Abstract
The p300 and CREB binding protein (CBP) transcriptional coactivators interact with a variety of transcription factors and regulate their activity. Among the interactions that have been described, the COOH-terminal region of p300 binds to cyclin E-cyclin-dependent kinase 2 (cyclin E-Cdk2) and TFIIB, as well as to the E1A gene products of adenovirus. Inhibition of Cdk activity by Cdk inhibitors, such as p21 or p27, potentiates NF-κB activity and provides a mechanism to coordinate cell cycle progression with the transcription of genes expressed during growth arrest. In this report, we analyze the specific domains of p300 required for the binding of p300 to cyclin E-Cdk2, TFIIB, and E1A and the ability of these proteins to interact with p300, alone or in combination. 12S E1A, an inhibitor of p300-dependent transcription, reduces the binding of TFIIB, but not that of cyclin E-Cdk2, to p300. In contrast, 13S E1A, a pleiotropic transcriptional activator, does not inhibit TFIIB binding to p300, although it enhances the interaction of cyclin E-Cdk2 with p300. Modification of cyclin E-Cdk2 is most likely required for association with p300 since the interaction is observed only with cyclin E-Cdk2 purified from mammalian cells. Domain swap studies show that the cyclin homology domain of TFIIB is involved in interactions with p300, although the homologous region from cyclin E does not mediate this interaction. These findings suggest that p300 or CBP function is regulated by interactions of various proteins with a common coactivator domain.
The role of the p300 and CREB binding protein (CBP) transcriptional coactivators in the regulation of cellular gene expression is complex and involves the modulation of gene expression in cooperation with many transcription factors. The activation of many cellular transcription factors by the p300 and CBP coactivators, including CREB (23), Myb (9), Fos (3), Jun (5), Sap1α (19), NF-κB (34), p53 (15, 25), Stat 1 (44), Stat 2 (7), nuclear receptors (21), and myogenic transcription factors (11), has been widely demonstrated, but the mechanisms for coordinating the regulation of these different transcriptional activators are poorly understood. p300 and CBP transcriptional coactivators likely function, in part, through their histone acetylation property (4, 31), which is also shared by p300/CBP-associated factor, P/CAF (43). In addition, the p300/CBP transcriptional coactivators may provide a link between specific transcription factors and the general transcriptional machinery through binding to TFIIB and TATA binding protein (TBP) (1, 10, 23). In vitro experiments with the beta interferon enhanceosome have demonstrated that certain transcriptional activators recruit TFIIB and other general transcription factors to the enhanceosome, which allows for subsequent recruitment of a CBP-containing RNA polymerase II holoenzyme (22). Thus, multiple contacts between promoter-bound factors and the holoenzyme may be required for assembly of a stable transcription complex, and the interaction of p300/CBP with both specific and general transcription factors may facilitate this process.
Proteins that regulate cellular signaling and proliferation have also been found in association with p300/CBP. For instance, pp90Rsk and p160Src have been shown to contribute to the control of p300/CBP function (28, 29). In addition, cyclin E–cyclin-dependent kinase 2 (cyclin E-Cdk2), required for the G1/S transition of the cell cycle, has been found in association with p300/CBP (34). These complexes also seem to regulate p300 function, since inhibition of cyclin E-Cdk2 by the p21 cyclin-dependent kinase inhibitor activates NF-κB function through p300 (34). The mechanism for regulation of p300 by cyclin E-Cdk2 complexes, however, has not been determined.
The adenoviral E1A proteins also bind to and inhibit p300/CBP (41). In addition to disrupting cell proliferation, E1A controls gene expression of both early viral and certain cellular transcripts. The single E1A message is spliced to give rise to two products, the 13S and 12S proteins of E1A, which share two conserved regions, CR1 and CR2, while 13S E1A contains a third motif, CR3, that is not present in the 12S form (13, 20). The transcriptional activating potential of 13S E1A is well documented and is linked to the CR3 region, the portion of E1A that interacts with the TBP (13, 14, 20). 12S E1A, however, represses transcription of certain viral and cellular genes (8, 12, 17, 18, 38–40), although its mechanism remains unclear.
Here we investigate the molecular mechanisms underlying the regulation of p300 function by E1A, cyclin E-Cdk2, and TFIIB. In particular, we analyze the requirements for p300 binding by cyclin E-Cdk2, the 12S and 13S proteins of E1A, and TFIIB and compare the specificities of and levels of competition between these interactions. We conclude that TFIIB, cyclin E-Cdk2, and E1A all bind within a common region of p300 and that competition for binding to p300 suggests a mechanism for integrating a wide variety of cellular signals.
MATERIALS AND METHODS
Plasmids, cell culture, and nuclear extracts.
The COOH region of p300 was cloned into pBluescript as described previously (34), with the introduction of a Kozak sequence upstream of amino acid 1240. BstEII, StuI, and StyI sites in the p300 insert were utilized to introduce termination codons following residues 1906, 1710, and 1542, respectively. A DNA fragment encoding residues 1532 to 1900 of p300 was amplified by PCR and fused downstream of the glutathione S-transferase (GST) gene in pGEX-CD to generate pGEX-p300 (residues 1532 to 1900) containing the cyclin E-Cdk2, TFIIB, and E1A binding sites. PCR products containing the 13S and 12S E1A cDNAs were cloned into pGEX-6P-1 (Pharmacia) to generate GST-13S E1A and GST-12S E1A.
Human TFIIB was cloned by reverse transcription-PCR from Jurkat cell mRNA by using SuperScript II Moloney murine leukemia virus reverse transcriptase (GIBCO BRL) and Pwo polymerase (Boehringer Mannheim) and ligated into the HindIII and BamHI sites of pBluescript SK in the T7 orientation to generate pBS-TFIIB. The TFIIB cDNA was also cloned into pGEX-CD for expression of GST-TFIIB. A PstI site was introduced into the TFIIB sequence by PCR to generate TFIIB-cyclin E (114/125) containing the N terminus of TFIIB (residues 1 to 114) fused to the C terminus of cyclin E (residues 125 to 395), which includes the cyclin box. The TFIIB-cyclin E (114/125) plasmid was used to generate a truncated TFIIB-cyclin E fusion by PCR with primers corresponding to the N-terminal TFIIB fragment (HindIII) and to the cyclin homology domain of cyclin E (XmaI). This fragment containing the TFIIB N terminus (residues 1 to 114) fused to the cyclin homology domain of cyclin E was cloned into pBluescript SK in the T7 orientation to generate pBS-TFIIB/Cyclin E cyclin homology domain.
GST proteins were expressed in BL21 (DE3) cells, and extracts were prepared as described elsewhere (36). GST fusion proteins were purified by using glutathione-Sepharose beads (Pharmacia) and washed three times with immunoprecipitation (IP) buffer (34). In vitro-translated proteins (T7 TNT rabbit reticulocyte transcription and translation system [Promega]) were incubated with purified GST proteins in IP buffer for 2 h, and the complexes were washed three times with IP buffer. Complexes were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 8% polyacrylamide). For binding of GST proteins to Cdk2 from nuclear extracts, purified GST proteins were incubated with 50 μg of Jurkat cell nuclear extract and washed three times with IP buffer. Jurkat human T leukemia cells were maintained in RPMI medium (GIBCO BRL) containing 5% fetal bovine serum, 50 μg of l-glutamine per ml, and 50 U of penicillin and streptomycin per ml. Nuclear extracts were prepared as previously described (33). Complexes were analyzed by SDS–8% PAGE, followed by Western blot analysis, by using an antibody to Cdk2 (SC-163; Santa Cruz). Cdk2 antibodies were used at a concentration of 0.5 μg/ml, and a secondary anti-rabbit antibody (Cappel) linked to horseradish peroxidase was used at a dilution of 1:8,000. Proteins were visualized with an enhanced chemiluminescence system (Amersham).
For binding to in vitro-translated proteins, immunoprecipitations were performed on 100 μg of Jurkat cell nuclear extracts with antibodies to Cdk2 (SC-6248; Santa Cruz) or TFIIB (SC-225; Santa Cruz). Complexes were washed three times with IP buffer, incubated for 2 h with in vitro-translated proteins, washed three additional times, and subjected to SDS-PAGE analysis. For immunoprecipitations of p300 followed by Western blot analysis with antibodies to Cdk2 or for immunoprecipitations of Cdk2 followed by Western blot analysis with antibodies to Cdk2, cyclin E (SC-198; Santa Cruz), and p300, 3 mg of Jurkat cell nuclear extract was used.
Competition assays.
GST proteins were purified as described above and eluted from glutathione-Sepharose beads by incubation with 10 mM reduced glutathione in IP buffer. Immunoprecipitations and binding of in vitro-translated proteins were performed as described above, except that at the time of addition of the in vitro-translated protein, increasing amounts of the eluted GST proteins were added.
RESULTS
A common region of p300 binds to Cdk, TFIIB, and E1A proteins.
To examine combinations of proteins that bind to p300, we compared the levels of binding of p300 to TFIIB, the 12S and 13S proteins of adenovirus E1A, and cyclin E-Cdk2 complexes. TFIIB and cyclin E-Cdk2 have been shown to interact with the COOH-terminal region of CBP and p300, respectively (23, 34). The specific requirement of amino acid residues 1767 to 1816 of p300 for binding to 12S E1A has been previously characterized (2).
To define the regions of p300 that bind to cyclin E-Cdk2, TFIIB, and the 12S and 13S proteins of E1A, these proteins were incubated with various truncations of in vitro-transcribed and -translated COOH-terminal p300. GST fusion proteins of TFIIB, 12S E1A, or 13S E1A were incubated with these translated radiolabeled COOH-terminal p300 fragments that spanned amino acids 1240 to 2414, 1240 to 1906, 1240 to 1710, and 1240 to 1542. TFIIB bound to all four p300 fragments, but the interaction was substantially less with the shortest p300 fragment, truncated at amino acid 1542 (Fig. 1A), than with a GST control. Both the 12S and 13S proteins of E1A also bound to these p300 fragments, and binding was still pronounced even with the shortest fragment (Fig. 1B). The binding of cyclin E-Cdk2 complexes to p300 was tested by incubating the in vitro-translated p300 fragments with immunoprecipitated cyclin E-Cdk2 complexes. Binding of cyclin E-Cdk2 to p300 most resembled the binding of 12S and 13S E1A proteins to p300 (Fig. 1C), suggesting that these proteins may recognize common epitopes of p300. While interactions of cyclin E-Cdk2, 12S and 13S E1A proteins, and TFIIB with p300 seem complex and may involve multiple domains, the localization of binding to similar regions of p300 suggested possible competition between these p300 binding proteins. In addition, since cyclin E-Cdk2, TFIIB, and 12S and 13S E1A proteins all bound to even the smallest carboxy-terminal p300 fragment, the possibility of competition among these proteins for binding to this p300 region was strengthened.
FIG. 1.
TFIIB, 12S and 13S E1A proteins, and cyclin E-Cdk2 bind to similar regions of p300. (A) TFIIB binds to p300 carboxy-terminal fragments ending at amino acids 2414, 1906, and 1710. In vitro-translated, carboxy-terminal p300 fragments were incubated with GST (lanes 2, 5, 8, and 11) or GST-TFIIB (lanes 3, 6, 9, and 12). The in vitro-translated p300 fragments each began at amino acid 1240. Complexes were subjected to SDS–8% PAGE analysis, and 10% of the in vitro translation reaction mixture was loaded in lanes 1, 4, 7, and 10. (B) GST-12S and -13S E1A proteins bind to p300 carboxy-terminal fragments ending at amino acids 2414, 1906, and 1542. In vitro-translated, carboxy-terminal p300 fragments were incubated with a control GST (lanes 14, 18, 22, and 26), with GST-13S E1A (lanes 15, 19, 23, and 27), or with GST-12S E1A (lanes 16, 20, 24, and 28); this was followed by SDS–8% PAGE analysis. Lanes 13, 17, 21, and 25 contain 10% of each in vitro translation reaction product. (C) Immunoprecipitated Cdk2 complexes bind to p300 carboxy-terminal fragments ending at amino acids 2414, 1906, and 1542. Products of immunoprecipitations with control antibodies (lanes 30, 33, 36, and 39) or antibodies to Cdk2 (lanes 31, 34, 37, and 40) were incubated with in vitro-translated p300 fragments and subjected to SDS–8% PAGE analysis. Lanes 29, 32, 35, and 38 contain 10% of the in vitro-translated p300 fragments. Arrows labeled a, b, c, and d indicate the positions of carboxy-terminal p300 fragments ending at residues 2414, 1906, 1710, and 1542, respectively.
Competitive binding of cyclin E-Cdk2 and TFIIB to p300 and effects of 12S and 13S E1A proteins on p300 binding activities.
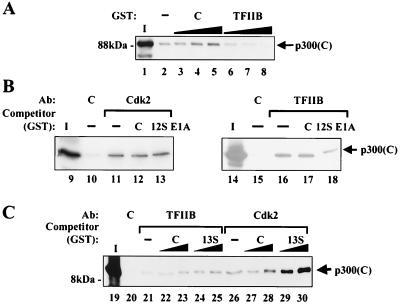
To examine the possibility that these proteins may affect the binding of one another to p300, competition experiments were performed. Immunoprecipitated cyclin E-Cdk2 was incubated with in vitro-translated COOH-terminal p300 and increasing amounts of control GST or GST-TFIIB. Addition of GST-TFIIB specifically inhibited the binding of cyclin E-Cdk2 to p300 (Fig. 2A), suggesting that p300 binds to TFIIB and cyclin E-Cdk2 through an overlapping site.
FIG. 2.
TFIIB and 13S E1A affect the binding of cyclin E-Cdk2 complexes to p300, while 12S E1A affects the binding of TFIIB to p300. (A) TFIIB inhibits interactions between cyclin E-Cdk2 and p300. Products of immunoprecipitations performed with antibodies to Cdk2 were incubated with increasing amounts of purified GST (lanes 3 to 5) or GST-TFIIB (lanes 6 to 8), as indicated, followed by the addition of an in vitro-translated carboxy-terminal p300 fragment spanning amino acids 1240 to 1906 [p300(C)]. Complexes were analyzed by SDS–8% PAGE. Lane 1 contains 10% of the in vitro-translated p300 sample, and lane 2 shows the control immunoprecipitate which contains no competitor GST protein. (B) 12S E1A weakly inhibits the interaction of p300 with TFIIB but has no effect on the interaction of p300 with cyclin E-Cdk2. (Left panel) Immunoprecipitations were performed with control antibodies (lane 10) or antibodies to Cdk2 (lanes 11 to 13). GST-12S E1A (lane 13) or an amount of GST exceeding that of GST-12S E1A in lane 13 (lane 12) were added to immunoprecipitates prior to incubation with in vitro-translated COOH-terminal p300 (amino acids 1240 to 2412) (similar results were obtained with p300 [amino acids 1240 to 1906; data not shown]). The control immunoprecipitate that lacks GST or GST-12S E1A is shown in lane 11. Lane 9 contains 10% of the p300 in vitro translation product. (Right panel) Immunoprecipitations were performed with control antibodies (lane 15) or antibodies to TFIIB (lanes 16 to 18). GST-12S E1A (lane 18) was added to immunoprecipitates prior to incubation with in vitro-translated, COOH-terminal p300 (amino acids 1240 to 1906). The control immunoprecipitate that lacks GST or GST-12S E1A is shown in lane 16. Lane 14 contains 10% of the p300 in vitro translation reaction product. (C) 13S E1A enhances the interaction between cyclin E-Cdk2 and p300 but has no effect on TFIIB-p300 complexes. Immunoprecipitations were performed with a control antibody (lane 20), an antibody to TFIIB (lanes 21 to 25), or antibodies to Cdk2 (lanes 26 to 30). Increasing amounts of control GST (lanes 22, 23, 27, and 28) or GST-13S E1A (lanes 24, 25, 29, and 30), as indicated, were added to immunoprecipitates prior to incubation with in vitro-translated, carboxy-terminal p300 (amino acids 1240 to 1906). The control immunoprecipitates that lack the addition of GST or GST-13S E1A are shown for TFIIB (lane 21) and Cdk2 (lane 26). Lane 19 contains 10% of the p300 in vitro translation reaction product. Ab, antibody.
12S E1A and 13S E1A were tested in the competition assay with immunoprecipitated cyclin E-Cdk2 and TFIIB. Interestingly, 12S E1A inhibited TFIIB binding to p300 but did not affect the cyclin E-Cdk2 interaction with p300 (Fig. 2B). In contrast, 13S E1A enhanced the binding of cyclin E-Cdk2 to p300 but had no effect on TFIIB binding to p300 (Fig. 2C). Densitometric analysis revealed that p300 binding to cyclin E-Cdk2 in the presence of GST-13S E1A was increased more than threefold relative to the binding observed in the presence of the control GST. These findings suggested that different forms of E1A may function to inhibit p300 activity by inhibiting the binding of p300 to the basal transcriptional machinery, as in the case of 12S E1A, or by enhancing interactions between p300 and cyclin E-Cdk2 complexes, as in the case of 13S E1A.
Differences in specificity of TFIIB and homologous cyclin E regions for interaction with p300.
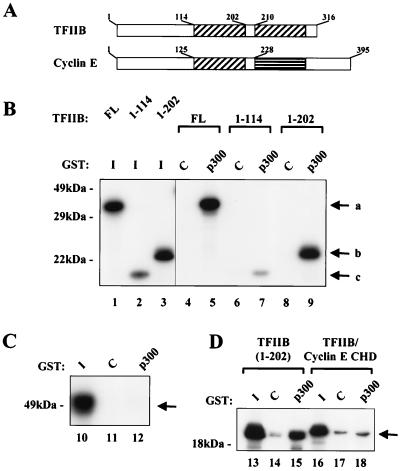
The ability of TFIIB to compete with cyclin E-Cdk2 for binding to p300 suggested that these proteins may share similar domains that interact with p300. The regions of TFIIB required for binding to CBP have been previously mapped to both NH2-terminal and COOH-terminal domains (23). The TFIIB NH2-terminal region contains a putative zinc-binding domain. The COOH-terminal region bears two 84-amino-acid imperfect direct repeats that form a protease-resistant core. This core domain is able to form a stable complex with TBP and DNA but cannot support further assembly of the basal transcription complex (6, 26, 42). In addition, the repeats are similar in their three-dimensional structure to a region in cyclin A, known as the cyclin box or cyclin homology domain (30) (Fig. 3A). Cyclins B, D1, and E contain homologous cyclin box sequences (16). Interactions of the cyclin E cyclin homology domain with p21 seem to resemble those demonstrated with the p27 cyclin-dependent kinase inhibitor and cyclin A (35); this, together with the similarity in the TFIIB and cyclin A structures, suggested that the cyclin boxes in TFIIB and cyclin E may have similar functions. We first examined the contribution of the domains of TFIIB to the TFIIB-p300 interaction. In vitro-translated deletion mutants of TFIIB were incubated with a GST-p300 fusion protein containing amino acids 1532 to 1900 of p300 or a control GST protein (Fig. 3B). The amino-terminal TFIIB fragment showed a substantial reduction in p300 binding compared to that of full-length TFIIB or the TFIIB fragment lacking the second repeat, suggesting a minimal requirement of both the NH2-terminal zinc binding region and the first direct cyclin repeat for optimal binding of TFIIB to p300. When in vitro-translated cyclin E was incubated with GST-p300, no binding was detected (Fig. 3C), indicating a difference in specificity of these gene products despite the presence of related cyclin boxes in these proteins.
FIG. 3.
The cyclin homology domain of TFIIB but not cyclin E interacts with p300. (A) Both TFIIB and cyclin E contain cyclin homology domains. The two cyclin homology domains in TFIIB and the one in cyclin E are indicated by hatched boxes. The amino acid end points of the relevant domains in both proteins are indicated. (B) The cyclin homology domain and the amino terminus of TFIIB interact with p300. In vitro-translated fragments of TFIIB, including the entire protein (FL), a form truncated at amino acid 114 (1-114), and a form truncated at amino acid 202 (1-202), were incubated with control GST (C) or GST-p300 (p300). Complexes were analyzed by SDS–12% PAGE, and lanes 1 to 3 were used to show 10% of the in vitro-translated products. The GST-p300 fragment contained amino acids 1532 to 1900. Arrows labeled a, b, and c indicate the positions of TFIIB amino acids 1 to 316, 1 to 202, and 1 to 114, respectively. (C) Cyclin E alone does not bind to p300. In vitro-translated cyclin E was incubated with control GST (lane 11) or GST-p300 (lane 12). Complexes were examined by SDS–12% PAGE. Lane 10 contains 10% of the in vitro-translated cyclin E. (D) A chimeric protein containing the amino terminus of TFIIB and the cyclin homology domain of cyclin E does not bind to p300. Control GST (lanes 14 and 17) or GST-p300 (lanes 15 and 18) was incubated with the in vitro-translated TFIIB fragment (amino acids 1 to 202) or a similar fragment in which the TFIIB cyclin homology domain was replaced with the cyclin E cyclin homology domain (TFIIB/cyclin E CHD). Complexes were analyzed by SDS–12% PAGE, and 10% of the input in vitro-translated proteins are shown in lanes 13 and 16.
To further define the contribution of the TFIIB domains to p300 binding, chimeric TFIIB-cyclin E vectors were constructed. Substitution of the first cyclin E repeat for the first TFIIB repeat eliminated the binding of TFIIB to p300 (Fig. 3D), demonstrating that the cyclin E cyclin box does not bind to p300. Interestingly, addition of the cyclin E repeat to TFIIB inhibited the ability of the NH2-terminal p300 binding domain of TFIIB to interact with p300. The structural integrity of the chimeric protein was confirmed through its ability to interact with the p21 cyclin-dependent kinase inhibitor (data not shown).
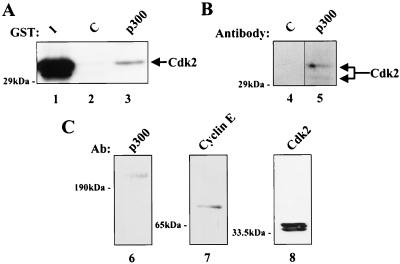
The lack of binding of cyclin E to p300 suggested that interactions of p300 with cyclin E-Cdk2 complexes may require regions of both cyclin E and Cdk2 in order to provide the binding motif for p300. We have previously demonstrated binding of p300 to immunoprecipitated cyclin E (34). In experiments using in vitro-translated cyclin E and Cdk2 and GST-p300 (amino acids 1532 to 1902), however, interactions between Cdk2, cyclin E, and p300 were not observed (data not shown). This finding suggested that intact cyclin E-Cdk2 from a cellular source may be required for complex formation. To examine this possibility, GST-p300 (amino acids 1532 to 1902) was incubated with nuclear extracts from Jurkat T lymphocytes, and Western blot analysis was performed to test for the presence of Cdk2 (Fig. 4A). Cdk2 was readily detected in the presence of GST-p300 but not in a control GST sample. Cdk2 was also found in p300 immunoprecipitates from Jurkat cell nuclear extracts (Fig. 4B), and both p300 and cyclin E coimmunoprecipitated with Cdk2 (Fig. 4C), confirming the presence of a p300-cyclin E-Cdk2 ternary complex in vivo. These results suggest that the cyclin E-Cdk2-p300 interaction requires specific protein-complex conformations or posttranslational modifications not present in in vitro-translated products.
FIG. 4.
The binding of p300 to cyclin E-Cdk2 requires cyclin E-Cdk2 from a cellular source. (A) GST-p300 binds Cdk2 from nuclear extracts. GST (lane 2) or GST-p300 (lane 3) was incubated with nuclear extracts from Jurkat T lymphocytes. Protein complexes were analyzed by SDS–12% PAGE followed by Western blotting using antibodies to Cdk2. Lane 1 contains 10% of the input nuclear extract. (B) Cdk2 and p300 coimmunoprecipitate in nuclear extracts. Immunoprecipitations were performed on Jurkat cell nuclear extracts with control antibodies (lane 4) or antibodies to p300 (lane 5). Protein complexes were analyzed by SDS–8% PAGE, followed by Western blotting with antibodies to Cdk2. (C) Cdk2, cyclin E, and p300 coimmunoprecipitate in nuclear extracts. Jurkat cell nuclear extracts were immunoprecipitated with antibodies to Cdk2. Protein complexes were resolved by SDS-PAGE, followed by Western blotting with antibodies to p300 (lane 6), cyclin E (lane 7), or Cdk2 (lane 8). Ab, antibody.
DISCUSSION
In this study, we show that E1A, cyclin E-Cdk2, and TFIIB each bind to overlapping COOH-terminal domains of p300, suggesting that E1A and cyclin E-Cdk2 may affect the interaction of p300 with TFIIB and regulate the link between specific and general transcription factors. While the 12S and 13S proteins of E1A, cyclin E-Cdk2, and TFIIB bind to a common region of p300, they appear to interact with p300 in different ways. TFIIB competes with cyclin E-Cdk2 for binding to p300, whereas the 13S form of E1A enhances the binding of cyclin E-Cdk2. 12S E1A inhibits the binding of TFIIB to p300, while 13S E1A has no effect on the p300-TFIIB interaction. Although TFIIB and cyclin E share a structurally similar cyclin homology domain, substitution of the cyclin E cyclin homology domain for the first TFIIB cyclin homology domain eliminates binding of TFIIB to p300. Cyclin E-Cdk2 binding to p300 occurs only with cyclin E-Cdk2 complexes from nuclear extracts, suggesting that this interaction requires additional factors or modifications not present in vitro. The ability of proteins involved in p300 regulation to alter the interactions of p300 with a critical general transcription factor suggests that p300 functions, in part, by linking cell cycle regulators and general transcription factors.
Our observations of cooperative and competitive interactions of cyclin E-Cdk2 and TFIIB with p300 provide mechanistic explanations for several previously described functional activities of these proteins. For instance, we have demonstrated that expression of the p21 cyclin-dependent kinase inhibitor activates human immunodeficiency virus transcription through NF-κB and p300 (34). This finding suggested that inhibition of cyclin E-Cdk2 complexes activates NF-κB through p300 and that active cyclin E-Cdk2 antagonizes this activation. We have also shown that p21 specifically inhibits cyclin E-Cdk2 complexes associated with p300-Rel A and CBP-Rel A complexes, confirming that one mechanism for p21 activation of NF-κB through p300-CBP is by its inhibition of associated cyclin E-Cdk2 complexes. While active cyclin E-Cdk2 complexes seem to inhibit p300-CBP function, TFIIB contributes to the activation of transcription by p300-CBP, as demonstrated by its involvement in the recruitment of a CBP-containing RNA polymerase II holoenzyme to the beta interferon enhancer (22). Thus, the observation reported here that TFIIB and cyclin E-Cdk2 complexes compete for binding to a common region of p300 provides an explanation for the opposing effects of TFIIB and cyclin E-Cdk2 complexes on p300 activity. This example of competitive interactions at the COOH terminus of p300 could be a mechanism that occurs with additional regulatory proteins to control a variety of promoters dependent on p300.
Although the binding of TFIIB and cyclin E-Cdk2 to p300 is competitive, cyclin E-Cdk2 and TFIIB differ in the biochemical basis for their interaction with p300. The binding of TFIIB involves, in part, its cyclin homology domain, but the corresponding region of cyclin E alone cannot facilitate p300 binding. This result is consistent with the findings of others who have shown that sequences within both the amino- and carboxy-terminal regions of TFIIB are necessary for its interaction with CBP (23). Intact cyclin E-Cdk2 complexes from nuclear extracts are required for interactions with p300, suggesting that a specific conformation or posttranslational modification of cyclin E-Cdk2 or additional polypeptides are needed to mediate interactions between cyclin E-Cdk2 and p300. Cdk2 function is modulated at specific cell cycle phases by phosphorylation and dephosphorylation at certain threonine and tyrosine residues (27). The requirement of the assembled cyclin E-Cdk2 complex and an additional role of phosphorylation of critical residues may ensure the formation of cyclin E-Cdk2-p300 complexes at distinct times for proper regulation of certain genes.
The 13S form of E1A enhances the binding of p300 by cyclin E-Cdk2. 13S E1A alters the effects of p300 on cell cycle control and activates the transcription of viral and cellular genes, presumably through interactions with TBP (14, 24). The mechanism for modulation of cell cycle control by p300 in the presence of 13S E1A may lie in its ability to increase cyclin E-Cdk2 association with p300, while the ability of 13S E1A to bind TBP may provide an alternative pathway by which to enhance transcription, for example, as observed in activation of NF-κB-independent human immunodeficiency virus transcription (32).
12S E1A inhibits the interaction of p300 with the general transcription factor TFIIB. This result suggests a model for 12S E1A-mediated repression whereby E1A inhibits gene expression by preventing p300 from interacting with a component of the general transcription machinery. Consistent with this hypothesis, overexpression of p300 eliminates repression by 12S E1A, presumably because additional p300 molecules titrate E1A, thus restoring the p300-TFIIB interaction (37). Further support for this model comes from experiments using an E2 DNA binding domain-p300 fusion protein that activates transcription from E2 binding sites. Arany et al. have shown that activation by p300-E2 is repressed by 12S E1A and that mutations in 12S E1A or p300 that impair the p300-E1A interaction also eliminate E1A-mediated repression of p300-E2 activity (2). The repressing activity of 12S E1A resides in the NH2-terminal portion of the protein, a region common to both the 12S and 13S forms. It is possible that CR3, the domain specific to 13S E1A, masks the TFIIB-competing activity found in the NH2 terminus.
The ability of viral and cell cycle regulatory proteins to regulate p300 function suggests that these proteins may alter the interaction of p300-CBP with other key regulatory proteins. In fact, E1A competes with the P/CAF histone acetylase for binding to p300. Conversely, E1A does not inhibit intrinsic p300-CBP histone acetyltransferase activity. Thus, E1A may inhibit p300 by one or both of two possible mechanisms: (i) by altering histone acetylation through competition with P/CAF and (ii) by altering the connections between p300 and the basal machinery by regulating levels of cyclin E-Cdk2 and TFIIB bound to p300. The ability of p300 to integrate diverse signal transduction pathways and activities of viral proteins may lie in the selective binding of p300 to specific subsets of proteins at defined times during cell growth and differentiation.
ACKNOWLEDGMENTS
We thank Donna Gschwend and Nancy Barrett for their assistance with preparation of the manuscript and figures. L.K.F. was supported by postdoctoral fellowships from the University of Michigan Immunopathology Training Program and the Cancer Research Institute.
REFERENCES
- 1.Abraham S E, Lobo S, Yaciuk P, Wang H-G H, Moran E. p300, and p300-associated proteins, are components of TATA-binding protein (TBP) complexes. Oncogene. 1993;8:1639–1647. [PubMed] [Google Scholar]
- 2.Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 3.Bannister A J, Kouzarides T. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J. 1995;14:4758–4762. doi: 10.1002/j.1460-2075.1995.tb00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 5.Bannister A J, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. [PubMed] [Google Scholar]
- 6.Barberis A, Muller C, Harrison S C, Ptashne M. Delineation of two functional regions of transcription factor TFIIB. Proc Natl Acad Sci USA. 1993;90:5628–5632. doi: 10.1073/pnas.90.12.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Eckner R, Grossman S, Oldfield E, Arany Z, D’Andrea A, Livingston D M. Cooperation of Stat2 and p300/CBP in signalling induced by interferon-α. Nature. 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- 8.Borrelli E, Hen R, Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984;312:608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- 9.Dai P, Akimaru H, Tanaka Y, Hou D, Yasukawa T, Kanei-Ishii C, Takahashi T, Ishii S. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 1996;10:528–540. doi: 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- 10.Dallas P B, Yaciuk P, Moran E. Characterization of monoclonal antibodies raised against p300: both p300 and CBP are present in intracellular TBP complexes. J Virol. 1997;71:1726–1731. doi: 10.1128/jvi.71.2.1726-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckner R, Yao T-P, Oldread E, Livingston D M. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 1996;10:2478–2490. doi: 10.1101/gad.10.19.2478. [DOI] [PubMed] [Google Scholar]
- 12.Enkemann S A, Konieczny S F, Taparow E J. Adenovirus 5 E1A represses muscle-specific enhancers and inhibits expression of the myogenic regulatory factor genes, MoD1 and myogenin. Cell Growth Differ. 1990;1:375–382. [PubMed] [Google Scholar]
- 13.Flint J, Shenk T. Viral transactivating proteins. Annu Rev Genet. 1997;31:177–212. doi: 10.1146/annurev.genet.31.1.177. [DOI] [PubMed] [Google Scholar]
- 14.Geisberg J V, Lee W S, Berk A J, Ricciardi R P. The zinc finger region of the adenovirus E1A transactivating domain complexes with the TATA box binding protein. Proc Natl Acad Sci USA. 1994;91:2488–2492. doi: 10.1073/pnas.91.7.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu W, Shi X-L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 16.Hadwiger J A, Wittenberg C, Richardson H E, de Barros Lopes M, Reed S I. A family of cyclin homologs that control the G1 phase in yeast. Proc Natl Acad Sci USA. 1989;86:6255–6259. doi: 10.1073/pnas.86.16.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hen R, Borrelli E, Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adevirus-2 E1A products. Science. 1985;230:1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- 18.Hen R, Borrelli E, Fromental C, Sassone-Corsi P, Chambon P. A mutated polyoma virus enhancer which is active in undifferentiated embryonal carcinoma cells is not repressed by adenovirus-2 E1A products. Nature. 1986;321:249–251. doi: 10.1038/321249a0. [DOI] [PubMed] [Google Scholar]
- 19.Janknecht R, Nordheim A. Regulation of the c-fos promoter by the ternary complex factor Sap-1α and its coactivator CBP. Oncogene. 1996;12:1961–1969. [PubMed] [Google Scholar]
- 20.Jones N. Transcriptional modulation by the adenovirus E1A gene. Curr Top Microbiol Immunol. 1995;199:59–80. doi: 10.1007/978-3-642-79586-2_4. [DOI] [PubMed] [Google Scholar]
- 21.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim T K, Kim T H, Maniatis T. Efficient recruitment of TFIIB and CBP-RNA polymerase II holoenzyme by an interferon-β enhanceosome in vitro. Proc Natl Acad Sci USA. 1998;95:12191–12196. doi: 10.1073/pnas.95.21.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwok R P S, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G E, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 24.Lee W S, Kao C C, Bryant G O, Liu X, Berk A J. Adenovirus E1A activation domain binds the basic repeat in the TATA box transcription factor. Cell. 1991;67:365–376. doi: 10.1016/0092-8674(91)90188-5. [DOI] [PubMed] [Google Scholar]
- 25.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 26.Malik S, Lee D K, Roeder R G. Potential RNA polymerase II-induced interactions of transcription factor TFIIB. Mol Cell Biol. 1993;13:6253–6259. doi: 10.1128/mcb.13.10.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima T, Fukamizu A, Takahachi J, Gage F H, Fisher T, Blenis J, Montminy M R. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima T, Fukamizu A, Takahashi J, Gage F H, Fisher T, Blenis J, Montminy M. The signal-dependent coactivator CBP is a nuclear target for pp90. Cell. 1996;86:465–474. doi: 10.1016/s0092-8674(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 30.Nikolov D B, Chen H, Halay E D, Usheva A A, Hisatake K, Lee D K, Roeder R G, Burley S K. Crystal structure of a TFIIB-TBP-TATA-element ternary complex. Nature. 1995;377:119–128. doi: 10.1038/377119a0. [DOI] [PubMed] [Google Scholar]
- 31.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 32.Parker S F, Felzien L K, Perkins N D, Imperiale M J, Nabel G J. Distinct domains of adenovirus E1A interact with specific cellular factors to differentially modulate human immunodeficiency virus transcription. J Virol. 1997;71:2004–2012. doi: 10.1128/jvi.71.3.2004-2012.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkins N D, Agranoff A B, Duckett C S, Nabel G J. The transcription factor AP-2 regulates human immunodeficiency virus-1 gene expression. J Virol. 1994;68:6820–6823. doi: 10.1128/jvi.68.10.6820-6823.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 co-activator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 35.Russo A A, Jeffrey P D, Paten A K, Massague J, Pavletich N P. Crystal structure of the p21Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 36.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 37.Smits P H, de Wit L, van der Eb A J, Zantema A. The adenovirus E1A-associated 300 kDa adaptor protein counteracts the inhibition of the collagenase promoter by E1A and represses transformation. Oncogene. 1996;12:1529–1535. [PubMed] [Google Scholar]
- 38.Stein R W, Corrigan M, Yaciuk P, Whelan J, Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990;64:4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velcich A, Ziff E. Adenovirus E1A proteins repress transcription from the SV40 early promoter. Cell. 1985;40:705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- 40.Ventura A M, Arens M Q, Srinivasan A, Chinnadurai G. Silencing of human immunodeficiency virus long terminal repeat expression by an adenovirus E1A mutant. Proc Natl Acad Sci USA. 1990;87:1310–1314. doi: 10.1073/pnas.87.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whyte P, Williamson N M, Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989;56:67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita S, Hisatake K, Kokubo T, Doi K, Roeder R G, Horikoshi M, Nakatani Y. Transcription factor TFIIB sites important for interaction with promoter-bound TFIID. Science. 1993;261:463–466. doi: 10.1126/science.8332911. [DOI] [PubMed] [Google Scholar]
- 43.Yang X-J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J J, Vinkemeier U, Gu W, Chakravarti D, Horvath C M, Darnell J E., Jr Two contact regions between Stat1 and CBP/p300 in interferon gamma signaling. Proc Natl Acad Sci USA. 1996;93:15092–15096. doi: 10.1073/pnas.93.26.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]