Abstract
Cells receive enormous amounts of information from their environment. How they act on this information - by migrating, expressing genes, or relaying signals to other cells - comprises much of the regulatory and self-organizational complexity found across biology. The ”parts list” involved in cell signaling is generally well established, but how do these parts work together to decode signals and produce appropriate responses? This fundamental question is being increasingly addressed with optogenetic tools: light-sensitive proteins that enable biologists to manipulate the interaction, localization, and activity state of proteins with high spatial and temporal precision. Here we will summarize how optogenetics is being used in the pursuit of an answer to this question, outlining the current suite of optogenetic tools available to the researcher and calling attention to studies that increase our understanding of and improve our ability to engineer biology.
Keywords: optogenetics, cell signaling, developmental biology, synthetic biology
1. INTRODUCTION
Traditionally, cellular phenomena are studied at the level of their primary components. Genes and proteins are the language of biological inquiry, and by understanding the parts we hope to gain insight into the functioning of the whole. While this approach has led to an overflowing encyclopedia detailing the cell’s constituent pieces, it is a poor level of abstraction to understand the operation of a cell, like trying to parse the output of a computer program by observing the changing states of the computer’s transistors. One must also study higher levels of abstraction, looking not just at the carriers of information but also at the patterns of information flow and at the actions these patterns execute (1).
David Marr, in his 1982 seminal work on visual perception, proposed analyzing information-processing systems by subdividing them into three tiers: computation, algorithm, and implementation (2). The highest level of abstraction in this framework is the computation, i.e., the desired outcome of a given operation. For example, bacterial chemotaxis may be said to operate under the computation “if the concentration of chemoattractant is increasing, stay the course” (3). The middle level, the algorithm, consists of the specific input-output relationship that achieves the computation. To move towards a chemoattractant, bacteria incorporate an integral feedback controller that maintains flagellar motor activity if chemoattractant concentrations are transiently increasing (4, 5). The most concrete level, the implementation, describes the physical elements that are carrying out the prescribed algorithm. This is the “wetware” of biological systems, the chemotaxis receptors and downstream components that are chemically interacting to elicit the computational goal. Each level of this hierarchy can be specified somewhat independently; several physical instantiations can manifest the same algorithm, and several algorithms can manifest the same computation.
A central barrier to the study and rational engineering of biological systems is our inadequate understanding of the computations and algorithms of cellular information processing. This is predominantly due to the fact that, through much of the history of cell biology, researchers have lacked the necessary tools to deliver time- and space-varying inputs to living cells (indeed, bacterial chemotaxis is one notable exception, where ramps and sinusoidal chemoattractant stimuli have long been part of the experimental toolbox) (6). The optogenetics revolution of the last decade has changed this. We now have an abundance of engineered photo-controllable proteins that allow for the direct and near-instantaneous regulation of a cellular process, providing the control required to interrogate cell behavior at different levels of abstraction. To understand the computation cells perform, we can deliver light inputs to photoactivatable signaling pathways and monitor the resultant cellular response. To narrow down the algorithm used to perform this cellular computation, we can systematically probe individual signaling steps to deduce the architecture of more complex networks. After generations of studying the biological equivalent of transistors, we are now poised to read and write the code of the cell.
In this review, we will both cover the current experimental paradigms in the optogenetic analysis of natural cellular systems and emphasize how this analysis can be coupled with traditional engineering principles to design biological entities de novo. Neuronal control using light-sensitive ion channels, which transformed the field of neuroscience over a decade ago, has received extensive review in the literature and so will not be expounded on here (7). We begin by summarizing the photosensitive proteins found in nature and detailing how these proteins can be used to generate a diverse range of optogenetic tools. We then transition into mammalian cell biology, highlighting how optogenetics can be used to uncover and control the processing mechanisms that translate extracellular signals into cell fate decisions. We next call attention to the discoveries in developmental biology that have been afforded by optogenetic control, namely the interpretation of cell fate decisions and the sculpting of embryological form. Finally, we highlight some emerging applications: how optogenetics can be used to engineer multicellular structures and can be combined with in silico feedback control to direct cellular processes.
2. THE OPTOGENETIC TOOLBOX
Numerous optogenetic tools have been engineered from naturally occurring photosensitive protein domains. These domains, adopted from plants, bacteria, and fungi, have been extensively engineered to allow for the manipulation of protein activity, subcellular localization, and protein-protein interactions with high spatiotemporal precision. Here, we present a brief overview of some of the major classes of photosensitive proteins and the resulting optogenetic tools. For readers who are interested in learning more, we also highly recommend browsing the OptoBase website, a continually-updated database of optogenetic tools and the studies that use them (8).
2.1. Photosensitive Proteins
2.1.1. Rhodopsins.
Likely the most well-known photosensitive building blocks, channelrhodopsins were among the first proteins to be used in optogenetic studies. Channelrhodopsin-1 (ChR1) and channelrhodopsin-2 (ChR2) from Chlamydomonas reinhardtii are blue light-gated channels that mediate the transfer of cations across the cell membrane. These and other light-controlled opsins, while having revolutionized neuroscience and our understanding of neurological diseases and treatments by enabling researchers to activate specific populations of neurons and monitor changes in behavior (7, 9), have seen limited application in non-excitatory cells where transmembrane voltage plays a less prominent signaling role. As a result, they will not be extensively reviewed here, and we refer the reader to past reviews for further information (10, 11). Nevertheless, the success of the neuronal optogenetics program opened the door to using light-controlled proteins to manipulate cell behavior across broad cellular contexts.
2.1.2. Light-Oxygen-Voltage Sensing Proteins.
One of the first families of photosensitive proteins outside of rhodopsins to be widely applied in cell biology research was light-oxygen-voltage (LOV) sensing proteins. LOV proteins are naturally found in a number of plant, fungal, and bacterial species, where they regulate diverse cellular processes in response to blue light (12). One LOV domain that has been the subject of particularly detailed study, the second LOV domain from Avena sativa Phototropin 1 (AsLOV2), is instructive to examine to better understand the family as a whole. In addition to a folded core of about 100 amino acids, AsLOV2 includes a C-terminal alpha helical domain, the Jα-helix (13). After illumination with blue light, a cysteine residue on LOV forms a covalent bond with an associated flavin mononucleotide (FMN) co-factor, initiating a structural change in the folded domain that propagates to the Jα-helix and results in its displacement away from the core. The covalent bond formed is energetically unfavorable (14), causing the Jα-helix to return to its dark state conformation over time. Although the details vary, all of the pieces described here, a folded domain, flavin co-factor and light-dissociated helix, are broadly conserved across the LOV family.
Two important characteristics of LOV domains deserve explicit note. One is their capacity for extreme variations in the time required for dark-state reversion: depending on mutations around the chromophore, the lifetime of the light-activated state can be tuned from seconds to days. This variation can be found in both natural (15) and engineered (16) LOV domains. Second, LOV domains are widespread in nature, where they are found coupled to diverse protein functions. Naturally-occurring LOV domains have been described that undergo light-induced dimerization (17), kinase activation (18), intercalation into the plasma membrane (19), DNA binding (20), and even RNA hairpin binding (21). It is very likely that additional light-coupled functions of LOV domains remain to be discovered.
2.1.3. Cryptochromes.
Like LOV domains, cryptochromes incorporate a flavin cofactor (FAD) and are photoactivated by blue light (400-500 nm). Relatives of cryptochromes occur in many different cellular contexts (DNA repair in bacteria; phototropism in plants; the metazoan circadian clock). Hints of their utility as optogenetic tools came from observations of the Arabidopsis thaliana flavoprotein Cryptochrome 2 (Cry2), wherein Liu et al. found that Cry2 binds to the transcriptional regulator, CIB1 (cryptochrome-interacting basic-helix-loop-helix), in a blue light-dependent manner (22). Cry2 also clusters in response to blue light, forming “photo-bodies” in the nucleus that are thought to regulate transcription (23). Although Cry2 has been widely deployed as an optogenetic tool, its active-state structure and photochemistry are still the focus of intense study (24-28). Nevertheless, the 498 amino acid photolyase homology domain of Cry2 (Cry2PHR) can be used reliably for light-induced heterodimerization (wth CIB1) or homo-oligomerization in many cellular contexts, making it one of the most widely-used components in the optogenetic toolbox.
2.1.4. Phytochromes.
What about optogenetic control with activation wavelengths other than blue light? This capability is provided by a third major superfamily of light-sensitive proteins: the phytochromes. Phytochromes convert between two stable forms, one red-absorbing (Pr) and one far-red absorbing (Pfr), upon illumination with two wavelengths. Photoconversion arises from the light-induced isomerization of a bilin chromophore that is covalently ligated to the phytochrome.
Three features make phytochromes very different from cryptochromes and LOV domains. One is that they are photochromic: the two states of a phytochrome (Pr and Pfr) are each photosensitive, and photon-absorption in either state causes isomerization of the chromophore that can occur many times per second and scales with the administered magnitude of light intensity. It is thus possible to control the rate of on/off photoswitching with these two wavelengths. Second, the extended, flexible structure of the bilin chromophore endows it with the ability to absorb light at very different wavelengths depending on the identity of specific amino acid contacts holding it in place (29, 30). As a result, natural phytochrome variants exist that span the entire visible spectrum, and engineered phytochrome-based optogenetic systems are beginning to be developed that sense wavelengths ranging from blue to far-red (31). Finally, unlike cryptochromes and LOV domains that harness ubiquitous flavin chromophores, the bilin cofactors required by phytochromes are often only found in particular organisms and must be purified or synthesized to function in other contexts.
One important phytochrome in optogenetics research has been the Arabidopsis thaliana Phytochrome B (PhyB) protein and its binding partners PIF3 and PIF6 (32, 33). Ni et al. demonstrated that activation of PhyB with red light results in binding to the transcriptional regulator phytochrome interaction factor 3 (PIF3), and that conversion to the inactive isoform with far red light results in dissociation; analogous logic applies to PIF6 (34). Other phytochromes exhibit similar light-regulated binding: a bacterial phytochrome, BphP1, binds to PpsR2 in a light dependent manner (35), and the cyanobacterial phytochrome 1 (Cph1) forms homodimers predominately in the Pfr state (36).
2.1.5. Beyond LOV, Cry, and Phy: other photosensitive domains in the optogenetics toolbox.
The suite of optogenetic tools has expanded well beyond this core triad of photosensitive proteins. Here, we remark on a few additional families of light-sensitive domains that have been harnessed to control cellular processes. The aptly-named “blue light using flavin” (BLUF) domains are small (roughly 100 amino acid) light-sensitive domains with rapid activation and deactivation kinetics. One member of this family, PixD, is well-suited for controlling protein oligomerization. PixD forms multimeric complexes with its non-light-sensitive binding partner PixE in the dark (37). Upon blue light exposure, this complex breaks apart into PixD dimers and PixE monomers that reassemble into oligomers within seconds after returning to darkness. Other light-sensitive domains undergo irreversible transitions, which can be desirable in certain contexts where light delivery or phototoxicity prove challenging. Notable examples include the UV receptor UVR8, which dissociates from homodimer to monomer upon UV light exposure (38) and the cobalamin binding domain of CarH, which dissociates from a tetrameric to monomeric state upon chromophore cleavage by green light (540 nm) (39). Photoactive yellow protein (PYP), a light-sensitive protein from Halorhodospira halophila, undergoes a profound yet reversible unfolding event in response to blue light (40) that is beginning to be repurposed for optogenetic control (41).
A final recent development has been the emergence of engineered fluorescent proteins as optogenetic tools. Photoswitchable and photoconvertible fluorescent proteins exhibit conformational changes in response to specific wavelengths of light. While these have traditionally been harnessed to change spectral characteristics, they can also be used to control other biochemical events. The engineering of proteins Dronpa (42), which changes from dark to green-fluorescent upon illumination with violet light, and mMaple (43), which changes from green fluorescent to red fluorescent upon illumination with violet light, will be discussed below.
2.2. From Photosensitive Proteins to Optogenetic Tools
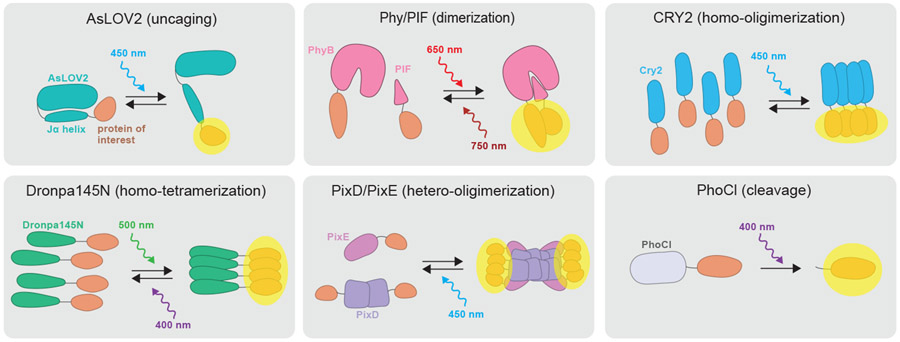
Of course, cataloguing the diverse repertoire of light-sensitive domains is not enough – each of these natural photosensitive protein domains must be repurposed (and sometimes altered) to create a functional optogenetic tool. Over the past decade many light-sensitive proteins have been refined through 2-3 cycles of design, allowing for precise control over the activity and localization of a vast catalog of proteins (Figure 1, Table 1). Here we will describe some of the most useful classes of tools: those that enable light-dependent uncaging, photocleavage, protein dimerization, higher-order clustering, and allosteric control.
Figure 1.
Schematics illustrating several mechanisms of action achievable with optogenetic tools including uncaging (AsLOV2), dimerization (Phy/PIF), homo-oligomerization (Cry2), homo-tetramerization (Dronpa), hetero-oligomerization (PixD/PixE), and photocleavage (PhoCl).
Table 1.
Summary of optogenetic tools
| Mechanism | Tool | λon/off | activation/reversion time | Notes |
|---|---|---|---|---|
| Channel opening | ChR2 | 480 nm | milliseconds/milliseconds | Used extensively in neuroscience |
| Uncaging | AsLOV2 | 450 nm | < 1 sec /tens of seconds | Functionalized by mutating the Jα helix to present various motifs. |
| PYP | 450 nm | < 1 sec / seconds | ||
| Heterodimerization | Cry2/CIB1 | 450 nm | < 1 sec / minutes | Cry2 can also homo-oligomerize |
| iLID/SspB | 450 nm | < 1 sec / tens of seconds | iLID nano, micro, and milli variants available with tunable affinity | |
| TULIPs | 450 nm | < 1 sec / tens of seconds | ||
| Magnets | 450 nm | < 1 sec / variable | For all LOV domains, mutations can tune kinetics from seconds to hours | |
| FKF1/GI | 450 nm | < minutes / tens of hours | ||
| LOVTRAP | dark/450 nm | < 1 sec / tens of seconds | Binds AsLOV2 in dark state | |
| PhyB/PIF | 650 nm/750 nm | < 1 sec / < 1 sec | Requires addition of exogenous bilin chromophore (PCB or PΦB) | |
| BphP1/PpsR2 | 760 nm/640 nm | < 1 sec / < 1 sec | Reversion in the dark state also occurs spontaneously in minutes | |
| UVR8/COP1 | 300 nm | < 1 sec / none | Dimerization is irreversible | |
| Homodimerization | VVD | 450 nm | <1 sec / hours | |
| pdDronpa1 | 500 nm/400 nm | < 1 sec / < 1 sec | ||
| EL222 | 450 nm | < 1 sec / < 1 sec | Homodimers bind specific DNA sequence | |
| Cph1 | 660 nm/740 nm | < 1 sec / < 1 sec | Requires addition of exogenous bilin chromophore (PCB or PΦB) | |
| Oligomerization | Cry2PHR | 450 nm | < 1 sec / minutes | Rapid photoactivation but clustering kinetics depend on expression level |
| Dronpa145N | 500 nm/400 nm | < 1 sec / < 1 sec | Proteins form tetramer after light activation, Dronpa145N also forms heterodimer with Dronpa145K | |
| PixD/PiXE | dark/450 nm | < 1 sec / tens of seconds | Forms approx. 10:4 stoichiometry oligomers in the dark | |
| Cleavage | PhoCl | 400 nm | minutes / none | Cleavage is irreversible |
2.2.1. Uncaging.
Photo-uncaging occurs when a light-dependent conformational change exposes an active region of the photosensitive protein (either a folded surface or a disordered linear motif). By introducing mutations at the exposed site, it is then possible to trigger responses ranging from changes in subcellular localization to specific protein interactions. AsLOV2 has been a runaway success in optogenetic uncaging applications because its Jα-helix switches from a folded domain to a disordered 20 amino acid peptide upon illumination. Crucially, the sequence of those 20 amino acids can be altered to encode a wide variety of linear motifs if key alpha helical contacts are retained. In this manner, variants of AsLOV2 have been engineered for many applications including light-triggered nuclear import (44, 45), nuclear export (46, 47), protein degradation (48), and protein binding (49, 50).
Photo-uncaging can also be adapted to regulate the activity of proteins of interest if light-sensitive domains can be switched between occluding and exposing a functionally-required surface. Zhou and colleagues described such a general strategy for protein kinases, flanking an active kinase domain with N- and C-terminal fusions of the pdDronpa1 homodimerization domains (42). In response to cyan light, pdDronpa1 dissociates to expose the protein’s kinase domain, whereas magenta light reverses activation. This approach proved to be broadly applicable and has led to photoactivatable alleles of the kinases MEK1, MEK2, RAF1, and CDK5, variants of which have been successfully deployed in many cellular contexts (51).
2.2.2. Photocleavage.
To date, only one optogenetic tool, PhoCl, has been developed to undergo photocleavage of the peptide sequence upon illumination. PhoCl is based on an engineered variant of the green-to-red photoconvertible protein mMaple (43). The mMaple chromophore undergoes a β-elimination reaction upon illumination with violet light that results in cleavage of the polypeptide backbone. This cleavage produces N and C terminal fragments that, in mMaple, remain associated. However, by circularly permuting this protein sequence, the researchers generated an mMaple variant where the N and C terminal fragments spontaneously and irreversibly dissociate upon violet light irradiation. By creating fusion proteins that include a localization tag, PhoCl, and a protein of interest, proteins were redistributed throughout the cell in a light-dependent manner.
2.2.3. Dimerization.
Many optogenetic tools rely on light-induced dimerization between natural or engineered domains. Indeed, one of the first optogenetic tools ever reported (even predating the term “optogenetics”) was constructed from PhyB and PIF3, each fused to one half of a split Gal4p transcription factor, for light-inducible gene expression in S. cerevisiae (52). Several years later, a series of papers developed light-switchable dimerization in mammalian cells using Cry2/CIB1 (53), PhyB/PIF6 (54), and an engineered interaction between AsLOV2 and a cognate PDZ domain (50). Since that time, each of these light-inducible dimerization systems has seen numerous improvements, extending their dynamic range between dark and lit states (49, 55), preventing off-pathway interactions such as Cry2 clustering (56), and enabling biosynthesis of the PCB chromophore required for PhyB photoswitching (57). Light induced dimerization systems have been employed for a variety of applications, including the creation of split transcription factors and enzymes (e.g. Cre recombinase) (58, 59) as well as the translocation of proteins on and off the plasma membrane (50, 53, 54).
2.2.4. Oligomerization.
Protein clustering plays a crucial role in many biological events, from the activation of cell surface receptors to the assembly of transcription factors for driving gene transcription. Taking an analogous approach to the dimerization systems described above, light-sensitive oligomerization systems have been developed by fusing proteins of interest to photosensitive domains that reversibly oligomerize upon light stimulation.
Cry2 has long been at the center of the optogenetic clustering universe. Bugaj et al. showed that Cry2 forms light-induced punctae at the cell surface that can be used to control receptor clustering (60) and subsequent studies expanded this platform to other receptors and cytosolic cargo (61). Point mutations in Cry2 were also discovered that enhance this clustering effect in the Cry2olig and Cry2clust systems (62, 63). The success of Cry2 optogenetic clustering has also spurred approaches using other naturally-occurring domains that undergo light-sensitive oligomerization, including the PixD/PixE system (64). A second useful strategy has been to co-localize multiple optogenetic heterodimerization domains, either as head-to-tail domain fusions in the iPOLYMER system (65) or as fusions to a naturally-oligomeric protein in the LARIAT system (61). The ability to oligomerize proteins in a light-dependent manner has enabled studies of cell-surface receptor signaling, which is often triggered by clustering (66, 67).
In recent years, protein phase separation has emerged as a fundamental process in many cellular contexts. Reflecting this development, one important direction in optogenetic protein clustering has been the development of tools that combine nucleation of small oligomeric seeds with the growth of liquid-like protein droplets. The first optogenetic tool designed specifically to address protein phase separation was the optoDroplet system developed by Shin et al. (68). Intrinsically disordered protein regions (IDRs) known to phase separate were fused to Cry2, dramatically increasing the rate and overall extent of clustering upon illumination. IDR fusions continued to show remarkable efficacy when combined with PixD/E- or ferritin-based clusters (69, 70), and have since been combined with CRISPR/Cas9 technology to engineer light-controlled protein condensates that form at predefined genetic loci (71). Yet another approach for light-activated biomolecular condensate formation is the SPLIT system developed by Reed et al. (72). The SPLIT system makes use of the photocleavable protein, PhoCl, for triggered removal of a solubilization tag from a phase-separating IDR.
2.2.5. Allosteric control by optogenetic domain insertion.
A final exciting development in optogenetic control, pioneered by the Hahn laboratory, involves inserting a light-sensitive domain into a solvent-exposed loop in a target protein of interest (73). AsLOV2 has turned out to be ideally suited for this application, as its N and C termini he very near one another in the dark state but are displaced upon illumination. This provides a light-induced ‘hairpin’ that can be opened to potentially alter the conformation of the loop in which AsLOV2 is fused. This approach was originally applied across a range of protein families (a kinase, a G protein, and two GTP exchange factors), with subsequent reports suggesting that it generalizes further to additional contexts. For instance, two recent reports demonstrated that AsLOV2 insertion can be used to regulate binding between nanobodies/monobodies and their cognate targets, achieving up to a 330-fold change in affinity upon illumination (74, 75).
3. OPTOGENETICS FOR DECONSTRUCTING CELLULAR SIGNAL PROCESSING
Even though optogenetic control is more than a decade old, the idea of using light to control intracellular processes in living cells still has a ring of science fiction to it. The practical appeal is also straightforward: light is a near-ideal stimulus, as most cellular processes are not naturally sensitive to light (provided that photoxicity limits are not exceeded), and it can be turned on and off rapidly or focused with high spatial precision. Here, we will describe how optogenetic inputs at different nodes can reveal information about cellular algorithms and network architecture, how time-varying light inputs can be used to define dynamic cellular computation, and how optogenetics has impacted our understanding of spatially-regulated cellular processes.
3.1. Light Inputs for Dissecting the Algorithms of Cellular Signal Processing
Molecular studies of cell signaling networks have revealed a tangled web of crosstalk, feedback and feed-forward connections through which information is processed and cell fate decisions are made (Figure 2a). Exposure to a single extracellular ligand might activate half a dozen intracellular pathways, each turning on and off with its own dynamics. While these experiments can be highly informative of which nodes are involved in a cellular response, we are left without intuition for how pathways are organized (i.e., what overall network architecture dictates each pathway’s observed response). Moreover, the standard tools of genetic manipulation, knock-out or overexpression at specific signaling steps are of limited utility because they tend to cause long-term changes in signaling levels that trigger compensatory processes or fundamentally alter the cell’s state. Ideally, a cell biologist would like to deliver precise, acute, and reversible stimuli to specific nodes to map network responses.
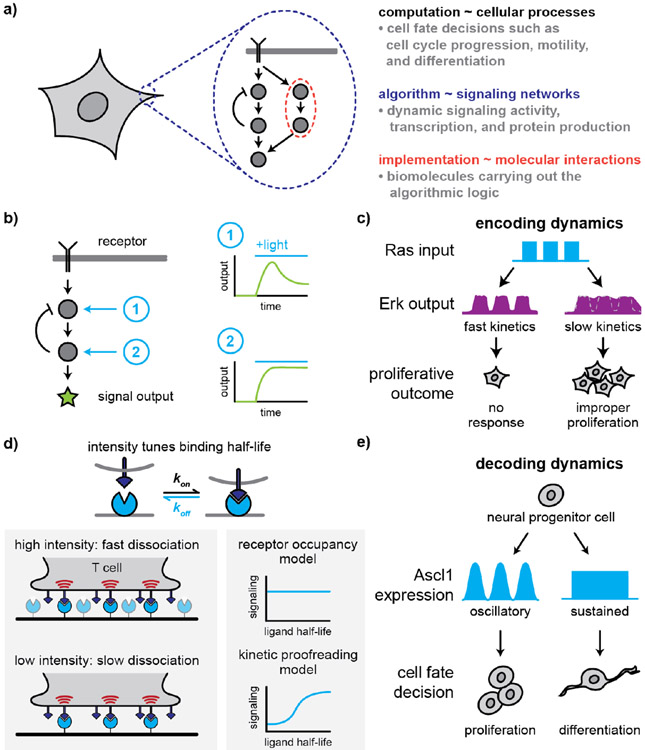
Figure 2.
(a) As information processing systems, cells can be viewed through the lens of Marr’s hierarchy: molecular interactions (implementation) comprise the signaling networks (algorithm) that cells employ to regulate cellular processes (computation). (b) Systematically stimulating signaling nodes along a pathway allows one to identify regulatory mechanisms that influence signaling outputs. (c) Alterations in Ras/Erk signal encoding can result in slow Erk activation kinetics, leading to increasingly sustained Erk activation and increased proliferation. (d) Light-regulated ligand-receptor interactions can be used to tune ligand binding half-life independent of other aspects of ligand-receptor interactions, a strategy that was used to dissect mechanisms of T cell receptor activation. (e) The dynamics of gene expression regulate cell fate decisions, such as in neural progenitor cells that proliferate in response to oscillatory Ascl1 expression, and differentiate in response to sustained Ascl1 expression.
One promising approach to solving this problem is to be able to “walk up and down” a pathway, applying activating inputs at successive nodes. Then, by mapping response dynamics as the input node is varied, one might define where regulatory interactions are connected to regulate signaling (Figure 2b). A series of recent studies have adopted this strategy to various mammalian cell signaling networks. For instance, work by Graziano and colleagues compared signaling dynamics in neutrophil chemotaxis after receptor stimulation or optogenetic PI 3-kinase (PI3K) activation, revealing an adaptation module that functions downstream of PI3K and that is masked by additional, redundant circuits when the cell is stimulated at the receptor level (76). More recently, DeFelice and colleagues studied NF-κB signaling downstream of Toll-like receptor 4 (TLR4) and Interleukin-1 receptor (IL-1R) in mouse embryonic fibroblasts (MEFs) (77). In both cases, prior stimulation of either receptor prevents activation by a subsequent stimulus, but where in the pathway does this inhibitory effect occur? To address this question, the authors developed Cry2 fusions of the intracellular proteins MyD88 and TRAF6 that could be activated by light-dependent clustering. Combinations of light and ligand stimulation revealed that inhibition occurred downstream of MyD88 but upstream of TRAF6, leading the authors to identify a dose-sensing autoinhibitory feedback loop through IRAK1 that limits signal transmission to NF-κB. As additional signaling nodes are brought under optogenetic control, we can envision many future studies that take analogous approaches to determine which feedback and feed-forward connections dictate each pathway’s combinatorial and dynamic responses.
3.2. Cellular Computation: Time-Varying Inputs Reveal Role of Signaling Dynamics
Cells are exposed to constantly-varying external environments; perhaps unsurprisingly, their intracellular life is similarly dynamic. The recent development of live-cell signaling biosensors revealed that many major metazoan signaling pathways undergo complex time-varying responses (e.g. pulses, oscillations, or traveling waves) in response to certain stimuli (78, 79). These states were long invisible to the experimentalist, as population-level measurements at a single time point tend to average together asynchronous responses between individual cells. What is now missing is a “code book” for determining which signaling dynamics trigger each cellular response. Is a pulse of activity interpreted differently than a constant input, and if so, is it the duration, the amplitude, or the area under the curve that matters? This is essentially a question at the level of Marr’s computation: we must define the logic with which signaling pathway activity triggers different responses.
One model signaling pathway has served as a hub for optogenetic studies of signaling dynamics: the Erk mitogen-activated protein kinase (MAPK) cascade downstream of receptor tyrosine kinases (RTKs). It is one of the first-identified examples of signaling dynamics: different durations of Erk signaling have long been proposed to drive distinct cellular responses (80, 81), and live-cell biosensors have revealed complex, pulsatile dynamics in many cellular contexts (82, 83). It has also been a hub for optogenetic control, with light-sensitive tools available to activate the pathway at virtually every node including the pathway’s cell surface receptors, the small G protein Ras, and the kinases Raf and MEK that culminate in Erk phosphorylation and activation (42, 84, 85).
In 2013, Ras was first placed under optogenetic control using the OptoSOS system, where a red illumination drove a PIF-tagged Ras activator (SOScat) to a membrane-localized PhyB anchor (85). The authors were then able to apply dynamic light inputs to define which stimuli were transmitted, and which were filtered out by this intracellular pathway. They found that the pathway faithfully transmitted dynamics from 4 min to at least 2 hours, arguing that any complex dynamic filtering is performed outside of the core Ras-to-Erk module. But if the Erk pathway is a simple low-pass filter, transmitting all inputs across a broad range of timescales, where might dynamic discrimination be carried out? In a follow-up study, Wilson et al. hypothesized that dynamic filtering might be implemented downstream of Erk, by transcription factors and target genes (86). To test this hypothesis, we tagged five “immediate-early” genes with live-cell biosensors of transcription and protein accumulation to compare their induction kinetics with the dynamics of the upstream Erk pathway. This experiment revealed that even long-term, constant Erk stimuli only resulted in a transient pulse of target gene expression, adapting back to baseline within 1 hour. In contrast, short repeated pulses of Erk activation were able to bypass adaptation, resulting in repeated pulses of transcription. Thus, while the Ras/Erk pathway faithfully transmits a broad range of dynamics, its target genes can act as band-pass filters, activated strongly at certain pulse frequencies.
Several optogenetic studies have gone further to directly test whether the frequency of Erk pulses is important for orchestrating cellular responses. A study of non-small cell lung carcinoma cell lines revealed that signal transmission through the Ras/Erk pathway is altered by cancer-associated BRaf mutations and BRaf-activating drugs, extending the time spent active in response to transient light inputs (87) (Figure 2c). Under drug-induced BRaf activation, even brief Ras-level input pulses lead to sustained responses, increasing the expression of Cyclin D and triggering cell proliferation. Most recently, a drug screen for kinase inhibitors that modulate Erk dynamics revealed that increasing Erk pulse frequency also increases cell proliferation in primary epidermal stem cells (88). Based on the observation that pulsatile Erk patterns drive phenotypic changes in both normal and diseased cells, it is likely that a deeper understanding of the gene regulatory networks that “decode” Erk dynamics will provide further valuable insights.
Optogenetics has also driven insights in a second classic model system for stimulus dynamics: dissecting kinetic proofreading downstream of T cell receptors (TCRs). T cells are tasked with sensing foreign antigens presented as peptide-MHC complexes on antigen-presenting cells (pMHCs). Although foreign pMHCs bind with higher affinity to the TCR, they are vastly outnumbered by native peptide-MHC complexes, raising an open question: how do T cells rapidly respond to rare foreign peptides while ignoring a potentially much larger number of receptor interactions with self peptides? One model suggests that T cell activation is determined by the duration of individual pMHC-TCR complexes, rather than overall levels of receptor occupancy, so even a small number of high-affinity binding events could robustly and specifically drive T cell activation. As a direct test of this hypothesis, two separate groups recently engineered optogenetic TCRs whose duration in complex could be directly varied using pulsed or constant-intensity light (89, 90). Their strategies differed: one laboratory used the Phy/PIF system, where the intensity of red light changed the rate of Phy photoconversion between PIF-bound and PIF-unbound states; the other use a light-dissociable dimer between AsLOV2 and an engineered dark-state binder, Zdk (91). Both studies reached consistent conclusions: increasing the ligand binding half-life drove a corresponding increase in downstream signaling, even while controlling for overall receptor occupancy (Figure 2d). It remains to be seen whether similar principles might hold for other classes of cell surface receptors, and to define what steps in the T cell response network sense the duration of ligand binding.
Signaling dynamics are widespread, and optogenetic approaches are gaining traction across an ever-broader range of contexts. Intracellular calcium release also occurs in brief pulses, and Hannanta-anan and Chow used melanopsin for light-gated calcium release to determine dynamics regulate the activity of the calcium-dependent transcription factor NFAT (92). By varying both the frequency and duty cycle of stimulation, they found that NFAT integrates cumulative signaling activity rather than decoding the frequency of calcium pulses, as was previously thought. Optogenetic tools can also be used to completely bypass upstream signaling activity and directly assess the effects of dynamic target gene induction. In a beautiful example of this approach, Imayoshi et al. studied the effects of oscillatory versus sustained gene expression in mouse neural progenitor cells (NPCs) (Figure 2e). Placing the transcription of Ascl1, a regulator of neuronal differentiation, under optogenetic control, their study revealed that oscillatory Ascl1 expression induced proliferation of NPCs, whereas sustaining Ascl1 expression led NPCs to adopt a neuronal phenotype (93).
3.3. Spatially Probing Cellular Signal Processing
Cell signaling unfolds not just in time, but also in space. Organelles compartmentalize signaling, and a polarized cytoskeleton is essential for cell movement and asymmetry (e.g. apical-basal polarity). Cells within a tissue also coordinate their activities by paracrine signaling to drive wound repair, collective cell migration and tissue morphogenesis. From the length scales of molecules to tissues, our ability to study spatial aspects of biological systems requires tools that can be turned on and off with high spatial precision. Optogenetics is ideally suited for such studies, as even straightforward single-photon illumination can be focused with micron-scale precision, enabling the experimentalist to study localized signaling at scales ranging from fine subcellular manipulation to tissue-scale control.
Indeed, cytoskeletal researchers were among the first to enthusiastically adopt optogenetic tools. Cell polarization is controlled in part by the localized activation of Rho GTPases such as Rac1, RhoA, and Cdc42, and PI3K, which together can locally alter the balance of membrane protrusion and actomyosin contractility. Some of the earliest successes of optogenetic control involved engineering light-regulated variants of these proteins. Wu et al. demonstrated that a fusion between Rac1 and AsLOV2, termed PA-Rac, leads to rapid RhoA inhibition at the site of Rac1 activation, providing compelling evidence that RhoA is inhibited in regions where Rac1 is active (94). Furthermore, localized Rac1 stimulation elicited protrusions at the site of stimulation, and retraction of the cellular edge opposite to the site of stimulation, suggesting that localized Rac1 activity is sufficient for front-back cell polarization. In parallel, light-induced dimerization was used to activate Rac, Rho, Cdc42, PI3K, and lipid phosphatases at specific membrane sites, providing a broad palette of tools to paint localized cytoskeletal activity (54, 95, 96). More recently, the light-dissociable interaction between Zdk1 and AsLOV2 was used to create a light-activated cofilin (97) and a light-inactivated microtubule binding protein EB1 (98), demonstrating the sufficiency of each for locally directing cell movement.
Optogenetics has also helped clarify the role of cytoskeletal signaling in multicellular contexts. Cavanaugh and colleagues employed optogenetics to understand how the dynamics of RhoA activation influence the remodeling of junctions in epithelial monolayers (99). By modulating the duration of RhoA stimulation, Cavanaugh and colleagues found that short pulses of RhoA stimulation at cell-cell junctions decreases their length in a reversible manner. However, RhoA pulses longer than 5 min irreversibly shortened junctions to a saturating extent of 80% the original junction length, through a mechanism involving E-cadherin internalization. Mathematical modeling predicted that sequential pulses of RhoA stimulation could shorten junctions beyond the 80% saturation point, which was confirmed experimentally using optogenetics. Similar optogenetic tools for RhoA have been used to understand how this key regulator influences tissue contractility and progression through mitosis (100-102). For instance, while Uroz et al. found that light-induced RhoA activation increased the time cells required for mitotic rounding, relaxing tissues through the optogenetic inhibition of RhoA led to a decrease in the time required for mitotic rounding (102).
What about even larger tissue length scales? Waves of mechanical and biochemical information can travel across hundreds of cell diameters in a tissue to coordinate long-range effects like collective migration or tissue growth (79). One breathtaking example is the recent discovery of propagating waves of Erk activity in vivo. Using a live-cell biosensor of Erk, Hiratsuka et al. observed traveling waves of activity that propagate across mouse skin and emanate from a wound site (103). Cells were also observed to move towards the source of waves, hinting at a possible role for propagating Erk waves in collective cell migration and wound healing (104-106). Optogenetics has been instrumental in causally testing this hypothesis: activating Erk signaling in an epithelial sheet using a traveling wave of light revealed movement toward the wave source (105) and reorientation of front-rear cell polarity (106), although it remains to be seen whether Erk waves are sufficient to orchestrate long-range cell migration. More broadly, light-based control over spatial signaling could be useful for testing these patterns’ sufficiency for producing collective cell behaviors, and for providing a strategy to predictably control tissue-scale responses.
4. RESOLVING AND RECONSTRUCTING MULTICELLULAR SYSTEMS
A biological engineer, reading a developmental biology textbook for the first time, might think of an audacious goal. Can she deliver a signal to just a cell of interest in its native developmental context, telling that cell to move to a particular location, differentiate into a particular cell type, and then carry out a user-defined function? Having such targeted control could be revolutionary for correcting developmental defects, programming novel tissues and organs for biotechnologies, and teaching us about the control of cell identity and behavior. Optogenetic tools enable major steps towards this goal, allowing researchers to deliver signals to specific tissues or even subcellular positions in a live, developing embryo. Here we will summarize some recent advances in optogenetic studies of developmental signaling, organized in two major themes: how upstream signals are interpreted by the embryo to trigger specific cell fate programs, and how cells collectively move, grow, and shrink to form tissues with predictable shapes.
4.1. Optogenetics for Dissecting Lineage-Commitment Programs
The past few years have witnessed an explosion of optogenetic tools employed in the study of developing organisms. Take the Drosophila embryo as an example: studies have now demonstrated at least some degree of optogenetic control over all three major spatial cues in the early embryo – Bicoid, Dorsal and Erk (107-109), as well as other important pathways (e.g. Delta-Notch signaling) (67) and genes (e.g. Zelda) (110). The story is similar in vertebrate embryos, with studies demonstrating embryo-wide control over Ras/Erk, Wnt, and TGF-β/SMAD signaling (51, 111, 112). These tools provide powerful control over when, where, and how a signal is delivered in a developing embryo, thus providing a means of deciphering the highly context-dependent process of cell fate decision-making.
4.1.1. The where….
The application of optogenetics to the study of embryogenesis has gone beyond proof-of-principle, revealing previously unattainable details of signaling interpretation and cell fate control. These tools allow us to determine when during the developmental timeline a particular upstream cue is capable of triggering a cell fate program of interest, thus defining a signal’s temporal window of sensitivity. Sako et al. uncovered such a relationship in the zebrafish embryo using a light-controlled Activin receptor (112). A Nodal gradient is believed to provide positional information within the zebrafish gastrula, coding for the specification of either prechordal plate (ppl) or endoderm cells. By activating the receptor within tight temporal windows, the researchers discovered a duration-dependent phenomenon in which short Nodal signaling induced endoderm formation and longer signaling induced ppl formation.
An inverted approach, relying on light-inducible clustering or degradation to inactivate upstream factors, has proven useful for understanding developmental gene expression in Drosophila. For example, Huang et al. developed a light-inactivatable Bicoid morphogen to study gap gene expression requirements (107). The authors fused Bicoid to Cry2 for light-inducible clustering, which through an as-yet-unclear mechanism turns the fusion protein into a dominant-negative suppressor of Bicoid activity. Varying the illumination window revealed that at anterior positions, where Bicoid levels are naturally highest, brief lapses in signaling were sufficient to compromise gap gene expression. This sensitivity decreased as one moved down the A-P axis, with the mesothorax only requiring Bicoid activity in a narrow window between nuclear cycle 13 and early cycle 14. A similar approach was taken by Viswanathan et al. in their study of mesectoderm specification in the gastrulating Drosophila embryo (67). Cry2-based optogenetic clustering of Delta acutely inhibited Notch cleavage, enabling the researchers to examine the relationship between the temporal window of Notch signaling and the downstream expression of the mesectoderm transcription factor sim. Individual cells exhibited a switch-like mechanism, turning on sim when Delta activity exceeded a certain threshold. The duration of the Delta activation window affected the time at which each cell reached this threshold, however, and thus manifested unique sim activation kinetics at the tissue scale. Finally, a light-degradable Dorsal transcription factor revealed that expression of the target gene snail only requires Dorsal activity prior to nuclear cycle 14, the point at which Dorsal levels normally peak (108). While each of these studies reveals unique insights, together they paint a recurring picture: that target gene expression is decided by more complex functions than simply the instantaneous concentration of an upstream signal. How this complex interpretation is achieved in each case remains an open question.
4.1.2. The when….
The aforementioned opto-Bicoid paper hints at a second strength of optogenetics in developmental biology: the ability to map the spatial location of cells in an embryo to their unique interpretation of cell fate signals. This capability has been employed predominantly through the use of the OptoSOS system, used to modulate Ras/Erk signaling, during Drosophila development. Erk activity is patterned as two outward-to-inward gradients in the fly embryo, with maximal activity at the anterior and posterior poles. In a series of papers, Johnson and colleagues interrogated the consequences of perturbing this pattern, either by increasing the terminal dose of Erk activity, applying it in regions other than the termini, or “erasing and replacing” the endogenous Erk gradient with a fully synthetic, light-based pattern (109, 113, 114). In short, Johnson et al. found that the embryo was remarkably robust to changes in Erk patterning at the termini, with embryos developing normally even when Erk was activated in great excess of endogenous levels. Most surprising, a simple all-or-none pattern of light supplied to OptoSOS embryos deficient for the normal graded Erk patterning was sufficient to generate viable organisms. Indeed, approximately 30% of these “opto-rescued” embryos were able to hatch from the microscope and lead normal lives, including mating and laying eggs whose embryos exhibited the same lethal lack of terminal signaling that, save for the light pattern, would have killed their mothers. In contrast, whereas the embryonic poles are robust to Erk dose well above normal physiological levels, even a small 40 μm strip of illumination in the center of the embryo resulted in almost complete embryonic lethality.
That simple light patterns can suffice is also revealed by a study of non-canonical Wnt signaling during zebrafish morphogenesis (115). Non-canonical Wnt signaling is critical for directed cell migration in developmental processes, e.g., for the coordinated internalization of ppl cells toward the animal pole of the gastrula. By expressing an optogenetically-controlled variant of the non-canonical Wnt receptor Fz7 in MZfz7a/b double mutants, the researchers revealed that uniform photoactivation of the embryo was sufficient to fully rescue the pattern and directionality of ppl movement. This demonstrated a permissive role of non-canonical Wnt signaling in this mesenchymal cell movement, in contrast to the instructive role of the same pathway in polarizing epithelial migration. This combination of robustness and sensitivity underscores the context-dependence with which a single signal may be processed in the embryo.
4.1.3. The how….
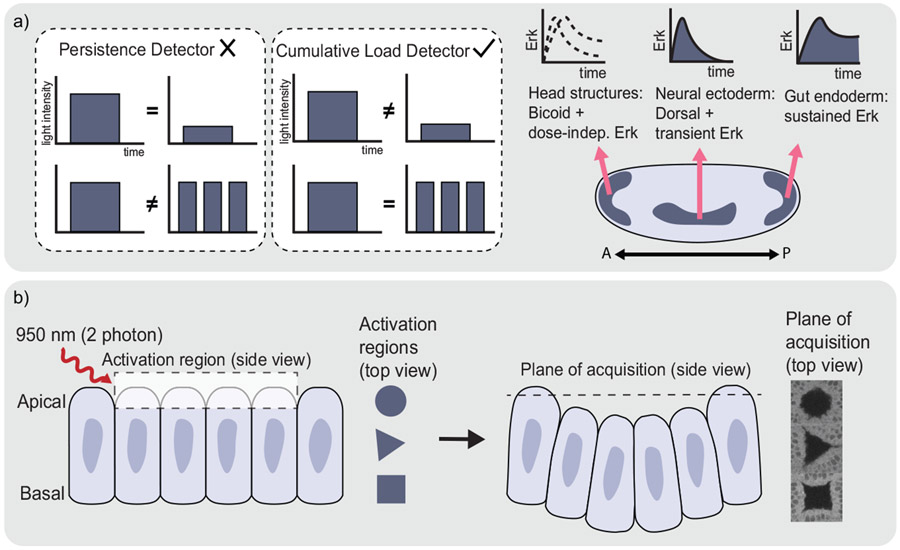
The preceding studies have addressed the when and where of signaling requirements, but what about the how: what is the logic by which pathway activity is interpreted into specific cell fate decisions? Johnson and Toettcher explored this question, using the OptoSOS system to investigate the role of Erk dynamics in Drosophila cell fate decision-making (113). Erk signaling is required to specify two separate cell types – gut endoderm or neural ectoderm – at different positions in the embryo. How this one signal could direct two distinct cellular responses was unknown. The authors found that, as the dose of optogenetic Erk activation increased, cells first induced a neural ectoderm gene expression program before switching to a gut endoderm program. By delivering various light pulse sequences, the authors established that the total integrated dose of Erk activity determined cell fate, in contrast to prior indications that the duration of a single Erk pulse might serve as the discriminating factor (116) (Figure 3a). Although the mechanistic basis for this translation from signal interpretation into target gene expression remains elusive, an exciting study by Keenan et al. provides a first clue. The researchers found that optogenetic Erk activation can trigger unbinding of the transcriptional repressor Capicua (Cic) from DNA with astonishing speed, activating gene expression within minutes, and suggesting that signal processing occurs downstream of this initial transcriptional response (117). We await further studies to close this temporal gap and relate minutes-timescale gene expression to the hours-timescale selection of cell fate.
Figure 3.
Optogenetics has allowed for a deeper understanding of and control over developmental signaling. (a) The iLID-based OptoSOS system was used to unravel an Erk-dependent cell fate specification event in the Drosophila embryo. To distinguish between two proposed signal-decoding mechanisms, pairs of photoactivation regimes were either administered with the same uninterrupted signal duration but different amplitude or vice versa (left side of the panel). By inducing these signals in individual embryos and observing the phenotypic response, the decoding mechanism was found to act based on the cumulative load of Erk administered. This finding led to a more complete picture of how a single morphogen, Erk, can specify multiple cell types in the Drosophila embryo (right side of panel). Figure adapted from (113). (b) Optogenetic localization of RhoGEF2 to the apical membrane of Drosophila cells allows for the precise induction of morphological movements. Here, light-activated apical constriction is sufficient to induce local tissue invagination in a user-defined pattern. Figure adapted from (118).
4.2. Light-Guided Morphogenesis
In tandem with proper specification of cell fate, the growing embryo must undergo a miraculous feat of self-organization to reproducibly generate an organism’s complex final form. The robustness of this morphogenesis, choreographed amongst the inherent noisiness of biological systems, is a testament to the tight regulation of the signals shaping development. To probe this precise regulatory landscape requires an equally precise tool, one that can modulate signaling strength in real time and with subcellular precision. Optogenetics has proven to be of great use in this domain, allowing researchers to examine how factors such as protein localization, physical organization, and local force disruption can influence global morphological movements.
4.2.1. Probing and directing tissue movements.
Cells integrate a complex array of cues to initiate a morphological response. Probing certain cues, for example the downregulation of the molecular motor myosin-II at the basal membrane of invaginating cells, requires tight spatial control over subcellular protein localization. To examine this phenomenon, Krueger et al. developed a CRY2-RhoGEF fusion that can be localized with subcellular precision in a Drosophila embryo (119). Models had suggested that basal membrane relaxation via myosin-II downregulation was necessary for tissue invagination, however studies were limited by the lack of genetic tools to direct this protein localization. Using two-photon excitation to precisely modulate myosin-II levels at multiple stages of ventral furrow formation, the researchers demonstrated that basal relaxation was required both before and after the commencement of tissue bending for proper furrow development. One can also harness these tools to precisely interfere with tissue contractility and disrupt an endogenous morphogenetic program. This capability was first demonstrated by Guglielmi et al. using a photoactivatable inositol polyphosphate 5-phosphatase OCRL (CRY2-OCRL), which depletes PI(4,5)P2 and actin when recruited to the plasma membrane and thus inhibits contractility (120). By directing CRY2-OCRL to the apical membrane on a subpopulation of cells during Drosophila ventral furrow formation, the researchers defined the minimum size of the contractile region required for tissue invagination. Similarly, localizing a dominant-negative Rho1 construct to the plasma membrane is sufficient to block Drosophila cephalic furrow formation, with even a thin strip of light-based inhibition preventing the dorsal and ventral cephalic furrows from aligning with one another (121). This myosin-dependent disruption of furrow linearity suggests that continuous mechanical coupling is required for robust morphogenesis.
Once the optogenetic inputs sufficient to elicit a morphological response are determined, the opportunity arises to use light to sculpt tissue into a user-defined structure. Izquierdo et al. demonstrated this capability, showing that epithelial folding can be optogenetically induced on a Drosophila embryo even where morphological movements are normally absent (118). Their experiments made use of an optogenetic RhoGEF2, recruited to just the apical membrane using two-photon stimulation. The researchers were able to induce local tissue invagination in an arbitrary shape, absent of any endogenous instructions (Figure 3b).
4.2.2. Effects of the mechanical microenvironment.
The dependence of these morphological phenomena on both internal and external cellular organization has also been investigated using optogenetics tools. The effects of internal organization were addressed by Krueger et al. in a study of the link between actomyosin network geometry and tissue contraction (122). The researchers again used their CRY2-RhoGEF fusion, here to activate basal myosin-II during various stages of Drosophila cellularization. Actomyosin organizes into a hexagonal topology during the early stages of cellularization, transitioning to a ring-like topology once the plasma membrane has internalized from the apical surface. Through optogenetic stimulation, Krueger et al. showed that hexagonally-organized actomyosin fibers are insensitive to basal myosin-II upregulation, whereas fibers in a circular geometry are responsive to myosin-II activity and contract upon induction. Conversely, Qin et al. examined the effects of external organization on signal interpretation, in particular how cell-cell and cell-matrix adhesions affect basal myosin-II oscillations in the Drosophila ovary (123). These oscillations are critical for tissue elongation in the egg chamber, however their dependence on a cell’s mechanical interactions were unclear. Using a light-inducible protein trap to sequester tagged adhesion proteins and inactivate them in real-time (124), the researchers were able to validate their genetic findings by showing that tissue elongation is directly controlled by cell-matrix adhesion activity but only weakly influenced by cell-cell adhesions.
5. OPTOGENETICS FOR CELL AND TISSUE ENGINEERING
The precision that makes optogenetics so well-suited for discovering the finer details of developmental signaling is equally desirable for the engineering of biological systems. This engineering may take many forms, including synthetic morphogenesis, where cells are programmed to organize into multicellular structures, or of optogenetic feedback control, where individual cells are monitored and signaling inputs are modulated to attain a given output set-point. Here we transition from “reading” to “writing” the code of the cell, using optogenetics not just to discover the inner workings of biology but to modify the computations and algorithms taking place.
5.1. Synthetic Morphogenesis
All of the tissue-scale studies highlighted thus far have probed signaling phenomena within a developing organism. Converging from the opposite direction, synthetic morphogenesis aims to engineer multicellular complexity using either individual cells or synthetic “protocells” as the basic building blocks (125). Making this vision a reality will require control over multiple levels of interaction, including cell-cell contractility and 3-D pattern formation. In one early example of synthetic tissue control, Sakar et al. designed functional muscle micro-tissues that could undergo light-inducible contraction (126). Equipped with a photoactivatable Channelrhodopsin-2, the stimulated tissues exhibited force and tension parameters comparable to those of spontaneous muscle contractility. Likewise, Staddon et al. probed the mechanosensitive remodeling of human epithelial cells using a photoactivatable RhoA (127). By modulating the frequency of actomyosin contractility, the researchers uncovered a mechanical ratcheting system in which epithelial junctions are most efficiently rearranged under high-amplitude actomyosin activation with long rest periods between successive pulses (see (99) for a mechanistic look at this phenomenon). We can envision the quantitative model that accompanies this result being of exceptional value in the rational shaping of engineered tissues.
One of the most challenging aspects of synthetic morphogenesis will be to design structures with hierarchical complexity and reproducible patterning. This will require us to both harness the natural self-organizing capacity of multicellular systems and to impart arbitrary non-native patterns at will. In an example of spontaneous self-assembly, co-cultures of wild-type and optoWnt-expressing embryonic stem cells were found to segregate into reproducible structures upon global illumination (128). In 3-D spheroids, wild-type cells organized in an epithelium-like manner within the central lumen, while cells with a photoactivated Wnt pathway sorted to the exterior and developed protrusions that interacted with the surrounding matrix. Some studies have taken a more synthetic approach, engineering cells or protocells to express light-inducible dimerization pairs and reversibly form multicellular structures. Chervyachkova et al. functionalized colloids to present two mutually-exclusive optogenetic dimerization pairs: iLID/Nano or nMagHigh/pMagHigh, which could be sorted into two distinct populations upon illumination (129). This approach also extended to live cells, where Mueller et al. demonstrated that the frequency of dimer activation is the main controller of aggregate morphology (130). The structures appeared tightly-packed under high-frequency activation and branched under low-frequency activation, analogous to colloidal aggregation under thermodynamic and kinetic control, respectively. These exciting first steps for light-regulated synthetic morphogenesis will be further enhanced by coupling optogenetic control with other synthetic circuits (131), allowing for the creation of far more sophisticated structures than have been produced to date. This will require some form of in vivo / in silico feedback control, as highlighted in the following section.
5.2. Regulating Cellular Processes Using Light-Based Feedback Control
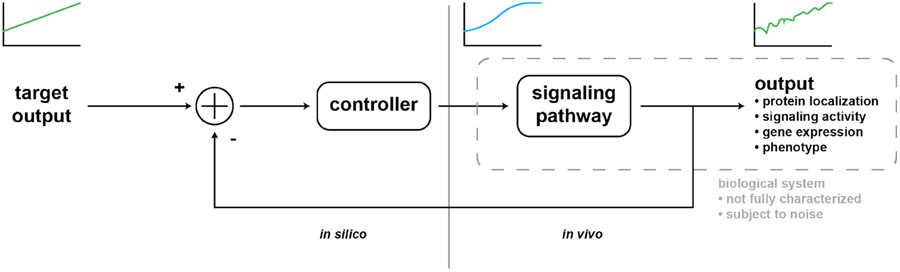
As our understanding of cellular signal processing grows, so will the benefit of controlling signaling processes. While cell engineering is poised to revolutionize medicine and biotechnology, it suffers from challenges in designing stable, predictable synthetic circuits. The picture is also complicated by cell-to-cell variability and intracellular noise. Closed-loop feedback control, where an input is iteratively updated in response to real-time measurements of the cell’s output, could be used to deliver a vast array of dynamic stimuli with high precision while circumventing noise and unwanted cellular regulation (e.g. desensitization to inputs over time) that may distort a cell’s response to the input signal (Figure 4). Here, we highlight some successes in optogenetic feedback control, where measurements of cell state are used to update light inputs for precision control of biological processes.
Figure 4.
In silico feedback control aims to produce a target output by measuring a system’s output, determining the offset from the target output, and updating the input accordingly. When applied to biological systems, dynamically-varying outputs can be achieved despite complex signal processing and inherent noise.
What type of optogenetic feedback controllers are appropriate and efficacious for cellular systems? One early effort utilized proportional-integral (PI) feedback control (95), where a light input is simply updated in response to both the instantaneous mismatch between the experimentally-measured response and a desired value (the proportional term) and the past history of such mismatches (the integral term). This type of feedback control proved useful for driving both constant and time-varying responses and stabilizing PI3K signaling to the same set point, even after perturbation by pathway-altering drugs. In parallel, Milias-Argeitis et al. developed a model predictive controller (MPC) (132) to regulate gene expression at the cell population level, using periodic flow cytometry measurements to characterize current gene expression levels. In a subsequent study, Milias-Argeitis extended their approach to regulate the production of methionine synthase (MetE), an essential enzyme for methionine biosynthesis, enabling the authors to set a user-defined growth rate for bacterial cultures (133).
Light-based feedback control is now being used to dissect complex signaling pathways. Harrigan and colleagues developed an experimental pipeline for replacing endogenous regulatory proteins in the yeast pheromone response pathway with their light-controlled counterparts, and then determined when they were required for regulating pathway output by placing them under in silico feedback control (134). This pipeline, named Closed Loop Optogenetic Compensation (CLOC), was used on three different pheromone response regulators, each of which displayed distinct dynamic requirements to restore signaling to wild-type levels. The dynamic requirements that CLOC yields could, in theory, be compared to the dynamics of regulator expression that occur naturally within a biological system of interest. Moreover, the modular nature of the authors’ Cry2-based gene expression system allows for CLOC to be generalized to a variety of other biological systems.
A computational optogenetic control system need not simply drive a fixed output – it can also be used to implement any desired signaling logic and test for cellular responses. As an example of imparting non-native signaling logic onto multicellular systems, Perkins et al. set out to implement a simple biochemical network resembling Notch-Delta mutual inhibition and connect it into living cells (135). Using a lawn of non-interacting yeast cells expressing an optogenetic transcriptional activator, the researchers computed the light input to each cell based on the current states of all neighboring cells. They found that the in silico cell-cell communication network could switch cells between a uniform level of gene expression and a “checkerboard” pattern of alternating bright and dim cells by altering the parameters of the simulated biochemical pathway. Such experiments, which necessitate the high spatiotemporal control presented by optogenetics, could prove a very useful prototyping step to rapidly evaluate many biochemical networks before physical implementation. It also offers a powerful approach for the direct study of cellular algorithms: because alter-native algorithms can be directly tested in an experimental context, their capacity to elicit biologically-relevant outcomes can be objectively evaluated.
6. CONCLUSION
Cellular optogenetics has already begun to redefine what experiments are possible in biological systems. By systematically applying different light patterns we can now map how upstream signals are decoded into changes in cell state. By applying inputs at different nodes in a pathway we can reveal hidden features of the underlying biochemical network. And by pointing these tools at a developing embryo we can deduce how cells sense their position, choose appropriate fates, and move to produce a living organism. Light-controlled proteins open the door to a closer link between biology and engineering: we may now envision performing real-time feedback control or system identification on any biological system for which optogenetic inputs exist, or even implementing user-defined signaling logic in software while feeding its output back into a living cell.
Nevertheless, there are still many challenges in deploying optogenetics within cell biology, developmental biology, and biological engineering. One pressing challenge is a better suite of tools for multi-color control. Most widely-used optogenetic domains (Cry, LOV and BLUF) require a flavin cofactor, leading to near-complete spectral overlap between these tools and making it virtually impossible to separately trigger Cry2-based clustering and LOV-based nuclear transport in the same cell, for example. Fortunately, phytochromes have proven to be especially spectrally flexible, with the development of orthogonal phytochrome-based tools at various excitation wavelengths presenting one exciting future avenue for multi-color control (31).
A second major unsolved problem, especially for developmental and multicellular applications, is the need for precise 3D optogenetic stimulation. Conventional methods rely on single-photon excitation using a light source placed outside a tissue, at best creating a column of light-activated cells along the entire illumination path. Two-photon excitation is an attractive solution for limiting photoactivation to a single 3D position, with the degree of activation depending nonlinearly on light intensity (136). Some domains, however, are very weakly excited by two-photon illumination (e.g. AsLOV2). Recent approaches that hold promise for precise 3D optogenetic activation include fusing a two-photon-excitable fluorescent protein donor and an optogenetic acceptor so that energy can be transferred between them by Förster resonance energy transfer (FRET) (137), as well as “primed conversion”, a phenomenon in which two photons of differing wavelength must be absorbed sequentially for optogenetic activation of certain photoconvertible fluorescent proteins (138). By delivering each wavelength from a different light source, it should be possible to obtain primed conversion and optogenetic activation only in regions where the two beams intersect.
During its early years, cellular optogenetics was limited primarily to proofs of principle: demonstrating that light could be used to activate a protein of interest or change its localization. The field has matured since then and now resides firmly in a second phase – one in which a researcher can expect the tools to work, and the merit of a study is determined by what they learn about a biological system. This substrate – of interesting biological questions and precise perturbative tools – offers the hope of a third phase, where biologists and engineers can work together to control a biological system for therapy, to garner a deeper understanding of its operation, or simply because they can.
ACKNOWLEDGMENTS
We thank Celeste M. Nelson and all members of the Toettcher laboratory for valuable discussion, insights and comments. This work was supported by NIH grant DP2EB024247 and NSF CAREER Award 1750663 (to J.E.T.), the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-2039656 (P.E.F. and E.J.U.), and the National Institutes of Natural Sciences Japan (E.H.R. and K.A.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- (1).Hartwell LH, Hopfield JJ, Leibler S, and Murray AW (1999). From molecular to modular cell biology. Nature 402, C47–C52. [DOI] [PubMed] [Google Scholar]
- (2).Marr D, Vision: A computational investigation into the human representation and processing of visual information; The MIT Press: Cambridge, MA, 1982. [Google Scholar]
- (3).Berg HC, Random walks in biology; Princeton University Press: 1993. [Google Scholar]
- (4).Yi T-M, Huang Y, Simon MI, and Doyle J (2000). Robust perfect adaptation in bacterial chemotaxis through integral feedback control. Proceedings of the National Academy of Sciences 97, 4649–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Alon U, Surette MG, Barkai N, and Leibler S (1999). Robustness in bacterial chemotaxis. Nature 397, 168–171. [DOI] [PubMed] [Google Scholar]
- (6).Segall JE, Block SM, and Berg HC (1986). Temporal comparisons in bacterial chemotaxis. Proceedings of the National Academy of Sciences 83, 8987–8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Fenno L, Yizhar O, and Deisseroth K (2011). The Development and Application of Optogenetics. Annu. Rev. of Neurosci 34, 389–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kolar K, Knobloch C, Stork H, Zindaric M, and Weber W (2018). OptoBase: A Web Platform for Molecular Optogenetics. ACS Synth. Biol 7, 1825–1828. [DOI] [PubMed] [Google Scholar]
- (9).Tye KM, and Deisseroth K (2012). Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neuroscience 13, 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, Magnuson J, Hegemann P, and Diesseroth K (2011). The Microbial Opsin Family of Optogenetic Tools. Cell 147, 1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Govorunova EG, Sineshchekov OA, Li H, and Spudich JL (2017). Microbial Rhodopsins: Diversity, Mechanisms, and Optogenetic Applications. Annu. Rev. Biochem 86, 845–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Crosson S, Rajagopal S, and Moffat K (2003). The LOV Domain Family: Photoresponsive Signaling Modules Coupled to Diverse Output Domains. Biochemistry 42, 2–10. [DOI] [PubMed] [Google Scholar]
- (13).Harper SM, Neil LC, and Gardner KH (2003). Structural basis of a phototropin light switch. Science 301, 1541–1544. [DOI] [PubMed] [Google Scholar]
- (14).Yao X, Rosen MK, and Gardner KH (2008). Estimation of the available free energy in a LOV2-Jαphotoswitch. Nat. Chem. Biol 4, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Sawa M, Nusinow DA, Kay SA, and Imaizumi T (2007). FKF1 and GIGAN-TEA Complex Formation Is Required for Day-Length Measurement in Arabidopsis. Science 318, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Zoltowski BD, Vaccaro B, and Crane BR (2009). Mechanism-based tuning of a LOV domain photoreceptor. Nat. Chem. Biol 5, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Zoltowski BD, and Crane BR (2008). Light Activation of the LOV Protein Vivid Generates a Rapidly Exchanging Dimer. Biochemistry 47, 7012–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Harper SM, Christie JM, and Gardner KH (2004). Disruption of the LOVJαHelix Interaction Activates Phototropin Kinase Activity. Biochemistry 43, 16184–16192. [DOI] [PubMed] [Google Scholar]
- (19).Glantz ST, Berlew EE, Jaber Z, Schuster BS, Gardner KH, and Chow BY (2018). Directly light-regulated binding of RGS-LOV photoreceptors to anionic membrane phospholipids. PNAS 115, E7720–E7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Nash AI, McNulty R, Shillitob ME, Swartz TE, Bogomolnic RA, Lueckeb H, and Gardner KH (2011). Structural basis of photosensitivity in a bacterial light-oxygen-voltage/helix-turn-helix (LOV-HTH) DNA-binding protein. PNAS 108, 9449–9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Weber AM et al. (2019). A blue light receptor that mediates RNA binding and translational regulation. Nat. Chem. Biol 15, 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Liu H, Yu X, Li K, Klejnot J, Yang H, Lisiero D, and Lin C (2008). Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322, 1535–1539. [DOI] [PubMed] [Google Scholar]
- (23).Zuo Z, Meng Y, Yu X, Zhang Z, Feng D, Sun S, Liu B, and Lin C (2012). A Study of the Blue-Light-Dependent Phosphorylation, Degradation, and Photobody Formation of Arabidopsis CRY2. Mol. Plant 5, 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ma L, Guan Z, Wang Q, Yan X, Wang J, Wang Z, Cao J, Zhang D, Gong X, and Yin P (2020). Structural insights into the photoactivation of Arabidopsis CRY2. Nature Plants, DOI: 10.1038/s41477-020-00800-1. [DOI] [PubMed] [Google Scholar]
- (25).Ma L, Wang X, Guan Z, Wang L, Wang Y, Zheng L, Gong Z, Shen C, Wang J, Zhang D, Liu Z, and Yin P (2020). Structural insights into BIC-mediated inactivation of Arabidopsis cryptochrome 2. Nat. Struct. Mol. Biol 27, 472–479. [DOI] [PubMed] [Google Scholar]
- (26).Liu B, Liu H, Zhong D, and Lin C (2010). Searching for a photocycle of the cryptochrome photoreceptors. Curr. Opin. Plant Biol 13, 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Li P, Wang Q, Yu X, Liu H, Yang H, Zhao C, Liu X, Tan C, Klejnot J, Zhong D, and Lin C (2011). Arabidopsis cryptochrome 2 (CRY2) functions by the photoactivation mechanism distinct from the tryptophan (trp) triad-dependent photoreduction. PNAS 108, 20844–20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Giovani B, Byrdin M, Ahmad M, and Brettel K (2003). Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nat. Struct. Mol. Biol 10, 489–490. [DOI] [PubMed] [Google Scholar]
- (29).Rockwell NC, Martin SS, Feoktistova K, and C. LJ (2011). Diverse two-cysteine photocycles in phytochromes and cyanobacteriochromes. PNAS 108, 11854–11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Fushimi K, and Narikawa R (2019). Cyanobacteriochromes: photoreceptors covering the entire UV-to-visible spectrum. Curr. Opin. Struct. Biol 57, 39–46. [DOI] [PubMed] [Google Scholar]
- (31).Fushimi K, Hasegawa M, Ito T, Rockwell NC, Enomoto G, Win N, Lagarias JC, Ikeuchi M, and Narikawa R (2020). Evolution-inspired design of multicolored photoswitches from a single cyanobacteriochrome scaffold. PNAS 117, 15573–15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, and Wagner D (1995). Phytochromes: Photosensory Perception and Signal Transduction. Science 268, 675–680. [DOI] [PubMed] [Google Scholar]
- (33).Burgie ES, Bussell AN, Walker JM, Dubiel K, and Vierstra RD (2014). Crystal structure of the photosensing module from a red/far-red light-absorbing plant phytochrome. PNAS 111, 10179–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ni M, Tepperman JM, and Quail PH (1999). Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400, 781–784. [DOI] [PubMed] [Google Scholar]
- (35).Kaberniuk AA, Shemetov AA, and Verkhusha VV (2016). A bacterial phytochrome-based optogenetic system controllable with near-infrared light. Nat. Methods 13, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Strauss HM, Schmieder P, and Hughes J (2005). Light-dependent dimerisation in the N-terminal sensory module of cyanobacterial phytochrome 1. FEBS Lett. 579, 3970–3974. [DOI] [PubMed] [Google Scholar]
- (37).Yuan H, and Bauer CE (2008). PixE promotes dark oligomerization of the BLUF photoreceptor PixD. PNAS 105, 11715–11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Cloix C, Kaiserli E, Heilmann M, Baxter KJ, Brown BA, O’Hara A, Smith BO, Christie JM, and Jenkins GI (2012). C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein. PNAS 109, 16366–16370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kainrath S, Stadler M, Reichhart E, Distel M, and Janovjak H (2017). Green-Light-Induced Inactivation of Receptor Signaling Using Cobalamin-Binding Domains. Angew. Chem. Int 56, 4608–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Woolley GA, and Morgan SA (2010). A photoswitchable DNA-binding protein based on a truncated GCN4-photoactive yellow protein chimer. Photochem. Photobiol. Sci 9, 1320–1326. [DOI] [PubMed] [Google Scholar]
- (41).Reis JM, Xu X, McDonald S, Woloschuk RM, Jaikaran ASI, Vizeacoumar FS, Woolley GA, and Uppalapati M (2018). Discovering Selective Binders for Photoswitchable Proteins Using Phage Display. ACS Synth. Biol 7, 2355–2364. [DOI] [PubMed] [Google Scholar]
- (42).Zhou XX, Chung HK, Lam AJ, and Lin MZ (2012). Optical control of protein activity by fluorescent protein domains. Science 338, 810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Zhang W, Lohman AW, Zhuravlova Y, Lu X, Wiens MD, Hoi H, Yaganoglu S, Mohr MA, Kitova EN, Klassen JS, Pantazis P, Thompson RJ, and Campbell RE (2017). Optogenetic control with a photocleavable protein, PhoCl. Nat. Methods 14, 391–394. [DOI] [PubMed] [Google Scholar]
- (44).Niopek D, Benzinger D, Roensch J, Draebing T, Wehler P, Eils R, and Di Ventura B (2014). Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. Nat. Commun 5, DOI: 10.1038/ncomms5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yumerefendi H, Dickinson DJ, Wang H, Zimmerman SP, Bear JE, Goldstein B, Hahn K, and Kuhlman B (2015). Control of Protein Activity and Cell Fate Specification via Light-Mediated Nuclear Translocation. PLoS One 10, e0128443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Niopek D, Wehler P, Roensch J, Eils R, and Di Ventura B (2016). Optogenetic control of nuclear protein export. Nat. Commun 7, DOI: 10.1038/ncomms10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Yumerefendi H, Lerner AM, Zimmerman SP, Hahn K, Bear JE, Strahl BD, and Kuhlman B (2016). Light-induced nuclear export reveals rapid dynamics of epigenetic modifications. Nat. Chem. Biol 12, 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Renicke C, Schuster D, Usherenko S, Essen L, and Taxis C (2013). A LOV2 Domain-Based Optogenetic Tool to Control Protein Degradation and Cellular Function. Chem. Biol 20, 619–626. [DOI] [PubMed] [Google Scholar]
- (49).Guntas G, Hallett RA, Zimmerman SP, Williams T, Yumerefendi H, Bear JE, and Kuhlman B (2015). Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. PNAS 112, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Strickland D, Lin Y, Wagner E, Hope CM, Zayner J, Antoniou C, Sos-nick TR, Weiss EL, and Glotzer M (2012). TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Patel AL, Yeung E, McGuire SE, Wu AY, Toettcher JE, Burdine RD, and Shvartsman SY (2019). Optimizing photoswitchable MEK. Proceedings of the National Academy of Sciences 116, 25756–25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Shimizu-Sato S, Huq E, Tepperman JM, and Quail PH (2002). A light-switchable gene promoter system. Nat. Biotechnol 20, 1041–1044. [DOI] [PubMed] [Google Scholar]
- (53).Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, and Tucker CL (2010). Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods 7, 973–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Levskaya A, Weiner OD, Lim WA, and Voigt CA (2009). Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Kawano F, Suzuki H, Furuya A, and Sato M (2015). Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat. Commun 6, 6256. [DOI] [PubMed] [Google Scholar]
- (56).Duan L, Hope J, Ong Q, Lou HY, Kim N, McCarthy C, Acero V, Lin MZ, and Cui B (2017). Understanding CRY2 interactions for optical control of intracellular signaling. Nat. Commun 8, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Uda Y, Miura H, Goto Y, and Aoki K (2020). Improvement of phycocyanobilin synthesis for genetically encoded phytochrome-based optogenetics. bioRxiv, 908343. [DOI] [PubMed] [Google Scholar]
- (58).Kawano F, Okazaki R, Yazawa M, and Sato M (2016). A photoactivatable Cre–loxP recombination system for optogenetic genome engineering. Nat. Chem. Biol 12, 1059–1064. [DOI] [PubMed] [Google Scholar]
- (59).Meador K, Wysoczynski CL, Norris AJ, Aoto J, Bruchas MR, and Tucker CL (2019). Achieving tight control of a photoactivatable Cre recombinase gene switch: new design strategies and functional characterization in mammalian cells and rodent. Nucleic Acids Research 47, e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, and Schaffer DV (2013). Optogenetic protein clustering and signaling activation in mammalian cells. Nat. Methods 10, 249–252. [DOI] [PubMed] [Google Scholar]
- (61).Lee S, Park H, Kyung T, Kim NY, Kim S, Kim J, and Heo WD (2014). Reversible protein inactivation by optogenetic trapping in cells. Nat. Methods 11, 633–636. [DOI] [PubMed] [Google Scholar]
- (62).Taslimi A, Vrana JD, Chen D, Borinskaya S, Mayer BJ, Kennedy MJ, and Tucker CL (2014). An optimized optogenetic clustering tool for probing protein interaction and function. Nat. Commun 5, 4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Park H, Kim NY, Lee S, Kim N, Kim J, and Heo WD (2017). Optogenetic protein clustering through fluorescent protein tagging and extension of CRY2. Nat. Commun 8, DOI: 10.1038/s41467-017-00060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Masuda S, Nakatani Y, Ren S, and Tanaka M (2013). Blue light-mediated manipulation of transcription factor activity in vivo. ACS Chem. Biol 8, 2649–2653. [DOI] [PubMed] [Google Scholar]
- (65).Nakamura H. et al. (2018). Intracellular production of hydrogels and synthetic RNA granules by multivalent molecular interactions. Nat. Mater 17, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Woo D, Seo Y, Jung H, Kim S, Kim N, Park S-M, Lee H, Lee S, Cho K-H, and Do Heo W (2019). Locally activating TrkB receptor generates actin waves and specifies axonal fate. Cell Chem. Biol 26, 1652–1663. [DOI] [PubMed] [Google Scholar]
- (67).Viswanathan R, Necakov A, Trylinski M, Harish RK, Krueger D, Esposito E, Schweisguth F, Neveu P, and De Renzis S (2019). Optogenetic inhibition of Delta reveals digital Notch signalling output during tissue differentiation. EMBO Reports 20, e47999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Shin Y, Berry J, Pannucci N, Haataja MP, Toettcher JE, and Brangwynne CP (2017). Spatiotemporal Control of Intracellular Phase Transitions Using Light-Activated optoDroplets. Cell 168, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Dine E, Gil AA, Uribe G, Brangwynne CP, and Toettcher JE (2018). Protein Phase Separation Provides Long-Term Memory of Transient Spatial Stimuli. Cell Syst. 6, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Bracha D, Walls MT, Wei MT, Zhu L, Kurian M, Avalos JL, Toettcher JE, and Brangwynne CP (2018). Mapping Local and Global Liquid Phase Behavior in Living Cells Using Photo-Oligomerizable Seeds. Cell 175, 1467–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Shin Y, Chang YC, Lee DSW, Berry J, Sanders DW, Ronceray P, Wingreen NS, Haataja M, and Brangwynne CP (2018). Liquid Nuclear Condensates Mechanically Sense and Restructure the Genome. Cell 175, 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Reed EH, Schuster BS, Good MC, and Hammer DA (2020). SPLIT: Stable Protein Coacervation Using a Light Induced Transition. ACS Synth. Biol 9, 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Dagliyan O, Tarnawski M, Chu P, Shirvanyants D, Schlichting I, Dokholyan NV, and Hahn KM (2016). Engineering extrinsic disorder to control protein activity in living cells. Science 354, 1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Gil AA, Carrasco-López C, Zhu L, Zhao EM, Ravindran PT, Wilson MZ, Goglia AG, Avalos JL, and Toettcher JE (2020). Optogenetic control of protein binding using light-switchable nanobodies. Nat. Commun 11, DOI: 10.1038/s41467-020-17836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Carrasco-López C, Zhao EM, Gil AA, Alam N, Toettcher JE, and Avalos JL (2020). Development of light-responsive protein binding in the monobody non-immunoglobulin scaffold. Nat. Commun 11, DOI: 10.1038/s41467-020-17837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Graziano BR, Gong D, Anderson KE, Pipathsouk A, Goldberg AR, and Weiner OD (2017). A module for Rac temporal signal integration revealed with optogenetics. Journal of Cell Biology 216, 2515–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).DeFelice MM, Clark HR, Hughey JJ, Maayan I, Kudo T, Gutschow MV, Covert MW, and Regot S (2019). NF-κB signaling dynamics is controlled by a dose-sensing autoregulatory loop. Sci. Signal 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Purvis JE, and Lahav G (2013). Encoding and decoding cellular information through signaling dynamics. Cell 152, 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Deneke VE, and Di Talia S (2018). Chemical waves in cell and developmental biology. Journal of Cell Biology 217, 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Marshall C. (1995). Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185. [DOI] [PubMed] [Google Scholar]
- (81).Santos SD, Verveer PJ, and Bastiaens PI (2007). Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nature cell biology 9, 324–330. [DOI] [PubMed] [Google Scholar]
- (82).Albeck JG, Mills GB, and Brugge JS (2013). Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Molecular cell 49, 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Aoki K, Kumagai Y, Sakurai A, Komatsu N, Fujita Y, Shionyu C, and Matsuda M (2013). Stochastic ERK activation induced by noise and cell-to-cell propagation regulates cell density-dependent proliferation. Molecular cell 52, 529–540. [DOI] [PubMed] [Google Scholar]
- (84).Kim N, Kim JM, Lee M, Kim CY, Chang K-Y, and Do Heo W (2014). Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chem. Biol 21, 903–912. [DOI] [PubMed] [Google Scholar]
- (85).Toettcher JE, Weiner OD, and Lim WA (2013). Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Wilson MZ, Ravindran PT, Lim WA, and Toettcher JE (2017). Tracing information flow from Erk to target gene induction reveals mechanisms of dynamic and combinatorial control. Mol. Cell 67, 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Bugaj L, Sabnis A, Mitchell A, Garbarino J, Toettcher JE, Bivona T, and Lim W (2018). Cancer mutations and targeted drugs can disrupt dynamic signal encoding by the Ras-Erk pathway. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Goglia AG, Wilson MZ, Jena SG, Silbert J, Basta LP, Devenport D, and Toettcher JE (2020). A live-cell screen for altered Erk dynamics reveals principles of proliferative control. Cell Syst. 10, 240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Tischer DK, and Weiner OD (2019). Light-based tuning of ligand half-life supports kinetic proofreading model of T cell signaling. Elife 8, e42498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Yousefi OS, Günther M, Hörner M, Chalupsky J, Wess M, Brandl SM, Smith RW, Fleck C, Kunkel T, Zurbriggen MD, et al. (2019). Optogenetic control shows that kinetic proofreading regulates the activity of the T cell receptor. Elife 8, e42475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Wang H, Vilela M, Winkler A, Tarnawski M, Schlichting I, Yumerefendi H, Kuhlman B, Liu R, Danuser G, and Hahn KM (2016). LOVTRAP: an optogenetic system for photoinduced protein dissociation. Nat. Methods 13, 755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Hannanta-anan P, and Chow BY (2016). Optogenetic control of calcium oscillation waveform defines NFAT as an integrator of calcium load. Cell systems 2, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Imayoshi I, Isomura A, Harima Y, Kawaguchi K, Kori H, Miyachi H, Fujiwara T, Ishidate F, and Kageyama R (2013). Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science 342, 1203–1208. [DOI] [PubMed] [Google Scholar]
- (94).Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, and Hahn KM (2009). A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Toettcher JE, Gong D, Lim WA, and Weiner OD (2011). Light-based feedback for controlling intracellular signaling dynamics. Nat. Methods 8, 837–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Idevall-Hagren O, Dickson EJ, Hille B, Toomre DK, and De Camilli P (2012). Optogenetic control of phosphoinositide metabolism. Proceedings of the National Academy of Sciences 109, E2316–E2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Stone OJ, Pankow N, Liu B, Sharma VP, Eddy RJ, Wang H, Putz AT, Teets FD, Kuhlman B, Condeelis JS, et al. (2019). Optogenetic control of cofilin and αTAT in living cells using Z-lock. Nat. Chem. Biol 15, 1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).van Haren J, Charafeddine RA, Ettinger A, Wang H, Hahn KM, and Wittmann T (2018). Local control of intracellular microtubule dynamics by EB1 photodissociation. Nat. Cell Biol 20, 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Cavanaugh KE, Staddon MF, Munro E, Banerjee S, and Gardel ML (2020). RhoA mediates epithelial cell shape changes via mechanosensitive endocytosis. Dev. Cell 52, 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Oakes PW, Wagner E, Brand CA, Probst D, Linke M, Schwarz US, Glotzer M, and Gardel ML (2017). Optogenetic control of RhoA reveals zyxin-mediated elasticity of stress fibres. Nature communications 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Valon L, Marin-Llauradó A, Wyatt T, Charras G, and Trepat X (2017). Optogenetic control of cellular forces and mechanotransduction. Nat. Commun 8, 14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Uroz M, Wistorf S, Serra-Picamal X, Conte V, Sales-Pardo M, Roca-Cusachs P, Guimerà R, and Trepat X (2018). Regulation of cell cycle progression by cell–cell and cell–matrix forces. Nat. Cell Biol 20, 646–654. [DOI] [PubMed] [Google Scholar]
- (103).Hiratsuka T, Fujita Y, Naoki H, Aoki K, Kamioka Y, and Matsuda M (2015). Intercellular propagation of extracellular signal-regulated kinase activation revealed by in vivo imaging of mouse skin. Elife 4, e05178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Nikolic DL, Boettiger AN, Bar-Sagi D, Carbeck JD, and Shvartsman SY (2006). Role of boundary conditions in an experimental model of epithelial wound healing. Am. J. Physiol. Cell Physiol 291, C68–C75. [DOI] [PubMed] [Google Scholar]
- (105).Aoki K, Kondo Y, Naoki H, Hiratsuka T, Itoh RE, and Matsuda M (2017). Propagating wave of ERK activation orients collective cell migration. Dev. Cell 43, 305–317. [DOI] [PubMed] [Google Scholar]
- (106).Hino N, Rossetti L, Marin-Llauradó A, Aoki K, Trepat X, Matsuda M, and Hirashima T (2020). ERK-mediated mechanochemical waves direct collective cell polarization. Dev. Cell 53, 646–660. [DOI] [PubMed] [Google Scholar]
- (107).Huang A, Amourda C, Zhang S, Tolwinski NS, and Saunders TE (2017). Decoding temporal interpretation of the morphogen Bicoid in the early Drosophila embryo. Elife 6, e26258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Irizarry J, McGehee J, Kim G, Stein D, and Stathopoulos A (2020). Twist-dependent ratchet functioning downstream from Dorsal revealed using a light-inducible degron. Genes & Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Johnson HE, Goyal Y, Pannucci NL, Schüpbach T, Shvartsman SY, and Toettcher JE (2017). The spatiotemporal limits of developmental Erk signaling. Dev. Cell 40, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).McDaniel SL, Gibson TJ, Schulz KN, Garcia MF, Nevil M, Jain SU, Lewis PW, Zaret KS, and Harrison MM (2019). Continued activity of the pioneer factor Zelda is required to drive zygotic genome activation. Molecular cell 74, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Kaur P, Saunders TE, and Tolwinski NS (2017). Coupling optogenetics and light-sheet microscopy, a method to study Wnt signaling during embryogenesis. Scientific Reports 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Sako K, Pradhan SJ, Barone V, Inglés-Prieto Á, Müller P, Ruprecht V, Čapek D, Galande S, Janovjak H, and Heisenberg C-P (2016). Optogenetic control of nodal signaling reveals a temporal pattern of nodal signaling regulating cell fate specification during gastrulation. Cell Reports 16, 866–877. [DOI] [PubMed] [Google Scholar]
- (113).Johnson HE, and Toettcher JE (2019). Signaling dynamics control cell fate in the early Drosophila embryo. Dev. Cell 48, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Johnson HE, Djabrayan NJ, Shvartsman SY, and Toettcher JE (2020). Optogenetic Rescue of a Patterning Mutant. Current Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Čapek D, Smutny M, Tichy A-M, Morri M, Janovjak H, and Heisenberg C-P (2019). Light-activated Frizzled7 reveals a permissive role of non-canonical wnt signaling in mesendoderm cell migration. Elife 8, e42093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Murphy LO, Smith S, Chen R-H, Fingar DC, and Blenis J (2002). Molecular interpretation of ERK signal duration by immediate early gene products. Nature cell biology 4, 556–564. [DOI] [PubMed] [Google Scholar]
- (117).Keenan SE, Blythe SA, Marmion RA, Djabrayan NJ-V, Wieschaus EF, and Shvartsman SY (2020). Rapid Dynamics of Signal-Dependent Transcriptional Repression by Capicua. Dev. Cell 52, 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Izquierdo E, Quinkler T, and De Renzis S (2018). Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nat. Commun 9, 2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Krueger D, Tardivo P, Nguyen C, and De Renzis S (2018). Downregulation of basal myosin-II is required for cell shape changes and tissue invagination. EMBO J. 37, e100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Guglielmi G, Barry JD, Huber W, and De Renzis S (2015). An optogenetic method to modulate cell contractility during tissue morphogenesis. Dev. Cell 35, 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Eritano AS, Bromley CL, Albero AB, Schütz L, Wen F-L, Takeda M, Fukaya T, Sami MM, Shibata T, Lemke S, et al. (2020). Tissue-scale mechanical coupling reduces morphogenetic noise to ensure precision during epithelial folding. Dev. Cell 53, 212–228. [DOI] [PubMed] [Google Scholar]
- (122).Krueger D, Quinkler T, Mortensen SA, Sachse C, and De Renzis S (2019). Cross-linker–mediated regulation of actin network organization controls tissue morphogenesis. J. Cell Biol 218, 2743–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Qin X, Park BO, Liu J, Chen B, Choesmel-Cadamuro V, Belguise K, Do Heo W, and Wang X (2017). Cell-matrix adhesion and cell-cell adhesion differentially control basal myosin oscillation and Drosophila egg chamber elongation. Nat. Commun 8, 14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Lee S, Park H, Kyung T, Kim NY, Kim S, Kim J, and Do Heo W (2014). Reversible protein inactivation by optogenetic trapping in cells. Nat. Methods 11, 633–636. [DOI] [PubMed] [Google Scholar]
- (125).Teague BP, Guye P, and Weiss R (2016). Synthetic morphogenesis. Cold Spring Harb. Perspect. Biol 8, a023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Sakar MS, Neal D, Boudou T, Borochin MA, Li Y, Weiss R, Kamm RD, Chen CS, and Asada HH (2012). Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab Chip 12, 4976–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Staddon MF, Cavanaugh KE, Munro EM, Gardel ML, and Banerjee S (2019). Mechanosensitive Junction Remodeling Promotes Robust Epithelial Morphogenesis. Biophys. J 117, 1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Repina NA, Bao X, Zimmermann JA, Joy DA, Kane RS, and Schaffer DV (2019). Optogenetic control of Wnt signaling for modeling early embryogenic patterning with human pluripotent stem cells. bioRxiv, 665695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Chervyachkova E, and Wegner SV (2018). Reversible Social Self-Sorting of Colloidal Cell-Mimics with Blue Light Switchable Proteins. ACS Synth. Biol 7, 1817–1824. [DOI] [PubMed] [Google Scholar]
- (130).Mueller M, Rasoulinejad S, Garg S, and Wegner SV (2019). The Importance of Cell–Cell Interaction Dynamics in Bottom-Up Tissue Engineering: Concepts of Colloidal Self-Assembly in the Fabrication of Multicellular Architectures. Nano Lett. 20, 2257–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Toda S, Blauch LR, Tang SK, Morsut L, and Lim WA (2018). Programming self-organizing multicellular structures with synthetic cell-cell signaling. Science 361, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Milias-Argeitis A, Summers S, Stewart-Ornstein J, Zuleta I, Pincus D, El-Samad H, Khammash M, and Lygeros J (2011). In silico feedback for in vivo regulation of a gene expression circuit. Nat. Biotechnol 29, 1114–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Milias-Argeitis A, Rullan M, Aoki SK, Buchmann P, and Khammash M (2016). Automated optogenetic feedback control for precise and robust regulation of gene expression and cell growth. Nat. Commun 7, 12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Harrigan P, Madhani HD, and El-Samad H (2018). Real-time genetic compensation defines the dynamic demands of feedback control. Cell 175, 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Perkins ML, Benzinger D, Arcak M, and Khammash M (2020). Cell-in-the-loop pattern formation with optogenetically emulated cell-to-cell signaling. Nat. Commun 11, 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Benninger RK, and Piston DW (2013). Two-photon excitation microscopy for the study of living cells and tissues. Curr. Protoc. Cell Biol 59, 4.11.1–4.11.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Kinjo T, Terai K, Horita S, Nomura N, Sumiyama K, Togashi K, Iwata S, and Matsuda M (2019). FRET-assisted photoactivation of flavoproteins for in vivo two-photon optogenetics. Nat. Methods 16, 1029–1036. [DOI] [PubMed] [Google Scholar]
- (138).Dempsey WP, Georgieva L, Helbling PM, Sonay AY, Truong TV, Haffner M, and Pantazis P (2015). In vivo single-cell labeling by confined primed conversion. Nat. Methods 12, 645–648. [DOI] [PubMed] [Google Scholar]