Abstract
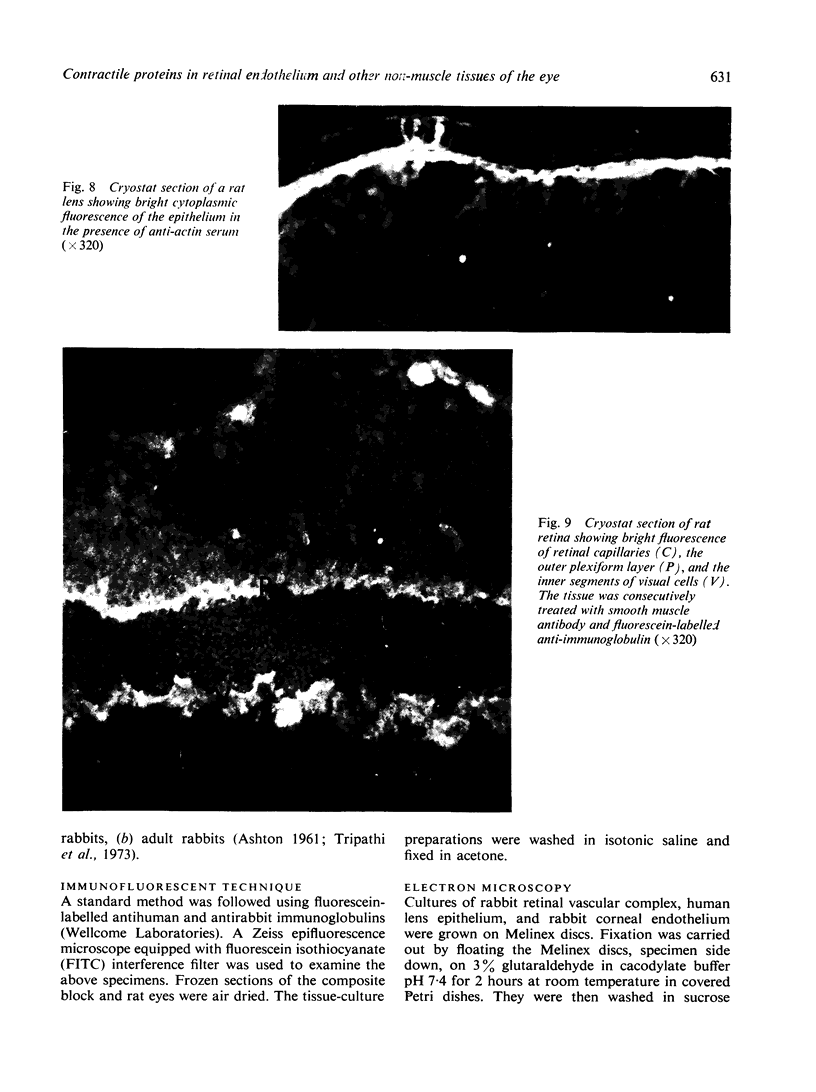
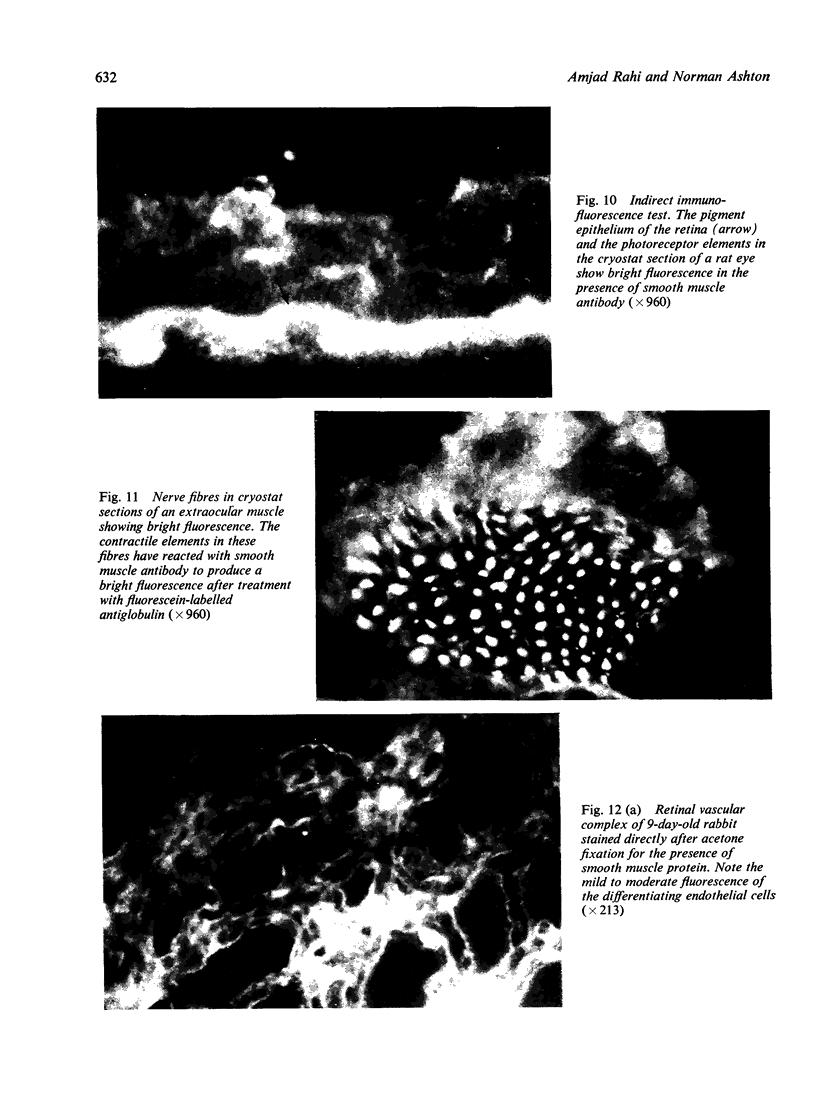
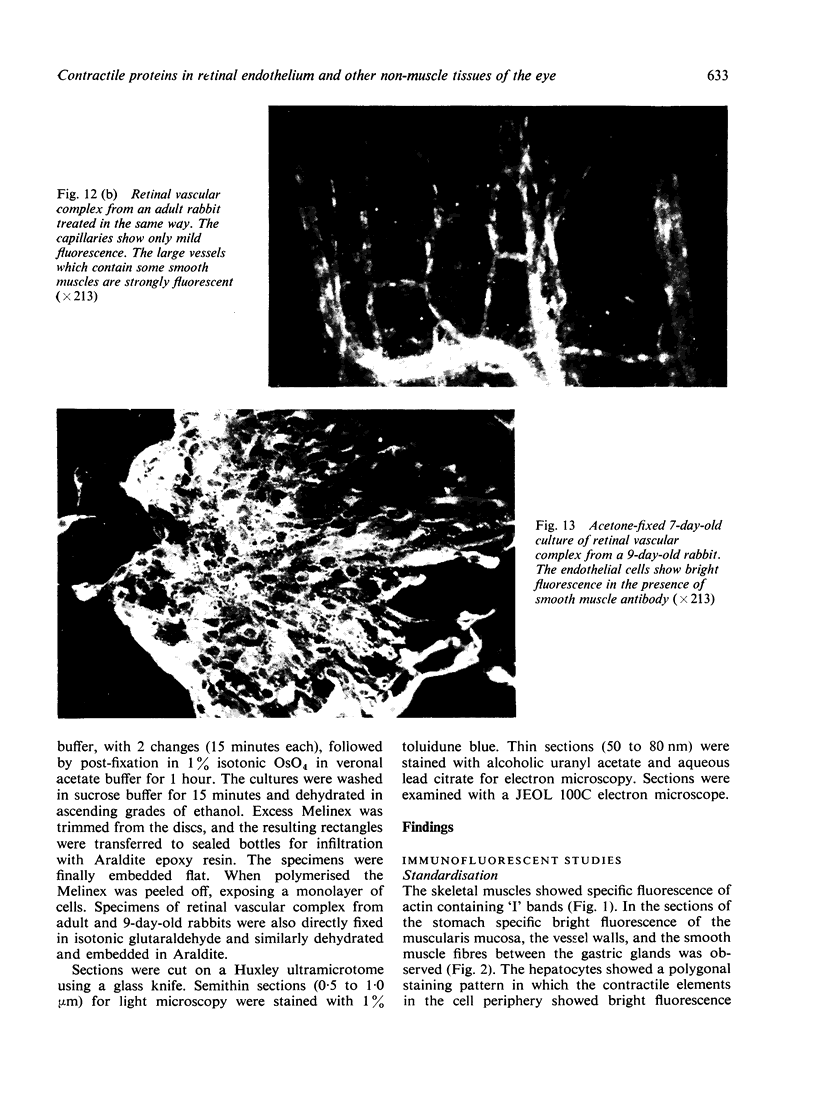
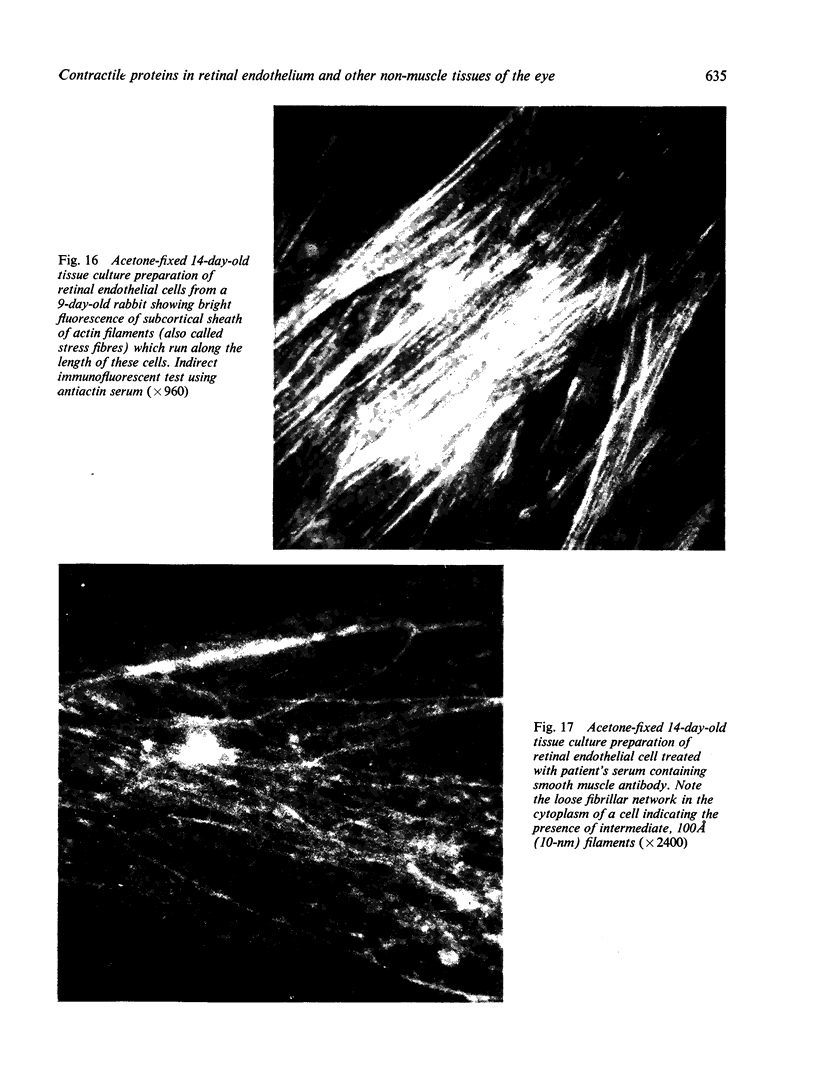
The present study is the first investigation to demonstrate, by employing the combined approach of immunologically and electron microscope methods, the presence of actin-like contractile proteins in the mammalian retina, the corneal epithelium and endothelium, the iris, and the ciliary body, and to confirm their presence in lens epithelium. This is also the first report to demonstrate by these methods the presence of microfilaments and intermediate filaments in retinal vascular endothelium. Since we have shown that actin filaments are especially abundant in immature retinal endothelial cells, the question of their function arises, and we have discussed their possible relevance to the closure of immature retinal vessels when exposed to hyperoxia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON N. Neovascularization in ocular disease. Trans Ophthalmol Soc U K. 1961;81:145–161. [PubMed] [Google Scholar]
- ASHTON N., WARD B., SERPELL G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954 Jul;38(7):397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. A. Actin filaments in normal and migrating corneal epithelial cells. Invest Ophthalmol Vis Sci. 1977 Feb;16(2):161–166. [PubMed] [Google Scholar]
- Ashton N., Tripathi B., Knight G. Effect of oxygen on the developing retinal vessels of the rabbit. II. In vivo experiments. Exp Eye Res. 1972 Nov;14(3):221–232. doi: 10.1016/0014-4835(72)90007-3. [DOI] [PubMed] [Google Scholar]
- Ashton N., Tripathi R. The problem of selective pericyte injury. Bibl Anat. 1977;(16 Pt 2):19–23. [PubMed] [Google Scholar]
- Becker C. G., Murphy G. E. Demonstration of contractile protein in endothelium and cells of the heart valves, endocardium, intima, arteriosclerotic plaques, and Aschoff bodies of rheumatic heart disease. Am J Pathol. 1969 Apr;55(1):1–37. [PMC free article] [PubMed] [Google Scholar]
- Burnside B., Laties A. M. Actin filaments in apical projections of the primate pigmented epithelial cell. Invest Ophthalmol. 1976 Jul;15(7):570–575. [PubMed] [Google Scholar]
- Burnside M. B. Possible roles of microtubules and actin filaments in retinal pigmented epithelium. Exp Eye Res. 1976 Aug;23(2):257–275. doi: 10.1016/0014-4835(76)90208-6. [DOI] [PubMed] [Google Scholar]
- Cecio A. Ultrastructural features of cytofilaments within mammalian endothelial cells. Z Zellforsch Mikrosk Anat. 1967;83(1):40–48. doi: 10.1007/BF00334737. [DOI] [PubMed] [Google Scholar]
- Feeney L., Mixon R. N. An in vitro model of phagocytosis in bovine and human retinal pigment epithelium. Exp Eye Res. 1976 May;22(5):533–548. doi: 10.1016/0014-4835(76)90190-1. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Trenchev P., Holborow E. J. Increase of contractile proteins in human cancer cells. Lancet. 1975 Oct 25;2(7939):796–797. doi: 10.1016/s0140-6736(75)80008-0. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The use of heavy meromyosin binding as an ultrastructural cytochemical method for localizing and determining the possible functions of actin-like microfilaments in nonmuscle cells. J Histochem Cytochem. 1975 Jul;23(7):529–542. doi: 10.1177/23.7.1095652. [DOI] [PubMed] [Google Scholar]
- Grierson I., Lee W. R. Pressure-induced changes in the ultrastructure of the endothelium lining Schlemm's canal. Am J Ophthalmol. 1975 Nov;80(5):863–884. doi: 10.1016/0002-9394(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Holborow E. J., Trenchev P. S., Dorling J., Webb J. Demostration of smooth muscle contractile protein antigens in liver and epithelial cells. Ann N Y Acad Sci. 1975 Jun 30;254:489–504. doi: 10.1111/j.1749-6632.1975.tb29196.x. [DOI] [PubMed] [Google Scholar]
- Kurki P., Linder E., Virtanen I., Stenman S. Human smooth muscle autoantibodies reacting with intermediate (100 A) filaments. Nature. 1977 Jul 21;268(5617):240–241. doi: 10.1038/268240a0. [DOI] [PubMed] [Google Scholar]
- Kuwabara T. Microtubules in the lens. Arch Ophthalmol. 1968 Feb;79(2):189–195. doi: 10.1001/archopht.1968.03850040191017. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Immunofluorescence studies on the structure of actin filaments in tissue culture cells. J Histochem Cytochem. 1975 Jul;23(7):507–528. doi: 10.1177/23.7.1095651. [DOI] [PubMed] [Google Scholar]
- Lonchampt M. O., Laurent M., Courtois Y., Trenchev P., Hughes R. C. Microtubules and microfilaments of bovine lens epithelial cells: electron microscopy and immunofluorescence staining with specific antibodies. Exp Eye Res. 1976 Nov;23(5):505–518. doi: 10.1016/0014-4835(76)90159-7. [DOI] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J., Webster H. de F., Wollberg M. Cell elongation in the cultured embryonic chick lens epithelium with and without protein synthesis. Involvement of microtubules. J Cell Biol. 1972 Oct;55(1):82–92. doi: 10.1083/jcb.55.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Rafferty N. S., Esson E. A. An electron-microscope study of adult mouse lens: some ultrastructural specializations. J Ultrastruct Res. 1974 Feb;46(2):239–253. doi: 10.1016/s0022-5320(74)80059-6. [DOI] [PubMed] [Google Scholar]
- Rahi A. H., Holborow E. J., Perkins E. S., Gungen Y. Y., Dinning W. J. Immunological investigations in uveitis. Trans Ophthalmol Soc U K. 1976 Apr;96(1):113–122. [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Trenchev P., Dorling J., Webb J., Holborow E. J. Localization of smooth muscle-like contractile proteins in kidney by immunoelectron microscopy. J Anat. 1976 Feb;121(Pt 1):85–95. [PMC free article] [PubMed] [Google Scholar]
- Trenchev P., Holborow E. J. The specificity of anti-actin serum. Immunology. 1976 Oct;31(4):509–517. [PMC free article] [PubMed] [Google Scholar]
- Tripathi B., Ashton N., Knight G. Effect of oxygen on the developing retinal vessels of the rabbit. 3. Mode of growth of rabbit retinal vessels in tissue culture. Exp Eye Res. 1973 Mar;15(3):321–351. doi: 10.1016/0014-4835(73)90149-8. [DOI] [PubMed] [Google Scholar]
- Tripathi B., Knight G., Ashton N. Effect of oxygen on the developing retinal vessels of the rabbit. IV. Effect of hyperoxia on rabbit retinal vessels in tissue culture. Exp Eye Res. 1974 Nov;19(5):449–475. doi: 10.1016/0014-4835(74)90053-0. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]