Abstract
The double stranded RNA binding protein Adad1 (adenosine deaminase domain containing 1) is a member of the adenosine deaminase acting on RNAs (Adar) protein family with germ cell-specific expression. In mice, Adad1 is necessary for sperm differentiation, however its function outside of mammals has not been investigated. Here, through an N-ethyl-N-nitrosourea (ENU) based forward genetic screen, we identified an adad1 mutant zebrafish line that develops as sterile males. Further histological examination revealed complete lack of germ cells in adult mutant fish, however germ cells populated the gonad, proliferated, and entered meiosis in larval and juvenile fish. Although meiosis was initiated in adad1 mutant testes, the spermatocytes failed to progress beyond the zygotene stage. Thus, Adad1 is essential for meiosis and germline maintenance in zebrafish. We tested if spermatogonial stem cells were affected using nanos2 RNA FISH and a label retaining cell (LRC) assay, and found that the mutant testes had fewer LRCs and nanos2-expressing cells compared to wild-type siblings, suggesting that failure to maintain the spermatogonial stem cells resulted in germ cell loss by adulthood. To identify potential molecular processes regulated by Adad1, we sequenced bulk mRNA from mutants and wild-type testes and found mis-regulation of genes involved in RNA stability and modification, pointing to a potential broader role in post-transcriptional regulation. Our findings suggest that the RNA regulatory protein Adad1 is required for fertility through regulation of spermatogonial stem cell maintenance in zebrafish.
Author summary
Infertility is a serious problem for millions of couples who wish to have children. Globally more than 10% of couples suffer from infertility due to genetic, epigenetic, and environmental factors. Among these about 50% of cases occur due to genetic factors such as aneuploidy and genetic mutations affecting development of the gametes (i.e. sperm and eggs). Although many genes are known to be involved in germ cell development, genetic causes of infertility are still largely unexplained. Therefore, it is imperative to investigate genes involved in reproductive processes. In this study, we report that the adad1 gene is essential for germ cell maintenance and fertility in zebrafish. Our analysis of zebrafish adad1 mutants demonstrates that it is required for maintenance of the germline stem cells and for completion of meiosis. This is in contrast to mouse Adad1, which functions later in gamete development to regulate differentiation of haploid sperm. Our work on zebrafish adad1 has uncovered previously unknown roles of adad1 function in germline stem cell maintenance.
Introduction
Reproduction in multicellular organisms generally depends on the differentiation of gametes from germline stem cells. In many organisms, fertility is maintained throughout life through continuous replenishment of the germ cells. The testis of most animals, including zebrafish and humans, contain spermatogonial stem cells (SSCs) which enable them to maintain continuous sperm production. SSCs are undifferentiated male germ cells which have the ability to self-renew in order to maintain a stem cell population, as well as to produce progenitor cells that differentiate into sperm [1,2]. How the SSC population is established and maintained throughout life is not completely understood and is critical to understanding infertility.
The process of spermatogenesis is similar among vertebrates, except that where anamniote vertebrates (fishes and amphibians) have a cystic mode of spermatogenesis, amniotes (reptiles, birds, and mammals) have non-cystic type spermatogenesis [3]. In the zebrafish, which have cystic spermatogenesis, each cyst is derived from a single spermatogonial cell that is associated with a Sertoli cell [4]. Both cell types divide to produce a cyst of synchronously differentiating germ cells surrounded by Sertoli cells. Generally, in fishes, single spermatogonial cells (Asingle) have been assumed to be the SSCs. Although it is likely that Asingle are SSCs, it is possible that additional spermatogonial type A cells, also have stem cell capacity, as has been shown in mice [1,5,6]. In mouse testes spermatogonia type Asingle, Apaired, and Aaligned can all act as SSCs–Apaired and Aaligned after fragmentation into Asingle [7]. In support of this, in zebrafish, the germ line stem cell marker, nanos2, is expressed in single spermatogonia and spermatogonia in cysts of up to four cells suggesting that Asingle−A4 are the SSCs in zebrafish [8,9].
Zebrafish spermatogonia cells enter meiosis after nine rounds of mitotic divisions [4]. Meiotic prophase I can be sub-divided into leptotene, zygotene, pachytene, and diplotene stage [10]. In the leptotene stage, chromosomes start to condense and synaptonemal complex protein Sycp3 starts to associate with chromosome ends near the telomeres. During the late leptotene to early zygotene stage, all telomeres are associated with the nuclear envelope and cluster to one side of the nuclear envelope to form a zygotene bouquet. It has been hypothesized that meiotic bouquet formation facilitates pairing of homologous chromosomes. In zygonema, Sycp1 begins to associate with chromosomes near the telomeres, following the extension of Sycp3 along chromosomes as the zygotene stage progresses. In the pachytene stage, all homologs synapse together and begin to exchange their DNA via recombination. This stage is characterized by end-to-end association of Sycp1 and Sycp3 on the chromosomes, marking a fully formed synaptonemal complex. Homologs start separating from each other in the diplotene stage followed by the homologue segregation and cell division. Completion of meiosis II and spermiogenesis gives rise to haploid sperm. Disruption of meiosis or spermiogenic phases can cause failed or abnormal gamete formation and infertility.
Post-transcriptional RNA regulation is critical for many aspects of spermatogenesis. RNA binding proteins (RBPs) form ribonucleoprotein complexes which are important for post-transcriptional regulation of RNAs such as translational control, RNA stability, RNA biogenesis, and modifications of RNAs [11]. During spermatogenesis, RBPs play critical roles in all stages of spermatogenesis. For example, RBPs function to protect genome integrity through destabilization of transposon RNAs, for translational regulation of mRNAs encoding proteins that function during transcriptionally silent stages (i.e. during spermiogenesis), and to regulate RNA stability during transition from one spermatogenic stage to the next [12]. The adult human testis has the highest number of tissue-specific RBPs suggesting a particularly important role of post-transcriptional RNA regulation as a means to control processes specific to the male germ line [13]. As such, understanding the function of germline-specific RBPs is critical to uncovering developmental mechanisms that govern spermatogenesis.
Here, through an ENU mutagenesis screen we identified a zebrafish mutant line (t30103) that failed to maintain germ cells during adulthood. These mutants had germ cells in larval and juvenile stages but rapidly lost the germ cells at adulthood. Through positional cloning and whole exome sequencing, we identified a missense mutation in the adenosine deaminase domain containing 1 (adad1) gene that was responsible for the germ cell loss in this mutant line. The adad1 gene encodes a germ cell-specific dsRNA binding protein and is conserved in vertebrates. The Adad1 protein has a deaminase domain similar to Adar (adenosine deaminase acting on RNA) RNA editases, however key catalytic residues are missing in Adad1 and RNA editing activity has not been detected suggesting that Adad1 regulates RNAs through a different mechanism [14,15]. We found that zebrafish adad1 mutants had defects in SSC maintenance, as well as in meiosis indicating that adad1 functions in multiple stages of spermatogenesis. Furthermore, transcriptomics analysis showed that RNA processing and modification pathways were disrupted in the absence of Adad1 function. Our work identified Adad1 as a putative RNA regulatory protein necessary for germline maintenance and fertility in zebrafish.
Results
adad1 is necessary for germline maintenance in zebrafish
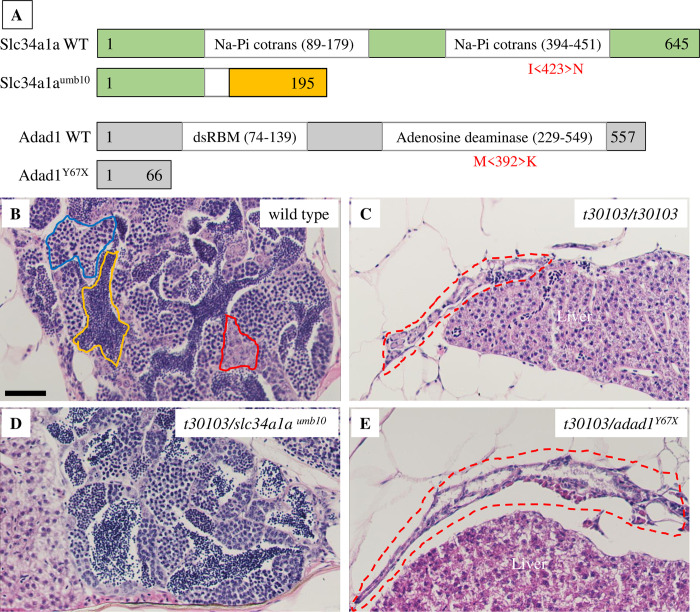
To identify unknown regulators of germline development, we conducted an ENU based mutagenesis screen and identified a zebrafish mutant line (named t30103) which developed only as males and failed to maintain germ cells during adulthood (Fig 1). To identify candidate mutations that cause this phenotype, we performed whole exome sequencing and genetic mapping. Analysis of the exome sequencing data identified two candidate mutations that were genetically linked to the t30103 phenotype. These two mutations were located on chromosome 14 of the zebrafish genome, approximately 1 Mbp apart from each other. One mutation was found in the slc34a1a (solute carrier family 34 member 1) gene, which would result in a change of amino acid 423 from Isoleucine to Asparagine (slc34a1aI423N). The other mutation affected the adad1 (adenosine deaminase domain containing 1) gene and resulted in amino acid 392 to change from Methionine to Lysine (adad1M392K) (Figs 1A and S1). Expression of both slc34a1a and adad1 was detected in zebrafish testes by RT-PCR, therefore both candidate mutations could potentially result in testes defects (Figs 2 and S2).
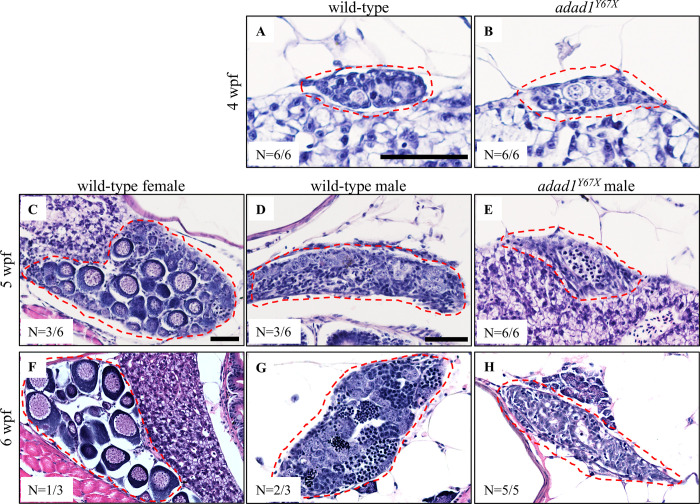
Fig 1. Mutations disrupting the adad1 gene cause germ cell loss in adult zebrafish.
A: Graphical representation of Slc34a1a and Adad1 wild-type and mutant proteins. Missense mutations are shown in red below wild-type protein schematics in the approximate position they occur in the protein. Representative images of truncated proteins resulting from nonsense mutations are shown. The frameshifted region of slc34a1aumb10 is colored orange. Numbers denote the number of amino acids in each protein. Abbreviations: Na-Pi cotrans (sodium dependent phosphate co-transporter domain), dsRBM (double-stranded RNA binding motif), WT (wild type). B-E: Hematoxylin-eosin staining of testes from adult wild type (B), t30103 homozygous mutant (C), slc34a1aumb10 trans-heterozygous to t30103 (D), and adad1Y67X trans-heterozygous to t30103 (E). In the wild-type testis spermatogonia, spermatocytes, and mature sperm are marked by red, blue, and yellow outlines, respectively. The t30103 homozygous and adad1Y67X/t30103 trans-heterozygous testes are small and lack germ cells (red dotted outlines in C and E). Conversely, there is no visible difference between wild-type and slc34a1aumb10/t30103 trans-heterozygous testes. Scale bar: 50 μm.
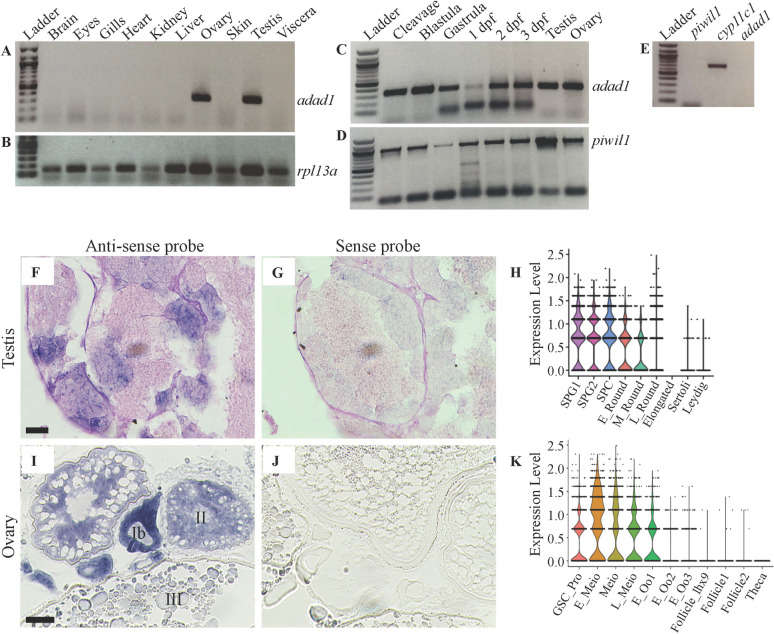
Fig 2. Adad1 mRNA is expressed in both sexes and is germline specific.
A-D: RT-PCR assaying adad1 expression in wild-type tissues. RT-PCR on adult tissues demonstrates that adad1 is expressed only in ovary and testis (A), whereas the control gene rpl13a is expressed in all organs tested (B). During embryonic and larval development adad1 is expressed in all stages tested (C), similar to the germ cell marker piwil1 (D). E: RT-PCR on testes lacking germ cells revealed that adad1 is not expressed in somatic cells of the testis. Piwil1 expression was not detected confirming that germ cells are not present while the Leydig cell marker gene cyp11c1 confirmed the presence of testes tissue. F-K: In situ hybridization (ISH) and single cell RNA sequencing data showing adad1 is expressed in germ cells of adult ovaries and testes. (F-H) In testes adad1 expression was highest in spermatogonia, spermatocytes, and less expressed in early spermatids. Testes were counterstained with periodic acid-Schiff. (I-K) In ovaries adad1 expression was found in oogonia through early follicle stages. Scale bars: 20 μm for testes and 50 μm for ovaries. Abbreviations: SPG1 = spermatogonia cluster 1; SPG2 = spermatogonia cluster 2; SPC = spermatocytes; E_Round = early round spermatids; M_Round = middle round spermatids; L_Round = late round spermatids; Elongated = elongated spermatids; GSC_Pro = germline stem cell and progenitor cells; E_Meio = early meiosis, Meio = Meiosis, L_Meiosis = Late meiosis; E_Oo1 = early follicle stage oocyte cluster 1; E_Oo2 = early follicle stage oocyte cluster 2; E_Oo3 = early follicle stage oocyte cluster 3; Follicle_Lhx9 = Lx9-expressing follicle cells; Follicle1 = follicle cells cluster 1; Follicle2 = Follicle cells cluster 2; Theca = Theca cells.
To identify the causative mutation of the t30103 line, we performed phenotypic and complementation analysis with independently derived mutations affecting each candidate gene (Fig 1A). Using CRISPR/Cas9 genome editing targeting slc34a1a, we generated a 17 bp deletion that caused a frameshift and premature stop codon after amino acid 195 (slc34a1aumb10), which would disrupt the transmembrane domains of the encoded channel protein (Figs 1A and S2). We obtained the adad1sa14397 mutant line from the Zebrafish International Resource Center. This line carries an ENU-induced nonsense mutation at codon 67 and will be referred to as adad1Y67X henceforth (Figs 1A and S1). We performed genetic complementation analysis to test which t30103 candidate mutation caused the germ cell defects. The slc34a1aumb10 homozygous fish and t30103/slc34a1aumb10 trans-heterozygotes were adult viable, developed as both males and females, and the adult males had normal testes histology (Figs 1D and S2). By contrast, t30103/adad1Y67X trans-heterozygous fish were all male and displayed a tiny testis without any germ cells at adulthood, similar to t30103 homozygotes (Fig 1C and 1E). These data confirm that the adad1 mutation causes the testis defect in the t30103 mutant line. We will refer to the adad1t30103 allele as adad1M392K and adad1sa14397 as adad1Y67X based on their effects on the encoded protein to more easily distinguish the two alleles.
Zebrafish adad1 is expressed in both sexes and is germline specific
To test which organs express the adad1 gene, we performed RT-PCR using cDNA from brains, eyes, gills, hearts, kidneys, livers, ovaries, skin, testes, and viscera. The RT-PCR results showed that adad1 is gonad specific, as no other organs besides testes and ovaries expressed this gene (Fig 2A and 2B). We also asked whether adad1 is germ cell specific in testes by performing RT-PCR on cDNA generated from testes devoid of germ cells. Adad1 was not detected in germ cell depleted testes whereas the Leydig cell-expressed gene cyp11c1 was detected (Fig 2E). This result suggests that adad1 is germ cell specific in male zebrafish. We could not perform a similar experiment with germ cell-depleted ovaries because zebrafish can only develop as males when lacking germ cells [16,17]. To ask whether adad1 is expressed during embryonic or early larval stages, we performed RT-PCR using cDNA from whole embryos and larvae of the cleavage, blastula, gastrula, 1 day post fertilization (dpf), 2 dpf, and 3 dpf. Adad1 expression was detected in all stages tested (Fig 2C and 2D). Next, to see which type of germ cells expressed adad1 mRNA, we performed in situ hybridization (ISH) on testis and ovary sections of adult zebrafish and analyzed adad1 expression in published single cell RNA sequencing (scRNAseq) data from adult testes and 40 dpf ovaries [18,19]. In testes, adad1 was expressed in spermatogonia, spermatocytes, and early spermatids, but not in the mature sperm (Fig 2F–2H). The adult ovary ISH showed high adad1 expression in stage Ib and stage II oocytes, and faint expression in later stage oocytes, with no apparent expression in somatic cells (Fig 2I and 2J). In the 40 dpf ovary scRNAseq data, adad1 expression was detected in stem cells through early oogenesis (Fig 2K). The ovary scRNAseq includes germ cells up to early 1b oocytes only [18], so adad1 expression in later stage oocytes could not be verified. Overall, these data show that zebrafish adad1 is expressed in germ cells of both sexes.
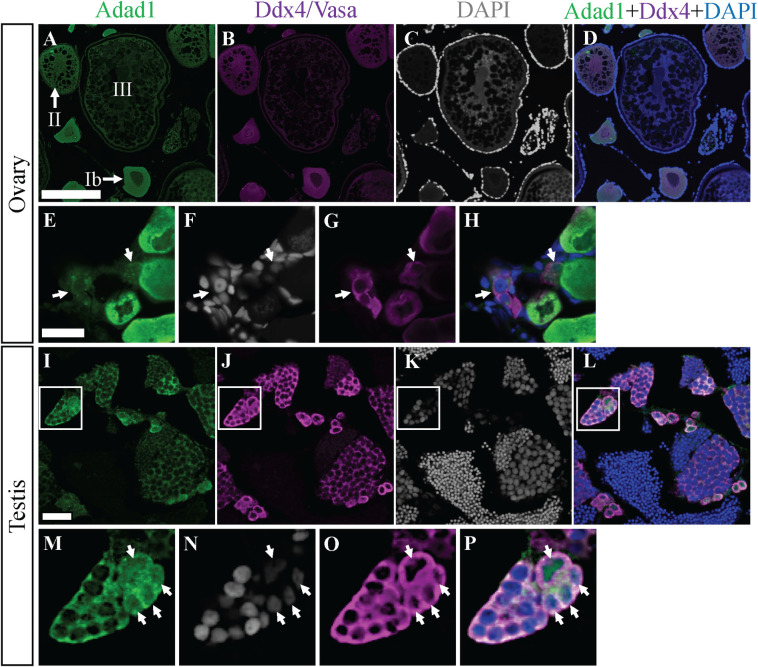
To enhance our understanding of Adad1, we made a polyclonal antibody against zebrafish Adad1 protein. We tested specificity of the antibody through western blot and immunofluorescence (IF) using mutant and wild-type testes (S3 Fig). In both experiments, mutant testes were collected from juvenile fish prior to the time at which germ cells are absent, as evident from detection of the germ cell-specific protein Vasa/Ddx4 (DEAD-box RNA helicase protein 4) (S3 Fig). By western blot, we detected a predicted 62 kDa sized protein in wild-type testes extracts, whereas no bands were detected in testes extracts from the adad1Y67X mutants (S3I Fig). Similarly, no protein was detected by IF on adad1Y67X mutant testis while protein was visible in wild-type and adad1M392K testes (Figs 3I–3L and S3A–S3H), indicating that the antibody is specific for Adad1 and that the Adad1M392K mutant protein is expressed at detectable levels. Analysis of IF performed on wild-type adult testis and ovary sections showed germ cell-specific expression of Adad1 protein in both sexes (Fig 3). The IF on ovary sections demonstrated that Adad1 is expressed moderately in pre-follicle-stages, highly expressed in stage Ib and stage II, and has little to no expression in later stage oocytes (Fig 3A–3H). In the testis, Adad1 was expressed highly in spermatogonia and moderately in the spermatocytes but undetectable in the sperm (Fig 3I–3P). Interestingly, we observed both nuclear and cytoplasmic localization of Adad1 in early spermatogonia (1 cell and cyst of up to 4 cells) and in oogonia/oocytes in nests or cysts (Fig 3E–3H and 3M–3P, arrows), whereas later stage germ cells showed primarily cytoplasmic localization. Because nanos2 is expressed in single SSCs and spermatogonia in cysts of up to four cells, we attempted to test if nanos2-expressing cells had nuclear Adad1 protein. However, Adad1 nuclear localization was less clear when samples were processed for nanos2 fluorescent in situ hybridization (FISH), likely due to the different fixatives used, which made it difficult to assess the overlap between nanos2 expression and Adad2 sub-cellular localization. We next asked if the Adad1M392K mutation affected protein localization. IF detection of the Adad1M392K mutant protein on testes sections showed that the nuclear localization in early stage spermatogonia cells was not disrupted by this mutation (S3E–S3H Fig, arrows). The observed differences in Adad1 subcellular localization suggests that Adad1 may play a unique role in early stage spermatogonia and oogonia, which includes the putative stem cells, compared to later stage germ cells. A more in-depth investigation of the cell population with Adad1 nuclear localization will require refinement of the nanos2 FISH combined with Adad1 IF to more precisely determine the relationship between nanos2-expressing SSCs and cells with Adad1 nuclear localization.
Fig 3. Adad1 protein expression in adult ovaries and testes.
A-H: In the ovary, Adad1 is expressed in the cytoplasm of prefollicle stages through stage II oocytes (A and E) similar to the Ddx4/Vasa protein (B and G). Similar levels of Adad1 were seen in nuclei and cytoplasm of pre-follicle stage germ cells (E-H, arrows). Images in panels A-D and E-H are from different samples I-L: In the testis, Adad1is expressed in the cytoplasm of spermatogonia and spermatocytes but not in mature sperm (I), overlapping with Ddx4/Vasa expression (J). Magnification of the insets shows that Adad1 is both nuclear and cytoplasmic in single spermatogonia and spermatogonia in cysts of 2–4 cells (M-P, arrows). Scale bars: 200 μm for A-D and I-L, 20 μm for E-H.
Adad1 is essential for spermatogenesis and oogenesis in zebrafish
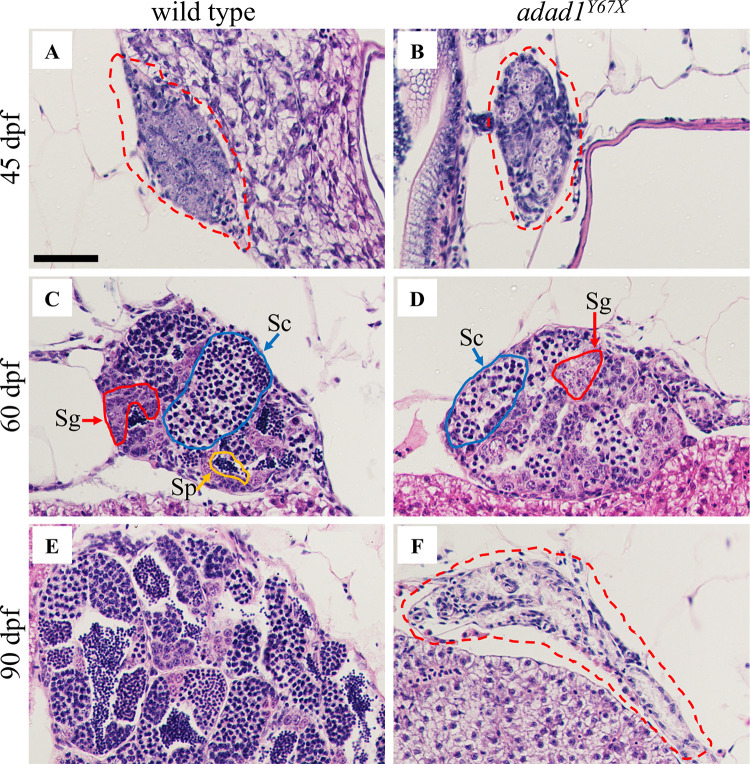
To identify the developmental stage at which adad1 mutants lost their germ cells, we did histology on gonads from juvenile (45 and 60 dpf) and adult (90 dpf) mutant and wild-type fish (Fig 4). Histological analysis showed that adad1 mutants had germ cells at 45 dpf similar to the wild types (Fig 4A and 4B). At 60 dpf, both mutant and wild-type testes had abundant germ cells, however wild-type fish had some mature sperm, but mutant testes lacked sperm (Fig 4C and 4D). To rule out the possibility of developmental differences in adad1 mutants due to growth delays, we measured the standard length of the fish prior to fixation and found them to be comparable between mutants and wild types. These results suggest that adad1 is dispensable for embryonic and early larval germ cell development and for the initiation of spermatogenesis, but is required to produce mature sperm. The histology of testes from 90 dpf mutant fish were completely devoid of germ cells in contrast to wild-type testes which had a robust germ cell population undergoing spermatogenesis (Fig 4E and 4F). These results indicate that adad1 mutants lost their germ cells during the juvenile to adult stage transition.
Fig 4. Histology of wild-type and adad1Y67X mutant gonads at 45, 60, and 90 dpf.
A-B: At 45 dpf, germ cells are present in both the wild-type (N = 8) (A) and mutant (N = 8) (B) testes. C-D: At 60 dpf, spermatogonia (Sg, red outline) and spermatocytes (Sc, blue outline) are present in both wild-type (N = 10) (C) and mutant (D), but the mutant lacks mature sperm (N = 10) (Sp, yellow outline). E-F: At 90 dpf, the wild-type testis is larger with abundant sperm (N = 6) (E) whereas the mutant testis is small and devoid of germ cells (N = 6) (F). Dotted red lines outline the gonads in A, B and F. Scale bar: 50 μm.
We also tested whether adad1 mutant males were capable of inducing spawning in females. Breeding experiments showed that adad1 mutant males (N = 3) successfully induced wild-type females to release eggs even though mutant males were unable to fertilize them (Table 1). These data suggest that adad1 mutants have normal male mating behavior.
Table 1. Fertility test of adad1Y67X mutant fish.
| Genotype | Number of crosses | Numbers of egg per cross (Mean ± SD) | Percentage of fertilized eggs (Mean± SD) |
|---|---|---|---|
| adad1 (+/-) | 3 | 137.5 ±56.71 | 91.28 ±8.52 |
| adad1 (-/-) | 3 | 149.67 ±34.08 | 0.0 ± 0.0 |
Since all adad1 mutants developed as males, we wondered whether or not mutants could initiate female development. Zebrafish manifest their gonadal sex at around 30 dpf, although this can vary within different zebrafish facilities. Before this age, the zebrafish gonad remains undifferentiated and passes through a juvenile ovary-stage, where immature oocytes are present, before finally differentiating into either an ovary or testes [20]. Thus, all zebrafish display a juvenile ovary prior to gonadal sex differentiation. To ask if adad1 mutant fish could initiate oogenesis, we did histology at 28, 35, and 42 dpf. Because the precise timing of zebrafish sex differentiation can be variable, we compared histology of mutant and wild-type siblings at each time point, using wild-type siblings to determine the developmental stage of the population. Histology at 28 dpf showed that the gonads were still in an undifferentiated state and had not initiated oogenesis in either the mutant (N = 6) or wild-type (N = 6) fish (Fig 5A and 5B). However, histology at 35 dpf showed that all adad1 mutants (N = 6) were developing as males, whereas their wild-type siblings were developing both as males or females (Fig 5C–5E). We found similar results for the 42 dpf fish (Fig 5F–5H). These results suggest that adad1 mutants failed to initiate oogenesis, therefore this gene is necessary for both female and male germ cell development.
Fig 5. Hematoxylin-eosin staining on 28, 35, and 42 dpf wild-type and mutant gonads.
A-B: At 28 dpf, gonads are undifferentiated in wild-type (A) and adad1Y67X mutant (B) fish. C-H: At 35 and 42 dpf, wild-type fish were developing as either female (C, F) or male (D, G), whereas mutant fish were developing only as males (E, H). Gonads are marked by dotted red outlines in all images. Scale bar: 50 μm.
Adad1 is needed to complete the zygotene stage of meiosis prophase I
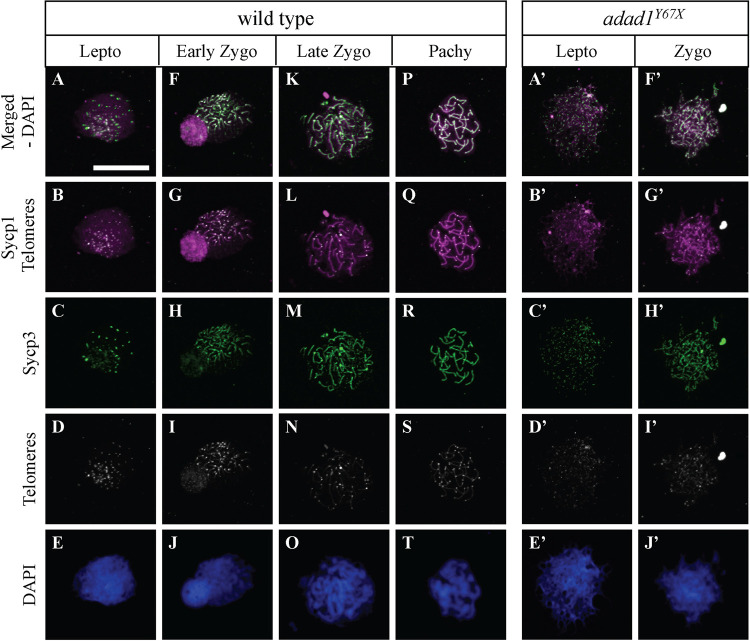
Since we observed that adad1 mutants produced spermatocytes but failed to make mature sperm (Fig 4D), we asked what stage of meiosis the spermatocytes could reach. Therefore, we performed IF on testes sections and on chromosome spreads to visualize key events in meiotic prophase I. IF labeling the Synaptonemal complex protein 3 (Sycp3) on testes section revealed the presence of three distinct cell populations in both the mutant and wild-type testes (Fig 6). In wild-type testes, leptotene stage nuclei had few Sycp3 dots, early zygotene/bouquet stage nuclei had bright Sycp3 concentrated on one side, and late zygotene/pachytene stage cells had Sycp3 evenly dispersed across the nucleus (Fig 6A–6C). The presence of similar cell populations in the mutants suggests that adad1 mutant spermatocytes can reach at least the zygotene stage (Fig 6D–6F). However, co-labelling with nuclear stain DAPI showed that the mutant testis lacked mature sperm, as observed in histological sections (Figs 4D and 6E). To better define the meiotic stage at which adad1 mutant spermatocytes stop developing, we did IF for Sycp1 and Sycp3 together with telomere fluorescence in situ hybridization (Telo-FISH) on nuclear spreads (Fig 7). In wild types, leptotene stage cells are characterized by Sycp3 expression near chromosome ends with Sycp1 protein displaying either few or no visible puncta (Fig 7A–7E). In the early zygotene stage cells, telomeres cluster together to form the meiotic bouquet where both Sycp1 and Sycp3 begin to load on chromosomes as homologous chromosomes start pairing (Fig 7F–7J). Late zygotene stage cells have elongated Sycp1 and Sycp3 lines as synapsis extends toward the interstitial region of the chromosomes (Fig 7K–7O). The pachytene stage cells have Sycp1 and Sycp3 labelling from end to end of homologous chromosomes (Fig 7P–7T) [10]. In adad1 mutants, we could see leptotene stage cells similar to the wild types (Fig 7A’–7E’). We also found that some adad1 mutant spermatocytes had Sycp1 and Sycp3 loaded onto chromosomes, indicating that synapsis and pairing were initiated, characteristic of zygotene stage cells (Fig 7F’–7J’). However, we did not find any mutant spermatocytes in the pachytene stage (N = 61 total wild-type nuclei: 28 leptotene, 20 zygotene, 13 pachytene; and 110 total mutant nuclei: 81 leptotene, 29 zygotene). These observations suggest that adad1 mutant spermatocytes initiated meiotic prophase I but failed to reach the pachytene stage, therefore Adad1 is necessary to complete meiotic prophase I in zebrafish.
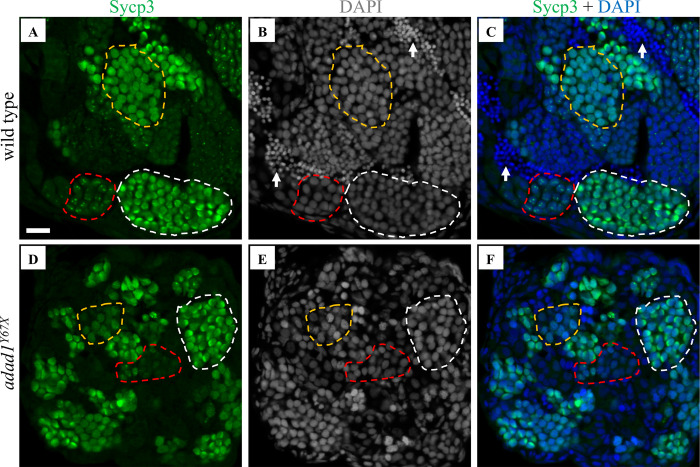
Fig 6. Immunostaining with meiotic marker Sycp3.
A-F: Sycp3 immunofluorescence (green) and DAPI nuclear stain (white in B, E and blue in C, F) on 60 dpf wild-type (A-C) and adadY67X testis (D-F) showed the presence of leptotene (red dotted outline), early zygotene or bouquet (white dotted outline), and late zygotene/pachytene (yellow dotted outline) stage cells in both wild-type (A-C) and adad1 mutant (D-F) testes. N = 9 for each genotype. Arrows point to sperm. Scale bar: 20 μm.
Fig 7. Telomere and synaptonemal complex protein labelling on meiotic chromosome spreads.
A-T: Wild-type chromosome spreads have leptotene (A-E), zygotene (F-O), and pachytene (P-T) stage cells. A’-J’: Chromosome spreads from adad1Y67X testes lack pachytene stage cells. Telo-FISH (white), IF for Sycp1 (magenta) and Sycp3 (green), and DAPI nuclear staining (blue). Scale bar: 10 μm.
Adad1 is required for spermatogonial stem cell maintenance in zebrafish
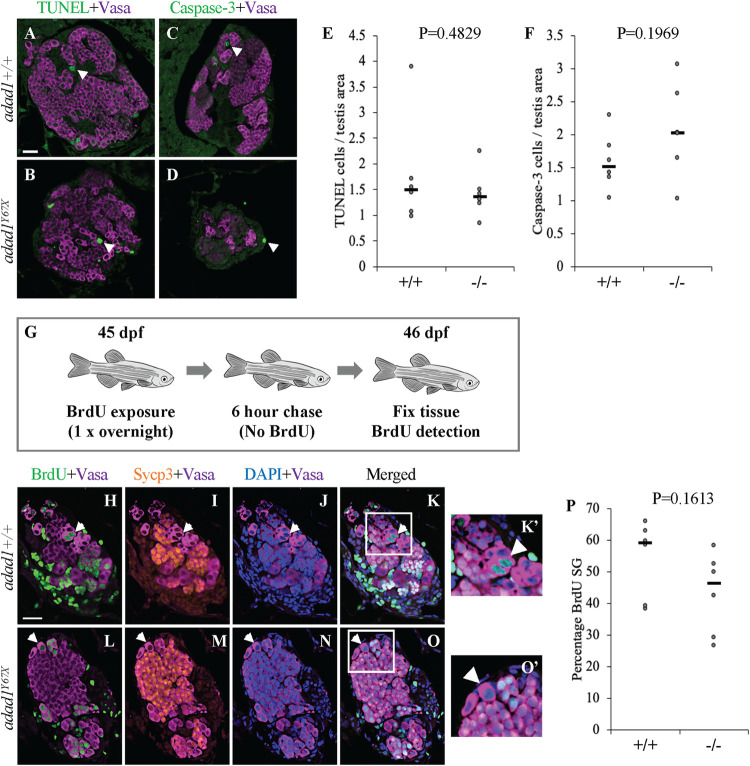
To identify the cellular processes leading to germ cell loss at the juvenile to adult transition in adad1 mutants, we assayed cell death, cell proliferation, and stem cells. Using a TUNEL assay and cleaved-Caspase-3 staining, we found no significant difference in the number of apoptotic cells between the mutant and wild-type testes (N = 6 testes per genotype per experiment) (Fig 8A–8F). Interestingly, almost all cells which were positive for TUNEL and Caspase-3 were negative for Ddx4/Vasa, indicating that germ cells lost Ddx4/Vasa expression during apoptosis. To test whether cell proliferation was reduced in adad1 mutant spermatogonia, we labelled mitotic cells using BrdU together with Ddx4/Vasa and Sycp3 antibody labeling (Fig 8G–8P). The cells which were positive for Ddx4/Vasa and BrdU, but negative for Sycp3 were considered proliferating spermatogonia (mitotic germ cells in the testis). We found no significant difference in the percentage of proliferating spermatogonia between mutants and wild types (N = 6) (Fig 8P). These results suggest that adad1 is neither required to prevent apoptosis nor to regulate spermatogonia proliferation in zebrafish.
Fig 8. Apoptosis and proliferation are not affected in adad1 mutants.
A-F: Cell death assays in 60 dpf adad1Y67X wild-type and mutant testes. Neither TUNEL assays (A,B,E) nor cleaved-Caspase 3 immunofluorescence (B,D,F) showed a significant difference between wild-type and mutant testes. Arrowheads indicate examples of TUNEL and cleaved-Caspase3 positive cells. G-P: Cell proliferation assay in 45 dpf testes using BrdU labeling. Fish were treated overnight with BrdU then waited for 6 hours without BrdU prior to fixation (G). Immunostaining was done with BrdU, Ddx4/Vasa, and Sycp3 antibodies to detect proliferating spermatogonia. Spermatogonia were identified as Ddx4/Vasa positive and Sycp3 negative (arrowheads, H-O). Quantification of the percentage of BrdU positive spermatogonia showed no significant difference between wild-types and mutants (P). SG = spermatogonia. Scale bar: 20 μm.
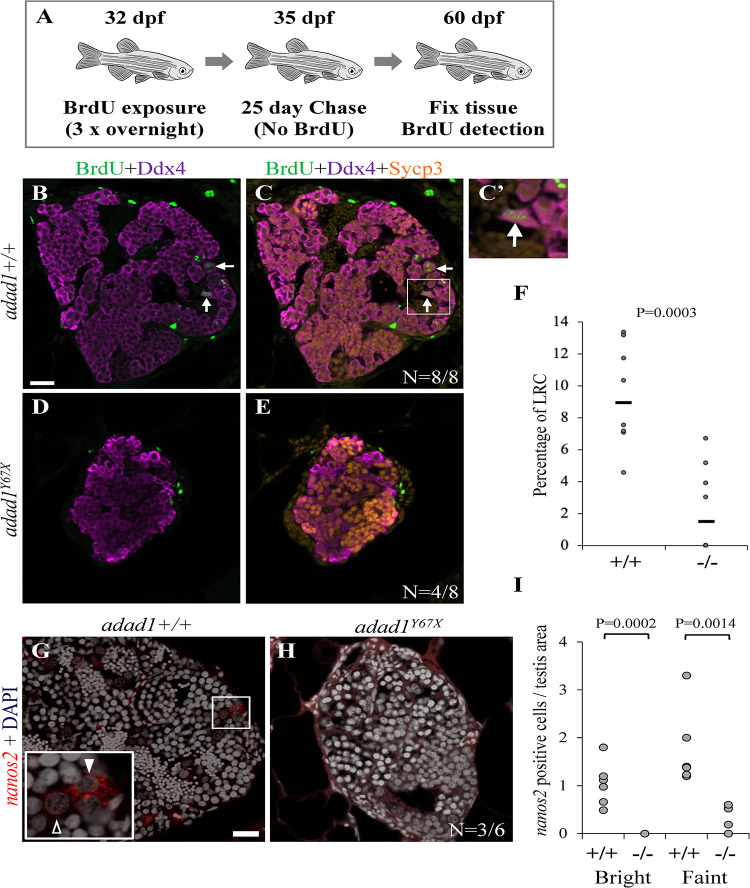
We next asked whether establishment or maintenance of SSCs was defective in the adad1 mutants by performing label retaining cell (LRC) assays, which assays for long term BrdU labeling. LRC assays have been used as a reliable method to identify quiescent stem cells in zebrafish testes [21]. To identify LRCs in the testis, 32 dpf fish were treated with BrdU for three consecutive nights followed by a 25 day chase period (Fig 9A). During the chase period, all differentiating spermatogonia completed spermatogenesis and were no longer present in the testes at the end of the chase period leaving only quiescent SSCs retaining BrdU. BrdU detection was combined with Ddx4/Vasa, and Sycp3 antibody labeling to identify spermatogonial cells (Ddx4 positive, Sycp3 negative). In the wild type, LRCs were present in all testes (N = 8 fish), indicating the presence of the SSCs in juvenile testes at 60 dpf (Fig 9B and 9C). On the other side, mutant testes had fewer LRCs than wild-type testes and half of the testes had no detectable LRCs (N = 8 fish) (Fig 9D–9F). These data show that adad1 has a likely role in regulating SSCs.
Fig 9. Label retaining cell assays (LRC) to identify spermatogonial stem cells.
A: 32 dpf fish were treated with BrdU three times for about 16 hours per treatment (overnight) followed by a 25 day chase where fish were in water with no BrdU. At the end of the chase period, fish were fixed and processed for BrdU detection. B-F: Wild-type testes all had LRCs (B-C, arrows) with median percentage of 9.34% out of the total spermatogonia (N = 8 fish) (F). Four out of eight mutant (adad1Y67X) testes had no LRCs (D-E) and the median percentage of LRCs of all mutant samples was less than the wild-types (F). G-I: Fluorescent in situ hybridization for nanos2 in wild-type and adad2Y67X mutant testis. Wild-type testes had cells with bright (solid arrowhead) and faint (outlined arrowhead) nanos2 label (N = 6) (G and I). Mutant testes had either no visible nanos2 labeled cells (N = 3/6) (H and I) or had only faint nanos2 labeled cells (N = 3/6) (I). Scale bar: 20 μm.
Because the LRCs represent only a subset of SSCs, we also assayed the SSCs using nanos2 RNA FISH, which is considered to be an SSC marker. We performed FISH on testes from 60 dpf old wild-type and mutant fish (N = 6 for each genotype) (Fig 9G and 9H). We found two types of nanos2 positive cells (bright and faint staining) in the wild-type testes, as previously described [22]. However, in the adad1Y67X mutant testes we detected only faint nanos2 positive cells, and these were fewer in number compared to the wild types (Fig 9G–9I). The absence of bright nanos2 staining in the mutant suggests that SSCs are broadly affected by loss of adad1 function. Together, our LRC and nanos2 staining experiments suggest that Adad1 is required to maintain SSCs in zebrafish.
Adad1 is involved in regulation of DNA damage response and repair, RNA regulatory processes, and expression of specific stem cell and pluripotency genes
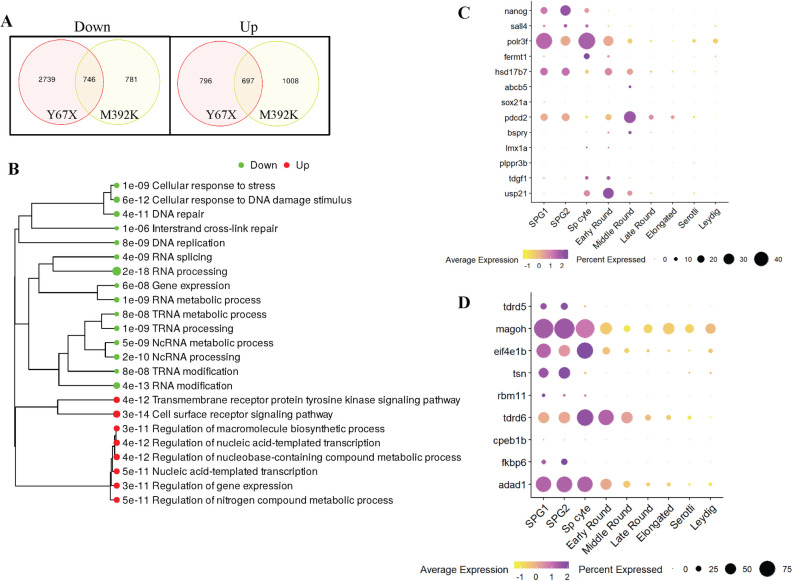
To identify differentially expressed genes (DEG) and potential molecular pathways through which Adad1 regulates germ cell development, we performed bulk RNA-seq from 45 dpf mutant and wild-type testes. We chose 45 dpf because the number of germ cells in the mutants are typically comparable to the wild types at this age. To identify wild-type males, we used adad1Y67X;Tg[piwil1:eGFP] and adad1M392K;Tg[ddx4:eGFP] lines, which can be used to sex juvenile fish based on GFP expression [23–25]. RNA-seq was done from testes of each the missense and non-sense mutant lines to identify common mis-regulated genes. For each mutant line, we identified differentially expressed genes between mutant and wild-type sibling testes through DESeq2 with a fold change >1,5 and false discovery rate (FDR) <0.05. We then compared the DEGs from each line to identify those transcripts that were dysregulated in both lines. The transcriptomic analysis identified 746 down-regulated and 697 up-regulated transcripts that were shared between both adad1 mutant testes (Fig 10A). Interestingly, we found that genes with well-known roles in germ cell development and maintenance were generally not dysregulated in both mutant alleles (e.g. vasa/ddx4, dazl, dnd1, ziwi/piwil1, nanos2 etc) (S1 Table). This was primarily apparent in the adad1Y67X allele, whereas adad1M392K had several such genes downregulated. We attribute this discrepancy to differences in the developmental progression of the phenotypes when the tissue was collected: based on our observations of germ cell GFP expression when collecting mutant testes, adad1M392K mutants had apparently fewer germ cells than adadY67X testes. Therefore, we believe that the downregulated expression of multiple germ cell-expressed genes in the adad1M392K is likely a secondary effect of germ cell reduction. However, it is also possible that differences in RNA expression between the two alleles are due to the nature of each allele as one (adad1M392K) is predicted to produce full length protein with an intact dsRNA binding domain, which is supported by the presence of Adad1 antibody staining in adad1M392K mutants (S3 Fig). We focused on genes that were dysregulated in both mutant lines for further analysis. Pathway analysis on the shared dysregulated genes revealed that DNA repair, RNA processing and modification, noncoding RNA processing, tRNA metabolic processing pathways were downregulated in the absence of Adad1 (Fig 10B). We found downregulation of several genes with known roles in stem cells and pluripotency (Tables 2 and S1), DNA repair and meiotic recombination (Tables 3 and S1), meiosis (Tables 4 and S1), and RNA modifications (Tables 5 and S1) in the mutant testes.
Fig 10. Bulk RNAseq from wild-type and mutant testes.
A: Venn diagrams showing numbers of differentially expressed genes in each mutant line compared to their wild-type siblings using fold change >1.5 and false discovery rate (FDR) <0.05 as the cutoff. B: Gene ontogeny pathway analysis performed on genes that were dysregulated in both adad1 alleles showing down- and up-regulated pathways in the mutant testes. C-D: Dot plot analysis of adult testes scRNAseq data shows expression pattern of genes listed in Tables 2(C) and 3(D).
Table 2. Pluripotency and stem cell genes downregulated in adad1 mutants.
| Gene | Known Functions | Reference |
|---|---|---|
| usp21 | Maintains stemness of mouse embryonic stem cells (ESC) via stabilizing Nanog | [26] |
| tdgf1 | Component of Nodal signaling which maintains germ cell pluripotency | [27] |
| plppr3b | SSC marker in human | [28,29] |
| lmx1a | Regulator of stem cell niche in Drosophila ovary | [30] |
| bspry | Maintains pluripotency of mouse ESC | [31] |
| pdcd2 | Needed for ovarian stem cells in Drosophila | [32] |
| sox21a | Regulates stem cell fate in mice | [33] |
| abcb5 | Stem cell gene needed for cornea development and repair | [34] |
| hsd17b7 | Associated with stemness and oncogenicity in keratinocytes | [35] |
| fermt1 | Epidermal stem cell maintenance and integrin activation | [36] |
| polr3f | Regulates pluripotency in human ESC | [37] |
| sall4 | Expressed and functions in mouse SSC | [38–40] |
| nanog | Inhibits primordial germ cell proliferation in zebrafish | [41] |
Table 3. DNA repair and meiotic recombination genes downregulated in adad1 mutants.
| Gene | Known Functions | Reference |
|---|---|---|
| rad51ap1 | Protects cells from adverse effects of DSB | [42] |
| hsf2bp | Recruit recombinase to DSB sites | [43] |
| swsap1 | Promotes early events of meiotic recombination | [44] |
| mei4 | Regulates meiotic DSB formation | [45] |
| helq | Promotes Rad51 paralogue-dependent repair | [46] |
| rad51b | Required for DNA recombination and repair | [47] |
| mcm8 | Needed for homologous recombination | [48] |
| nudt1 | Oxidative DNA repair protein | [49] |
| trip13 | Required for meiotic recombination | [50] |
| ercc1 | Maintains integrity of germ line DNA | [51] |
| bard1 | Localize to synaptonemal complex and regulates recombination | [52] |
| eme1 | Holiday junction resolution and DNA repair | [53] |
| trdmt1 | Participates in DNA damage repair | [54] |
Table 4. Meiotic genes downregulated in adad1 mutants.
| Gene | Known Functions | Reference |
|---|---|---|
| rbp1 | Component of retinoic acid signaling and meiosis | [55] |
| sumo2a | SUMOylates synaptonemal complex proteins in meiosis | [56] |
| ccna1 | Cyclin A1 is needed for male meiosis | [57] |
| zar1 | Needed for female meiosis in zebrafish | [58] |
| fkbp6 | Required for pairing of homologous chromosomes | [59] |
| hsd17b12a | Plays role in female meiosis and fertility | [60] |
Table 5. RNA binding and modification genes downregulated in adad1 mutants.
| Gene | Known Functions | Reference |
|---|---|---|
| fkbp6 | Associated with piRNA biogenesis and transposon silencing | [61] |
| cpeb1b | Regulates mRNA translation during oocyte maturation | [62] |
| tdrd6 | Regulates miRNA expression | [63] |
| rbm11 | Involved in alternate splicing of mRNA | [64] |
| tsn | Binds meiotic RNA | [65] |
| eif4e1b | Regulates maternal mRNA translation | [66] |
| magoh | A component of exon-exon junction complex | [67] |
| tdrd5 | Binds piRNA and is required for transposon silencing | [68] |
We used available scRNAseq data to determine the expression pattern of the genes listed in Tables 2–5 in adult testes and 40 dpf ovaries [18,19]. In the testes scRNAseq data, two spermatogonial clusters were identified, SPG1 and SPG2, with SPG1 presumed to be earlier stages [19]. However, cells with enriched nanos2 expression were not identified in these data suggesting that SSCs were not well-represented in this experiment (S4 Fig). Nevertheless, expression of nearly all genes listed in Tables 2–5 was detected in testes and ovaries and were either exclusively detected or highly enriched in germ cells (Figs 10C and 10D and S5). Several genes showed a similar expression pattern to adad1 in the testis, which was enriched in spermatogonial cells and spermatocytes (Fig 10C), including polr3f, magoh, eif4e1b, and trip13 (Fig 10C and 10D and S5) Among the pluripotency and stem cell genes, hsd17b7, which is involved in steroid and cholesterol synthesis, showed enrichment in both spermatogonial cells and oogonial stem cells/progenitor cells (GSC-Pro), while nanog was enriched in spermatogonial cells (Figs 10C and S5A). Among genes involved in RNA regulation, fkbp6, tsn, and rbm11 showed enriched expression in both spermatogonial and GSC-Pro cells (Figs 10D and S5B). These results suggest that adad1 function is needed for normal expression of some germ-line stem cell genes in one or both sexes, which may be due to reduced stem cell numbers or reduced mRNA levels within these cells. Because nanos2 was not down-regulated in the adad2 mutant testes in our RNAseq experiment, which was done with 45 dpf testes, it is more likely that these data represent dysregulated gene expression rather than loss of stem cells.
Discussion
In this study, we have shown that the zebrafish Adad1 RNA binding protein has multiple roles in germ cell development. In the testis, Adad1 functions in germline maintenance through regulating SSC function and is also necessary for completion of meiotic prophase I. We propose that Adad1 carries out these roles through regulation of specific RNAs that function in these processes. Our work revealed essential functions of Adad1 in fish, thus increased our understanding of this RNA-binding protein in vertebrates.
Regulation of spermatogonial stem cells (SSC) by Adad1
We identified adad1 mutant zebrafish and found that adad1 functions to maintain the SSCs. We applied an established label retaining cell (LRC) assay to identify quiescence stem cells and FISH for the germline stem cell marker, nanos2, in zebrafish testes [9,21]. These experiments showed that by 60 dpf adad1 mutants either lacked or had reduced numbers of SSCs compared to their wild-type siblings. Because some mutant testes contained LRCs at the time fish were assayed, we believe that SSCs were initially established in mutants but failed to be maintained. It is also possible that adad1 mutant SSCs divide less frequently than wild-type SSCs leading to fewer BrdU labeled SSCs after the BrdU treatment. Previously faint and bright nanos2 expressing cells were characterized in 14 dpf zebrafish gonads [22]. We detected both faint and bright nanos2 expressing cells in 60 dpf wild-type testes (Fig 10G–10I) suggesting that these may represent different states of the SSCs. Half of the adad1 mutant testes had nanos2 expressing cells, however only a relatively small number of faint nanos2 expressing cells was detected (Fig 10I). By contrast, at 45 dpf, bulk RNAseq showed that nanos2 expression levels were similar between the mutant and wild-type testes in both mutant alleles (S1 Table). Together these data suggest that the SSCs are initially established and are present in normal quantities in 45 dpf mutant testes but are reduced or absent by 60 dpf. SSCs could be lost through multiple mechanism including reduced proliferation rates and/or loss of stem cell character leading to differentiation. Although adad1 is expressed in all spermatogonia, proliferation was normal in adad1 mutant spermatogonia indicating that failure to maintain the SSCs is likely the primary cause of germ cell loss in these mutants.
The establishment and maintenance of SSCs has not been well characterized in fish. Nanos2, which is a conserved germline stem cell marker in vertebrates, is expressed in A-type single spermatogonia (Asingle) and spermatogonia in cysts of up to four cells (A4) in zebrafish [6–9,69]. The nanos2 gene is required for maintenance and regeneration of germ cells in zebrafish pointing to an essential role in stem cell function [70]. Therefore, Asingle−A4 spermatogonia can be presumed to be the stem cells in zebrafish testes. Although Adad1 protein was localized only in the cytoplasm of spermatocytes and most spermatogonia, it was also localized in nuclei in Asingle−A4 spermatogonia (Fig 3). These expression data further support a role of Adad1 in regulating zebrafish SSCs and suggests that nuclear Adad1 may be important for stem cell function. Our transcriptomic data points to dysregulation of genes associated with stem cells and pluripotency (Table 2). For instance, sall4, which is a well-known SSC marker in mammals, was downregulated in the adad1 mutant testes (Table 2) and was expressed in spermatogonial cells in wild-type testes according to scRNAseq experiments (Fig 10C) [38–40]. We also found downregulation of plppr3b in mutants, which is a recently identified SSC marker in humans (Table 2) [28,29]. Although the expression of plppr3b was not well represented in the testis scRNAseq data, nanos2 expression was also not detected above background levels, thus genes that are specifically expressed in SSCs are likely not well represented in these data. Furthermore, the pluripotency factor nanog, which is expressed in zebrafish spermatogonia, showed reduced expression in adad1 mutants and was expressed in spermatogonia of wild-type testes (Fig 10C) [41]. Although, nanog is generally not associated with spermatogonial cells in mice, it is expressed in spermtogonia of several other fish species, including medaka (Oryzias latipes), farmed carp (Labeo rohita), and Japanese flounder (Paralichthys olivaceus) [71–73]. Therefore, it is possible that nanog plays a conserved role in regulating spermatogonial cells in fishes. These findings suggest that in the absence of functional Adad1, expression of genes with potential roles in stem cell regulation were disrupted in zebrafish testes, which contributes to germline loss in this vertebrate model.
Meiosis defects and failure of female development in zebrafish
Because adad1 mutants initially contained germ cells in larval-juvenile stages, we were able to discover an essential role of adad1 in meiosis. The adad1 mutant spermatocytes initiated pairing and synapsis of homologous chromosomes, but failed to complete the process and reach the pachytene stage. We propose that Adad1 binds with RNAs important for meiotic prophase I, thus regulates the process directly, however other possibilities exist, such as regulation of RNAs that indirectly impacts meiosis.
The necessity of Adad1 in meiotic prophase I is further supported by the lack of female development in the mutant lines. Domesticated zebrafish do not have sex chromosomes, and their gonads remain as bipotential until 2–3 wpf [74,75]. The bipotential gonads produce meiotic cells that initiate oogenesis. As testes undergo sex differentiation these early oocytes are depleted and spermatogenesis begins [20]. Disruption of meiosis in zebrafish gonads leads to male sexual development. This outcome is likely due to failed development of the ovarian follicle in meiotic mutants. In ovaries, the transition from the cyst/nest stage to formation of ovarian follicles occurs around the pachytene stage [76]. Thus, defects that disrupt meiotic prophase I typically lead to failed folliculogenesis and male development. Several studies reported a link between meiotic prophase I failure in early oocytes and lack of female development in zebrafish [58,77–83]. Zebrafish mutants disrupting genes encoding synaptonemal complex proteins sycp1, sycp2, and sycp3 as well as the meiotic cohesion component smc1b, developed as 100% sterile males due to failures in meiotic prophase I [77–79,82]. Mutations that specifically effect female meiosis, such as mutations in zar1 and rad21l1, failed to develop as female or were male biased, respectively [58,80]. In these mutants, males were normal indicating a requirement in female meiosis but not spermatogenesis [58,80]. We demonstrated that adad1 mutant spermatocytes failed to progress beyond the zygotene stage and that ovarian follicles were never formed in the bipotential gonad stage. Therefore, it is likely that adad1 mutant zebrafish failed to develop as females due to failure in meiotic prophase I.
RNA-binding proteins and germline maintenance in zebrafish
Post-transcriptional regulation by RNA binding proteins (RBPs) is critical for multiple aspects of germ cell development. [11,12]. The functions of RBPs varies and can include translational control, localization, stability, and modifications of RNAs [11,12]. Several studies have shown that the conserved germline RBPs Dazl (deleted in azoospermia like), Vasa/Ddx4 (DEAD-box RNA helicase protein 4), Nanos2, and Nanos3 are necessary for germline stem cell establishment or maintenance in zebrafish [8,22,70,84,85]. In dazl mutants, the germ cells failed to undergo cystic proliferation and the transition from PGCs to germline stem cells was disrupted leading to a failure to establish germline stem cells [22]. Zebrafish ddx4/vasa, nanos2, and nanos3 mutants all established germline stem cells but failed to maintain them [8,70,84,85]. The nanos3 gene is only required for germline stem cell maintenance in ovaries as male mutants were fertile with histologically normal testes [8]. By contrast, dazl, ddx4/vasa, and nanos2 are required in both sexes [22,70,84]. Here we demonstrated that the zebrafish RBP Adad1 has a critical role in SSC maintenance and progression of meiosis I. We assayed for germline stem cells in juvenile adad1 mutants using a label retaining cell assay and nanos2 FISH and found that germline stem cells were either reduced or absent by 60 dpf. Thus, similar to ddx4/vasa and nanos2, adad1 functions in stem cell maintenance in the testis. We could not assess the role of adad1 in ovarian germline stem cells as oogenesis was never initiated in adad1 mutants due to the meiotic defects. However, because adad1 is expressed in both sexes, it may regulate both spermatogonial and oogonial stem cells. Together, these studies indicate that Adad1 is among the multiple RBPs, which are essential for germline stem cell function in zebrafish pointing to critical roles of post-transcriptional processes in germline stem cell regulation.
The two adad1 mutant alleles investigated in this study had indistinguishable phenotypes yet one (adad1Y67X) is predicted to give rise to a truncated protein without the dsRBM, while the other (adad1M392K) produces a full length protein with only a single amino acid change within the adenosine deaminase domain. IF using Adad1 antibodies confirmed that the adad1Y67X mutants lacked full-length protein, as the antibody epitope is at the C-terminus of the protein, whereas adad1M392K produced Adad1 protein that exhibited similar subcellular localization as the wild-type protein. This indicates that the adenosine deaminase domain is necessary for protein function despite the predicted lack of deaminase catalytic activity. This prediction is based on the absence of key catalytic residues that are known to be necessary for Adar deaminase activity and the failure to detect defects in RNA editing in mouse Adad1 mutant testes [14,15]. One possibility is that the Adad1 deaminase domain facilitates binding to specific RNA targets. This is supported by data from the related ADAR2 protein showing that the deaminase domain can bind and edit RNA without the dsRBMs in vitro, albeit with decreased efficiency [86]. Therefore, the Adad1M392K mutant protein may have reduced or abolished RNA binding capacity or may have disrupted RNA binding specificity causing loss of function.
Similarities and differences in Adad1 expression and function in vertebrates
The vertebrate specific Adad1 protein has an N-terminal double stranded RNA binding motif (dsRBM) suggesting that it functions in RNA regulation. Adad1 is a member of the Adar (adenosine deaminase acting on RNA) protein family due to the presence of a deaminase domain at its C-terminus (Fig 1). However, the Adad1 deaminase domain is probably inactive because the amino acid residues known to be required for deaminase activity are not conserved in either zebrafish or mammalian Adad1 [14,87]. Moreover, RNA editing in mouse Adad1 mutant testes was unaffected, indicating that the deaminase domain is not involved in RNA editing [15]. The mouse Adad1 protein (also known as Tenr), was first identified due to in vitro binding to the Protamine 1 (Prm1) mRNA, however additional target RNAs have not been reported [88]. As zebrafish do not have protamines, this RNA target is not conserved across these species [89]. Hence, although adad1 is a predicted RNA regulator, the molecular function of Adad1 remains elusive.
Adad1 is expressed in zebrafish and mammalian germ cells, however differences exist in the precise cell-types in which it is expressed. We found that zebrafish adad1 is expressed in both testis and ovary, however it is testis specific in humans (proteinatlas.org) and mice [15,90]. We found that zebrafish Adad1 is expressed in both spermatogonia and spermatocytes in the testis (Fig 3), whereas its expression is limited to meiotic and post-meiotic cells in the mammalian testes [15,90]. The expression of Adad1 in earlier developmental stages in the zebrafish testis compared to mammals suggests that zebrafish Adad1 regulates early stages of spermatogenesis, as discussed below. Nevertheless, the germline specific expression of Adad1 is conserved from zebrafish to human.
Although both zebrafish and mouse Adad1 have essential roles in male germ cell development, they function in different spermatogenic stages. We demonstrated that loss of adad1 function triggered germ cell loss due to failure of SSCs maintenance in zebrafish. In addition, these mutants failed to complete meiotic prophase I and therefore never produced sperm. Our results are in harmony with previous studies on mice which reported that Adad1/Tenr deficient male mice are infertile due to abnormal sperm development [14,15]. However, Adad1/Tenr mutant mice only display abnormal sperm morphology and appear to have normal spermatogonial cell maintenance and meiosis. These abnormal sperm have difficulty binding to oocytes and penetrating the zona pellucida resulting in infertility [14]. Whether or not zebrafish adad1 has roles in spermiogenesis similar to those reported in mice cannot be investigated as the earlier defects preclude the ability to investigate spermiogenesis. The human ADAD1 gene has also been linked to impaired fertility in men. Reduced testicular or sperm ADAD1 expression was found in male patients and was particularly associated with non-obstructive azoospermia suggesting a role in spermatogenesis [91,92]. Overall, these observations suggest that Adad1 functions to regulate spermatogenesis in humans, mice, and zebrafish, although additional roles exist in zebrafish compared to those described in mice.
Materials and methods
Ethics statement
Approval for all animal procedures was attained by the UMass Boston Institutional Animal Care and Use Committee (IACUC), protocol #35.
Zebrafish Lines
Zebrafish were raised and maintained in a recirculating system under standard conditions. Zebrafish lines used in this study were: Tue (wild type), slc34a1aumb10, adad1t30103, adad1sa14397, Tg[piwil1:eGFP]uc1 [24], Tg[ddx4:eGFP] [23].
The adad1t30103 mutant line was identified in an N-Ethyl-N-Nitrosourea (ENU) mutagenesis screen for defects affecting adult zebrafish gonads, as described previously [93,94]. Briefly, wild-type male zebrafish of Tuebingen (Tue) background were treated with ENU. The males were then crossed with females to make F1 families, which were then in-crossed to generate F2 families. Finally, F2 siblings were crossed to obtain homozygous F3 mutants which were raised to adulthood and screened for anomalous gonad phenotypes. The adad1t30103 allele harbors a missense mutation resulting in a Methionine to Lysine change in the protein at amino acid 392, we therefore refer to this mutation as adad1M392K for simplicity. The predicted mRNA sequence of this allele was confirmed by sequencing cDNA derived from this mutant line.
The adad1sa14397 nonsense mutant line was generated by the zebrafish mutation project [95] and provided by the Zebrafish International Resource Center (ID: sa14397). This mutant carries a premature stop codon after codon 66, we therefore refer to this mutation as adad1Y67X. The predicted mRNA sequence of this allele was confirmed by sequencing cDNA derived from this mutant line.
The slc34a1aumb10 mutants were generated using CRISPR-Cas9 genome editing in the wild-type Tue background. We designed a crRNA (5’-TGTGATCCTGTCCAACCCGG-3’) targeting exon5 of slc34a1a using the online tool CRISPRscan (https://www.crisprscan.org/). The crRNA and universal tracrRNA were purchased from Integrated DNA Technologies (IDT). To make the gRNA complex, the crRNA and tracrRNA were mixed and annealed according to the manufacturer’s instructions. The gRNA (5 pg/embryo) and mRNA encoding Cas9 protein (150 pg/embryo, System Biosciences) were injected into one-cell stage embryos. Injected embryos were raised to adulthood and crossed with Tue fish to identify germline mutations. We found F1 embryos carrying multiple different deletions in the gRNA target region from three different parents. However, for further analysis we used the mutant with a 17 bp deletion which caused a premature stop codon after amino acid 195.
Positional cloning and whole exome sequencing
To identify the heritable mutation of the t30103 line, we employed whole exome sequencing (WES) as previously described [93] and mapping according to methods described in Bowen et al. [96]. Linkage of genomic regions with high mapping scores were tested using SSLP markers and a region on the left arm of chromosome 14 was confirmed to be linked to the gonad phenotype. Two missense mutations were identified within the linked interval, one affecting the scl34a1a gene and one affecting the adad1 gene. Genetic complementation tests between t30103 and either slc34a1aumb10 or adad1sa14397 revealed that the adad1 mutation caused the gonad phenotypes. For complementation tests, adad1 trans-heterozygous fish were generated by crossing t30103+/- males with adad1sa14397+/- females. Similarly, t30103+/- males were crossed with slc34a1aumb10+/- females to establish t30103/slc34a1a trans-heterozygous fish. When the fish reached adulthood, fertility tests and gonad histology were performed to test the phenotype.
Genotyping
To genotype adad1M392K, the target region was amplified by primers KS552 and KS553, followed by MslI digestion. The digested PCR product was run on 7% acrylamide gel to distinguish mutant (564 bp) and wild-type (473 bp) bands. Genotyping of the adad1Y67X mutation was done by PCR amplification using primers KNI203 and KNI204, followed by MseI digestion and electrophoresis on 7% acrylamide gel. The mutant band was 140 bp, whereas the wild type was 166 bp. Primers KS554 and KS555 were used to amplify the region of slc34a1a harboring the slc34a1aI423N variant. This amplicon includes an intronic size polymorphism resulting in 482 bp size mutant and a 582 bp wild-type PCR product. The slc34a1a PCR product was run on a 2% agarose gel to separate the mutant and wild-type bands. The slc34a1aumb10 mutation was genotyped by amplifying the gRNA target region using primers KNI201 and KNI202, followed by 7% acrylamide gel electrophoresis to distinguish the mutant band (186 bp) from the wild type (203 bp). Sanger sequencing was done on PCR products to confirm all the mutations. The name and sequence of the genotyping primers is listed in S1 Table.
Fertility tests
The fertility test of adad1M392K/Y67X trans-heterozygotes (N = 6) and adad1Y67X homozygous mutants (N = 3) were done by pairing mutant males with wild-type Tue females, since the mutants were all male phenotypically. The following morning, embryos were collected and monitored under the dissecting microscope to see the developmental stage. Embryos were considered to be fertilized if they had undergone normal appearing cell divisions. Eggs that did not appear to be developing were raised up to 24 hours to confirm that they were not fertilized, by which time all of them died and became cloudy.
Histology
Histology was performed as previously described [97]. Briefly, fish were euthanized by tricaine overdose and the torso was isolated and fixed in Bouin’s solution overnight at room temperature. Following several dehydration steps, the fixed tissues were embedded in paraffin for sectioning. A rotating microtome was used to obtain 5 μm thick sections and the sections were stained following modified Harris’s hematoxylin-eosin staining protocol. After staining, the tissue was covered using Permount as a mounting medium.
RT-PCR
Total RNA was isolated from zebrafish testes or ovaries using TRIzol-reagent, following the manufacture’s protocol (Fisher Scientific). Isolated RNA was treated with TURBO DNase (Fisher Scientific) to remove any genomic DNA contamination and cleaned via LiCl precipitation. The first strand cDNA was synthesized using oligo-dT primers and AMV-Reverse Transcriptase (New England Biolabs). The piwil1 gene is expressed in the germ cells of both sexes and was used as germline control. Rpl13a expression was used as a control for other tissues. The primers used for RT-PCR are listed in S3 Table. To generate a zebrafish testes devoid of germ cells for RT-PCR using male somatic gonadal tissue, we injected a morpholino targeting the dead end gene into 1-cell embryos, as previously described [98].
In situ hybridization (ISH)
To make the adad1 ISH probe, a 341 bp cDNA fragment was amplified using adad1 RT-PCR primers KNI207 and KNI208. The cDNA was sub-cloned into pGEMT easy vector (Promega), and Sanger sequencing was done to confirm the right insert. The sense and anti-sense probes were generated using a DIG RNA labelling kit (Roche). ISH on 4% paraformaldehyde (PFA) fixed, paraffin embedded tissue sections was performed as previously described [93] To visualize the probe, alkaline phosphatase conjugated anti-DIG antibody (Roche), and BM purple substrate were used.
Adad1 antibody generation and western blotting
Polyclonal antibodies were raised in rabbits to the c-terminal peptide sequence 534-KSYLERKGYGQWVEKPPISDHFSI-557 by Pacific Biosciences, CA. Antibodies used in this study were purified by affinity chromatography. To test the antibody through western blot, we isolated total protein from 45 dpf adad1Y67X mutant and wild-type testes in RIPA buffer (Thermo Fisher Scientific). After denaturation through heat and 100 mM DTT, the samples were run on 10% SDS-PAGE and transferred onto a PVDF membrane. The membrane was blocked in 5% milk in TBST for 1 hour at room temperature. Primary antibodies were diluted in TBST as follows: rabbit Adad1 at 1:500, rabbit Ddx4/Vasa at 1:2000 [99], mouse α-Tubulin at 1:1000 (T9026, Sigma-Aldrich). The membrane was incubated in primary antibodies overnight at 4°C. After 4 x 5 minutes washes in TBST, incubation in the secondary antibodies (Li-COR Biosciences) were done for 1 hour at room temperature. Finally, the membrane was washed 4 x 5 minutes in TBST before imaging in the Odyssey CLx instrument (Li-COR Biosciences).
BrdU incorporation experiments
For cell proliferation assays, 45 dpf mutant and wild-type fish were kept in BrdU solution (3 mg/mL) overnight at 27°C. The next morning, the BrdU solution was removed, and fish were kept in fresh water for 6 hours before euthanizing. The torsos were fixed in 4% PFA, embedded and sectioned in paraffin, and processed for immunofluorescence (IF). For label retaining cell (LRC) assays, 32 dpf non-genotyped fish were kept in BrdU solution (3 mg/mL) for three consecutive nights. Between treatments the fish were kept in fresh fish water to avoid BrdU toxicity. After a 25 days chase period, tissue was collected for genotyping and the mutant and wild-type fish were fixed and processed for IF as described below.
Immunofluorescence and cell death assay
The process of tissue fixation and antigen retrieval for IF was done as previously described [78]. TUNEL staining on 60 dpf paraffin embedded testes sections were performed using a fluorescein In Situ Cell Death Detection Kit (11684795910, Roche). Immunolabelling with Ddx4/Vasa antibody was done before the TUNEL staining. Primary antibodies were diluted in the blocking buffer (1% goat serum in PBST) as follows: rabbit Adad1 at 1:500, chicken Ddx4/Vasa at 1:2000 [83], rabbit Sycp3 at 1:200 (NB300-232SS, Novus Biologicals), mouse BrdU at 1:200 (5292S, Cell Signaling Technology), rabbit Caspase-3 at 1:1000 (C8487, Sigma-Aldrich). The testes sections were incubated overnight with the primary antibodies at 4°C. Incubation with appropriate secondary antibody (Thermo Fisher Scientific) was done at room temperature for 1 hour. Following 10 minutes incubation with nuclear stain DAPI, sections were washed 3x5 minutes in PBST and 1x5 minutes in miliQ water. Finally, the sections were covered with Fluoroshield (Sigma-Aldrich) and kept at 4°C before imaging.
Chromosome spreads and staining
Meiotic chromosome spreads from 8 wpf mutant and wild-type testes were prepared as previously described [78,100]. Two experiments were performed each using 5 wild-type and 15 mutant fish. Telomere staining was done using a TelC-Cy3 probe (PNA Bio) following a previously described method [101]. Antibody labeling of chromosome spreads was done as described above.
Fluorescent in situ hybridization (FISH) for nanos2
An HCR probe for zebrafish nanos2 was purchased from Molecular Instruments according to Liu et al 2022 [18]. The tissue was prepared according to Liu et al 2022 [18] and the staining was done following the manufacture’s guideline (MI-Protocol-HCRv3-Zebrafish). Briefly, the torsos of 60 dpf old adad1Y67X mutants and wild-type fish were fixed in neutral alcohol buffer (10% formalin, 29.5% ethanol, 50 mM Na2HPO4 pH 7.2) overnight at 4°C. The following morning, the torsos were washed 3x10 minutes in PBST at 4°C. After dehydration in 30, 50, 70, 95, and 100% ethanol, the tissue was embedded in paraffin to obtain 5 μM thick section. Following rehydration steps, the sections were incubated in 5 μg/ml proteinase K solution for 10 minutes at room temperature. To avoid over-digestion, the sections were quickly rinsed in PBST 3 times. The rest of the process was similar to the manufacture’s guideline except that we used 5 pmol nanos2 probe instead of 2 pmol.
Imaging
We used a confocal laser scanning microscope (Zeiss LSM 880) to capture images of the IF and telomere staining. The images were further analyzed through Fiji/ImageJ. To quantify cell numbers, we used 3 sections for each biological replicate (6–8 replicates for each genotype). Student’s t-test (p<0.05) was employed to see whether difference between the mutant and wild-type samples were significant or not.
RNA sequencing and data analysis
Total RNA was isolated from 45 dpf adad1Y67X Tg[piwil1:eGFP]uc1 and adad1M392K Tg[ddx4:eGFP] testes using the RNeasy micro kit (Qiagen). Since the testes were very small at this age, 5 testes were pooled together for each biological replicate (4 biological replicates for each genotype–i.e. 4 pools of adad1Y67X mutants and 4 of wild-type siblings; 4 pools from adadM392K mutants and 4 of wild-type siblings). Isolated RNA was sent to Genewiz at Azenta Life Sciences, NJ for library preparation with poly-A selection method and 150-bp paired end sequencing on an Illumina HiSeq platform. We assessed the quality of the raw sequence data using FastQC, low quality and adapter sequences were trimmed with TrimGalore. Trimmed reads were aligned to the GRCz11 assembly of the zebrafish genome (Ensembl) using STAR and the gene annotations published by the Lawson lab [102]. Reads were counted using HTseq and analyzed for differential gene expression (DEG) and gene ontology (GO) using the iDEP v95 pipeline [103], with a DeSeq2 cut off value for fold change of >1.5, and false discovery rate (FDR) of less than 0.05. RNAseq data can be found at https://www.ncbi.nlm.nih.gov/bioproject/, project ID PRJNA980839
Single cell RNA seq data analysis
Adult testes and 40 dpf ovaries scRNAseq data was extracted and regenerated from Quian et al and Liu et. al, respectively [18,19]. The ovaries data were accessed from the singlecell.broadinstitute.org and testes data from GEO accession view. Data preprocessing and clustering was done using Seurat 4.3.0. Genes that were not expressed in a minimum of 5 cells, and cells with less than 200 mapped transcripts were excluded from downstream analysis. Cells with higher than 5% mitochondrial relative to all transcripts were also excluded from downstream analysis. To achieve this, the parameters we set for the Seurat clustering were as follows—min.cell = 5, min.feature = 200, max.feature = 10000, pc.num = 18, variable.features.n = 1000, mt.per = 5, regress.type = 1. Plots were generated using ggplot2 from scaled and normalized datasets. Code is available at https://figshare.com/projects/Adad1_scRNA_analysis/173724.
Supporting information
(PDF)
(PDF)
(PDF)
The nonsense mutation (sa14397) is located in exon 3 where a T<A mutation caused a premature stop codon (left panel). The missense mutation (t30103) is in exon 9 where a T<A mutation resulted in a Methionine to Lysine residue change (right panel).
(PDF)
The scl34a1aumb10 mutation is a 17 bp deletion (top panel). The guide-RNA target sequence, including the PAM, is highlighted in yellow. RT-PCR detected slc34a1a expression in adult testes (left panel). Homozygous slc34a1aumb10 mutants exhibited normal testes histology. Scale bar:50 μm.
(PDF)
A-D: Immunolabeling of Adad1 antibody on 60 dpf adad1Y67X mutant testis. No protein was detected as expected since the antibody epitope is at the C-terminus of the protein (A). Co-labeling with Ddx4/Vasa showed the presence of germ cells in the mutant testis (B). E-H: Immunolabeling of Adad1 on 45 dpf adad1M393K mutant testis (E) co-labeled with Ddx4/Vasa (F). Arrows point to cells with nuclear and cytoplasmic Adad1 localization. I: Western blot showing that the antibody recognized the 62 kDa size Adad1 protein in wild-type testes extracts but not in testes extracts from adad1Y67X mutants. The wild-type sample was loaded in two lanes: the left lane had twice the volume as the right lane. Ddx4/Vasa was detected in the mutant sample indicating that germ cells were present. Scale bar: 20 μm.
(PDF)
The nanos2 gene was not enriched in spermatogonial cell clusters SPG1 or SPG2 whereas known spermatogonia-expressed genes ddx4 and dazl were enriched in both.
(PDF)
A-D: Dot plot analysis of scRNAseq data from adult testes and 40dpf ovaries. Genes that are listed in Tables 2–4 are shown.
(PDF)
Acknowledgments
We would like to thank Sean Burgess (UC Davis) for providing Sycp1 and Ddx4/Vasa (raised in chicken) antibodies. We are also grateful to Holger Knaut (NYU) for generously giving Ddx4/Vasa antibody (raised in rabbit). Special thanks to Leo Harris for the zebrafish drawings used in Figs 8 and 9. The adad1sa14397 mutant line was provided by the International Zebrafish Resource Center (ZIRC). The mutagenesis screen in which the adad1t30103 mutant was isolated was supported by the European Commissions, ZF-MODELS 6th framework program (EC Contract LSHG-CT-2003-503496).
Data Availability
RNA sequencing data has been submitted to NCBI BioProjects. Identifier: PRJNA980839. scRNAseq analysis code can be found at https://figshare.com/projects/Adad1_scRNA_analysis/173724.
Funding Statement
This work was supported by NIH-NICHD grant # R15HD107594 awarded to KRS and NIH-NICHD grant# R03HD097433 awarded to KRS and KH. KNI was supported by a Doctoral Dissertation Grant awarded by the University of Massachusetts Boston and a College of Science and Mathematics Doctoral Research Fellowship. AA was supported by the University of Massachusetts Boston – Dana Farber/Harvard Cancer Center U54 Partnership Grant, NCI-NIH #’s U54 CA156734-11 (UMass Boston) and U54 CA156732-11 (DF/HCC). The funders had no role in study design data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Lacerda SM dos SN, Costa GMJ, de França LR. Biology and identity of fish spermatogonial stem cell. Gen Comp Endocrinol. 2014;207: 56–65. doi: 10.1016/j.ygcen.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 2.Mäkelä J-A, Hobbs RM. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction. 2019;158: R169–R187. doi: 10.1530/REP-18-0476 [DOI] [PubMed] [Google Scholar]
- 3.Pudney J. Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech. 1995;32: 459–497. doi: 10.1002/jemt.1070320602 [DOI] [PubMed] [Google Scholar]
- 4.Leal MC, Cardoso ER, Nóbrega RH, Batlouni SR, Bogerd J, França LR, et al. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol Reprod. 2009;81: 177–187. doi: 10.1095/biolreprod.109.076299 [DOI] [PubMed] [Google Scholar]
- 5.Fayomi AP, Orwig KE. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018;29: 207–214. doi: 10.1016/j.scr.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song H, Park HJ, Lee WY, Lee KH. Models and Molecular Markers of Spermatogonial Stem Cells in Vertebrates: To Find Models in Nonmammals. Stem Cells Int. 2022;2022. doi: 10.1155/2022/4755514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional Hierarchy and Reversibility Within the Murine Spermatogenic Stem Cell Compartment. Science (80-). 2010;328: 62–67. doi: 10.1126/science.1182868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beer RL, Draper BW. Nanos3 maintains germline stem cells and expression of the conserved germline stem cell gene nanos2 in the zebrafish ovary. Dev Biol. 2013;374: 308–318. doi: 10.1016/j.ydbio.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 9.Draper BW. Identification of Germ-Line Stem Cells in Zebrafish. Methods in molecular biology (Clifton, NJ). 2017. pp. 103–113. doi: 10.1007/978-1-4939-4017-2_8 [DOI] [PubMed] [Google Scholar]
- 10.Imai Y, Olaya I, Sakai N, Burgess SM. Meiotic Chromosome Dynamics in Zebrafish. Front Cell Dev Biol. 2021;9: 1–18. doi: 10.3389/fcell.2021.757445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trcek T, Lehmann R. All about the RNA after all. Elife. 2017;6: e24106. doi: 10.7554/eLife.24106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan M, Kumar L, Li Y, Baptissart M. Post-transcriptional regulation in spermatogenesis: all RNA pathways lead to healthy sperm. Cell Mol Life Sci. 2021;78: 8049–8071. doi: 10.1007/s00018-021-04012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15: 829–845. doi: 10.1038/nrg3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly CM, Dearth AT, Braun RE. Disruption of murine Tenr results in teratospermia and male infertility. Dev Biol. 2005;278: 13–21. doi: 10.1016/j.ydbio.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 15.Snyder E, Chukrallah L, Seltzer K, Goodwin L, Braun RE. ADAD1 and ADAD2, testis-specific adenosine deaminase domain-containing proteins, are required for male fertility. Sci Rep. 2020;10. doi: 10.1038/s41598-020-67834-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slanchev K, Stebler J, de la Cueva-Méndez G, Raz E. Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci U S A. 2005. doi: 10.1073/pnas.0407475102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegfried KR, Nüsslein-Volhard C. Germ line control of female sex determination in zebrafish. Dev Biol. 2008;324: 277–287. doi: 10.1016/j.ydbio.2008.09.025 [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Kossack ME, McFaul ME, Christensen L, Siebert S, Wyatt SR, et al. Single-cell transcriptome reveals insights into the development and function of the zebrafish ovary. Elife. 2022;11. doi: 10.7554/eLife.76014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian P, Kang J, Liu D, Xie G. Single Cell Transcriptome Sequencing of Zebrafish Testis Revealed Novel Spermatogenesis Marker Genes and Stronger Leydig-Germ Cell Paracrine Interactions. Front Genet. 2022;13. doi: 10.3389/fgene.2022.851719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi H. Juvenile Hermaphroditism in the Zebrafish, Brachydanio rerio. Bull Fac Fish Hokkaido Univ. 1977;28: 57–65. Available: http://www.mendeley.com/catalog/juvenile-hermaphroditism-zebrafish-brachydanio-rerio/ [Google Scholar]
- 21.Nóbrega RH, Greebe CD, van de Kant H, Bogerd J, de França LR, Schulz RW. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS One. 2010;5: 1–16. doi: 10.1371/journal.pone.0012808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertho S, Clapp M, Banisch TU, Bandemer J, Raz E, Marlow FL. Zebrafish dazl regulates cystogenesis and germline stem cell specification during the primordial germ cell to germline stem cell transition. Dev. 2021;148. doi: 10.1242/dev.187773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krøvel AV, Olsen LC. Expression of a vas::EGFP transgene in primordial germ cells of the zebrafish. Mech Dev. 2002;116: 141–50. Available: http://www.ncbi.nlm.nih.gov/pubmed/12128213 doi: 10.1016/s0925-4773(02)00154-5 [DOI] [PubMed] [Google Scholar]
- 24.Leu DH, Draper BW. The ziwi promoter drives germline-specific gene expression in zebrafish. Dev Dyn. 2010;239: 2714–2721. doi: 10.1002/dvdy.22404 [DOI] [PubMed] [Google Scholar]
- 25.Wang XG, Bartfai R, Sleptsova-Freidrich I, Orban L. The timing and extent of ‘juvenile ovary’phase are highly variable during zebrafish testis differentiation. J Fish Biol. 2007;70: 33–44. [Google Scholar]
- 26.Jin J, Liu J, Chen C, Liu Z, Jiang C, Chu H, et al. The deubiquitinase USP21 maintains the stemness of mouse embryonic stem cells via stabilization of Nanog. Nat Commun. 2016;7: 1–15. doi: 10.1038/ncomms13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiller CM, Feng CW, Jackson A, Gillis AJM, Rolland AD, Looijenga LHJ, et al. Endogenous Nodal signaling regulates germ cell potency during mammalian testis development. Dev. 2012;139: 4123–4132. doi: 10.1242/dev.083006 [DOI] [PubMed] [Google Scholar]
- 28.Tan K, Song H-W, Thompson M, Munyoki S, Sukhwani M, Hsieh T-C, et al. Transcriptome profiling reveals signaling conditions dictating human spermatogonia fate in vitro. Proc Natl Acad Sci. 2020;117: 17832–17841. doi: 10.1073/pnas.2000362117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sohni A, Tan K, Song HW, Burow D, de Rooij DG, Laurent L, et al. The Neonatal and Adult Human Testis Defined at the Single-Cell Level. Cell Rep. 2019;26: 1501–1517.e4. doi: 10.1016/j.celrep.2019.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allbee AW, Rincon-Limas DE, Biteau B. Lmx1a is required for the development of the ovarian stem cell niche in Drosophila. Development. 2018;145: dev163394. doi: 10.1242/dev.163394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda M, Inoue F, Ohkoshi K, Yokoyama S, Tatemizo A, Tokunaga T, et al. B-box and SPRY domain containing protein (BSPRY) is associated with the maintenance of mouse embryonic stem cell pluripotency and early embryonic development. J Reprod Dev. 2012; 2009–2011. doi: 10.1262/jrd.2011-009 [DOI] [PubMed] [Google Scholar]
- 32.Minakhina S, Changela N, Steward R. Zfrp8/PDCD2 is required in ovarian stem cells and interacts with the piRNA pathway machinery. Development. 2014;141: 259–268. doi: 10.1242/dev.101410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallanna SK, Ormsbee BD, Iacovino M, Gilmore JM, Cox JL, Kyba M, et al. Proteomic analysis of Sox2-associated proteins during early stages of mouse embryonic stem cell differentiation identifies Sox21 as a novel regulator of stem cell fate. Stem Cells. 2010;28: 1715–1727. doi: 10.1002/stem.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511: 353–357. doi: 10.1038/nature13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Tassone B, Ostano P, Katarkar A, Proust T, Joseph J, et al. HSD17B7 gene in self-renewal and oncogenicity of keratinocytes from Black versus White populations. EMBO Mol Med. 2021;13: e14133. doi: 10.15252/emmm.202114133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai-Cheong JE, Tanaka A, Parsons M, McGrath JA. Mutations in FERMT1 in Kindler syndrome implicate a role for fermitin family homolog 1 in epidermal stem cell maintenance and integrin activation. J Invest Dermatol. 2009;129: S81–S81. [Google Scholar]
- 37.Wong RC-B, Pollan S, Fong H, Ibrahim A, Smith EL, Ho M, et al. A novel role for an RNA polymerase III subunit POLR3G in regulating pluripotency in human embryonic stem cells. Stem Cells. 2011;29: 1517–1527. doi: 10.1002/stem.714 [DOI] [PubMed] [Google Scholar]
- 38.Gassei K, Orwig KE. SALL4 expression in gonocytes and spermatogonial clones of postnatal mouse testes. PLoS One. 2013;8: e53976. doi: 10.1371/journal.pone.0053976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hobbs RM, Fagoonee S, Papa A, Webster K, Altruda F, Nishinakamura R, et al. Functional antagonism between Sall4 and Plzf defines germline progenitors. Cell Stem Cell. 2012;10: 284–298. doi: 10.1016/j.stem.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan A-L, La HM, Legrand JMD, Mäkelä J-A, Eichenlaub M, De Seram M, et al. Germline stem cell activity is sustained by SALL4-dependent silencing of distinct tumor suppressor genes. Stem Cell Reports. 2017;9: 956–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Liu Y, Ye D, Li J, Liu J, Deng F. Knockdown of zebrafish Nanog increases primordial germ cells during early embryonic development. Dev Growth Differ. 2016;58: 355–366. doi: 10.1111/dgd.12279 [DOI] [PubMed] [Google Scholar]
- 42.Modesti M, Budzowska M, Baldeyron C, Demmers JAA, Ghirlando R, Kanaar R. RAD51AP1 is a structure-specific DNA binding protein that stimulates joint molecule formation during RAD51-mediated homologous recombination. Mol Cell. 2007;28: 468–481. doi: 10.1016/j.molcel.2007.08.025 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Fujiwara Y, Yamamoto S, Shibuya H. A meiosis-specific BRCA2 binding protein recruits recombinases to DNA double-strand breaks to ensure homologous recombination. Nat Commun. 2019;10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abreu CM, Prakash R, Romanienko PJ, Roig I, Keeney S, Jasin M. Shu complex SWS1-SWSAP1 promotes early steps in mouse meiotic recombination. Nat Commun. 2018;9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar R, Ghyselinck N, Ishiguro K, Watanabe Y, Kouznetsova A, Höög C, et al. MEI4–a central player in the regulation of meiotic DNA double-strand break formation in the mouse. J Cell Sci. 2015;128: 1800–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adelman CA, Lolo RL, Birkbak NJ, Murina O, Matsuzaki K, Horejsi Z, et al. HELQ promotes RAD51 paralogue-dependent repair to avert germ cell loss and tumorigenesis. Nature. 2013;502: 381–384. doi: 10.1038/nature12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Setton J, Selenica P, Mukherjee S, Shah R, Pecorari I, McMillan B, et al. Germline RAD51B variants confer susceptibility to breast and ovarian cancers deficient in homologous recombination. NPJ breast cancer. 2021;7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutzmann M, Grey C, Traver S, Ganier O, Maya-Mendoza A, Ranisavljevic N, et al. MCM8-and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol Cell. 2012;47: 523–534. doi: 10.1016/j.molcel.2012.05.048 [DOI] [PubMed] [Google Scholar]
- 49.Garre P, Briceño V, Xicola RM, Doyle BJ, de la Hoya M, Sanz J, et al. Analysis of the Oxidative Damage Repair Genes NUDT1, OGG1, and MUTYH in Patients from Mismatch Repair Proficient HNPCC Families (MSS-HNPCC) BER Pathway in MSS-HNPCC. Clin Cancer Res. 2011;17: 1701–1712. [DOI] [PubMed] [Google Scholar]
- 50.Roig I, Dowdle JA, Toth A, de Rooij DG, Jasin M, Keeney S. Mouse TRIP13/PCH2 is required for recombination and normal higher-order chromosome structure during meiosis. PLoS Genet. 2010;6: e1001062. doi: 10.1371/journal.pgen.1001062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsia K-T, Millar MR, King S, Selfridge J, Redhead NJ, Melton DW, et al. DNA repair gene Ercc1 is essential for normal spermatogenesis and oogenesis and for functional integrity of germ cell DNA in the mouse. 2003. doi: 10.1242/dev.00221 [DOI] [PubMed] [Google Scholar]
- 52.Li Q, Saito TT, Martinez-Garcia M, Deshong AJ, Nadarajan S, Lawrence KS, et al. The tumor suppressor BRCA1-BARD1 complex localizes to the synaptonemal complex and regulates recombination under meiotic dysfunction in Caenorhabditis elegans. PLoS Genet. 2018;14: e1007701. doi: 10.1371/journal.pgen.1007701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castor D, Nair N, Déclais A-C, Lachaud C, Toth R, Macartney TJ, et al. Cooperative control of holliday junction resolution and DNA repair by the SLX1 and MUS81-EME1 nucleases. Mol Cell. 2013;52: 221–233. doi: 10.1016/j.molcel.2013.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sha C, Chen L, Lin L, Li T, Wei H, Yang M, et al. TRDMT1 participates in the DNA damage repair of granulosa cells in premature ovarian failure. Aging (Albany NY). 2021;13: 15193. doi: 10.18632/aging.203080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gewiss RL, Law NC, Helsel AR, Shelden EA, Griswold MD. Two distinct Sertoli cell states are regulated via germ cell crosstalk. Biol Reprod. 2021;105: 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown PW, Hwang K, Schlegel PN, Morris PL. Small ubiquitin-related modifier (SUMO)-1, SUMO-2/3 and SUMOylation are involved with centromeric heterochromatin of chromosomes 9 and 1 and proteins of the synaptonemal complex during meiosis in men. Hum Reprod. 2008;23: 2850–2857. doi: 10.1093/humrep/den300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu D, Matzuk MM, Sung WK, Guo Q, Wang P. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20: 377–380. doi: 10.1038/3855 [DOI] [PubMed] [Google Scholar]
- 58.Miao L, Yuan Y, Cheng F, Fang J, Zhou F, Ma W, et al. Translation repression by maternal RNA binding protein Zar1 is essential for early oogenesis in zebrafish. Dev. 2017;144: 128–138. doi: 10.1242/dev.144642 [DOI] [PubMed] [Google Scholar]
- 59.Crackower MA, Kolas NK, Noguchi J, Sarao R, Kikuchi K, Kaneko H, et al. Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science (80-). 2003;300: 1291–1295. doi: 10.1126/science.1083022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kemiläinen H, Adam M, Mäki-Jouppila J, Damdimopoulou P, Damdimopoulos AE, Kere J, et al. The hydroxysteroid (17β) dehydrogenase family gene HSD17B12 is involved in the prostaglandin synthesis pathway, the ovarian function, and regulation of fertility. Endocrinology. 2016;157: 3719–3730. [DOI] [PubMed] [Google Scholar]
- 61.Xiol J, Cora E, Koglgruber R, Chuma S, Subramanian S, Hosokawa M, et al. A role for Fkbp6 and the chaperone machinery in piRNA amplification and transposon silencing. Mol Cell. 2012;47: 970–979. doi: 10.1016/j.molcel.2012.07.019 [DOI] [PubMed] [Google Scholar]
- 62.Sousa Martins JP, Liu X, Oke A, Arora R, Franciosi F, Viville S, et al. DAZL and CPEB1 regulate mRNA translation synergistically during oocyte maturation. J Cell Sci. 2016;129: 1271–1282. doi: 10.1242/jcs.179218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vasileva A, Tiedau D, Firooznia A, Müller-Reichert T, Jessberger R. Tdrd6 is required for spermiogenesis, chromatoid body architecture, and regulation of miRNA expression. Curr Biol. 2009;19: 630–639. doi: 10.1016/j.cub.2009.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pedrotti S, Busa R, Compagnucci C, Sette C. The RNA recognition motif protein RBM11 is a novel tissue-specific splicing regulator. Nucleic Acids Res. 2012;40: 1021–1032. doi: 10.1093/nar/gkr819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho YS, Iguchi N, Yang J, Handel MA, Hecht NB. Meiotic messenger RNA and noncoding RNA targets of the RNA-binding protein Translin (TSN) in mouse testis. Biol Reprod. 2005;73: 840–847. doi: 10.1095/biolreprod.105.042788 [DOI] [PubMed] [Google Scholar]
- 66.Yang G, Xin Q, Feng I, Dean J. Germ-cell specific eIF4E1B regulates maternal RNA translation to ensure zygotic genome activation. bioRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palacios IM. RNA processing: splicing and the cytoplasmic localisation of mRNA. Curr Biol. 2002;12: R50–R52. doi: 10.1016/s0960-9822(01)00671-6 [DOI] [PubMed] [Google Scholar]
- 68.Yabuta Y, Ohta H, Abe T, Kurimoto K, Chuma S, Saitou M. TDRD5 is required for retrotransposon silencing, chromatoid body assembly, and spermiogenesis in mice. J Cell Biol. 2011;192: 781–795. doi: 10.1083/jcb.201009043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saga Y. Function of Nanos2 in the male germ cell lineage in mice. Cell Mol life Sci. 2010;67: 3815–3822. doi: 10.1007/s00018-010-0456-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao Z, Mao X, Luo L. Germline stem cells drive ovary regeneration in zebrafish. Cell Rep. 2019;26: 1709–1717. doi: 10.1016/j.celrep.2019.01.061 [DOI] [PubMed] [Google Scholar]
- 71.Camp E, Sánchez-Sánchez A V., García-España A, DeSalle R, Odqvist L, O’Connor JE, et al. Nanog regulates proliferation during early fish development. Stem Cells. 2009;27: 2081–2091. doi: 10.1002/stem.133 [DOI] [PubMed] [Google Scholar]
- 72.Patra SK, Vemulawada C, Soren MM, Sundaray JK, Panda MK, Barman HK. Molecular characterization and expression patterns of Nanog gene validating its involvement in the embryonic development and maintenance of spermatogonial stem cells of farmed carp, Labeo rohita. J Anim Sci Biotechnol. 2018;9: 1–17. doi: 10.1186/s40104-018-0260-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao J, Wang J, Jiang J, Fan L, Wang W, Liu J, et al. Identification and characterization of a nanog homolog in Japanese flounder (Paralichthys olivaceus). Gene. 2013;531: 411–421. doi: 10.1016/j.gene.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 74.Liew WC, Orbán L. Zebrafish sex: a complicated affair. Brief Funct Genomics. 2014;13: 172–87. doi: 10.1093/bfgp/elt041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kossack ME, Draper BW. Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio). Curr Top Dev Biol. 2019;134: 119–149. doi: 10.1016/bs.ctdb.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elkouby YM, Mullins MC. Methods for the analysis of early oogenesis in Zebrafish. Dev Biol. 2016. [cited 14 Aug 2017]. doi: 10.1016/j.ydbio.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takemoto K, Imai Y, Saito K, Kawasaki T, Carlton PM, Ishiguro KI, et al. Sycp2 is essential for synaptonemal complex assembly, early meiotic recombination and homologous pairing in zebrafish spermatocytes. PLoS Genet. 2020;16: 1–29. doi: 10.1371/journal.pgen.1008640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Islam KN, Modi MM, Siegfried KR. The Zebrafish Meiotic Cohesin Complex Protein Smc1b Is Required for Key Events in Meiotic Prophase I. Front Cell Dev Biol. 2021;9: 1–17. doi: 10.3389/fcell.2021.714245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imai Y, Saito K, Takemoto K, Velilla F, Kawasaki T, Ishiguro KI, et al. Sycp1 Is Not Required for Subtelomeric DNA Double-Strand Breaks but Is Required for Homologous Alignment in Zebrafish Spermatocytes. Front Cell Dev Biol. 2021;9: 1–19. doi: 10.3389/fcell.2021.664377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blokhina YP, Frees MA, Nguyen A, Sharifi M, Chu DB, Bispo K, et al. Rad21l1 cohesin subunit is dispensable for spermatogenesis but not oogenesis in zebrafish. Cohen PE, editor. PLOS Genet. 2021;17: e1009127. doi: 10.1371/journal.pgen.1009127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang R, Dodd A, Lai D, McNabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin (Shanghai). 2007;39: 384–90. Available: http://www.ncbi.nlm.nih.gov/pubmed/17492136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan YJ, Tong SK, Hsu CW, Weng JH, Chung BC. Zebrafish Establish Female Germ Cell Identity by Advancing Cell Proliferation and Meiosis. Front Cell Dev Biol. 2022;10: 1–11. doi: 10.3389/fcell.2022.866267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blokhina YP, Nguyen AD, Draper BW, Burgess SM. The telomere bouquet is a hub where meiotic double-strand breaks, synapsis, and stable homolog juxtaposition are coordinated in the zebrafish, Danio rerio. PLoS Genet. 2019;15. doi: 10.1371/journal.pgen.1007730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hartung O, Forbes MM, Marlow FL. Zebrafish vasa is required for germ-cell differentiation and maintenance. Mol Reprod Dev. 2014;81: 946–961. doi: 10.1002/mrd.22414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Draper BW, McCallum CM, Moens CB. nanos1 is required to maintain oocyte production in adult zebrafish. Dev Biol. 2007;305: 589–598. doi: 10.1016/j.ydbio.2007.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomas JM, Beal PA. How do ADARs bind RNA? New protein-RNA structures illuminate substrate recognition by the RNA editing ADARs. BioEssays. John Wiley and Sons Inc.; 2017. doi: 10.1002/bies.201600187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Macbeth MR, Schubert HL, VanDemark AP, Lingam AT, Hill CP, Bass BL. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science (80-). 2005;309: 1534–1539. doi: 10.1126/science.1113150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schumacher JM, Lee K, Edelhoff S, Braun RE. Spnr, a murine RNA-binding protein that is localized to cytoplasmic microtubules. J Cell Biol. 1995;129: 1023–1032. doi: 10.1083/jcb.129.4.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu S-F, Zhang H, Cairns BR. Genes for embryo development are packaged in blocks of multivalent chromatin in zebrafish sperm. Genome Res. 2011;21: 578–589. doi: 10.1101/gr.113167.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schumacher JM, Lee K, Edelhoff S, Braun RE. Distribution of Tenr, an RNA-binding protein, in a lattice-like network within the spermatid nucleus in the mouse. Biol Reprod. 1995;52: 1274–1283. doi: 10.1095/biolreprod52.6.1274 [DOI] [PubMed] [Google Scholar]
- 91.Omolaoye TS, Hachim MY, du Plessis SS. Using publicly available transcriptomic data to identify mechanistic and diagnostic biomarkers in azoospermia and overall male infertility. Sci Rep. 2022;12: 1–17. doi: 10.1038/s41598-022-06476-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bansal SK, Gupta N, Sankhwar SN, Rajender S. Differential genes expression between fertile and infertile spermatozoa revealed by transcriptome analysis. PLoS One. 2015;10. doi: 10.1371/journal.pone.0127007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Webster KA, Henke K, Ingalls DM, Nahrin A, Harris MP, Siegfried KR. Cyclin-dependent kinase 21 is a novel regulator of proliferation and meiosis in the male germline of zebrafish. Reproduction. 2019;157: 383–398. doi: 10.1530/REP-18-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saito K, Siegfried KR, Nüsslein-Volhard C, Sakai N. Isolation and cytogenetic characterization of zebrafish meiotic prophase I mutants. Dev Dyn. 2011;240: 1779–1792. doi: 10.1002/dvdy.22661 [DOI] [PubMed] [Google Scholar]
- 95.Kettleborough RNW, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496: 494–497. doi: 10.1038/nature11992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bowen ME, Henke K, Siegfried KR, Warman ML, Harris MP. Efficient mapping and cloning of mutations in zebrafish by low-coverage whole-genome sequencing. Genetics. 2012;190: 1017–24. doi: 10.1534/genetics.111.136069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siegfried KR, Steinfeld JS. Histological Analysis of Gonads in Zebrafish. Methods in Molecular Biology. Humana Press Inc.; 2021. pp. 253–263. doi: 10.1007/978-1-0716-0970-5_20 [DOI] [PubMed] [Google Scholar]
- 98.Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, Lovell-Badge R, et al. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol. 2003;13: 1429–34. Available: http://www.ncbi.nlm.nih.gov/pubmed/12932328 doi: 10.1016/s0960-9822(03)00537-2 [DOI] [PubMed] [Google Scholar]
- 99.Knaut H, Pelegri F, Bohmann K, Schwarz H, Nüsslein-Volhard C. Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J Cell Biol. 2000;149: 875–88. Available: http://www.ncbi.nlm.nih.gov/pubmed/10811828 doi: 10.1083/jcb.149.4.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blokhina YP, Olaya I, Burgess SM. Preparation of meiotic chromosome spreads from zebrafish spermatocytes. J Vis Exp. 2020;2020. doi: 10.3791/60671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saito K, Sakai C, Kawasaki T, Sakai N. Telomere distribution pattern and synapsis initiation during spermatogenesis in zebrafish. Dev Dyn. 2014;243: 1448–1456. doi: 10.1002/dvdy.24166 [DOI] [PubMed] [Google Scholar]
- 102.Lawson ND, Li R, Shin M, Grosse A, Yukselen O, Stone OA, et al. An improved zebrafish transcriptome annotation for sensitive and comprehensive detection of cell type-specific genes. Elife. 2020;9: e55792. doi: 10.7554/eLife.55792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ge SX, Son EW, Yao R. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics. 2018;19: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
The nonsense mutation (sa14397) is located in exon 3 where a T<A mutation caused a premature stop codon (left panel). The missense mutation (t30103) is in exon 9 where a T<A mutation resulted in a Methionine to Lysine residue change (right panel).
(PDF)
The scl34a1aumb10 mutation is a 17 bp deletion (top panel). The guide-RNA target sequence, including the PAM, is highlighted in yellow. RT-PCR detected slc34a1a expression in adult testes (left panel). Homozygous slc34a1aumb10 mutants exhibited normal testes histology. Scale bar:50 μm.
(PDF)
A-D: Immunolabeling of Adad1 antibody on 60 dpf adad1Y67X mutant testis. No protein was detected as expected since the antibody epitope is at the C-terminus of the protein (A). Co-labeling with Ddx4/Vasa showed the presence of germ cells in the mutant testis (B). E-H: Immunolabeling of Adad1 on 45 dpf adad1M393K mutant testis (E) co-labeled with Ddx4/Vasa (F). Arrows point to cells with nuclear and cytoplasmic Adad1 localization. I: Western blot showing that the antibody recognized the 62 kDa size Adad1 protein in wild-type testes extracts but not in testes extracts from adad1Y67X mutants. The wild-type sample was loaded in two lanes: the left lane had twice the volume as the right lane. Ddx4/Vasa was detected in the mutant sample indicating that germ cells were present. Scale bar: 20 μm.
(PDF)
The nanos2 gene was not enriched in spermatogonial cell clusters SPG1 or SPG2 whereas known spermatogonia-expressed genes ddx4 and dazl were enriched in both.
(PDF)
A-D: Dot plot analysis of scRNAseq data from adult testes and 40dpf ovaries. Genes that are listed in Tables 2–4 are shown.
(PDF)
Data Availability Statement
RNA sequencing data has been submitted to NCBI BioProjects. Identifier: PRJNA980839. scRNAseq analysis code can be found at https://figshare.com/projects/Adad1_scRNA_analysis/173724.