Abstract
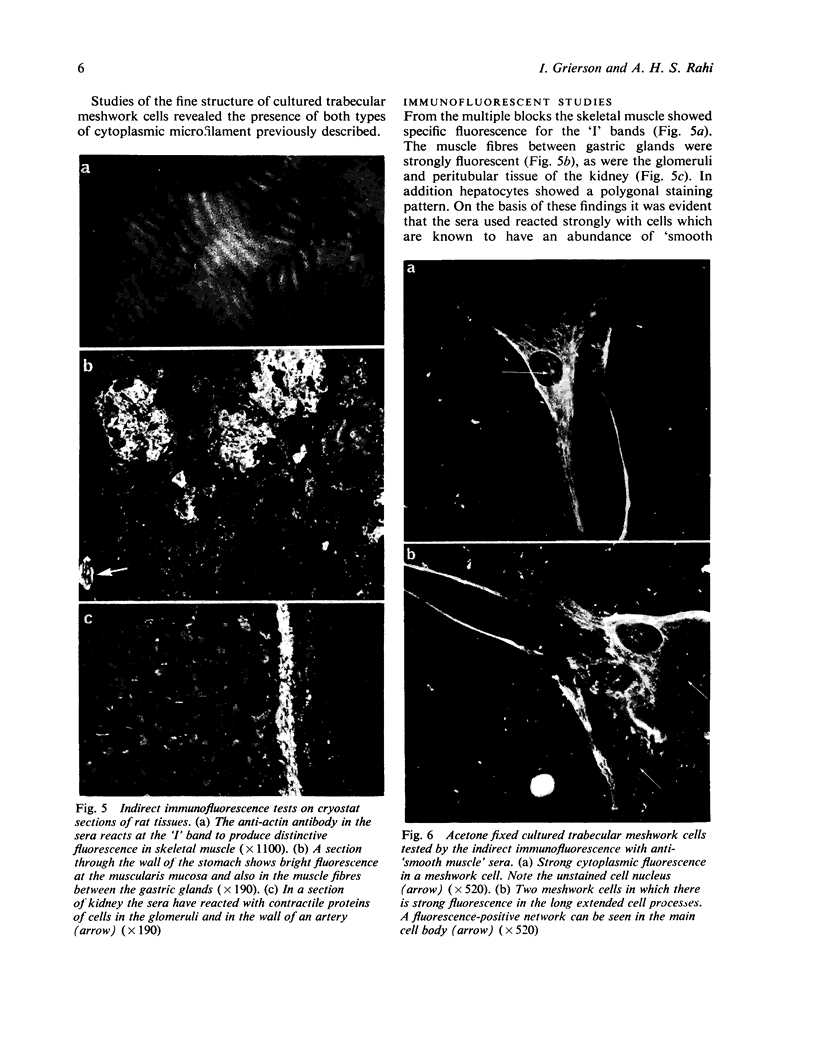
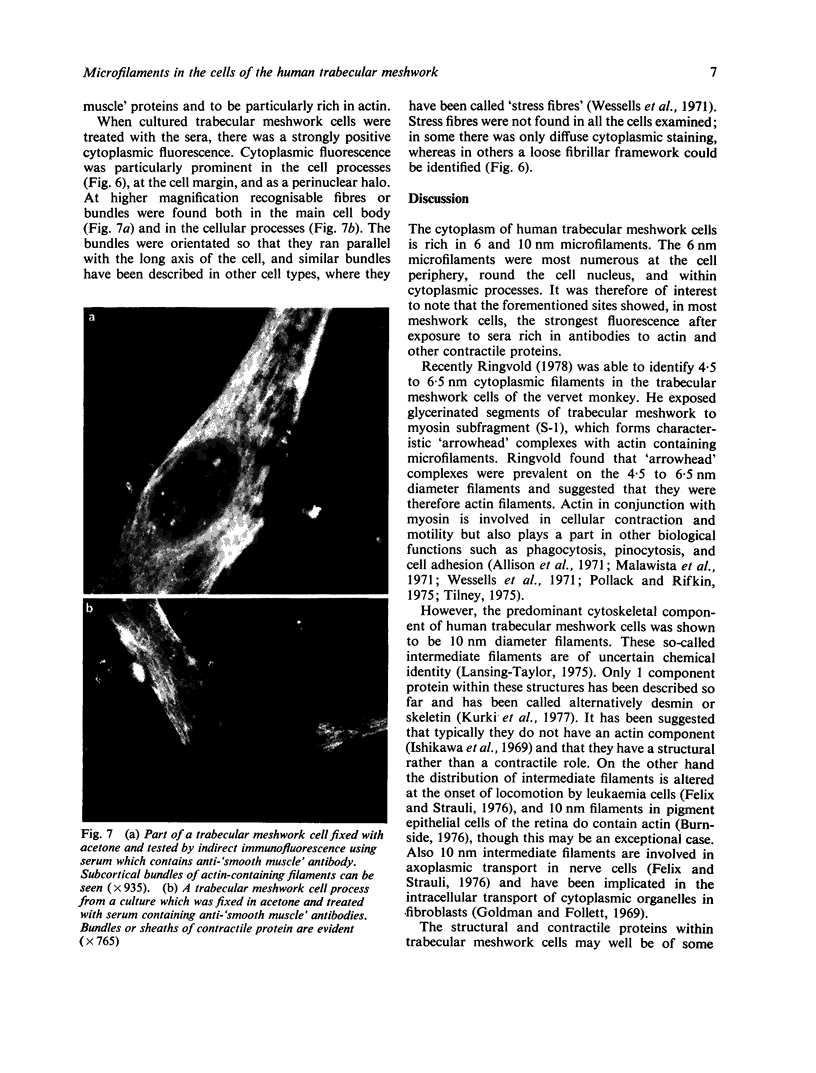
In this present study the results are presented of a combined ultrastructural and immunofluorescent investigation of 'smooth muscle' elements within the cytoplasm of human trabecular meshwork cells. The cytoplasm of human meshwork cells both in vivo and in vitro is replete with 10 nm intermediate filaments and also contains smaller 6 nm filaments which are particularly prominent in the cell processes. By immunofluorescence using sera rich in antibodies to contractile proteins, particularly actin, cultured meshwork cells showed strong cytoplasmic fluorescence. On occasion the cytoplasmic fluorescence was diffuse, but more often recognisable bundless (stress fibres) or a loose fibrillar framework was found. The possible role of structural and contractile cellular proteins in trabecular function was discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- Bill A. Editorial: The drainage of aqueous humor. Invest Ophthalmol. 1975 Jan;14(1):1–3. [PubMed] [Google Scholar]
- Burnside M. B. Possible roles of microtubules and actin filaments in retinal pigmented epithelium. Exp Eye Res. 1976 Aug;23(2):257–275. doi: 10.1016/0014-4835(76)90208-6. [DOI] [PubMed] [Google Scholar]
- Felix H., Strauli P. Different distribution pattern of 100-A filaments in resting and locomotive leukaemia cells. Nature. 1976 Jun 17;261(5561):604–606. doi: 10.1038/261604a0. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Follett E. A. The structure of the major cell processes of isolated BHK21 fibroblasts. Exp Cell Res. 1969 Oct;57(2):263–276. doi: 10.1016/0014-4827(69)90150-5. [DOI] [PubMed] [Google Scholar]
- Grierson I., Lee W. R., Abraham S. Effects of pilocarpine on the morphology of the human outflow apparatus. Br J Ophthalmol. 1978 May;62(5):302–313. doi: 10.1136/bjo.62.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson I., Lee W. R. Erythrocyte phagocytosis in the human trabecular meshwork. Br J Ophthalmol. 1973 Jun;57(6):400–415. doi: 10.1136/bjo.57.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson I., Lee W. R. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (1) Pressure effects within the near-physiological range (8-30 mmHg). Exp Eye Res. 1975 Jun;20(6):505–521. doi: 10.1016/0014-4835(75)90218-3. [DOI] [PubMed] [Google Scholar]
- Inomata H., Bill A., Smelser G. K. Aqueous humor pathways through the trabecular meshwork and into Schlemm's canal in the cynomolgus monkey (Macaca irus). An electron microscopic study. Am J Ophthalmol. 1972 May;73(5):760–789. doi: 10.1016/0002-9394(72)90394-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. L., Bárány E. H. Cytochalasin B reversibly increases outflow facility in the eye of the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1977 Jan;16(1):47–53. [PubMed] [Google Scholar]
- Kayes J. Pressure gradient changes on the trabecular meshwork of monkeys. Am J Ophthalmol. 1975 Apr;79(4):549–556. doi: 10.1016/0002-9394(75)90791-6. [DOI] [PubMed] [Google Scholar]
- Kurki P., Linder E., Virtanen I., Stenman S. Human smooth muscle autoantibodies reacting with intermediate (100 A) filaments. Nature. 1977 Jul 21;268(5617):240–241. doi: 10.1038/268240a0. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Gee J. B., Bensch K. G. Cytochalasin B reversibly inhibits phagocytosis: functional, metabolic, and ultrastructural effects in human blood leukocytes and rabbit alveolar macrophages. Yale J Biol Med. 1971 Dec;44(3):286–300. [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Rahi A. H., Holborow E. J., Perkins E. S., Gungen Y. Y., Dinning W. J. Immunological investigations in uveitis. Trans Ophthalmol Soc U K. 1976 Apr;96(1):113–122. [PubMed] [Google Scholar]
- Rahi A., Ashton N. Contractile proteins in retinal endothelium and other non-muscle tissues of the eye. Br J Ophthalmol. 1978 Sep;62(9):627–643. doi: 10.1136/bjo.62.9.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringvold A. Actin filaments in trabecular endothelial cells in eyes of the vervet monkey. (Cercopithecus aethiops). Acta Ophthalmol (Copenh) 1978 Apr;56(2):217–225. doi: 10.1111/j.1755-3768.1978.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Tripathi B., Ashton N., Knight G. Effect of oxygen on the developing retinal vessels of the rabbit. 3. Mode of growth of rabbit retinal vessels in tissue culture. Exp Eye Res. 1973 Mar;15(3):321–351. doi: 10.1016/0014-4835(73)90149-8. [DOI] [PubMed] [Google Scholar]
- Van Buskirk E. M., Leure-duPree A. E. Pathophysiology and electron microscopy of melanomalytic glaucoma. Am J Ophthalmol. 1978 Feb;85(2):160–166. doi: 10.1016/s0002-9394(14)75942-2. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]