Abstract
Human growth hormone (GH) is the indispensable hormone for the maintenance of normal physiological functions of the human body, including the growth, development, metabolism, and even immunoregulation. The GH is synthesized, secreted, and stored by somatotroph cells in adenohypophysis. Abnormal GH is associated with various GH-related diseases, such as acromegaly, dwarfism, diabetes, and cancer. Currently, some studies found there are dozens or even hundreds of GH proteoforms in tissue and serum as well as a series of GH-binding protein (GHBP) proteoforms and GH receptor (GHR) proteoforms were also identified. The structure-function relationship of protein hormone proteoforms is significantly important to reveal their overall physiological and pathophysiological mechanisms. We propose the use of proteoformics to study the relationship between every GH proteoform and different physiological/pathophysiological states to clarify the pathogenic mechanism of GH-related disease such as pituitary neuroendocrine tumor and conduct precise molecular classification to promote predictive preventive personalized medicine (PPPM / 3P medicine). This article reviews GH proteoformics in GH-related disease such as pituitary neuroendocrine tumor, which has the potential role to provide novel insight into pathogenic mechanism, discover novel therapeutic targets, identify effective GH proteoform biomarker for patient stratification, predictive diagnosis, and prognostic assessment, improve therapy method, and further accelerate the development of 3P medicine.
Keywords: Growth hormone proteoform, Growth hormone proteoformic pattern, Growth hormone receptor, Growth hormone receptor proteoform, Growth hormone-binding protein, Growth hormone-binding protein proteoform, Proteoform, Proteoformics, Growth hormone-related disease, Pituitary neuroendocrine tumor, Patient stratification, Predictive diagnosis, Prognostic assessment, Target therapy, Personalized medical service, Primary and secondary care, Healthcare efficacy, Individual outcomes, Predictive preventive personalized medicine (PPPM / 3P medicine)
Introduction
Brief description of growth hormone
The human growth hormone (GH), namely somatotropin, was first isolated in the 1930s, it is about eight decades since today [1]. A series of functions of GH were revealed, including promoting growth and development, regulating metabolism, and immunology [2, 3]. While if the GH in the body is disturbed, the body will suffer from various GH-related diseases, such as acromegaly, dwarfism, diabetes, and cancer [4]. Furthermore, an increasing number of GH proteoforms in pituitary tissue and serum were identified in plenty of studies [5–15], and the types and constitution of GH would dynamically vary from different physiological/pathological states of organism. However, the exact relationships between GH proteoforms and different physiological/pathological states remain unclear. This review proposes that the use of “proteoformics” to systematically study the function of every GH proteoform and the relationship between every GH proteoform and different physiological/pathological states to promote the development of predictive preventive personalized medicine (PPPM; 3P medicine). This review conducted a systematic and comprehensive summary of the proteoforms of human GH, GH-binding protein (GHBP), and GH receptor (GHR) to find specific biomarkers for GH-related diseases towards the development of 3P medicine.
Structure and functions of growth hormone
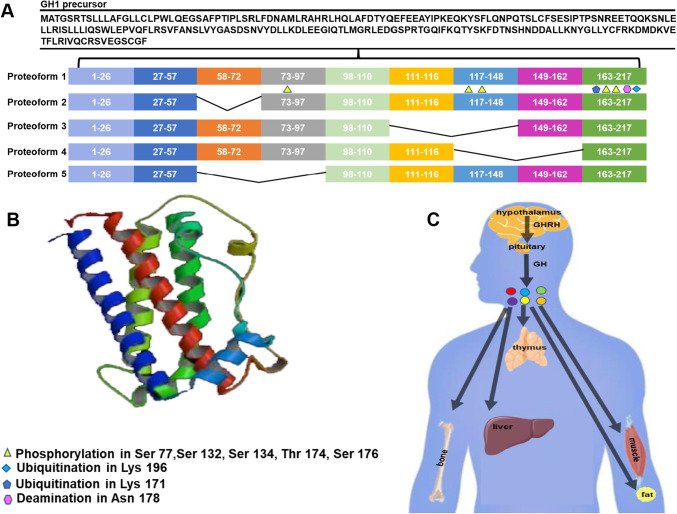
Human GH is a protein hormone with a single peptide chain, belonging to the somatotropin/prolactin (PRL) family, which is produced by GH-secreting cells, with eosinophilic granules, in the anterior pituitary [16, 17]. The human GH genes locate on chromosome 17q23.3 in humans [18, 19]. They include five highly homologous and structurally similar genes GH-N (GH normal gene; GH1 gene), GH-V (GH variant gene; GH2 gene), CS-A (chorionic somatomammotropin A), CS-B (chorionic somatomammotropin B), and CS-L (chorionic somatomammotropin-like gene) [16, 19, 20]. GH-N is expressed in the pituitary gland and the other four genes are expressed in placenta paralogs [16, 19, 20]. The GH-N cDNA encodes GH precursor with 217 amino acids (sequence 1–217), which includes a signal peptide at amino acid sequence positions 1–26 (Fig. 1A and B). There are two main mature forms of GH-22 kD and 20 kD [16, 21, 22]. The 22 kD GH owns 191 amino acids, and is the main existence form in the pituitary, which is necessary for human growth and development. In addition, the 20 kD GH owns 176 amino acids, with a lack in amino acid sequence positions 32 to 46, accounting for about 5 ~ 10% of total serum GH, which is produced by alternative splicing of mRNA [16, 21, 22]. Besides the two monomeric forms in pituitary tissue and serum, there are some other kinds of forms, including their fragments, homo- and heteropolymers, and complexes with binding proteins as well as receptors [3]. Currently, it has been found that GH owns more than one hundred of forms in circulation. In fact, the expression products of GH gene make up a protein family, rather than a single protein [3].
Fig. 1.
The structure and function of human growth hormone (hGH). A hGH amino acid sequence and its splicing variants. Data originates from Swiss-Prot No: P01241. The curve line represents the removed sequence after splicing. B The 3D structure of hGH. Data originates from PROTEIN DATA BANK (PDB). C Hypothalamic-pituitary-GH axis and the main targeted organs of hGH
GHR is a member of the GH/PRL/cytokine receptor superfamily, and its signaling is mainly transduced through the nonreceptor tyrosine kinase pathway [23]. The initiation of the pathway is started with the binding of GH to GHRs, then the dimerization of GHRs to activate JAK2 (Janus kinase 2), one of nonreceptor tyrosine kinases. Once activated, JAK2 tyrosyl-phosphorylates both itself and the cytoplasmic domain of GHR. The GHR-JAK2 complex can recruit and activate the signaling molecules including Stat (signal transducers and activators of transcription factors) and IRSs (insulin receptor substrates) 1 and 2, and further modulate a series of cellular functions. GH has a wide variation of physiological functions, and the implementation of its function cannot be achieved without GHR. GH affects almost all tissues and cell types in human body, including bone, muscle, fat [24], hair follicle [25], reproductive [26] and immune systems [27, 28], and even the hematopoietic system [29] and central nervous system [16, 30, 31] (Fig. 1C). The two basic functions of hGH are to stimulate the growth of cells, tissues, and organs, and regulate metabolism of nutrients [16]. On the one hand, it promotes the growth and development of the body through increasing the size and number of somatic cells as well as protein synthesis. On the other hand, for the regulation of metabolism, the overall effect of GH on protein metabolism is to promote anabolism, mainly through promoting amino acid transport into cells to increase protein synthesis and inhibit protein consumption [32]. Noteworthily, the effect of GH on protein synthesis is in harmony with its effect on growth. Furthermore, GH is a lipolysis hormone because it promotes fat degradation [24]. The effect of GH on glucose metabolism is secondary to its mobilization of fat. The increase of free fatty acids in the blood can inhibit glucose uptake by skeletal muscle and adipose tissues to reduce glucose consumption and increase blood glucose level, which is manifested as “anti-insulin” effect. GH also increases blood sugar by reducing the sensitivity of peripheral tissue to insulin.
Moreover, GH can promote the secretion of thymosin by thymic stromal cells, stimulate the production of antibodies by B lymphocytes, improve the activities of natural killer cells (NK cells) and macrophages, and thus participate in the function regulation of the immune system in the human body [28]. GH also has the effect of anti-aging [33, 34] and regulates emotional and behavioral activities [35]. GH is also one of the important stress hormones secreted by adenohypophysis.
The concept of proteoform and proteoformics
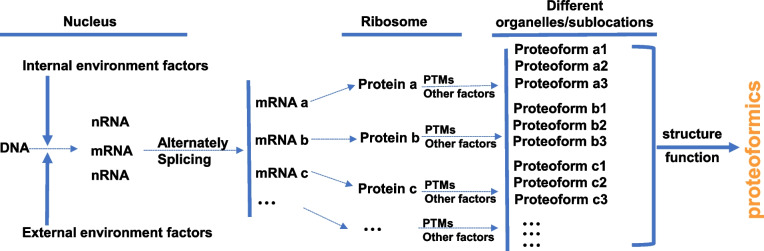
With the development of molecular biology, the human genome project identified ~ 20,300 genes, rather than ~ 100,000 genes as initially predicted [36, 37]. This showed that the cause of the diversity of biological functions is not huge number of genes but protein variety [38]. The diversity can originate from allelic variations on the DNA level, alternative splicing of RNA transcripts, epigenetic modifications on DNA and RNA levels, and a serious of post-translational modifications (PTMs) on the protein level (Fig. 2) [37, 39]. These events work together to produce different proteoforms originating from a single gene, which can involve in a wide range of biological processes, such as regulations of gene expression, energy metabolism, and cell signal transduction.
Fig. 2.
The formation of proteoform and concept of proteoformics. PTM: post-translational modification. Modified from Zhan et al. [37] with copyright permission from MDPI publisher open access article, copyright year 2019. Modified form Zhan et al. [39] with copyright permission from rom Hapres publisher open access article, copyright year 2018
In 2012, Smith and Kelleher first proposed the term “proteoform” to designate all of the different molecular forms in which the protein products of a single gene, including changes due to genetic variations, alternatively spliced RNA transcripts, and post-translational modifications [40].
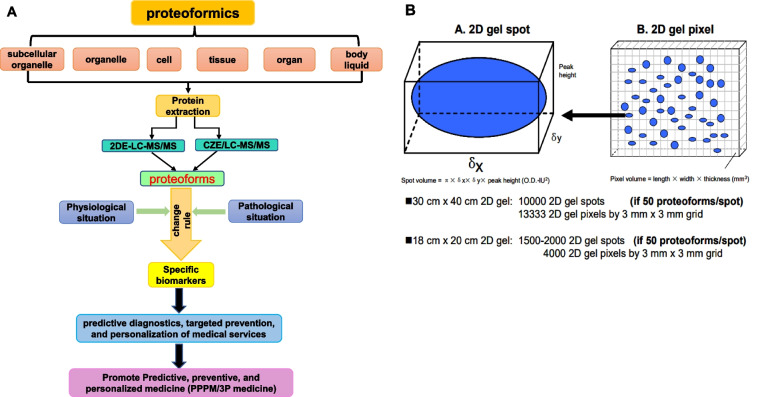
With the development of two-dimensional gel electrophoresis (2DGE) and mass spectrometry (MS), many proteoforms were identified, thus the concept of “proteoform” is enriched. The complete definition for a proteoform is that a proteoform is composed of its amino acid sequence, PTMs, spatial conformation, cofactors, binding partners, localization, and a function [37, 39, 41] (Fig. 2). A protein is an umbrella term for all proteoforms encoded by the same gene [41]. Proteoform is an objective substance and the term “proteoform” has been used for more than 10 years [40]. The term “proteomics” is commonly looked as the theories and methods to study “proteome” whose components are traditionally thought as the canonical “proteins” although currently ones look the “proteoforms” as the basic units of a proteome, and still use the term “proteomics” as the theories and methods to study proteoforms. In fact, to emphasize the important scientific merit of the term “proteoform,” it is necessary to propose a new term “proteoformics” that is the theories and methods to study proteoforms in a proteome (Fig. 2) [42]. The aim of “proteoformics” is to study the proteoform compositions and their change rules in a cell, tissue, organ, and organism as well as the effects of proteoforms on physiological/pathological processes in human body (Fig. 3A).
Fig. 3.
The methodology of proteoformics. A The general procedure and application of proteoformics. B The 2DE analysis of proteoforms. Modified from Zhan et al. [37] with copyright permission from MDPI publisher open access article, copyright year 2019. 2-DE two-dimensional gel electrophoresis, LC liquid chromatography, MS/MS tandem mass spectrometry, PTM post-translational modification
GH proteoforms
For human GH, currently, more than one hundred of its proteoforms have been identified in different tissues and sera [5, 9–15]. The reasons for the formation of GH proteoforms are currently described as gene variation, alternative splicing, and PTMs, and they are enriched with the development of proteoformics. The gene to code GH include five forms GH-N, GH-V, CS-A, CS-B, and CS-L, and they have expression tendency [3, 16, 18–20, 22]. GH-N is expressed in the pituitary gland, and the other four genes are expressed in the placenta. Due to alternative splicing, there are five forms of GH [3]. The GH isoform 1 is the most canonical form, with an amino acid sequence of 1-217. Differences from canonical form of GH, isoform 2 owns a missing of amino acid sequence 58-72, isoform 3 owns a missing of amino acid sequence 111–148, isoform 4 owns a missing of amino acid sequence 117–162, and isoform 5 owns a missing of amino acid sequence 58–97 (Fig. 1A). Furthermore, GH contains a signal peptide with the amino acid sequence 1–26. Every isoform of GH contains two forms - the removal and non-removal of signal peptide. In addition, a number of PTMs are also identified [5, 9]; for example, phosphorylation is characterized at residues Ser77, Ser132, Ser134, Thr174, and Ser176, ubiquitination at residue Lys196, acetylation at residue Lys171, and deamination at residue Asn178; and more kinds of PTMs and PTM-sites will be found. All of these PTMs together make up the diversity of GH but the specific physiological function of every GH proteoform has not been elucidated.
GH proteoforms and diseases
The maintenance of homeostasis of GH levels is significantly essential to perform physiological functions of the human body. Whereas, once GH levels are disturbed, a series of GH-related disorders appears. During childhood, hyposecretion of GH will cause dwarfism, whose characteristics are slow growth, skeletal hypoplasia, and accompanying with sexual organ agenesis or lack of secondary sex characteristics [43–46]. While hypersecretion of GH can cause hyperplasia of bone and cartilage, metabolic disorders, and complications [47]. For the adult, the disorder of GH, which is mainly caused by PitNETs, can lead to acromegaly with a typical acromegaly facies, hypertrophy of hands and feet, skin hypertrophy, visceral enlargement, and various metabolic diseases and so on.
Techniques to study GH proteoforms
It is estimated that the number of human proteoforms exceeds one million [37]. It is very urgent to find an effective method to achieve a high-throughput test of all proteoforms. Currently, there are two main strategies to study proteoforms: top-down mass spectrometry-based strategy (Top-down MS), and two-dimensional gel electrophoresis in combination with liquid chromatography-mass spectrometry strategy (2DE-LC/MS) (Fig. 3). Each strategy has its own advantages and disadvantages in the analysis of proteoforms [37].
-
(i)
Top-down MS: the general procedure of top-down MS is that, first, protein separation technology, such as capillary zone electrophoresis (CZE) and LC, is used to separate proteoforms; second, the separated proteoforms are analyzed with MS/MS; and third, MS/MS data are used to identify the amino acid sequence and PTMs against protein database (Fig. 3A) [48, 49]. The drawback of this method is that its analytical throughout is not high. Currently, the maximum analytical throughput is ~ 30,000 proteoforms derived from 1690 human genes [50]. Although this throughput is nearly 10 times more than previous studies, it is still much far from the number of all proteoforms in the human body.
-
(ii)
2DE-LC/MS: the general procedure of 2DE-LC/MS strategy is that, first, 2DE is used to separate proteoforms per their isoelectric point (pI) and relative mass (Mr); second, the separated proteoforms are digested with trypsin, followed by MS/MS identification; third, MS/MS data are used to identify the amino acid sequence and PTMs against protein database [37, 51]. Because each 2D gel spot includes over 50 to several hundreds of proteoforms, and 2DE can separate low-abundance proteoforms, 2DE-LC/MS has great potential for high-throughput analysis of proteoforms (Fig. 3B) [51].
GH proteoforms in pituitary diseases
GH proteoforms in pituitary tissues
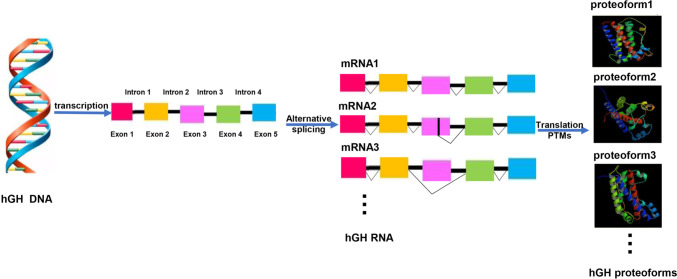
GH presents a variety of proteoforms, including monomeric [52–54], an oligomeric series of dimeric to pentameric forms [55], and segments/fragments, with different modifications, such as glycosylation, phosphorylation, acetylation, ubiquitination, and deamination [9, 10, 52, 53]. All of these factors cause the human GH heterogeneity in structure and function, which might be significant to clarify various GH functions in various physiological/pathological conditions (Fig. 4).
Fig. 4.
The formation of hGH proteoforms. The broken line represents the splicing methods of hGH exons. Data originates from Swiss-Prot No: P01241
Different GH proteoforms are identified in the pituitary gland, and their functions are also clarified partially [5–10, 56, 57]. In 1990s, the 17 kD GH fragment (amino acid sequence 44–191) and the 5 kD GH fragment (amino acid sequence 1–43) were identified in pituitary and plasma in several studies, and the 17kD GH was found to involve in glucose intolerance [6–8, 56]. Later with the development of 2DE and mass spectrometry, more GH proteoforms were identified. In 2009, 9 kD and 12 kD GH fragments, 20 kD GH, 22 kD GH, and 23 kD GH with glycosylation were identified in the pituitary, and different GH proteoforms show different abundance [10]. Furthermore, PitNET has a high prevalence and causes huge pain for patients, but its pathogenesis mechanism has not been clarified. In 2005 and 2021, two systematic studies on GH proteoforms were conducted [5, 9]. At first, a total of 24 human GH proteoforms [5] were identified in normal pituitary with 2DGE coupled with mass spectrometry, and those GH proteoforms showed significantly different abundance. Moreover, phosphorylation at residues Ser-77, Ser-132, and Ser-176, and deamination at residue Asn-178 were identified in GH. These findings not only enrich the GH proteoform atlas but also provide a new perspective to further explore the action mechanisms of GH in physiological/pathological conditions of the human body (Fig. 5A and B) [5]. Later in 2021 [9], a total of 46 GH proteoforms in GH-secreting PitNETs and 35 GH proteoforms in control pituitaries were identified with 2DE in combination with LC-ESI-MS/MS, MALDI-TOF-MS, and MALDI-TOF-TOF-MS/MS (Fig. 5C). Further analysis of every GH proteoform found that 11 GH proteoforms only existed in GH-secreting PitNETs but not in control pituitaries, and the remaining 35 GH proteoforms existed in both GH-secreting PitNETs and control pituitaries and had different abundance differences in tumors and controls. Different PTMs (acetylation, ubiquitination, phosphorylation, and deamination) were also characterized in GH proteoforms between GH-secreting PitNETs and controls. For example, phosphorylation has been characterized in GH proteoforms with MS/MS analysis (Fig. 6), and MS/MS analysis can accurately discriminate phosphorylated peptide and non-phosphorylated peptide that were derived from GH proteoforms (Fig. 7). This study provides the first human GH proteoform atlas and their partial PTMs in GH-secreting PitNETs compared to normal control pituitary tissues, which provides novel perspective to predict, prevent, treat, and conduct prognostic assessment for effective medical service for GH-secreting PitNETs, especially insight into development of feasible GH proteoformic pattern biomarkers for prediction, prevention, diagnosis, patient stratification, and prognostic assessment of GH-secreting PitNETs and GH-related diseases in the context of PPPM practice [9].
Fig. 5.
The hGH proteoforms identified in normal pituitary and GH-secreting PitNETs. A 2DE image of GH proteoforms in human pituitary tissues. A total of 24 hGH proteoforms were identified with 2DE and MS, and labeled in 2DE map. Modified from Zhan et al. [5] with copyright permission from Wiley publisher, copyright year 2005. B. The MALDI-TOF-MS PMF of GH proteoform in spot 6 in Fig. 5A. T means the autodigestion fragments of trypsin. M* means oxidized Met; C# means carbamidomethyl-Cys; N@ means deamidated Asn. Modified from Zhan et al. [5] with copyright permission from Wiley publisher, copyright year 2005. C 2DE image of GH proteoforms in GH-secreting PitNETs relative to control pituitary tissues. (a) 2DE image of GH proteoforms in GH-secreting PitNETs. (b) 2DE image of GH proteoforms in control pituitary tissues. (c) Western blot image of GH proteoforms in GH-secreting PitNETs. (d) Western blot image of GH proteoforms in control pituitary tissues. Modified from Li et al. [9] with copyright permission from Springer publisher open access article, copyright year 2021. IEF isoelectric focusing, GH growth hormone, 2DGE two-dimensional gel electrophoresis, m/z mass to charge
Fig. 6.
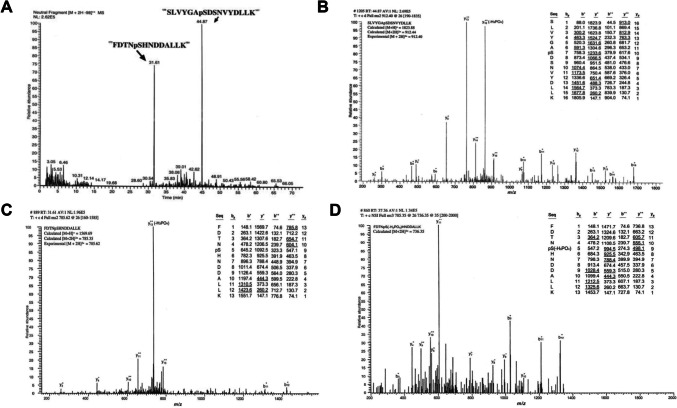
Phosphosites identified in hGH in spot 6 in Fig. 5A. A Neutral loss scanning of phospopeptides with LC-MS/MS data (only ion [M + 2H–98]2+ generated a signal after neutral loss scanning of phosphate group). B MS2 spectrum of phosphopeptide 126SLVYGApSDSNVYDLLK141 (pS = phosphorylated Ser; RT = 44.87 min in (A)). C MS2 spectrum of phosphopeptide 172FDTNpSHNDDALLK184 (pS = phosphorylated Ser; RT = 31.61 min in (A)). D MS3 spectrum of the fragment ion [M + 2H–98]2+ at m/z 736.35 in (C). RT retention time, m/z mass to charge. Modified from Zhan et al. [5] with copyright permission from Wiley publisher, copyright year 2005
Fig. 7.
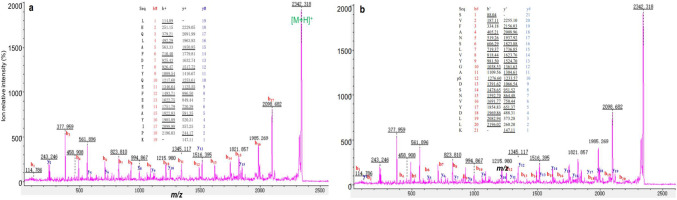
MS/MS analysis discriminates two different tryptic peptides with the same [M + H]+ values, which are derived from hGH in spot 36 in Fig. 5C(a). a Tryptic peptide 46LHOLAFDTYQEFEEAYIPK64. b Phosphopeptide 121SVFANSLVYGApSDSNVYDLLK141(pS = phosphorylated Ser). m/z mass to charge. Modified from Li et al. [9] with copyright permission from Springer publisher open access article, copyright year 2021
GH proteoforms in serum
The type and concentration of different GH proteoforms in sera are not invariable but change constantly with different physiological/pathological states [3]. Therefore, exploring GH proteoformic pattern in different diseases is of great significance, and the specific GH proteoforms related to specific diseases have great potential for the development of pattern biomarkers. Currently, the number of GH proteoforms in plasma is more than one hundred [3], and the forms of GH proteoforms include monomers, fragments, homopolymers, heteropolymers, and their combinations with GHBPs and other binding proteins [11–13]. For GH monomers, the main forms include 22 kD GH proteoforms, 20 kD GH proteoforms, and acidic GH proteoforms, probably including GH proteoforms with deamidation and N-acylation, and their ratio is about 75%, 16%, and 9%, respectively [14, 15, 52–54]. In addition to monomeric GH proteoforms, oligomeric forms, with an oligomeric series of dimeric to pentameric GH, are also identified in sera [55]. Furthermore, it is significantly worth noting that serum GH is not consistent, but varies dynamically with time and physiological/pathophysiological states. Therefore, it is very reasonable to infer different patterns of GH proteoforms that can play various functions to fit a variety of situations that an organism needs to face.
Currently, the 22 kD GH proteoforms and 20 kD GH proteoforms are the most studied, and both of them bind to and activate GHR. However, they exhibit different signal transmission characteristics; the 22 kD GH proteoforms exhibit stronger receptor-binding ability and signaling transmission ability than the 20 kD GH proteoforms [57]. This finding showed that different serum proteoforms might exhibit various effects for the growth and development of the human body and the progression of GH-related diseases. Therefore, it is urgent to systematically study GH proteoforms in sera and draw GH proteoform atlas, further clarify the influence of every GH proteoform and every cluster of different proportion of GH proteoforms to conduct an accurate molecular typing of different GH proteoforms corresponding to clinical characteristics of GH-related diseases to better clarify mechanisms of their occurrences and progression.
GH proteoforms and GH-binding proteins in serum
GH-binding proteins (GHBPs) were initially characterized in two independent studies in 1986 [58, 59]. The researchers found that 125I-labeled GH can specifically bind to a protein in human plasma, whose pI is of about 5 and Mr is about 60–65 kD [3, 58].
GHBPs mainly include two forms, namely high-affinity GHBP (HGHBP) and low-affinity GHBP (LGHBP) [60–67]. HGHBP is a heat-labile, high-affinity, low-capacity binding protein, which specifically binds to 22 kD GH, with pI = about 5.0 and Mr = about 61 kD [3, 56, 60]. Whereas, LGHBP is a heat-stabilized, low-affinity, high-capacity binding protein, which specifically binds to 20 kD GH, with pI = about 7.1 and Mr = about 100 kD [62, 63].
GHBP origins from the shed of ectodomain of GHR by specific proteolytic enzymes. Among these enzymes, TACE (tumor necrosis factor-alpha converting enzyme) is necessary for GHR proteolysis and GHBP generation [68, 69]. GHBP is widely distributed in body fluids, binding about averaged 50.1% (range, 39–59%) 22kD GH but averaged 28.5% (range, 26–31%) 20 kD GH [61, 65]. It can maintain relatively stable concentration and half-life of GH in plasma by regulating the proportion of free GH and binding GH by inhibiting GH binding to receptors. Furthermore, it can also regulate metabolism, transport, and biological function of GH [66, 67]. It significantly demonstrates the importance of GHBP to the implementation of GH function. The identification of many GH proteoforms [9, 52–55] provides a basis to speculate that there are many different GHBP proteoforms besides HGHBP and LGHBP, which can bind a great number of GH proteoforms, so as to conduct a variety of functions that every GH proteoform owned. Further studies are necessary to identify GHBP proteoform atlas and reveal the crosstalk between GH proteoforms and GHBP proteoforms.
The relationship between GH proteoforms and the invasiveness of GH-secreted PitNETs
GH-secreted PitNETs are usually benign, but some of them are invasive and can cause serious outcomes similar to malignant tumors. The invasive tendency is accounted for 49.7% of all GH-secreted PitNETs [70, 71]. Not only the normal anterior pituitary gland can synthesize and secret GH, but the tumor cells of GH-secreted PitNETs can also synthesize and secret GH, and the GH synthesized and secreted by GH-secreted PitNETs is closely related to its invasive behavior [71–75]. Acromegaly is caused by excessive secretion of GH and is commonly caused by GH-secreted PitNETs [75]. A meta-analysis of surgical therapy of GH-secreted PitNETs found that preoperative GH level is an important outcome predictor of the remission rate of acromegaly [73]. GH-secreted PitNETs are divided into two subtypes, namely sparse granular type (SG) and dense granular type (DG) [73]. Studies showed that SG are more often associated with aggressive features, such as local invasion and cavernous sinus invasion, and when preoperative GH levels were correlated with histological subtypes of GH-secreted PitNETs, the tendency was found for SG tumors to be associated with lower basal GH levels. Although most studies have shown that SG adenomas are more often associated with invasive features, histological subtypes of SG and DG do not absolutely predict invasive behavior. There are various hypotheses about the reasons for the invasive behavior to grow under the saddle of GH-secreted PitNETs [74], including the effect of GH itself on sella thinning, GH proteoforms of anterior pituitary, and the promotion of bone degradation by GH [74]. All of these show GH proteoforms are highly related to the invasive behavior of GH-secreted PitNETs. Therefore, exploration of GH proteoform atlas between invasive and non-invasive GH-secreted PitNETs will further clarify the relationship between GH proteoforms and the invasive behavior of GH-secreted PitNETs.
Future perspectives
Serum GH proteoforms and GHR proteoforms in target organs
GH has a series of functions-mainly promote the growth and development, modulate metabolism, and regulate immunity [16, 24, 27–32]. However, serum GH cannot perform its functions unless it binds to human GHR in target organs. The main targeted organs of GH include the liver, bone, muscle, and immune system [16, 27–29, 32]. Thus, GHR is vitally crucial to transduction of downstream signals of GH and the implementation of different GH function. Therefore, it is very reasonable to infer that there are different GHR proteoforms in different targeted organs corresponding to various functions.
There are nine exons encoding GHR in chromosome 5 [76–78]. Among them, the exons 2 to 7 encode extracellular part of GHR, binding specifically to GH. Currently, two human GHR mRNAs are identified, one contains exon 3 and the other excludes exon 3, and their expressions are tissue-specific, thereby exon 3 might influence receptor signaling [76–78]. The dysfunction of GHR gene is found to relate to GH-related diseases. Laron-type dwarfism is an autosomal recessive inheritance, with the characteristics of high concentration GH and low concentration IGF-1 (insulin-like growth factor-1) in the circulation [78]. Characterization of GHR gene from nine patients with Laron-type dwarfism showed that two patients have a deletion of a large portion of the hormone-binding domain of GHR gene, showing the defect of GHR gene is tightly related to Laron-type dwarfism [78]. Another analysis of the GHR-gene RNA transcripts revealed that Laron-dwarfism is caused by GHR gene abnormality, and it may vary from family to family [79].
All of these studies indicated that there is not just one form of GHR, and different GHR proteoforms have different functions, and GHR proteoforms have a close relationship with GH-related diseases. However, currently, only several GHR proteoforms have been characterized, and their exact physiological functions and their relationships to GH-related disease have not been well clarified. Therefore, it should be laid emphasis on finding more GHR proteoforms and clarifying their physiological effects and pathogenesis of GH-related diseases to promote the development of 3P medicine in GH-related diseases.
Serum GH proteoforms and GHR proteoforms in pituitary tissues for invasive behavior in GH-secreting PitNETs
Currently, some studies have found GH concentration in serum is related to the invasive behavior and therapeutic effect of GH-secreted PitNETs [73, 74]. GH proteoforms in GH-secreting PitNETs show a significant difference, including species, abundance, and PTMs compared to normal pituitary glands [5, 9]. Moreover, different GHBP proteoforms and GHR proteoforms also show close relationship with GH signal transduction and GH-related diseases [3, 66, 67, 78, 79]. Based on these studies, we can reasonably infer that serum GH proteoforms and GHR proteoforms in pituitary tissues may play an essential role in tumorigenesis, development and its invasive behavior of GH-secreting PitNETs, and more studies are needed to explore the relationship among GH proteoforms, GHR proteoforms in pituitary tissues, and PitNET invasive behavior, which will reveal the mechanism of invasive behavior of GH-secreting PitNETs, develop targeted drugs, and find specific biomarkers to achieve precise patient stratification and proper therapy methods, promoting 3P medicine of GH-secreting PitNETs.
Clinical problem-driven study design for GH proteoforms
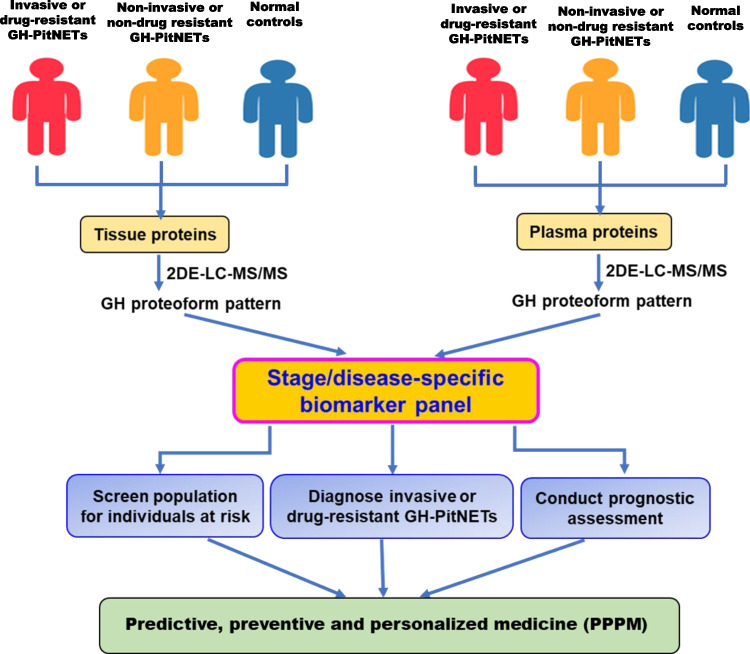
The judgement of invasiveness and drug resistance of PitNETs is an intractable clinical problem. We emphasize the following studies on GH proteoforms: (i) it is a problem-driven urgent work to find key molecules that are involved in the invasive behaviors of PitNETs. It is a valid study to compare the differences of GH proteoforms among invasive PitNETs, non-invasive PitNETs, and normal control pituitary tissues or patient plasmas, which has huge potential for the development of targeted drugs to treat invasive PitNETs (Fig. 8). (ii) Currently, no specific drugs are used to treat PitNETs, especially invasive PitNETs; only several drugs are used to lower the secretion of hormones or relief clinical symptoms; however, a relatively high rate of drug resistance occurs in the actual clinical practice. Thereby, the comparison of GH proteforms among drug-resistant PitNETs, drug-sensitive PitNETs, and normal control pituitary tissues or patient plasmas is necessary to reveal the molecular mechanism of drug resistance, and guide precise clinical medication (Fig. 8). (iii) The comparison of GH proteoforms between PitNET tissue and plasma from the same patient can identify the same proteoforms between tumor tissue and plasma, which might be developed as biomarkers to screen invasive and drug-resistant patients for proper treatment, this screening can be achieved with non-invasive serological examination (Fig. 8). (iv) The previous publications demonstrate that many post-translation modifications, including glycosylation, acetylation, ubiquitination, phosphorylation, and deamination occur in GH proteoforms. However, the functions of these modifications remain unclear. It is necessary to study the functions and action mechanism of each specific modification with multiple methodologies such as knock out, knock down, and overexpression of specific GH gene. (v) GH is involved in cell signal transduction, which might participate in the occurrence and progression of GH-secreted PitNETs. It is of great significance to clarify the functions of every GH proteoform and its relationship with GH-related diseases. (vi) 2DE in combination with western blotting has a powerful capability to detect and separate different GH proteoforms according to their pI and Mr. However, how to purify and enrich each GH proteoform is still a technique bottleneck for top-down MS analysis. It is necessary to develop a new method to purify and enrich every GH proteoform. (vii) The structure of a biomolecule decides its specific function. It is worth studying the structures of every GH proteoform in future GH proteoformics to in-depth reveal the functions and mechanism of each GH proteoform in GH-related disease.
Fig. 8.
The experimental flow-chart of GH proteoformics analysis among invasive vs. non-invasive GH-secreted PitNETs compared to controls in tissues and plasma, or among drug-resistant vs. non-drug-resistant GH-secreted PitNETs compared to controls in tissue and plasma. Red shape means invasive or drug-resistant GH-secreted PitNETs. Orange shape means non-invasive or non-drug resistant GH-secreted PitNETs. Blue shape means normal controls. 2DE two-dimensional gel electrophoresis, LC liquid chromatography, MS/MS tandem mass spectrometry
Conclusions and expert recommendation in the framework of 3P medicine
GH, known as one of the most indispensable hormones for the growth and metabolism of human body, is identified to have a variety of proteoforms, and different GH proteoforms might have different functions and roles, and might have different relationships with different GH-related diseases. It is a significantly urgent work to clarify the function of every GH proteoform and its relationship with GH-related diseases. The clarification of different function of GH proteoforms will help to find specific biomarkers to predict the risk of diseases, achieve early diagnosis and classification of diseases, and further conduct proper treatment for the best prognosis. Therefore, GH proteoformics offers great potential for insight into molecular mechanisms of GH-related diseases, and construction of effective biomarkers for predictive diagnosis, prognostic assessment, and even discovery of novel therapeutic targets in the framework of PPPM.
We strongly recommend GH proteoformics studies in GH-related diseases towards PPPM practice. GH is encoded by GH gene. However, many factors such as PTMs, alternative splicing, conformation, binding proteins, and others cause different GH proteoforms at the protein level under the different pathophysiological conditions. We hypothesize that GH proteoforms are different in GH-related diseases vs. normal controls, invasive vs. non-invasive PitNETs, and drug-resistant vs. drug-nonresistant PitNEts. The abnormal GH proteoforms or abnormal GH proteoform patterns are the important resource for insight into molecular mechanisms of GH-related diseases, invasive PitNETs, and drug-resistant PitNETs, discovery of effective therapeutic targets, and construction of GH proteoform biomarkers for patient stratification, and personalized medical services towards PPPM. The GH proteoformics offers the PPPM innovation in the following three aspects.
-
(i)
Predictive approach: GH proteoformics is a completely innovative concept, which enables to clarify the fine structure and function of GH in GH-related diseases, such as GH-secreting PitNETs. Invasive behavior is the challenging clinical problem. Currently, the GH concentration in plasma has been found to be related to the invasiveness of GH-secreted PitNETs [71–75]. The higher the plasma GH level, the more likely the GH-secreted PitNETAs is to be invasive. Moreover, per-operative plasma GH half-life is also found to be related to the completeness of surgery in acromegaly [80]. However, previous studies were only conducted from the overall level of plasma GH. If more in-depth and detailed studies can be conducted from the level of GH proteoform and GH proteoform pattern unique to invasive GH-secreted PitNETs are found, it would be very inspiring to use the unique GH proteoform pattern to predict the invasiveness of GH-secreted PitNETs, guide doctors to make clinical decisions based on the judgement of tumor invasiveness, and conduct the prognosis of patients as the follow-up indicator. 2DE-LC/MS is an effective method to array and identify GH proteoform pattern changes in invasive vs. non-invasive PitNETs. The changed GH proteoform pattern might be the effective predictive biomarkers for early-stage predictive diagnosis and prognostic assessment of invasive behaviors of PitNETs towards the innovative predictive approach.
-
(ii)
Targeted prevention: different GH proteoforms derived from the same GH gene are related to different GH-related diseases. For example, identification of any GH proteoform specific to invasive behaviors of PitNETs, and if this GH proteoform is involved in the molecular mechanism of PitNET invasive behaviors, then it is possible to develop the inhibitors or transform them, further reducing or eliminating the invasiveness and maximizing the benefits to the patients, which will be the innovative therapeutic target for targeted prevention and targeted therapy of invasive GH-secreting PitNETs. In addition, the GH must bind to its receptor in the targeted cells, targeted tissues and targeted organs to implement its function. If the tumor is aggressive, the GHR proteoforms may be changed. Therefore, if the unique GHR proteoform pattern of invasiveness is found, it is possible to develop the inhibitors or transform them, further delaying or reversing the patient’s clinical symptoms. it is possible to develop the inhibitors or transform them, further reducing or eliminating the invasiveness and maximizing the patient's recovery.
-
(iii)
Personalization of medical services: the 2DE pattern of GH proteoforms can be developed as effective biomarkers for patient stratification, predictive diagnosis, prognostic assessment, and personalized treatment towards innovative personalization of medical services. If GH proteoform pattern unique to invasive GH-secreted PitNETs are found, it would be used to predict the invasiveness of GH-secreted PitNETs and guide doctors to the make clinical treatment accurately based on the judgement of tumor invasiveness, further providing personal medical services.
In summary, quantitative GH proteoformics can detect, identify, and quantify GH proteoforms, and GH proteoform pattern alterations, which is an innovative area for GH-related diseases, such as PitNETs. The abnormal GH proteoforms, or GH proteoform pattern will provide novel insight into molecular mechanisms, therapeutic targets, and discovery of effective biomarkers of GH-related diseases towards predictive diagnosis, targeted prevention, and personalized medical service. This present GH proteoformics demonstrates an innovative, GH proteoform-based state of the art contributing to the paradigm shift from reactive medicine to PPPM in human GH-related diseases.
Abbreviations
- CZE
Capillary zone electrophoresis
- GHBP
Growth hormone-binding proteins
- GHR
Growth hormone receptor
- hGH
Human growth hormone
- HGHBP
High-affinity GHBP
- JAK2
Janus kinase 2
- IRSs
Insulin receptor substrates
- LC
Liquid chromatography
- LGHBP
Low-affinity GHBP
- MS
Mass spectrometry
- MS/MS
Tandem mass spectrometry
- Mr
Molecular weight
- pI
Isoelectric point
- PPPM / 3PM
Predictive preventive personalized medicine
- PTMs
Post-translational modifications
- Stat
Activators of transcription factors
- TACE
Tumor necrosis factor-alpha converting enzyme
- 2DGE
Two-dimensional gel electrophoresis
Author contribution
L.Y. collected and analyzed literature and wrote the manuscript. C.L. and T.S. participated in partial literature analysis and reviewed the manuscript. X.Z. conceived the concept, designed the manuscript, coordinated, and critically revised manuscript, and was responsible for the corresponding works. All authors approved the final manuscript.
Funding
The authors acknowledge the financial support from the Shandong Provincial Natural Science Foundation (ZR2021MH156 to X.Z.), Shandong Provincial Taishan Scholar Engineering Project Special Funds (NO.tstp20221143 to X.Z.), the Shandong First Medical University Talent Introduction Funds (to X.Z.), the Shandong First Medical University High-level Scientific Research Achievement Cultivation Funding Program (to X.Z.), and China National Nature Scientific Funds (82203592).
Data availability
All data and materials are provided in this article and supplemental materials, which can be available publicly.
Code availability
Not applicable.
Declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li CH, Evans HM. The isolaton of pitutiary growth homrone. Science. 1944;99(2566):183–184. doi: 10.1126/science.99.2566.183. [DOI] [PubMed] [Google Scholar]
- 2.de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 3.Baumann G. Growth hormone heterogeneity: genes, isohormones, variants, and binding proteins. Endocr Rev. 1991;12(4):424–449. doi: 10.1210/edrv-12-4-424. [DOI] [PubMed] [Google Scholar]
- 4.Basu R, Nahar K, Kulkarni P, Kerekes O, Sattler M, Hall Z, et al. A novel peptide antagonist of the human growth hormone receptor. J Biol Chem. 2021;296:100588. doi: 10.1016/j.jbc.2021.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan X, Giorgianni F, Desiderio DM. Proteomics analysis of growth hormone isoforms in the human pituitary. Proteomics. 2005;5(5):1228–1241. doi: 10.1002/pmic.200400987. [DOI] [PubMed] [Google Scholar]
- 6.Sinha YN, Jacobsen BP, Lewis UJ. Antibodies to newly recognized murine 13–18 KDa pituitary peptides crossreact with growth hormone and prolactin from several species, including man. Biochem Biophys Res Commun. 1989;163(1):386–393. doi: 10.1016/0006-291x(89)92147-5. [DOI] [PubMed] [Google Scholar]
- 7.Sinha YN, Jacobsen BP. Human growth hormone (hGH)-(44–191), a reportedly diabetogenic fragment of hGH, circulates in human blood: measurement by radioimmunoassay. J Clin Endocrinol Metab. 1994;78(6):1411–1418. doi: 10.1210/jcem.78.6.8200944. [DOI] [PubMed] [Google Scholar]
- 8.López-Guajardo CC, Armstrong LS, Jordan L, Staten NR, Krivi GG, Martinez AO, et al. Generation, characterization and utilization of anti-human growth hormone 1–43, (hGH1-43), monoclonal antibodies in an ELISA. J Immunol Methods. 1998;215(1–2):179–185. doi: 10.1016/s0022-1759(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Wang X, Yang C, Wen S, Li J, Li N, et al. Human growth hormone proteoform pattern changes in pituitary adenomas: potential biomarkers for 3P medical approaches. EPMA J. 2021;12(1):67–89. doi: 10.1007/s13167-021-00232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler M, Thomas A, Püschel K, Schänzer W, Thevis M. Identification of human pituitary growth hormone variants by mass spectrometry. J Proteome Res. 2009;8(2):1071–1076. doi: 10.1021/pr800945b. [DOI] [PubMed] [Google Scholar]
- 11.Reiter EO, Morris AH, MacGillivray MH, Weber D. Variable estimates of serum growth hormone concentrations by different radioassay systems. J Clin Endocrinol Metab. 1988;66(1):68–71. doi: 10.1210/jcem-66-1-68. [DOI] [PubMed] [Google Scholar]
- 12.Felder RA, Holl RW, Martha P, Jr, Bauler G, Hellman P, Wills MR, et al. Influence of matrix on concentrations of somatotropin measured in serum with commercial immunoradiometric assays. Clin Chem. 1989;35(7):1423–1426. doi: 10.1093/clinchem/35.7.1423. [DOI] [PubMed] [Google Scholar]
- 13.Celniker AC, Chen AB, Wert RM, Jr, Sherman BM. Variability in the quantitation of circulating growth hormone using commercial immunoassays. J Clin Endocrinol Metab. 1989;68(2):469–476. doi: 10.1210/jcem-68-2-469. [DOI] [PubMed] [Google Scholar]
- 14.Baumann G, Winter RJ, Shaw M. Circulating molecular variants of growth hormone in childhood. Pediatr Res. 1987;22(1):21–2. doi: 10.1203/00006450-198707000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Baumann G, Stolar MW. Molecular forms of human growth hormone secreted in vivo: nonspecificity of secretory stimuli. J Clin Endocrinol Metab. 1986;62(4):789–790. doi: 10.1210/jcem-62-4-789. [DOI] [PubMed] [Google Scholar]
- 16.Strobl JS, Thomas MJ. Human growth hormone. Pharmacol Rev. 1994;46(1):1–34. [PubMed] [Google Scholar]
- 17.Carter-Su C, Schwartz J, Smit LS. Molecular mechanism of growth hormone action. Annu Rev Physiol. 1996;58:187–207. doi: 10.1146/annurev.ph.58.030196.001155. [DOI] [PubMed] [Google Scholar]
- 18.Owerbach D, Rutter WJ, Martial JA, Baxter JD, Shows TB. Genes for growth hormone, chorionic somatomammotropin, and growth hormone-like gene on chromosome 17 in humans. Science. 1980;209(4453):289–292. doi: 10.1126/science.7384802. [DOI] [PubMed] [Google Scholar]
- 19.Barsh GS, Seeburg PH, Gelinas RE. The human growth hormone gene family: structure and evolution of the chromosomal locus. Nucleic Acids Res. 1983;11(12):3939–3958. doi: 10.1093/nar/11.12.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen EY, Liao YC, Smith DH, Barrera-Saldaña HA, Gelinas RE, Seeburg PH. The human growth hormone locus: nucleotide sequence, biology, and evolution. Genomics. 1989;4(4):479–497. doi: 10.1016/0888-7543(89)90271-1. [DOI] [PubMed] [Google Scholar]
- 21.Sedman L, Padhukasahasram B, Kelgo P, Laan M. Complex signatures of locus-specific selective pressures and gene conversion on Human Growth Hormone/Chorionic Somatomammotropin genes. Hum Mutat. 2008;29(10):1181–1193. doi: 10.1002/humu.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frankenne F, Scippo ML, Van Beeumen J, Igout A, Hennen G. Identification of placental human growth hormone as the growth hormone-V gene expression product. J Clin Endocrinol Metab. 1990;71(1):15–18. doi: 10.1210/jcem-71-1-15. [DOI] [PubMed] [Google Scholar]
- 23.Campbell GS. Growth-hormone signal transduction. J Pediatr. 1997;131(1 Pt 2):S42–S44. doi: 10.1016/s0022-3476(97)70010-6. [DOI] [PubMed] [Google Scholar]
- 24.Nam SY, Marcus C. Growth hormone and adipocyte function in obesity. Horm Res. 2000;53(Suppl 1):87–97. doi: 10.1159/000053211. [DOI] [PubMed] [Google Scholar]
- 25.Horesh EJ, Chéret J, Paus R. Growth hormone and the human hair follicle. Int J Mol Sci. 2021;22(24). 10.3390/ijms222413205. [DOI] [PMC free article] [PubMed]
- 26.Chang CW, Sung YW, Hsueh YW, Chen YY, Ho M, Hsu HC, et al. Growth hormone in fertility and infertility: mechanisms of action and clinical applications. Front Endocrinol (Lausanne) 2022;13:1040503. doi: 10.3389/fendo.2022.1040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kooijman R, Gerlo S, Coppens A, Hooghe-Peters EL. Growth hormone and prolactin expression in the immune system. Ann N Y Acad Sci. 2000;917:534–540. doi: 10.1111/j.1749-6632.2000.tb05418.x. [DOI] [PubMed] [Google Scholar]
- 28.Velkeniers B, Dogusan Z, Naessens F, Hooghe R, Hooghe-Peters EL. Prolactin, growth hormone and the immune system in humans. Cell Mol Life Sci. 1998;54(10):1102–1108. doi: 10.1007/s000180050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy WJ, Tsarfaty G, Longo DL. Growth hormone exerts hematopoietic growth-promoting effects in vivo and partially counteracts the myelosuppressive effects of azidothymidine. Blood. 1992;80(6):1443–1447. doi: 10.1182/blood.V80.6.1443.1443. [DOI] [PubMed] [Google Scholar]
- 30.Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. 2015;68:76–81. doi: 10.1016/j.exger.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyberg F, Hallberg M. Growth hormone and cognitive function. Nat Rev Endocrinol. 2013;9(6):357–365. doi: 10.1038/nrendo.2013.78. [DOI] [PubMed] [Google Scholar]
- 32.Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 33.Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323(1):1–6. doi: 10.1056/nejm199007053230101. [DOI] [PubMed] [Google Scholar]
- 34.Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981;67(5):1361–1369. doi: 10.1172/jci110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler T, Harvey P, Cardozo L, Zhu YS, Mosa A, Tanzi E, et al. Epilepsy, depression, and growth hormone. Epilepsy Behav. 2019;94:297–300. doi: 10.1016/j.yebeh.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruitt KD, Tatusova T, Maglott DR. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007;35(Database issue):D61–5. 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed]
- 37.Zhan X, Li B, Zhan X, Schlüter H, Jungblut PR, Coorssen JR. Innovating the concept and practice of two-dimensional gel electrophoresis in the analysis of proteomes at the proteoform level. Proteomes. 2019;7(4). 10.3390/proteomes7040036. [DOI] [PMC free article] [PubMed]
- 38.Schlüter H, Apweiler R, Holzhütter HG, Jungblut PR. Finding one’s way in proteomics: a protein species nomenclature. Chem Cent J. 2009;3:11. doi: 10.1186/1752-153x-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan X, Li N, Zhan X, Qian S. Revival of 2DE-LC/MS in proteomics and its potential for large-scale study of human proteoforms. Med One. 2018;3:e180008. doi: 10.20900/mo.20180008. [DOI] [Google Scholar]
- 40.Smith LM, Kelleher NL. Proteoform: a single term describing protein complexity. Nat Methods. 2013;10(3):186–187. doi: 10.1038/nmeth.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Desiderio DM, Zhan X. The use of mass spectrometry in a proteome-centered multiomics study of human pituitary adenomas. Mass Spectrom Rev. 2022;41(6):964–1013. doi: 10.1002/mas.21710. [DOI] [PubMed] [Google Scholar]
- 42.Zhan X, Su J, Yang L. Editorial: Biomolecular modifications in endocrine-related cancers. Front Endocrinol (Lausanne) 2023;14:1133629. doi: 10.3389/fendo.2023.1133629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quitmann JH, Rohenkohl AC, Kammerer U, Schöfl C, Bullinger M, Dörr HG. Quality of life of young adults after a growth hormone therapy with childhood onset. Dtsch Med Wochenschr. 2014;139(46):2335–2338. doi: 10.1055/s-0034-1387314. [DOI] [PubMed] [Google Scholar]
- 44.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(6):1587–1609. doi: 10.1210/jc.2011-0179. [DOI] [PubMed] [Google Scholar]
- 45.Castro C, Trivin C, Souberbielle JC, Zerah M, Brauner R. Growth hormone deficiency: permanence and diagnosis in young adults. Horm Res. 2002;58(4):165–171. doi: 10.1159/000065489. [DOI] [PubMed] [Google Scholar]
- 46.de Boer H, Blok GJ, Van der Veen EA. Clinical aspects of growth hormone deficiency in adults. Endocr Rev. 1995;16(1):63–86. doi: 10.1210/edrv-16-1-63. [DOI] [PubMed] [Google Scholar]
- 47.Vilar L, Vilar CF, Lyra R, Lyra R, Naves LA. Acromegaly: clinical features at diagnosis. Pituitary. 2017;20(1):22–32. doi: 10.1007/s11102-016-0772-8. [DOI] [PubMed] [Google Scholar]
- 48.Gomes FP, Diedrich JK, Saviola AJ, Memili E, Moura AA, Yates JR., 3rd EThcD and 213 nm UVPD for top-down analysis of bovine seminal plasma proteoforms on electrophoretic and chromatographic time frames. Anal Chem. 2020;92(4):2979–2987. doi: 10.1021/acs.analchem.9b03856. [DOI] [PubMed] [Google Scholar]
- 49.Melby JA, Jin Y, Lin Z, Tucholski T, Wu Z, Gregorich ZR, et al. Top-down proteomics reveals myofilament proteoform heterogeneity among various rat skeletal muscle tissues. J Proteome Res. 2020;19(1):446–454. doi: 10.1021/acs.jproteome.9b00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melani RD, Gerbasi VR, Anderson LC, Sikora JW, Toby TK, Hutton JE, et al. The blood proteoform atlas: a reference map of proteoforms in human hematopoietic cells. Science. 2022;375(6579):411–418. doi: 10.1126/science.aaz5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan X, Yang H, Peng F, Li J, Mu Y, Long Y, et al. How many proteins can be identified in a 2DE gel spot within an analysis of a complex human cancer tissue proteome? Electrophoresis. 2018;39(7):965–980. doi: 10.1002/elps.201700330. [DOI] [PubMed] [Google Scholar]
- 52.Baumann G, MacCart JG, Amburn K. The molecular nature of circulating growth hormone in normal and acromegalic man: evidence for a principal and minor monomeric forms. J Clin Endocrinol Metab. 1983;56(5):946–952. doi: 10.1210/jcem-56-5-946. [DOI] [PubMed] [Google Scholar]
- 53.Baumann G, Stolar MW, Amburn K. Molecular forms of circulating growth hormone during spontaneous secretory episodes and in the basal state. J Clin Endocrinol Metab. 1985;60(6):1216–1220. doi: 10.1210/jcem-60-6-1216. [DOI] [PubMed] [Google Scholar]
- 54.Markoff E, Lee DW, Culler FL, Jones KL, Lewis UJ. Release of the 22,000- and the 20,000-dalton variants of growth hormone in vivo and in vitro by human anterior pituitary cells. J Clin Endocrinol Metab. 1986;62(4):664–669. doi: 10.1210/jcem-62-4-664. [DOI] [PubMed] [Google Scholar]
- 55.Stolar MW, Amburn K, Baumann G. Plasma, “big” and “big-big” growth hormone (GH) in man: an oligomeric series composed of structurally diverse GH monomers. J Clin Endocrinol Metab. 1984;59(2):212–218. doi: 10.1210/jcem-59-2-212. [DOI] [PubMed] [Google Scholar]
- 56.Hettiarachchi M, Watkinson A, Leung KC, Sinha YN, Ho KK, Kraegen EW. Human growth hormone fragment (hGH44-91) produces insulin resistance and hyperinsulinemia but is less potent than 22 kDa hGH in the rat. Endocrine. 1997;6(1):47–52. doi: 10.1007/bf02738801. [DOI] [PubMed] [Google Scholar]
- 57.Yao-Xia L, Jing-Yan C, Xia-Lian T, Ping C, Min Z. The 20kDa and 22kDa forms of human growth hormone (hGH) exhibit different intracellular signalling profiles and properties. Gen Comp Endocrinol. 2017;248:49–54. doi: 10.1016/j.ygcen.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Baumann G, Stolar MW, Amburn K, Barsano CP, DeVries BC. A specific growth hormone-binding protein in human plasma: initial characterization. J Clin Endocrinol Metab. 1986;62(1):134–141. doi: 10.1210/jcem-62-1-134. [DOI] [PubMed] [Google Scholar]
- 59.Herington AC, Ymer S, Stevenson J. Identification and characterization of specific binding proteins for growth hormone in normal human sera. J Clin Invest. 1986;77(6):1817–1823. doi: 10.1172/jci112507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baumann G. Growth hormone binding protein 2001. J Pediatr Endocrinol Metab. 2001;14(4):355–375. doi: 10.1515/jpem.2001.14.4.355. [DOI] [PubMed] [Google Scholar]
- 61.Baumann G, Amburn K, Shaw MA. The circulating growth hormone (GH)-binding protein complex: a major constituent of plasma GH in man. Endocrinology. 1988;122(3):976–984. doi: 10.1210/endo-122-3-976. [DOI] [PubMed] [Google Scholar]
- 62.Baumann G, Shaw MA. Plasma transport of the 20,000-dalton variant of human growth hormone (20K): evidence for a 20K-specific binding site. J Clin Endocrinol Metab. 1990;71(5):1339–1343. doi: 10.1210/jcem-71-5-1339. [DOI] [PubMed] [Google Scholar]
- 63.Baumann G, Shaw MA. A second, lower affinity growth hormone-binding protein in human plasma. J Clin Endocrinol Metab. 1990;70(3):680–686. doi: 10.1210/jcem-70-3-680. [DOI] [PubMed] [Google Scholar]
- 64.Baumann G, Shaw MA, Amburn K. Circulating growth hormone binding proteins. J Endocrinol Invest. 1994;17(1):67–81. doi: 10.1007/bf03344965. [DOI] [PubMed] [Google Scholar]
- 65.Baumann G, Vance ML, Shaw MA, Thorner MO. Plasma transport of human growth hormone in vivo. J Clin Endocrinol Metab. 1990;71(2):470–473. doi: 10.1210/jcem-71-2-470. [DOI] [PubMed] [Google Scholar]
- 66.Lim L, Spencer SA, McKay P, Waters MJ. Regulation of growth hormone (GH) bioactivity by a recombinant human GH-binding protein. Endocrinology. 1990;127(3):1287–1291. doi: 10.1210/endo-127-3-1287. [DOI] [PubMed] [Google Scholar]
- 67.Mannor DA, Winer LM, Shaw MA, Baumann G. Plasma growth hormone (GH)-binding proteins: effect on GH binding to receptors and GH action. J Clin Endocrinol Metab. 1991;73(1):30–34. doi: 10.1210/jcem-73-1-30. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Jiang J, Black RA, Baumann G, Frank SJ. Tumor necrosis factor-alpha converting enzyme (TACE) is a growth hormone binding protein (GHBP) sheddase: the metalloprotease TACE/ADAM-17 is critical for (PMA-induced) GH receptor proteolysis and GHBP generation. Endocrinology. 2000;141(12):4342–4348. doi: 10.1210/endo.141.12.7858. [DOI] [PubMed] [Google Scholar]
- 69.Schantl JA, Roza M, Van Kerkhof P, Strous GJ. The growth hormone receptor interacts with its sheddase, the tumour necrosis factor-alpha-converting enzyme (TACE) Biochem J. 2004;377(Pt 2):379–384. doi: 10.1042/bj20031321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin H, Zheng X, Tang X, Zang Z, Li B, He S, et al. Potential biomarkers and lncRNA-mRNA regulatory networks in invasive growth hormone-secreting pituitary adenomas. J Endocrinol Invest. 2021;44(9):1947–1959. doi: 10.1007/s40618-021-01510-x. [DOI] [PubMed] [Google Scholar]
- 71.Cuevas-Ramos D, Carmichael JD, Cooper O, Bonert VS, Gertych A, Mamelak AN, et al. A structural and functional acromegaly classification. J Clin Endocrinol Metab. 2015;100(1):122–131. doi: 10.1210/jc.2014-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donoho DA, Bose N, Zada G, Carmichael JD. Management of aggressive growth hormone secreting pituitary adenomas. Pituitary. 2017;20(1):169–178. doi: 10.1007/s11102-016-0781-7. [DOI] [PubMed] [Google Scholar]
- 73.Lu T, Yu C, Ni H, Liang W, Yan H, Jin W. Expression of the long non-coding RNA H19 and MALAT-1 in growth hormone-secreting pituitary adenomas and its relationship to tumor behavior. Int J Dev Neurosci. 2018;67:46–50. doi: 10.1016/j.ijdevneu.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Pangal DJ, Wishart D, Shiroishi MS, Ruzevick J, Carmichael JD, Zada G. Growth hormone secreting pituitary adenomas show distinct extrasellar extension patterns compared to nonfunctional pituitary adenomas. Pituitary. 2022;25(3):480–485. doi: 10.1007/s11102-022-01217-z. [DOI] [PubMed] [Google Scholar]
- 75.Kiseljak-Vassiliades K, Carlson NE, Borges MT, Kleinschmidt-DeMasters BK, Lillehei KO, Kerr JM, et al. Growth hormone tumor histological subtypes predict response to surgical and medical therapy. Endocrine. 2015;49(1):231–241. doi: 10.1007/s12020-014-0383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Urbanek M, MacLeod JN, Cooke NE, Liebhaber SA. Expression of a human growth hormone (hGH) receptor isoform is predicted by tissue-specific alternative splicing of exon 3 of the hGH receptor gene transcript. Mol Endocrinol. 1992;6(2):279–287. doi: 10.1210/mend.6.2.1569971. [DOI] [PubMed] [Google Scholar]
- 77.Sobrier ML, Duquesnoy P, Duriez B, Amselem S, Goossens M. Expression and binding properties of two isoforms of the human growth hormone receptor. FEBS Lett. 1993;319(1–2):16–20. doi: 10.1016/0014-5793(93)80028-s. [DOI] [PubMed] [Google Scholar]
- 78.Godowski PJ, Leung DW, Meacham LR, Galgani JP, Hellmiss R, Keret R, et al. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron-type dwarfism. Proc Natl Acad Sci U S A. 1989;86(20):8083–8087. doi: 10.1073/pnas.86.20.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amselem S, Duquesnoy P, Attree O, Novelli G, Bousnina S, Postel-Vinay MC, et al. Laron dwarfism and mutations of the growth hormone-receptor gene. N Engl J Med. 1989;321(15):989–995. doi: 10.1056/nejm198910123211501. [DOI] [PubMed] [Google Scholar]
- 80.van den Berg G, van Dulken H, Frölich M, Meinders AE, Roelfsema F. Can intra-operative GH measurement in acromegalic subjects predict completeness of surgery? Clin Endocrinol (Oxf) 1998;49(1):45–51. doi: 10.1046/j.1365-2265.1998.00436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are provided in this article and supplemental materials, which can be available publicly.
Not applicable.