Abstract
Background and Objectives
Ischemic stroke despite a direct oral anticoagulant (DOAC) is increasingly common and portends a high risk of subsequent ischemic stroke. The efficacy and safety of antithrombotic regimens after the condition are unclear. We aimed to compare the outcomes of patients with ischemic stroke despite DOACs with and without an alternative antithrombotic regimen and determine the risk factors of recurrent ischemic stroke while on anticoagulation.
Methods
In a population-based, propensity score–weighted, retrospective cohort study, we compared the clinical outcomes of DOAC-to-warfarin switch, DOAC-to-DOAC switch (DOACswitch), or addition of antiplatelet agents, with those of unchanged DOAC regimen (DOACsame) among patients with nonvalvular atrial fibrillation (NVAF) who developed the first ischemic stroke despite a DOAC from January 1, 2015, to December 31, 2020, in Hong Kong. The primary outcome was recurrent ischemic stroke. Secondary outcomes were intracranial hemorrhage, acute coronary syndrome, and death. We performed competing risk regression analyses to compare the clinical endpoints and determined the predictors of recurrent ischemic stroke in an unweighted multivariable logistic regression model.
Results
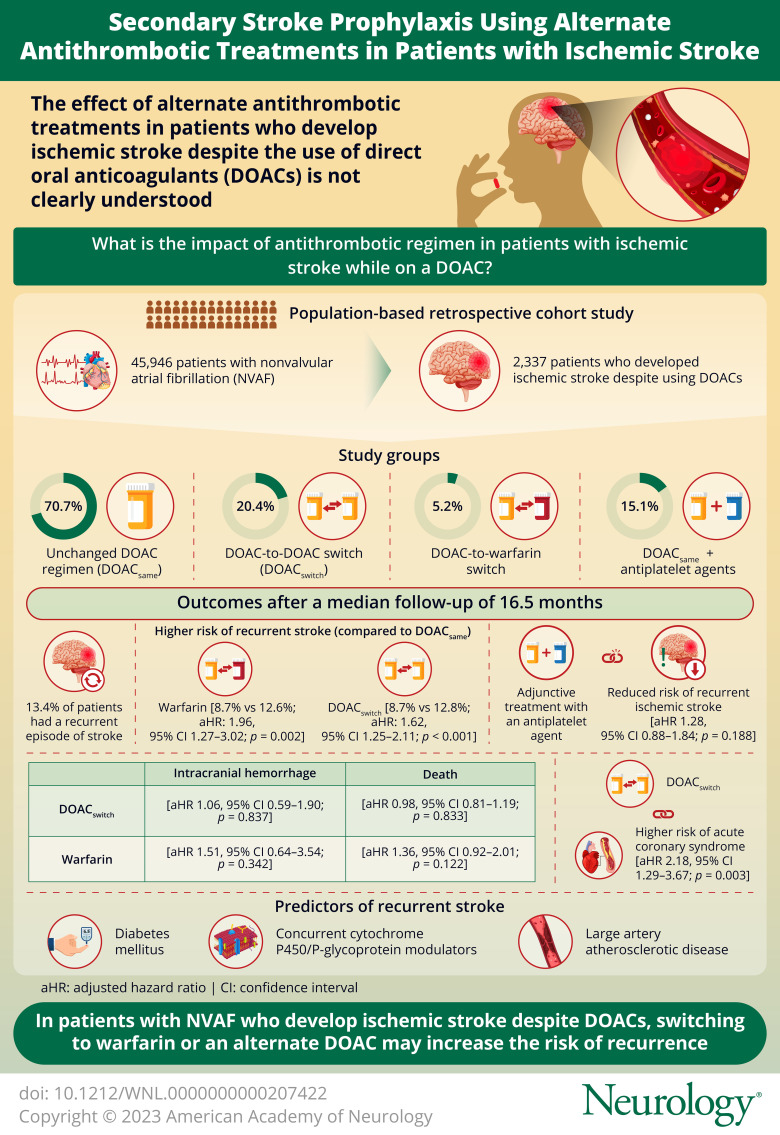
During the 6-year study period, among 45,946 patients with AF on a DOAC as stroke prophylaxis, 2,908 patients developed ischemic stroke despite a DOAC. A total of 2,337 patients with NVAF were included in the final analyses. Compared with DOACsame, warfarin (aHR 1.96, 95% CI 1.27–3.02, p = 0.002) and DOACswitch (aHR 1.62, 95% CI 1.25–2.11, p < 0.001) were associated with an increased risk of recurrent ischemic stroke. In the DOACsame group, adjunctive antiplatelet agent was not associated with a reduced risk of recurrent ischemic stroke. Diabetes mellitus, concurrent cytochrome P450/P-glycoprotein (CYP/P-gp) modulators, and large artery atherosclerotic disease (LAD) were predictors of recurrent ischemic stroke.
Discussion
In patients with NVAF with ischemic stroke despite a DOAC, the increased risk of recurrent ischemic stroke with switching to warfarin called for caution against such practice, while the increased ischemic stroke with DOAC-to-DOAC switch demands further studies. Adjunctive antiplatelet agent did not seem to reduce ischemic stroke relapse. Because diabetes mellitus, the use of CYP/P-gp modulators, and LAD were predictors of recurrent ischemic stroke, further investigations should evaluate whether strict glycemic control, DOAC level monitoring, and routine screening for carotid and intracranial atherosclerosis may reduce ischemic stroke recurrence in these patients.
Classification of Evidence
This study provides Class II evidence that in patients with NVAF experiencing an ischemic stroke while being treated with a DOAC, continuing treatment with that DOAC is more effective at preventing recurrent ischemic stroke than switching to a different DOAC or to warfarin.
Direct oral anticoagulants (DOACs) effectively prevent ischemic stroke in patients with nonvalvular atrial fibrillation (NVAF).1 Yet, ischemic stroke despite DOACs still occurred in 1%–2% of patients with AF in pivotal randomized controlled trials; and up to 30% of patients with AF who developed ischemic stroke were on oral anticoagulants at stroke onset.2-6 Because the condition often precluded intravenous thrombolytic therapy and predicted a high risk of recurrence,7 it is critical to improve secondary stroke prevention for patients with ischemic stroke despite DOACs.
In practice, there are broadly 3 alternative antithrombotic regimens after ischemic stroke despite DOACs: (1) switching between the 4 DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban); (2) switching to warfarin; and (3) addition of antiplatelet agents. These therapeutic options were highly variable across different regions. For instance, while most German stroke neurologists favored switching between DOACs,8 in an Irish study, thrombotic events during DOAC usage commonly prompted switching to warfarin treatment.9 Nonetheless, in an epidemiologic study, adjustment of oral anticoagulant therapy collectively did not reduce subsequent ischemic stroke risk.7 Because ischemic stroke despite DOACs is increasingly common due to surging DOAC prescriptions,10-12 investigating the outcomes of patients on alternative antithrombotic regimens after an episode of ischemic stroke despite DOACs may inform treatment decisions.
In this population-based analysis, we compared the clinical outcomes of patients with NVAF with ischemic stroke despite DOACs who were kept on the same DOAC regimen, with those given alternative DOAC, warfarin, or adjunctive antiplatelet agent, with a primary research question of whether switching the original DOAC to another DOAC or warfarin was associated with a reduced risk of recurrent ischemic stroke.
Methods
Study Design and Data Source
We performed a population-based retrospective cohort study and recruited all patients with ischemic stroke despite DOACs admitted to public hospitals in Hong Kong from January 1, 2015, to December 31, 2020. Clinical parameters were retrieved through the Clinical Data Analysis and Reporting System (CDARS), a territory-wide database that contains clinical information of more than 90% of the 7.5-million population in Hong Kong. The CDARS had a coding accuracy of 85%–100% according to previous studies.13 We followed the STROBE reporting guidelines.
Study Subjects
We enrolled patients with NVAF who developed ischemic stroke while on apixaban, dabigatran, edoxaban, or rivaroxaban during the 6-year study period. We recorded the baseline clinical and laboratory parameters (see Covariates section). Based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM), diagnoses of AF, ischemic stroke, intracranial hemorrhage, congestive heart failure, hypertension, diabetes mellitus (DM), peripheral vascular disease, acute coronary syndrome (ACS), hyperlipidemia, and arterial and venous thromboembolism were retrieved. We collected any documented history of small vessel disease and large intracranial or extracranial artery atherosclerotic disease (LAD). We recorded the use of antiplatelet agents (clopidogrel, aspirin, cilostazol, and ticagrelor), intensity of statins (none, low, moderate, and high14), cytochrome P450 or P-glycoprotein (CYP/P-gp) modulators (phenytoin, valproate, carbamazepine, amiodarone, dronedarone, rifampicin, cyclosporin, and verapamil), and nonsteroidal anti-inflammatory drugs (NSAIDs). CHADS2-Vasc and HAS-BLED scores after the first ischemic stroke despite DOACs were calculated.15 We excluded patients with active malignancy, mitral stenosis, left ventricular thrombus, valvular replacement, left atrial appendage occlusion, atrial septal defect, polycythemia ruba vera, essential thrombocytosis, and thrombophilia. We also excluded patients who had anticoagulation discontinued after the stroke episode or with an inappropriate DOAC dosage for body weight, renal function, and age at stroke onset. eTable 1 (links.lww.com/WNL/C839) in the Supplement listed the ICD9-CM codes used in the study.
Ischemic Stroke Despite DOACs
Ischemic stroke despite DOACs was defined as an episode of acute ischemic stroke during DOAC usage. Anticoagulation type and dosage immediately before and after the first ischemic stroke despite DOACs were retrieved. Anticoagulation therapy after the first episode of ischemic stroke despite DOACs was classified into (1) unchanged group (DOACsame); (2) DOAC-to-warfarin group; or (3) DOAC-to-another DOAC group (DOACswitch), that is, switching to another DOAC. We also retrieved antiplatelet use before and within 8 weeks of the index ischemic stroke event during DOAC usage.
Outcome Definitions
The primary outcome was recurrent ischemic stroke after the first episode of ischemic stroke despite DOACs. Secondary outcomes were intracranial hemorrhage, ACS, and all-cause death.
Covariates
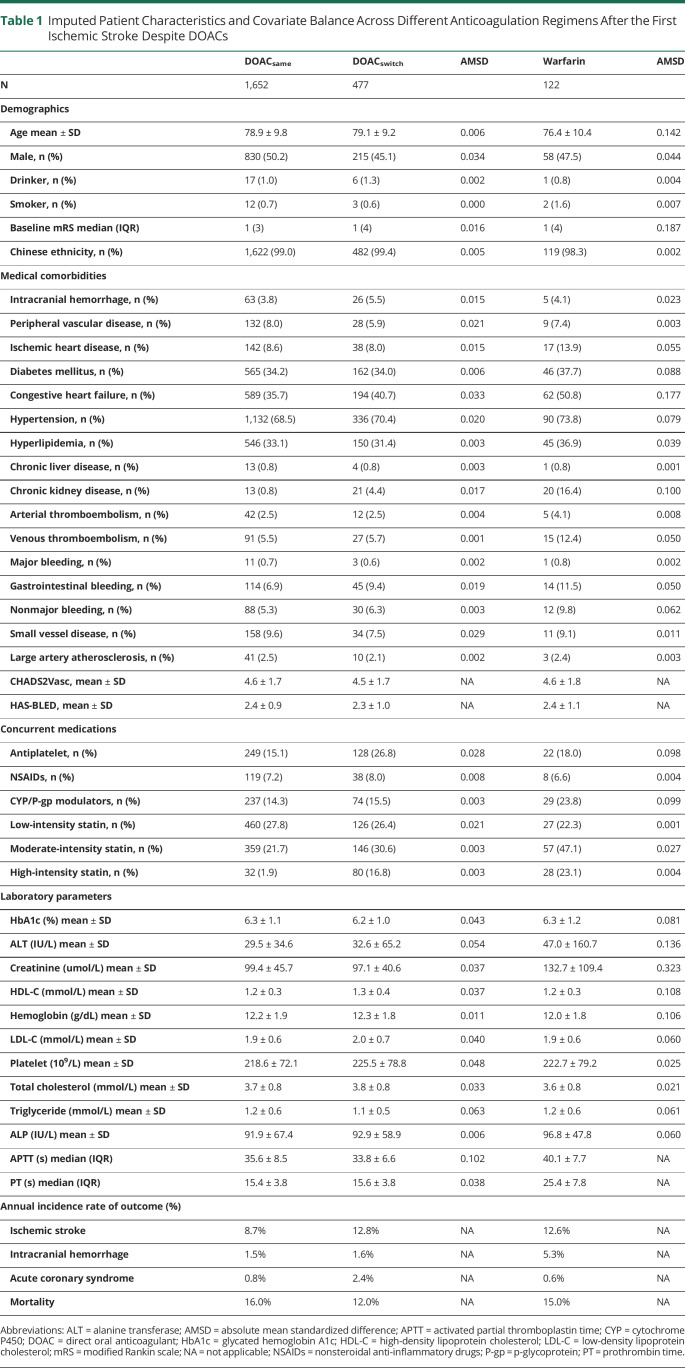
We included 5 demographic variables (age, sex, a history of smoking or drinking, and modified Rankin scale after the first ischemic stroke despite DOACs), 12 medical comorbidities (intracranial hemorrhage, congestive heart failure, hypertension, DM, ischemic heart disease, arterial and venous thromboembolism, peripheral vascular disease, chronic kidney and liver disease, small vessel disease, and LAD), 9 baseline laboratory parameters (total cholesterol, low-density and high-density lipoprotein cholesterol, triglycerides, glycated hemoglobin A1c, creatinine, alanine transaminase, hemoglobin, and platelet count), and 5 classes of medications (CYP or P-gp modulators, NSAIDs, antiplatelet agents and statins) in our propensity score (PS) weighting model, and secondary unweighted analyses.
Standard Protocol Approvals, Registrations, and Patient Consents
The institutional review board approved the study (Joint CUHK-NTEC Clinical Research Ethics Committee Reference No. 2021.349). The need for written informed consent was waived because of the de-identified data.
Statistical Analyses
To minimize the effects of confounding variables, we developed a PS weighting model for comparisons between the average treatment effect of each oral anticoagulant strategy (warfarin, DOACswitch) with the reference group (DOACsame) by inverse probability of treatment weighting. We estimated the PS of each patient by generalized boosted models (GBMs) that included the covariates mentioned earlier. The optimal iteration of GBMs was determined by 4 stopping rules previously described16; the stopping rule with the best covariate balance and effective sample size was selected. We adjusted covariates that failed to achieve an absolute standardized mean difference of <0.1 in a doubly robust model. Time to event was determined as the time lapse (in months) between the date of first ischemic stroke despite DOACs and the date of the outcome events, while that for patients without outcome occurrence was defined as the time lapse between the date of first ischemic stroke despite DOACs and the earliest date among (1) study end, (2) death, and (3) discontinuation of oral anticoagulation. The Fine-Gray regression model was used to analyze the primary and secondary endpoints with death as a competing risk, while Cox regression was used to analyze all-cause death. We used the Gray method to estimate the cumulative incidence of the primary and secondary endpoints and the Kaplan-Meier method to estimate the cumulative incidence of death. The Bonferroni method was used to correct for multiple testing. A 2-sided test with a p value <0.05 was considered statistically significant.
Secondary Analyses
We performed 3 planned secondary analyses.
Antiplatelet Therapy
To evaluate the safety and efficacy of antiplatelet therapy, we performed a PS-weighted analysis for patients with new adjunctive antiplatelet therapy within 8 weeks of the index ischemic stroke despite DOACs, vs patients with no antiplatelet therapy before and after the event. We confined the analysis to the DOACsame group.
DOAC Regimen After the Index Ischemic Stroke During DOACs
In an exploratory analysis, we compared the outcomes of patients who received 5 mg of apixaban twice daily (API10), 110 mg of dabigatran twice daily (DAB220), 150 mg of dabigatran twice daily (DAB300), 60 mg of edoxaban daily (EDO60), or 20 mg of rivaroxaban (RIV20) daily after the first ischemic stroke despite DOACs, regardless of the initial DOAC regimen in a PS-weighted analysis. We excluded patients who took 2.5 mg of apixaban twice daily, 30 mg of edoxaban daily, or 15 mg of rivaroxaban daily in this analysis owing to the higher intrinsic risk of cerebrovascular events from the advanced age, renal impairment, and low body weight that could bias the comparison.4,17 We included 110 mg of dabigatran twice daily in this comparison because the regimen was considered a standard dosage locally, that is, dabigatran recipients who received DAB220 may otherwise be eligible for DAB300, in contrast to European summary of product characteristics (SmPC) on dabigatran dosage.18
Risk Factors Associated With Recurrent Ischemic Stroke
To identify the risk factors of recurrent ischemic stroke, we compared the demographics, medical comorbidities, laboratory parameters, and medications among patients with and without recurrent ischemic stroke in an unweighted logistic regression analysis. We limited this analysis to the DOACsame group. Covariates that were clinically relevant or reached a p value ≤0.25 in the univariate logistic regression model were subjected to a final multivariate logistic regression model. Schoenfeld residual plots were used to determine the variability of the computed hazard ratios with time.
Normally distributed continuous variables, non-normally distributed continuous variables, and categorical variables were expressed as mean ± SD, median (interquartile range), and number (percentage), respectively. Assuming missing baseline data to be missing at random, we substituted missing data with values computed by multiple imputation with chained equations that created 20 complete datasets after the first 20 iterations. In the descending order of missing information, missing data on HbA1c (17.9%), lipid profile (13.8%), alkaline phosphatase (0.1%), alanine transferase (0.04%), hemoglobin (0.04%), and platelet (0.04%) were imputed and kept within reasonable ranges. We performed sensitivity analyses using patients with complete data. All statistical analyses were performed with RStudio V.1.4.1717.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
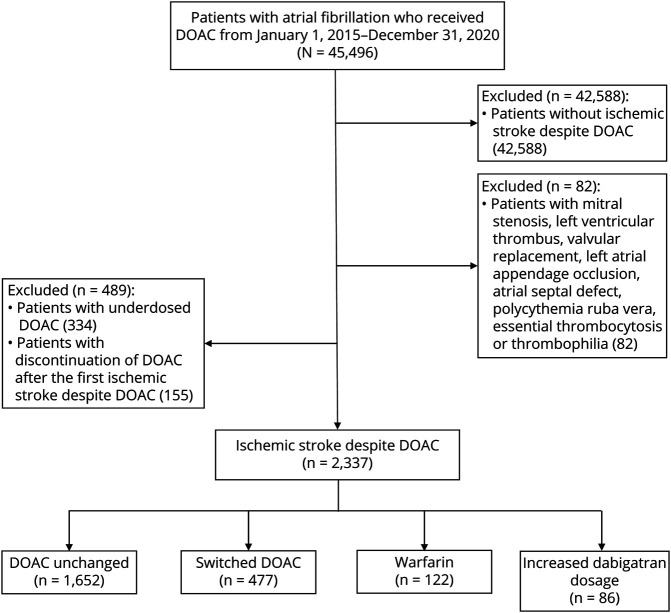
From January 1, 2015, to December 31, 2020, 2,908 of 45,496 patients with AF developed ischemic stroke despite DOACs. We excluded 571 patients with valvular AF, thrombophilia, underdosed DOACs, or discontinuation of anticoagulation after the first ischemic stroke despite DOACs (Figure 1). Among 2,337 patients who were included in the final analyses, 1,652 (70.7%) patients remained on the same DOAC regimen (DOACsame), 477 (20.4%) patients received an alternative DOAC (DOACswitch), 122 (5.2%) patients were prescribed warfarin, and 86 (3.7%) patients stepped up from 110 mg of dabigatran twice daily to 150 mg of dabigatran twice daily. We excluded patients with an increased dabigatran dosage from the primary analysis because of the small sample size. Over a median follow-up period of 16.5 months, 315 (13.4%) patients experienced another episode of ischemic stroke.
Figure 1. Study Flow Diagram.
Primary Analysis
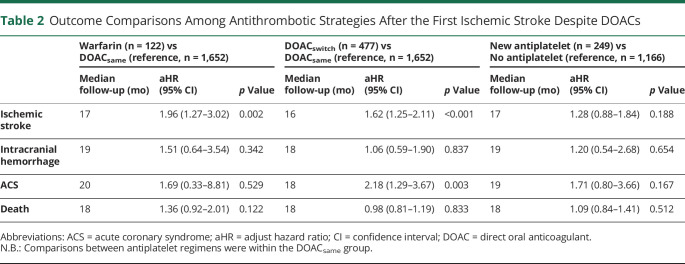
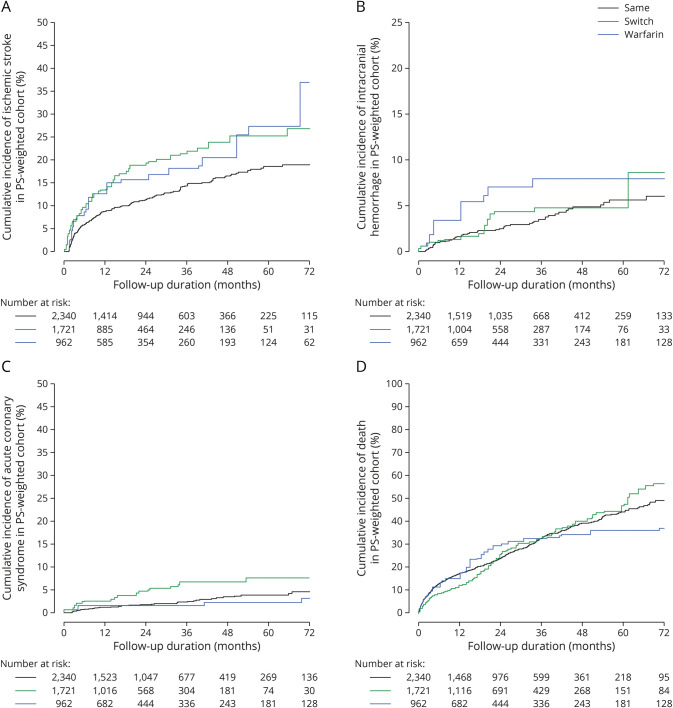
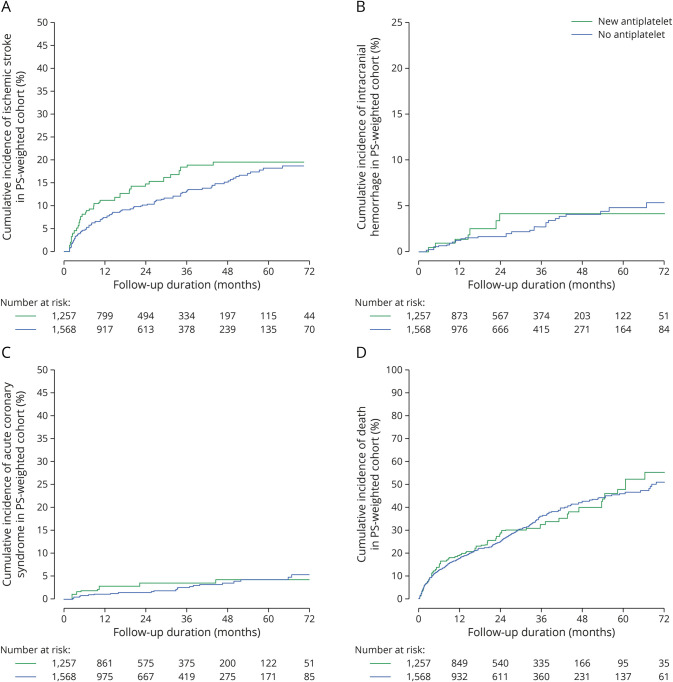
Compared with DOACsame, warfarin (adjusted hazard ratio [aHR] 1.96, 95% confidence interval [CI] 1.29–3.02, p = 0.002) and DOACswitch (aHR 1.62, 95% CI 1.25–2.11, p < 0.001) were associated with an increased risk of recurrent ischemic stroke (Figure 1A). DOACswitch was also associated with an increased risk of ACS (aHR 2.18, 95% CI 1.29–6.67 p = 0.003, Figure 1C). DOACswitch and warfarin groups were not associated with intracranial hemorrhage or death (Tables 1 and 2, Figure 2, A–D).
Table 1.
Imputed Patient Characteristics and Covariate Balance Across Different Anticoagulation Regimens After the First Ischemic Stroke Despite DOACs
Table 2.
Outcome Comparisons Among Antithrombotic Strategies After the First Ischemic Stroke Despite DOACs
Figure 2. Cumulative Incidence of (A) Ischemic Stroke, (B) Intracranial Hemorrhage, (C) Acute Coronary Syndrome, and (D) Death in Patients With Ischemic Stroke Despite Direct Oral Anticoagulant With Different Anticoagulation Strategies.
Secondary Analyses
Adjunctive Antiplatelet Therapy
Among 1,652 patients in the DOACsame group, 249 (15.1%) patients were started on antiplatelet agents within 8 weeks of the first ischemic stroke despite DOACs (eTable 2, links.lww.com/WNL/C839 in the Supplement), and 1,166 (70.6%) patients did not receive any antiplatelet therapy. Adjunctive antiplatelet treatment did not reduce the risk of recurrent ischemic stroke (aHR 1.28, 95% CI 0.88–1.84, p = 0.188), intracranial hemorrhage (aHR 1.20, 95% CI 0.54–2.68, p = 0.654), ACS (aHR 1.71, 95% CI 0.80–3.66, p = 0.167), or death (aHR 1.09, 95% CI 0.84–1.41, p = 0.512) (Table 2, Figure 3, A–D).
Figure 3. Cumulative Incidence of (A) Ischemic Stroke, (B) Intracranial Hemorrhage, (C) Acute Coronary Syndrome, and (D) Death in Patients With Ischemic Stroke Despite Direct Oral Anticoagulant With or Without Newly Added Antiplatelet Therapy.
Associated Factors of Recurrent Ischemic Stroke
After adjusting covariates that were clinically relevant or reached a p value of ≤0.25 in the univariate logistic regression analyses (eTable 3, links.lww.com/WNL/C839 in the Supplement), our multivariable logistic regression analysis within the DOACsame group revealed that advanced age (aHR 1.02, 95% CI 1.00–1.03, p = 0.029), a history of DM (aHR 1.49 95% CI 0.73–1.20, p = 0.002), the use of CYP/P-gp modulators (aHR 1.39, 95% CI 1.05–1.84, p = 0.021), and LAD (aHR 2.84, 95% CI 1.16–6.91, p = 0.021) were associated with recurrent ischemic stroke (eTable 4). Schoenfeld residual tests suggested that these risk factors did not vary significantly with time (p = 0.131; eFigure 1).
Between-DOAC Comparison
After the first ischemic stroke despite DOACs, 371 (16.4%) patients received API10, 516 (22.9%) received DAB220, 261 (11.5%) received DAB300, and 297 (13.2%) received RIV20. Edoxaban was excluded because of the small sample size (n = 47). Covariate balance was listed in eTable 5 (links.lww.com/WNL/C839) with API10 as the reference group. The risks of recurrent ischemic stroke were comparable among the 4 DOAC regimens. Compared with API10, DAB220 was associated with a lower risk of intracranial hemorrhage (aHR 0.27, 95% CI 0.15–0.99, p = 0.019), and RIV20 was associated with a higher risk of ACS (aHR 6.20, 95% CI 1.41–27.30, p = 0.016). DAB300 was associated with a lower risk of all-cause death (aHR 0.42, 95% CI 0.24–0.73, p = 0.003) (eTable 6, eFigure 2, A–D).
Sensitivity Analyses
Sensitivity analyses of 1,848 patients without any missing data yielded similar findings: warfarin and DOACswitch were associated with an increased risk of recurrent ischemic stroke. Addition of antiplatelet after the first ischemic stroke despite DOACs was not associated with a lower risk of subsequent ischemic stroke. eTables 7–12 (links.lww.com/WNL/C839) in the Supplement listed the details of sensitivity analyses.
Classification of Evidence
This study provides Class II evidence that in patients with NVAF experiencing an ischemic stroke while being treated with a DOAC, continuing treatment with that DOAC is more effective at preventing recurrent ischemic stroke than switching to a different DOAC or to warfarin.
Discussion
In this population-based study of patients with NVAF with an episode of ischemic stroke despite DOACs, we found the following: (1) Switching from the original DOAC to warfarin or another DOAC was associated with an increased risk of recurrent ischemic stroke. (2) Addition of antiplatelet agents did not reduce the risk of recurrent ischemic stroke. (3) Recurrent ischemic stroke while on oral anticoagulation was associated with advanced age, DM, concurrent use of CYP/P-gp inducers, and large artery atherosclerosis.
A key finding of our study is that switching from DOAC to warfarin in patients with NVAF may increase the risk of ischemic stroke. Although the longer half-life, ease of therapeutic drug monitoring, and data availability for intracranial atherosclerotic disease might favor switching to warfarin,19 real-world studies consistently revealed a higher thromboembolic risk with warfarin compared with that with DOAC.20 The lower time within therapeutic range in warfarin recipients might play a role in the increased recurrent ischemic stroke risk observed.21 Because switching to warfarin after a recurrent thrombotic episode with DOACs is popular,9,22 our study results caution against such practice unless strong indications such as renal insufficiency,18 antiphospholipid syndrome,23 or valvular AF are present.18
Of interest, DOAC-to-DOAC switch was associated with an increased risk of recurrent ischemic stroke compared with an unchanged DOAC regimen with well-balanced covariates. The underlying mechanism was unclear. Selection bias may result from unmeasured covariates. We postulate that DOAC adjustment could have been more frequent among patients with ischemic stroke risk unexplained by conventional cardiovascular risk factors. For example, the presence of left atrial appendage thrombus,24 cardiomyopathies,25 or pharmacogenomic susceptibility such as ABCB1 and CES1 allelic variants may predispose to cardioembolic ischemic strokes.26,27 The resultant cortical or wedge-shaped infarct topography may prompt DOAC switching after a verdict of “DOAC failure.”28 Of note, the higher risk of ACS with DOAC switching might reflect the unmeasured thromboembolic risk and subsequent coronary embolism. Future prospective studies on heart-brain axis and pharmacogenomic assessment should evaluate whether these factors would have influenced the decision on DOAC switching.
Adjunctive antiplatelet therapy to DOAC did not seem to reduce the risk of ischemic stroke. Although recent studies showed the efficacy of aspirin in combination with rivaroxaban for cardiovascular protection in atherosclerotic diseases,29,30 our study suggested that this “dual pathway inhibition” strategy might not be similarly effective in a population purely consisted of patients with NVAF. Some studies even found a higher risk of major cardiovascular events in patients with AF with dual antithrombotic therapy.31,32 However, these comparisons were confounded by the higher prevalence of cardiovascular risk factors in patients who received dual antithrombotic therapy,31 when compared with our cohort with a low incidence of ischemic heart disease of less than 4% in both groups. Overall, our study did not find a clinical benefit of adjunctive antiplatelet therapy in patients with NVAF who developed ischemic stroke despite DOACs.
In the unweighted multivariable regression analysis, DM was associated with an increased risk of recurrent ischemic stroke. Although DM is modifiable and predicted ischemic stroke in patients with AF,15 the mean glycated hemoglobin A1c levels were not significantly different among DOAC recipients with and without recurrent cerebral ischemia in our cohort. Another study also found that most patients with ischemic stroke despite DOACs had satisfactory glycemic control at stroke onset.33 Further longitudinal studies should determine whether a stringent long-term glycemic control may lower the risk of recurrent ischemic stroke among DOAC recipients. Apart from DM, the use of CYP/P-gp modulators also independently predicted the risk of recurrent ischemic stroke. Due to the small sample size (n = 157), we were unable to stratify individual drugs for further analysis. Nevertheless, DOAC level monitoring maybe necessary in these patients to detect underanticoagulation from drug-drug interactions between DOAC and CYP/P-gp modulators.16,34
In our exploratory comparison of different DOAC regimens, all 4 drug regimens had a comparable risk of recurrent ischemic stroke. The lower risk of intracranial hemorrhage with 110 mg of dabigatran twice daily was similar to that observed in population-based studies that included patients without ischemic stroke despite DOACs.35 This observation may be related to the preservation of blood-brain barrier function through direct thrombin inhibition.36 On the contrary, the reason behind the lower risk of ischemic heart disease with 5 mg of apixaban twice daily compared with 20 mg of rivaroxaban daily is unclear because previous studies reported only a lower risk of major cardiovascular events with DOAC compared with warfarin without robust comparisons among DOACs.37,38 Although the pharmacologic mechanisms behind these observations are yet to be confirmed, our findings hinted the importance of personalized DOAC therapy based on individual risk profile. The lower all-cause death with 150 mg of dabigatran twice daily, however, could be biased by younger age, better functional status, and renal function. It should also be noted that there were no prespecified dosage adjustment criteria for dabigatran in patients with normal renal function during the study period. Patients on 110 mg of dabigatran twice daily might otherwise be eligible for the 150 mg of dabigatran twice daily regimen. In comparison with the European SmPC recommendation on 110 mg of dabigatran twice daily in patients older than 80 years, on concomitant verapamil or with high bleeding risks,18 patients who received 110 mg of dabigatran twice daily in our cohort may be younger with lower bleeding propensities. Therefore, caution should be exercised in extrapolating our study findings on dabigatran recipients under the European dosing guidelines.
Last, to put our findings into perspective, antithrombotic regimen is only part of the secondary stroke prophylaxis. A comprehensive cardiovascular workup and stringent cardiovascular risk factor control may be equally important in patients with ischemic stroke despite DOACs, as reflected by the risk of recurrent ischemic stroke in patients with LAD.
Our study has several limitations. First, limited by the retrospective study design, unmeasured confounders maybe present. Factors such as DOAC level, compliance, and competing stroke etiologies could have biased the comparisons. Although we attempted to retrieve competing stroke etiologies, 12%–14% of ischemic stroke patients received an incomplete cardiovascular workup for ischemic stroke according to our previous study.39 Future prospective studies or clinical trials with specific coagulation assays, drug adherence measures, heart-brain axis assessment, and causative classification of stroke are warranted to evaluate whether these factors might influence the treatment decisions and the subsequent stroke risk after an episode of ischemic stroke despite DOACs.27 Second, the sample size of warfarin group was relatively small, and the time within therapeutic range was not measured. Although the incidence of intracranial hemorrhage was numerically higher in the warfarin group than in the DOACsame group, the sample size may not be powered to detect statistical significance. Third, we did not evaluate the outcomes of patients who underwent left atrial appendage occlusion, which is also a potential intervention for patients with ischemic stroke despite DOAC. Last, because 98% of our study population were ethnic Chinese, the study findings remained to be confirmed in other ethnicities, given the potential disparities in pharmacogenomics and ischemic stroke etiologies.26,39
To conclude, in patients with NVAF who developed ischemic stroke despite DOACs, the increased risk of recurrent ischemic stroke with switching to warfarin called for caution against such practice, while the increased ischemic stroke with DOAC-to-DOAC switch demands further studies. Adjunctive antiplatelet agent did not seem to reduce ischemic stroke relapse. Because DM, the use of CYP/P-gp modulators, and LAD were predictors of recurrent ischemic stroke, further investigations should evaluate whether strict glycemic control, DOAC level monitoring, and routine screening for carotid and intracranial atherosclerosis may reduce ischemic stroke recurrence in these patients.
Acknowledgment
The authors thank the contributions from Dr. Robert Dan (The Chinese University of Hong Kong), Mrs. Sophia Dan (The Chinese University of Hong Kong), Ms. Anki Miu (The Chinese University of Hong Kong), Ms. Trista Hung (The Chinese University of Hong Kong), and Kwok Tak Seng Center for Stroke Research and Intervention (The Chinese University of Hong Kong). Dr. Robert Dan provided general advice for the study. Mrs. Sophia Dan provided general advice for the study. Ms Anki Miu provided clerical assistance for the study. Ms Trista Hung provided clerical assistance for the study. Kwok Tak Seng Center for Stroke Research and Intervention provided clerical assistance for the study.
Glossary
- ACS
acute coronary syndrome
- aHR
adjusted hazard ratio
- CI
confidence interval
- DM
diabetes mellitus
- DOAC
direct oral anticoagulant
- GBM
generalized boosted models
- LAD
large artery atherosclerotic disease
- NVAF
nonvalvular atrial fibrillation
- PS
propensity score
- SmPC
summary of product characteristics
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Infographic: links.lww.com/WNL/C950
Study Funding
The authors report no targeted funding.
Disclosure
Y.M.B. Ip, H. Ko, L. Lau, A. Yao, G.L.H. Wong, T.C.F. Yip, X. Leng, H. Chan, H. Chan, V. Mok, Y. Soo, and T.W. Leung report no disclosures relevant to the article. G. Lau received grants from Croucher Foundation, Research Fund Secretariat of the Food and Health Bureau, Innovation and Technology Bureau, Research Grants Council, Amgen, Boehringer Ingelheim, Eisai and Pfizer; and consultation fees from Amgen, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi, all outside the submitted work. D. Seiffge received research funding from the Bangerter Rhyner Foundation and AstraZeneca. He served on advisory boards, consultancy, or speaker bureau for AsztaZeneca, Bioxodes, VarmX, Javeline, and Vifor. All fees were paid to his employer and used for research funding. All outside the submitted work. Go to Neurology.org/N for full disclosures.
References
- 1.Lee JJ, Ha ACT, Dorian P, Verma M, Goodman SG, Friedrich JO. Meta-analysis of safety and efficacy of direct oral anticoagulants versus warfarin according to time in therapeutic range in atrial fibrillation. Am J Cardiol. 2021;140:62-68. [DOI] [PubMed] [Google Scholar]
- 2.Stretz C, Wu TY, Wilson D, et al. . Ischaemic stroke in anticoagulated patients with atrial fibrillation. J Neurol Neurosurg Psychiatry. 2021;92(11):1164-1172. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, et al. . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, et al. . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, et al. . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. [DOI] [PubMed] [Google Scholar]
- 6.Giugliano RP, Ruff CT, Braunwald E, et al. . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. [DOI] [PubMed] [Google Scholar]
- 7.Seiffge DJ, De Marchis GM, Koga M, et al. . Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. 2020;87(5):677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fastner C, Szabo K, Samartzi M, et al. . Treatment standards for direct oral anticoagulants in patients with acute ischemic stroke and non-valvular atrial fibrillation: a survey among German stroke units. PLoS One. 2022;17(2):e0264122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett A, Moore M, Ferrins P, Thornton P, Murphy P, Quinn J. From a direct oral anticoagulant to warfarin: reasons why patients switch. Ir J Med Sci. 2018;187(3):719-721. [DOI] [PubMed] [Google Scholar]
- 10.Yu Z, Yu L, Shan C. Trends of ambulatory oral anticoagulant prescription in five major cities of China, 2012-2017. BMC Health Serv Res. 2020;20(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huiart L, Ferdynus C, Renoux C, et al. . Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population-based cross-sectional study in the French health insurance databases. BMJ Open. 2018;8(3):e018180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale ZD, Kong X, Haymart B, et al. . Prescribing trends of atrial fibrillation patients who switched from warfarin to a direct oral anticoagulant. J Thromb Thrombolysis. 2017;43(2):283-288. [DOI] [PubMed] [Google Scholar]
- 13.Wong AY, Root A, Douglas IJ, et al. . Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926. [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48(3):438-445. [DOI] [PubMed] [Google Scholar]
- 15.Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865. [DOI] [PubMed] [Google Scholar]
- 16.Ip BY, Ko H, Wong GLH, et al. . Thromboembolic risks with concurrent direct oral anticoagulants and antiseizure medications: a population-based analysis. CNS Drugs. 2022;36(12):1313-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okumura K, Tomita H, Nakai M, et al. . Risk factors associated with ischemic stroke in Japanese patients with nonvalvular atrial fibrillation. JAMA Netw Open. 2020;3(4):e202881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffel J, Collins R, Antz M, et al. . 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612-1676. [DOI] [PubMed] [Google Scholar]
- 19.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. . Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305-1316. [DOI] [PubMed] [Google Scholar]
- 20.Graham DJ, Baro E, Zhang R, et al. . Comparative stroke, bleeding, and mortality risks in older medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596-604.e11. [DOI] [PubMed] [Google Scholar]
- 21.Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246-254. [DOI] [PubMed] [Google Scholar]
- 22.Manzoor BS, Walton SM, Sharp LK, Galanter WL, Lee TA, Nutescu EA. High number of newly initiated direct oral anticoagulant users switch to alternate anticoagulant therapy. J Thromb Thrombolysis. 2017;44(4):435-441. [DOI] [PubMed] [Google Scholar]
- 23.Pengo V, Denas G, Zoppellaro G, et al. . Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132(13):1365-1371. [DOI] [PubMed] [Google Scholar]
- 24.Backhaus JF, Pflaumbaum A, Krogias C, et al. . Short- and long-term outcome of patients with spontaneous echo contrast or thrombus in the left atrial appendage in the era of the direct acting anticoagulants. Clin Res Cardiol. 2021;110(11):1811-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahsili-Fahadan P, Geocadin RG. Heart-brain axis: effects of neurologic injury on cardiovascular function. Circ Res. 2017;120(3):559-572. [DOI] [PubMed] [Google Scholar]
- 26.Kanuri SH, Kreutz RP. Pharmacogenomics of novel direct oral anticoagulants: newly identified genes and genetic variants. J Pers Med. 2019;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arsava EM, Ballabio E, Benner T, et al. . The Causative Classification of Stroke system: an international reliability and optimization study. Neurology. 2010;75(14):1277-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. . Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41. [DOI] [PubMed] [Google Scholar]
- 29.Eikelboom JW, Connolly SJ, Bosch J, et al. . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. [DOI] [PubMed] [Google Scholar]
- 30.Anand SS, Bosch J, Eikelboom JW, et al. . Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219-229. [DOI] [PubMed] [Google Scholar]
- 31.Said A, Keeney S, Matka M, Hafeez A, George J, Halalau A. Concomitant use of direct oral anticoagulants and aspirin versus direct oral anticoagulants alone in atrial fibrillation and flutter: a retrospective cohort. BMC Cardiovasc Disord. 2020;20(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Danik SB, Altman RK, et al. . Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation: a meta-analysis of randomized controlled trials. Cardiol Rev. 2016;24(5):218-223. [DOI] [PubMed] [Google Scholar]
- 33.Szeto CLC, Hui KF. Residual stroke risk in patients with atrial fibrillation treated with non-vitamin K oral anticoagulants: an 8-year retrospective cohort study. Cerebrovasc Dis Extra. 2021;11(1):9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galgani A, Palleria C, Iannone LF, et al. . Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu W, Ye Z, Chen S, et al. . Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients. Stroke. 2021;52(4):1225-1233. [DOI] [PubMed] [Google Scholar]
- 36.Puech C, Delavenne X, He Z, Forest V, Mismetti P, Perek N. Direct oral anticoagulants are associated with limited damage of endothelial cells of the blood-brain barrier mediated by the thrombin/PAR-1 pathway. Brain Res. 2019;1719:57-63. [DOI] [PubMed] [Google Scholar]
- 37.Amin A, Garcia Reeves AB, Li X, et al. . Effectiveness and safety of oral anticoagulants in older adults with non-valvular atrial fibrillation and heart failure. PLoS One. 2019;14(3):e0213614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CJ, Gerds TA, Carlson N, et al. . Risk of myocardial infarction in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(1):17-26. [DOI] [PubMed] [Google Scholar]
- 39.Ip B, Au L, Chan A, et al. . Evolving ischemic stroke subtypes in 15 years: a hospital-based observational study. Int J Stroke. 2022;17(4):444-454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.