Abstract
Neurodevelopmental impairment is a common and important long-term morbidity among infants with congenital heart disease (CHD). More than half of those with complex CHD will demonstrate some form of neurodevelopmental, neurocognitive, and/or psychosocial dysfunction requiring specialized care and impacting long-term quality of life. Preventing brain injury and treating long-term neurological sequelae in this high-risk clinical population is imperative for improving neurodevelopmental and psychosocial outcomes. Thus, cardiac neurodevelopmental care is now at the forefront of clinical and research efforts. Initial research primarily focused on neurocritical care and operative strategies to mitigate brain injury. As the field has evolved, investigations have shifted to understanding the prenatal, genetic, and environmental contributions to impaired neurodevelopment. This review summarizes the recent literature detailing the brain abnormalities affecting neurodevelopment in children with CHD, the impact of genetics on neurodevelopmental outcomes, and the best practices for neonatal neurocritical care, focusing on developmental care and parental support as new areas of importance. A framework is also provided for the infrastructure and resources needed to support CHD families across the continuum of care settings.
Table of Contents Summary:
This review summarizes brain abnormalities affecting neurodevelopment in children with congenital heart disease, impact of genetics, and best neonatal neurocritical care practices for optimizing outcomes.
INTRODUCTION
Advances in medical and surgical management of infants with congenital heart disease (CHD) have improved survival and contributed to a rapidly growing population of affected children, adolescents, and adults.1 These successes have created an urgent need to address the long-term sequelae of CHD, with neurodevelopmental impairment now recognized as the most common morbidity experienced by infants who undergo cardiac surgery.2 The seminal work of the Boston Circulatory Arrest Trial initially highlighted the importance of neurodevelopmental outcomes in this population.3 Since then, it has become well-documented that nearly half of children with complex CHD experience neurodevelopmental and psychosocial impairments that impact their health-related quality of life.2, 4-11 By adolescence, 65% receive educational and/or psychosocial services.10 Critically, these deficits persist into adulthood, affecting educational achievement, quality of life, employment, and insurance status (Table 1).12, 13
Table 1.
Summary of Neurodevelopmental Outcomes by Age and Domain
| Age Group | Domains Assessed | Outcome | Missing Domain(s) |
|---|---|---|---|
| Infants (birth to 12 months) |
General Cognition Motor Skills Language Memory |
Scores in normal range Deficits observed Scores in low-normal range Deficits observed |
Executive Function Audiology |
| Toddlers (1-3.5 years) |
General Cognition Motor Skills Language Behavioral-emotional Audiology |
Deficits observed Deficits observed Deficits observed Deficits observed Scores in normal range |
Executive Function Memory |
| Preschoolers (3.5-5 years) |
General Cognition Academic Achievement Motor Language Processing Speed ADHD Symptoms Behavioral-Emotional Audiology |
Scores in low-normal range Scores in normal range Deficits observed Deficits observed for more complex measures Deficits observed Deficits observed Deficits observed Slight deficits observed |
Executive Function Memory |
| School-age children (5-12 years) |
General Cognition Academic Achievement Language Speech Motor Skills Memory Executive Function Attention (sustained) ADHD Symptoms Visuospatial Skills Behavioral-Emotional Audiology |
Scores in low-normal range Deficits observed Scores in low-normal range Deficits observed Deficits observed Deficits observed Deficits observed Deficits observed Deficits observed Deficits observed Deficits observed Scores in normal range |
Some domains in single studies only, difficult to generalize Quality of life |
| Adolescents (13-18 years) |
Academic Achievement Executive Function Memory Visuospatial Skills Attention (ADHD Symptoms) Behavioral-Emotional |
Deficits observed Deficits observed Deficits observed Deficits observed Deficits observed Deficits observed |
Motor skills Audiology Quality of life |
Reprinted from Congenital Heart Disease and Neurodevelopment, 1st Edition, Kharitonova, M. and Marino B.S., An Emergent Phenotype: A Critical Review of Neurodevelopmental Outcomes for Complex Congenital Heart Disease Survivors During Infancy, Childhood, and Adolescence, pages 55-87, 2016 with permission from Elsevier.
While optimization of neurodevelopmental outcomes initially centered on neurocritical care and operative strategies, prenatal, genetic, and environmental contributions have become important areas of focus as the field has evolved (Figure 1). Concomitantly, there has been increasing recognition of the need for infrastructure and resources to support CHD families as they navigate the continuum of neurodevelopmental care settings, including the transition to adult providers. This review summarizes the literature across these critical aspects of cardiac neurodevelopmental care.
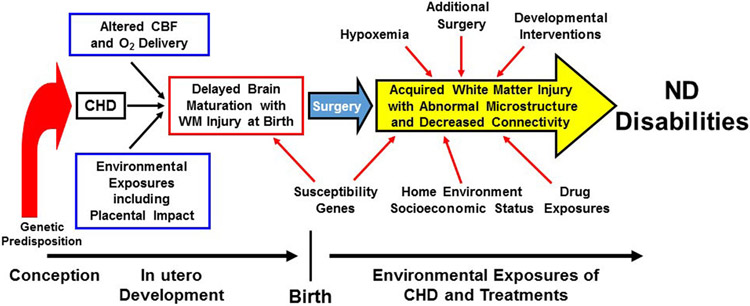
Figure 1.
Risk Factors for Neurological and Neurodevelopmental Abnormalities. Schematic representation of prenatal, perioperative, and social/environmental factors that contribute to neurodevelopmental disabilities in CHD. Prenatally, abnormal cardiac anatomy can alter blood flow and oxygenation to the developing brain, leading to impaired brain development. Concurrently, other pregnancy exposures including abnormal placental development and function and parental well-being can also have a negative impact on the brain. Genetic disruption of brain development may also occur via underlying genetics syndromes or pathogenic variants. Susceptibility genes (i.e., apolipoprotein E ε2) can further contribute to postoperative neurodevelopmental deficits. A variety of physiologic/intensive care exposures, such as hypoxemia, additional surgery, and drug exposure are important predictors in the neurocritical care arena. Finally, home environment and socioeconomic status can have a positive or negative influence, depending on the infant’s environment, whereas developmental care interventions that begin in the intensive care unit and continue after discharge may have neurodevelopmental benefit. CBF, cerebral blood flow; ND, neurodevelopment; WM, white matter. Adapted with permission from J. William Gaynor.
The research strategy used to inform this review involved searching Pubmed MeSH settings to identify peer reviewed articles on neurodevelopmental and psychiatric outcomes in CHD published since 2000. Additional studies were included based on the authors’ personal knowledge (including publications before 2000). Non-full text and non-English publications were excluded. Authors independently reviewed studies and included/excluded based on the evidence strength. This manuscript is part of a larger series of articles simultaneously published as a Supplement in Pediatrics by the Neonatal Cardiac Care Collaborative (NeoC3). Please refer to the Executive Committee introductory paper for discussion on Class of Recommendations/Level of Evidence (LOE), writing committee organization, and document review and approval.
BRAIN INJURY AND ALTERED BRAIN DEVELOPMENT
Prenatal Brain Abnormalities
Recent literature has characterized the timing and patterns of brain injury and altered brain development in CHD (Figure 2). Over the last decade, it has become clear that brain development is altered in utero in CHD fetuses.14 These abnormalities persist through adulthood without evidence of “catch up” growth.15-17 Prenatal aberrations in brain development are evident on ultrasound and magnetic resonance imaging (MRI) by the second and third trimesters of pregnancy.18-23 A large, population-based study of ultrasound biometry identified CHD-related brain alterations by 20 weeks gestation.18 Smaller fetal MRI cohorts have shown altered cortical development at 22-25 weeks19, 20 preceding reductions in brain volumes that occur by 30 weeks.14, 19
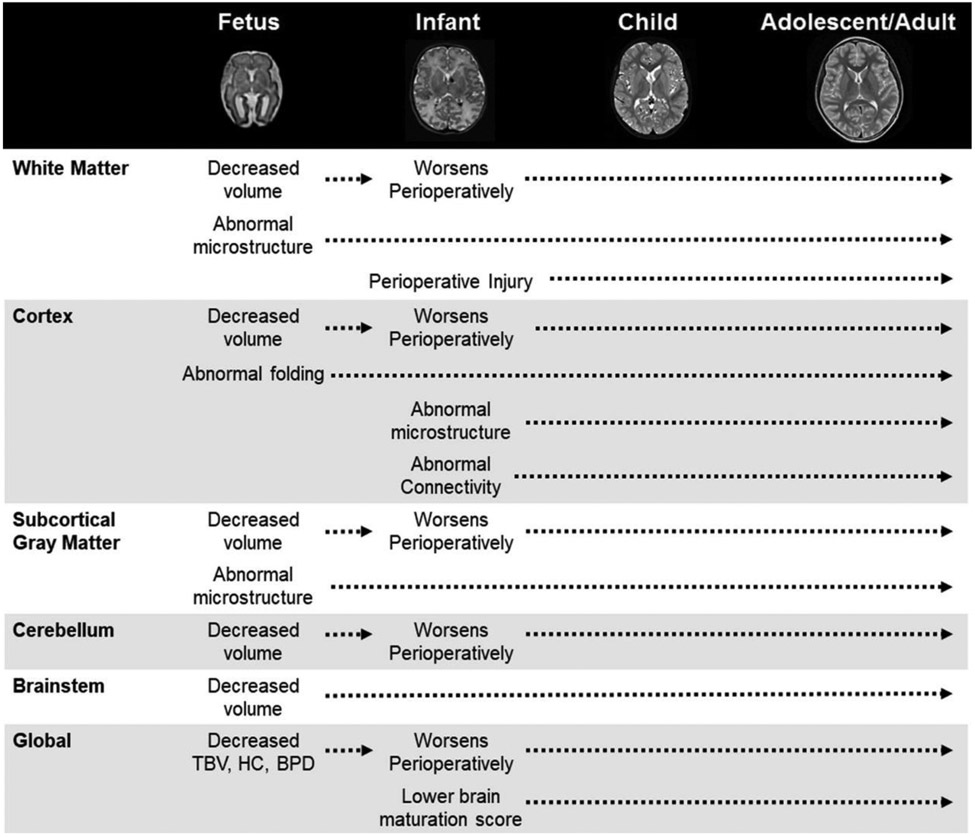
Figure 2.
Timing and Pattern of Neuroimaging Abnormalities. This timeline depicts abnormalities identified across key brain regions in neuroimaging studies of brain development in patients with CHD, beginning during the fetal period and extending into adolescence/adulthood. Representative T2-weighted images are provided for each developmental epoch depicting the significant change in brain size and structure that occurs during this period. As demonstrated, brain abnormalities in CHD are global in nature, involving white and gray matter regions at the micro- and macro-structural level. BPD, biparietal diameter; HC, head circumference; TBV, total brain volume.
This in utero vulnerability is not surprising given the negative impact of aberrant cardiac anatomy on fetal cerebral hemodynamics and substrate delivery (e.g., glucose, oxygen) during a period of rapid brain development. Aerobic glycolysis is a critical component of typical brain development throughout this window,24 with increased oxygen delivery facilitating synapse formation, gyrification, sulcation, and increased brain weight from the second to third trimester.25 Normal fetal-placental-cardiac hemodynamics are essential for supplying oxygen and nutrients to the fetus, and placental abnormalities, including decreased weight, vascular abnormalities, and altered perfusion, are prevalent in CHD.26-29 Pro-angiogenic genetic variants are also common and associated with altered placental function and smaller birth head circumference.30 Abnormal cardiac anatomy may also disrupt cerebral oxygen/nutrient delivery via hypoxia and/or altered cerebral blood flow.31 In one cohort, CHD fetuses demonstrated 10% and 32% reductions in cerebral oxygen saturation and consumption, respectively, with both associated with smaller brain size.23 Further, CHD fetuses have elevated lactate on MR spectroscopy, indicative of cerebral anaerobic metabolism.14
Preterm models indicate the oligodendroglial lineage plays an important role in this impact of CHD-related hypoxia on brain development.32 Selective vulnerability of pre-myelinating oligodendrocytes to hypoxia/hypoperfusion causes arrested white matter maturation and hypomyelination. Cortical abnormalities follow, driven by impairments in thalamocortical and cortico-cortical connectivity. Intrauterine and perinatal chronic hypoxia models mimicking CHD physiology also demonstrate decreased neuronal progenitor cells in the subventricular zone, as well as hypomyelination and abnormal cortical development.33, 34 Similar findings are evident in neuropathological specimens from CHD infants.34
Postnatal Brain Abnormalities
Up to 40% of CHD infants who undergo cardiothoracic surgery have preoperative brain injury, and another third display new injury postoperatively.35-38 The most common pattern is white matter injury (WMI), though stroke/infarct also occur.35, 38 Infants with abnormal brain development on prenatal and/or preoperative MRI are at heighted risk of perioperative injury.35, 36, 39 Further, moderate-severe injury on pre- and/or post-operative MRI is associated with slower brain growth over the 2-3 weeks surrounding cardiac surgery40 suggesting a “two-hit” phenomenon. Data linking WMI with neurodevelopmental deficits have been mixed, but moderate-severe injury is clearly associated with worse neurodevelopmental outcomes.38
Longer-term brain abnormalities in CHD include reductions in white and gray matter volumes and abnormal gyrification, sulcation, microstructure, metabolism, and cerebral connectivity, many of which are already present preoperatively (Figure 2).15-17, 35, 41-51 Due in part to these abnormalities, children with complex CHD have high rates of deficits across academic and neurodevelopmental performance domains, altered language and motor skills, and lower health-related quality of life.2, 10, 13, 15-17, 35, 41-43, 51-57 These data highlight the complexity of brain development in CHD, where fetal brain abnormalities predispose the infant to perioperative injury and ongoing aberrations in brain development that impact neurodevelopmental and psychiatric outcomes long after cardiac surgery.2, 13
CLINICAL UTILITY OF IMAGING
Initial data suggest abnormal brain development on fetal MRI is associated with increased perioperative brain injury risk.39, 58 However, the expertise and resources required for fetal MRI currently prevent its routine clinical application to obtain detailed brain measures. Postnatal imaging is more accessible and commonly used. Many centers routinely perform cranial ultrasound screening pre-operatively in all CHD infants. Cranial ultrasound is 82% sensitive and 93% specific for intraventricular hemorrhage when compared to clinically acquired computed tomography or MRI.60 This same study showed the odds of developing intraventricular hemorrhage increased 1.3-fold for each week decrease in gestational age, resulting in an incidence >50% for very preterm CHD infants, although the majority was low-grade hemorrhage. However, ultrasound may miss many abnormalities seen on MRI, particularly WMI.59 There are currently no conclusive data supporting routine MRI to guide critical care approaches or surgical timing, with only one cohort reporting that clinically-silent preoperative lesions do not worsen postoperatively.61 Neuroimaging studies are key components of ongoing research efforts that may inform future clinical practice. In the interim, detailed neurological examinations and consultation with neurology and/or dedicated neurocritical care services may facilitate decision-making regarding neuroimaging.
Recommendations:
The utility of routine clinical fetal or postnatal MRI in CHD is unclear. However, regular, detailed neurological examinations before and after cardiac surgery can be useful to identify infants who would benefit from neuroimaging (Class IIa/LOE C-EO).
Cranial ultrasound may be considered to screen for intraventricular hemorrhage or major structural abnormalities in all CHD infants but may not be useful for WMI (Class IIb, LOE C-LD).
When available, consultation with pediatric neurology and/or neurocritical care services can be beneficial for facilitating optimal neuroimaging use (Class IIa/LOE C-EO).
GENETIC INFLUENCES ON NEURODEVELOPMENTAL OUTCOME
CHD occurs in common and rare genetic disorders and syndromes (Table 2), some of which are known to be associated with neurodevelopmental dysfunction, while other genetic abnormalities have less clear neurodevelopmental implications.62 Genetic factors have been previously recognized as major neurodevelopmental determinants in CHD, independent of cardiac defect or surgical repair.52, 63 These include greater risk of motor and cognitive delays in early childhood and school-age64-67 and greater intellectual impairments, poorer academic achievement, worse executive functioning, increased autism symptoms, and lower health-related quality of life in adolescence.56, 68, 69 These relationships likely begin in the prenatal period and may be independent of or additive to the effects of hemodynamic alterations. Recently, impaired head growth in CHD fetuses showed better correlation with the presence of a genetic syndrome and/or extracardiac anomaly than abnormal fetal hemodynamics.70
Table 2.
Genetic Syndromes Associated with CHD
| Common Syndromes | Rare Syndromes |
|---|---|
| Trisomy 21 | LEOPARD syndrome |
| Trisomy 18 | Kabuki syndrome |
| Trisomy 13 | Costello syndrome |
| 22q11 deletion syndrome | PHACES syndrome |
| Turner syndrome | Rubenstein – Taybi syndrome |
| Williams syndrome | Ellis- can Crevald syndrome |
| Jacobsen syndrome | Townes- Brocks syndrome |
| Alagille syndrome | Smith- Lemli -Opitz syndrome |
| Holt Oram syndrome | Goldenhar syndrome |
| Heterotaxy | Keutel syndrome |
| CHARGE syndrome | Cat eye syndrome |
| Cornelia de Lange syndrome | Char syndrome |
| Noonan syndrome | Simpson- Golabi- Behmel syndrome |
| VACTERL | Fryns syndrome |
| Adams- Oliver syndrome | |
| Ritscher-Schinzel syndrome |
An increasing focus on the genetic underpinnings of CHD have established that genetic variants outside of known syndromes are also important. For example, genotyping apolipoprotein E, a cholesterol metabolism regulator involved in neuronal and white matter repair, may be valuable because of associations between the ε2 allele and motor and behavioral deficits in CHD.71, 72 New sequencing technologies and strategies for analyzing genetic data also provide key insights. Studies utilizing rare variant burden analyses implicate neurotransmitter, axon guidance, and RASopathy gene pathways in neurodevelopmental disability associated with CHD.73 Further, novel and known pathogenic copy-number variants have been associated with motor delay,74 and damaging de novo variants in high-heart, high-brain expressing genes are upregulated in CHD patients with neurodevelopmental disability.75 Importantly, adolescents with these variants demonstrate atypical left hemisphere sulcal development on MRI, 76 with similar abnormalities in CHD fetuses.20 These data suggest genetic pathways for abnormal brain development in CHD.
Early diagnosis of genetic disorders is a key component of assessing neurodevelopmental risk and optimizing outcomes.2, 62 Genetic evaluation begins prenatally for CHD as reviewed by Haxel and colleagues elsewhere in this issue. A Scientific Statement from the American Heart Association (AHA) provides recommendations for clinical genetic testing, including karyotype, chromosomal microarray, and targeted testing, as well as ethical considerations and use of genetic counselors.62 Testing results and limitations of the testing should be discussed amongst clinicians and genetic specialists, communicated to parents, and documented in the medical record. Genetic specialists can also address cost effectiveness and financial burden.
Recommendations:
Prenatal and/or postnatal genetic testing, including karyotype and chromosomal microarray, can be effective for informing neurodevelopmental risk in CHD infants (Class IIa/LOE B-NR).
Additional targeted genetic testing may be considered based upon individual phenotypes; the role of routine expanded genetic sequencing remains unclear (Class IIb/LOE C-LD).
Vigilant neurodevelopmental surveillance should be incorporated into routine care for CHD infants with genetic syndromes (Class I/LOE B-NR).
NEONATAL NEURODEVELOPMENTAL CARE
Prenatal Diagnosis and Delivery Planning
Infants with a prenatal diagnosis of CHD demonstrate less frequent and less severe brain injury and better brain development compared to those with a postnatal diagnosis.77 Prenatal diagnosis also correlates with improved cognition in children with transposition of the great arteries.78 This neuroprotective effect is presumably related to the expectant care provided for the known diagnosis postnatally, leading to more favorable postnatal hemodynamics.38 Gestational age at birth is also important for CHD infants. Elective delivery at<39 weeks gestation is associated with increased mortality, morbidity, and worse outcomes.79 Lower gestational age also increases the likelihood and severity of brain injury and abnormal brain development80-82 and is a key predictor of adverse motor, cognitive, language, and psychiatric outcomes.79, 83, 84 The AHA Scientific Statement on fetal cardiac medicine supports multidisciplinary approaches to avoid elective delivery at <39 weeks, to create specialized care plans that optimize postnatal hemodynamics, and to consider maternal complications and fetal well-being in delivery decision-making.85 Additional data and recommendations regarding delivery timing are discussed by Haxel and colleagues elsewhere in this issue.
Intensive Care, Perioperative Management, and Neuromonitoring
Cardiac intensive care strategies and operative management are modifiable variables that contribute to optimizing neurodevelopmental outcomes. Risk factors for perioperative brain injury and worse neurodevelopmental outcomes include hypoxemia; impaired cerebral oxygenation and hemodynamics; longer time from birth to surgery; prolonged cardiopulmonary bypass and circulatory arrest; larger base deficit during cardiopulmonary bypass; inadequate levels of hypothermia while on cardiopulmonary bypass and/or hyperthermia during the early postoperative period; greater hemodilution on cardiopulmonary bypass; pre- and postoperative cardiac arrest and the need for cardiopulmonary resuscitation; utilization of pre- or postoperative extracorporeal membrane oxygenation (ECMO) support; and single ventricle physiology and aortic arch obstruction.35, 86-97 Cardiac surgery-related brain injury appears to occur via hypoxic/ischemic effects on oligodendrocyte precursor cells, which is exacerbated by prior hypoxic exposures and alters white matter development.98-100 Intensive care unit events and postoperative complications that increase length of stay and/or result in repetitive anesthetic exposure contribute to worse neurodevelopmental outcomes. These include higher inotropic score, extubation failure, infection (e.g., bacteremia/sepsis, mediastinitis), arrhythmias, postoperative seizures, electroencephalogram (EEG) abnormalities, ECMO, and additional cardiac and non-cardiac operations (e.g., residual lesions requiring re-intervention, gastrostomy tube).10, 52, 101-110
In an attempt to minimize perioperative neurological injury risk, many centers have adopted the use of continuous, non-invasive monitors that provide surrogate markers of oxygen delivery, such as near-infrared spectroscopy (NIRS). This technology provides information on regional tissue oxygenation, expressed as an oxygen saturation (rSO2). In the days after birth, cerebral oxygenation declines and reaches a threshold where WMI risk likely increases.89 In one cohort, lower cerebral rSO2 intraoperatively predicted acute neurologic injury postoperatively.111 Reduced rSO2 variability in the immediate postoperative period may reflect impaired cerebral autoregulation leading to poor outcomes.112 Further, utilizing NIRS in combination with blood lactate postoperatively enhances prediction of mortality and poor neurodevelopmental outcomes.113 Perioperative neurological management may also include monitoring for seizure activity. Approximately 10-30% of CHD infants have electrographic seizures perioperatively, and up to 60% have abnormal background patterns.114-119 Brain injury and altered brain development are associated with abnormal EEG, and new postoperative seizures may suggest new brain injury.116, 119, 120 While amplitude-integrated EEG (aEEG) can detect seizures and provide trends in background activity, conventional video EEG remains the standard of care. Adverse neurodevelopmental outcomes are seen in children who have seizures on conventional EEG,102, 121 whereas background patterns, but not seizures, relate to outcome for aEEG.114 Similar to neuroimaging, there are currently no standardized recommendations for use of NIRS or EEG in all CHD infants, although the American Clinical Neurophysiology Society recommends EEG monitoring in neonates undergoing early cardiopulmonary bypass.122 Despite data showing an association of reduced rSO2 and seizures with poorer neurodevelopmental outcomes, there is not yet evidence that incorporation of these monitoring strategies improves neurodevelopmental outcomes.
While perioperative management is critical for mitigating neurologic risk, operative and postoperative factors explain ≤5% of neurodevelopmental outcome variance, whereas pre-operative and patient-specific factors account for ~25%.97 Non-modifiable factors associated with adverse neurodevelopmental outcomes include lower maternal education (e.g., less than high school compared to graduate school), lower socioeconomic status, genetic disorders/variants, prematurity, and cardiac diagnosis.97 These data highlight the need to optimize current practices while exploring new neuroprotective strategies.
Developmental Care in the Intensive Care Unit
CHD infants require surgical interventions during periods of developmental immaturity. Therefore, in addition to providing optimal medical care, the critical care environment must foster autonomic and behavioral subsystem development.123 Implementation of developmental and kangaroo care improves outcomes in premature infants.124-126 Recently, individualized, family-centered developmental care has been identified as a promising neuroprotective model for the cardiac intensive care environment.127 Straightforward modifications include cycled-lighting to maintain circadian rhythms; music exposure to minimize noxious stimulation; physiologic positioning with flexion, spinal alignment, swaddling; and non-pharmacologic comfort measures.128-133 Early engagement with therapy services is often essential for instituting these modifications.132 Kangaroo care has not only been shown to be safe for CHD infants, but actually improves cardiopulmonary status following extubation and optimizes feeding tolerance.134-137
Oromotor development and coordination of sucking and feeding are among the earliest manifestations of motor control.138 Feeding dysfunction in CHD can lead to impaired somatic growth, which remains critical to motor and cognitive outcomes.135 Furthermore, CHD infants who breastfeed have higher weight-for-age scores,139 likely secondary to fewer episodes of desaturation and temperature decreases.140 Breastfeeding allows mothers to actively participate in their child’s care, leading to improved maternal-child bonding.141, 142 Orally fed CHD infants demonstrate more rapid white and gray matter brain maturation compared to their tube-fed counterparts,135 emphasizing the importance of practices facilitating feeding via bottle or breast.
Parental Well-being in the Intensive Care Unit
Growing evidence suggests the long-term behavioral, social, and emotional difficulties of children with CHD may be partially attributable to parental mental health beginning prenatally.143-145 A systematic review found 80% of CHD parents report trauma symptoms, 25-50% report elevated depression and/or anxiety symptoms, and 30-80% report severe psychological distress.146 Prenatal exposure to maternal psychological distress has recently been associated with altered fetal brain metabolism, hippocampal growth, and cortical development in healthy pregnancies.147 Further, abnormal hippocampal and cerebellar development are seen in CHD fetuses exposed to maternal stress and anxiety.148 In the hospital setting, parental stressors include feelings of inadequate preparation and knowledge, concerns about outcomes, infant appearance, the intensive care environment, altered parental roles, financial burdens, and inadequate support.149, 150 Importantly, stress is experienced differently by parents. While mothers tend to focus on the baby, fathers tend to focus on supporting mother and child.150
Parental mental health interventions are necessary to support CHD families and optimize neurodevelopment. A recent review of parental mental health during intensive care identified only five trials of infants with congenital anomalies (four included CHD; two included fathers).151 Although available evidence is minimal, these data suggest interventions are efficacious for reducing parental anxiety and improving maternal coping, mother-infant attachment, parenting confidence, clinical care satisfaction, and infant development.151
Transition to Home
To date, no data have been published linking neurodevelopmental outcomes to the discharge process and transition to home. However, this is a period of unanticipated physical and emotional transitions where greater parental education and support are needed.152, 153 Use of discharge specialists increases parents’ perceived discharge readiness.154 Parental education on referral for early intervention services and outpatient therapies should be a priority at discharge to facilitate this transition. Support of parental well-being during this period is also critical.
Recommendations:
Intensive care management should minimize clinical complications and exposure to clinical factors that increase neurodevelopmental risk (Class I/LOE B-NR).
Incorporation of NIRS and EEG into intensive care management may be considered to identify CHD infants at higher neurological risk (Class IIb/LOE C-LD).
Incorporation of developmental care approaches, including appropriate positioning, cycled lighting, noxious stimuli minimization, non-pharmacologic comfort measure use, kangaroo care, and oral/breastfeeding, may be considered in the intensive care unit to optimize neurodevelopmental outcomes (Class IIb/LOE C-LD).
Screening for parental psychological distress may be reasonable in the intensive care environment as a component of neurodevelopmental care (Class IIb/LOE C-LD).
CONTINUUM OF NEURODEVELOPMENTAL CARE THORUGHOUT CHILDHOOD
Childhood Outcomes
Children with CHD have a distinct neurodevelopmental profile of early motor deficits, cognitive delays, language impairments, executive dysfunction, inattention, emotional and behavioral disorders, and issues with social cognition.2, 13 Up to 50% display impairments across one or more domains.7 Increasing neurodevelopmental risk occurs with increasing CHD severity.7 However, significant variation in neurodevelopmental outcomes is present among those with the same cardiac diagnosis,155 likely due to patient-specific risk factors outlined in the AHA and American Academy of Pediatrics (AAP) Scientific Statement on CHD outcomes (Table 3).2
Table 3.
Categories of Pediatric CHD Patients at High Risk for Developmental Disorders or Disabilities
| 1. Neonates/infants requiring open heart surgery (cyanotic and acyanotic types), for example, HLHS, IAA, PA/IVS, TAPVC, TGA, TOF, tricuspid atresia. |
| 2. Children with other cyanotic heart lesions not requiring open heart surgery during the neonatal or infant period, for example, TOF with PA and MAPCA(s), TOF with shunt without use of CPB, Ebstein anomaly. |
| 3. Any combination of CHD and the following comorbidities: |
| 3.1. Prematurity (<37 wk) |
| 3.2. Developmental delay recognized in infancy |
| 3.3. Suspected genetic abnormality or syndrome associated with DD |
| 3.4. History of mechanical support (ECMO or VAD use) |
| 3.5. Heart transplantation |
| 3.6. Cardiopulmonary resuscitation at any point |
| 3.7. Prolonged hospitalization (postoperative LOS >2-wk in the hospital) |
| 3.8. Perioperative seizures related to CHD surgery |
| 3.9. Significant abnormalities on neuroimaging or microcephaly* |
| 4. Other conditions determined at the discretion of the medical home providers |
CHD indicates congenital heart disease; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; PA/IVS, pulmonary atresia with intact ventricular septum; TA, truncus arteriosus; TAPVC, total anomalous pulmonary venous connection; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; PA, pulmonary atresia; MAPCA, major aortopulmonary collateral arteries; CPB, cardiopulmonary bypass; DD, developmental disorder or disability; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device; and LOS, length of stay.
Normative data by sex, including percentiles and z scores, are available from the World Health Organization (www.who.int/childgrowth; accessed February 2010).
Reprinted with permission
Circulation.2012;126:1143-1172
© 2012 American Heart Association, Inc.
It is important to recognize the dynamic nature of neurodevelopment in children with CHD.156 Motor delay tends to be prevalent in the first year of life and may improve during early childhood.157, 158 However, even at age 10 years, deficits are still common, particularly within adaptive motor function, static balance, and movement quality.159 Infant surgery and exposure to the intensive care environment are key factors affecting motor development.160 Lower socioeconomic status and maternal education are associated with worsening fine motor skills.161 Cognitive and language impairments are also present early, but generally do not demonstrate improvement, and may worsen, particularly in children with genetic syndromes and lower socioeconomic status.157, 158, 161, 162 Of interest, a cognitively stimulating home environment is associated with better cognitive development,163 whereas parental stress is associated with worse cognition.164 At school-age, cognitive ability typically falls in the low-normal range.165 Lower scores in language, attention, and executive function measures are common.8 In a recent cohort, parent-reported executive dysfunction was present in >60% of school-aged children166 and strongly correlated with health-related quality of life.57, 167 Working memory and flexibility appear to be notably problematic, though behavioral dysregulation is prominent in children born preterm, of male sex, or with arch obstruction.166 Behavioral and emotional deficits become more evident throughout childhood and are associated with impairments in other domains, including motor and language development.159, 168, 169
Screening and Evaluation of Childhood Neurodevelopment
While neurodevelopmental disabilities are common, deficits are mild for many CHD children and may not be detected without formal testing.64 The dynamic nature of neurodevelopmental outcomes and evolution of children’s risk category highlight the need for continual surveillance and use of early intervention services.170 The AHA/AAP 2012 Scientific Statement provides guidelines for neurodevelopmental evaluation in CHD, using a medical home model that includes the family, primary care providers, and specialty services.2 Consistent with general AAP recommendations, primary care providers are the foundation for the medical home, providing neurodevelopmental surveillance at every visit, standardized developmental screening at 9, 18, and 30 months of age, and autism screening at 18 and 24 months.2, 171, 172 CHD-specific guidelines expand these recommendations by incorporating a risk assessment for all children with CHD.2 Those identified as high-risk should be referred for formal developmental and medical evaluations (Table 3). Developmental evaluation includes referral to early intervention/special education services and multidisciplinary cardiac neurodevelopmental follow-up programs. The medical evaluation includes a developmental history, growth and feeding assessment, motor and audiologic examinations, evaluation by a developmental pediatrician or pediatric neurologist and, if indicated, a geneticist. Medical and developmental re-evaluation is recommended between ages 12-24 months, 3-5 years, and 11-12 years.2 A variety of neurodevelopmental assessment tools are available across ages and have been summarized in two recent publications from the Cardiac Neurodevelopmental Outcome Collaborative.173, 174 An important component of formal neurodevelopmental evaluation across all ages is the communication of results, and any limitations of the testing (e.g., related to cooperation of the child), between clinicians and the family, as well as documentation of these discussions in the medical record.
Navigating the School System
Navigating school systems and providing school-based interventions are essential components of neurodevelopmental care (Table 4).175, 176 Neuropsychological or educational evaluations are undertaken to facilitate individualized education plans (IEPs) and 504 plans.177 Testing is repeated every 2-3 years, with IEPs and 504 plans updated annually to provide home-school communication. Homebound instruction can be established for medically-indicated absences. It is beneficial to have an education specialist, or school liaison, facilitate the interaction between the parents/guardians and the school system to provide support and implement recommendations.178 The education specialist facilitates school-based therapies and curriculum modifications, which include extra time for tests/homework, note taking, recording classes, reading or scribing during tests, and quiet testing environments.179 Education of school staff regarding the child’s medical condition, disability risk, and learning needs is another important component. Recently, children with CHD with executive dysfunction were found to receive similar school services as those without difficulties.166 This highlights the critical need for continued education amongst schools and the benefit of school liaisons, who can encourage parents to become empowered advocates. As cardiac neurodevelopmental programs evolve, education specialists/school liaisons will be an important component of care that can be supported through a number of mechanisms including local health and school systems.
Table 4.
School Based Interventions to Support Learning Needs
| Type of Intervention | Examples |
|---|---|
| Regular evaluations including psychological, education, and speech and language |
|
| Collaboration between education liaison in the neurodevelopmental clinic and school setting to share diagnostic information and recommendations |
|
| Development of formal educational plans |
|
| Homebound Instruction |
|
| Development of formal medical plan |
|
| Development of accommodation and modification plans |
|
| School based interventions |
|
| Vocational skill training or programming | |
| Advocacy assistance |
|
Created with assistance from Vicki Baker, MS, LPC, St. Louis Children’s Hospital
Optimizing Family Support
Caring for a child with CHD can place significant strain on the family dynamic as parents navigate the complexity of their child’s care. Implementation of targeted support strategies for mothers, fathers, and siblings are essential and span medical, classroom, and home arenas (Table 5). Navigating early intervention referrals and routine neurodevelopmental screening can be challenging. A recent cohort from the Single Ventricle Reconstruction Extension Study identified that less than half of children were receiving early intervention services at age one year, and over one-third never received services despite high rates of neurodevelopmental delay.170 These findings highlight referral patterns and the need to support parents. Multidisciplinary cardiac neurodevelopmental follow-up programs can provide a collaborative platform for primary care providers to utilize as a “home base” for accessing early interventions, rehabilitative services, behavioral management, counseling, school-based support, and care transitions. Family-specific support typically focuses on parental wellbeing and support networks and can be provided by primary care providers and/or cardiac neurodevelopmental follow-up programs,151, 180, 181 with referral to mental health specialists as indicated. Recent data have identified that parents of children with medical complexity are often unsure of where to find community help or resources for mental health needs, suggesting that policy makers and health care organizations consider family mental health as a component of improving health systems.182
Table 5.
Toolkit for Supporting Parents
| Neurodevelopmental Assessment |
| Psychosocial Assessment |
|
| Care Coordination |
|
| Care Transition |
|
| Social Support Networks |
|
| Financial Assistance |
|
Recommendations:
Developmental surveillance by primary care providers at every well child visit can be beneficial for all children with CHD. Standardized developmental screening can also be beneficial at 9, 18, and 30 months, with autism screening at 18 and 24 months. Primary care providers and/or subspecialists can perform this screening (Class IIa/LOE C-LD).
- A risk assessment should be performed in children with CHD and those who meet the below criteria should be considered high-risk for neurodevelopmental disabilities (Class I/LOE A):
- Neonates/infants requiring open heart surgery
- Children with cyanotic heart conditions not requiring open heart surgery during neonatal period/infancy
- CHD with comorbidities (Table 3)
- Other conditions at discretion of medical home providers
Children with CHD who meet high-risk criteria can be referred to multidisciplinary cardiac neurodevelopmental follow-up and early intervention programs for developmental evaluation and to relevant providers for medical evaluation of growth, feeding, and audiologic assessment (Class IIa/LOE B-NR).
Medical and developmental re-evaluation by the primary care provider and/or a subspecialist can be beneficial between ages 12-24 months, 3-5 years, and 11-12 years (Class IIa/LOE C-LD).
Education specialists can be beneficial for facilitating the interaction between parents/guardians and the school system and school-based interventions (Class IIa/LOE C-EO).
Screening for parental stress and mental health problems, and referral for psychosocial intervention, during neurodevelopmental assessments may be reasonable to promote better family functioning and outcomes (Class IIb/LOE C-LD).
ADOLESCENTS AND ADULTS WITH CHD
Improving survival for patients with even severe cardiac lesions has led to a larger population of adults than children with CHD,1 resulting in a rapid evolution of adolescent and adult CHD neurodevelopmental care. Neurodevelopmental disability occurs twice as commonly in adolescents with CHD than typically-developing children.183 These deficits span working memory, perceptual reasoning, cognitive flexibility, and executive and motor function domains183, 184 (Table 1) and correlate with CHD complexity.185 The persistence of neurodevelopmental disability into adulthood, particularly in those with severe CHD, has been associated with higher unemployment, reliance on disability, and lower education levels.186 Evaluation of these deficits encompasses use of formal assessment tools.187 Efforts to improve outcomes include targeted childhood interventions, medical management of inattention/hyperactivity, and career counseling.188
In addition to neurodevelopmental disabilities, adolescents and adults with CHD have an increased incidence of comorbid psychiatric disorders including anxiety, depression, post-traumatic stress disorder, attention difficulties, adjustment disorder, and early onset dementia.189, 190 Up to 69% of CHD patients with mood or anxiety disorders do not receive psychotherapy or psychotropic drugs,191 highlighting the need for lowering thresholds for referral to and/or adoption of psychologists into comprehensive programs.192 Given this high prevalence and limitations in access to therapies, it is essential that routine care include screening for these disorders coupled with standardized treatment referral pathways. Approaches for mental health care in pediatric practices193 and psychosocial screening tools for adolescents and young adults,194 are available from the AAP. Additional tools may be useful for adults with CHD.195, 196
The complex co-morbidities found in the adult CHD population make it imperative that care is transitioned from pediatric- to adult-centered providers. Currently there are many barriers to this transition (Table 6). It is estimated that only 48% of CHD adolescents successfully transfer to adult centers.197 The most recent AHA recommendations include a three-step process spanning many years.198 The pre-transition period occurs throughout childhood and is dedicated to creating a foundation for lifelong care. Ideally, at age 12-14, patients advance to a transition curriculum with inclusion of residual hemodynamic concerns, insurance planning, future education, and employment goals. The third phase is transfer, when responsibility shifts from the pediatric to adult provider, ideally by age 21. A well-planned and coordinated effort is paramount to success. Each individual should have a detailed plan including their specific cardiac physiology, medical history, previous interventions, medication list, diagnostic studies, functional status, and comorbidities. Successful models include a dedicated staff member who assumes primary responsibility199 and use of resources including mobile applications and webpages, such as gottransition.org.200
Table 6.
Barriers to Transition from Pediatric to Adult Centers
| Domain | Barriers to Transition | Recommendation |
|---|---|---|
| Health Care System |
|
|
| Neurocognitive |
|
|
| Psychosocial |
|
|
| Relationships |
|
|
Recommendations:
Screening for neurodevelopmental and psychological disorders should continue in adults with CHD and be coupled with standardized treatment pathways (Class I/LOE C-LD).
Transition of care from the pediatric to adult clinical care arena through the medical home model should use a structured plan including parental partnership (Class I/LOE C-LD).
CONCLUSIONS
Neurodevelopmental care has become a critical component of treating children with CHD. Brain injury and abnormal brain development occur through multifactorial pathways that begin prenatally and underlie longstanding neurodevelopmental deficits. A multifaceted, longitudinal family-based approach to screening, evaluation, and treatment of neurodevelopmental disabilities is essential for optimizing neurodevelopmental outcomes. Recognition of high-risk clinical factors, incorporation of parental and family support, and implementation of developmental care strategies in the intensive care environment are important aspects of clinical practice. Genetic testing, neuroimaging, NIRS, EEG, and parental mental health screening may be useful in evaluating neurodevelopmental risk and are areas of ongoing investigation. Beyond the inpatient setting, routinely screening and evaluating neurodevelopment throughout childhood and adolescence, incorporating school liaisons in neurodevelopmental programs, and providing support and resources as children transition to adult care providers all facilitate a comprehensive longitudinal neurodevelopmental approach.
Funding/Support:
The Neonatal Heart Society (NHS) contributed an educational grant to the project, NeoC3. The NHS, on a regular basis, applies and receives several unrestrictive educational grants for several internal projects from the following organizations and companies: Abbott Formula, Mead Johnson, Cheisi, Mallinckrodt, Prolacta, and Medtronic. This work was also supported by the National Institutes of Health (K23HL141602, CMO and P50HD103525, CDS).
Role of Funder:
The grants received from industry partners were utilized solely to offset the cost of publishing this supplement in Pediatrics. The industry supporters did not suggest manuscript content, nor did they participate in any way to the writing or editing of the manuscript.
Abbreviations:
- CHD
congenital heart disease
- ECMO
extracorporeal membrane oxygenation
- EEG
electroencephalogram
- IEP
individualized education plans
- MRI
magnetic resonance imaging
- WMI
white matter injury
Footnotes
Financial Disclosure Statement:
The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest:
The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N and Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–56. [DOI] [PubMed] [Google Scholar]
- 2.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH Jr., Li J, Smith SE, Bellinger DC, Mahle WT, American Heart Association Congenital Heart Defects Committee CoCDitYCoCN and Stroke C. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–72. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, Barnes PD, Holmes GL, Hickey PR, Strand RD and et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–55. [DOI] [PubMed] [Google Scholar]
- 4.Marino BS, Tomlinson RS, Wernovsky G, Drotar D, Newburger JW, Mahony L, Mussatto K, Tong E, Cohen M, Andersen C, Shera D, Khoury PR, Wray J, Gaynor JW, Helfaer MA, Kazak AE, Shea JA and Pediatric Cardiac Quality of Life Inventory Testing Study C. Validation of the pediatric cardiac quality of life inventory. Pediatrics. 2010;126:498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wray J, Brown K, Franklin R, Cassedy A and Marino BS. Assessing the generalisability of the pediatric cardiac quality of life inventory in the United Kingdom. Cardiol Young. 2014;24:220–8. [DOI] [PubMed] [Google Scholar]
- 6.Mellion K, Uzark K, Cassedy A, Drotar D, Wernovsky G, Newburger JW, Mahony L, Mussatto K, Cohen M, Limbers C, Marino BS and Pediatric Cardiac Quality of Life Inventory Testing Study C. Health-related quality of life outcomes in children and adolescents with congenital heart disease. J Pediatr. 2014;164:781–788 e1. [DOI] [PubMed] [Google Scholar]
- 7.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16 Suppl 1:92–104. [DOI] [PubMed] [Google Scholar]
- 8.Miatton M, De Wolf D, Francois K, Thiery E and Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J Pediatr. 2007;151:73–8, 78 e1. [DOI] [PubMed] [Google Scholar]
- 9.Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B and Tchervenkov C. Developmental and functional outcomes at school entry in children with congenital heart defects. J Pediatr. 2008;153:55–60. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL Jr., Dunbar-Masterson C, Rappaport LA, Wernovsky G, Jonas RA and Newburger JW. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg CS, Lu M, Sleeper LA, Mahle WT, Gaynor JW, Williams IA, Mussatto KA, Ohye RG, Graham EM, Frank DU, Jacobs JP, Krawczeski C, Lambert L, Lewis A, Pemberton VL, Sananes R, Sood E, Wechsler SB, Bellinger DC, Newburger JW and Pediatric Heart Network I. Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr. 2014;165:490–496 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr., Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD, Smith SC Jr., Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Page RL, Riegel B, Tarkington LG, Yancy CW, American College of C, American Heart Association Task Force on Practice G, American Society of E, Heart Rhythm S, International Society for Adult Congenital Heart D, Society for Cardiovascular A, Interventions and Society of Thoracic S. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–263. [DOI] [PubMed] [Google Scholar]
- 13.Marelli A, Miller SP, Marino BS, Jefferson AL and Newburger JW. Brain in Congenital Heart Disease Across the Lifespan: The Cumulative Burden of Injury. Circulation. 2016;133:1951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr., Guizard N, McGrath E, Geva J, Annese D, Dunbar-Masterson C, Trainor B, Laussen PC and Du Plessis AJ. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontes K, Rohlicek CV, Saint-Martin C, Gilbert G, Easson K, Majnemer A, Marelli A, Chakravarty MM and Brossard-Racine M. Hippocampal alterations and functional correlates in adolescents and young adults with congenital heart disease. Hum Brain Mapp. 2019;40:3548–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollins CK, Watson CG, Asaro LA, Wypij D, Vajapeyam S, Bellinger DC, DeMaso DR, Robertson RL Jr., Newburger JW and Rivkin MJ. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr. 2014;165:936–44 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W and Latal B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2013;137:268–276. [DOI] [PubMed] [Google Scholar]
- 18.Lauridsen MH, Uldbjerg N, Petersen OB, Vestergaard EM, Matthiesen NB, Henriksen TB, Ostergaard JR and Hjortdal VE. Fetal Heart Defects and Measures of Cerebral Size. J Pediatr. 2019;210:146–153. [DOI] [PubMed] [Google Scholar]
- 19.Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, Gholipour A, Kudelski D, Warfield SK, McCarter RJ, Robertson RL Jr., Evans AC, Newburger JW and Limperopoulos C. Delayed cortical development in fetuses with complex congenital heart disease. Cerebral cortex. 2013;23:2932–43. [DOI] [PubMed] [Google Scholar]
- 20.Ortinau CM, Rollins CK, Gholipour A, Yun HJ, Marshall M, Gagoski B, Afacan O, Friedman K, Tworetzky W, Warfield SK, Newburger JW, Inder TE, Grant PE and Im K. Early-emerging sulcal patterns are atypical in fetuses with congenital heart disease. Cerebral cortex. 2018;29:3605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masoller N, Martinez JM, Gomez O, Bennasar M, Crispi F, Sanz-Cortes M, Egana-Ugrinovic G, Bartrons J, Puerto B and Gratacos E. Evidence of second-trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet Gynecol. 2014;44:182–7. [DOI] [PubMed] [Google Scholar]
- 22.Schellen C, Ernst S, Gruber GM, Mlczoch E, Weber M, Brugger PC, Ulm B, Langs G, Salzer-Muhar U, Prayer D and Kasprian G. Fetal MRI detects early alterations of brain development in Tetralogy of Fallot. Am J Obstet Gynecol. 2015;213:392 e1–7. [DOI] [PubMed] [Google Scholar]
- 23.Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, Grosse-Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, Hickey E, Miller S and Seed M. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal MS, Iannotti LL and Raichle ME. Brain Nutrition: A Life Span Approach. Annu Rev Nutr. 2018;38:381–399. [DOI] [PubMed] [Google Scholar]
- 25.du Plessis AJ. Cerebral blood flow and metabolism in the developing fetus. Clin Perinatol. 2009;36:531–48. [DOI] [PubMed] [Google Scholar]
- 26.Matthiesen NB, Henriksen TB, Agergaard P, Gaynor JW, Bach CC, Hjortdal VE and Ostergaard JR. Congenital Heart Defects and Indices of Placental and Fetal Growth in a Nationwide Study of 924 422 Liveborn Infants. Circulation. 2016;134:1546–1556. [DOI] [PubMed] [Google Scholar]
- 27.Rychik J, Goff D, McKay E, Mott A, Tian Z, Licht DJ and Gaynor JW. Characterization of the Placenta in the Newborn with Congenital Heart Disease: Distinctions Based on Type of Cardiac Malformation. Pediatric cardiology. 2018;39:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones HN, Olbrych SK, Smith KL, Cnota JF, Habli M, Ramos-Gonzales O, Owens KJ, Hinton AC, Polzin WJ, Muglia LJ and Hinton RB. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta. 2015;36:1078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zun Z, Zaharchuk G, Andescavage NN, Donofrio MT and Limperopoulos C. Non-Invasive Placental Perfusion Imaging in Pregnancies Complicated by Fetal Heart Disease Using Velocity-Selective Arterial Spin Labeled MRI. Sci Rep. 2017;7:16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell MW, Moldenhauer JS, Rychik J, Burnham NB, Zullo E, Parry SI, Simmons RA, Elovitz MA, Nicolson SC, Linn RL, Johnson MP, Yu S, Sampson MG, Hakonarson H and Gaynor JW. Damaging Variants in Proangiogenic Genes Impair Growth in Fetuses with Cardiac Defects. J Pediatr. 2019;213:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudolph AM. Impaired cerebral development in fetuses with congenital cardiovascular malformations: Is it the result of inadequate glucose supply? Pediatr Res. 2016;80:172–7. [DOI] [PubMed] [Google Scholar]
- 32.Volpe JJ. Encephalopathy of congenital heart disease- destructive and developmental effects intertwined. J Pediatr. 2014;164:962–5. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence KM, McGovern PE, Mejaddam A, Rossidis AC, Baumgarten H, Kim A, Grinspan JB, Licht DJ, Didier RA, Vossough A, Radaelli E, Rychik J, Song L, Peranteau WH, Davey MG, Flake AW and Gaynor JW. Chronic intrauterine hypoxia alters neurodevelopment in fetal sheep. J Thorac Cardiovasc Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton PD, Korotcova L, Lewis BK, Bhuvanendran S, Ramachandra SD, Zurakowski D, Zhang J, Mori S, Frank JA, Jonas RA, Gallo V and Ishibashi N. Abnormal neurogenesis and cortical growth in congenital heart disease. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, Finucane K, Brizard C, Dance B and Shekerdemian LS. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–9. [DOI] [PubMed] [Google Scholar]
- 36.Dimitropoulos A, McQuillen PS, Sethi V, Moosa A, Chau V, Xu D, Brant R, Azakie A, Campbell A, Barkovich AJ, Poskitt KJ and Miller SP. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claessens NHP, Algra SO, Ouwehand TL, Jansen NJG, Schappin R, Haas F, Eijsermans MJC, de Vries LS, Benders M and Utrecht CHDLSG. Perioperative neonatal brain injury is associated with worse school-age neurodevelopment in children with critical congenital heart disease. Dev Med Child Neurol. 2018. [DOI] [PubMed] [Google Scholar]
- 38.Peyvandi S, Chau V, Guo T, Xu D, Glass HC, Synnes A, Poskitt K, Barkovich AJ, Miller SP and McQuillen PS. Neonatal Brain Injury and Timing of Neurodevelopmental Assessment in Patients With Congenital Heart Disease. J Am Coll Cardiol. 2018;71:1986–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claessens NHP, Khalili N, Isgum I, Ter Heide H, Steenhuis TJ, Turk E, Jansen NJG, de Vries LS, Breur J, de Heus R and Benders M. Brain and CSF Volumes in Fetuses and Neonates with Antenatal Diagnosis of Critical Congenital Heart Disease: A Longitudinal MRI Study. AJNR Am J Neuroradiol. 2019;40:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyvandi S, Kim H, Lau J, Barkovich AJ, Campbell A, Miller S, Xu D and McQuillen P. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. The Journal of thoracic and cardiovascular surgery. 2018;155:291–300. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heye KN, Knirsch W, Latal B, Scheer I, Wetterling K, Hahn A, Akinturk H, Schranz D, Beck I, R OGT and Reich B. Reduction of brain volumes after neonatal cardiopulmonary bypass surgery in single-ventricle congenital heart disease before Fontan completion. Pediatr Res. 2018;83:63–70. [DOI] [PubMed] [Google Scholar]
- 42.Munoz-Lopez M, Hoskote A, Chadwick MJ, Dzieciol AM, Gadian DG, Chong K, Banks T, de Haan M, Baldeweg T, Mishkin M and Vargha-Khadem F. Hippocampal damage and memory impairment in congenital cyanotic heart disease. Hippocampus. 2017;27:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson CG, Asaro LA, Wypij D, Robertson RL Jr., Newburger JW and Rivkin MJ. Altered Gray Matter in Adolescents with d-Transposition of the Great Arteries. J Pediatr. 2016;169:36–43 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW and Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36; discussion 536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ and Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. [DOI] [PubMed] [Google Scholar]
- 46.Watson CG, Stopp C, Newburger JW and Rivkin MJ. Graph theory analysis of cortical thickness networks in adolescents with d-transposition of the great arteries. Brain Behav. 2018;8:e00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Asis-Cruz J, Donofrio MT, Vezina G and Limperopoulos C. Aberrant brain functional connectivity in newborns with congenital heart disease before cardiac surgery. Neuroimage Clin. 2018;17:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Rhein M, Buchmann A, Hagmann C, Dave H, Bernet V, Scheer I, Knirsch W, Latal B, Heart and Brain Research G. Severe Congenital Heart Defects Are Associated with Global Reduction of Neonatal Brain Volumes. The Journal of pediatrics. 2015;167:1259–63 e1. [DOI] [PubMed] [Google Scholar]
- 49.Ortinau C, Alexopoulos D, Dierker D, Van Essen D, Beca J and Inder T. Cortical folding is altered before surgery in infants with congenital heart disease. The Journal of pediatrics. 2013;163:1507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly CJ, Christiaens D, Batalle D, Makropoulos A, Cordero-Grande L, Steinweg JK, O'Muircheartaigh J, Khan H, Lee G, Victor S, Alexander DC, Zhang H, Simpson J, Hajnal JV, Edwards AD, Rutherford MA and Counsell SJ. Abnormal Microstructural Development of the Cerebral Cortex in Neonates With Congenital Heart Disease Is Associated With Impaired Cerebral Oxygen Delivery. J Am Heart Assoc. 2019;8:e009893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonthrone AF, Dimitrova R, Chew A, Kelly CJ, Cordero-Grande L, Carney O, Egloff A, Hughes E, Vecchiato K, Simpson J, Hajnal JV, Pushparajah K, Victor S, Nosarti C, Rutherford MA, Edwards AD, O'Muircheartaigh J and Counsell SJ. Individualized brain development and cognitive outcome in infants with congenital heart disease. Brain Commun. 2021;3:fcab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, Nord AS, Clancy RR, Nicolson SC and Spray TL. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133:1344–53, 1353 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butler SC, Sadhwani A, Stopp C, Singer J, Wypij D, Dunbar-Masterson C, Ware J and Newburger JW. Neurodevelopmental assessment of infants with congenital heart disease in the early postoperative period. Congenit Heart Dis. 2019;14:236–245. [DOI] [PubMed] [Google Scholar]
- 54.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW and Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–67. [DOI] [PubMed] [Google Scholar]
- 55.Bean Jaworski JL, White MT, DeMaso DR, Newburger JW, Bellinger DC and Cassidy AR. Visuospatial processing in adolescents with critical congenital heart disease: Organization, integration, and implications for academic achievement. Child Neuropsychol. 2018;24:451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellinger DC, Watson CG, Rivkin MJ, Robertson RL, Roberts AE, Stopp C, Dunbar-Masterson C, Bernson D, DeMaso DR, Wypij D and Newburger JW. Neuropsychological Status and Structural Brain Imaging in Adolescents With Single Ventricle Who Underwent the Fontan Procedure. J Am Heart Assoc. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerstle M, Beebe DW, Drotar D, Cassedy A and Marino BS. Executive Functioning and School Performance among Pediatric Survivors of Complex Congenital Heart Disease. J Pediatr. 2016;173:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brossard-Racine M, du Plessis A, Vezina G, Robertson R, Donofrio M, Tworetzky W and Limperopoulos C. Brain Injury in Neonates with Complex Congenital Heart Disease: What Is the Predictive Value of MRI in the Fetal Period? AJNR Am J Neuroradiol. 2016;37:1338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rios DR, Welty SE, Gunn JK, Beca J, Minard CG, Goldsworthy M, Coleman L, Hunter JV, Andropoulos DB and Shekerdemian LS. Usefulness of routine head ultrasound scans before surgery for congenital heart disease. Pediatrics. 2013;131:e1765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortinau CM, Anadkat JS, Smyser CD and Eghtesady P. Intraventricular Hemorrhage in Moderate to Severe Congenital Heart Disease. Pediatr Crit Care Med. 2018;19:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Block AJ, McQuillen PS, Chau V, Glass H, Poskitt KJ, Barkovich AJ, Esch M, Soulikias W, Azakie A, Campbell A and Miller SP. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2010;140:550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pierpont ME, Brueckner M, Chung WK, Garg V, Lacro RV, McGuire AL, Mital S, Priest JR, Pu WT, Roberts A, Ware SM, Gelb BD, Russell MW, American Heart Association Council on Cardiovascular Disease in the Y, Council on C, Stroke N, Council on G and Precision M. Genetic Basis for Congenital Heart Disease: Revisited: A Scientific Statement From the American Heart Association. Circulation. 2018;138:e653–e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alsaied T, Marino BS, Esbensen AJ, Anixt JS, Epstein JN and Cnota JF. Does Congenital Heart Disease Affect Neurodevelopmental Outcomes in Children with Down Syndrome? Congenit Heart Dis. 2016;11:26–33. [DOI] [PubMed] [Google Scholar]
- 64.Brosig CL, Bear L, Allen S, Hoffmann RG, Pan A, Frommelt M and Mussatto KA. Preschool Neurodevelopmental Outcomes in Children with Congenital Heart Disease. J Pediatr. 2017;183:80–86 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naef N, Liamlahi R, Beck I, Bernet V, Dave H, Knirsch W and Latal B. Neurodevelopmental Profiles of Children with Congenital Heart Disease at School Age. J Pediatr. 2017;188:75–81. [DOI] [PubMed] [Google Scholar]
- 66.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE and Mital S. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, Beca J, Donofrio MT, Duncan K, Ghanayem NS, Goldberg CS, Hovels-Gurich H, Ichida F, Jacobs JP, Justo R, Latal B, Li JS, Mahle WT, McQuillen PS, Menon SC, Pemberton VL, Pike NA, Pizarro C, Shekerdemian LS, Synnes A, Williams I, Bellinger DC, Newburger JW and International Cardiac Collaborative on Neurodevelopment I. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bellinger DC, Rivkin MJ, DeMaso D, Robertson RL, Stopp C, Dunbar-Masterson C, Wypij D and Newburger JW. Adolescents with tetralogy of Fallot: neuropsychological assessment and structural brain imaging. Cardiology in the young. 2015;25:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neal AE, Stopp C, Wypij D, Bellinger DC, Dunbar-Masterson C, DeMaso DR and Newburger JW. Predictors of health-related quality of life in adolescents with tetralogy of Fallot. The Journal of pediatrics. 2015;166:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Nisselrooij AEL, Jansen FAR, van Geloven N, Linskens IH, Pajkrt E, Clur SA, Rammeloo LA, Rozendaal L, van Lith JMM, Blom NA and Haak MC. Impact of extracardiac pathology on head growth in fetuses with congenital heart defect. Ultrasound Obstet Gynecol. 2020;55:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaynor JW, Kim DS, Arrington CB, Atz AM, Bellinger DC, Burt AA, Ghanayem NS, Jacobs JP, Lee TM, Lewis AB, Mahle WT, Marino BS, Miller SG, Newburger JW, Pizarro C, Ravishankar C, Santani AB, Wilder NS, Jarvik GP, Mital S and Russell MW. Validation of association of the apolipoprotein E epsilon2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants. J Thorac Cardiovasc Surg. 2014;148:2560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaynor JW, Nord AS, Wernovsky G, Bernbaum J, Solot CB, Burnham N, Zackai E, Heagerty PJ, Clancy RR, Nicolson SC, Jarvik GP and Gerdes M. Apolipoprotein E genotype modifies the risk of behavior problems after infant cardiac surgery. Pediatrics. 2009;124:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blue GM, Ip E, Walker K, Kirk EP, Loughran-Fowlds A, Sholler GF, Dunwoodie SL, Harvey RP, Giannoulatou E, Badawi N and Winlaw DS. Genetic burden and associations with adverse neurodevelopment in neonates with congenital heart disease. Am Heart J. 2018;201:33–39. [DOI] [PubMed] [Google Scholar]
- 74.Carey AS, Liang L, Edwards J, Brandt T, Mei H, Sharp AJ, Hsu DT, Newburger JW, Ohye RG, Chung WK, Russell MW, Rosenfeld JA, Shaffer LG, Parides MK, Edelmann L and Gelb BD. Effect of copy number variants on outcomes for infants with single ventricle heart defects. Circ Cardiovasc Genet. 2013;6:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Homsy J, Zaidi S, Shen Y, Ware JS, Samocha KE, Karczewski KJ, DePalma SR, McKean D, Wakimoto H, Gorham J, Jin SC, Deanfield J, Giardini A, Porter GA Jr., Kim R, Bilguvar K, Lopez-Giraldez F, Tikhonova I, Mane S, Romano-Adesman A, Qi H, Vardarajan B, Ma L, Daly M, Roberts AE, Russell MW, Mital S, Newburger JW, Gaynor JW, Breitbart RE, Iossifov I, Ronemus M, Sanders SJ, Kaltman JR, Seidman JG, Brueckner M, Gelb BD, Goldmuntz E, Lifton RP, Seidman CE and Chung WK. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015;350:1262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morton SU, Maleyeff L, Wypij D, Yun HJ, Newburger JW, Bellinger DC, Roberts AE, Rivkin MJ, Seidman JG, Seidman CE, Grant PE and Im K. Abnormal Left-Hemispheric Sulcal Patterns Correlate with Neurodevelopmental Outcomes in Subjects with Single Ventricular Congenital Heart Disease. Cereb Cortex. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peyvandi S, De Santiago V, Chakkarapani E, Chau V, Campbell A, Poskitt KJ, Xu D, Barkovich AJ, Miller S and McQuillen P. Association of Prenatal Diagnosis of Critical Congenital Heart Disease With Postnatal Brain Development and the Risk of Brain Injury. JAMA Pediatr. 2016;170:e154450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calderon J, Angeard N, Moutier S, Plumet MH, Jambaque I and Bonnet D. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. J Pediatr. 2012;161:94–8 e1. [DOI] [PubMed] [Google Scholar]
- 79.Goff DA, Luan X, Gerdes M, Bernbaum J, D'Agostino JA, Rychik J, Wernovsky G, Licht DJ, Nicolson SC, Clancy RR, Spray TL and Gaynor JW. Younger gestational age is associated with worse neurodevelopmental outcomes after cardiac surgery in infancy. J Thorac Cardiovasc Surg. 2012;143:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Costello JM, Polito A, Brown DW, McElrath TF, Graham DA, Thiagarajan RR, Bacha EA, Allan CK, Cohen JN and Laussen PC. Birth before 39 weeks' gestation is associated with worse outcomes in neonates with heart disease. Pediatrics. 2010;126:277–84. [DOI] [PubMed] [Google Scholar]
- 81.Guo T, Chau V, Peyvandi S, Latal B, McQuillen PS, Knirsch W, Synnes A, Feldmann M, Naef N, Chakravarty MM, De Petrillo A, Duerden EG, Barkovich AJ and Miller SP. White matter injury in term neonates with congenital heart diseases: Topology & comparison with preterm newborns. Neuroimage. 2019;185:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paquette LB, Votava-Smith JK, Ceschin R, Nagasunder AC, Jackson HA, Bluml S, Wisnowski JL and Panigrahy A. Abnormal development of thalamic microstructure in premature neonates with congenital heart disease. Pediatr Cardiol. 2015;36:960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gunn JK, Beca J, Hunt RW, Goldsworthy M, Brizard CP, Finucane K, Donath S and Shekerdemian LS. Perioperative risk factors for impaired neurodevelopment after cardiac surgery in early infancy. Arch Dis Child. 2016;101:1010–1016. [DOI] [PubMed] [Google Scholar]
- 84.Calderon J, Stopp C, Wypij D, DeMaso DR, Rivkin M, Newburger JW and Bellinger DC. Early-Term Birth in Single-Ventricle Congenital Heart Disease After the Fontan Procedure: Neurodevelopmental and Psychiatric Outcomes. J Pediatr. 2016;179:96–103. [DOI] [PubMed] [Google Scholar]
- 85.Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, Lacey S, Lee W, Michelfelder EC Sr., Rempel GR, Silverman NH, Spray TL, Strasburger JF, Tworetzky W, Rychik J, American Heart Association Adults With Congenital Heart Disease Joint Committee of the Council on Cardiovascular Disease in the Y, Council on Clinical Cardiology CoCS, Anesthesia, Council on C and Stroke N. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–242. [DOI] [PubMed] [Google Scholar]
- 86.Petit CJ, Rome JJ, Wernovsky G, Mason SE, Shera DM, Nicolson SC, Montenegro LM, Tabbutt S, Zimmerman RA and Licht DJ. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman MF, Saunders AM, Nicolson SC, Spray TL and Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. [DOI] [PubMed] [Google Scholar]
- 88.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, Azakie A, Karl T and Miller SP. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–41. [DOI] [PubMed] [Google Scholar]
- 89.Lynch JM, Buckley EM, Schwab PJ, McCarthy AL, Winters ME, Busch DR, Xiao R, Goff DA, Nicolson SC, Montenegro LM, Fuller S, Gaynor JW, Spray TL, Yodh AG, Naim MY and Licht DJ. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2014;148:2181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hovels-Gurich HH, Seghaye MC, Schnitker R, Wiesner M, Huber W, Minkenberg R, Kotlarek F, Messmer BJ and Von Bernuth G. Long-term neurodevelopmental outcomes in school-aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg. 2002;124:448–58. [DOI] [PubMed] [Google Scholar]
- 91.Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G, Lin M and Bellinger DC. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–403. [DOI] [PubMed] [Google Scholar]
- 92.Hirsch JC, Jacobs ML, Andropoulos D, Austin EH, Jacobs JP, Licht DJ, Pigula F, Tweddell JS and Gaynor JW. Protecting the infant brain during cardiac surgery: a systematic review. Ann Thorac Surg. 2012;94:1365–73; discussion 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kirklin J B-BB. Cardiopulmonary Bypass. New York: Churchill Livingstone; 1993. [Google Scholar]
- 94.Stephen CR DS, Hall KD, Knox PR, North WC. Body temperature regulation during anesthesia in infants and children: Constant monitoring of body temperature helps to prevent complications associated with hypothermia and hyperthermia. JAMA. 1960;174:1579–1585. [Google Scholar]
- 95.Lim JM, Porayette P, Marini D, Chau V, Au-Young SH, Saini A, Ly LG, Blaser S, Shroff M, Branson HM, Sananes R, Hickey EJ, Gaynor JW, Van Arsdell G, Miller SP and Seed M. Associations Between Age at Arterial Switch Operation, Brain Growth, and Development in Infants With Transposition of the Great Arteries. Circulation. 2019;139:2728–2738. [DOI] [PubMed] [Google Scholar]
- 96.Namachivayam SP, d'Udekem Y, Millar J, Cheung MM and Butt W. Survival status and functional outcome of children who required prolonged intensive care after cardiac surgery. J Thorac Cardiovasc Surg. 2016;152:1104–1112 e3. [DOI] [PubMed] [Google Scholar]
- 97.International Cardiac Collaborative on Neurodevelopment I. Impact of Operative and Postoperative Factors on Neurodevelopmental Outcomes After Cardiac Operations. Ann Thorac Surg. 2016;102:843–849. [DOI] [PubMed] [Google Scholar]
- 98.Ishibashi N, Scafidi J, Murata A, Korotcova L, Zurakowski D, Gallo V and Jonas RA. White matter protection in congenital heart surgery. Circulation. 2012;125:859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stinnett GR, Lin S, Korotcov AV, Korotcova L, Morton PD, Ramachandra SD, Pham A, Kumar S, Agematsu K, Zurakowski D, Wang PC, Jonas RA and Ishibashi N. Microstructural Alterations and Oligodendrocyte Dysmaturation in White Matter After Cardiopulmonary Bypass in a Juvenile Porcine Model. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agematsu K, Korotcova L, Scafidi J, Gallo V, Jonas RA and Ishibashi N. Effects of preoperative hypoxia on white matter injury associated with cardiopulmonary bypass in a rodent hypoxic and brain slice model. Pediatr Res. 2014;75:618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guerra GG, Robertson CM, Alton GY, Joffe AR, Cave DA, Dinu IA, Creighton DE, Ross DB, Rebeyka IM and Western Canadian Complex Pediatric Therapies Follow-up G. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Paediatric anaesthesia. 2011;21:932–41. [DOI] [PubMed] [Google Scholar]
- 102.Rappaport LA, Wypij D, Bellinger DC, Helmers SL, Holmes GL, Barnes PD, Wernovsky G, Kuban KC, Jonas RA and Newburger JW. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998;97:773–9. [DOI] [PubMed] [Google Scholar]
- 103.Gaynor JW, Jarvik GP, Gerdes M, Kim DS, Rajagopalan R, Bernbaum J, Wernovsky G, Nicolson SC, Spray TL and Clancy RR. Postoperative electroencephalographic seizures are associated with deficits in executive function and social behaviors at 4 years of age following cardiac surgery in infancy. J Thorac Cardiovasc Surg. 2013;146:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tabbutt S, Nord AS, Jarvik GP, Bernbaum J, Wernovsky G, Gerdes M, Zackai E, Clancy RR, Nicolson SC, Spray TL and Gaynor JW. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics. 2008;121:476–83. [DOI] [PubMed] [Google Scholar]
- 105.Newburger JW, Wypij D, Bellinger DC, du Plessis AJ, Kuban KC, Rappaport LA, Almirall D, Wessel DL, Jonas RA and Wernovsky G. Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr. 2003;143:67–73. [DOI] [PubMed] [Google Scholar]
- 106.Rhodes JF, Blaufox AD, Seiden HS, Asnes JD, Gross RP, Rhodes JP, Griepp RB and Rossi AF. Cardiac arrest in infants after congenital heart surgery. Circulation. 1999;100:II194–9. [DOI] [PubMed] [Google Scholar]
- 107.Kurachek SC, Newth CJ, Quasney MW, Rice T, Sachdeva RC, Patel NR, Takano J, Easterling L, Scanlon M, Musa N, Brilli RJ, Wells D, Park GS, Penfil S, Bysani KG, Nares MA, Lowrie L, Billow M, Chiochetti E and Lindgren B. Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med. 2003;31:2657–64. [DOI] [PubMed] [Google Scholar]
- 108.Barker GM, O'Brien SM, Welke KF, Jacobs ML, Jacobs JP, Benjamin DK Jr., Peterson ED, Jaggers J and Li JS. Major infection after pediatric cardiac surgery: a risk estimation model. Ann Thorac Surg. 2010;89:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wheeler DS, Dent CL, Manning PB and Nelson DP. Factors prolonging length of stay in the cardiac intensive care unit following the arterial switch operation. Cardiol Young. 2008;18:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diaz LK, Gaynor JW, Koh SJ, Ittenbach RF, Gerdes M, Bernbaum JC, Zackai EH, Clancy RR, Rehman MA, Pennington JW, Burnham N, Spray TL and Nicolson SC. Increasing cumulative exposure to volatile anesthetic agents is associated with poorer neurodevelopmental outcomes in children with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2016;152:482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Austin EH 3rd, Edmonds HL Jr., Auden SM, Seremet V, Niznik G, Sehic A, Sowell MK, Cheppo CD and Corlett KM. Benefit of neurophysiologic monitoring for pediatric cardiac surgery. J Thorac Cardiovasc Surg. 1997;114:707–15, 717; discussion 715-6. [DOI] [PubMed] [Google Scholar]
- 112.Spaeder MC, Klugman D, Skurow-Todd K, Glass P, Jonas RA and Donofrio MT. Perioperative Near-Infrared Spectroscopy Monitoring in Neonates With Congenital Heart Disease: Relationship of Cerebral Tissue Oxygenation Index Variability With Neurodevelopmental Outcome. Pediatr Crit Care Med. 2017;18:213–218. [DOI] [PubMed] [Google Scholar]
- 113.Aly SA, Zurakowski D, Glass P, Skurow-Todd K, Jonas RA and Donofrio MT. Cerebral tissue oxygenation index and lactate at 24 hours postoperative predict survival and neurodevelopmental outcome after neonatal cardiac surgery. Congenit Heart Dis. 2017;12:188–195. [DOI] [PubMed] [Google Scholar]
- 114.Gunn JK, Beca J, Hunt RW, Olischar M and Shekerdemian LS. Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med. 2012;38:1539–47. [DOI] [PubMed] [Google Scholar]
- 115.Gunn JK, Beca J, Penny DJ, Horton SB, d'Udekem YA, Brizard CP, Finucane K, Olischar M, Hunt RW and Shekerdemian LS. Amplitude-integrated electroencephalography and brain injury in infants undergoing Norwood-type operations. Ann Thorac Surg. 2012;93:170–6. [DOI] [PubMed] [Google Scholar]
- 116.Claessens NHP, Noorlag L, Weeke LC, Toet MC, Breur J, Algra SO, Schouten ANJ, Haas F, Groenendaal F, Benders M, Jansen NJG and de Vries LS. Amplitude-Integrated Electroencephalography for Early Recognition of Brain Injury in Neonates with Critical Congenital Heart Disease. J Pediatr. 2018;202:199–205 e1. [DOI] [PubMed] [Google Scholar]
- 117.Mebius MJ, Oostdijk NJE, Kuik SJ, Bos AF, Berger RMF, Bilardo CM, Kooi EMW and Ter Horst HJ. Amplitude-integrated electroencephalography during the first 72 h after birth in neonates diagnosed prenatally with congenital heart disease. Pediatr Res. 2018;83:798–803. [DOI] [PubMed] [Google Scholar]
- 118.Naim MY, Gaynor JW, Chen J, Nicolson SC, Fuller S, Spray TL, Dlugos DJ, Clancy RR, Costa LV, Licht DJ, Xiao R, Meldrum H and Abend NS. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–78; discussion 178-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mulkey SB, Yap VL, Bai S, Ramakrishnaiah RH, Glasier CM, Bornemeier RA, Schmitz ML and Bhutta AT. Amplitude-integrated EEG in newborns with critical congenital heart disease predicts preoperative brain magnetic resonance imaging findings. Pediatr Neurol. 2015;52:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Birca A, Vakorin VA, Porayette P, Madathil S, Chau V, Seed M, Doesburg SM, Blaser S, Nita DA, Sharma R, Duerden EG, Hickey EJ, Miller SP and Hahn CD. Interplay of brain structure and function in neonatal congenital heart disease. Ann Clin Transl Neurol. 2016;3:708–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gaynor JW, Nicolson SC, Jarvik GP, Wernovsky G, Montenegro LM, Burnham NB, Hartman DM, Louie A, Spray TL and Clancy RR. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J Thorac Cardiovasc Surg. 2005;130:1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, Nguyen S, Wusthoff CJ and Clancy RR. The American Clinical Neurophysiology Society's Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011;28:611–7. [DOI] [PubMed] [Google Scholar]
- 123.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C and Tchervenkov C. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. The Journal of pediatrics. 2000;137:638–645. [DOI] [PubMed] [Google Scholar]
- 124.Kleberg A, Westrup B and Stjernqvist K. Developmental outcome, child behaviour and mother–child interaction at 3 years of age following Newborn Individualized Developmental Care and Intervention Program (NIDCAP) intervention. Early human development. 2000;60:123–135. [DOI] [PubMed] [Google Scholar]
- 125.Byers JF. Components of developmental care and the evidence for their use in the NICU. MCN: The American Journal of Maternal/Child Nursing. 2003;28:174–180. [DOI] [PubMed] [Google Scholar]
- 126.Lester BM, Salisbury AL, Hawes K, Dansereau LM, Bigsby R, Laptook A, Taub M, Lagasse LL, Vohr BR and Padbury JF. 18-month follow-up of infants cared for in a single-family room neonatal intensive care unit. The Journal of pediatrics. 2016;177:84–89. [DOI] [PubMed] [Google Scholar]
- 127.Lisanti AJ, Vittner D, Medoff-Cooper B, Fogel J, Wernovsky G and Butler S. Individualized Family-Centered Developmental Care: An Essential Model to Address the Unique Needs of Infants With Congenital Heart Disease. J Cardiovasc Nurs. 2019;34:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Altimier L and Phillips RM. The neonatal integrative developmental care model: seven neuroprotective core measures for family-centered developmental care. Newborn and Infant Nursing Reviews. 2013;13:9–22. [Google Scholar]
- 129.Andropoulos DB, Easley RB, Brady K, McKenzie ED, Heinle JS, Dickerson HA, Shekerdemian L, Meador M, Eisenman C and Hunter JV. Changing expectations for neurological outcomes after the neonatal arterial switch operation. The Annals of thoracic surgery. 2012;94:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grasty MA, Ittenbach RF, Knightly C, Solot CB, Gerdes M, Bernbaum JC, Wernovsky G, Spray TL, Nicolson SC and Clancy RR. Hearing loss after cardiac surgery in infancy: An unintended consequence of life-saving care. The Journal of pediatrics. 2018;192:144–151. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Madlinger-Lewis L, Reynolds L, Zarem C, Crapnell T, Inder T and Pineda R. The effects of alternative positioning on preterm infants in the neonatal intensive care unit: a randomized clinical trial. Research in developmental disabilities. 2014;35:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sweeney JK and Gutierrez T. Musculoskeletal implications of preterm infant positioning in the NICU. The Journal of perinatal & neonatal nursing. 2002;16:58–70. [DOI] [PubMed] [Google Scholar]
- 133.Harrison TM, Brown R, Duffey T, Frey C, Bailey J, Nist MD, Renner L and Fitch J. Effects of Massage on Post-Operative Pain in Infants with Complex Congenital Heart Disease. Nurs Res. 2020. [DOI] [PubMed] [Google Scholar]
- 134.Gazzolo D, Masetti P and Meli M. Kangaroo care improves post-extubation cardiorespiratory parameters in infants after open heart surgery. Acta Paediatr. 2000;89:728–9. [DOI] [PubMed] [Google Scholar]
- 135.Hsieh A, Tabbutt S, Xu D, Barkovich AJ, Miller S, McQuillen P and Peyvandi S. Impact of Perioperative Brain Injury and Development on Feeding Modality in Infants With Single Ventricle Heart Disease. J Am Heart Assoc. 2019;8:e012291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lisanti AJ, Buoni A, Steigerwalt M, Daly M, McNelis S and Spatz DL. Kangaroo Care for Hospitalized Infants with Congenital Heart Disease. MCN Am J Matern Child Nurs. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Broge MJ, Steurer LM and Ercole PM. The Feasibility of Kangaroo Care and the Effect on Maternal Attachment for Neonates in a Pediatric Cardiac Intensive Care Unit. Adv Neonatal Care. 2021;21:E52–E59. [DOI] [PubMed] [Google Scholar]
- 138.McFarland DH and Tremblay P. Clinical implications of cross-system interactions. Seminars in speech and language. 2006;27:300–9. [DOI] [PubMed] [Google Scholar]
- 139.Davis D, Davis S, Cotman K, Worley S, Londrico D, Kenny D and Harrison A. Feeding difficulties and growth delay in children with hypoplastic left heart syndrome versus d-transposition of the great arteries. Pediatric cardiology. 2008;29:328–333. [DOI] [PubMed] [Google Scholar]
- 140.Medoff-Cooper B, Naim M, Torowicz D and Mott A. Feeding, growth, and nutrition in children with congenitally malformed hearts. Cardiology in the Young. 2010;20:149–153. [DOI] [PubMed] [Google Scholar]
- 141.Lambert JM and Watters NE. Breastfeeding the infant/child with a cardiac defect: an informal survey. Journal of Human Lactation. 1998;14:151–155. [DOI] [PubMed] [Google Scholar]
- 142.Steltzer MM, Sussman-Karten K, Kuzdeba HB, Mott S and Connor JA. Creating opportunities for optimal nutritional experiences for infants with complex congenital heart disease. Journal of Pediatric Health Care. 2016;30:599–605. [DOI] [PubMed] [Google Scholar]
- 143.Woolf-King SE, Arnold E, Weiss S and Teitel D. “There's no acknowledgement of what this does to people”: A qualitative exploration of mental health among parents of children with critical congenital heart defects. Journal of clinical nursing. 2018;27:2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ernst MM, Marino BS, Cassedy A, Piazza-Waggoner C, Franklin RC, Brown K and Wray J. Biopsychosocial Predictors of Quality of Life Outcomes in Pediatric Congenital Heart Disease. Pediatr Cardiol. 2018;39:79–88. [DOI] [PubMed] [Google Scholar]
- 145.Wray J, Cassedy A, Ernst MM, Franklin RC, Brown K and Marino BS. Psychosocial functioning of parents of children with heart disease-describing the landscape. Eur J Pediatr. 2018;177:1811–1821. [DOI] [PubMed] [Google Scholar]
- 146.Woolf-King SE, Anger A, Arnold EA, Weiss SJ and Teitel D. Mental Health Among Parents of Children With Critical Congenital Heart Defects: A Systematic Review. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu Y, Lu YC, Jacobs M, Pradhan S, Kapse K, Zhao L, Niforatos-Andescavage N, Vezina G, du Plessis AJ and Limperopoulos C. Association of Prenatal Maternal Psychological Distress With Fetal Brain Growth, Metabolism, and Cortical Maturation. JAMA Netw Open. 2020;3:e1919940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu Y, Kapse K, Jacobs M, Niforatos-Andescavage N, Donofrio MT, Krishnan A, Vezina G, Wessel D, du Plessis A and Limperopoulos C. Association of Maternal Psychological Distress With In Utero Brain Development in Fetuses With Congenital Heart Disease. JAMA Pediatr. 2020:e195316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lisanti AJ, Allen LR, Kelly L and Medoff-Cooper B. Maternal Stress and Anxiety in the Pediatric Cardiac Intensive Care Unit. Am J Crit Care. 2017;26:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sood E, Karpyn A, Demianczyk AC, Ryan J, Delaplane EA, Neely T, Frazier AH and Kazak AE. Mothers and Fathers Experience Stress of Congenital Heart Disease Differently: Recommendations for Pediatric Critical Care. Pediatr Crit Care Med. 2018;19:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kasparian NA, Kan JM, Sood E, Wray J, Pincus HA and Newburger JW. Mental health care for parents of babies with congenital heart disease during intensive care unit admission: Systematic review and statement of best practice. Early Hum Dev. 2019;139:104837. [DOI] [PubMed] [Google Scholar]
- 152.Gaskin KL, Barron DJ and Daniels A. Parents' preparedness for their infants' discharge following first-stage cardiac surgery: development of a parental early warning tool. Cardiol Young. 2016;26:1414–24. [DOI] [PubMed] [Google Scholar]
- 153.Gaskin KL. Patterns of Transition Experience for Parents Going Home from Hospital with their Infant after First Stage Surgery for Complex Congenital Heart Disease. J Pediatr Nurs. 2017. [DOI] [PubMed] [Google Scholar]
- 154.Vigna K, Balakas K, Steurer LM and Ercole PM. Improving the Discharge to Home Experience for Pediatric Heart Center Patients and Families. J Pediatr Nurs. 2018. [DOI] [PubMed] [Google Scholar]
- 155.Wernovsky G and Licht DJ. Neurodevelopmental Outcomes in Children With Congenital Heart Disease-What Can We Impact? Pediatr Crit Care Med. 2016;17:S232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brosig CL, Bear L, Allen S, Simpson P, Zhang L, Frommelt M and Mussatto KA. Neurodevelopmental outcomes at 2 and 4 years in children with congenital heart disease. Congenit Heart Dis. 2018;13:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Snookes SH, Gunn JK, Eldridge BJ, Donath SM, Hunt RW, Galea MP and Shekerdemian L. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics. 2010;125:e818–27. [DOI] [PubMed] [Google Scholar]
- 158.Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y and Brosig C. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133:e570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Teixeira J, Caflisch J, Chaouch A, Beck I, Feldmann M, Polentarutti S, Balmer C and Latal B. Motor and visuomotor function in 10-year-old children with congenital heart disease: association with behaviour. Cardiology in the young. 2021:1–6. [DOI] [PubMed] [Google Scholar]
- 160.Uzark K, Smith C, Donohue J, Yu S and Romano JC. Infant Motor Skills After a Cardiac Operation: The Need for Developmental Monitoring and Care. Ann Thorac Surg. 2017;104:681–686. [DOI] [PubMed] [Google Scholar]
- 161.Bucholz EM, Sleeper LA, Sananes R, Brosig CL, Goldberg CS, Pasquali SK and Newburger JW. Trajectories in Neurodevelopmental, Health-Related Quality of Life, and Functional Status Outcomes by Socioeconomic Status and Maternal Education in Children with Single Ventricle Heart Disease. The Journal of pediatrics. 2021;229:289–293 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Naef N, Wehrle F, Rousson V and Latal B. Cohort and Individual Neurodevelopmental Stability between 1 and 6 Years of Age in Children with Congenital Heart Disease. J Pediatr. 2019;215:83–89 e2. [DOI] [PubMed] [Google Scholar]
- 163.Bonthrone AF, Chew A, Kelly CJ, Almedom L, Simpson J, Victor S, Edwards AD, Rutherford MA, Nosarti C and Counsell SJ. Cognitive function in toddlers with congenital heart disease: The impact of a stimulating home environment. Infancy. 2021;26:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Roberts SD, Kazazian V, Ford MK, Marini D, Miller SP, Chau V, Seed M, Ly LG, Williams TS and Sananes R. The association between parent stress, coping and mental health, and neurodevelopmental outcomes of infants with congenital heart disease. Clin Neuropsychol. 2021;35:948–972. [DOI] [PubMed] [Google Scholar]
- 165.Wernovsky G, Stiles KM, Gauvreau K, Gentles TL, duPlessis AJ, Bellinger DC, Walsh AZ, Burnett J, Jonas RA, Mayer JE Jr. and Newburger JW. Cognitive development after the Fontan operation. Circulation. 2000;102:883–9. [DOI] [PubMed] [Google Scholar]
- 166.Sanz JH, Berl MM, Armour AC, Wang J, Cheng YI and Donofrio MT. Prevalence and pattern of executive dysfunction in school age children with congenital heart disease. Congenit Heart Dis. 2017;12:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sanz JH, Wang J, Berl MM, Armour AC, Cheng YI and Donofrio MT. Executive Function and Psychosocial Quality of Life in School Age Children with Congenital Heart Disease. J Pediatr. 2018;202:63–69. [DOI] [PubMed] [Google Scholar]
- 168.Jilek E, Shields A, Zhang L, Simpson P, Bear L, Martins SA, Mussatto KA and Brosig CL. Predictors of behavioural and emotional outcomes in toddlers with congenital heart disease. Cardiology in the young. 2021:1–6. [DOI] [PubMed] [Google Scholar]
- 169.Sananes R, Goldberg CS, Newburger JW, Hu C, Trachtenberg F, Gaynor JW, Mahle WT, Miller T, Uzark K, Mussatto KA, Pizarro C, Jacobs JP, Cnota J, Atz AM, Lai WW, Burns KM, Milazzo A, Votava-Smith J, Brosig CL and investigators PHN. Six-Year Neurodevelopmental Outcomes for Children With Single-Ventricle Physiology. Pediatrics. 2021;147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mussatto KA, Hollenbeck-Pringle D, Trachtenberg F, Sood E, Sananes R, Pike NA, Lambert LM, Mahle WT, Goldberg DJ, Goldberg CS, Dunbar-Masterson C, Otto M, Marino BS, Bartle BH, Williams IA, Jacobs JP, Zyblewski SC and Pemberton VL. Utilisation of early intervention services in young children with hypoplastic left heart syndrome. Cardiol Young. 2018;28:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lipkin PH, Macias MM, Council On Children With Disabilities SOD and Behavioral P. Promoting Optimal Development: Identifying Infants and Young Children With Developmental Disorders Through Developmental Surveillance and Screening. Pediatrics. 2020;145. [DOI] [PubMed] [Google Scholar]
- 172.Council on Children With D, Section on Developmental Behavioral P, Bright Futures Steering C and Medical Home Initiatives for Children With Special Needs Project Advisory C. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. 2006;118:405–20. [DOI] [PubMed] [Google Scholar]
- 173.Ware J, Butcher JL, Latal B, Sadhwani A, Rollins CK, Brosig Soto CL, Butler SC, Eiler-Sims PB, Ullman Shade CV and Wernovsky G. Neurodevelopmental evaluation strategies for children with congenital heart disease aged birth through 5 years: recommendations from the cardiac neurodevelopmental outcome collaborative. Cardiol Young. 2020;30:1609–1622. [DOI] [PubMed] [Google Scholar]
- 174.Ilardi D, Sanz JH, Cassidy AR, Sananes R, Rollins CK, Ullman Shade C, Carroll G and Bellinger DC. Neurodevelopmental evaluation for school-age children with congenital heart disease: recommendations from the cardiac neurodevelopmental outcome collaborative. Cardiol Young. 2020;30:1623–1636. [DOI] [PubMed] [Google Scholar]
- 175.Kendall L, Sloper P, Lewin RJ and Parsons JM. The views of parents concerning the planning of services for rehabilitation of families of children with congenital cardiac disease. Cardiol Young. 2003;13:20–7. [DOI] [PubMed] [Google Scholar]
- 176.Northman L, Ross S, Morris M and Tarquini S. Supporting pediatric cancer survivors with neurocognitive late effects: a model of care. J Pediatr Oncol Nurs. 2015;32:134–42. [DOI] [PubMed] [Google Scholar]
- 177.U.S. Department of Education OoCR. Protecting Students With Disabilities. 2020. [Google Scholar]
- 178.Chambers MA, Miller DT and Ullrich NJ. School liaison program supporting children with neurofibromatosis type 1: a model of care for children with chronic disease. Genet Med. 2018;20:785–788. [DOI] [PubMed] [Google Scholar]
- 179.America LDAo. Accommodations, Techniques and Aids For Learning. 2020. [Google Scholar]
- 180.Barlow JH and Ellard DR. The psychosocial well-being of children with chronic disease, their parents and siblings: an overview of the research evidence base. Child Care Health Dev. 2006;32:19–31. [DOI] [PubMed] [Google Scholar]
- 181.McCusker CG, Armstrong MP, Mullen M, Doherty NN and Casey FA. A sibling-controlled, prospective study of outcomes at home and school in children with severe congenital heart disease. Cardiol Young. 2013;23:507–16. [DOI] [PubMed] [Google Scholar]
- 182.Bayer ND, Wang H, Yu JA, Kuo DZ, Halterman JS and Li Y. A National Mental Health Profile of Parents of Children With Medical Complexity. Pediatrics. 2021;148. [DOI] [PubMed] [Google Scholar]
- 183.Cassidy AR, White MT, DeMaso DR, Newburger JW and Bellinger DC. Executive function in children and adolescents with critical cyanotic congenital heart disease. Journal of the International Neuropsychological Society. 2015;21:34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schaefer C, von Rhein M, Knirsch W, Huber R, Natalucci G, Caflisch J, Landolt MA and Latal B. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Developmental Medicine & Child Neurology. 2013;55:1143–1149. [DOI] [PubMed] [Google Scholar]
- 185.Karsdorp PA, Everaerd W, Kindt M and Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. Journal of pediatric psychology. 2006;32:527–541. [DOI] [PubMed] [Google Scholar]
- 186.Pfitzer C, Helm PC, Rosenthal L-M, Walker C, Ferentzi H, Bauer UM, Berger F and Schmitt KR. Educational level and employment status in adults with congenital heart disease. Cardiology in the Young. 2018;28:32–38. [DOI] [PubMed] [Google Scholar]
- 187.Ilardi D SJ, Cassidy AR, Sananes R, Rollins CK, Ullman Shade C, Carroll G, Bellinger DC. Neurodevelopmental Evaluation for School-Age Children with Congenital Heart Disease: Recommendations from the Cardiac Neurodevelopmental Outcome Collaborative Cardiol Young. 2020, in press. [DOI] [PubMed] [Google Scholar]
- 188.Crossland DS, Jackson SP, Lyall R, Burn J and O'sullivan JJ. Employment and advice regarding careers for adults with congenital heart disease. Cardiology in the Young. 2005;15:391–395. [DOI] [PubMed] [Google Scholar]
- 189.Deng LX, Khan AM, Drajpuch D, Fuller S, Ludmir J, Mascio CE, Partington SL, Qadeer A, Tobin L and Kovacs AH. Prevalence and correlates of post-traumatic stress disorder in adults with congenital heart disease. The American journal of cardiology. 2016;117:853–857. [DOI] [PubMed] [Google Scholar]
- 190.Bagge CN, Henderson VW, Laursen HB, Adelborg K, Olsen M and Madsen NL. Risk of dementia in adults with congenital heart disease: population-based cohort study. Circulation. 2018;137:1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kovacs AH, Saidi AS, Kuhl EA, Sears SF, Silversides C, Harrison JL, Ong L, Colman J, Oechslin E and Nolan RP. Depression and anxiety in adult congenital heart disease: predictors and prevalence. International journal of cardiology. 2009;137:158–164. [DOI] [PubMed] [Google Scholar]
- 192.Ferguson M and Kovacs AH. An integrated adult congenital heart disease psychology service. Congenital heart disease. 2016;11:444–451. [DOI] [PubMed] [Google Scholar]
- 193.Foy JM, Green CM, Earls MF, Committee On Psychosocial Aspects Of C and Family Health MHLWG. Mental Health Competencies for Pediatric Practice. Pediatrics. 2019;144. [DOI] [PubMed] [Google Scholar]
- 194. https://www.aap.org/mentalhealth.
- 195.Moon JR, Huh J, Song J, Kang IS, Park SW, Chang SA, Yang JH and Jun TG. The Center for Epidemiologic Studies Depression Scale is an adequate screening instrument for depression and anxiety disorder in adults with congential heart disease. Health Qual Life Outcomes. 2017;15:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Siu AL, Force USPST, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Ebell M, Garcia FA, Gillman M, Herzstein J, Kemper AR, Krist AH, Kurth AE, Owens DK, Phillips WR, Phipps MG and Pignone MP. Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380–7. [DOI] [PubMed] [Google Scholar]
- 197.Reid GJ, Irvine MJ, McCrindle BW, Sananes R, Ritvo PG, Siu SC and Webb GD. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113:e197–e205. [DOI] [PubMed] [Google Scholar]
- 198.Sable C, Foster E, Uzark K, Bjornsen K, Canobbio MM, Connolly HM, Graham TP, Gurvitz MZ, Kovacs A and Meadows AK. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123:1454–1485. [DOI] [PubMed] [Google Scholar]
- 199.Jalkut MK and Allen PJ. Transition from pediatric to adult health care for adolescents with congenital heart disease: a review of the literature and clinical implications. Pediatric nursing. 2009;35. [PubMed] [Google Scholar]
- 200.Health Resources and Services Administration of the U.S. Department of Health and Human Services, GotTransition.org. COPYRIGHT © 2014-2020 GOT TRANSITION®.
- 201.Laing S and Badawi N. Best practice guidelines: Supporting parents in the NICU. Early Hum Dev. 2019;139:104911. [DOI] [PubMed] [Google Scholar]
- 202.Council on Children with D and Medical Home Implementation Project Advisory C. Patient- and family-centered care coordination: a framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133:e1451–60. [DOI] [PubMed] [Google Scholar]
- 203.American Academy of Pediatrics, National Resource Center for Patient/Family-Centered Medical Home.
- 204.Sisters by Heart. 2020. [Google Scholar]
- 205.Pediatric Congenital Heart Association. [Google Scholar]
- 206.Mended Hearts. 2020. [Google Scholar]
- 207.National Heart, Lung, and Blood Institute, Health Topics, Congenital Heart Defects. [Google Scholar]
- 208.Center for Disease Control and Prevention, Congenital Heart Defects. [Google Scholar]
- 209.American Academy of Pediatrics, Congenital Heart Public Health Consortium. 2020. [Google Scholar]
- 210.American Heart Association, Health Topics, Congenital Heart Defects. 2020. [Google Scholar]