Abstract
Attention Deficit Hyperactivity Disorder (ADHD) is an increasingly prevalent neuropsychiatric disorder characterized by hyperactivity, inattention, and impulsivity. Symptoms emerge from underlying deficiencies in neurocircuitry, and recent research has suggested a role played by the gut microbiome. The gut microbiome is an ecosystem of interdependent taxa involved in an exponentially complex web of interactions, plus host gene and reaction pathways, some of which involve neurotransmitters with roles in ADHD neurocircuitry. Studies have analyzed the ADHD gut microbiome using macroscale metrics such as diversity and differential abundance, and have proposed several taxa as elevated or reduced in ADHD compared to Control. Few studies have delved into the complex underlying dynamics ultimately responsible for the emergence of such metrics, leaving a largely incomplete, sometimes contradictory, and ultimately inconclusive picture. We aim to help complete this picture by venturing beyond taxa abundances and into taxa relationships (i.e. cooperation and competition), using a publicly available gut microbiome dataset (targeted 16S, v3-4 region, qPCR) from an observational, case-control study of 30 Control (15 female, 15 male) and 28 ADHD (15 female, 13 male) undergraduate students. We first perform the same macroscale analyses prevalent in ADHD gut microbiome literature (diversity, differential abundance, and composition) to observe the degree of correspondence, or any new trends. We then estimate two-way ecological relationships by producing Control and ADHD Microbial Co-occurrence Networks (MCNs), using SparCC correlations (p ≤ 0.01). We perform community detection to find clusters of taxa estimated to mutually cooperate along with their centroids, and centrality calculations to estimate taxa most vital to overall gut ecology. We finally summarize our results, providing conjectures on how they can guide future experiments, some methods for improving our experiments, and general implications for the field.
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a significant mental health problem with a current 3.4% prevalence worldwide [1]. In the United States, ADHD affects one in 10 children (a 43% increase over the last 15 years) [2], and 3–16% of adults [3] with that percentage increasing over the past 20 years. Individuals with ADHD face many practical challenges, including risk for low academic achievement, lower employment status, and incarceration [4]. Symptoms of hyperactivity, impulsivity, and inattention characterize ADHD [5]. Underlying ADHD behavioral symptoms are deficits in the neurocognitive mechanisms of both executive function (EF) and emotional regulation (ER) [6]. EF refers to a set of cognitive control processes, including attention (specifically, one’s ability to focus on relevant information while suppressing irrelevant distractors), memory, and motor skills [7]. ER generally ascribes to one’s ability to effectively cope with emotionally charged circumstances (both negative and positive). These deficits include and extend beyond prefrontal-striatal networks [8]. As just one example, during attention-based tasks, reduced activation in the right dorsal attention network (including the right dorsolateral prefrontal cortex, the basal ganglia and thalamus, parietal lobe, and precuneus) has been reported [9]. Many medications have been developed to combat the disorder by influencing the underlying neurocircuitry [10].
The pathogenesis of ADHD is thought to be multifactorial, with heritability estimates at roughly 70–90% [11]. These genetic connections suggest some dependency on underlying metabolic reactions, directly or indirectly involving gene products. In the meantime, the new and exciting field of microbiome research has made its way into the mental health domain. Our gut is home to a plethora of bacteria, fungi, and other microbial organisms, whose collective genomes comprise our gut microbiome. Studies estimate that the average number of bacterial cells in humans matches or exceeds that of host cells [12, 13]. Each bacterium has unique genetic material that produces different sets of metabolites, which interact with each other and host metabolites downstream [14], creating a complex host-microbiome web of interactions. It has become increasingly important to pay attention to the symbiotic relationship between the gut microbiome and brain development and function, often referred to as the gut-brain-microbiome axis [15]. This axis is a bidirectional communication network, providing gut microbiota and metabolites an avenue for influencing brain development and function [16–20].
The fact that individuals with ADHD suffer from gastrointestinal (GI) dysfunction, including childhood digestive difficulties and low-grade inflammation [21] as well as constipation [22, 23], suggests a potential role of the gut microbiome in this disorder. For example, the plasma levels of the cytokine TNF-α were found to be significantly lower in ADHD children compared to healthy controls, and these levels were also found to be negatively correlated with gut microbiome diversity in these same samples [24]. Plasma short-chain fatty acid (SCFA) levels were also found as deficient in ADHD patients (both children and adults, [25]). This is particularly interesting because SCFAs are produced during bacterial fermentation and have been hypothesized to improve neuro-immunoendicrine functionality [26], and speculated to have a mediational role in microbiota-gut-brain crosstalk [27].
It has also been proposed that gut microbiota may affect our neurobiology by directly or indirectly altering the levels of neurotransmitters, including dopamine and serotonin (5-HT) [28], which fuel brain regions that mediate cognition and emotion. Although serotonin is also produced in the brain, up to 90% of serotonin is synthesized in the gut [29]. Connections between the gut microbiome and neurotransmitters, EF/ER, and neuropsychiatric disorders (NPDs) are already well-established. In rodents, anxiety and social behavior have been linked to the gut microbiome that can be attributed to altered neurotransmission in the hippocampus and amygdala [30]. In humans, associations between microbiome composition and ER have been shown [20]. It has also been established that the gut microbiome can release dopamine and 5-HT, impacting ER [31, 32]. Connections on the cognitive axis related to EF are less well-established in humans, though some theories are beginning to emerge [33]. In humans, dopamine influences EF [34]. In rodents, the gut microbiome is linked to dopamine [35], and EF-like behavior [36]. The Autism Spectrum Disorder (ASD) [37], which is associated with impaired EF [38], has been linked to the gut microbiome [39]. In animal studies, the gut microbiome has been associated with anxiety-related disorders such as depression [40–45]. People with stress-related diseases have responded positively to probiotics [46, 47]. Connections between the gut microbiome and another neuropsychiatric disorder (NPD) characterized by EF/ER dysfunction such as ADHD would further support the impact of the gut microbiome on EF/ER. It could also help to explain the large amount of symptomatic overlap that exists between ADHD with other NPDs, particularly ASD [48–50], and could even provide differentiating factors [51] to help address the current diagnosis challenges due to this overlap [52], and new potential options for treatment [53].
There are limited studies that implicate the gut microbiome on clinically diagnosed ADHD, and recent efforts have been made to survey and summarize their results [54–57]. Two in particular [55, 56] contained findings from multiple published studies involving ADHD and the gut microbiome. Based on this, we make the following observations about the current state of ADHD and gut microbiome research:
1. Diversity results are contradictory and inconclusive
Table 1 shows that even closely age-matched gut microbiome studies using the same Shannon index [58] to measure alpha-diversity produce contradictory results when comparing Control and ADHD cohorts. With respect to Beta-Diversity in these same studies, some report a difference between Control and ADHD samples while others do not.
Table 1. Closely age-matched alpha- and beta-diversity results.
| Age | Alpha-Diversity (Shannon Index [58]) | Beta-Diversity |
|---|---|---|
| Mean: 11.9 [59] | Higher in Control | Difference |
| Mean: 9.3 [60] | Higher in ADHD | No Difference |
| 6–10 [61] | No Change | No Difference |
| 10 and 15 [62] | Higher in ADHD for 15, No Change for 10 |
No Difference |
| 20.2 [63] | No Change | Difference (depends on metric) |
Alpha- and beta-diversity results from ADHD gut microbiome studies of similar age groups.
2. Results that estimate overall degree of differentiation between ADHD microbiome datasets compared to Control are inconclusive
One ADHD gut microbiome study [59] attempted the unsupervised method non-parametric multi-dimensional scaling (NMDS, [64]), but could not differentiate the two groups. Limited studies have further decomposed ADHD samples by subscale but these focus on diversity and distinguishing taxa, noting Inattention (elevated genus Dialister and reduced genus Phascolarctobacterium [63]) and Hyperactivity (lower alpha-diversity and elevated genus Parabacteroides [59]) properties.
3. Many microbial taxa have been proposed as elevated or reduced in ADHD compared to healthy controls, some contradictory, others mixed depending on taxonomic level, and others inconclusive
Table 2 summarizes these results. Taxa proposed as elevated in Control are colored orange, those proposed as elevated in ADHD samples are colored purple, and those with contradictory results are colored grey with bold text. Each includes corresponding literature citations. Note taxa have been grouped by the next highest taxonomic level (for example, genera Ruminococcus, Coprococcus, and Lachnoclostridium are all of the Lachnospiraceae family). Taxa in bold and italics had conflicting results over member taxa, in just italics (i.e., with family Veillonellaceae, member genus Dialister was reported elevated in Control, member species V. parvula was reported elevated in ADHD).
Table 2. Taxa proposed as elevated/reduced in ADHD.
| Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|
| Actinobacteria | Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium[65], [66] | |
| Coriobacteria | Coriobacteriales | Coriobacteriaceae | Eggerthella [66] | ||
| Fusobacteria | Fusobacteria | Fusobacteriales | Fusobacteriaceae | Fusobacterium [60] | |
| Firmicutes | Clostridia | Clostridiales [66, 67] | Lachnospiraceae [68] | Ruminococcus | R. gnavus [68] |
| Coprococcus [63] | C. eutactus [66] | ||||
| Lachnoclostridium [61] | |||||
| Ruminococcaceae [68] | Faecalibacterium [61, 68] | F. prausnitzii [68] | |||
| Clostridiaceae | Intestinibacter [63] | ||||
| Catabacteriaceae [59] | |||||
| Veillonellaceae [68] | Veillonella | V. parvula [68] | |||
| Dialister [61] | |||||
| Peptostreptococcaceae [61] | |||||
| Peptococcaceae [61] | |||||
| Bacilli | Lactobacillales | Lactobacillaceae | Lactobacillus [60] | ||
| Enterococcaceae [68] | Enterococcus [68] | ||||
| Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae [59, 66] | ||
| Prevotellaceae [59] | Prevotella [59, 63] | ||||
| Paraprevotella | P. xylaniphila [68] | ||||
| Odoribacteriaceae [68] | Odoribacter [68] | O. splanchicus [68] | |||
| Rikenellaceae [66] | |||||
| Bacteroidaceae [59] | Bacteroides [59, 65] | B. uniformis [60] | |||
| B. ovatus [60] | |||||
| B. caccae [68] | |||||
| B. coprocola [60] | |||||
| Parabacteroides [59] | |||||
| Proteobacteria | Betaproteobacteria | Neisseriales | Neisseriaceae [59] | Neisseria [59] | |
| Burkholderiales | Sutterellaceae | Sutterella | S. stercoricanis [60] | ||
| Alcaligenaceae [61] | |||||
| Deltaproteobacteria | Desulfovibriales | Desulfovibrionaceae | Desulfovibrio [67] | ||
| Gammaproteobacteria | Pseudomondales | Moraxellaceae [61] | |||
| Xanthomonadales | Xanthomonadaceae [61] | ||||
Taxa that have been proposed as elevated in Control (orange) and ADHD (purple). Taxa labelled grey with bold text had conflicting results over multiple studies. Taxa in bold and italics had mixed results over member sub-classifications (in normal italics).
Table 2 shows several taxa families with mixed results over member genera and species, including Bacteroidaceae, Prevotellaceae, and Veillonellaceae (these mixed results were actually within the same study). Conflicting results were reported for the following taxa:
Order clostridiales (Phylum: Firmicutes)
This order was reported as reduced in ADHD samples compared to Controls in a study with average ages of 19.5 and 27.1 in the respective sample sets [66], but a later Genome-Wide Association (GWAS, [69]) study found Clostridiales as elevated in ADHD samples compared to Controls [67].
Family Porphyromonadaceae (Phylum: Bacteroidetes)
The same study (with average ages of 19.5 and 27.1 [66]) reported elevated Porphyromonadaceae in ADHD samples compared to Controls, but a second gender-matched study [59] with similar mean respective ages (11.9 and 13.7) reported taxa of this family as reduced in ADHD samples compared to Controls.
Family Bacteroides (Phylum: Bacteroidetes)
The same study (mean ages 11.9 and 13.7, [59]) found members of this genus as elevated in ADHD samples compared to Controls. Another involving 18 to 24 month olds [65] found members of this genus as lower in ADHD samples compared to Controls (though it should be noted, these children did undergo probiotic intervention).
Genus Bifidobacterium (Phylum: Actinobacteria)
Perhaps no greater mystery currently exists than the role of genus Bifidobacterium. One Dutch study found a nominal increase in Bifidobacterium in ADHD with average ADHD and Control subject ages of 19.5 and 27.1 years, respectively [66]. A longitudinal study (3 months, six months and 13 years) made a somewhat contradictory observation of reduced Bifidobacterium in ADHD samples during infancy, but not at age 13 [65]. A third study [70] reported reduced Bifidobacterium (specifically species B. longum and B. adolescentis) in ADHD children (mean age: 9.3) that actually reversed after micro-nutrient treatment, where elevated Bifidobacterium was observed at high ADHD-Rating Scale IV (ADHD-RS-IV, [71]) scores.
The current picture of the role played by the gut microbiome in ADHD is therefore still unclear. Most of the effort to connect ADHD to the gut microbiome has involved (1) macroscale population metrics such as diversity, and/or (2) taxa abundances. These properties are in reality emergent from a complex and interdependent interaction web involving taxa, their gene products, and those of the host [72]. Diversity and abundance therefore ignore many underlying details behind their measurements, helping to explain the current incomplete picture. Venturing deeper into this web is critical to completing more of this picture. Two studies have attempted this task, both using multi-omics. One [68] reported differences ADHD neurotransmitter pathways. A second [66] uncovered a connection between Bifidobacterium and cyclohexadienyl dehydratase (CDT) abundances.
We have thus far only scratched the surface of this large and exponentially complex interaction web, and every completed piece has value. Multi-omics will continue to be critical, bridging an important gap between taxa, products, and metabolic reactions. We aim to complete another piece, that involves ecological relationships between taxa. Microbial taxa have been shown to demonstrate a wide variety of ecological relationships, including cooperation [73, 74] and competition [75], that ultimately impact collective functionality of the ecosystem and macroscale properties [72]. We estimate these relationships for Control and ADHD datasets and report results; including relationships, communities, driver taxa (or ‘centroids’) of these communities, and taxa central to overall gut ecology. Results can offer guidance on potential taxa to target for further multi-omics or laboratory experiments. The ultimate goal is to increase depth of knowledge about connections between the influence of the gut microbiome on an NPD that impacts millions of individuals worldwide.
This work involves two parts, conducted on a publicly available, gender-matched dataset of 16S gut microbiome sequences. The first involves performing the same macroscale analyses currently prevalent in ADHD gut microbiome literature, to note how this dataset compares, as well as any new and interesting trends. Metrics will include alpha- and beta-diversity, Sparse Partial Least Squares Discriminant Analysis (sPLS-DA, [76]) to estimate Control and ADHD differentiation degree, various methods for differential abundance analysis [77–80] and Qiime [81] normalized abundance compositional profiles.
In the second part we estimate ecological relationships [82] within Control and ADHD gut microbiomes. We first use Microbial Co-occurrence Networks (MCNs, [83]) to estimate these relationships [84], and then perform cluster analysis using the Affinity Propagation (AP, [85]) algorithm to discover communities of mutually supporting taxa, as well as driver or ‘centroid’ nodes of these communities. Finally we perform centrality analysis using the Ablatio Triadum (ATria, [86]) algorithm, to estimate taxa most significant to the overall ecosystem.
Materials and methods
We provide more details on the methods we use for analysis. Our entire downstream analysis pipeline has been built using Plugin-Based Microbiome Analysis (PluMA, [87]) version 2.0 and is available for download within its publicly available pipeline pool, (pipeline ADHD, available open-source at http://biorg.cs.fiu.edu/pluma/pipelines.html), along with processed sequence data. Source code for PluMA is available open source under the MIT software License at http://biorg.cs.fiu.edu/pluma. Supplementary material, including all alpha- and beta-diversity output, taxa plots, differential abundance output, networks, clusters, and central nodes are available in computer-readable TXT and CSV formats at http://biorg.cs.fiu.edu/pluma/ADHD.html.
Cohort
Our study starts from a publicly available dataset (Accession Number: PRJNA656791) of raw gut microbiome samples (observational, case-control) from an undergraduate student population. Members of this population completed the Adult ADHD Self Report Scale (ASRS) and were classified as “Control” or “ADHD” following the published practice [88] of scoring at least 17 on one of the two subscales (inattention or hyperactivity). A subset of patients were then randomly selected from each group (32 Control, 29 ADHD) for gut microbiome sampling. Full sequencing details are provided in the BioProject description; 16S rRNA (V3-V4 region) sequencing was used, following steps corresponding to standard Illumina protocols [89]. We include details of the experiment that we were able to gather from both the BioProject and its referenced protocols [90–92] using STORMS [93] format in S1 Table. Each deidentified sample provides gender (NCIT:C17357, self-reported) and ADHD assessment (EFO:0007860) based on ASRS score (Control, ADHD Combined, ADHD Inattentive, or ADHD Hyperactivity) in its title. The project released 61 samples, but recommended excluding 3 based on outlier analysis (Mahalanobis distance, confidence level = 97.5%) leaving 58 samples: 30 Control and 28 ADHD, with 15 females in both groups. We summarize statistics in Table 3. Tetachoric correlation [94] revealed little to no association (-0.05) between gender and group classification (Control or ADHD).
Table 3. Cohort analysis.
| Groups | Total No | Gender | Total No | Inattentiveness | Hyperactivity | Combined |
|---|---|---|---|---|---|---|
| ASRS Scores ± SD | ||||||
| Control | 30 | Female | 15 | 11.2 ± 4.3 | 9.6 ± 4.9 | 20.8 ± 7.2 |
| Male | 15 | 9.8 ± 3.4 | 9.9 ± 4.4 | 19.7 ± 6.8 | ||
| ADHD | 28 | Female | 15 | 22.6 ± 6.8 | 19.5 ± 5.3 | 42.1 ± 10.1 |
| Male | 13 | 18.2 ± 3.8 | 17.3 ± 4.7 | 35.5 ± 6.1 | ||
Cohort analysis. Samples are from an undergraduate student population.
The study used ASRS as its method for case-control classification, and much of our analyses apply protocols which assume discrete classification schemes for samples. It is important to note however, that while the ASRS can be a useful screening tool for ADHD [95] and has been shown to achieve accuracy rates above 90% [96], it in the end does not offer an official diagnosis. Therefore, an immediate next step must involve a transition to continuous analysis protocols, operating on the ASRS scores (and possibly subscales as well) as purely continuous data. We present these results as preliminary work, with the expectation of an immediate segue into this continuous analysis as future work. We elaborate on these points in the discussion.
Sequences were trimmed using Trimmomatic-0.33 [97] then assembled into a complete amplicon sequence using SeqPrep [98]. Chimeric amplicon sequences were removed using USearch 6.1 [99] in reference mode against a curated RDP database [100], along with all sequences with length under 320bp. Finally for each sample, a set of 50000 sequences were randomly sampled to reduce uneven sampling bias. To establish an initial set of Operational Taxonomic Units (OTUs) we took these sequences and compiled, clustered, and analyzed them using Qiime 1.9.1 [81] open reference clustering (UClust, [99]) with a similarity threshold of 97% (GreenGenes version 13.8 reference database [101]). Each of these packages are available as PluMA plugins. Finally, we removed all singletons. S2 Table shows the average number of sequences retained in each sample after each preprocessing step.
Ethics Statement
As mentioned, we commenced this study from a publicly available dataset that was released in fully deidentified form and no master key was provided, blocking any possible route for tracing a sample back to an individual. IRB consent was therefore verbally waived for our study, by the FIU Office of Research Integrity.
Part I. Traditional macroscale analyses
We first perform macroscale analyses on this ADHD dataset that have been performed on other ADHD datasets, compare and contrast our results with those in the literature, and take note of any new and interesting observations.
Diversity analysis
Alpha- and beta-diversity plots were constructed using Qiime (version 1.9.1). Input was provided in the form of raw abundances, sampled at a depth of 15000. For alpha-diversity, we used default metrics: unique taxa count, Chao1 [102], and PD_whole_tree (phylogenetic diversity), with default parameters. We confirm results using a non-parametric t-test (999 Monte Carlo permutations and Bonferroni [103] correction for p-values). For beta-diversity, we plot unweighted and weighted Unifrac distance [104], confirming results through an ADONIS [105] statistical test on their respective distance matrices (999 permutations).
Differential abundance analysis
We conduct this using two methods, that produce different types of classification. The first uses Sparse Partial Least Squares Discriminant Analysis (sPLS-DA, [76]), a sparse version of the Partial Least Squares (PLS, [106]) method (confidence = 95%), as a supervised dimension reduction method for determining differentiation degree between Control and ADHD samples with respect to taxa abundance [107]. We use centered log-ratio (CLR, [108]) transformed abundances to map to an unbound space and reduce potential compositional effects. The second attempts to uncover specific microbial taxa that are elevated/reduced in Control and ADHD samples. For this, we take a consensus approach [109] using four methods: (1) Linear discriminant analysis Effect Size (LEfSe,[77]) (p ≤ 0.05, LDA effect size > 2) with Bonferroni [103] correction, (2) DESeq2 [78] with Benjamini-Hochberg [110] False Discovery Rate (FDR) correction (p ≤ 0.05), ANOVA-like Differential Gene Expression Analysis of Single-Organism and Meta-RNA-Seq (ALDEx2, [79]) (p ≤ 0.05, also with Benjamini-Hochberg correction), and Analysis of Compositions of Microbiomes with Bias Correction (ANCOM-BC, [80]) (p ≤ 0.05, Holm-Bonferroni [111] correction). Taxa were classified at the lowest possible taxonomic level.
Taxa plots
We use Qiime 1.9.1 [81] to generate taxa bar graphs, producing one bar per sample broken down by taxa percentages (this directly corresponds to our normalized data). We produce taxa plots at all levels of the taxonomic tree (from phylum to genus), plus one at the lowest possible level of classification.
Part II: Ecological relationships
Co-occurrence network analysis
We computed correlations based on log-ratio transformed taxa absolute abundances using SparCC [112] (p ≤ 0.01) using 100 permutations and 5 iterations, and built Microbial Co-occurrence Networks (MCNs, [83]) using taxa as nodes and correlations as edges. MCNs were visualized using Cytoscape (version 3.9.1, [113]) with layout produced by Fruchterman-Reingold [114].
Clustering
MCNs were clustered using Affinity Propagation (AP, [85]) with a damping coefficient of 0.5 and 200 convergence iterations. AP has been shown to operate efficiently and successfully on signed and weighted biological networks without requiring an initial cluster count estimate, and additionally computes the most representative or centroid node for each cluster.
Centrality analysis
We use Ablatio Triadium (ATria, [86]) for evaluating the importance, or centrality, of taxa in our MCNs. ATria computes centrality for signed and weighted networks through a modified economic payment model [115] that calculates the influence of a node on all other nodes. ATria provides an alternative perspective by considering relationships (not relative abundance) when computing centrality, and unlike LEfSe does not compare sample sets. ATria produces a ranked list of important taxa and runs iteratively; once a taxon is found as central, ATria removes this taxon and its dependencies using social network theory [116]. Then it runs again to produce the next most important taxon, repeating until no edges are left. Taxa not found as important are simply not ranked.
We analyze these ecological relationships at all taxonomic levels starting from phylum. We first observe the upper three levels (phylum, class, and order) for an overview of relationships between consistently abundant taxa and an observation of general trends and behaviors. We then move to the lower three levels (family, genus and lowest possible classification level) which provide a finer level of granularity and enough taxa to perform meaningful community analyses, facilitating a vision of the key players within these dynamics. The lowest possible classification level will attempt to classify at a species level, but because of the lack of variability in the 16S gene at the species level only a limited number of taxa will be classifiable at an appropriate level of confidence. When species-level classification is not possible, the next highest level (genus or family) will be used. To be clear, these networks are based on correlations, which does not imply causation. Results from our analyses should therefore be interpreted as providing guidance and potential targets for future downstream (i.e. pathway or multi-omics) analysis, that ultimately will require lab experimentation for full verification.
Prevalence filtering
Once we venture past macroscale community metrics (alpha- and beta-diversity), we apply a prevalence filter to remove scarce taxa and restrict our analysis to core microbiome members of both groups (Control and ADHD) by adopting a prevalence threshold of 50% [117, 118]. By restricting our analysis to taxa that are not scarce, our sample size (30 and 28, 1.07 control to case ratio) achieves a power size of 88% given a two-sided confidence interval of 95% when comparing specific taxa between Control and ADHD sets (computed using EpiInfo version 7.2.5, STATCALC package), critical especially for differential abundance analysis. Additionally for co-occurrence networks, a prevalence threshold is important for avoiding spurious clusters of correlated scarce taxa that are simply not appearing across most samples, and 50% is estimated to be a moderate to conservative threshold [119]. A survey across multiple correlation algorithms (including SparCC) also found a performance decline (defined as the ratio of true to false positives) once sparsity (the percentage of zeroes in correlated taxa) exceeded 50% [120]. S2 Table shows that this prevalence filter still maintains 91% of sequence counts on average per sample.
With this prevalence filter the final set of taxa will differ slightly for Control and ADHD, as some taxa will meet the prevalence threshold in only one of the two groups. This will create bias in differential abundance calculations, as these taxa will have an artificial zero abundance in one group but not the other. To avoid this bias, we keep taxa that meet the prevalence threshold in one of the two groups in both groups for all differential abundance calculations (SPLS-DA, LEfSe, DESeq, ALDEx2 and ANCOM-BC). For all other methods that do not perform differential abundance calculations between the two groups (taxa plots and co-occurrence networks), we use the prevalence filter as defined above.
Results
Part I. Traditional macroscale analyses
Diversity
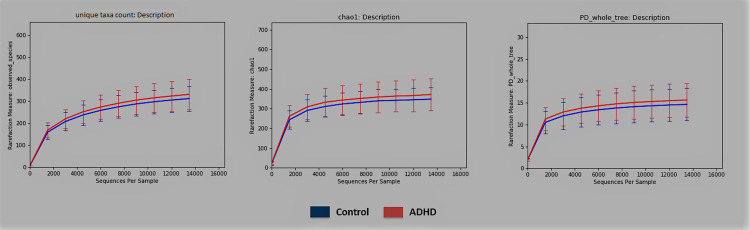
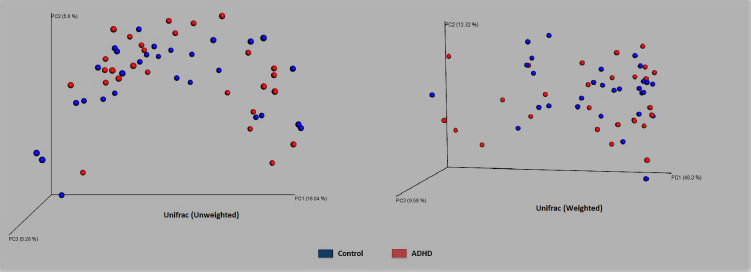
Qiime [81] alpha- and beta-diversity results produced no conclusive differences between ADHD and Control. Fig 1 shows no Alpha diversity difference within error bars for ADHD using all three metrics: unique taxa count, Chao1 [102], and PD_whole_tree (phylogenetic diversity), which was later confirmed by a non-parameteric t-test (respective t = -1.14, -1.17, -0.967; p-values: 0.2664, 0.246, 0.309). Beta-diversity with unweighted and weighted Unifrac [104] distance also shows no separation (Fig 2), confirmed by p-values computed from an ADONIS [105] statistical test (respective F = 0.847, 0.581; p-values = 0.707, 0.671 respectively). This lack of alpha- and beta- diversity differences matches several results from other datasets [61, 62, 66].
Fig 1. Alpha-diversity.
Alpha-diversity of Control (blue) and ADHD (red) samples using (in order) the count of unique taxa, Chao1 richness [102], and phylogenetic diversity, with error bars.
Fig 2. Beta-diversity.
Beta-diversity of Control (blue) and ADHD (red) samples computed using unweighted and weighted Unifrac [104] distance.
Differential abundance
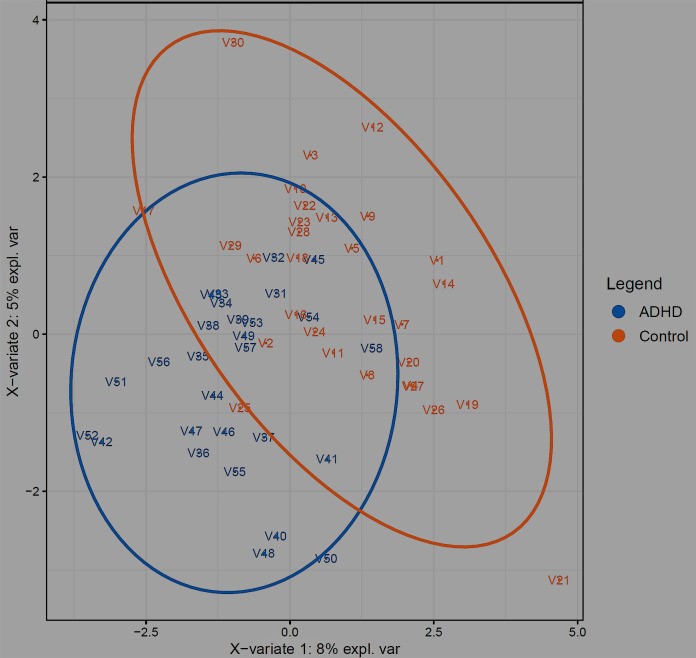
One differential abundance technique involves dimensionality reduction, attempting to determine differentiation degree between datasets, by accounting for all variables in each set [121] but displaying in a reduced dimensional space using principal component analysis. Unsupervised and supervised approaches can be used, with supervised having prior sample classification knowledge (i.e., Control or ADHD). When applying a CLR transform to our data in Fig 3 (ellipse confidence level = 95%), we see that even our supervised method Sparse Partial Least Squares Discriminant Analysis (sPLS-DA, [76]) is unable to convincingly differentiate Control vs. ADHD. Table 4 confirms this, as classification error rates of the two principal components for general Control vs. ADHD range from 40 to 50%. This is even with a supervised method, which needs to be taken with a grain of salt anyway, as supervised methods have a priori sample category knowledge and have sometimes been shown to differentiate completely random data [107].
Fig 3. Differential analysis.
Results of running sPLS-DA [76] on microbiome abundance data (ellipse confidence level 95%), comparing Control (orange) and ADHD (blue) groups.
Table 4. Principal component error rates.
| Principal Component | Largest Predicted Score | Distance to Centroid (Euclidean) | Distance to Centroid (Mahalanobis) |
|---|---|---|---|
| 1 | 0.476 | 0.481 | 0.481 |
| 2 | 0.453 | 0.457 | 0.452 |
Classification error rates of the top two principal components in Fig 3.
Techniques for dimensionality reduction like sPLS-DA estimate the degree of differentiation between two sets (e.g., Control and ADHD). For more targetted future lab studies, it may also be useful to estimate specific taxa that are elevated in one set of samples (e.g., Control) compared to another set of samples (e.g., ADHD). As differential abundance techniques have been shown to vary widely in their results [109], we use four techniques (LEfSe [77], DESeq2 [78], ALDEx2 [79] and ANCOM-BC [80]) with a range of different correction mechanisms for their p-values (Bonferroni [103], Benjamini-Hochberg [110], and Holm-Bonferroni [111]) though keep the same p-value threshold of 0.05.
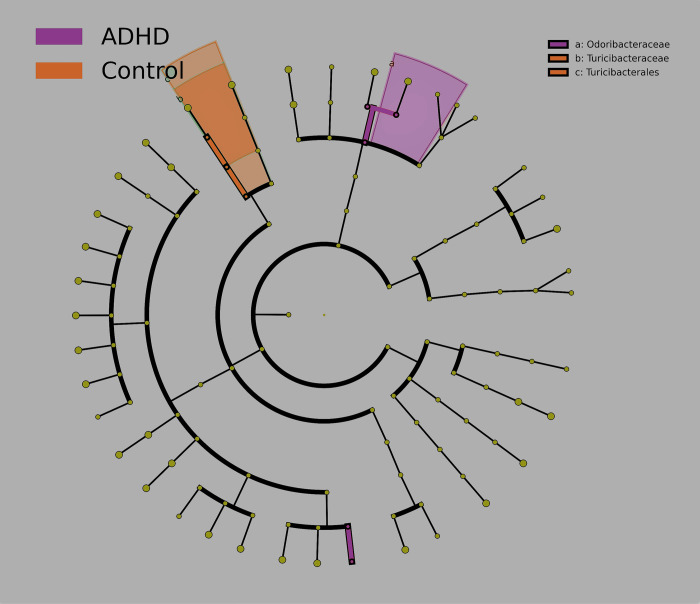
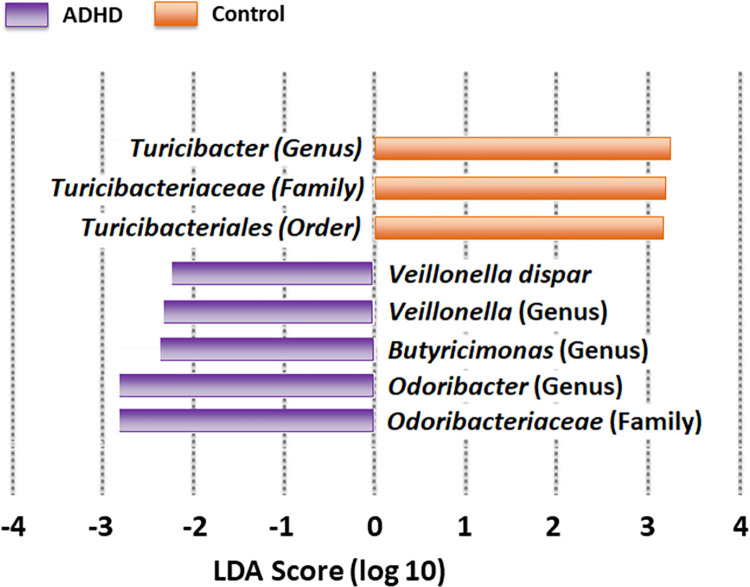
We first show results for LEfSe (p ≤ 0.05, Bonferroni correction) both as a cladogram (Fig 4) and a bar graph (Fig 5). LEfSe has identified orange taxa as elevated for Control, and purple taxa as elevated for ADHD. Taxa produced by LEfSe will be classified at the lowest taxonomic level possible.
Fig 4. Differential abundance (Cladogram).
Taxa reported by LEfSe as elevated for Control (orange) and ADHD (purple) groups, produced by LEfSe [77] (p ≤ 0.05, Bonferroni correction). LEfSe-reported taxa are plotted on a cladogram, with each concentric circle representing a phylogenetic classification level (innermost = phylum). Shared areas represent distinctive regions of the phylogenetic tree.
Fig 5. Differential abundance (Bar graph).
LEfSe-reported taxa ordered by Linear Discriminant Analysis (LDA, [122]). A higher magnitude indicates more reliable differentiation.
The cladogram (Fig 4) shows these taxa on the phylogenetic tree, highlighting those closely related. Fig 4 shows one Bacteroidetes family (Odoribacteriaceae) distinguishing ADHD, while Firmicutes (family Turicibacteriaceae and its order Turicibacterales) distinguishing Control. The bar graph (Fig 5) uses Linear Discriminant Analysis (LDA, [122]) to order by differentiation degree, expanding to include genera and species. ADHD continues to be predominated by Bacteroidetes taxa, again including Odoribacteriaceae and its genera Odoribacter and Butyricimonas, supporting earlier claims of Odoribacteriaceae as ADHD-elevated [68]. Veillonella and member V. dispar were also reported as elevated in ADHD. Control continues to be predominated by Firmicutes taxa (now including genus Turicibacter).
We also run three other methods: DESeq2 [78] and ALDEx2 [79] (both with Benjamini-Hochberg [110] corrected p ≤ 0.05, and ANCOM-BC [80] with a Holm-Bonferroni corrected p ≤ 0.05. Table 5 shows our results. In addition to p-values we include the applicable effect size–Linear Discriminant Analysis (LDA) for LEfSe, Log-Fold Change (LFC) for DESeq2, and Effect Size (EFF) for ALDEx2, computed as the ratio between the group difference and the larger of the two internal group variations. Note ANCOM-BC did not report any differentially abundant taxa. Turicibacter and Butyricimonas (in bold) are the only genera reported more than one of these methods, and Turicibacter was actually repoted by three (LEfSe, DESeq2 and ALDEx2).
Table 5. Taxa reported by various differential abundance methods throughout our analysis.
| Taxonomic Level | Taxon | Algorithm | Control/ADHD | Effect Size and p-value |
|---|---|---|---|---|
| Family | Clostridiaceae 1 | ALDEx2 | Control | EFF = 7.90, p = 0.016 |
| Genus | Coprobacillus | DESeq2 | ADHD | LFC = 2.00, p = 0.044 |
| Genus | Odoribacter | LEfSe | ADHD | LDA = 2.68, p = 0.027 |
| Genus | Turicibacter | LEfSe | Control | LDA = 3.04, p = 0.028 |
| DESeq2 | LFC = 2.12, p = 0.007 | |||
| ALDEx2 | EFF = 1.75, p = 0.033 | |||
| Genus | Haemophilus parainfluenzae | DESeq2 | Control | LFC = 0.572, p = 0.042 |
| Genus | Butyricimonas | LEfSe | ADHD | LDA = 2.37, p = 0.019 |
| ALDEx2 | EFF = 3.15, p = 0.032 | |||
| Lowest Possible | V. dispar | LEfSe | ADHD | LDA = 2.31, p = 0.039 |
Taxa reported as elevated by various differential abundance methods, when comparing Control vs ADHD, and further broken down by subscale. LDA = Linear Discriminant Analysis effect size (for LEfSe), LFC = Log Fold Change (for DESeq2), EFF = Effect Size (for ALDEx2).
Genus Odoribacter has also been previously reported as ADHD-elevated [68]. Although species H. parainfluenzae has not been observed as Control-elevated in any ADHD gut microbiome studies, its genus (Haemophilus) has [63]. This same study [68] found family Veillonellaceae as Control-elevated and member species V. parvula as differentially abundant in ADHD. Our LEfSe analysis found Veillonellaceae members Veillonella and V. dispar as differentially abundant in ADHD samples. Although Turicibacter has never previously reported as a elevated or reduced in any ADHD gut microbiome study, it has been reported in one involving depression in mice [42]. Metabolically in mice, Turicibacter signals the gut to produce serotonin (5-HT) [123], which influences ER [124]. Both ADHD and depression are characterized by ER neurocircuitry deficiencies. LEfSe did not report any EF-associated taxa. This may be largely because EF is more strongly regulated by dopamine [124], for which the gut only produces roughly 50% [125], compared to 90% of 5-HT [29].
Taxa plots
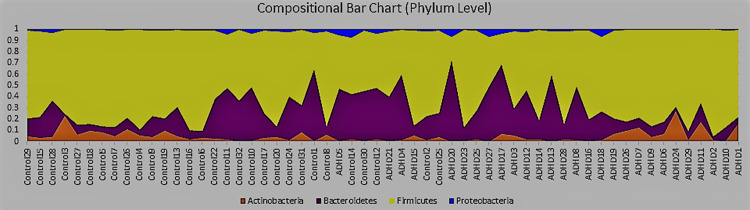
Taxa bar plots visualize taxa relative abundances [126]. We generated these at all phylogenetic tree levels beginning with phylum (Fig 6). Samples on the x-axis are ordered by increasing ASRS score, and the y-axis represents relative abundance.
Fig 6. Taxa plots, phylum level.
Plots of taxa relative abundance for each subject, generated using Qiime [81], conducted at the phylum level. Subjects are ordered by increasing Adult ADHD Self Report Scale (ASRS) score, with the y-axis representing relative abundance.
A typical gut microbiome profile [127] is observed, dominated by Firmicutes and Bacteroidetes phyla, followed by Actinobacteria and Proteobacteria. Control has slightly elevated Firmicutes (70–66%), mirroring an earlier study [66] that importantly [128] also sequenced the same 16S V3-V4 region. Slightly contrary to this same study, which reported this difference to be largely occupied by an ADHD Actinobacteria increase, ours was mostly occupied by an ADHD Bacteroidetes increase (from 22% to 25%). Yet Actinobacteria remains mysterious in Fig 6, elevated at very high ASRS scores, but also at very low scores. Bacteroidetes and Proteobacteria also appear reduced at these same extremes. These seemingly contradictory results create challenges in drawing meaningful conclusions with respect to role(s) played by these phyla. Yet they capture our interest, especially given the earlier reported anomalous behavior of an Actinobacteria genus, Bifidobacterium, at high and low ASRS-IV scores [70].
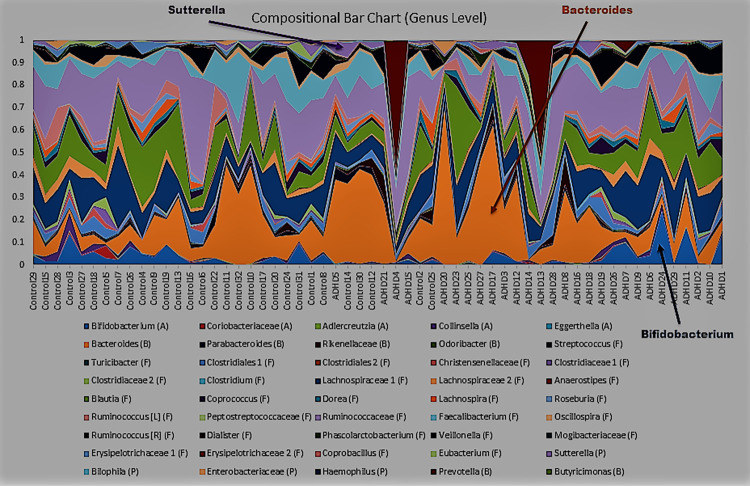
Class and order levels produced bar charts similar to Fig 6; we include these as S1 and S2 Figs. Levels below order often had too many taxa to clearly view dynamics. We include the genus level (Fig 7), family and species as S3 and S4 Figs), as the genus level includes Bifidobacterium. And indeed, it turns out, Bifidobacterium (blue, bottom) has elevated abundances high and low ASRS scores, appearing most responsible for this same behavior in its phylum Actinobacteria (Fig 6). Bacteroides (orange, middle) is a highly abundant genus that also mirrors the behavior of its phylum (Bacteroidetes, Fig 6), increasing in the middle and decreasing at extremes. Proteobacteria is more difficult to observe given its low relative abundance (1–2%), though genus Sutterella (lilac, top) also appears to follow this trend. These three observations are verified in S5–S7 Figs.
Fig 7. Taxa plots, genus level.
Plots of taxa relative abundance for each subject, generated using Qiime [81], conducted at the genus level. Subjects are ordered by increasing Adult ADHD Self Report Scale (ASRS) score, with the y-axis representing relative abundance.
This is not the first time these taxa have generated interest. Many Actinobacteria, and especially Bifidobacterium, have been used as probiotics and are considered elements of a healthy gut [129–134]. As discussed, genus Bacteroides and its family Bacteroidaceae, as well as several member species, have been reported differentially abundant in ADHD [59, 60, 65, 68]; some elevated, others reduced. Some have argued Bacteroides to be the most important "window" to understanding the human gut [135]. Species Sutterella stercoricanis was also reported as ADHD elevated [60]. These same taxa make multiple appearances in studies involving other NPDs as well. Bacteoridaceae was reported as the most differentially abundant LEfSe Major Depressive Disorder (MDD) taxon in one study [45]. Another reported elevated Bacteroides and reduced Bifidobacterium in anxiety [136]. Sutterella is elevated in Autism Spectrum Disorder (ASD) [137], a condition with so much symptomatic overlap with ADHD that an ASD+ADHD phenotype has been established [138].
Discussion
These analyses produced a few interesting preliminary observations, but once again their birds-eye view limited the depth we could pursue. Our taxa plots showed a perfect example: even though there was a visible trend between ASRS score and Bifidobacterium, Bacteroides, and Sutterella abundances, no definitive conclusions could be observed. Fundamentally macroscale behaviors emerge from microscale interactions. We attempt to unlock some of these mysteries by now exploring ecological relationships.
Microbial ecological relationships take many forms. They can be positive or negative, mutual (cooperation [73, 74] or competition [75]) or one-way (commensalism [139] or amensalism [140]). In particular, two-way relationships (cooperation and competition) can be approximated using correlations [84]. We use SparCC [112] to compute correlations, which has advantages in reducing compositional effects within relative abundances. We build Microbial Co-occurrence Networks (MCNs, [83]) using taxa as nodes and SparCC correlations as edges, and perform community detection on these networks using the clustering algorithm Affinity Propagation (AP, [85]). Finally, we use Ablatio Triadium (ATria, [86]) as a centrality algorithm to produce a ranked list of important taxa in each MCN. ATria is specifically designed for signed and weighted networks, incorporating both social network [117] and economic theory [116] in its calculations. It is also iterative, removing dependencies of a central node before computing the next most central.
When computing correlations, we use a p-value threshold of 0.01, the generally recommended value for disease studies [141], to increase the confidence of our results, as the historically accepted threshold of 0.05 has come under recent question [142, 143]. Because our study is exploratory and meant to guide future experimentation, we chose to err on the side of false positives as opposed to false negatives, and increase the strength of the threshold as opposed to correcting the p-value. Our p-value threshold of 0.01 produced correlations with magnitude moderate (0.4, [144]) or stronger more than 95% of the time (279 out of 293 total over all MCNs). We therefore report all of these candidate relationships in our MCNs, each of which can be subsequently experimentally verified in future studies.
During our analyses we sometimes use “cooperation” to refer to a positive SparCC correlation and “competition” when referring to a negative. We emphasize, however, that correlations are an estimate of ecological relationships, that ultimately require further downstream analysis (multi-omics and experimental verification) before establishing official conclusions. With the underlying web of interactions being exponentially complex and large-scale laboratory experiments potentially costly, our results can provide guidance regarding target taxa and avenues to pursue.
Part II. Ecological relationships
Upper levels: Phylum, class, and order
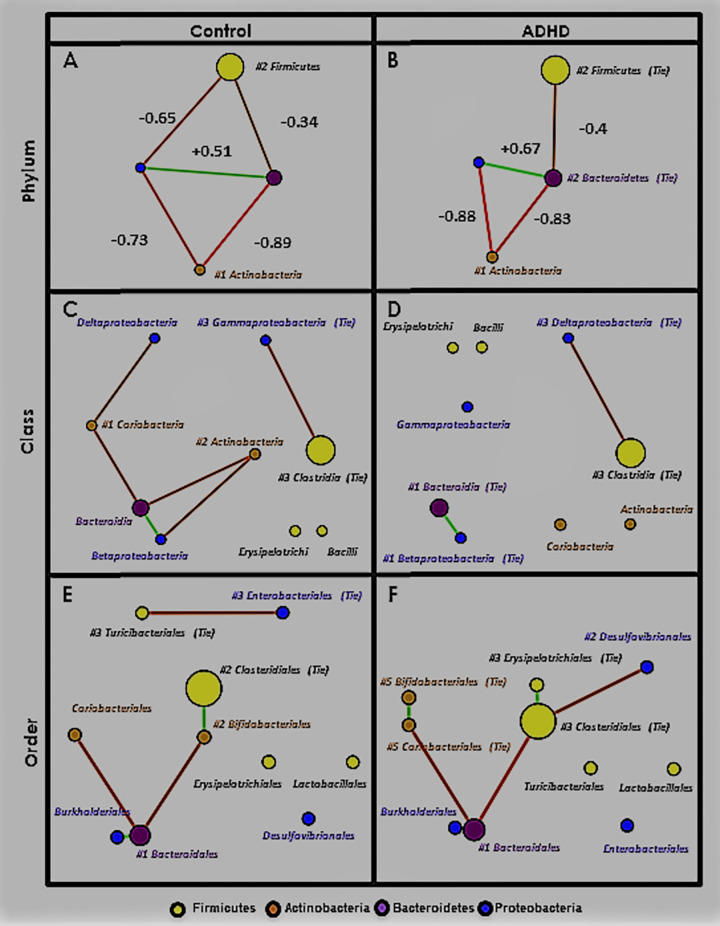
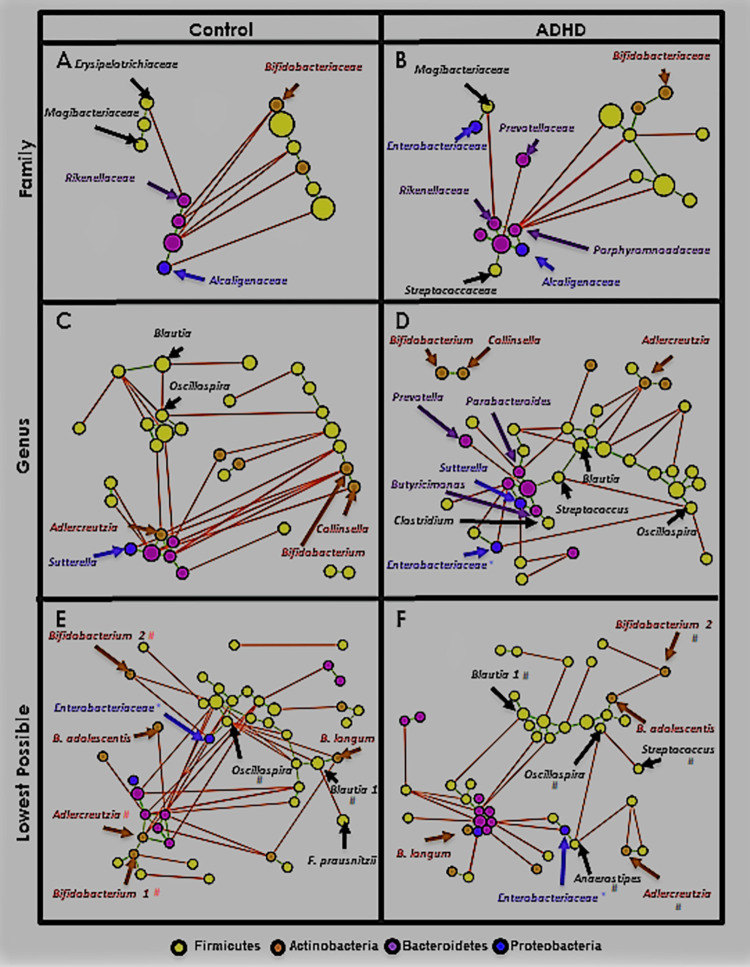
Fig 8 shows MCNs at the phylum (Fig 8(A) and 8(B)), class (Fig 8(C) and 8(D)), and order (Fig 8(E) and 8(F)) levels. Taxa (nodes) in all MCNs are colored by phylum (legend at the bottom of Fig 8). Node size is proportional to relative abundance (larger = higher). Correlation (edge) color represents sign; green indicates positive (est. cooperation) and red indicates negative (est. competition). Edge thickness is proportional to correlation magnitude (thicker = stronger). Networks are visualized using the Fruchterman-Reingold algorithm [114], which spatially orients nodes based on edge weight (closer = more positive). Nodes are labeled with their taxon and provided with ATria centrality ranking if found important (format: #rank, T = Tie). At the phylum level only (Fig 8(A) and 8(B)), we label each edge with its correlation value. Phylum-level MCNs (Fig 8(A) and 8(B)) show SparCC appears to handle compositional effects well, as despite collectively encompassing about 95% of both populations, Firmicutes and Bacteroidetes are only weakly negatively correlated.
Fig 8. Upper-level Microbial Co-occurrence Networks (MCNs).
MCNs at the phylum (A), class (B), and order (C) taxonomic levels, visualized using Cytoscape [57], and oriented by Fruchterman-Reingold [58]. Nodes represent taxa, colored by phylum (yellow = Firmicutes, purple = Bacteroidetes, brown = Actinobacteria, blue = Proteobacteria) with size directionally proportional to abundance. The co-occurrences are distinguished by those that co-habit (green edges) and co-avoid (red edges). SparCC [112] correlation (p≤0.01) was used as edge weight and also the parameter for Fruchterman-Reingold when determining edge length (larger = closer). SparCC correlations are shown at the phylum level. All taxa found as important by ATria are denoted by a pound sign (#) followed by its rank (ties indicated).
Table 6 shows every correlation in all three MCNs, and its sign, + (green) or–(red). Correlations that appear only in Control are highlighted orange, only in ADHD highlighted purple, and in both highlighted grey. Correlations at each taxonomic level are grouped by their next highest level classification; for example in row 1: phyla Actinobacteria and Bacteroidetes were negatively correlated in both phylum-level MCNs (Fig 8(A) and 8(B)), member classes Actinobacteria and Bacteroidia were negatively correlated only in Control (Fig 8(C)), as were member orders Bifidobacteriales and Bacteroidales (Fig 8(E)). White, italicized correlations were not present in either MCN, but a correlation among descendants was; for example in row 3: phyla Actinobacteria and Firmicutes were not correlated in either MCN, nor were member classes Actinobacteria and Clostridia, but member orders Bifidobacteriales and Clostridiales were positively correlated in Control (Fig 8(E)).
Table 6. Upper-level taxa correlations.
| Phylum | Class | Order | |||
|---|---|---|---|---|---|
| Actinobacteria-Bacteroidetes | - | Actinobacteria-Bacteroidia | - | Bifdobacteriales-Bacteroidales | - |
| Coriobacteria-Bacteroidia | - | Coriobacteriales-Bacteroidales | - | ||
| Actinobacteria-Proteobacteria | - | Actinobacteria-Betaproteobacteria | - | ||
| Coriobacteria-Deltaproteobacteria | - | ||||
| Actinobacteria-Firmicutes | Actinobacteria-Clostridia | Bifidobacteriales-Clostridales | + | ||
| Proteobacteria-Firmicutes | - | Gammaproteobacteria-Bacilli | Enterobacteriales-Turicibacteriales | - | |
| Gammaproteobacteria-Clostridia | - | ||||
| Deltaproteobacteria-Clostridia | - | Desulfovibrionales-Clostridiales | - | ||
| Proteobacteria-Bacteroidetes | + | Betaproteobacteria-Bacteroidia | + | Burkholderiales-Bacteroidales | + |
| Bacteroidetes-Firmicutes | - | Bacteroida-Clostridia | Bacteroidales-Clostridiales | - | |
Upper level taxa correlations (p ≤ 0.01), grouped by taxonomic classification. Orange = only found in Control, purple = only found in ADHD, grey = found in both. +(green) = positive correlation, -(red) = negative correlation.
Table 7 shows collective ATria results, similarly grouped. At each level, taxa found equally important in both MCNs are highlighted grey; taxa found more important in Control light orange, and only important in Control dark orange (analogous case for ADHD and purple). Taxa ranked as first or tied for first in either MCN are bold.
Table 7. Upper-level ATria rankings.
| Phylum | Class | Order |
|---|---|---|
| Actinobacteria (#1/#1) | Actinobacteria (#2/NR) | Bifidobacteriales (#T2/T5) |
| Coriobacteria (#1/NR) | Coriobacteriales (NR/#T5) | |
| Bacteroidetes (NR/#T2) | Bacteroidia (NR/#T1) | Bacteroidales (#1/#1) |
| Firmicutes (#2/#T2) | Bacilli (NR/NR) | Turicibacteriales (#T3/NR) |
| Clostridia (#T3/#T3) | Clostridiales (#T2/#T3) | |
| Erysipelotrichia (NR/NR) | Erysipelotrichiales (NR/#T3) | |
| Proteobacteria (NR/NR) | Betaproteobacteria (NR/#T1) | |
| Deltaproteobacteria (NR/#T3) | Desulfovibrionales (NR/#2) | |
| Gammaproteobacteria (#T3/NR) | Enterobacteriales (#T3/NR) |
Upper-level ATria results, grouped by taxonomic classification. Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1.
Taxa bar plots are mirrored here: ADHD showed elevated Bacteroidetes at the expense of Firmicutes, and these taxa are negatively correlated in both MCNs (Fig 8(A) and 8(B)). But while Firmicutes and Bacteroidetes dominate both populations (largest nodes, Fig 8(A) and 8(B)) as is typical in the gut microbiome [127], SparCC and ATria estimate a far less abundant phylum, Actinobacteria (roughly 4% of both populations), as most important to their overall gut ecology. In both MCNs (Fig 8(A) and 8(B)), phylum Actinobacteria has the strongest negative correlations and ATria ranks it first (Table 7).
We make three more observations at these upper taxonomic levels, that we keep in mind when moving to the lower:
(A) A core Proteobacteria-Bacteroidetes positive correlation (est. cooperation) forms. Table 6 shows this, with Proteobacteria and Bacteroidetes (the only positive correlation in either phylum-level MCN), member classes Betaproteobacteria and Bacteroidia, and member orders Burkholderiales and Bacteroidiales.
(B) In Control, taxa in (A) have more negative edges with Actinobacteria (est. competition), especially order Bifidobacteriales. The highest magnitude negative edges in both phylum-level MCNs (Fig 8(A) and 8(B)) involve Actinobacteria with Proteobacteria and Bacteroidetes. Yet while the two consistently dependent Actinobacteria classes (Actinobacteria and Coriobacteria) continue this same dynamic with Bacteroidia (Bacteroidetes) and Betaproteobacteria (Proteobacteria) in Control (Fig 8(C) and Table 6), they are completely disconnected in ADHD (Fig 8(D)). Worth noting, this is despite their average relative abundance being nearly the same in Control/ADHD: Coriobacteria 1.5/1.1%, and Actinobacteria 3.2/3.7%. Further, ATria ranks Actinobacteria and Coriobacteria as the top two Control taxa (Table 7). In ADHD, Bacteroidia and Betaproteobacteria are the top two (Table 7), and the MCN shows no negative edges (est. competition) at all involving these taxa (Fig 8(D)).
The order level reveals Bifidobacteriales (Actinobacteria) may be more responsible for this difference than Coriobacteriales (Coriobacteria). While Bifidobacteriales and Coriobacteriales both continue their negative correlations with Bacteroidales (Bacteroidia) in Control, only Coriobacteriales does in ADHD. Table 6 actually shows all edges involving Bifidobacteriales to be exclusive to Control, now including a positive correlation with Clostridiales (the most abundant Firmicute). An increased participation of order Bifidobacteriales thus emerges as a distinguishing feature of Control, which is further supported by ATria (Table 7), which ranks Bifidobacteriales higher (tied for second) in Control, and Coriobacteriales only in ADHD.
(C) A shift in Firmicutes-Proteobacteria dynamics. This begins immediately at the phylum level (Fig 8(A)) with Control having a negative correlation (-0.65) that is absent in ADHD (Fig 8(B)). The most abundant Firmicute class (Clostridia) is negatively correlated with different Proteobacteria classes; Gammaproteobacteria in Control (Fig 8(C)), Deltaproteobacteria in ADHD (Fig 8(D)), and the latter continues at the order level (Fig 8(F)) with Clostridiales (Clostridia) and Desulfovibrionales (Deltaproteobacteria). In Control (Fig 8(E)), a negative correlation emerges between order Enterobacteriales (class Gammaproteobacteria) and LEfSe-reported order Turicibacteriales (class Bacilli).
Summary
Upper-level analysis revealed increased Actinobacteria participation in Control gut ecology, especially order Bifidobacteriales. Much of this involved negative correlations with a core of positively correlated Bacteroidetes (order Bacteroidales) and Proteobacteria (order Burkholderiales). Recalling our taxa plots and anomalous behavior involving genera Bifidobacterium (Bifidobacteriales), Bacteroides (Bacteroidales), and Sutterella (Burkholderiales), we are now interested in exploring these dynamics at lower taxonomic levels. We will continue to observe Firmicutes-Proteobacteria dynamics, as despite a still unclear picture, a clear distinction is shown between Control and ADHD.
Lower levels: Family, genus, and lowest possible
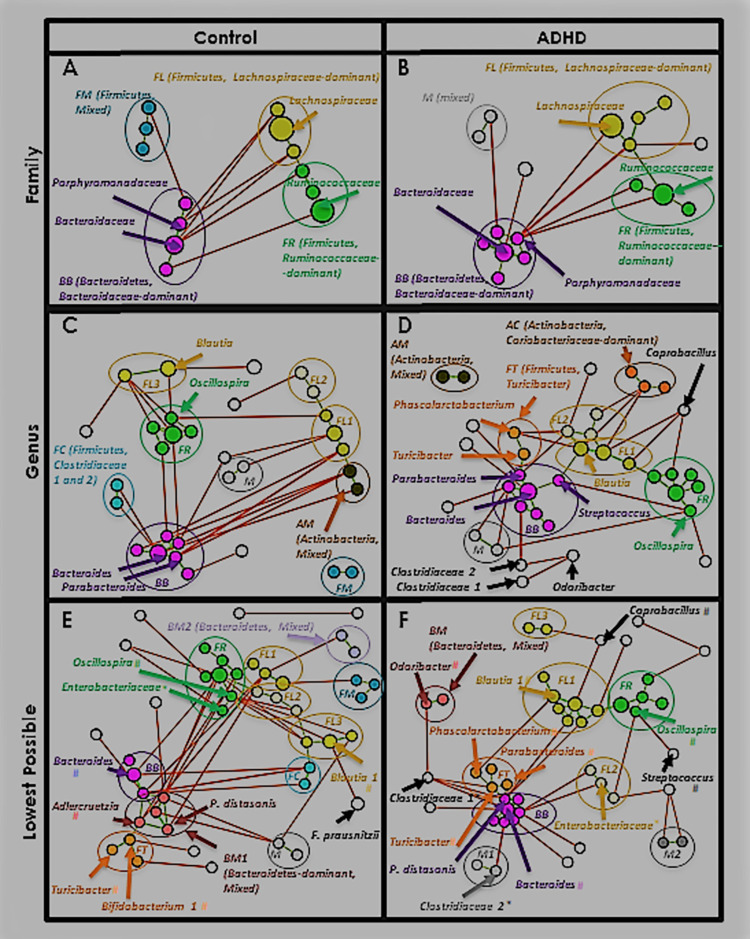
Fig 9 shows Control and ADHD MCNs at family (Fig 9(A) and 9(B)), genus (Fig 9(C) and 9(D)), and lowest possible taxonomic classification levels (Fig 9(E) and 9(F)). In this latter MCN each taxon is classified at the species level if possible (rare with 16S), otherwise more commonly the genus level is used. Schemes regarding color, node size, and edge thickness are the same as Fig 8. Since the MCNs are now larger we do not label every node, only those that we reference in our analyses. We also extend Table 6 to include correlations from every taxonomic level, but as this is also very large we include it as S3 Table and extract only relevant portions to our discussion. We perform a similar task with ATria, and S4 Table.
Fig 9. Lower-level MCNs.
MCNs at the family (A), genus (B), and species (C) taxonomic levels. Network visual properties, including node and edge size, color, and orientation, are the same as Fig 8. Taxa noted throughout our analyses are labeled (* = family-level taxon, # = genus-level taxon).
Fig 9 shows taxa separating into a group of primarily Bacteroidetes (dark purple, lower left), and another of primarily Firmicutes (yellow, upper right). Enough taxa are also now present to perform meaningful community analysis. Fig 10 shows the same MCNs as Fig 9, after running Affinity Propagation (AP, [112]) and coloring by cluster. At the family level (Fig 10(A) and 10(B)) four clusters form. One is dominated by Bacteroidetes, family Bacteroidaceae (BB, magenta). Two are dominated by Firmicutes, one family Lachnospiraceae (FL, gold), and the other family Ruminococcaceae (FR, green). In Control (Fig 10(A)) the fourth cluster consists of three mixed-family Firmicutes (FM, dark teal). In ADHD (Fig 10(B)) two of these are absent and the Proteobacteria family Enterobacteriaceae is present, leaving it no longer Firmicutes-dominant (M, grey).
Fig 10. Clusters.
Same MCNs as Fig 9, after clustering with the affinity propagation (AP) algorithm [85]. Family-level clusters are each given a unique color, and labeled with their dominant phylum and member family. New clusters that form at each lower taxonomic level are labeled, colored with shades corresponding to their dominant phylum/family when applicable—i.e. at the genus level FL1-FL3 are different shades of gold (family-level FL). Taxa noted throughout our analyses are labeled (* = family-level taxon, # = genus-level taxon).
Clusters BB and FR remain at the genus level (Fig 10(C) and 10(D)). Several Firmicutes, family Lachnospiraceae-dominant clusters emerge, referred to as FL1, FL2, etc. (gold shades). A mixed-family Actinobacteria cluster of genera Bifidobacterium and Collinsella forms in both MCNs (AM, brown), and an Actinobacteria, family Coriobacteriaceae-dominated cluster forms in ADHD (Fig 10(D), AC, burnt sienna). A small group of two Clostridiaceae family composes cluster FC in Control (Fig 10(C), aqua). In ADHD (Fig 10(D)), a cluster (orange) emerges as the only Firmicutes-dominant cluster with positive correlations to cluster BB. This eventually becomes present in both lowest-level MCNs (Fig 10(E) and 10(F)) with core member Control-elevated genus Turicibacter, so we call this cluster FT.
At the lowest level we kept cluster names as consistent as possible with genus-level membership (for example, a cluster mostly comprised of FL2 genus-level taxa would also be named FL2 at the lowest level). Both MCNs (Fig 10(E) and 10(F)) now include a mixed-family, Bacteroidetes-dominant cluster BM1 (pink), and Control includes a second (BM2, orchid). S5–S7 Tables list all clusters, members, and centroids at all levels. As with earlier tables, we will extract portions relevant to our discussion.
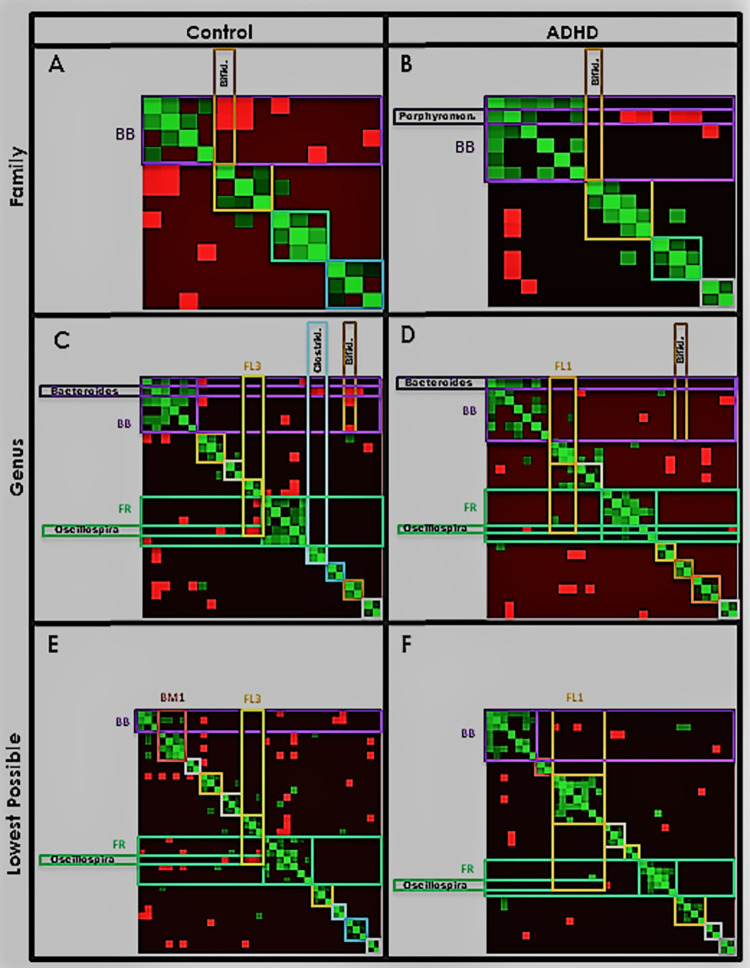
Finally, to measure cluster size, tightness, and interactions, we produce a heatmap of taxa correlations (Fig 11) with taxa ordered on the x- and y-axes by Fig 10 cluster. Green/red intensity at each point (x, y) denotes the degree of positive/negative correlation between taxa x and y (symmetric, by definition). Clusters appear as rough squares of positive (green) correlations on the diagonal. We outline each box with the same color as its corresponding Fig 10 cluster.
Fig 11. Heatmaps.
Heatmap representation of taxa correlations (green = positive, red = negative), with taxa organized on each axis by cluster (symmetric matrix). The area corresponding to the intersection of each cluster with itself is outlined with a box using the corresponding cluster color in Fig 10. Taxa and clusters noted throughout our analyses are labeled on the axes.
We first continue to pursue observations (A)-(C) from the upper taxonomic levels. Afterwards, we discuss any new and interesting trends.
(A) A core Proteobacteria-Bacteroidetes positive correlation (est. cooperation) forms. Recall the orders involved in this correlation were Burkholderiales (Proteobacteria) and Bacteroidales (Bacteroidetes). This corresponds to cluster BB, with genus Sutterella and multiple Bacteroidales taxa. In ADHD this cluster is larger and includes more Bacteroidales plus some Firmicutes, and nearly all members are positively correlated with its centroid Bacteroides. Additionally it has fewer negative correlations (est. competition) with other clusters.
Cluster BB is the only cluster with order Burkholderiales and Bacteroidales descendants. Table 8 shows all correlations involving Burkholderiales and Bacteroidales lineages, organized and shaded using the same scheme as Table 6. One core positive correlation survives all six taxonomic levels in Control and ADHD (12 MCNs total, the only correlation in our entire dataset with this property). This occurs between genera Sutterella and Bacteroides. Several others involving Sutterella and its family Alcaligenaceae with cluster BB members are present only in ADHD–support for a larger cluster BB in ADHD. Alcaligenaceae/Sutterella are immediately visible in Fig 9, as the only Proteobacteria (royal blue) among a slew of Bacteroidetes (dark purple).
Table 8. Burkholderiales-Bacteroidales correlations.
| Phylum | Class | Order | Family | Genus | Species | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteobacteria-Bacteroidetes | + | Betaproteobacteria-Bacteroidia | + | Burkholderiales-Bacteroidales |
+ | Alcaligenaceae-Bacteroidaceae | + | Sutterella-Bacteroides | + | Sutterella-Bacteroides | + |
| Sutterella- B.uniformis | + | ||||||||||
| Alcaligenaceae-Porphyromonadaceae | + | Sutterella-Parabacteroides | Sutterella-P. distasonis | + | |||||||
| Alcaligenaceae-Odoribacteriaceae | + | Sutterella-Butyricimonas | + | Sutterella-Butyricimonas | + | ||||||
| Alcaligenaceae-Rikenellaceae | Sutterella-Rikenellaceae | + | |||||||||
Correlations between order Burkholderiales-Bacteroidales lineages, shaded using the same scheme as Table 6 (grey present in both MCNs, purple only ADHD).
Fig 10 also illustrates the increase in ADHD cluster BB size, as do the heatmaps (Fig 11, magenta square). Table 9 quantifies differences in node and edge count.
Table 9. Cluster BB size.
| Taxonomic Level | Family | Genus | Lowest | |||
|---|---|---|---|---|---|---|
| MCN | Control | ADHD | Control | ADHD | Control | ADHD |
| Cluster BB size: Taxa (+ Edges) | 4 (3) | 6 (7) | 6 (7) | 7 (9) | 3 (2) | 7 (11) |
Control and ADHD cluster BB size. Notation: Taxa (edges).
Table 9 shows cluster BB size to mysteriously drop in Control from the genus to the lowest level, from six taxa down to three. A closer look at Fig 10(C) and 10(E) shows several genus-level BB members may be joining a mixed-family, Bacteroidetes-dominant cluster (BM1, pink) at the lowest level. Table 10, which shows BB and Control BM1 members, confirms this. Core BB members are shown in bold, while italicized members are unique to Control or ADHD. Taxa of genus-level Control cluster BB members Odoribacter, Adlercruetzia, Parabacteroides (P. distasonis) and Bacteroides (B. ovatus) compose Control cluster BM1 at the lowest level.
Table 10. Bacteroidaceae/Bacteroides dominant clusters.
| Control | ADHD | |||||
|---|---|---|---|---|---|---|
| Level | Community | Cluster Type | Taxon | Phylum | Taxon | Phylum |
| Family | Bacteroidetes-dominant (B) | Bacteroidaceae-dominant (BB) | Bacteroidaceae | Bacteroidaceae* | ||
| Porphyromonadaceae* | Porphyromonadaceae | |||||
| Alcaligenaceae | Alcaligenaceae | |||||
| Rikenellaceae | Rikenellaceae | |||||
| Odoribacteraceae | ||||||
| Streptococcaceae | ||||||
| Level | Community | Cluster Type | Taxon | Phylum | Taxon | Phylum |
| Genus | Bacteroidetes-dominant (B) | Bacteroidaceae-dominant (BB) | Bacteroides | Bacteroides* | ||
| Parabacteroides* | Parabacteroides | |||||
| Sutterella | Sutterella | |||||
| Rikenellaceae | Rikenellaceae | |||||
| Odoribacter | Butyricimonas | |||||
| Adlercruetzia | Streptococcus | |||||
| Clostridium | ||||||
| Level | Community | Cluster Type | Taxon | Phylum | Taxon | Phylum |
| Species | Bacteroidetes-dominant (B) | Bacteroidaceae-dominant (BB) | Bacteroides* | Bacteroides* | ||
| Bacteroides uniformis | Bacteroides uniformis | |||||
| Sutterella | Sutterella | |||||
| Parabacteroides distasonis | ||||||
| Rikenellaceae | ||||||
| Butyricimonas | ||||||
| Bifidobacterium longum | ||||||
| Bacteroidetes, Mixed (BM1) | Odoribacter | |||||
| Bacteroides ovatus | ||||||
| Parabacteroides distasonis* | ||||||
| Adlercruetzia | ||||||
Bacteroides, Bacteroidaceae dominant clusters (BB) and Bacteroidetes, Mixed family (BM1) cluster in Control. Core taxa are bold, taxa exclusive to one MCN (Control or ADHD) are italicized, and centroids are marked with an asterisk (*). Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown-Actinobacteria, blue = Proteobacteria.
Table 11 supports weakened connections between BB and BM1 taxa in Control, showing higher intra-correlation values (0.61 and 0.62) relative to inter-correlation (0.44).
Table 11. Bacteroidaceae-dominant community intra- and inter-correlations.
| Control BB (Intra) | Control BM1 (Intra) | Control BB-BM1 (Inter) | ADHD BB (Intra) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | Edges | Mean Correlation | Taxa | Edges | Mean Correlation | Taxa | Edges | Mean Correlation | Taxa | Edges | Mean Correlation |
| 3 | 2 | 0.61 ± 0.15 | 4 | 4 | 0.62 ± 0.1 | 4 | 3 | 0.44 ± 0.02 | 7 | 11 | 0.56 ± 0.12 |
Intra-correlations between cluster BB members, and BB-BM1 inter-correlations in Control.
Table 10 also shows cluster BB members that differ between the MCNs. Cluster BB gains a different Actinobacteria–species B. longum (ADHD) and genus Adlercruetzia (Control, eventually joining BM1). The presence of Firmicutes (yellow) is exclusive to ADHD, including family Streptococcaceae and member genus Streptococcus, plus genus Clostridium. Family Odoribacteriaceae (reported by LEfSe as ADHD-elevated) joins cluster BB only in ADHD, and the sole Clostridium connection to cluster BB is with genus Butyricimonas (Fig 9(D)), a taxon reported as ADHD differentially abundant by two methods (LEfSe and ALDEx2).
Table 10 also indicates BB/BM1 centroids, which we see across the board for ADHD are genus Bacteroides and its family Bacteroidaceae. In Control this belongs to Porphyromonadaceae (family) and descendant Parabacteroides (genus), until the BB-BM1 “split” where genus Bacteroides becomes centroid of BB and species P. distasonis of BM1. Table 12 shows connectivity of each of these taxa within their corresponding cluster. Percentagewise, in ADHD Bacteroidaceae/Bacteroides is a much stronger centroid; in fact over all levels only one cluster BB taxon was not positively correlated (Clostridium, genus level). Particularly given the ADHD cluster BB size increase, this could imply a significant role of Bacteroidaceae/Bacteroides in stabilizing a large ADHD Bacteroidetes-dominant community (would require additional experiments to verify).
Table 12. Cluster BB centroid connectivity.
| Level | Family | Genus | Lowest | |||
|---|---|---|---|---|---|---|
| MCN | Control | ADHD | Control | ADHD | Control | ADHD |
| Bacteroidaceae/Bacteroides | 2/3 (66%) | 5/5 (100%) | 3/5 (60%) | 5/6 (84%) | 2/2 (100%) | 6/6 (100%) |
| Porphyromonadaceae/Parabacteroides/P. distasonis* | 2/3 (66%) | 2/5 (40%) | 4/5 (80%) | 3/6 (50%) | 3/3* (100%) | 3/6 (50%) |
Connectivity between the centroid of cluster BB (* = BM1) and the rest of its cluster members.
Interestingly ATria (Table 13) shows Bacteroidaceae/Bacteroides and lineages to nearly always have higher importance in Control, supporting a more “global” importance to overall gut ecology as opposed to a more local importance (cluster BB) in ADHD. MCNs agree, as in ADHD Bacteroidaceae/Bacteroides have few connections outside cluster BB (Fig 10(B), 10(D) and 10(F)). In Control (Fig 10(A), 10(C) and 10(E)) Bacteroidaceae/Bacteroides have many external connections, mostly negative (est. competition).
Table 13. Bacteroidetes rankings.
| Phylum | Class | Order | Family | Genus | Lowest Possible |
|---|---|---|---|---|---|
| Bacteroidetes (NR/#T2) | Bacteroidia (NR/#1) | Bacteroidales (#1/#1) | Bacteroidaceae (#T9/#T12) | Bacteroides (#10/#T23) | B. uniformis (#12/#7) |
| B. ovatus (#2/#T20) | |||||
| Bacteroides (#T23/#T28) | |||||
| Odoribacteriaceae (NR/#2) | Odoribacter (#9/#T21) | Odoribacter (#3/#T20) | |||
| Butyricimonas (NR/#4) | Butyricimonas (NR/#17) | ||||
| Porphyromonadaceae (#T7/#1) | Parabacteroides (#T12/NR) | Parabacteroides (#T19/#T24) | |||
| P. distasonis (NR/#T24) | |||||
| Prevotellaceae (NR/#5) | Prevotella (NR/#18) | ||||
| Rikenellaceae (#T7/#3) | Rikenellaceae (#T12/#8) | Rikenellaceae (#T19/#8) |
ATria rankings of Bacteroidetes taxa. Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1.
Control MCNs (Fig 10(A), 10(C) and 10(E) and heatmaps) (Fig 11(A), 11(C) and 11(E), magenta rectangle) show negative correlations (red) to be fairly evenly distributed among cluster BB taxa. By contrast in ADHD (Figs 10–11(B)), nearly all cluster BB negative correlations are localized to family Porphyromonadaceae (ranked #1 by ATria). Fig 9(B) shows Porphyromonadaceae to be the sole cluster BB member negatively correlated with the Firmicutes-dominant portion (Fig 9(B), upper right, collectively more than 70% of the population).
Table 14 shows that for all MCNs, in Control more than two-thirds of cluster BB had negative correlations with members of other clusters, compared to less than half in ADHD. Negative edge count was also almost always higher for Control, despite a smaller cluster BB. Collectively these results show that in Control cluster BB is smaller, and more connected to other clusters, primarily through negative correlations (est. competition). In ADHD cluster BB is larger, and more isolated.
Table 14. Negative correlations between cluster BB and other clusters.
| Level | Family | Genus | Lowest | |||
|---|---|---|---|---|---|---|
| MCN | Control | ADHD | Control | ADHD | Control | ADHD |
| Cluster BB (-) Edges with Other Clusters (Participation Rate) | 7 (100%) | 5 (33%) | 10 (88%) | 4 (43%) | 5 (67%) | 5 (43%) |
Amount of negative correlations between members of Bacteroidaceae-dominant cluster BB and other clusters. Notation: Number (participation rate).
Table 15 provides a few final interesting observations for various Bacteroidetes taxa.
Table 15. Additional Bacteroidetes observations.
| Taxa | Observation |
|---|---|
| Family Odoribacteriaceae | ADHD-elevated, ADHD cluster BB member, only ranked in ADHD. |
| Family Prevotellaceae and member genus Prevotella | ADHD negative correlation with Bacteroidaceae/Bacteroides is the only negative correlation between two Bacteroidetes taxa in any MCN. Only ranked in ADHD, |
| Family Rikenellaceae | Ranked in every MCN at every level, and always higher for ADHD. |
Additional observations we make for some other Bacteroidetes taxa.
(B) In Control, taxa in (A) have more negative edges with Actinobacteria (est. competition), especially Bifidobacteriales. We now know taxa from (A) to correspond to cluster BB, which in both MCNs contained one core Proteobacteria (family Alcaligenaceae/genus Sutterella) and otherwise primarily Bacteroidetes. We also observed cluster BB taxa to have far more negative correlations (est. competition) with other clusters in Control. We now see if this is also true with order Bifidobacteriales lineages, including genus Bifidobacterium. Our analysis in fact reveals that negative correlations between Bifidobacterium or any parent/descendant with any Bacteroidetes or Proteobacteria are exclusive to Control and absent in ADHD.
Table 16 shows all correlations involving Bifidobacterium and its lineages, grouped and colored as in previous tables. Not only are negative Bacteroidetes correlations exclusive to Control (orange), but these taxa include the most abundant Bacteroidetes family/genus Bacteroidaceae/Bacteroides (ADHD cluster BB centroid), as well as family/genus Porphyromonadaceae/Parabacteroides (Control cluster BB centroid). Another appears at the lowest level between soecues B. adolescentis and B. ovatus. With Proteobacteria, negative Bifidobacterium correlations are also observed with genus Sutterella (core cluster BB member) and family Enterobacteriaceae, also only in Control. Heatmaps confirm family/genus Bifidobacteriaceae/Bifidobacterium to be negatively correlated with cluster BB taxa only in Control (Fig 11(A)–11(D), intersection of brown and magenta rectangles). By contrast, the only ADHD correlation is positive and within cluster BB (species B. longum with species B. uniformis).
Table 16. Bifidobacterium correlations.
| Phylum | Class | Order | Family | Genus | Lowest Possible | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria-Bacteroidetes | - | Actinobacteria- Bacteroidia | - | Bifidobacteriales-Bacteroidales | - | Bifidobacteriaceae–Bacteroidaceae | - | Bifidobactrium -Bacteroides | - | B. adolescentis–B. ovatus | - |
| B. longum–B.uniformis | + | ||||||||||
| Bifidobacteriaceae–Porphyromonadaceae | - | Bifidobacterium—Parabacteroides | - | ||||||||
| Actinobacteria Proteobacteria | - | Actinobacteria -Betaproteobacteria | - | Bifidobacteriales-Burkholderiales | Bifidobacteriaceae-Alcaligenaceae | Bifidobacterium– Sutterella |
- | ||||
| Actinobacteria-Gammaproteobacteria | Bifidobacteriales- Enterobacteriales | Bifidobacteriaceae-Enterobacteriaceae | Bifidobacterium-Enterobacteriaceae | Bifidobacterium 2 –Enterobacteriaceae | - | ||||||
| Actinobacteria- Firmicutes | Actinobacteria- Bacilli | Bifidobacteriales-Turicibacteriales | Bifidobacteriaceae-Turicibacteriaceae | Bifidobacterium-Turicibacter | Bifidobacterium 1 –Turicibacter | + | |||||
| Actinobacteria-Clostridia | Bifidobacteriales -Clostridiales | + | Bifidobacteriaceae- Lachnospiraceae | + | Bifidobacterium -Lachnospiraceae 2 | + | Bifidobacterium 1 –Blautia 2 | - | |||
| Bifidobacterium 1 –Ruminococcus [L] | + | ||||||||||
| B. longum–Blautia 1 | + | ||||||||||
| Bifidobacteriales–Clostridiales | Bifidobacterium-Clostridiales | B. adolescentis–Clostridiales 1 | + | ||||||||
| Bifidobacterium-Ruminococcaceae | Bifidobacterium 2 –Ruminococcaceae | - | |||||||||
| B. longum–Oscillospira | - | ||||||||||
| Bifidobacteriaceae–Veillonellaceae | Bifidobacterium-Veillonella | Bifidobacterium 2 –Dialister | - | ||||||||
| Actinobacteria-Actinobacteria | Actinobacteria-Actinobacteria | Bifidobacteriales–Bifidobacteriales | Bifidobacteriaceae–Bifidobacteriaceae | Bifidobacterium-Bifidobacterium | Bifidobacterium 2 –B. adolescentis | - | |||||
| Actinobacteria-Coriobacteria | Bifidobacteriales-Coriobacteriales | + | Bifidobacteriaceae-Coriobacteriaceae | + | Bifidobacterium-Adlercruetzia | Bifidobacterium 1 -Adlercruetzia | + | ||||
| Bifidobacterium-Collinsella | + | ||||||||||
| Bifidobacterium-Eggerthella | B. longum–E.lenta | - | |||||||||
Correlations involving Bifidobacterium and its lineages. Orange = only found in Control, purple = only found in ADHD, grey = found in both. +(green) = positive correlation, -(red) = negative correlation.
Table 16 also shows Bifidobacterium to even have far more Firmicutes connections (positive and negative) in Control. Collectively 24 correlations were observed in Control, compared to 9 in ADHD, supporting an overall increase in Bifidobacterium participation in Control. ATria (Table 17) also almost uniformly ranks Bifidobacterium and its lineages higher in Control. Again, this is despite Bifidobacterium average abundances being relatively the same (slightly higher in ADHD in fact, 3.6% to 3.2%).
Table 17. Bifidobacterium rankings.
| Phylum | Class | Order | Family | Genus | Lowest Possible |
|---|---|---|---|---|---|
| Actinobacteria (#1/#1) | Actinobacteria (#2/NR) | Bifidobacteriales (#T2/ #T5) | Bifidobacteriaceae (#5/NR) | Bifidobacterium (NR/#T19) | Bifidobacterium 1 (#10/NR) |
| Bifidobacterium 2 (#1/11) | |||||
| B. longum (#15/NR) |
ATria rankings of Bifidobacterium and lineages. Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1.
(C) A Shift in Firmicutes-Proteobacteria dynamics. Only two Proteobacteria families/genera were consistently present. One was genus Sutterella (family Alcaligenaceae), already noted as a core cluster BB member. The other is family Enterobacteriaceae, which our analysis supports being mostly responsible for this shift.
Table 18 shows all Proteobacteria-Firmicutes correlations. A couple of negative correlations can be seen involving family/genus Alcaligenaceae/Sutterella, with Firmicutes families Ruminococcaceae (Control) and Clostridiaceae (both). Far more significant are the differences involving family Enterobacteriaceae. One is its negative correlation with genus Oscillospira in ADHD (genus level), that becomes a positive correlation with Oscillospira in Control (lowest level). This is the only time, over all twelve MCNs, where a correlation sign changed between the same two taxa in Control vs. ADHD.
Table 18. Proteobacteria-Firmicutes correlations.
| Phylum | Class | Order | Family | Genus | Lowest Possible | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteobacteria- Firmicutes | - | Betaproteobacteria—Clostridia | Burkholderiales-Clostridiales | Alcaligenaceae–Ruminococcaceae | - | ||||||
| Alcaligenaceae—Clostridiaceae- | - | Sutterella–Clostridiaceae 2 | - | Sutterella–Clostridiaceae 2 | - | ||||||
| Gammaproteobacteria—Bacilli | Enterobacteriales—Turicibacteriales | - | |||||||||
| Gammaproteobacteria–Clostridia | - | Enterobacteriales-Clostridiales | Enterobacteriaceae–Mogibacteriaceae | + | |||||||
| Enterobacteriaceae-Lachnospiraceae | Enterobacteriaceae–Anaerostipes | Enterobacteriaceae-Anaerostipes | + | ||||||||
| Ruminococcaceae-Enterobacteriaceae | Enterobacteriaceae–Oscillospira | - | Enterobacteriaceae–Oscillospira | + | |||||||
| Gammaproteobacteria—Erysipelotrichi | Enterobacteriales-Erysipelotrichiales | Enterobacteriaceae-Erysipelotrichiaceae | Enterobacteriaceae–Erysipelotrichiaceae 2 | + | Enterobacteriaceae–Erysipelotrichiaceae 2 | + | |||||
| Deltaproteobacteria—Clostridia | - | Desulfovibrionales–Clostridiales | - | ||||||||
Correlations between Proteobacteria and Firmicutes taxa. Orange = only found in Control, purple = only found in ADHD, grey = found in both. +(green) = positive correlation, -(red) = negative correlation.
Interesting shifts involving family Enterobacteriaceae and various Firmicutes occur even at the family level, however. A small mixed-family, Firmicutes-dominant cluster FM forms (Fig 10(A), upper left), consisting of families Mogibacteriaceae, Christensenellaceae, and Erysipelotrichiaceae (Table 19). In ADHD, Enterobacteriaceae instead joins family Mogibacteriaceae to form a small two-taxon mixed cluster M (Fig 10(B), upper left, and Table 19).
Table 19. Mixed-family clusters.
| Control | ADHD | ||||||
|---|---|---|---|---|---|---|---|
| Community | Cluster Type | Cluster | Taxon | Phy | Cluster | Taxon | Phy |
| Firmicutes-dominant (F) | Mixed (FM) | FM | Mogibacteriaceae | ||||
| FM | Christensellaceae* | ||||||
| FM | Erysipelotrichiaceae | ||||||
| Mixed (M) | N/A | M | Mogibacteriaceae | ||||
| M | Enterobacteriaceae | ||||||
Control and ADHD clusters consisting of taxa from multiple families. Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown-Actinobacteria, blue = Proteobacteria.
Dynamics of FM and M taxa change between the MCNs. Fig 10(A) and 10(B) shows a distinguishing core FM/M feature is the negative correlation with family Rikenellaceae of cluster BB, but the taxon involved changes from family Erysipelotrichiaceae in Control to family Mogibacteriaceae in ADHD. Table 20 (ATria) shows the two taxa from Control cluster FM “replaced” by family Enterobacteriaceae in ADHD cluster M, families Christensenellaceae and Erysipelotrichiaceae, are only ranked in Control, and family Mogibacteriaceae only ranked in ADHD. This applied across all descendants, with the one notable exception being genus Coprobacillus (family Erysipelotrichiaceae), ranked #1 for ADHD at the genus and lowest levels (the only taxon to be ranked #1 in two MCNs). Recall Coprobacillus was also reported by DESeq2 as elevated in ADHD compared to Control. Other members of the Erysipelotrichaceae family (including the family itself) were only ranked by ATria in the case of Control. We label it in Fig 10(D) and 10(F), noting its negative correlations with multiple Firmicutes-dominant clusters.
Table 20. Mixed-family cluster member rankings.
| Phylum | Class | Order | Family | Genus | Lowest Possible |
|---|---|---|---|---|---|
| Firmicutes (#2/#T2) | Clostridia (#T3/#T3) | Clostridiales (#T2/#3) | Christensenellaceae (#2/NR) | Christensenellaceae (NR/NR) | Christensenellaceae (#18/NR) |
| Mogibacteriaceae (NR/#T6) | Mogibacteriaceae (NR/#6) | ||||
| Erysipelotrichia (NR/NR) | Erysipelotrichales (NR/#T3) | Erysipelotrichaceae (#3/NR) | Coprobacillus (NR/#1) | Coprobacillus (#T31/#1) | |
| Erysipelotrichaceae 1 (#6/NR) | Erysipelotrichaceae 1 (#16/NR) | ||||
| Erysipelotrichaceae 2 (#11/NR) | |||||
| Eubacterium (#T14/NR) | E. dolicum (#T29/NR) | ||||
| Proteobacteria (NR/NR) | Gammaproteobacteria (#T3/NR) | Enterobacteriales (#T3/NR) | Enterobacteriaceae (NR/#T6) | Enterobacteriaceae (NR/#7) | Enterobacteriaceae (NR/#T26) |
Members of mixed family clusters from Table 19, their lineages, and ATria rankings. Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1.
Family Enterobacteriaceae was also only ranked in ADHD, across all three lower levels. Its Oscillospira positive correlation (Table 18) is the only Control correlation involving Enterobacteriaceae, and Enterobacteriaceae actually joins genus Oscillospira’s cluster (FR, Fig 10(E)) in Control. The sign change takes place at the genus level in ADHD (Fig 9(D)), where Oscillospira and Enterobacteriaceae are negatively correlated. Although this correlation did not persist to the lowest level (Fig 9(F)), Enterobacteriaceae is still positively correlated with genus Anaerostipes, a taxon negatively correlated with Oscillospira across the board. We therefore observe Enterobacteriaceae dynamics to shift from a state that favors Oscillospira cooperation in Control, to Oscillospira competition in ADHD. The role of Enterobaceriaceae in gut ecology has historically been controversial [145], with both beneficial [146] and pathogenic [147] properties emerging. Gut dysbiosis has actually been shown to trigger horizontal gene transfer between the two types [148].
New observations
We make the following new observations at the lower levels.
(D) LEfSe reported taxa: Genera Turicibacter and Odoribacter. Earlier we noted ADHD-elevated family Odoribacteriaceae as an ADHD cluster BB member (Table 15). We now observe two remaining LEfSe-reported genera: Turicibacter (Control) and Odoribacter (ADHD).
Cluster FT (Fig 10, orange) was the only Firmicutes-dominant cluster with members positively correlated with any Bacteroidetes-dominant cluster (BB in ADHD, BM1 in Control). We named this cluster FT because of core member genus Turicibacter. Turicibacter (Firmicutes, Control-elevated), which joins genus Phascolarctobacterium (Firmicutes, reduced in Inattention, [63]) to form FT at the genus level in ADHD (Fig 10(D)), where it is not present in Control. At the lowest level, FT is slightly larger (by one taxon) in ADHD. Supplementing the earlier trend of less cluster BB negative correlations (est. competition) in ADHD, this also supports the presence of a larger cluster with positive correlations (est. cooperation) as well, with Turicibacter as its centroid (Table 21).
Table 21. FT members.
| Control | ADHD | |||||
|---|---|---|---|---|---|---|
| Level | Community | Cluster Type | Taxon | Phy | Taxon | Phy |
| Family | Firmicutes-dominant (F) | Turicibacter-core (FT) | Not present. | Turicibacter | ||
| Phascolarctobacterium | ||||||
| Level | Community | Sub-Community | Taxon | Phy | Taxon | Phy |
| Genus | Firmicutes-dominant (F) | Turicibacter-core (FT) | Turicibacter | Turicibacter* | ||
| Ruminococcus [L] | Phascolarctobacterium | |||||
| Bifidobacterium 2* | Clostridiales 2 | |||||
| Parabacteroides | ||||||
Cluster FT (Firmicutes-dominant, Turicibacter-core) members. Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown-Actinobacteria, blue = Proteobacteria.
In ADHD Turicibacter provides the sole genus-level (Fig 10(D)) FT-BB positive correlation, with genus Parabacteroides (Bacteroidetes, previously reported elevated in Hyperactivity, [59]). At the lowest level (Fig 10(F)) Parabacteroides joins FT, and along with Turicibacter forms FT-BB positive correlations, with member species P. distasonis. Interestingly in Control (Fig 10(E)), the FT-BB positive correlation does not involve Firmicutes or Bacteroidetes taxa at all, but rather two Actinobacteria–Bifidobacterium 1 (FT centroid), and Adlercruetzia (BB). This continues our observed increases in Actinobacteria and particularly Bifidobacterium involvement in Control gut ecology.
Cluster FC forms in Control (Fig 10(C) and 10(E), aqua) and contains two family Clostridiaceae taxa. In both MCNs these taxa negatively correlate with multiple cluster BB members, and in ADHD (Fig 10(F)) family Clostridiaceae 1 has negative correlations with BB centroid genus Bacteroides plus taxa involved in FT-BB cooperation: species P. distasonis, and FT centroid genus Turicibacter. In both MCNs, they participate in correlations that favor cluster BB competition (especially the more abundant Clostridiaceae 1).
Exclusive to ADHD is a negative correlation (est. competition) between these family Clostridiaceae taxa and ADHD-elevated genus Odoribacter–both at the genus level (Fig 10(D)), and family Clostridiaceae 1 at the lowest level (Fig 10(F)). Genus Odoribacter was reported by LEfSe as elevated in ADHD, and this negative correlation implies that an increase in Odoribacter abundance will decrease family Clostridiaceae 1. ALDEx2 reported Clostridiaceae 1 as differentially abundant in Control, and upon further inspection Clostridiaceae 1 average relative abundance is indeed reduced by a factor of two in ADHD vs. Control. Cooperation between families Clostridiaceae 1 and 2 in Control (forming FC) is also absent in ADHD.
(E) Changes in the role of genus Adlercruetzia (Actinobacteria). In contrast to genus Bifidobacterium (Bifidobacteriaceae), Adlercruetzia is a member of the other consistently present Actinobacteria family, Coriobacteriaceae. While the distinguishing feature of family/genus Bifidobacteriaceae/Bifidobacterium was increased Control participation, the distinguishing feature of family Coriobacteriaceae appears to be changes in cluster membership. In fact over all Coriobacteriaceae descendants, only once (Collinsella, genus level, cluster AM, Fig 10(C) and 10(D)) were any in the same Control and ADHD cluster. Table 22 also shows ATria results to be more mixed for family Coriobacteriaceae, compared to family Bifidobacteriaceae (Table 19).
Table 22. Coriobacteriaceae rankings.
| Phylum | Class | Order | Family | Genus | Lowest Possible |
|---|---|---|---|---|---|
| Actinobacteria (#1/#1) | Coriobacteria (#1/NR) | Coriobacteriales (NR/#T5) | Coriobacteriaceae (NR/#4) | Adlercruetzia (NR/#17) | Adlercruetzia (#14/NR) |
| Collinsella (NR/#T19) | C. aerofaciens (NR/#15) | ||||
| Coriobacteriaceae (#18/NR) | |||||
| Eggerthella (#4/NR) | E. lenta (#T25/NR) |
ATria rankings of Coriobacteriaceae and its lineages. Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1.
We earlier noted genus Adlercruetzia as the Actinobacteria member of cluster BB/BM1 in Control, and (along with genus Bifidobacterium 1) connecting clusters FT and BB. Table 23 shows that outside of Bifidobacterium 1, its positive correlations in Control were entirely with Bacteroidetes taxa (all BB/BM1 members). By contrast in ADHD, Adlercruetzia relationships mostly occur with Firmicutes, including a cluster membership with genus/species Eubacterium/E. dolicum. Several negative correlations are seen between genus Adlercruetzia and different Firmicutes, with no overlap between Control and ADHD. This suggests genus Adlercruetzia may play a significantly different role in Control and ADHD gut ecologies.
Table 23. Adlercruetzia correlations.
| Phylum | Class | Order | Family | Genus | Lowest Possible | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria-Bacteroidetes | - | Coriobacteria-Bacteroidia | - | Coriobacteriales -Bacteroidales | - | Coriobacteriaceae–Bacteroidaceae | - | Adlercreutzia- Bacteroides | + | Adlercreutzia- B.uniformis | + |
| Adlercreutiza-Parabacteroides | + | Adlercreutzia- P.distasonis | + | ||||||||
| Adlercreutzia- Odoribacter | + | ||||||||||
| Actinobacteria-Firmicutes | Actinobacteria-Clostridia | Coriobacteriales- Costridiales | Coriobacteriaceae- Erysipelotrichaceae- | Adlercreutzia- Eubacterium | + | Adlercreutzia- E. dolicum | + | ||||
| Coriobacteriaceae- Lachnospiraceae | Adlercreutzia -Blautia | - | |||||||||
| Adlercreutzia–Lachnospira | - | Adlercreutzia—Lachnospira | - | ||||||||
| Adlercreutzia—Lachnospiraceae 1 | - | ||||||||||
| Adlercreutzia—Lachnospiraceae 2 | Adlercreutzia—Lachnospiraceae 2 | - | |||||||||
| Coriobacteriaceae- Ruminococcaceae | Adlercreutzia–Ruminococcus | - | Adlercreutzia–Ruminococcus | - | |||||||
| Actinobacteria-Actinobacteria | Actinobacteria-Coriobacteria | Bifidobacteriales-Coriobacteriales | + | Bifidobacteriaceae-Coriobacteriaceae | + | Bifidobacterium-Adlercruetzia | Bifidobacterium 1 -Adlercruetzia | + | |||
| Coriobacteria—Coriobacteria | Coriobacteriales—Coriobacteriales | Coriobacteriaceae-Coriobacteriaceae | Adlercruetzia-Coriobacteriaceae | + | |||||||
Correlations involving genus Adlercreutzia and its lineages. Orange = only found in Control, purple = only found in ADHD, grey = found in both. +(green) = positive correlation, -(red) = negative correlation.
(F) Bacteroidetes-Firmicutes positive correlations (est. cooperation) are entirely exclusive to ADHD, and absent in Control. Table 24 shows all Bacteroidetes-Firmicutes positive correlations. They are entirely limited to ADHD, and with one exception (genus Clostridium) involve class Bacilli descendants.
Table 24. Bacteroidetes-Firmicutes positive correlations.
| Phylum | Class | Order | Family | Genus | Lowest Possible | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacteroidetes-Firmicutes | - | Bacteroidia -Bacilli | Bacteroidales Lactobacillales | Bacteroidaceae Streptococcaceae | + | Bacteroides -Streptococcus | + | |||
| Porphyromonadaceae Turicibacteriaceae- | Parabacteroides -Turicibacter | + | Parabacteroides-Turicibacter | + | ||||||
| Parabacteroides -Turicibacter | + | P.distasonis -Turicibacter | + | |||||||
| Bacteroidales–Clostridiales | - | Odoribacteriaceae–Clostridiaceae | Butyricimonas -Clostridium | + | ||||||
Bacteroidetes-Firmicutes positive correlations, over all MCNs. Orange = only found in Control, purple = only found in ADHD, grey = found in both. +(green) = positive correlation, -(red) = negative correlation.
We have already seen most of these, including genera Clostridium and Butyricimonas, and the ADHD FT-BB connections involving genera Turicibacter and Parabacteroides, and species P.distasonis. We now analyze the remaining top row, between genera Bacteroides (family Bacteroidaceae) and Streptococcus (family Streptococcaceae).
Firmicutes taxa were only ever present in cluster BB in ADHD, and we earlier noted family Streptococcaceae and its genus Streptococcus as two of those taxa. Their cluster BB positive correlation was with centroid family/genus Bacteroidaceae/Bacteroides. Additionally cluster BB had almost no negative correlations (est. competition) with FL/FR (collectively 70% of the population) in ADHD, compared to a significant amount in Control.
What makes genus Streptococcus interesting for ADHD is that across all MCNs, it forms the only positive correlation between cluster BB and FL/FR (Fig 10(D)). In other words, in addition to estimating significantly less BB-(FL/FR) competition in ADHD, our MCNs also estimate cooperation only in ADHD, between genera Streptococcus (BB) and Blautia (FL1).
Fig 10(D) and 10(F) also show Streptococcus to be negatively correlated with genus Oscillospira in ADHD, a taxon we noted earlier its correlation sign change with family Enterobacteriaceae. ATria (Table 25) also only ranks family/genus Streptococcaceae/Streptococcus as important in ADHD.
Table 25. Streptococcaceae/Streptococcus rankings.
| Phylum | Class | Order | Family | Genus | Lowest Possible |
|---|---|---|---|---|---|
| Firmicutes (#2/#T2) | Bacilli (NR/NR) | Lactobacillales (NR/NR) | Streptococcaceae (NR/#T12) | Streptococcus (NR/#T23) |
ATria rankings of family/genus Streptococcaceae/Streptococcus. Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1.
(G) A shift in genera Blautia-Oscillospira dynamics, and their respective clusters. Thus far genus Oscillospira has been noted for two ADHD-exclusive negative correlations, with taxa only ranked in ADHD: family Enterobacteriaceae and genus Streptococcus. Enterobacteriaceae-Oscillospira was the only correlation to ever change sign from Control (positive) to ADHD (negative). Streptococcus was noted for its correlation with genus Blautia, the sole positive correlation between the largest Bacteroidetes-dominant cluster (BB) and Firmicutes-dominant clusters (FL/FR) in any MCN.
Previous studies have indicated butyrate-producing Oscillospira as a healthy gut taxon [149], specifically associated with leanness [150]. Blautia is actually a taxon that has been associated with obesity [151]. And interestingly in the Control MCN (Fig 10(C) and 10(E)) Blautia and Oscillospira are negatively correlated, but not in ADHD (Fig 10(D) and 10(F)).
Since obesity has been associated with ADHD [152], the shift in Enterobacteriaceae (Oscillospira cooperation in Control, competition in ADHD) and Streptococcus (Blautia cooperation and Oscillospira competition in ADHD) correlations become interesting, favoring Blautia cooperation and Oscillospira competition. Indeed correlation can never imply causation and further experimental verification is required. But ATria results (Table 26) also support this, ranking Blautia higher in ADHD and Oscillospira in Control.
Table 26. Blautia and Oscillospira rankings.
| Phylum | Class | Order | Family | Genus | Lowest Possible |
|---|---|---|---|---|---|
| Firmicutes (#2/#T2) | Clostridia (#T3/#T3) | Clostridiales (#T2/#3) | Lachnospiraceae (NR/NR) | Blautia (#T16/#13) | Blautia 1* (NR/#19) |
| Ruminococcaceae (#4/#T8) | Oscillospira (#3/#10) | Oscillospira (#5/#9) |
Blautia and Oscillospira ATria rankings (plus lineages). Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1. * = The lowest level had two Blautia taxa; we assumed the more abundant (Blautia 1, overall 9.3% relative abundance vs 0.6%, composing 93% of the Blautia population).
In fact our heatmap (Fig 11(C)–11(F)) shows by intersecting Oscillospira’s row (small green rectangle) with the columns of Blautia’s cluster (gold rectangles, Control FL3, ADHD FL1) that Oscillospira is negatively correlated with Blautia’s entire cluster in Control, and these correlations are completely absent in ADHD.
The lowest level MCNs (Fig 10(E) and 10(F)) also show Blautia’s cluster as larger in ADHD, and Oscillospira’s cluster as larger in Control. Table 27 contains members of these clusters. Blautia and Oscillospira each belong to a cluster dominated by its respective family: Lachnospiraceae (FL), and Ruminococcaceae (FR). Oscillospira is a core FR member and at the lowest level, we see the Control FR cluster (with family Enterobacteriaceae now a member). Blautia is consistently a member of the same cluster as both family Lachnospiraceae taxa in ADHD, comparably larger than its FL3 Control cluster.
Table 27. Blautia and Oscillospira clusters.
| Control | ADHD | |||||||
|---|---|---|---|---|---|---|---|---|
| Level | Community | Cluster Type | Cluster | Taxon | Phy | Cluster | Taxon | Phy |
| Lowest | Firmicutes-dominant, | Lachnospiraceae-dominant (FL) | FL3 | Blautia 1* | FL1 | Lachnospiraceae 1 | ||
| FL3 | Dorea 2 | FL1 | Lachnospiraceae 2* | |||||
| FL3 | Bifidobacterium longum | FL1 | Coprococcus | |||||
| FL1 | Ruminococcus [L] | |||||||
| FL1 | Blautia 1 | |||||||
| FL1 | Dorea 2 | |||||||
| FL1 | Faecalibacterium prausnitzii | |||||||
| Ruminococcaceae-dominant (FR) | FR | Ruminococcaceae* | FR | Ruminococcaceae | ||||
| FR | Ruminococcus [R] | FR | Ruminococcus [R] | |||||
| FR | Oscillospira | FR | Oscillospira | |||||
| FR | Clostridiales 1 | FR | Clostridiales 1* | |||||
| FR | Coprobacillus | FR | Bifidobacterium adolescentis | |||||
| FR | Enterobacteriaceae | |||||||
| FR | Lachnospira | |||||||
Clusters involving Blautia and Oscillospira. Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown = Actinobacteria, blue = Proteobacteria.
Heatmaps also indicate increased participation of Oscillospira’s cluster (FR) in Control (large green rectangle, Fig 11(C)–11(F)), including negative correlations with cluster BB that are absent in ADHD, yet another example of reduced ADHD cluster BB competition. In the MCNs, Fruchterman-Reingold places cluster FR (green) in a much more central position in Control (Fig 10(C) vs 10(D), and 10(E) vs. 10(F)). The negative correlations between Oscillospira and Blautia’s entire cluster FL3 (Figs 10(C) and 11(E)) are also evident, almost separating FL3 from the MCN. In ADHD Blautia’s cluster FL1 (gold) occupies a much more central position (Fig 10(D) and 10(F)), with increased ADHD size particularly noticeable at the lowest level (Fig 10(F)).
ATria (Table 28) indicates a general increased importance of Blautia’s family (Lachnospiraceae) in ADHD, and Oscillospira’s family (Ruminococcaceae) in Control. A couple of noteworthy taxa follow this trend. Species Faecalibacterium prausnitzii (Ruminococcaceae), an anti-inflammatory bacterium [153] touted as a next-generation probiotic [154], is only ranked in Control. Species Ruminococcus gnavus (Lachnospiraceae), known to produce an inflammatory polysaccharide [155], is only ranked in ADHD.
Table 28. Lachnospiraceae and Ruminococcaceae rankings.
| Phylum | Class | Order | Family | Genus | Lowest Possible |
|---|---|---|---|---|---|
| Firmicutes (#2/#T2) | Clostridia (#T3/#T3) | Clostridales (#T2/#3) | Lachnospiraceae (NR/NR) | Anaerostipes (#T19/NR) | Anaerostipes (#6/#T26) |
| Blautia (#T16/#13) | Blautia 1 (NR/#19) | ||||
| Blautia 2 (#9/#T22) | |||||
| Coprococcus (NR/#5) | Coprococcus (#T21/#6) | ||||
| Dorea (#T14/#12) | Dorea 2 (#13/NR) | ||||
| Lachnospira (NR/#16) | Lachnospira (#T27/#5) | ||||
| Lachnospiraceae 1 (#19/#15) | Lachnospiraceae 1 (#17/#2) | ||||
| Lachnospiraceae 2 (#7/NR) | Lachnospiraceae 2 (#8/#14) | ||||
| Roseburia (NR/NR) | Roseburia 1 (NR/#T28) | ||||
| Roseburia 2(#T25/#T22) | |||||
| Ruminococcus (#1/#11) | R. gnavus (NR/#12) | ||||
| Ruminococcaceae (#4/#T8) | Faecalibacterium (#T16/#2) | F. prausnitzii (#11/NR) | |||
| Oscillospira (#3/#10) | Oscillospira (#5/#9) | ||||
| Ruminococcaceae (#8/#9) | Ruminococcaceae (#4/#18) | ||||
| Ruminococcus (#2/NR) | Ruminococcus (#T21/NR) |
ATria rankings of Lachnospiraceae and Ruminococcaceae taxa. Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1.
Summary
Four clusters were consistently present in both Control and ADHD MCNs. Three are Firmicutes-dominant (FL, FR, FT) and one is Bacteroidetes-dominant (BB). Table 29 shows their attributes, and summarizes observations we made about each.
Table 29. Largest, consistently present clusters.
| Cluster | Attribute | Observation |
|---|---|---|
| BB | Largest Bacteroidetes-dominant cluster | Larger in ADHD, with more internal cooperation and less external competition. |
| FL(1,2,…) | Multiple Firmicutes, family Lachnospiraceae-dominant clusters | One large, centrally located cluster emerges in ADHD (FL1). Others are small, about the same size, and more disconnected (all are this way in Control). |
| FR | Firmicutes, family Ruminococcaceae-dominant | Smaller and less centrally located in ADHD. |
| FT | Firmicutes, core member genus Turicibacter | Slightly larger in ADHD. |
Clusters that we note as being consistently present in Control and ADHD MCNs, and observations.
Table 30 summarizes correlations between members of these clusters. Other than the one exception in ADHD involving genera Streptococcus and Blautia: FT is the only Firmicutes-dominant cluster with taxa positively correlated with Bacteroidetes-dominant cluster (BB) members, and all correlations involving FL/FR (largest Firmicute-dominant clusters) and BB taxa are negative. FT is completely disconnected from FL/FR except some ADHD competition. FL-FR competition only happens in Control.
Table 30. Cluster member interactions.
| Cluster 1 | Cluster 2 | Observation |
|---|---|---|
| BB | FL | Always (-), with one exception in ADHD (Streptococcus-Blautia) |
| BB | FR | Always (-) |
| BB* | FT | Always (+) |
| FL | FR | Generally (+). Some (-) in Control (all involve either Ruminococcus (FL) or Oscillospira (FR)) |
| FL | FT | Generally disconnected. Some (-) in ADHD (all involve Phascolarcobacterium (FT)) |
| FR | FT | Always disconnected. |
Interactions between taxa from Table 29 clusters (* = In Control, this took place with BM1 after the BB “split”).
Finally, we summarize taxa (Table 31) and relationships (Table 32) that we noted throughout our analyses.
Table 31. Taxa noted throughout our analysis.
| Level | Taxon | Observation |
|---|---|---|
| Genus | Adlercruetzia | Role change from Control (Bacteroidetes cooperation) to ADHD (E. dolichum cooperation). Competition with different Firmicutes (in ADHD,). |
| Genus | Bacteroides | Centroid (with nearly 100% connectivity) of cluster BB in ADHD. |
| Genus | Bifidobacterium | Higher participation in Control (mostly competition). Competition with all Bacteroidetes or Proteobacteria taxa is entirely exclusive to Control, including multiple members and centroid of cluster BB. Cooperation (small amount) is entirely exclusive to ADHD.Ranked higher in Control than ADHD nearly 100% of the time, including a #1 ranking at the lowest taxonomic level. |
| Genus | Coprobacillus | DESeq2-elevated ADHD taxon, and ranked #1 for ADHD in two MCNs (genus and lowest possible). Competes with multiple Lachnospiraceae taxa, including the most abundant. |
| Family | Enterobacteriaceae | Involved with Firmicutes-Proteobacteria shifts. Only ranked in ADHD. |
| Species | F. prausnitzii | Probiotic species only ranked in Control |
| Family | Lachnospiraceae | Most abundant family, generally ranked higher in ADHD |
| Genus | Phascolarctobacterium | Only FT member connected to another Firmicute-dominant cluster (competition). Only ranked in ADHD. |
| Family | Porphyromonadaceae | #1 ADHD family, only cluster BB member to compete with FL/FR. |
| Family | Rikenellaceae | Previously reported as ADHD-elevated. Ranked by ATria as important in all six lower level MCNs, and always higher in ADHD. |
| Genus | Ruminococcus [L] | #1 Control genus, involved in FL-FR competition (only observed in Control). |
| Species | R. gnavus | Produces inflammatory biosaccharide, only ranked in ADHD |
| Family | Ruminococcaceae | Second-most abundant family, generally ranked higher in Control |
| Genus | Turicibacter | Reported as differentially abundant (Control) by three methods (LEfSe, DESeq and ALDEx2), core member (centroid in ADHD) of FT. |
Summary of notable taxa throughout our ecological analyses, and observations.
Table 32. Relationships noted throughout our analyses.
| Taxonomic Level | Relationship(s) | Reason |
|---|---|---|
| Genus | Bacteroides-Sutterella (+, both) | Only core correlation consistent across both sample sets at all levels (12 MCNs). |
| Genus | Bacteroides-Prevotella (-, ADHD) | Only competition involving two Bacteroidetes taxa. |
| Genus | Bacteroides-Streptococcus (+, ADHD) | Streptococcus is one of only two Firmicutes genera to join cluster BB, through this connection. |
| Genus | Butyricimonas-Clostridium (+, ADHD) | Butyricimonas was only ranked by ATria in ADHD, and reported as ADHD differentially abundant by two methods (LEfSe and ALDEx2). Clostridium is one of only two Firmicutes to join cluster BB, through this connection. |
| Family-Genus | Clostridiaceae-Odoricibacter (-, ADHD) | Odoribacter was reported as ADHD-elevated by LEfSe. In ADHD competes with Clostridiaceae taxa that compete with multiple cluster BB members (including its centroid). Clostridiaceae was reported as ADHD-reduced (Control-elevated) by ALDEx2. |
| Family-Genus | Enterobacteriaceae-Oscillospira (+, Control; -, ADHD) | Only correlation ever to change sign from Control to ADHD. Taxa involved are in the same cluster in Control. |
| Genus |
Blautia-Oscillospira (-, Control) Streptococcus-Blauta (+, ADHD) Streptococcus-Oscillospira (-, ADHD) |
Blautia is associated with obesity and Oscillospira with leanness. Oscillospira (FR) competes with every member of Blautia’s cluster (FL3) in Control. FL-FR competition only happens in Control. In ADHD Streptococcus cooperates with Blautia (obesity) and competes with Oscillospira (leanness) Streptococcus-Blautia is the only time a cluster BB member (largest Bacteroidetes-dominant) ever cooperates with taxa from FL or FR (largest Firmicutes-dominant, collectively over 70% of the population). Streptococcus is only ranked in ADHD, Blautia is ranked higher in ADHD, Oscillospira is ranked higher in Control. Blautia’s cluster (FL1) is larger and more central in ADHD.). Oscillospira’s (FR) is larger and more central in Control. |
Summary of notable relationships throughout our ecological analyses, and observations.
Discussion
Traditional analysis methods (i.e. diversity and composition) prevalent in current ADHD gut microbiome literature provide a macroscale representation of a complex ecosystem. Conducting some of these approaches on equal-sized, gender-balanced undergraduate Control and ADHD gut microbiome datasets produced many results that corresponded with this literature, plus a potentially new Control-elevated genus, Turicibacter (reported by LEfSe, DESeq2 and ALDEx2). Current literature, as well as our results, suggest this macroscale perspective leaves a largely incomplete picture due to its neglect of underlying complexity. Our goal was to complete more of this picture by venturing deeper, by analyzing two-way ecological relationships (cooperation and competition), plus community detection, and centrality.
Our results provide a deeper meaning to those from the macroscale. Previous differential abundance studies have reported collectively inconclusive results with respect to Bifidobacterium [65, 66] and Bacteroides [59, 65, 70]. We experienced similar issues with our taxa plots, where anomalous results involving elevated genus Bifidobacterium and reduced genera Bacteroides and Sutterella at ASRS extremes (high and low) imposed significant challenges when interpreting results. Our MCNs estimate that a Bacteroidetes-dominant community (cluster BB) forms in both microbiomes, with Bacteroides and Sutterella both core members, that in ADHD is larger and resides in conditions that favor its cooperation, as opposed to competition in Control. Several cluster BB members have previously been reported as ADHD-elevated, including Butyricimonas (reported by two of our differential abundance methods), Parabacteroides (for hyperactivity, [59]), Bacteroides uniformis [60], Rikenellaceae [66], and Odoribacteriaceae [68], plus two member descendants (Bacteroides ovatus and Sutterella stercoricanis, [60]), making its increased size, tightness, and more favorable environment observed in our ADHD MCN interesting. Moreover our MCNs estimate a shift in roles played by Bacteroides and Bifidobacterium between Control and ADHD microbiomes. In ADHD, Bacteroides was estimated as the centroid (driver) taxon for cluster BB, having cooperative with nearly every member. And Bifidobacterium shifted from exclusively competitive relationships with cluster BB members (including its most abundant and centroid) in Control, to exclusively a cooperative relationship in ADHD—with Bacteroides uniformis, one of the taxa previously reported as ADHD-elevated [60]. In addition to these relationships with cluster BB our MCNs estimated Bifidobacterium and its lineages to be much more active in Control gut ecology (24 relationships) compared to ADHD (9 relationships), with ATria ranking Bifidobacterium higher in Control more than 85% of the time. Overall, these results suggest potentially significant differential roles played by both Bifidobacterium and Bacteroides in the Control and ADHD gut ecosystems.
Potential roles played by taxa reported by our differential abundance analysis also became observable. Our MCNs estimated genus Odoribacter, reported by our analysis and another [68] as ADHD-elevated, to also compete with two family Clostridiaceae taxa, one of which (Clostridiaceae 1) was reported by ALDEx2 as ADHD-reduced (subsequently we found, by a factor of two). Clostridiaceae 1, in turn, was estimated to compete with multiple taxa from cluster BB (much larger in ADHD) in both of our MCNs. Exploring candidate metabolic reactions involved within this triangle may therefore also be important for future experimentation. Butyricimonas, reported by two of our differential analysis methods (LEfSe and ALDEx2) as ADHD-elevated, joined cluster BB in ADHD and provided the sole cooperative relationship with Clostridium, a taxon otherwise completely disconnected from both MCNs. Coprobacillus, reported by DESeq2 as ADHD-elevated, was ranked #1 twice by ATria in the ADHD MCN, which estimated competitive relationships with abundant Lachnospiraceae taxa, reported by another study as Control-elevated [68].
New interesting taxa and communities also emerged. Cluster FT (cooperative with cluster BB) was larger in ADHD. Cluster FR (Family Ruminococcaceae-dominant, competitive with cluster BB) was smaller in ADHD. Ruminococcaceae taxa (previously reported as ADHD-reduced [68]) were also almost universally less central in ADHD, including the probiotic Faecalibacterium prausnitzii (only ranked by ATria in Control), which was also reported as ADHD-reduced in two other studies [61, 68]. Our MCNs also showed inflammatory polysaccharide Ruminococcus gnavus (only ranked by ATria in ADHD), to have ADHD-exclusive competition with F. prausnitzii. Ruminococcaceae member genus Oscillospira was also estimated to have ADHD-exclusive competition, with Proteobacteria family Enterobacteriaceae (cooperative and fellow FR member in Control), and cluster BB member Firmicute Streptococcus. In particular, the shift in relationship between Enterobacteriaceae and Oscillospira from cooperation (Control) to competition (ADHD), as well as the dynamic involved in the triangle composed of genera Streptococcus, Blautia, and Oscillospira (Table 32, last row), emerged as noteworthy potential targets for further exploration.
This exploration can add deeper meaning to these results through pursuit of additional studies targeting some of these taxa and relationships, including multi-omics [156] and/or physical laboratory experiments. Fundamentally, ecological relationships manifest through internal interplay within the underlying web of interactions [157]. Cooperation could take place for example if two taxa produce a nutrient that the other consumes; competition could take place if two taxa consume a nutrient that neither produces. Coupling taxa to metabolites they produce and consume and analyzing pathways can help elucidate underlying mechanisms behind these ecological relationships. These pathways can then be searched for neurotransmitters to establish ADHD connections. Clustering could also be performed by functional pathway, enabling a more direct association between microbial genes and specialized biological pathways involving neurotransmitters that influence ADHD.
While results from our analysis could be used as guidance for more in-depth analysis of any ADHD dataset (present or future), it is of course possible (and likely) that changes within attributes of the target population can impact ecological relationships. We are merely laying the groundwork, and with very few studies even attempting this level of analysis [66], an enormous breadth of knowledge remains and many future improvements to our analyses are possible. Published metadata for this dataset was somewhat limited. Future studies involving ADHD and the gut microbiome should account for factors such as BMI [158], demographics [159], ethnicity [160], use of medication/probiotics [61], use of antibiotics [161], diet [162] and gastrointestinal issues [163], age [164], and official diagnosis (beyond ASRS). Additionally although we did strenghten the threshold of our p-value for correlations in our MCNs, future work could incorporate multiple hypothesis corrections and sensitivity analysis for ATria for more thorough statistical relevance validation. More meaning to relationships in our MCNs can also be uncovered, through causality studies. Causality would give direction to edges, enabling detection of both two- and one-way (i.e. commensalism [139], amensalism [140]) relationships. This can be achieved through for example Bayesian Networks [165], which detect relationships where a taxon is conditionally dependent on another. Conditional dependence also eliminates spurious edges that can occur with correlations; for example, two entities that co-occur with a mutual entity will naturally tend to co-occur [116] (this was also a dependency removed by ATria after finding a central node). Sazal et al. [166] have already verified such networks as a predictor for oral microbiome colonization order. Time can also factor into ecological relationships because while sometimes these relationships are constant in microbiomes [167], they can also be transitive [168] or even time-varying [169]. DBNs that account for time have already been used to predict long-term infant gut behavior [170]. Higher-level network metrics such as modularity [171] and vulnerability [172] would provide another potential avenue for comparing and contrasting Control and ADHD MCNs. Amplicon Sequence Variants (ASVs, [173]) can be used in place of the current Operational Taxonomic Units (OTUs) that are generated by similarity-based clustering. ASVs exhibit more reliability at lower levels of the taxonomic tree and can improve the granularity of our MCNs, achieving more species- and sometimes even strain-level classifications.
Also given that ASRS does not offer an official diagnosis and this metadata did provide ASRS scores, an immediate future goal should be a migration from case-control to continuous data analysis with respect to ASRS. Our taxa plots, which observed abundances of core taxa with respect to ASRS score, have already started this process. Some of our other analysis protocols (i.e. alpha- and beta-diversity) will trivially propagate. While differential abundance analysis will ultimately be difficult without explicit sample classifications, networks have potential given some of the strategies mentioned above. For example, multi-omic network techniques such as marginal correlation analysis have already proven useful for correlating heterogeneous datasets [174], making them potentially applicable to estimating correlation between microbial abundance and ASRS scores. Applying Bayesian techniques to such networks could assist in estimating the conditional presence or absence of a microbe-microbe correlation on another dependence (i.e., high or low ASRS score).
A more complete understanding of ADHD and the gut microbiome will best equip the community to make the right decisions when administering treatment(s). Our results, coupled with those in the literature, suggest that the gut microbiota cannot afford to be ignored when it comes to ADHD, and treatments directly targeting the gut microbiome have potential. Encouraging results have been uncovered for gluten and casein-free diets [53], Microbiota Transfer Therapy (MTT, [175, 176]), and probiotics [177] with ASD. Our results also indicate that the gut microbiome is an ecosystem, and any changes to one single element will likely impact other members. Additionally since the human gut microbiome is widely varied across individuals [178], personalized medicine should be used when developing such treatments.
Supporting information
Bar plot of taxa relative abundance, at the class level. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Bar plot of taxa relative abundance, at the order level. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Bar plot of taxa relative abundance, at the family level. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Bar plot of taxa relative abundance, using the lowest possible classification level. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Relative abundance plot of Bifidobacterium. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Relative abundance plot of Bacteroides. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Relative abundance plot of Sutterella. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Data obtained from the openly available BioProject (PRJNA656791), along with cited protocols [90–92].
(DOCX)
Average number of sequences retained after every preprocessing step.
(DOCX)
Every correlation in all MCNs, grouped by taxonomy. Orange = only found in Control, purple = only found in ADHD, grey = found in both. +(green) = positive correlation, -(red) = negative correlation.
(DOCX)
ATria rankings of all taxa over all MCNs, grouped by taxonomy. Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1.
(DOCX)
Communities within Control and ADHD family-level MCNs. Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown = Actinobacteria, blue = Proteobacteria.
(DOCX)
Communities within Control and ADHD genus-level MCNs. Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown = Actinobacteria, blue = Proteobacteria.
(DOCX)
Communities within Control and ADHD MCNs using the lowest possible level of taxonomic classification. Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown-Actinobacteria, blue = Proteobacteria.
(DOCX)
Acknowledgments
The authors would like to thank colleagues from the Bioinformatics Research Group (BioRG) and the Stollstorff Lab for many useful discussions.
Data Availability
The dataset is publicly available in the NCBI database (Accession Number: PRJNA656791). Our entire downstream analysis pipeline has been built using Plugin-Based Microbiome Analysis (PluMA, (76)) and is available for download within its publicly available pipeline pool (http://biorg.cs.fiu.edu/pluma/pipelines.html).
Funding Statement
The work of GN and KM was supported by the National Institute of Health (nih.gov) [Award #580 1R15AI128714-01]. The work of GN was also supported by the Department of Defense (defense.gov) [Contract #581 W911NF-16-1-0494] and National Institute of Justice (nij.ojp.gov) [Award #582 2017-NE-BX-0001]. TC received support from NVIDIA (nvidia.com) and Florida International University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015. Mar;56(3):345–65. doi: 10.1111/jcpp.12381 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) [Internet]. Available from: http://www.cdc.gov/ncbddd/adhd/data.html
- 3.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. The Lancet. 2005. Jul;366(9481):237–48. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher J, Wolfe B. Long-term consequences of childhood ADHD on criminal activities. J Ment Health Policy Econ. 2009. Sep;12(3):119–38. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders [Internet]. Fifth Edition. American Psychiatric Association; 2013. [cited 2020 Mar 27]. Available from: https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 [Google Scholar]
- 6.Woods KE, Danko CM, Chronis-Tuscano A. ADHD in Children and Adolescents. In: Oxford Research Encyclopedia of Psychology [Internet]. Oxford University Press; 2016. [cited 2020 Mar 27]. Available from: https://oxfordre.com/psychology/view/10.1093/acrefore/9780190236557.001.0001/acrefore-9780190236557-e-75 [Google Scholar]
- 7.Oliveira CR de, Pedron AC, Gurgel LG, Reppold CT, Fonseca RP. Executive functions and sustained attention:Comparison between age groups of 19–39 and 40–59 years old. Dement neuropsychol. 2012. Mar;6(1):29–34. doi: 10.1590/S1980-57642012DN06010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends in Cognitive Sciences. 2012. Jan;16(1):17–26. doi: 10.1016/j.tics.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubia K. Cognitive Neuroscience of Attention Deficit Hyperactivity Disorder (ADHD) and Its Clinical Translation. Front Hum Neurosci. 2018. Mar 29;12:100. doi: 10.3389/fnhum.2018.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through Alpha-2 adrenoceptor and Dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1(1):2. doi: 10.1186/1744-9081-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson H, Chang Z, D’Onofrio BM, Lichtenstein P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol Med. 2014. Jul;44(10):2223–9. doi: 10.1017/S0033291713002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luckey TD. Introduction to intestinal microecology. The American Journal of Clinical Nutrition. 1972. Dec 1;25(12):1292–4. doi: 10.1093/ajcn/25.12.1292 [DOI] [PubMed] [Google Scholar]
- 13.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016. Aug 19;14(8):e1002533. doi: 10.1371/journal.pbio.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manor O, Levy R, Borenstein E. Mapping the inner workings of the microbiome: Genomic- and metagenomic-based study of metabolism and metabolic interactions in the human microbiome. Cell Metabolism. 2014. Nov;20(5):742–52. doi: 10.1016/j.cmet.2014.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-gut-microbiome Axis. Cellular and Molecular Gastroenterology and Hepatology. 2018;6(2):133–48. doi: 10.1016/j.jcmgh.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romijn JA, Corssmit EP, Havekes LM, Pijl H. Gut-brain axis. Current Opinion in Clinical Nutrition and Metabolic Care. 2008. Jul;11(4):518–21. doi: 10.1097/MCO.0b013e328302c9b0 [DOI] [PubMed] [Google Scholar]
- 17.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009. May;6(5):306–14. doi: 10.1038/nrgastro.2009.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011. Sep 20;108(38):16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: How gut microbes shape human behavior. J Psychiatr Res. 2015. Apr;63:1–9. doi: 10.1016/j.jpsychires.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 20.Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. Neurobiology of Stress. 2016. Oct;4:23–33. doi: 10.1016/j.ynstr.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ming X, Chen N, Ray C, Brewer G, Kornitzer J, Steer RA. A gut feeling: A hypothesis of the role of the microbiome in Attention-Deficit/Hyperactivity Disorders. Child Neurology Open. 2018. Jan;5:2329048X1878679. doi: 10.1177/2329048X18786799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duel BP, Steinberg-Epstein R, Hill M, Lerner M. A survey of voiding dysfunction in children with Attention Deficit-Hyperactivity Disorder. Journal of Urology. 2003. Oct;170(4 Part 2):1521–4. doi: 10.1097/01.ju.0000091219.46560.7b [DOI] [PubMed] [Google Scholar]
- 23.McKeown C, Hisle-Gorman E, Eide M, Gorman GH, Nylund CM. Association of constipation and fecal incontinence with Attention-Deficit/Hyperactivity Disorder. PEDIATRICS. 2013. Nov 1;132(5):e1210–5. doi: 10.1542/peds.2013-1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LJ, Li SC, Li SW, Kuo HC, Lee SY, Huang LH, et al. Gut microbiota and plasma cytokine levels in patients with attention-deficit/hyperactivity disorder. Transl Psychiatry. 2022. Feb 23;12(1):76. doi: 10.1038/s41398-022-01844-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang LL, Stiernborg M, Skott E, Gillberg T, Landberg R, Giacobini M, et al. Lower plasma concentrations of short-chain fatty acids (SCFAs) in patients with ADHD. Journal of Psychiatric Research. 2022. Dec;156:36–43. doi: 10.1016/j.jpsychires.2022.09.042 [DOI] [PubMed] [Google Scholar]
- 26.Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol. 2020. Jan 31;11:25. doi: 10.3389/fendo.2020.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019. Aug;16(8):461–78. doi: 10.1038/s41575-019-0157-3 [DOI] [PubMed] [Google Scholar]
- 28.Galland L. The gut microbiome and the brain. Journal of Medicinal Food. 2014. Dec;17(12):1261–72. doi: 10.1089/jmf.2014.7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettersson G. The neural control of the serotonin content in mammalian enterochromaffin cells. Acta Physiol Scand Suppl. 1979;470:1–30. [PubMed] [Google Scholar]
- 30.Luczynski P, Whelan SO, O’Sullivan C, Clarke G, Shanahan F, Dinan TG, et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Gaspar P, editor. Eur J Neurosci. 2016. Nov;44(9):2654–66. doi: 10.1111/ejn.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013. Jun;18(6):666–73. doi: 10.1038/mp.2012.77 [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity. 2015. Aug;48:186–94. doi: 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 33.Roman P, Rueda-Ruzafa L, Cardona D, Cortes-Rodríguez A. Gut–brain axis in the executive function of autism spectrum disorder: Behavioural Pharmacology. 2018. Oct;29(7):654–63. [DOI] [PubMed] [Google Scholar]
- 34.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: Beyond working memory. Psychopharmacology. 2006. Oct 6;188(4):567–85. doi: 10.1007/s00213-006-0404-5 [DOI] [PubMed] [Google Scholar]
- 35.Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences. 2011. Feb 15;108(7):3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needham BD, Tang W, Wu WL. Searching for the gut microbial contributing factors to social behavior in rodent models of autism spectrum disorder: Gut microbiota and social behavior. Devel Neurobio. 2018. May;78(5):474–99. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Han Y, Dy ABC, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci. 2017. Apr 28;11:120. doi: 10.3389/fncel.2017.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell J, editor. Autism as an executive disorder. Oxford; New York: Oxford University Press; 1997. 313 p. [Google Scholar]
- 39.Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. Journal of Medical Microbiology. 2005. Oct 1;54(10):987–91. doi: 10.1099/jmm.0.46101-0 [DOI] [PubMed] [Google Scholar]
- 40.Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013. Sep;25(9):733–e575. doi: 10.1111/nmo.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain, Behavior, and Immunity. 2015. Aug;48:165–73. doi: 10.1016/j.bbi.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 42.Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016. Jun;21(6):797–805. doi: 10.1038/mp.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inserra A, Rogers GB, Licinio J, Wong ML. The microbiota-inflammasome hypothesis of major depression. BioEssays. 2018. Sep;40(9):1800027. doi: 10.1002/bies.201800027 [DOI] [PubMed] [Google Scholar]
- 44.Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. Journal of Affective Disorders. 2016. Sep;202:254–7. [DOI] [PubMed] [Google Scholar]
- 45.Chen J jun, Zheng P, Liu Y yun, Zhong X gang, Wang H yang, Guo Y jie, et al. Sex differences in gut microbiota in patients with major depressive disorder. NDT. 2018. Feb;Volume 14:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer C, Vassar M. The fragility of probiotic Bifidobacterium longum NCC3001 use for depression in patients with Irritable Bowel Syndrome. Gastroenterology. 2018. Feb;154(3):764. [DOI] [PubMed] [Google Scholar]
- 47.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011. Mar 14;105(5):755–64. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein S, Schwebach AJ. The comorbidity of Pervasive Developmental Disorder and Attention Deficit Hyperactivity Disorder: results of a retrospective chart review. J Autism Dev Disord. 2004. Jun;34(3):329–39. doi: 10.1023/b:jadd.0000029554.46570.68 [DOI] [PubMed] [Google Scholar]
- 49.Reiersen AM, Todd RD. Co-occurrence of ADHD and autism spectrum disorders: phenomenology and treatment. Expert Rev Neurother. 2008. Apr;8(4):657–69. doi: 10.1586/14737175.8.4.657 [DOI] [PubMed] [Google Scholar]
- 50.Gadow KD, DeVincent CJ, Pomeroy J. ADHD symptom subtypes in children with pervasive developmental disorder. J Autism Dev Disord. 2006. Feb;36(2):271–83. doi: 10.1007/s10803-005-0060-3 [DOI] [PubMed] [Google Scholar]
- 51.Mayes SD, Calhoun SL, Mayes RD, Molitoris S. Autism and ADHD: Overlapping and discriminating symptoms. Research in Autism Spectrum Disorders. 2012. Jan;6(1):277–85. [Google Scholar]
- 52.Doherty JL, Owen MJ. Genomic insights into the overlap between psychiatric disorders: Implications for research and clinical practice. Genome Med. 2014;6(4):29. doi: 10.1186/gm546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grimaldi R, Gibson GR, Vulevic J, Giallourou N, Castro-Mejía JL, Hansen LH, et al. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome. 2018. Dec;6(1):133. doi: 10.1186/s40168-018-0523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathee K, Cickovski T, Deoraj A, Stollstorff M, Narasimhan G. The gut microbiome and neuropsychiatric disorders: implications for attention deficit hyperactivity disorder (ADHD). Journal of Medical Microbiology. 2020. Jan 1;69(1):14–24. doi: 10.1099/jmm.0.001112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Checa-Ros A, Jeréz-Calero A, Molina-Carballo A, Campoy C, Muñoz-Hoyos A. Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients. 2021. Jan 16;13(1):249. doi: 10.3390/nu13010249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalenik A, Kardaś K, Rahnama A, Sirojć K, Wolańczyk T. Gut microbiota and probiotic therapy in ADHD: A review of current knowledge. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2021. Aug;110:110277. doi: 10.1016/j.pnpbp.2021.110277 [DOI] [PubMed] [Google Scholar]
- 57.Sukmajaya AC, Lusida MI, Soetjipto, Setiawati Y. Systematic review of gut microbiota and attention-deficit hyperactivity disorder (ADHD). Ann Gen Psychiatry. 2021. Dec;20(1):12. doi: 10.1186/s12991-021-00330-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shannon CE. A Mathematical Theory of Communication. Bell System Technical Journal. 1948. Jul;27(3):379–423. [Google Scholar]
- 59.Prehn-Kristensen A, Zimmermann A, Tittmann L, Lieb W, Schreiber S, Baving L, et al. Reduced microbiome alpha diversity in young patients with ADHD. Yao YG, editor. PLoS ONE. 2018. Jul 12;13(7):e0200728. doi: 10.1371/journal.pone.0200728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang LJ, Yang CY, Chou WJ, Lee MJ, Chou MC, Kuo HC, et al. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2020. Mar;29(3):287–97. doi: 10.1007/s00787-019-01352-2 [DOI] [PubMed] [Google Scholar]
- 61.Jiang HY, Zhou YY, Zhou GL, Li YC, Yuan J, Li XH, et al. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav Brain Res. 2018. 16;347:408–13. [DOI] [PubMed] [Google Scholar]
- 62.Casas L, Karvonen AM, Kirjavainen PV, Täubel M, Hyytiäinen H, Jayaprakash B, et al. Early life home microbiome and hyperactivity/inattention in school-age children. Sci Rep. 2019. Dec;9(1):17355. doi: 10.1038/s41598-019-53527-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szopinska-Tokov J, Dam S, Naaijen J, Konstanti P, Rommelse N, Belzer C, et al. Investigating the gut microbiota composition of individuals with Attention-Deficit/Hyperactivity Disorder and association with symptoms. Microorganisms. 2020. Mar 13;8(3). doi: 10.3390/microorganisms8030406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kruskal JB. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika. 1964. Mar;29(1):1–27. [Google Scholar]
- 65.Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr Res. 2015. Jun;77(6):823–8. doi: 10.1038/pr.2015.51 [DOI] [PubMed] [Google Scholar]
- 66.Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. Hashimoto K, editor. PLoS ONE. 2017. Sep 1;12(9):e0183509. doi: 10.1371/journal.pone.0183509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng S, Han B, Ding M, Wen Y, Ma M, Zhang L, et al. Identifying psychiatric disorder-associated gut microbiota using microbiota-related gene set enrichment analysis. Briefings in Bioinformatics. 2020. May 21;21(3):1016–22. doi: 10.1093/bib/bbz034 [DOI] [PubMed] [Google Scholar]
- 68.Wan L, Ge WR, Zhang S, Sun YL, Wang B, Yang G. Case-Control Study of the Effects of Gut Microbiota Composition on Neurotransmitter Metabolic Pathways in Children With Attention Deficit Hyperactivity Disorder. Front Neurosci. 2020. Feb 18;14:127. doi: 10.3389/fnins.2020.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uffelmann E, Huang QQ, Munung NS, de Vries J, Okada Y, Martin AR, et al. Genome-wide association studies. Nat Rev Methods Primers. 2021. Aug 26;1(1):59. [Google Scholar]
- 70.Stevens AJ, Purcell RV, Darling KA, Eggleston MJF, Kennedy MA, Rucklidge JJ. Human gut microbiome changes during a 10 week Randomised Control Trial for micronutrient supplementation in children with attention deficit hyperactivity disorder. Sci Rep. 2019. Dec;9(1):10128. doi: 10.1038/s41598-019-46146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation. New York, NY, US: Guilford Press; 1998. viii, 79 p. (ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation.). [Google Scholar]
- 72.Coyte KZ, Rao C, Rakoff-Nahoum S, Foster KR. Ecological rules for the assembly of microbiome communities. Gordo I, editor. PLoS Biol. 2021. Feb 19;19(2):e3001116. doi: 10.1371/journal.pbio.3001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. Journal of bacteriology. 1997;179(12):4043–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xavier KB, Bassler BL. Regulation of uptake and processing of the quorum-sensing autoinducer AI-2 in Escherichia coli. JB. 2005. Jan 1;187(1):238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engelmoer DJP, Behm JE, Toby Kiers E. Intense competition between arbuscular mycorrhizal mutualists in an in vitro root microbiome negatively affects total fungal abundance. Mol Ecol. 2014. Mar;23(6):1584–93. [DOI] [PubMed] [Google Scholar]
- 76.Lê Cao KA, Boitard S, Besse P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinformatics. 2011. Dec;12(1):253. doi: 10.1186/1471-2105-12-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014. Dec;15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB. ANOVA-Like Differential Expression (ALDEx) Analysis for Mixed Population RNA-Seq. Parkinson J, editor. PLoS ONE. 2013. Jul 2;8(7):e67019. doi: 10.1371/journal.pone.0067019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin H, Peddada SD. Analysis of compositions of microbiomes with bias correction. Nat Commun. 2020. Dec;11(1):3514. doi: 10.1038/s41467-020-17041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010. May;7(5):335–6. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tasnim N, Abulizi N, Pither J, Hart MM, Gibson DL. Linking the Gut Microbial Ecosystem with the Environment: Does Gut Health Depend on Where We Live? Front Microbiol. 2017. Oct 6;8:1935. doi: 10.3389/fmicb.2017.01935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fernandez M, Riveros JD, Campos M, Mathee K, Narasimhan G. Microbial “social networks.” BMC Genomics. 2015;16(Suppl 11):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, et al. Microbial co-occurrence relationships in the human microbiome. Ouzounis CA, editor. PLoS Comput Biol. 2012. Jul 12;8(7):e1002606. doi: 10.1371/journal.pcbi.1002606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frey BJ, Dueck D. Clustering by passing messages between data points. Science. 2007. Feb 16;315(5814):972–6. doi: 10.1126/science.1136800 [DOI] [PubMed] [Google Scholar]
- 86.Cickovski T, Peake E, Aguiar-Pulido V, Narasimhan G. ATria: A novel centrality algorithm applied to biological networks. BMC Bioinformatics. 2017. Jun;18(S8):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cickovski T, Narasimhan G. Constructing lightweight and flexible pipelines using Plugin-Based Microbiome Analysis (PluMA). Bioinformatics. 2018. Sep 1;34(17):2881–8. doi: 10.1093/bioinformatics/bty198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rösler M, Casas M, Konofal E, Buitelaar J. Attention deficit hyperactivity disorder in adults. The World Journal of Biological Psychiatry. 2010. Aug;11(5):684–98. doi: 10.3109/15622975.2010.483249 [DOI] [PubMed] [Google Scholar]
- 89.Goodrich JK, Di Rienzi SC, Poole AC, Koren O, Walters WA, Caporaso JG, et al. Conducting a microbiome study. Cell. 2014. Jul;158(2):250–62. doi: 10.1016/j.cell.2014.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013. Sep 1;79(17):5112–20. doi: 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.16S Metagenomic Sequencing Library Preparation: Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System [Internet]. 2021. [cited 2023 Apr 18]. Available from: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf [Google Scholar]
- 92.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011. Aug 15;27(16):2194–200. doi: 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mirzayi C, Renson A, Genomic Standards Consortium, Massive Analysis and Quality Control Society, Furlanello C, Sansone SA, et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat Med. 2021. Nov;27(11):1885–92. doi: 10.1038/s41591-021-01552-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cureton EE. Tetrachoric Correlation by the Camp Approximation. Educational and Psychological Measurement. 1968. Jul;28(2):239–44. [Google Scholar]
- 95.Kessler RC, Adler LA, Gruber MJ, Sarawate CA, Spencer T, Van Brunt DL. Validity of the World Health Organization Adult ADHD Self-Report Scale (ASRS) Screener in a representative sample of health plan members. Int J Methods Psychiatr Res. 2007. Jun;16(2):52–65. doi: 10.1002/mpr.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brevik EJ, Lundervold AJ, Haavik J, Posserud M. Validity and accuracy of the Adult Attention‐Deficit/Hyperactivity Disorder (ADHD) Self‐Report Scale (ASRS) and the Wender Utah Rating Scale (WURS) symptom checklists in discriminating between adults with and without ADHD. Brain Behav [Internet]. 2020. Jun [cited 2023 Apr 9];10(6). Available from: doi: 10.1002/brb3.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014. Aug 1;30(15):2114–20. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.John J. SeqPrep [Internet]. 2019. Available from: https://github.com/jstjohn/SeqPrep [Google Scholar]
- 99.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010. Oct 1;26(19):2460–1. doi: 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 100.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucl Acids Res. 2014. Jan;42(D1):D633–42. doi: 10.1093/nar/gkt1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology. 2006. Jul 1;72(7):5069–72. doi: 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chao A. Species Estimation and Applications. In: Kotz S, Read CB, Balakrishnan N, Vidakovic B, Johnson NL, editors. Encyclopedia of Statistical Sciences [Internet]. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2006. [cited 2021 Jan 6]. p. ess5051. Available from: http://doi.wiley.com/10.1002/0471667196.ess5051 [Google Scholar]
- 103.Bonferroni C. Teoria statistica delle classi e calcolo delle probabilità. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze 8: 3–62.—Open Access Library [Internet]. 2020. [cited 2020 Jan 24]. Google Scholar. 1936; [Google Scholar]
- 104.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011. Feb;5(2):169–72. doi: 10.1038/ismej.2010.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992. Jun 1;131(2):479–91. doi: 10.1093/genetics/131.2.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barker M, Rayens W. Partial least squares for discrimination. J Chemometrics. 2003. Mar 24;17(3):166–73. [Google Scholar]
- 107.Ruiz-Perez D, Guan H, Madhivanan P, Mathee K, Narasimhan G. So you think you can PLS-DA? In: 2018 IEEE 8th International Conference on Computational Advances in Bio and Medical Sciences (ICCABS) [Internet]. Las Vegas, NV: IEEE; 2018. [cited 2020 Apr 3]. p. 1–1. Available from: https://ieeexplore.ieee.org/document/8542038/ [Google Scholar]
- 108.Quinn TP, Erb I, Gloor G, Notredame C, Richardson MF, Crowley TM. A field guide for the compositional analysis of any-omics data. GigaScience. 2019. Sep 1;8(9):giz107. doi: 10.1093/gigascience/giz107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nearing JT, Douglas GM, Hayes MG, MacDonald J, Desai DK, Allward N, et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat Commun. 2022. Jan 17;13(1):342. doi: 10.1038/s41467-022-28034-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995. Jan;57(1):289–300. [Google Scholar]
- 111.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 112.Friedman J, Alm EJ. Inferring Correlation Networks from Genomic Survey Data. von Mering C, editor. PLoS Comput Biol. 2012. Sep 20;8(9):e1002687. doi: 10.1371/journal.pcbi.1002687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shannon P. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Research. 2003. Nov 1;13(11):2498–504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Softw: Pract Exper. 1991. Nov;21(11):1129–64. [Google Scholar]
- 115.Jackson MO, Wolinsky A. A strategic model of social and economic networks. Journal of Economic Theory. 1996. Oct;71(1):44–74. [Google Scholar]
- 116.Easley D, Kleinberg J. Networks, crowds, and markets: Reasoning about a highly connected world. New York: Cambridge University Press; 2010. 727 p. [Google Scholar]
- 117.Toro‐Valdivieso C, Toro F, Stubbs S, Castro‐Nallar E, Blacklaws B. Patterns of the fecal microbiota in the Juan Fernández fur seal (Arctocephalus philippii). MicrobiologyOpen [Internet]. 2021. Aug [cited 2023 Jul 25];10(4). Available from: https://onlinelibrary.wiley.com/doi/10.1002/mbo3.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salonen A, Salojärvi J, Lahti L, De Vos WM. The adult intestinal core microbiota is determined by analysis depth and health status. Clinical Microbiology and Infection. 2012. Jul;18:16–20. doi: 10.1111/j.1469-0691.2012.03855.x [DOI] [PubMed] [Google Scholar]
- 119.Lam TJ, Ye Y. Meta-analysis of microbiome association networks reveal patterns of dysbiosis in diseased microbiomes. Sci Rep. 2022. Oct 19;12(1):17482. doi: 10.1038/s41598-022-22541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weiss S, Van Treuren W, Lozupone C, Faust K, Friedman J, Deng Y, et al. Correlation detection strategies in microbial data sets vary widely in sensitivity and precision. ISME J. 2016. Jul;10(7):1669–81. doi: 10.1038/ismej.2015.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klecka WR. Discriminant analysis. Beverly Hills, Calif: Sage Publications; 1980. 71 p. (Quantitative applications in the social sciences). [Google Scholar]
- 122.Fisher RA. The use of multiple measurements in taxonomic problems. Annals of Eugenics. 1936. Sep;7(2):179–88. [Google Scholar]
- 123.Fung TC, Vuong HE, Luna CDG, Pronovost GN, Aleksandrova AA, Riley NG, et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol. 2019. Dec;4(12):2064–73. doi: 10.1038/s41564-019-0540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nikolas M, Friderici K, Waldman I, Jernigan K, Nigg JT. Gene × environment interactions for ADHD: Synergistic effect of 5HTTLPR genotype and youth appraisals of inter-parental conflict. Behav Brain Funct. 2010;6(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xue R, Zhang H, Pan J, Du Z, Zhou W, Zhang Z, et al. Peripheral dopamine controlled by gut microbes inhibits invariant natural killer T cell-mediated Hepatitis. Front Immunol. 2018. Oct 17;9:2398. doi: 10.3389/fimmu.2018.02398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aitchison J. The statistical analysis of compositional data. Caldwell, N.J: Blackburn Press; 2003. 1 p. [Google Scholar]
- 127.D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015. Dec 7;451(Pt A):97–102. doi: 10.1016/j.cca.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 128.Bukin YuS, Galachyants YuP, Morozov IV, Bukin SV, Zakharenko AS, Zemskaya TI. The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci Data. 2019. Mar;6(1):190007. doi: 10.1038/sdata.2019.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents—physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005. Sep;22(6):495–512. doi: 10.1111/j.1365-2036.2005.02615.x [DOI] [PubMed] [Google Scholar]
- 130.Russell DA, Ross RP, Fitzgerald GF, Stanton C. Metabolic activities and probiotic potential of bifidobacteria. International Journal of Food Microbiology. 2011. Sep;149(1):88–105. doi: 10.1016/j.ijfoodmicro.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 131.Sharma M, Wasan A, Sharma RK. Recent developments in probiotics: An emphasis on Bifidobacterium. Food Bioscience. 2021. Jun;41:100993. [Google Scholar]
- 132.Zavaglia AG, Kociubinski G, Pérez P, De Antoni G. Isolation and Characterization of Bifidobacterium Strains for Probiotic Formulation. Journal of Food Protection. 1998. Jul 1;61(7):865–73. doi: 10.4315/0362-028x-61.7.865 [DOI] [PubMed] [Google Scholar]
- 133.Invernici MM, Salvador SL, Silva PHF, Soares MSM, Casarin R, Palioto DB, et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J Clin Periodontol. 2018. Oct;45(10):1198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Usta-Gorgun B, Yilmaz-Ersan L. Short-chain fatty acids production by Bifidobacterium species in the presence of salep. Electronic Journal of Biotechnology. 2020. Sep;47:29–35. [Google Scholar]
- 135.Wexler AG, Goodman AL. An insider’s perspective: Bacteroides as a window into the microbiome. Nat Microbiol. 2017. May;2(5):17026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mason BL, Li Q, Minhajuddin A, Czysz AH, Coughlin LA, Hussain SK, et al. Reduced anti-inflammatory gut microbiota are associated with depression and anhedonia. Journal of Affective Disorders. 2020. Apr;266:394–401. doi: 10.1016/j.jad.2020.01.137 [DOI] [PubMed] [Google Scholar]
- 137.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Craig F, Lamanna AL, Margari F, Matera E, Simone M, Margari L. Overlap between Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder: Searching for distinctive/common clinical features: Overlap between ASD and ADHD. Autism Research. 2015. Jun;8(3):328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Telesford K, Ochoa-Repáraz J, Kasper LH. Gut commensalism, cytokines, and central nervous system demyelination. Journal of Interferon & Cytokine Research. 2014. Aug;34(8):605–14. doi: 10.1089/jir.2013.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sahney S, Benton MJ, Ferry PA. Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land. Biol Lett. 2010. Aug 23;6(4):544–7. doi: 10.1098/rsbl.2009.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jafari M, Ansari-Pour N. Why, When and How to Adjust Your P Values? Cell J [Internet]. 2018. Aug [cited 2023 Apr 12];20(4). Available from: doi: 10.22074/cellj.2019.5992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Di Leo G, Sardanelli F. Statistical significance: p value, 0.05 threshold, and applications to radiomics—reasons for a conservative approach. Eur Radiol Exp. 2020. Dec;4(1):18. doi: 10.1186/s41747-020-0145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neumann NM, Plastino A, Pinto Junior JA, Freitas AA. Is p -value 0.05 enough? A study on the statistical evaluation of classifiers. The Knowledge Engineering Review. 2020;36:e1. [Google Scholar]
- 144.Dancey CP, Reidy J. Statistics without maths for psychology. Pearson Education; 2007. [Google Scholar]
- 145.Martinson JNV, Pinkham NV, Peters GW, Cho H, Heng J, Rauch M, et al. Rethinking gut microbiome residency and the Enterobacteriaceae in healthy human adults. ISME J. 2019. Sep;13(9):2306–18. doi: 10.1038/s41396-019-0435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, et al. Enterobacteriaceae Act in Concert with the Gut Microbiota to Induce Spontaneous and Maternally Transmitted Colitis. Cell Host & Microbe. 2010. Sep;8(3):292–300. doi: 10.1016/j.chom.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu J, Yan Q, Luo F, Shang D, Wu D, Zhang H, et al. Acute cholecystitis associated with infection of Enterobacteriaceae from gut microbiota. Clinical Microbiology and Infection. 2015. Sep;21(9):851.e1–851.e9. doi: 10.1016/j.cmi.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 148.Stecher B, Denzler R, Maier L, Bernet F, Sanders MJ, Pickard DJ, et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proceedings of the National Academy of Sciences. 2012. Jan 24;109(4):1269–74. doi: 10.1073/pnas.1113246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gophna U, Konikoff T, Nielsen HB. Oscillospira and related bacteria—From metagenomic species to metabolic features: Metabolism of Oscillospira. Environ Microbiol. 2017. Mar;19(3):835–41. [DOI] [PubMed] [Google Scholar]
- 150.Konikoff T, Gophna U. Oscillospira: a Central, Enigmatic Component of the Human Gut Microbiota. Trends in Microbiology. 2016. Jul;24(7):523–4. doi: 10.1016/j.tim.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 151.Ozato N, Saito S, Yamaguchi T, Katashima M, Tokuda I, Sawada K, et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. npj Biofilms Microbiomes. 2019. Dec;5(1):28. doi: 10.1038/s41522-019-0101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cortese S. The association between ADHD and obesity: Intriguing, progressively more investigated, but still puzzling. Brain Sciences. 2019. Sep 27;9(10):256. doi: 10.3390/brainsci9100256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proceedings of the National Academy of Sciences. 2008. Oct 28;105(43):16731–6. doi: 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He X, Zhao S, Li Y. Faecalibacterium prausnitzii: A Next-Generation Probiotic in Gut Disease Improvement. Chen T, editor. Canadian Journal of Infectious Diseases and Medical Microbiology. 2021. Mar 5;2021:1–10. [Google Scholar]
- 155.Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci USA. 2019. Jun 25;116(26):12672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aguiar-Pulido V, Huang W, Suarez-Ulloa V, Cickovski T, Mathee K, Narasimhan G. Metagenomics, metatranscriptomics, and metabolomics approaches for microbiome analysis: Supplementary issue: Bioinformatics methods and applications for big metagenomics data. Evolutionary Bioinformatics. 2016. Jan;12s1:EBO.S36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ponomarova O, Patil KR. Metabolic interactions in microbial communities: Untangling the Gordian knot. Current Opinion in Microbiology. 2015. Oct;27:37–44. doi: 10.1016/j.mib.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 158.Aoun A, Darwish F, Hamod N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. pnf. 2020. Jun 30;25(2):113–23. doi: 10.3746/pnf.2020.25.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.The Milieu Intérieur Consortium, Scepanovic P, Hodel F, Mondot S, Partula V, Byrd A, et al. A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome. 2019. Dec;7(1):130. doi: 10.1186/s40168-019-0747-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gaulke CA, Sharpton TJ. The influence of ethnicity and geography on human gut microbiome composition. Nat Med. 2018. Oct;24(10):1495–6. doi: 10.1038/s41591-018-0210-8 [DOI] [PubMed] [Google Scholar]
- 161.Iizumi T, Battaglia T, Ruiz V, Perez Perez GI. Gut Microbiome and Antibiotics. Archives of Medical Research. 2017. Nov;48(8):727–34. doi: 10.1016/j.arcmed.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 162.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014. Jan;505(7484):559–63. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Verlaet AAJ, Noriega DB, Hermans N, Savelkoul HFJ. Nutrition, immunological mechanisms and dietary immunomodulation in ADHD. Eur Child Adolesc Psychiatry. 2014. Jul;23(7):519–29. doi: 10.1007/s00787-014-0522-2 [DOI] [PubMed] [Google Scholar]
- 164.Pallikkuth S, Mendez R, Russell K, Sirupangi T, Kvistad D, Pahwa R, et al. Age Associated Microbiome and Microbial Metabolites Modulation and Its Association With Systemic Inflammation in a Rhesus Macaque Model. Front Immunol. 2021. Oct 19;12:748397. doi: 10.3389/fimmu.2021.748397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pearl J. Causality: models, reasoning, and inference. Cambridge, U.K.; New York: Cambridge University Press; 2000. 384 p. [Google Scholar]
- 166.Sazal MR, Ruiz-Perez D, Cickovski T, Narasimhan G. Inferring relationships in microbiomes from Signed Bayesian Networks. In: 2018 IEEE 8th International Conference on Computational Advances in Bio and Medical Sciences (ICCABS) [Internet]. Las Vegas, NV: IEEE; 2018. [cited 2020 Mar 27]. p. 1–1. Available from: https://ieeexplore.ieee.org/document/8542086/ [Google Scholar]
- 167.Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, et al. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host & Microbe. 2012. Nov;12(5):611–22. doi: 10.1016/j.chom.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 168.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-Induced Dysbiosis of the Intestinal Microbiota and the Effects on Immunity and Disease. Nutrients. 2012. Aug 21;4(8):1095–119. doi: 10.3390/nu4081095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cho I, Blaser MJ. The human microbiome: At the interface of health and disease. Nat Rev Genet. 2012. Apr;13(4):260–70. doi: 10.1038/nrg3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McGeachie MJ, Sordillo JE, Gibson T, Weinstock GM, Liu YY, Gold DR, et al. Longitudinal prediction of the infant gut microbiome with dynamic Bayesian networks. Sci Rep. 2016. Apr;6(1):20359. doi: 10.1038/srep20359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Newman MEJ, Girvan M. Finding and evaluating community structure in networks. Phys Rev E. 2004. Feb 26;69(2):026113. doi: 10.1103/PhysRevE.69.026113 [DOI] [PubMed] [Google Scholar]
- 172.Bozzo E, Franceschet M, Rinaldi F. Vulnerability and power on networks. Net Sci. 2015. Jun;3(2):196–226. [Google Scholar]
- 173.Callahan BJ, McMurdie PJ, Holmes SP. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017. Dec;11(12):2639–43. doi: 10.1038/ismej.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Heintz-Buschart A, May P, Laczny CC, Lebrun LA, Bellora C, Krishna A, et al. Integrated multi-omics of the human gut microbiome in a case study of familial type 1 diabetes. Nat Microbiol. 2017. Jan;2(1):16180. [DOI] [PubMed] [Google Scholar]
- 175.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome. 2017. Dec;5(1):10. doi: 10.1186/s40168-016-0225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Adams J, Krajmalnik-Brown R, Kang D, Sadowski M, Khoruts A, Borody T. Method for treating Autism Spectrum Disorder and associated symptoms. 20190134144, 2019. [Google Scholar]
- 177.Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HSA, Saad K, et al. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutritional Neuroscience. 2018. Oct 21;21(9):676–81. doi: 10.1080/1028415X.2017.1347746 [DOI] [PubMed] [Google Scholar]
- 178.Flint HJ. Variability and Stability of the Human Gut Microbiome. In: Why Gut Microbes Matter [Internet]. Cham: Springer International Publishing; 2020. [cited 2021 Jun 13]. p. 63–79. (Fascinating Life Sciences). Available from: http://link.springer.com/10.1007/978-3-030-43246-1_6 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bar plot of taxa relative abundance, at the class level. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Bar plot of taxa relative abundance, at the order level. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Bar plot of taxa relative abundance, at the family level. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Bar plot of taxa relative abundance, using the lowest possible classification level. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Relative abundance plot of Bifidobacterium. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Relative abundance plot of Bacteroides. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Relative abundance plot of Sutterella. Samples are ordered on the x-axis by increasing ASRS score.
(PNG)
Data obtained from the openly available BioProject (PRJNA656791), along with cited protocols [90–92].
(DOCX)
Average number of sequences retained after every preprocessing step.
(DOCX)
Every correlation in all MCNs, grouped by taxonomy. Orange = only found in Control, purple = only found in ADHD, grey = found in both. +(green) = positive correlation, -(red) = negative correlation.
(DOCX)
ATria rankings of all taxa over all MCNs, grouped by taxonomy. Dark orange = only ranked in Control, dark purple = only ranked in ADHD, light orange = higher ranked in Control, light purple = higher ranked in ADHD, grey = evenly ranked. Bold taxa are ranked #1.
(DOCX)
Communities within Control and ADHD family-level MCNs. Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown = Actinobacteria, blue = Proteobacteria.
(DOCX)
Communities within Control and ADHD genus-level MCNs. Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown = Actinobacteria, blue = Proteobacteria.
(DOCX)
Communities within Control and ADHD MCNs using the lowest possible level of taxonomic classification. Phylum: purple = Bacteroidetes, yellow = Firmicutes, brown-Actinobacteria, blue = Proteobacteria.
(DOCX)
Data Availability Statement
The dataset is publicly available in the NCBI database (Accession Number: PRJNA656791). Our entire downstream analysis pipeline has been built using Plugin-Based Microbiome Analysis (PluMA, (76)) and is available for download within its publicly available pipeline pool (http://biorg.cs.fiu.edu/pluma/pipelines.html).