Abstract
We previously described a control element in the granulocyte-macrophage colony-stimulating factor (GM-CSF) enhancer that is necessary and sufficient to mediate both transcriptional activation in response to T-cell stimuli and transcriptional repression by 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] through the vitamin D3 receptor (VDR). This DNA element is a composite site that is recognized by both Fos-Jun and NFAT1; it is directly bound by VDR in the absence of a retinoid X receptor as an apparent monomer, and it is bound in a unique tertiary conformation. We describe here the mechanism by which VDR elicits its transcriptional inhibitory effect. Firstly, VDR outcompetes NFAT1 for binding to the composite site. Overexpression of NFAT1 in vivo by transient transfection is able to relieve the 1,25(OH)2D3-dependent repression. Secondly, VDR stabilizes the binding of a Jun-Fos heterodimer to the adjacent AP-1 portion of the element. This appears to occur through a direct interaction between VDR and c-Jun, as demonstrated in vitro by direct glutathione S-transferase coprecipitation assays. In vivo, overexpression of c-Jun, but not c-Fos, leads to a rescue of the 1,25(OH)2D3-mediated repression. Transfected FLAG-VDR bound to the NFAT1–AP-1 DNA binding element can be selectively precipitated from nuclear extracts that are made from cells treated with activating agents in the presence of 1,25(OH)2D3. VDR is not detected in the complex in the absence of the ligand. Thus, VDR acts selectively on the two components required for activation of this promoter/enhancer: it competes with NFAT1 for binding to the composite site, positioning itself adjacent to Jun-Fos on the DNA. Co-occupancy apparently leads to an inhibitory effect on c-Jun’s transactivation function. These two events mediated by VDR effectively block the NFAT1–AP-1 activation complex, resulting in an attenuation of activated GM-CSF transcription.
Although the physiological processes regulated by 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] are numerous and diverse, we are just beginning to understand the mechanisms through which this secosteroid elicits its many biological effects. The classical functions of 1,25(OH)2D3 include the regulation of calcium absorption in the intestine, maintenance of mineral homeostasis in the kidney, and regulation of bone remodeling (10, 25, 33). 1,25(OH)2D3 also plays important roles in what may be considered nonclassical target systems. For example, it is a potent differentiating factor for hematopoietic processes; an antiproliferative agent for many cancer cell lines, such as breast, prostate, and colon cancer; and an immunosuppressive agent (1, 3, 5, 34).
1,25(OH)2D3 signals by binding to a transcription factor, the vitamin D3 receptor (VDR) (reviewed in references 16 and 27). This protein is a member of the steroid-nuclear receptor superfamily, whose members include receptors that bind glucocorticoids, sex steroids, thyroid hormones, retinoids, fatty acids, and eicosanoids. Activation of a 1,25(OH)2D3-dependent target gene occurs through a ligand-dependent association of the receptor with coactivator proteins (31) and selective binding of a VDR-retinoid X receptor (RXR) heterodimer to a vitamin D responsive element (VDRE) (8, 9, 19, 20). Positive VDREs are typically comprised of two direct repeat elements containing the sequence PuG(G/T)TCA, spaced by three nucleotides (DR-3). Target genes whose promoters contain such VDREs include the mouse osteopontin, human and rat osteocalcin, and human p21 genes (18, 23, 25, 29, 30).
VDR also mediates a 1,25(OH)2D3-dependent transcriptional repressive function (2, 12, 14, 22, 26, 36). Several target genes that are repressed by 1,25(OH)2D3 have been identified, and interestingly, the architecture of some so-called “negative VDREs” (nVDREs) is divergent from that of the aforementioned DR-3-type positive VDREs in both sequence and organization. Such nVDREs have been characterized in the promoters of the human parathyroid, interleukin-2 (IL-2), and granulocyte-macrophage colony-stimulating factor (GM-CSF) genes (2, 12, 36). Direct VDR binding to these nVDREs has been clearly demonstrated, often independent of RXR and, in the case of GM-CSF, as a conformationally altered, monomeric species (36). This suggests that 1,25(OH)2D3-mediated transrepression of specific target genes may occur through mechanisms distinct from that established for 1,25(OH)2D3-mediated activation.
The initial detection of VDR in activated T cells led to the postulation that 1,25(OH)2D3 played a role in the immune response. In the presence of 1,25(OH)2D3, a decrease in the proliferation of T lymphocytes is observed (34). This immunosuppressive effect correlated with a decrease in the mRNA levels of the primary response cytokine IL-2, as well as with a decrease in interferon-γ and GM-CSF mRNA levels (5, 35). Previous studies in our laboratory demonstrated that the direct transcriptional repression of the IL-2 and GM-CSF genes contributes to the overall immunosuppressive effects of 1,25(OH)2D3 (2, 36).
GM-CSF is a glycoprotein which signals through a cell surface receptor of the hematopoietin receptor family. It is synthesized in activated T cells, activated macrophages, endothelial cells, and fibroblasts (17). GM-CSF was initially identified as both a proliferation and a differentiation factor for granulocytes and monocytes, and it was later shown to also function as a survival factor for these mature cell types. The activation of the GM-CSF gene is mediated through a 716-bp region of the enhancer that was initially identified as a DNase I hypersensitive site induced upon treatment with T-cell-activating agents (11). This fragment can function as a strong, inducible enhancer when it is fused to the 600-bp GM-CSF promoter, and within this sequence are four binding sites for AP-1 (11). In addition, one of these four sites resembles a composite site consisting of NFAT1 and AP-1 binding elements that are similar to those found in the IL-2 enhancer as well as in other cytokine promoters (28, 32). Our laboratory demonstrated that this composite site in the IL-2 enhancer is responsible for mediating the 1,25(OH)2D3 repression of activated IL-2 transcription, primarily through direct inhibition of the NFAT–AP-1 complex by VDR (2). We subsequently showed that the distinct NFAT–AP-1 site in the GM-CSF enhancer also mediates repression by 1,25(OH)2D3 (36), and it was the intent of this study to determine the mode by which VDR accomplishes this.
We report here that 1,25(OH)2D3-mediated repression of the GM-CSF locus involves a two-hit mechanism that targets both NFAT1 and Jun-Fos. In the first hit, VDR outcompetes NFAT1 for binding to a composite site that consists of a novel nVDRE and a consensus NFAT1 binding site. Overexpression of NFAT1 in vivo by transient transfection is able to partially relieve the 1,25(OH)2D3-dependent repression. In the second hit, VDR stabilizes the binding of a Jun-Fos heterodimer to an adjacent AP-1 site; this appears to occur through a direct interaction between VDR and c-Jun. Overexpression of c-Jun, but not c-Fos, leads to a rescue of the 1,25(OH)2D3-mediated repression. Given the scope of genes transactivated by NFAT1 and AP-1 in immunocompetent cells, the results described here may provide a mechanistic basis for how steroid and nuclear receptors elicit immunosuppressive responses.
MATERIALS AND METHODS
Overexpression and purification of proteins.
VDR and glutathione S-transferase (GST)-VDR overexpression and purification have been previously described (8, 31). NFATXS was overexpressed in Escherichia coli with a pET19b His-tagged vector (pQE31-NFATXS[1-297], generously provided by Anjana Rao). A 100-ml culture was grown to an optical density at 600 nm of 0.7 and induced with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for an additional 3 h at 37°C. Cells were centrifuged at 4,000 rpm in an SS-34 rotor for 10 min, and the cell pellet was lysed in lysis buffer (8 M urea, 5 mM β-mercaptoethanol, 0.1 mM sodium phosphate, 10 mM Tris-HCl [pH 8.0]) and incubated at 4°C for 10 min with gentle rocking. The resuspended pellet was then sonicated three times for 20-s intervals and was spun at 10,000 rpm in an SS-34 rotor for 10 min at 4°C to pellet the cell membrane. The supernatant was incubated with 300 μl of Ni-nitrilotriacetic acid-agarose beads (Qiagen, Chatsworth, Calif.) which was preequilibrated in lysis buffer for 2 h at 4°C with gentle rocking. Beads were pelleted at 5,000 rpm for 5 min and washed three times with lysis buffer plus 10 mM imidazole. Bound proteins were eluted from the beads in lysis buffer plus 200 mM imidazole. Eluted proteins were dialyzed overnight at 4°C in a stepwise manner against a buffer containing 20 mM HEPES (pH 7.4), 1 mM dithiothreitol (DTT), 100 mM NaCl, 2 mM EDTA, 20% glycerol, 2 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg of aprotinin per ml, 20 μM leupeptin, and decreasing concentrations of urea. Jun and Fos proteins were generously provided by K. R. Yamamoto and B. Maler (University of California, San Francisco).
Oligonucleotides and plasmids.
The NFAT–AP-1 site at position 550 within the 716-bp region of the GM-CSF enhancer (GM550) was synthesized as complementary oligonucleotides of the sequence 5′-GATCTCTTATTATGACTCTTGCTTTCCTCCTTTCA-3′ (top strand). An oligonucleotide that contained only the nVDRE sequence was also synthesized to determine if VDR was able to bind this site independent of the flanking sequence, nVDRE, which consists of the sequence 5′-TCGATCGTGTTGTAGAGCTTTCCTATGTCA-3′. Cytomegalovirus (CMV)-c-Jun and CMV-c-Fos were generously provided by K. R. Yamamoto. N3GMCSF-Luc and CMV-VDR were previously described (2, 36).
Electrophoretic mobility shift analysis (EMSA).
VDR DNA binding was assessed by gel mobility shift electrophoresis with complementary strands of synthetic oligonucleotides. Overexpressed, purified NFATXS, c-Jun, c-Fos, and VDR were preincubated individually or in various combinations with 12 fmol of duplex probe for 20 min at room temperature together with 50 μg of poly(dI-dC) per ml in binding buffer (20 mM Tris-HCl [pH 7.9], 1 mM EDTA, 50 mM KCl, 10% glycerol, 0.05% Nonidet P-40 [NP-40], and 1 mM DTT). Protein-DNA complexes were resolved by electrophoresis on 10% nondenaturing acrylamide gels run in 0.5× Tris-borate-EDTA at a constant voltage of 250 V at 4°C. Gels were dried and subjected to autoradiography.
Cell transfection and reporter assays.
The T-cell line Jurkat was transfected by the electroporation method with BTX (San Diego, Calif.) 0.2-μm-diameter cuvettes. Cells were grown in RPMI medium containing sodium pyruvate, glutamine, and penicillin-streptomycin to a final concentration of 100 μg/ml. Fetal calf serum was added to 10%, and cells were maintained at a density of approximately 8 × 105 cells/ml. For transfection, cells were washed in RPMI medium and resuspended in this medium to a density of 3 × 107 cells/200 μl. Each transfection reaction mixture contained 5 μg of reporter plasmid, 1.25 μg of internal control plasmid, 500 ng of producer plasmid, and 5 × 106 cells. All reactions were done in triplicate and with two treatments. Therefore, six reactions were transfected per BTX cuvette in a final volume of 200 μl. BTX settings were as follows: capacitance (C) = 1,700 μF, resistance (R) = 72 Ω, and charging voltage (S) = 126 V. After electroporation, cells were incubated for 30 min, and the contents of each cuvette was then added to 6 ml of RPMI medium containing sodium pyruvate, glutamine, penicillin-streptomycin, and charcoal-stripped fetal calf serum to 10%. Cells were plated as 1 ml of transfection mix added to 14 ml of the same stripped serum-containing medium, and they were incubated for 24 h (5% CO2; 37°C). At 24 h posttransfection, cells were treated for 9 h in one of the following ways: (i) no treatment, (ii) addition of the activating agents phorbol myristate acetate (PMA; Sigma, St. Louis, Mo.) (50 ng/ml) and phytohemagglutinin (PHA; Sigma) (2 μg/ml), (iii) addition of 5 × 10−8 M 1,25(OH)2D3 (Biomol, Plymouth Meeting, Pa.), or (iv) addition of the activating agents and 1,25(OH)2D3. Cells were harvested, and extracts were normalized to protein concentration as well as to β-galactosidase activity produced off the internal control plasmid CMV-β-gal included in each transfection. Equal amounts of total cell extract were added to luciferase assays, and results were quantitated as relative light units with a luminometer.
Nuclear extract preparation.
Jurkat cells were grown as described above. Two hundred milliliters of cells at a density of 8 × 105 cells/ml was treated with the activating agents PMA (50 ng/ml) and PHA (2 μg/ml) in the presence or absence of 2.4 × 10−8 M 1,25(OH)2D3 for the time indicated above. Cells were harvested by pelleting at 1,700 rpm at 4°C in a clinical tabletop centrifuge. Cell pellets were washed in phosphate-buffered saline (PBS) and respun. Cell pellets were resuspended gently in 3 pellet volumes of buffer A (10 mM HEPES, 15 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 0.1 mM PMSF). Cells were pelleted and resuspended in 1 ml of buffer A plus 0.2% NP-40, incubated for 5 min, and centrifuged at 1,700 rpm at 4°C for 10 min. The pellets were resuspended in 315 μl of buffer C (50 mM HEPES [pH 7.8], 50 mM KCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM PMSF, 10% glycerol). Thirty-five microliters of 3 M (NH4)2SO4 (pH 7.9) in buffer C was added to each sample and was incubated at 4°C for 35 min with gentle rocking. Samples were centrifuged at 55,000 rpm in a TL100.2 rotor for 30 min at 4°C. The supernatants were collected, an equal volume of 3 M (NH4)2SO4 (pH 7.9) was added to each, and samples were incubated for 30 min. The samples were centrifuged at 55,000 rpm for 10 min. The pellets were resuspended in 100 μl of buffer C and were frozen in liquid nitrogen in 5-μl aliquots.
GST affinity binding assay.
Immobilized GST-VDR fusion proteins or GST alone (as a negative control) was incubated with increasing concentrations of purified c-Jun or c-Fos protein in 1× binding buffer (10% glycerol, 20 mM Tris-HCl [pH 7.9], 50 mM KCl, 1 mM DTT, 0.05% NP-40) for 2 h at 4°C with gentle rocking. The beads were washed three times in 1× binding buffer supplemented with 450 to 750 mM KCl. After the final wash, the beads were resuspended in sodium dodecyl sulfate (SDS)-loading buffer, boiled for 5 min, and loaded on an SDS–10% polyacrylamide gel. The gels were subsequently transferred onto polyvinylidene difluoride membranes (NEN, Boston, Mass.) for 30 min at 10 V with the semidry transfer system (Bio-Rad, Richmond, Calif.). Blots were blocked at 4°C overnight in 5% nonfat dry milk in PBS–0.1% Tween and were incubated with anti-c-Jun rabbit polyclonal antibody (Oncogene Science, Cambridge, Mass.) at a 1:1,000 dilution or with anti-c-Fos rabbit antibody (Affinity BioReagents, Golden, Colo.) at a 1:5,000 dilution at room temperature for 1 h. Blots were rinsed three times and were washed three times for 5 min in PBS–0.1% Tween. A secondary antibody, horseradish peroxidase-linked anti-rabbit immunoglobulin (Amersham, Little Chalfont, England), was used at a 1:3,000 dilution in 5% nonfat dry milk in PBS–0.1% Tween for 20 min at room temperature. Washes were performed as described above, and blots were developed with ECL Western blotting detection reagent according to the manufacturer’s protocol (Amersham).
Immunoprecipitation of DNA-bound complex.
The method used for immunoprecipitation of the VDR-DNA complex was adopted from Cella et al. (6). Briefly, 6 μg of nuclear extract was mixed with a [γ-32P]ATP-labeled oligonucleotide (approximately 15,000 cpm), which was synthesized as a complementary oligonucleotide of the sequence 5′-GATCTCTTATTATGACTCTTGCTTTCCTCCTTTCA-3′. Following a 10-min incubation at room temperature, 2 μl of anti-FLAG M2 antibody (Eastman Kodak Co., New Haven, Conn.) was added for an additional 10 min at room temperature. The complexes were precipitated with 10 μl of protein A-agarose beads (Santa Cruz Biotechnology, Santa Cruz, Calif.) for 30 min on ice. Samples were washed three times with TNE buffer (10 mM Tris-Cl [pH 7.4], 1 mM EDTA, 0.2 M NaCl), and the immunoprecipitated material was counted by scintillation.
RESULTS
A composite NFAT1–AP-1 site in the GM-CSF enhancer contains an overlapping VDR binding element.
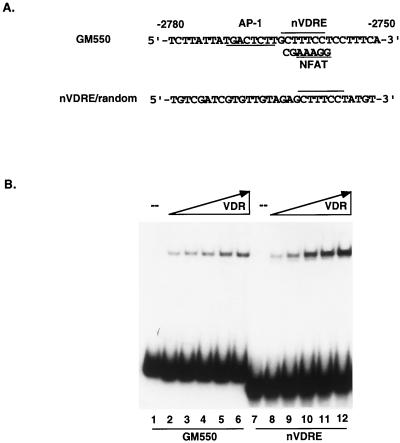
We previously described a control element in the GM-CSF enhancer that is necessary and sufficient to mediate both transcriptional activation in response to T-cell stimuli, such as PMA and PHA, and transcriptional repression by 1,25(OH)2D3 through VDR. The element, shown in Fig. 1A and called GM550, is a composite site that is recognized by both Fos-Jun and NFAT1, and it is also directly bound by VDR in the absence of RXR as an apparent monomer (36). Using a series of mutant oligonucleotides, we defined the minimal VDR recognition site within the composite GM550 element as a noncanonical 7-bp sequence (bases −2758 to −2764 in the GM-CSF enhancer, here called the nVDRE [Fig. 1A]). The nVDRE was further examined in order to determine if this core sequence could still function as a high-affinity binding site for VDR in the absence of adjacent sites, such as the AP-1 element. A synthetic oligonucleotide consisting of the 7-bp nVDRE synthesized in the context of randomized DNA sequence was tested for binding with recombinant, purified VDR with the EMSA. As shown in Fig. 1B, VDR’s ability to bind this site was independent of flanking sequences. As a control, the flanking randomized sequence without the nVDRE was also tested, but no specific VDR binding was detected (data not shown). Thus, this 7-bp sequence overlapping the NFAT1 element constitutes a novel binding site for VDR. Based on our previous work, this site preferentially recognizes VDR in a monomeric form with a unique tertiary structure (36).
FIG. 1.
(A) The GM550 element contains a composite site consisting of overlapping NFAT and VDR binding sites (nVDRE) and a proximal AP-1 site. Also depicted is a 30-bp synthetic oligonucleotide that contains the 7-bp nVDRE core sequence in the context of a random nonspecific sequence (nVDRE/random). (B) VDR binds the 7-bp nVDRE independent of flanking GM550 sequences. A comparison of VDR binding to GM550 and nVDRE/random is depicted. Purified VDR, ranging from 20 to 100 ng, was incubated with 12 fmol of either radiolabeled GM550 oligonucleotide or nVDRE/random.
VDR and NFAT1 compete for binding to the GM550 element.
The delineation of a VDR binding site to a 7-bp sequence overlapping the NFAT1 binding site, together with the observation that cyclosporin A affects activated GM-CSF gene expression (11), suggested that a mechanism for VDR-mediated transrepression of GM-CSF-activated transcription might be competition for DNA binding between NFAT1 and VDR. In this scenario, VDR and NFAT1 would bind to the GM550 element in a mutually exclusive manner, since the elements overlap.
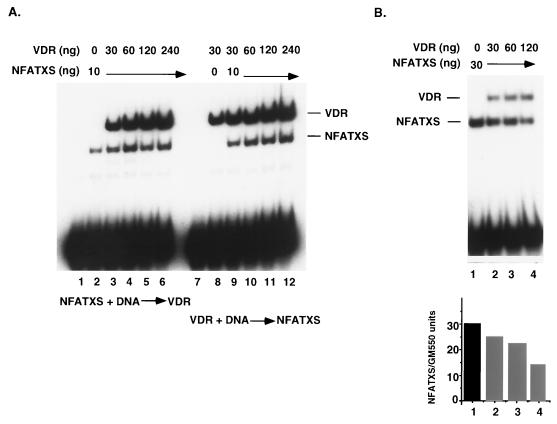
In order to directly test this possibility, a gel mobility shift assay was performed with full-length VDR and a derivative of NFAT1, NFATXS (32). This 297-amino-acid fragment contains the minimal region required for DNA binding and for complex formation with Jun and Fos. It is located centrally in the protein (residues 396 to 693) and has limited homology to the c-Rel DNA binding domain. EMSA carried out with either of these proteins demonstrated high-affinity binding to the GM550 element (Fig. 2A, lanes 2 and 8). Simultaneous addition of both NFATXS and VDR at equimolar concentrations yielded NFATXS-GM550 and VDR-GM550 binding complexes, but a higher-order complex consisting of VDR, NFATXS, and GM550 was never observed under these conditions. This was true irrespective of the order of addition and the concentration of the two proteins within the range used (Fig. 2A, compare lanes 3 to 6 and 9 to 12). Identical results were observed when the VDR concentration was held constant and titrated in NFATXS (data not shown).
FIG. 2.
VDR and NFAT1 do not co-occupy the GM550 element. (A) Mutually exclusive binding of VDR and NFATXS to GM550. Fifteen femtomoles of radiolabeled GM550 element was incubated with the indicated amounts of recombinant VDR and NFATXS, where the order of addition was reversed, as indicated (lanes 1 to 6 and 7 to 12). At excess concentrations of DNA, NFATXS binding is independent of VDR. (B) VDR and NFATXS compete for binding to the GM550 site at limiting DNA concentrations. Increasing amounts of VDR were incubated with a constant amount of NFATXS (30 ng) in a binding reaction in which the radiolabeled GM550 element was limiting (5 fmol). The quantitation of the NFATXS-shifted species below the gel indicates that 120 ng of VDR resulted in a >50% decrease in NFATXS binding.
In these experiments, a 50-fold molar excess of protein to DNA was used, but a considerable amount of unbound probe was still detected. Since the probe is in such excess, competition for binding between VDR and NFATXS might be detected only by using limiting amounts of probe. Therefore, 5 fmol of a GM550 probe was incubated with a constant amount of NFATXS and increasing amounts of VDR. The results in Fig. 2B demonstrate that the fraction of NFATXS bound to the GM550 element diminished by half in the presence of 120 ng of VDR (lane 4). This is consistent with a DNA binding competition between the two proteins, although it could also result from protein-protein interactions off the DNA. In order to test the latter possibility, we performed pull-down assays in which His-tagged NFATXS was immobilized to Ni-nitrilotriacetic acid-agarose beads and used in an attempt to coprecipitate VDR. No interaction was detected in this manner or in the reverse experiment, when GST-VDR was used to coprecipitate NFATXS (data not shown). Thus, the effect observed in Fig. 2B most likely is due to a competition for two overlapping DNA binding sites, i.e., the 5-bp NFAT1 binding site contained within the 7-bp core nVDRE, whereby co-occupancy is not possible.
Overexpression of NFAT1 partially relieves the 1,25(OH)2D3-mediated repression.
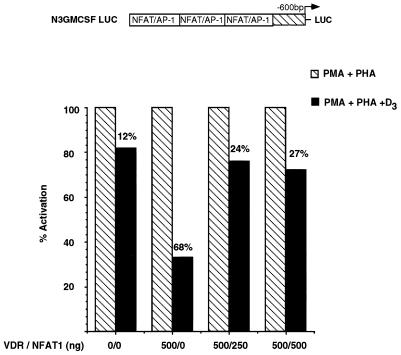
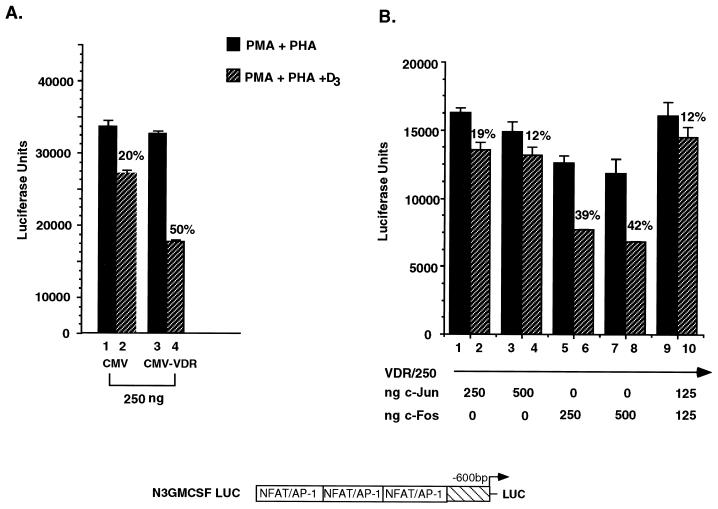
If VDR-mediated transrepression stems from competition with NFAT1 for binding to the GM550 element, it would be predicted that overexpression of NFAT1 protein in vivo might relieve the repression. To test this, a plasmid overexpressing the full-length NFAT1 coding sequence, pLGP3mNFAT1-A (28), was used to cotransfect Jurkat cells together with a VDR producer plasmid and a reporter containing the GM550 element reiterated three times (called N3GMCSF) (36). The cells were treated with activating agents in the absence or presence of 1,25(OH)2D3, and luciferase activity from the N3GMCSF reporter was assayed. As shown in Fig. 3, overexpression of 500 ng of CMV-VDR resulted in a 68% repression of activated transcription in response to 2 × 10−8 M 1,25(OH)2D3. Co-overexpression of 250 or 500 ng of the NFAT1 plasmid gave significant relief of the VDR- and 1,25(OH)2D3-dependent repression. It is worth noting, however, that the repression was only partially relieved, suggesting that additional components of the transactivation complex are targets of VDR repression. Interestingly, overexpression of NFAT1 alone did not augment activation levels in response to activating agents (data not shown). This implies a requirement for both components of the GM-CSF activator complex, namely NFAT1 and Jun-Fos, in order to activate the expression of this gene.
FIG. 3.
Overexpression of NFAT1 in T cells partially relieves 1,25(OH)2D3-mediated repression of the N3GMCSF reporter. Jurkat cells were transiently transfected with the reporter plasmid depicted above the histogram, together with VDR and NFAT1 overexpression plasmids under the control of the CMV promoter at the indicated ratios. A 0 indicates the endogenous level of the respective factor. Cells were activated with PMA and PHA in the presence or absence of 1,25(OH)2D3 for 8 h; cells were then harvested, and luciferase activity was assayed. Activation levels independent of 1,25(OH)2D3 treatment were set to 100%. Shown are results of a representative experiment carried out in triplicate and repeated five times. All values were normalized to protein concentration as well as to β-galactosidase activity produced off the internal control plasmid. LUC, luciferase-encoding sequence.
VDR stabilizes the AP-1 complex on the negative element.
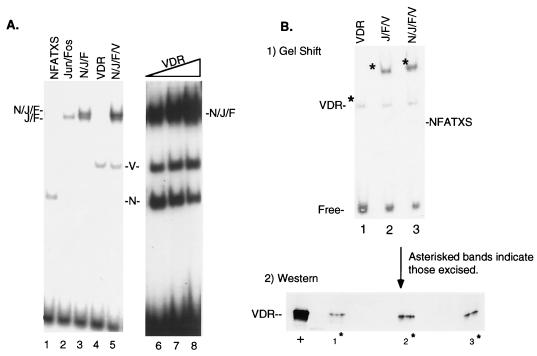
VDR antagonism of NFAT1 DNA binding on the GM550 element appears to be one component of the 1,25(OH)2D3 transrepression mechanism. The presence of an adjacent AP-1 site (Fig. 1A) that is occupied in vivo during activation of the GM-CSF promoter/enhancer suggests that an additional target of VDR could be the Jun-Fos heterodimer. We therefore decided to examine putative interactions between VDR and the AP-1 components and to explore how the interplay of these factors might also affect the transrepression of GM-CSF gene expression. Figure 4A shows the DNA binding profile of purified Jun, Fos, NFAT1, and VDR proteins (all overexpressed in E. coli) bound to the GM550 element. NFATXS bound with high affinity to the negative element (lane 1), independent of AP-1 occupancy. Likewise, the c-Jun-c-Fos heterodimer bound to the negative element in the absence of NFATXS (lane 2). The combination of these factors led to a higher-order complex which migrates as a doublet (lane 3) under our EMSA conditions. The loss of the NFAT shift in lane 3 corresponded to the gain of the slowest-migrating band, a ternary NFAT-Jun-Fos complex. Unexpectedly, the addition of VDR led to an increase in the apparent affinity of the largest complex, yet it did not result in a significant change in the overall mobility of the complex (Fig. 4A, lane 5). Increasing the amount of VDR in the presence of NFAT, Jun, and Fos increased the intensity of the slowest-shifted species, but it did not lead to a change in the mobility of the higher-order complex (Fig. 4A, lanes 6 to 8). Consistent with the results shown in Fig. 2, an increase in the level of VDR was accompanied by a decrease in the observed NFATXS-GM550 complex (Fig. 4A, lanes 6 to 8). Moreover, the apparent stabilizing effect of VDR on the higher-order complex did not require NFATXS, in that identical results were obtained when only Jun, Fos, and VDR were included in the binding reactions (data not shown).
FIG. 4.
VDR stabilizes the AP-1 complex on the GM550 element. (A) VDR stabilizes the Jun-Fos-GM550 ternary complex in the presence or absence of NFATXS. Twelve nanograms of c-Jun protein (J) and 6 ng of c-Fos protein (F) were used in lanes 2, 3, 5, and 6 to 8. Ten nanograms of NFATXS (N) was used in lanes 1, 3, 5, and 6 to 8, and 30 ng of VDR (V) was used in lanes 4 and 5. A titration of VDR was performed (lanes 6 to 8) in an attempt to detect an enhancement in the mobility shift of the upper complex. (B) VDR is present in the Jun-Fos DNA-bound complex. The top panel shows the DNA binding profile of the indicated purified proteins in a complex with a GM550 probe, using the amounts used for panel A. The asterisks denote those complexes which were excised as gel fragments, eluted, and run in an SDS-polyacrylamide gel. The bottom panel shows an immunoblot analysis of the SDS gel probed with an anti-VDR monoclonal antibody (Affinity BioReagents). Lane numbers correspond to the same lanes on the gel mobility shift from which the complexes were excised. +, purified VDR loaded directly onto the SDS gel.
The apparent stabilization of the Jun-Fos complex by VDR was suggestive of a putative interaction between the three proteins, at least when bound to the GM550 element. If such an interaction was occurring, one would expect to detect VDR in a DNA-bound complex with Jun and Fos. In order to detect VDR directly in this complex, DNA-bound complexes resolved in a gel shift (Fig. 4B, top panel) were excised and loaded onto an SDS-polyacrylamide gel which was immunoblotted for the presence of VDR (Fig. 4B, bottom panel). Lanes 2 and 3 in Fig. 4B clearly demonstrate the presence of VDR in the Jun-Fos complex bound to the GM550 element. Again, the addition of NFATXS had no effect on the ability of VDR to associate in the higher-order complex with Jun and Fos. Taken together, these results strongly suggest that while VDR competes with NFAT1 for a binding site, it co-occupies this element with Jun-Fos and actually stabilizes its association with the DNA.
Overexpression of c-Jun prevents 1,25(OH)2D3-mediated repression.
The DNA binding data shown in Fig. 4 suggests some kind of functional interplay between VDR and Jun and/or Fos, presumably resulting in an inability by Jun-Fos to transactivate. As we observed with NFAT1, transient overexpression of Jun, Fos, or both proteins might lead to a rescue of the 1,25(OH)2D3-mediated repression of the activated transcription of GM-CSF. The results of such an experiment are shown in Fig. 5. Transfection of 250 ng of CMV-VDR resulted in approximately 50% repression of activated transcription (Fig. 5A, lanes 3 and 4). Transfection of an equal amount of CMV-Jun rescued the 1,25(OH)2D3-mediated repression (Fig. 5B, lanes 1 and 2). In contrast, overexpression of c-Fos had very little effect (less than 10%) on 1,25(OH)2D3-mediated repression (lanes 5 and 6). Consistent with this, the effect observed with c-Fos and c-Jun together (Fig. 5B, lanes 9 and 10) was identical to that with c-Jun alone. It is important to note that the effect of overexpressing either c-Jun or c-Fos led to a diminution of the absolute activation levels in response to the activating agents PMA and PHA (Fig. 5, compare the y axis in panel B versus the y axis in panel A); this is most likely due to general squelching. Although this level of activation was reduced by 40%, it still represents a 10-fold induction over the basal level of activity in the absence of any activating agents. In order to rule out a general squelching effect of transfecting overexpression plasmids, the experiment shown in Fig. 5B was repeated with endogenous levels of VDR and significantly lower levels of overexpressed c-Jun and c-Fos. Results obtained from this experiment recapitulated those shown in Fig. 5B; low amounts of c-Jun, but not c-Fos, rescued endogenous VDR-mediated repression to the same extent as was observed when all three proteins were overexpressed (data not shown).
FIG. 5.
Overexpression of Jun prevents 1,25(OH)2D3-mediated transrepression of the GM-CSF locus. Jurkat cells were transiently transfected with the indicated plasmids and were activated with PMA and PHA in the presence or absence of 10−8 M 1,25(OH)2D3. (A) A total of 250 ng of CMV-VDR leads to 50% repression. (Note that this is half the amount of VDR that was used in Fig. 3.) (B) CMV-Jun, but not CMV-Fos, blocks 1,25(OH)2D3-mediated repression. The indicated amount of plasmid DNAs were used in each transfection. Note that overall activation levels are decreased in the presence of overexpressed c-Jun and c-Fos plasmids, most likely due to general squelching (compare the average luciferase (LUC) units in panel B, 15,000, with that in panel A, 30,000). All values were normalized to protein concentration as well as to β-galactosidase activity produced off the internal control plasmid.
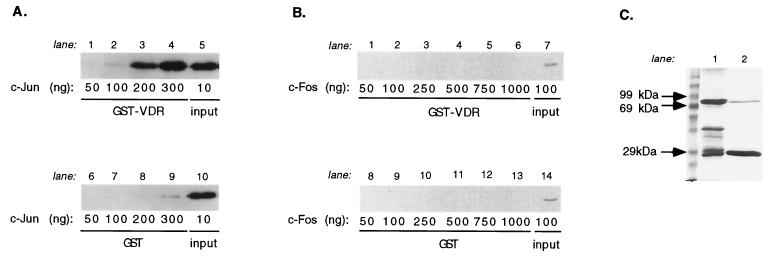
VDR interacts with c-Jun in vitro.
The effects of VDR on the stabilization of AP-1 binding and the relief of transrepression by c-Jun (but not c-Fos) in vivo suggested that VDR might interact with the Jun-Fos complex, perhaps specifically with c-Jun. To test this prediction, we carried out GST pull-down experiments, using VDR as the bait. As is evident from the gels shown in Fig. 6A and B, GST-VDR interacted selectively with c-Jun, but not c-Fos, over a concentration range of proteins. In both cases, there was no interaction detected with GST alone. Thus, VDR may stabilize the AP-1 complex at the GM550 site by interacting directly with c-Jun and, in the process, inhibit the transactivation function of c-Jun.
FIG. 6.
VDR interacts directly with c-Jun, but not c-Fos, in an in vitro interaction assay. (A) VDR interactions with c-Jun. Lanes 1 to 4, glutathione-agarose beads containing immobilized GST-VDR coincubated with 50 to 300 ng of c-Jun; lane 5, input c-Jun protein; lanes 6 to 9, immobilized GST incubated with identical amounts of added purified c-Jun protein; lane 10, input c-Jun. (B) VDR does not interact with c-Fos. Lanes 1 to 6, immobilized GST-VDR incubated with 50 to 1,000 ng of c-Fos protein; lane 7, input c-Fos protein; lanes 8 to 13, immobilized GST incubated with identical amounts of added purified c-Fos protein; lane 14, 100 ng of input c-Fos. Visualization of c-Jun and c-Fos proteins was by Western blot analysis with specific antibodies raised against the two proteins. (Note that detection of c-Fos required greater amounts of protein than detection of c-Jun due to its lower-affinity antibody; therefore, the c-Fos titration was taken out to 1,000 ng.) (C) GST-VDR (lane 1) and GST (lane 2) baits shown at the amounts used in both pull-down series (2.5 μg).
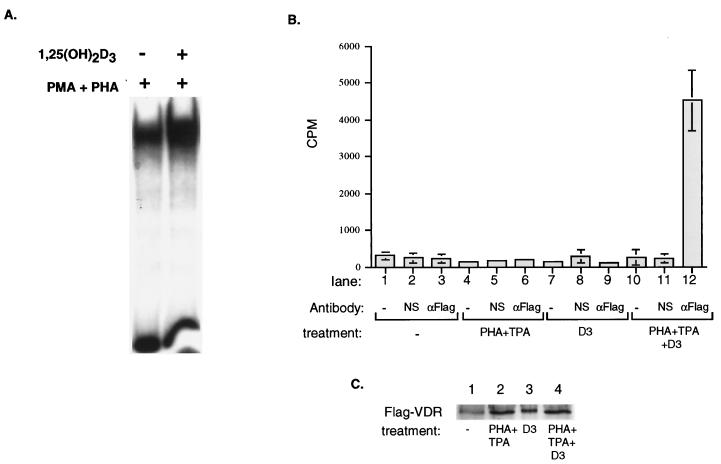
1,25(OH)2D3 leads to VDR occupancy on the GM550 element in nuclear extracts from activated T cells.
The results presented in this work demonstrate an antagonistic effect of VDR on the Jun–Fos–NFAT1-GM550 ternary complex through competition for NFAT1 DNA binding by VDR and simultaneously through stabilization of the AP-1 complex by direct interaction of VDR with c-Jun. While the antagonistic effects on GM-CSF transcription are consistently observed upon treatment of T cells with 1,25(OH)2D3, our mechanistic interpretations were derived by using purified components in vitro. We therefore made nuclear extracts from Jurkat cells which were treated with activating agents in the absence or presence of 1,25(OH)2D3 to determine if VDR could be detected as part of the PMA- and PHA-inducible DNA binding complex after treatment with 1,25(OH)2D3. The results of this experiment are shown in Fig. 7. A PHA- and PMA-inducible complex was detected after 4 h; this complex was further enhanced in the presence of 1,25(OH)2D3 (Fig. 7A). This result is in agreement with the stabilization effect by VDR on Jun-Fos binding that we observed with purified proteins (Fig. 4). In order to directly demonstrate the ligand-dependent presence of VDR in a complex on DNA derived from nuclear extracts, a modified pull-down assay was utilized. In this approach, the GM550 element was radiolabeled and incubated with nuclear extracts derived from variously treated Jurkat cells and then immunoprecipitated via its interaction with VDR. As shown in Fig. 7B, GM550 DNA-bound VDR could be selectively precipitated from VDR-FLAG transfected nuclear extracts only in the presence of activating agents and 1,25(OH)2D3 (lane 12). Importantly, VDR was not detected in a DNA-bound form from unactivated nuclear extracts, activated nuclear extracts in the absence of 1,25(OH)2D3, or 1,25(OH)2D3-treated nuclear extracts in the absence of activating agents (Fig. 7B, lanes 3, 6, and 9). These results indicate that VDR occupies the GM550 element in nuclear extracts in a fully ligand-dependent manner, leading to an inhibition of activated GM-CSF transcription.
FIG. 7.
Nuclear extract derived from 1,25(OH)2D3-treated cells demonstrates that VDR is bound to the GM550 element. (A) 1,25(OH)2D3 alters an endogenous GM550 binding complex in nuclear extracts from activated Jurkat T cells. Nuclear extracts were prepared from activated T cells which were untreated or treated with 1,25(OH)2D3. Three milligrams of nuclear extract was incubated with 12 fmol of radiolabeled GM550. Complexes were separated on 4% nondenaturing acrylamide gels supplemented with glycerol. (B) VDR binds to the GM550 element in extracts from activated cells in the presence of 1,25(OH)2D3. Jurkat cells were transfected with FLAG-VDR and were either mock treated (lanes 1 to 3) or treated with 1,25(OH)2D3 (lanes 7 to 9), PHA-tetradecanoyl phorbol acetate (TPA) in the absence of ligand (lanes 4 to 6), or PHA-tetradecanoyl phorbol acetate in the presence of 1,25(OH)2D3 (lanes 10 to 12). Nuclear extracts were adjusted for FLAG-VDR expression levels (as determined by a Western blot quantitation in panel C) and were incubated with an end-labeled GM550 DNA element and either an anti-FLAG antibody (lanes 3, 6, 9, and 12), a nonspecific antibody (lanes 2, 5, 8, and 11), or no antibody (lanes 1, 4, 7, and 10). Protein-DNA-antibody complexes were precipitated with agarose A beads, washed, and counted. Precipitated counts per minute are shown; values are the means of three independent experiments carried out in triplicate. (C) Western blot analysis of adjusted FLAG-VDR expression levels in nuclear extracts as used for panel B.
DISCUSSION
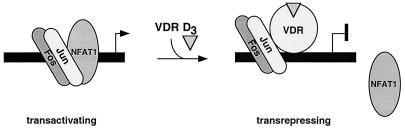
The GM550 element we previously identified as a target for 1,25(OH)2D3-mediated transrepression is a short region within the vast GM-CSF enhancer. It contains binding sites for three key transcriptional regulatory proteins, namely, NFAT1, Jun, and Fos, that are critical to its responsiveness to activating agents. In addition, VDR can also bind to this element. However, it does so in a manner completely distinct from how it recognizes positive, classical vitamin D response elements: it binds to the GM550 element as a conformationally altered monomeric species, independent of its heterodimeric partner RXR. This allosteric effect renders VDR transcriptionally silent, and it is reflected in the VDR binding site itself. The nVDRE is not arranged in the typical direct repeat fashion of hormone-responsive elements, and it does not contain the canonical AGGTCA core sequence (Fig. 1A). Since within the GM550 element the nVDRE actually overlaps an NFAT binding site, one possible mechanism for VDR-mediated transrepression is simple steric hindrance, in which a transcriptionally inert VDR monomer blocks the association of NFAT1 protein to one half of the NFAT1–AP-1 site. Here, we have presented evidence demonstrating that VDR indeed competes with NFAT1 for DNA binding to these overlapping sites, resulting in mutually exclusive occupancy by either NFAT1 or VDR but never occupancy by both proteins (Fig. 8).
FIG. 8.
A two-hit mechanism for repression of GM-CSF-activated transcription by VDR.
The exclusion of NFAT1 occupancy in the GM-CSF promoter should lead to complete repression of this locus based on promoter sensitivity to the immunosuppressive drug cyclosporin A (11). Cyclosporin A elicits its immunosuppressive effects by targeting cytoplasmically localized NFAT, inhibiting its dephosphorylation by calcineurin and its subsequent nuclear localization (4, 15). VDR inhibition of NFAT DNA binding ought to have resulted in the repression of GM-CSF gene expression to a similar extent as inhibition by cyclosporin A did. However, in an experiment in which NFAT1 was overexpressed in order to outcompete VDR, activation levels were not restored to 100% (Fig. 3). This implies that NFAT1 is not the sole component for activation and, by extension, that VDR must be targeting an additional factor or factors to achieve maximal transrepression. That factor appears to be Jun of the AP-1 binding complex, which we propose to be the second part of VDR’s two-hit mechanism for repression, as shown in Fig. 8.
We anticipated an inhibitory effect of VDR on Jun-Fos DNA binding, thereby completely disrupting the activation complex. It seemed reasonable that the occupancy of VDR at the NFAT-nVDRE composite element at the expense of NFAT1 could also indirectly inhibit Jun-Fos binding at the neighboring AP-1 site, since NFAT1 acts to potentiate Jun-Fos binding. Surprisingly, VDR had a stabilizing effect on the Jun-Fos-GM550 ternary complex (Fig. 4). The ability of VDR to stabilize the Jun-Fos complex initially seems counterintuitive. However, the stabilization of the AP-1 complex by VDR is not without precedent. Many repressors can co-occupy with activators and prevent activator function. Examples of this type of activator masking can be found in a wide range of eukaryotes, from flies to humans. The Drosophila protein Krüppel selectively binds to and represses the activity of a reporter construct consisting of Krüppel and Sp1 binding sites. Krüppel cooccupies both sites, masking the glutamine-rich activator domain of Sp1 (24). In mammalian cells, this type of activator masking as a means of repression is best exemplified by the proliferin gene. Simultaneous occupancy of AP-1 and the glucocorticoid receptor at a composite negative glucocorticoid responsive element in the proliferin promoter leads to repression of AP-1 transactivation. Although both factors bind to the DNA, the glucocorticoid receptor is able to prevent AP-1 from transactivating by locking it in an inactive state (13).
The stabilization of Jun-Fos by VDR predicted a co-occupancy of VDR and the transactivating Jun-Fos heterodimer on the GM550 element in a quaternary complex. These results suggest a model in which VDR would somehow have to lock AP-1 in an “off” state, such that it could no longer transactivate, perhaps by a direct physical interaction and/or by precluding a productive interaction with a coactivator or basal factor. We have in fact demonstrated here a selective interaction between purified c-Jun and VDR proteins utilizing a GST pull-down assay (Fig. 6). In addition to this in vitro result, we have overexpressed c-Jun, c-Fos, and both proteins together in a transient transfection and assayed 1,25(OH)2D3-dependent repression. Overexpression of c-Jun, but not c-Fos, led to a rescue of the VDR repression. These results do not distinguish between a Jun-VDR interaction in solution and the same interaction on DNA. However, the elucidation of the crystal structure of a quaternary complex consisting of Jun, Fos, NFAT1, and the NFAT–AP-1 site from the IL-2 locus lends some insight into the organization of these factors on DNA (7). In this structure, preferential orientation of the Jun-Fos heterodimer, predicted by Leonard et al. (21), was observed, where Jun always occupies the proximal half of the AP-1 recognition element with respect to the NFAT site. Such an orientational constraint would never allow Fos to come into close proximity with NFAT. In our model of 1,25(OH)2D3-mediated repression, shown in Fig. 8, a monomeric VDR molecule bound to the NFAT binding site interacts with the bound AP-1 complex. VDR bound to the NFAT-nVDRE composite site would position itself spatially adjacent to Jun on the DNA (provided that an extrapolation of the NFAT-AP-1 structure on the IL-2 site applies to that in GM-CSF). This organization possibly explains why overexpression of c-Jun is capable of blocking the 1,25(OH)2D3 negative effects, yet c-Fos overexpression cannot. It is likely that VDR simply contacts Jun because Jun is within its grasp.
Why would a repressor (VDR) allow an activator protein (Jun-Fos) to remain bound to its binding site in the enhancer? One possibility is that by stabilizing the Jun-Fos complex on DNA, VDR is decreasing the off rate of a component of the activator complex. The net effect would be to shift the equilibrium to an inactive DNA-bound state, thereby preventing reactivation by the activator complex. Another possibility is that it indirectly decreases the available pools of Jun-Fos so as to prevent the activation of other AP-1 responsive cytokine genes.
Repression of cytokine gene transcription by VDR serves to explain the molecular mechanisms underlying the immunosuppressive effects of 1,25(OH)2D3. The work presented here provides a molecular framework to understand the interplay between VDR, NFAT1, and AP-1. We have delineated a mechanism of how VDR transduces the appropriate signal to a specific cytokine locus based on promoter architecture. Moreover, our studies suggest that the NFAT–AP-1 site may serve as a hallmark for the identification of negative regulatory elements for VDR and other nuclear receptors.
ACKNOWLEDGMENTS
We thank A. Rao and K. R. Yamamoto for plasmids, B. Maler and K. R. Yamamoto for Jun and Fos proteins, and C. Rachez for critically reading this paper. We are also grateful to R. Benezra and J. Massagué for helpful discussions and insights.
This work was supported by NIH grant DK454460 to L.P.F. and NIH grant CA08748 to Sloan-Kettering. T.L.T. was a Sloan-Kettering Institute Rudin Scholar.
REFERENCES
- 1.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alroy I, Towers T L, Freedman L P. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol. 1995;15:5789–5799. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-Shavit Z, Teitelbaum S L, Reitsma P, Hall A, Pegg L E, Trial J, Kahn A J. Induction of monocytic differentiation and bone resorption by 1,25 dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1983;80:5907–5911. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beals C R, Clipstone N A, Ho S N, Crabtree G R. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 1997;11:824–834. doi: 10.1101/gad.11.7.824. [DOI] [PubMed] [Google Scholar]
- 5.Bhalla A K, Amento E P, Krane S M. Differential effects of 1,25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell Immunol. 1986;98:311–322. doi: 10.1016/0008-8749(86)90291-1. [DOI] [PubMed] [Google Scholar]
- 6.Cella N, Groner B, Hynes N E. Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells. Mol Cell Biol. 1998;18:1783–1792. doi: 10.1128/mcb.18.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Glover J N M, Hogan P G, Rao A, Harrison S C. Structure of the DNA-binding domains from NFAT, Fos, and Jun bound specifically to DNA. Nature. 1998;392:42–48. doi: 10.1038/32100. [DOI] [PubMed] [Google Scholar]
- 8.Cheskis B, Freedman L P. Ligand modulates the conversion of DNA-bound vitamin D3 receptor (VDR) homodimers into VDR-retinoid X receptor heterodimers. Mol Cell Biol. 1994;14:3329–3338. doi: 10.1128/mcb.14.5.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheskis B, Freedman L P. Modulation of nuclear receptor interactions by ligands: kinetic analysis using surface plasmon resonance. Biochemistry. 1996;35:3309–3318. doi: 10.1021/bi952283r. [DOI] [PubMed] [Google Scholar]
- 10.Christakos S, Raval-Pandya M, Wernyj R P, Yang W. Genomic mechanisms involved in the pleiotropic actions of 1,25-dihydroxyvitamin D3. Biochem J. 1996;316:361–371. doi: 10.1042/bj3160361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cockerill P N, Shannon M F, Bert A G, Ryan G R, Vadas M A. Granulocyte-macrophage colony-stimulating factor/interleukin-3 locus is regulated by an inducible cyclosporin A-sensitive enhancer. Proc Natl Acad Sci USA. 1993;90:2466–2470. doi: 10.1073/pnas.90.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demay M B, DeLuca H F, Kronenberg H M. Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1992;89:8097–8101. doi: 10.1073/pnas.89.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond M I, Miner J N, Yoshinaga S K, Yamamoto K R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990;249:1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- 14.Ezura Y, Tournay O, Nifuji A, Noda M. Identification of a novel suppressive vitamin D response sequence in the 5′-flanking region of murine Id1 gene. J Biol Chem. 1997;272:29865–29872. doi: 10.1074/jbc.272.47.29865. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan W M, Corthesy B, Bram R J, Crabtree G R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–806. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 16.Freedman L P, Lemon B D. Structural and functional determinants of DNA-binding and dimerization by the vitamin D receptor. In: Feldman D, editor. Vitamin D. San Diego, Calif: Academic Press; 1997. pp. 127–148. [Google Scholar]
- 17.Gasson J C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–1145. [PubMed] [Google Scholar]
- 18.Kerner S A, Scoot R A, Pike J W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci USA. 1989;86:4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kliewer S A, Umesono K, Mangelsdorf D J, Evans R M. Retinoid X receptor interacts with nuclear receptors in retinoic acid thyroid hormone, and vitamin D3 signalling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemon B D, Freedman L P. Selective effects of ligands on vitamin D3 receptor- and retinoid X receptor-mediated gene activation in vivo. Mol Cell Biol. 1996;16:1006–1016. doi: 10.1128/mcb.16.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard D A, Rajaram N, Kerppola T K. Structural basis of DNA bending and oriented heterodimer binding by the basic leucine zipper domains of Fos and Jun. Proc Natl Acad Sci USA. 1997;94:4913–4918. doi: 10.1073/pnas.94.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Gardner D. Negative regulation of the human atrial natriuretic peptide gene by 1,25-dihydroxyvitamin D3. J Biol Chem. 1994;269:4934–4939. [PubMed] [Google Scholar]
- 23.Liao J, Ozono K, Sone T, McDonnell D P, Pike J W. Vitamin D receptor interaction with specific DNA requires a nuclear protein and 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1990;87:9751–9755. doi: 10.1073/pnas.87.24.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licht J D, Ro M, English M A, Grossel M, Hansen U. Selective repression of transcriptional activators at a distance by the Drosophila Kruppel protein. Proc Natl Acad Sci USA. 1993;90:11361–11365. doi: 10.1073/pnas.90.23.11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M, Lee M H, Cohen M, Bommakanti M, Freedman L P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 26.Liu S M, Koszewski N, Lupez M, Malluche H H, Olivera A, Russell J. Characterization of a response element in the 5′-flanking region of the avian (chicken) PTH gene that mediates negative regulation of gene transcription by 1,25-dihydroxyvitamin D3 and binds the vitamin D3 receptor. Mol Endocrinol. 1996;10:206–215. doi: 10.1210/mend.10.2.8825560. [DOI] [PubMed] [Google Scholar]
- 27.Lowe K E, Maiyar A C, Norman A W. Vitamin D-mediated gene expression. Crit Rev Eukaryot Gene Expr. 1992;2:65–109. [PubMed] [Google Scholar]
- 28.Luo C, Burgeon E, Carew J A, McCaffrey P G, Badalian T M, Lane W S, Hogan P G, Rao A. Recombinant NFAT1 (NFATp) is regulated by calcineurin in T cells and mediates transcription of several cytokine genes. Mol Cell Biol. 1996;16:3955–3966. doi: 10.1128/mcb.16.7.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison N A, Shine J, Fragonas J C, Verkest V, McMenemy M L, Eisman J A. 1,25-Dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcinin gene. Science. 1989;246:1158–1161. doi: 10.1126/science.2588000. [DOI] [PubMed] [Google Scholar]
- 30.Noda M, Vogel R L, Craig A M, Prahl J, DeLuca H F, Denhardt D T. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (Spp-1 or osteopontin) gene expression. Proc Natl Acad Sci USA. 1990;87:9995–9999. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rachez C R, Suldan Z, Ward J, Chang C P, Burakov D, Freedman L P. A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev. 1998;12:1787–1800. doi: 10.1101/gad.12.12.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 33.Reichel H, Koeffler H P, Norman A. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320:980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- 34.Rigby W F C, Denome S, Fanger M W. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. J Clin Investig. 1987;79:1659–1664. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobler A, Gasson J, Reichel H, Norman A W, Koeffler H P. Granulocyte-macrophage colony-stimulating factor sensitive and receptor-mediated regulation by 1,25-dihydroxyvitamin D3 in normal human peripheral blood lymphocytes. J Clin Investig. 1987;79:1700–1705. doi: 10.1172/JCI113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Towers T L, Freedman L P. Granulocyte-macrophage colony stimulating factor gene transcription is directly repressed by the vitamin D3 receptor: implications for allosteric influences on nuclear receptor structure and function by a DNA element. J Biol Chem. 1998;273:10338–10348. doi: 10.1074/jbc.273.17.10338. [DOI] [PubMed] [Google Scholar]