Abstract
Preterm birth (PTB) is the leading cause of neonatal morbidity and mortality. The vaginal microbiome has been associated with PTB, yet the mechanisms underlying this association are not fully understood. Understanding microbial genetic adaptations to selective pressures, especially those related to the host, may yield insights into these associations. Here, we analyze metagenomic data from 705 vaginal samples collected during pregnancy from 40 women who delivered preterm spontaneously and 135 term controls from the Multi-Omic Microbiome Study-Pregnancy Initiative. We find that the vaginal microbiome of pregnancies that ended preterm exhibited unique genetic profiles. It was more genetically diverse at the species level, a result which we validate in an additional cohort, and harbored a higher richness and diversity of antimicrobial resistance genes, likely promoted by transduction. Interestingly, we find that Gardnerella species drove this higher genetic diversity, particularly during the first half of the pregnancy. We further present evidence that Gardnerella spp. underwent more frequent recombination and stronger purifying selection in genes involved in lipid metabolism. Overall, our population genetics analyses reveal associations between the vaginal microbiome and PTB and suggest that evolutionary processes acting on vaginal microbes may play a role in adverse pregnancy outcomes such as PTB.
Subject terms: Metagenomics, Reproductive disorders, Microbial genetics
Here, Liao et al. analyze the vaginal microbiome during pregnancy, and find a unique population genetic structure associated with preterm birth, suggesting that evolutionary processes acting on vaginal bacteria may play a role in prematurity.
Introduction
Preterm birth (PTB), childbirth at <37 weeks of gestation, is the leading cause of neonatal morbidity and mortality1. Each year, approximately 15 million infants are born preterm globally, over 500,000 of them in the US2. Preterm infants are at a high risk of respiratory, gastrointestinal and neurodevelopmental complications3. While a number of maternal, fetal, and environmental factors have been associated with PTB1,4,5, its etiopathology remains largely unknown, and early diagnosis and effective therapeutics are still lacking.
Over the past decades, growing evidence has pointed to potential involvement of the vaginal microbiome in PTB6–10. This involvement has so far been mostly characterized as an ecological process, meaning changes in microbial abundances and vaginal community states. For instance, increased richness and diversity of microbial communities and the presence of particular community state types (CST), have been repeatedly associated with PTB6,9,11–15. In addition, vaginal microbiomes of women who delivered preterm appear to be less stable during pregnancy, with some studies reporting a significant decrease in the richness and diversity of these microbial communities during pregnancy6,12.
Multiple endogenous factors, such as hormonal changes, nutrient availability and microbial interactions, and exogenous factors, such as genital infections, antibiotic treatment and exposure to xenobiotics, could trigger ecological processes and alter the vaginal microbial composition16,17. These factors may also act as selective pressures that affect genetic variation in the microbial populations that make the vaginal microbiome. Such adaptive evolution in the vaginal environment, even during pregnancy, is highly plausible given the high mutation rates, short generation times, and large population sizes of microbes18. They are further supported by observations of rapid adaptation to environmental changes in other human-associated microbial ecosystems19–21. The way by which vaginal microbes respond to various selective pressures may, in turn, affect the host, including pregnancy outcomes. Therefore, a comprehensive investigation of the genetic diversity of the vaginal microbiome at the population level, which we term “microdiversity”, and the underlying evolutionary forces that shape it, holds promise for a better understanding of the etiopathology of PTB.
Here, we performed an in-depth population genetics analysis and characterized the population structure of the vaginal microbiome along pregnancy and in the context of preterm birth. We used metagenomic sequencing data from 705 vaginal samples collected longitudinally during pregnancy as part of the Multi-Omic Microbiome Study-Pregnancy Initiative (MOMS-PI6). Our analyses include samples from 40 women who subsequently experienced spontaneous preterm birth (sPTB) and 135 women who had a term birth (TB). We show that the vaginal microbiome of pregnancies that ended preterm exhibits higher nucleotide diversity at the species level and higher antimicrobial resistance potential. We find that this higher nucleotide diversity is driven by Gardnerella spp., a group of central vaginal pathobionts, especially during the first half of pregnancy, and suggest that this may be related to optimization of growth rates in this taxon. We further identify a strong association between evolutionary signatures and sPTB in Gardnerella spp., including more frequent homologous recombination and stronger purifying selection. Overall, our results reveal strong associations between the vaginal microbiome and sPTB at the population genetics level, and suggest that evolutionary processes acting on the vaginal microbiome may play a critical role in sPTB, and potentially also in other adverse pregnancy outcomes.
Results
The phylogenetic composition of the vaginal microbiome associates with sPTB
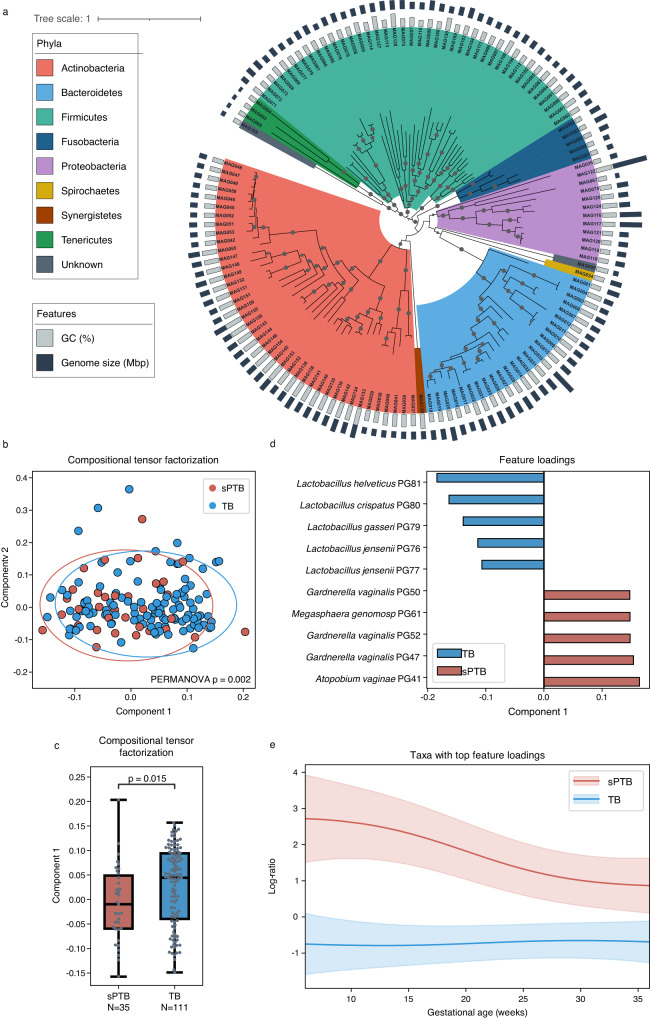
We assembled a total of 1078 metagenome-assembled genomes (MAGs) with at least medium quality22 (>50% completeness, <10% contamination; Supplementary Data 1; Methods) from previously published6 metagenomic reads generated from 705 vaginal samples6. These samples were collected from 175 women visiting maternity clinics in Virginia and Washington at various time points along pregnancy6, with an average of 3.36 and 3.21 samples for women delivering preterm and at term, respectively (Supplementary Table 1). We clustered these MAGs into 157 species-level phylogroups at the level of 95% average nucleotide identity (ANI), which roughly corresponds to the species level23; and selected the most complete MAG with the least contamination as the representative for each phylogroup. These representative MAGs were 86 ± 14% (mean ± SD) complete and 1.1 ± 1.8% contaminated, with 93 (59% of 157) of them estimated to have high quality22 (>90% completeness and <5% contamination; Supplementary Data 1). Taxonomic assignment of these representative MAGs (Methods) revealed that the phylogroups represent at least 8 phyla, with genome size (adjusted by completeness) ranging from 0.6 to 7.4 Mbps and GC content ranging from 25.3% to 69.7% (Fig. 1a, Supplementary Data 1). Actinobacteria had the most phylogroups detected in the samples, followed by Firmicutes and Bacteroidetes (Fig. 1a).
Fig. 1. The composition of the vaginal microbiome associated with sPTB.
a Phylogenetic tree of non-redundant MAGs representing 132 species-level phylogroups differing by at least 95% average nucleotide identity (ANI) based on concatenated amino acid (AA) sequences of 120 marker genes. Representative MAGs of 25 phylogroups had <60% of marker genes AA sequence identified and were not included in the tree. Gray nodes indicate a bootstrap value >80. The tree is rooted by midpoint and annotated by the GC content and genome size of the representative MAGs. b Compositional tensor factorization (CTF) analysis showing microbiome composition trajectories over gestational ages separated by pregnancy outcomes using the top two ordination axes (Component 1 and Component 2). Each dot represents a subject. c Component 1 in the CTF analysis compared between sPTB and TB. Box, IQR; line, median; whiskers, 1.5*IQR; p, two-sided Mann-Whitney. d Feature rankings of phylogroups colored by preterm birth (sPTB) and term birth (TB) based on Component 1 in the CTF analysis. e Log ratio of top and bottom taxa from (d) over time, separated to PTB and TB samples. Line, mean; shaded area, 95% CI.
Of note, 12 of these species-level phylogroups (PG042-PG053) were assigned to Gardnerella vaginalis according to CheckM24, supporting the existence of multiple genotypes at the species level within the ‘species’ G. vaginalis25,26. To better resolve the classification of these G. vaginalis phylogroups, we compared the average nucleotide identity (ANI) for the representative MAGs of these phylogroups against updated reference genomes for Gardnerella, including G. vaginalis, G. piotti, G. leopoldii, G. swidsinskii, and nine species remained to be characterized (gs2-3 and gs7-13)25; gs-2-3 and gs7-13 correspond to group 2-3 and 7-13 shown in Fig. 1 in ref. 25. The ANI analysis shows that PG043 represents G. vaginalis, PG044 represents G. swidsinskii, PG042 represents G. piotti, and PG046, PG049, PG051 and PG053 represent G. gs7, G. gs8, G. gs13 and G. gs12, respectively (Supplementary Fig. 1). The remaining phylogroups (PG045, PG047, PG048, PG050, and PG052) do not cluster with any reference species and may represent previously undocumented species of Gardnerella. Here, we refer to phylogroups PG042-PG053 as Gardnerella spp.
To understand if the temporal dynamics of the vaginal microbiome is associated with sPTB, we employed compositional tensor factorization (CTF)27 to assess temporal changes to the composition of the microbiome during pregnancy. This analysis shows a significant separation of women by pregnancy outcomes (PERMANOVA F = 8.0492; p = 0.002; Fig. 1b-e) based on the dynamics of their microbiome composition over time (Fig. 1e), and specifically observed for component 1 in the CTF analysis (Mann-Whitney p = 0.015; Fig. 1c). We further found that the top features contributing to this difference belong to Lactobacillus helveticus (PG081), Lactobacillus crispatus (PG080), Lactobacillus gasseri (PG079), and Lactobacillus jensenii (PG076 and PG077) that are associated with TB and Megasphaera genomosp. (PG061), Gardnerella spp. (PG047, PG050, PG052), and Atopobium vaginae (PG041) that are associated with PTB (Fig. 1d); these species were previously found to be associated with pregnancy outcomes6,9,28. These results suggest that the vaginal microbiome has a different temporal trajectory during pregnancies ending preterm, consistent with previous findings6,7, and with Gardnerella as an important factor. Overall, our results demonstrate that de-novo metagenomic analysis replicates and expands previous findings with respect to associations between the composition of the vaginal microbiome and sPTB.
Next, we sought to examine the diversity of microbial strains detected within species, and its association with sPTB. We performed the analysis on all phylogroups and found that the strains of M. genomosp. showed significantly higher ANI between women who delivered preterm, compared to a null distribution calculated based on ANIs from any two randomly selected women (Permutation p = 0.002, adjusted P < 0.05; Methods), a relationship not observed between women who delivered at term (p = 0.208; Supplementary Fig. 2). This result indicates that M. genomosp. were more closely related than expected by chance across women who delivered preterm. It suggests that sPTB-associated vaginal conditions across women may be more conserved, harboring a group of significantly closely related M. genomosp. strains, compared to TB-associated vaginal conditions.
The microdiversity of the vaginal microbiome is higher in the first half of pregnancies that ended preterm, driven by Gardnerella species
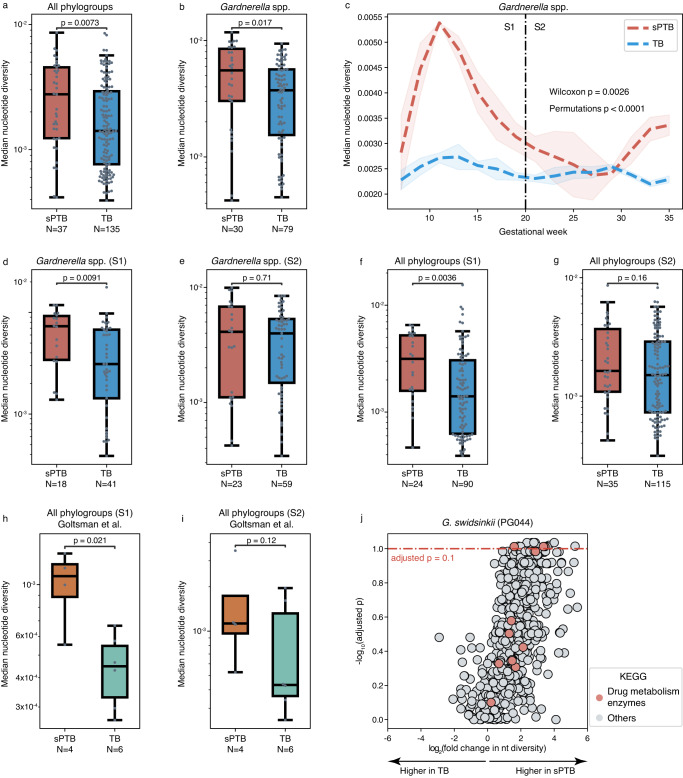
Human microbes can adapt to host-induced environmental changes (e.g., diet, antibiotics) through genetic variations29. Therefore, the microbial populations of the same species in different hosts can have a different genetic structure, which provides them a competitive advantage. These genetic differences, in turn, may be related to the phenotype of the host. To understand the genetic structure of microbial populations in the vaginal environment and its association with pregnancy outcomes, we calculated the nucleotide diversity for each identified phylogroup. Overall, vaginal microbial populations had a significantly higher genome-wide nucleotide diversity in sPTB than in TB (median along pregnancy; Mann-Whitney p = 0.0073; Fig. 2a). Stratifying by phylogroups, we found that this difference was mainly driven by Gardnerella spp. (p = 0.017; Fig. 2b). G. piotti (PG042), G. swidsinskii (PG044), and G. gs13 (PG051) and a potentially new Gardnerella spp. (PG045), along with a phylogroup of Atopobium vaginae, a suspected vaginal pathobiont30, showed significantly higher genome-wide nucleotide diversity in sPTB (P < 0.05, adjusted P < 0.1 for all; Supplementary Fig. 3a). These results imply that microbial populations composed of more diverse strains from the same species, and particularly Gardnerella spp., are growing in the vaginal environment associated with sPTB.
Fig. 2. Microdiversity patterns of the vaginal microbiome are associated with sPTB.
a, b A comparison of median genome-wide nucleotide diversity along pregnancy between sPTB and TB, displayed for all phylogroups (a) and Gardnerella spp. (b). p, two-sided Mann-Whitney. c Trajectory of median nucleotide diversity of Gardnerella spp. along pregnancy. S1 - first half of pregnancy; S2 - second half of pregnancy. The shaded area depicts mean ± s.d./n. p, one-sided Wilcoxon and permutation. d, e A comparison of median genome-wide nucleotide diversity of Gardnerella spp. between sPTB and TB, displayed for pregnancy S1 (d) and S2 (e). p, two-sided Mann-Whitney. f, g A comparison of median genome-wide nucleotide diversity of all phylogroups between sPTB and TB, displayed for pregnancy S1 (f) and S2 (g). p, two-sided Mann-Whitney. h, i Same as d–e, using data from the independent cohort of Goltsman et al.32. p, one-sided Mann-Whitney. j Volcano plot illustrating the significance (two-sided Mann-Whitney; y-axis) of difference between nucleotide diversity (fold change; x-axis) in sPTB and TB of every gene in G. swidsinskii (PG044). Genes above the red dashed line have P < 0.05 and an adjusted P < 0.1. Genes belonging to KEGG pathways that were significantly enriched in genes showing significant nucleotide diversity differences are color-coded (adjusted P < 0.1). Box, IQR; line, median; whiskers, 1.5*IQR.
To understand how the nucleotide diversity of Gardnerella spp. changes over time during pregnancy, we analyzed temporal trajectories of term and preterm pregnancies. To this end, we pooled the data from all women in each group, binned pregnancy weeks and used splines to smooth the temporal curves (Methods). We found a significant difference between the temporal trajectories of Gardnerella spp. nucleotide diversity in pregnancies ending at term and preterm (Permutation test <0.001 (ref. 31), Wilcoxon signed-rank P < 0.003; Methods; Fig. 2c). Specifically, we found that the nucleotide diversity of Gardnerella spp. increased at the beginning of pregnancies which ended preterm, with a peak at around gestational week 13, and then dropped to its initial value at around gestational week 20 (Fig. 2c). In comparison, nucleotide diversity of Gardnerella spp. in TB remained relatively stable (Fig. 2c). Given that gestational week 20 is the middle of a full-term pregnancy, we subsequently analyzed samples with respect to two time periods - first half (0–19 gestational week) and second half of pregnancy (20–37 gestational week; 37 was chosen to ensure a similar time range for both sPTB and TB). As expected, the nucleotide diversity of Gardnerella spp.in sPTB was also significantly higher in sPTB in the first half of pregnancy (median along first half; Mann-Whitney U p = 0.0091; Fig. 2d), but not in the second half (p = 0.71; Fig. 2e). We further found that nucleotide diversity had a significantly stronger correlation with synonymous mutations than with nonsynonymous mutations across Gardnerella spp. (paired t-test p = 0.0011; Supplementary Fig. 3b), suggesting a more important role of purifying selection in shaping genomic diversity. Overall, these results suggest that genetic diversity of Gardnerella spp. in the first half of pregnancy is important to birth outcomes, and could perhaps be used as a biomarker for early diagnosis of sPTB.
Analyzing microdiversity across all phylogroups in the two halves of pregnancy, we again found that it was significantly higher in sPTB in the first half of pregnancy (median along first half; Mann-Whitney U p = 0.0036; Fig. 2f), but not in the second half (p = 0.16; Fig. 2g). Notably, we were able to replicate this analysis using data from an additional dataset of vaginal metagenomic sequencing from ten pregnant individuals32 (median along first and second half; one-sided Mann-Whitney U p = 0.021 and p = 0.12, respectively; Fig. 2h, i). Finally, clinical interventions for risk of preterm birth (e.g., receiving cerclage or progesterone) may alter the microbiome composition, and thus confound our findings. To examine this potential bias, we repeated our analysis on 161 woman who received neither progesterone nor cerclage. Once more, we found that it was significantly higher in sPTB in the first half of pregnancy (p = 0.028; Supplementary Fig. 4a), but not in the second half (p = 0.12; Supplementary Fig. 4b). Overall, our results demonstrate an increased nucleotide diversity in the vaginal microbiome during pregnancies that ended preterm, in a way that replicates across studies and is not biased by common clinical interventions for prevention of preterm birth.
To understand if any particular genes are driving the association between sPTB and the microdiversity of Gardnerella spp. in the first half of pregnancy, we further analyzed nucleotide diversity at the gene level for these species. We identified 21 and 47 genes (out of 825 and 531) in G. swidsinskii (PG044) and G. vaginalis (PG043), respectively, that showed significantly different nucleotide diversity between sPTB and TB (median along the first half of pregnancy; Mann-Whitney P < 0.05, adjusted P < 0.1 for all). These genes included one gene encoding the putative tail-component of bacteriophage HK97-gp10 (p = 0.0012) and one gene encoding putative AbiEii toxin, Type IV toxin–antitoxin system (p = 5 × 10−4), which might be involved in the interaction with maternal health33,34. To further identify what functions were related to these associations, we then performed functional enrichment analysis (Methods) using the eggNOG functional annotation of genes (Supplementary Data 2). We found that the KEGG pathway ‘drug metabolism - other enzymes’ (ko00983) was significantly enriched among genes from G. swidsinskii (PG044) that had significantly higher microdiversity (P < 0.05, adjusted P < 0.1; Fig. 2j). This result suggests that the more diverse gene pool in G. swidsinskii (PG044) detected in sPTB may be associated with adaptation to drugs present in the environment. This may be consistent with our recent finding that xenobiotics detected in the vaginal environment are strongly associated with sPTB35.
To verify that the higher nucleotide diversity we observed in sPTB pregnancies was not caused by sampling or sequencing bias, we compared the read count and quality of MAGs obtained from sPTB and TB samples. If this higher diversity is the result of a higher read count in sPTB samples or more complete MAGs, we would expect read count and MAG completeness to be higher in sPTB samples. Instead, we found the completeness and contamination of MAGs assembled from sPTB samples were not significantly different from TB (Mann-Whitney p = 0.71 and 0.73, respectively; Supplementary Fig. 5a, b). Next, we assessed the correlation between the number of reads mapped to each phylogroup and its genome-wide diversity. If a higher diversity is caused by more reads mapped to the MAG representing the phylogroup, we would expect a positive correlation between these two measurements. However, only 3 phylogroups (PG064, a Dialister spp.; PG102, a Peptoniphilus spp.; and PG122, a Bradyrhizobium spp., which was likely either a contaminant or misidentified) had a significant positive correlation between read counts and nucleotide diversity (Spearman ρ = 0.70, 0.54, and 0.42, respectively; non-adjusted p = 0.035, 0.024, and 0.00015, respectively). In 98% of phylogroups, we did not observe a statistically significant positive correlation (median [IQR] Spearman correlation of −0.067 [−0.19, −0.13]). None of the four Gardnerella spp. Phylogroups that showed significantly higher nucleotide diversity in sPTB pregnancies in Supplementary Fig. 3 were significantly positively correlated (Spearman ρ = −0.00, −0.12, −0.02, and −0.35 and p = 0.96, 0.091, 0.77, and 0.00045 for PG042, PG044, PG045, and PG051, respectively; Supplementary Fig. 5c).
Finally, as we have observed a significantly higher read count in sPTB samples (Mann-Whitney p = 0.0004 and 0.061 for samples and subjects, respectively; Supplementary Fig. 5d, e, respectively), we subsampled an identical number of reads (105) from each sample, retaining 75% of samples, and repeated our analyses of nucleotide diversity. As with the first analysis (Fig. 2), nucleotide diversity was significantly higher in sPTB pregnancies across all phylogroups, and particularly in Gardnerella spp.(Mann-Whitney p = 0.015 and p = 0.0043, respectively; Supplementary Fig. 5f, g, respectively). Similarly, in the first half of pregnancy, the nucleotide diversity of Gardnerella spp.was significantly higher in sPTB (p = 0.026; Supplementary Fig. 5h), while in the second half, there was no significant difference (p = 0.22; Supplementary Fig. 5i). We further subsampled an identical number of reads (5000) mapped to Gardnerella spp. from each sample and repeated the analysis in Fig. 2b. A significant higher nucleotide diversity in sPTB pregnancies in Gardnerella spp. is still detected (p = 0.028; Supplementary Fig. 5j), indicating this association is not biased by higher coverage of reads mapped to Gardnerella spp. Overall, these results confirm that the sPTB-associated nucleotide diversity we observed was not biased by technical artifacts.
Evolutionary forces acting on Gardnerella species are associated with pregnancy outcomes
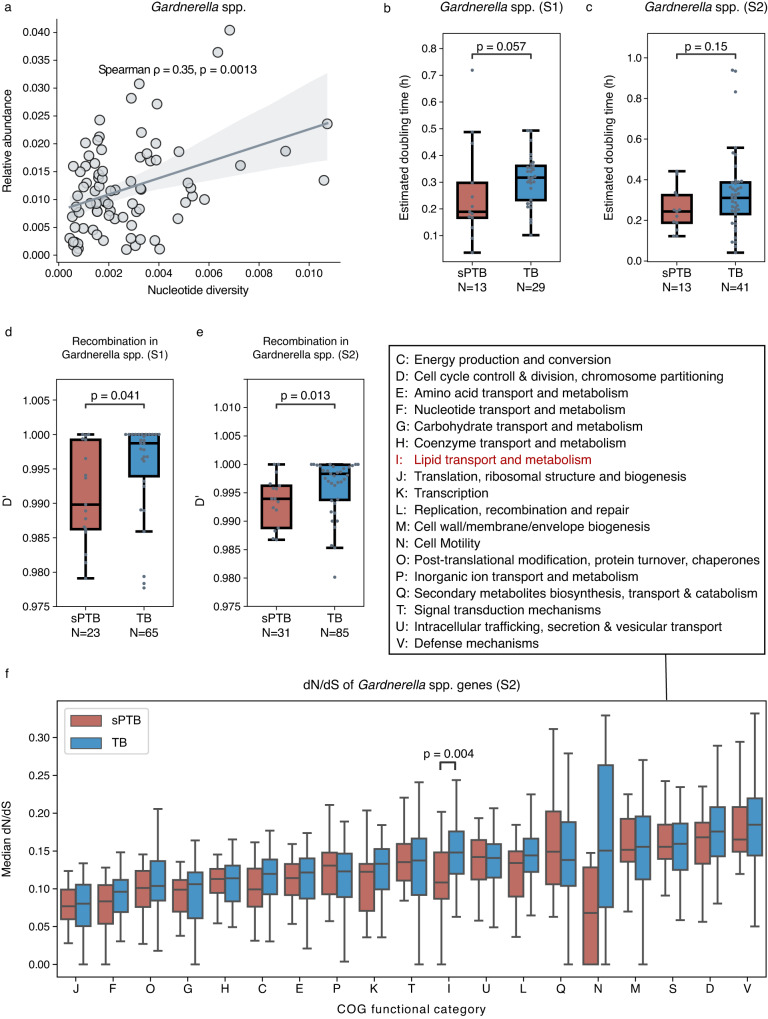
Adaptation should increase the fitness of an organism, its ability to survive and reproduce in a given environment. To better understand if the Gardnerella spp. populations with higher genetic diversity grow better in the vaginal environment associated with sPTB, we inferred fitness using two measures: relative abundance and growth rate. Indeed, we found that nucleotide diversity in these species was positively correlated with relative abundance (Spearman ρ = 0.35, p = 0.0013; Fig. 3a). This correlation was not observed in other phylogroups (Supplementary Fig. 6a). L. crispatus (PG080) and L. iners (PG086) even showed a significantly negative correlation (ρ = −0.39 and −0.32, p = 0.026 and 0.0014, respectively; Supplementary Fig. 6b, c). We additionally used gRodon36 to predict the maximal growth rate of microbes based on codon usage bias in highly expressed genes encoding ribosomal proteins. We found that in the first half of pregnancy, Gardnerella spp. had a somewhat higher, albeit not statistically significant, maximal growth rate in sPTB pregnancies (Mann-Whitney p = 0.057; Fig. 3b), while in the second half of pregnancy, the difference was diminished (p = 0.15; Fig. 3c). No significant difference in the maximal growth rate was observed in non-Gardnerella phylogroups (P > 0.05 for all). The in situ growth rates37,38 of Gardnerella were not significantly different between the groups (p = 0.14). These results suggest that the sPTB-associated genetic diversity observed in Gardnerella spp. may be related to the optimization for faster growth in the sPTB-associated vaginal environment.
Fig. 3. Evolutionary forces on Gardnerella spp.
a Spearman correlation between median genome-wide nucleotide diversity and relative abundance of Gardnerella spp. along pregnancy. The line and the shaded area depict the best-fit trendline and the 95% confidence interval (mean ± 1.96 s.e.m.) of the linear regression. b, c Predicted maximal doubling time (gRodon36) of Gardnerella spp. compared between sPTB and TB, displayed for the first (b, S1) and second (c, S2) halves of pregnancy. d, e Median D’ of Gardnerella spp compared between sPTB and TB, displayed for the first (S1, d) and second (S2, e) halves of pregnancy. Lower D’ indicates more frequent recombination. f dN/dS of Gardnerella spp genes compared between sPTB and TB by COG functional categories, displayed for the second half of pregnancy (S2). dN/dS closer to 0 indicates stronger purifying selection. N = 40 and 135 for sPTB and TB, respectively, included in this panel. Box, IQR; line, median; whiskers, 1.5*IQR; p, two-sided Mann-Whitney.
Microbial population structure is influenced by various evolutionary processes including selection and homologous recombination39. Competence, a mechanism of horizontal gene transfer which involves homologous recombination, has been identified in Gardnerella spp40. To better interpret the significant differences we observed in the microdiversity patterns of Gardnerella spp. between sPTB and TB, we quantified the degree of homologous recombination using the normalized coefficient of linkage disequilibrium between alleles at two loci, D’. A value of D’ closer to 0 indicates a higher degree of recombination41. Interestingly, we found that the median D’ of Gardnerella spp. was significantly smaller in sPTB pregnancies in both the first (Mann-Whitney p = 0.041; Fig. 3d) and second halves of pregnancy (p = 0.013; Fig. 3e), and the same was also observed for the D’ of three specific Gardnerella spp., G. piotti (PG042), G. gs7 (PG046), and PG047, in the first half of pregnancy (P < 0.05, adjusted P < 0.1 for all; Supplementary Fig. 7a). No significant difference in recombination was observed in non-Gardnerella phylogroups (adjusted P > 0.1 for all). These results suggest that Gardnerella spp. tends to have more frequent recombination in women who delivered preterm during both halves of pregnancy.
Next, we quantified the degree of selection using dN/dS in this species (Methods). This measure quantifies the ratio between synonymous and non-synonymous mutations, and hence offers insight into the type of selection, with values close to zero indicating purifying selection, and values higher than one indicating positive selection42. dN/dS is calculated in relation to the reference, and can therefore detect selection on mutations that have already been fixed within the population43. Consistent with the gut and ocean microbiomes44–46, purifying selection is predominant across all genes of the vaginal microbiome (dN/dS « 1; median [IQR] dN/dS of 0.17 [0.10, 0.29]; Supplementary Fig. 7b). While the median dN/dS of all Gardnerella spp. genes was not significantly different between sPTB and TB pregnancies (Mann-Whitney U p = 0.48), we detected some differences when examining high-level functions (COG categories47) within each half of pregnancy. In the first half, the median dN/dS of Gardnerella spp. genes was somewhat lower in sPTB pregnancies for inorganic ion transport and metabolism, lipid transport and metabolism, secondary structure, and cell wall/membrane/envelope biogenesis, though this was not statistically significant after adjusting for multiple testing (COG categories “P”, “I”, “Q”, and “M”, respectively; Mann-Whitney P < 0.05, adjusted P > 0.1 for all; Supplementary Fig. 7c). In the second half of pregnancy, the median dN/dS was significantly lower in sPTB pregnancies for lipid transport and metabolism and cell motility (COG categories “I” “N”; Mann-Whitney p = 0.0040 and p = 0.04, adjusted p = 0.07 and 0.40, respectively; Fig. 3f). No significant difference in the selection based on dN/dS was observed in non-Gardnerella phylogroups (P > 0.05 for all). Our results suggest that Gardnerella spp. genes involved in lipid transport and metabolism may undergo stronger purifying selection in sPTB. As purifying selection maintains the fitness of organisms by constantly sweeping away deleterious mutations and conserving functions, Gardnerella spp. may benefit from this stronger purifying selection targeting lipid functioning when growing in the sPTB-associated vaginal environment during pregnancy.
sPTB-associated vaginal microbiomes have a higher antibiotic-resistance potential
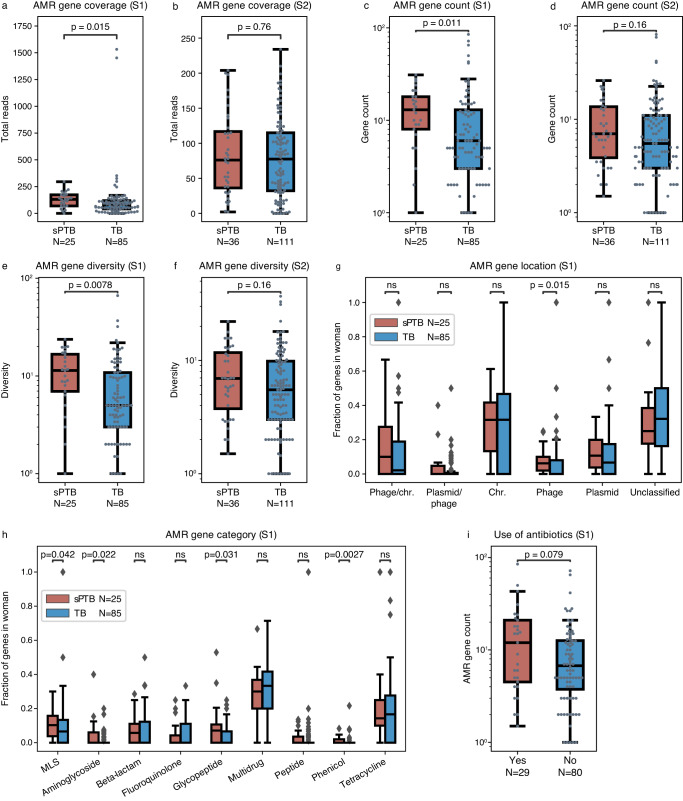
Antibiotics are widely used during pregnancy, sometimes even topically in the vagina48. This exposure may promote antimicrobial resistance (AMR). To assess if antibiotic-resistance potential in the vaginal microbiome is associated with sPTB, we subsampled an identical number of reads (105) from each sample and mapped them to the Comprehensive Antibiotic Resistance Database49. The total number of reads mapped to AMR reference genes was significantly higher in the first half of sPTB pregnancies (Mann-Whitney U p = 0.015; Fig. 4a), but not in the second half (p = 0.76; Fig. 4b). In addition, to assess the difference of specific AMR genes between the vaginal microbiomes of sPTB and TB, we identified AMR genes in the genomic assemblies. A significantly higher median count and Shannon-Wiener diversity of AMR genes were detected in vaginal microbes sampled at the first half of pregnancies that ended preterm (3-times higher on average; Mann-Whitney U p = 0.011 and p = 0.0078, respectively; Fig. 4c, e, respectively), yet this difference was not detected in the second half (p = 0.16 for both; Fig. 4d, f, respectively). Exploring the source of these genes, we found a significantly higher median fraction of phage-borne AMR genes in the microbiomes of women who delivered preterm (one-sided p = 0.016, adjusted p = 0.093.; Fig. 4g), suggesting transduction may promote the higher median count and diversity of AMR genes observed in the first half of sPTB pregnancies (Fig. 4c, e). Among the 9 AMR gene categories that had genes present in at least 10% of women, phenicol, aminoglycoside, glycopeptide, and MLS resistance genes showed a significantly higher median fraction in the sPTB microbiome (P < 0.05, adjusted P < 0.1 for all; Fig. 4h). These results suggest a unique antibiotic resistance profile associated with the first half of sPTB pregnancies, potentially indicative of usage of specific antibiotics. Indeed, we detected a somewhat higher richness of AMR genes along the first half of pregnancy in the 29 women who used antibiotics in the past 6 months before pregnancy than those who did not (Mann-Whitney U p = 0.079; Fig. 4i). This is also consistent with our observation that genes with sPTB-associated nucleotide diversity were enriched for drug metabolism in G. swidsinskii (PG044) (Fig. 2d).
Fig. 4. Antimicrobial resistance (AMR) gene profiles of the vaginal microbiome are associated with sPTB.
a, b Total subsampled reads (105) mapped to AMR genes compared between sPTB and TB, in the first (S1, a) and second (S2, b) halves of pregnancy. c, d Median count (along period) of AMR genes compared between sPTB and TB, in the first (S1, c) and second (S2, d) halves of pregnancy. e, f Median Shannon-Wiener diversity (along period) of AMR genes compared between sPTB and TB, in the first (S1, e) and second (S2, f) halves of pregnancy. g Fraction of AMR genes originating in different locations, shown as median along the first half of each pregnancy. Chr.: chromosome. h Fraction of AMR genes belonging to different resistance categories, shown as median along the first half of each pregnancy. MLS, macrolide, lincosamide and streptogramin B. i AMR gene richness in the first half of pregnancy (S1) compared between women who used and did not use antibiotics in the 6 months before pregnancy. Box, IQR; line, median; whiskers, 1.5*IQR; p, two-sided Mann-Whitney U.
As a higher risk of preterm birth has been reported to be associated with bacterial vaginosis (BV)50–52, and antibiotics used to treat BV may change the AMR genetic profiles of the vaginal microbiome, our findings in AMR genes might be potentially confounded by BV. To assess this potential bias, we compared AMR gene count between women with and without BV as well as between women with and without BV history. We found that the AMR gene count was not significantly different between women with and without BV for both halves of pregnancy (p = 0.38 and 0.5, respectively; Supplementary Fig. 8a). Similarly, it was not different between women with and without BV history (p = 0.45 and 0.1, respectively; Supplementary Fig. 8b). These results suggest that the association between AMR gene and pregnancy outcomes is independent of BV.
To check if the strong association between sPTB and the AMR potential of the vaginal microbiome is contributed by a particular phylogroup, we performed a similar analysis for each phylogroup. We found, however, that none of them showed a significant difference in the median count and diversity of AMR genes between sPTB and TB (Mann-Whitney U P > 0.05 for all). This result suggests that the higher AMR potential associated with sPTB may be a property of the vaginal microbiome as an ecosystem. However, this lack of association could also be driven by underestimation of AMR genes due to the limitation of MAG binning methods in recovering mobile genetic elements53.
Discussion
Microbial genomes can exhibit large variations even within the same species, as a result of adaptation to various environments54. Associations between the vaginal microbiome and preterm birth have been widely reported7,8,12,35,52. However, there is still much left to explore regarding potential mechanisms underlying host-microbiome interactions in this context. Here, by leveraging publicly available metagenomic data6, we provide a population genetic view of the vaginal microbiome during pregnancy. We identify a number of microbial features including population nucleotide diversity, selection metrics, and antibiotic resistance potential that are associated with sPTB. Interestingly, we find that the higher population nucleotide diversity is driven by Gardnerella spp. during the first half of pregnancy. This species appears to undergo more intense changes in the population structure contributed by recombination and purifying selection in pregnancies which ended preterm. We also show evidence that this sPTB-associated genetic pattern of Gardnerella spp. may be related to optimization of growth rates in vaginal conditions linked to sPTB. Our results are indicative of adaptation of the vaginal microbiota to the host, which in turn may influence pregnancy outcomes.
Our findings regarding a relationship between ecological processes in the pregnancy vaginal microbiome and subsequent preterm birth are consistent with previous studies6,7,11,12,14,52,55. We add to these studies by exploring an additional layer of microbial variability associated with sPTB - microbial genetic diversity. It is known that genomic variation within species can result in phenotypic diversity and adaptations to different environments54. These adaptations, in turn, can affect host phenotypes such as disease outcomes56. Such associations between microbial genomic variation and host phenotypes have been reported in the gut microbiome45,57,58. Our study suggests that this phenomenon also occurs in the vaginal ecosystem, and that it may be associated with pregnancy outcomes. Nethertheless, the associations between microbial genetic diversity and pregnancy outcomes we detect might also be a consequence of different processes or unmeasured confounders that act on both variables (e.g., certain drugs or exogenous chemical compounds are that drive inflammation), and while we find this unlikely, this should be determined by future studies.
Interestingly, we found that the association of genetic diversity and sPTB was largely driven by Gardnerella spp., a group of species commonly associated with BV50–52. A number of studies reported a higher abundance of these species in sPTB pregnancies6,9,52,59,60. A recent preprint also demonstrated a higher number of Gardnerella clades in sPTB61. We show that Gardnerella spp. populations with more genetically diverse strains may also be associated with sPTB. In addition, we found that this taxon has the capacity to grow 1.5 times faster in pregnancies that ended preterm, consistent with an overall higher relative transcriptional rate of G. vaginalis which was previously reported6. These more genetically diverse strains appear to have adapted to the vaginal environment associated with sPTB, exhibiting higher fitness. Notably, the higher genetic diversity associated with sPTB in Gardnerella spp. was detected during the first half of the pregnancy (<20 gestational week) rather than the second half. The enriched nucleotide diversity during the first half of pregnancy might be related to the change of human chorionic gonadotropin (HCG), which peaks at roughly the same time as G. vaginalis microdiversity as we observed in Fig. 2c, and was suggested to play an immunomodulatory role in humans62,63. To our knowledge, however, the effect of HCG on the vaginal ecosystem is not well established yet. Most potential biomarkers of sPTB (e.g., serum alpha-fetoprotein64 were so far identified using samples from the second trimester of pregnancy (gestational week 14–27). Our results suggest that high resolution analysis of microbiome samples from even earlier stages of pregnancy (<week 20) may yield informative biomarkers of pregnancy outcomes.
As in the human gut microbiome19–21, we show evidence that adaptive evolution also occurs in the vaginal microbiome. Several environmental factors affecting the vaginal ecosystem, such as pH, neutrophil levels, and xenobiotics, have been reported to be associated with sPTB35,65. These environmental factors may act as selective stressors that lead to different evolutionary patterns in the vaginal microbiome. Indeed, we detected more frequent homologous recombination and stronger purifying selection within Gardnerella spp. during pregnancies that end preterm. Homologous recombination is a critical mechanism speeding adaptation by increasing fixation probability of beneficial mutations66 and reducing clonal interference (i.e., competition between beneficial mutations) in bacteria67. Purifying selection also contributes to adaptation by sweeping away deleterious mutations and conserving functions, such as in oligotrophic nutrient conditions44,68. Notably, we found that sPTB-associated purifying selection is particularly strong on genes involved in lipid transportation and metabolism. This is consistent with previous identification of lipid metabolites (e.g., monoacylglycerols and sphingolipids) as signatures of sPTB35,69. Whether this stronger purifying selection targeting lipid transportation and metabolisms in pregnancy that ended preterm leads to changes in the concentrations of lipid metabolites however requires further experimental testing. As both recombination and purifying selection can reduce genetic diversity, sPTB-associated recombination and purifying selection along pregnancy may explain the higher nucleotide diversity of Gardnerella spp. in sPTB in the first half of pregnancy compared to the second half.
Antibiotics are common selective stresses acting on the human microbiome29 and have been associated with preterm birth70. We detected higher count and diversity of AMR genes associated with sPTB, which our analysis suggests to be facilitated by prophages in preterm vaginal microbiomes. While multiple phages (e.g., Siphoviridae, Myoviridae, and Microviridae) have been detected in the vagina of pregnant women, their association with sPTB is rarely studied71. Our results imply a potentially important role of bacteria-phage interactions in pregnancy outcomes via transferring of AMR genes. We also found that genes related to phenicol and aminoglycoside resistance were more abundant in vaginal microbiomes during pregnancies that ended preterm. While both antibiotics have been frequently used to treat gynecologic infection for decades72, and some phenicols (e.g., chloramphenicol) are thought to be safe for use during pregnancy73 aminoglycoside is teratogenic. Previous studies reported that exposure to antibiotics could change the composition of the vaginal microbiome48,74, indicating an ecological effect. In comparison, our results may suggest adaptation of the vaginal microbiome to more frequent antibiotics usage in women who delivered preterm, leading to an enrichment of AMR genes as well as higher nucleotide diversity in Gardnerella spp. genes encoding enzymes for drug metabolism. While this hypothesis requires further study, it is further supported by the fact that a higher proportion of women who delivered preterm (31%) had used antibiotics in the past 6 months before pregnancy than women who delivered at term (23%).
Despite its findings, our study is limited by low sequencing depth (median bacterial read count <5 × 105) and inconsistent sampling frequency during pregnancy (1 to 8 samples per pregnancy, with an average of 4). These limitations lead to high sparsity in the features analyzed, preventing a more in-depth temporal and predictive analysis of the link between population genetics of the vaginal microbiome and sPTB. Our results warrant a high-resolution investigation of the vaginal metagenome, with frequent sampling and high sequencing depth.
In summary, through in-depth population genomic analyses, our study identified genetic and functional associations between the vaginal microbiome and preterm birth. We revealed evidence of microbial genetic adaptation to the host environment linked to preterm birth and highlighted the importance of microbial evolutionary processes to adverse pregnancy outcomes, particularly in Gardnerella spp. Future investigation on the pressures driving the sPTB-associated microbial adaptation is warranted to fully understand the molecular mechanisms underlying preterm birth.
Methods
Sample selection and metagenomic data
We analyzed published metagenomic sequencing data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI) PTB case-control study6 as well as publicly available data from ref. 32 for validation. The metagenomic sequencing data and clinical covariates from ref. 6 were obtained from dbGaP (study no. 20280; accession ID phs001523.v1.p1) and directly from Virginia Commonwealth University. MOMS-PI participants provided written informed consent allowing for their data to be shared and used in future research studies. The analyses performed here were approved by the IRB of Columbia University, approval number AAAS5367.
MOMS-PI recruited a majority of women identifying as Black as described in ref. 6. We obtained 135 vaginal samples collected longitudinally during pregnancy from 40 women who eventually delivered preterm spontaneously (sPTB; mean ± sd) age 26 ± 5.68) and 570 vaginal samples from 135 women who delivered at term (TB; age 25.9 ± 5.43). Samples were sequenced paired-end, to a mean ± sd depth of 717,887 ± 1,536,354 (mean ± s.d.) non-human reads. Fettweis et al. 20196 only included in this dataset term births after 39 weeks of gestation, with the intention of avoiding complications associated with early term birth6. Our study therefore uses the same definitions: spontaneous preterm birth is defined as live birth between 23 and 37 gestational weeks without medical indication, and term birth is defined as live birth at or after 39 gestational weeks.
On average, 3.36 and 3.21 samples were collected for each sPTB and TB woman, respectively (Mann-Whitney U p = 0.83); 1.68 and 1.51 samples were collected for the first half of pregnancy for each sPTB and TB woman, respectively (p = 0.30); and 2.34 and 2.14 samples were collected for the second half of pregnancy for each sPTB and TB woman, respectively (p = 0.27) (Supplementary Table 1). The average gestational age at the first sample being collected for sPTB and TB women is 17.38 and 16.09, respectively (p = 0.45), while for the last sample being collected for sPTB and TB women is 31.23 and 32.31, respectively (p = 0.72) (Supplementary Table 1). In addition, none of these women delivered at <20 gestational weeks (Supplementary Fig. 9a). These indicate that dropping out due to late miscarriage/early PTB is not a concern to bias our findings in this study.
To check for the presence of some potential confounders for vaginal microbiome-sPTB associations, we calculated propensity scores75 for each subject based on income, age, and race using a logistic regression model. We found that propensity scores for both sPTB and TB subjects exhibited a similar distribution (Kolmogorov–Smirnov test p = 0.21), suggesting the associations we detect are not likely to be confounded with these variables (Supplementary Fig. 9b). These results suggest a negligible confounding effect of income, age, and race in this study on microbiome-sPTB associations. We note, however, that population studies such as the one performed by Fettweis et al.6 can be exposed to selection bias, via access to medical care and other reasons. Experimental procedures for data generation are described by Fettweis et al6.
Metagenomic assembly, genomic binning, genome annotation, and relative abundance
Our analysis follows the accepted standards used in refs. 76–79, using the ATLAS pipeline80 (v. 2.4.4). Bases with quality scores <25, raw reads <50 bp lengths, and sequencing adapters were removed using Trimmomatic v.0.3981. Reads mapped to human and PhiX genome sequences were removed by mapping with Bowtie2 v.2.3.5.182. Assembly and binning were done with ATLAS: filtered reads were assembled using metaSPAdes v.3.15.283, and contigs were binned into metagenome-assembled genomes (MAGs) using MetaBAT2 v.2.14.0 (ref. 84) with a minimum contig length of 1500. Quality, GC content, genome size, and taxonomy of MAGs were estimated using CheckM v.1.0.924. MAGs were de-replicated using dRep v.3.2.085 with an average nucleotide identity (ANI) of 0.95, minimum completeness of 50%, and maximum genome contamination of 10%. The MAG with the highest dRep score within each 95% ANI cluster, termed here as a phylogroup, was selected as the representative MAGs for that phylogroup. Genes were predicted using Prodigal v.2.6.386 and annotated using EggNOG v.5.087. Filtered reads were mapped to representative MAGs using Bowtie2 v.2.3.5.188. The relative abundance of each representative MAG was calculated by dividing the number of reads that mapped to that MAG, corrected to the genome size and completeness, by the total number of reads in each sample.
Phylogeny, ANI, and dendrogram
Amino acid (AA) sequences of 120 marker genes were called and aligned for representative MAGs using GTDB-Tk v.1.5.1 (ref. 89). MAGs with <60% of AA in the alignment were excluded in the phylogenetic tree construction. The best evolutionary model LG + G + I (the Le Gascuel model + gamma distribution + invariant sites) was identified using prottest3 v.3.4.2 (ref. 90) and 500 bootstraps were used for tree construction using RAxML v.8.2.12 (ref. 91). The tree was rooted by midpoint and visualized in iTol v.6.3 (ref. 92).
Pairwise ANI of MAGs for each phylogroup as well as for representative MAGs annotated as G. vaginalis (PG42-53) and reference genomes of 13 Gardnerella species defined in ref. 25 was calculated using pyani v.0.2. Dendrogram of ANI was constructed using the complete-linkage clustering method in the vegan package (v.2.6-4) in R v.3.6.0.
Microdiversity profiling, growth rate estimation, and antimicrobial resistance genes
Population microdiversity metrics including genome-wide nucleotide diversity, gene-wide nucleotide diversity, linkage disequilibrium measures (D’) and selection measures (dN/dS), were calculated using InStrain v1.0.043 using the 157 representative MAGs as the reference database. Maximal growth rate was estimated for each MAG using gRodon36 v.1. Antimicrobial resistance (AMR) genes were detected in assemblies and MAGs using PathoFact v.1.0 (ref. 93) with default parameters.
Temporal analysis
To generate the trajectories representing the change in nucleotide diversity over time in term and preterm deliveries, we pooled the temporal data of Gardnerella spp. from all women in each group (term and preterm). When we had more than one observation per gestational week, we took the median value across samples. We then binned the temporal data into bins of 3 weeks, except for the first bin which spanned weeks 1-7, and took the median of each bin as a summary. To smooth the observed binned data we applied splines, which is a special function defined piecewise by polynomials for data smoothing. To compare between the temporal trajectories of preterm and term, we performed a permutation test, in which we generated a null distribution of euclidean distances by shuffling the these trajectories 104 times and comparing to the euclidean distance in the original data31.
Functional enrichment analysis
To identify COG/KEGG pathways that were enriched in genes showing significant difference in nucleotide diversity between sPTB and TB, the frequency of each COG/KEGG category was first calculated from significant genes (observed frequency). Then, the frequency of each COG/KEGG category was calculated from an identical number of genes randomly selected from all genes (expected frequency). This process was repeated 10,000 times. The null hypothesis was that the observed frequency of COG category is smaller than the expectation. For each COG, probability P of the null hypothesis was calculated using the formula: p = |[xi ∈ x: xi > = k]| / 10000, where […] denotes a multiset, x = (x1, x2, …, xn) is a list of expected values, and k is the observed value.
Statistics & reproducibility
This was a secondary analysis of data obtained from a case-control analysis of an observational cohort6. Sample size follows the original study and no sample size calculation was performed for this study. No data was excluded from this analysis. As this was a secondary analysis of an observational study, there was no allocation to intervention, and hence no randomization or blinding to allocation. A STORMS checklist is provided as Supplementary Data 3. A different number of samples was available for each woman in the database. In our analyses, we therefore used the median along pregnancy (or its first or second half). The false-discovery rate procedure (FDR) of Benjamini and Hochberg (BH)94 was used to correct for multiple testing. Adjusted P < 0.1 was used as the significance cutoff.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank members of the Korem lab for useful discussions. This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health under award number R01HD106017, the Program for Mathematical Genomics at Columbia University, and the CIFAR Azrieli Global Scholarship in the Humans & the Microbiome Program (T.K.). The dataset used was obtained from dbGaP (phs001523), using data provided by Gregory A. Buck, Ph.D. and colleagues and supported by NICHD (U54 HD080784) (G.A.B.).
Author contributions
J.L. and T.K. designed the study. J.L., L.S., and J.A.U analyzed the data with input from T.K., M.S., B.Z. and G.A.B. J.L. wrote the manuscript with input from L.S., T.K., M.S., B.Z. and G.A.B. G.A.B. assisted with data access and acquisition.
Peer review
Peer review information
Nature Communications thanks Matthew Olm, Marina Sirota, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Data availability
The raw sequencing data and metadata were obtained from ref. 6. Data are available under restricted access for ethical and privacy concerns from dbGAP under accession phs001523. Access can be obtained by a data access request to dbGaP, under the purview of the data access committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development [HD-DAC@mail.nih.gov]. All processed features generated in this study are available95 at Zenodo, 10.5281/zenodo.8150902. The validation dataset32 is available in SRA, under accession PRJNA288562.
Code availability
Code to replicate all analyses is available96 from https://github.com/korem-lab/MOMs-PI_microdiversity_2023.
Competing interests
G.A.B. is a member of the Scientific Advisory Board of Juno, LTD., a startup biotech firm focused on using the vaginal microbiome to address issues of women’s gynecologic and reproductive health. Juno had no involvement in the current study. Other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jingqiu Liao, Liat Shenhav.
Contributor Information
Jingqiu Liao, Email: liaoj@vt.edu.
Tal Korem, Email: tal.korem@columbia.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-40719-7.
References
- 1.Tiensuu H, et al. Risk of spontaneous preterm birth and fetal growth associates with fetal SLIT2. PLoS Genet. 2019;15:e1008107. doi: 10.1371/journal.pgen.1008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walani SR. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 2020;150:31–33. doi: 10.1002/ijgo.13195. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong X, et al. Genome-wide approach identifies a novel gene-maternal pre-pregnancy BMI interaction on preterm birth. Nat. Commun. 2017;8:15608. doi: 10.1038/ncomms15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong, X. et al. Genome-wide association study identifies a novel maternal gene × stress interaction associated with spontaneous preterm birth. Pediatr. Res. 89, 1549–1556 (2021). [DOI] [PMC free article] [PubMed]
- 6.Fettweis JM, et al. The vaginal microbiome and preterm birth. Nat. Med. 2019;25:1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiGiulio DB, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl Acad. Sci. USA. 2015;112:11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravel J, et al. Vaginal microbiome of reproductive-age women. Proc. Natl Acad. Sci. USA. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabatabaei N, et al. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: a case-control study. BJOG. 2019;126:349–358. doi: 10.1111/1471-0528.15299. [DOI] [PubMed] [Google Scholar]
- 10.Chu DM, Seferovic M, Pace RM, Aagaard KM. The microbiome in preterm birth. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018;52:103–113. doi: 10.1016/j.bpobgyn.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Freitas AC, Bocking A, Hill JE, Money DM. & VOGUE Research Group. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome. 2018;6:117. doi: 10.1186/s40168-018-0502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stout MJ, et al. Early pregnancy vaginal microbiome trends and preterm birth. Am. J. Obstet. Gynecol. 2017;217:356.e1–356.e18. doi: 10.1016/j.ajog.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyman RW, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci. 2014;21:32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feehily C, et al. Shotgun sequencing of the vaginal microbiome reveals both a species and functional potential signature of preterm birth. NPJ Biofilms Microbiomes. 2020;6:50. doi: 10.1038/s41522-020-00162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosti I, Lyalina S, Pollard KS, Butte AJ, Sirota M. Meta-Analysis of Vaginal Microbiome Data Provides New Insights Into Preterm Birth. Front. Microbiol. 2020;11:476. doi: 10.3389/fmicb.2020.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta P, Singh MP, Goyal K. Diversity of Vaginal Microbiome in Pregnancy: Deciphering the Obscurity. Front. Public Health. 2020;8:326. doi: 10.3389/fpubh.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceccarani C, et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019;9:14095. doi: 10.1038/s41598-019-50410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chase, A. B., Weihe, C. & Martiny, J. B. H. Adaptive differentiation and rapid evolution of a soil bacterium along a climate gradient. Proc. Natl. Acad. Sci. USA. 118, e2101254118 (2021). [DOI] [PMC free article] [PubMed]
- 19.Zhao S, et al. Adaptive Evolution within Gut Microbiomes of Healthy People. Cell Host Microbe. 2019;25:656–667.e8. doi: 10.1016/j.chom.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garud NR, Pollard KS. Population Genetics in the Human Microbiome. Trends Genet. 2020;36:53–67. doi: 10.1016/j.tig.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Garud NR, Good BH, Hallatschek O, Pollard KS. Evolutionary dynamics of bacteria in the gut microbiome within and across hosts. PLoS Biol. 2019;17:e3000102. doi: 10.1371/journal.pbio.3000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murovec B, Deutsch L, Stres B. Computational Framework for High-Quality Production and Large-Scale Evolutionary Analysis of Metagenome Assembled Genomes. Mol. Biol. Evol. 2020;37:593–598. doi: 10.1093/molbev/msz237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olm MR, et al. Consistent Metagenome-Derived Metrics Verify and Delineate Bacterial Species Boundaries. mSystems. 2020;5:e00731–19. doi: 10.1128/mSystems.00731-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaneechoutte M, et al. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int. J. Syst. Evol. Microbiol. 2019;69:679–687. doi: 10.1099/ijsem.0.003200. [DOI] [PubMed] [Google Scholar]
- 26.Hill JE, Albert AYK. & the VOGUE Research Group. Resolution and Cooccurrence Patterns of Gardnerella leopoldii, G. swidsinskii, G. piotii, and G. vaginalis within the Vaginal Microbiome. Infect. Immun. 2019;87:e00532–19. doi: 10.1128/IAI.00532-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martino C, et al. Context-aware dimensionality reduction deconvolutes gut microbial community dynamics. Nat. Biotechnol. 2021;39:165–168. doi: 10.1038/s41587-020-0660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendz GL, Petersen R, Quinlivan JA, Kaakoush NO. Potential involvement of Campylobacter curvus and Haemophilus parainfluenzae in preterm birth. BMJ Case Rep. 2014;2014:bcr2014205282. doi: 10.1136/bcr-2014-205282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki, T. A. & Ley, R. E. The role of the microbiota in human genetic adaptation. Science370, eaaz6827 (2020). [DOI] [PubMed]
- 30.Ferris MJ, et al. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect. Dis. 2004;4:5. doi: 10.1186/1471-2334-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danielsson P-E. Euclidean distance mapping. Computer Graph. Image Process. 1980;14:227–248. doi: 10.1016/0146-664X(80)90054-4. [DOI] [Google Scholar]
- 32.Goltsman DSA, et al. Metagenomic analysis with strain-level resolution reveals fine-scale variation in the human pregnancy microbiome. Genome Res. 2018;28:1467–1480. doi: 10.1101/gr.236000.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manrique P, Dills M, Young MJ. The Human Gut Phage Community and Its Implications for Health and Disease. Viruses. 2017;9:141. doi: 10.3390/v9060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J. Infect. Dis. 2014;210:338–343. doi: 10.1093/infdis/jiu089. [DOI] [PubMed] [Google Scholar]
- 35.Kindschuh WF, et al. Preterm birth is associated with xenobiotics and predicted by the vaginal metabolome. Nat. Microbiol. 2023;8:246–259. doi: 10.1038/s41564-022-01293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weissman JL, Hou S, Fuhrman JA. Estimating maximal microbial growth rates from cultures, metagenomes, and single cells via codon usage patterns. Proc. Natl Acad. Sci. USA. 2021;118:e2016810118. doi: 10.1073/pnas.2016810118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korem T, et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science. 2015;4:1101–1106. doi: 10.1126/science.aac4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph TA, Chlenski P, Litman A, Korem T, Pe’er I. Accurate and robust inference of microbial growth dynamics from metagenomic sequencing reveals personalized growth rates. Genome Res. 2022;32:558–568. doi: 10.1101/gr.275533.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Achtman M, Wagner M. Microbial diversity and the genetic nature of microbial species. Nat. Rev. Microbiol. 2008;6:431–440. doi: 10.1038/nrmicro1872. [DOI] [PubMed] [Google Scholar]
- 40.Bohr LL, Mortimer TD, Pepperell CS. Lateral Gene Transfer Shapes Diversity of spp. Front. Cell. Infect. Microbiol. 2020;10:293. doi: 10.3389/fcimb.2020.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudson, R. R. Linkage disequilibrium and recombination. Handbook of statistical genetics (John Wiley & Sons, Ltd, 2004).
- 42.Kryazhimskiy S, Plotkin JB. The population genetics of dN/dS. PLoS Genet. 2008;4:e1000304. doi: 10.1371/journal.pgen.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olm MR, et al. inStrain profiles population microdiversity from metagenomic data and sensitively detects shared microbial strains. Nat. Biotechnol. 2021;39:727–736. doi: 10.1038/s41587-020-00797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenhav L, Zeevi D. Resource conservation manifests in the genetic code. Science. 2020;370:683–687. doi: 10.1126/science.aaz9642. [DOI] [PubMed] [Google Scholar]
- 45.Schloissnig S, et al. Genomic variation landscape of the human gut microbiome. Nature. 2013;493:45–50. doi: 10.1038/nature11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He M, et al. Evolutionary dynamics of Clostridium difficile over short and long time scales. Proc. Natl Acad. Sci. USA. 2010;107:7527–7532. doi: 10.1073/pnas.0914322107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stokholm J, et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin. Microbiol. Infect. 2014;20:629–635. doi: 10.1111/1469-0691.12411. [DOI] [PubMed] [Google Scholar]
- 49.Alcock BP, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:D517–D525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pace RM, et al. Complex species and strain ecology of the vaginal microbiome from pregnancy to postpartum and association with preterm birth. Med. 2021;2:1027–1049. doi: 10.1016/j.medj.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown RG, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9. doi: 10.1186/s12916-017-0999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Callahan BJ, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl Acad. Sci. USA. 2017;114:9966–9971. doi: 10.1073/pnas.1705899114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maguire, F. et al. Metagenome-assembled genome binning methods with short reads disproportionately fail for plasmids and genomic Islands. Microb Genom6, mgen000436 (2020). [DOI] [PMC free article] [PubMed]
- 54.Liao J, et al. Nationwide genomic atlas of soil-dwelling Listeria reveals effects of selection and population ecology on pangenome evolution. Nat. Microbiol. 2021;6:1021–1030. doi: 10.1038/s41564-021-00935-7. [DOI] [PubMed] [Google Scholar]
- 55.Haque MM, Merchant M, Kumar PN, Dutta A, Mande SS. First-trimester vaginal microbiome diversity: A potential indicator of preterm delivery risk. Sci. Rep. 2017;7:16145. doi: 10.1038/s41598-017-16352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leung, J. M., Graham, A. L. & Knowles, S. C. L. Parasite-Microbiota Interactions With the Vertebrate Gut: Synthesis Through an Ecological Lens. Front. Microbiol.9, 843 (2018). [DOI] [PMC free article] [PubMed]
- 57.Morowitz, M. J. et al. Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. Proc. Natl Acad. Sci. USA.108, 1128–1133 (2011). [DOI] [PMC free article] [PubMed]
- 58.Zeevi, D. et al. Structural variation in the gut microbiome associates with host health. Nature568, 43–48 (2019). [DOI] [PubMed]
- 59.Menard JP, et al. High vaginal concentrations of Atopobium vaginae and Gardnerella vaginalis in women undergoing preterm labor. Obstet. Gynecol. 2010;115:134–140. doi: 10.1097/AOG.0b013e3181c391d7. [DOI] [PubMed] [Google Scholar]
- 60.Kumar S, et al. The Vaginal Microbial Signatures of Preterm Birth Delivery in Indian Women. Front. Cell. Infect. Microbiol. 2021;11:622474. doi: 10.3389/fcimb.2021.622474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berman, H. L., Aliaga Goltsman, D. S., Anderson, M., Relman, D. A. & Callahan, B. J. Gardnerella diversity and ecology in pregnancy and preterm birth. bioRxiv10.1101/2023.02.03.527032 (2023). [DOI] [PMC free article] [PubMed]
- 62.Schumacher A, et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J. Immunol. 2009;182:5488–5497. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- 63.Polese B, et al. The Endocrine Milieu and CD4 T-Lymphocyte Polarization during Pregnancy. Front. Endocrinol. 2014;5:106. doi: 10.3389/fendo.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan W, Chen L, Bernal AL. Is elevated maternal serum alpha-fetoprotein in the second trimester of pregnancy associated with increased preterm birth risk? Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;145:57–64. doi: 10.1016/j.ejogrb.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 65.Simhan HN, Caritis SN, Krohn MA, Hillier SL. Elevated vaginal pH and neutrophils are associated strongly with early spontaneous preterm birth. Am. J. Obstet. Gynecol. 2003;189:1150–1154. doi: 10.1067/S0002-9378(03)00582-9. [DOI] [PubMed] [Google Scholar]
- 66.Otto SP, Barton NH. The evolution of recombination: removing the limits to natural selection. Genetics. 1997;147:879–906. doi: 10.1093/genetics/147.2.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooper TF. Recombination speeds adaptation by reducing competition between beneficial mutations in populations of Escherichia coli. PLoS Biol. 2007;5:e225. doi: 10.1371/journal.pbio.0050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Gutierrez CA, Aylward FO. Strong Purifying Selection Is Associated with Genome Streamlining in Epipelagic Marinimicrobia. Genome Biol. Evol. 2019;11:2887–2894. doi: 10.1093/gbe/evz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerson KD, et al. A non-optimal cervicovaginal microbiota in pregnancy is associated with a distinct metabolomic signature among non-Hispanic Black individuals. Sci. Rep. 2021;11:22794. doi: 10.1038/s41598-021-02304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terzic M, et al. Periodontal Pathogens and Preterm Birth: Current Knowledge and Further Interventions. Pathogens. 2021;10:730. doi: 10.3390/pathogens10060730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.da Costa AC, et al. Identification of bacteriophages in the vagina of pregnant women: a descriptive study. BJOG. 2021;128:976–982. doi: 10.1111/1471-0528.16528. [DOI] [PubMed] [Google Scholar]
- 72.Bargaza RA, Cunha BA. Aminoglycosides in gynecology. Int. Urogynecol. J. 1992;3:197–207. doi: 10.1007/BF00455001. [DOI] [Google Scholar]
- 73.Amstey, M. S. Chloramphenicol therapy in pregnancy. Clin. Infect. Dis.30, 237 (2000). [DOI] [PubMed]
- 74.Rick A-M, et al. Group B Streptococci Colonization in Pregnant Guatemalan Women: Prevalence, Risk Factors, and Vaginal Microbiome. Open Forum Infect. Dis. 2017;4:ofx020. doi: 10.1093/ofid/ofx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 76.Gálvez EJC, et al. Distinct Polysaccharide Utilization Determines Interspecies Competition between Intestinal Prevotella spp. Cell Host Microbe. 2020;28:838–852.e6. doi: 10.1016/j.chom.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Yang S, Liebner S, Svenning MM, Tveit AT. Decoupling of microbial community dynamics and functions in Arctic peat soil exposed to short term warming. Mol. Ecol. 2021;30:5094–5104. doi: 10.1111/mec.16118. [DOI] [PubMed] [Google Scholar]
- 78.Kieser S, Zdobnov EM, Trajkovski M. Comprehensive mouse microbiota genome catalog reveals major difference to its human counterpart. PLoS Comput. Biol. 2022;18:e1009947. doi: 10.1371/journal.pcbi.1009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chevalier C, et al. Warmth Prevents Bone Loss Through the Gut Microbiota. Cell Metab. 2020;32:575–590.e7. doi: 10.1016/j.cmet.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kieser S, Brown J, Zdobnov EM, Trajkovski M, McCue LA. ATLAS: a Snakemake workflow for assembly, annotation, and genomic binning of metagenome sequence data. BMC Bioinforma. 2020;21:257. doi: 10.1186/s12859-020-03585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nurk S, Meleshko D, Korobeynikov A, Pevzner P. A. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 2017;27:824–834. doi: 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang DD, et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ. 2019;7:e7359. doi: 10.7717/peerj.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olm MR, Brown CT, Brooks B, Banfield JF. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 2017;11:2864–2868. doi: 10.1038/ismej.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hyatt D, et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinforma. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huerta-Cepas J, et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019;47:D309–D314. doi: 10.1093/nar/gky1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Danecek P, et al. Twelve years of SAMtools and BCFtools. Gigascience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaumeil P-A, Mussig AJ, Hugenholtz P, Parks DH. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics. 2019;36:1925–1927. doi: 10.1093/bioinformatics/btz848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Nies L, et al. PathoFact: a pipeline for the prediction of virulence factors and antimicrobial resistance genes in metagenomic data. Microbiome. 2021;9:49. doi: 10.1186/s40168-020-00993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57:289–300. [Google Scholar]
- 95.Liao, J., Shenhav L. & Korem T. Processed data of vaginal microbiome microdiversity from the MOMS-PI dataset. Zenodo10.5281/zenodo.8150902 (2023).
- 96.Liao, J., Shenhav L. & Korem T. Code for “Microdiversity of the Vaginal Microbiome is Associated with Preterm Birth”. GitHub10.5281/zenodo.8150916 (2023). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The raw sequencing data and metadata were obtained from ref. 6. Data are available under restricted access for ethical and privacy concerns from dbGAP under accession phs001523. Access can be obtained by a data access request to dbGaP, under the purview of the data access committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development [HD-DAC@mail.nih.gov]. All processed features generated in this study are available95 at Zenodo, 10.5281/zenodo.8150902. The validation dataset32 is available in SRA, under accession PRJNA288562.
Code to replicate all analyses is available96 from https://github.com/korem-lab/MOMs-PI_microdiversity_2023.