Abstract
The Polycomb group (Pc-G) genes encode proteins that assemble into complexes implicated in the epigenetic maintenance of heritable patterns of expression of developmental genes, a function largely conserved from Drosophila to mammals and plants. The Pc-G is thought to act at the chromatin level to silence expression of target genes; however, little is known about the molecular basis of this repression. In keeping with the evidence that Pc-G homologs in higher vertebrates exist in related pairs, we report here the isolation of XPc1, a second Polycomb homolog in Xenopus laevis. We show that XPc1 message is maternally deposited in a translationally masked form in Xenopus oocytes, with XPc1 protein first appearing in embryonic nuclei shortly after the blastula stage. XPc1 acts as a transcriptional repressor in vivo when tethered to a promoter in Xenopus embryos. We find that XPc1-mediated repression can be only partially alleviated by an increase in transcription factor dosage and that inhibition of deacetylase activity by trichostatin A treatment has no effect on XPc1 repression, suggesting that histone deacetylation does not form the basis for Pc-G-mediated repression in our assay.
The diversity required in higher organisms to give rise to the multitude of differentiated cell types is generated early in embryonic development. In Drosophila, the best-studied example, the maintenance of homeotic patterns of expression is dependent on the interplay of two antagonistic groups of proteins, the Polycomb group (Pc-G) and the Trithorax group (Trx-G) (49), with both groups comprising a large number of genetically identified loci (31, 33). The role of the Pc-G in the regulation of homeotic gene expression is one of repression: Pc-G mutants exhibit posteriorly directed homeotic transformations arising from the anteriorly ectopic expression of homeotic genes (47, 70, 85). Conversely, the Trithorax group acts to maintain transcriptional activity of homeotic genes (78, 79).
The developmental functions of the Pc-G appear to be conserved throughout evolution, as evidenced by the isolation of Pc-G homologs in mammals (67), Xenopus laevis (61), chicken (87), plants (24, 25), and, recently, Caenorhabditis elegans (30, 35). In addition, the Polycomb phenotype can be rescued by expression of the murine M33 Polycomb homolog in mutant flies, providing further evidence for functional conservation (45). The genetic analysis of Pc-G function in null knockout mice has been particularly informative in that respect. The range of phenotypes observed in Pc-G knockout mice included posterior transformations in the axial skeleton, as well as neurological and hematopoietic defects, all consistent with a role for Pc-G in mammalian Hox gene regulation (67). These diverse phenotypes provide strong evidence for extensive developmental functions of Pc-G homologs in mice, at least some of which are conserved between mammals and Drosophila.
Despite our increasing knowledge regarding the developmental functions of the Pc-G, little is known about the underlying biochemical mechanisms. There is evidence suggesting that Pc-G members exist in large nuclear multimeric protein complexes in Drosophila (8, 20, 60) as well as in mammalian cells (1, 2, 26, 28, 63, 64, 66, 69, 82). There also appears to be considerable heterogeneity in Pc-G complex composition, distribution, and, potentially, function (8, 28, 69, 77, 82). Largely on the basis of genetic evidence, parallels have been drawn between Pc-G-mediated repression and position effect variegation (PEV), a heterochromatin-mediated repressive phenomenon in Drosophila (43, 56). This association between the Pc-G and PEV was further strengthened at the molecular level by the identification of the chromodomain, an N-terminal conserved protein motif originally shown to be shared by Polycomb, a key Pc-G member, and heterochromatin protein 1 (HP1), a component of constitutive heterochromatin and a modifier of PEV (50). The chromodomain has been subsequently shown to be present in an expanding class of heterogeneous proteins, which appear to have chromosomal functions in common, although not always related to transcriptional repression (10, 34).
Based primarily on the parallels drawn between Polycomb and HP1, it has been suggested that the Pc-G acts at the chromatin level by forming a multimeric repressive protein complex that spreads over entire domains, reminiscent of heterochromatin in PEV (42, 43, 46, 47, 56). Pc-G function might therefore provide an epigenetic means for faithfully fixing and propagating the precise patterns of expression of target genes, through multiple rounds of cell division in embryonic development and tissue differentiation (10, 11, 55). Precisely how this is achieved at the molecular level remains unknown. Apart from the heterochromatin-like assembly model, one that is largely superseded by recent evidence (6, 8, 74), a number of other models have been proposed for Pc-G function. These include compartmentalization of Pc-G target genes to nuclear subdomains of transcriptional inactivity (8, 48); Pc-G-mediated sequestration of gene regulatory elements, such as enhancers and promoters, by looping out intervening DNA (6, 55, 56); or localized remodelling of nucleosome density (54). The localized modification of histones, such as deacetylation, was also recently proposed to play a role in Pc-G mediated repression (10, 32a, 55).
The model system of X. laevis offers some advantages in the study of the developmental regulation of chromatin components and their impact on gene activity. For example, the developmentally regulated release of masked maternal mRNA encoding histone H1 (14, 76, 86a) leads to the progressive accumulation of the somatic linker histone through gastrulation. This causes the selective repression of oocyte-type 5S rRNA genes (7, 32) and restrictions in the capacity of ectodermal cells to change their fate to mesoderm (73, 83). As a step in employing Xenopus in the study of Pc-G function, we report here the isolation of XPc1, a second Polycomb homolog in X. laevis. We find XPc1 to be distinct from the previously described XPc homolog (61) and closely related to the mouse M33 homolog. XPc1 gene expression is developmentally regulated, and tethered XPc1 acts as a transcriptional repressor in vivo in Xenopus embryos. Significantly, we found that deacetylase inhibition by trichostatin A (TSA) treatment did not have any effect on XPc1 repression in Xenopus embryos.
MATERIALS AND METHODS
Isolation of XPc1 cDNA.
A fragment corresponding to an X. laevis Polycomb homolog was initially generated by reverse transcription-PCR from ovary poly(A)+ RNA. Degenerate primers accounting for codon bias in X. laevis were designed by using the cDNA for the mouse M33 Pc homolog as a template (53). The 5′ primer (5′-GCC/T GCC/T GAG/A TGC/T ATC/T CTG AGC AAG-3′) corresponds to M33 nucleotides 37 to 60, just inside the sequence encoding the conserved N-terminal chromodomain. The 3′ primer (5′-GAT C/GAG GTT A/GGC TGT C/GAC ATG T/CGT-3′) corresponds to M33 nucleotides 1477 to 1500 derived from the sequence encoding the C-terminal domain of homology (53). For both primers, the degenerate nucleotides are separated from the M33 sequence by a slash. From the M33 cDNA sequence, the predicted amplifiable fragment obtained by using these primers is 1,463 nucleotides, covering the majority of the coding sequence. Adult ovary poly(A)+ RNA was isolated by using the Promega RNAgents kit according to the manufacturer’s instructions. Approximately 1 μg of oligo(dT)-primed poly(A)+ RNA was used for first-strand synthesis with avian myeloblastosis virus reverse transcriptase according to the instructions of the manufacturer (Promega, Madison Wis.). Approximately one-fifth of the ovary cDNA was used as a template in each PCR with the degenerate primers at the stringent annealing temperature of 60°C. The predominant fragment amplified under these conditions was in the predicted ∼1.5-kb size range. This fragment was gel purified and cloned into vector pGEM-T (Promega), and its identity was confirmed by partial DNA sequencing of both ends. A PstI fragment containing the chromodomain was then used to screen a Xenopus stage VI oocyte cDNA library constructed in Uni-ZAP XR (Stratagene, La Jolla, Calif.). An excess of 1.25 × 106 phage plaques were transferred and immobilized on Hybond-N+ filters (Amersham, Amersham, United Kingdom) and were hybridized overnight at 65°C in 3× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS)–10× Denhardt’s solution–10% dextran sulfate–50 μg of sonicated salmon sperm competitor DNA per ml. The filters were then washed several times at 65°C in 0.3× SSC–0.1% SDS for 30 min each. A number of candidate positive clones were isolated following successive rounds of screening and were partially sequenced and arranged into overlapping cDNAs. Two of these clones were used to assemble the full 4.37-kb XPc1 cDNA. Both strands of the full cDNA were completely sequenced by the DNA Sequencing Core Facility at the Interdisciplinary Center for Biotechnology Research of the University of Florida (Gainesville).
Northern blot analysis.
Total RNAs from oocytes isolated from collagenase-treated ovary (71), adult tissues, and embryos from different developmental stages was prepared by using RNA STAT-60 according to the instructions of the supplier (Tel-Test B, Friendswood, Tex.). Expression of XPc1, H1°, and H1C mRNAs was assayed by standard Northern blot hybridization techniques with approximately 5 μg of total RNA for each sample (3a, 33a).
Ribonucleoprotein fractionation by density gradient centrifugation.
Extract from 100 oocytes or embryos was fractionated on a Nycodenz (Nycomed, Oslo, Norway) density gradient (41). Fractions were stripped of proteins by phenol-chloroform extraction, and RNA was recovered by ethanol precipitation. RNA pellets were dissolved in a small volume of water, and fractionation profiles of specific mRNAs were determined by Northern blot hybridization as described above.
XPc1 antibody production and Western blot analysis.
Affinity-purified XPc1 antibodies were obtained from rabbits immunized with the synthetic peptide RNPRPRDSHPVPQKKAPA, corresponding to amino acids 125 to 142 of XPc1. Peptide synthesis, purification, and coupling; rabbit immunizations; initial serum screening; and antibody affinity purification were all carried out by Quality Control Biochemicals, Inc. (Hopkinton, Mass.). For XPc1 protein detection by immunoblotting, extracts were prepared (37), and proteins were separated by SDS-polyacrylamide gel electrophoresis on an 8% Tris-glycine–SDS gel and electroblotted onto Hybond-ECL nitrocellulose filters (Amersham). Filters were routinely incubated at 4°C overnight with a 100-fold dilution of XPc1 antibody. Detection was by chemiluminescence with SuperSignal (Pierce, Rockford, Ill.).
Isolation of nuclei from Xenopus embryos.
Nuclei were prepared (4). Briefly, 50 to 100 embryos from different developmental stages were homogenized in 300 μl of E-1 buffer (110 mM KCl, 50 mM Tris-HCl [pH 7.4], 5 mM MgCl2, 0.1 mM spermine, 0.1 mM EDTA, 2 mM dithiothreitol, 0.4 mM phenylmethylsulfonyl fluoride)–0.25 M sucrose, and the final volume was adjusted to 500 μl with the same buffer. To this homogenate, 2.4 ml of E-1–2.2 M sucrose buffer was added, mixed, and then layered onto a 150-μl cushion of E-1–2.2 M sucrose buffer in a polyallomer centrifuge tube (13 by 51 mm; Beckman, Fullerton, Calif.). Nuclei were pelleted by centrifugation at 130,000 × g and 4°C for 2 h with a TLA100.3 rotor in a Sorvall TLA benchtop ultracentrifuge. Pelleted nuclei were resuspended in 250 μl of nuclear buffer (25% glycerol, 25 mM Tris-HCl [pH 7.4], 70 mM KCl, 5 mM MgCl2, 0.2 mM EDTA, 2 mM dithiothreitol, 0.4 mM phenylmethylsulfonyl fluoride), and residual yolk protein was removed by washing the nuclei twice by pelleting through a 10-μl cushion of 80% glycerol at 6,000 rpm in a Microfuge at 4°C for 10 min. In order to remove most of the melanin granules that copellet with the nuclei during the ultracentrifugation step, nuclear pellets were resuspended in 500 μl of ice-cold phosphate-buffered saline and repelleted at 1,500 rpm for 5 min in a benchtop centrifuge at 4°C. Nuclei were then resuspended in 50 to 100 μl of nuclear buffer and lysed by sonication.
In vitro transcription.
All in vitro-transcribed polyadenylated RNAs were generated by using the SP6 transcription vector pSP64 (Promega), which was modified by the insertion of a linker in the unique EcoRI site downstream of the poly(A) stretch. This linker provides additional restriction sites for linearizing the vector prior to transcription (the modified vector was kindly donated by Melissa Stolow). Capped RNA transcripts were generated by using the SP6 mMessage mMachine kit according to the instructions of the manufacturer (Ambion, Austin, Tex.). For in vitro transcription, the XPc1 cDNA was cloned into the HindIII/HincII sites of the modified pSP64. N-terminally tagged XPc1 was constructed by amplifying three copies of the HindIII-fitted hemagglutinin (HA) epitope tag from plasmid pSM491 (81) and cloning it into the HindIII site of XPc1pSP64. The GAL4 DNA binding domain (DBD) was cloned into the HindIII site of XPc1pSP64 by Deep Vent DNA polymerase (New England Biolabs, Beverly, Mass.) amplification with specific primers fitted with HindIII restriction sites. This results in an in-frame fusion of the GAL4 DBD to the N terminus of XPc1. In addition, the GAL4 DBD alone was cloned into the HindIII/PstI sites of the modified pSP64 vector by PCR with Deep Vent DNA polymerase and primers fitted with the appropriate restriction sites. The in vitro transcription construct for Xenopus heat shock factor (HSF) (75) has been previously described (38). The FLAG-tagged histone H1 construct has been previously described (7). Constructs were checked for correct in-frame fusions by DNA sequencing. In addition, in vitro-transcribed RNAs were microinjected into oocytes and checked by Western blotting with anti-XPc1, anti-GAL4 (Babco, Richmond, Calif.), anti-HA (Boehringer Mannheim, Indianapolis, Ind.), and anti-FLAG M2 (Kodak IBI, Rochester, N.Y.) antibodies.
XPc1 phosphorylation and nuclear localization.
In vitro-transcribed RNA for full-length XPc1 was injected into oocytes as described below. Following incubation to allow for protein synthesis, germinal vesicles (GVs) were manually dissected and the remaining oocyte cytoplasms were collected. Alkaline phosphatase assays were carried out as described previously (52). Briefly, protein extracts were precipitated with 10% trichloroacetic acid, and pellets were washed twice with cold acetone. Pellets were then resuspended in 30 mM triethanolamine (pH 8.25)–0.1 mM EDTA–1 mM MgCl2–0.2% SDS buffer and divided into two aliquots. To one aliquot 10 U of alkaline phosphatase (Boehringer Mannheim) was added, followed by incubation at 37°C for 2 h. The second aliquot was mock treated by incubation in the same way but with no phosphatase addition.
Embryo and oocyte microinjections.
For embryo microinjections, mature Xenopus females were injected with 1,000 IU of human chorionic gonadotropin (Sigma, St. Louis, Mo.). After 12 h at 18 to 22°C, the females were stripped of eggs which were fertilized in vitro (39). Fertilized eggs were dejellied in 2% cysteine (pH 8.0), followed by several washes in MMR buffer (62). Washed fertilized eggs were placed in 1× MMR–5% Ficoll (Pharmacia, Piscataway, N.J.) and allowed to begin their first cleavage division before they were microinjected. In all experiments, 500 to 800 pg of DNA was injected in a total volume of 13.8 nl per embryo, either alone or with in vitro-synthesized RNA. In all cases, a maximum of approximately 1 ng of each in vitro-transcribed RNA was coinjected with the DNA. Injected embryos were placed in 0.1× MMR–2% Ficoll and incubated at 25°C until harvested at various developmental stages for analysis. For TSA treatments, embryos were placed in medium with 30 or 90 nM TSA immediately after microinjection. TSA was purchased from Wako Pure Chemical Industries (Tokyo, Japan) and dissolved in ethanol at a stock concentration of 30 μM. The H10 chloramphenicol acetyltransferase (CAT), cytomegalovirus CAT, hsp70CAT, and G5hsp70CAT reporter plasmids have been previously described (3a, 33a, 52, 59). Oocyte microinjections were as previously described (3a). Microinjection of RPD3 mRNA into oocytes and embryos was exactly as described earlier (7, 86a).
RNA analysis by primer extension.
For the XPc1 tethering repression assays, healthy injected embryos were collected at various times after fertilization and homogenized as described previously (37). Primer extension analysis with RNA equivalent to three embryos was carried out as previously described (80). Three primers were utilized: first, we used a CAT-specific primer (37) which gives rise to a 167-nucleotide extension product from the hsp70 promoter; second, to assay H1° transcription, we used a 26-mer (5′-GTCAAGCCCTGACTCGCAATGGCTTC-3′) that hybridizes in the noncoding region of the H1° gene; and finally, as an RNA recovery and loading control, we used a histone H4 primer (5′-GAG GCC GGA GAT GCG CTT GAC-3′) which hybridizes to endogenous H4 RNA, giving rise to a 182-nucleotide extended product. All microinjections and RNA analyses were carried out at least in duplicate with reproducible results (relative transcription levels varied less than 5%).
RESULTS
Isolation and sequence analysis of a second Xenopus Polycomb homolog.
In order to isolate a Polycomb homolog in Xenopus, we employed reverse transcription-PCR with a set of degenerate primers that were designed by using the mouse M33 homolog as a template (53). A cDNA fragment of the predicted size was amplified from adult ovary mRNA and cloned. Partial sequencing confirmed that the cloned cDNA fragment was a putative Xenopus Polycomb homolog. This fragment was used as a probe to screen a Xenopus oocyte cDNA library. This resulted in the isolation of several overlapping clones that were used to assemble a 4,380-bp cDNA clone (GenBank Accession number AF101438). This clone has a single 1,416-bp open reading frame coding for a predicted 472-amino-acid protein. Interestingly, the majority (approximately 2.9 kb) of the cDNA is 3′ untranslated sequence, suggesting that posttranscriptional control of gene expression may occur.
Amino acid analysis revealed that the predicted protein is a Polycomb homolog. First, it possesses an evolutionarily conserved N-terminal chromodomain that is 60% identical (75.5% similar, with conservative changes included) to the Drosophila Pc chromodomain and 93% identical to the mouse M33 chromodomain (Fig. 1A, B, and C). The chromodomain of the protein reported here clearly belongs to the Polycomb class, as evidenced, for example, by the presence of the ILDPRLL amino acid identity which is found immediately downstream of the chromodomain in Polycomb homologs (Fig. 1A and B). In addition, Pearce et al. (53) identified a short region of C-terminal homology (the COOH box) which is shared between Polycomb homologs and which is also present in the protein reported here (Fig. 1D). Finally, from an extensive analysis of chromodomain-containing proteins, Koonin et al. (34) proposed that proteins in the Polycomb class share a similar overall structure, in which the conserved N- and C-terminal domains (the chromodomain and COOH box, respectively) are contained within short globular domains separated by a long nonglobular central region. The predicted protein reported here fully conforms to the proposed structure. We conclude, therefore, that the cDNA we isolated codes for a true Xenopus Polycomb homolog that we will henceforth refer to as XPc1 (for Xenopus Polycomb homolog 1) (see also below).
FIG. 1.
(A and B) Amino acid sequence comparison of XPc1 to Xenopus XPc2 (A) and mouse MPc1 (B) Polycomb homologs. The conserved chromodomains and COOH boxes are shaded. Conserved putative nuclear localization signals are underlined. The sequence of the synthetic peptide used to raise antibodies against XPc1 is shown in boldface in panel A; the polyserine stretch conserved between XPc1 and M33 is in boldface in panel B. (C) Amino acid alignments of the conserved chromodomains of higher vertebrate Polycomb homologs and of Drosophila Pc. The shaded areas indicate identity with the indicated consensus sequence. Lowercase letters indicate amino acids that deviate from the consensus sequence. (D) Amino acid alignments of the C-terminal COOH boxes of vertebrate Polycomb homologs and Drosophila Pc. The shaded areas in the COOH-box indicate identity with the consensus sequence, and lowercase letters indicate residues that are not identical to the consensus. Additional sequences beyond the conserved COOH box are boxed (XPc1, MPc1, and hPc1) or underlined (XPc2, MPc2, hPc2) in order to illustrate the extended C-terminal homologies which allow the classification of the vertebrate Polycomb homologs into two classes. MPc1 is M33 (53), and hPc1 is CBX2 (23) (see text for details). All sequence analyses were done by using the Wisconsin Genetics Computer Group package.
The isolation of another Polycomb homolog in Xenopus (XPc) has been reported previously (61). Sequence comparison of our XPc1 homolog with that isolated by Reijnen et al. (61) revealed surprisingly little identity between the two proteins outside the conserved N-terminal chromodomain and C-terminal COOH box (Fig. 1A). Overall, the two proteins have only 28.5% identity, or 43.8% similarity when conservative amino acid changes are included. Both Xenopus homologs are similarly conserved with Drosophila Pc (45% similarity for XPc versus 42% similarity for XPc1). By contrast, XPc1 is much more closely related to the mouse M33 homolog (53), with homology extending well beyond the conserved N- and C-terminal motifs (Fig. 1B). It is striking, for example, that both XPc1 and M33 contain a conserved polyserine stretch near the N terminus of the protein, which is not present in any other Polycomb homolog (Fig. 1B). Overall, XPc1 and M33 have 53% identity, or 67.5% similarity. XPc1 and M33 also exhibit similar degrees of homology to the other mammalian Polycomb homologs. For example, they are both closely related to the human homolog CBX2 (23) (recently renamed hPc1 [64]). By contrast, both M33 and XPc1 are more distantly related to the recently described mammalian homologs MPc2 (2) and hPc2 (64). The Xenopus Polycomb homolog described by Reijnen et al. (61) is instead more closely related to MPc2 and hPc2 (2, 64), and we would therefore like to rename it XPc2. XPc2, MPc2, and hPc2 share a characteristic short amino acid motif, YVTV, immediately following the COOH box, which is not present in XPc1, M33 (MPc1), or CBX2 (hPc1). They instead have more extensive regions of sequence identity extending further upstream and downstream of the COOH box. Although overall sequence identity has already served to divide vertebrate Polycomb homologs into two classes (63), we suggest that the blocks of extended C-terminal homology could serve as a further, more specific, means of distinguishing between them.
XPc1 gene expression: masked maternal mRNA and XPc1 accumulation in embryonic nuclei.
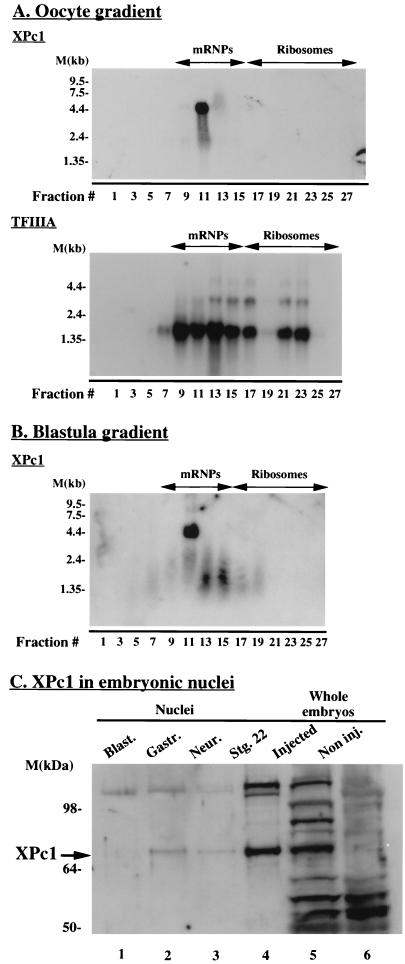
We determined the temporal patterns of expression and tissue distribution of the XPc1 transcript by Northern blot analysis. With the XPc1-coding region as a probe, an approximately 4.5-kb transcript is detected in all samples assayed; this size is consistent with that of the cloned XPc1 cDNA. The message for XPc1 appears to be abundant in the early stages of oocyte development and remains abundant in unfertilized eggs and early in embryonic development (data not shown). The abundance of XPc1 message appears to decline around gastrulation, remaining low during neurulation and moderately increasing again around the tailbud stage and in subsequent developmental stages. The relative abundances of XPc1 mRNA in oocytes, eggs, and embryonic stages prior to the midblastula transition (MBT) strongly suggests that the XPc1 mRNA in these stages is of maternal origin, as has also been suggested for XPc2 (61). The overall pattern of XPc1 expression during Xenopus development is similar to that observed for XPc2 (61). It is estimated that as much as 80% of the maternal mRNA synthesized in Xenopus oocytes is masked, complexed with storage messenger ribonucleoproteins (mRNPs), only to be translationally mobilized in the early stages of embryonic development (12). It should be emphasized that the oocyte exhibits highly selective tissue-specific patterns of gene expression. In general, mRNAs that are synthesized and sequestered in masked form encode proteins whose functions help determine differentiated states of gene expression in somatic cells (73, 83). The fact that the XPc1 cDNA contains a large (∼2.9-kb) 3′ untranslated region (3′ UTR) and the presence of a long U tract (37 bases) in the 3′ UTR led us to examine whether XPc1 is a maternal mRNA that is masked in the oocyte and translationally activated late in development (72). We found that the XPc1 mRNA in Xenopus oocytes is exclusively associated with the translationally inactive storage mRNPs (Fig. 2A, upper panel). It is striking that the XPc1 message appears to be very tightly associated with one particular fraction (fraction 11) (Fig. 2A, upper panel). This may reflect an intimate association of XPc1 mRNA with a component(s) of the mRNP complexes. By contrast, the mRNA for the translationally active transcription factor TFIIIA cofractionates with mRNP and ribosomal fractions (Fig. 2A, lower panel), as previously observed (76). The masking of XPc1 message in the mRNP complex is maintained to the blastula stage (Fig. 2B). This is remarkably late in development for a masked maternal mRNA and may reflect the distance of the U tract from the 3′ end of the mRNA (72). Our results suggest that XPc1 protein will begin to accumulate in Xenopus embryos only after the blastula stage is complete.
FIG. 2.
(A) XPc1 mRNA masking in Xenopus oocytes. Total mRNA from stage VI Xenopus oocytes was fractionated on a Nycodenz density gradient. Fractions were analyzed by Northern blot hybridization. The blot in the top panel shows the XPc1 message to be associated with translationally inactive storage mRNPs. As a control, the same filter was stripped and reprobed with TFIIIA to show the association of this message with ribosomes as well as with mRNPs (bottom panel). (B) Same as panel A except that mRNPs were fractionated from blastula-stage embryos (6 h postfertilization). (C) Western blot analysis with anti-XPc1 antibody to show that XPc1 protein (arrow) is first detected in em- bryonic nuclei after the blastula stage. Lanes 1 to 4, nuclear extracts from different developmental stages (10 h postfertilization). Blast., blastula; Gastr., gastrula; Neur., neurula; Stg., stage. Lane 5, total embryonic protein extract from embryos (10 h postfertilization) microinjected with XPc1 in vitro-transcribed RNA. Lane 6, noninjected (Non inj.) total embryo extract. M, markers.
In order to determine the timing of the appearance of XPc1 protein during Xenopus development and as a means of extending the observations on XPc1 mRNA masking, we raised a rabbit polyclonal antibody against a synthetic peptide derived from the N terminus of the predicted amino acid sequence of XPc1 (see Materials and Methods). This peptide shows little homology with the XPc2 protein (Fig. 1A). The antibody against the synthetic peptide detects a band of approximately 70 kDa, or more, in protein extracts from embryos (8 to 9 h postfertilization) injected with in vitro-transcribed XPc1 RNA (Fig. 2C, lane 5). No band in this size range is detected in noninjected embryos (Fig. 2C, lane 6) or in oocytes (not shown). This suggested that XPc1, if at all present, is not very abundant in embryos and prompted us to test embryonic nuclei from different developmental stages. In addition, our antibody was raised against a synthetic peptide and recognizes several other proteins in whole embryonic extracts, so restricting our analysis to nuclear proteins would enable us to focus on XPc1. Endogenous XPc1 protein was not detected in the oocyte or in the GV (the oocyte’s nucleus). This is in good agreement with our finding that XPc1 mRNA is stored in a translationally inactive form in the oocyte (Fig. 2A). In addition, XPc1 protein did not appear to be present in embryonic nuclei at the MBT, the stage at which the zygotic genome becomes transcriptionally active, which is consistent with the mRNP masking data (Fig. 2B and Fig. 2C, lane 1). A protein band of a size similar to that detected in XPc1-injected embryos first becomes detectable in gastrula-stage nuclei (Fig. 2C, lane 2) and persists up to the early tailbud stage (Fig. 2C, lane 4), which is the latest developmental time point tested in this experiment. At approximately 70 kDa, the XPc1 protein detected in embryonic nuclei, as well as in injected embryos, is appreciably larger than the predicted 52 kDa. This is consistent with the sizes observed for all Polycomb homologs characterized to date (2, 51, 64) and has been attributed to a high content of charged amino acids, posttranslational modifications, or both (51). We next wished to examine whether the regulation of XPc1 uptake into nuclei reflected a deficiency in nuclear import of XPc1 in oocytes and early embryos or whether oocytes were competent to accumulate XPc1 in nuclei. We also wished to examine whether the aberrant mobility of XPc1 (Fig. 2C) reflects posttranslational modification.
XPc1 synthesized from exogenous mRNA microinjected into oocyte cytoplasm readily accumulates in the GVs of injected oocytes (data not shown). This result indicates that the XPc1 protein is competent for nuclear import. It should be noted that masking of maternal mRNA synthesized in vivo depends on both transcription and association with the FRGY2 protein (7). Thus, naked mRNA injected into oocytes is translated. The XPc1 protein that accumulates in oocyte nuclei and cytoplasm migrates aberrantly with an apparent size of at least 70 kDa, as seen in embryos (Fig. 2C and data not shown). Alkaline phosphatase treatment of nuclear and cytoplasmic XPc1 results in an increase in electrophoretic mobility (data not shown). This result indicates that the XPc1 is a phosphoprotein. Thus, the aberrant mobility of XPc1 can be partially explained by phosphorylation, and the protein synthesized in oocytes is competent for nuclear uptake. We next focused our attention on the mechanism of XPc1-mediated transcriptional repression.
XPc1 is a transcriptional repressor in Xenopus embryos.
Polycomb has not been shown to bind to DNA directly (42). However, immunostaining analysis in Drosophila salivary glands has shown that it associates with several (over 100) loci in polytene chromosomes (88). We tested whether injected HA epitope-tagged XPc1 could be detected in embryonic chromatin. As a control for chromatin localization, we coinjected FLAG-tagged histone H1 in vitro-transcribed RNA. Embryos were collected at the early gastrula stage and treated with limiting amounts of micrococcal nuclease. Embryonic chromatin of different nucleosomal lengths was fractionated by sucrose gradient centrifugation (22), and individual fractions were assayed for the lengths of nucleosomal fragments (data not shown). By assaying for the fractionation profiles of HA-XPc1 and H1-FLAG, we found that XPc1 fractionated almost exclusively with the soluble, nucleosome-free fractions, whereas H1-FLAG could clearly be detected in the polynucleosomal chromatin fractions (data not shown). From this we conclude that injected XPc1 cannot be detected in the bulk chromatin of Xenopus embryos.
Polycomb has been genetically characterized as acting as a transcriptional repressor, yet it does not bind to DNA directly. When fused to a DBD and artificially tethered to a promoter, however, Polycomb and its higher vertebrate homologs have been shown to act as repressors in transiently (9, 63, 64, 66) or stably (2) transfected cells and in transgenic flies (45). We wished to determine whether XPc1 would also act as a transcriptional repressor in Xenopus embryonic chromatin when tethered to a promoter. We fused the yeast GAL4 DBD (amino acid residues 1 to 147) to the N terminus of XPc1 so as not to interfere with transcriptional repression, which appears to be mediated by the C-terminal domain of the protein (9, 44, 64, 66). The reporter we chose to use for our transcriptional assays was that of the Xenopus hsp70 promoter (5). The transcriptional regulation and cis-acting requirements of this promoter have been characterized in a chromatin context for Xenopus oocytes, embryos, and somatic cells (37–40). Furthermore, the hsp70 promoter can be induced to high levels of transcription in all of these systems by a simple heat shock treatment (37). In the absence of heat shock, the hsp70 promoter is capable of high levels of transcription in oocytes by exogenous addition of Xenopus HSF (38, 75). We used a reporter plasmid in which five copies of the GAL4 DNA binding sites were cloned in tandem immediately upstream of the hsp70 promoter, which was fused to a CAT reporter gene (G5hsp70CAT).
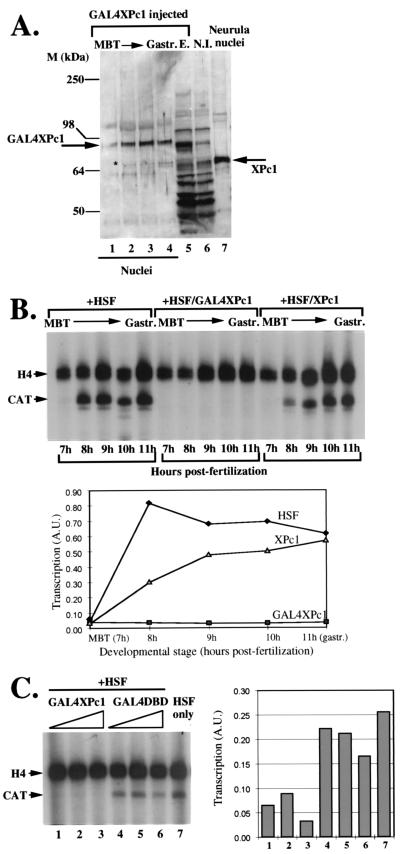
We employed Xenopus embryos to examine the influence of GAL4XPc1 on transcription. We first tested the localization of GAL4XPc1 in the nuclei of microinjected embryos. In Fig. 3A (lane 1), it is evident that microinjected GAL4XPc1 is efficiently localized in embryonic nuclei, even at developmental time points when endogenous XPc1 is not detectable. We next tested the transcriptional regulation of the G5hsp70CAT reporter plasmid in embryos by coinjecting the DNA with in vitro-transcribed HSF mRNA (Fig. 3B). No transcription from the hsp70 promoter can be detected at the MBT (Fig. 3B, lane 1), possibly due to the transcriptional constraints to which the zygotic genome is subjected up to that stage of development (3, 39, 57, 58). However, robust levels of hsp70 transcription appear shortly after the blastula stage (Fig. 3B, lane 2) and persist up to the gastrula stage (Fig. 3B, lane 5). Tethering GAL4XPc1 to the hsp70 promoter almost completely abolishes transcription at all developmental time points tested (Fig. 3B, lanes 6 to 10) even in the continued presence of HSF. Coinjection of XPc1 RNA without the GAL4 DBD tether leads to only a slight reduction in hsp70 transcriptional levels (Fig. 3B, lanes 11 to 15). This is probably a nonspecific effect due to the sensitivity of Xenopus embryos to the dosage of microinjected nucleic acids (62). We conclude from this experiment that tethering GAL4XPc1 to the hsp70 promoter efficiently represses transcription following zygotic gene activation at the MBT (27). The results after removal of the GAL4 DBD tether suggest that this repression is specific and not due to the indiscriminate overexpression of proteins in microinjected embryos. The repressive effect of GAL4XPc1 is not peculiar to the hsp70 promoter, as we observed a similar repression in embryos when GAL4XPc1 was tethered to a herpes simplex virus thymidine kinase promoter construct (data not shown).
FIG. 3.
Transcriptional repression by tethering of GAL4XPc1 to an hsp70CAT reporter construct in Xenopus embryos. (A) The GAL4XPc1 fusion protein is efficiently localized in embryonic nuclei. Lanes 1 to 4, nuclear extracts at developmental stages between the MBT and gastrula (Gastr.) from embryos microinjected with GAL4XPc1 RNA. Lane numbers indicate hours postfertilization. The XPc1 antibody used detects endogenous XPc1 as well as the GAL4XPc1 fusion protein (arrows). Lane 5, total protein extract (E.) from embryos microinjected with GAL4XPc1. Lane 6, noninjected (N.I.) total extract control. Lane 7, noninjected nuclear extract from neurula embryos, used as a positive control for detecting endogenous XPc1. M, markers. (B) Primer extension analysis of transcription from the microinjected hsp70CAT reporter during early Xenopus development is shown in lanes 1 to 5. Microinjected embryos were collected and analyzed at hourly time points between the MBT (approximately 7 h postfertilization) and the gastrula stage (approximately 11 h postfertilization). In order to drive detectable levels of hsp70 transcription, in vitro-transcribed Xenopus HSF RNA was coinjected with the reporter plasmid in all experiments. The effect of tethering of GAL4XPc1 to the hsp70 promoter is shown in lanes 6 to 10. Lanes 11 to 15 show the absence of significant repression from the hsp70 promoter when XPc1 is coinjected without the tether of the GAL4 DBD. A.U., arbitrary units. (C) Effects of titrating GAL4XPc1 (lanes 1 to 3) versus the GAL4 DBD (lanes 4 to 6) on hsp70 transcription in microinjected embryos. Approximately equal amounts of in vitro-transcribed GAL4XPc1 or GAL4 DBD RNA were injected. Due to the size difference between the two RNAs and assuming similar translation rates, the GAL4 DBD is in approximately a fourfold molar excess compared to GAL4XPc1 in these experiments. Lane 7, hsp70CAT-HSF coinjection control.
To assess whether the repressive effect on the hsp70 promoter was due to a nonspecific squelching effect mediated by the GAL4 DBD fused to XPc1, we injected increasing, but equivalent, amounts of GAL4XPc1 or GAL4 DBD in vitro-transcribed RNAs (Fig. 3C). Transcriptional activity was assayed in embryos at 10 h postfertilization. It can be seen that whereas GAL4XPc1 almost completely repressed hsp70 transcription at all RNA concentrations injected, the GAL4 DBD had only a very moderate effect on transcription at the highest concentration (Fig. 3C). It should be noted that due to the size difference for the two RNAs, the GAL4 DBD in these injections is in a fourfold molar excess compared to GAL4XPc1. We reason from this that the GAL4 DBD does not significantly contribute to the observed repression by GAL4XPc1. In addition, our GAL4XPc1 titration indicates that relatively low levels of mRNA can serve to repress transcription, and our Western blotting analysis indicates that XPc1 levels are raised less than 10-fold over those present in control embryos. From the evidence presented in Fig. 3B and C, we conclude that the GAL4XPc1-mediated repression of the hsp70 promoter in Xenopus embryos is specific to the XPc1 part of the fusion protein.
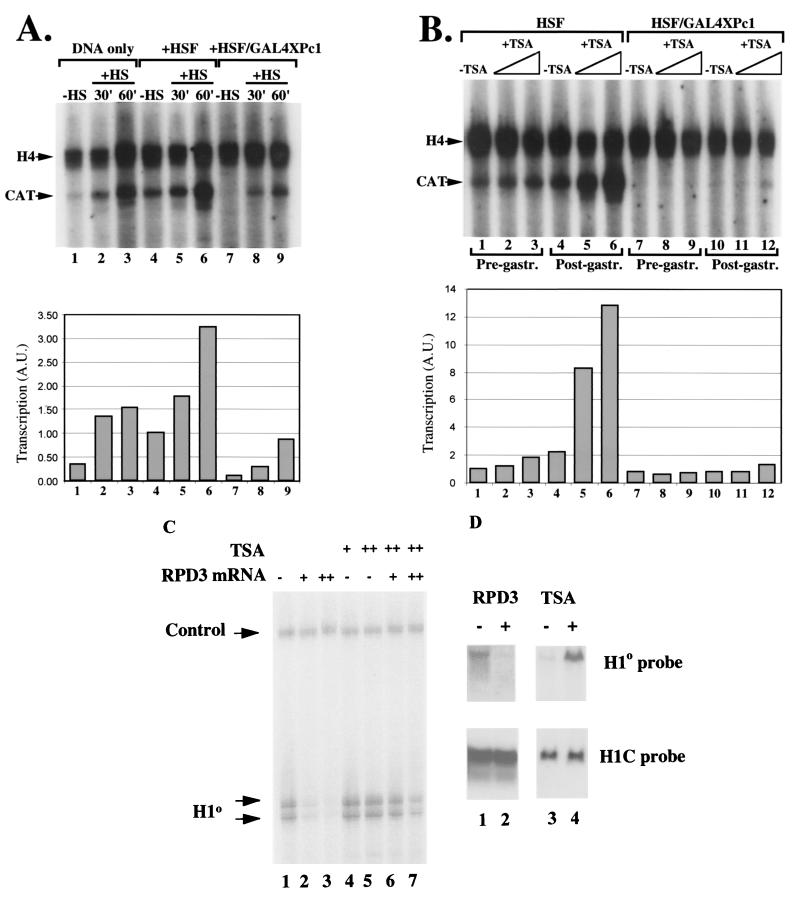
It has been previously shown that heat shock at 34°C induces high levels of transcription from an hsp70 promoter injected in embryos (36, 38). In general, heat shock mobilizes endogenous HSF residing in the cell in an inactive complexed form. We wanted to test whether the heat shock-inducible increase in the dosage of HSF would be sufficient to alleviate the repressive effect of GAL4XPc1 on the hsp70 promoter. As a control for heat shock, we injected G5hsp70CAT plasmid DNA alone (Fig. 4A, lanes 1 to 3). As previously seen (37), there is little hsp70 transcription in the absence of exogenously added HSF (Fig. 4A, lane 1). Heat shock for as little as 30 min, however, is sufficient to induce high levels of hsp70 transcription (lane 2), which increases further with prolonged heat shock treatment (lane 3). Coinjection of HSF mRNA together with the G5hsp70CAT reporter further enhances the heat shock response (Fig. 4A, lanes 5 and 6). By contrast, tethering GAL4XPc1 reduces the heat shock responsiveness of the hsp70 promoter such that only a moderate increase in transcription is observed (Fig. 4A, lanes 8 and 9), which, even after prolonged heat shock treatment, does not reach control non-heat-shock-induced levels (compare lane 4 to lane 9). Increasing the dosage of the HSF transcription factor, therefore, does not fully reverse the repressive effect of GAL4XPc1 on the hsp70 promoter in Xenopus embryos.
FIG. 4.
GAL4XPc1-mediated repression of transcription is maintained in the presence of elevated levels of HSF and in the presence of inhibitors of histone deacetylase. (A) Effect of heat shock (HS) on GAL4XPc1-mediated repression in microinjected embryos. Lanes 1 to 3, inducibility of the hsp70 promoter in the absence of any coinjected HSF after 30 (lane 2) or 60 (lane 3) min of heat shock. Lanes 4 to 6, enhanced heat shock induction of the hsp70 promoter when coinjected with HSF RNA. Lanes 7 to 9, heat shock is not sufficient to restore hsp70 transcription to control levels when GAL4XPc1 is tethered to the promoter. A.U., arbitrary units. (B) Effect of TSA treatment on GAL4XPc1-mediated repression. Microinjected embryos were treated with 30 nM (lanes 2, 5, 8, and 11) or 90 nM (lanes 3, 6, 9, and 12) TSA and harvested for analysis before (stage 10, lanes 1 to 3 and 7 to 9) or after (stage 13, lanes 4 to 6 and 10 to 12) gastrulation (gastr.). For all experiments, unless otherwise indicated, embryos were harvested for analysis at approximately the initial gastrula stage (stage 10). A histone H4 primer was included in all primer extensions as a control for RNA recovery and loading. (C) Expression of RPD3 represses transcription from the H1° promoter, and TSA relieves this repression. Oocytes were injected with double-stranded DNA of H1° with or without an increasing amount of RPD3 mRNA (0.5 ng in lanes 2 and 6 and 1 ng in lanes 3 and 7). The oocytes were assayed by primer extension. The oocytes were incubated overnight in the presence of 30 nM (+) or 90 nM (++) TSA or in the absence of TSA (−). The positions of the extension products of the H1° transcript and of the endogenous H4 control are indicated. (D) Lanes 1 and 2, fertilized eggs were microinjected with 1 ng of RPD3 mRNA (+) or water (−) and allowed to develop. Lanes 3 and 4, fertilized eggs were incubated in the absence (−) or presence (+) of 30 nM TSA. Total RNA was isolated from 10 embryos and electrophoresed on a 1% agarose gel. The blot was probed with [α-32P]dCTP random-prime-labeled full-length H1° or H1C coding regions and washed stringently.
We next tested whether inhibition of the RPD3 family of histone deacetylases (33b) by TSA was sufficient to reverse XPc1-mediated repression. Three lines of evidence led us to carry out this experiment. First, recent work in our laboratory showed that treatment with low levels of TSA could induce high levels of hsp70 transcription in Xenopus oocytes, comparable to those achieved by heat shock (40). Second, it was recently shown that the transcriptional repression observed in Schizosaccharomyces pombe centromeric heterochromatin domains could be alleviated by treatment with TSA (19). The repressive effect in S. pombe heterochromatin is mediated by swi6, a chromodomain-containing homolog of HP1 (18). Third, the Drosophila dMi-2 protein is a Hunchback-interacting protein that functions in Polycomb-mediated repression (32a), and Xenopus Mi-2 is part of a histone deacetylase complex (83b). Bearing in mind the parallels that have often been drawn between heterochromatic and Pc-G repression, it was suggested that deacetylation may also play a role in Pc-G-mediated repression (10, 33a). We therefore tested whether TSA treatment was sufficient to lift the GAL4XPc1-mediated repression on the hsp70 promoter in Xenopus embryos. Immediately following microinjection, embryos were treated with two concentrations of TSA, 30 and 90 nM. Deacetylase activity is effectively inhibited at both TSA concentrations in embryos (83a) (see Fig. 4C and D below). Surprisingly, both concentrations of TSA had little effect on hsp70 transcription prior to gastrulation (Fig. 4B, lanes 1 to 3, 8 h postfertilization). After gastrulation (14 h postfertilization), we observed an induction of hsp70 transcription, the levels of which were proportional to the TSA concentration used (Fig. 4B, lanes 4 to 6). By contrast, TSA treatment appears to have practically no effect on GAL4XPc1-mediated repression, before or after gastrulation (Fig. 4B, lanes 7 to 12). In order to control for the effectiveness of TSA in inhibiting the RPD3 class of histone deacetylases inhibitors during early embryogenesis, we made use of the H1° promoter (3a, 33a). We tested the role of Xenopus RPD3 in H1° promoter regulation by examining transcription in oocytes microinjected with increasing amounts of RPD3 mRNA (Fig. 4C, lanes 1 to 3). Increasing amounts of RPD3 mRNA elevate deacetylase activity in oocytes and repress transcription from the H1° promoter. This repression is relieved in the presence of TSA (Fig. 4C, lanes 4 to 7). Similar results are obtained with embryos when microinjection of RPD3 mRNA selectively represses H1° mRNA transcription from the endogenous chromosomes (Fig. 4D, lanes 1 and 2), whereas addition of TSA to embryos as for Fig. 4B selectively activates H1° transcription (Fig. 4D, lanes 3 and 4) (3a). We conclude that deacetylation is unlikely to play a significant role in Polycomb-mediated transcriptional repression in our in vivo assay with Xenopus embryos.
DISCUSSION
We report here the isolation and characterization of XPc1, a new chromodomain homolog in X. laevis. XPc1 is the second Polycomb homolog to be isolated in Xenopus, in agreement with the accumulating evidence that Pc-G vertebrate homologs exist in pairs (2, 63, 64). It is also clear that Pc-G homolog pairs, although related, are distinct proteins (2, 26, 61, 63, 64). On the basis of sequence homology, the vertebrate Polycomb homologs can be grouped in two classes (64). Whereas the N-terminal chromodomains are similar (Fig. 1C), we note that C-terminal homologies extending beyond the COOH box can serve as another criterion to further distinguish between the two classes of homologs (Fig. 1D). The divergence in the C termini of vertebrate homologs may be of functional significance. Detailed analysis of the molecular basis of Polycomb mutants in Drosophila revealed that many of the mutations were clustered in the conserved C-terminal domain of Polycomb (21). The C-terminal domain does not appear to be necessary for targeting Polycomb to its normal chromosomal binding sites (21); it is, however, indispensable for transcriptional repression (9, 44, 64, 66). These observations have led to the suggestion that the C-terminal domain is important for protein-protein interactions in recruiting a repressive complex. Whether the differences in the C-terminal domains of the vertebrate Polycomb homologs also reflect functional distinctions remains to be seen.
Developmental regulation of XPc1.
The expression analysis of XPc1 showed high levels of its transcript in the ovary and in immature Xenopus oocytes, which is strongly suggestive of a maternal deposition of XPc1 mRNA. Maternal deposition of Pc mRNA has also been observed in Drosophila oogenesis (51), where genetic evidence has indeed shown a maternal component in the developmental functions of Polycomb and other Pc-G members (13, 29, 51). As in Drosophila oocytes (51), we failed to detect any XPc1 protein in Xenopus oocytes. In fact, we find that maternally deposited XPc1 Polycomb message is stored in the oocyte in a translationally inactive form (Fig. 2A). There are features in the XPc1 3′ UTR, including the long U tract, that function as embryonic cytoplasmic polyadenylation elements and are consistent with the delayed activation of translation (Fig. 2B) (72). There are interesting parallels between the expression of XPc1 and the regulation of histone H1 protein synthesis during early Xenopus development. In this case, masked maternal H1 mRNA is released for translation during the early cleavage divisions, with a normal stoichiometry of H1 relative to core histones being achieved at gastrulation (14, 76, 80). The progressive accumulation of histone H1 leads to the selective repression of oocyte-type 5S rRNA genes (7, 32) and the loss of mesodermal competence (73, 83). Thus, changes in chromatin composition influence the patterning of the Xenopus embryo into distinct cell lineages.
Transcriptional repression by XPc1.
We find that XPc1 acts as transcriptional repressor in vivo in Xenopus embryos when tethered to a promoter by virtue of an N-terminal fusion to the GAL4 DBD (Fig. 3 and 4). Repression by tethering to a promoter has been reported for many Pc-G members, mostly in transfected cells (2, 9, 63, 64, 66). However, the in vivo repressive effect of tethered Polycomb during development has been previously described only for transgenic Drosophila embryos (44). Our results, therefore, document for the first time the fact that a Polycomb homolog will also repress transcription during embryonic development in higher vertebrates. XPc1-mediated repression is first observed shortly after the MBT stage, when the hsp70 reporter promoter becomes transcriptionally active. This is consistent with the presence of GAL4XPc1 protein in the nucleus and suggests that all factors required for XPc1-mediated repression, for example, other Pc-G members, are also present in the nucleus at this stage. Repression persisted in all of the early developmental stages we tested and extended beyond gastrulation. In Drosophila, repression persists in a promoter-specific manner even after the GAL4Pc fusion protein has decayed (44). Presently we do not know whether this is also the case for our assay in Xenopus embryos, since GAL4XPc1 protein is present in embryonic nuclei in all developmental stages tested.
Using our in vivo repression assay with Xenopus embryos, we found that increasing the HSF transcription factor dosage by heat shock only partially alleviates XPc1-mediated repression and even then, hsp70 expression does not reach wild-type, non-heat-shock-induced levels (Fig. 4A). This suggests that at least in this reporter system, GAL4XPc1 exerts a dominant repressive effect over the inductive effects of HSF. This is in contrast to results obtained with Drosophila in vivo repression assays. Zink and Paro (89) have used naturally occurring Polycomb response elements and GAL4 DNA binding sites in transgenic constructs to show that a large increase in the amount of the GAL4 transcription factor can completely reverse the Pc-G-mediated repression in reporter constructs. Furthermore, GAL4 in high doses will also replace Polycomb binding from reporter transgenes in polytene chromosomes (89). Several factors could account for the differences observed between the two assays. For example, GAL4 may be a more powerful transcription factor than HSF, thus completely overcoming Pc-G-mediated repression. Alternatively, the GAL4 DNA binding sites present in our reporter construct may be more effective than the Polycomb response element in anchoring and maintaining GAL4XPc1 to the promoter.
We established that inhibition of the RPD3 family of histone deacetylases (33b) by TSA treatment does not affect repression mediated by XPc1 when artificially tethered to a promoter, thus suggesting that, at least in this type of assay, deacetylation is unlikely to play a role in Polycomb-mediated repression (Fig. 4B). Control experiments indicate that RPD3-mediated repression of H1° gene expression within the endogenous chromosomes of the Xenopus embryo is relieved by TSA (Fig. 4C and D). It was recently postulated that Pc-G repression was mediated by the recruitment of histone deacetylases to the Pc-G complex (32a, 55). Our results suggest that whatever complex assembles and mediates the repression observed in our tethering assay, it does not involve an essential deacetylase activity. Ekwall et al. showed recently that TSA treatment causes derepression of heterochromatic silencing in S. pombe (19). TSA treatment also induces removal from centromeric heterochromatin of swi6, a chromodomain-containing HP1-like protein essential for centromeric function and a mediator of heterochromatic silencing (18, 19). Bearing in mind the parallels that have often been drawn between HP1-mediated heterochromatic repression and Pc-G-mediated repression, it may be tempting to suggest that, just like for swi6 silencing in S. pombe, Pc-G repression also employs deacetylation. Our data in fact indicate the opposite, suggesting that the molecular bases for heterochromatin silencing and Pc-G repression may be different. This is not the first time that despite the postulated mechanistic parallels, differences have been observed at the molecular level between the two silencing phenomena. In Drosophila, transgenes integrated in pericentric heterochromatin have been suggested to have altered chromatin structures consistent with a tighter packaging in nucleosomes (84). By contrast, Pc-G gene targets in the homeotic loci in Drosophila did not appear to have altered chromatin structures, as evidenced by restriction enzyme accessibility, in the presence or absence of Polycomb protein (65). Alternatively, the influence of acetylation on silencing mediated by chromodomain proteins may be a phenomenon peculiar to centromeric heterochromatin. Wallrath and Elgin (84) found that genetically induced histone hyperacetylation, while derepressing centromeric silenced domains (17), did not affect telomeric silencing, even though HP1 also appears to be a component of telomeric heterochromatin. In that respect, it would be interesting to test in our assay whether in Xenopus embryos HP1 tethered to a promoter represses transcription in a TSA-reversible manner.
By drawing parallels again between the HP1-like swi6 protein and Polycomb, it was recently proposed that chromodomain proteins may serve to faithfully protect and propagate epigenetic states set up in a highly localized manner by modifications in chromatin structure, for example, deacetylation (10). According to this model, therefore, targeted deacetylation could provide the imprint for the establishment of epigenetically regulated transcriptional states which will then be maintained by chromodomain protein complexes through several mitotic and, surprisingly, meiotic cycles (10, 11). Our results cannot distinguish whether deacetylation is required for the establishment of repressive states, since tethering a chromodomain protein to a promoter bypasses this step altogether. However, we presented evidence suggesting that, once established, deacetylation may not be involved in the maintenance of repression mediated by Polycomb complexes. This may not be the case in HP1 chromodomain-mediated repression (19). The presence of a chromodomain therefore may signify a common mechanism for specifically targeting protein complexes involved in the epigenetic maintenance of transcriptional states but may not be involved in the molecular mechanism(s) by which the effects on transcription are achieved.
ACKNOWLEDGMENTS
We are indebted to Nicoletta Landsberger for reagents and invaluable help.
J.S. has been supported by an HFSPO long-term postdoctoral fellowship.
REFERENCES
- 1.Alkema M J, Bronk M, Verhoeven E, Otte A, van’t Veer L J, Berns A, van Lohuizen M. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian Polycomb complex. Genes Dev. 1997;11:226–240. doi: 10.1101/gad.11.2.226. [DOI] [PubMed] [Google Scholar]
- 2.Alkema M J, Jacobs J, Voncken J W, Jenkins N A, Copeland N G, Satijn D P, Otte A P, Berns A, van Lohuizen M. MPc2, a new murine homolog of the Drosophila Polycomb protein is a member of the mouse Polycomb transcriptional repressor complex. J Mol Biol. 1997;273:993–1003. doi: 10.1006/jmbi.1997.1372. [DOI] [PubMed] [Google Scholar]
- 3.Almouzni G, Wolffe A P. Constraints on transcriptional activator function contribute to transcriptional quiescence during early Xenopus embryogenesis. EMBO J. 1995;14:1752–1765. doi: 10.1002/j.1460-2075.1995.tb07164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Almouzni G, Khochbin S, Dimitrov S, Wolffe A P. Histone acetylation influences both gene expression and development of Xenopus laevis. Dev Biol. 1994;165:654–669. doi: 10.1006/dbio.1994.1283. [DOI] [PubMed] [Google Scholar]
- 4.Andrews M T, Brown D D. Transient activation of oocyte 5S RNA genes in Xenopus embryos by raising the level of the trans-acting factor TFIIIA. Cell. 1987;51:445–453. doi: 10.1016/0092-8674(87)90640-4. [DOI] [PubMed] [Google Scholar]
- 5.Bienz M. Xenopus hsp 70 genes are constitutively expressed in injected oocytes. EMBO J. 1984;3:2477–2483. doi: 10.1002/j.1460-2075.1984.tb02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bienz M, Müller J. Transcriptional silencing of homeotic genes in Drosophila. Bioessays. 1995;17:775–784. doi: 10.1002/bies.950170907. [DOI] [PubMed] [Google Scholar]
- 7.Bouvet P, Dimitrov S, Wolffe A P. Specific regulation of Xenopus chromosomal 5S rRNA gene transcription in vivo by histone H1. Genes Dev. 1994;8:1147–1159. doi: 10.1101/gad.8.10.1147. [DOI] [PubMed] [Google Scholar]
- 8.Buchenau P, Hodgson J, Strutt H, Arndt-Jovin D J. The distribution of Polycomb-group proteins during cell division and development in Drosophila embryos: impact on models for silencing. J Cell Biol. 1998;141:469–481. doi: 10.1083/jcb.141.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunker C A, Kingston R E. Transcriptional repression by Drosophila and mammalian Polycomb group proteins in transfected mammalian cells. Mol Cell Biol. 1994;14:1721–1732. doi: 10.1128/mcb.14.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalli G, Paro R. Chromo-domain proteins: linking chromatin structure to epigenetic regulation. Curr Opin Cell Biol. 1998;10:354–360. doi: 10.1016/s0955-0674(98)80011-2. [DOI] [PubMed] [Google Scholar]
- 11.Cavalli G, Paro R. The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell. 1998;93:505–518. doi: 10.1016/s0092-8674(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 12.Davidson E H. Gene activity in early development. Orlando, Fla: Academic Press, Inc.; 1986. [Google Scholar]
- 13.Denell R E. Homeosis in Drosophila: evidence of a maternal effect of the Polycomb locus. Dev Genet. 1982;3:103–113. [Google Scholar]
- 14.Dimitrov S, Almouzni G, Dasso M, Wolffe A P. Chromatin transitions during early Xenopus embryogenesis: changes in histone H4 acetylation and in linker histone type. Dev Biol. 1993;160:214–227. doi: 10.1006/dbio.1993.1299. [DOI] [PubMed] [Google Scholar]
- 15.Dombradi V, Axton J M, Barker H M, Cohen P T. Protein phosphatase 1 activity in Drosophila mutants with abnormalities in mitosis and chromosome condensation. FEBS Lett. 1990;275:39–43. doi: 10.1016/0014-5793(90)81434-p. [DOI] [PubMed] [Google Scholar]
- 16.Dombradi V, Cohen P T. Protein phosphorylation is involved in the regulation of chromatin condensation during interphase. FEBS Lett. 1992;312:21–26. doi: 10.1016/0014-5793(92)81402-8. [DOI] [PubMed] [Google Scholar]
- 17.Dorn R, Heymann S, Lindigkeit R, Reuter G. Supressor mutation of position-effect variegation in Drosophila melanogaster affecting chromatin properties. Chromosoma. 1986;93:398–403. [Google Scholar]
- 18.Ekwall K, Javerzat J P, Lorentz A, Schmidt H, Cranston G, Allshire R. The chromodomain protein Swi6: a key component at fission yeast centromeres. Science. 1995;269:1429–1431. doi: 10.1126/science.7660126. [DOI] [PubMed] [Google Scholar]
- 19.Ekwall K, Olsson T, Turner B M, Cranston G, Allshire R C. Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres. Cell. 1997;91:1021–1032. doi: 10.1016/s0092-8674(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 20.Franke A, DeCamillis M, Zink D, Cheng N, Brock H W, Paro R. Polycomb and polyhomeotic are constituents of a multimeric protein complex in chromatin of Drosophila melanogaster. EMBO J. 1992;11:2941–2950. doi: 10.1002/j.1460-2075.1992.tb05364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke A, Messmer S, Paro R. Mapping functional domains of the Polycomb protein of Drosophila melanogaster. Chromosome Res. 1995;3:351–360. doi: 10.1007/BF00710016. [DOI] [PubMed] [Google Scholar]
- 22.Freeman L, Kurumizaka H, Wolffe A P. Functional domains for assembly of histones H3 and H4 into the chromatin of Xenopus embryos. Proc Natl Acad Sci USA. 1996;93:12780–12785. doi: 10.1073/pnas.93.23.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gecz J, Gaunt S J, Passage E, Burton R D, Cudrey C, Pearce J J, Fontes M. Assignment of a Polycomb-like chromobox gene (CBX2) to human chromosome 17q25. Genomics. 1995;26:130–133. doi: 10.1016/0888-7543(95)80091-y. [DOI] [PubMed] [Google Scholar]
- 24.Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz E M, Coupland G. A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature. 1997;386:44–51. doi: 10.1038/386044a0. [DOI] [PubMed] [Google Scholar]
- 25.Grossniklaus U, Vielle-Calzada J P, Hoeppner M A, Gagliano W B. Maternal control of embryogenesis by MEDEA, a Polycomb group gene in Arabidopsis. Science. 1998;280:446–450. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- 26.Gunster M J, Satijn D P, Hamer K M, den Blaauwen J L, de Bruijn D, Alkema M J, van Lohuizen M, van Driel R, Otte A P. Identification and characterization of interactions between the vertebrate polycomb-group protein BMI1 and human homologs of polyhomeotic. Mol Cell Biol. 1997;17:2326–2335. doi: 10.1128/mcb.17.4.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hair A, Prioleau M N, Vassetzky Y, Méchali M. Control of gene expression in Xenopus early development. Dev Genet. 1998;22:122–131. doi: 10.1002/(SICI)1520-6408(1998)22:2<122::AID-DVG2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto N, Brock H W, Nomura M, Kyba M, Hodgson J, Fujita Y, Takihara Y, Shimada K, Higashinakagawa T. RAE28, BMI1, and M33 are members of heterogeneous multimeric mammalian Polycomb group complexes. Biochem Biophys Res Commun. 1998;245:356–365. doi: 10.1006/bbrc.1998.8438. [DOI] [PubMed] [Google Scholar]
- 29.Haynie J L. The maternal and zygotic roles of the gene Polycomb in embryonic determination in Drosophila melanogaster. Dev Biol. 1983;100:399–411. doi: 10.1016/0012-1606(83)90234-8. [DOI] [PubMed] [Google Scholar]
- 30.Holdeman R, Nehrt S, Strome S. MES-2, a maternal protein essential for viability of the germline in Caenorhabditis elegans, is homologous to a Drosophila Polycomb group protein. Development. 1998;125:2457–2467. doi: 10.1242/dev.125.13.2457. [DOI] [PubMed] [Google Scholar]
- 31.Jürgens G. A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature. 1985;316:153–155. [Google Scholar]
- 32.Kandolf H. The H1A histone variant is an in vivo repressor of oocyte-type 5S gene transcription in Xenopus laevis embryos. Proc Natl Acad Sci USA. 1994;91:7257–7261. doi: 10.1073/pnas.91.15.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Kehle J, Benchle D, Treuheit S, Christen B, Kennison J A, Bienz M, Muller J. dMi-2, a Hunchback-interacting protein that functions in Polycomb repression. Science. 1998;282:1897–1900. doi: 10.1126/science.282.5395.1897. [DOI] [PubMed] [Google Scholar]
- 33.Kennison J A. Transcriptional activation of Drosophila homeotic genes from distant regulatory elements. Trends Genet. 1993;9:75–79. doi: 10.1016/0168-9525(93)90227-9. [DOI] [PubMed] [Google Scholar]
- 33a.Khochbin S, Wolffe A P. Developmental regulation and butyrate inducible transcription of the Xenopus histone H1° promoter. Gene. 1993;128:173–180. doi: 10.1016/0378-1119(93)90560-p. [DOI] [PubMed] [Google Scholar]
- 33b.Khochbin S, Wolffe A P. The origin and utility of histone deacetylases. FEBS Lett. 1997;419:157–160. doi: 10.1016/s0014-5793(97)01423-3. [DOI] [PubMed] [Google Scholar]
- 34.Koonin E V, Zhou S, Lucchesi J C. The chromo superfamily: new members, duplication of the chromo domain and possible role in delivering transcription regulators to chromatin. Nucleic Acids Res. 1995;23:4229–4233. doi: 10.1093/nar/23.21.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korf I, Fan Y, Strome S. The Polycomb group in Caenorhabditis elegans and maternal control of germline development. Development. 1998;125:2469–2478. doi: 10.1242/dev.125.13.2469. [DOI] [PubMed] [Google Scholar]
- 36.Krone P H, Heikkila J J. Expression of microinjected hsp 70/CAT and hsp 30/CAT chimeric genes in developing Xenopus laevis embryos. Development. 1989;106:271–281. doi: 10.1242/dev.106.2.271. [DOI] [PubMed] [Google Scholar]
- 37.Landsberger N, Ranjan M, Almouzni G, Stump D, Wolffe A P. The heat shock response in Xenopus oocytes, embryos, and somatic cells: a regulatory role for chromatin. Dev Biol. 1995;170:62–74. doi: 10.1006/dbio.1995.1195. [DOI] [PubMed] [Google Scholar]
- 38.Landsberger N, Wolffe A P. Role of chromatin and Xenopus laevis heat shock transcription factor in regulation of transcription from the X. laevis hsp70 promoter in vivo. Mol Cell Biol. 1995;15:6013–6024. doi: 10.1128/mcb.15.11.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landsberger N, Wolffe A P. Remodeling of regulatory nucleoprotein complexes on the Xenopus hsp70 promoter during meiotic maturation of the Xenopus oocyte. EMBO J. 1997;16:4361–4373. doi: 10.1093/emboj/16.14.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Herrler M, Landsberger N, Kaludov N, Ogryzko V V, Nakatani Y, Wolffe A P. Xenopus NF-Y pre-sets chromatin to potentiate p300 and acetylation-responsive transcription from the Xenopus hsp70 promoter in vivo. EMBO J. 1998;17:6300–6315. doi: 10.1093/emboj/17.21.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meric F, Matsumoto K, Wolffe A P. Regulated unmasking of in vivo synthesized maternal mRNA at oocyte maturation. A role for the chaperone nucleoplasmin. J Biol Chem. 1997;272:12840–12846. doi: 10.1074/jbc.272.19.12840. [DOI] [PubMed] [Google Scholar]
- 42.Messmer S, Franke A, Paro R. Analysis of the functional role of the Polycomb chromo domain in Drosophila melanogaster. Genes Dev. 1992;6:1241–1254. doi: 10.1101/gad.6.7.1241. [DOI] [PubMed] [Google Scholar]
- 43.Moehrle A, Paro R. Spreading the silence: epigenetic transcriptional regulation during Drosophila development. Dev Genet. 1994;15:478–484. doi: 10.1002/dvg.1020150606. [DOI] [PubMed] [Google Scholar]
- 44.Müller J. Transcriptional silencing by the Polycomb protein in Drosophila embryos. EMBO J. 1995;14:1209–1220. doi: 10.1002/j.1460-2075.1995.tb07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Müller J, Gaunt S, Lawrence P A. Function of the Polycomb protein is conserved in mice and flies. Development. 1995;121:2847–2852. doi: 10.1242/dev.121.9.2847. [DOI] [PubMed] [Google Scholar]
- 46.Orlando V, Paro R. Mapping Polycomb-repressed domains in the bithorax complex using in vivo formaldehyde cross-linked chromatin. Cell. 1993;75:1187–1198. doi: 10.1016/0092-8674(93)90328-n. [DOI] [PubMed] [Google Scholar]
- 47.Paro R. Imprinting a determined state into the chromatin of Drosophila. Trends Genet. 1990;6:416–421. doi: 10.1016/0168-9525(90)90303-n. [DOI] [PubMed] [Google Scholar]
- 48.Paro R. Mechanisms of heritable gene repression during development of Drosophila. Curr Opin Cell Biol. 1993;5:999–1005. doi: 10.1016/0955-0674(93)90084-4. [DOI] [PubMed] [Google Scholar]
- 49.Paro R, Harte P J. The role of Polycomb Group and Trithorax Group chromatin complexes in the maintenance of determined cells rates. In: Russo V E A, Martienssen R A, Riggs A D, editors. Epigenetic mechanisms of gene regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 507–528. [Google Scholar]
- 50.Paro R, Hogness D S. The Polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc Natl Acad Sci USA. 1991;88:263–267. doi: 10.1073/pnas.88.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paro R, Zink B. The Polycomb gene is differentially regulated during oogenesis and embryogenesis of Drosophila melanogaster. Mech Dev. 1993;40:37–46. doi: 10.1016/0925-4773(93)90086-d. [DOI] [PubMed] [Google Scholar]
- 52.Parthun M R, Jaehning J A. A transcriptionally active form of GAL4 is phosphorylated and associated with GAL80. Mol Cell Biol. 1992;12:4981–4987. doi: 10.1128/mcb.12.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearce J J, Singh P B, Gaunt S J. The mouse has a Polycomb-like chromobox gene. Development. 1992;114:921–929. doi: 10.1242/dev.114.4.921. [DOI] [PubMed] [Google Scholar]
- 54.Pirrotta V. PcG complexes and chromatin silencing. Curr Opin Genet Dev. 1997;7:249–258. doi: 10.1016/s0959-437x(97)80135-9. [DOI] [PubMed] [Google Scholar]
- 55.Pirrotta V. Polycombing the genome: PcG, trxG, and chromatin silencing. Cell. 1998;93:333–336. doi: 10.1016/s0092-8674(00)81162-9. [DOI] [PubMed] [Google Scholar]
- 56.Pirrotta V, Rastelli L. White gene expression, repressive chromatin domains and homeotic gene regulation in Drosophila. Bioessays. 1994;16:549–556. doi: 10.1002/bies.950160808. [DOI] [PubMed] [Google Scholar]
- 57.Prioleau M N, Huet J, Séntenac A, Méchali M. Competition between chromatin and transcription complex assembly regulates gene expression during early development. Cell. 1994;77:439–449. doi: 10.1016/0092-8674(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 58.Prioleau M N, Buckle R S, Méchali M. Programming of a repressed but committed chromatin structure during early development. EMBO J. 1995;14:5073–5084. doi: 10.1002/j.1460-2075.1995.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ranjan M, Tafuri S R, Wolffe A P. Masking mRNA from translation in somatic cells. Genes Dev. 1993;7:1725–1736. doi: 10.1101/gad.7.9.1725. [DOI] [PubMed] [Google Scholar]
- 60.Rastelli L, Chan C S, Pirrotta V. Related chromosome binding sites for zeste, suppressors of zeste and Polycomb group proteins in Drosophila and their dependence on Enhancer of zeste function. EMBO J. 1993;12:1513–1522. doi: 10.1002/j.1460-2075.1993.tb05795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reijnen M J, Hamer K M, den Blaauwen J L, Lambrechts C, Schoneveld I, van Driel R, Otte A P. Polycomb and bmi-1 homologs are expressed in overlapping patterns in Xenopus embryos and are able to interact with each other. Mech Dev. 1995;53:35–46. doi: 10.1016/0925-4773(95)00422-x. [DOI] [PubMed] [Google Scholar]
- 62.Sargent T D, Mathers P H. Analysis of class II gene regulation. Methods Cell Biol. 1991;36:347–364. doi: 10.1016/s0091-679x(08)60287-3. [DOI] [PubMed] [Google Scholar]
- 63.Satijn D P, Gunster M J, van der Vlag J, Hamer K M, Schul W, Alkema M J, Saurin A J, Freemont P S, van Driel R, Otte A P. RING1 is associated with the Polycomb group protein complex and acts as a transcriptional repressor. Mol Cell Biol. 1997;17:4105–4113. doi: 10.1128/mcb.17.7.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Satijn D P, Olson D J, van der Vlag J, Hamer K M, Lambrechts C, Masselink H, Gunster M J, Sewalt R G, van Driel R, Otte A P. Interference with the expression of a novel human Polycomb protein, hPc2, results in cellular transformation and apoptosis. Mol Cell Biol. 1997;17:6076–6086. doi: 10.1128/mcb.17.10.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlossherr J, Eggert H, Paro R, Cremer S, Jack R S. Gene inactivation in Drosophila mediated by the Polycomb gene product or by position-effect variegation does not involve major changes in the accessibility of the chromatin fibre. Mol Gen Genet. 1994;243:453–462. doi: 10.1007/BF00280476. [DOI] [PubMed] [Google Scholar]
- 66.Schoorlemmer J, Marcos-Gutierrez C, Were F, Martinez R, Garcia E, Satijn D P, Otte A P, Vidal M. Ring1A is a transcriptional repressor that interacts with the Polycomb-M33 protein and is expressed at rhombomere boundaries in the mouse hindbrain. EMBO J. 1997;16:5930–5942. doi: 10.1093/emboj/16.19.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schumacher A, Magnuson T. Murine Polycomb- and trithorax-group genes regulate homeotic pathways and beyond. Trends Genet. 1997;13:167–170. [PubMed] [Google Scholar]
- 68.Schumacher A, Faust C, Magnuson T. Positional cloning of a global regulator of anterior-posterior patterning in mice. Nature. 1996;384:648. doi: 10.1038/384648a0. [DOI] [PubMed] [Google Scholar]
- 69.Sewalt R G, van der Vlag J, Gunster M J, Hamer K M, den Blaauwen J L, Satijn D P, Hendrix T, van Driel R, Otte A P. Characterization of interactions between the mammalian Polycomb-group proteins Enx1/EZH2 and EED suggests the existence of different mammalian Polycomb-group protein complexes. Mol Cell Biol. 1998;18:3586–3595. doi: 10.1128/mcb.18.6.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simon J, Chiang A, Bender W. Ten different Polycomb group genes are required for spatial control of the AbdA and AbdB homeotic products. Development. 1992;114:493–505. doi: 10.1242/dev.114.2.493. [DOI] [PubMed] [Google Scholar]
- 71.Smith L D, Xu W, Varnold R L. Oogenesis and oocyte isolation. Methods Cell Biol. 1991;36:45–60. doi: 10.1016/s0091-679x(08)60272-1. [DOI] [PubMed] [Google Scholar]
- 72.Stebbins-Boaz B, Richter J D. Translational control during early development. Crit Rev Eukaryot Gene Expr. 1997;7:73–94. doi: 10.1615/critreveukargeneexpr.v7.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 73.Steinbach O C, Wolffe A P, Rupp R A. Somatic linker histones cause loss of mesodermal competence in Xenopus. Nature. 1997;389:395–399. doi: 10.1038/38755. [DOI] [PubMed] [Google Scholar]
- 74.Strutt H, Cavalli G, Paro R. Co-localization of Polycomb protein and GAGA factor on regulatory elements responsible for the maintenance of homeotic gene expression. EMBO J. 1997;16:3621–3632. doi: 10.1093/emboj/16.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stump D G, Landsberger N, Wolffe A P. The cDNA encoding Xenopus laevis heat-shock factor 1 (XHSF1): nucleotide and deduced amino-acid sequences, and properties of the encoded protein. Gene. 1995;160:207–211. doi: 10.1016/0378-1119(95)00176-7. [DOI] [PubMed] [Google Scholar]
- 76.Tafuri S R, Wolffe A P. Selective recruitment of masked maternal mRNA from messenger ribonucleoprotein particles containing FRGY2 (mRNP4) J Biol Chem. 1993;268:24255–24261. [PubMed] [Google Scholar]
- 77.Takihara Y, Tomotsune D, Shirai M, Katoh-Fukui Y, Nishii K, Motaleb M A, Nomura M, Tsuchiya R, Fujita Y, Shibata Y, Higashinakagawa T, Shimada K. Targeted disruption of the mouse homologue of the Drosophila polyhomeotic gene leads to altered anteroposterior patterning and neural crest defects. Development. 1997;124:3673–3682. doi: 10.1242/dev.124.19.3673. [DOI] [PubMed] [Google Scholar]
- 78.Tamkun J W. The role of brahma and related proteins in transcription and development. Curr Opin Genet Dev. 1995;5:473–477. doi: 10.1016/0959-437x(95)90051-h. [DOI] [PubMed] [Google Scholar]
- 79.Tamkun J W, Deuring R, Scott M P, Kissinger M, Pattatucci A M, Kaufman T C, Kennison J A. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992;68:561–572. doi: 10.1016/0092-8674(92)90191-e. [DOI] [PubMed] [Google Scholar]
- 80.Toyoda T, Wolffe A P. Characterization of RNA polymerase II-dependent transcription in Xenopus extracts. Dev Biol. 1992;153:150–157. doi: 10.1016/0012-1606(92)90099-3. [DOI] [PubMed] [Google Scholar]
- 81.Tyers M, Tokiwa G, Nash R, Futcher B. The Cln3-Cdc28 kinase complex of S. cerevisiae is regulated by proteolysis and phosphorylation. EMBO J. 1992;11:1773–1784. doi: 10.1002/j.1460-2075.1992.tb05229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Lohuizen M, Tijms M, Voncken J W, Schumacher A, Magnuson T, Wientjens E. Interaction of mouse Polycomb-group (Pc-G) proteins Enx1 and Enx2 with Eed: indication for separate Pc-G complexes. Mol Cell Biol. 1998;18:3572–3579. doi: 10.1128/mcb.18.6.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vermaak D, Steinbach O C, Dimitrov S, Rupp R A W, Wolffe A P. The globular domain of histone H1 is sufficient to direct specific gene repression in early Xenopus embryos. Curr Biol. 1998;23:533–536. doi: 10.1016/s0960-9822(98)70206-4. [DOI] [PubMed] [Google Scholar]
- 83a.Wade, P. Personal communication.
- 83b.Wade P A, Jones P L, Vermaak D, Wolffe A P. The multiple subunit Mi-2 histone deacetylase from Xenopus laevis contains a Snf2 superfamily ATPase. Curr Biol. 1998;8:843–846. doi: 10.1016/s0960-9822(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 84.Wallrath L L, Elgin S C. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 1995;9:1263–1277. doi: 10.1101/gad.9.10.1263. [DOI] [PubMed] [Google Scholar]
- 85.Wedeen C, Harding K, Levine M. Spatial regulation of Antennapedia and bithorax gene expression by the Polycomb locus in Drosophila. Cell. 1986;44:739–748. doi: 10.1016/0092-8674(86)90840-8. [DOI] [PubMed] [Google Scholar]
- 86.Wong J, Patterton D, Imhof A, Guschin D, Shi Y-B, Wolffe A P. Distinct requirements for chromatin assembly in transcriptional repression by thyroid hormone receptor and histone deacetylase. EMBO J. 1998;17:520–534. doi: 10.1093/emboj/17.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86a.Woodland H R, Flynn J M, Wyllie A J. Utilization of stored mRNA in Xenopus embryos and its replacement by newly synthesized transcripts: histone H1 synthesis using interspecies hybrids. Cell. 1979;18:165–171. doi: 10.1016/0092-8674(79)90365-9. [DOI] [PubMed] [Google Scholar]
- 87.Yamaguchi K, Hidema S, Mizuno S. Chicken chromobox proteins: cDNA cloning of CHCB1, -2, -3 and their relation to W-heterochromatin. Exp Cell Res. 1998;242:303–314. doi: 10.1006/excr.1997.4082. [DOI] [PubMed] [Google Scholar]
- 88.Zink B, Paro R. In vivo binding pattern of a trans-regulator of homeotic genes in Drosophila melanogaster. Nature. 1989;337:468–471. doi: 10.1038/337468a0. [DOI] [PubMed] [Google Scholar]
- 89.Zink B, Paro R. Drosophila Polycomb-group regulated chromatin inhibits the accessibility of a trans-activator to its target DNA. EMBO J. 1995;14:5660–5671. doi: 10.1002/j.1460-2075.1995.tb00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]