Abstract
Increasing evidence indicated that mitophagy might play a crucial role in the occurrence and progression of liver diseases. In order to enhance our understanding of the intricate relationship between mitophagy and liver diseases, a comprehensive bibliometric analysis of the existing literature in this field was conducted. This analysis aimed to identify key trends, potential areas of future research, and forecast the development of this specific field. We systematically searched the Web of Science Core Collection (WoSCC) for publications related to mitophagy in liver diseases from 2000 to 2022. We conducted the bibliometric analysis and data visualization through VOSviewer and CiteSpace. The analysis of publication growth revealed a substantial increase in articles published in this field over the past years, indicating mitophagy's growing interest and significance in liver diseases. China and USA emerged as the leading contributors in the number of papers, with 294 and 194 independent papers, respectively. Exploring the mechanism of mitophagy in the initiation and procession of liver diseases was the main content of studies in this field, and Parkin-independent mediated mitophagy has attracted much attention recently. “Lipid metabolism,” “cell death,” “liver fibrosis” and “oxidative stress” were the primary keywords clusters. Additionally, “nlrp3 inflammasome”, “toxicity” and “nonalcoholic steatohepatitis” were emerging research hotspots in this area and have the potential to continue to be focal areas of investigation in the future. This study represents the first systematic bibliometric analysis of research on mitophagy in liver diseases conducted over the past 20 years. By providing an overview of the existing literature and identifying current research trends, this analysis sheds light on the critical areas of investigation and paves the way for future studies in this field.
Keywords: Liver diseases, Mitophagy, NLRP3 inflammasome, Bibliometric, CiteSpace, VOSviewer
1. Introduction
The liver is the biggest solid organ of the body and performs numerous roles in effective physiological functions. Liver diseases are significant global causes of mortality, resulting in an estimated annual death toll of approximately 2 million individuals [1]. Among these individuals, chronic liver diseases such as cirrhosis and liver cancer account for the majority of cases [2,3]. Therefore, understanding the underlying mechanisms of the initiation and progression of liver diseases is crucial for disease prevention, therapy, and ultimately reducing global mortality. Meanwhile, the liver is a dynamic organ with abundant mitochondria that play essential roles in liver metabolism, detoxification, storage and release of energy. Mitochondrial dysfunction in the liver can impair these functions, leading to the pathological status of the liver. Mitochondrial energy metabolism is vital to maintain the metabolic processes of hepatocytes and the macronutrient metabolism of the liver [4,5]. Currently, multiple studies have demonstrated that dysfunctional mitochondria are found in various liver diseases, including liver injury, nonalcoholic fatty liver disease (NAFLD), cirrhosis, and even hepatocellular carcinoma (HCC) [6,7].
Mitophagy, a selective form of autophagy, plays a crucial role in maintaining mitochondrial quality and abundance control [8]. De Duve C and Wattiaux R first discovered the phenomenon of mitochondria caught and formed autophagic vacuoles in 1966 [9]. And Lemasters JJ first proposed the term "mitophagy" to emphasize this specifically targeted degradation process [10]. Since the process of injured mitochondria phagocytosed by autophagic vesicles was discovered and the term mitophagy was proposed, extensive research has been conducted to elucidate the underlying mechanisms. Mitochondria are central to energy production, the substrates' metabolism and apoptosis regulation [11]. Various factors, including oxidative stress, inflammation, and ischemia/reperfusion(I/R) Injury, could induce mitochondrial damage. When mitochondrial dysfunction occurs, mitophagy is believed to eliminate and degrade damaged mitochondria. Inhibition of mitophagy results in the accumulation of dysfunctional mitochondria, disrupting cellular homeostasis and leading to excessive production of reactive oxygen species (ROS), ultimately exacerbating cell death and organ injury [12]. Furthermore, mitochondria play a pivotal role in immune responses, providing a platform for appropriate activation of innate immunity [13]. Damaged mitochondria could trigger immune signaling pathways. Pathological mitochondria release mitochondrial DNA (mtDNA), which acts as a danger-associated molecular pattern (DAMP), binding to cytosolic NLRP3 and activating the NLRP3 inflammasome [14,15]. The liver is susceptible to external stimuli, such as infection, chronic alcoholism, drug injury, or hypoxia, which can lead to hepatic pathological conditions and further induce mitochondrial dysfunction as well as hepatotoxicity. Consequently, inflammatory cytokines are released. Mitophagy plays a critical role in the pathogenesis of liver diseases, including liver injury, fatty liver diseases, viral hepatitis, and liver cancer [16]. Modulation of mitophagy could affect the state of hepatotoxicity and inflammation. For instance, overexpression of PINK1 promotes mitophagy, ameliorates hepatic I/R injury, inhibits NLRP3 activation, and reverses the Kupffer cells-mediated inflammatory damage to hepatocytes [14]. Additionally, BBCL-B-dependent mitophagy can regulate apoptosis in hepatic stellate cells(HSCs) and alleviate liver fibrosis [17]. Manipulating mitophagy can also affect the metabolism of hepatocellular carcinoma cells, consequently affecting tumor growth [18,19]. Thus, maintaining a functional mitophagy system may hold promise for treating liver diseases.
Bibliometric analysis is an emerging method for analyzing the literature in a scientific field, providing a quantitative and qualitative assessment of developing trends [20,21]. It encompasses not only the analysis of citations in a specific area of research but also considers a comprehensive range of cited references associated with the studies. Moreover, other characteristics, including journal distribution, the cooperative relationships among countries, institutions, and authors, are also meaningful for researchers to understand the hotspots of a study. Bibliometric analysis has been widely applied in various studies, including those focused on hypertension, proteolysis targeting chimera (PROTAC) technology, Crohn's disease (CD), among others [22,23]. However, to date, no relative bibliometric analysis has been conducted to study the relationship between mitophagy and liver diseases.
The objective of this study was to perform a comprehensive bibliometric analysis of 623 published papers pertaining to mitophagy in liver diseases over the past two decades. The research aimed to summarize the publication characteristics and trends in this field. By undertaking this analysis, we seek to provide a systematic understanding of the subject and identify potential therapeutic strategies targeting the mitophagy pathway in liver diseases.
2. Materials and methods
2.1. Data sources and search strategies
The systematic literature search and filter conditions on mitophagy in liver diseases were based on the Web of Science Core Collection (WoSCC) on October 27, 2022. As the high-quality database accepted by many scholars, WoSCC was considered the most suitable for bibliometrics and served the most comprehensive information (17)(18). The search formula was (TS = ("liver" OR "livers" OR "liver tissue" OR "livers" OR "liver hepatic" OR "hepatic" OR "hepatocyte" OR "hepar")) AND TS = ("mitophagy" OR "mitochondrial autophagy"), and document search was conducted in one day (October 27, 2022) to avoid publication and citation differences. A total of 662 articles were acquired and started screening procedures. Our search was limited to systematic reviews and original articles. We excluded meeting abstracts (n = 21), online publications (n = 2), editorial materials (n = 12), and others (n = 3). Further, non-English article (n = 1) was excluded, too. Two researchers (JL and YS C) operated the above steps independently and any differences were discussed. Ultimately, we included 623 publications; the screening procedures are shown in Fig. 1. All information on publications can be found in Supplementary Table 1.
Fig. 1.
Flow chart of the screening process.
2.2. Bibliometric analysis and visualization
Bibliometric analysis and all data visualization were performed utilizing VOSviewer (version 1.6.18), CiteSpace (version 6.1.R3), the R package “bibliometrix” (version 4.0.1), online analysis Charticulator (https://charticulator.com/) and Microsoft Office Excel 2021.
VOSviewer (version 1.6.18), a Java-based bibliometric analysis tool, could extract and visualize key information from all literature (19). We used VOSviewer in our study to visualize co-authorship of countries, authors, institutions, co-citation of journals, references, and co-occurrence of keywords. Node size and color represented the counts and clusters of these items, and the thickness of links connecting nodes indicated the strength of correlation.
CiteSpace (version 6.1.R3) developed by Professors Chen C is another visualization tool for Bibliometric analysis (20). Using this tool, we conducted the clustered network map of co-cited references and keywords by the logarithmic likelihood ratio (LLR) algorithm. Q > 0.3 means that the community structure is consequential, and mean silhouette >0.5 indicates that clustering is credible. Moreover, we used it to make the dual-map overlay of journals on the research of mitophagy in liver diseases and the timeline diagram of co-cited references and keywords, which offered us a better understanding of the current state and prospects in this research field. Besides, the bursts of co-cited references and keywords were applied to identify the hotspots and new research trends in mitophagy on liver diseases.
The R package “bibliometrix” (version 4.0.1) (https://www.bibliometrix.org) was used to calculate the annual growth rate of publications and build a global distribution of country scientific production (21). Microsoft Office Excel 2021 was applied to perform quantitative data analysis and make a radar map of publications. And the online analysis Charticulator (https://charticulator.com/) was employed to conduct a string chart of collaboration among countries and institutions.
3. Results
3.1. Temporal trends of overall publications and citations
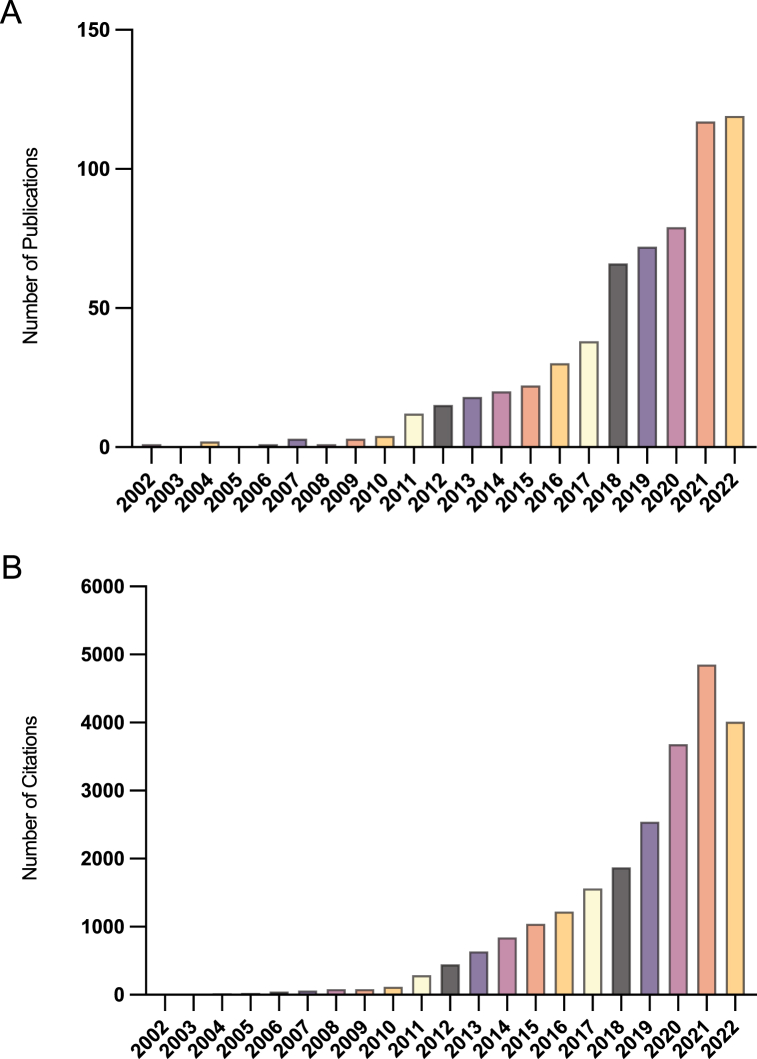
Based on the retrieval strategy described above, a total of 623 qualified studies, comprising 487 original research articles and 136 reviews, were identified. The histogram (Fig. 2A) displayed the global annual publications on mitophagy in liver diseases from 2002 to 2022. The publication growth curve rapidly increased, with an average growth rate of 26.99%. Since its first reported in 2002 [24], the research field of mitophagy in liver diseases has garnered increasing attention from researchers. From 2002 to 2010, the number of publications in this area remained relatively low, with less than 10 publications per year. However, over the subsequent 10 years, there was a notable surge in publications, rising from 12 in 2011 to 79 in 2020. Notably, the number of publications experienced a substantial increase in 2018, nearly doubling that of 2017, and surpassed 100 in both 2021 and 2022. These findings indicate a growing research focus on this field. With the increase in the number of publications, the citations also rose (Fig. 2B). In total, the studies included in the analysis were cited 23,448 times, with a mean citation rate of 37.64 times. The year-to-year variations in publications and citations reflect the rapid progress of studies on mitophagy in liver diseases, highlighting the need for further exploration in this area.
Fig. 2.
Global publications and citations of research on mitophagy in liver diseases from 2002 to 2022. (A)Annual publication trends worldwide; (B) Annual citation trends worldwide.
3.2. Countries/regions analysis:
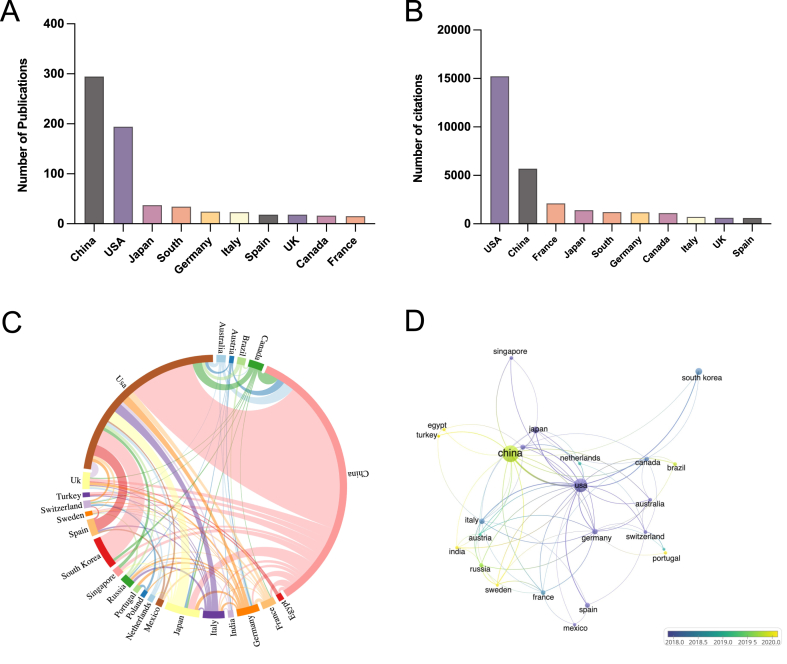
From 2002 to 2022, scholars from 50 countries/regions contributed to the publications on mitophagy research in liver diseases, with most high-yield countries in Asia, North America, and Europe. The top 10 productive countries are listed in Table 1. China led in the number of papers (n = 294, 47.19%), followed by the USA (n = 194, 31.14%) (Fig. 3A). While China published 100 more articles than the USA, the citations of China were only one-third of that of the USA. In terms of citations, the USA had the highest citation frequency of 15,230 times, followed by China with 5684 times and France with 2109 times (Fig. 3B). Notably, although France did not have the highest number of publications or citations, it had the highest average citation/publication ratio (140.60), surpassing that of the USA (78.51) and China (19.33). Therefore, improving the quality of articles is crucial in addition to increasing the number of publications. Furthermore, a network of cooperative relationships among countries was established using a string chart (Fig. 3C). The thickness of the lines represented the strength of collaboration, while a wider margin indicated a greater number of documents published by the country. This map demonstrated that China and USA had the tightest cooperative relationship among these countries. And centered on China and USA, extensive cooperation of countries worldwide had been conducted. Finally, a time-related visualization map revealed that the USA had initially established cooperation with other countries and more recently initiated collaboration with China (Fig. 3D).
Table 1.
The top 10 most productive countries on studies of mitophagy in liver diseases.
| Rank | Country | Publications | Citations | Average Citation/Publication |
|---|---|---|---|---|
| 1 | China | 294 | 5684 | 19.33 |
| 2 | USA | 194 | 15,230 | 78.51 |
| 3 | Japan | 37 | 1413 | 38.19 |
| 4 | South Korea | 34 | 1207 | 35.50 |
| 5 | Germany | 24 | 1188 | 49.50 |
| 6 | Italy | 23 | 702 | 30.52 |
| 7 | Spain | 18 | 612 | 34.00 |
| 8 | UK | 18 | 622 | 34.56 |
| 9 | Canada | 16 | 1114 | 69.63 |
| 10 | France | 15 | 2109 | 140.60 |
Fig. 3.
Countries/Region's analysis. (A) Publications of top 10 countries; (B) Citations of top 10 countries; (C) String chart of cooperation among countries. The links between countries represented cooperative relationships, and a thicker line indicated stronger cooperation. Wider margin represents more documents published by the country; (D) Time-related visualization map among countries. The nodes represented countries, and the links between nodes indicated the link strength of a co-authorship relation. Node and link color reflected each country's corresponding average appearing year (AAY). Based on the color gradient in the lower right corner, yellow represented the relatively recently published countries, while purple represented the earlier published countries.
3.3. Institutions and authors analysis:
Since 2002, 3878 authors from 859 institutions have contributed to the study of mitophagy in liver diseases. The top ten productive institutions are listed in Table 2, with six out of ten located in China (Fig. 4A). The University of Kansas, located in the USA, emerged as the institution with the highest number of publications and the most citations, with 31 articles and a total citation frequency of 1694 times. Subsequently, to visualize the collaboration among institutions, we constructed a string chart based on 21 institutions that published at least 7 articles (Fig. 4B). As shown in Fig. 4B, Fudan University (FUDAN) had a close partnership with Shanghai Jiao Tong University (SJTU) and the University of Wyoming (UWyo). Similarly, active cooperation was observed among the University of Kansas (KU), the University of Pittsburgh (PITT), and the University of Washington (UWashington).
Table 2.
The top 10 most productive institutions in studies of mitophagy in liver diseases.
| Rank | Institution | Abbreviation | Publications | Citations | Average Citation/Publication |
|---|---|---|---|---|---|
| 1 | University of Kansas | KU | 31 | 1694 | 54.65 |
| 2 | Fudan University | FUDAN | 18 | 631 | 35.06 |
| 3 | Shanghai Jiao Tong University | SJTU | 18 | 329 | 18.28 |
| 4 | University of Pittsburgh | PITT | 16 | 1251 | 78.19 |
| 5 | Capital Medical University | CCMU | 15 | 365 | 24.33 |
| 6 | Chinese Academy of Sciences | UCAS | 13 | 259 | 19.92 |
| 7 | Huazhong University of Science and Technology | HUST | 11 | 464 | 42.18 |
| 8 | Northeast Agricultural University | NEAU | 10 | 59 | 5.90 |
| 9 | University of Missouri System |
UM | 10 | 300 | 30.00 |
| 10 | Sungkyunkwan University | SKKU | 9 | 359 | 39.89 |
Fig. 4.
Authors and institutions analysis. (A) Radar map displayed the sum of publications from the top 10 productive institutions; (B) String chart of cooperation among institutions. The links between institutions represented cooperative relationships, and a thicker line indicated a stronger cooperation. Margin represented documents published by the institutions; (C) Network visualization showing the relationship among authors. The node and line color represented the cluster it belonged to; (D) Top-Authors’ production over time. The bubble size represents the number of publications written by each author in a given year, and the intensity of the color indicates the total number of citations (TC) per year. CCMU, Capital Medical University; UCAS, Chinese Academy of Sciences; CBNU, Chungbuk National University; FUDAN, Fudan University; HUST, Huazhong University of Science and Technology; JHU, Johns Hopkins University; MUSC, Medical University of South Carolina; NJMU, Nanjing Medical University; QU, Qingdao University; SJTU, Shanghai Jiao Tong University; SCAU, South China Agricultural University; SYSU, SUN YAT-SENUNIVERSITY; USD, University of San Diego; UF, University of Florida; KU, University of Kansas; UM, University of Missouri; UNC, University of North Carolina; PITT, University of Pittsburgh; UWyo, University of Wyoming; UWashington, University of Washington.
Regarding author analysis, the top 14 productive authors are listed in Table 3. Ding WX from the University of Kansas Medical Center emerged as the most productive and cited author, with 25 publications and 1651 citations, respectively. Moreover, we depicted a network visualization map of author collaborative analysis that included 12 authors with at least 5 publications (Fig. 4C). The cooperation among authors could be roughly divided into two clusters, with Ding WX and Thyfault JP serving as hubs. Both of them were affiliated with the University of Kansas, which aligns with the results of cooperation among institutions. Additionally, the top 10 authors’ production over time is displayed in Fig. 4D. Lemasters JJ was the earliest to start the research on mitophagy in liver diseases in 2002 and continued until 2018. Almost studies of the top 10 productive authors were published in the past decade. Studying the research of influential scholars, such as the most productive or the most cited authors, could provide us with directions and guidelines [25].
Table 3.
The top 14 productive authors on studies of mitophagy in liver diseases.
| Rank | Author | Affiliation | Publications | Citations | Average Citation/Publication |
|---|---|---|---|---|---|
| 1 | Ding, Wen-Xing | University of Kansas Medical Center | 25 | 1651 | 66.04 |
| 2 | Ni, Hong-Min | University of Kansas Medical Center | 13 | 1276 | 98.15 |
| 3 | Lemasters, John J | University of North Carolina | 9 | 891 | 99.00 |
| 4 | Jaeschke, Hartmut | University of Kansas Medical Center | 8 | 636 | 79.50 |
| 5 | Lee,Sun-Mee | Sungkyunkwan University | 8 | 298 | 37.25 |
| 6 | Ren, Jun | University of Wyoming College of Health Sciences | 8 | 411 | 51.38 |
| 7 | Thyfault, John P | University of Kansas Medical Center | 8 | 92 | 11.50 |
| 8 | Williams, Jessica A | University of Kansas Medical Center | 8 | 422 | 52.75 |
| 9 | Eid, Nabil | Osaka Medical College | 6 | 204 | 34.00 |
| 10 | Ibdah, Jamal A | University of Missouri | 6 | 240 | 40.00 |
| 11 | Ito, Yuko | Osaka Medical College | 6 | 204 | 34.00 |
| 12 | Otsuki, Yoshinori | Osaka Medical College | 6 | 204 | 34.00 |
| 13 | Tang, Zhaoxin | South China Agricultural University | 6 | 126 | 21.00 |
| 14 | Yang, Fan | Chengdu Fifth People's Hospital | 6 | 124 | 20.67 |
3.4. Journals and Co-cited journals analysis
Publications related to mitophagy in liver diseases were featured in 219 journals. Table 4 presents the top 10 journals with at least 10 articles. The International Journal of Molecular Sciences was the most prolific (published 24 articles, IF = 6.20), followed by Autophagy (published 15 articles, IF = 13.39) (Fig. 5A). Autophagy was the most cited journal (1602 times), followed by Hepatology (803 times, IF = 17.298) and Redox Biology (792 times, IF = 10.787). All of the top 10 journals had an Impact Factor (IF) above five. A network visualization of journals is shown in Fig. 5B. Additionally, we depicted the network visualization of the top 20 co-cited journals, which could be roughly classified into two clusters (Fig. 5C). The red nodes on the left primarily consisted of mechanistic studies, while the green nodes on the right represented clinical studies. Several highly cited articles were from renowned journals in the field of mitophagy in liver diseases, such as Science, Nature, Autophagy, Hepatology, and Journal of Hepatology.
Table 4.
The top 10 journals associated with studies of mitophagy in liver diseases.
| Rank | Journal | Publications | Citations | IF | Q | Average Citation/Publication |
|---|---|---|---|---|---|---|
| 1 | International Journal of Molecular Sciences | 24 | 521 | 6.208 | Q1 | 21.71 |
| 2 | Autophagy | 15 | 1062 | 13.391 | Q1 | 70.80 |
| 3 | Cells | 14 | 214 | 7.666 | Q2 | 15.29 |
| 4 | Frontiers in Pharmacology | 12 | 140 | 5.988 | Q1 | 11.67 |
| 5 | Cell Death & Disease | 11 | 182 | 9.696 | Q1 | 16.55 |
| 6 | Hepatology | 11 | 803 | 17.298 | Q1 | 73.00 |
| 7 | Nutrients | 11 | 118 | 6.706 | Q1 | 10.73 |
| 8 | Redox Biology | 11 | 792 | 10.787 | Q1 | 72.00 |
| 9 | Journal of Biological Chemistry | 10 | 616 | 5.485 | Q2 | 61.60 |
| 10 | Oxidative Medicine and Cellular Longevity | 10 | 180 | 7.31 | Q2 | 18.00 |
Fig. 5.
Journals and Co-Cited Journals analysis. (A) Radar map displayed the sum of publications from the top 10 productive journals; (B) Network visualization showing the relationship among journals. The node and line color represented the cluster it belonged to; (C) Network visualization showing the relationship among co-cited journals. The node and line color represented the cluster it belonged to; (D) The dual-map overlay of journals on the research of mitophagy in liver diseases. The citing journals were on the left, the cited journals were on the right, and the colored path represents the citation relationship.
To further analyze the scientific portfolio and interconnections among journals, the dual-map overlay of journals designed by Chen and Leydesdorff was utilized [26]. This dual-map, shown in Fig. 5D, provides insights into the citation patterns and interconnections among all journals worldwide. The left side represents citing journals, while the right side represents cited journals. Each curve originating from the left node to the right node indicates the citation link. Among these links, the thickest link colored orange was the primary citation path, representing that papers published in Molecular/Biology/Genetics journals were often cited by researchers in Molecular/Biology/Immunology journals. This dual-map provided a comprehensive view of the interconnections among journals on mitophagy in liver diseases, assisting researchers in identifying potential collaborations and opportunities for further research.
3.5. Co-cited reference clusters analysis
The co-cited relationships refer to the occurrence of multiple articles being cited by one or more follow-on publications simultaneously [27]. Analyzing the conceptual clusters and co-cited networks could help us understand the knowledge structure and cutting-edge areas of research. Therefore, we conducted an analysis of co-cited references in the context of mitophagy in liver diseases. Studies in this field cited a total of 31,600 references over the past two decades. The top 10 most cited pieces of literature are shown in Table 5. We utilized VOSviewer to generate a visualization analysis showing the relationship among 22 co-cited references that reached the threshold of over 30 occurrences (Fig. 6A). The “Narendra D, 2008, Journal of Cell Biology” reference displayed active co-cited relationships with other references. In addition, the co-cited references were grouped into 18 clusters generated by CiteSpace: labeled as#0 (drug-induced liver injury), #1 (mitochondrial spheroid formation), #2 (mitochondrial dysfunction), #3 (autophagy), #4 (liver cancer), and so on (Fig. 6B). More detailed information about these clusters can be found in Table 6. We used the logarithmic likelihood ratio (LLR) algorithm to produce cluster labels. The modularity Q was 0.7901, and the weight means silhouette of 0.9183, indicating reasonable cluster quality. Fig. 6C depicts the timeline diagram of 18 clusters of co-cited references. Accordingly, studies between mitophagy and liver diseases were recently concerned by scholars, and previous studies paid more attention to autophagy. Additionally, the references burst analysis was conducted to gain further insight into the development of research on mitophagy in liver diseases. The top 10 references with the highest burstiness were examined (Fig. 6D). The blue line represented the timeline, and the red intervals on the blue line indicated the burst duration. According to Fig. 6D, the paper entitled “Mechanisms of Mitophagy” published by Richard JY et al., in 2011 exhibited the strongest burstiness (10.83) [28]. Another study titled “Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice” by Wang H et al., in 2019 had a burst strength of 7.45, making it the most recent paper with persistent burstiness [29].
Table 5.
The top 10 co-cited references on studies of mitophagy in liver diseases.
| Rank | First Author | Citations | Year | Journal | Annotation | DOI |
|---|---|---|---|---|---|---|
| 1 | Narendra D | 74 | 2008 | Journal of Cell Biology | Parkin promotes autophagy of damaged mitochondria | 10.1083/jcb.200809125 |
| 2 | Youle RJ | 66 | 2011 | Nature Reviews Molecular Cell Biology | Mechanisms of mitophagy | 10.1038/nrm3028 |
| 3 | Geisler S | 56 | 2010 | Nature Cell Biology | VDAC1 and p62/SQSTM1 regulate PINK1/Parkin-mediated mitophagy | 10.1038/ncb2012 |
| 4 | Lazarou M | 53 | 2015 | Nature | Ubiquitin phosphorylation plays a role in mitophagy | 10.1038/nature14893 |
| 5 | Singh R | 49 | 2009 | Nature | Autophagy regulates lipid metabolism | 10.1038/nature07976 |
| 6 | Williams Ja | 49 | 2015 | American Journal of Physiology-Gastrointestinal and Liver Physiology | Parkin is an important mediator of protection against alcohol-induced mitochondrial damage | 10.1152/ajpgi.00108.2015 |
| 7 | Lemasters Jj | 47 | 2005 | Rejuvenation Research | Proposing the term "mitophagy" to emphasize the selective mitochondrial autophagy | 10.1089/rej.2005.8.3 |
| 8 | Matsuda N | 43 | 2010 | Journal of Cell Biology | PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates mitophagy | 10.1083/jcb.200910140 |
| 9 | Narendra Dp | 43 | 2010 | PLOS Biology | Interaction between PINK1 and Parkin | 10.1371/journal.pbio.1000298 |
| 10 | Twig G | 42 | 2008 | EMBO Journal | Fission followed by selective fusion segregates dysfunctional mitochondria | 10.1038/sj.emboj.7601963 |
Fig. 6.
Co-cited references analysis. (A) Network visualization showing the relationship among 22 co-cited references that reach the threshold of over 30 occurrences. The node and line color represented the cluster it belonged to; (B) The clustered network map of co-cited references. Different colors represented different clusters; (C) The timeline diagram of the top 18 clusters of co-cited references; (D) The top 10 burst references with the strongest citation bursts. Year indicates the year in which the keyword first appeared and is reflected in the cyan line, Begin and End indicate the starting and ending years of the keyword as a frontier reflected in the red line, and Strength indicates the emergent strength.
Table 6.
The co-cited references cluster analysis on studies of mitophagy in liver diseases.
| ClusterID | Size | Mean (Year) | Top terms |
|---|---|---|---|
| 0 | 110 | 2017 | drug-induced liver injury |
| 1 | 108 | 2010 | mitochondrial spheroid formation |
| 2 | 91 | 2018 | mitochondrial dysfunction |
| 3 | 85 | 2002 | autophagy |
| 4 | 77 | 2017 | liver cancer |
| 5 | 45 | 2008 | autophagosome formation |
| 6 | 44 | 2013 | adipose tissue remodeling |
| 7 | 42 | 2012 | ethanol-induced mitophagy |
| 8 | 32 | 2012 | amp-activated protein kinase alpha |
| 12 | 8 | 2000 | injury |
| 14 | 7 | 2015 | human gastric cancer line |
| 17 | 4 | 2010 | treatment |
3.6. Keywords analysis
A total of 2880 keywords were extracted from the title and abstract of 623 publications. We used VOSviewer to visualize the network and time-related patterns of 73 keywords that reached the threshold of over 15 occurrences (Fig. 7A B). The most frequently occurring keyword was “mitophagy”, followed by “autophagy” and “oxidative stress”. These 73 keywords could be roughly divided into four clusters. Red mainly included the phenotype, green was the pathological condition, blue represented processes of mitophagy, and yellow focused on molecular mechanisms. Furthermore, 2880 keywords were classified into 11 clusters based on the logarithmic likelihood ratio (LLR) algorithm (Fig. 7C), with the modularity Q being 0.3835 and weight means silhouette of 0.7327. Q > 0.3 meant that the community structure was consequential and meant silhouette >0.5 indicated that clustering was credible. According to 11 clusters, we constructed a timeline map (Fig. 7D), which revealed that lipid metabolism and hepatocellular carcinoma were recent areas of study. While Oxidative stress and fission were the topics that had the most extended duration. More detailed results are listed in Table 7. All these results proved that scholars had performed extensive studies in this sphere and are likely to continue doing so. Finally, we list the top 25 keywords with the strongest burstiness in Fig. 8. “Permeability transition” and “mitochondrial permeability transition" began at the earliest and featured prominently for a long time. The keyword “degradation” exhibited the strongest burstiness (7.7), indicating a heightened focus on mechanistic studies in this field in recent years. Additionally, keywords such as “nlrp3 inflammasome”, “fibrosis”, “toxicity”, “liver disease", and "nonalcoholic steatohepatitis" have attracted increasing attention from investigators. A review highlighting the relationship between mitophagy and inflammation [13] indicates that investigators are now placing greater emphasis on clinical and microenvironments research.
Fig. 7.
Keywords analysis. (A) Network visualization showing the relationship among 73 keywords that reach the threshold of over 15 occurrences. The node and line color represented the cluster it belonged to; (B) Time-related visualization map among 73 keywords. Node and link color reflected each keyword's corresponding average appearing year (AAY). Based on the color gradient in the lower right corner, yellow represented the relatively recently published keywords, while purple represented the earlier published keywords; (C) The clustered network map of keywords; (D) The timeline diagram of the top 11 clusters of keywords.
Table 7.
The keyword cluster analysis on studies of mitophagy in liver diseases.
| ClusterID | Size | Mean (Year) | Top terms |
|---|---|---|---|
| 0 | 75 | 2018 | lipid metabolism |
| 1 | 68 | 2008 | cell death |
| 2 | 47 | 2017 | liver fibrosis |
| 3 | 45 | 2017 | fission |
| 4 | 42 | 2012 | oxidative stress |
| 5 | 42 | 2012 | protein |
| 6 | 33 | 2016 | hepatocellular carcinoma |
| 7 | 32 | 2012 | inhibition |
| 8 | 31 | 2013 | thyroid hormone |
| 9 | 31 | 2016 | mechanism |
| 10 | 10 | 2011 | scleroderma |
Fig. 8.
Top 25 burst keywords with the strongest citation bursts. Year indicates the year in which the keyword first appeared and is reflected in the cyan line; Begin and End indicate the starting and ending years of the keyword as a frontier reflected in the red line, and Strength indicates the emergent strength.
4. Discussion
Liver dysfunction is a pressing global issue, causing great harm to human health, yet the underlying mechanism and treatment remain incomplete. We conducted a comprehensive bibliometric analysis of 623 articles on mitophagy in liver diseases published between 2002 and 2022 to address this issue. By utilizing VOSviewer, CiteSpace, and the R package, we employed scientific quantitative analysis and visualization techniques to explore the hotspots, development trends, and frontiers in this research field. Our analysis revealed that before 2020, research on mitophagy in liver diseases received limited attention, with only a few studies published during the period. However, since 2011, the number of publications has rapidly increased and continues to persist. Notably, a review written by Danielle Glick in 2010 and an article by Daniel F Egan in 2011 emerged as the two most highly cited references among the 623 publications, with 1882 and 1722 citations, respectively [30,31]. These influential papers have played a crucial role in shaping the development of this research field. Our analysis also identified mitophagy in liver diseases as a current and future research hotspot. The number of publications reflects the popularity of this field, while the post-publication citation count represents the impact and influence of the papers within the scientific community [32]. Among the 50 countries contributing to publications, China had the highest number of literature outputs, followed by the USA. However, the USA achieved the highest number of citations, with China ranking second but receiving less than half the number of citations compared to the USA (Fig. 3). These findings underscore the importance of not only the quantity but also the quality of research papers. Furthermore, China and the USA were at the center of the international cooperation arena, with a higher quantity and output quality in this area. However, there is limited interaction and collaboration among other countries, suggesting that it would be beneficial to eliminate academic barriers and strengthen cooperation between countries to promote mitophagy development in liver diseases.
Among the top 10 productive institutions, the University of Kansas emerged as the institution with the highest number of papers (Table 2). This result was primarily due to the significant contribution of the top author affiliated with the University of Kansas. And Fig. 4B depicts the cooperation among 21 institutions, revealing the solid interconnections and collaborations among these institutions. This result highlights the importance of interdisciplinary collaboration and the potential for mutual success by transcending organizational boundaries. The top 10 authors' production over time is shown in Fig. 4D, with the majority of authors focusing on this research field in the recent decade. Lemasters JJ was the first to begin investigating this area in 2002. His early work focused on elucidating the role of changes in mitochondrial inner membrane permeabilization in cell death, apoptosis, and autophagy, and creating a new term “necrapoptosis” to define this death process [33]. Another significant contribution by Lemasters JJ was the introduction of the term “mitophagy” for the first time in 2005 to describe the selective clearance of mitochondria during the process of mitochondrial degradation [10]. Ding WX, another prominent scholar in this field, has the greatest number of publications and citations. Recently, he conducted a study on mitochondrial function in alcoholic hepatitis and found that intracellular mitochondrial maladaptation impaired mitophagy, leading to abnormal innate immune response and aggravated liver injury [34]. Over the past decade, studies on mitophagy in liver diseases have exhibited a consistent upward trend, indicating the continued growth and significance of research in this area.
The analysis of journals and co-cited journals provides valuable insight into the publications’ landscape and assists scholars in finding appropriate journals for their research. In our study, we identified a total of 219 journals that have published articles in this field. Notably, the top 10 journals accounted for nearly a quarter of all publications (23.6%). These included highly influential journals such as Autophagy (IF = 13.39) and Hepatology (IF = 17.298), indicating that research in this field is concentrated in reputable journals and holds promising prospects. Among the journals issuing 31,600 co-cited references, we observed several top journals within the area, including Nature, Science, Autophagy, Hepatology, and others. This result demonstrates that the research on mitophagy in liver diseases is an integral and indispensable part of the broader scientific community. To further investigate the distribution of academic journals, we conducted a dual-map overlay analysis. The results depicted in Fig. 5D revealed that papers from journals related to Molecular/Biology/Genetics were frequently cited by literature in Molecular/Biology/Immunology journals. This suggests a shift in focus within the field of mitophagy in liver diseases from molecular mechanisms to immunology.
In addition to journal analysis, examining the quality and quantity of co-cited references provides insight into the foundations and fundamental research in the field [35]. The PINK1-Parkin-mediated pathway and the Parkin-independent pathway play crucial roles in the initiation of mitophagy. Although the PINK1-Parkin-mediated pathway has been extensively studied, recent research has demonstrated that the outer mitochondrial membrane (OMM)-localized receptors such as BNIP3L/NIX, BNIP3, and FUNDC1 can initiate mitophagy through Parkin-independent pathway. We selected the top 10 co-cited references in Table 5. And upon reviewing their abstracts or even full texts, we discovered that most studies among these references focus on PINK1/Parkin-mediated mitophagy. However, we identified references frequently cited in specific years by conducting a burstiness analysis on co-cited references. Interestingly, research associated with the PINK1-Parkin-mediated pathway accounted for the majority before 2016, while after 2016, scholars shifted their attention to the Parkin-independent pathway [36,37]. This phenomenon implies that the center of studies has shifted from PINK1/Parkin-mediated mitophagy to Parkin-independent mitophagy, making the latter a recent research hotspot.
Apart from references with citation bursts, the keywords of co-occurrence analysis can also help scholars grasp the development trends and hotspots in the field of research on mitophagy in liver diseases. Based on the visualization map of the top 73 keywords, we can effectively depict the studies' development and focal points (Fig. 7A B). In the studies of mitophagy in liver diseases, early researchers may study relating more mindfully to the state of mitophagy than focusing on clinical symptoms, such as keywords “permeability transition”, “degradation”, “oxidative stress”, “fusion” and “cell-death”. As research progressed, scholars gradually shifted their attention toward investigating the molecular mechanisms of mitophagy. Keywords “pink1”, “ubiquitin”, “ros”, and “receptor” well represented the hotspots throughout this period. And recent investigations have garnered attention to the role of mitophagy in liver disease pathogenesis, with keywords in this development period including “liver fibrosis”, “fatty liver diseases”, “inflammation”, “liver injury”, and “NAFLD”. Mitophagy, serving as a protective mechanism in liver diseases by clearing the damaged mitochondria, necessitates further exploration. Activation of mitophagy has been shown to inhibit HSCs activation and alleviate hepatic fibrosis [38]. The PINK1/Parkin pathway activated by thyroid hormone in hepatocytes may be another barrier against liver cancer incidence and development [39]. Some studies demonstrated that PINK1/Parkin-mediated mitophagy might be critical in attenuating liver injury in NAFLD. Genetic ablation of macrophage stimulating 1 (Mst1) proved it could alleviate hepatocyte injury through PINK1/Parkin-mediated mitophagy [40]. Selenoprotein M, a key thioredoxin-like enzyme, had been shown to relieve hepatic injury in NAFLD, likely attributed to its regulation of Parkin-mediated mitophagy through the AMPKα1–MFN2 pathway [41]. Furthermore, certain substances could alleviate hepatic steatosis via BNIP3-mediated mitophagy [42]. As a selective autophagy process targeting mitochondria, mitophagy plays a crucial role in negatively regulating I/R injury. Pterostilbene was proven to prevent hepatic I/R injury, and this protective effect was abolished when cells were transfected with PINK1 siRNA, indicating the potential positive regulation of PINK1-mediated mitophagy in hepatic I/R injury [43]. Additionally, in another research, overexpression of Pink1 was found to attenuate I/R injury by inhibiting NLRP3 inflammasome activation and clearing mitochondria ROS [14]. Although current studies on mitophagy in liver diseases are predominantly in the basic research stage and have not yet progressed to clinical trials, mitophagy in liver diseases may be a new potential target for future prevention and treatment. However, further investigations are necessary to unravel the intricate relationship between mitophagy and liver diseases comprehensively.
In addition to the keywords co-occurrence network analysis, we also conducted keyword clustering analysis, timeline diagram, and bursts analysis to show the research trends and ground-breaking advances in the research on mitophagy in liver diseases (Fig. 7 8). Our analysis revealed that research in this field has primarily focused on four main areas: phenotype analysis, processes of mitophagy, molecular mechanisms of mitophagy, and pathological conditions of the liver. Phenotype analysis involves examining the observable characteristics of mitophagy in liver diseases, such as permeability transition, degradation, oxidative stress, fusion, and cell death, which were identified as early research focuses in our co-occurrence analysis. Conversely, recent studies have increasingly shifted their attention to exploring the molecular mechanisms underlying the processes of mitophagy. These processes encompass the recognition, sequestration, and degradation of damaged mitochondria, which have become prominent areas of investigation. Key research hotspots in this area include pink1, ubiquitin, ros, and receptor. Pathological conditions of the liver, including liver fibrosis, fatty liver diseases, inflammation, liver injury, and NAFLD, have also been identified as research hotspots in the co-occurrence analysis. These conditions are associated with alterations in the mitophagy process and provide potential targets for future therapeutic interventions. All of these not only portray the development trends in the past but also outline potential research hotspots for the future.
Mitochondrial damage mainly manifests as the mitochondrial permeability transition (MPT) in the early stage, inducing uncoupling of oxidative phosphorylation and reduction of ATP generation [44]. Earlier studies were interested in connecting mitochondrial dysfunction with apoptosis and cell death. Just as Fig. 8 showed, with burst keywords such as “permeability transition”, “mitochondrial permeability transition”, and “apoptosis” in the first decade in this field. In the initiation of liver diseases, impaired energy metabolism and mitochondrial dysfunction were significant factors. Mitochondrial damage can lead to ATP depletion, promoting caspase-and ATP-dependent apoptosis and necrosis [44]. Overexpression of Atg7 or Beclin-1 to stimulate mitophagy has been shown to improve ATP production and prevent MPT-dependent hepatocyte necrosis and apoptosis after I/R injury [45]. The MPT of dysfunctional mitochondria may be the leading cause of hepatocyte apoptosis and necrosis in many liver diseases, such as I/R injury and ethanol-induced hepatotoxicity. And degradation of damaged mitochondria may play an important role in ethanol metabolism and decrease hepatocyte apoptosis induced by ethanol [46]. Although recent studies on the relationship between mitophagy and apoptosis have been limited, there is still much to explore in terms of their mechanisms and potential therapeutic interventions.
Notably, inflammation taking part in the processes of onset and progression in liver diseases has become a focus in the recent two years. Nod-like receptors (NLRs), a special class of intracellular proteins playing an important role in regulating the innate immune response, becomes the burst keyword (Fig. 7 8). The mtROS or mtDNA released from injured mitochondria as sources of DAMPs could activate NLRP3 inflammasome and further drive the innate immune response [47]. Certain NLRs, including NLRP2, NLRP3, and NLRC4, can be assembled into an inflammasome complex with apoptosis-associated speck-like protein containing a CARD (ASC) and pro-cysteinyl aspartate specific proteinase-1 (pro-caspase-1) [48]. NLRP3 is the most classical inflammasome associated with liver injury in nonalcoholic fatty liver disease, alcoholic liver disease, liver fibrosis, and cancer [49]. Damaged mitochondria release mtDNA and become stimulatory molecules that stimulate Toll-like receptors (TLRs), then trigger NLRP3 inflammasome assembly. The NLRP3 inflammasome next activates KCs and initiates an innate immune response, Casp1 activation, as well as IL-18 and IL-1β maturation [50]. Mitochondria play a pivotal role in this process. Overexpression of PINK1 enhanced mitophagy, then inhibited activation of NLRP3 and relieved KC-mediated inflammatory injury to the liver [14]. However, the impairments of mitophagy activated NLRP3 inflammasome activation and further aggravated hepatocyte injury [51]. In addition, the deficiency of XBP1, an important component in endoplasmic reticulum stress, could promote ROS/NLRP3/caspase-1/GSDMD-mediated pyroptosis via inhibition of mitophagy and induce the activation of macrophage inflammatory response by the release of mtDNA [52]. It is evident that mitophagy plays a vital role in regulating immune response, making it a significant target in treating liver injury. Despite considerable knowledge regarding the involvement of the NLRP3 inflammasome in liver diseases, understanding how mitophagy in hepatocytes precisely regulates and controls the NLRP3 inflammasome remains limited.
To sum up, mitophagy participates in the initiation and progression of liver diseases and holds potential as a new therapeutic target for treating liver diseases. Through bibliometrics analysis, we have gained a comprehensive understanding of the dynamic changes, development trends, and study hotspots in this field, enabling us to predict future research trends. This study represents the first systematic analysis of research on mitophagy in liver diseases using bibliometric analysis, providing a more holistic insight into the frontiers and hotspots in this field. Nevertheless, there are certain limitations. Firstly, the data was retrieved solely from the WoSCC database, potentially excluding relevant literature published in other databases. Secondly, non-English papers that were excluded could also be important. Additionally, the limited period considered in the analysis may have underestimated the impact of recent studies due to their relatively low citation counts. Finally, bibliometric analysis conducted by VOSviewer and CtieSpace may ignore some important information due to machine algorithm cannot undertake full-text analysis.
5. Conclusion
Our study based on bibliometric analysis provides valuable insights into the trends and focal points of research on mitophagy in liver diseases. The results indicated that the publications in this field showed a fast-increasing trend over the years. China emerges as the leading contributor in terms of the number of papers, while the USA stands out with the highest citations. International Journal of Molecular Sciences exhibits the highest publication output in this area. Notably, the mechanisms of mitophagy in lipid metabolism-associated diseases attracted scholars’ attention in past years; liver fibrosis and cancer also had a substantial number of related studies. While PINK1/Parkin-mediated pathway has been proven to be the main pathway in mitophagy, there is growing interest in exploring Parkin-independent pathways. Furthermore, our analysis suggests that the regulation between mitophagy and the NLRP3 inflammasome in liver diseases holds promise as a new research focus, offering potential avenues for further investigation.
Author contribution statement
Jie Lin, Yushun Chang: Conceived and designed the experiments; Performed the experiments; Wrote the paper; Qiuxia Gun, Jinyao Dai: Performed the experiments; Analyzed and interpreted the data; Junjie Nan, Ziyan Wang: Performed the experiments; Wrote the paper; Jiachen Chen, Danyang Zhong: Analyzed and interpreted the data; Meiling Hu, Enjie Zhou: Contributed reagents, materials, analysis tools or data; Wrote the paper; Xiujun Cai, Yifan Wang: Conceived and designed the experiments.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the WoSCC database and all colleagues for their contributions and commitment to this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18843.
Contributor Information
YiFan Wang, Email: anwyf@zju.edu.cn.
XiuJun Cai, Email: srrsh_cxj@zju.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J. Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatol. Baltim. Md. 2021;73(1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet Lond. Engl. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 4.Soboll S. Regulation of energy metabolism in liver. J. Bioenerg. Biomembr. 1995;27:571–582. doi: 10.1007/BF02111655. [DOI] [PubMed] [Google Scholar]
- 5.Trefts E., Gannon M., Wasserman D.H. The liver. Curr. Biol. CB. 2017;27:R1147–R1151. doi: 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y., Guo Z.-W., Li J., Li A.-H., Huo T.-G. Insight into the regulation of NLRP3 inflammasome activation by mitochondria in liver injury and the protective role of natural products. Biomed. Pharmacother. Biomedecine Pharmacother. 2022;156 doi: 10.1016/j.biopha.2022.113968. [DOI] [PubMed] [Google Scholar]
- 7.Mansouri A., Gattolliat C.-H., Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155:629–647. doi: 10.1053/j.gastro.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 8.Youle R.J. Mitochondria-Striking a balance between host and endosymbiont. Science. 2019;365 doi: 10.1126/science.aaw9855. eaaw9855. [DOI] [PubMed] [Google Scholar]
- 9.De Duve C., Wattiaux R. Functions of lysosomes. Annu. Rev. Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 10.Lemasters J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 11.Green D.R., Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 12.Yao R.-Q., Ren C., Xia Z.-F., Yao Y.-M. Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy. 2021;17:385–401. doi: 10.1080/15548627.2020.1725377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54:437–453. doi: 10.1016/j.immuni.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y., Tang Y., Lu J., Zhang W., Zhu Y., Zhang S., Ma G., Jiang P., Zhang W. PINK1-mediated mitophagy protects against hepatic ischemia/reperfusion injury by restraining NLRP3 inflammasome activation. Free Radic. Biol. Med. 2020;160:871–886. doi: 10.1016/j.freeradbiomed.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Xian H., Watari K., Sanchez-Lopez E., Offenberger J., Onyuru J., Sampath H., Ying W., Hoffman H.M., Shadel G.S., Karin M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55:1370–1385.e8. doi: 10.1016/j.immuni.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke P.-Y. Mitophagy in the pathogenesis of liver diseases. Cells. 2020;9:E831. doi: 10.3390/cells9040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Q., Xie X.-L., Wang M.-M., Yin J., Tian J.-M., Jiang X.-Y., Zhang D., Han J., Bai Y., Cui Z.-J., Jiang H.-Q. The role of the apoptosis-related protein BCL-B in the regulation of mitophagy in hepatic stellate cells during the regression of liver fibrosis. Exp. Mol. Med. 2019;51:1–13. doi: 10.1038/s12276-018-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Zhou L., Li H., Sun T., Wen X., Li X., Meng Y., Li Y., Liu M., Liu S., Kim S.-J., Xiao J., Li L., Zhang S., Li W., Cohen P., Hoffman A.R., Hu J.-F., Cui J. Nuclear-encoded lncRNA MALAT1 epigenetically controls metabolic reprogramming in HCC cells through the mitophagy pathway. Mol. Ther. Nucleic Acids. 2021;23:264–276. doi: 10.1016/j.omtn.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng J., Zhou J., Wu Y., Shen H.-M., Peng T., Lu G.-D. Targeting mitophagy as a novel therapeutic approach in liver cancer. Autophagy. 2022:1–2. doi: 10.1080/15548627.2022.2157547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S., Zhang Y., Dai W., Qi S., Tian W., Gu X., Chen X., Yu W., Tian J., Su D. Publication trends and hot spots in postoperative cognitive dysfunction research: a 20-year bibliometric analysis. J. Clin. Anesth. 2020;67 doi: 10.1016/j.jclinane.2020.110012. [DOI] [PubMed] [Google Scholar]
- 21.Wu F., Gao J., Kang J., Wang X., Niu Q., Liu J., Zhang L. Knowledge mapping of exosomes in autoimmune diseases: a bibliometric analysis (2002-2021) Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.939433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devos P., Menard J. Bibliometric analysis of research relating to hypertension reported over the period 1997-2016. J. Hypertens. 2019;37:2116–2122. doi: 10.1097/HJH.0000000000002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D., Yu D., Li Y., Yang R. A bibliometric analysis of PROTAC from 2001 to 2021. Eur. J. Med. Chem. 2022;244 doi: 10.1016/j.ejmech.2022.114838. [DOI] [PubMed] [Google Scholar]
- 24.Lemasters J.J., Qian T., He L.H., Kim J.S., Elmore S.P., Cascio W.E., Brenner D.A. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid. Redox Signal. 2002;4:769–781. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- 25.Cheng P., Tang H., Dong Y., Liu K., Jiang P., Liu Y. Knowledge mapping of research on land use change and food security: a visual analysis using CiteSpace and VOSviewer. Int. J. Environ. Res. Public. Health. 2021;18 doi: 10.3390/ijerph182413065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C., Leydesdorff L. Patterns of connections and movements in dual-map overlays: a new method of publication portfolio analysis. J. Assoc. Inf. Sci. Technol. 2014;65:334–351. doi: 10.1002/asi.22968. [DOI] [Google Scholar]
- 27.Shao B., Qin Y.-F., Ren S.-H., Peng Q.-F., Qin H., Wang Z.-B., Wang H., Li G.-M., Zhu Y.-L., Sun C.-L., Zhang J.-Y., Li X., Wang H. Structural and temporal dynamics of mesenchymal stem cells in liver diseases from 2001 to 2021: a bibliometric analysis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.859972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H., Ni H.-M., Chao X., Ma X., Rodriguez Y.A., Chavan H., Wang S., Krishnamurthy P., Dobrowsky R., Xu D.-X., Jaeschke H., Ding W.-X. Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice. Redox Biol. 2019;22 doi: 10.1016/j.redox.2019.101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egan D.F., Shackelford D.B., Mihaylova M.M., Gelino S., Kohnz R.A., Mair W., Vasquez D.S., Joshi A., Gwinn D.M., Taylor R., Asara J.M., Fitzpatrick J., Dillin A., Viollet B., Kundu M., Hansen M., Shaw R.J. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann Nielsen M., Jaeger K.A. How to write and publish a successful scientific article. Ultraschall Med. Stuttg. Ger. 1980. 2007;28:472–474. doi: 10.1055/s-2007-963559. [DOI] [PubMed] [Google Scholar]
- 33.Lemasters J.J., Qian T., He L., Kim J.-S., Elmore S.P., Cascio W.E., Brenner D.A. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid. Redox Signal. 2002;4:769–781. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- 34.Ma X., Chen A., Melo L., Clemente-Sanchez A., Chao X., Ahmadi A.R., Peiffer B., Sun Z., Sesaki H., Li T., Wang X., Liu W., Bataller R., Ni H.-M., Ding W.-X. Loss of hepatic drp1 exacerbates alcoholic hepatitis by inducing megamitochondria and mitochondrial maladaptation. Hepatol. Baltim. Md. 2022 doi: 10.1002/hep.32604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mo W., Li Q., Zhou H., Shi X., Yang H., Xiao Z., Wei J., Lv X. Bibliometric analysis of global research trends on pyroptosis in lung disease. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.978552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin Q., Li R., Hu N., Xin T., Zhu P., Hu S., Ma S., Zhu H., Ren J., Zhou H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576–587. doi: 10.1016/j.redox.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou H., Wang J., Zhu P., Zhu H., Toan S., Hu S., Ren J., Chen Y. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2α. Basic Res. Cardiol. 2018;113:23. doi: 10.1007/s00395-018-0682-1. [DOI] [PubMed] [Google Scholar]
- 38.Dou S.-D., Zhang J.-N., Xie X.-L., Liu T., Hu J.-L., Jiang X.-Y., Wang M.-M., Jiang H.-D. MitoQ inhibits hepatic stellate cell activation and liver fibrosis by enhancing PINK1/parkin-mediated mitophagy. Open Med. Wars. Pol. 2021;16:1718–1727. doi: 10.1515/med-2021-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chi H.-C., Chen S.-L., Lin S.-L., Tsai C.-Y., Chuang W.-Y., Lin Y.-H., Huang Y.-H., Tsai M.-M., Yeh C.-T., Lin K.-H. Thyroid hormone protects hepatocytes from HBx-induced carcinogenesis by enhancing mitochondrial turnover. Oncogene. 2017;36:5274–5284. doi: 10.1038/onc.2017.136. [DOI] [PubMed] [Google Scholar]
- 40.Zhou T., Chang L., Luo Y., Zhou Y., Zhang J. Mst1 inhibition attenuates non-alcoholic fatty liver disease via reversing Parkin-related mitophagy. Redox Biol. 2019;21 doi: 10.1016/j.redox.2019.101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai J., Huang J., Yang J., Chen X., Zhang H., Zhu Y., Liu Q., Zhang Z. The protective effect of selenoprotein M on non-alcoholic fatty liver disease: the role of the AMPKα1-MFN2 pathway and Parkin mitophagy. Cell. Mol. Life Sci. CMLS. 2022;79:354. doi: 10.1007/s00018-022-04385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong L.-L., Yang S., Zhang W., Han F.-F., Lv Y.-L., Wan Z.-R., Liu H., Jia Y.-J., Xuan L.-L., Liu L.-H. Akebia saponin D alleviates hepatic steatosis through BNip3 induced mitophagy. J. Pharmacol. Sci. 2018;136:189–195. doi: 10.1016/j.jphs.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Shi Q., Zhao G., Wei S., Guo C., Wu X., Zhao R.C., Di G. Pterostilbene alleviates liver ischemia/reperfusion injury via PINK1-mediated mitophagy. J. Pharmacol. Sci. 2022;148:19–30. doi: 10.1016/j.jphs.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Kim J.-S., Qian T., Lemasters J.J. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003;124:494–503. doi: 10.1053/gast.2003.50059. [DOI] [PubMed] [Google Scholar]
- 45.Kim J.-S., Nitta T., Mohuczy D., O'Malley K.A., Moldawer L.L., Dunn W.A., Behrns K.E. Impaired autophagy: a mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatol. Baltim. Md. 2008;47:1725–1736. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding W.-X., Li M., Chen X., Ni H.-M., Lin C.-W., Gao W., Lu B., Stolz D.B., Clemens D.L., Yin X.-M. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 48.Kanneganti T.-D., Lamkanfi M., Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Yang L., Mizuochi T., Shivakumar P., Mourya R., Luo Z., Gutta S., Bezerra J.A. Regulation of epithelial injury and bile duct obstruction by NLRP3 and IL-1R1 in experimental biliary atresia. J. Hepatol. 2018;69:1136–1144. doi: 10.1016/j.jhep.2018.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gurung P., Lukens J.R., Kanneganti T.-D. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol. Med. 2015;21:193–201. doi: 10.1016/j.molmed.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang N.-P., Liu X.-J., Xie L., Shen X.-Z., Wu J. Impaired mitophagy triggers NLRP3 inflammasome activation during the progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. Lab. Investig. J. Tech. Methods Pathol. 2019;99:749–763. doi: 10.1038/s41374-018-0177-6. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z., Wang M., Wang X., Bu Q., Wang Q., Su W., Li L., Zhou H., Lu L. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022;52 doi: 10.1016/j.redox.2022.102305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.