Abstract
Royal jelly (RJ) is a bee product produced by young adult worker bees, composed of water, proteins, carbohydrates and lipids, rich in bioactive components with therapeutic properties, such as free fatty acids, mainly 10-hydroxy-trans-2-decenoic acid (10-H2DA) and 10-hydroxydecanoic acid (10-HDA), and major royal jelly proteins (MRJPs), as well as flavonoids, most flavones and flavonols, hormones, vitamins and minerals. In vitro, non-clinical and clinical studies have confirmed its vital role as an antioxidant and anti-inflammatory. This narrative review discusses the possible effects of royal jelly on preventing common complications of non-communicable diseases (NCDs), such as inflammation, oxidative stress and intestinal dysbiosis, from the viewpoint of predictive, preventive and personalised medicine (PPPM/3PM). It is concluded that RJ, predictively, can be used as a non-pharmacological therapy to prevent and mitigate complications related to NCDs, and the treatment must be personalised.
Keywords: Royal jelly, Non-communicable disease, Oxidative stress, Inflammation, Dysbiosis, Predictive preventive personalised medicine (PPPM/3 PM)
Introduction
Non-communicable diseases (NCDs), specifically chronic kidney disease, cardiovascular diseases, diabetes, cancer and chronic respiratory diseases, are responsible for 17 million premature deaths yearly, causing nearly three-quarters of deaths worldwide [1]. By 2023, the NCD mortality rate would be 510.54 (per 100,000 population), whilst the global mean NCD deaths would be 75.26% of the total deaths [2].
NCDs are associated with some complications, such as inflammation, oxidative stress and gut dysbiosis [3]. Oxidative stress is mainly caused by a high production of reactive oxygen species (ROS) in the cells (most by macrophages) and low antioxidant levels. Oxidative stress is closely associated with inflammation since it can stimulate the expression of genes involved with nuclear transcription factors that can increase cytokine production [4].
Some unhealthy lifestyle is heavily linked to the causes and progression of NCDs, also known as lifestyle diseases. Prioritising a balanced diet, especially concerning a non-pharmacological treatment surrounding the “food as medicine” concept, is one of the steps to prevent and control NCDs, reducing the need for expensive therapies [1, 5]. Bioactive nutraceuticals found in foods could improve health in those diseases [6].
Royal jelly is a bee product used for feed of honeybee larvae. The worker bees are fed royal jelly until the third day of life, whilst the selected female that will become the queen bee is fed royal jelly throughout her life. The worker bees live around 40 days, whilst the queen bees are around 5–6 years old. The nutritional transformation of immature female larvae into a fertile queen bee is usually linked to the benefits of royal jelly nutrients, including the longevity of the queen [7, 8].
Royal jelly (RJ), an acidic emulsion produced by young adult worker Apis mellifera bee specie, which comes from the secretion of bee hypopharyngeal and mandibular glands, has been studied as antimicrobial, anti-inflammatory, anticancer, bio-stimulating, antiaging, immuno-modulating and antioxidant besides several others [8, 9]. Due to the several biological effects already described, royal jelly can be used as a nutrient and as medicine (apitherapy), covering a great range of applications from sexual dysfunctions, fertility and menopause discomforts until longevity, including heart, blood circulation, nervous system, respiratory, dermatological and several other diseases [8]. These properties are due to their composition, based on amounts of major royal jelly proteins (MRJPs) and lipids, mainly the fatty acids 10-H2DA (10-hydroxt-trans-decenoic acid) and 10-HDA (10-hydroxydecanoic acid), besides peptides, amino acids, flavonoids, polyphenols, vitamins and minerals [7, 8, 10].
In vitro and in vivo studies have shown that RJ could modulate inflammation mechanisms by reducing nuclear factor-kB (NF-κB) and tumour necrosis factor (TNF-α) [11–14]. Moreover, royal jelly upregulates the Nrf2 expression, a master of antioxidants, mitigating inflammatory and oxidative burden [15–17].
Therefore, an integrative, personalised, evidence-based medical approach is essential for advancing health care. It is necessary to consider risk factors, promote prevention and adopt a collaborative approach between disciplines and health professionals. Advanced and innovative technologies are also encouraged to improve the health and well-being of patients [18].
In this context, this narrative review aims to provide an overview of the effects of royal jelly as a nutritional strategy for patients with NCDs, using the concept of “food as medicine” and its consideration in the context of predictive, preventive and personalised medicine (PPPM), helpful in preventing and treating diseases.
Royal jelly: origin and increasing value in pharmaco-business
Bee products have been used since the ancient world. The first use registered was when Greeks utilised a part of RJ to produce “ambrosia”, giving immortality to the Olympus god [19, 20]. RJ’s function in bee society was first discovered by Aristotle when he studied RJ’s effects on the queen bee. In addition, RJ was used as a cosmetic by Cleopatra in ancient Egypt. In China, human medicine has used RJ for a long time for its health-protecting properties. The name of this substance came from a French scientist René Antoine de Réaumur (1683–1757), who correlated the queen bee food with its exceptional growth. Since the 60s, RJ properties have been investigated and used for human health [20].
No official data about RJ production exists, but the honey market is estimated at US$2.9 billion in 2020 in the USA. China is a big RJ producer and has grown considerably, from 200 tons per year in 1980 to 3500 tons per year in 2010 [21]. Other countries are responsible for RJ production, such as Korea, Taiwan, Japan, Mexico, Spain, Greece and France [10].
After fertilising flowers, the pollen digestion by bees results in the raw material for RJ production, which is secreted by Apis mellifera (3- to 12-day-old) worker (nurses) bees from hypopharyngeal and mandibular glands and used to feed the queen bee in larval, young and adult stages bee larvae, and also the worker bees until the 3 days of life. This feeding concept differentiates honeybee caste designation and honeybee larvae into queens due to the protein in royal jelly called royalactin [22].
Royal jelly composition
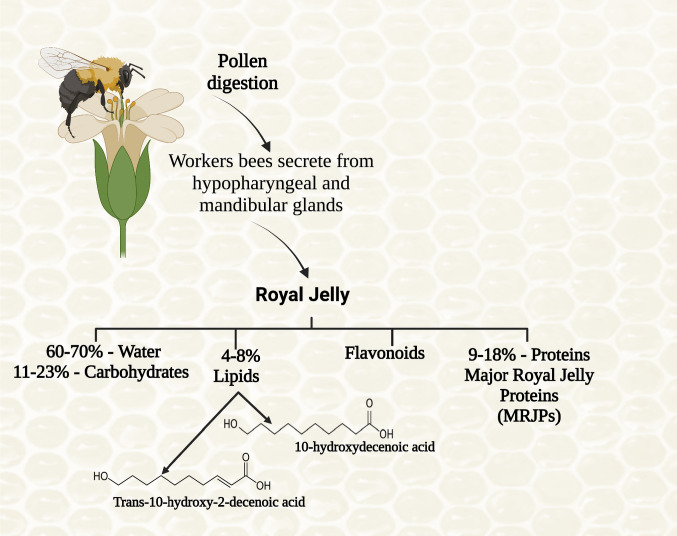
Considered a superfood, with a nutrient-dense natural item, it is heat and light susceptible, going through oxidation with air contact. RJ is known as “milky-white” because of its white or yellowish creamy substance. Its unique characteristics are its gelatinous-viscous sour and sweet taste and a slightly sour and pungent smell of phenol [23]. It is slightly acidic (pH 3.5–4.5), containing water (50–70%), carbohydrates (7–18%), proteins (9–18%) and lipids (4–8%). However, the most attractive attention of RJ is the exciting characteristic of fatty acid composition, with a rare and unusual structure. Around 90% of lipids are short chains of carboxylic acids—8 up to 12 (animal or plant materials usually contain 14–20 carbon atoms). This carboxylic acid is mainly the unsaturated hydroxyl fatty acid, 10-hydroxy-trans-2-decenoic acid (10-H2DA) (corresponds to around 50% of total FAs), often referred to as “royal jelly acid”, followed by saturated hydroxyl fatty acid, 10-hydroxydecanoic acid (10-HDA), which has never been detected in another natural or bee product [7, 24]; together, 10-H2DA and 10-HDA sum around > 60–80% of total FAs, and a dicarboxylic fatty acid, sebacic acid (SEA), in small amount is also found in RJ (Fig. 1).
Fig. 1.
Royal jelly composition. Created with BioRender.com
After ingestion, 10-HDA is metabolised into SEA, seen in urine samples and human plasma. However, when RJ is ingested without enzyme treatment (protease), the hydroxy fatty acids are not in the plasma [25].
Besides the fatty acids, some researchers attribute the protein fraction to the characteristic of the most important one in royal jelly since it is responsible for a specific physiological role in queen honeybee development and includes numerous essential amino acids. It presents a family of proteins called major royal jelly proteins (MRJPs), and several were already described as MRJP1, MRJP2 and so on [26].
Concerning micronutrients, it contains around 1.5% mineral salts (mainly copper, zinc, iron, calcium, manganese, potassium and sodium salts) and vitamins (B1, B2, B3, B5, B6, B7, B9, inositol and traces of vitamin C) [27, 28]. It is also relevant to keep in mind that in the fresh royal jelly, the hormones were also quantified: testosterone (0.20 ± 0.03), progesterone (4.61 ± 0.26), prolactin (70.8 ± 20.0) and estradiol (52.0 ± 6.0), in all cases, in nmoles/100 g [8].
Uthaibutra et al. (2014) estimated that RJ has gallic acid contents of 6.68 ± 0.60 mg gallic acid/g relative to total phenolic content [29]. However, it has already been clarified that harvest time is directly related to the content of phenolic compounds and flavonoids present in royal jelly. Therefore, the faster the harvest, the greater the antioxidant potential [30]. The main flavonoids found in royal jelly are hesperetin, isosakuranetin and naringenin from the flavanone group; acacetin, apigenin and their glycosides, chrysin and luteolin glycoside from the flavone group; isorhamnetin and kaempferol glycosides which are isoflavonols; and coumestrol, formononetin and genistein which are isoflavonoids [31, 32].
Carboxylic and phenolic groups form phenolic acids. The most commonly found in royal jelly are pinobanksin and organic acids and their esters, which comprise octanoic acids, 2-hexenedioic acid, dodecanoic acid, 1,2-benzenedicarboxylic acid and benzoic acid [33, 34].
Besides that, many aromatic components have also been found, such as pyrocatechol, hydroquinone, 2-methoxy-p-cresol, methyl salicylate, methyl benzoate, benzaldehyde, phenol, 2-methoxyphenol, toluene, benzoic acid, 4-hydroxyhydrocinnamic acid, 4-hydroxy-3-methoxyphenylethanol, p-coumaric acid, caffeic acid and nicotinic acid [35].
The presence of phenol groups provides the antiradical property [36]. Therefore, due to its biological properties, such as anti-inflammatory [37], antioxidant [38], cardioprotective [39], antimicrobial [40], antidiabetic [41], anticancer [42] and renoprotective actions [43], RJ has mainly been used in health foods, commercial medical products and cosmetics worldwide [44].
As previously informed, royal jelly is an unstable product. Besides the oxidation process that can suffer, it can also spoil or deteriorate and lose its commercial value if improper storage is used. The sensibility to temperature is the most important and impacting aspect. One alternative to a better and more convenient consumption of royal jelly is to transform it into a powder using freeze-drying or lyophilisation to avoid fast degradation. Some countries, such as Brazil and Japan, established minimal requirements of authenticity and quality for lyophilised royal jelly. In the case of Brazilian regulation, the maximum water content was established at 8%w/w. The minimum values of lipids, proteins and carbohydrates were defined as 3%w/w, 27%w/w and 27% w/w, and with the sum of 10-HDA, 10-H2DA and 10-HDAA minimum values of 5% w/w in the dry basis [45]. In the case of Japan, the standard was done based on Apilac product (the mixture of 6 parts of frozen RJ added to 1 part of dried glucose-lactose (1:1) with 50 mg/kg of l-ascorbic acid as an antioxidant being dried until 4% humidity) [8]. It must contain at least 4.0% and 8.0% of 10-H2DA (JP XVII). According to Ramadan and Al-Ghamdi [26], lyophilised royal jelly contains < 5% water, 27–41% protein, 22–31% carbohydrate and 15–30% fat. The quality and identity requirements must be followed to try to guarantee the effects of royal jelly. It is particularly interesting in the case of RJ in powder since it is usually the focus of adulteration that can substantially compromise the biological effects of this critical product [26].
Anti-inflammatory and antioxidant properties of RJ
The current literature points out that bee products, including honey, propolis, pollen and royal jelly, can mitigate the oxidative stress observed in various pathological conditions because they are sources of natural antioxidants [46]. Flavonoids and phenolic acids can scavenge ROS and prevent oxidative damage [47]. Some researchers demonstrated that three tyrosyl dipeptides (Lys-Tyr, Arg-Tyr and Tyr-Tyr) of RJ had high antioxidant activity in vitro model scavenging free radicals by a hydroxyl group [48].
In addition, the presence of hydroxydicarboxylic fatty acids, mainly 10-HDA described exclusively in royal jelly, also contributes to the antioxidant capacity of royal jelly [38]. Sugiyama et al. [7] attempted to explain the inhibitory antioxidant mechanism of 10-HDA by assessing its ability to inhibit nitric oxide (NO) production. For this, they encouraged the generation of NO in a RAW264 murine macrophage cell line from lipopolysaccharides (LPS), which can induce the production of interferon-β (IFN-β) and other factors involved with the induction of iNOS. Finally, it was confirmed that 10-HDA inhibited NO generation and attenuated the activation of the IFN-β-mediated nuclear factor NF-kB and the tumour necrosis factor α (TNF-α) [7].
From this, there is also an insight that, in addition to the antioxidant capacity of royal jelly, it is possible to observe an anti-inflammatory potential. According to Mihajlovic et al. [49], the hydroxydicarboxylic fatty acids present in royal jelly are the central modulators of the inflammatory cascade. These authors, when evaluating the effects of a dose of 500 µM of 10-HDA on dendritic cells derived from human monocytes (Mo-DCs) incited by LPS, observed that there was inhibition of the maturation of Mo-DCs and the production of the inflammatory cytokines IL-8, IL-12 and TNF-α [49].
Thus, it was elucidated that the anti-inflammatory potential of royal jelly revolves around the attenuation of the transcription of inflammatory cytokines from the modulation of the NF-kB signalling pathway.
In basal situations, NF-kB is coupled to its inhibitory protein IκB, which keeps it inactivated in the cytoplasm. After stimuli, such as induction by LPS, phosphorylation of IκB occurs, and consequent release of NF-kB to the nucleus, where the p65 subunit is phosphorylated, and activation of target genes for the transcription of pro-inflammatory factor occurs [50]. However, NF-kB activation can also occur through interferons, such as IFN-β, involving activation of the phosphatidylinositol 3-kinase (PI-3 K) and of the Akt, which, when active, induce NF-kB activation interferon-dependent, and when inactive suppress NF-kB activation [51]. As Sugiyama et al. [52] proposed, 10-H2DA, the primary fatty acid of RJ, would suppress the expression and transcription of IκB mRNA and inhibit the activation of PI-3 K/Akt, causing the inactivation of NF-kB mediated by IFN-β.
Corroborating this, it was also observed that RJ could exert anti-inflammatory activity by stimulating the NRF2 pathway, an endogenous defence transcription factor of the organism [16]. This mechanism is explained due to the ability of a fatty acid to increase the translocation of Nrf2 from the cytoplasm to the nucleus to bind to the element of antioxidant response (ARE), which consequently activates the transcription of genes encoding antioxidant enzymes, increasing the expression of these enzymes [53]. Aslan et al. (2023) confirmed in a study with albino Wistar rats for 8 weeks that 100 mg/kg of RJ effectively increases the expression of NRF2 and consequently was associated with increased levels of the antioxidant enzymes glutathione and catalase. These authors also observed that the intervention with RJ reduced malondialdehyde levels, a lipid peroxidation parameter, and the NF-κB expression levels.
Other studies on royal jelly’s antioxidant and anti-inflammatory capacity, in vitro and in vivo, are summarised in Table 1. Figure 2 illustrates the primary mechanisms that RJ promotes inflammation and oxidative stress reduction and increases antioxidant enzyme synthesis.
Table 1.
Studies involving the antioxidant and anti-inflammatory capacity of royal jelly
| References | Sample | Intervention | Results |
|---|---|---|---|
| In vitro studies | |||
| [179] | Human lung cancer cell lines (A549) | Treatment with 30 μM 10-HDA for 3, 6, 12, 24, and 36 h |
↑ p-p38, p-JNK, and IκB expression ↓ p-ERK, p-STAT3, and NF-κB expression |
| [180] | Primary bone marrow cells (1 × 105 cells/cm2) | Different doses of RJ (12.5–50 ng/mL) for 3 days | ↓ NF-κB receptor activator |
| [181] | Microglial BV-2 and N9 cell lines | Different doses of 10-HDAA (1–4 mM) from RJ + LPS for 1 h |
↓ iNOS, NO levels and levels of pro-inflammatory mediators ↓ NLRP3 inflammatory pathway, from P53 modulation |
| [53] | Human neuroblastoma SH-SY5Y cells | Treatment with 50 µM HPO-DAEE (4-hydroperoxy-2-decenoic acid ethyl ester, a synthesised RJ fatty acid derivative), for 4 h |
↑ HO-1 by promoted phosphorylation of eIF2α ↑ Nuclear accumulation of Nrf2 and activated ARE |
| [123] | WiDR human adenocarcinoma cell (BCRC 60157) | Different doses of 10-HDA (0.1 to 5 mM) from RJ for 24 h |
↓ IL-8, IL-1β, TNF-α and NF-κB ↑ IL-1Ra |
| [24] | Murine macrophage RAW 264.7 cells | Different doses of 10-H2DA, 10-HDAA and SEA, from RJ for 24 h |
10-H2DA, 10-HDAA e SEA: ↓ IL-6, IL-10 and NO SEA: ↓ TNF-α |
| Animal studies | |||
| [42] | 60 male Wistar rats | (1) control group; (2) RJ (300 mg/kg); (3) Vit E (180 mg/kg); (4) 30 mg/kg of dimethylhydrazine (DMH); (5) RJ (300 mg/kg) + DMH; (6) Vit E (180 mg/kg) + DMH |
DMH + RJ: ↓ hs-CRP, MDA levels ↑ CAT, SOD |
| [182] | 42 adult albino rats | (1) DOX (3 mg/kg) for 6 weeks; (2) DOX + 500 mg/kg/d of honey orally; (3) DOX + 100 mg/kg/d of RJ; 4) DOX + 50 mg/kg/d of propolis; (5) DOX + oral honey, RJ and propolis for 21 days |
DOX + RJ: ↓ MDA and TNF-α levels ↓ PARP-1 and Bcl-2 protein expression ↑ SOD and GPX levels ↑ Caspase-3 |
| [183] | 48 male BALB/c mice | 8 groups: saline, 3 different doses of RJ (100, 150, and 200 mg/kg/d), nicotine (1.5 mg/kg), and 3 different groups of Nic + RJ (1.5 mg/kg of Nic + 100, 150, and 200 mg/kg of RJ) |
After treatment with RJ regardless of dosage: ↑ Nfr2 and Bcl-2 gene expression ↓ MDA levels, NO, Caspase-3 and P53 gene expression |
| [184] | 250 zebrafish—Danio rerio | 5 different gelatine and casein-based diets were prepared containing RJ in the ratios of D1 (%0.00), D2 (%0.10), D3 (%0.40), D4 (%1.60) and D5 (%6.40), for 8 weeks | ↑ mRNA levels and enzymatic activities of CAT, GR, SOD, GPx and GST |
| [15] | 42 males Wistar albino rats | (1) Control; (2) RJ (100 mg/kg); (3) fluorine (F) (50 mg/kg); (4) fluorine (100 mg/kg); (5) F50 + RJ; (6) F100 + RJ for 8 weeks |
↓ MDA levels, Bcl-2, Gsk-3 and NF-κB protein expression ↑ GSH, caspase-3, caspase-9, caspase-6, Bax, BDNF and Nrf2 protein levels |
| [134] | Healthy male BALB/c mice | 4 groups: low-dose RJ (1000 mg/kg/d), medium-dose RJ (3000 mg/kg/d) and high-dose RJ (9000 mg/kg/d) and a control group for 30 days | ↑ IL-10 and levels of antioxidant activities in the liver and kidney |
| [185] | 36 male BALB/c mice | (1) normal saline; (2) RJ (100 mg/kg/d RJ; (3) testicular injury induced by nicotine (NIC) (0.50 mg/kg/d); (4) NIC (1 mg/kg/d); (5): NIC (0.50 mg/kg/d) + RJ; (6) NIC (1 mg/kg/d) + RJ for 35 days |
↓ MDA levels, p53 and Caspase-3 m-RNA ↑ TAC and CAT |
| [186] | 30 Wistar rats with exposing 40 Watt UV-B lamps for 2 h/day in 14 day | RJ cream application with doses of 2.5%, 5%, and 10%; negative control with vaseline; and normal control |
The higher the RJ dose: ↑ Nrf2 levels ↓ NF-kB and TNF-α levels |
| [16] | 28 Swiss male mice | (1) control group; (2) RJ (85 mg/kg); (3) CdCl2 (6.5 mg/kg); (4) RJ 1 h before CdCl2 injection for 1 week |
RJ pretreatment restored the oxidant/antioxidant balance: ↑ GSH content and the activities of GPx, GR, SOD, and CAT ↑ mRNA expression of GPx1, GR, SOD2, and CAT ↑ Nrf2 expression |
| Human studies | |||
| [187] | 20 high-level swimmers | 400 mg of RJ and 60 mg of CoQ10 or placebo, once daily for 10 days |
↓ Lipid peroxidation products (Diene conjugates and Schiff bases) ↓ Creatine kinase activity |
| [188] | 72 overweight adults aged 25–50 | RJ (333 mg of lyophilised organic—standardised to a minimum of 4% 10-HDA) or placebo for 8 weeks |
↓ TC and hs-CRP ↑ Adiponectin, leptin and serum TAC |
| [189] | 46 type 2 diabetic patients, aged 25–65 | RJ (1000 mg) or placebo (glycerin) 3 × /d for 8 weeks |
↓ HOMA-IR ↑ TAC |
Abbreviatios: RJ, royal jelly; 10-HDA, 10-hydroxy-2-decenoic acid; 10-HDAA, 10-hydroxydecanoic acid; TC, total cholesterol; hsCRP, high-sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment—insulin resistance; TAC, total antioxidant capacity; CAT, catalase; MDA, malondialdehyde; GSH, glutathione; TXL, taxol; NO, oxide nitric; NF-Κb, nuclear factor-Κb; TNF-α, tumour necrosis factor-α; IL, interleukin; iNOS, induced nitric oxide synthase; NLRP3, NOD-like receptor pyrin domain-containing-3; 10-H2DA, trans-10-hydroxy-2-decenoic acid; SEA, sebacic acid; GR, glutathione reductase; SOD, superoxide dismutase; GPx, glutathione peroxidase; GST, glutathione S-transferase; CdCl2, cadmium chloride; eIF2α, eukaryotic initiation factor 2α; ARE, antioxidant response element; Nrf2, NF-E2-related factor 2; HO-1, heme oxygenase-1
Fig. 2.
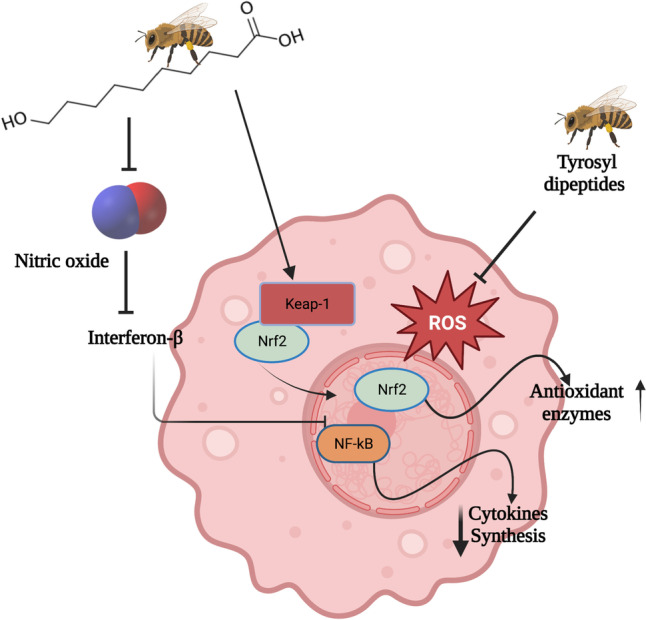
The royal jelly mechanism mitigates inflammation and oxidative stress and increases antioxidant enzyme synthesis. Created with BioRender.com
Beneficial effects of RJ on the multi-faceted mitochondrial functions
Mitochondrial function is vital to cellular health and functioning in the context of PPPM/3PM, since mitochondria are the organelles responsible for cellular energy production and play critical roles in regulating metabolism and cellular stress response. Mitochondrial dysfunction has been linked to various diseases, including metabolic disorders, neurodegenerative diseases, cancer and ageing. In this context, mitochondrial dysfunction can occur due to several factors, including genetic mutations, oxidative stress, mitochondrial DNA damage, nutritional deficiencies and exposure to toxins and drugs [54].
Mitochondrial dysfunction can lead to energy imbalance and ROS growth, triggering cell damage, decreased ATP production, resulting in lack of energy in cells and impaired metabolic functions, and metabolic disorders such as insulin resistance, dyslipidemia and accumulation of toxic substances [55].
Studies have shown that mitochondrial dysfunction plays a crucial role in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease because the accumulation of abnormal proteins in these diseases, such as beta-amyloid and alpha-synuclein, can lead to mitochondrial dysfunction, resulting in oxidative stress, impaired energy metabolism and cell death [56, 57].
Mitochondrial dysfunction has been implicated in the pathogenesis of type 2 diabetes. With reduced mitochondrial function and oxidative capacity, decreased ATP production and ROS accumulation occur, contributing to insulin resistance and impaired pancreatic beta cell function, leading to hyperglycemia and the development of type 2 diabetes [58].
Furthermore, mitochondrial dysfunction compromises the heart’s contractile efficiency, increases the production of ROS and promotes cardiomyocyte apoptosis. These events contribute to the progression of cardiovascular diseases and worsen the prognosis of patients [59]. In ageing, the accumulation of mitochondrial DNA damage over time leads to reduced mitochondrial function, decreased ATP production and increased oxidative stress. These changes contribute to cellular ageing and are associated with the development of age-related diseases such as cancer, cardiovascular and neurodegenerative diseases [59].
The analysis of mitochondrial function includes mitochondrial DNA integrity, dynamics, oxidative stress and mitochondrial stress response capacity, which can provide a detailed view of mitochondrial health and can be used to identify early dysfunctions and monitor the effectiveness of personalised interventions [54].
Scientific studies have explored the possible effects of RJ on mitochondrial health, and some preliminary results are promising, showing improvement of mitochondrial biogenesis by AMPK activation [60]; antioxidant protection against oxidative stress due to the critical content of RJ antioxidant compounds, which help to neutralise ROS [16, 61]; and RJ may have a potentially protective effect against mitochondria-mediated apoptosis by modulating molecular mechanisms related to apoptosis, such as the Mfn2 protein and the Bax/Bcl2 ratio [62].
Whilst these preliminary studies are promising, it is essential to note that research on the effects of RJ on mitochondrial health is still in its early stages. More investigations, including human clinical studies, are needed to fully understand the mechanisms of action and determine the effectiveness of RJ in this context.
Royal jelly and its antidiabetic potential
Diabetes mellitus is an NCD and a global public health problem. Its prevalence increases each year dramatically, and between 2030 and 2040, about 439 and 642 million people will be diagnosed with diabetes mellitus [63]. In 2045, this number will increase to 700 million people [64]. Diabetes mellitus is characterised by glucose and insulin metabolism disturbances, generating chronic hyperglycemia [65, 66]. Chronic hyperglycemia increases oxidative stress and inflammation associated with macrovascular and microvascular complications, such as diabetic nephropathy, cardiovascular disease, renopathy and diabetic foot [67, 68].
Therapy strategies provide the patient with a better quality of life and control of the complications of diabetes, including insulin and oral hypoglycaemic agents [69]. Also, adjuvant nutritional strategies have been widely used, showing anti-inflammatory and antioxidant effects, such as cinnamon, curcumin, psyllium fibre and polyphenols [70–72].
In this sense, RJ has also been a target of experimental and clinical studies due to its antidiabetic potential. RJ is vital in scavenging free radicals and improving insulin resistance [9]. The antioxidant action may be related to three tyrosyl dipeptides in the RJ molecule, which have high antioxidant activity due to the hydroxyl group of their hydrogen atom [48]. Activation of detoxifying enzymes, such as glutathione-s-transferase and glutathione peroxide genes, has also been associated with RJ [33, 73].
In addition, RJ appears to increase glucose absorption by improving insulin signalling and activating the AMP-activated protein kinase (AMPK) pathway in the skeletal and hepatic muscle through increased adiponectin secretion, reducing gluconeogenesis and lipogenesis, leading to a decrease in glucose production and an improvement in the glycemic index [74, 75].
10-H2DA stimulates the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/glycogen synthase kinase 3β (GSK3β) signalling pathway [76]. This pathway is critical in the metabolic control of glucose, with insulin activation leading to glycogen synthase activation and consequent reduction in blood glucose, showing a significant hypoglycemic action [76].
Furthermore, RJ is essential as an anti-hypercholesterolemic by decreasing very low-density lipoprotein (VLDL) and triglyceride plasma levels [77]. 10-H2DA can modulate apolipoprotein (Apo) A–I, and the Apo B/Apo A–I ratio, reducing the risk of cardiovascular disease in diabetic patients [41]. RJ seems to increase the mRNA and protein expression of thermogenic uncoupling protein 1 (UCP1), mitochondrial cytochrome c oxidase subunit IV (COX-IV) Nbat, promoting thermogenic effect, reducing body fat and improving the glucose homeostasis [78]. Table 2 shows the studies with RJ in diabetes.
Table 2.
Summary of in vivo studies and clinical trials involving royal jelly supplementation and effects on diabetes mellitus
| References | Sample | Intervention | Results |
|---|---|---|---|
| Animal studies | |||
| [76] | STZ induced DM + HFD in mice | 100 mg/kg of 10-H2DA for 4 weeks |
↓ fasting blood glucose, ↑ insulin levels, ↑ area of pancreatic islets ↑ SOD, CAT and GPx activities ↓ lipid peroxidation; ↓ NF-κB nuclear translocation, ↓ IL-6 and TNF-α ↑ PI3K, AKT, and GSK3β protein levels |
| [77] | STZ induced DM in rats | 2 g/daily of RJ by gavage for 28 days |
↓ plasma VLDL and TG ↓ fasting blood glucose and HbA1c |
| [78] | Mice fed with HFD | HFD with 5% of RJ for 10 weeks |
↓ fasting blood glucose and insulin ↓ HOMA-IR ↑ UCP1 mRNA and protein expression in BAT ↑ Cox-IV mRNA and protein expression in BAT |
| [74] | Obese/diabetic KK-Ay mice | 10 mg/kg of RJ by gavage for 4 weeks |
↑ AdipoQ and AdipoR1 expression ↑ Pampk expression ↓ hyperglycaemia ↔ insulin resistance |
| [190] | STZ induced DM in rats | 100 mg/kg of RJ administered orally |
↓ serum levels of AST, ALT, ALP and fasting blood glucose ↑ CAT and FRAP ↓ MDA |
| Human studies | |||
| [189] | 46 T2D patients | 1000 mg RJ 3 × /d for 8 weeks | ↓ HOMA-IR |
| [41] | 50 T2D patients | 1000 mg RJ 3 × /d for 8 weeks | ↓ fasting blood glucose |
| [73] | 50 T2D female patients | 1000 mg RJ / daily in soft gel or placebo |
↓ fasting blood glucose and HbA1c ↑ insulin concentration ↑ erythrocyte superoxide dismutase and GPx ↓ MDA |
Abbreviatios: STZ, streptozotocin; HFD, high-fat diet; T2D, type 2 diabetes; 10H2DA, 10-hydroxy-2-decenoic acid; DM, diabetes mellitus; RJ, royal jelly; HbA1c, glycated haemoglobin; TG, triglycerides; VLDL, very low-density lipoprotein cholesterol; SOD, superoxidase dismutase; GPx, glutathione peroxidase; MDA, malondialdehyde; HOMA-IR, homeostasis model assessment-insulin resistance; BAT, thermogenic capacities of brown; UCP1, thermogenic uncoupling protein 1; COX-IV, mitochondrial cytochrome c oxidase subunit IV; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; CAT, catalase; FRAP, ferric reducing antioxidant power; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; GSK3β, glycogen synthase kinase 3 beta; AdipoQ, adiponectin gene; AdipoR1, adiponectin receptor-1 gene; pAMPK, phosphorylated AMP-activated protein kinase; NF-κB, nuclear factor kappa B; IL, interleukin; TNF-α, tumour necrosis factor-alpha
Protective effects of RJ on cardiovascular diseases
Cardiovascular diseases (CVDs) persist as the leading cause of mortality worldwide. A dominant cause of CVD is atherosclerosis. CVDs are responsible for a reduced quality of life, despite the advances in managing CVD risk factors [79, 80]. Excessive ROS production is responsible for cardiovascular disorders, such as ischemia/reperfusion injury, cardiac hypertrophy, atherosclerosis, heart failure and myocardial infarction [81–83].
A multidisciplinary and personalised approach is essential for preventing CVDs such as ischemic stroke. Since the traditional approach to investigating these cases is not always sufficient to determine the root cause of the stroke, a more comprehensive assessment is necessary since traditional risk factors, such as high blood pressure, diabetes and dyslipidaemia, may not be sufficient to explain the stroke. Within this approach, the PPPM principles aim to identify risk factors and underlying causes through a detailed analysis of each stroke case, considering genetic factors, medical history and individual lifestyle. This allows for a more comprehensive understanding of stroke aetiology [84].
In this context, there is growing evidence linking healthy dietary habits with a reduced risk of developing or reversing the progression of CVD. These effects could be attributed to some foods, single nutrients or bioactive compounds [5, 85]. Royal jelly has beneficial effects in the prophylaxis and treatment of CVD risk factors, as seen in Table 3 [86].
Table 3.
Studies involving royal jelly and cardiovascular diseases
| References | Sample | Intervention | Results |
|---|---|---|---|
| In vitro studies | |||
| [90] | Isolated aortic rings from male Japanese white rabbits | Treatment with cumulative doses (0.01–10 mg/mL) of RJ |
↑ vasorelaxation of the isolated aortic rings ↑ NO production ↓ NE-induced intracellular Ca2+ releases and high K+-induced extracellular Ca2+ influx in denuded aortic rings |
| [91] | Thoracic aorta and superior mesenteric artery (contracted with phenylephrine) from male Wistar rats | Fresh RJ (0.0001–0.3 mg/mL), ACh (10−10 to 10−6 M) in the presence or absence of inhibitors L-NAME (10−4 M, 20 min) or atropine (10−5 M, 15 min) | ↑ vasorelaxation in a concentration-dependent manner of isolated rat aortas and superior mesenteric arteries |
| [191] | Mouse (C57BL/6 strain) VSMC | MRJP1 group: Mrjp1 gene was inserted into mouse VSMC and Control group | ↓ Cell contraction, migration, and proliferation |
| [192] | Mice aortic VSMCs |
Control, glycosylated MRJP1 (P +), deglycosylated MRJP1 (P), and glycans (L) All treatments were divided into subgroups: with or without AngII for 16 h |
P + : ↓α-SMA vs. control, P and L; P + inhibited the migration of VSMCs |
| Animal studies | |||
| [193] | 32 Wistar rats |
1) MRJ (15 mg/kg/d); 2) PRJ (15 mg/kg/d); 3) CRJ (15 mg/kg/d);4) captopril (50 mg/kg/d) 5) metabolic syndrome model—no treatment; 6) placebo (distilled water) |
Continuous intake of RJ prevented elevation in BP values |
| [15] | 42 rats | Control group; RJ group (100 mg of RJ/kg); F50 group (50 mg of fluoride/kg); F100 group (100 mg of fluoride/kg); F50 + RJ group (50 mg of fluoride/kg + 100 mg of RJ/kg); F100 + RJ group (100 mg of fluoride/kg + 100 mg of RJ/kg) for 8 weeks |
In heart tissue: RJ in all groups: ↓ MDA; F50 + RJ and F100 + RJ groups: ↑ GSH level, CAT activity, caspase-3, caspase-9, caspase-6, Bax, BDNF and Nrf-2 proteins levels; ↓ Bcl-2, GSK-3 and NF-κB expression |
| [90] | Male SHR and WKY | WKY-control group; SHR-control group; SHR-RJ group: received 1 g/kg of RJ daily for 4 weeks |
↓SBP and DBP in SHR; ↑NO levels in SHR |
|
[91] *Experiment 1 |
10-week-old male Wistar rats | Control group and RJ group (100 mg/kg) for 6 days |
↑ Tail blood flow and mass ↔ velocity, SBP or HR |
|
[91] *Experiment 2 |
9-week-old male Wistar rats |
Control group; l-NAME group (0.5 mg/mL); l-NAME + RJ (100 mg/kg) |
RJ: ↔ Blood flow, mass, velocity, SBP and HR in L-NAME-treated rats |
| [39] | Healthy adult male Wistar rats | Control group; TXL group (7.5 mg/kg); T1 group (TXL—50 mg RJ/kg); T2 group (TXL + 100 mg RJ/kg); T3 group (TXL + 150 mg RJ/kg); T4 group: RJ (100 mg/kg) for 4 weeks |
In heart tissue: ↓ MDA and NO levels; ↓CK-BM level and necrosis; In serum: ↑ total antioxidant capacity |
| Human studies | |||
| [194] | 88 healthy volunteers between 20 and 60 years | 690 mg of RJ/day (equivalent to 2040 mg of fresh royal jelly) or placebo for 4 weeks |
RJ group: ↑RH-PAT index |
| [195] | 40 subjects w/ mild hypercholesterolemia | 9 capsules (350 mg of RJ)/day or placebo for 3 months |
RJ group: ↓ TC and LDL-c ↑DHEA-S |
| [196] | 36 postmenopausal healthy women aged 53–66 years | 150 mg of RJ/day for 3 months |
↑ HDL-C ↓ LDL-C and TC |
| [94] | 15 healthy adult volunteers | 6 g RJ/day or placebo for 4 weeks | RJ group ↓ serum TC and ↓ serum LDL by lowering small VLDL levels |
Abbreviatios: α-SMA, anti-α-smooth muscle actin; BDNF, brain-derived neurotrophic factor; BW, body weight; CK-BM, creatine kinase; CRJ, control royal jelly; DBP, diastolic blood pressure; DHEA-S, dehydroepiandrosterone sulphate; FATP3, fatty acid transport protein 3; FGF21, fibroblast growth factor 21; HDL, high-density lipoprotein; HepG2, cells of the human hepatoblastoma cell line; HMG-CoA reductase, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase; IVY, Ile-Val-Tyr; IY, Ile-Tyr peptide; LDL-c, low-density lipoprotein cholesterol; LDLR, low-density lipoprotein receptor; L-NAME, L-NG-nitro arginine methyl ester; MDA, malondialdehyde; MRJ, mucuna royal jelly; MRJP1, major royal jelly protein 1; NO, nitric oxide; Nrf-2, nuclear factor erythroid 2-related factor 2; PCT-RJ, RJ hydrolyzed by peptide, chymotrypsin and trypsin; PRJ, pollen royal jelly; ProRJ, protease N treated royal jelly; RJ, royal jelly; RH-PAT, reactive hyperemia peripheral arterial tonometry; SBP, systolic blood pressure; SHR, spontaneously hypertensive rats; SQLE, squalene epoxidase; SREBP-1, sterol regulatory element binding protein 1; TC, total cholesterol; VLDL, very-low-density lipoprotein; VSMCs, vascular smooth muscle cells; WKY, Wistar Kyoto rats; TXL, paclitaxel—chemotherapeutic agent
It has been shown in the literature that RJ exhibited antihypertensive effects, but the exact mechanism has not been elucidated [87–90]. The antihypertensive effect of RJ may be linked to its capacity to increase NO production. Studies show that RJ has a muscarinic receptor agonist, potentially acetylcholine, which causes vasodilation via NO/cGMP (cyclic 3′,5′‐guanosine monophosphate) pathway and through calcium channels [90, 91].
Experimental studies have shown that RJ can ameliorate dyslipidemia [92, 93]. However, the mechanisms by which RJ exerts its hypocholesterolemic effects are still under investigation. One possible mechanism is the large number of proteins in RJ, which may decrease plasma levels of cholesterol, LDL and small VLDL, reduce cholesterol biosynthesis enzyme, influence hepatic lipoprotein receptors that regulated VLDL uptake and suppress of hepatic sterol synthesis [94]. Royal jelly can up-regulate cholesterol 7-α-hydroxylase (CYP7A1), an enzyme associated with receptors that synthesise very low-density lipoproteins (LDL-c) [95]. A recent meta-analysis found that RJ reduces total cholesterol and increases HDL serum levels in studies with a long-term follow-up period. Otherwise, the same study indicates that triglycerides and LDL serum levels did not significantly ameliorate [96]. Also, RJ seems to block the reabsorption of bile acids, reducing cholesterol plasma levels [97].
Lastly, endothelin-1 is a vasoconstrictor peptide produced by endothelial cells and plays a vital role in regulating cardiovascular function, with conversion to a PPPM approach [98]. In this sense, in a study with hypertensive rats, treatment with a combination of propolis, royal jelly and bee venom at daily oral doses of 0.5, 1.0 and 2.0 mg/kg decreased serum levels of endothelin-1 and TNF-β NF-kB and biomarkers of oxidative stress, being considered a preventive factor and potential treatment for CVD [99].
Despite the cardioprotective potential of RJ, there is a lack of specific studies on how RJ can prevent the onset or progression of CVDs. The findings in the literature point to different protective effects of RJ on important risk factors for CVDs, which reinforces the importance of further studies to elucidate the mechanisms behind the already demonstrated effects and explore more that still need to be well established.
Can royal jelly be a promising strategy for treating chronic kidney disease patients?
Chronic kidney disease (CKD) is a significant global health burden and is increasing in prevalence [100]. The uremic phenotype is marked by elevated oxidative stress, persistent low-grade inflammation, gut dysbiosis and premature ageing, contributing to poor health status and premature mortality in CKD, where cardiovascular disease is the leading cause [100–102].
Several factors, including excess ROS and a pro-inflammatory phenotype, explain chronic inflammation in CKD. The deregulation of pro-inflammatory and anti-inflammatory factors, such as those mediated by NF-kB and Nrf2, plays an essential role in the aggravation and progression of kidney damage [103, 104].
Accumulated evidence encourages using additional non-drug therapeutic strategies such as foods, nutrients and bioactive compounds with anti-inflammatory and antioxidant effects in managing the changes found in CKD [105–108].
Based on what has been exposed so far, RJ may be a promising strategy to be included as an adjuvant treatment for CKD. There are no published studies on patients with CKD. Only one clinical trial conducted by Ohba et al. [109] is in progress and will supplement 3600 mg/day of RJ in patients on haemodialysis for 24 months. Taking into account, animal studies with models related to kidney damage have demonstrated that RJ can improve markers of kidney function [43, 110] and alleviate oxidative stress and inflammation through upregulation of Nrf2 and downregulation of NF-kB [111], leading to increased antioxidant enzymes, reduction of lipid peroxidation [43] and production of inflammatory cytokines [112]. Thus, more studies, especially clinical trials, are needed to elucidate the possible effects of RJ on patients with CKD. Table 4 summarises the animal and one human study with models of kidney damage induced using RJ supplementation.
Table 4.
Summary of in vivo studies involving royal jelly supplementation and renal effects
| References | Sample | Intervention | Results |
|---|---|---|---|
| In vivo | |||
| [61] | Fluoride-induced nephrotoxicity in rats | RJ (100 mg/kg) + fluorine (100 or 50 mg/kg) for 8 weeks |
↓ MDA in renal tissue ↑ CAT activity and GSH in renal tissue ↑ caspase-3, caspase-6, caspase-9 and Bcl-2 proteins |
| [43] | Moxifloxacin-induced renal and hepatic injury in rats | RJ (100 mg/kg/d) or ECH (40 mg/kg/d) or RJ + ECH for 30 days |
RJ group: ↓ creatinine, urea, K+, and Ca2+ ↑ GSH and ↓ MDA in renal tissue ↓ renal tissue KIM-1 contents Attenuated MOX-induced histopathological changes and reduced caspase-3 in renal tissue |
| [197] | Silver nanoparticles-induced liver and kidney inflammation in rats | Groups: 30 mg/kg of nano-silver + 100 mg/kg of RJ; 100 mg/kg of RJ alone; control group; 30 mg/kg of nano-silver for 28 days |
RJ alone: ↓ kidney IL-6 when compared to control group RJ and nano-silver groups: ↓ kidney IL-1β and IL-33 levels NS + RJ group: ↑ IL-33 and IL-1 β levels in kidney tissue compared to nano-silver group |
| [110] | Diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats | Diclofenac (50 mg/kg/d) for 7 days + RJ (150 or 300 mg/kg/d) for 30 days |
RJ (150 and 300 mg/kg): ↓ blood urea and creatinine ↑ PGE2 and ↓ MPO in renal tissue |
| [111] | Cadmium-induced nephrotoxicity in male mice | 85 mg/kg RJ 2 h prior to i.p. injection of 6.5 mg/kg CdCl2 daily for 7 days |
↓ Cadmium, urea, creatinine and uric acid levels ↓ kidney weight ↓ Lipid peroxidation, NO ↑ Glutathione levels ↑ Nrf2, HO-1, and NQO1 protein expressions and ↓ NF-κB protein expression ↓ Bax and caspase-3 expressions ↓ kidney levels of TNF-α and IL-1β |
| [198] | Renal ischemia/reperfusion –induced in rats | 300 mg/kg of RJ before bilateral renal ischemia for 30 min followed by 24 h of reperfusion for 15 days |
↓ blood urea nitrogen, creatinine ↓ kidney malondialdehyde, ↓ glomerular diameter, leukocyte infiltration, levels of TNF-α, adhesion molecule-1 expression, and NO |
| [43] | Cisplatin-induced nephrotoxicity in rats | 100 mg/kg of RJ or 20 mg/kg of bee honey orally 1 h before intraperitoneal cisplatin (2 × /week) for 10 weeks | ↓ urea, creatinine and uric acid in the RJ group |
| [112] | Ethylene glycol induced renal urolithiasis and inflammation in rats | 100 mg/kg of RJ by oral gavage for 4 weeks | ↓ IL–1 and IL-18 in renal tissue |
| Clinical trial | |||
| [199] | Cisplatin-induced nephrotoxicity in patients with cancer | 2 capsules of RJ or bee honey (80 g) daily 3 days before the onset of cisplatin chemotherapy and continued during the regimen of the 2 cycles (21 days each) ± 50 days |
RJ group: ↔ urea and creatinine serum levels |
Abbreviatios: RJ, royal jelly; NO, nitric oxide; MPO, myeloperoxidase; PGE2, prostaglandin-E2; ECH, Echinacea; MDA, malondialdehyde; KIM-1, kidney injury molecule-1
The role of royal jelly in cancer treatment
Significant changes in cancer treatment in terms of PPPM are occurring. In addition to conventional treatments for the disease, health care, in general, is part of this new perspective. During the development of the PPPM, new rapid, specific and sensitive methods are approached for early cancer detection, contributing to more efficient patient management. In addition, this management will contribute to patients’ quality of life through the interaction of a multidisciplinary team [113].
In this sense, since the emergence and development of cancer are directly related to inflammation, using anti-inflammatory agents that can prevent its appearance and increase the effectiveness of cancer treatment is necessary [114, 115]. As in other products derived from beekeeping, the properties of RJ are due, amongst other factors, to the presence of phenolic structures capable of stabilising free radicals and reducing ROS and oxidative stress [46, 116].
Amongst phenolics, flavonoids play an essential role in cancer prevention. They can act by stimulating the action of antioxidant enzymes that protect cells from damage, such as superoxide dismutase (SODs), catalase (CAT), cyclooxygenase-2 (COX-2) and glutathione (GSH), in addition to stimulating anti-inflammatory pathways such as that of NRF2 [75, 117]. This further highlights the vital role of an adequate and balanced diet in preventing and treating cancer.
It is also necessary to draw attention to the essential role of the immune system as a natural mechanism to fight cancer. In addition to acting to protect the body through the action of natural killer cells, preventing cancer from installing, when a cell becomes cancerous, there is recognition of the antigens/membrane proteins of this cell by the immune system, which starts to attack, blocking its action and promoting its lysis [118–120]. Despite its essential role, the immune system often fails to act as it should, given the location, progression and characteristics of the disease [119, 120].
One of the mechanisms by which RJ can mitigate cancer is also through modulation of the immune system [121]. The RJ 10-HDA compound can inhibit the cellular activation of NF-κB and inflammatory cytokines, such as TNFα, IL-1β and IL-8, and even increase IL-1Ra, an interleukin 1 antagonist, with a dose–response effect [121–123]. Interleukin-1 receptor (IL-1R) is expressed in several types of cells and acts as a receptor/ligand for IL-1α and IL-1 β, both with inflammatory potential. This receptor plays a vital role in the emergence of some types of cancer since, with the binding of interleukin 1, it regulates cell signalling pathways such as NF-kB and MAP kinase, which are pro-proliferative and pro-tumorigenic [124, 125].
Some scientific findings have also discussed the role of hormonal modulation in the onset of cancer. In this context, oestrogen, for example, plays an essential role in the emergence of breast cancer since binding to receptors on these cells stimulates its development [126, 127]. In addition to endogenous factors, environmental/synthetic compounds may also play a role. Bisphenol A (BPA), a compound used in the manufacture of polycarbonate, is found in most plastic products [128, 129] and has recently gained prominence. In addition to other risks, studies point to a probable relationship between BPA consumption and the onset of cancer, especially oestrogen-dependent ones such as breast cancer, since it is an endocrine disruptor [128, 129].
BPA can accelerate cell ageing by decreasing telomerase activity, generating telomere shortening with the upregulation of telomerase reverse transcriptase. Furthermore, BPA binds to oestrogen receptors, increasing the risk of cell proliferation of cancerous cell lines that are hormone-dependent [128, 129].
It is suggested that RJ can interrupt the damage caused by BPA in breast cancer cells, inhibiting their proliferative activity. In these cases, it can suppress the binding between oestrogen and cancer cells and thus interfere with cell signalling that stimulates cell lineage proliferation (MCF-7) [122, 130, 131]. Despite this, there is still a need for more robust studies that prove this theory, as well as the dose and time that would be ideal.
RJ can also mitigate the levels of prostaglandin E (PGE-2), which, in addition to being a carcinogenesis stimulator, is also an apoptosis inhibitor, modulating the cellular response [132–134]. Also, RJ can increase macrophage colony-stimulating factor (M-CSF) levels, suppress TNF-α production dose-dependently and influence the picture of anorexia and fatigue in cancer, minimising involuntary weight loss, common in these patients [135].
In addition to potentially mitigating the development of some types of cancer and possible symptoms, as previously mentioned, RJ has also been able to reduce mucositis. Mucositis can be defined as an inflammation of the epithelium, which arises as a result of chemotherapy and radiotherapy treatments or a combination of both and manifests itself as an increased sensitivity in areas of the gastrointestinal mucosa, which in more severe cases leads to wounds and prevents the feeding [136, 137]. Given the high burden of drug therapy that cancer patients undergo, associating non-pharmacological adjuvant treatments such as RJ can be interesting.
In addition to its anti-inflammatory potential, RJ plays an important role in preventing the progression of mucositis due to its antimicrobial and bactericidal capacity, which prevents pathogenic bacteria from adhering to the site and worsening inflammation [123, 138, 139]. It was observed that the healing of wounds resulting from mucositis was facilitated using RJ [136, 139–141]. Such effects seem to be primarily related to the ability to reduce inflammation and modulation of the immune system through the reduction of activated macrophages [136, 139–141]. Its antioxidant and anti-inflammatory capacity neutralise free radicals and decrease the production of pro-inflammatory cytokines, promoting wound healing [138, 139].
Studies are still needed to clarify better the different mechanisms of action of royal jelly on mucositis and robust data on its effectiveness in prophylaxis and treatment. Furthermore, it is essential to remember the allergenic potential of RJ, which should always be considered when recommending its use.
Table 5 presents the studies that evaluated the effects of RJ on cancer. Although its mechanisms of action have not yet been fully elucidated, RJ can be a promising adjuvant agent in cancer treatment. More studies are needed to evaluate the dose × response.
Table 5.
Studies involving royal jelly and its effects on cancer
| References | Sample | Intervention | Results |
|---|---|---|---|
| In vitro studies | |||
| [123] | WiDR human adenocarcinoma cell (BCRC 60157) | Control group and cells treated with 10-HDA (0.1, 0.5, 1, 2, 3, and 5 mM) for 24 h |
10-HDA (3 mM):↓ IL-8, IL-1β, TNF-α, NF-κB Dose response 10-HDA ↑ IL-1Ra ↓ Staphylococcus aureus, Streptococcus alactolyticus ( +), Staphylococcus intermedius B( +), Staphylococcus xylosus ( +), Pseudomonas aeruginosa ( −), Salmonella choleraesuis ( −), Vibrio parahaemolyticus ( −), Escherichia coli (hemolytic) |
| [200] | Human colorectal adenocarcinoma cells (CaCo2) | Cells treated with 10-HDA and human HuIFN-αN3 |
RJ antiproliferative activity of 2.0 HuIFN-αN3 antiproliferative activity of 2.5 10-HDA antiproliferative activity of 1.5 RJ + HuIFN-αN3 (2:1) antiproliferative activity of 3.8 ↓ GSH ↑MDA |
| Animal studies | |||
| [201] | 56 female Swiss albino mice induced with the animal model of EST |
Mice: control; normal saline; CP (50 mg/kg); Non-EST mice: RJ (200 or 400 mg/kg); EST mice: RJ (200 or 400 mg/kg) for 2 weeks |
Both doses of RJ (200 and 400 mg): ↓ Tumour size and markers (AFP and CEA) ↓ LPO and NO, ↓ TNF-α, ↓ Bcl ↑ GPx, CAT, SOD ↑ Caspase-3, Bax genes |
| [42] | 60 rats and HT-29 cells |
Control group (saline); DMH (30 mg/kg); Vitamin E (180 mg/kg); DMH + RJ (300 mg/kg); DMH + vitamin E (180 mg/kg) |
RJ Group ↓ PCNA, ↓ PDGF, ↓ CEA ↓ Congestion, necrosis, inflammation and cell proliferation |
| Human Studies | |||
| [14] | Patients with metastatic renal cell carcinoma 33 patients, aged 54–79 | Capsules containing 900 mg of RJ or placebo, 3 × /d for 3 months |
↓ Tumour size ↓ TNF-α e TGF-β ↓ Anorexia and fadiga |
| [135] | Patients with renal cell carcinoma treated with TKIs | Capsules with 800 mg of RJ 3 times/d or placebo for 3 months |
Week 2 to 4: ↑ TGF-β, ↑ M-CSF ↓ TNF-α ↔ after 12 weeks |
| [140] | 103 patients undergoing radiotherapy and chemotherapy | 1 g of RJ divided in 2 times/d as long as the symptoms persist or standard treatment | ↓ Healing time in all grades of mucositis |
Abbreviatios: RJ, royal jelly; PCNA, proliferating cell nuclear antigen; PDGF, platelet-derived growth fator; CEA, carcinoembryonic antigen; DMH, dimethylhydrazine; EST, Ehrlich solid tumours; TKIs, tyrosine kinase inhibitors; M-CSF, macrophage colony stimulating factor; HuIFN-αN3, human interferon-alpha; CaCo2, human colorectal adenocarcinoma cells; 10-HAD, 10-hydroxy-2-decenoic acid; HuIFN-αN3, interferon-alpha; AP, antiproliferative effect
New promising therapeutic avenues of royal jelly in neurological diseases
Positive results have been found against neurodegenerative diseases, cognitive performance, increased life expectancy and improvement of specific behaviours after RJ consumption [142].
Some components can be pointed out as responsible for the results of studies that relate RJ to neuronal capacity. 10-H2DA can stimulate the differentiation of neuronal stem cells [143], probably due to its similarity with the omega 3, docosahexaenoic acid, which acts in the neurogenesis of glial cells [144, 145]; further studies are needed to confirm this hypothesis.
It is also important to note that vitamins and minerals account for up to 3% of the composition of RJ [146]. Amongst them, those of the B complex, particularly pantothenic acid (B5), are highlighted and related to neuronal function [146, 147]. The protective effects of this group of vitamins are due to their antioxidant capacity that neutralises free radicals. They also act as cofactors in the synthesis reactions of some neurotransmitters and ATP when they form the acetyl-coenzyme A (acetyl-CoA) molecule, which is significant in cellular respiration [148–150]. Complex B also reduces homocysteine, which, when elevated, is related to adverse neuronal outcomes, improving cognitive function [147].
Acetylcholine (ACH) is a neurotransmitter derived from the reaction of choline with acetyl-CoA in nerve endings in the central and peripheral nervous system [151, 152]. This neurotransmitter is also found in the composition of RJ (1 mg/g). It has been linked to preventing neurodegenerative disorders and diseases and improving memory and cognitive functioning [146, 153]. Cholinergic neurons are susceptible to coenzyme A, and its increase by external sources such as RJ helps maintain their optimal levels [151, 152].
Deficiency of B vitamins and neurotransmitters such as acetylcholine has been observed in some diseases of a neurodegenerative nature, such as Alzheimer’s disease (AD), dementia with Lewy bodies and Parkinson’s [148, 154, 155]. One of the possible treatments for AD involves increasing the availability of acetylcholine in the brain [156].
RJ contains nucleotides, DNA and RNA-forming subunits, such as adenosine and guanidine, and is a phosphate source that gives rise to ADP and ATP molecules, which are fundamental in energy metabolism [147, 157]. Adenosine N1-oxide, the active component present in royal jelly, can be 20 × more active than adenosine monophosphate (AMP) and demonstrates, in addition to neurogenic effects, an affinity for the CNS, stimulating neurite growth and inducing differentiation of PC12 cells in neurons with a role in the sympathetic nervous system (SNS). The activity of adenosine N1-oxide is achieved through A2A adenosine receptors present in the brain, regulating apoptosis [146, 147]. There was also an improvement in neuronal function and regeneration of essential cells in the process of cognition and memory, present in the hippocampus, an action similar to that of the nerve growth factor (NFG), a signalling protein that stimulates the development of neurons [46, 146, 147, 158]. RJ may even reduce apoptosis in the hippocampus of mice by reducing caspase-3 activation, inhibiting JNK phosphorylation and negatively regulating the bax/bcl2 ratio and pro-apoptotic pathway, and further increasing the cAMP/PKA/CREB/BDNF pathway, generating better memory [159].
The ingestion of RJ by the queen bees causes an endocrine stimulus to the development of their ovaries, which allows that unlike the sterile workers, the queen bees are fertile and perpetuate the species [146, 160]. RJ, based on its lipid compounds, mimics the activity of oestrogen, improving the blood–brain barrier and binding to nuclear and membrane receptors compatible with it in the brain, such as ERα and ERβ, which are mainly expressed in the hypothalamus, amygdala, hippocampus and cortex [149, 161, 162].
More results from studies performed in rats and humans regarding the neuroprotective effect of RJ can be found in Table 6.
Table 6.
Studies involving the neuroprotective properties of royal jelly
| References | Sample | Intervention | Results |
|---|---|---|---|
| In vitro studies | |||
| [202] | Human neuroblastoma SH-SY5Y cells (ATCC® CRL-2266™) | H2O2- and glutamate-damaged cells: treated or pretreated with RJH (200 µg protein/mL) | ↓ cell death from the H2O2- and glutamate-induced cell damage |
| [203] | Mouse neuroblastoma N2a cells and N2a/APP695 | Cells were treated with RJP fractions |
RJPs (3 and 9 μg/mL): ↓ the Aβ1-40 and Aβ1-42 levels, ↓ BACE1 mRNA expression |
| Animal studies | |||
| [204] | Adult male Wistar rats | Non-stress group; control stress group (saline); stress group with EE; stress group with RJ; stress group with EE + RJ for 14 days |
RJ: ↓ corticosterone levels ↑ BDNF levels in the hippocampus and PFC in stressed rats RJ prevented the detrimental effects of stress on anxiety-like behaviours and memory processes |
| [202] | Adult male Wistar rats | (1) Control; (2) donepezil (1 mg/kg); groups 3–5: RJH (10, 50, to 100 mg/kg/d) for 2 weeks through the experimental period and 2 weeks after inducing MCAO |
RJH: ↓ Escape latency ↓ escape latency in 14 days after MCAO ↑ working memory ↓ AChE activity in the hippocampus after rtMCAO |
| [205] | Male Wistar rats | (1) Control; (2) STZ (icv); (3) Ringer’s solution (icv) + RJ (og); (4) STZ (icv) + RJ (og). From day 7 after the surgery until day 21: treatment with RJ or distilled water |
RJ: ↑ beneficial effects in animals injured by STZ, ↑ retention time for working spatial memory ↑ proliferation of new neurons in the hippocampus ↓ neurodegeneration and oxidative stress level |
| [16] | Adult male Swiss mice | (1) Control; (2) CdCl2 (6.5 mg/kg); (3) CdCl2 (85 mg); (4) 85 mg RJ/kg 2 h before IP-injection with 6.5 mg/kg CdCl2 for 7 1 week |
RJ: was able to mitigate CdCl2-increased Cd concentration in cortical tissue Pre-treatment with RJ: ↓ LPO, NO, iNOS TNF-α and IL-1β; levels; Ameliorated Cd-induced oxidative damage in cortical tissue; ↑ GSH-Px, GSH-R, SOD and CAT activity; ↑ Nrf2 expression; ↑ mRNA Bcl-2; ↓ mRNA expression levels of Bax and caspase-3 ↓ cortical Cd-produced damage |
| [206] | Female WHBE rabbits | Sham group; HCD group; OVX + HCD group; OVX + HCD + RJ group (400 mg RJ/kg/d) for 12 weeks |
RJ: ↑ behavioural deficits of OVX cholesterol-fed rabbits and brain structure ↓ blood lipids, Aβ, AchE and MDA levels, Evans blue in brain ↓ BACE1, and RAGE mRNA expression ↑ LRP-1 expression, activities of ChAT and SOD ↑ HRV and BRS in OVX cholesterol-fed rabbits |
| [159] | APP/PS1 transgenic mice with a C57BL/6 background and age-matched C57BL/6 mice |
4 groups with IGAS treatments: (1) Wt group: saline; (2) Tg group: APP/PS1 transgenic mice that received saline; (3) TgRJ group: APP/PSI Tg mice that received RJ (300 mg/kg/d); (4)WtRJ group: Wt mice received RJ. 3 months of intervention |
RJ: ↓ caspase-3 activation; Inhibited JNK phosphorylation and negatively regulated the bax/bcl2 ratio and pro-apoptotic pathway ↑ cAMP/PKA/CREB/BDNF pathway, generating better memory and learning results in mice that consumed RJ |
| [207] | Male Sprague–Dawley rat pups | Group I: 0.4 mL distilled water; Group II: RJ (300 mg/kg); Group III: CLO (0.4 ml/kg); Group IV: tartrazine (500 mg/kg); Group V: RJ (300 mg/kg) + tartrazine (500 mg/kg); Group VI: CLO (0.4 ml/kg) + tartrazine (500 mg/kg) |
RJ in the group V: ↑ GABA, CAT, SOD and GSH levels in brain tissue; ↓ MDA in brain tissue; ↓ cells w/ pyknotic nuclei and less ssDNA positive cells in the cerebral cortex when compared with the group IV |
| Human studies | |||
| [208] | 42 healthy postmenopausal women | Enzyme-treated RJ: 800 mg of protease-digested lyophilised powder of RJ daily and placebo for12 weeks | Improve the anxiety score and backache and low back pain score |
Abbreviatios: Aβ, amyloid-beta; Aβ1-40, beta-amyloid 40; Aβ1-42, beta-amyloid 42; AChE, acetylcholinesterase; AMP, adenosine monophosphate; APP, amyloid precursor protein; BACE1, beta-site APP cleaving enzyme 1; Bax, Bcl-2-like protein 4; BBB, blood–brain barrier; Bcl-2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; BRS, baroreflex sensitivity; BW, body weight; CAT, catalase; Cd, cadmium; CdCl2, cadmium chloride; ChAT, choline acetyltransferase; CLO, cod liver oil; CNPase, 2ʹ,3ʹ-cyclic nucleotide 3ʹ-phosphodiesterase; CREB, cAMP-response element-binding protein; CTR, control; EE, environmental enrichment; ERK1/2, extracellular signal‑regulated protein kinase; GABA, gamma amino butyric acid; GDNF, glial cell line-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; GSH, glutathione; GSH, glutathione peroxidase; GSH-R, glutathione reductase; HCD, high cholesterol diet; HDEA, 10-hydroxy-trans-2-decenoic acid; HRV, heart rate variability; icv, injected intracerebroventricularly; IGAS, intragastric; IP, intraperitoneal; LDL, low-density lipoprotein; LPO, lipid peroxidation; LRP-1, LDL receptor-related protein 1; MCAO, middle cerebral artery occlusion; MDA, malonaldehyde; N2a/APP695, N2a cells stably expressing human APP genes; NO, nitric oxide; Non-ST, non-stress; Nrf2, nuclear factor erythroid 2-related factor 2; NSCs, neural stem/progenitor cells; og, oral gavage; OVX, ovariectomy; PFC, prefrontal cortex; PERJ, PBS-extract of RJ; PG, propylene glycol; RAGE, receptor for advanced glycation end products; RJ, royal jelly; RJH, royal jelly hydrolysate; RJPs, royal jelly peptides; Rt.MCAO, occlusion of the right middle cerebral artery; SOD, superoxide dismutase; ssDNA, single-stranded DNA; SOD, superoxide dismutase; STZ, streptozotocin; ST, stress groups; Tg, transgenic; TNF-α, tumour necrosis factor-α; Tuj1, neuron-specific class III β-tubulin; IL-1β, interleukin-1β; VaD, vascular dementia; WHBE, white hair and black eyes; Wt, wild-type
Royal jelly could be an ally to re-establish the balance of the gut microbiota
The gut microbiota is a complex microbial community of microorganisms that coexist in the host organism [163]. The most abundant bacterial domain has around 1000 to 1200 intestinal bacteria species [164, 165]. Approximately 90% of the human gut microbiota comprises the phyla Firmicutes and Bacteroidetes, whilst the rest comprises Actinobacteria, Proteobacteria, Fusobacteria, Verrucomicrobia and others [166]. Genetic variations in gut microbiota composition may occur because of several factors, including sex, age, medication, socioeconomic disparities, diet and diseases [167–171]. Indeed, this compositional variation can affect host-microbe interactions influencing health and disease [134].
Gut microbiota imbalance and toxins dysbiosis-derived have been associated with several NCDs, and nutritional strategies are the first driver to modulate the gut microbiota [172].
RJ has an antibacterial property and can be a new strategy to be included in the list of treatments such as pre, pro and symbiotics. In 2003, Eshraghi and Seifollahi demonstrated that RJ inhibited the growth of more than 30 bacterial species in vitro [173]. Moreover, an animal model study observed that a moderate dose of RJ (3 g/kg/day) for 30 days decreased the abundance of the phylum Proteobacteria and increased the abundance of the genera Lachnospiraceae_NK4A136_group and Bacteroides in healthy mice [134]. Corroborating, it was reported that 2.0 g/kg of RJ in dextran sulphate sodium (DSS)–induced colitis for 31 days decreased intestinal permeability due to increased expression of tight-junction proteins. Moreover, RJ increased the expression of MUC2, a protein related to the mucin layer. The authors suggested that these results might occur because RJ decreases the relative abundance of Proteobacteria, improving the mucosal barrier. Besides, RJ upregulated the beneficial genus Muribaculum [174]. Recently, Wang et al. [175] found that major royal jelly proteins (MRJPs) affect the gut microbiota composition of mice. The MRJP enriched the diversity of the gut microbiota in mice. Moreover, they observed mice fed with a high dose of MRJP 0.5 g/kg/day for 30 days presented a significantly higher abundance of the phylum Bacteroidetes, which they suggested to be involved with the improvement of the immunity [175].
Although there is evidence regarding the beneficial effects of RJ on gut microbiota modulation, all studies are in animal models. Given the potential role of RJ in the gut microbiota composition, it could be an ally to re-establish the balance of the gut microbiome composition and improve the host’s health. More studies are needed to enhance the knowledge about the effect of RJ on gut microbiota.
Perspectives and preliminary conclusions
Predictive medicine
Predictive medicine seeks to identify risks for developing NCDs, such as cardiovascular disease, chronic kidney diseases, diabetes and cancer. Chronic inflammation and oxidative stress are biological processes that lead to the development of these diseases and play a key role in modulating these processes [176]. Healthy foods, such as fruits, vegetables, legumes, nuts and seeds, can reduce chronic inflammation and oxidative stress, and RJ is presented here as one of these foods that can be part of a healthy diet. A particular value of RJ in reducing complications in patients with chronic non-communicable diseases can be considered in the framework of predictive, preventive and personalised medicine (PPPM). Furthermore, personalised nutrition can be a powerful tool in predictive medicine.
Preventive medicine
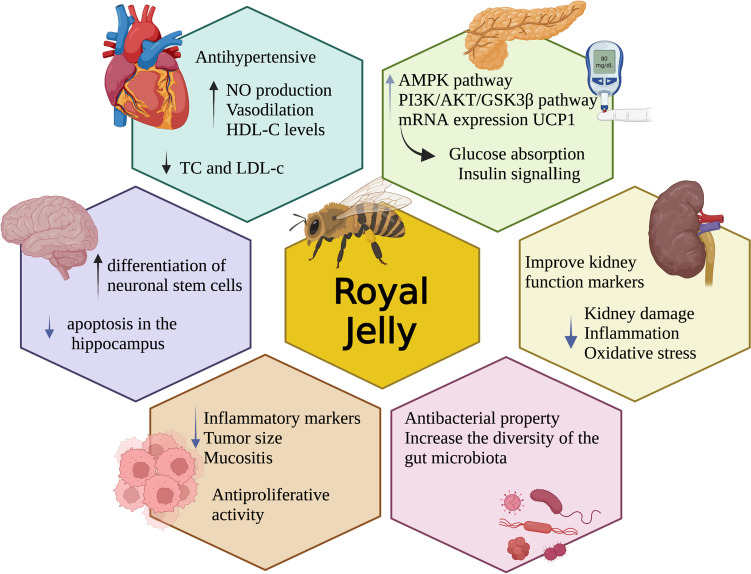
There is evidence that RJ can be an excellent option to mitigate inflammation, increasing the expression of Nrf2 and antioxidants and reducing the NF-kB and synthesis of cytokines in patients with non-communicable diseases. Figure 3 summarises the benefits of royal jelly for NCDs such as cancer, neurological disease, CVD, diabetes and CKD, including gut dysbiosis. Nutritional interventions can cover terms of individualization for the best possible care for the individual in ethical terms of medicine, which is a right of any patient in health care [177, 178]. However, despite many studies, we still need more data to determine a prescription’s exact dose. Nevertheless, if nature shows that it is suitable for a queen’s honeybees, why wouldn’t it be good for the simple human being?
Fig. 3.
Effects of royal jelly for NCDs such as cancer, neurological disease, CVD, diabetes and CKD, including gut dysbiosis. Created with BioRender.com
Future of personalised medicine
There is a growing interest in a healthy lifestyle, and a significantly improved health economy can bring benefits of the proposed PPPM paradigm shift; also, close collaboration between all stakeholders is essential for implementing the concepts in daily practice [84].
In this sense, personalised and individualised nutrition can be used to prevent or treat disease-related complications. Nutritional treatment should be approached according to the characteristics of the disease, being beneficial for the patient in terms of quality of life, prognosis and reduction of mortality. RJ is a bee product rich in bioactive compounds, specific fatty acids and proteins with therapeutic properties, playing a vital role as an antioxidant and anti-inflammatory. Thus, RJ can be used as a non-pharmacological therapy in nutritional treatment to mitigate NCD complications, such as diabetes, cancer and cardiovascular and chronic kidney diseases. In the future, it is believed that the use of RJ can be part of personalised nutrition to improve the immunological health of patients. In this context, PPPM can help identify the problem and find a solution to mitigate complications related to inflammation and oxidative stress present in NCDs through the concept of “food as medicine” [105].
Author contribution
All authors contributed to the review conception and design. The first draft of the manuscript was written by Beatriz Germer Baptista, Márcia Ribeiro, Livia Alvarenga Ligia Lima, Isadora Britto and Julie Kemp; and Ludmila Cardozo, Andresa A. Berretta and Denise Mafra corrected the versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Conselho Nacional de Pesquisa (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Heads of state commit to noncommunicable disease global compact to save 50 million lives by 2030. 2022; Available from: https://www.who.int/news/item/21-09-2022-heads-of-state-commit-to-noncommunicable-disease-global-compact-to-save-50-million-lives-by-2030. Accessed 27 Jan 2023
- 2.Wang Y, Wang J. Modelling and prediction of global non-communicable diseases. BMC Public Health. 2020;20:1–13. doi: 10.1186/s12889-020-08890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med Nature Res. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soomro S. Oxidative stress and inflammation. Open J Immunol Scientific Research Publishing Inc. 2019;09:1–20. doi: 10.4236/oji.2019.91001. [DOI] [Google Scholar]
- 5.Mafra D, Borges NA, Lindholm B, Shiels PG, Evenepoel P, Stenvinkel P. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat Rev Nephrol. 2021;17:153–71. [DOI] [PubMed]
- 6.Câmara JS, Albuquerque BR, Aguiar J, Corrêa RCG, Gonçalves JL, Granato D, et al. Food bioactive compounds and emerging techniques for their extraction: polyphenols as a case study. Foods. 2021;10:1–34. doi: 10.3390/foods10010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugiyama T, Takahashi K, Mori H. Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the innate immune responses Endocr Metab Immune Disord Drug Targets. United Arab Emirates. 2012;12:368–76. doi: 10.2174/187153012803832530. [DOI] [PubMed] [Google Scholar]
- 8.Bogdanov S. The royal jelly book. Royal jelly and bee brood: harvest, composition, quality. Bee Prod Sci. 2017;1:1–13. [Google Scholar]
- 9.Kocot J, Kiełczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: possible medical application. Oxid Med Cell Longev. 2018;2018:7074209. [DOI] [PMC free article] [PubMed]
- 10.Kanelis D, Tananaki C, Liolios V, Dimou M, Goras G, Rodopoulou MA, et al. A suggestion for royal jelly specifications. Arh Hig Rada Toksikol. 2015;66:275–284. doi: 10.1515/aiht-2015-66-2651. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Serie MM, Habashy NH. Two purified proteins from royal jelly with in vitro dual anti-hepatic damage potency: major royal jelly protein 2 and its novel isoform X1. Int J Biol. 2019;128:782–95. doi: 10.1016/j.ijbiomac.2019.01.210. [DOI] [PubMed] [Google Scholar]
- 12.Ali FEM, Saad Eldien HM, Mostafa NAM, Almaeen AH, Marzouk MRA, Eid KM, et al. The impact of royal jelly against hepatic ischemia/reperfusion-induced hepatocyte damage in rats: the role of cytoglobin, Nrf-2/HO-1/COX-4, and P38-MAPK/NF-κB-p65/TNF-α Signaling Pathways. Curr Mol Pharmacol. 2021;14:88–100. doi: 10.2174/1874467213666200514223829. [DOI] [PubMed] [Google Scholar]
- 13.Aslan A, Beyaz S, Gok O, Can MI, Parlak G, Ozercan IH, et al. Royal jelly abrogates flouride-induced oxidative damage in rat heart tissue by activating of the nrf-2/NF-κB and bcl-2/bax pathway. Toxicol Mech Methods. 2021;31:644–54. doi: 10.1080/15376516.2021.1950249. [DOI] [PubMed] [Google Scholar]
- 14.Miyata Y, Araki K, Ohba K, Mastuo T, Nakamura Y, Yuno T, et al. Oral intake of royal jelly improves anti-cancer effects and suppresses adverse events of molecular targeted therapy by regulating TNF-α and TGF-β in renal cell carcinoma: a preliminary study based on a randomized double-blind clinical trial. Mol Clin Oncol. 2020;13:1–8. doi: 10.3892/mco.2020.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aslan A, Beyaz S, Gok O, Can MI, Parlak G, Ozercan IH, et al. Royal jelly abrogates flouride-induced oxidative damage in rat heart tissue by activating of the nrf-2/NF-κB and bcl-2/bax pathway. Toxicol Mech Methods. 2021;31:644–654. doi: 10.1080/15376516.2021.1950249. [DOI] [PubMed] [Google Scholar]
- 16.Almeer RS, Soliman D, Kassab RB, AlBasher GI, Alarifi S, Alkahtani S, et al. Royal jelly abrogates cadmium-induced oxidative challenge in mouse testes: involvement of the Nrf2 pathway. Int J Mol Sci. 2018;19:3979. doi: 10.3390/ijms19123979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkhetkan W, Thitiorul S, Jansom C, Ratanavalachai T. Molecular and cytogenetic effects of Thai royal jelly: modulation through c-MYC, h-TERT, NRF2, HO-1, BCL2, BAX and cyclins in human lymphocytes in vitro. Mutagenesis. 2017;32:525–531. doi: 10.1093/mutage/gex020. [DOI] [PubMed] [Google Scholar]
- 18.Golubnitschaja O, Watson ID, Topic E, Sandberg S, Ferrari M, Costigliola V. Position paper of the EPMA and EFLM: a global vision of the consolidated promotion of an integrative medical approach to advance health care. EPMA J. 2013;4:12. doi: 10.1186/1878-5085-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denisow B, Denisow-Pietrzyk M. Biological and therapeutic properties of bee pollen: a review. J Sci Food Agric. 2016;96:4303–4309. doi: 10.1002/jsfa.7729. [DOI] [PubMed] [Google Scholar]
- 20.Fratini F, Cilia G, Mancini S, Felicioli A. Royal Jelly: an ancient remedy with remarkable antibacterial properties. Microbiol Res. 2016;192:130–41. doi: 10.1016/j.micres.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Cao LF, Zheng HQ, Pirk CWW, Hu FL, Xu ZW. High royal jelly-producing honeybees (Apis mellifera ligustica) (Hymenoptera: Apidae) in China. J Econ Entomol. 2016;109:510–514. doi: 10.1093/jee/tow013. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Huang C, Xue Y. Contribution of lipids in honeybee (Apis mellifera) royal jelly to health. J Med Food. 2013;16:96–102. doi: 10.1089/jmf.2012.2425. [DOI] [PubMed] [Google Scholar]
- 23.Khalifa SAM, Elashal MH, Yosri N, Du M, Musharraf SG, Nahar L, et al. Bee pollen: Current status and therapeutic potential. Nutrients. 2021;13:1–15. doi: 10.3390/nu13061876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y-F, Wang K, Zhang Y-Z, Zheng Y-F, Hu F-L. In vitro anti-inflammatory effects of three fatty acids from royal jelly. Mediat Inflamm. 2016;2016:3583684. doi: 10.1155/2016/3583684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaga M, Tani H, Yamaki A, Tatefuji T, Hashimoto K. Metabolism and pharmacokinetics of medium chain fatty acids after oral administration of royal jelly to healthy subjects. RSC Adv Royal Soc Chem. 2019;9:15392–15401. doi: 10.1039/C9RA02991E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramadan MF, Al-Ghamdi A. Bioactive compounds and health-promoting properties of royal jelly: a review. J Funct Foods. 2012;4:39–52. doi: 10.1016/j.jff.2011.12.007. [DOI] [Google Scholar]
- 27.Melliou E, Chinou I. Chemistry and bioactivity of royal jelly from Greece. J Agric Food Chem. 2005;53:8987–8992. doi: 10.1021/jf051550p. [DOI] [PubMed] [Google Scholar]
- 28.Isdorov VA, Bakier S, Grzech I. Gas chromatographic–mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J Chromatogr B. 2012;885-886:109–116. doi: 10.1016/j.jchromb.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Uthaibutra V, Kaewkod T, Prapawilai P, Pandith H. Yingmanee Tragoolpua Inhibition of skin pathogenic bacteria, antioxidant and anti-inflammatory activity of royal jelly from northern Thailand. Molecules. 2023;28(3):28. doi: 10.3390/molecules28030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J-R, Yang Y-C, Shi L-S, Peng C-C. Antioxidant properties of royal jelly associated with larval age and time of harvest. J Agric Food Chem. 2008;56:11447–11452. doi: 10.1021/jf802494e. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Amoedo LH, De Almeida-Muradian LB. Physicochemical composition of pure and adulterated royal jelly. Quim Nova. 2007;30:257–259. doi: 10.1590/S0100-40422007000200002. [DOI] [Google Scholar]
- 32.López-Gutiérrez N, Aguilera-Luiz MDM, Romero-González R, Vidal JLM, Garrido Frenich A. Fast analysis of polyphenols in royal jelly products using automated TurboFlowTM-liquid chromatography-Orbitrap high resolution mass spectrometry. J Chromatogr B. 2014;973:17–28. doi: 10.1016/j.jchromb.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 33.Kanbur M, Eraslan G, Silici S, Karabacak M. Effects of sodium fluoride exposure on some biochemical parameters in mice: evaluation of the ameliorative effect of royal jelly applications on these parameters. Food Chem Toxicol an Int J Publ Br Ind Biol Res Assoc. 2009;47:1184–9. doi: 10.1016/j.fct.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Negri G, Teixeira EW, Alves MLTMF, De Camargo CarmelloMoreti AC, Otsuk IP, Borguini RG, et al. Hydroxycinnamic acid amide derivatives, phenolic compounds and antioxidant activities of extracts of pollen samples from Southeast Brazil. J Agric Food Chem. 2011;59:5516–22. doi: 10.1021/jf200602k. [DOI] [PubMed] [Google Scholar]
- 35.Durazzo A, Lucarini M, Plutino M, Lucini L, Aromolo R, Martinelli E, et al. Bee products: a representation of biodiversity, sustainability, and health. Life. 2021;11:1–32. doi: 10.3390/life11090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arct J, Pytkowska K. Flavonoids as components of biologically active cosmeceuticals. Clin Dermatol. 2008;26:347–357. doi: 10.1016/j.clindermatol.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Manzo LP, De-Faria FM, Dunder RJ, Rabelo-Socca EA, Consonni SR, De Almeida ACA, et al. Royal jelly and its dual role in TNBS colitis in mice. Sci World J. 2015;2015:1–7. doi: 10.1155/2015/956235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolayli S, Sahin H, Can Z, Yildiz O, Malkoc M, Asadov A. A member of complementary medicinal food: Anatolian royal jellies, their chemical compositions, and antioxidant properties. J Evidence-Based Complement Altern Med. 2016;21:43–48. doi: 10.1177/2156587215618832. [DOI] [PubMed] [Google Scholar]
- 39.Malekinejad H, Ahsan S, Delkhosh-Kasmaie F, Cheraghi H, Rezaei-Golmisheh A, Janbaz-Acyabar H. A cardioprotective effect of royal jelly on paclitaxel-induced cardio-toxicity in rats. Iran J Basic Med Sci. 2016;19:221–227. [PMC free article] [PubMed] [Google Scholar]
- 40.Klaudiny J, Bachanová K, Kohútová L, Dzúrová M, Kopernický J, Majtán J. Expression of larval jelly antimicrobial peptide defensin1 in Apis mellifera colonies. Biologia. 2012;67:200–211. doi: 10.2478/s11756-011-0153-8. [DOI] [Google Scholar]
- 41.Khoshpey B, Djazayeri S, Amiri F, Malek M, Hosseini AF, Hosseini S, et al. Effect of royal jelly intake on serum glucose, apolipoprotein A-I (ApoA-I), apolipoprotein B (ApoB) and ApoB/ApoA-I ratios in patients with type 2 diabetes: a randomized, double-blind clinical trial study. Can J Diabetes. 2016;40:324–8. doi: 10.1016/j.jcjd.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 42.ShakibKhoob M, Hosseini SM, Kazemi S. In vitro and in vivo antioxidant and anticancer potentials of royal jelly for dimethylhydrazine-induced colorectal cancer in Wistar rats. Oxid Med Cell Longev. 2022;2022:9506026. doi: 10.1155/2022/9506026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mostafa RE, Shaffie NM, Allam RM. Protective effects of royal jelly and Echinacea against moxifloxacin-induced renal and hepatic injury in rats. Drug Chem Toxicol. 2022;1–10. [DOI] [PubMed]
- 44.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73:117–124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 45.Brazil. Regulamentos Técnicos de Identidade e Qualidade de apitoxina, cera de abelha, geléia real, geléia real liofilizada, pólen apícola, própolis e extrato de própolis. BRAZIL. 2001;97:11.
- 46.Kocot J, Kiełczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: possible medical application. Oxid Med Cell Longev. 2018;2018:7074209. doi: 10.1155/2018/7074209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Paula R, Rabalski I, Messia MC, Abdel-Aal E-SM, Marconi E. Effect of processing on phenolic acids composition and radical scavenging capacity of barley pasta. Food Res Int. 2017;102:136–43. doi: 10.1016/j.foodres.2017.09.088. [DOI] [PubMed] [Google Scholar]
- 48.Guo H, Kouzuma Y, Yonekura M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food. 2009;113:238–45. doi: 10.1016/j.foodchem.2008.06.081. [DOI] [Google Scholar]
- 49.Mihajlovic D, Rajkovic I, Chinou I, Colic M. Dose-dependent immunomodulatory effects of 10-hydroxy-2-decenoic acid on human monocyte-derived dendritic cells. J Funct Foods. 2013;5:838–846. doi: 10.1016/j.jff.2013.01.031. [DOI] [Google Scholar]
- 50.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Yang CH, Murti A, Pfeffer SR, Kim JG, Donner DB, Pfeffer LM. Interferon alpha /beta promotes cell survival by activating nuclear factor kappa B through phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2001;276:13756–13761. doi: 10.1074/jbc.M011006200. [DOI] [PubMed] [Google Scholar]
- 52.Sugiyama T, Takahashi K, Kuzumaki A, Tokoro S, Neri P, Mori H. Inhibitory mechanism of 10-hydroxy-trans-2-decenoic acid (royal jelly acid) against lipopolysaccharide- and interferon-β-induced nitric oxide production. Inflammation. 2013;36:372–378. doi: 10.1007/s10753-012-9556-0. [DOI] [PubMed] [Google Scholar]
- 53.Inoue Y, Hara H, Mitsugi Y, Yamaguchi E, Kamiya T, Itoh A, et al. 4-Hydroperoxy-2-decenoic acid ethyl ester protects against 6-hydroxydopamine-induced cell death via activation of Nrf2-ARE and eIF2α-ATF4 pathways. Neurochem Int. 2018;112:288–296. doi: 10.1016/j.neuint.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 54.Koklesova L, Mazurakova A, Samec M, Kudela E, Biringer K, Kubatka P, et al. Mitochondrial health quality control: measurements and interpretation in the framework of predictive, preventive, and personalized medicine. EPMA J. 2022;13:177–193. doi: 10.1007/s13167-022-00281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Videla LA, Marimán A, Ramos B, José Silva M, Del Campo A. Standpoints in mitochondrial dysfunction: underlying mechanisms in search of therapeutic strategies. Mitochondrion. 2022;63:9–22. doi: 10.1016/j.mito.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Rai SN, Singh C, Singh A, Singh MP, Singh BK. Mitochondrial dysfunction: a potential therapeutic target to treat Alzheimer’s disease. Mol Neurobiol. 2020;57:3075–3088. doi: 10.1007/s12035-020-01945-y. [DOI] [PubMed] [Google Scholar]
- 57.Eldeeb MA, Thomas RA, Ragheb MA, Fallahi A, Fon EA. Mitochondrial quality control in health and in Parkinson’s disease. Physiol Rev. 2022;102:1721–1755. doi: 10.1152/physrev.00041.2021. [DOI] [PubMed] [Google Scholar]
- 58.Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol. 2017;11:637–645. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle. 2017;8:349–369. doi: 10.1002/jcsm.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi Y, Hijikata K, Seike K, Nakano S, Banjo M, Sato Y, et al. Effects of royal jelly administration on endurance training-induced mitochondrial adaptations in skeletal muscle. Nutrients. 2018;10:1735. doi: 10.3390/nu10111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aslan A, Beyaz S, Gok O, Can MI, Parlak G, Gundogdu R, et al. Protective effect of royal jelly on fluoride-induced nephrotoxicity in rats via the some protein biomarkers signalling pathways: a new approach for kidney damage. Biomarkers. 2022;27:637–47. doi: 10.1080/1354750X.2022.2093977. [DOI] [PubMed] [Google Scholar]
- 62.Hashem KS, Elkelawy AMMH, Abd-Allah S, Helmy NA. Involvement of Mfn2, Bcl2/Bax signaling and mitochondrial viability in the potential protective effect of royal jelly against mitochondria-mediated ovarian apoptosis by cisplatin in rats. Iran J Basic Med Sci. 2020;23:515–526. doi: 10.22038/ijbms.2020.40401.9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 64.Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin North Am. 2021;50:337–55. [DOI] [PubMed]
- 65.Ali O. Principles of diabetes mellitus: third edition. Princ Diabetes Mellit. 2017:1–1066.
- 66.DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Prim. 2015;1:15019. doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 67.Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol an Off J Polish Physiol Soc. 2019;70:809–818. doi: 10.26402/jpp.2019.6.01. [DOI] [PubMed] [Google Scholar]
- 68.Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan SY, Mei Wong JL, Sim YJ, Wong SS, Mohamed Elhassan SA, Tan SH, et al. Type 1 and 2 diabetes mellitus: a review on current treatment approach and gene therapy as potential intervention. Diabetes Metab Syndr. 2019;13:364–372. doi: 10.1016/j.dsx.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Cao H, Ou J, Chen L, Zhang Y, Szkudelski T, Delmas D, et al. Dietary polyphenols and type 2 diabetes: human study and clinical trial. Crit Rev Food Sci Nutr. 2019;59:3371–9. doi: 10.1080/10408398.2018.1492900. [DOI] [PubMed] [Google Scholar]
- 71.Pivari F, Mingione A, Brasacchio C, Soldati L. Curcumin and type 2 diabetes mellitus: prevention and treatment. Nutrients. 2019;11:1837. doi: 10.3390/nu11081837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma S, Mandal A, Kant R, Jachak S, Jagzape M. Is cinnamon efficacious for glycaemic control in type-2 diabetes mellitus? J Pak Med Assoc. 2020;70:2065–2069. [PubMed] [Google Scholar]
- 73.Pourmoradian S, Mahdavi R, Mobasseri M, Faramarzi E, Mobasseri M. Effects of royal jelly supplementation on glycemic control and oxidative stress factors in type 2 diabetic female: a randomized clinical trial. Chin J Integr Med. 2014;20:347–352. doi: 10.1007/s11655-014-1804-8. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida M, Hayashi K, Watadani R, Okano Y, Tanimura K, Kotoh J, et al. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J Vet Med Sci. 2017;79:299–307. doi: 10.1292/jvms.16-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maleki V, Jafari-Vayghan H, Saleh-Ghadimi S, Adibian M, Kheirouri S, Alizadeh M. Effects of royal jelly on metabolic variables in diabetes mellitus: a systematic review. Complement Ther Med. 2019;43:20–27. doi: 10.1016/j.ctim.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 76.Hu X, Liu Z, Lu Y, Chi X, Han K, Wang H, et al. Glucose metabolism enhancement by 10-hydroxy-2-decenoic acid via the PI3K/AKT signaling pathway in high-fat-diet/streptozotocin induced type 2 diabetic mice. Food Funct England. 2022;13:9931–9946. doi: 10.1039/D1FO03818D. [DOI] [PubMed] [Google Scholar]
- 77.Nohair SF. Al Antidiabetic efficacy of a honey-royal jelly mixture: biochemical study in rats. Int J Health Sci (Qassim) 2021;15:4–9. [PMC free article] [PubMed] [Google Scholar]
- 78.Yoneshiro T, Kaede R, Nagaya K, Aoyama J, Saito M, Okamatsu-Ogura Y, et al. Royal jelly ameliorates diet-induced obesity and glucose intolerance by promoting brown adipose tissue thermogenesis in mice. Obes Res Clin Pract. 2018;12:127–137. doi: 10.1016/j.orcp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 79.GBD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study. Lancet. 2017;2018(392):1736–88. [DOI] [PMC free article] [PubMed]
- 80.GBD. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study. Lancet. 2017;2018(392):1859–922. [DOI] [PMC free article] [PubMed]
- 81.Jain AK, Mehra NK, Swarnakar NK. Role of antioxidants for the treatment of cardiovascular diseases: challenges and opportunities. Curr Pharm Des. 2015;21:4441–4455. doi: 10.2174/1381612821666150803151758. [DOI] [PubMed] [Google Scholar]
- 82.Frostegård J. Immunity atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lind L, Ingelsson M, Sundstrom J, Ärnlöv J. Impact of risk factors for major cardiovascular diseases: a comparison of life-time observational and Mendelian randomisation findings. Open Hear. 2021;8:e001735. [DOI] [PMC free article] [PubMed]
- 84.Golubnitschaja O, Liskova A, Koklesova L, Samec M, Biringer K, Büsselberg D, et al. Caution, “normal” BMI: health risks associated with potentially masked individual underweight-EPMA Position Paper 2021. EPMA J. 2021;12:243–264. doi: 10.1007/s13167-021-00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Badimon L, Chagas P, Chiva-Blanch G. Diet and cardiovascular disease: effects of foods and nutrients in classical and emerging cardiovascular risk factors. Curr Med Chem. 2019;26:3639–3651. doi: 10.2174/0929867324666170428103206. [DOI] [PubMed] [Google Scholar]
- 86.Olas B. Bee products as interesting natural agents for the prevention and treatment of common cardiovascular diseases. Nutrients. 2022;14:2267. [DOI] [PMC free article] [PubMed]
- 87.Matsui T, Yukiyoshi A, Doi S, Sugimoto H, Yamada H, Matsumoto K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J Nutr Biochem. 2002;13:80–86. doi: 10.1016/S0955-2863(01)00198-X. [DOI] [PubMed] [Google Scholar]
- 88.Tokunaga K, Yoshida C, Suzuki K, Maruyama H, Futamura Y, Araki Y, et al. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol Pharm Bull. 2004;27:189–192. doi: 10.1248/bpb.27.189. [DOI] [PubMed] [Google Scholar]
- 89.Takaki-Doi S, Hashimoto K, Yamamura M, Kamei C. Antihypertensive activities of royal jelly protein hydrolysate and its fractions in spontaneously hypertensive rats. Acta Med Okayama. 2009;63:57–64. doi: 10.18926/AMO/31859. [DOI] [PubMed] [Google Scholar]
- 90.Pan Y, Rong Y, You M, Ma Q, Chen M, Hu F. Royal jelly causes hypotension and vasodilation induced by increasing nitric oxide production. Food Sci Nutr. 2019;7:1361–1370. doi: 10.1002/fsn3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang Y, Kagota S, Maruyama K, Oonishi Y, Miyauchi-Wakuda S, Ito Y, et al. Royal jelly increases peripheral circulation by inducing vasorelaxation through nitric oxide production under healthy conditions. Biomed Pharmacother. 2018;106:1210–1219. doi: 10.1016/j.biopha.2018.07.047. [DOI] [PubMed] [Google Scholar]
- 92.Patel SA, Winkel M, Ali MK, Narayan KMV, Mehta NK. Cardiovascular mortality associated with 5 leading risk factors: national and state preventable fractions estimated from survey data. Ann Intern Med. 2015;163:245–253. doi: 10.7326/M14-1753. [DOI] [PubMed] [Google Scholar]
- 93.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 94.Guo H, Saiga A, Sato M, Miyazawa I, Shibata M, Takahata Y, et al. Royal jelly supplementation improves lipoprotein metabolism in humans. J Nutr Sci Vitaminol. 2007;53:345–348. doi: 10.3177/jnsv.53.345. [DOI] [PubMed] [Google Scholar]
- 95.Kamakura M, Moriyama T, Sakaki T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J Pharm Pharmacol. 2006;58:1683–1689. doi: 10.1211/jpp.58.12.0017. [DOI] [PubMed] [Google Scholar]
- 96.Hadi A, Najafgholizadeh A, Aydenlu ES, Shafiei Z, Pirivand F, Golpour S, et al. Royal jelly is an effective and relatively safe alternative approach to blood lipid modulation: a meta-analysis. J Funct Foods. 2018;41:202–209. doi: 10.1016/j.jff.2017.12.005. [DOI] [Google Scholar]
- 97.Kashima Y, Kanematsu S, Asai S, Kusada M, Watanabe S, Kawashima T, et al. Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS One. 2014;9:e105073. doi: 10.1371/journal.pone.0105073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Torres Crigna A, Link B, Samec M, Giordano FA, Kubatka P, Golubnitschaja O. Endothelin-1 axes in the framework of predictive, preventive and personalised (3P) medicine. EPMA J. 2021;12:265–305. doi: 10.1007/s13167-021-00248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abd El-Hakam FE-Z, Abo Laban G, Badr El-Din S, Abd El-Hamid H, Farouk MH. Apitherapy combination improvement of blood pressure cardiovascular protection, and antioxidant and anti-inflammatory responses in dexamethasone model hypertensive rats. Sci Rep. 2022;12:20765. doi: 10.1038/s41598-022-24727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bikbov B, Purcell C, Levey A, Smith M, Abdoli A, Abebe M. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–33. [DOI] [PMC free article] [PubMed]
- 101.Ebert T, Pawelzik SC, Witasp A, Arefin S, Hobson S, Kublickiene K, et al. Inflammation and premature ageing in chronic kidney disease. Toxins. 2020;12:227. doi: 10.3390/toxins12040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rysz J, Franczyk B, Ławiński J, Olszewski R, Ciałkowska-Rysz A, Gluba-Brzózka A. The impact of CKD on uremic toxins and gut microbiota. Toxins. 2021;13:252. [DOI] [PMC free article] [PubMed]
- 103.Stenvinkel P, Chertow GM, Devarajan P, Levin A, Andreoli SP, Bangalore S, et al. Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int Reports. 2021;6:1775–1787. doi: 10.1016/j.ekir.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pedruzzi LM, Cardozo LFMF, Daleprane JB, Stockler-Pinto MB, Monteiro EB, Leite MJ, et al. Systemic inflammation and oxidative stress in hemodialysis patients are associated with down-regulation of Nrf2. J Nephrol. 2015;28:495–501. doi: 10.1007/s40620-014-0162-0. [DOI] [PubMed] [Google Scholar]
- 105.Mafra D, Borges NA, Lindholm B, Shiels PG, Evenepoel P, Stenvinkel P. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat Rev. 2021;17:153–71. doi: 10.1038/s41581-020-00345-8. [DOI] [PubMed] [Google Scholar]
- 106.Kruse NT. Nutraceuticals as a potential adjunct therapy toward improving vascular health in CKD. Am J Physiol Regul Integr Comp Physiol. 2019;317:R719–32. doi: 10.1152/ajpregu.00152.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gobe GC, Wojcikowski K. Nontraditional (non-Western pharmaceutical) treatments for chronic kidney disease. Clin Nephrol. 2020;93:49–54. doi: 10.5414/CNP92S108. [DOI] [PubMed] [Google Scholar]
- 108.Mafra D, Ugochukwu SA, Borges NA, Cardozo LFMF, Stenvinkel P, Shiels PG. Food for healthier aging: power on your plate. Crit Rev Food Sci Nutr. 2022;1–14. [DOI] [PubMed]
- 109.Ohba K, Miyata Y, Shinzato T, Funakoshi S, Maeda K, Matsuo T, et al. Effect of oral intake of royal jelly on endothelium function in hemodialysis patients: study protocol for multicenter, double-blind, randomized control trial. Trials. 2021;22:950. doi: 10.1186/s13063-021-05926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mostafa RE, El-Marasy SA, Abdel Jaleel GA, Bakeer RM. Protective effect of royal jelly against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats. Heliyon. 2020;6:e03330. doi: 10.1016/j.heliyon.2020.e03330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Almeer RS, Kassab RB, AlBasher GI, Alarifi S, Alkahtani S, Ali D, et al. Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol Biol Rep Netherlands. 2019;46:119–131. doi: 10.1007/s11033-018-4451-x. [DOI] [PubMed] [Google Scholar]
- 112.Aslan Z, Aksoy L. Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int Braz J Urol. 2015;41:1008–1013. doi: 10.1590/S1677-5538.IBJU.2014.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, et al. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6:9. doi: 10.1186/s13167-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bui TM, Wiesolek HL, Sumagin R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108:787–799. doi: 10.1002/JLB.2MR0220-549R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18:261–279. doi: 10.1038/s41571-020-00459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nabas Z, Haddadin MSY, Haddadin J, Nazer IK. Chemical composition of royal jelly and effects of synbiotic with two different locally isolated probiotic strains on antioxidant activities. Polish J Food Nutr Sci. 2014;64:171–180. doi: 10.2478/pjfns-2013-0015. [DOI] [Google Scholar]
- 117.Ponte LGS, Pavan ICB, Mancini MCS, Da Silva LGS, Morelli AP, Severino MB, et al. The hallmarks of flavonoids in cancer. Molecules. 2021;26:1–55. doi: 10.3390/molecules26072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huntington ND, Cursons J, Rautela J. The cancer-natural killer cell immunity cycle. Nat Rev Cancer. 2020;20:437–454. doi: 10.1038/s41568-020-0272-z. [DOI] [PubMed] [Google Scholar]
- 119.Ikeda H, Togashi Y. Aging, cancer, and antitumor immunity. Int J Clin Oncol. 2022;27:316–322. doi: 10.1007/s10147-021-01913-z. [DOI] [PubMed] [Google Scholar]
- 120.Wu S-Y, Fu T, Jiang Y-Z, Shao Z-M. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19:120. doi: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El-Seedi HR, Eid N, Abd El-Wahed AA, Rateb ME, Afifi HS, Algethami AF, et al. Honey bee products: preclinical and clinical studies of their anti-inflammatory and immunomodulatory properties. Front Nutr. 2022;8:761267. doi: 10.3389/fnut.2021.761267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miyata Y, Sakai H. Anti-cancer and protective effects of royal jelly for therapy-induced toxicities in malignancies. Int J Mol Sci. 2018;19:3270. [DOI] [PMC free article] [PubMed]
- 123.Yang Y-C, Chou W-M, Widowati DA, Lin I-P, Peng C-C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement Altern Med. 2018;18:202. doi: 10.1186/s12906-018-2267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang W, Borcherding N, Kolb R. IL-1 signaling in tumor microenvironment. Adv Exp Med Biol. 2020;1240:1–23. doi: 10.1007/978-3-030-38315-2_1. [DOI] [PubMed] [Google Scholar]
- 125.Ibrahimi R, Ibrahimi M, Jamalzei B, Akbari ME, Navari M, Moossavi M, et al. Association between interleukin-1 receptor antagonist (IL-1ra) VNTR, gene polymorphism and breast cancer susceptibility in Iranian population: experimental and web-based analysis. Int J Immunogenet. 2022;49:254–259. doi: 10.1111/iji.12584. [DOI] [PubMed] [Google Scholar]
- 126.Rothenberger NJ, Somasundaram A, Stabile LP. The role of the estrogen pathway in the tumor microenvironment. Int J Mol Sci. 2018;19:611. doi: 10.3390/ijms19020611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao Y, Wang X, Liu Y, Wang H-Y, Xiang J. The effects of estrogen on targeted cancer therapy drugs. Pharmacol Res. 2022;177:106131. doi: 10.1016/j.phrs.2022.106131. [DOI] [PubMed] [Google Scholar]
- 128.Liu G, Cai W, Liu H, Jiang H, Bi Y, Wang H. The association of bisphenol A and phthalates with risk of breast cancer: a meta-analysis. Int J Environ Res Public Health. 2021;18:2375. [DOI] [PMC free article] [PubMed]
- 129.Engin AB, Engin A. The effect of environmental bisphenol A exposure on breast cancer associated with obesity. Environ Toxicol Pharmacol. 2021;81:103544. doi: 10.1016/j.etap.2020.103544. [DOI] [PubMed] [Google Scholar]
- 130.Ahmad S, Campos MG, Fratini F, Altaye SZ, Li J. New insights into the biological and pharmaceutical properties of royal jelly. Int J Mol Sci. 2020;21:382. [DOI] [PMC free article] [PubMed]
- 131.Wu S, Wei X, Jiang J, Shang L, Hao W. Effects of bisphenol A on the proliferation and cell cycle of HBL-100 cells. Food Chem Toxicol an Int J Publ Br Ind Biol Res Assoc. 2012;50:3100–5. doi: 10.1016/j.fct.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 132.Wang Q, Morris RJ, Bode AM, Zhang T. Prostaglandin pathways: opportunities for cancer prevention and therapy. Cancer Res. 2022;82:949–965. doi: 10.1158/0008-5472.CAN-21-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bincoletto C, Eberlin S, Figueiredo CAV, Luengo MB, Queiroz MLS. Effects produced by royal Jelly on haematopoiesis: relation with host resistance against Ehrlich ascites tumour challenge. Int Immunopharmacol. 2005;5:679–688. doi: 10.1016/j.intimp.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 134.Chi X, Liu Z, Wang H, Wang Y, Wei W, Xu B. Royal jelly enhanced the antioxidant activities and modulated the gut microbiota in healthy mice. J Food Biochem. 2021;45:e13701. doi: 10.1111/jfbc.13701. [DOI] [PubMed] [Google Scholar]
- 135.Yuno T, Miyata Y, Mukae Y, Otsubo A, Mitsunari K, Matsuo T, et al. Mechanisms underlying the inhibition of tyrosine kinase inhibitor-induced anorexia and fatigue by royal jelly in renal cell carcinoma patients and the correlation between macrophage colony stimulating factor and inflammatory mediators. Med Sci. 2020;8:43. doi: 10.3390/medsci8040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Münstedt K, Männle H. Using bee products for the prevention and treatment of oral mucositis induced by cancer treatment. Molecules. 2019;24:3023. doi: 10.3390/molecules24173023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bolton L. Managing oral mucositis in patients with cancer. Wounds a Compend Clin Res Pract. 2021;33:136–8. doi: 10.25270/wnds/2021.136138. [DOI] [PubMed] [Google Scholar]
- 138.Severo MLB, Thieme S, Silveira FM, Tavares RPM, Gonzaga AKG, Zucolotto SM, et al. Comparative study of royal jelly, propolis, and photobiomodulation therapies in 5-fluorouracil-related oral mucositis in rats. Support care cancer Off J Multinatl Assoc Support Care Cancer. 2022;30:2723–34. doi: 10.1007/s00520-021-06660-5. [DOI] [PubMed] [Google Scholar]
- 139.Yamauchi K, Kogashiwa Y, Moro Y, Kohno N. The effect of topical application of royal jelly on chemoradiotherapy-induced mucositis in head and neck cancer: a preliminary study. Int J Otolaryngol. 2014;2014:974967. doi: 10.1155/2014/974967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Erdem O, Güngörmüş Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist Nurs Pract. 2014;28:242–246. doi: 10.1097/HNP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 141.Daugėlaitė G, Užkuraitytė K, Jagelavičienė E, Filipauskas A. Prevention and treatment of chemotherapy and radiotherapy induced oral mucositis. Medicina. 2019;55:25. doi: 10.3390/medicina55020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pasupuleti VR, Sammugam L, Ramesh N, Gan SH. Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev. 2017;2017:1259510. doi: 10.1155/2017/1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cornara L, Biagi M, Xiao J, Burlando B. Therapeutic properties of bioactive compounds from different honeybee products. Front Pharmacol. 2017;8:412. doi: 10.3389/fphar.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Borsini A, Nicolaou A, Camacho-Muñoz D, Kendall AC, Di Benedetto MG, Giacobbe J, et al. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: relevance for major depression and for human hippocampal neurogenesis. Mol Psychiatry. 2021;26:6773–6788. doi: 10.1038/s41380-021-01160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodríguez-Iglesias N, Nadjar A, Sierra A, Valero J. Susceptibility of female mice to the dietary omega-3/omega-6 fatty-acid ratio: effects on adult hippocampal neurogenesis and glia. Int J Mol Sci. 2022;23:3399. doi: 10.3390/ijms23063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bălan A, Moga MA, Dima L, Toma S, Elena Neculau A, Anastasiu CV. Royal jelly-a traditional and natural remedy for postmenopausal symptoms and aging-related pathologies. Molecules. 2020;25:3291. doi: 10.3390/molecules25143291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kunugi H, Mohammed Ali A. Royal jelly and its components promote healthy aging and longevity: from animal models to humans. Int J Mol Sci. 2019;20:4662. doi: 10.3390/ijms20194662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Botchway BOA, Moore MK, Akinleye FO, Iyer IC, Fang M. Nutrition: review on the possible treatment for Alzheimer’s disease. J Alzheimers Dis. 2018;61:867–883. doi: 10.3233/JAD-170874. [DOI] [PubMed] [Google Scholar]
- 149.Wang Z, Zhu W, Xing Y, Jia J, Tang Y. B vitamins and prevention of cognitive decline and incident dementia: a systematic review and meta-analysis. Nutr Rev. 2022;80:931–949. doi: 10.1093/nutrit/nuab057. [DOI] [PubMed] [Google Scholar]
- 150.Mikkelsen K, Apostolopoulos V. B vitamins and ageing. Subcell Biochem United States. 2018;90:451–470. doi: 10.1007/978-981-13-2835-0_15. [DOI] [PubMed] [Google Scholar]
- 151.Sun Q, Zhang J, Li A, Yao M, Liu G, Chen S, et al. Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer’s disease. Nat Commun England. 2022;13:998. doi: 10.1038/s41467-022-28493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsetlin VI. Acetylcholine and acetylcholine receptors: textbook knowledge and new data. Biomolecules. 2020;10:852. [DOI] [PMC free article] [PubMed]
- 153.Kunugi H, Ali AM. Royal jelly and its components promote healthy aging and longevity: from animal models to humans. Int J Mol Sci. 2019;20:1–26. doi: 10.3390/ijms20194662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Calderón-Ospina CA, Nava-Mesa MO. B Vitamins in the nervous system: current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther. 2020;26:5–13. doi: 10.1111/cns.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scholefield M, Church SJ, Xu J, Patassini S, Hooper NM, Unwin RD, et al. Substantively lowered levels of pantothenic acid (vitamin B5) in several regions of the human brain in Parkinson’s disease dementia. Metabolites. 2021;11:569. doi: 10.3390/metabo11090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Athar T, Al Balushi K, Khan SA. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol Biol Rep. 2021;48:5629–5645. doi: 10.1007/s11033-021-06512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ab Hamid N, Abu Bakar AB, Mat Zain AA, Nik Hussain NH, Othman ZA, Zakaria Z, et al. Composition of royal jelly (RJ) and its anti-androgenic effect on reproductive parameters in a polycystic ovarian syndrome (PCOS) animal model. Antioxidants (Basel, Switzerland). Switzerland; 2020;9. [DOI] [PMC free article] [PubMed]
- 158.Hattori N, Nomoto H, Fukumitsu H, Mishima S, Furukawa S. AMP N(1)-oxide, a unique compound of royal jelly, induces neurite outgrowth from PC12 cells via signaling by protein kinase A independent of that by mitogen-activated protein kinase. Evid Based Complement Alternat Med. 2010;7:63–8. doi: 10.1093/ecam/nem146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.You M, Pan Y, Liu Y, Chen Y, Wu Y, Si J, et al. Royal jelly alleviates cognitive deficits and β-amyloid accumulation in APP/PS1 mouse model via activation of the cAMP/PKA/CREB/BDNF pathway and inhibition of neuronal apoptosis. Front Aging Neurosci. 2018;10:428. doi: 10.3389/fnagi.2018.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abdelnour SA, Abd El-Hack ME, Alagawany M, Taha AE, Elnesr SS, AbdElmonem OM, et al. Useful impacts of royal jelly on reproductive sides, fertility rate and sperm traits of animals. J Anim Physiol Anim Nutr. 2020;104:1798–808. doi: 10.1111/jpn.13303. [DOI] [PubMed] [Google Scholar]
- 161.Russell JK, Jones CK, Newhouse PA. The role of estrogen in brain and cognitive aging. Neurother J Am Soc Exp Neurother. 2019;16:649–65. doi: 10.1007/s13311-019-00766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Borrás C, Ferrando M, Inglés M, Gambini J, Lopez-Grueso R, Edo R, et al. Estrogen replacement therapy induces antioxidant and longevity-related genes in women after medically induced menopause. Oxid Med Cell Longev. 2021;2021:8101615. doi: 10.1155/2021/8101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pascale A, Marchesi N, Marelli C, Coppola A, Luzi L, Govoni S, et al. Microbiota and metabolic diseases. Endocrine. 2018;61:357–371. doi: 10.1007/s12020-018-1605-5. [DOI] [PubMed] [Google Scholar]
- 164.Kovatcheva-Datchary P, Tremaroli V, Backhed F. The prokaryotes: human microbiology. 4th ed. Prokaryotes Hum Microbiol. 2013;1–554.
- 165.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Allaband C, McDonald D, Vázquez-Baeza Y, Minich JJ, Tripathi A, Brenner DA, et al. Microbiome 101: studying, analyzing, and interpreting gut microbiome data for clinicians. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2019;17:218–30. doi: 10.1016/j.cgh.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cresci GA, Bawden E. Gut microbiome: what we do and don’t know. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2015;30:734–46. doi: 10.1177/0884533615609899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim YS, Unno T, Kim BY, Park MS. Sex differences in gut microbiota. World J Mens Health. 2020;38:48–60. doi: 10.5534/wjmh.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81:e00036–17. doi: 10.1128/MMBR.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nobre JG, Alpuim CD. “Sociobiome”: how do socioeconomic factors influence gut microbiota and enhance pathology susceptibility? - a mini-review. Front Gastroenterol. 2022;1:1–6. doi: 10.3389/fgstr.2022.1020190. [DOI] [Google Scholar]
- 172.Mafra D, Borges N, Alvarenga L, Esgalhado M, Cardozo L, Lindholm B, et al. Dietary components that may influence the disturbed gut microbiota in chronic kidney disease. Nutrients. 2019;11:1–23. doi: 10.3390/nu11030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eshraghi S, Seifollahi F. Antibacterial effects of royal jelly on different strains of bacteria. Iran J Public Health [Internet]. 2003;32:25–30. Available from: http://www.doaj.org/doaj?func=openurl&issn=22516085&date=2003&volume=32&issue=1&spage=25&genre=article. Accessed 27 Jan 2023
- 174.Guo J, Ma B, Wang Z, Chen Y, Tian W, Dong Y. Royal jelly protected against dextran-sulfate-sodium-induced colitis by improving the colonic mucosal barrier and gut microbiota. Nutrients. 2022;14:2069. doi: 10.3390/nu14102069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang W, Li X, Li D, Pan F, Fang X, Peng W, et al. Effects of major royal jelly proteins on the immune response and gut microbiota composition in cyclophosphamide-treated mice. Nutrients MDPI. 2023;15:974. doi: 10.3390/nu15040974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang W, Yan Y, Guo Z, Hou H, Garcia M, Tan X, et al. All around suboptimal health - a joint position paper of the suboptimal health study consortium and european association for predictive, preventive and personalised medicine. EPMA J. 2021;12:403–433. doi: 10.1007/s13167-021-00253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lemke HU, Golubnitschaja O. Towards personal health care with model-guided medicine: long-term PPPM-related strategies and realisation opportunities within “Horizon 2020”. EPMA J. 2014;5:8. doi: 10.1186/1878-5085-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;2016(7):23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lin XM, Liu S Bin, Luo YH, Xu WT, Zhang Y, Zhang T, et al. 10-HDA induces ROS-mediated apoptosis in A549 human lung cancer cells by regulating the MAPK, STAT3, NF-κB, and TGF-β1 signaling pathways. Biomed Res Int. 2020;3042636. [DOI] [PMC free article] [PubMed]
- 180.Tsuchiya Y, Hayashi M, Nagamatsu K, Ono T, Kamakura M, Iwata T, et al. The key royal jelly component 10-hydroxy-2-decenoic acid protects against bone loss by inhibiting NF-κB signaling downstream of FFAR4. J Biol Chem. 2020;295:12224–12232. doi: 10.1074/jbc.RA120.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.You M, Miao Z, Sienkiewicz O, Jiang X, Zhao X, Hu F. 10-Hydroxydecanoic acid inhibits LPS-induced inflammation by targeting p53 in microglial cells. Int Immunopharmacol. 2020;84:106501. doi: 10.1016/j.intimp.2020.106501. [DOI] [PubMed] [Google Scholar]
- 182.Mohamed HK, Mobasher MA, Ebiya RA, Hassen MT, Hagag HM, El-Sayed R, et al. Anti-inflammatory, anti-apoptotic, and antioxidant roles of honey, royal jelly, and propolis in suppressing nephrotoxicity induced by doxorubicin in male albino rats. Antioxidants. 2022;11:1029. doi: 10.3390/antiox11051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nazar-Zadeh M, Jalili C, Nikgoftar Fathi A, Ghanbari A, Bakhtiari M. Royal-jelly-based apitherapy can attenuate damages to male reproductive parameter following nicotine administration. Anim Model Exp Med. 2022;5:133–140. doi: 10.1002/ame2.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aksakal E, Ekinci D, Supuran CT. Dietary inclusion of royal jelly modulates gene expression and activity of oxidative stress enzymes in zebrafish. J Enzyme Inhib Med Chem. 2021;36:885–894. doi: 10.1080/14756366.2021.1900167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Azad F, Nejati V, Shalizar-Jalali A, Najafi G, Rahmani F. Antioxidant and anti-apoptotic effects of royal jelly against nicotine-induced testicular injury in mice. Environ Toxicol. 2019;34:708–718. doi: 10.1002/tox.22737. [DOI] [PubMed] [Google Scholar]
- 186.Fatmawati F, Erizka E, Hidayat R. Royal jelly (bee product) decreases inflammatory response in Wistar rats induced with ultraviolet radiation. Open access Maced J Med Sci North. 2019;7:2723–7. doi: 10.3889/oamjms.2019.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ovchinnikov AN, Paoli A, Seleznev VV, Deryugina AV. Royal jelly plus coenzyme Q10 supplementation improves high-intensity interval exercise performance via changes in plasmatic and salivary biomarkers of oxidative stress and muscle damage in swimmers: a randomized, double-blind, placebo-controlled pilot t. J Int Soc Sports Nutr. 2022;19:239–57. doi: 10.1080/15502783.2022.2086015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Petelin A, Kenig S, Kopinč R, Deželak M, ČerneličBizjak M, Jenko Pražnikar Z. Effects of royal jelly administration on lipid profile, satiety, inflammation, and antioxidant capacity in asymptomatic overweight adults. Evid Based Complement Alternat Med. 2019;2019:4969720. doi: 10.1155/2019/4969720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shidfar F, Jazayeri S, Mousavi SN, Malek M, Hosseini AF, Khoshpey B. Does supplementation with royal jelly improve oxidative stress and insulin resistance in type 2 diabetic patients? Iran J Public Health. 2015;44:797–803. [PMC free article] [PubMed] [Google Scholar]
- 190.Ghanbari E, Nejati V, Khazaei M. Improvement in serum biochemical alterations and oxidative stress of liver and pancreas following use of royal jelly in streptozotocin-induced diabetic rats. Cell J. 2016;18:362–370. doi: 10.22074/cellj.2016.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fan P, Han B, Feng M, Fang Y, Zhang L, Hu H, et al. Functional and proteomic investigations reveal major royal jelly protein 1 associated with anti-hypertension activity in mouse vascular smooth muscle cells. Sci Rep. 2016;6:30230. doi: 10.1038/srep30230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Feng M, Fang Y, Han B, Xu X, Fan P, Hao Y, et al. In-depth N-glycosylation reveals species-specific modifications and functions of the royal jelly protein from western (Apis mellifera) and eastern honeybees (Apis cerana) J Proteome Res. 2015;14:5327–5340. doi: 10.1021/acs.jproteome.5b00829. [DOI] [PubMed] [Google Scholar]
- 193.Escamilla KIA, Ordóñez YBM, Sandoval-Peraza VM, Fernández JJA, Ancona DAB. Anti-hypertensive activity in vitro and in vivo on royal jelly produced by different diets. Emirates J Food Agric. 2022;34:9–15. [Google Scholar]
- 194.Fujisue K, Yamamoto E, Sueta D, Arima Y, Hirakawa K, Tabata N, et al. A randomized, double-blind comparison study of royal jelly to augment vascular endothelial function in healthy volunteers. J Atheroscler Thromb. 2022;29:1285–1294. doi: 10.5551/jat.63044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chiu H-F, Chen B-K, Lu Y-Y, Han Y-C, Shen Y-C, Venkatakrishnan K, et al. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharm Biol. 2017;55:497–502. doi: 10.1080/13880209.2016.1253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lambrinoudaki I, Augoulea A, Rizos D, Politi M, Tsoltos N, Moros M, et al. Greek-origin royal jelly improves the lipid profile of postmenopausal women. Gynecol Endocrinol. 2016;32:835–839. doi: 10.1080/09513590.2016.1188281. [DOI] [PubMed] [Google Scholar]
- 197.Pourmobini H, Kazemi Arababadi M, Salahshoor MR, Roshankhah S, Taghavi MM, Taghipour Z, et al. The effect of royal jelly and silver nanoparticles on liver and kidney inflammation. Avicenna J Phytomedicine. 2021;11:218–223. [PMC free article] [PubMed] [Google Scholar]
- 198.Salahshoor MR, Jalili C, Roshankhah S. Can royal jelly protect against renal ischemia/reperfusion injury in rats? Chin J Physiol. 2019;62:131–137. doi: 10.4103/CJP.CJP_36_19. [DOI] [PubMed] [Google Scholar]
- 199.Osama H, Abdullah A, Gamal B, Emad D, Sayed D, Hussein E, et al. Effect of honey and royal jelly against cisplatin-induced nephrotoxicity in patients with cancer. J Am Coll Nutr. 2017;36:342–346. doi: 10.1080/07315724.2017.1292157. [DOI] [PubMed] [Google Scholar]
- 200.Filipič B, Gradišnik L, Rihar K, Šooš E, Pereyra A, Potokar J. The influence of royal jelly and human interferon-alpha (HuIFN-αN3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro. Arh Hig Rada Toksikol. 2015;66:269–274. doi: 10.1515/aiht-2015-66-2632. [DOI] [PubMed] [Google Scholar]
- 201.Albalawi AE, Althobaiti NA, Alrdahe SS, Alhasani RH, Alaryani FS, Binmowyna MN. Antitumor activity of royal jelly and its cellular mechanisms against Ehrlich solid tumor in mice. Biomed Res Int. 2022:7233997. [DOI] [PMC free article] [PubMed]
- 202.Sirinupong N, Chansuwan W, Kaewkaen P. Hydrolase-treated royal jelly attenuates H(2)O(2)- and glutamate-induced SH-SY5Y cell damage and promotes cognitive enhancement in a rat model of vascular dementia. Int J food Sci. 2021;2021:2213814. doi: 10.1155/2021/2213814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang X, Yu Y, Sun P, Fan Z, Zhang W, Feng C. Royal jelly peptides: potential inhibitors of β-secretase in N2a/APP695swe cells. Sci Rep. 2019;9:168. doi: 10.1038/s41598-018-35801-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ghorbanpour AM, Saboor M, Panahizadeh R, Saadati H, Dadkhah M. Combined effects of royal jelly and environmental enrichment against stress-induced cognitive and behavioral alterations in male rats: behavioral and molecular studies. Nutr Neurosci. 2022;25:1860–71. doi: 10.1080/1028415X.2021.1909205. [DOI] [PubMed] [Google Scholar]
- 205.de Guardia Souza Silva ET, de Val Paulo MEF, da Silva JRM, da Silva Alves A, Britto LRG, Xavier GF, et al. Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer’s disease. Heliyon. 2022;6:e03281. doi: 10.1016/j.heliyon.2020.e03281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pan Y, Xu J, Jin P, Yang Q, Zhu K, You M, et al. Royal jelly ameliorates behavioral deficits, cholinergic system deficiency, and autonomic nervous dysfunction in ovariectomized cholesterol-fed rabbits. Molecules. 2019;24:1149. doi: 10.3390/molecules24061149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mohamed AAR, Galal AAA, Elewa YHA. Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochem. 2015;117:649–58. doi: 10.1016/j.acthis.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 208.Asama T, Matsuzaki H, Fukushima S, Tatefuji T, Hashimoto K, Takeda T. Royal jelly supplementation improves menopausal symptoms such as backache, low back pain, and anxiety in postmenopausal Japanese women. Evid Based Complement Alternat Med. 2018;2018:4868412. doi: 10.1155/2018/4868412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.