Abstract
Chronic pain affects more than 50 million Americans. Treatments remain inadequate, in large part, because the pathophysiological mechanisms underlying the development of chronic pain remain poorly understood. Pain biomarkers could potentially identify and measure biological pathways and phenotypical expressions that are altered by pain, provide insight into biological treatment targets, and help identify at-risk patients who might benefit from early intervention. Biomarkers are used to diagnose, track, and treat other diseases, but no validated clinical biomarkers exist yet for chronic pain. To address this problem, the National Institutes of Health Common Fund launched the Acute to Chronic Pain Signatures (A2CPS) program to evaluate candidate biomarkers, develop them into biosignatures, and discover novel biomarkers for chronification of pain after surgery. This article discusses candidate biomarkers identified by A2CPS for evaluation, including genomic, proteomic, metabolomic, lipidomic, neuroimaging, psychophysical, psychological, and behavioral measures. Acute to Chronic Pain Signatures will provide the most comprehensive investigation of biomarkers for the transition to chronic postsurgical pain undertaken to date. Data and analytic resources generatedby A2CPS will be shared with the scientific community in hopes that other investigators will extract valuable insights beyond A2CPS's initial findings. This article will review the identified biomarkers and rationale for including them, the current state of the science on biomarkers of the transition from acute to chronic pain, gaps in the literature, and how A2CPS will address these gaps.
Keywords: Pain, Chronic pain, Postsurgical pain, Biomarker, Biosignatures, Omics, Brain imaging, Psychosocial
1. Introduction
The worldwide epidemic of chronic pain has reached crisis proportions. In the United States, more than 50 million Americans are affected by chronic pain,37 and an estimated 10 to 20 million Americans experience high-impact chronic pain or chronic pain with major activity restrictions.20 Chronic pain is now the leading cause of disability in the United States, with an economic burden exceeding $560 billion in annual healthcare costs and reduced productivity.37 In addition, chronic pain is associated with a cascade of other adverse effects that impact health and quality of life, families, and society. Current pharmacological and nonpharmacological treatments have limited effectiveness in reducing chronic pain, with almost three-quarters of individuals with chronic pain reporting inadequate pain control.28,33,110 The growing incidence of chronic pain is also associated with increased exposure to prescription opioids and development of opioid use disorder and overdose in some individuals.105
In 2010, the Affordable Care Act mandated the US Department of Health and Human Services and the Institute of Medicine (IOM) to investigate pain as a public health problem. Their 2011 report,37 titled Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research, identified a health crisis that was previously widely underappreciated by policymakers, healthcare professionals, and the public. However, the intervening 11 years have seen insufficient progress in pain prevention and treatment. In the wake of the IOM report, experts convened to map out a National Pain Strategy19 and Federal Pain Research Strategy (FPRS).20 A major conclusion of the FPRS was that the transition from acute to chronic pain demands immediate and intensive research. In 2018, the National Institutes of Health (NIH) announced the Helping to End Addiction Long-Term (HEAL) Initiative18 to combat the opioid crisis through pain and addiction research. To inform HEAL, the NIH led a workshop titled Discovery and Validation of Biomarkers to Develop Non-Addictive Therapeutics for Pain,74 grounded in the realization that identification of biomarkers and biosignatures for chronic pain will provide a crucial step toward understanding mechanisms underlying the transition to chronic pain and thereby improving prevention and treatment. To address this critical problem, the NIH Common Fund within the Office of the Director, which supports high-risk, innovative research with the potential for extraordinary impact, launched the Acute to Chronic Pain Signatures Program (A2CPS) program.
A comprehensive understanding of the transition to chronic pain will require biomarkers and signatures gleaned from patients with many different forms of chronic pain. One of the most promising areas, however, is chronic postsurgical pain (CPSP). Studying patients before and after the controlled injury of surgery provides a natural way to examine predictors of pain chronicity and examine biological and psychological correlates of pain and resilience. This is the focus of the A2CPS program. Although many people recover within weeks of surgery, 10% to 70% of individuals develop chronic pain; estimates vary widely based on the type of surgery.9,77 As with other forms of chronic pain, mechanisms underlying the transition to chronic pain after surgery remain understudied, and risk factors were insufficiently characterized. The inability to identify the biopsychosocial mechanisms underlying the development of chronic pain poses a major barrier to developing effective new therapies. Acute to Chronic Pain Signatures applies a team science, consortium-based approach to validate and discover biomarkers and biosignatures for the transition to chronic pain using cutting-edge techniques across multiple domains. The goals of the consortium are to validate existing biomarkers (previously identified in small-scale human studies), develop biosignatures, and discover novel biomarkers and biosignatures that identify risk or resilience for the development of chronic pain. This article will review the existing literature for evidence of biomarkers of the transition from acute to chronic pain and highlight how the A2CPS study will enable us to define biomarkers and biosignatures for the transition from acute to chronic pain.
2. The need for biomarkers and biosignatures
Although pain is a symptom of many diseases, chronic pain is a disease in its own right with distinct pathophysiological processes.37 Emerging evidence implicates structural and functional changes in the brain and spinal cord, peripheral nervous system, and immune system.4,89,112 However, objective readouts of pathophysiology, called biomarkers, have not been identified with sufficient precision and reliability to be useful in clinical settings. The use of biomarkers has revolutionized medicine and become standard practice to assist in diagnosis, treatment, and monitoring of many diseases. For example, echocardiograms and cardiac biochemical markers are routinely used to diagnose heart disease, and imaging is routinely used to diagnose stroke. Biomarkers can also identify disease subtypes to define diagnostic categories and treatments based on pathophysiology. The field of oncology is leading the way toward precision medicine; for example, cancer biomarkers, ie, tumor-associated antigens, have improved disease detection and diagnosis as well as development and use of antibody-based therapies.85 By contrast, diagnosis and treatment of pain relies principally on self-reported symptoms because biological tests for pathophysiological mechanisms that support individualized treatments are currently not available. Importantly, pain biomarkers are not intended to serve as a replacement for self-reported pain but rather to identify biological processes linked to pain. These processes can point to underlying causes of pain, enhance diagnosis and treatment, and spur the development and implementation of novel therapeutics.22
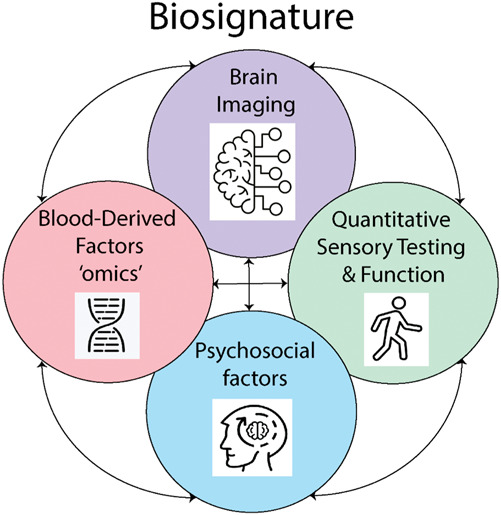
The Food and Drug Administration's Biomarkers, Endpoints and other Tools (BEST) resource34 defines multiple types of biomarkers (Table 1), including (1) susceptibility/risk biomarkers, indicating a predisposition for developing future disease; (2) prognostic biomarkers, indicating risk for disease recurrence or progression; (3) predictive biomarkers, which indicate whether a person is likely to respond to a particular treatment; and (4) monitoring biomarkers, indicating status of the disease. A single measure can serve in multiple biomarker roles. For example, a biomarker may be developed as a prognostic biomarker, but it may identify an important mechanistic cause of pain and later be validated as a predictive or monitoring biomarker. Acute to Chronic Pain Signatures focuses on susceptibility/risk and prognostic biomarkers, which can help identify mechanistic targets for the development of future treatments and identify individuals most in need of preventive care or additional treatments. Acute to Chronic Pain Signatures also takes an expanded view of the term “biomarker” and includes psychosocial measures (eg, depression, fatigue, anxiety) as well, which serve as indirect indicators of complex biological processes. Such indicators may have important prognostic value and can be assessed in a cost-effective manner. As pain is often driven jointly by multiple processes acting in concert, biosignatures composed of multiple biomarkers are particularly promising (Fig. 1). Although multiple potential biomarkers have been discovered, as reviewed below, each individual with a given disease may have a unique biosignature. Pain-related biomarkers and biosignatures could, therefore, inform mechanistic hypotheses of pain pathophysiology, potential therapeutic targets, and a means for monitoring disease progression and treatment.
Table 1.
Types of biomarkers as outlines in by Food and Drug Administration task force.
| Biomarker type | Definition | Example |
|---|---|---|
| Susceptibility/risk | Potential for developing a disease or medical condition | APOE for Alzheimer disease |
| Diagnostic | Detect or confirm disease/condition | Rheumatoid factor for autoimmune disease |
| Monitoring | Measured repeatedly for assessing state of the disease | Prostate-specific antigen (PSA), prostate cancer |
| Predictive | Likelihood of experiencing a favorable or unfavorable response to treatment | BRCA1/2 for response to ADP-ribose-PARP inhibitors |
| Response | Show a biological response has occurred when exposed to a treatment | C-reactive protein (CRP) for effective inflammatory disease treatment |
| Safety | Prescence of toxicity as an adverse event | Creatinine for muscle damage |
APOE, apolipoprotein E; BRCA, BReast CAncer Gene; ADP, adenosine diphosphate; PARP, Poly-ADP ribose polymerase.
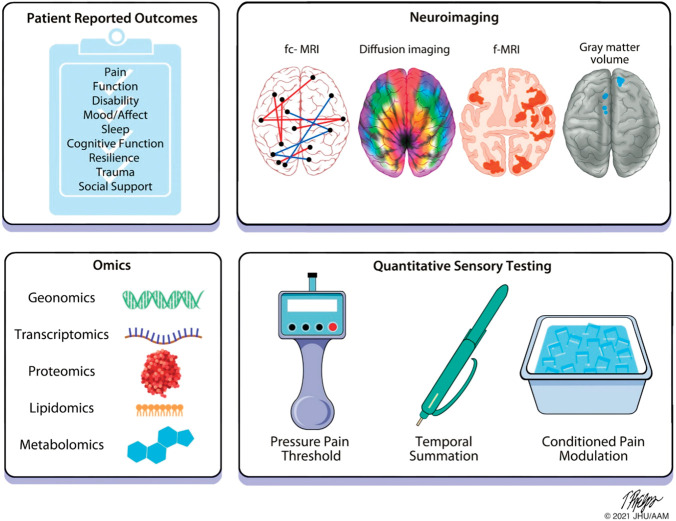
Figure 1.
Schematic diagram showing different types of biomarkers combined to form a biosignature. Single biomarkers may be measured across multiple domains, including brain imaging, quantitative sensory testing and function, blood-derived factors, and psychosocial measures. Combining biomarkers from within the same category and from multiple categories would produce a biosignature.
By design, A2CPS will include biomarkers that might differ at baseline (pre-surgery) between those who develop chronic postsurgical pain and those who do not and biomarkers that might suggest someone with chronic pain will fare better or worse. Acute to Chronic Pain Signatures will consider a wide array of types of markers—multiple omics platforms, brain imaging, psychophysical measures, and patient-reported outcomes (PROs). Acute to Chronic Pain Signatures focus is not on developing clinical tools but on identifying biosignatures that can inform risk for the development of chronic pain.
3. Pain biomarkers associated with transition from acute to chronic pain
Biopsychosocial factors are widely understood to contribute to the development of and recovery from chronic pain. As such, biomarkers (or biosignatures) of pain need to include biological readouts and those related to psychosocial and behavioral factors. Individuals may have preexisting factors that increase or decrease risk of transitioning to chronic pain after injury, including surgery. The injury itself sets up a cascade of events that may interact with preexisting factors to further promote transition to chronic pain. A large body of literature has reported neuroimaging signals,4 quantitative sensory testing (QST),73 and multiple PROs31 that predict the transition to chronic pain and indicate increased or decreased risk, including pain intensity, anxiety, depression, and resilience. Although these studies identified key constructs primarily related to pain intensity, psychosocial factors, and brain signatures, most studies use relatively small sample sizes and consider a limited number of potential predictors.
A combination of disability with psychological factors, pain, and trauma has been proposed as a key factor in the transition to chronic pain,117 suggesting that combinations of factors will be critical to fully understanding the transition from acute to chronic pain. By contrast, few studies have examined biological samples in humans, with most of those focusing on genetic predictors and cytokines. Preclinical studies have identified other potential biological mechanistic targets across the immune, endocrine, and nervous systems that contribute to the transition to chronic pain, but these have yet to be explored in humans.
Markers that predict chronic pain may differ from predictors of disability and function associated with chronic pain. As an example, predictors of chronic pain after spinal surgery include age, education, pain, comorbidities, pain catastrophizing, anxiety and depression, whereas predictors of disability include employment and neuroticism but not socioeconomic factors, comorbidities, pain, or psychological factors.36 Similarly, exposure to traumatic life events and depression predict chronic pain after acute spinal pain, whereas depression and negative pain beliefs predict disability.117
Predictors of pain and disability can be classified as modifiable and nonmodifiable. Certainly, nonmodifiable factors such as age and sex contribute to the transition from acute to chronic pain in important ways. This review, however, will focus primarily on modifiable predictors of chronic pain from domains across the biopsychosocial spectrum. Although this review focuses on predictors of pain, it should be kept in mind that disability and function are key factors in recovery after surgery or injury and thus may need to be considered as part of the outcome for each individual.
3.1. Pain
Pain is among the strongest predictors of the transition from acute to chronic pain. This includes a variety of pain measures. In multiple populations, pain itself either before surgery or in the acute postoperative period predicts the development of chronic pain after surgery with strong effect sizes, including having pain at rest and with movement,6,16 widespread pain or multiple regions of pain, and acute pain trajectories.2,14,17,23,31,45,51,63,87,102,108,114 Interestingly, in people with knee arthroplasty (KA), those with severe pain with movement before surgery showed 10-fold greater likelihood of moderate to severe pain 6 months after surgery.63 Several systematic reviews support preoperative pain, postoperative pain, and widespread pain as predictors of chronic postsurgical pain after a variety of surgeries.51,67,107
3.2. Physical function and disability
Disability and function can contribute to poor outcomes and predict development of chronic pain and disability after surgery or acute injury.17,26,35,51,100,117 In the transition to chronic pain in a large acute back pain population, severe disability predicted chronic pain at 6 months (odds ratio, 1.82).92
3.3. Patient-reported outcomes
Psychological factors have become increasingly recognized as important predictors of the transition from acute to chronic pain with particularly strong evidence for anxiety, depression, pain catastrophizing, and fear of movement as predictive factors.2,11,12,23,27,28,40,51,52,63,76,86,95,99,111,114 Poor social support and less-solicitous response of others are associated with a greater risk for transition to chronic pain.43,79 Systematic reviews also show strong evidence for pain catastrophizing, depression, anxiety, psychological distress, and mental health and to a lesser extent fear of movement and self-efficacy, as risk factors.31,51 Thus, it is critically important to consider a variety of psychosocial factors in the transition from acute to chronic pain.
Other patient-reported outcomes that play a role in the transition from acute to chronic pain include sleep dysfunction, fatigue, comorbidities, multisensory sensitivity, and past trauma. Sleep dysfunction is common in those with acute and chronic pain, and the degree of sleep dysfunction predicts transition to chronic pain.54,115 Recently, heightened sensitivity80,83,106 to multiple nonnoxious sensations has been identified as a risk factor for chronic overlapping pain conditions (COPCs). Conversely, low multisensory sensitivity was a marker for reduced risk of COPCs.106 Early childhood and adult trauma, including physical and sexual abuse, neglect, and parental separation also increase the risk for the development of chronic pain.1,13,82,91,93,117
Resilience—the capacity to recover from an adverse event—has emerged as an important and complex factor that protects against the development of pain and disability. Indeed, studies show that people with greater resilience show less disability and greater health-related quality of life.55
3.4. Biological factors as predictors
In contrast to psychosocial factors, few studies have investigated biological markers of the transition from acute to chronic pain. Development of a variety of omics platforms enables examination of a variety of biological factors. These platforms allow analysis of biological factors from variations in the genetic code to changes in the levels of RNAs, proteins, lipids, or metabolites. Despite these advances, few studies have employed these platforms to examine underlying biological factors that contribute to the transition from acute to chronic pain. Furthermore, the studies that have examined these factors have considered only a few genes or proteins in a relatively small number of individuals.
3.4.1. Genetics
Over the past 2 decades, researchers have made a concerted effort to understand the genetic components of pain phenotypes. Chronic pain conditions are strongly heritable, varying between 25% and 50% depending on the specific phenotype, and a considerable proportion of the risk of developing a pain condition is genetically influenced.55 Candidate gene studies have identified signaling molecules and receptors known to interact with nociceptive pathways, including the catechol-O-methyltransferase (COMT),14,51,59,67,90 the μ-opioid receptor 1 (OPRM1),40,45the potassium channel KCNS1,15 guanosine trisphosphate cyclohydrolase (GCH1), the voltage-gated calcium channel CACNG, the cholinergic receptor CHRNA6, the purinergic receptor P2X7R, cytokine-associated genes, human leucocyte antigens, the dopamine receptor DRD2, and the transcriptional regulator ataxin 1 (ATXN1).15 These are associated with various pain phenotypes; for example, COMT and OPRM1 have been associated with postsurgical pain.40,67 A number of other potential genes have also been associated with pain phenotypes, including those encoding the ATP-binding cassette subfamily B member 1 (ABCB1)6,81 and brain-derived neurotrophic factor (BDNF).21,86,91,93 However, it has yet to be determined whether these predict the transition to chronic pain.
However, these pain-associated genes were identified in candidate gene studies of limited sample size. In recent years, researchers have tested these and many other variants in genome-wide association studies (GWAS), with tests of 1 million or more single-nucleotide polymorphisms (SNP) in studies of hundreds of thousands of participants. Genome-wide association studies have identified SNPs linked to chronic back pain,96 chronic widespread pain,70 temporomandibular disorder (TMD),59 multisite chronic pain,42 and knee pain,59 among other conditions. Although these studies have uncovered hundreds of new pain-linked genetic variants, remarkably, none of them have been in candidate genes like COMT, OPRM1, DRD2, and other commonly studied variants. Although genes for some conditions, like widespread pain, appear to be specifically enriched in brain tissue and linked to neuroinflammation,44 the effect sizes for individual genes are small. The genetic influences on common chronic pain conditions studied with GWAS to date appear to be polygenic: large numbers of genes with small effects and possibly complex interactions among them underlie genetic risk for pain. These findings of high polygenicity and small effect sizes of individual genes echo GWAS studies for virtually all other complex traits. One recent report, using the UK Biobank data developed a common risk score that predicted development of chronic pain in pain-free individuals, which included a polygenic risk score as well as an inflammatory blood marker (C-reactive protein [CRP]), a brain-based pain signature, and psychosocial variables.97 The A2CPS project tests a set of gene candidates, largely for historical value to provide additional evidence based on previously generated hypotheses in other pain conditions. In addition, approximately 660,000 human gene variants will be tested, including a broad set of human gene variants, as well as variants associated with pain or other pain-relevant phenotypes. Genetic information may contribute to a pain biosignature with prognostic value and inform analyses of RNA, protein, and other molecular measures.
3.4.2. Extracellular RNA
Extracellular RNAs (exRNAs) are produced and secreted by all known cell types. ExRNAs are rich in micro RNAs (miRNAs) and are known to be associated with extracellular vesicles (EVs), ribonucleoprotein complexes (RNPs), lipoprotein particles (LPPs), and other macromolecular complexes.64 Although their functions are not fully understood, they have been shown to play a role in a variety of biological processes mediating both short-range and long-range communication between the cells.64,69 Recent reports implicate exRNA in several chronic pain conditions, including complex regional pain syndrome, spinal pain, endometriosis, and irritable bowel syndrome.24,60,66,75,109,118 A variety of different miRNAs have been identified and associated with pain. For example, miR-199a and miR-122 were increased in the serum of those with endometriosis and discriminated between individuals with severe and mild pain.109 On the other hand, miR-223 is associated with decreased risk after lumbar disk herniation.60 These prior studies, with small samples sizes between 1 and 41, were not comprehensive in terms of miRNA profiling or pain phenotyping. The A2CPS will use small RNA sequencing (small RNAseq) for miRNA measurement in an untargeted/discovery approach and reverse transcription quantitative polymerase chain reaction (RT-qPCR) to validate the existing literature and discover novel exRNAs and their role in the transition to chronic pain.
3.4.3. Proteins
Proteins, including neurotransmitters, receptors, and cytokines, play a critical role in the generation and maintenance of pain.3,30,34,101 Numerous proteins have been identified in a variety of pain conditions, yet our understanding of those that contribute to the transition to chronic pain are limited. Inflammatory markers have been the most well studied, resulting in strong effect sizes for CRP, tumor necrosis factor-alpha (TNFα), interleukin-6 (IL6), and interleukin-12 (IL12).81,88,90,94 Two months after total knee replacement, those with reductions in pain showed a decrease in substance P and greater increases in anti-inflammatory cytokines like IL-10 and IL-12 when compared with those without changes in pain.88 Other smaller studies revealed changes in a variety of immune factors, including inflammatory cytokines like Il-1β, MIP-1β, leptin, and TNFα.27,46 In addition to the role of immune factors in the generation of chronic pain, a recent study in individuals with acute low back pain using whole-genome transcriptomics of peripheral immune cells suggests that resolution of pain involves active inflammatory processes in the recovery phase involving neutrophil-derived immune factors.68 Although there is strong evidence for immunological modulation of the transition from acute to chronic pain, studies investigating other protein classes that could potentially impact the development of chronic pain are lacking. Furthermore, most studies examined a few immune markers in relatively small sample sizes. The A2CPS will use a proteomics and Luminex approach to examine immune-related and nonimmune proteins and will include protein abundances beyond their genetic expression level, including posttranslational modifications and testing multidimensional cellular networks and systems.
3.4.4. Lipids and metabolites
Lipids play essential roles in cellular functions, including forming cellular barriers and membrane matrices, signaling, and energy depots, and have recently been postulated to play a role in nociceptive processing and resolution of pain.3,50 Metabolites are the intermediate products of metabolic reactions and cellular metabolism and thus provide insight into underlying cellular processes. Emerging studies show changes in metabolites in a variety of chronic pain conditions.5,98 A few studies have begun to examine lipids and metabolites as markers of the transition from acute to chronic pain. In women undergoing hysterectomy surgery, changes in lipid metabolites, including phosphatidylcholines, lysophosphatidylcholines, and lysophosphatidylethanolamines, were associated with increased risk for chronic pain, and fucose and pregnenolone sulfate were associated with decreased risk.78 In a large study of 461 individuals with total knee replacement, presurgical ratios of metabolites related to inflammation, and muscle breakdown predicted recovery from chronic pain.19 Thus, there is emerging evidence for the role of metabolites and lipids as markers for the transition to chronic pain. The A2CPS will use lipidomics and metabolomics to examine blood-derived changes and their relationship to chronic postsurgical pain.
3.5. Quantitative sensory testing
Altered central nervous system (CNS) activity has been proposed to predict the transition from acute to chronic pain, specifically increased excitability and decreased inhibition.62,116 Three measures were identified with sufficient effect sizes to be included as primary biomarkers, including pressure pain threshold and temporal summation to gauge excitability, and conditioned pain modulation to measure CNS inhibition. Decreased conditioned pain modulation and the change in conditioned pain modulation at 3 months predicts the development of chronic pain after surgery or musculoskeletal injury.29,104,116 Lower pressure pain thresholds predict transition to chronic pain after an acute musculoskeletal injury or surgery,29,49,53,113 and enhanced excitability measured with temporal summation predicts transition to chronic pain after total joint replacement.41,71,72 Although an indirect measure, QST provides an important glimpse into the activity of the CNS before and after the transition to chronic pain.
3.6. Brain imaging
Brain pathophysiology associated with chronic pain may be detected with neuroimaging, particularly when imaging is combined with machine-learning techniques designed to identify predictive measures embedded in rich and complex data sets. Magnetic resonance imaging (MRI) is a primary tool for neuroimaging studies and can offer a wealth of imaging-based markers for investigating the acute-to-chronic pain transition. Magnetic resonance imaging allows for the assessment of multiple imaging modalities within a single patient session,103 including those related to macroscopic brain structure using structural MRI (sMRI), structural connectivity using diffusion MRI (dMRI), molecular properties using MR spectroscopy (MRS), and dynamic changes in activity and connectivity as a function of stimuli, tasks, and mental states using functional MRI (fMRI) and functional connectivity MRI (fc-fMRI). With respect to the transition from acute to chronic pain, Apkarian et al. followed patients with acute back pain for more than a year and found that baseline measures of (1) white matter fractional anisotropy (FA), (2) gray matter volume of medial prefrontal cortex, (3) corticostriatal functional connectivity, and (4) hippocampal functional connectivity predicted the development of chronic pain 1 year later.4,7,8,58,61,103 Thus, brain imaging before surgery or during the recovery period could provide a signal that could predict the transition to chronic pain.
4. Acute to chronic pain signatures
Acute to chronic pain signatures will develop a biosignature of multiple biomarkers that predict the transition from acute to chronic pain across the biopsychosocial spectrum among a cohort of participants undergoing knee replacement or thoracic surgery to determine risk or resilience for developing chronic pain in a large sample, with a goal of 1400 per cohort (Table 2). Putative biomarkers will be collected from multiple sources, including direct report from the participants and electronic health record extraction, and more objective measures of physical function, QST, brain imaging, and omics. As pain is multifactorial, incorporation of candidate biomarkers across multiple biopsychosocial domains provides a means for evaluating interactions across domains to comprehensively understand the transition to chronic pain.
Table 2.
Acute to chronic pain signatures goals.
| Goal 1 will use a candidate approach to examine whether putative biomarkers across multiple domains—clinical, biospecimen, patient-reported outcomes, and brain structure/function—individually predict susceptibility or resilience to the development of chronic pain at 6 mo after surgery |
| Goal 2 will develop biosignature(s) using the candidate biomarkers, evaluated in isolation for goal 1, to determine if combinations of biomarkers improve the prediction from acute to chronic pain after surgery |
| Goal 3 is exploratory and will use a discovery-validation approach to define novel putative biomarkers and biosignatures across multiple domains—clinical, biospecimen, psychosocial, and brain structure/function—that predict the susceptibility and resilience to the development of chronic pain at 6 mo. The approach will include biomarkers (1) defined from prior basic work using a targeted approach and (2) identified using a discovery approach |
4.1. Selection of candidate and exploratory biomarkers
Selection of candidate and exploratory biomarkers were based on several factors, including cost, participant burden, research assistant time, and strength of the evidence related to chronic pain and pain transition. Before the publication of the funding opportunity announcement (FOA), the National Institutes of Health selected omics platforms that would be cost-effective and could be sufficiently powered with the proposed sample size. Cost-efficient and high-throughput omics were selected across a spectrum from genes to proteins and their metabolites. Budget limitations precluded the use of GWAS and RNA sequencing. Genome-wide association studies typically require larger samples (>5000) because of small effects, and RNA sequencing is currently cost-prohibitive for the A2CPS sample size.
During the initial planning year, the consortium determined broad categories of biomarkers: PROs, brain imaging, quantitative sensory testing, and omics. Work groups were formed to identify potential primary and secondary biomarkers from each of these categories from the literature. These working groups included consortium members, NIH staff, and external consultants. Throughout this process, a patient consultant was actively involved bringing information valuable insight into the patient experience in research including subject burden. Once identified, effect sizes for each proposed biomarker were estimated from the literature, and the statistical analysis team assessed power and scientific value based on the literature. The MRI protocol was designed to include multiple relevant image types, including anatomical structure, white matter tactography, and resting state and pain-related functional MRI. The protocol was designed to minimize participant burden and be completed within one hour, including patient placement and instruction, with time to repeat the structural image if needed. For PROs, once the constructs were identified, the selected surveys reflected validated instruments, but often shorter versions of surveys were selected to minimize participant burden. Other potential measures were considered but ultimately not chosen including activity monitoring, electroencephalography, autonomic activity, motion capture, and more detailed examination of immune cells in the blood or cytokines in synovial fluid of the knee. Although these methods can readily be performed on their own in specialized studies, including them alongside other prioritized measures was considered to impose a high participant burden.
The A2CPS consortium identified candidate biomarkers based on the strength of existing data in the literature as the foundation for developing biosignatures for chronic pain (Fig. 2; Table 3). Two landmark prospective studies examined diverse biomarker domains resulting in transformative discoveries for understanding of pain that were incorporated into A2CPS: the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) and the Multidisciplinary Approach to the Study of Pelvic Pain (MAPP).48,57 The PROs selected in A2CPS have the strongest prior evidence encompassing pain and other symptoms, psychosocial factors, and function. The PROs included the HEAL common data elements, along with additional measures to address each candidate biomarker. Neuroimaging biomarkers were similarly guided by prior studies4 and measure brain morphology and structural and functional connectivity using MRI. Quantitative sensory testing will assess altered nervous system activity, including increased excitability and decreased inhibition, which are proposed to predict the transition from acute to chronic pain.73 High-throughput molecular screening techniques (omics) will be used to analyze genetic variants, extracellular RNA, proteins, lipids, and metabolites in blood samples with the aim of comprehensively assessing biological molecules and processes as candidate and exploratory biomarkers, enabling a broad understanding of the flow of information from the genomic level through functional consequences.65 The measures used for each candidate biomarker are outlined in detail elsewhere.10
Figure 2.
A conceptual framework for the biomarkers collected as part of the A2CPS program. These biomarkers span (1) patient-reported outcomes (PROs) that include a variety of symptoms and psychosocial factors, (2) brain imaging, (3) quantitative sensory testing (QST), and (4) biological factors across genetics, extracellular RNA, proteins, and metabolites. The PROs include factors related to the person and the pain condition as well as external factors that can influence pain. Brain imaging will use multiple techniques to assess functional connectivity, white matter tractography and diffusion imaging, and pattern responses to nociceptive stimuli. QST will be used to test for sensitivity to noxious stimuli, central excitability, and central inhibition. A biosignature for risk for development of, or resilience to, chronic pain after surgery will be developed by combining variables across the biopsychosocial continuum of outcomes. A2CPS, Acute to Chronic Pain Signatures; fc-fMRI, functional connectivity-magnetic resonance imaging; MRI, magnetic resonance imaging. Figure copyright by Johns Hopkins University.
Table 3.
Primary and secondary biomarkers.
| Domain | Category | Primary biomarker | Secondary biomarkers |
|---|---|---|---|
| Patient-reported outcomes and behavior | Pain | General pain intensity Local pain intensity Widespread body pain Movement-evoked pain Pain trajectory |
Pain duration Pain impact/quality of life Pain interference |
| Psychological | Anxiety Depression Pain catastrophizing Fear of movement |
Personality traits: Neuroticism Agreeableness Conscientiousness Openness Extraversion |
|
| Other | Disability Perceived physical function Performance physical function (KA) Sleep Cognitive dysfunction Trauma history Resilience Social support |
Physical activity Multisensory sensitivity Expectation Global impression of change Fatigue Neuropathic pain symptoms Acute pain Fibromyalgia diagnostic criteria |
|
| Patient characteristics | Age Sex/gender Ethnicity/race Education Relationship status Income Disability insurance Comorbidities Opioid use Opioid likeability Opioid or other substance misuse Other treatments—pharmacologic Other treatments—nonpharmacologic Smoking Surgery and hospital stay details |
||
| Omics | Proteomics | C-reactive protein Soluble glycoprotein 130 Tumor necrosis factor-alpha Interleukin-6 Interleukin-12 |
Interferon-gamma Interleukin-17 Interleukin-1beta Heparin-binding epidermal growth factor Monocyte chemoattractant protein-1 (MCP-1/CCL2) Interleukin-4 Interleukin-5 Interleukin-10 Interleukin-13 Brain derived neurotrophic factor Nerve growth factor Leptin Adiponectin |
| Genetics | Catechol-O-methyltransferase haplotype (rs4680) Mu-opioid receptor (rs1799971) ATP-binding cassette subfamily B1 (rs1045642) Brain-derived neurotrophic factor (rs6265, rs1491850) |
Interleukin-6 (rs2069845) Interleukin-13 (rs1295686) Tumor necrosis factor-alpha (rs18800610) Serotonergic signaling pathway (rs9316233, rs4776783, rs12439516, rs2276008, rs6928, rs3813928) T-cell receptor pathway (rs10500205, rs216535, rs306083, rs3797739, rs2070995, rs3756612, rs815815, rs790250) Voltage-gated potassium channel subunit KV9.1 (rs734784) Nuclear receptor subfamily-3 group-C member 1 (rs2963155) GTP cyclohydrolase 1 (rs998259) |
|
| Extracellular RNA | miR-223-3p | ||
| Lipidomics | Palmitoylethanolamide (NAE 16:0), an endocannabinoid 2-Arachidonoylglycerol sphingomyelin (d18:1/16:0) Ceramide (d18:1/16:0) Phosphoinositol (18:0/20:4) |
||
| Metabolomics | Threonic acid Nonanoic acid Hypoxanthine, a marker for redox status, ischemia Inosine, a purine marker for adenosine metabolism Kynurenic acid, a tryptophan/serotonin metabolite |
||
| Quantitative sensory testing | Pressure pain threshold (surgical site) Temporal summation (surgical site) Conditioned pain modulation |
Pressure pain threshold (control site) Temporal summation (control site) Pressure cuff pain sensitivity Dynamic mechanical allodynia (brush-evoked pain at the surgical site) |
|
| Brain imaging | Gray matter volume of medial prefrontal cortex Structural integrity in Am-NAc-mPFC network Core default mode network (DMN) (vmPFC/CC)—somatosensory (dplNS/S1) Core DMN (vmPFC/CC)—Nac/ventral striatum Core DMN (vmPFC/CC)—Anterior/middle insula Hub disruption Evoked response (pressure cuff) in neurologic pain signature Evoked response (pressure cuff) in Frontostriatal systems related to descending/central pain modulation and self-regulation (vmPFC, NAc) |
Gray matter density Gray matter morphometry Gray matter volume hippocampus Gray matter volume of amygdala Node degree—Hippocampus, dlPFC Lateral thalamus functional connectivity with periaqueductal gray Fractional anisotropy in superior longitudinal fasciculus, internal and external capsule Local pattern responses in nociceptive regions Local pattern responses in context-related regions Response to pain offset in reward systems |
Am, amygdala; ATP, adenosine trisphosphate; CC, cingulate cortex; dlPFC, dorsolateral prefrontal cortex; dpINS, dorsoposterior insula; GTP, guanosine trisphosphate, KA, knee arthroplasty; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; S1, somatosensory cortex 1; vmPFC, ventromedial prefrontal cortex.
4.2. Protocol
Working in collaboration with NIH and diverse team members (Fig. 3), A2CPS developed a protocol to examine biomarkers in 2 different surgical populations: (1) KA and (2) thoracic surgery10 (Fig. 4). These 2 populations allow investigation of one condition that typically features existing pain before surgery (KA), and one typically without pain (thoracic surgery) to identify factors related or unrelated to current pain status in the transition to chronic pain. From a clinical intervention perspective, identifying factors before surgery and in the postoperative recovery period is critical to developing approaches to mitigate the development of chronic pain. The proposed sample size (n = 1400 per cohort) is sufficiently powered to assess 39 putative primary biomarkers. Primary, secondary, and exploratory outcomes will be measured 6 months after the surgery, when it is expected that the initial surgical insult should be fully healed. Although the primary outcome will be mean daily pain 6 months after surgery, disability and opioid use are recognized as key outcomes in determining success after surgery (Fig. 4). The study is currently in the active recruitment phase. The last date of enrollment is currently planned for August of 2025 with the final subject completing 6 months later in January 2026. This will be followed by data cleaning and analysis to be completed by July 2027.
Figure 3.
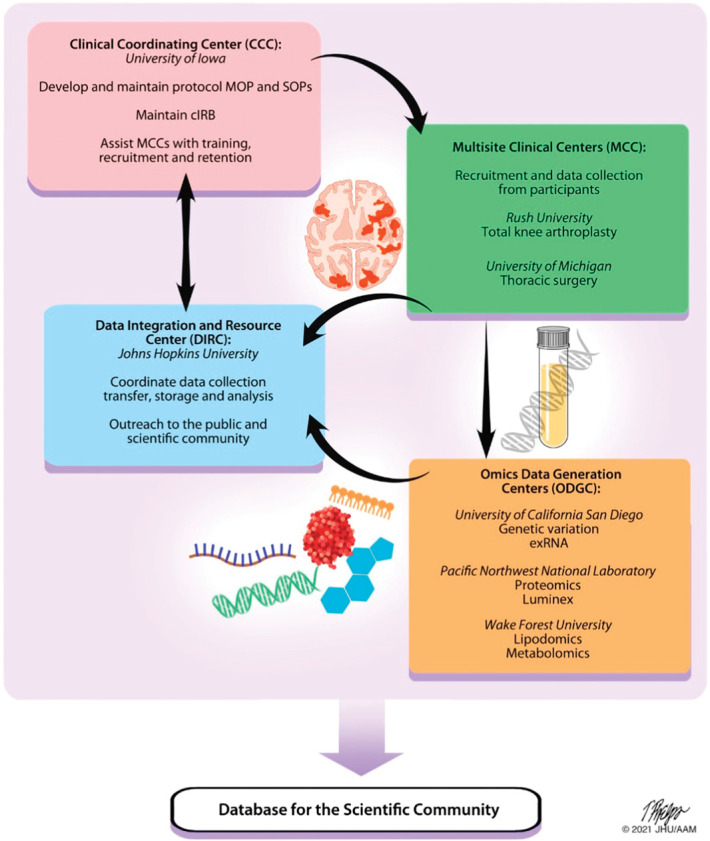
The A2CPS Consortium structure consists of 4 main components spread across multiple sites, including a Clinical Coordinating Center (CCC), 2 Multisite Clinical Centers (MCCs), a Data Integration and Resource Center (DIRC), and 3 Omics Data Generation Centers (ODGCs). Each component has specific tasks supporting the collection and generation of data; these groups work together to accomplish the goals of the Consortium with collaboration from the NIH. Data generated from the A2CPS will be made available to the scientific community for further study. The 2 MCCs are responsible for participant recruitment, enrollment, and data collection. The DIRC combines biostatisticians, informaticians, and database experts with pain scientists into a single integrated team who integrate efforts of all funded components of the Consortium and serves as a community-wide nexus for protocol, assay, and data standards. The DIRC also leads an outreach component, including a public website (www.a2cps.org), a portal for Consortium members, and will provide user-friendly, publicly accessible data to the scientific community for novel discovery approaches. The CCC leads development and maintenance of procedures and is responsible for coordinating with the central Institutional Review Board (cIRB). Finally, 3 ODGCs each focus on different omics analyses, including genetic variants and exRNA, proteomics, and lipidomics and metabolomics. In addition to the funded components of the Consortium, volunteer external Program Consultants, composed of senior scientists invited by the NIH, are responsible for providing NIH with their individual opinion of progress toward the goals of the program. Volunteer biomarker experts, invited by NIH, provided feedback on selection of biomarkers early in the program, and 2 patient representatives, invited by the NIH, provide ongoing feedback on clinical protocols, recruitment, and retention to ensure the participants' needs are best considered. The Consortium is led by a Steering Committee with leadership representation from each component and the NIH. A2CPS, Acute to Chronic Pain Signatures; NIH, National Institutes of Health. Figure copyright by Johns Hopkins University.
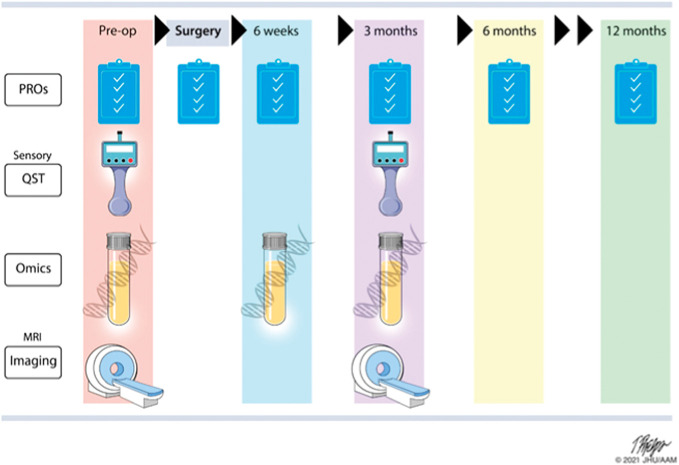
Figure 4.
General protocol for A2CPS: Assessments are collected across a 12-month period with PROs, QST, omics, and imaging data collected from all subjects before surgery. After surgery, daily pain trajectories for the first month are collected. PRO biomarkers are collected at 4 to 6 weeks and 3 months postsurgery. QST, imaging and blood draws for omics are also collected at 3 months postsurgery. The primary, secondary, and exploratory outcomes are collected from all participants at 6 months, and there is a short 12-month optional follow-up questionnaire. A2CPS, Acute to Chronic Pain Signatures; MRI, magnetic resonance imaging; PRO, patient-reported outcome; QST, quantitative sensory testing. Figure copyright by Johns Hopkins University.
4.3. Data analysis plan
The complexity and dimensionality of the selected biomarkers will require novel statistical and computational methodology, which will enable the development of a comprehensive biosignature and exploration of novel, as-yet undiscovered biomarkers for the prediction of transition or resilience to chronic pain. The A2CPS data analysis plan will use a candidate approach to examine whether each of the putative biomarkers identified across multiple domains (psychosocial, omics, and circuits) individually or in combination (as a biosignature) predict susceptibility or resilience to the development of chronic pain at 6 months after an acute painful event. First, we will consider each primary biomarker in isolation in preregistered analyses, testing its prognostic value for predicting the primary outcome, and reporting results for all biomarkers. Then, we will consider secondary biomarkers and their relationships with the primary outcome.
In addition to tests of a single, prespecified biomarker, we will use a predictive modeling approach, identifying a combination of biomarkers optimized to predict the primary outcome based on a constrained set of variables related to each biomarker individual items related to a biomarker, such as individual inflammatory cytokines, individual depression inventory items, or voxels in the prefrontal cortex, and test the prognostic effect size using both cross-validation and independent data (ie, 10-fold cross-validation of a discovery set composed of 66% of the available sample, and replication on an independent test set composed of 33% of the available sample, stratified on primary outcome, and other key variables). This allows us to move beyond prespecified aggregate summaries of multi-item measures, which were not designed for prognosis and may need to be refined.
Second, we will use predictive modeling to create multimodal biosignatures across different biomarkers and types of data. This will allow us to identify (1) a model across the set of primary biomarkers that is optimally prognostic of the primary outcome, (2) an optimized model across all primary and secondary biomarkers, (3) optimized biomarkers across all measures in a particular modality (eg, exRNA, structural MRI, functional MRI, proteomics, metabolomics), and (4) an optimized model across all available measures. Auxiliary data sets will be used to develop candidate algorithms suited to the data, and limited optimization of the algorithms and feature-selection methods will be performed on the discovery data set (70% of patients), with the holdout test set (30% of patients) used only to test the final models in each category. Biosignatures will generally involve stacked (hierarchical) models that use output from predictive models based on individual biomarkers (using, eg, penalized regression) as input into a second-level model that includes their joint effects and potential interactions (using, eg, random forests). Models will include a baseline set of covariates (ie, sex, age, pre-surgical baseline pain) and compare prognostic accuracy with the baseline-only model. Analyses of some biomarker types will require specific baseline covariates, including batch effects for blood and scanner site, head size, and head movement for neuroimaging, and multiplicative error models will be considered to account for batch effects on scaling as well as additive effects. Model pruning analyses will focus on finding combinations of biomarkers that require as few data collection modalities as possible. Planned exploratory analyses include exploring alternative outcomes (eg, pain interference and function), testing for biological mediators of exposure–outcome relationships (eg, pathways from psychosocial risk factors to inflammation or brain changes to pain chronification), and investigating how changes in brain measures, psychosocial assessments, and omics measures track changes in reported pain from baseline to 6 months.
5. Future directions and impact
The data collected by A2CPS will be available to the research community through a repository for additional hypothesis-driven research and data exploration after publication of the original study goals. The study was designed with the highest rigor using validated assessments, HEAL common data elements, and harmonization of data collection across sites. Using Findable, Accessible, Interoperable and Reusable (FAIR) principles, the A2CPS data set will provide an invaluable resource to researchers outside the consortium.
After promising biomarkers are identified, these can be scaled to test generalizability in a more clinically relevant setting. Findings from this study will result in a large amount of publicly available data. We expect these could lead to additional mechanistic studies examining the role of identified markers relevant to pain, new potential targets for interventions, and implementation of existing interventions aimed at modifying target biomarker(s). The data could lead to a more mechanistically based approach to pain management. For example, if anxiety or depression is a key predictor, interventions aimed at these mood disorders could be implemented before surgery. Indeed, a pilot study applying Acceptance Commitment Therapy (ACT) before surgery in at-risk individuals results in faster pain recovery and less opioid use.23
Use of biosignatures can be used to develop a risk score for chronic pain that could subsequently be tested with targeted interventions to determine their role in the transition to chronic pain, similar to the previously developed distress risk assessment method (DRAM), which evaluates psychological distress in patients before surgery and predicts poorer outcomes after spinal surgery and other medical procedures.56,84 Putting such predictive markers to practical use in the clinic may require a greater level of outreach and education to surgeons and other clinicians to discuss the potential risks and benefits of surgery to an individual patient. The final A2CPS data set will be available for machine learning and related analytical techniques requiring large data sets. Such analysis could allow investigators to determine the prognostic potential of each biomarker with greater certainty and identify linkages between biomarker domains such as psychosocial with neuroimaging, or gene variant with proteomics or metabolomics. For example, one line of research has linked genotype for translocator protein (TSPO) with pain ratings and pain-evoked functional connectivity measured by MRI.47 This is not to suggest that patients would routinely receive imaging, but such brain imaging-based signatures can lend a better understanding to the mechanistic underpinnings of other markers. Generation of these new models of disease risk and resilience will drive future mechanistic and intervention studies to transform our understanding and potential treatment of chronic pain. Such signatures may differ between individuals and understanding these subtypes of patients could help inform more individualized care.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
A list of consortium member can be found at: http://links.lww.com/PAIN/B871.
Supplementary Material
Acknowledgements
The authors acknowledge the University of Iowa for coordination and implementation of the single institutional review board model, the External Program Consultants, and the Patient Representatives for their contributions to the design and implementation of the project.
The A2CPS Consortium is supported by the National Institutes of Health Common Fund, which is managed by the Office of the Director/Office of Strategic Coordination (OSC). Consortium components include Clinical Coordinating Center (U01NS077179), Data Integration and Resource Center (U01NS077352), Omics Data Generation Centers (U54DA049116, U54DA049115, U54DA09113), Multisite Clinical Center 1 (UM1NS112874), and Multisite Clinical Center 2 (UM1NS118922).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Tor D. Wager, Email: tor.d.wager@dartmouth.edu.
Stephani P. Sutherland, Email: sutherland@nasw.org.
Patricia A. Labosky, Email: patricia.labosky@nih.gov.
Tessa Balach, Email: tbalach@bsd.uchicago.edu.
Emine O. Bayman, Email: emine-bayman@uiowa.edu.
Giovanni Berardi, Email: giovanni-berardi@uiowa.edu.
Chad M. Brummett, Email: cbrummet@med.umich.edu.
John Burns, Email: john_burns@rush.edu.
Asokumar Buvanendran, Email: asokumar_buvanendran@rush.edu.
Brian Caffo, Email: bcaffo@gmail.com.
Vince D. Calhoun, Email: vcalhoun@gsu.edu.
Daniel Clauw, Email: dclauw@umich.edu.
Andrew Chang, Email: andrwchg@umich.edu.
Christopher S. Coffey, Email: christopher-coffey@uiowa.edu.
Dana L. Dailey, Email: dana-dailey@uiowa.edu.
Dixie Ecklund, Email: dixie-ecklund@uiowa.edu.
Oliver Fiehn, Email: ofiehn@ucdavis.edu.
Kathleen M. Fisch, Email: kfisch@health.ucsd.edu.
Laura A. Frey Law, Email: laura-freylaw@uiowa.edu.
Richard E. Harris, Email: reharris@med.umich.edu.
Steven E. Harte, Email: seharte@med.umich.edu.
Timothy D. Howard, Email: tdhoward@wakehealth.edu.
Joshua Jacobs, Email: joshua.jacobs@rushortho.com.
Jon M. Jacobs, Email: jon.jacobs@pnnl.gov.
Kristen Jepsen, Email: kjepsen@health.ucsd.edu.
Nicolas Johnston, Email: nic.johnston@nih.gov.
Carl D. Langefeld, Email: clangefe@wakehealth.edu.
Louise C. Laurent, Email: llaurent@health.ucsd.edu.
Rebecca Lenzi, Email: rebecca.lenzi@nih.gov.
Martin A. Lindquist, Email: mlindqui@jhsph.edu.
Anna Lokshin, Email: lokshina@pitt.edu.
Ari Kahn, Email: akahn@tacc.utexas.edu.
Robert J. McCarthy, Email: robert_j_mccarthy@rush.edu.
Michael Olivier, Email: molivier@wakehealth.edu.
Linda Porter, Email: porterl@ninds.nih.gov.
Wei-Jun Qian, Email: weijun.qian@pnnl.gov.
Cheryse A. Sankar, Email: cheryse.sankar@nih.gov.
John Satterlee, Email: satterleej@nida.nih.gov.
Adam C. Swensen, Email: adam.swensen@pnnl.gov.
Carol G.T. Vance, Email: carol-vance@uiowa.edu.
Jennifer Waljee, Email: filip@med.umich.edu.
Laura D. Wandner, Email: laura.wandner@nih.gov.
David A. Williams, Email: daveawms@umich.edu.
Richard L. Wixson, Email: rwixson@northshore.org.
Xiaohong Joe Zhou, Email: xjzhou@uic.edu.
References
- [1].Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, Cuneo JG. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosomatic Med 2014;76:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Althaus A, Arránz Becker O, Neugebauer E. Distinguishing between pain intensity and pain resolution: using acute post-surgical pain trajectories to predict chronic post-surgical pain. Eur J Pain 2014;18:513–21. [DOI] [PubMed] [Google Scholar]
- [3].Antunes-Martins A, Perkins JR, Lees J, Hildebrandt T, Orengo C, Bennett DL. Systems biology approaches to finding novel pain mediators. Wiley Interdiscip Rev Syst Biol Med 2013;5:11–35. [DOI] [PubMed] [Google Scholar]
- [4].Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol 2013;26:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aroke EN, Powell-Roach KL. The metabolomics of chronic pain conditions: a systematic review. Biol Res Nurs 2020;22:458–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].As-Sanie S, Till SR, Schrepf AD, Griffith KC, Tsodikov A, Missmer SA, Clauw DJ, Brummett CM. Incidence and predictors of persistent pelvic pain following hysterectomy in women with chronic pelvic pain. Am J Obstet Gynecol 2021;225:568.e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 2010;66:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15:1117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain 2014;15:887–97. [DOI] [PubMed] [Google Scholar]
- [10].Berardi G, Frey-Law L, Sluka KA, Bayman EO, Coffey CS, Ecklund D, Vance CGT, Dailey DL, Burns J, Buvanendran A, McCarthy RJ, Jacobs J, Zhou XJ, Wixson R, Balach T, Brummett CM, Clauw D, Colquhoun D, Harte SE, Harris RE, Williams DA, Chang AC, Waljee J, Fisch KM, Jepsen K, Laurent LC, Olivier M, Langefeld CD, Howard TD, Fiehn O, Jacobs JM, Dakup P, Qian WJ, Swensen AC, Lokshin A, Lindquist M, Caffo BS, Crainiceanu C, Zeger S, Kahn A, Wager T, Taub M, Ford J, Sutherland SP, Wandner LD. Multi-site observational study to assess biomarkers for susceptibility or resilience to chronic pain: the acute to chronic pain signatures (A2CPS) study protocol. Front Med (Lausanne) 2022;9:849214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bérubé M, Choinière M, Laflamme YG, Gélinas C. Acute to chronic pain transition in extremity trauma: a narrative review for future preventive interventions (part 2). Int J Orthop Trauma Nurs 2017;24:59–67. [DOI] [PubMed] [Google Scholar]
- [12].Biddle SJH, Batterham AM. High-intensity interval exercise training for public health: a big HIT or shall we HIT it on the head? Int J Behav Nutr Phys Activity 2015;12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brown RC, Plener PL, Braehler E, Fegert JM, Huber-Lang M. Associations of adverse childhood experiences and bullying on physical pain in the general population of Germany. J Pain Res 2018;11:3099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bruce J, Thornton AJ, Scott NW, Marfizo S, Powell R, Johnston M, Wells M, Heys SD, Thompson AM. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Br J Cancer 2012;107:937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bruehl S, Denton JS, Lonergan D, Koran ME, Chont M, Sobey C, Fernando S, Bush WS, Mishra P, Thornton-Wells TA. Associations between KCNJ6 (GIRK2) gene polymorphisms and pain-related phenotypes. PAIN 2013;154:2853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol 2015;67:1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Buvanendran A, Della Valle CJ, Kroin JS, Shah M, Moric M, Tuman KJ, McCarthy RJ. Acute postoperative pain is an independent predictor of chronic postsurgical pain following total knee arthroplasty at 6 months: a prospective cohort study. Reg Anesth Pain Med 2019;44:e100036. [DOI] [PubMed] [Google Scholar]
- [18].Collins FS, Koroshetz WJ, Volkow ND. Helping to end addiction over the long-term: the research plan for the NIH HEAL initiative. JAMA 2018;320:129–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Costello CA, Hu T, Liu M, Zhang W, Furey A, Fan Z, Rahman P, Randell EW, Zhai G. Metabolomics signature for non-responders to total joint replacement surgery in primary osteoarthritis patients: the newfoundland osteoarthritis study. J Orthop Res 2020;38:793–802. [DOI] [PubMed] [Google Scholar]
- [20].Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal weekly Rep 2018;67:1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dave AJ, Selzer F, Losina E, Usiskin I, Collins JE, Lee YC, Band P, Dalury DF, Iorio R, Kindsfater K, Katz JN. The association of pre-operative body pain diagram scores with pain outcomes following total knee arthroplasty. Osteoarthritis Cartilage 2017;25:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Davis KD, Aghaeepour N, Ahn AH, Angst MS, Borsook D, Brenton A, Burczynski ME, Crean C, Edwards R, Gaudilliere B, Hergenroeder GW, Iadarola MJ, Iyengar S, Jiang Y, Kong JT, Mackey S, Saab CY, Sang CN, Scholz J, Segerdahl M, Tracey I, Veasley C, Wang J, Wager TD, Wasan AD, Pelleymounter MA. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol 2020;16:381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dindo L, Zimmerman MB, Hadlandsmyth K, StMarie B, Embree J, Marchman J, Tripp-Reimer T, Rakel B. Acceptance and commitment therapy for prevention of chronic postsurgical pain and opioid use in at-risk veterans: a pilot randomized controlled study. J Pain 2018;19:1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Douglas SR, Shenoda BB, Qureshi RA, Sacan A, Alexander GM, Perreault M, Barrett JE, Aradillas-Lopez E, Schwartzman RJ, Ajit SK. Analgesic response to intravenous ketamine is linked to a circulating microRNA signature in female patients with complex regional pain syndrome. J Pain 2015;16:814–24. [DOI] [PubMed] [Google Scholar]
- [25].Edwards RR, Dolman AJ, Michna E, Katz JN, Nedeljkovic SS, Janfaza D, Isaac Z, Martel MO, Jamison RN, Wasan AD. Changes in pain sensitivity and pain modulation during oral opioid treatment: the impact of negative affect. Pain Med 2016;17:1882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Epping-Jordan JE, Wahlgren DR, Williams RA, Pruitt SD, Slater MA, Patterson TL, Grant I, Webster JS, Atkinson JH. Transition to chronic pain in men with low back pain: predictive relationships among pain intensity, disability, and depressive symptoms. Health Psychol 1998;17:421–7. [DOI] [PubMed] [Google Scholar]
- [27].Gandhi R, Santone D, Takahashi M, Dessouki O, Mahomed NN. Inflammatory predictors of ongoing pain 2 years following knee replacement surgery. Knee 2013;20:316–8. [DOI] [PubMed] [Google Scholar]
- [28].Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev 2017;4:CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. PAIN 2019;160:1920–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gerdle B, Ghafouri B. Proteomic studies of common chronic pain conditions—a systematic review and associated network analyses. Expert Rev Proteomics 2020;17:483–505. [DOI] [PubMed] [Google Scholar]
- [31].Giusti EM, Lacerenza M, Manzoni GM, Castelnuovo G. Psychological and psychosocial predictors of chronic postsurgical pain: a systematic review and meta-analysis. PAIN 2021;162:10–30. [DOI] [PubMed] [Google Scholar]
- [32].Gomez-Varela D, Barry AM, Schmidt M. Proteome-based systems biology in chronic pain. J Proteomics 2019;190:1–11. [DOI] [PubMed] [Google Scholar]
- [33].Gregori D, Giacovelli G, Minto C, Barbetta B, Gualtieri F, Azzolina D, Vaghi P, Rovati LC. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA 2018;320:2564–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Group F-NBW. BEST (biomarkers, EndpointS, and other tools) resource. Silver Spring (MD), Bethesda (MD): Food and Drug Administration (US), National Institutes of Health (US), 2016. [Google Scholar]
- [35].Hadlandsmyth K, Sabic E, Zimmerman MB, Sluka KA, Herr KA, Clark CR, Noiseux NO, Callaghan JJ, Geasland KM, Embree JL, Rakel BA. Relationships among pain intensity, pain-related distress, and psychological distress in pre-surgical total knee arthroplasty patients: a secondary analysis. Psychol Health Med 2017;22:552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Halicka M, Duarte R, Catherall S, Maden M, Coetsee M, Wilby M, Brown C. Systematic review and meta-analysis of predictors of return to work after spinal surgery for chronic low back and leg pain. J Pain 2022;23:1318–42. [DOI] [PubMed] [Google Scholar]
- [37].Institute of Medicine Committee on Advancing Pain Research C, Education. The national academies collection: reports funded by National Institutes of Health. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press (US) Copyright © 2011, National Academy of Sciences, 2011. [Google Scholar]
- [38].Interagency Pain Research Coordinating Committee. A comprehensive population health-level stragy for pain. NIH. Available at: https://www.iprcc.nih.gov/node/5/national-pain-strategy-report. [Google Scholar]
- [39].Interagency Pain Research Coordinating Committee. Federal pain research strategy. NIH, 2017. Available at: https://www.iprcc.nih.gov/sites/default/files/documents/NationalPainStrategy_508C.pdf [Google Scholar]
- [40].Iversen MD, Daltroy LH, Fossel AH, Katz JN. The prognostic importance of patient pre-operative expectations of surgery for lumbar spinal stenosis. Patient Educ Couns 1998;34:169–78. [DOI] [PubMed] [Google Scholar]
- [41].Izumi M, Petersen KK, Laursen MB, Arendt-Nielsen L, Graven-Nielsen T. Facilitated temporal summation of pain correlates with clinical pain intensity after hip arthroplasty. PAIN 2017;158:323–32. [DOI] [PubMed] [Google Scholar]
- [42].Johnston KJA, Adams MJ, Nicholl BI, Ward J, Strawbridge RJ, Ferguson A, McIntosh AM, Bailey MES, Smith DJ. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet 2019;15:e1008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009;9:723–44. [DOI] [PubMed] [Google Scholar]
- [44].Khoury S, Parisien M, Thompson SJ, Vachon-Presseau E, Roy M, Martinsen AE, Winsvold BS, Mundal IP, Zwart JA, Kania A, Mogil JS, Diatchenko L. Genome-wide analysis identifies impaired axonogenesis in chronic overlapping pain conditions. Brain 2022;145:1111–23. [DOI] [PubMed] [Google Scholar]
- [45].Kim DH, Pearson-Chauhan KM, McCarthy RJ, Buvanendran A. Predictive factors for developing chronic pain after total knee arthroplasty. J Arthroplasty 2018;33:3372–8. [DOI] [PubMed] [Google Scholar]
- [46].Klyne DM, Barbe MF, van den Hoorn W, Hodges PW. ISSLS prize in clinical science 2018: longitudinal analysis of inflammatory, psychological, and sleep-related factors following an acute low back pain episode-the good, the bad, and the ugly. Eur Spine J 2018;27:763–77. [DOI] [PubMed] [Google Scholar]
- [47].Kosek E, Martinsen S, Gerdle B, Mannerkorpi K, Lofgren M, Bileviciute-Ljungar I, Fransson P, Schalling M, Ingvar M, Ernberg M, Jensen KB. The translocator protein gene is associated with symptom severity and cerebral pain processing in fibromyalgia. Brain Behav Immun 2016;58:218–27. [DOI] [PubMed] [Google Scholar]
- [48].Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV, Mayer EA, Lai HH, Krieger JN, Kreder KJ, Afari N, Andriole GL, Bradley CS, Griffith JW, Klumpp DJ, Hong BA, Lutgendorf SK, Buchwald D, Yang CC, Mackey S, Pontari MA, Hanno P, Kusek JW, Mullins C, Clemens JQ; The MAPP Research Network Study Group. The MAPP research network: design, patient characterization and operations. BMC Urol 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].LeResche L, Turner JA, Saunders K, Shortreed SM, Von Korff M. Psychophysical tests as predictors of back pain chronicity in primary care. J Pain 2013;14:1663–70. [DOI] [PubMed] [Google Scholar]
- [50].Leuti A, Fava M, Pellegrini N, Maccarrone M. Role of specialized pro-resolving mediators in neuropathic pain. Front Pharmacol 2021;12:717993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth 2015;114:551–61. [DOI] [PubMed] [Google Scholar]
- [52].Linton SJ. A review of psychological risk factors in back and neck pain. Spine 2000;25:1148–56. [DOI] [PubMed] [Google Scholar]
- [53].Luna IE, Kehlet H, Petersen MA, Aasvang EK. Clinical, nociceptive and psychological profiling to predict acute pain after total knee arthroplasty. Acta Anaesthesiol Scand 2017;61:676–87. [DOI] [PubMed] [Google Scholar]
- [54].Luo Z-Y, Li L-L, Wang D, Wang H-Y, Pei F-X, Zhou Z-K. Preoperative sleep quality affects postoperative pain and function after total joint arthroplasty: a prospective cohort study. J Orthop Surg Res 2019;14:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Magaldi RJ, Staff I, Stovall AE, Stohler SA, Lewis CG. Impact of resilience on outcomes of total knee arthroplasty. J Arthroplasty 2019;34:2620–3.e1. [DOI] [PubMed] [Google Scholar]
- [56].Main CJ, Wood PL, Hollis S, Spanswick CC, Waddell G. The distress and risk assessment method. A simple patient classification to identify distress and evaluate the risk of poor outcome. Spine (Phila Pa 1976) 1992;17:42–52. [DOI] [PubMed] [Google Scholar]
- [57].Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C, Ohrbach R, Weir B, Slade GD. Orofacial pain prospective evaluation and risk assessment study—the OPPERA study. J Pain 2011;12:T4–T11.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian VA. Brain white matter structural properties predict transition to chronic pain. PAIN 2013;154:2160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Meng W, Adams MJ, Palmer CNA, Shi J, Auton A, Ryan KA, Jordan JM, Mitchell BD, Jackson RD, Yau MS, McIntosh AM, Smith BH. Genome-wide association study of knee pain identifies associations with GDF5 and COL27A1 in UK Biobank. Commun Biol 2019;2:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Moen A, Jacobsen D, Phuyal S, Legfeldt A, Haugen F, Roe C, Gjerstad J. MicroRNA-223 demonstrated experimentally in exosome-like vesicles is associated with decreased risk of persistent pain after lumbar disc herniation. J Transl Med 2017;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Musto AE, Walker CP, Petasis NA, Bazan NG. Hippocampal neuro-networks and dendritic spine perturbations in epileptogenesis are attenuated by neuroprotectin d1. PLoS One 2015;10:e0116543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, Nevitt M, Bradley L, Felson DT. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis 2015;74:682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Noiseux NO, Callaghan JJ, Clark CR, Zimmerman MB, Sluka KA, Rakel BA. Preoperative predictors of pain following total knee arthroplasty. J Arthroplasty 2014;29:1383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].O'Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol 2020;21:585–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Olivier M, Asmis R, Hawkins GA, Howard TD, Cox LA. The need for multi-omics biomarker signatures in precision medicine. Int J Mol Sci 2019;20:4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Orlova IA, Alexander GM, Qureshi RA, Sacan A, Graziano A, Barrett JE, Schwartzman RJ, Ajit SK. MicroRNA modulation in complex regional pain syndrome. J Transl Med 2011;9:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Papadomanolakis-Pakis N, Uhrbrand P, Haroutounian S, Nikolajsen L. Prognostic prediction models for chronic postsurgical pain in adults: a systematic review. PAIN 2021;162:2644–57. [DOI] [PubMed] [Google Scholar]
- [68].Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, Grant AV, Huising J, Verma V, Meloto CB, Silva JR, Dutra GGS, Markova T, Dang H, Tessier PA, Slade GD, Nackley AG, Ghasemlou N, Mogil JS, Allegri M, Diatchenko L. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med 2022;14:eabj9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Patton JG, Franklin JL, Weaver AM, Vickers K, Zhang B, Coffey RJ, Mark Ansel K, Blelloch R, Goga A, Huang B, L'Etoille N, Raffai RL, Lai CP, Krichevsky AM, Mateescu B, Greiner VJ, Hunter C, Voinnet O, McManus MT. Biogenesis, delivery, and function of extracellular RNA. J Extracell Vesicles 2015;4:27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Peters MJ, Broer L, Willemen HL, Eiriksdottir G, Hocking LJ, Holliday KL, Horan MA, Meulenbelt I, Neogi T, Popham M, Schmidt CO, Soni A, Valdes AM, Amin N, Dennison EM, Eijkelkamp N, Harris TB, Hart DJ, Hofman A, Huygen FJ, Jameson KA, Jones GT, Launer LJ, Kerkhof HJ, de Kruijf M, McBeth J, Kloppenburg M, Ollier WE, Oostra B, Payton A, Rivadeneira F, Smith BH, Smith AV, Stolk L, Teumer A, Thomson W, Uitterlinden AG, Wang K, van Wingerden SH, Arden NK, Cooper C, Felson D, Gudnason V, Macfarlane GJ, Pendleton N, Slagboom PE, Spector TD, Völzke H, Kavelaars A, van Duijn CM, Williams FM, van Meurs JB. Genome-wide association study meta-analysis of chronic widespread pain: evidence for involvement of the 5p15.2 region. Ann Rheum Dis 2013;72:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. PAIN 2015;156:55–61. [DOI] [PubMed] [Google Scholar]
- [72].Petersen KK, Simonsen O, Laursen MB, Arendt-Nielsen L. The role of preoperative radiologic severity, sensory testing, and temporal summation on chronic postoperative pain following total knee arthroplasty. Clin J Pain 2018;34:193–7. [DOI] [PubMed] [Google Scholar]
- [73].Petersen KK, Vaegter HB, Stubhaug A, Wolff A, Scammell BE, Arendt-Nielsen L, Larsen DB. The predictive value of quantitative sensory testing: a systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. PAIN 2021;162:31–44. [DOI] [PubMed] [Google Scholar]
- [74].Price TJ, Basbaum AI, Bresnahan J, Chambers JF, De Koninck Y, Edwards RR, Ji RR, Katz J, Kavelaars A, Levine JD, Porter L, Schechter N, Sluka KA, Terman GW, Wager TD, Yaksh TL, Dworkin RH. Transition to chronic pain: opportunities for novel therapeutics. Nat Rev Neurosci 2018;19:383–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Qureshi RA, Tian Y, McDonald MK, Capasso KE, Douglas SR, Gao R, Orlova IA, Barrett JE, Ajit SK, Sacan A. Circulating microRNA signatures in rodent models of pain. Mol Neurobiol 2016;53:3416–27. [DOI] [PubMed] [Google Scholar]
- [76].Rakel BA, Blodgett NP, Zimmerman BM, Logsden-Sackett N, Clark C, Noiseux N, Callaghan J, Herr K, Geasland K, Yang X, Sluka KA. Predictors of postoperative movement and resting pain following total knee replacement. PAIN 2012;153:2192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Richebe P, Capdevila X, Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology 2018;129:590–607. [DOI] [PubMed] [Google Scholar]
- [78].Sasamoto N, Zeleznik OA, Vitonis AF, Missmer SA, Laufer MR, Avila-Pacheco J, Clish CB, Terry KL. Presurgical blood metabolites and risk of postsurgical pelvic pain in young patients with endometriosis. Fertil Sterility 2022;117:1235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schade V, Semmer N, Main CJ, Hora J, Boos N. The impact of clinical, morphological, psychosocial and work-related factors on the outcome of lumbar discectomy. PAIN 1999;80:239–49. [DOI] [PubMed] [Google Scholar]
- [80].Schrepf A, Hellman KM, Bohnert AM, Williams DA, Tu FF. Generalized sensory sensitivity is associated with comorbid pain symptoms: a replication study in women with dysmenorrhea. PAIN 2023;164:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Schrepf A, Kaplan CM, Ichesco E, Larkin T, Harte SE, Harris RE, Murray AD, Waiter GD, Clauw DJ, Basu N. A multi-modal MRI study of the central response to inflammation in rheumatoid arthritis. Nat Commun 2018;9:2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schrepf A, Naliboff B, Williams DA, Stephens-Shields AJ, Landis JR, Gupta A, Mayer E, Rodriguez LV, Lai H, Luo Y, Bradley C, Kreder K, Lutgendorf SK. Adverse childhood experiences and symptoms of urologic chronic pelvic pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Ann Behav Med 2018;52:865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Schrepf A, Williams DA, Gallop R, Naliboff BD, Basu N, Kaplan C, Harper DE, Landis JR, Clemens JQ, Strachan E, Griffith JW, Afari N, Hassett A, Pontari MA, Clauw DJ, Harte SE. Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP Research Network Study. PAIN 2018;159:2002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Serrano-Garcia A, Fernandez-Gonzalez M, Betegon-Nicolas J, Villar-Perez J, Lozano-Munoz A, Hernandez-Encinas J, Fernandez-Bances I, Esteban-Blanco M, Seco-Calvo JA. Evaluation of dram score as a predictor of poor postoperative outcome in spine surgery. J Clin Med 2020;9:3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shah A, Rauth S, Aithal A, Kaur S, Ganguly K, Orzechowski C, Varshney GC, Jain M, Batra SK. The current landscape of antibody-based therapies in solid malignancies. Theranostics 2021;11:1493–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Shaw WS, Means-Christensen AJ, Slater MA, Webster JS, Patterson TL, Grant I, Garfin SR, Wahlgren DR, Patel S, Atkinson JH. Psychiatric disorders and risk of transition to chronicity in men with first onset low back pain. Pain Med 2010;11:1391–400. [DOI] [PubMed] [Google Scholar]
- [87].Singh JA, Gabriel S, Lewallen D. The impact of gender, age, and preoperative pain severity on pain after TKA. Clin Orthop Relat Res 2008;466:2717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Singh JA, Noorbaloochi S, Knutson KL. Cytokine and neuropeptide levels are associated with pain relief in patients with chronically painful total knee arthroplasty: a pilot study. BMC Musculoskelet Disord 2017;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016;338:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis 2013;72:535–40. [DOI] [PubMed] [Google Scholar]
- [91].Steine IM, Winje D, Krystal JH, Bjorvatn B, Milde AM, Grønli J, Nordhus IH, Pallesen S. Cumulative childhood maltreatment and its dose-response relation with adult symptomatology: findings in a sample of adult survivors of sexual abuse. Child Abuse Neglect 2017;65:99–111. [DOI] [PubMed] [Google Scholar]
- [92].Stevans JM, Delitto A, Khoja SS, Patterson CG, Smith CN, Schneider MJ, Freburger JK, Greco CM, Freel JA, Sowa GA, Wasan AD, Brennan GP, Hunter SJ, Minick KI, Wegener ST, Ephraim PL, Friedman M, Beneciuk JM, George SZ, Saper RB. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open 2021;4:e2037371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Stickley A, Koyanagi A, Kawakami N. Childhood adversities and adult-onset chronic pain: results from the world mental health survey, Japan. Eur J Pain 2015;19:1418–27. [DOI] [PubMed] [Google Scholar]
- [94].Stürmer T, Raum E, Buchner M, Gebhardt K, Schiltenwolf M, Richter W, Brenner H. Pain and high sensitivity C reactive protein in patients with chronic low back pain and acute sciatic pain. Ann Rheum Dis 2005;64:921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sullivan MJ, Thibault P, Andrikonyte J, Butler H, Catchlove R, Larivière C. Psychological influences on repetition-induced summation of activity-related pain in patients with chronic low back pain. PAIN 2009;141:70–8. [DOI] [PubMed] [Google Scholar]
- [96].Suri P, Palmer MR, Tsepilov YA, Freidin MB, Boer CG, Yau MS, Evans DS, Gelemanovic A, Bartz TM, Nethander M, Arbeeva L, Karssen L, Neogi T, Campbell A, Mellstrom D, Ohlsson C, Marshall LM, Orwoll E, Uitterlinden A, Rotter JI, Lauc G, Psaty BM, Karlsson MK, Lane NE, Jarvik GP, Polasek O, Hochberg M, Jordan JM, Van Meurs JBJ, Jackson R, Nielson CM, Mitchell BD, Smith BH, Hayward C, Smith NL, Aulchenko YS, Williams FMK. Genome-wide meta-analysis of 158,000 individuals of European ancestry identifies three loci associated with chronic back pain. PLoS Genet 2018;14:e1007601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tanguay-Sabourin C, Fillingim M, Parisien M, Guglietti GV, Zare A, Norman J, Da-ano R, Perez J, Thompson SJ, Martel MO, Roy M, Diatchenko L, Vachon-Presseau E. A data-driven biopsychosocial framework determining the spreading of chronic pain. medRxiv 2022. doi: 10.1101/2022.07.22.22277850. [DOI] [Google Scholar]
- [98].Teckchandani S, Nagana Gowda GA, Raftery D, Curatolo M. Metabolomics in chronic pain research. Eur J Pain 2021;25:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain 2012;28:819–41. [DOI] [PubMed] [Google Scholar]
- [100].Thomazeau J, Rouquette A, Martinez V, Rabuel C, Prince N, Laplanche JL, Nizard R, Bergmann JF, Perrot S, Lloret-Linares C. Acute pain Factors predictive of post-operative pain and opioid requirement in multimodal analgesia following knee replacement. Eur J Pain 2016;20:822–32. [DOI] [PubMed] [Google Scholar]
- [101].Thudium CS, Lofvall H, Karsdal MA, Bay-Jensen AC, Bihlet AR. Protein biomarkers associated with pain mechanisms in osteoarthritis. J Proteomics 2019;190:55–66. [DOI] [PubMed] [Google Scholar]
- [102].Tork S, Faleris J, Engemann A, Deister C, DeVinney E, Valerio IL. Application of a porcine small intestine submucosa nerve cap for prevention of neuromas and associated pain. Tissue Eng Part A 2020;26:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Vachon-Presseau E, Tétreault P, Petre B, Huang L, Berger SE, Torbey S, Baria AT, Mansour AR, Hashmi JA, Griffith JW, Comasco E, Schnitzer TJ, Baliki MN, Apkarian AV. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016;139:1958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Valencia C, Fillingim RB, Bishop M, Wu SS, Wright TW, Moser M, Farmer K, George SZ. Investigation of central pain processing in postoperative shoulder pain and disability. Clin J Pain 2014;30:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Volkow ND, Collins FS. The role of science in addressing the opioid crisis. N Engl J Med 2017;377:391–4. [DOI] [PubMed] [Google Scholar]
- [106].Wang D, Frey-Law LA. Multisensory sensitivity differentiates between multiple chronic pain conditions and pain-free individuals. PAIN 2023;164:e91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wang L, Guyatt GH, Kennedy SA, Romerosa B, Kwon HY, Kaushal A, Chang Y, Craigie S, de Almeida CPB, Couban RJ, Parascandalo SR, Izhar Z, Reid S, Khan JS, McGillion M, Busse JW. Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. CMAJ 2016;188:E352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang O, Lee SW, O'Doherty J, Seymour B, Yoshida W. Model-based and model-free pain avoidance learning. Brain Neurosci Adv 2018;2:2398212818772964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang WT, Zhao YN, Han BW, Hong SJ, Chen YQ. Circulating microRNAs identified in a genome-wide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. J Clin Endocrinol Metab 2013;98:281–9. [DOI] [PubMed] [Google Scholar]
- [110].Williams ACdC, Fisher E, Hearn L, Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2020;8:CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].`Wirth B, Humphreys BK, Peterson C. Importance of psychological factors for the recovery from a first episode of acute non-specific neck pain—a longitudinal observational study. Chiropract Man Ther 2016;24:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Woolf CJ. Capturing novel non-opioid pain targets. Biol Psychiatry 2020;87:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wylde V, Palmer S, Learmonth ID, Dieppe P. The association between pre-operative pain sensitisation and chronic pain after knee replacement: an exploratory study. Osteoarthritis Cartilage 2013;21:1253–6. [DOI] [PubMed] [Google Scholar]
- [114].Yang MMH, Hartley RL, Leung AA, Ronksley PE, Jetté N, Casha S, Riva-Cambrin J. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open 2019;9:e025091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yang XH, Zhang BL, Gu YH, Zhan XL, Guo LL, Jin HM. Association of sleep disorders, chronic pain, and fatigue with survival in patients with chronic kidney disease: a meta-analysis of clinical trials. Sleep Med 2018;51:59–65. [DOI] [PubMed] [Google Scholar]
- [116].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. PAIN 2008;138:22–8. [DOI] [PubMed] [Google Scholar]
- [117].Young Casey C, Greenberg MA, Nicassio PM, Harpin RE, Hubbard D. Transition from acute to chronic pain and disability: a model including cognitive, affective, and trauma factors. PAIN 2008;134:69–79. [DOI] [PubMed] [Google Scholar]
- [118].Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010;59:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A list of consortium member can be found at: http://links.lww.com/PAIN/B871.