Abstract
Background and Objectives
Sleep disordered breathing (SDB) has been related to amyloid deposition and an increased dementia risk. However, how SDB relates to medial temporal lobe neurodegeneration and subsequent episodic memory impairment is unclear. Our objective was to investigate the impact of amyloid positivity on the associations between SDB severity, medial temporal lobe subregions, and episodic memory performance in cognitively unimpaired older adults.
Methods
Data were acquired between 2016 and 2020 in the context of the Age-Well randomized controlled trial of the Medit-Aging European project. Participants older than 65 years who were free of neurologic, psychiatric, or chronic medical diseases were recruited from the community. They completed a neuropsychological evaluation, in-home polysomnography, a Florbetapir PET, and an MRI, including a specific high-resolution assessment of the medial temporal lobe and hippocampal subfields. Multiple linear regressions were conducted to test interactions between amyloid status and SDB severity on the volume of MTL subregions, controlling for age, sex, education, and the ApoE4 status. Secondary analyses aimed at investigating the links between SDB, MTL subregional atrophy, and episodic memory performance at baseline and at a mean follow-up of 20.66 months in the whole cohort and in subgroups stratified according to amyloid status.
Results
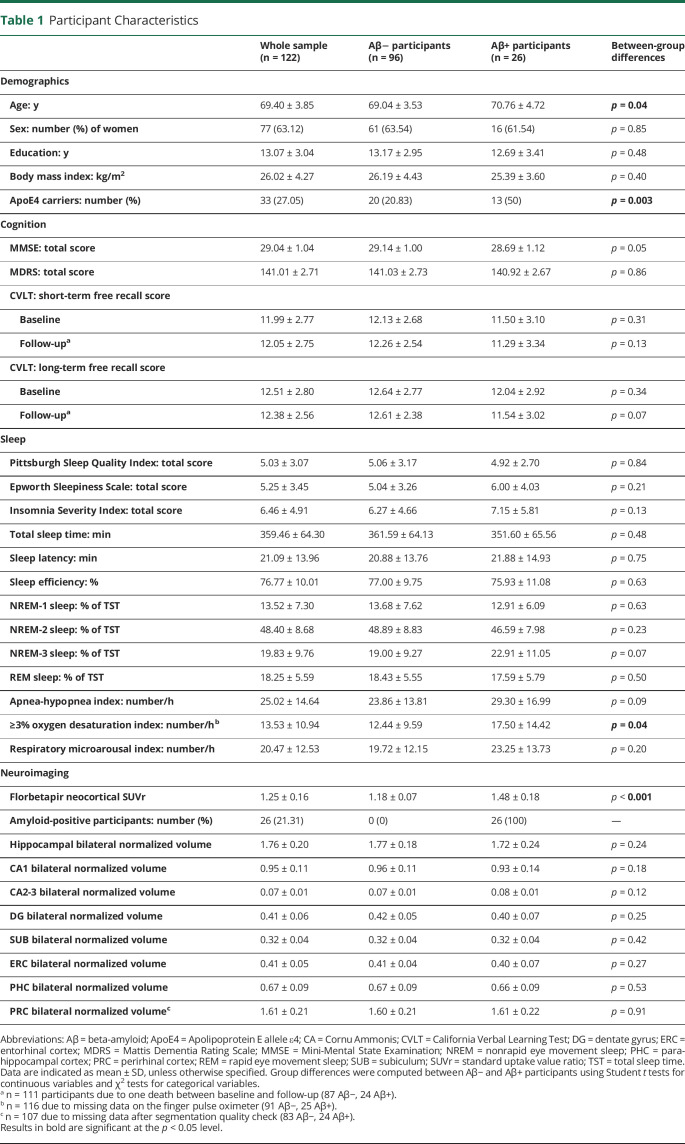
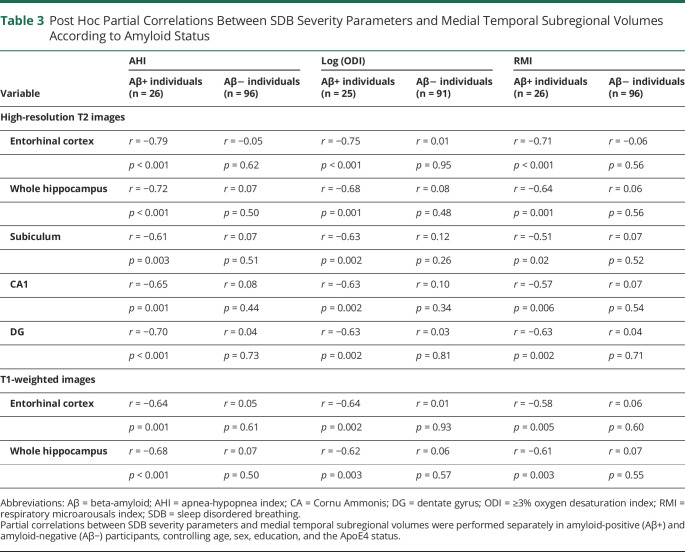
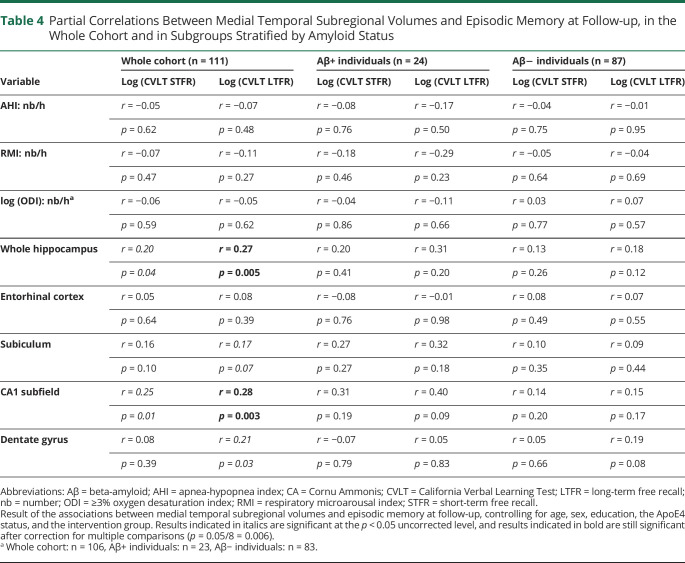
We included 122 cognitively intact community-dwelling older adults (mean age ± SD: 69.40 ± 3.85 years, 77 women, 26 Aβ+ individuals) in baseline analyses and 111 at follow-up. The apnea-hypopnea index interacted with entorhinal (β = −0.81, p < 0.001, pη2 = 0.19), whole hippocampal (β = −0.61, p < 0.001, pη2 = 0.10), subiculum (β = −0.56, p = 0.002, pη2 = 0.08), CA1 (β = −0.55, p = 0.002, pη2 = 0.08), and DG (β = −0.53, p = 0.003, pη2 = 0.08) volumes such that a higher sleep apnea severity was related to lower MTL subregion volumes in amyloid-positive individuals, but not in those who were amyloid negative. In the whole cohort, lower whole hippocampal (r = 0.27, p = 0.005) and CA1 (r = 0.28, p = 0.003) volumes at baseline were associated with worse episodic memory performance at follow-up.
Discussion
Overall, we showed that SDB was associated with MTL atrophy in cognitively asymptomatic older adults engaged in the Alzheimer continuum, which may increase the risk of developing memory impairment over time.
Trial Registration Information
ClinicalTrials.gov Identifier: NCT02977819.
Sleep disordered breathing (SDB), characterized by recurrent upper airway collapse during sleep, is largely prevalent and underdiagnosed in older adults.1,2 SDB-induced intermittent hypoxia and sleep fragmentation are believed to trigger neurodegeneration3 and increase the risk of cognitive decline and dementia.4 Previous studies have demonstrated that older adults with SDB exhibit higher amyloid deposition notably in posterior cortical regions.5-7 Of importance, SDB may exacerbate amyloid pathology over time and interact with Alzheimer disease pathology to precipitate cognitive decline.7-9 We have previously shown that cognitively healthy older adults with moderate-to-severe SDB exhibit greater amyloid deposition, gray matter (GM) volume, perfusion, and metabolism in the precuneus and posterior cingulate cortices.6 Contrary to our expectations and to previous reports,10-14 we did not observe GM loss in the medial temporal lobe (MTL), which is known to play a key role in episodic memory,15,16 and to be atrophied in Alzheimer disease, mainly (but potentially not exclusively) because of tau pathology spreading.17-19 However, the MTL is composed of several subregions, namely the entorhinal, perirhinal, and parahippocampal cortices and the hippocampus, which can itself be divided into distinct subfields, including the subiculum (SUB), Cornu Ammonis (CA1 to CA3), and dentate gyrus (DG). MTL subregions are not homogeneously affected by Alzheimer disease pathology in predementia stages. Previous studies have reported an early involvement of perirhinal and entorhinal cortices and an early vulnerability of the CA1 subfield.20-22 Of interest, animal studies have revealed that some MTL structures are particularly vulnerable to hypoxia, such as the hippocampus and CA1 subfield.23-25 Yet the links between SDB and MTL volume integrity in older populations still need to be clarified. If most studies have reported SDB-related decreases in GM volume in MTL regions including the hippocampus,11-13 others have rather showed increases in the hippocampal volume26,27 or no link with MTL atrophy.6,28 First, these discrepancies are likely partly due to methodological limitations, with voxel-wise volumetry being less suitable to reveal subregional and focal neurodegeneration in small brain regions. Thus, segmentations of MTL subregions on high-resolution MRI sequences may be better suited to assess the earliest GM changes associated with SDB. Second, SDB consequences may differ depending on its severity or the presence of underlying pathology.29 In Alzheimer disease, the development and spreading of amyloid pathology is known to precede by several years and believed to promote neurodegenerative processes and cognitive deficits.30-32 Therefore, SDB-related neurodegeneration may be only evident, or more strongly measurable, once amyloid deposition becomes significant. Meanwhile, SDB alone may not be enough to lead to significant atrophy in amyloid-negative individuals or could be associated with increases in GM volume due to inflammatory processes or edema, as previously suggested.33,34 Further investigating whether and in which conditions SDB affects MTL atrophy is crucial, given the involvement of these structures in episodic memory processes and cognitive deterioration, because SDB could represent a modifiable risk factor of cognitive decline. Moreover, treating SDB may have the potential to improve cognition.7
Our primary objective was to investigate the associations between SDB severity and the volume of MTL substructures, including hippocampal subfields specifically segmented on a high-resolution T2-weighted MRI sequence of the MTL, according to amyloid status, in cognitively healthy community-dwelling older adults. Our secondary objective was to assess the links between SDB and MTL subregion volumes with episodic memory performance, both cross-sectionally and longitudinally. Sensitivity and specificity analyses notably aimed at (1) investigating whether disrupted sleep architecture (e.g., reduced deep sleep and greater light sleep) mediated the associations between sleep apnea severity and MTL integrity and (2) verifying whether our results were influenced by sex.
We hypothesized that SDB would be associated with GM atrophy in regions that are early affected by Alzheimer disease pathology and/or particularly sensitive to hypoxia (i.e., perirhinal, entorhinal, and CA1 regions), especially in amyloid-positive participants. Last, we expected MTL atrophy and/or SDB severity to be associated with worse episodic memory performance.
Methods
Study Design
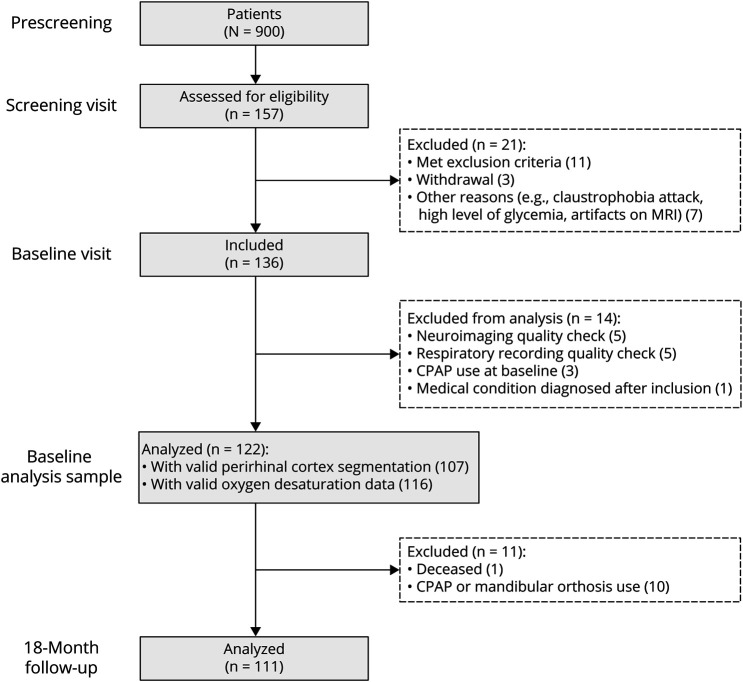
Participants included in this study were enrolled in the Age-Well randomized controlled trial (RCT) of the Medit-Aging European project,35 sponsored by the French National Institute of Health and Medical Research (INSERM). As previously described,6 between 2016 and 2018, we recruited cognitively unimpaired older adults from the community aged older than 65 years with no evidence of major neurologic and psychiatric disorders, chronic diseases, or current medication interfering with cognition (Figure 1 and Table 1). Participants treated for SDB at baseline were excluded from the analysis sample. Baseline examinations consisted in a detailed neuropsychological assessment, polysomnography recording, structural MRI, 18F-Florbetapir PET scan, and Apolipoprotein E ε4 (ApoE4) genotyping, performed within a mean (SD) time interval of 32.24 (17.32) days. Participants were randomized after baseline in 1 of the 3 following groups: meditation-based intervention, foreign language training, or passive control arm. Interventions lasted for 18 months. Then, they underwent the same neuropsychological assessment than at baseline, within a mean (SD) time interval of 20.66 (0.76) months between cognitive evaluations. Because we did not aim at investigating the effect of interventions, longitudinal analyses were controlled for the intervention group. Moreover, participants who presented with an apnea-hypopnea index >15 and a respiratory arousal index >10 were referred to a sleep medicine specialist through their general practitioner. Participants who started continuous positive airway pressure treatment after baseline were excluded from analyses using follow-up data (Figure 1).
Figure 1. Flowchart of the Study.
CPAP = continuous positive airway pressure.
Table 1.
Participant Characteristics
Polysomnography Recording
Polysomnography was performed in the home environment using a portable device (Siesta, Compumedics, Australia) with 20 EEG electrodes (Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, Cz, T3, T4, P3, P4, Pz, O1, O2, vertex ground, and a bimastoid reference), an electrooculogram, electrocardiogram, chin electromyogram, thoracic and abdominal belts, nasal and oral thermistors, and a finger pulse oximeter. The EEG processing and scoring protocol have been described elsewhere.6 Recordings were scored following the American Academy of Sleep Medicine rules,36 and sleep apnea was defined by a ≥90% drop of nasal pressure for at least 10 seconds, whereas sleep hypopnea was characterized by a ≥30% drop of nasal pressure for a minimum of 10 seconds, associated with an arousal or a ≥3% oxygen desaturation.36 We extracted 3 standard variables reflecting sleep apnea severity: the apnea-hypopnea index (AHI; the sum of apneas and hypopneas per hour of sleep), the oxygen desaturation index (ODI; the number of oxygen desaturations superior to 3% per hour of sleep), and the respiratory microarousal index (RMI; the number of respiratory arousals per hour of sleep) (Table 1). In our cohort, 91 of 122 participants had moderate-to-severe SDB (AHI ≥15/h), but were poorly symptomatic, with only 11 of 91 exhibiting excessive daytime sleepiness (Epworth Sleepiness Scale score ≥10).
Neuroimaging Examinations
Participants underwent structural MRI and a dual-phase Florbetapir-PET scanning at the Cyceron Center (Caen, France) on a Philips Achieva 3T scanner (Eindhoven, The Netherlands) and a Discovery RX VCT 64 PET-CT scanner (General Electric Healthcare, Milwaukee, WI), respectively. Acquisition details have been previously reported6,35 and are available in eMethods, links.lww.com/WNL/C821.
Structural MRI
T1-weighted images were segmented using fluid attenuated inversion recovery (FLAIR) images, spatially normalized to the Montreal Neurologic Institute (MNI) template, modulated using the SPM12 segmentation procedure37 and smoothed with an 8-mm full-width at half-maximum Gaussian filter. Images were then masked to exclude non-GM voxels from the analyses.
In addition, 2 high-resolution T2-weighted structural images were acquired perpendicularly to the long axis of the hippocampus (repetition time [TR] = 5,310 ms; echo time [TE] = 110 ms; flip angle = 90°; 23 slices; slice thickness = 2.5 mm; field of view (FOV) = 140 × 111 mm2; matrix = 352 × 352; in-plane resolution = 0.398 × 0.398 mm2, acquisition time = 3 minutes 43). The 2 high-resolution T2-weighted structural images of the MTL were coregistered and averaged using SPM12. Hippocampal subfields (CA1, CA2, CA3, DG, SUB) and extrahippocampal region (entorhinal cortex [ERC], Brodmann areas 35 and 36, and parahippocampal cortex [PHC]) volumes were estimated automatically on averaged high-resolution T2-weighted images using the Automated Segmentation for Hippocampal Subfields (ASHS) software together with a custom atlas.38 We used a custom atlas because recent observations based on ex vivo MRI and histology data showed that the dark band, a hypointense line appearing within the hippocampal GM on T2 images frequently used in segmentation protocols as the boundary between DG and SUB/CA, is composed of the stratum lacunosum moleculare.39 Thus, dark band voxels should be labeled as SUB/CA only, but were split between SUB/CA and DG in the original atlas provided with ASHS (ashs_atlas_upennpmc_20170810).38 Therefore, we manually edited the segmentations from the original atlas (29 subjects ranging from normal cognition to mild Cognitive Impairment) to label all dark band voxels as SUB/CA and generated a new atlas. Leave-one-out cross-validation analyses showed similar accuracy when compared with the original atlas (Dice coefficients: CA1 = 0.82, CA2 = 0.56, CA3 = 0.53, DG = 0.80, SUB = 0.76, ERC = 0.79, BA35 = 0.72, BA36 = 0.78, PHC = 0.78). For complementary analyses, the ERC, perirhinal cortex (PRC), PHC, and whole hippocampus were also segmented on T1-weighted images using a specific atlas (ashsT1_atlas_upennpmc_07202018).22 All MR images and segmentations were visually inspected, and failed segmentations were manually edited when feasible or discarded (see eMethods, links.lww.com/WNL/C821 for additional details). For statistical analyses, left and right hemispheres were averaged for each subregion to limit the number of statistical tests. Moreover, CA2 and CA3, on one hand, and BA35 and BA36, on the other hand, were pooled together and referred to as CA2-3 region and the PRC, respectively, hereafter. Of note, we verified a posteriori whether BA35 and BA36 showed different associations with SDB parameters, and because it was not the case, we displayed only the results with the whole PRC. Total hippocampal volume was obtained by summing CA1, CA2-3, DG, and SUB regions. Bilateral averaged volumes (mm3) were normalized to the total intracranial volume (TIV; obtained from SPM12) to compensate for interindividual variability in head size, as follows: averaged bilateral volume/(TIV*1,000).
Florbetapir-PET
PET images were coregistered on their corresponding anatomical MRI. Resulting images were then normalized to the MNI template using deformation parameters derived from the anatomical MRI, scaled using cerebellar GM as a reference, smoothed at 10 mm and masked to exclude non-GM voxels. Individual neocortical amyloid standard uptake value ratio (SUVr) was extracted from normalized and scaled Florbetapir-PET images using a neocortical GM mask excluding the cerebellum, occipital and sensory motor cortices, hippocampi, amygdala, and basal nuclei.6 The threshold for amyloid positivity was defined as >0.99 and corresponded to the 99.9th percentile of the neocortical SUVr distribution among 45 healthy young individuals younger than 40 years from the Imagerie Multimodale de la maladie d'Alzheimer Précoce (IMAP) cohort.6
Neuropsychological Assessment
Participants underwent a neuropsychological assessment of global cognitive functioning, processing speed, attention, working memory, executive functions, and episodic memory, detailed elsewhere.6,35 Verbal episodic memory was assessed using the French version of the California Verbal Learning Test (CVLT).40 Short-term and long-term free recall scores were used in analyses.
Statistical Analyses
Before statistical analyses, the distribution of all variables was visually inspected and checked for normality using Shapiro-Wilk tests. All non-normal variables were log transformed. We first assessed the associations between SDB parameters and MTL subregions according to amyloid status by performing multivariable linear regressions with the volume of each MTL subregion as dependent variables and SDB parameters and amyloid status as independent variables, with age, sex, education, and the ApoE4 status as covariates. Separate models were conducted for each MTL subregion and each SDB parameter, and an interaction term was added between SDB parameters and amyloid status. When the interaction was significant, post hoc partial correlations were conducted separately in amyloid-positive and amyloid-negative individuals. Adding the body mass index as a covariate in regression models did not change the results, so it was not included in the final model to avoid statistical overfitting.
Then, we investigated the links between SDB parameters and SDB-associated MTL regions and episodic memory performance at baseline and follow-up. We thus performed partial correlations between SDB parameters and/or MTL subregion volumes and both the log-transformed short-term and long-term free recalls of the CVLT at the 2 time points, controlling for age, sex, education, ApoE4 status, and the intervention group for longitudinal analyses. These analyses were performed in the whole cohort and in subgroups stratified by amyloid status. Of note, CVLT scores at follow-up (i.e., performed after the interventions) did not statistically differ between the passive control, English-learning, and meditation groups (eFigure 1, links.lww.com/WNL/C821).
Finally, we performed several sensitivity and specificity analyses to ensure the robustness of our results. First, we replicated multiple regression analyses between SDB parameters and MTL subregion volumes derived from T1-weighted images, rather than high-resolution MRI sequences. We also checked whether our results were affected by laterality by replicating partial correlations between SDB severity parameters and left and right MTL volumes separately, rather than bilateral volumes, in amyloid-positive and amyloid-negative participants, controlling for age, sex, education, and ApoE4 status. Second, to evaluate the specificity of our main results (i.e., to verify whether the links between SDB and atrophy were restricted or not to MTL subregions), we performed voxel-wise multivariable regressions between SDB parameters and GM volume, controlling for the same covariates, using a p < 0.005 (uncorrected) threshold combined with p < 0.05 family-wise error (FWE) cluster-level threshold.
Third, we investigated the influence of sex on our results by conducting partial correlations between SDB parameters and MTL subregion volumes in sex-stratified subgroups (in the whole sample and subgroups separated according to amyloid status), controlling for age, education, and the ApoE4 status.
Finally, to verify whether the associations between sleep apnea severity and MTL integrity were mediated by alterations in sleep architecture (e.g., reduced deep sleep and increased lighter sleep and nocturnal awakenings), we assessed the associations between sleep architecture variables and both (1) SDB parameters and (2) MTL subregion volumes, controlling for age, sex, education, and the ApoE4 status. In case of significant associations, mediation analyses were conducted to confirm the mediating role of sleep architecture.
For all analyses, we reported only results surviving the correction for multiple comparisons (p = 0.05/number of comparisons, see table legends for details).
Standard Protocol Approvals, Registrations, and Patient Consents
All participants included in the Age-Well RCT of the Medit-Aging European project gave their written informed consent before the examinations. The Age-Well RCT was approved by the ethics committee (IMAP : Imagerie Multimodale de la maladie d'Alzheimer Précoce [CPP] Nord-Ouest III, Caen; trial registration number: EudraCT: 2016- 002441-36; IDRCB: 2016-A01767-44; ClinicalTrials.gov Identifier: NCT02977819).35
Data Availability
Data are made available on request following a formal data sharing agreement and approval by the consortium and executive committee (silversantestudy.eu/2020/09/25/data-sharing). The material can be mobilized, under the conditions and modalities defined in the Medit-Aging Charter, by any research team belonging to an Academic for conducting a scientific research project relating to the scientific theme of mental health and well-being in older people. The material may also be mobilized by nonacademic third parties, under conditions, particularly financial, which will be established by a separate agreement between INSERM and by the said third party. Data sharing policies described in the Medit-Aging Charter are in compliance with our ethical approval and guidelines from our funding body.
Results
Participant Characteristics
Of the 136 participants included in the Age-Well RCT at baseline, 122 were included in the analysis sample (Figure 1). Their demographical and clinical characteristics are detailed in Table 1. The mean age of the sample was 69.40 ± 3.85 years, with 77 (63.12%) women and 33 (27.05%) ApoE4 carriers. Twenty-six (21.31%) participants were amyloid positive, and 96 were amyloid negative. Amyloid-positive participants were significantly older and more susceptible to be ApoE4 carriers but did not differ from amyloid-negative individuals for sex ratio, education level, memory performance, MTL subregion volumes, and sleep data (Table 1).
Associations Between MTL Subregional Volumes and SDB According to Amyloid Status
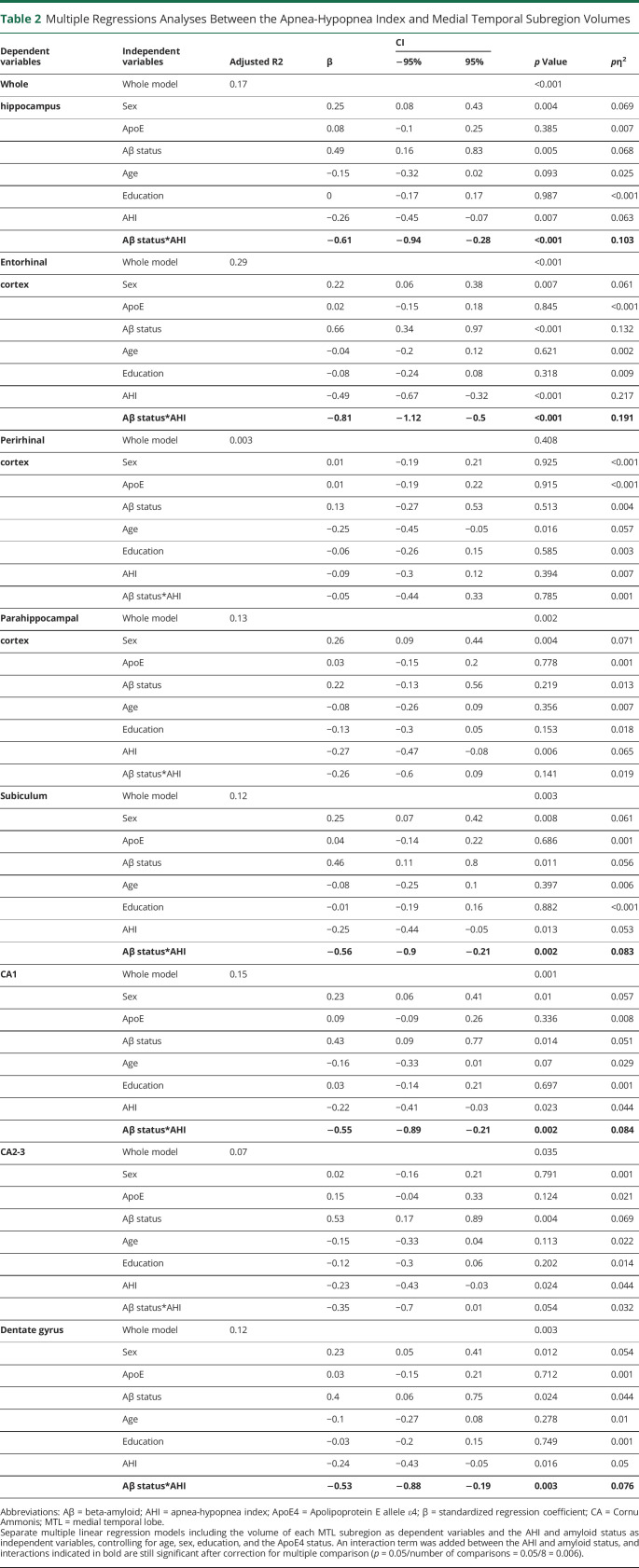
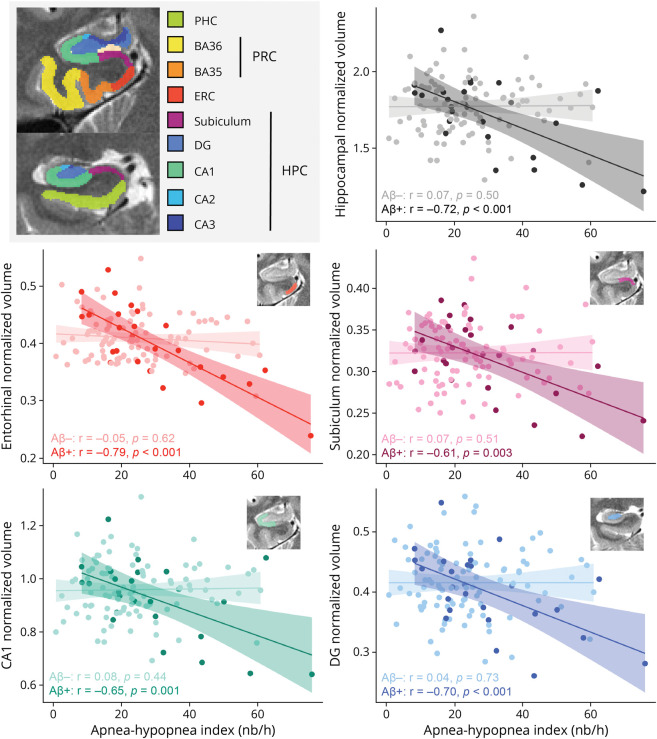
The AHI and amyloid status significantly interacted on the volumes of the ERC (β = −0.81 [95% CI −1.12 to −0.50]; p < 0.001; pη2 = 0.19), whole hippocampus (β = −0.61 [95% CI −0.94 to −0.28]; p < 0.001; pη2 = 0.10), SUB (β = −0.56 [95% CI −0.90 to −0.22]; p = 0.002; pη2 = 0.08), CA1 (β = −0.55 [95% CI −0.89 to −0.22]; p = 0.002; pη2 = 0.08), and DG (β = −0.53 [95% CI −0.88 to −0.19]; p = 0.003; pη2 = 0.08) extracted from high-resolution T2-weighted MTL images (Table 2). Post hoc analyses showed that a higher AHI was significantly associated with lower GM volume in the ERC (r = −0.79, p < 0.001), whole hippocampus (r = −0.72, p < 0.001), SUB (r = −0.61, p = 0.001), CA1 (r = −0.65, p = 0.001), and DG (r = −0.70, p < 0.001), only in amyloid-positive participants (Figure 2 and Table 3). Similarly, a greater ODI was associated with lower ERC, whole hippocampus, SUB, and CA1 volumes only in amyloid-positive participants (eTable 1, links.lww.com/WNL/C821 and Table 3). The associations with the RMI were restricted to the ERC and whole hippocampus (eTable 2, links.lww.com/WNL/C821).
Table 2.
Multiple Regressions Analyses Between the Apnea-Hypopnea Index and Medial Temporal Subregion Volumes
Figure 2. Significant Associations Between the Apnea-Hypopnea Index and Medial Temporal Subregion Volumes According to Amyloid Status.
Scatterplots of partial correlations between the AHI and medial temporal subregional volumes, according to amyloid status. Detailed statistics are summarized in Table 3. Aβ = beta-amyloid; AHI = apnea-hypopnea index; BA = Brodmann area; CA = Cornu Ammonis; DG = dentate gyrus; ERC = entorhinal cortex; HPC = hippocampus; PHC = parahippocampal gyrus; PRC = perirhinal cortex.
Table 3.
Post Hoc Partial Correlations Between SDB Severity Parameters and Medial Temporal Subregional Volumes According to Amyloid Status
Replication analyses performed using hippocampal, ERC, PRC, and PHC volumes measured on T1-weighted images confirmed that greater AHI, ODI, and RMI were associated with lower volumes of the ERC and hippocampus only in amyloid-positive participants (eTable 3, links.lww.com/WNL/C821 and Table 3). Moreover, the associations between SDB severity and WH, ERC, SUB, CA1, and DG were all bilateral because both left and right hemisphere volumes were significantly associated with SDB severity, while the links with PHC were only significant in the right hemisphere (eTable 4, links.lww.com/WNL/C821).
Associations Between SDB and Whole-Brain GM Volume in Amyloid-Positive Participants
To test whether the negative associations between SDB parameters and MTL subregion atrophy in amyloid-positive participants were restricted to the MTL or part of a larger pattern of neurodegeneration, we performed voxel-wise multiple regressions between SDB parameters and GM volume (eFigure 2 and eTable 5, links.lww.com/WNL/C821). Greater AHI was associated with lower GM volume in the left superior temporal pole, hippocampus, PHC, and amygdala (p < 0.005 unc., k = 613, T = 3.85) and right superior temporal pole, amygdala and PHC (p < 0.005 unc., k = 191, T = 3.59). A greater ODI was associated with lower volume in the right superior temporal pole, amygdala, and PHC (p < 0.005 unc., k = 181, T = 4.00). The RMI was not significantly associated with GM volume.
Links With Episodic Memory at Baseline and Follow-up
In the whole cohort at baseline, CVLT scores were neither associated with SDB parameters nor with the volume of SDB-associated MTL subregions (eTable 6, links.lww.com/WNL/C821). However, baseline whole hippocampal (r = 0.27, p = 0.005) and CA1 volumes (r = 0.28, p = 0.003) were positively associated with long-term free recall scores at follow-up such that lower MTL subregional volumes at baseline were associated with poorer episodic memory performance at follow-up (Table 4). No significant associations were observed between SDB parameters at baseline and memory scores at follow-up. Moreover, no significant associations were observed in subgroups stratified by amyloid status.
Table 4.
Partial Correlations Between Medial Temporal Subregional Volumes and Episodic Memory at Follow-up, in the Whole Cohort and in Subgroups Stratified by Amyloid Status
Sex-Stratified Analyses
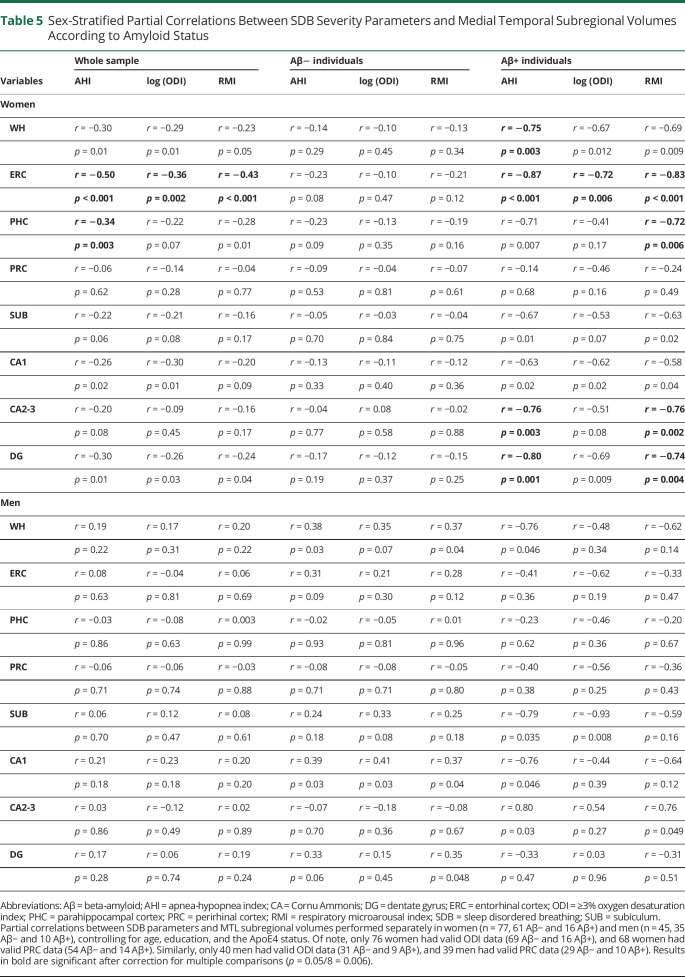
Differences in demographical, sleep, and MTL data between men and women are summarized in eTable 7, links.lww.com/WNL/C821. Male participants exhibited greater AHI scores and lower whole hippocampal, ERC, PHC, SUB, CA1, and DG volumes. Female participants had greater memory scores at all time points despite overall lower education level. Sex-stratified analyses revealed that the AHI was negatively associated with GM volume in the whole hippocampus (r = −0.30, p = 0.01), ERC (r = −0.50, p < 0.001), PHC (r = −0.34, p = 0.003), CA1 (r = −0.26, p = 0.02), and DG (r = −0.30, p = 0.01), only in women (Table 5). Similar associations were obtained with the ODI and RMI. When stratifying female participants according to amyloid status, these associations were significant only in amyloid-positive women (Table 5). Marginal associations were observed in male participants (Table 5).
Table 5.
Sex-Stratified Partial Correlations Between SDB Severity Parameters and Medial Temporal Subregional Volumes According to Amyloid Status
Associations With Sleep Architecture
The AHI was positively associated with N1 percentage (r = 0.41, p < 0.001) and the number of nocturnal awakenings per hour of sleep (r = 0.25, p = 0.007) and negatively associated with both N3 (r = −0.26, p = 0.005) and REM sleep (r = −0.30, p = 0.001) percentage (eTable 8, links.lww.com/WNL/C821). Similar associations were obtained with the ODI and RMI, although more marginally for the ODI because it was only related to N1 and REM sleep percentage (eTable 8, links.lww.com/WNL/C821). However, no significant associations were obtained between MTL subregion volume and sleep architecture (eTable 8, links.lww.com/WNL/C821).
Discussion
This study shows that greater SDB severity, mainly reflected by a higher AHI and ODI, was associated with reduced GM volume in the ERC and hippocampus in amyloid-positive individuals only. Specifically, hippocampal subfields involved in these associations included the SUB, CA1, and DG subfields. Moreover, lower hippocampal and CA1 volumes at baseline were associated with poorer episodic memory performance at follow-up. Complementary analyses show that the links between SDB and GM atrophy were largely bilateral, restricted to temporal cortical areas, not due to changes in sleep architecture, and that women may be particularly vulnerable to the adverse effects of sleep apnea on the MTL.
Because SDB has been associated with a greater risk of cognitive decline,4 a number of studies investigated SDB-related brain alterations. Some studies have previously demonstrated that SDB is associated with hippocampal damage.11-14,41 Our results demonstrate that MTL subregions are particularly vulnerable to SDB, including the ERC and within the hippocampus, the SUB, CA1, and DG subfields. Alzheimer disease is known to affect the ERC before CA1 and SUB subfields due to the spreading of tau pathology in early stages of the disease.17-19 Of interest, MTL subregions are also known to be sensitive to hypoxia, a major consequence of SDB. Animal studies indicate that CA1 may be the most vulnerable region to hypoxia within the MTL,23-25,42 while other subfields such as CA3 are spared. Compared with those located in CA3, CA1 pyramidal neurons seem to be selectively vulnerable to oxidative stress because of cellular and molecular specificities such as (1) a preexisting pattern of high basal expression of genes associated to metabolic stress, (2) high intrinsic levels of oxidative stress and reactive oxygen and nitrogen species, (3) a high concentration of glutamatergic receptors, which may be overstimulated in case of excessive glutamate release, and (4) lower adenosine triphosphate (ATP) and energy reserves, etc.24,43 Other potential mechanisms for this selective vulnerability are more structural, including larger neuron size and potentially lower levels of vascularization in CA1 and lower angiogenesis-related gene expression.24,43 In humans, a recent postmortem study has shown that greater SDB severity was related to cortical thinning in the DG, CA1, and ERC.10 In addition, in children with SDB, lower mean diffusivity in the DG has been associated with a higher AHI and lower verbal learning and memory scores.44 It has been shown that hypoxia affects multiple stages of adult neurogenesis in the DG, leading to a reduction of newly generated adult-born neurons.45 If adult neurogenesis is known to decline with aging and to be largely reduced in Alzheimer disease,46 it is still possible that hypoxia may affect DG neurogenesis in our sample, explaining its vulnerability to SDB, although this mechanism may be relatively marginal.
Our results suggest that some individuals may be more vulnerable to the adverse effects of sleep apnea. Indeed, we demonstrate that participants who are engaged in the Alzheimer pathologic continuum (i.e., amyloid positive) exhibit a specific vulnerability to SDB, which amyloid-negative participants did not show. The fact that no association between SDB and MTL integrity was found in amyloid-negative participants contrasts with some previous reports showing SDB-related hippocampal changes in younger populations who are unlikely to exhibit significant Alzheimer disease pathology (i.e., young middle-aged individuals and children with obstructive sleep apnea).44,47,48 We can speculate that SDB may exacerbate Alzheimer disease pathology in all participants, but amyloid-negative individuals (1) may not experience SDB for enough time to exhibit neurodegeneration yet or (2) may be more resilient to the adverse effects of SDB than amyloid-positive individuals. Of importance, in our cohort, only a small proportion of participants with moderate-to-severe SDB exhibited symptoms of excessive daytime sleepiness (11 of 91 participants). In addition, they were overall highly educated, had no cognitive deficits, and may therefore present with a high cognitive reserve. However, amyloid-negative participants may still be at risk of neurodegeneration over the long term. Moreover, additional analyses showed that women (notably those with significant levels of amyloid pathology) seemed particularly vulnerable to the adverse effects of SDB, while greater SDB severity was not significantly associated with MTL volume changes in men. If these results are preliminary and pend replication in larger samples, they are in line with recent reports showing that the links between SDB and cognitive decline may be stronger in women.49
Finally, we found that lower baseline CA1 and whole hippocampus volumes were associated with poorer episodic memory performance measured approximately 21 months later in the whole cohort. This result provides preliminary evidence that, although neither SDB nor MTL structure are significantly associated with cognition cross-sectionally, the adverse effects of SDB on the MTL in amyloid-positive individuals could ultimately result in lower episodic memory performance later. It is not surprising to observe associations between hippocampal atrophy and poorer episodic memory because we show that SDB affected regions of the MTL that are crucial for episodic memory formation and sleep-dependent consolidation.15,16 Furthermore, the neurodegeneration of MTL regions due to tau pathology is known to start early in the course of Alzheimer disease and to underlie memory deficits. In particular, CA1 atrophy has been associated with memory deficits in patients with mild cognitive impairment and Alzheimer disease.50,51 Given that our population was composed of cognitively healthy older participants, we surmise that longitudinal designs may be more powerful to detect subtle cognitive changes compared with cross-sectional analyses.
The strengths of our study include the combination of polysomnography, a high-resolution imaging and careful segmentation of MTL subregions, and longitudinal cognitive assessment. However, our study is not without limitations. First, the same CVLT version was used at baseline and follow-up. If the time interval was long between these 2 time points (i.e., 20.66 ± 0.76 months), suggesting a rather modest test/retest effect, we cannot exclude that this may have minimized memory decline measurements at follow-up. Second, the results of sex-stratified analyses should be replicated in larger samples because our sample size was limited, especially when separating the groups according to amyloid status. Third, the polysomnography setup did not include leg electrodes, preventing us from evaluating and controlling for periodic limb movements. Overall, this study shows that SDB in cognitively unimpaired amyloid-positive individuals may exacerbate neurodegeneration in MTL subregions, which may in turn foster memory decline. Further studies should evaluate the impact of tau pathology and assess the potentially beneficial effects of SDB treatment.
Acknowledgment
The authors thank all participants, Franck Doidy, Alison Mary, Sébastien Polvent, Aurélia Cognet, the Cyceron neuroimaging staff, Euclid team, and the sponsor (Institut National de la Santé et de la Recherche Médicale).
Glossary
- AHI
apnea-hypopnea index
- ApoE4
Apolipoprotein E ε4
- ASHS
Automated Segmentation for Hippocampal Subfields
- CVLT
California Verbal Learning Test
- DG
dentate gyrus
- ERC
entorhinal cortex
- GM
gray matter
- INSERM
Institute of Health and Medical Research
- MNI
Montreal Neurologic Institute
- MTL
medial temporal lobe
- ODI
oxygen desaturation index
- PHC
parahippocampal cortex
- PRC
perirhinal cortex
- RCT
randomized controlled trial
- RMI
respiratory microarousals index
- SDB
Sleep disordered breathing
- SUB
subiculum
- SUVr
standard uptake value ratio
- TIV
total intracranial volume
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
CME Course: NPub.org/cmelist
Study Funding
The Age-Well randomized clinical trial is part of the Medit-Aging project and is funded through the European Union's Horizon 2020 Research and Innovation Program (grant 667696), Institut National de la Santé et de la Recherche Médicale (INSERM), Région Normandie, and Fondation d’Entreprise MMA des Entrepreneurs du Futur. C. André was funded by the Institut National de la Santé et de la Recherche Médicale (INSERM), Région Normandie, and the Fonds Européen de Développement Régional (FEDER). G. Rauchs obtained funding from Fondation Vaincre Alzheimer (grant 13732), Fondation Planiol, and France Alzheimer (project 1714). P. Yushkevich was funded by NIH grants R01 AG056014 and RF1 AG069474. The funders and sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Senaratna C, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 2.Morrell MJ, Finn L, McMillan A, Peppard PE. The impact of ageing and sex on the association between sleepiness and sleep disordered breathing. Eur Respir J. 2012;40(2):386–393. doi: 10.1183/09031936.00177411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93(12):1778–1794. doi: 10.1002/jnr.23634 [DOI] [PubMed] [Google Scholar]
- 4.Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol. 2017;74(10):1237–1245. doi: 10.1001/jamaneurol.2017.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yun C-H, Lee H-Y, Lee SK, et al. Amyloid burden in obstructive sleep apnea. J Alzheimers Dis. 2017;59(1):21–29. doi: 10.3233/jad-161047 [DOI] [PubMed] [Google Scholar]
- 6.André C, Rehel S, Kuhn E, et al. Association of sleep-disordered breathing with Alzheimer disease biomarkers in community-dwelling older adults. JAMA Neurol. 2020;77(6):716–724. doi: 10.1001/jamaneurol.2020.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullins AE, Kam K, Parekh A, Bubu OM, Osorio RS, Varga AW. Obstructive sleep apnea and its treatment in aging: effects on Alzheimer's disease biomarkers, cognition, brain structure and neurophysiology. Neurobiol Dis. 2020;145:105054. doi: 10.1016/j.nbd.2020.105054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bubu OM, Umasabor‐Bubu OQ, Turner AD, et al. Self‐reported obstructive sleep apnea, amyloid and tau burden, and Alzheimer's disease time‐dependent progression. Alzheimers Demen. 2021;17(2):226–245. doi: 10.1002/alz.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma RA, Varga AW, Bubu OM, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly. A longitudinal study. Am J Respir Crit Care Med. 2018;197(7):933–943. doi: 10.1164/rccm.201704-0704oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen JE, Benediktsdóttir B, Gislason T, Robinson SR. Neuropathological investigation of cell layer thickness and myelination in the hippocampus of people with obstructive sleep apnea. Sleep. 2019;42(1). doi: 10.1093/sleep/zsy199 [DOI] [PubMed] [Google Scholar]
- 11.Weng HH, Tsai YH, Chen CF, et al. Mapping gray matter reductions in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Sleep. 2014;37(1):167–175. doi: 10.5665/sleep.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahmasian M, Rosenzweig I, Eickhoff SB, et al. Structural and functional neural adaptations in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Neurosci Biobehav Rev. 2016;65:142–156. doi: 10.1016/j.neubiorev.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Tang S, Lyu X, Yang C, Chen X. Structural and functional brain alterations in obstructive sleep apnea: a multimodal meta-analysis. Sleep Med. 2019;54:195–204. doi: 10.1016/j.sleep.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 14.Macey PM, Prasad JP, Ogren JA, et al. Sex-specific hippocampus volume changes in obstructive sleep apnea. Neuroimage Clin. 2018;20:305–317. doi: 10.1016/j.nicl.2018.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47(8-9):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028 [DOI] [PubMed] [Google Scholar]
- 16.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12(10):585–601. doi: 10.1038/nrn3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–757. doi: 10.1212/wnl.58.5.750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Souza LC, Chupin M, Lamari F, et al. CSF tau markers are correlated with hippocampal volume in Alzheimer's disease. Neurobiol Aging. 2012;33(7):1253–1257. doi: 10.1016/j.neurobiolaging.2011.02.022 [DOI] [PubMed] [Google Scholar]
- 19.Das SR, Xie L, Wisse LEM, et al. Longitudinal and cross-sectional structural magnetic resonance imaging correlates of AV-1451 uptake. Neurobiol Aging. 2018;66:49–58. doi: 10.1016/j.neurobiolaging.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pini L, Pievani M, Bocchetta M, et al. Brain atrophy in Alzheimer's Disease and aging. Ageing Res Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 21.de Flores R, la Joie R, Chételat G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer's disease. Neuroscience. 2015;309:29–50. doi: 10.1016/j.neuroscience.2015.08.033 [DOI] [PubMed] [Google Scholar]
- 22.Xie L, Wisse LEM, Pluta J, et al. Automated segmentation of medial temporal lobe subregions on in vivo T1-weighted MRI in early stages of Alzheimer's disease. Hum Brain Mapp. 2019;40(12):3431–3451. doi: 10.1002/hbm.24607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartsch T, Döhring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836–1845. doi: 10.1038/jcbfm.2015.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt-Kastner R. Genomic approach to selective vulnerability of the hippocampus in brain ischemia-hypoxia. Neuroscience. 2015;309:259–279. doi: 10.1016/j.neuroscience.2015.08.034 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40(3):599–636. doi: 10.1016/0306-4522(91)90001-5 [DOI] [PubMed] [Google Scholar]
- 26.Rosenzweig I, Kempton MJ, Crum WR, et al. Hippocampal hypertrophy and sleep apnea: a role for the ischemic preconditioning? PLoS One. 2013;8(12):e83173. doi: 10.1371/journal.pone.0083173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cross NE, Memarian N, Duffy SL, et al. Structural brain correlates of obstructive sleep apnoea in older adults at risk for dementia. Eur Respir J. 2018;52(1):1800740. doi: 10.1183/13993003.00740-2018 [DOI] [PubMed] [Google Scholar]
- 28.Baril AA, Gagnon K, Brayet P, et al. Gray matter hypertrophy and thickening with obstructive sleep apnea in middle-aged and older adults. Am J Respir Crit Care Med. 2017;195(11):1509–1518. doi: 10.1164/rccm.201606-1271oc [DOI] [PubMed] [Google Scholar]
- 29.Legault J, Thompson C, Martineau-Dussault MÈ, et al. Obstructive sleep apnea and cognitive decline: a review of potential vulnerability and protective factors. Brain Sci MDPI AG. 2021;11(6):706. doi: 10.3390/brainsci11060706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/s1474-4422(13)70044-9 [DOI] [PubMed] [Google Scholar]
- 31.Villemagne VL, Pike KE, Chételat G, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–192. doi: 10.1002/ana.22248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Demen. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martineau-Dussault MÈ, André C, Daneault V, et al. Medial temporal lobe and obstructive sleep apnea: effect of sex, age, cognitive status and free-water. Neuroimage Clin. 2022;36:103235. doi: 10.1016/j.nicl.2022.103235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baril AA, Martineau-Dussault MÈ, Sanchez E, et al. Obstructive sleep apnea and the brain: a focus on gray and white matter structure. Curr Neurol Neurosci Rep. 2021;21(3):11. doi: 10.1007/s11910-021-01094-2 [DOI] [PubMed] [Google Scholar]
- 35.Poisnel G, Arenaza‐Urquijo E, Collette F, et al. The Age‐Well randomized controlled trial of the Medit‐Ageing European project: effect of meditation or foreign language training on brain and mental health in older adults. Alzheimers Dement (N Y). 2018;4(1):714–723. doi: 10.1016/j.trci.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (version 2.4). J Clin Sleep Med. 2017;13(05):665–666. doi: 10.5664/jcsm.6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.fil.ion.ucl.ac.uk.
- 38.Yushkevich PA, Pluta JB, Wang H, et al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp. 2015;36(1):258–287. doi: 10.1002/hbm.22627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flores R, Berron D, Ding SL, et al. Characterization of hippocampal subfields using ex vivo MRI and histology data: lessons for in vivo segmentation. Hippocampus. 2020;30(6):545–564. doi: 10.1002/hipo.23172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poitrenaud J, Deweer B, Kalafat M, van der Linden M. Adaptation en langue française du California Verbal Learning Test. Les Éditions du Centre de psychologie appliquée; 2007. [Google Scholar]
- 41.Marchi NA, Ramponi C, Hirotsu C, et al. Mean oxygen saturation during sleep is related to specific brain atrophy pattern. Ann Neurol. 2020;87(6):921–930. doi: 10.1002/ana.25728 [DOI] [PubMed] [Google Scholar]
- 42.Wilde GJC, Pringle AK, Wright P, Iannotti F. Differential vulnerability of the CA1 and CA3 subfields of the hippocampus to superoxide and hydroxyl radicals in vitro. J Neurochem. 2002;69(2):883–886. doi: 10.1046/j.1471-4159.1997.69020883.x [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cha J, Zea-Hernandez JA, Sin S, et al. The effects of obstructive sleep apnea syndrome on the dentate gyrus and learning and memory in children. J Neurosci. 2017;37(16):4280–4288. doi: 10.1523/jneurosci.3583-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khuu MA, Pagan CM, Nallamothu T, et al. Intermittent hypoxia disrupts adult neurogenesis and synaptic plasticity in the dentate gyrus. J Neurosci. 2019;39(7):1320–1331. doi: 10.1523/jneurosci.1359-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med. 2019;25(4):554–560. doi: 10.1038/s41591-019-0375-9 [DOI] [PubMed] [Google Scholar]
- 47.Kheirandish-Gozal L, Sahib AK, Macey PM, Philby MF, Gozal D, Kumar R. Regional brain tissue integrity in pediatric obstructive sleep apnea. Neurosci Lett. 2018;682:118–123. doi: 10.1016/j.neulet.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183(10):1419–1426. doi: 10.1164/rccm.201005-0693oc [DOI] [PubMed] [Google Scholar]
- 49.Thompson C, Legault J, Moullec G, et al. Association between risk of obstructive sleep apnea, inflammation and cognition after 45 years old in the Canadian Longitudinal Study on Aging. Sleep Med. 2022;91:21–30. doi: 10.1016/j.sleep.2022.02.006 [DOI] [PubMed] [Google Scholar]
- 50.Apostolova LG, Morra JH, Green AE, et al. Automated 3D mapping of baseline and 12-month associations between three verbal memory measures and hippocampal atrophy in 490 ADNI subjects. Neuroimage. 2010;51(1):488–499. doi: 10.1016/j.neuroimage.2009.12.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fouquet M, Desgranges B, la Joie R, et al. Role of hippocampal CA1 atrophy in memory encoding deficits in amnestic Mild Cognitive Impairment. Neuroimage. 2012;59(4):3309–3315. doi: 10.1016/j.neuroimage.2011.11.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are made available on request following a formal data sharing agreement and approval by the consortium and executive committee (silversantestudy.eu/2020/09/25/data-sharing). The material can be mobilized, under the conditions and modalities defined in the Medit-Aging Charter, by any research team belonging to an Academic for conducting a scientific research project relating to the scientific theme of mental health and well-being in older people. The material may also be mobilized by nonacademic third parties, under conditions, particularly financial, which will be established by a separate agreement between INSERM and by the said third party. Data sharing policies described in the Medit-Aging Charter are in compliance with our ethical approval and guidelines from our funding body.