Abstract
Short-chain fatty acids (SCFAs) are the main metabolites produced by bacterial fermentation of dietary fibre in the gastrointestinal tract. The absorption of SCFAs is mediated by substrate transporters, such as monocarboxylate transporter 1 and sodium-coupled monocarboxylate transporter 1, which promote cellular metabolism. An increasing number of studies have implicated metabolites produced by microorganisms as crucial executors of diet-based microbial influence on the host. SCFAs are important fuels for intestinal epithelial cells (IECs) and represent a major carbon flux from the diet, that is decomposed by the gut microbiota. SCFAs play a vital role in multiple molecular biological processes, such as promoting the secretion of glucagon-like peptide-1 by IECs to inhibit the elevation of blood glucose, increasing the expression of G protein-coupled receptors such as GPR41 and GPR43, and inhibiting histone deacetylases, which participate in the regulation of the proliferation, differentiation, and function of IECs. SCFAs affect intestinal motility, barrier function, and host metabolism. Furthermore, SCFAs play important regulatory roles in local, intermediate, and peripheral metabolisms. Acetate, propionate, and butyrate are the major SCFAs, they are involved in the regulation of immunity, apoptosis, inflammation, and lipid metabolism. Herein, we review the diverse functional roles of this major class of bacterial metabolites and reflect on their ability to affect intestine, metabolic, and other diseases.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-023-01219-9.
Keywords: Short-chain fatty acids, Gut microbiota, Inflammation, Metabolism, Immunity
Background
The human intestine contains a complex and diverse symbiotic microbial system that is mainly composed of bacteria, fungi, viruses, archaea, and protozoans [1]. Microbiota-derived metabolites are crucial mediators of host-microbial interactions. Recent studies have shown that the main metabolites of intestinal microbiota include short-chain fatty acids (SCFAs), secondary bile acids, trimethylamine, lipopolysaccharides (LPS), imidazopropionic acid, branched-chain amino acids, and indole and its derivatives, which affect metabolism, immunity, and tumour development [1, 2]. SCFAs are mainly produced by the intestinal microbiota from indigestible carbohydrates and host secretions via anaerobic fermentation and are one of the most important metabolite categories involved in the regulation of several biological functions [3, 4].
SCFAs regulate the structure of intestinal microbiota [5], enhance the function of the intestinal epithelial barrier [6], and are beneficial in delaying disease progression through a variety of ways, such as those in type 2 diabetes (T2D) [7], obesity [8], chronic kidney disease (CKD) [9], hypertension [9], inflammatory bowel disease (IBD) [10] and colorectal cancer (CRC) [7, 9–14]. Two signal transduction mechanisms mediate SCFA function; inhibition of histone deacetylase (HDAC) and activation of G protein-coupled receptors (GPCRs) [15]. HDAC-mediated epigenetic modifications play a vital role in gene expression [16]; thus, the inhibition of HDAC induced by SCFA affects the progression of diseases that include metabolic diseases [17], immune diseases [18], and cancer [19]. However, our understanding of SCFA-mediated inhibition remains incomplete [20]. GPCRs, particularly GPR43 (also known as free fatty acid receptor 2, FFAR2), GPR41 (FFAR3), and GPR109A (also known as hydroxycarboxylic acid receptor 2, HCAR2), have been identified as receptors for SCFAs. These GPCRs play important roles in the regulation of metabolism and inflammation [15, 21–24]. In this review, we summarise the classification, source, and role of SCFAs in diseases. The information is intended to provide a perspective for future studies of impact of SCFAs on diseases.
Source and function of SCFAs
Saturated fatty acids with a chain length of 1 to 6 carbon atoms are defined as SCFAs [3, 25]. The most abundant SCFAs in the intestine are acetate, propionate, and butyrate [26]. SCFAs act on many cell types to regulate important biological processes, including host metabolism, intestinal function, and immunity [4, 27–31]. In the human colon and faeces, the molar ratio of acetate: propionate: butyrate is approximately 60:20:20 [21, 32]. Each SCFA is produced via bacterial fermentation. Therefore, the main reason for the different proportions of acetate, propionate, and butyrate was the catabolism of the different bacteria [26]. Cross-feeding with acetate- and propionate-producing bacteria, such as Akkermansia muciniphila, and butyrate-producing bacteria, such as Faecalibacterium prausnitzii, can improve the levels of intestinal SCFAs and have good preventive and therapeutic effects on inflammation and tumours [33]. This review focusses on the sources and functions of acetate, propionate, and butyrate.
Acetate
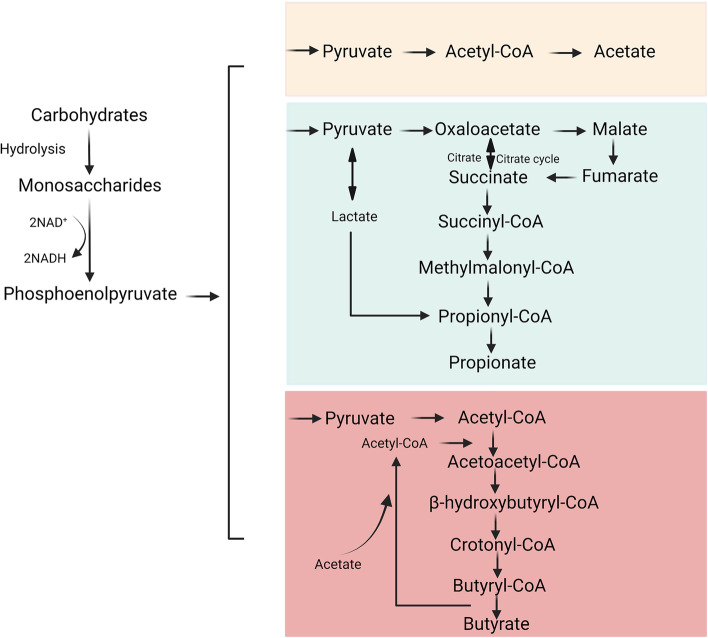
Acetate is mainly produced by anaerobic bacteria that digest dietary fibres in the animal colon. Several kinds of bacteria, such as A. muciniphila and Bacteroides spp. produce acetic acid through fermentation (Table 1) [21, 27, 34–36]. This process is mainly converted to acetate by acetyl-CoA produced by glycolysis, which is also enzymatically converted to butyrate by butyryl-CoA:acetyl-CoA transferase (Fig. 1) [21]. The highest concentration of this fermentation product is found in the proximal colon, where it is absorbed by intestinal epithelial cells (IECs) or transported to the blood through the intestinal epithelium and then quickly absorbed by the liver via the hepatic portal vein [15, 37]. In the blood, acetate exists as a free acid that is metabolised mainly in the liver [38], brain [39], heart [40], and muscles [41]. Acetate also has a wide range of effects on tissues and organs [42], is an important biofuel and nutrient source for tumour cells, and is involved in lipid synthesis [43]. In mice, fructose in the diet is converted to acetate by intestinal microbes, thereby providing acetyl-CoA for fat production [38]. In the brains of germ-free mice, acetate drives microglial maturation and regulates metabolic homeostasis [39]. Acetate maintains energy balance and metabolic homeostasis, resists oxidation and mitochondrial stress, affects immunity, and controls body weight and insulin sensitivity by affecting lipid metabolism and glucose homeostasis in animals and humans [38, 44–47]. Taken together, these data suggest that acetate is essential for lipid metabolism, weight control, neurological diseases, and tumour development of tumors. However, further studies are needed to explore the mechanisms by which acetate functions in these diseases and to determine the individual metabolic differences in the application of acetate.
Table 1.
Sources of acetate, propionate, and butyrate
| SCFAs | Origin | References |
|---|---|---|
| Acetate | Akkermansia muciniphila, Bacteroides spp., Bifidobacterium spp., Prevotella spp., Ruminococcus spp., Escherichia coli, Blautia hydrogenotrophica, Clostridium spp., and Streptococcus spp. | [21, 27, 34–36] |
| Propionate | Bacteroides uniformis, Bacteroides vulgatus, Prevotellacopr, Alistipes putredinis, Roseburia inulinivorans, Eubacterium hallii, Blautiaobeum, Coprococcuscatus, Dialisterinvisus Phascolarctobacterium succinatutens, Akkermansia muciniphila, Dialister spp., Veillonella spp., Megasphaera elsdenii, Coprococcuscatus, Bacteroides spp., Salmonella spp., Roseburia inulinivorans, and Ruminococcus obeum | [21, 34, 48–51] |
| Butyrate | Clostridium clusters IV and XIVa, Faecalibacterium prausnitzii, Ruminococcus bromii, Lachnospiraceae, Eubacterium rectale, Roseburia spp., Roseburia inulinivorans, Roseburia intestinalis, Eubacterium hallii, Anaerostipes hadrus, Anaerostipes spp., Coprococcus eutactus, Coprococcus comes, Coprococcuscatus, Subdoligranulum variabile, Eubacterium biforme, Actinobacteria, Fusobacteria, Spirochaetes, and Thermotogae | [34, 48, 50, 52–55] |
Fig. 1.
Schematic diagram of carbohydrate fermentation pathways producing acetate, propionate and butyrate
Propionate
Propionate is primarily derived from carbohydrate metabolism during glycolysis, mainly through the succinate pathway [48] (Fig. 1), as indicated by the widespread distribution of the methylmalonyl-CoA decarboxylase (mmdA) gene in Bacteroidetes and many Negativicutes [56]. Bacteroides and gram-negative bacteria, such as A. muciniphila, and Roseburia inulinivorans, are frequent human coliforms (Table 1) [21, 34, 48–51, 57].These microorganisms are involved in maintaining human health [34, 48, 56]. Zhang et al. described that propionate in chickens increased the secretion of glucagon-like peptide-1 (GLP-1) by IECs, inhibited hepatocyte adipogenesis, and reduced fat deposition in vitro [57]. Propionate also participates in liver gluconeogenesis in rats by acting on hydroxy-methylglutaryl-CoA reductase (3-hydroxy-3-methylglutaryl-CoA, HMG-CoA) to inhibit the synthesis of cholesterol in the liver [58, 59]. As a GPR41 agonist, propionate targets the enteric nervous system and exerts neuroprotective effects in mice with 6-hydroxydopamine-induced Parkinson’s disease (PD) [60]. Studies have suggested that T lymphocytes are sensitive to immunological challenges in the body, which is further supported by animal studies showing that propionate supplementation is either neutral or beneficial for host immune activity against bacteria and viruses [61]. In addition, propionate has a vascular protective effect in NMRI mice; it first acts on the immune system to inhibit the activity of helper T cells, and then on the cardiovascular system to reduce cardiovascular damage from hypertension [62, 63]. In C57BL/6 J mice, propionate inhibits intestinal cholesterol absorption, regulates dyslipidaemia, and reduces the area of aortic atherosclerosis [64]. These findings indicate crucial roles of propionate in lipid metabolism, the nervous system, and cardiovascular diseases. Although studies have shown that propionate is neutral or beneficial to the host by destroying bacteria and viruses, current knowledge is insufficient to explain how propionate supplementation affects immune responses to specific pathogens.
Butyrate
Many commensal bacteria such as Clostridium clusters IV and XIVa, and F. prausnitzii, promote the synthesis of butyrate, which is then absorbed by human host IECs [14, 25, 65] (Table 1) [34, 48, 50, 52–55]. As an important metabolite off gut microbes, butyrate plays a critical role in the maintenance of energy supply in colon cells [27]. Butyrate is produced from carbohydrates via glycolysis via the combination of two acetyl-CoA molecules to form acetoacetyl-CoA, followed by a stepwise reduction to butyryl-CoA. The final step in the formation of butyrate from butyryl-CoA involves two different approaches; either the butyryl-CoA:acetate CoA-transferase route or the phospho-butyrate and butyrate kinase pathways (Fig. 1) [48]. Butyrate restores the epithelial barrier of the airway by inhibiting interleukin (IL)-6 production and by reducing IL-4 and zonula occludens protein 1 (ZO-1) expression in 16HBE human bronchial epithelial cells, which induces extracellular signal-regulated protein kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) phosphorylation [66]. Butyrate provides energy for normal IECs, whereas cancerous colon cells depend on glucose as their main energy source due to Warburg effect. Butyrate accumulates and acts as an HDAC inhibitor preventing the proliferation of HCT116 colon cancer cells [67]. Butyrate participates in maintaining the integrity of colonic mucosa in BALB/c mice, resisting colitis, and preventing the occurrence and development of cancer by regulating cell proliferation, apoptosis, and differentiation [27, 42, 68]. In intestinal diseases, butyrate enhances intestinal barrier function and suppresses intestinal inflammatory responses in mice by interacting with GPCRs and inhibiting HDACs [69]. These findings indicate that butyrate improves colonic inflammation and inhibits the occurrence and development of colon cancer, mainly by enhancing intestinal barrier integrity.
Receptors for SCFAs
GPCRs are the largest family of receptors in mammals, comprising seven transmembrane domains. These receptors participate in the regulation of almost all cellular physiological functions in vivo [70]. GPCRs can bind to chemicals in the extracellular environment, such as hormones, neurotransmitters, chemokines, sugars, and lipids [71]. GPR41 and GPR43 are considered the most important SCFA receptors in the GPCR family [72]. A recent study demonstrated that SCFAs can also activate GPR109A. Unlike GPR41 and GPR43, GPR109A is activated by longer SCFAs, mainly C4 [73]. Different SCFAs receptors have different affinities for each other. In humans, the affinity ranking of GPR41 is C3 = C4 = C5 > C2 > C1, and that of GPR43 is C2 = C3 > C4 > C5 = C1 [71, 74]. GPCRs are reportedly associated with metabolic diseases [75], neurological diseases [76], inflammation [77], cardiovascular disease [78], and cancer [79]. Therefore, GPCRs have attracted attention as potential therapeutic targets for various of diseases.
GPR41
GPR41 is a Gi/o-coupled receptor for SCFAs with ligands including acetate (C2), propionate (C3) and butyrate (C4) [80]. Recent findings have shown that SCFAs produced by microbial fermentation act as signalling molecules through receptors, such as GPR41 [81]. Thus, the gut microbiome plays a key role in host physiological and pathological processes via these receptors. Exogenous supplementation with SCFAs reduces liver fat content and improves liver metabolism by inhibiting the expression of lipid synthesis genes in GPR41−/− mice [82]. The effect of propionate on allergic inflammation is dependent on GPR41 but not on GPR43. Activation of GPR41 by propionic acid treatment leads to changes in bone marrow haematopoietic function in mice, characterised by enhanced production of macrophages and dendritic cell precursors, followed by implantation of highly phagocytic dendritic cells in the lungs, which shape the immune environment of the lungs and influences the severity of allergic inflammation [83]. In addition, SCFAs promote IL-22 production by human CD4+T and innate lymphoid cells through GPR41, thereby protecting the intestinal tract from inflammation and maintaining intestinal homeostasis [18]. Therefore, GPR41, as an SCFA receptor, plays an important role in immune, inflammatory, metabolic, and other diseases, and is worthy of further exploration.
GPR43
GPR43 is activated by acetate (C2), propionate (C3), and butyrate (C4) [80]. GPR43 is a Gi/o- and Gq double-coupled receptor expressed in intestinal endocrine cells, such as L cells [84]. GPR43 is also present in immune cells, such as monocytes, neutrophils, eosinophils, and regulatory T cells (Tregs) [85–87]. Levels of regenerating islet-derived protein 3 gamma (RegIIIγ) and β-defensin expression levels are decreased in IECs of GPR43−/− mice. The oral administration of SCFAs in wild-type mice maintains intestinal homeostasis by activating the mammalian target of rapamycin (mTOR) and signal transducer and activator of transcription 3 (STAT3) pathways in IECs to produce antimicrobial peptides [88]. In T2D and diabetic nephropathy mouse models induced by high fat diet (HFD) and streptozolectin, GPR43 activation inhibits hyperglycaemia-induced oxidative stress and nuclear factor-κB (NF-κB) activation, enhances the interaction between β-arrestin-2 and I-κBα, and reduces kidney damage. Silencing of GPR43 by short interfering RNA inhibits this effect in mouse glomerular mesangial cells [89]. GPR43-deficient mice beccome obese under normal diet conditions, whereas mice specifically expressing GPR43 in adipose tissue remain slim even when fed a HFD. SCFA mediated GPR43 activation inhibits insulin signallings in adipose cells, thus inhibiting fat accumulation in adipose tissue and promoting lipid and glucose metabolism in other tissues. These findings implicate GPR43 as a sensor of excess dietary energy, thus controlling energy utilisation and maintaining metabolic balance [90]. GPR43 is expressed in adipose tissue, intestinal tract, and islet cells, and plays important roles in various physiological functions.
GPR109A
GPR109A was originally identified as a nicotinic acid receptor that is activated by β-hydroxybutyrate and butyrate but not by acetate and propionate [91]. GPR109A is a Gi/o coupling protein expressed in colonic epithelial cells. The protein is downregulated in germ-free mice [92]. Butyrate alleviates osteolysis in mice by activating its receptor GPR109A, thereby inhibiting the activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome induced by titanium particles [93]. Obese mice treated with butyrate displayed improved glucose metabolism and inhibited adipose tissue inflammation via GPR109A activation. Thus, targeting GPR109A to reduce metabolic and inflammatory dysfunctions is a potential new approach for treating obesity [94]. GPR109A activated by butyrate promotes the anti-inflammatory properties of colon macrophages and dendritic cells in C57BL/6 mice, induces the differentiation of Tregs and IL-10-producing T cells, and inhibits colon inflammation and carcinogenesis [95]. The collective findings demonstrate that GPR109A is expressed in many organs and cells and is beneficial for metabolism and immunity. The mechanism requires further study.
SCFAs and diseases
SCFAs are closely associated with the occurrence and development of metabolic diseases, inflammation, and tumours (Fig. 2). An increasing number of studies have indicated that SCFAs act on membrane protein receptors GPCRs [96], and intracellular enzymes in IECs, or enter the circulatory system to regulate energy, glucose, and lipid metabolism [97]. SCFAs may function in three ways. The first is via interactions with GPR41, GPR43, and GPR109A, which are expressed in various organs that include the intestine, kidney, and heart [32]. The interactions are involved in the stimulation of phospholipase-Cβ, leading to the release of intracellular Ca2+ and activation of protein kinase C, in addition to cAMP accumulation and protein kinase A and ERK activation [98]. The second way is via transcriptional regulation and post-translational modifications. SCFAs, particularly butyrate, act as HDAC inhibitors [99, 100], promoting gene expression and regulating cell metabolism, differentiation, and proliferation [67]. In the third way, SCFAs enter cells through transporters such as the monocarboxylate transporter (MCT1) and sodium-coupled MCT (SMCT1) [3], which regulate cellular glucose metabolism, lipid metabolism, and immune function [21]. In this review, we summarise the mechanisms through which SCFAs ameliorate multiple diseases. The intent is to provide a perspective for future studies exploring the effects of SCFAs on diseases.
Fig. 2.
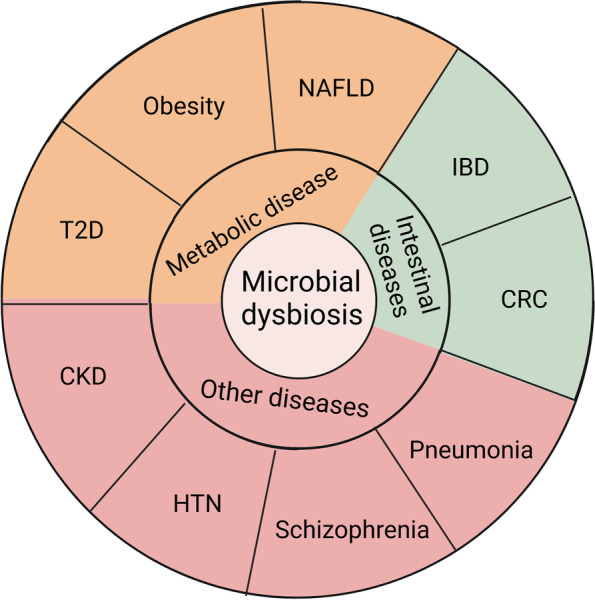
Impact of intestinal microbes on human diseases. T2D: type 2 diabetes; NAFLD: non-alcoholic fatty liver disease; IBD: inflammatory bowel disease; CRC: colorectal cancer; HTN: hypertension; and CKD: chronic kidney disease
Intestinal diseases
IBD
IBD, which includes ulcerative colitis and Crohn’s disease, is an idiopathic intestinal inflammatory disease of the ileum, rectum, and colon [101]. Both IBD subtypes are characterised by repeated cycles of epithelial damage, infiltration of inflammatory cells into the lamina propria, and failure of immune regulation to control the inflammatory response, resulting in recurrent cycles of chronic inflammatory remission and relapse in the gastrointestinal tract [102]. An imbalance in the intestinal microbiome is closely associated with IBD. SCFA-producing bacteria, such as F. prausnitzii, and Roseburia intestinalis, are significantly reduced in patients with IBD (Table 2) [21, 103–105]. Their reductions lead to impaired crosstalk between bacterial and immune cells. The concentrations of acetate and propionate are significantly reduced in the intestinal lumen of IBD patients [104]. SCFAs have important immunomodulatory effects due to their regulation of innate and adaptive immune cell generation, function, and trafficking, which play beneficial roles in IBD [102].
Table 2.
Intestinal microbiota imbalance in various diseases
| Disease | Microorganism (elevated) | Microorganism (reduced) | References |
|---|---|---|---|
| IBD | Bifidobacterium, Staphylococcus, Enterococcus, Lactobacillus, Pseudomonas, Klebsiella, and Proteus genera | Bacteroidetes, Faecalibacterium prausnitzii, Roseburia intestinalis, Clostridium leptum, and Akkermansia muciniphila, Actinobacteria, Fusobacteria, Proteobacteria, Spirochaetes, Thermotogae, Butyricicoccus pullicaecorum, and Rhominis | [21, 103–105] |
| CRC | Bacteroides sp., C. cocleatum, Collinsella spp, S. xylosus, P. excrementihominis, Muribaculum spp, A. equolifaciens, P. goldsteinii, F. aecalibaculum spp, B. faecis, Escherichia coli, and Bacteroides fragilis | Akkermansia muciniphila, B. pseudolongum, L. johnsonii, Olsenella spp, Prevotellasp, Colidextribacter spp, Bacillus spp, B. acidifaciens, L. reuteri, and E. faecalis | [106, 107] |
| Obesity | Firmicutes | Bacteroides, and Bifidobacteria | [108–111] |
| T2D | Proteobacteria, Lachnospiraceae, Ruminococcaceae, and Acinetobacter baumanii | Bacteroides | [112–114] |
| NAFLD | Anaerococcus, Ruminococcus, Peptoniphilus, Dorea, Bradyrhizobium, Propionibacterium acnes, Bateroides, Mucispirillum, Desulfovibrio, Anaerotruncus, and Desulfovibrionaceae | Bacteroides, Lactobacillus curvatus, L. plantarum, Bifidobacterium spp., Rikenellaceae, Oscillospira, Prevotella, Bifidobacterium | [115–118] |
| Pneumonia and respiratory diseases | Coprobacillus, Clostridium ramosum, and C. hathewayi | Faecalibacterium prausnitzii, Bacteroides dorei, B. thetaiotaomicron, B. massiliensis, and B. ovatus | [119, 120] |
| CKD | Firmicutes, Proteobacteria phyla, Akkermansia muciniphila, Ruminococcus, Romboutsia, and Actinobacteria | Bacteroides, Faecalibacterium prausnitzii, Enterobacter, Enterococcus, Bifidobacterium, Roseburia, Clostridium, Coprococcu, and Lactobacilli | [12, 106, 121, 122] |
| Hypertension | Bacteroides, Klebsiella, Parabacteroides, Desulfovibrio, Lachnospiraceae, Ruminococcus, Actinobacteria, and Phascolarcto bacterium | Proteobacteria, Ruminococcaceae, Roseburia, Faecalibacterium spp., Lactobacillus, Oscillibacter, Lachnospira, Prevotella, and Alistipes | [13, 29, 123–125] |
| Neurologic disorders | Streptococcus vestibularis, Akkermansia muciniphila, Bacteroides plebeius, Veillonellaparvula, Clostridium symbiosum, Eubacterium siraeum, Cronobacter sakazakii/turicensis, S. vestibularis, Alkaliphilus oremlandii, Enterococcus faecium, Bifidobacterium longum, Ruminococcaceae, Bacteroides, Coprococcussp., Anaerococcus, and Lactobacillus fermentum | Proteobacteria, Faecalibacterium prausnitzii, Haemophilus spp., Sutterella spp., Clostridium spp., Gemmiger, Roseburia, Lachnospira, and Anaerostipes | [126–128] |
Hung et al. showed that pretreatment of Caco-2 cells with acetate and propionate inhibited tumour necrosis factor-alpha (TNF-α)-induced inflammation by suppressing the activation of NF-κB p65 [129], spleen tyrosine kinase, and mitogen-activated protein kinase (MAPK) [130]. SCFAs, such as acetate, propionate, and butyrate, inhibit AKT and NF-κB by activating cell surface GPCRs. Inhibition of the NF-κB p65 signalling pathway reduces colon inflammation induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) in mice and ameliorates intestinal epithelial barrier dysfunction [131]. In a mouse model of colitis, butyrate treatment induced the differentiation of naïve T cells into Tregs by enhancing promoter histone H3 acetylation and a conserved noncoding sequence region in forkhead box protein 3 (Foxp3) [132]. In addition, SCFAs promote the expression of Foxp3 by activating GPR43 on T cells and induce the polarisation of intestinal T cells to Tregs in mice with colitis to inhibit the inflammatory response [133]. IBD is often accompanied by intestinal bleeding; Holtug et al. described that the concentration of SCFAs in the colons of IBD patients without intestinal bleeding was normal, while different concentrations of SCFAs were evident in the colons of IBD patients with bleeding [134]. In vitro co-culture of blood and faeces revealed that acetate and propionate levels were significantly reduced, whereas long-chain FAs, such as valerate, were significantly increased. The findings suggest that bleeding, as a risk factor in patients with IBD, contributes to the imbalance of SCFAs and accelerates the progression of IBD [134]. Concentrations of SCFAs can be decreased in IBD patients, and supplementation with SCFAs can send signals through GPCRs on the cell surface to activate signalling cascades that modulate immune function, thereby suppressing intestinal inflammation (Fig. 3). SCFAs are host-beneficial microbial products that can be supplemented through diet [21]. No significant adverse effects were observed in studies on SCFAs in IBD [104, 131, 135, 136].
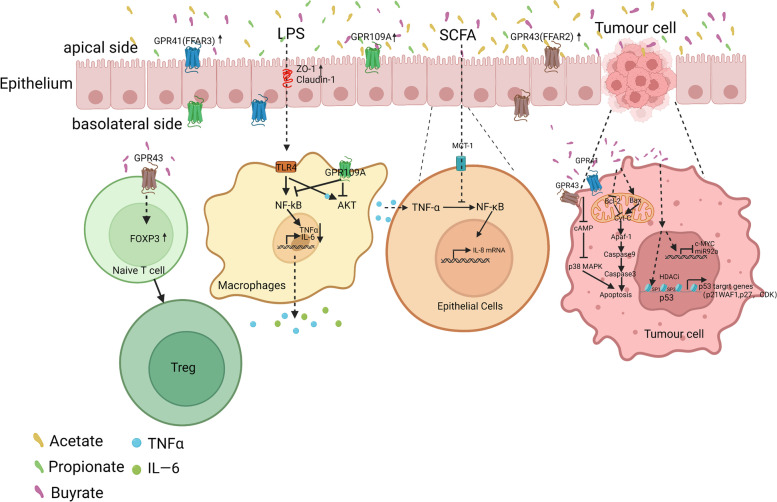
Fig. 3.
SCFAs alleviate IBD and CRC by increasing intestinal barrier function and reducing endotoxin levels in the blood. SCFAs increase the intestinal barrier function, reduce the entry of lipopolysaccharide (LPS) into the blood, induces naive T cells to differentiate into Tregs, inhibit the production of tumour necrosis factor-alpha (TNF-α) and interleukin (IL)-6 by intestinal macrophages, and inhibit the production of IL-8 by normal intestinal epithelial cells (IECs) to alleviate inflammation. Butyrate promotes CRC cell apoptosis by inhibiting C-AMP/P38-MAPK and increasing Bax/Bcl-2 ratio. Other abbreviations are: SCFA: short-chain fatty acid; GPCR: G protein-coupled receptor; FFAR: free fatty acid receptor; IBD: inflammatory bowel disease; CRC: colorectal cancer; AKT: protein kinase B; TLR4: Toll-like receptors 4; and HDACi: histone deacetylase inhibitor
CRC
CRC has the third-highest incidence among gastrointestinal tumours [137]. The main types of CRCs are adenocarcinoma, mucinous adenocarcinoma, and undifferentiated carcinoma [138]. CRC is associated with HFD, stress, antibiotics, synthetic food dyes, monosodium glutamate, titanium dioxide, physical inactivity and/or sedentary behaviour, environmental factors, and other factors [139]. Among these, HFD is a key factor in CRC owing to the westernisation of the global diet, which involves high intake of red and processed meat, high fructose corn syrup, and unhealthy cooking methods [140]. Current research focuses on the role of different intestinal contents, such as fibres, vitamins, and metabolite imbalances in CRC [141]. The abundance of Escherichia coli and Bacteroides fragilis is reportedly higher in the intestines of patients with CRC, whereas the abundance of typical SCFA-producing bacteria, such as A. muciniphila, are significantly reduced (Table 2) [106, 107]. SCFAs are produced by catabolic fibre metabolism; butyrate is the main energy source for IECs. Butyrate inhibits the occurrence and development of colon cancer by regulating the expression of tumour suppressor genes and promoting apoptosis [142].
As an energy metabolite, butyrate promotes the proliferation of normal colon cells. Due to the Warburg effect, cancerous colonocytes rely on glucose as their primary energy source [67]. Therefore, butyrate accumulates in cancer cells, and high concentrations of butyrate acts as an inhibitor of HDAC and epigenetic inhibit the proliferation of cancer cells [143]. In HCT-116 cells, butyrate promotes pyruvate kinase isozyme 2 (PKM2) dephosphorylation and tetramerization to activate PKM2. This reprogrammes the metabolism of CRC cells, inhibits the Warburg effect, and promote energy metabolism [144]. Interestingly, in one study, the butyrate producer Fusobacterium nucleatum did not act as expected to inhibit colon cancer [145]. The authors reported that infection of HCT116 cells with F. nucleatum activated the Toll-like receptors 4 (TLR4)/ myeloid differentiation primary response 88 (MYD88)/NFκB signalling pathway, thereby increasing the expression of microRNA (miRNA) 21 (miR21) and promoting the malignant phenotype of colon cancer cells [145]. In patients with colon cancer, F. nucleatum activates the transcription of long noncoding RNA (lncRNA) ENO1 Intronic Transcript 1 (ENO1-IT1) by up-regulating the binding efficiency of transcription factor SP1 to the promoter region of lncRNA ENO1-IT1 and promoting glucose metabolism in CRC cells to induce cancer [146]. These findings suggest that the butyrate-producing bacterium F. nucleatum mainly promotes the development of colon cancer through other mechanisms, while the inhibitory effect of butyrate is weakened.
As an HDAC inhibitor, butyrate upregulates various signalling pathways, such as Janus kinase 2 (JAK2)/STAT, vascular endothelial growth factor, protein kinase C/WNT, and miRNA [147]. Butyrate promotes hyperacetylation of p53, which in turn increases the transcription and translation of p53 in RKO CRC cells [148]. Simultaneously, butyrate also promotes the acetylation of Sp1 and Sp3 in colon cancer HT-29 cells and further upregulates the expression of p53 targets (p21WAF1, p27, and cyclin-dependent kinases), induces cell cycle arrest, and promotes cancer cell apoptosis [149]. In addition, butyrate upregulates the expression of apoptosis-inducing factor 1, mitochondrial (AIFM1) to induce apoptosis in HT29 CRC cells [150]. In HCT-116 cells, butyrate inhibits the proto-oncogene c-Myc [151], thereby suppressing the transcription of miR-92a, promoting cell differentiation and apoptosis, and exerting anti-tumour effects in vivo [152, 153]. In a male BALB/c mouse model of colitis-associated CRC, tumours were induced by azoxymethane and dextran sodium sulfate. Combined administration of SCFAs can inhibit the expression of pro-inflammatory cytokines, including IL-6, IL-17, TNF-α, thereby preventing tumour development and reducing colon inflammation [154]. Therefore, butyrate induces CRC cell apoptosis by increasing the expression of apoptosis-inducing factors and tumour suppressor genes by acting as an HDAC inhibitor (Fig. 3). In the future, it will be interesting to test whether the addition of butyrate or administration of butyrate-producing bacteria foods, such as omega-3 polyunsaturated fatty acids, to the diet, will effectively prevent CRC and reduce the incidence of CRC.
Metabolic diseases
Metabolic diseases include obesity, T2D, non-alcoholic fatty liver disease (NAFLD), thyroid disease, hyperuricemia, hyperlipidemia, and others. [155, 156]. The gut microbiota and SCFAs affect host metabolism, and dysbiosis is believed to be one of the main causes of common metabolic diseases in humans [2]. In mice, propionate and butyrate produced by the consumption of soluble dietary fibres activate intestinal gluconeogenesis (IGN) through a complementary mechanism [157]. IGN has beneficial effects on glucose and energy homeostasis, promotes metabolism, and regulates body weight and blood sugar levels. Butyrate activates IGN gene expression through a cAMP-dependent mechanism. Furthermore, as the substrate of IGN, propionate promotes IGN gene expression by activating the gut-brain neural circuit [157]. SCFAs promote IGN production to prevent metabolic diseases in mice [158]. Most studies investigating the effect of the gut microbiota and its metabolites on metabolic diseases have focussed on obesity, T2D, and NAFLD [156, 159], with few reports on thyroid diseases and hyperuricaemia. Therefore, the intestinal microbiota and its metabolites have relatively broad therapeutic prospects in the research of metabolic diseases, such as thyroid disease and hyperuricaemia. However, this review focusses on three widely studied metabolic diseases: obesity, T2D, and NAFLD.
Obesity
Obesity is a global health concern. HFD and high-carbohydrate diets reduce the diversity and abundance of human intestinal microbes, leading to fat accumulation and obesity [160]. Studies have demonstrated that the ratio of Firmicutes/Bacteroidetes in the intestinal lumen of obese patients increases, whereas the abundance of the SCFA-producing bacteria Bacteroides and Bifidobacteria decreases (Table 2) [108–111, 161–163]. The host gains extra energy from SCFAs through the activity of gut microbiota [164, 165]. SCFAs are key factors involved in obesity resistance in both animals and humans [108].
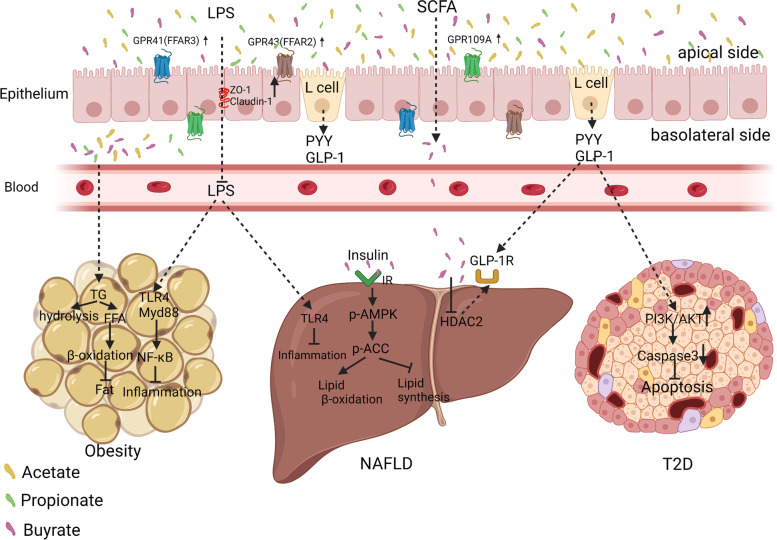
In one study, 3T3-L1 mouse embryonic fibroblasts (preadipocytes) treated with acetate and propionate displayed expressions of GPR43, peroxisome proliferator-activated receptor-gamma 2 (PPAR-γ2), and leptin, which in turn promoted lipolysis metabolism [166]. In germ-free mice, inulin reportedly can restore the flora imbalance caused by HFD and promotes the production of SCFAs and IL-22, thereby preventing metabolic syndrome [167]. Lu et al. found that dietary supplementation with SCFAs, such as acetate, propionate, butyrate, or their mixtures, increased the expressions of GPR43 and GPR41 in adipose tissue, promoted the hydrolysis of triglycerides and promoted oxidation of free fatty acids in adipose tissue to produce brown fat, and reduced body weight in an HFD-fed mouse model [110]. In addition, supplementation of HFD mice with butyrate in increased the expression of PPAR-γ coactivator-1 alpha (PGC-1α), activated AMPK and p38, and enhanced insulin sensitivity [168]. Ganoderma lucidum is a medicinal mushroom that has been used to promote health, prolong life, and prevent diseases in Asian countries for more than 2000years [169]. The sporoderm-broken spores of G. lucidum (BSGL) increase the production of SCFAs and the expression of GPR43 in C57BL/6 J mice, promote the expression of ileal tight junction proteins and antibacterial peptides, improve endotoxaemia, and significantly reduce the upregulation of TLR4/Myd88/NF-κB signalling in adipose tissue induced by HFD (Fig. 4) [170]. Acute administration of inulin-propionate (which can be metabolised by the microbiota into propionate in the colon) to overweight adults significantly increases postprandial GLP-1 and peptide YY levels through the action of GPR43, promoting glucose decomposition and weight loss [108]. Reducing caloric intake and long-term propionate supplementation can significantly reduce weight gain [108]. Farup et al. tested the levels of SCFAs in the faeces of 90 patients undergoing bariatric surgery. The total SCFA levels were decreased 6 months after surgery, including those of acetate, propionate, butyrate, and branched-chain SCFAs [171]. The collective findings demonstrate the reduced SCFA content in the intestinal tract of obese patients, enhanced intestinal barrier function following SCFA supplementation, reduced endotoxaemia, promoted oxidative decomposition of free fatty acids, and reduced fat accumulation. However, few in-depth mechanistic studies have investigated the role of SCFAs in the treatment of obesity. Such studies are warranted.
Fig. 4.
Intestinal microbiota metabolites acetate, propionate, and butyrate alleviates obesity, non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes (T2D). Short-chain fatty acids (SCFAs) increase the intestinal barrier function by elevating the expression of zonula occludens-1 (ZO-1) and claudin-1 in IECs, preventing lipopolysaccharide (LPS) from entering the blood from the intestine, and further inhibiting adipose tissue inflammation. SCFAs promote the production of glucagon-like peptide-1 (GLP-1) in L cells; GLP-1 enters the liver and binds to the GLP-1 receptor on the surface of hepatocytes, promoting hepatic β-oxidation by activation of AMPK, thereby relieving fatty liver. GLP-1 activates PI3K/AKT signalling in pancreatic islets, inhibits apoptosis, and alleviates T2D. Other abbreviations are: GPCR: G protein-coupled receptors; FFAR: free fatty acid receptor; NF-κB: nuclear factor kappa-B; TG: triacylglycerol; TLR4: Toll-like receptor 4; p-ACC: phospho-acetyl-CoA carboxylase; p-AMPK: phospho-adenosine monophosphate-activated protein kinase; PYY: Peptide YY; and IECs: intestinal epithelial cells
T2D
T2D is a metabolic disorder characterised by unbalanced blood sugar and lipid levels. Unbalanced gut microbiota is a factor in the rapid progress of T2D insulin resistance and account for approximately 90% of diabetes cases worldwide [172]. The abundance of Proteobacteria and the ratio of Firmicutes/Bacteroidetes are reportedly higher in T2D patients than in healthy subjects, whereas the abundance of SCFA-producing Bacteroides is reduced (Table 2) [112–114]. Levels of SCFAs, bile acids, and lipids in T2D patients are significantly dysregulated [112]. An increasing number of studies have shown that SCFAs play important roles in the restoration of insulin sensitivity [11].
Acetate and butyrate activate GPR43 and GPR41 on the surface of rat intestinal cells, promoting the secretion of insulin, GLP-1, and peptide YY, thereby regulating blood lipid energy metabolism and reducing peripheral blood glucose levels [173]. A human genome-driven increase in gut butyrate production is associated with improved insulin response after an oral glucose tolerance test, whereas abnormalities in the production or absorption of propionate are causally related to an increased risk of T2D [174]. Probiotics increase the activities of GPR43/41, proglucagon, and proconvertase 1/3, enhance insulin secretion through GLP-1 secretion triggered by glucose, and activate the phosphoinositide 3-kinase (PI3K)/AKT pathway to inhibit pancreatic cell apoptosis [174]. The findings show that SCFAs can reduce peripheral blood glucose levels and improve insulin resistance by increasing the levels of GPCRs and promoting the secretion of insulin, GLP-1, and peptide YY (Fig. 4). However, suitable doses and acceptable exposure times for SCFA treatment in T2D are undefined. In addition, whether there are time- and dose-dependent effects for SCFA treatment remains uncharacterized.
NAFLD
The gut-liver axis refers to the bidirectional relationship between the gut and its microbiota and the liver, resulting from the integration of signals generated by dietary, genetic, and environmental factors [175]. This reciprocal interaction is established by the portal vein, which enables the transport of gut-derived products directly to the liver and the liver feedback route for bile secretion into the intestine [176]. The abundance of the SCFA-producing bacteria Bacteroides, Lactobacillus curvatus, and L. plantarum in the gut of NAFLD patients is significantly decreased (Table 2) [115–118]. Growing evidence supports the pathogenic role of microbe-derived metabolites, such as trimethylamine, secondary bile acids, SCFAs, and ethanol, in the pathogenesis of NAFLD [175]. Acetate provides a substrate for liver fat synthesis to promote fat production, whereas propionate can alter liver metabolism and reduce lipid storage, thus playing an important role in patients with NAFLD [177].
Expression of the GLP-1 receptor in the liver of NAFLD patients is significantly downregulated, and butyrate supplementation enhances the expression of the GLP-1 receptor in the livers of mice with NAFLD by inhibiting HDAC-2, which in turn promotes energy metabolism and inhibits lipid accumulation [178]. It also enhances insulin sensitivity, activates AMPK to promote the expression of fatty acid oxidation genes in hepatocytes, and reduces fat deposition in NFLAD mice [179]. In an HFD-induced NAFLD mouse model, dietary supplementation with sodium butyrate reportedly increased the abundance of Christensenellaceae, Blautia, and Lactobacillus in the intestine, forming a beneficial positive feedback cycle by producing more butyric acid [180, 181]. In another study, butyrate attenuated NAFLD-induced intestinal mucosal injury by increasing the expression of ZO-1 in the intestinal tract of mice, thereby preventing the migration of enterotoxins to the liver and inhibiting liver inflammation [182]. In humans, acute administration of inulin-propionate (which can be metabolised by the microbiota into propionate in the colon) significantly increases postprandial GLP-1 and peptide YY levels and decreases hepatic lipid deposition through the action of GPR43 [108]. Taken together, the findings demonstrate that SCFAs can promote liver energy metabolism to reduce fat deposition, enhance intestinal barrier function, and reduce ectopic toxins in the liver. These actions have protected against the development of NFLAD (Fig. 4).
Other diseases
Pneumonia and respiratory diseases
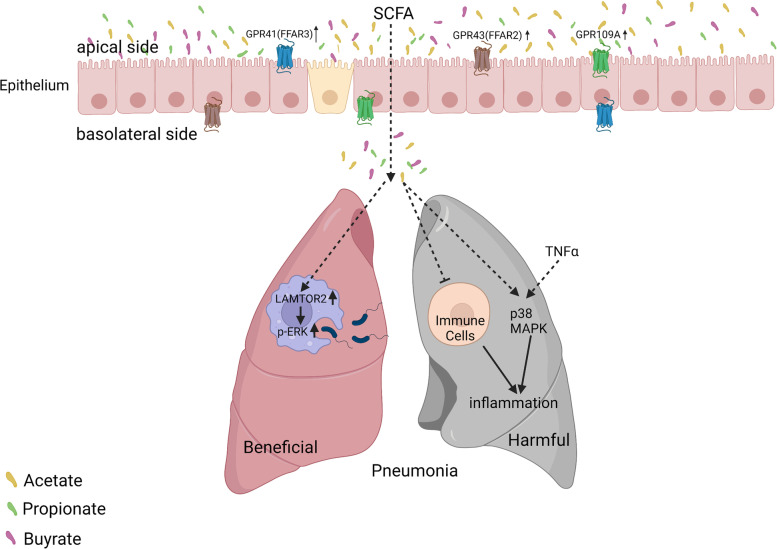
Pneumonia is an infectious inflammation of the alveoli, airways, and lung interstitium. It is primarily caused by infections with bacteria, viruses, or other pathogens [183]. Normal upper respiratory tract and intestinal microbiota prevent pneumonia by preventing the colonisation of potentially pathogenic bacteria and regulating the immune response [184]. Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), primarily infects the respiratory system and affects other organs, such as the gastrointestinal tract [185]. Recent studies have reported altered gut microbiota in SARS-CoV-2 infections, characterised by the depletion of probiotics (butyrate-producing) [119, 186], such as several genera of the family Ruminococcaceae and Lachnospiraceae [187]. Gut microbiota are also disturbed in patients with other forms of pneumonia and respiratory diseases (Table 2) [188]. Mice fed a high-fibre diet have increased circulating SCFA levels, which protect the lungs from allergic inflammation, whereas low SCFA levels are associated with increased allergic airway disease [83].
The role of SCFAs in pneumonia remains unclear. Propionate and acetate are important contributors to the gut-lung axis and may influence the immune response of piglets [189]. Propionate reshapes the mouse lung immune environment via GPR41 and reduces the severity of lung inflammation [83]. Furthermore, acetate and GPR43 increase bacterial clearance in mouse lung macrophages by upregulating late endosomal/lysosomal adaptor, MAPK, and mTOR activator 2 (LAMTOR2), which was further identified as an antibacterial effector and shown to facilitate phagosome-lysosome fusion and ERK phosphorylation [190]. However, not all studies have agreed on the anti-inflammatory effects of SCFAs. Rutting et al. investigated whether SCFAs inhibited TNFα-induced inflammation in primary human lung fibroblasts and airway smooth muscle cells in vitro and found that the combination of propionate and TNFα further promotes the phosphorylation of p38 MAPK and enhances inflammation [191]. Mammalian enteral malnutrition can aggravate allergic lung inflammation via T cell-and DC-dependent mechanisms that are inhibited by SCFAs [192]. In humans, SCFAs inhibit alveolar macrophage polymorphonuclear leukocyte phagocytosis by opsonised Staphylococcus and may cause anaerobic infections (Fig. 5) [193]. The role of SCFAs in pneumonia and respiratory diseases remains unclear. Whether SCFAs play a role in pneumonia and respiratory diseases depends on the patient’s nutritional status. Further research is required to clarify the role of SCFAs in respiratory diseases.
Fig. 5.
Beneficial and detrimental effects of SCFAs on pneumonia and other respiratory diseases. Short-chain fatty acids (SCFAs) promote the phagocytosis of Klebsiella. pneumoniae by pulmonary macrophages to clear lung bacteria. SCFAs can also inhibit lung immune cell function and promote inflammation. Other abbreviations are: GPCR: G protein-coupled receptors; FFAR: free fatty acid receptor; p-ERK: phosphorylated extracellular signal-regulated kinase; TNF-α: tumour necrosis factor-alpha; MAPK: mitogen-activated protein kinase
CKD
In CKD, impaired renal function leads to the accumulation of uraemic toxins in the intestine, causing changes in bacterial composition and faecal metabolites [194]. Altered metabolites undergo positive feedback, which allows endotoxins to translocate into the blood, thereby enhancing local kidney inflammation, exacerbating kidney damage, and affecting CKD prognosis [9]. In patients with end-stage renal disease, the abundance of Brachybacterium and Catenibacterium increases in the colon, whereas those of Lactobacillaceae and Prevotellaceae decrease [195]. The administration of dietary fibre to mice with nephropathy increased the abundance of the SCFA-producing bacteria Prevotella and Bifidobacterium (Table 2) [12, 106, 121, 122]. Complex interactions exist among the brain, intestines, microbiota, and kidneys in patients with CKD and hypertension [154]. The pathogenesis of these diseases can be explained by the brain-gut-kidney axis [9]. Increased concentrations of SCFAs in faeces and blood circulation are associated with decreased levels of inflammatory cytokines and chemokines in the kidney via GPR43 or GPR109A [196].
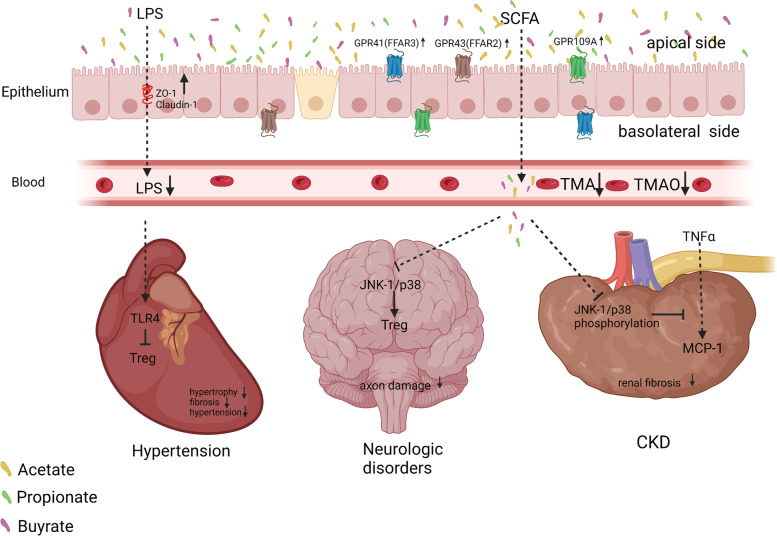
SCFAs, especially propionate, attenuate the expression of monocyte chemotactic protein-1 stimulated by TNF-α by inhibiting the phosphorylation of p38 and JNK in human renal cortical epithelial cells, thereby inhibiting renal inflammation and fibrosis [197]. Clinical studies have shown that butyrate levels in healthy volunteers are three times higher than those in CKD patients [12]. Administration of butyrate to rats with nephropathy improves renal fibrosis, reduces trimethylamine and trimethylamine N-oxide in the serum and faeces, and improves CKD progression in rats [12]. Diabetic mice given a high-fibre diet showed improved intestinal microecology, increased intestinal and systemic SCFAs, and inhibited kidney injury caused by GPR43 and GPR109A [121, 198]. These findings indicate that an imbalance in the intestinal microbiota of patients with CKD affects their SCFA metabolites, and the reduction of propionate and butyrate enhances the progression of CKD (Fig. 6). Therefore, supplementing SCFAs directly or modulating the gut microbiota that favours the production of SCFAs through dietary fibre or nutritional therapy may have a positive impact on the management of chronic renal failure.
Fig. 6.
Short-chain fatty acids (SCFAs) alleviate hypertension, neurologic disorders, and chronic kidney disease (CKD) by modulating immunity. SCFAs alleviates hypertension by reducing lipopolysaccharide (LPS) entry into the blood and inhibiting cardiac Treg cells. SCFAs can also alleviate neurological diseases by reducing axonal damage via the inhibition of the c-Jun N-terminal kinase 1 (JNK-1)/p-38 pathway. In addition, SCFAs alleviate renal fibrosis by inhibiting the phosphorylation of JNK-1/p-38 pathway. Other abbreviations are: GPCR: G protein-coupled receptors; FFAR: free fatty acid receptor; and MCP-1: monocyte chemotactic protein-1
Hypertension
Hypertension is the most common chronic disease and the most important risk factor for cardiovascular and cerebrovascular diseases [199]. The composition of the gut microbiome is dysregulated in hypertensive patients, while SCFA-producing bacteria, such as Roseburia spp. and Faecalibacterium prausnitzii, are decreased (Table 2) [13, 29, 123–125].
Oral gavage application of acetate and butyrate in hypertensive rats inhibits the vascular LPS/TLR4 pathway, increased the infiltration of Treg cells into the vascular system, and reduced the number of Firmicutes in the ratio of Firmicutes/Bacteroides [200]. In a mouse model of angiotensin II-induced hypertension, mice administered a propionate gavage showed significantly reduced cardiac hypertrophy, fibrosis, vascular dysfunction, and hypertension [62]. Studies have shown that the cardioprotective effect of SCFAs in C57BL/6 J mice is mediated by the SCFA cognate receptor GPR43/GPR109A, which modulates L-3,4-dihydroxyphenylalanine levels and the abundance of Tregs regulated by DNA methylation [201]. Supplementation with SCFA in mice prevented dietary fibre deficiency-induced upregulation of calmodulin-dependent protein kinase II and NLRP3 inflammasome activation in atrial tissue [202]. Compared to individuals with normal blood pressure, faeces of hypertensive individuals display higher levels of acetate, butyrate, and propionate [203], whereas their levels in the plasma are lower [124, 204, 205]. Thus, SCFA-producing bacteria in the intestines of hypertensive patients and the content of SCFAs in the plasma are reduced. Therefore, it is possible that the absorption of SCFAs in hypertensive patients is significantly reduced and that SCFAs are excreted in the faeces. These findings have shown that the levels of SCFAs in the plasma of hypertensive patients are low, and supplementation with SCFAs improves immunity and suppresses inflammatory responses in patients, thereby relieving symptoms such as cardiac hypertrophy and fibrosis (Fig. 6). However, most studies testing the effects of SCFAs on hypertension have focussed on phenotypic descriptions, and their specific molecular mechanisms remain unclear. Further research is needed to elucidate the potential effects and underlying mechanisms of SCFAs in hypertension, which will provide fundamental information for new approaches for the prevention and treatment of hypertension.
Neurologic disorders
The gut microbiota participates in the pathogenesis of neurological disorders through the gut-brain axis [206]. Compared to healthy volunteers, patients with neurological disorders, such as schizophrenia, PD, Alzheimer's disease, and autoimmune encephalomyelitis, have a significant imbalance in multiple characteristic bacteria in the gut, such as a significantly reduced abundance of the butyrate-producing bacterium F. prausnitzii (Table 2) [126–128, 207, 208]. Increased serum butyrate levels are associated with favourable treatment responses in drug-naïve first-episode schizophrenia patients [209]. SCFAs in the faeces of PD patients are reduced, and intestinal inflammation occurs, indicating that SCFAs may be candidate molecules and pathways for the pathogenesis of PD [210]. Dietary SCFAs help expand gut Tregs to modulate autoimmune responses by inhibiting the JNK1 and p38 pathways, thereby reducing axonal damage in patients with autoimmune encephalomyelitis [208]. The abundance of Clostridium spp. in the gut of patients with schizophrenia is significantly reduced, resulting in reduced production of SCFAs and aggravation of the disease [127]. Transplanting the faecal microbiota of patients with schizophrenia into antibiotic-treated mice can cause abnormal behaviours in the recipient animals, such as hyperactive psychomotor function and impaired learning and memory [211]. In another study, administration of a mixture of acetate, propionate, and butyrate to mice following a 3-week social defeat and overcrowding procedure alleviated the heightened stress-responsiveness and stress-induced increases in intestinal permeability while also decreasing anxiety-like behaviour in the open field test and decreasing depressive-like behaviour in the forced swim test [212]. To date, the roles of SCFAs in dietary intake and immune and metabolic outcomes have not been systematically studied in neurological disorders [213]. It remains unclear whether a high-fibre diet can ameliorate these diseases by increasing SCFA production. Whether SCFAs play a direct or indirect role in neurological disorders should be studied in detail (Fig. 6).
Therapeutic relevance
Faecal microbiota transplantation (FMT)
FMT is a treatment method that transfers a faecal suspension obtained from a healthy donor to the patient's digestive tract to restore the normal microbial composition and function of the intestinal tract [214, 215]. FMT could have applications in the treatment of many diseases, such as ulcerative colitis, irritable bowel syndrome, asthma, PD [214, 216–219]. The results of FMT depend on the donor, and the use of super donors with normal organisms and favourable specific bacterial characteristics is critical for successful treatment [216]. In several studies, after FMT administration in patients with IBD, the relative abundances of Eubacterium hallii and Odoribacter genera increased, but the relative abundances of Bacteroides, Helicobacter, and Clostridia decreased [220, 221]. E. hallii and Odoribacter are the main bacteria that produce SCFAs and increase their concentration in the colon [222, 223]. SCFAs inhibit inflammation in mice by interacting with GPR43 to improve inflammatory diseases, such as colitis, arthritis, and asthma [224]. In addition, SCFAs in mice modulate B-cell differentiation through the GPR43 receptor and relieve rheumatoid arthritis [225]. FMT administration increases the concentration of SCFAs in the colon [209], and the NF-κB pathway is regulated to inhibit inflammation [214]. At present, frozen stool processing promotes the clinical application of FMT, making it possible to establish an FMT library [216, 226]. However, the specific bacterial composition of FMTs and the mechanisms underlying of FMT treatment remain unclear. Given the apparent effectiveness of this treatment strategy, further research is required to elucidate the precise underlying mechanisms.
Dietary intervention
Dietary composition has the most significant effect on gut microbes [227, 228]. Different types of diets can change the composition of microorganisms, increase the ratio of harmful bacteria to their metabolites, and induce chronic metabolic diseases, such as obesity, and T2D [229, 230]. Healthy eating habits, which include consuming plenty of fresh fruits, vegetables, fish, extra virgin olive oil, and whole grains, can effectively prevent these diseases, whereas refined and processed foods such as sweet, fried foods, processed meats, and refined grains can increase the risk of illness [230, 231]. Mice fed a high-fibre diet showed increased levels of SCFAs and were protected against allergic inflammation in the lungs, whereasthose fed a low-fibre diet showed decreased levels of SCFAs and increased allergic airway disease [83]. Dietary fibres alter intestinal SCFA levels, maintain mucosal homeostasis and intestinal epithelial integrity, promote the growth of Tregs, and inhibit the expression of inflammatory cytokines to prevent and/or ameliorate diseases [232]. A low-calorie, low-protein, low-carbohydrate HFD was adopted as a fast-mimicking diet; this diet may promote cell regeneration by reducing the activity of protein kinase A and mTOR, inducing the expression of Sox2 and Ngn3, and restoring insulin generation, secretion, and glucose homeostasis in T2D mouse models and T1D patients [233]. Eating habits, that regulate physical health are more feasible and have certain advantages over drugs, surgical, and healthcare products.
Prebiotic/probiotic applications
In recent years, research on prebiotics and probiotics has attracted considerable attention [234]. Their mechanisms of action are complex and diverse and are usually strain- and compound-specific [235]. Probiotics can change the microenvironment of the gastrointestinal tract, compete with pathogenic bacteria for nutrients, and inhibit the growth of pathogenic bacteria by producing strain-specific antimicrobial compounds [236, 237].
In the human body, probiotic effector molecules can directly interact with receptors in the intestinal epithelium, enteroendocrine cells, immune cells, and vagus nerve afferent fibres to enhance the local effects on the intestinal tract, such as the integrity of the intestinal barrier, inflammation, immunity, and the endocrine and enteric nervous systems [236]. In addition, probiotics-based interventions promote an increase in SCFA levels, insulin sensitivity, fat decomposition, and body metabolism [238]. SCFAs are involved in regulating human emotions and cognitive abilities and affect mental function through the gut-brain axis [3]. Changes in microbial composition and metabolite concentration caused by administration of prebiotics affect host epithelial, immune, neurological, and endocrine signals and thus mediate health benefits that include improved intestinal function, immune response, glucose and lipid metabolism, bone health, appetite, and regulation of satiety [239, 240]. Therefore, prebiotics and probiotics provide health benefits to the host and reduce side effects when applied in clinical settings and are expected to become effective treatments for many diseases.
Conclusion
SCFAs are important metabolites produced by gut microbes. As the second-largest genome and ninth-largest system in the human body, the intestinal microbiota is critical for maintaining health. Bacterial imbalances and their metabolites lead to various diseases, including obesity, T2D, NAFLD, CKD, IBD, CRC, pneumonia, and schizophrenia. SCFAs affect the occurrence and development of various diseases in several ways. Among the diseases covered in this review, SCFAs mainly exert their effects by enhancing intestinal barrier function, inhibiting the inflammatory response, promoting apoptosis, increasing the expression of GPCRs, affecting histone acetylation, and regulating immunity. Currently, the mechanisms by which SCFAs function in various diseases are not fully understood. In the present review, we clarify the mechanisms of action SCFAs in various diseases. The therapeutic effects of faecal bacterial transplantation, dietary intervention, and probiotic/prebiotic supplementation on diseases through the regulation of microbial metabolites are described.
Acknowledgements
The Figures in this review were created with BioRender.com.
Abbreviations
- BSGL
Broken spores of Ganoderma lucidum
- CKD
Chronic kidney disease
- COVID-19
Coronavirus disease 2019
- CRC
Colorectal cancer
- ERK
Extracellular signal-regulated kinase
- FMT
Fecal microbiota transplantation
- FOXP3
Forkhead box protein 3,
- GLP-1
Glucagon-like peptide-1
- GPCRs
G protein-coupled receptors
- HCAR2
Hydroxycarboxylic acid receptor 2
- HDAC
Histone deacetylase
- HFD
High-fat diet
- HMG-CoA
3-Hydroxy-3-methylglutaryl-CoA
- IBD
Inflammatory bowel disease
- IECs
Intestinal epithelial cells
- IGN
Intestinal gluconeogenesis
- IL
Interleukin
- LAMTOR2
Late endosomal/lysosomal adaptor, MAPK, and mTOR activator 2
- JAK2
Janus kinase 2
- LPS
Lipopolysaccharide
- lncRNA
Long noncoding RNA
- MAPK
Mitogen-activated protein kinase
- MCT1
Monocarboxylate transporter 1
- miRNA
MicroRNA
- mTOR
Mammalianarget of rapamycin
- mmdA
Methylmalonyl-CoA decarboxylase
- MYD88
Myeloid differentiation primary response 88
- NAFLD
Non-alcoholic fatty liver disease
- NF-κB
Nuclear factor-κB
- NLRP3
NLR family pyrin domain containing 3
- PD
Parkinson’s disease
- PI3K
Phosphoinositide 3-kinase
- PKM2
Pyruvate kinase isozyme 2
- PPAR-γ2
Peroxisome proliferator-activated receptor-γ2
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SCFAs
Short-chain fatty acids
- SMCT1
Sodium-coupled monocarboxylate transporter 1
- STAT3
Signal transducer and activator of transcription 3
- TLR4
Toll-like receptors 4
- TNF-α
Tumor necrosis factor-α
- Treg
Regulatory T cells
- T2D
Type 2 diabetes
- ZO-1
Zonula occludens protein 1
Authors’ contributions
DZ wrote the manuscript and designed the figures. YPJ, YNZ, YL, LTG, HHS, and MDL edited and assisted with the development of the manuscript throughout the writing process. HLZ, YSW, and ZXX edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No 82020108024), International Cooperation project of the Department of Science and Technology of Jilin Province (20210402005GH) and Health Commission of Jilin Province (No: 2020J033).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hong-Lan Zhou, Email: hlzhou@jlu.edu.cn.
Yi-Shu Wang, Email: wangys@jlu.edu.cn.
Zhi-Xiang Xu, Email: zhixiangxu08@gmail.com.
References
- 1.Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70(6):1174–1182. doi: 10.1136/gutjnl-2020-323071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 3.Dalile B, et al. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 4.Hu J, et al. Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr. 2018;58(8):1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita M, et al. Gut microbiota-derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res. 2021;81(15):4014–4026. doi: 10.1158/0008-5472.CAN-20-4090. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y, et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur J Nutr. 2021;60(4):2217–2230. doi: 10.1007/s00394-020-02414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 8.Tagliamonte S, et al. Mediterranean diet consumption affects the endocannabinoid system in overweight and obese subjects: possible links with gut microbiome, insulin resistance and inflammation. Eur J Nutr. 2021;60(7):3703–3716. doi: 10.1007/s00394-021-02538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang T, et al. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14(7):442–456. doi: 10.1038/s41581-018-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M, et al. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52(1):1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda) 2016;31(4):283–293. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, et al. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci (Lond) 2019;133(17):1857–1870. doi: 10.1042/CS20190171. [DOI] [PubMed] [Google Scholar]
- 13.Verhaar BJH, et al. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients. 2020;12(10):2982. doi: 10.3390/nu12102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691–706. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan J, et al. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 16.Bieliauskas AV, Pflum MK. Isoform-selective histone deacetylase inhibitors. Chem Soc Rev. 2008;37(7):1402–1413. doi: 10.1039/b703830p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hori Y, Kikuchi K. Chemical tools with fluorescence switches for verifying epigenetic modifications. Acc Chem Res. 2019;52(10):2849–2857. doi: 10.1021/acs.accounts.9b00349. [DOI] [PubMed] [Google Scholar]
- 18.Yang W, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11(1):4457. doi: 10.1038/s41467-020-18262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topper MJ, et al. Epigenetic therapy ties MYC depletion to reversing immune evasion and treating lung cancer. Cell. 2017;171(6):1284–1300.e21. doi: 10.1016/j.cell.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzaei R, et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed Pharmacother. 2021;139:111619. doi: 10.1016/j.biopha.2021.111619. [DOI] [PubMed] [Google Scholar]
- 21.Parada Venegas D, et al. Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Licciardi PV, Ververis K, Karagiannis TC. Histone deacetylase inhibition and dietary short-chain Fatty acids. ISRN Allergy. 2011;2011:869647. doi: 10.5402/2011/869647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldecker M, et al. Histone-deacetylase inhibition and butyrate formation: fecal slurry incubations with apple pectin and apple juice extracts. Nutrition. 2008;24(4):366–374. doi: 10.1016/j.nut.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Luu M, Visekruna A. Short-chain fatty acids: Bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol. 2019;49(6):842–848. doi: 10.1002/eji.201848009. [DOI] [PubMed] [Google Scholar]
- 25.van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29(8):700–712. doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Deleu S, et al. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine. 2021;66:103293. doi: 10.1016/j.ebiom.2021.103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh A, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 28.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95(1):50–60. doi: 10.5740/jaoacint.SGE_Macfarlane. [DOI] [PubMed] [Google Scholar]
- 29.Bier A, et al. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients. 2018;10(9):1154. doi: 10.3390/nu10091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CB, et al. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol. 2011;56(7):650–654. doi: 10.1016/j.archoralbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CH. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol Immunol. 2021;18(5):1161–1171. doi: 10.1038/s41423-020-00625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Gallausiaux C, et al. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 33.Belzer C, et al. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and Vitamin B(12) production by intestinal symbionts. mBio. 2017;8(5):e00770–17. doi: 10.1128/mBio.00770-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng H, et al. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci. 2019;20(5):1214. doi: 10.3390/ijms20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 36.Rey FE, et al. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem. 2010;285(29):22082–22090. doi: 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579(7800):586–591. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erny D, et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021;33(11):2260–2276.e7. doi: 10.1016/j.cmet.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Murashige D, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370(6514):364–368. doi: 10.1126/science.abc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frampton J, et al. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metab. 2020;2(9):840–848. doi: 10.1038/s42255-020-0188-7. [DOI] [PubMed] [Google Scholar]
- 42.Donohoe DR, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4(12):1387–1397. doi: 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schug ZT, Vande Voorde J, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer. 2016;16(11):708–717. doi: 10.1038/nrc.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao S, et al. Modulation of microbially derived short-chain fatty acids on intestinal homeostasis, metabolism, and neuropsychiatric disorder. Appl Microbiol Biotechnol. 2020;104(2):589–601. doi: 10.1007/s00253-019-10312-4. [DOI] [PubMed] [Google Scholar]
- 45.Hu S, et al. Acetate and butyrate improve β-cell metabolism and mitochondrial respiration under oxidative stress. Int J Mol Sci. 2020;21(4):1542. doi: 10.3390/ijms21041542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernández MAG, et al. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11(8):1943. doi: 10.3390/nu11081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bose S, Ramesh V, Locasale JW. Acetate metabolism in physiology, cancer, and beyond. Trends Cell Biol. 2019;29(9):695–703. doi: 10.1016/j.tcb.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 49.Markowiak-Kopeć P, Śliżewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12(4):1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagano Y, Itoh K, Honda K. The induction of Treg cells by gut-indigenous Clostridium. Curr Opin Immunol. 2012;24(4):392–397. doi: 10.1016/j.coi.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Derrien M, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(Pt 5):1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 52.Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther. 2016;164:144–151. doi: 10.1016/j.pharmthera.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boets E, et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595(2):541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belenguer A, et al. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72(5):3593–3599. doi: 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louis P, et al. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12(2):304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 56.Reichardt N, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. Isme j. 2014;8(6):1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang JM, et al. SCFAs-Induced GLP-1 secretion links the regulation of gut microbiome on hepatic lipogenesis in chickens. Front Microbiol. 2019;10:2176. doi: 10.3389/fmicb.2019.02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bueld JE, Bannenberg G, Netter KJ. Effects of propionic acid and pravastatin on HMG-CoA reductase activity in relation to forestomach lesions in the rat. Pharmacol Toxicol. 1996;78(4):229–234. doi: 10.1111/j.1600-0773.1996.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 59.Tong LT, et al. Effects of dietary hull-less barley β-glucan on the cholesterol metabolism of hypercholesterolemic hamsters. Food Chem. 2015;169:344–349. doi: 10.1016/j.foodchem.2014.07.157. [DOI] [PubMed] [Google Scholar]
- 60.Hou YF, et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson's disease. Microbiome. 2021;9(1):34. doi: 10.1186/s40168-020-00988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tobin D, Vige R, Calder PC. Review: the nutritional management of multiple sclerosis with propionate. Front Immunol. 2021;12:676016. doi: 10.3389/fimmu.2021.676016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartolomaeus H, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duscha A, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180(6):1067–1080.e16. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 64.Haghikia A, et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J. 2022;43(6):518–533. doi: 10.1093/eurheartj/ehab644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brame JE, et al. The potential of outdoor environments to supply beneficial butyrate-producing bacteria to humans. Sci Total Environ. 2021;777:146063. doi: 10.1016/j.scitotenv.2021.146063. [DOI] [PubMed] [Google Scholar]
- 66.Richards LB, et al. Butyrate and propionate restore the cytokine and house dust mite compromised barrier function of human bronchial airway epithelial cells. Int J Mol Sci. 2020;22(1):65. doi: 10.3390/ijms22010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Donohoe DR, et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 2012;48(4):612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang G, et al. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Br J Pharmacol. 2022;179(1):159–178. doi: 10.1111/bph.15693. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, et al. Butyrate in energy metabolism: there is still more to learn. Trends Endocrinol Metab. 2021;32(3):159–169. doi: 10.1016/j.tem.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 70.Kimura I, et al. Free fatty acid receptors in health and disease. Physiol Rev. 2020;100(1):171–210. doi: 10.1152/physrev.00041.2018. [DOI] [PubMed] [Google Scholar]
- 71.He J, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21(17):6356. doi: 10.3390/ijms21176356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kasubuchi M, et al. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Offermanns S. Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol Metab. 2017;28(3):227–236. doi: 10.1016/j.tem.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 74.Milligan G, Stoddart LA, Smith NJ. Agonism and allosterism: the pharmacology of the free fatty acid receptors FFA2 and FFA3. Br J Pharmacol. 2009;158(1):146–153. doi: 10.1111/j.1476-5381.2009.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barella LF, Jain S, Pydi SP. G protein-coupled receptors: role in metabolic disorders. Front Endocrinol (Lausanne) 2022;13:984253. doi: 10.3389/fendo.2022.984253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang J, et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat Med. 2017;23(4):450–460. doi: 10.1038/nm.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong CK, et al. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. 2022;34(10):1514–1531.e7. doi: 10.1016/j.cmet.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Nemet I, et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180(5):862–877.e22. doi: 10.1016/j.cell.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chaudhary PK, Kim S. An insight into GPCR and G-proteins as cancer drivers. Cells. 2021;10(12):3288. doi: 10.3390/cells10123288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown AJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 81.Li M, et al. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front Pharmacol. 2018;9:533. doi: 10.3389/fphar.2018.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimizu H, et al. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Sci Rep. 2019;9(1):16574. doi: 10.1038/s41598-019-53242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trompette A, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 84.Graaf C, et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: a long march to therapeutic successes. Pharmacol Rev. 2016;68(4):954–1013. doi: 10.1124/pr.115.011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang J, et al. Secreted M-ficolin anchors onto monocyte transmembrane G protein-coupled receptor 43 and cross talks with plasma C-reactive protein to mediate immune signaling and regulate host defense. J Immunol. 2010;185(11):6899–6910. doi: 10.4049/jimmunol.1001225. [DOI] [PubMed] [Google Scholar]
- 86.Wu H, et al. Gut microbial metabolites induce donor-specific tolerance of kidney allografts through induction of T regulatory cells by short-chain fatty acids. J Am Soc Nephrol. 2020;31(7):1445–1461. doi: 10.1681/ASN.2019080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vieira AT, et al. A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of Gout. Arthritis Rheumatol. 2015;67(6):1646–1656. doi: 10.1002/art.39107. [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11(3):752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang W, et al. Short-chain fatty acids ameliorate diabetic nephropathy via GPR43-mediated inhibition of oxidative stress and NF-κB signaling. Oxid Med Cell Longev. 2020;2020:4074832. doi: 10.1155/2020/4074832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kimura I, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmed K, Tunaru S, Offermanns S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci. 2009;30(11):557–562. doi: 10.1016/j.tips.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Cresci GA, et al. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg. 2010;14(3):449–461. doi: 10.1007/s11605-009-1045-x. [DOI] [PubMed] [Google Scholar]
- 93.Wu Y, et al. Melatonin alleviates titanium nanoparticles induced osteolysis via activation of butyrate/GPR109A signaling pathway. J Nanobiotechnology. 2021;19(1):170. doi: 10.1186/s12951-021-00915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato FT, et al. Tributyrin attenuates metabolic and inflammatory changes associated with obesity through a GPR109A-dependent mechanism. Cells. 2020;9(9):2007. doi: 10.3390/cells9092007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh N, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hosseinkhani F, et al. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes. 2021;13(1):1–22. doi: 10.1080/19490976.2021.1882927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amiri P, et al. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: a comprehensive narrative review. Front Pharmacol. 2021;12:837509. doi: 10.3389/fphar.2021.837509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7(2):79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 99.Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- 100.Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- 101.Lee M, Chang EB. Inflammatory Bowel Diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology. 2021;160(2):524–537. doi: 10.1053/j.gastro.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Russo E, et al. Immunomodulating activity and therapeutic effects of short chain fatty acids and tryptophan post-biotics in inflammatory bowel disease. Front Immunol. 2019;10:2754. doi: 10.3389/fimmu.2019.02754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salem F, et al. Gut microbiome in chronic rheumatic and inflammatory bowel diseases: Similarities and differences. United European Gastroenterol J. 2019;7(8):1008–1032. doi: 10.1177/2050640619867555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Machiels K, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 105.Bajer L, et al. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017;23(25):4548–4558. doi: 10.3748/wjg.v23.i25.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dejea CM, et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359(6375):592–597. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mager LF, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 108.Chambers ES, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rizzardi KF, et al. Firmicutes levels in the mouth reflect the gut condition with respect to obesity and early childhood caries. Front Cell Infect Microbiol. 2021;11:593734. doi: 10.3389/fcimb.2021.593734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu Y, et al. Short Chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep. 2016;6:37589. doi: 10.1038/srep37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu R, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 112.Zhao L, et al. Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine. 2019;66(3):526–537. doi: 10.1007/s12020-019-02103-8. [DOI] [PubMed] [Google Scholar]
- 113.Pedersen HK, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 114.Perera D, et al. Impaired host response and the presence of Acinetobacter baumannii in the serum microbiome of type-II diabetic patients. iScience. 2021;24(1):101941. doi: 10.1016/j.isci.2020.101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang X, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70(4):761–774. doi: 10.1136/gutjnl-2019-319664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20(4):461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xie C, Halegoua-DeMarzio D. Role of probiotics in non-alcoholic fatty liver disease: does gut microbiota matter? Nutrients. 2019;11(11):2837. doi: 10.3390/nu11112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ding Y, et al. Interactions between gut microbiota and non-alcoholic liver disease: the role of microbiota-derived metabolites. Pharmacol Res. 2019;141:521–529. doi: 10.1016/j.phrs.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zuo T, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ze X, et al. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. Isme j. 2012;6(8):1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hsu CN, et al. Blood pressure abnormalities associated with gut microbiota-derived short chain fatty acids in children with congenital anomalies of the kidney and urinary tract. J Clin Med. 2019;8(8):1090. doi: 10.3390/jcm8081090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang S, et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci Rep. 2017;7(1):2870. doi: 10.1038/s41598-017-02989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan Q, et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017;7:381. doi: 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Calderón-Pérez L, et al. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci Rep. 2020;10(1):6436. doi: 10.1038/s41598-020-63475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilck N, et al. Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature. 2017;551(7682):585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu F, et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun. 2020;11(1):1612. doi: 10.1038/s41467-020-15457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li J, et al. Clostridiales are predominant microbes that mediate psychiatric disorders. J Psychiatr Res. 2020;130:48–56. doi: 10.1016/j.jpsychires.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 128.Generoso JS, et al. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz J Psychiatry. 2021;43(3):293–305. doi: 10.1590/1516-4446-2020-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xie QS, et al. Short-chain fatty acids exert opposite effects on the expression and function of p-glycoprotein and breast cancer resistance protein in rat intestine. Acta Pharmacol Sin. 2021;42(3):470–481. doi: 10.1038/s41401-020-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hung TV, Suzuki T. Short-chain fatty acids suppress inflammatory reactions in caco-2 cells and mouse colons. J Agric Food Chem. 2018;66(1):108–117. doi: 10.1021/acs.jafc.7b04233. [DOI] [PubMed] [Google Scholar]
- 131.Chen G, et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a tnbs-induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 133.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Holtug K, Rasmussen HS, Mortensen PB. Mortensen, short chain fatty acids in inflammatory bowel disease. the effect of bacterial fermentation of blood. Scand J Clin Lab Invest. 1988;48(7):667–71. doi: 10.3109/00365518809085788. [DOI] [PubMed] [Google Scholar]
- 135.Li G, et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes. 2021;13(1):1968257. doi: 10.1080/19490976.2021.1968257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou L, et al. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm Bowel Dis. 2018;24(9):1926–1940. doi: 10.1093/ibd/izy182. [DOI] [PubMed] [Google Scholar]
- 137.Ternes D, et al. The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat Metab. 2022;4(4):458–475. doi: 10.1038/s42255-022-00558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garrett WS. The gut microbiota and colon cancer. Science. 2019;364(6446):1133–1135. doi: 10.1126/science.aaw2367. [DOI] [PubMed] [Google Scholar]
- 139.Hofseth LJ, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17(6):352–364. doi: 10.1038/s41575-019-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang J, et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. 2022;162(1):135–149.e2. doi: 10.1053/j.gastro.2021.08.041. [DOI] [PubMed] [Google Scholar]
- 141.Vernia F, et al. Dietary factors modulating colorectal carcinogenesis. Nutrients. 2021;13(1):143. doi: 10.3390/nu13010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wyatt M, Greathouse KL. Targeting dietary and microbial tryptophan-indole metabolism as therapeutic approaches to colon cancer. Nutrients. 2021;13(4):1189. doi: 10.3390/nu13041189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burgess DJ. Metabolism: Warburg behind the butyrate paradox? Nat Rev Cancer. 2012;12(12):798. doi: 10.1038/nrc3401. [DOI] [PubMed] [Google Scholar]
- 144.Li Q, et al. Butyrate suppresses the proliferation of colorectal cancer cells via targeting pyruvate kinase M2 and metabolic reprogramming. Mol Cell Proteomics. 2018;17(8):1531–1545. doi: 10.1074/mcp.RA118.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang Y, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of MicroRNA-21. Gastroenterology. 2017;152(4):851–866.e24. doi: 10.1053/j.gastro.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hong J, et al. F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer. Gut. 2021;70(11):2123–2137. doi: 10.1136/gutjnl-2020-322780. [DOI] [PubMed] [Google Scholar]
- 147.Chen J, Zhao KN, Vitetta L. Effects of intestinal microbial-elaborated butyrate on oncogenic signaling pathways. Nutrients. 2019;11(5):1026. doi: 10.3390/nu11051026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chang CC, et al. Butyrate supplementation regulates expression of chromosome segregation 1-like protein to reverse the genetic distortion caused by p53 mutations in colorectal cancer. Int J Oncol. 2022;60(6):64. doi: 10.3892/ijo.2022.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bai L, Merchant JL. Transcription factor ZBP-89 cooperates with histone acetyltransferase p300 during butyrate activation of p21waf1 transcription in human cells. J Biol Chem. 2000;275(39):30725–30733. doi: 10.1074/jbc.M004249200. [DOI] [PubMed] [Google Scholar]
- 150.K, B.A., et al., Short chain fatty acids enriched fermentation metabolites of soluble dietary fibre from Musa paradisiaca drives HT29 colon cancer cells to apoptosis. PLoS One, 2019. 14(5): p. e0216604. [DOI] [PMC free article] [PubMed]
- 151.Gu J, et al. Standardized Astragalus Mongholicus Bunge-Curcuma Aromatica Salisb. Extract Efficiently Suppresses Colon Cancer Progression Through Gut Microbiota Modification in CT26-Bearing Mice. Front Pharmacol. 2021;12:714322. doi: 10.3389/fphar.2021.714322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hu S, et al. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol Cancer. 2015;14:180. doi: 10.1186/s12943-015-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yu Q, et al. Sodium butyrate inhibits aerobic glycolysis of hepatocellular carcinoma cells via the c-myc/hexokinase 2 pathway. J Cell Mol Med. 2022;26(10):3031–3045. doi: 10.1111/jcmm.17322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tian Y, et al. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J Nutr Biochem. 2018;57:103–109. doi: 10.1016/j.jnutbio.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 155.Day EA, Ford RJ, Steinberg GR. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol Metab. 2017;28(8):545–560. doi: 10.1016/j.tem.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 156.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129(10):4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Vadder F, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 158.Vily-Petit J, et al. Improvement of energy metabolism associated with NUTRIOSE® soluble fiber, a dietary ingredient exhibiting prebiotic properties, requires intestinal gluconeogenesis. Food Res Int. 2023;167:112723. doi: 10.1016/j.foodres.2023.112723. [DOI] [PubMed] [Google Scholar]
- 159.Wu J, et al. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell. 2021;12(5):360–373. doi: 10.1007/s13238-020-00814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Y, et al. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp Mol Med. 2020;52(12):1959–1975. doi: 10.1038/s12276-020-00534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gallardo-Becerra L, et al. Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb Cell Fact. 2020;19(1):61. doi: 10.1186/s12934-020-01319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Komaroff AL. The microbiome and risk for obesity and diabetes. JAMA. 2017;317(4):355–356. doi: 10.1001/jama.2016.20099. [DOI] [PubMed] [Google Scholar]
- 163.Rivière A, et al. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ziętek M, et al. Implications of SCFAs on the parameters of the lipid and hepatic profile in pregnant women. Nutrients. 2021;13(6):1749. doi: 10.3390/nu13061749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc. 2015;74(3):227–234. doi: 10.1017/S0029665114001700. [DOI] [PubMed] [Google Scholar]
- 166.Hong YH, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146(12):5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 167.Zou J, et al. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe. 2018;23(1):41–53.e4. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gao Z, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bishop KS, et al. From 2000years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry. 2015;114:56–65. doi: 10.1016/j.phytochem.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 170.Sang T, et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr Polym. 2021;256:117594. doi: 10.1016/j.carbpol.2020.117594. [DOI] [PubMed] [Google Scholar]
- 171.Farup PG, Valeur J. Changes in faecal short-chain fatty acids after weight-loss interventions in subjects with morbid obesity. Nutrients. 2020;12(3):802. doi: 10.3390/nu12030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem. 2019;63:101–108. doi: 10.1016/j.jnutbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 173.Christiansen CB, et al. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–g65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 174.Sanna S, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51(4):600–605. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 176.Tripathi A, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chambers ES, et al. The effects of dietary supplementation with inulin and inulin-propionate ester on hepatic steatosis in adults with non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21(2):372–376. doi: 10.1111/dom.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhou D, Fan JG. Microbial metabolites in non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25(17):2019–2028. doi: 10.3748/wjg.v25.i17.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deng M, et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J Endocrinol. 2020;245(3):425–437. doi: 10.1530/JOE-20-0018. [DOI] [PubMed] [Google Scholar]
- 180.Zhao ZH, et al. Sodium butyrate supplementation inhibits hepatic steatosis by stimulating liver kinase B1 and insulin-induced gene. Cell Mol Gastroenterol Hepatol. 2021;12(3):857–871. doi: 10.1016/j.jcmgh.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou D, et al. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23(1):60–75. doi: 10.3748/wjg.v23.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu W, et al. A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: by changing gut barrier. Eur J Nutr. 2021;60(5):2317–2330. doi: 10.1007/s00394-020-02431-w. [DOI] [PubMed] [Google Scholar]
- 183.Dong J. Signaling pathways implicated in carbon nanotube-induced lung inflammation. Front Immunol. 2020;11:552613. doi: 10.3389/fimmu.2020.552613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thibeault C, Suttorp N, Opitz B. The microbiota in pneumonia: From protection to predisposition. Sci Transl Med. 2021;13(576):eaba0501. doi: 10.1126/scitranslmed.aba0501. [DOI] [PubMed] [Google Scholar]
- 185.Zhang F, et al. Prolonged impairment of short-chain fatty acid and L-Isoleucine biosynthesis in gut microbiome in patients with COVID-19. Gastroenterology. 2022;162(2):548–561.e4. doi: 10.1053/j.gastro.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zuo T, et al. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology. 2020;159(4):1302–1310.e5. doi: 10.1053/j.gastro.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gu S, et al. Alterations of the gut microbiota in patients with Coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. 2020;71(10):2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guo M, et al. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat Rev Gastroenterol Hepatol. 2021;18(4):269–283. doi: 10.1038/s41575-021-00416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang Y, et al. Deprivation of dietary fiber enhances susceptibility of piglets to lung immune stress. Front Nutr. 2022;9:827509. doi: 10.3389/fnut.2022.827509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wu T, et al. Microbiota-derived short-chain fatty acids promote LAMTOR2-mediated immune responses in macrophages. mSystems. 2020;5(6):e00587–20. doi: 10.1128/mSystems.00587-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rutting S, et al. Short-chain fatty acids increase TNFα-induced inflammation in primary human lung mesenchymal cells through the activation of p38 MAPK. Am J Physiol Lung Cell Mol Physiol. 2019;316(1):L157–l174. doi: 10.1152/ajplung.00306.2018. [DOI] [PubMed] [Google Scholar]
- 192.Cait A, et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018;11(3):785–795. doi: 10.1038/mi.2017.75. [DOI] [PubMed] [Google Scholar]
- 193.Eftimiadi C, et al. Short-chain fatty acids produced by anaerobic bacteria inhibit phagocytosis by human lung phagocytes. J Infect Dis. 1990;161(1):138–142. doi: 10.1093/infdis/161.1.138. [DOI] [PubMed] [Google Scholar]
- 194.Felizardo RJF, et al. The interplay among gut microbiota, hypertension and kidney diseases: the role of short-chain fatty acids. Pharmacol Res. 2019;141:366–377. doi: 10.1016/j.phrs.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 195.Vaziri ND, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 196.Li YJ, et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of G protein-coupled receptors GPR43 and GPR109A. J Am Soc Nephrol. 2020;31(6):1267–1281. doi: 10.1681/ASN.2019101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kobayashi M, et al. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun. 2017;486(2):499–505. doi: 10.1016/j.bbrc.2017.03.071. [DOI] [PubMed] [Google Scholar]
- 198.Sabatino A, et al. Intestinal microbiota in type 2 diabetes and chronic kidney disease. Curr Diab Rep. 2017;17(3):16. doi: 10.1007/s11892-017-0841-z. [DOI] [PubMed] [Google Scholar]
- 199.Kario K. Orthostatic hypertension-a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9(12):726–738. doi: 10.1038/nrneph.2013.224. [DOI] [PubMed] [Google Scholar]
- 200.Robles-Vera I, et al. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: role of short-chain fatty acids. Mol Nutr Food Res. 2020;64(6):e1900616. doi: 10.1002/mnfr.201900616. [DOI] [PubMed] [Google Scholar]
- 201.Kaye DM, et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. 2020;141(17):1393–1403. doi: 10.1161/CIRCULATIONAHA.119.043081. [DOI] [PubMed] [Google Scholar]
- 202.Zuo K, et al. Commensal microbe-derived SCFA alleviates atrial fibrillation via GPR43/NLRP3 signaling. Int J Biol Sci. 2022;18(10):4219–4232. doi: 10.7150/ijbs.70644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huart J, et al. Gut Microbiota and fecal levels of short-chain fatty acids differ upon 24-hour blood pressure levels in men. Hypertension. 2019;74(4):1005–1013. doi: 10.1161/HYPERTENSIONAHA.118.12588. [DOI] [PubMed] [Google Scholar]
- 204.Calderón-Pérez L, et al. Interplay between dietary phenolic compound intake and the human gut microbiome in hypertension: a cross-sectional study. Food Chem. 2021;344:128567. doi: 10.1016/j.foodchem.2020.128567. [DOI] [PubMed] [Google Scholar]
- 205.Guo Y, et al. Gut microbiota dysbiosis in human hypertension: a systematic review of observational studies. Front Cardiovasc Med. 2021;8:650227. doi: 10.3389/fcvm.2021.650227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cryan JF, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 207.Lombardi VC, et al. Nutritional modulation of the intestinal microbiota; future opportunities for the prevention and treatment of neuroimmune and neuroinflammatory disease. J Nutr Biochem. 2018;61:1–16. doi: 10.1016/j.jnutbio.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Haghikia A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43(4):817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 209.Li X, et al. The role of butyric acid in treatment response in drug-naïve first episode schizophrenia. Front Psychiatry. 2021;12:724664. doi: 10.3389/fpsyt.2021.724664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Aho VTE, et al. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson's disease. Mol Neurodegener. 2021;16(1):6. doi: 10.1186/s13024-021-00427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhu F, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2020;25(11):2905–2918. doi: 10.1038/s41380-019-0475-4. [DOI] [PubMed] [Google Scholar]
- 212.Kelly JR, et al. The role of the gut microbiome in the development of schizophrenia. Schizophr Res. 2021;234:4–23. doi: 10.1016/j.schres.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 213.Joseph J, et al. Modified mediterranean diet for enrichment of short chain fatty acids: potential adjunctive therapeutic to target immune and metabolic dysfunction in schizophrenia? Front Neurosci. 2017;11:155. doi: 10.3389/fnins.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Leonardi I, et al. Fungal trans-kingdom dynamics linked to responsiveness to Fecal Microbiota Transplantation (FMT) therapy in ulcerative colitis. Cell Host Microbe. 2020;27(5):823–829.e3. doi: 10.1016/j.chom.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.de Groot P, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69(3):502–512. doi: 10.1136/gutjnl-2019-318320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.El-Salhy M, Hausken T, Hatlebakk JG. Increasing the dose and/or repeating faecal microbiota transplantation (FMT) increases the response in patients with irritable bowel syndrome (IBS) Nutrients. 2019;11(6):1415. doi: 10.3390/nu11061415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sun MF, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 218.Zhang J, et al. Lactobacillus rhamnosus GG induced protective effect on allergic airway inflammation is associated with gut microbiota. Cell Immunol. 2018;332:77–84. doi: 10.1016/j.cellimm.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 219.Smits LP, et al. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145(5):946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 220.Kim JH, Kim K, Kim W. Gut microbiota restoration through fecal microbiota transplantation: a new atopic dermatitis therapy. Exp Mol Med. 2021;53(5):907–916. doi: 10.1038/s12276-021-00627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145(1):16–27. doi: 10.1016/j.jaci.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 222.Zhou MS, et al. Altered diversity and composition of gut microbiota in patients with allergic rhinitis. Microb Pathog. 2021;161(Pt A):105272. doi: 10.1016/j.micpath.2021.105272. [DOI] [PubMed] [Google Scholar]
- 223.Guo C, et al. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int J Biol Macromol. 2021;181:357–368. doi: 10.1016/j.ijbiomac.2021.03.137. [DOI] [PubMed] [Google Scholar]
- 224.Maslowski KM, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yao Y, et al. Short-chain fatty acids regulate B cells differentiation via the FFA2 receptor to alleviate rheumatoid arthritis. Br J Pharmacol. 2022;179(17):4315–4329. doi: 10.1111/bph.15852. [DOI] [PubMed] [Google Scholar]
- 226.Dorsaz S, et al. Changes in microbiota profiles after prolonged frozen storage of stool suspensions. Front Cell Infect Microbiol. 2020;10:77. doi: 10.3389/fcimb.2020.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 228.Shock T, et al. The interplay between diet, gut microbes, and host epigenetics in health and disease. J Nutr Biochem. 2021;95:108631. doi: 10.1016/j.jnutbio.2021.108631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362(6416):776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 230.Castro-Barquero S, et al. Dietary strategies for metabolic syndrome: a comprehensive review. Nutrients. 2020;12(10):2983. doi: 10.3390/nu12102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Opie RS, et al. A modified mediterranean dietary intervention for adults with major depression: dietary protocol and feasibility data from the SMILES trial. Nutr Neurosci. 2018;21(7):487–501. doi: 10.1080/1028415X.2017.1312841. [DOI] [PubMed] [Google Scholar]
- 232.Tan JK, Macia L, Mackay CR. Dietary fiber and SCFAs in the regulation of mucosal immunity. J Allergy Clin Immunol. 2023;151(2):361–370. doi: 10.1016/j.jaci.2022.11.007. [DOI] [PubMed] [Google Scholar]
- 233.Xu H, et al. Etiology of metabolic syndrome and dietary intervention. Int J Mol Sci, 2018. 20(1). [DOI] [PMC free article] [PubMed]
- 234.Salminen S, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cunningham M, et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021;29(8):667–685. doi: 10.1016/j.tim.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 236.Monteagudo-Mera A, et al. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol. 2019;103(16):6463–6472. doi: 10.1007/s00253-019-09978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wieërs G, et al. How probiotics affect the microbiota. Front Cell Infect Microbiol. 2019;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Müller M, et al. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci Rep. 2019;9(1):12515. doi: 10.1038/s41598-019-48775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gibson GR, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 240.Zheng DW, et al. Prebiotics-encapsulated probiotic spores regulate gut microbiota and suppress colon cancer. Adv Mater. 2020;32(45):e2004529. doi: 10.1002/adma.202004529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.