Abstract
Background
Perioperative interventions could enhance early mobilisation and physical function after hip fracture surgery.
Objective
Determine the effectiveness of perioperative interventions on early mobilisation and physical function after hip fracture.
Methods
Ovid MEDLINE, CINAHL, Embase, Scopus and Web of Science were searched from January 2000 to March 2022. English language experimental and quasi-experimental studies were included if patients were hospitalised for a fractured proximal femur with a mean age 65 years or older and reported measures of early mobilisation and physical function during the acute hospital admission. Data were pooled using a random effect meta-analysis.
Results
Twenty-eight studies were included from 1,327 citations. Studies were conducted in 26 countries on 8,192 participants with a mean age of 80 years. Pathways and models of care may provide a small increase in early mobilisation (standardised mean difference [SMD]: 0.20, 95% confidence interval [CI]: 0.01–0.39, I2 = 73%) and physical function (SMD: 0.07, 95% CI 0.00 to 0.15, I2 = 0%) and transcutaneous electrical nerve stimulation analgesia may provide a moderate improvement in function (SMD: 0.65, 95% CI: 0.24–1.05, I2 = 96%). The benefit of pre-operative mobilisation, multidisciplinary rehabilitation, recumbent cycling and clinical supervision on mobilisation and function remains uncertain. Evidence of no effect on mobilisation or function was identified for pre-emptive analgesia, intraoperative periarticular injections, continuous postoperative epidural infusion analgesia, occupational therapy training or nutritional supplements.
Conclusions
Perioperative interventions may improve early mobilisation and physical function after hip fracture surgery. Future studies are needed to model the causal mechanisms of perioperative interventions on mobilisation and function after hip fracture.
Keywords: surgery, pre-operative, post-operative, model of care, analgesia, systematic review, older people
Key Points
The primary goal of hip fracture surgery is to optimise health-related quality of life by alleviating pain and restoring physical function.
The effect of perioperative interventions on the ability to mobilise early postoperatively and restoration of physical function is relatively unknown.
Pathways, models of care and analgesia interventions may improve early mobilisation and physical function.
Introduction
Hip fracture is a life changing injury for older people that is associated with considerable morbidity, mortality, loss of independence and reduced health-related quality of life (HRQoL) [1–3]. Approximately 25% of hip fracture patients die within 12 months of injury; [4] of those who survive, around 40–70% fail to regain their previous level of physical function and 10–20% require new residential aged care facility accommodation [5]. By 2050, hip fractures are expected to affect 4.5 million people per year, representing considerable personal, health and societal cost from hospitalisation, rehabilitation and long-term support [6, 7].
The primary goal of treatment is to optimise HRQoL by alleviating pain and restoring physical function. For most hip fracture patients, this is best achieved with surgery followed by early mobilisation and rehabilitation [8, 9]. Early mobilisation is recommended in clinical practice guidelines usually by day 1 postoperatively [10, 11]. However, despite best efforts, only 20–50% of patients achieve first day walking and less than half receive physiotherapy for greater than two hours in the first 7 days after surgery [11, 12]. Factors thought to contribute to delay in first day walking include postoperative delirium, haemodynamic instability, pain, restricted weight bearing instructions, post-operative anaemia and patient expectations, [13] all of which are potentially amenable to interventions in the perioperative care period.
Perioperative interventions, such as analgesia regimens, timing of surgery and type of anaesthesia are recommended in clinical practice guidelines to address barriers to early mobilisation and optimise physical function outcomes [14–16]. However, the delivery of these perioperative interventions varies substantially between hospital sites and their impact on the ability to mobilise early postoperatively and restoration of physical function is not yet well understood. The aim of this systematic review is to determine the effectiveness of perioperative interventions on achieving early mobilisation and improving physical function after hip fracture.
Methods
A systematic review was undertaken and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses statement (PRISMA) [17]. The protocol was registered with Prospero (CRD42022313693) [18].
Search strategy and selection criteria
Five academic databases (Ovid MEDLINE, CINAHL, Embase, Scopus and Web of Science) were searched for peer-reviewed English language articles published between 1 January 2000 and 4 March 2022. Searches were supplemented by snowballing the reference lists of included articles, and relevant reviews and protocols identified, as well as forward citation tracking of articles citing those included in the review. The search strategy was created in collaboration with a medical librarian (Supplementary 1).
Study selection
Four reviewers (LT, SS, AC and NR) independently assessed the eligibility of title/abstract and full text articles for inclusion in pairs using Rayyan [19]. The inclusion criteria for the review are presented in Box 1. Any disagreements during screening were discussed and a third independent reviewer was consulted for the final decision where consensus could not be achieved (MS). Authors of studies where relevant data could not be obtained were contacted and excluded if no response was received.
Box 1.
Systematic review inclusion criteria
Participants
Mean age of 65 years and older and admitted to hospital with a fractured proximal femur.
Intervention
Perioperative interventions were defined as those delivered in preparation for surgery and during the operative period to recovery. This excluded the operation or procedure performed. Where one of the perioperative interventions also included early mobilisation, we considered the protocolised early mobilisation as the intervention, and whether it was achieved as the outcome.
Control
Any control conditions, including usual care or alternative interventions or exposures.
Outcome
Measures of both early mobilisation and physical function were included, considering the potential relationship between perioperative interventions on the ability to mobilise early and then improve eventual physical function outcomes.
Early mobilisation: Timing of commencement, proportion of patients mobilised early, or total amount of early postoperative mobilisation activity achieved postoperatively during the acute hospital admission.
Physical function: Any measure of function collected postoperatively during the acute hospital admission, including functional outcome assessments, distance walked and achievement of functional tasks, and level of independence during ambulation.
Types of study
Experimental and quasi-experimental studies.
Date of publication
Published between 1 January 2000 and 4 March 2022.
Language
English.
Data extraction
Data relating to study characteristics, interventions and outcomes were independently extracted to a customised Excel spreadsheet by reviewers in pairs (LT, SS, AC and NR), which was piloted before use. Risk of bias was assessed using the JBI Checklist for Randomised controlled trials (RCTs) or Checklist for Quasi-Experimental Studies (Supplement 2) [20]. Disagreements were resolved by discussion and a third independent reviewer was consulted for the final decision where consensus was not achieved (MS). Authors were contacted to request additional data as needed.
Data synthesis
Random effect meta-analysis was conducted where data were available for the same intervention and outcomes in two or more studies. Standardised mean difference (SMD) effect size was used for measures of early mobilisation and physical function with different scales and was interpreted according to Cohen’s d (0.2 = small, 0.5 = moderate and 0.8 = large) [21]. The mean and standard deviation were estimated for studies reporting medians and ranges, [22–26] using methods described by Wan et al. [27]. Dichotomous and continuous outcomes were combined using methods described by the Cochrane Handbook for Systematic Reviews of Interventions, which involved re-expressing odds ratios as SMDs [28]. Effect size directions were transformed to standardise positive effects as favouring the intervention and negative effects favouring the control. The I-squared statistic (I2) was used to represent heterogeneity in the study findings, with >50% considered substantial [29]. A leave-one-out sensitivity analysis was undertaken where overall heterogeneity levels were above 50% to further explore how each individual study affected the overall estimate of the rest of the studies. Analysis was performed using STATA Version 18 [30].
Early mobilisation: In studies where multiple measures of mobilisation were evaluated, time to first mobilisation was prioritised for the mobilisation outcome meta-analysis. Where not reported, number of patients mobilised on the earliest reported postoperative day (e.g. day 1) was used. Where activity during admission was reported, the number of mobilisation events was used instead of time spent mobilising.
Physical function: For studies with multiple functional outcomes assessed, the cumulated ambulation score (CAS) was prioritised as the functional outcome assessment for the meta-analysis. Totals or averages of functional outcomes over multiple inpatient days were used where reported; otherwise, the latest follow-up period was used (e.g. day of discharge). Distance walked was selected over achievement of tasks (e.g. walking beyond bedside chair) and ability to walk independently was selected over ability to walk with assistance. Ambulation capability over multiple distance categories were pooled to greater than 10 metres. The functional independence measure (FIM) motor function score was used over the locomotion sub-score.
A formal meta-regression was not planned, as it was anticipated that a small number of the included studies could be included in meta-analyses. A narrative synthesis was used to describe the data for the remaining studies.
Results
Study flow and characteristics of included studies
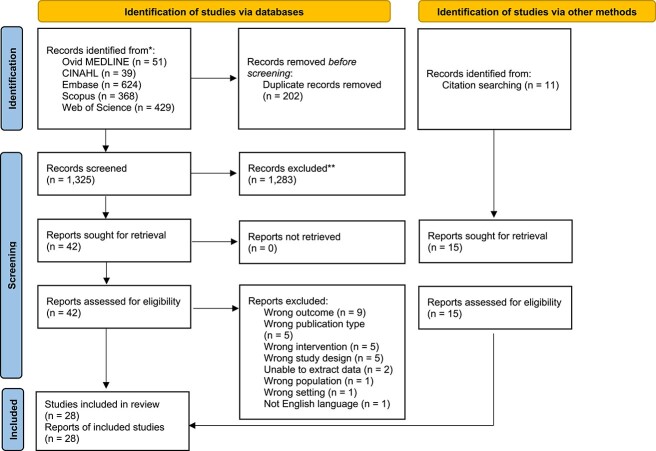
Twenty-eight studies were included in the review, from 1,327 identified citations (Figure 1). Eighteen studies were RCTs, five were non-randomised trials and five were controlled before and after studies. Studies were conducted in Sweden (n = 4), USA (n = 2), Australia (n = 3), China (n = 2), Denmark (n = 2), Japan (n = 2), Norway (n = 2), UK (n = 2), Korea (n = 1), Israel (n = 1), Italy (n = 1), Russia (n = 1), Spain (n = 1), Taiwan (n = 1), Ukraine (n = 1) and multinational (n = 2). Studies were conducted at a single (n = 22) or multiple hospital sites (n = 6). There were 8,192 participants across the included studies (range 41–781) with a mean age of 80 years. Interventions were grouped into six categories: analgesia, pathways and models of care, rehabilitation delivery modes, surgical protocols, nutritional supplements and clinical supervision. Early mobilisation outcomes were collected between postoperative days 1 and 7 or upon discharge. Physical function outcomes were collected between days 1 and 14 or upon discharge. Risk of bias assessment is reported in Supplement 2. Characteristics of the included studies are presented in Supplement 3, and the outcomes of perioperative interventions on early mobilisation and physical function after hip fracture surgery are presented in Supplement 4.
Figure 1.
PRISMA flow diagram of studies in the review.
Analgesia
Ten RCTs (n = 843) and one non-RCT (n = 103) reporting the effects of analgesia interventions in the perioperative period were identified. Six studies (n = 705) [22, 31–36] compared ultrasound guided peripheral nerve blocks to conventional pain management. Two studies (n = 104) [37, 38] compared active TENS to a sham device. One study (n = 82) [39] compared pre-emptive analgesic medication and intraoperative periarticular injections to standard care and another study (n = 55, 40) compared continuous postoperative epidural infusion to a placebo. Analgesia interventions had no clear overall effect on early mobilisation (SMD: 0.39, 95% CI: –0.35 to 1.13, I2 = 84%; Figure 2) or physical function (SMD: 0.64, 95% CI: –0.07 to 1.35, I2 = 96%; Figure 3). Subgroup analysis showed that TENS provided a moderate improvement in physical function (SMD: 0.65, 95% CI: 0.24–1.05, I2 = 96%; Figure 3). Leave-one-out sensitivity analysis suggested the absence of an effect persists irrespective of the omission of any one trial, with the exception of Foss et al. [40], where removed there was a large beneficial effect of analgesia interventions on physical function (Supplement 5-A and B).
Figure 2.
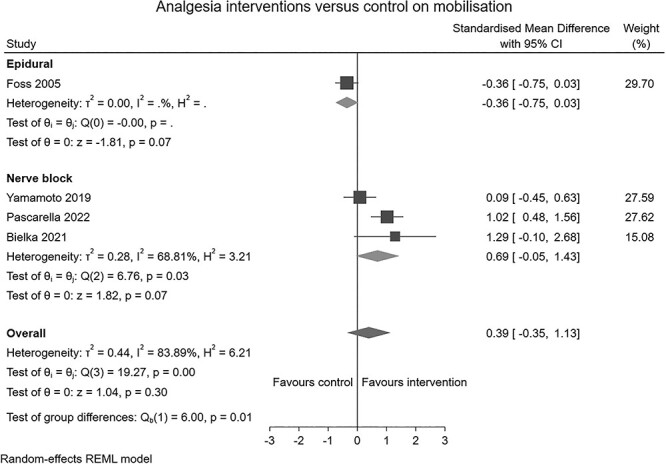
Forest plot of comparison: analgesia interventions versus control on mobilisation.
Figure 3.
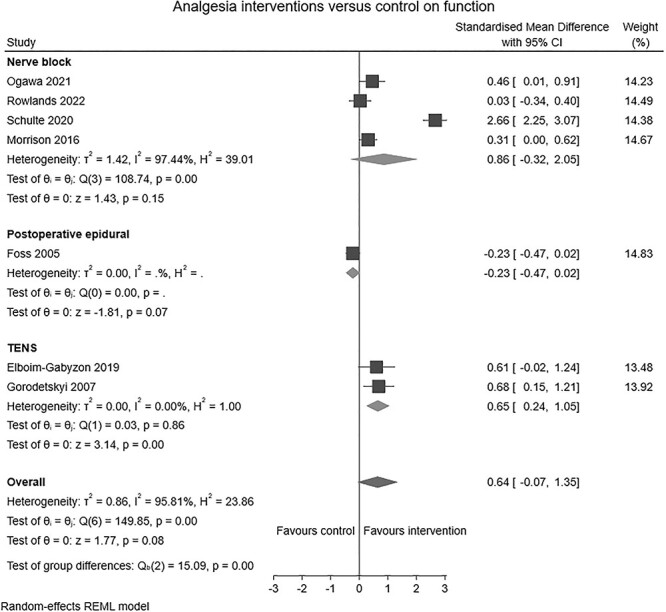
Forest plot of comparison: analgesia interventions versus control on function.
Pathways and models of care
Six RCTs (n = 1,210) [23, 41–45], one non-RCT (n = 244) [46] and two controlled before and after studies (n = 1,262) [47, 48] reporting the effects of perioperative pathways and models of care were identified. Four studies (n = 840) [23, 41, 42, 46] compared orthogeriatric models of care to conventional care. Five studies (n = 881) [43–45, 47, 48] compared Enhanced Recovery After Surgery (ERAS) care pathways to conventional postoperative care. Perioperative pathways and models of care produced a small positive effect on early mobilisation (SMD: 0.20, 95% CI: 0.01–0.39, I2 = 73%; Figure 4) and physical function (SMD: 0.07, 95% CI: 0.00–0.15, I2 = 0%; Figure 5). Leave-one-out sensitivity analysis suggested the small effect noted persisted following the removal of Gonzalez-Montalvo [46], Kang [43], Roberts [47] or Panella [45]. The effect did not persist following removal of remaining three studies (Supplement 5-C).
Figure 4.
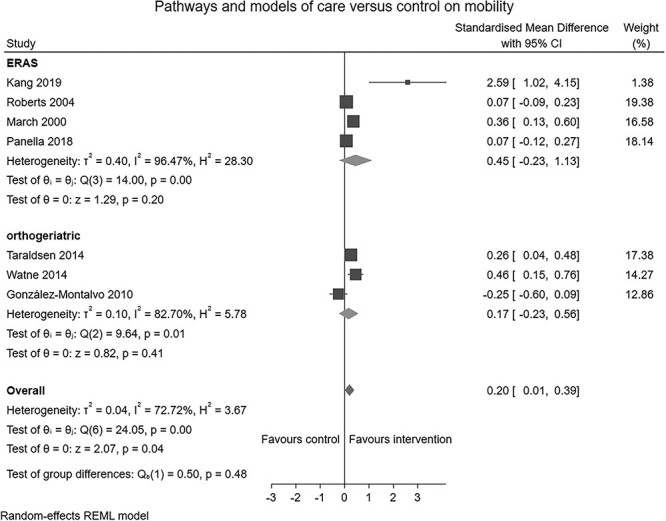
Forest plot of comparison: pathways and models of care versus control on mobilisation.
Figure 5.
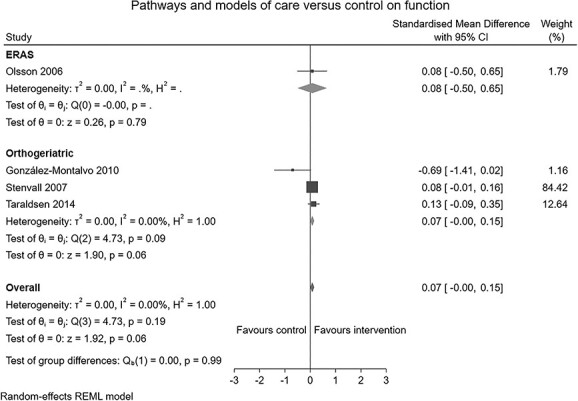
Forest plot of comparison: pathways and models of care versus control on function.
Rehabilitation delivery modes
One RCT (n = 100) [49] compared patients receiving occupational therapy training to those receiving conventional nursing care only, finding no significant difference in early mobilisation physical function. One RCT (n = 51) [24] compared recumbent cycling to usual care reporting similar between group function. One non-RCT (n = 150) [50] compared a preoperative mobilisation program with usual care finding significant improvements in physical function using the modified Barthel Index on admission day 3 and at discharge. One controlled before and after study (n = 155) [51] examined the effect of a multidisciplinary rehabilitation program compared to usual care, reporting significantly earlier ability to mobilise and less mobility decline (relative to pre-fracture mobility status).
Surgical protocols
One RCT (n = 2,970) [26] compared accelerated surgery (goal of surgery within 6 hours of diagnosis) to standard care, reporting reduced time to first mobilisation following surgery and no difference in physical function, time to first standing and weight bearing. One RCT (n = 162) [25] compared liberal transfusion thresholds to restrictive transfusion thresholds finding no significant difference in physical function on days 1–3.
Nutritional supplements
One controlled before and after study (n = 209) [52] compared nutritional supplements with usual care finding no significant improvement in walking assistance levels measured at day 5.
Clinical supervision
One controlled before and after study (n = 290) [53] compared the addition of direct face to face supervision of physiotherapists by an experienced orthopaedic physiotherapist external to the department to an existing reflective clinical supervision program. Patients receiving care from physiotherapists under the direct supervision program were more likely to mobilise day 1 and by day 2 postoperatively, and walked further day 5 with less assistance.
Discussion
This systematic review identified perioperative interventions that improved postoperative early mobilisation and physical function for hip fracture patients. TENS analgesia may provide a moderate improvement to physical function, and pathways and models of care may provide a small improvement in function, particularly orthogeriatric models. One study was identified supporting pre-operative mobilisation compared to bed rest for post-operative physical function when surgery was delayed beyond 48 hours. However, the average pre-operative waiting time in this study was greater than 6 days, limiting the generalisability to settings where operative waiting time is usually less than 48 hours. Other single studies supported improved early mobilisation for multidisciplinary rehabilitation delivery modes compared to usual care and direct clinical supervision of physiotherapists compared to usual reflective supervision. Multiple studies indicated less clear results for peripheral nerve blocks, and ERAS care pathways on early mobilisation and physical function, and orthogeriatric models and recumbent bike cycling on function. Single studies evaluating pre-emptive analgesia and intraoperative periarticular injections, continuous postoperative epidural infusion analgesia, indicated benefits for postoperative mobilisation and function. No improvement in physical function was identified from occupational therapy training rehabilitation delivery modes and nutritional supplements.
The proportion of patients achieving early mobilisation has been relatively resistant to improvement in the UK, Australia and New Zealand, despite increases in the proportion of hip fracture patients offered the opportunity to mobilise day 1 postoperatively [12, 54]. Several factors contribute to the inability to mobilise early, including the presence of delirium, low levels of pre-morbid mobility, older age, more days with a fever, urinary catheter or incontinence and non-use of anti-decubitus mattresses [13, 55]. Further barriers have been identified by treating physiotherapists, such as manual handling risks, patient declining rehabilitation, hypotension and pain [13]. Increasing opportunities for hip fracture patients to mobilise is important but other perioperative interventions are needed to overcome these barriers to improving physical function through early and ongoing rehabilitation in the acute phase postoperatively.
Peripheral nerve blocks and TENS analgesia interventions are thought to work by reducing reliance on opioids, which may avoid complications impacting the ability to mobilise including confusion, nausea, hemodynamic instability and chest infection [56, 57]. This mechanism is supported by Guay et al. who reported peripheral nerve blocks reduce pain, risk of acute confusion and probably reduce the risk of chest infection and time to first mobilisation [58]. There is a close bidirectional relationship between delirium and physical function [59], as immobility can be both a risk factor and a direct consequence of delirium [60–63]. Therefore, perioperative interventions that reduce the risk of delirium could also deliver dividends through earlier mobilisation and improved physical function.
Pathways and models of care may help to reduce variation by systematising the delivery of care [64]. Cooperation between orthopaedic surgeons, geriatricians and other multidisciplinary team members can lead to early identification of hip fracture patients for discharge planning and rehabilitation regimes [65]. Identifying and addressing comorbidities early is thought to optimise medical stability [66]; thereby reducing the risk of postoperative complications [67]. A study by Van Heghe et al. suggests that while there is evidence that orthogeriatric models of care reduce mortality and delirium and may reduce complications, the effect on functional outcome is inconsistent [68]. There is no ideal model identified to improve early postoperative mobilisation and physical function that is generalisable across different hospital sites with different contextual circumstances. The study by Snowdon et al. [53] evaluating clinical supervision of physiotherapists by an external senior orthopaedic physiotherapist could point to a potential mechanism for multidisciplinary team models improving mobility outcomes in an Australian context.
Mobilising hip fracture patients postoperatively can be resource intensive, often requiring two health professionals. Recumbent cycling offers an additional alternative mode to exercise that could be less resource intensive for therapists. However, the benefits on physical function were mixed, with the study authors suggesting benefits might not be expected to occur in the acute postoperative period and recommended a fully powered follow up trial [24]. Multidisciplinary rehabilitation programs were posited to offer more opportunities for early rehabilitation via nursing staff in addition to physiotherapists. Collaboration between members of the multidisciplinary team has been shown to result in fewer cases of death or loss of ability to live independently, although there is lower certainty of reductions in poorer functional outcomes at 12 months [69]. Pre-operative mobilisation targeted a different mechanism for improved post-operative early mobilisation, by preventing deconditioning and complications due to immobility while awaiting surgery. Immobilisation after fracture is a substantial contributor to poor prognosis and therefore efforts should be directed to improving time to surgery [70]. However, it is not uncommon for hip fracture patients to wait more than 48 hours for surgery [71]. Mobilisation during this pre-operative period could prevent functional deterioration, counter impaired ventilation and impaired cough reflex to reduce risk of pneumonia [72, 73], and prevent delirium and sleep disorders by helping to create a day and night routine [74].
Accelerated surgery reduces delirium, urinary tract infection and moderate to severe pain, which are closely related to early mobilisation [26]. However, the direction of this association between early mobilisation and these complications in the context of accelerated surgery is unclear. It is possible that early mobilisation itself reduces these complications rather than the absence of complications being a facilitator of early mobilisation. Foss et al. demonstrated that liberal transfusion thresholds did not affect physical function. Previous research has shown associations between postoperative anaemia and decreased mobilisation postoperatively [75–77]; however, correction via red blood cell transfusion has not previously been shown to improve rehabilitation outcomes [78].
Strengths and limitations
This review included only experimental and quasi-experimental study designs according to Cochrane Effective Practice and Organisation of Care study design criteria [79]. Most identified studies were RCTs, limiting the risk of confounding variables influencing the study findings. To ensure the capture of articles, forward and backward citation tracking snowballing of both included articles and other potentially relevant articles identified in the screening process was conducted. The included studies provided a relatively diverse sample (n = 8,192) from 26 countries and allowed comparisons across multiple studies evaluating similar perioperative interventions. However, the heterogeneity between methods, interventions and outcomes constrained our ability to examine pooled estimates across all included studies. Inclusive definitions of compared interventions and outcomes may have contributed to high levels of heterogeneity in our meta-analysis. Furthermore, some of the included studies did not restrict their inclusion criteria to older patient cohorts and reported on all patients over 18 years. These studies did not appear to have a younger average sample when compared to those with inclusion criteria selective of older age groups, but we were unable to examine the potential effect of age on the outcomes of interest. It is difficult to provided definitive recommendations for perioperative interventions with uncertain findings within and between studies, as well as those with only one study identified.
For analyses of analgesic approach, leave-one-out sensitivity analysis suggested the absence of an effect persists irrespective of the omission of any one trial. In contrast, the results of analyses of pathways and models of care varied with the omission of individual studies, but this did not appear to be related to underlying study quality.
Conclusion
The effect of several perioperative interventions on early mobilisation and physical function after hip fracture were identified in this systematic review. TENS, and orthogeriatric models and ERAS care pathways may improve physical function after hip fracture surgery. The benefit of peripheral nerve blocks, pre-operative mobilisation, multidisciplinary rehabilitation, recumbent cycling and clinical supervision is less certain. No improvement was identified for pre-emptive analgesia and intraoperative periarticular injections, continuous postoperative epidural infusion analgesia, occupational therapy training and nutritional supplements. Many barriers to early mobilisation are potential amenable to perioperative interventions. Yet, despite the importance of achieving early mobilisation and restoring physical function after hip fracture surgery, relatively few studies were identified. There is a lack of standardisation in outcome measurement and reporting practices that limits the ability to synthesise findings across studies. Future aetiologic studies are required to understand and model the causal mechanisms by which early mobilisation and physical function after hip fracture can be improved by perioperative interventions.
Supplementary Material
Acknowledgements
The authorship team wish to acknowledge the contributions of Sonia Singh to the screening of titles and abstracts for inclusion in this review.
Contributor Information
Mitchell N Sarkies, School of Health Sciences, Faculty of Medicine and Health, University of Sydney, Sydney NSW 2006, Australia.
Luke Testa, Australian Institute of Health Innovation, Faculty of Medicine, Health and Human Sciences, Macquarie University, Macquarie Park NSW 2113, Australia.
Ann Carrigan, Australian Institute of Health Innovation, Faculty of Medicine, Health and Human Sciences, Macquarie University, Macquarie Park NSW 2113, Australia.
Natalie Roberts, Australian Institute of Health Innovation, Faculty of Medicine, Health and Human Sciences, Macquarie University, Macquarie Park NSW 2113, Australia.
Rene Gray, James Paget University Hospital Foundation Trust, Norfolk NR31, UK.
Catherine Sherrington, Institute for Musculoskeletal Health, The University of Sydney and Sydney Local Health District, Sydney NSW 2006, Australia; School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney NSW 2006, Australia.
Rebecca Mitchell, Australian Institute of Health Innovation, Faculty of Medicine, Health and Human Sciences, Macquarie University, Macquarie Park NSW 2113, Australia.
Jacqueline C T Close, Falls, Balance and Injury Research Centre, Neuroscience Research Australia, Sydney NSW 2031, Australia; Prince of Wales Clinical School, University of New South Wales, Sydney NSW 2052, Australia.
Catherine McDougall, The University of Queensland, Brisbane 4072, Australia; The Prince Charles Hospital, Metro North Hospital and Health Service, Brisbane 4032, Australia.
Katie Sheehan, Department of Population Health Sciences, School of Life Course and Population Sciences, King’s College London, London WC2R, UK.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This study received funding from an NHMRC Investigator Grant (CIA Sarkies 2007970).
Data Availability Statement
Data is available from the corresponding author upon reasonable request.
References
- 1. Mitchell R, Draper B, Brodaty H. et al. An 11-year review of hip fracture hospitalisations, health outcomes, and predictors of access to in-hospital rehabilitation for adults ≥ 65 years living with and without dementia: a population-based cohort study. Osteoporos Int 2020; 31: 465–74. [DOI] [PubMed] [Google Scholar]
- 2. Griffin XL, Parsons N, Achten J, Fernandez M, Costa ML. Recovery of health-related quality of life in a United Kingdom hip fracture population. Bone Joint J 2015; 97-B: 372–82. [DOI] [PubMed] [Google Scholar]
- 3. Papadimitriou N, Tsilidis KK, Orfanos P. et al. Burden of hip fracture using disability-adjusted life-years: a pooled analysis of prospective cohorts in the CHANCES consortium. Lancet Glob Health 2017; 2: e239–46. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell R, Harvey L, Brodaty H, Draper B, Close J. One-year mortality after hip fracture in older individuals: the effects of delirium and dementia. Arch Gerontol Geriatr 2017; 72: 135–41. [DOI] [PubMed] [Google Scholar]
- 5. Dyer SM, Crotty M, Fairhall N. et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr 2016; 16: 158. 10.1186/s12877-016-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Veronese N, Maggi S. Epidemiology and social costs of hip fracture. Injury 2018; 49: 1458–60. [DOI] [PubMed] [Google Scholar]
- 7. Cooper C, Cole ZA, Holroyd CR. et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int 2011; 22: 1277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuckerman JD. Hip fracture. NEJM 1996; 334: 1519–25. [DOI] [PubMed] [Google Scholar]
- 9. Fairhall NJ, Dyer SM, Mak JCS. et al. Interventions for improving mobility after hip fracture surgery in adults. Cochrane Database Syst Rev 2022; 2022: CD001704. 10.1002/14651858.CD001704.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferris H, Brent L, Coughlan T. Early mobilisation reduces the risk of in-hospital mortality following hip fracture. Eur Geriatr Med 2020; 11: 527–33. [DOI] [PubMed] [Google Scholar]
- 11. Goubar A, Ayis S, Beaupre L. et al. The impact of the frequency, duration and type of physiotherapy on discharge after hip fracture surgery: a secondary analysis of UK national linked audit data. Osteoporos Int 2022; 33: 839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Australian and New Zealand Hip Fracture Registry . Annual Report of Hip Fracture Care 2021. Sydney. NSW: ANZHFR, 2022. [Google Scholar]
- 13. Said CM, Delahunt M, Ciavarella V. et al. Factors impacting early mobilization following hip fracture: an observational study. J Geriatr Phys Ther 2021; 44: 88–93. [DOI] [PubMed] [Google Scholar]
- 14. Australian Commission on Safety and Quality in Health Care . Hip Fracture Care Clinical Care Standard. ACSQHC. https://www.safetyandquality.gov.au/standards/clinical-care-standards/hip-fracture-care-clinical-care-standard (2016, date last accessed). [Google Scholar]
- 15. National Institute for Health Care Excellence . Hip Fracture: Management. Clinical Guideline CG124. NICE. https://www.nice.org.uk/guidance/cg124 (2011, date last accessed). [PubMed] [Google Scholar]
- 16. American Academy of Orthopaedic Surgeons . Management of Hip Fractures in Older Adults Evidence Based Clinical Practice Guideline. AAOS. https://www.aaos.org/hipfxcpg Published 12/03/2021 (2014, date last accessed). [DOI] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021; 10: 89. 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Testa L, Sarkies M, Sheehan K. Factors influencing mobilisation after hip fracture surgery: a systematic review. Prospero. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022313693 (8 August 2023, date last accessed).
- 19. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. JBI . Critical Appraisal Tools. Johanna Briggs Institute. https://jbi.global/critical-appraisal-tools (8 August 2023, date last accessed).
- 21. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hilsdale, NJ, USA: Lawrence Earlbaum Associates, 1988. [Google Scholar]
- 22. Schulte SS, Fernandez I, Van Tienderen R. et al. Impact of the fascia Iliaca block on pain, opioid consumption, and ambulation for patients with hip fractures: a prospective. Randomized Study J Orthop Trauma 2020; 34: 533–8. [DOI] [PubMed] [Google Scholar]
- 23. Watne LO, Torbergsen AC, Conroy S. et al. The effect of a pre- and postoperative orthogeriatric service on cognitive function in patients with hip fracture: randomized controlled trial. BMC Med 2014; 12: 63. 10.1186/1741-7015-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Said CM, Delahunt M, Hardidge A. et al. Recumbent cycling to improve outcomes in people with hip fracture: a feasibility randomized trial. BMC Geriatr 2022; 21: 394. 10.1186/s12877-021-02321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foss NB, Kristensen MT, Jensen PS, Palm H, Krasheninnikoff M, Kehlet H. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion 2009; 49: 227–34. [DOI] [PubMed] [Google Scholar]
- 26. The HIP ATTACK Investigators . Accelerated surgery versus standard care in HIP fracture (HIP ATTACK): an international, randomised, controlled trial. Lancet 2020; 395: 698–708. [DOI] [PubMed] [Google Scholar]
- 27. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. TJ HJPT, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2022. [Google Scholar]
- 29. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. StataCorp . Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC, 2023. [Google Scholar]
- 31. Bielka K, Kuchyn I, Tokar I, Artemenko V, Kashchii U. Psoas compartment block efficacy and safety for perioperative analgesia in the elderly with proximal femur fractures: a randomized controlled study. BMC Anesthesiol 2021; 21: 252. 10.1186/s12871-021-01473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morrison RS, Dickman E, Hwang U. et al. Regional nerve blocks improve pain and functional outcomes in hip fracture: a randomized controlled trial. JAGS 2016; 64: 2433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rowlands M, Walt GV, Bradley J. et al. Femoral nerve block intervention in neck of femur fracture (FINOF): a randomised controlled trial. BMJ Open 2018; 8: e019650. 10.1136/bmjopen-2017-019650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto N, Sakura S, Noda T. et al. Comparison of the postoperative analgesic efficacies of intravenous acetaminophen and fascia iliaca compartment block in hip fracture surgery: a randomised controlled trial. Injury 2019; 50: 1689–93. [DOI] [PubMed] [Google Scholar]
- 35. Ogawa T, Seki K, Tachibana T. et al. Early recovery of basic mobility under femoral nerve block after hip fracture surgery – a propensity score matched pilot study. Injury 2021; 52: 3382–7. [DOI] [PubMed] [Google Scholar]
- 36. Pascarella G, Costa F, Del Buono R. et al. Impact of the pericapsular nerve group (PENG) block on postoperative analgesia and functional recovery following total hip arthroplasty: a randomised, observer-masked, controlled trial. Anaesthesia 2021; 76: 1492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elboim-Gabyzon M, Andrawus Najjar S, Shtarker H. Effects of transcutaneous electrical nerve stimulation (TENS) on acute postoperative pain intensity and mobility after hip fracture: a double-blinded, randomized trial. Clin Interv Aging 2019; 14: 1841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gorodetskyi IG, Gorodnichenko AI, Tursin PS, Reshetnyak VK, Uskov ON. Non-invasive interactive neurostimulation in the post-operative recovery of patients with a trochanteric fracture of the femur. A randomised, controlled trial. J Bone Joint Surg Br 2007; 89: 1488–94. [DOI] [PubMed] [Google Scholar]
- 39. Kang H, Ha YC, Kim JY, Woo YC, Lee JS, Jang EC. Effectiveness of multimodal pain management after bipolar hemiarthroplasty for hip fracture: a randomized, controlled study. J Bone Joint Surg Am 2013; 95: 291–6. [DOI] [PubMed] [Google Scholar]
- 40. Foss NB, Kristensen MT, Kristensen BB, Jensen PS, Kehlet H. Effect of postoperative epidural analgesia on rehabilitation and pain after hip fracture surgery: a randomized, double-blind, placebo-controlled trial. Anesthesiology 2005; 102: 1197–204. 10.1097/00000542-200506000-00020. [DOI] [PubMed] [Google Scholar]
- 41. Taraldsen K, Sletvold O, Thingstad P. et al. Physical behavior and function early after hip fracture surgery in patients receiving comprehensive geriatric care or orthopedic care-a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2014; 69A: 338–45. [DOI] [PubMed] [Google Scholar]
- 42. Stenvall M, Olofsson B, Nyberg L, Lundström M, Gustafson Y. Improved performance in activities of daily living and mobility after a multidisciplinary postoperative rehabilitation in older people with femoral neck fracture: a randomized controlled trial with 1-year follow-up. J Rehabil Med 2007; 39: 232–8. [DOI] [PubMed] [Google Scholar]
- 43. Kang Y, Liu J, Chen H. et al. Enhanced recovery after surgery (ERAS) in elective intertrochanteric fracture patients result in reduced length of hospital stay (LOS) without compromising functional outcome. J Orthop Surg Res 2019; 14: 209. 10.1186/s13018-019-1238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olsson L-E, Karlsson J, Ekman I. The integrated care pathway reduced the number of hospital days by half: a prospective comparative study of patients with acute hip fracture. J Orthop Surg Res 2006; 1: 3. 10.1186/1749-799X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Panella M, Seys D, Sermeus W. et al. Minimal impact of a care pathway for geriatric hip fracture patients. Injury 2018; 49: 1581–6. [DOI] [PubMed] [Google Scholar]
- 46. Gonzalez-Montalvo JI, Alarcon T, Mauleon JL. et al. The orthogeriatric unit for acute patients: a new model of care that improves efficiency in the management of patients with hip fracture. Hip Int 2019; 20: 229–35. [DOI] [PubMed] [Google Scholar]
- 47. Roberts HC, Pickering RM, Onslow E. et al. The effectiveness of implementing a care pathway for femoral neck fracture in older people: a prospective controlled before and after study. Age Ageing 2004; 33: 178–84. [DOI] [PubMed] [Google Scholar]
- 48. March LM, Cameron ID, Cumming RG et al. Mortality and morbidity after hip fracture: can evidence based clinical pathways make a difference? J Rheumatol 2000; 27: 2227–31. [PubMed] [Google Scholar]
- 49. Hagsten B, Svensson O, Gardulf A. Early individualized postoperative occupational therapy training in 100 patients improves ADL after hip fracture: a randomized trial. Acta Orthop Scand 2004; 75: 177–83. [DOI] [PubMed] [Google Scholar]
- 50. Liu J, Chen L, Long C. et al. Effect of a preoperative mobilization program on perioperative complications and function recovery in older adults with femoral neck fracture. Geriatr Nurs 2022; 44: 69–75. [DOI] [PubMed] [Google Scholar]
- 51. Dai YT, Huang GS, Yang RS, Tsauo JY, Yang LH. Effectiveness of a multidisciplinary rehabilitation program in elderly patients with hip fractures. J Formos Med Assoc 2001; 100: 120–6. [PubMed] [Google Scholar]
- 52. Gunnarsson AK, Lönn K, Gunningberg L. Does nutritional intervention for patients with hip fractures reduce postoperative complications and improve rehabilitation? J Clin Nurs 2009; 18: 1325–33. [DOI] [PubMed] [Google Scholar]
- 53. Snowdon DA, Leggat S, Harding KE. et al. Direct supervision of physiotherapists improves compliance with clinical practice guidelines for patients with hip fracture: a controlled before-and-after study. Disabil Rehabil 2020; 42: 3825–32. [DOI] [PubMed] [Google Scholar]
- 54. NJR Editorial Board and contributors . National Joint Registry: 18th Annual Report. National Joint Registry. https://www.hqip.org.uk/wp-content/uploads/2021/11/njr-18th-annual-report-2021.pdf (8 August 2023, date last accessed).
- 55. Morri M, Forni C, Marchioni M, Bonetti E, Marseglia F, Cotti A. Which factors are independent predictors of early recovery of mobility in the older adults’ population after hip fracture? A cohort prognostic study. Arch Orthop Trauma Surg 2018; 138: 35–41. [DOI] [PubMed] [Google Scholar]
- 56. Saunders KW, Dunn KM, Merrill JO. et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med 2010; 25: 310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morrison SR, Magaziner J, McLaughlin MA. et al. The impact of post-operative pain on outcomes following hip fracture. Pain 2003; 103: 303–11. [DOI] [PubMed] [Google Scholar]
- 58. Guay J, Kopp S. Peripheral nerve blocks for hip fractures in adults. Cochrane Database Syst Rev 2020; 2021: CD001159. 10.1002/14651858.CD001159.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gual N, García-Salmones M, Brítez L. et al. The role of physical exercise and rehabilitation in delirium. Eur Geriatr Med 2020; 11: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord 1999; 10: 393–400. [DOI] [PubMed] [Google Scholar]
- 61. Laurila JV, Laakkonen M-L, Laurila JV, Timo SE, Reijo TS. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res 2008; 65: 249–54. [DOI] [PubMed] [Google Scholar]
- 62. Bickel H, Gradinger R, Kochs E, Förstl H. High risk of cognitive and functional decline after postoperative delirium. Dement Geriatr Cogn Disord 2008; 26: 26–31. [DOI] [PubMed] [Google Scholar]
- 63. Rudolph JL, Inouye SK, Jones RN. et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc 2010; 58: 643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tarazona-Santabalbina FJ, Belenguer-Varea Á, Rovira E. et al. Orthogeriatric care: improving patient outcomes. Clin Interv Aging 2016; 11: 843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moyet J, Deschasse G, Marquant B, Mertl P, Bloch F. Which is the optimal orthogeriatric care model to prevent mortality of elderly subjects post hip fractures? A systematic review and meta-analysis based on current clinical practice. Int Orthop 2019; 43: 1449–54. [DOI] [PubMed] [Google Scholar]
- 66. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc 2008; 56: 1349–56. [DOI] [PubMed] [Google Scholar]
- 67. Kammerlander C, Roth T, Friedman SM. et al. Ortho-geriatric service—a literature review comparing different models. Osteoporos Int 2010; 21: 637–46. [DOI] [PubMed] [Google Scholar]
- 68. Van Heghe A, Mordant G, Dupont J. et al. Effects of orthogeriatric care models on outcomes of hip fracture patients: a systematic review and meta-analysis. Calcif Tissue Int 2022; 110: 162–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Handoll HHG, Cameron ID, Mak JCS. et al. Multidisciplinary rehabilitation for older people with hip fractures. Cochrane Database Syst Rev 2021; 2021: CD007125. 10.1002/14651858.CD007125.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med 2000; 160: 2717–28. [DOI] [PubMed] [Google Scholar]
- 71. Klestil T, Röder C, Stotter C. et al. Impact of timing of surgery in elderly hip fracture patients: a systematic review and meta-analysis. Sci Rep 2018; 8: 13933. 10.1038/s41598-018-32098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Curtis LT. Prevention of hospital-acquired infections: review of non-pharmacological interventions. J Hosp Infect 2008; 69: 204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Janssens J-P, Krause K-H. Pneumonia in the very old. Lancet Infect Dis 2004; 4: 112–24. [DOI] [PubMed] [Google Scholar]
- 74. MLP M. Delirium. Ann Intern Med 2020; 173: ITC49–64. [DOI] [PubMed] [Google Scholar]
- 75. Halm EA, Wang JJ, Boockvar K. et al. The effect of perioperative anemia on clinical and functional outcomes in patients with hip fracture. J Orthop Trauma 2004; 18: 369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lawrence VA, Silverstein JH, Cornell JE, Pederson T, Noveck H, Carson JL. Higher Hb level is associated with better early functional recovery after hip fracture repair. Transfusion 2003; 43: 1717–22. [DOI] [PubMed] [Google Scholar]
- 77. Foss NB, Kristensen MT, Kehlet H. Anaemia impedes functional mobility after hip fracture surgery. Age Ageing 2008; 37: 173–8. [DOI] [PubMed] [Google Scholar]
- 78. Carson JL, Terrin ML, Barton FB. et al. A pilot randomized trial comparing symptomatic vs. hemoglobin-level- driven red blood cell transfusions following hip fracture. Transfusion 1998; 38: 522–9. [DOI] [PubMed] [Google Scholar]
- 79. Care. CEPOo . Reporting the effects of an intervention in EPOC reviews. EPOC Resources for review authors. Cochrane, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the corresponding author upon reasonable request.