Abstract
Blood-based biomarkers offer a major advance in the clinical evaluation of neurodegenerative diseases. Currently, research studies have reported robust assays of blood markers for the detection of amyloid and tau pathologies specific to Alzheimer disease (amyloid-β peptides, and p-tau) and nonspecific blood markers of neuronal (neurofilament light, β-synuclein, and ubiquitin-C-terminal-hydrolase-L1) and glial degeneration (glial fibrillary acidic protein) that can measure key pathophysiologic processes in several neurodegenerative diseases. In the near future, these markers may be used for screening, diagnosis, or disease and treatment response monitoring. Blood-based biomarkers for neurodegenerative diseases have been rapidly implemented in research, and they have the potential to enter clinical use soon in different clinical settings. In this review, we will describe the main developments and their potential implications for the general neurologist.
Introduction
In the past 2 decades, PET imaging and CSF biomarkers have offered the possibility of measuring core pathophysiologic processes in different neurodegenerative diseases in living individuals. The implementation of markers able to detect amyloid and tau pathologies in vivo has facilitated an earlier and more accurate diagnosis of Alzheimer disease (AD). Research in other neurodegenerative diseases, such as dementia with Lewy bodies and amyotrophic lateral sclerosis, has followed suit, and several central markers are being investigated for α-synuclein, TAR DNA-binding protein-43, and 4R and 3R tauopathies. However, the use of PET imaging is limited by cost and accessibility to specialized facilities, and the use of the CSF requires qualified personnel to perform lumbar punctures and specialized laboratories to perform assays. The recent development of sensitive technologies able to detect biomarkers of neurodegenerative diseases in blood has ushered in a new era. In research contexts, blood markers allow the detection of the pathophysiology of these conditions using an easily accessible sample that can be obtained repeatedly over time. Currently, there are robust blood markers for the detection of amyloid and tau pathologies specific to AD and nonspecific blood markers of neuronal and glial degeneration. These markers have the potential to be used in primary care and general neurology clinic settings to support screening, early diagnosis, disease monitoring, and eventually, treatment response monitoring. Blood biomarkers already have a key role in clinical trials, for screening, as measures of target engagement or to assess disease modification. For this review, we searched PubMed for studies reporting evidence of the use of blood biomarkers in neurodegenerative diseases until November 2022. We will cover the key aspects of blood biomarkers for the assessment of neurodegenerative diseases and how they can be instrumental in the future for the general neurologist. Some of the sections in this review, such as those focused on regulatory issues and cost-effectiveness, are not necessarily specific to neurodegenerative markers, but they might help the clinicians to familiarize with topics that are critical to the ultimate application of biomarkers.
A Brief History of Blood Biomarkers' Challenges and Successes
Historically, there was much skepticism about blood biomarkers in neurodegenerative diseases. For many decades, it was considered that blood markers were not useful in the diagnostic or prognostic assessment primarily due to poor reproducibility and low sensitivity of the assays. In addition, there was little conviction that peripheral markers could reflect bona fide brain changes. Regarding this, the field has progressed tremendously in the past few years, with the development of ultrasensitive technologies such as single-molecule array (Simoa) and electrochemiluminescence (ECL)–based assays, which can detect concentrations below the femtomolar (10−5 moles per liter) level and with enhancement in mass spectrometry that has enabled accurate, consistent, and high-sensitivity measurements of brain-derived proteins at very low concentrations. In the early 2010s, increased plasma concentrations of neurofilament light chain (NfL) were detected in patients with diverse neurodegenerative conditions compared with those in healthy controls and showed strong associations with their concentration in the CSF.1 Another major technological advance occurred in 2014 with the development of specific mass spectrometry methods for the reliable detection of β-amyloid (Aβ) in blood,2 which was further validated in a large AD cohort.3 Since then, several assays have improved their sensitivity for the detection of Aβ in the blood (see further).
Assay sensitivity aside, assessment of brain-derived proteins in the blood has been challenging for a variety of reasons. While some proteins, such as NfL, seem to be neuronal specific, many others, including Aβ, are highly expressed in other tissues, and thus their concentrations in the blood might not necessarily reflect neurologic changes. In addition, measurements of proteins in the blood may be complicated due to their rapid degradation by blood proteases, hepatic clearance, interference by peripheral antibodies, and/or binding to peripheral proteins, such as albumin.
The most rigorously studied blood biomarkers in neurodegenerative diseases are Aβ peptides (Aβ40 and Aβ42), NfL, total tau (t-tau), and phosphorylated tau (p-tau). Of these biomarkers, p-tau is emerging as the most consistent regarding sensitivity and specificity to detect AD in older adults. The first report on this marker was published in 2017,4 and since then, multiple studies have shown that plasma p-tau181 is associated with conversion from normal cognition to AD dementia, and it is highly correlated with its counterpart in the CSF and with amyloid and tau burden measured by PET imaging.5,6 Other isoforms of p-tau are also being investigated as useful markers, possibly reflecting different stages of tau pathology (see further). The correlation between the concentrations of the different p-tau assays is high among older adults, but relatively low in younger adults, possibly due to low concentrations of the biomarkers. This suggests that additional technological improvements with greater sensitivity and stability are necessary to identify these earliest alterations of AD in blood, a crucial step for the introduction of prevention treatments at the earliest stages of the disease.
Another challenge is the fact that the associations of AD-related neuropathology with the degree of cognitive impairment is weak in the oldest old,7 raising significant concern about the utility of isolated AD-specific blood-based biomarkers in this very high-risk population in whom multiple co-occurring degenerative pathologies is the rule rather than the exception. It is also worth noting that most studies that are centered on developing cutoffs for abnormal concentrations comprise highly selected participants, and population-based studies are needed to better determine the utility of these markers in the general population. This is particularly relevant in light of recent evidence from population-based cohorts suggesting effects of relatively common comorbidities (such as chronic kidney disease) on AD blood biomarkers levels.8,9 These results emphasize that the accuracy of AD biomarkers in the prediction of disease will likely improve as more knowledge is acquired, similarly to biomarkers for other diseases, such as myocardial infarction, which required a reiterative process of development until full optimization.
Technical and Regulatory Issues of Blood-Based Biomarkers for Neurodegenerative Diseases
The use of in vitro diagnostic (IVD) tests for clinical use is subject to a rigorous regulatory process. In the United States, laboratory testing is regulated through the Clinical Laboratory Improvement Amendments (CLIA) coordinated by the US Food and Drug Administration (FDA), the Center for Medicaid Services, and the Centers for Disease Control and Prevention.e1 Laboratory-developed tests are a subset of IVD tests that have been conceptualized, manufactured, and used within a single laboratory meeting the CLIA requirements and are also intended for clinical use.e1 The approval of an IVD test by regulatory agencies relies on existing scientific evidence supporting its safety and effectiveness. The decision follows a careful evaluation of data on analytical performance (reliability, replicability, and accuracy of the analyte measurements), and it also considers clinical performance and studies specific to the assay in the context of its intended use. A similar strategy is followed in the European Union, where all IVD devices are required to be CE marked. The CE marking certifies that the test complies with the in vitro Diagnostic Devices Directive (IVDD 98/79/EdC), currently in the process of being replaced by a new in vitro Diagnostic Medical Devices Regulation (IVDR 2017/746).
Research use only products are not subject to these stringent regulations and, although they may provide clinically relevant data, they are not qualified by law to be used as a diagnostic tool. Because preanalytical and analytical factors can largely affect the quality of plasma biomarker research, it is of utter importance that standards are followed in research evaluating the quality of data around plasma markers. Clinicians should be aware of this rigorous regulatory and approval process. The field should favor cautious incorporation of novel plasma markers into clinical practice, adopting only those with the best data supporting clinical use that was generated with methods compliant with the highest quality standards.
Blood-Based Biomarker Assays of Relevant Pathophysiologic Processes in Neurodegenerative Diseases
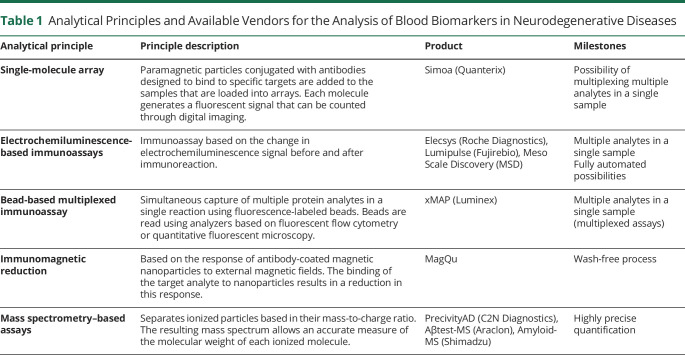
Several blood-based biomarker assays have recently shown promising value in the detection of key specific and nonspecific pathophysiologic processes of neurodegenerative dementias. The field is rapidly evolving, and the number of studies evaluating blood biomarkers in neurodegenerative diseases increases every day. Alzbiomarker is a live online database that curates and meta-analyzes published studies on the diagnostic performance of fluid biomarkers in AD and in other dementias.e2 Table 1 summarizes the most widely used techniques to detect blood biomarkers. Mass spectrometry–based assays allow highly accurate measurements. However, immunoassays with high accuracy have recently been developed and may present as a more accessible option because they allow high throughput results and ease of automation. Blood biomarkers can also be ranked based on their robustness,10 a measure of their capacity to remain unaffected by small alterations in the parameters of their methods of assessment, and that provides an indication of their reliability during normal usage.
Table 1.
Analytical Principles and Available Vendors for the Analysis of Blood Biomarkers in Neurodegenerative Diseases
Aβ Peptides
Aβ42 and Aβ40 peptides in plasma can be measured using ELISA-based assays, Simoa, ECL, bead-based multiplexed immunoassay, immunomagnetic reduction, and immunoprecipitation-based mass spectrometry. One of the limitations for the implementation of plasma amyloid markers in clinical use is that differences between patients with AD and controls (approximately 10%–15%) are significantly smaller than those observed in the CSF (approximately 40%–50%).12 Moreover, plasma Aβ42 assays have shown weaker correlations to each other, compared with CSF assays, and their results are more sensitive to changes in preanalytical conditions. The impact of these factors can be minimized by normalizing Aβ42 to Aβ40, and the Aβ42/Aβ40 ratio has shown better correlation with amyloid burden in PET than Aβ42 alone.3,11
In late 2020, C2N Diagnostics received US CLIA accreditation and EU CE Mark approval for PrecivityAD, which then became the first commercially available blood test to aid physicians in the diagnosis of brain amyloidosis. This test measures the Aβ42/Aβ40 concentration ratio and the apolipoprotein E isoform–specific peptide, which is equivalent to APOE genotype, in plasma samples, using a proprietary immunoprecipitation and liquid chromatography-tandem mass spectrometry platform. The result is provided as an amyloid probability score ranging from low to intermediate to high and indicates the probability that the patient has amyloid plaques. To date, the largest study of PrecivityAD accuracy (including Aβ42/Aβ40 ratio, ApoE, and age) for the diagnosis of brain amyloidosis, vs the gold standard of amyloid PET, showed good discrimination between participants with and without brain amyloidosis (area under the curve 0.88, 95% CI 0.85–0.91) among 686 participants with mild cognitive impairment (MCI) or mild dementia from 2 independent cohort studies.e3 While the test is performed in a CLIA-accredited laboratory and is available throughout the United States with the exception of New York State (which has distinct CLIA accreditation protocols), it is not yet US FDA approved, and the cost was $1,250 in 2022. The test cannot make a diagnosis of AD, although it can increase confidence in the clinical diagnosis of AD by aiding in the determination of likelihood of amyloid plaques being present in the brain. The test is currently marketed directly to practicing physicians and patients.
p-Tau
Assays measuring N‐terminal to mid-region fragments of p-tau in the blood have shown high accuracy to detect AD pathology in sporadic and familial AD and to differentiate AD from other neurodegenerative diseases.4,5,12-18 Large cohort studies have shown that p-tau in the blood has excellent accuracy to detect AD pathology along the disease and has a high correlation with both amyloid and tau PET.5,6,13 Assays that measure p-tau181 are commercially available using the Simoa technology and ECL-based assays. Prototype assays have been developed by different providers to measure other p-tau isoforms (p-tau217, p-tau231, p-tau205, and p-tau212). P-tau assays have shown moderate-to-strong correlation to each other19-21 and, due to the differences in platforms and test performance, it is unclear at this point whether one assay is superior to the others. In 2021, the US FDA granted the Breakthrough Device designation to the Simoa p-tau181 for aid in the evaluation of AD due to its potential to improve the diagnosis of this condition. This designation does not guarantee the approval for clinical use, but it acknowledges its diagnostic potential.
Recently, Eli Lilly began offering their plasma p-tau217 assay in their CLIA-accredited laboratory; however, it remains marketed for research purposes only. Janssen Research & Development has also designed a Simoa-based p-tau217 immunoassay. Several other commercial laboratories are pursuing CLIA accreditation for specific blood-based biomarkers of neurodegeneration, but it is unclear which, if any, will pursue research vs clinical applications. In March 2022, Eli Lilly entered into a collaboration agreement with Quanterix Corporation's CLIA-accredited Accelerator Laboratory in which they will work together on the further development of blood-based Simoa immunoassays for p-tau217, other blood-based Simoa immunoassays, and the future development of IVDs, which could certainly pave the way for future clinical applications.
NfL
NfL is a neuron-specific axonal protein, and plasma concentrations increase in a wide variety of neurologic conditions affecting either the central or the peripheral nervous system (neurodegenerative diseases, stroke, multiple sclerosis, traumatic brain injury, hypoxia and polyneuropathy, among others).e4
NfL concentrations in the plasma and serum can be measured using Simoa-based assays, and they show a high correlation with those in the CSF.17,e4 Although it is not specific for a particular etiology, it has been proposed as a potential candidate for the screening of neurologic damage and a useful tool to monitor disease progression in several neurodegenerative diseases.22,e4 In 2022, the US FDA granted the Breakthrough Device designation for Simoa NfL for the diagnosis of multiple sclerosis. Siemens also offers a NfL testing, which is CLIA certified but not US FDA approved.
GFAP and UCH-L1
Glial fibrillary acidic protein (GFAP) is mainly expressed in brain astrocytes. An increase in its concentration in the blood has been found in patients after mild traumatic brain injury (TBI).23,24 Recent studies have also shown that GFAP concentrations in the plasma are higher in patients with AD than in controls and are also associated with amyloid pathology.25-27 High concentrations of plasma GFAP have also been reported in patients with frontotemporal lobar degeneration (FTLD)–related syndromes but to a lesser extent than those in patients with AD.28,29
In early 2018, the US FDA approved the Banyan Brain Trauma Indicator (BTI) blood test under the Breakthrough Devices Program to aid in the initial evaluation of patients presenting acutely with mild TBI. BTI is an IVD chemiluminescent ELISA assay that provides semiquantitative measurement of GFAP and ubiquitin-C-terminal-hydrolase-L1 (UCH-L1) in the serum using the Synergy 2 Multimode Reader device. In early 2021, Abbott received FDA 510(k) clearance for another IVD for TBI, which provides semiquantitative measurement of GFAP and UCH-L1 in just 15 minutes from the plasma using its handheld i-STAT Alinity platform. The intended use is for ruling out evidence of intracranial trauma on head CT. A pivotal multisite study of 1,176 individuals with acute mild TBI referenced in the US FDA documentation found this Abbott TBI diagnostic to have 99.3% negative predictive value for ruling out intracranial trauma.23 Abbott is now developing its high throughput core laboratory ARCHITECT platform to run this assay and is also developing the i-STAT Alinity to function as a point-of-care test. GFAP can also be measured in the plasma using a commercially available Simoa-based assay.
β-Synuclein
The presynaptic protein β-synuclein has been proposed as a marker of synaptic degeneration detectable both in the CSF and plasma. Using quantitative mass spectrometry, higher concentrations of β-synuclein have been found in plasma samples of patients with AD30 and in Down syndrome population.31
Novel Technologies for Biomarker Quantification and Discovery
Highly sensitive immunoassays and mass spectrometry have been the main key tools for blood biomarker discovery and validation. New emerging analytical technologies for research, such as immunomagnetic reduction or real-time quaking-induced conversion, may allow hypersensitive measures of proteins implicated in neurodegeneration, such as non-AD tau or alpha-synuclein.e5,e6 Nanoneedle-based immunoassays may allow the detection of p-tau and other proteins with sensitivity in the femtomolar range in plasma or serum sample volumes as low as 5 μL.e7 Other platforms, such as Elecsys, are allowing CLIA-compliant quantification of biomarker panels compatible with routine clinical use on individual patients. Somamer-based proteomics or Olink-based technologies may allow cost-effective simultaneous interrogation of a large part of the human proteome.e8 Finally, progress in blood transcriptomics and functional proteomics techniques may allow exploring the role of epigenetics and posttranslational chemical modifications of proteins implicated in the pathophysiology of neurodegenerative diseases as diagnostic tools or targets of therapeutic interventions.
Potential Contexts of Use
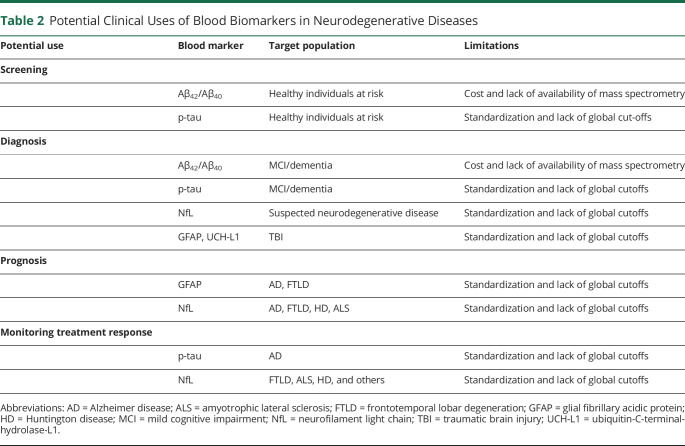
Current data support the potential high impact of blood-based biomarkers in a diversity of clinical contexts in neurodegenerative disorders. There are several previous examples of blood biomarkers that have improved the clinical practice of different diseases. Blood biomarkers have been used to aid with risk determination (e.g., total cholesterol), diagnosis (e.g., cardiac troponins), staging (e.g., creatinine), therapy response prediction (e.g., thyroperoxidase antibodies), treatment efficacy (e.g., thyroid stimulating hormone), monitoring progression (e.g., prostate-specific antigen), recurrence (e.g., erythrocyte sedimentation rate), and treatment compliance (e.g., glycosylated hemoglobin A1c). Blood biomarkers for neurodegenerative diseases, therefore, are expected to play a wide variety of roles in the clinical setting soon (Table 2). At this point, the precise application of blood-based biomarkers in clinical practice in the different neurodegenerative diseases remains unknown, and more clinical studies are needed. The Alzheimer Association has published a document of recommendations for their use in AD in the clinic and in clinical trials.32 In this section, we review their potential application in neurodegenerative conditions.
Table 2.
Potential Clinical Uses of Blood Biomarkers in Neurodegenerative Diseases
Screening in Symptomatic Individuals
Blood-based biomarkers are ideal tools to aid in the differential diagnosis of neurodegenerative disorders in the symptomatic phase. Plasma p-tau concentrations have shown high accuracy for the differentiation between AD and other forms of neurodegenerative diseases at early disease stages. Therefore, they could be used in clinical practice in diverse clinical settings to screen patients with MCI or dementia in whom AD is suspected. At this point, despite the high diagnostic value of plasma p-tau in different studies, the current diagnostic criteria require confirmation with CSF or amyloid PET.
High blood NfL concentrations also have value in distinguishing individuals with atypical parkinsonism due to FTLD from those caused by alpha-synucleinopathies.e9 Other studies have provided evidence that high plasma NfL may be used to distinguish patients with early-onset dementia from patients with behavioral symptoms due to primary psychiatric disorders.e10
Screening in Asymptomatic Individuals
Most neurodegenerative diseases have a long preclinical phase (usually decades) in which the main pathologic hallmarks are detectable in the absence of clinical signs. This window offers a possibility for early detection and intervention. In the primary care or general neurology setting, or as part of a clinical trial, blood-based biomarkers may become an important tool for early detection in these presymptomatic individuals. Decreases in plasma Aβ42/Aβ40 can identify cognitively healthy individuals at risk of progression of MCI or dementia.11 Elevations in plasma p-tau have been documented in cognitively normal individuals with evidence of AD pathophysiology. Similarly, plasma NfL predicts conversion to dementia, within 2 years, in asymptomatic individuals, in asymptomatic carriers of frontotemporal dementia–causing mutations, or in Down syndrome.33,34 These markers could be used in future for screening in the general population or in individuals at risk of neurodegenerative diseases who could be candidates to disease-modifying therapies, and some of them are already in use in clinical trials that enroll asymptomatic individuals. Plasma Aβ42/Aβ40 is currently being used as a screening tool in the phase 3 study of lecanemab in presymptomatic AD.e11 Plasma p-tau217 and p-tau181 are also being used as screening tools in phase 3 AD prevention studies with donanemabe12 and gantenerumab,e13 respectively.
Prognosis
Some cognitively healthy individuals or patients with MCI show little clinical progression in the short term, and enrollment of patients at high risk of progression identified with biomarkers may improve the design of clinical trials, providing higher chances of determining the clinical effectiveness of a candidate drug. Hence, there may be high clinical value for prospective clinical trials of disease-modifying therapies if blood biomarkers identify asymptomatic and symptomatic candidates with high potential for accelerated decline. For example, abnormal plasma Aβ42/Aβ40 is associated with a 15-fold increase in the risk of conversion from negative to positive amyloid PET in cognitively asymptomatic individuals.11 Similarly, high NfL identifies asymptomatic, prodromal, and fully symptomatic genetic FTLD individuals at high risk of clinical progression.34
Monitoring Progression
Evidence supporting the value of blood-based biomarkers to monitor disease progression in neurodegenerative diseases is derived from several large observational cohort studies. In the Alzheimer Disease Neuroimaging Initiative cohort, for example, longitudinal increases in plasma p-tau181 were associated with worse decline in memory, executive function, language, visuospatial function, amyloid burden by PET, regional brain hypometabolism, and with CSF Aβ42, p-tau181, and total tau concentrations.e14 Similarly, plasma NfL concentrations show trends for longitudinal intraindividual increases over time that correlated with decline in cognitive impairment.35 Similar associations between high plasma NfL concentrations and disease progression are evident in FTLD.36
Another remarkable aspect of the emerging blood biomarkers is their versatile association with diverse aspects of disease severity. For example, depending on the phenotype or the stage of the disease, blood NfL has been shown to correlate with global measures of disease severity, basic or instrumental functions, severity of neuropsychiatric symptoms, motor function, neuropsychological performance or global or regional brain volume in AD,35 primary progressive aphasia,37 progressive supranuclear palsy,38 FLTD,34,36 Down syndrome,39 and Huntington disease.40
Monitoring Treatment Response
Compelling evidence suggests that blood-based biomarker concentrations may normalize on effective treatment of neurodegenerative conditions and they could play a role in monitoring the response to prospective treatments (e.g., theragnostic markers). For example, plasma p-tau181 and p-tau217 are reduced in patients with AD treated with the monoclonal antiamyloid antibodies aducanumab or donanemab. These changes correlated with reductions in brain amyloid load and clinical benefit in clinical trials.41 For blood NfL, the most compelling evidence has emerged from multiple sclerosis research. A reduction of approximately 50% in plasma NfL concentrations was observed after treatment with dimethyl fumarate, with reductions that were evident as early as 6 months after treatment onset.42 Recent reports have also shown that effective treatment of spinal muscle atrophy with the recently approved antisense oligonucleotide nusinersen was associated with clinical benefits and rapid decreases in plasma NfL concentrations.43 All these studies support the prospect of using NfL as a marker of therapy response in neurodegenerative conditions in which treatments are actively being developed. NfL may be particularly valuable in combination with disease-specific biomarkers for monitoring meaningful response to therapy in populations with multiple comorbid brain pathologies in whom it is uncertain which pathology is primarily responsible for the clinical dementia syndrome.
Diversity and Personalized Medicine in the Era of Blood Biomarkers
Age, race, comorbidities, genetics, and exposures resulting from social and economic factors may all contribute to heterogeneity in clinical and biological features of neurodegenerative diseases. For this reason, studies of diverse populations will be critical for informing generalizability of emerging blood-based biomarkers, developing personalized approaches to diagnosis and disease monitoring, and developing pragmatic clinical algorithms and decision trees.
Age has been found to be associated with blood-based biomarkers for neurodegeneration, particularly plasma NfL and p-tau.8 Several studies have reported race effects on biofluid biomarkers of neurodegeneration. For example, prior CSF studies suggested that CSF p-tau may be less accurate for AD diagnosis in African Americans compared with White individuals,e15 particularly among APOEε4 carriers. Findings in studies of blood-based biomarkers are mixed. One large cohort study reported that while plasma Aβ42/Aβ40 was accurate for discrimination of brain amyloidosis among both African Americans and non-Hispanic White Americans, plasma p-tau181, p-tau231, and NfL were less accurate in African Americans.44 Another large multiethnic cohort study, however, reported high accuracy for plasma p-tau181 and p-tau217 for discriminating AD neuropathology among Black individuals.
One biological factor that has consistently been shown to affect blood biomarkers of neurodegeneration are medical comorbidities. Renal and hepatic function may moderate the association between blood-based biomarkers for AD and brain pathology. Several studies have found an association between renal or hepatic function and plasma Aβ, NfL, total-tau, and/or p-tau181 levels.9 In fact, a large cohort study of more than 1,600 older adults, half of whom were Mexican Americans, found that hypertension, dyslipidemia, and diabetes were each individually and additively associated with plasma NfL levels.45 The Systolic Blood Pressure Intervention Trial identified a strong association between longitudinal changes in plasma NfL (but not Aβ or total tau) and treatment-induced changes in renal function.46 In addition, p-tau181 and p-tau217 blood concentrations are also associated with chronic kidney disease and other comorbidities, such as hypertension, stroke, or myocardial infarction.8 These findings suggest that thresholds for some relevant blood-based biomarkers may need to be adjusted for certain medical comorbidities, such as renal function. Understanding associations and interactions of blood-based biomarkers with genetics is still at an early stage. One study has identified higher blood p-tau levels in cognitively normal APOEε4 carriers; however, this may simply represent the higher likelihood of preclinical brain amyloidosis in this population.8
Thus, more research is needed to parse out the complex relationship between age, race, genetics, neurodegeneration, and medical comorbidities. Resolving these questions will be critically important for informing appropriate use and diagnostic cutoffs for these blood-based biomarkers.
Limitations, Current Knowledge Gaps, and Future Directions
Preanalytical Factors
Different types of collection tubes can influence protein concentrations, with lithium heparin tubes having the highest concentrations for these biomarkers compared with EDTA K2, serum, or citrate tubes.47 Moreover, freezing and thawing affect differently the identification of these biomarkers—for example, in the plasma, Aβ42, NfL, t-tau, and Aβ42/Aβ40 are relatively stable after several freeze/thaw cycles, while in the serum, only NfL remains stable, and the concentrations of all others decrease significantly.47
Detection of Comorbid Brain Pathologies and Specific Biomarkers for Non-AD Dementias
Most neurodegenerative diseases, especially late-onset cases, are multiproteinopathies. Different pathologies contribute to particular phenotypes, and the severity of symptoms is influenced by the existence of other pathologic comorbidities. Current biomarkers reflect 1 pathophysiologic process, but they cannot inform whether this process is responsible for the main clinical syndrome. For example, associations of high plasma concentrations of p-tau217 with progressive supranuclear palsy or semantic variant primary progressive aphasia would suggest AD pathology coexisting with FTLD. Other studies relying on blood markers for the unbiased amyloid, tau, and neurodegeneration classification have shown the potential of these biomarkers in identifying individuals with dementia with Lewy bodies and comorbid AD pathology.17,47 Identification of multiproteinopathy will likely require the use of several biomarkers and a thorough clinical assessment to identify which of these processes is responsible for the main clinical syndrome.
Challenges in Biomarker Disclosure
An emerging challenge to be addressed as blood-based biomarkers enter clinical use is the understanding of the psychosocial impact of biomarker status disclosure, especially in asymptomatic people at risk of neurodegenerative disorders. One natural concern is the psychological distress associated with the awareness of an increased risk of dementia. Emerging data, however, suggest that disclosure conducted using well-established protocols is not associated with negative effects. In fact, asymptomatic individuals who learned about negative AD biomarker status through amyloid imaging reported less anxiety and concern, whereas patients who learned about a positive amyloid PET did not experience an increase in anxiety, depression, or suicidality and were more likely to engage in healthy lifestyles.49 Fluid biomarker result disclosure will become increasingly common, and future research should address the best uses in asymptomatic individuals and the safest possible ways to disclose results.
Assessing Cost-Effectiveness and Equitable Use of Fluid Biomarkers
Because blood biomarkers are potentially less expensive and more accessible than molecular imaging or CSF biomarkers, one would anticipate that their introduction to clinical practice would mitigate disparities and be especially beneficial to disadvantaged groups. An accessible and inexpensive blood biomarker could be more easily used and allow more equitable access to care. This, however, should be accomplished by the design of research projects that make findings generalizable by building cohorts that are representative of the entire population. How the benefits of blood biomarkers affect different racial or socioeconomic groups should be the subject of projects to assess the distributional cost-effectiveness of blood biomarkers.
Conclusions
The fast-growing field of blood-based biomarkers for neurodegenerative diseases has changed the way we approach these conditions. Although none has yet received regulatory approval for clinical use, their clinical value suggest they soon will enter clinical routine. Blood markers allow the detection of key pathophysiologic processes of these conditions that can be repeated over time providing valuable information about the underlying diagnosis, disease progression, or response to a therapy. These markers can be useful in specialized settings but probably will have more impact in less specialized settings, such as in general neurology or primary care. In these contexts, other specific biomarkers, such as CSF or molecular imaging markers, are less accessible, and the incorporation of blood biomarkers to the assessment together with clinical examination and imaging studies could facilitate an earlier referral to the specialist and an earlier and more accurate diagnosis. By contrast, for example, to cholesterol levels, which are used to guide treatment decisions, blood biomarkers for neurodegeneration are not anticipated to be substitutes for clinical judgment. This may change when effective medications to treat neurodegeneration evolve. Blood biomarkers are already being used in clinical trials to assess disease modification and as an entry criterion during screening. It is critical to advance in the harmonization of protocols, the understanding of the performance in real-world cohorts with systemic and brain comorbidities, and in the development of cutoffs to advance in their implementation in clinical routine. The rapid technological advances and our learnings from the CSF biomarker field should make this path easier. Ultimately, blood-based biomarkers can shorten the diagnostic process, provide an effective follow-up, and facilitate the approval of effective therapies for neurodegenerative diseases.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- BTI
Brain Trauma Indicator
- CLIA
Clinical Laboratory Improvement Amendments
- ECL
electrochemiluminescence
- FDA
US Food and Drug Administration
- FTLD
frontotemporal lobar degeneration
- GFAP
glial fibrillary acidic protein
- IVD
in vitro diagnostic
- MCI
mild cognitive impairment
- NfL
neurofilament light chain
- p-tau
phosphorylated tau
- t-tau
total tau
- TBI
traumatic brain injury
- UCH-L1
ubiquitin-C-terminal-hydrolase-L1
Appendix. Authors
Footnotes
See page e461
Study Funding
No targeted funding reported.
Disclosure
D. Alcolea participated in advisory boards from Fujirebio-Europe and Roche Diagnostics, received speaker honoraria from Fujirebio-Europe, Roche Diagnostics, Nutricia, Krka Farmacéutica S.L., Zambon S.A.U., and Esteve Pharmaceuticals S.A. and declares a filed patent application (WO2019175379 A1 markers of synaptopathy in neurodegenerative disease). M.S. Beeri reports no disclosures relevant to the manuscript. J.C. Rojas is the site PI for clinical trials sponsored by Eli-Lilly and Eisai and is supported by NIH/NIA K23AG059888. R.C. Gardner has received consulting fees from BrainBox Inc and is supported by the National Institute of Neurological Disorders and Stroke R01NS110944 and VA CSRD Merit CX002346. A. Lleó participated in advisory boards from Fujirebio-Europe, Grifols, Novartis, Roche Diagnostics, Otsuka Pharmaceutical, Nutricia, Zambón S.A.U., and Biogen, received speaker honoraria from Lilly, Biogen, KRKA, and Zambon and declares a filed patent application (WO2019175379 A1 markers of synaptopathy in neurodegenerative disease). Go to Neurology.org/N for full disclosures.
References
- 1.Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013;8(9):e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaneko N, Nakamura A, Washimi Y, et al. Novel plasma biomarker surrogating cerebral amyloid deposition. Proc Jpn Acad Ser B Phys Biol Sci. 2014;90(9):353-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature. 2018;554(7691):249-254. [DOI] [PubMed] [Google Scholar]
- 4.Tatebe H, Kasai T, Ohmichi T, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer's disease and down syndrome. Mol Neurodegener. 2017;12(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mielke MM, Hagen CE, Xu J, et al. Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14(8):989-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. EMBO Mol Med. 2019;11(12):e11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haroutunian V, Schnaider-Beeri M, Schmeidler J, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65(9):1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28(7):1398-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry K, Asken BM, Grab JD, et al. Hepatic and renal function impact concentrations of plasma biomarkers of neuropathology. Alzheimers Dement (Amst). 2022;14(1):e12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedet AL, Brum WS, Hansson O, et al. The accuracy and robustness of plasma biomarker models for amyloid PET positivity. Alzheimers Res Ther. 2022;14(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):E1647-E1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422-433. [DOI] [PubMed] [Google Scholar]
- 13.Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26(3):379-386. [DOI] [PubMed] [Google Scholar]
- 14.Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor A, Karikari TK, Poole T, et al. Plasma phospho-tau181 in presymptomatic and symptomatic familial Alzheimer's disease: a longitudinal cohort study. Mol Psychiatry. 2021;26(10):5967-5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lleó A, Zetterberg H, Pegueroles J, et al. Phosphorylated tau181 in plasma as a potential biomarker for Alzheimer's disease in adults with Down syndrome. Nat Commun. 2021;12(1):4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alcolea D, Delaby C, Muñoz L, et al. Use of plasma biomarkers for AT(N) classification of neurodegenerative dementias. J Neurol Neurosurg Psychiatry. 2021;92(11):1206-1214. [DOI] [PubMed] [Google Scholar]
- 18.Milà-Alomà M, Ashton NJ, Shekari M, et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's disease. Nat Med. 2022;28(9):1797-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayoumy S, Verberk IMW, den Dulk B, et al. Clinical and analytical comparison of six Simoa assays for plasma P-tau isoforms P-tau181, P-tau217, and P-tau231. Alzheimers Res Ther. 2021;13(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashton NJ, Puig‐Pijoan A, Milà‐Alomà M, et al. Plasma and CSF biomarkers in a memory clinic: head-to-head comparison of phosphorylated tau immunoassays. Alzheimers Dement. 2022. doi: 10.1002/alz.12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janelidze S, Bali D, Ashton NJ, et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer's disease. Brain. 2022;2022:awac333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palermo G, Mazzucchi S, Della Vecchia A, et al. Different clinical contexts of use of blood neurofilament light chain protein in the spectrum of neurodegenerative diseases. Mol Neurobiol. 2020;57(11):4667-4691. [DOI] [PubMed] [Google Scholar]
- 23.Biberthaler P, Musaelyan K, Krieg S, et al. Evaluation of acute glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 plasma levels in traumatic brain injury patients with and without intracranial lesions. Neurotrauma Rep. 2021;2(1):617-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korley FK, Datwyler SA, Jain S, et al. Comparison of GFAP and UCH-L1 measurements from two prototype assays: the Abbott i-STAT and ARCHITECT assays. Neurotrauma Rep. 2021;2(1):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verberk IMW, Thijssen E, Koelewijn J, et al. Combination of plasma amyloid beta(1-42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res Ther. 2020;12(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milà-Alomà M, Salvadó G, Gispert JD, et al. Amyloid beta, tau, synaptic, neurodegeneration, and glial biomarkers in the preclinical stage of the Alzheimer's continuum. Alzheimers Dement. 2020;16(10):1358-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee P, Pedrini S, Stoops E, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer's disease. Transl Psychiatry. 2021;11(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benussi A, Ashton NJ, Karikari TK, et al. Serum glial fibrillary acidic protein (GFAP) is a marker of disease severity in frontotemporal lobar degeneration. J Alzheimers Dis. 2020;77(3):1129-1141. [DOI] [PubMed] [Google Scholar]
- 29.Zhu N, Santos-Santos M, Illán-Gala I, et al. Plasma glial fibrillary acidic protein and neurofilament light chain for the diagnostic and prognostic evaluation of frontotemporal dementia. Transl Neurodegener. 2021;10(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oeckl P, Halbgebauer S, Anderl-Straub S, et al. Targeted mass spectrometry suggests beta-synuclein as synaptic blood marker in Alzheimer's disease. J Proteome Res. 2020;19(3):1310-1318. [DOI] [PubMed] [Google Scholar]
- 31.Oeckl P, Wagemann O, Halbgebauer S, et al. Serum beta-synuclein is higher in down syndrome and precedes rise of pTau181. Ann Neurol. 2022;92(1):6-10. [DOI] [PubMed] [Google Scholar]
- 32.Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18(12):2669-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carmona-Iragui M, Alcolea D, Barroeta I, et al. Diagnostic and prognostic performance and longitudinal changes in plasma neurofilament light chain concentrations in adults with Down syndrome: a cohort study. Lancet Neurol. 2021;20(8):605-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas JC, Wang P, Staffaroni AM, et al. Plasma neurofilament light for prediction of disease progression in familial frontotemporal lobar degeneration. Neurology. 2021;96(18):e2296-e2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Ende EL, Meeter LH, Poos JM, et al. Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol. 2019;18(12):1103-1111. [DOI] [PubMed] [Google Scholar]
- 37.Steinacker P, Semler E, Anderl-Straub S, et al. Neurofilament as a blood marker for diagnosis and monitoring of primary progressive aphasias. Neurology. 2017;88(10):961-969. [DOI] [PubMed] [Google Scholar]
- 38.Rojas JC, Karydas A, Bang J, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol. 2016;3:216-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fortea J, Vilaplana E, Carmona-Iragui M, et al. Clinical and biomarker changes of Alzheimer's disease in adults with Down syndrome: a cross-sectional study. Lancet. 2020;395(10242):1988-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrne LM, Rodrigues FB, Blennow K, et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington's disease: a retrospective cohort analysis. Lancet Neurol. 2017;16(8):601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197-210. [DOI] [PubMed] [Google Scholar]
- 42.Sejbaek T, Nielsen HH, Penner N, et al. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J Neurol Neurosurg Psychiatry. 2019;90(12):1324-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winter B, Guenther R, Ludolph AC, Hermann A, Otto M, Wurster CD. Neurofilaments and tau in CSF in an infant with SMA type 1 treated with nusinersen. J Neurol Neurosurg Psychiatry. 2019;90(9):1068-1069. [DOI] [PubMed] [Google Scholar]
- 44.Schindler SE, Karikari TK, Ashton NJ, et al. Effect of race on prediction of brain amyloidosis by plasma Aβ42/Aβ40, phosphorylated tau, and neurofilament light. Neurology. 2022;99(3):e245-e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Bryant S, Petersen M, Hall J, et al. Characterizing plasma NfL in a community-dwelling multi-ethnic cohort: results from the HABLE study. Alzheimers Dement. 2022;18(2):240-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pajewski NM, Elahi FM, Tamura MK, et al. Plasma amyloid beta, neurofilament light chain, and total tau in the Systolic Blood Pressure Intervention Trial (SPRINT). Alzheimers Dement. 2022;18(8):1472-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verberk IMW, Misdorp EO, Koelewijn J, et al. Characterization of pre‐analytical sample handling effects on a panel of Alzheimer's disease-related blood‐based biomarkers: results from the Standardization of Alzheimer's Blood Biomarkers (SABB) working group. Alzheimer’s Dement. 2022;18(8):1484-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van De Beek M, Ooms FAH, Ebenau JL, et al. Association of the ATN research framework with clinical profile, cognitive decline, and mortality in patients with dementia with Lewy bodies. Neurology. 2022;98(12):E1262-E1272. [DOI] [PubMed] [Google Scholar]
- 49.Largent EA, Harkins K, Van Dyck CH, Hachey S, Sankar P, Karlawish J. Cognitively unimpaired adults' reactions to disclosure of amyloid PET scan results. PLoS One. 2020;15(2):e0229137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- eReferences are listed at links.lww.com/WNL/C678.