare no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102137.
Appendix A. Supplementary data
References
- 1.Beauchamp M.R., Puterman E., Lubans D.R. Physical inactivity and mental health in late adolescence. JAMA Psychiatr. 2018;75(6):543–544. doi: 10.1001/jamapsychiatry.2018.0385. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . 2023. Coming of age: adolescent health.https://www.who.int/news-room/spotlight/coming-of-age-adolescent-health Published. [Google Scholar]
- 3.Polanczyk G.V., Salum G.A., Sugaya L.S., Caye A., Rohde L.A. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry Allied Discip. 2015;56(3):345–365. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- 4.Du Rietz E., Brikell I., Butwicka A., et al. Mapping phenotypic and aetiological associations between ADHD and physical conditions in adulthood in Sweden: a genetically informed register study. Lancet Psychiatry. 2021;8(9):774–783. doi: 10.1016/S2215-0366(21)00171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faraone S.V., Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562–575. doi: 10.1038/s41380-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demontis D., Walters R.K., Martin J., et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J.H., Kim J.Y., Lee J., et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry. 2020;7(11):955–970. doi: 10.1016/S2215-0366(20)30312-6. [DOI] [PubMed] [Google Scholar]
- 8.Hoogman M., Bralten J., Hibar D.P., et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-yysis. Lancet Psychiatry. 2017;4(4):310–319. doi: 10.1016/S2215-0366(17)30049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaidya C.J., Bunge S.A., Dudukovic N.M., Zalecki C.A., Elliott G.R., Gabrieli J.D.E. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2005;162(9):1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkley R.A. 2006. A theory of ADHD. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. [Google Scholar]
- 11.Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Bitsakou P., Psychogiou L., Thompson M., Sonuga-Barke E.J.S. Inhibitory deficits in attention-deficit/hyperactivity disorder are independent of basic processing efficiency and IQ. J Neural Transm. 2008;115(2):261–268. doi: 10.1007/s00702-007-0828-z. [DOI] [PubMed] [Google Scholar]
- 13.Rapport M.D., Bolden J., Kofler M.J., Sarver D.E., Raiker J.S., Alderson R.M. Hyperactivity in boys with attention-deficit/hyperactivity disorder (ADHD): a ubiquitous core symptom or manifestation of working memory deficits? J Abnorm Child Psychol. 2009;37(4):521–534. doi: 10.1007/s10802-008-9287-8. [DOI] [PubMed] [Google Scholar]
- 14.Banaschewski T., Ruppert S., Tannock R., et al. Colour perception in ADHD. J Child Psychol Psychiatry Allied Discip. 2006;47(6):568–572. doi: 10.1111/j.1469-7610.2005.01540.x. [DOI] [PubMed] [Google Scholar]
- 15.Coghill D.R., Rhodes S.M., Matthews K. The neuropsychological effects of chronic methylphenidate on drug-naive boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;62(9):954–962. doi: 10.1016/j.biopsych.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Toplak M.E., Dockstader C., Tannock R. Temporal information processing in ADHD: findings to date and new methods. J Neurosci Methods. 2006;151(1):15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Sonuga-Barke E., Bitsakou P., Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(4):345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Association AP . 5th ed. 2013. Diagnostic and statistical Manual of mental disorders: DSM-5TM. [Google Scholar]
- 19.Sharma A., Couture J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD) Ann Pharmacother. 2014;48(2):209–225. doi: 10.1177/1060028013510699. [DOI] [PubMed] [Google Scholar]
- 20.Wiener J. The ripple effect of ADHD in adolescents: self-perceptions and social relationships. Can J Sch Psychol. 2020;35(4):235–237. [Google Scholar]
- 21.Power T.J., Tresco K.E., Cassano M.C. School-based interventions for students with attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2009;11(5):407–414. doi: 10.1007/s11920-009-0061-6. [DOI] [PubMed] [Google Scholar]
- 22.Wolraich M.L., Hagan J.F., Allan C., et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4):1–25. doi: 10.1542/peds.2019-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young S., Myanthi Amarasinghe J. Practitioner Review: non-pharmacological treatments for ADHD: a lifespan approach. J Child Psychol Psychiatry Allied Discip. 2010;51(2):116–133. doi: 10.1111/j.1469-7610.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 24.Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126. [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Ayllon M., Cadenas-Sánchez C., Estévez-López F., et al. Role of physical activity and sedentary behavior in the mental health of preschoolers, children and adolescents: a systematic review and meta-analysis. Sport Med. 2019;49(9):1383–1410. doi: 10.1007/s40279-019-01099-5. [DOI] [PubMed] [Google Scholar]
- 26.Andermo S., Hallgren M., Nguyen T.T.D., et al. School-related physical activity interventions and mental health among children: a systematic review and meta-analysis. Sport Med Open. 2020;6(1):25. doi: 10.1186/s40798-020-00254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eime R.M., Young J.A., Harvey J.T., Charity M.J., Payne W.R. A systematic review of the psychological and social benefits of participation in sport for adults: informing development of a conceptual model of health through sport. Int J Behav Nutr Phys Act. 2013;10:135. doi: 10.1186/1479-5868-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu T., Li H., Colton J.P., Ge S., Li C. The BDNF Val66Met polymorphism, regular exercise, and cognition: a systematic review. West J Nurs Res. 2020;42(8):660–673. doi: 10.1177/0193945920907308. [DOI] [PubMed] [Google Scholar]
- 29.Conley M.I., Hindley I., Baskin-Sommers A., et al. The importance of social factors in the association between physical activity and depression in children. Child Adolesc Psychiatry Ment Health. 2020;14(1):1–15. doi: 10.1186/s13034-020-00335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang C., Brand S., Feldmeth A.K., Holsboer-Trachsler E., Pühse U., Gerber M. Increased self-reported and objectively assessed physical activity predict sleep quality among adolescents. Physiol Behav. 2013;120:46–53. doi: 10.1016/j.physbeh.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Lubans D., Richards J., Hillman C., et al. Physical activity for cognitive and mental health in youth: a systematic review of mechanisms. Pediatrics. 2016;138(3) doi: 10.1542/peds.2016-1642. [DOI] [PubMed] [Google Scholar]
- 32.North T.C., McCullagh P., Tran Z. Effect of exercise on depression. Exerc Sport Sci Rev. 1990;18:379–415. [PubMed] [Google Scholar]
- 33.Möhring W., Klupp S., Ludyga S., Grob A. Executive functions in children engaging in open and closed skilled sports. Psychol Sport Exerc. 2022;61 [Google Scholar]
- 34.Chang Y.K., Labban J.D., Gapin J.I., Etnier J.L. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 2012;1453(250):87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 35.Leahy A.A., Mavilidi M.F., Smith J.J., et al. Review of high-intensity interval training for cognitive and mental health in youth. Med Sci Sports Exerc. 2020;52(10):2224–2234. doi: 10.1249/MSS.0000000000002359. [DOI] [PubMed] [Google Scholar]
- 36.Ludyga S., Gerber M., Kamijo K. Exercise types and working memory components during development. Trends Cogn Sci. 2022;26(3):191–203. doi: 10.1016/j.tics.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Loprinzi P.D., Harper J., Ikuta T. The effects of aerobic exercise on corpus callosum integrity: systematic review. Physician Sportsmed. 2020;48(4):400–406. doi: 10.1080/00913847.2020.1758545. [DOI] [PubMed] [Google Scholar]
- 38.Valkenborghs S.R., Noetel M., Hillman C.H., et al. The impact of physical activity on brain structure and function in youth: a systematic review. Pediatrics. 2019;144(4) doi: 10.1542/peds.2018-4032. [DOI] [PubMed] [Google Scholar]
- 39.Krafft C.E., Schwarz N.F., Chi L., et al. An 8-month randomised controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity. 2014;22(1):232–242. doi: 10.1002/oby.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan A., Chan D., Lee H., Ng C.C., Yeo A.H.L. Reporting adherence, validity and physical activity measures of wearable activity trackers in medical research: a systematic review. Int J Med Inform. 2022;160 doi: 10.1016/j.ijmedinf.2022.104696. [DOI] [PubMed] [Google Scholar]
- 41.Ma Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull. 2008;24(4):265–270. doi: 10.1007/s12264-008-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erickson K.I., Hillman C., Stillman C.M., et al. Physical Activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. 2019;51(6):1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ioannidis J.P.A. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 2016;94(3):485–514. doi: 10.1111/1468-0009.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerrillo-Urbina A.J., García-Hermoso A., Sánchez-López M., Pardo-Guijarro M.J., Santos Gómez J.L., Martínez-Vizcaíno V. The effects of physical exercise in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis of randomised control trials. Child Care Health Dev. 2015;41(6):779–788. doi: 10.1111/cch.12255. [DOI] [PubMed] [Google Scholar]
- 45.Seiffer B., Hautzinger M., Ulrich R., Wolf S. The efficacy of physical activity for children with attention deficit hyperactivity disorder: a meta-analysis of randomised controlled trials. J Atten Disord. 2022;26(5):656–673. doi: 10.1177/10870547211017982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ioannidis J. Next-generation systematic reviews: prospective meta-analysis, individual-level data, networks and umbrella reviews. Br J Sports Med. 2017;51(20):1456–1458. doi: 10.1136/bjsports-2017-097621. [DOI] [PubMed] [Google Scholar]
- 47.Ioannidis J.P.A. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. C Can Med Assoc J. 2009;181(8):488–493. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fusar-Poli P., Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21(3):95–100. doi: 10.1136/ebmental-2018-300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker L.A., Higgins J.P. Cochrane Handbook for systematic Reviews of interventions. Version. Vol. 5. 2011. OAD. Overviews of reviews. [Google Scholar]
- 50.Faulkner G., Fagan M.J., Lee J. Umbrella reviews (systematic review of reviews) Int Rev Sport Exerc Psychol. 2022;15(1):73–90. [Google Scholar]
- 51.Dale L.P., Vanderloo L., Moore S., Faulkner G. Physical activity and depression, anxiety, and self-esteem in children and youth: an umbrella systematic review. Ment Health Phys Act. 2019;16(December 2018):66–79. [Google Scholar]
- 52.Ciria L.F., Román-Caballero R., Vadillo M.A., et al. An umbrella review of randomised control trials on the effects of physical exercise on cognition. Nat Hum Behav. 2023;7:928–941. doi: 10.1038/s41562-023-01554-4. [DOI] [PubMed] [Google Scholar]
- 53.Réol L.A. Is physical activity good medicine for children and youth with attention-deficit hyperactivity disorder? Which aspects could influence outcomes? An umbrella review. Int J Disabil Dev Educ. 2022;00(00):1–16. [Google Scholar]
- 54.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higgins J.P.T., Thomas J., Chandler J., et al. 2019. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 56.Liang X., Li R., Wong S.H.S., Sum R.K.W., Sit C.H.P. The impact of exercise interventions concerning executive functions of children and adolescents with attention-deficit/hyperactive disorder: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2021;18(1):68. doi: 10.1186/s12966-021-01135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun W., Yu M., Zhou X. Effects of physical exercise on attention deficit and other major symptoms in children with ADHD: a meta-analysis. Psychiatry Res. 2022;311 doi: 10.1016/j.psychres.2022.114509. [DOI] [PubMed] [Google Scholar]
- 58.Xie Y., Gao X., Song Y., et al. Effectiveness of physical activity intervention on ADHD symptoms: a systematic review and meta-analysis. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.706625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bustamante E.E., Balbim G.M., Ramer J.D., et al. Diverse multi-week physical activity programs reduce ADHD symptoms: a meta-analysis: 219. Med Sci Sport Exerc. 2022;54:51–52. [Google Scholar]
- 60.Sibbick E., Boat R., Sarkar M., Groom M., Cooper S.B. Acute effects of physical activity on cognitive function in children and adolescents with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Ment Health Phys Act. 2022;23 [Google Scholar]
- 61.Sung M.C., Ku B., Leung W., MacDonald M. The effect of physical activity interventions on executive function among people with neurodevelopmental disorders: a meta-analysis. J Autism Dev Disord. 2022;52(3):1030–1050. doi: 10.1007/s10803-021-05009-5. [DOI] [PubMed] [Google Scholar]
- 62.Welsch L., Alliott O., Kelly P., Fawkner S., Booth J., Niven A. The effect of physical activity interventions on executive functions in children with ADHD: a systematic review and meta-analysis. Ment Health Phys Act. 2021;20 [Google Scholar]
- 63.Zhang M., Liu Z., Ma H., Smith D.M. Chronic physical activity for attention deficit hyperactivity disorder and/or autism spectrum disorder in children: a meta-analysis of randomised controlled trials. Front Behav Neurosci. 2020;14 doi: 10.3389/fnbeh.2020.564886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grgic J., Grgic I., Pickering C., Schoenfeld B.J., Bishop D.J., Pedisic Z. Wake up and smell the coffee: caffeine supplementation and exercise performance an umbrella review of 21 published meta-analyses. Br J Sports Med. 2020;54(11):681–688. doi: 10.1136/bjsports-2018-100278. [DOI] [PubMed] [Google Scholar]
- 65.Lesinski M., Herz M., Schmelcher A., Granacher U. Effects of resistance training on physical fitness in healthy children and adolescents: an umbrella review. Sport Med. 2020;50(11):1901–1928. doi: 10.1007/s40279-020-01327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10(1):101. [Google Scholar]
- 67.Higgins J.P., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ioannidis J.P.A., Trikalinos T.A. An exploratory test for an excess of significant findings. Clin Trials. 2007;4(3):245–253. doi: 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- 70.Papatheodorou S.I., Tsilidis K.K., Evangelou E., Ioannidis J.P.A. Application of credibility ceilings probes the robustness of meta-Analyses of biomarkers and cancer risk. J Clin Epidemiol. 2015;68(2):163–174. doi: 10.1016/j.jclinepi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Aromataris E., Fernandez R., Godfrey C.M., Holly C., Khalil H., Tungpunkom P. Summarising systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 72.RStudio Team . 2020. RStudio. Published online. [Google Scholar]
- 73.Viechtbauer W. Conducting meta-analyses in R with the metafor. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 74.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagner J., Chelaru F., Kancherla J., et al. Metaviz: interactive statistical and visual analysis of metagenomic data. Nucleic Acids Res. 2018;46(6):2777–2787. doi: 10.1093/nar/gky136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hornik K. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2009;31(2):1–48. [Google Scholar]
- 77.Richardson M., Garner P., Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Heal. 2019;7(2):192–198. [Google Scholar]
- 78.Kim J.Y., Son M.J., Son C.Y., et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. The Lancet Psychiatry. 2019;6(7):590–600. doi: 10.1016/S2215-0366(19)30181-6. [DOI] [PubMed] [Google Scholar]
- 79.Cillekens B., Lang M., Van Mechelen W., et al. How does occupational physical activity influence health? An umbrella review of 23 health outcomes across 158 observational studies. Br J Sports Med. 2020;54(24):1474–1481. doi: 10.1136/bjsports-2020-102587. [DOI] [PubMed] [Google Scholar]
- 80.Pollock A., Farmer S.E., Brady M.C., et al. An algorithm was developed to assign GRADE levels of evidence to comparisons within systematic reviews. J Clin Epidemiol. 2016;70:106–110. doi: 10.1016/j.jclinepi.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoon M.C., Shin M.S., Kim T.S., et al. Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson's rats. Neurosci Lett. 2007;423(1):12–17. doi: 10.1016/j.neulet.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y., Zuo C., Xu Q., Hao L., Zhang Y. Attention-deficit/hyperactivity disorder is characterised by a delay in subcortical maturation. Prog Neuropsychopharmacology Biol Psychiatry. 2021;104:110044. doi: 10.1016/j.pnpbp.2020.110044. [DOI] [PubMed] [Google Scholar]
- 83.Shaw P., Eckstrand K., Sharp W., et al. Attention-deficit/hyperactivity disorder is characterised by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prince J. Catecholamine dysfunction in attention-deficit/hyperactivity disorder an update. J Clin Psychopharmacol. 2008;28:39–45. doi: 10.1097/JCP.0b013e318174f92a. [DOI] [PubMed] [Google Scholar]
- 85.De Crescenzo F., Cortese S., Adamo N., Janiri L. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4–11. doi: 10.1136/eb-2016-102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ng Q.X., Ho C.Y.X., Chan H.W., Yong B.Z.J., Yeo W.S. Managing childhood and adolescent attention-deficit/hyperactivity disorder (ADHD) with exercise: a systematic review. Complement Ther Med. 2017;34:123–128. doi: 10.1016/j.ctim.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 87.Kim H., Heo H.I., Kim D.H., et al. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci Lett. 2011;504(1):35–39. doi: 10.1016/j.neulet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 88.Ko I.G., Kim S.E., Kim T.W., et al. Swimming exercise alleviates the symptoms of attention-deficit hyperactivity disorder in spontaneous hypertensive rats. Mol Med Rep. 2013;8(2):393–400. doi: 10.3892/mmr.2013.1531. [DOI] [PubMed] [Google Scholar]
- 89.Mabandla M.V., Kellaway L.A., Daniels W.M.U., Russell V.A. Effect of exercise on dopamine neuron survival in prenatally stressed rats. Metab Brain Dis. 2009;24(4):525–539. doi: 10.1007/s11011-009-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sung Y.H., Kim S.C., Hong H.P., et al. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson's disease mice. Life Sci. 2012;91(25-26):1309–1316. doi: 10.1016/j.lfs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Halperin J.M., Bédard A.C.V., Curchack-Lichtin J.T. Preventive interventions for ADHD: a neurodevelopmental perspective. Neurotherapeutics. 2012;9(3):531–541. doi: 10.1007/s13311-012-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lambourne K., Tomporowski P. The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res. 2010;1341:12–24. doi: 10.1016/j.brainres.2010.03.091. [DOI] [PubMed] [Google Scholar]
- 93.Tsai C.L., Chen F.C., Pan C.Y., Wang C.H., Huang T.H., Chen T.C. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology. 2014;41:121–131. doi: 10.1016/j.psyneuen.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 94.Hung C.L., Huang C.J., Tsai Y.J., Chang Y.K., Hung T.M. Neuroelectric and behavioral effects of acute exercise on task switching in children with attention-deficit/hyperactivity disorder. Front Psychol. 2016;7:1–11. doi: 10.3389/fpsyg.2016.01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adolph K.E. Learning to move. Curr Dir Psychol Sci. 2008;17(3):213–218. doi: 10.1111/j.1467-8721.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Drollette E.S., Scudder M.R., Raine L.B., et al. Acute exercise facilitates brain function and cognition in children who need it most: an ERP study of individual differences in inhibitory control capacity. Dev Cogn Neurosci. 2014;7:53–64. doi: 10.1016/j.dcn.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kofler M.J., Rapport M.D., Sarver D.E., et al. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin Psychol Rev. 2013;33(6):795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.