Abstract
Collagenase 3 (MMP-13) is a recently identified member of the matrix metalloproteinase (MMP) gene family that is expressed at high levels in diverse human carcinomas and in articular cartilage from arthritic patients. In addition to its expression in pathological conditions, collagenase 3 has been detected in osteoblasts and hypertrophic chondrocytes during fetal ossification. In this work, we have evaluated the possibility that Cbfa1 (core binding factor 1), a transcription factor playing a major role in the expression of osteoblastic specific genes, is involved in the expression of collagenase 3 during bone formation. We have functionally characterized a Cbfa motif present in the promoter region of collagenase 3 gene and demonstrated, by cotransfection experiments and gel mobility shift assays, that this element is involved in the inducibility of the collagenase 3 promoter by Cbfa1 in osteoblastic and chondrocytic cells. Furthermore, overexpression of Cbfa1 in osteoblastic cells unable to produce collagenase 3 leads to the expression of this gene after stimulation with transforming growth factor β. Finally, we show that mutant mice deficient in Cbfa1, lacking mature osteoblasts but containing hypertrophic chondrocytes which are also a major source of collagenase 3, do not express this protease during fetal development. These results provide in vivo evidence that collagenase 3 is a target of the transcriptional activator Cbfa1 in these cells. On the basis of these transcriptional regulation studies, together with the potent proteolytic activity of collagenase 3 on diverse collagenous and noncollagenous bone and cartilage components, we proposed that this enzyme may play a key role in the process of bone formation and remodeling.
The human matrix metalloproteinases (MMPs) or matrixins are a family of structurally related neutral proteinases that are collectively capable of degrading essentially all extracellular matrix components (9). These enzymes play a major role in normal tissue-remodeling processes such as embryonic development, ovulation, and wound healing (44, 81). In addition, abnormal expression of these proteases may contribute to a variety of pathological conditions characterized by matrix destruction, including rheumatoid arthritis (52), atherosclerosis (25), and cancer invasion and metastasis (43, 72). Recently, and based on the hypothesis that samples of human tumor specimens could be an appropriate material to identify novel proteinases potentially involved in the spread of cancer, we have cloned from a breast carcinoma cDNA library a new member of the MMP family of enzymes that has been called collagenase 3 (MMP-13) (21, 55). Biochemical characterization of this enzyme has revealed that it degrades very efficiently the native helix of fibrillar collagens, with preferential activity on type II collagen. In addition, collagenase 3 may also act as a potent gelatinase, thus contributing to further degrade the initial cleavage products of collagenolysis to small fragments suitable for subsequent metabolism (33). Furthermore, recent studies have shown that collagenase 3 is also able to degrade the large cartilage proteoglycan aggrecan and other components of the extracellular matrix and basement membranes, including type IV collagen (19, 33, 35).
Analysis of the expression of collagenase 3 in human tissues has revealed that in addition to its presence in diverse malignant tumors including breast carcinomas (21, 26), chondrosarcomas (77), basal cell carcinomas of the skin (1), and head and neck carcinomas (13, 29), this enzyme is produced during fetal ossification (30, 70) and in destructive joint diseases such as osteoarthritis and rheumatoid arthritis (41, 49, 59). Recent studies have provided information on the mechanisms controlling human collagenase 3 expression in pathological conditions. Thus, we have reported that this gene is predominantly expressed in fibroblasts adjacent to invasive breast cancer cells, in response to diffusible factors released from the epithelial tumor cells (76). A search of molecular factors with ability to induce collagenase 3 expression in human fibroblasts has shown that interleukin-1 (IL-1), tetradecanoyl phorbol acetate (TPA), and transforming growth factor β (TGF-β) are able to up-regulate the expression of this gene (76, 78). Functional analysis of the collagenase 3 gene promoter region has revealed that the inductive effects of all of these factors on the expression of collagenase 3 are mediated in part by an AP-1 site present in the 5′-flanking region of this gene (56, 78). Similar studies using human chondrosarcoma cells have indicated that basic fibroblast growth factor (bFGF) may be a major in vivo modulator of collagenase 3 expression in these malignant tumors (77). Furthermore, different groups have reported that IL-1β and tumor necrosis factor alpha (TNF-α) may induce collagenase 3 expression in osteoarthritic cartilage (11, 59). However, in marked contrast to these data on human collagenase 3 expression in pathological conditions, very little information is available on the mechanisms mediating its expression in normal conditions and, more specifically, in the process of bone formation, in which high levels of collagenase 3 have been detected. Recent structural analysis of the 5′-flanking region of the human collagenase 3 gene (56) has shown that it contains a sequence motif located at positions −133 to −139 that exhibits striking similarity to a sequence motif called nuclear matrix protein 2 (NMP-2) binding site (8, 47) or osteoblast-specific element 2 (OSE2) (15, 17). This sequence, originally described as a structural element essential for the osteoblastic expression of osteocalcin, is recognized by a transcription factor of the runt domain gene family, called Cbfa1 or Osf2 (7, 15, 17, 69, 83), that plays a major role in the expression of different osteoblast-specific genes (6, 7, 17, 37, 53).
In this work we have evaluated the possibility that Cbfa1 is involved in the expression of collagenase 3 during bone formation. It was recently reported that parathyroid hormone (PTH) regulates the rat collagenase 3 promoter in osteoblastic cells through the cooperative interaction of an AP-1 site and a runt domain binding sequence recognized by runt domain proteins including Cbfa1 (67). Here, we provide in vitro and in vivo evidence that collagenase 3 is a target of Cbfa1 in osteoblastic and chondrocytic cells. In addition, on the basis of these transcriptional regulation studies, together with the potent proteolytic activity of collagenase 3 on bone and cartilage collagens, we propose that this enzyme may play a key role during fetal ossification.
MATERIALS AND METHODS
Materials.
Oligonucleotides were synthesized by the phosphoramidite method in an Applied Biosystems DNA synthesizer (model 392A) and used without further purification. Restriction endonucleases and other reagents used for molecular cloning were purchased from Boehringer Mannheim (Mannheim, Germany). Media for cell culture, fetal calf serum, and trypsin were obtained from GIBCO-BRL (Gaithersburg, Md.). Other supplements for cell culture (TPA, IL-1β, IL-6, epidermal growth factor [EGF], and TGF-β) were from Sigma. [α-32P]dCTP (3,000 Ci/mmol) and the random priming labeling kit were from Amersham International (Buckinghamshire, United Kingdom). The expression plasmid for Osf2/Cbfa1 (pCMV-Osf2/Cbfa1), which contains the cDNA encoding the Cbfa1 isoform with MASNSL as the N-terminal sequence (17, 73, 74, 83) was kindly provided by G. Karsenty (Department of Molecular Genetics, University of Texas M. D. Anderson Cancer Center). Antibodies against Cbfa1 (also called PEBP2αA) (42) were kindly provided by Y. Ito (Department of Viral Oncology, Institute for Virus Research, Kyoto University, Kyoto, Japan).
Cell culture.
Osteosarcoma cell lines U2OS, KHOS 321H, MG-63, and MC3T3 E1, chondrosarcoma cell lines SW1353 and RCS, and HeLa cells were obtained from the American Type Culture Collection (Rockville, Md.) or kindly provided by J. Kimura (Henry Ford Hospital, Detroit, Mich.). Cells were maintained at 37°C under 5% CO2 in Dulbecco’s modified Eagle’s medium supplemented with penicillin (100 IU/ml), streptomycin (100 μg/ml), and 10% fetal calf serum. MC3T3 E1 cells were grown in alpha minimal essential medium supplemented with 10% fetal calf serum.
Construction of luciferase fusion plasmids.
All plasmid constructs were prepared by standard methods (64). The promoterless basic plasmid pGL3 Basic (Promega Corp., Madison, Wis.) was used for cloning the different promoter fragments obtained from the human collagenase 3 gene at the 5′ end of the firefly luciferase gene. The different collagenase 3 promoter constructs (p1004-luc, p675-luc, p182-luc, and p83-luc) were generated by PCR amplification with specific oligonucleotides or by endonuclease restriction. p1004-luc was created by inserting a KpnI-BamHI fragment, extracted from plasmid pUC18 containing approximately 1 kb of collagenase 3 promoter, in pGL3 Basic digested with KpnI and BglII. p675-luc was generated by cloning an NheI-BamHI fragment from the same PUC18 vector into NheI-BglII-digested pGL3 Basic. p182-luc and p83-luc were PCR generated by using the 5′ primers 5′-AACAAGAGATGCTCTCA-3′ (nucleotides −182 to −166) and 5′-GTGACTAGGAAGTGGAAAC-3′ (nucleotides −83 to −65), respectively, and the same 3′ primer, 5′-GGTCTAGATTGAATGGTGATGCCTGG-3′ (nucleotides +10 to +27). To create the (Cbfa)8-p82-luc construct, oligonucleotides Cbfa′ direct (5′-AGCCACAAACCACACTCGGG-3′) and Cbfa′ reverse (5′-GTCCCGAGTGTGGTTTGTGG-3′) were annealed, tandemly ligated, and subsequently cloned in the XmaI site of plasmid p83-luc. A clone containing eight copies of the Cbfa element in the right orientation was selected. Constructs p1004-mutCbfa-luc, p182-mutCbfa-luc, p1004-mutAP1-luc, and (Cbfa)8-p82-mutAP1-luc were generated by site-directed mutagenesis, using mutCbfa and mutAP-1 direct and reverse primers (Table 1), following standard procedures. All collagenase 3 promoter PCR fragments were cloned in Klenow enzyme-filled BglII restriction site of pGL3 Basic. All constructs were verified by extensive restriction mapping and partial DNA sequencing using the dideoxy-chain termination method. pRL-TK (Promega), a plasmid containing the herpes simplex virus thymidine kinase promoter region upstream the cDNA encoding Renilla luciferase, was used as an internal control reporter vector. All recombinant plasmids used for transfection assays were purified by using a Qiagen plasmid kit (Qiagen Inc., Chatsworth, Calif.).
TABLE 1.
Oligonucleotides used in the functional analysis of the Cbfa element present in the collagenase 3 gene promoter
| Oligonucleotide | Sequencea |
|---|---|
| Cbfa direct | 5′-ATTCTACCACAAACCACACTCGGG-3′ |
| Cbfa reverse | 5′-CCCGAGTGTGGTTTGTGGTAGAAT-3′ |
| AP-1 direct | 5′-AAGTGATGACTCACCATTG-3′ |
| AP-1 reverse | 5′-CAATGGTGAGTCATCACTT-3′ |
| HRE direct | 5′-GGAAAGAACTATTTGTTCCAA-3′ |
| HRE reverse | 5′-CCTTGGAACAAATAGTTCTTT-3′ |
| mutCbfa direct | 5′-ATTCTACCACAAGACACACTCG-3′ |
| mutCbfa reverse | 5′-CCCGAGTGTGTCTTGTGGTAGA-3′ |
| mutAP-1 direct | 5′-AAGTGATTTCTCACCATTG-3′ |
| mutAP-1 reverse | 5′-CAATGGTGAGAAATCACTT-3′ |
Mutated nucleotides are indicated in boldface. HRE, hormone response element from the human pepsinogen C gene promoter (5).
DNA transfections and luciferase assays.
For each transfection experiment, cells were seeded at 2 × 105 cells/30-mm-diameter dish and transfected 18 h later with 0.7 μg of the indicated reporter plasmid DNA, 0.2 μg of the effector plasmid (pCMV-Cbfa1 or pcDNA3), and 0.05 μg of pRL-TK, using the LipofectAMINE Plus reagent (GIBCO-BRL) as specified by the manufacturer. Three hours after the start of transfection, serum-free DNA-containing medium was replaced by fresh growth medium with 2% serum. Transfected cells were harvested in passive lysis buffer (Promega) approximately 40 h after the start of transfection. Luciferase activity was measured with the Promega Dual-Luciferase reporter assay system as indicated by the manufacturer, using a Turner Designs model TD-20/20 Luminometer. Stimulation of firefly luciferase activity was based on at least three independent experiments.
Electrophoretic mobility shift DNA binding assay.
To obtain nuclear extracts, HeLa cells were previously transfected as described above with 1 μg of the corresponding effector plasmid. Nuclear extracts from the indicated cells were prepared as described by Schreiber et al. (66). DNA probes and competitors were complementary oligonucleotides (Table 1). Oligonucleotides were annealed, labeled with [γ-32P]ATP by T4 polynucleotide kinase, and further purified by Sephadex G-25 column chromatography (Pharmacia Biotech Inc.). Nuclear extracts (2 μg) were preincubated at 4°C for 15 min with the unlabeled competitor oligonucleotide or with specific antibodies against Cbfa1 in 25 mM Tris-HCl (pH 8.0)–60 mM KCl–5 mM MgCl2–1 mM EDTA–10% glycerol–1 mM dithiothreitol. The 25-min binding reaction was initiated by the addition of 2 μl (0.1 pmol) of 32P-labeled probe (5 × 106 cpm/pmol), in the presence of 10 μg of dried nonfat milk and 1 μg of poly(dI-dC). The amount of unlabeled competitor DNA added is indicated in the figure legends. Samples were electrophoresed on prerun 4% polyacrylamide gels containing 2.5% glycerol in 25 mM Tris–190 mM glycine–1 mM EDTA buffer at 200 V for 2 h at 4°C. Gels were dried and subjected to autoradiography.
Isolation of RNA and Northern blot analysis.
Total RNA from cells was isolated by the guanidinium isothiocyanate procedure of Chomczynski and Sacchi (14), separated by electrophoresis in 1.2% agarose-formaldehyde gels, and blotted onto Hybond N nylon filters (Amersham). Filters containing 20 μg of total RNA were prehybridized at 42°C for 3 h in 50% formamide–5× SSPE (1× SSPE is 150 mM NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.4])–10× Denhardt’s solution–2% sodium dodecyl sulfate–100 μg of denatured herring sperm DNA per ml and then hybridized with the indicated radiolabeled probe for 18 h under the same conditions. Filters were washed with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate for 2 h at 50°C and exposed to autoradiography. RNA integrity and equal loading were assessed by hybridization with an actin probe.
Reverse transcription-PCR (RT-PCR).
cDNA synthesis and PCR of total RNA were performed with a RNA-PCR kit from Perkin-Elmer. Reverse transcription was carried out for 30 min at 42°C with 500 ng of total RNA and random hexamers as primers in a total volume of 20 μl. For the amplification of human collagenase 3 cDNA, a 16-μl aliquot of each reverse transcription reaction was amplified in a volume of 50 μl with the oligonucleotides Int32 (5′-CCTCCTGGGCCAAATTATGGAG) and Int33 (5′-CAGCTCCGCATCAACCTGCTG) as primers. For β-actin amplification, 2-μl aliquots were amplified in the same way with the oligonucleotides 5′-GTGGGGCCGCTCTAGGCAC and 5′-TTTGATGTCACGCACGATTT. PCR was carried out in a GeneAmp 2400 PCR system (Perkin-Elmer Cetus) with cycles of 94°C (15 s), 60°C (15 s), and 72°C (30 s). To perform a semiquantitative analysis of the PCR product during the exponential phase of amplification, a 10-μl aliquot of each reaction product was removed after 24, 26, and 29 cycles and analyzed by agarose gel electrophoresis and Southern blotting using the corresponding specific probes. The radioactive signals obtained were quantified by electronic autoradiography in an InstantImager instrument (Packard, Meriden, Conn.).
Histological analysis.
Embryos were fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections of 5 μm were collected on Superfrost Plus (Menzel-Grazel) slides. Alkaline phosphatase activity was determined histochemically by incubation with a substrate solution containing 0.16 mg of 5-bromo-4-chloro-3 indolylphosphate and 0.33 mg of nitroblue tetrazolium per ml in 100 mM Tris–50 mM MgCl2 (pH 9.5) for 30 min. Tissue sections were counterstained with nuclear fast red (Merck), rinsed, and mounted.
In situ RNA hybridization.
Digoxigenin (DIG)-11-UTP-labeled single-stranded RNA probes were prepared with DIG RNA labeling mix and the corresponding T3 or T7 RNA polymerase (Boehringer Mannheim) according to the manufacturer’s instructions. The mouse collagenase 3 probe was a 700-bp fragment from the 3′ untranslated region cloned in pBluescript (Stratagene) vector. In situ hybridization was performed on paraffin-embedded tissue sections from Cbfa1−/−, Cbfa1+/− (37), and wild-type 18.5-day-postcoitum (dpc) mouse embryos essentially as described by Braissant and Wahli (12). Tissue sections were cut, placed on Superfrost Plus slides, postfixed in 4% paraformaldehyde in phosphate-buffered saline, rinsed in phosphate-buffered saline containing 0.1% active diethyl pyrocarbonate, and prehybridized for 2 h at 58°C in 50% formamide, 5× SSC, and 40 μg of salmon sperm DNA per ml. Hybridization was carried out at 58°C for 16 h in a humid chamber with 400 ng of DIG-labeled probe per ml diluted in the same solution used for prehybridization. After hybridization, the sections were successively washed in 2× SSC at room temperature for 30 min, 2× SSC for 1 h at 65°C, and 0.1× SSC at 65°C for 1 h. For the reaction of anti-DIG antibodies, slides were preincubated in buffer A (100 mM Tris, 150 mM NaCl [pH 7.5]) and then with alkaline phosphatase-coupled anti-DIG antibody (Boehringer Mannheim) diluted 1:5,000 in buffer A containing 0.5% Boehringer blocking reagent for 2 h at room temperature. The slides were washed in buffer A and then preincubated in buffer C (100 mM Tris, 50 mM MgCl2 [pH 9.5]). Alkaline phosphatase was then revealed as described above for 16 to 24 h at room temperature. The enzymatic reaction was stopped with Tris-EDTA for 15 min. The slides were rinsed in water for several hours and then dried, cleared in xylene, and mounted directly with Eukitt.
RESULTS
Functional characterization of a Cbfa1 element present in the promoter region of the human collagenase 3 gene.
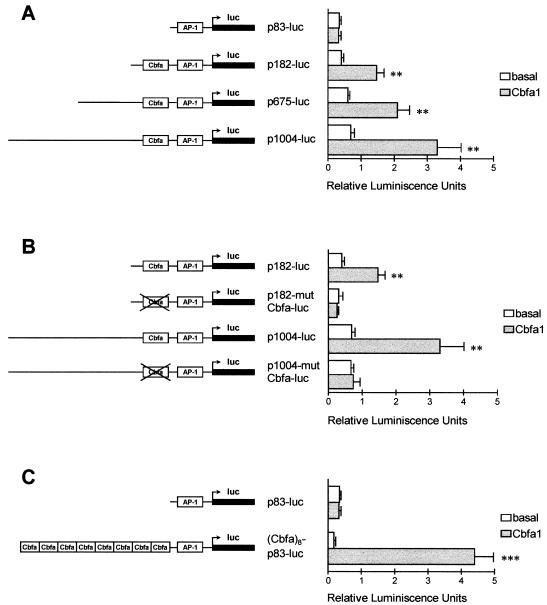
An analysis of the promoter region of the human collagenase 3 gene (56) has shown that it contains a motif located at positions −133 to −139, identical to the sequence of the element called Cbfa/NMP-2/OSE2 (8, 15, 17, 47). Similar motifs are present at equivalent positions in the promoter regions of mouse, rat, and rabbit collagenase 3 genes (57, 65, 79) (Fig. 1) but not in the corresponding regions of other MMP genes such as those encoding collagenase 1, gelatinases A and B, or stromelysins 1, 2, and 3 (2, 3, 24, 27, 28, 68). Since the presence of this sequence motif in the promoter region of the collagenase 3 gene was unique among MMP genes and could help to explain the production of human collagenase 3 by hypertrophic chondrocytes and osteoblasts during fetal ossification (30, 70), we were prompted to perform a functional analysis of the Cbfa element present in the promoter of this gene. To do that, we first examined by cotransfection experiments whether Cbfa1 protein is capable of stimulating collagenase 3 gene expression by transactivating through the Cbfa element both in nonosteoblastic cells and in bone-derived cells. Thus, we prepared a series of DNA constructs containing various lengths of the promoter inserted in front of the firefly luciferase gene. These constructs were cotransfected into HeLa cells together with plasmid pCMV-Osf2/Cbfa1, which contains the cDNA encoding the Cbfa1 isoform with MASNSL as N-terminal sequence, placed under transcriptional control of the cytomegalovirus promoter (17, 74). As shown in Fig. 2A, all collagenase 3 promoter constructs containing the Cbfa element were induced three- to fourfold by cotransfection with Cbfa1. By contrast, constructs lacking this element were not induced by cotransfection with the plasmid containing the cDNA for this transcription factor.
FIG. 1.
Nucleotide sequence comparison between human, rat, mouse, and rabbit collagenase 3 promoter regions and human, rat, and mouse osteocalcin promoter regions around the Cbfa element (boxed).
FIG. 2.
Functional analysis of the Cbfa element in the collagenase 3 promoter. (A) HeLa cells were cotransfected with several collagenase 3 promoter deletions fused to firefly luciferase (luc) reporter gene constructs and with pCMV-Cbfa1 (grey bars) or pcDNA3 (white bars) as the effector plasmid. Plasmid pRL-TK was used as an internal control of transfection efficiency as described in Materials and Methods. Values represent firefly luciferase-to-Renilla luciferase ratios. (B) Similarly, two Cbfa mutant constructs of different lengths (p1004-mutCbfa-luc and p182-mutCbfa-luc) were analyzed for transcriptional activity and compared to the wild-type constructs, in the presence or absence of Cbfa1. (C) Analysis of a plasmid containing eight copies of Cbfa element from the collagenase 3 promoter cloned upstream of the −83 promoter segment. Data are expressed as means ± standard errors of at least three independent experiments. Asterisks indicate significant differences from the control (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001).
Since these results showed that the Cbfa element could mediate the observed inducibility of the human collagenase 3 gene promoter by Cbfa1, we prepared additional constructs in which a double mutation (AACCACA→AGACACA) within this sequence motif was introduced. As shown in Fig. 2B, the activity of the different Cbfa mutant constructs was abolished independently of the length of the promoter region studied. These results confirm that collagenase 3 promoter activation by Cbfa1 is mediated by the Cbfa element. The Cbfa1 transcriptional activity on the Cbfa sequence identified in the collagenase 3 promoter was additionally assessed by cotransfections with a construct containing eight copies of Cbfa oligonucleotides cloned upstream of the 83-bp collagenase 3 promoter (Fig. 2C). Luciferase activity of this construct was stimulated 25-fold upon cotransfection with the Cbfa1 vector.
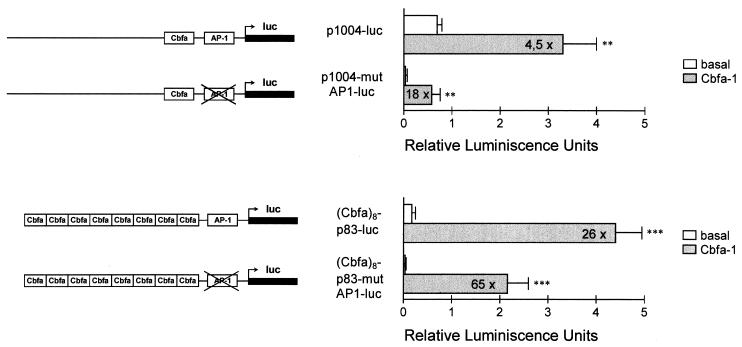
We next examined if transcriptional activation of the human collagenase 3 promoter by Cbfa1 was independent of the AP-1 element present in this promoter. This element has been found to mediate, at least in part, the induction of this MMP gene by diverse cytokines, growth factors, and tumor promoters (56, 78). To address this question, we made an inactivating AP-1 double mutation (TGACTCA→TTTCTCA) within the 1,004-bp collagenase 3 promoter construct as well as in the plasmid containing eight copies of Cbfa oligonucleotides cloned in front of the minimal 83-bp collagenase 3 promoter. These constructs were cotransfected in HeLa cells with the Cbfa1 expression vector, and transcriptional activity was determined as described above. As shown in Fig. 3, inactivation of the AP-1 element in both constructs resulted in a decrease in the basal activity of the collagenase 3 promoter, whereas cotransfection with the Cbfa1 transcription factor resulted in marked induction of promoter activity, 18- and 60-fold with p1004-mutAP1-luc and (Cbfa)8-p82-mutAP1-luc, respectively. Taken together, these results demonstrate that under these experimental conditions the Cbfa element present in the human collagenase 3 promoter may function independently of the AP-1 site.
FIG. 3.
Analysis of the role played by the AP-1 element in collagenase 3 promoter activation by Cbfa1 in HeLa cells. The complete collagenase 3 promoter construct or a construct containing eight copies of the Cbfa element linked to the p83-luc promoter and containing a double mutation in the AP-1 element (p1004-mutAP1-luc and Cbfa8-p83-mutAP1-luc) was transfected in HeLa cells, as described in the legend to Fig. 2 and assayed for luciferase (luc) activity. The corresponding wild-type constructs were also transfected in parallel experiments. Data are expressed as means ± standard errors of at least three independent experiments. Asterisks indicate significant differences from the control (∗∗, P < 0.01; ∗∗∗, P < 0.001).
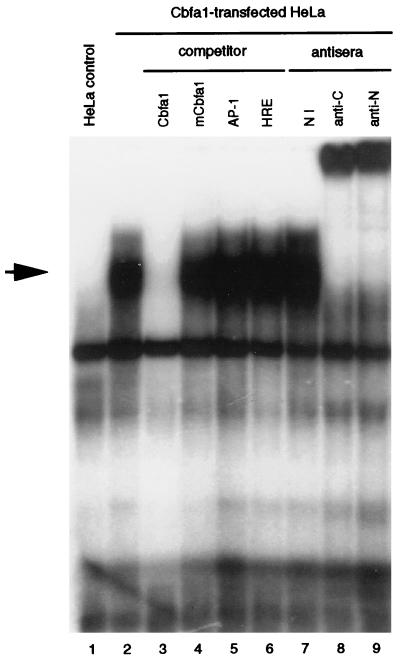
Analysis of binding of nuclear proteins from Cbfa1-transfected cells to the Cbfa element of the human collagenase 3 gene.
To further examine the transcriptional activity of Cbfa1 on the collagenase 3 promoter, we next performed a series of gel mobility shift assays with specific oligonucleotides and nuclear extracts prepared from diverse cell types. To this end, we first examined the DNA binding activity of nuclear extracts from HeLa cells transfected with the pCMV-Osf2/Cbfa1 vector or with a control plasmid (pcDNA3). A 20-bp synthetic oligonucleotide containing the Cbfa motif of the human collagenase 3 gene was radioactively labeled and incubated with nuclear extracts from transfected HeLa cells. After electrophoretic analysis, a strong protein-DNA complex was detected in nuclear extracts from cells transfected with plasmid pCMV-Osf2/Cbfa1 but not in control pcDNA3-transfected cells (Fig. 4). In addition, this complex was competed by an excess of nonlabeled Cbfa oligonucleotide and was supershifted when specific antibodies against Cbfa1 protein were added (Fig. 4). No variation was observed in the complex when the competitor was a molar excess of either mutant Cbfa, AP-1, or an unrelated HRE (hormone response element) oligonucleotide. Finally, it is noteworthy that these complexes were not observed when binding experiments were performed in similar conditions with nuclear extracts incubated in the presence of radiolabeled mutant Cbfa oligonucleotide (data not shown).
FIG. 4.
Electrophoretic mobility shift assay demonstrating specific binding of Cbfa1 to the Cbfa element in the collagenase 3 promoter. A complex (marked by an arrow) appears when nuclear extracts from HeLa cells transfected with pCMV-Cbfa1 are incubated with a labeled collagenase 3 promoter Cbfa element (lane 2). This complex is absent in control cells transfected with pcDNA3 (lane 1). To demonstrate the specificity of this binding, 20-fold molar excesses of different unlabeled probes (Cbfa [lane 3], mutant Cbfa1 [mCbfa1; lane 4], AP-1 [lane 5], and HRE [lane 6]) were added to the binding reaction as competitors. To assess the presence of Cbfa1 in the complexes, nuclear extracts were incubated with nonimmune serum (lane 7) or with specific antibodies against Cbfa1 (anti-αA1C17 [lane 8] and anti-αA1N35 [lane 9] [42]).
Functional relevance of Cbfa1 on collagenase 3 expression in human osteoblastic and chondrocytic cells.
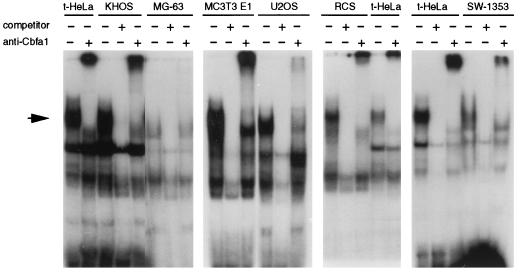
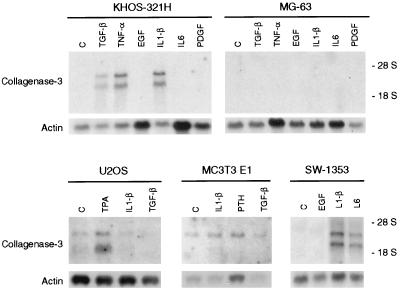
To extend the above observations for Cbfa1-transfected HeLa cells, we examined the possibility that the Cbfa binding activity was also present in nuclear extracts from different osteoblastic and chondrocytic cell lines. As shown in Fig. 5, nuclear extracts from KHOS 321H, U2OS, and MC3T3 E1 osteosarcoma cells and from SW1353 and RCS chondrosarcoma cells were able to bind labeled Cbfa oligonucleotides, generating a protein-DNA complex similar in mobility to the one produced by incubation with extracts from Cbfa1-transfected HeLa cells. This complex was also competed by an excess of nonlabeled Cbfa oligonucleotide, but not by a mutant Cbfa or AP-1 oligonucleotide, and was supershifted with antibodies against the Cbfa1 protein (Fig. 5 and data not shown). However, nuclear extracts from MG-63 osteosarcoma cells produced another specific but slightly faster-migrating protein-DNA complex that was not supershifted by the antibodies (Fig. 5). This complex, which was also observed in some of the studied cell lines, could represent the binding of other proteins, members or not of the Cbfa family, to the Cbfa1 element or sequences around this element. In summary, Cbfa binding studies indicate that different osteoblastic and chondrocytic cell lines have a variable ability to produce Cbfa1. The expression of collagenase 3 by these cells was also variable and dependent, in some cases, on stimulation with some cytokines and growth factors (Fig. 6 and data not shown). Thus, collagenase 3 expression could be detected by Northern blot analysis in U2OS and MC3T3 E1 cells in a constitutive fashion. In addition, KHOS 321H, SW1353, and RCS but not MG-63 cells were able to produce collagenase 3 transcripts after stimulation (Fig. 6). Expression of very low levels of collagenase-3 by MG-63 cells could be observed only by RT-PCR followed by Southern blot analysis.
FIG. 5.
Analysis of the presence of Cbfa1 binding activity in six different bone-derived cell lines. The binding assays were performed with KHOS 321H, U2OS, MC3T3 E1, MG-63, RCS, or SW1353 nuclear extracts and Cbfa1-transfected HeLa nuclear extracts (t-HeLa) as a reference. A 20-fold molar excess of unlabeled Cbfa oligonucleotide was used as competitor where indicated. Antiserum against Cbfa1 was added to the reaction mixture as indicated (the same results were obtained with anti-αA1N35 and anti-αA1C17).
FIG. 6.
Northern blot analysis of collagenase 3 expression in osteoblastic KHOS 321H, MG-63, U2OS, and MC3T3 E1 and chondrocytic SW1353 cell lines. Northern blot analysis was performed with 10 μg of total RNA from KHOS 312H, MG-63, U2OS, MC3T3 E1, or SW1353 cells incubated for 24 h in the presence of EGF (10 ng/ml), IL-1β (10 ng/ml), IL-6 (20 ng/ml), PTH (10−8 M), platelet-derived growth factor (PDGF; 10 ng/ml), TPA (10−6 M), TGF-β (5 ng/ml), TNF-α (20 ng/ml), or vehicle alone (C). Filters were hybridized with a collagenase 3 cDNA probe and with a β-actin probe to verify RNA loading.
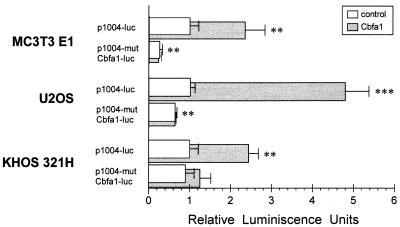
To further examine the functional relevance of Cbfa1 on collagenase 3 promoter activation through the Cbfa element in these osteoblastic and chondrocytic cells, functional assays of Cbfa1 activity on collagenase 3 promoter were performed by transfection of constructs containing a wild-type or Cbfa mutant element of this promoter and the luciferase reporter gene (Fig. 7). To first analyze endogenous Cbfa1 activity, basal transcriptional levels of the 1004- and 1004-mutCbfa-luc were compared in the transfected cells. Thus, comparison of the luciferase reporter activities of both transfected constructs (p1004-luc and p1004-mutCbfa-luc) revealed a decrease in luciferase activity of the Cbfa mutant plasmid to about 70% in MC3T3 E1 cells and to about 35% in U2OS cells. The decrease in luciferase activity was also observed in RCS and SW1352 cells transfected with the Cbfa mutant vector (50 and 20%, respectively [data not shown]). No variations in the basal luciferase activity of both constructs were observed in transfected KHOS 321H cells. These results suggest that the availability of functional endogenous Cbfa1 is variable within different cell lines and could explain the observed differences in collagenase 3 expression or inducibility. Thus, those osteosarcoma cell lines able to constitutively express collagenase 3 contain available endogenous Cbfa1 activity, while in cells like KHOS 321H, this activity could be repressed by the formation of complexes with proteins that might inhibit its transcriptional activity (4, 74) or by a different ability of these cells to perform putative posttranslational modifications required for full activity of this factor.
FIG. 7.
Functional analysis in osteoblastic cell lines of the Cbfa element present in the collagenase 3 promoter. MC3T3 E1, U2OS, and KHOS 321H cells were cotransfected with the collagenase 3 promoter construct p1004-luc or p1004-mutCbfa-luc fused to firefly luciferase reporter gene and with pCMV-Cbfa1 (grey bars) or pcDNA3 (white bars) as an effector plasmid. Plasmid pRL-TK was used as an internal control of transfection efficiency as described in Materials and Methods. Values represent firefly luciferase-to-Renilla luciferase ratios, normalized so that a relative activity of 1 was assigned to the basal activity of the wild-type construct in every cell line. Data are expressed as means ± standard errors of at least three independent experiments. Asterisks indicate significant differences from the control (∗∗, P < 0.01; ∗∗∗, P < 0.001).
Reinforcing the implication of Cbfa1 in collagenase 3 expression, basal luciferase activity was almost 2 orders of magnitude higher in MC3T3 E1 cells than in U2OS cells. This correlates with results of the above transfection experiments as well as with the results of electrophoretic mobility shift assays showing that MC3T3 E1 nuclear extracts seem to contain more Cbfa binding activity (Fig. 5). Moreover, cotransfection experiments with exogenous Cbfa1 gave consistent results (Fig. 7). Activity of the wild-type collagenase 3 construct was stimulated in all Cbfa1-transfected cell lines but to different extents. Thus, MC3T3 E1 cells, having more endogenous Cbfa1 activity, showed less inducibility of the wild-type collagenase 3 promoter by overexpressed Cbfa1, while U2OS cells displayed a higher response. In any case, this stimulation of activity was not observed in cells transfected with the Cbfa mutant construct.
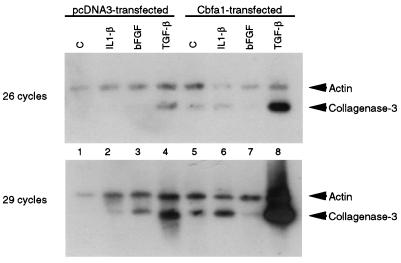
Finally, we examined whether overexpression of Cbfa1 into cells like MG-63, which do not produce significant amounts of this factor, followed by induction of the cells by cytokines or growth factors could affect expression of collagenase 3. Thus, we transiently transfected expression plasmid pCMV-Osf2/Cbfa1 into MG-63 cells and analyzed the ability of the transfected cells to express collagenase 3 mRNA. Total RNA from the transfected cells was prepared, and expression of collagenase 3 was studied by RT-PCR followed by Southern blot analysis in a semiquantitative assay. The results show that cells transiently transfected with a control plasmid (pcDNA3) expressed very low levels of collagenase 3 RNA, detectable only after stimulation with factors like TGF-β (Fig. 8, lanes 1 to 4). When cells were transfected with Cbfa1, collagenase 3 expression was also detected at low levels in control cells and after stimulation with IL-1β (lanes 5 to 7). By contrast, when cells transfected with the Cbfa1 plasmid were stimulated with TGF-β, a stronger band corresponding to collagenase 3 was detected (lane 8). This induction of Cbfa1-transfected cells by TGF-β was significantly (fourfold) higher than the effect on control MG-63 cells (lane 4), as measured in the semiquantitative assay. These results provide additional evidence that high levels of Cbfa1 favor expression of the collagenase 3 gene and also suggest that the presence of other factors such as TGF-β is required to achieve a full inducibility of the gene.
FIG. 8.
Effect of Cbfa1 and TGF-β on collagenase 3 expression in MG-63 osteosarcoma cells. Cells were transiently transfected with pcDNA3 (lanes 1 to 4) or pCMV-Osf2/Cbfa1 (lanes 5 to 8). Transfected cells were stimulated with vehicle alone, IL-1β (5 ng/ml), bFGF (5 ng/ml), or TGF-β (5 ng/ml) for 24 h. RNA from the stimulated transfected cells was further prepared and used for RT-PCR as described in Materials and Methods. Aliquots of samples were taken at 26 and 29 cycles of amplification. Samples were separated in agarose gel, transferred to nylon filters, and hybridized with collagenase 3 and actin probes. The results shown are from a representative experiment and were consistently reproducible in several independent experiments.
Analysis of collagenase 3 expression in mice deficient in Cbfa1.
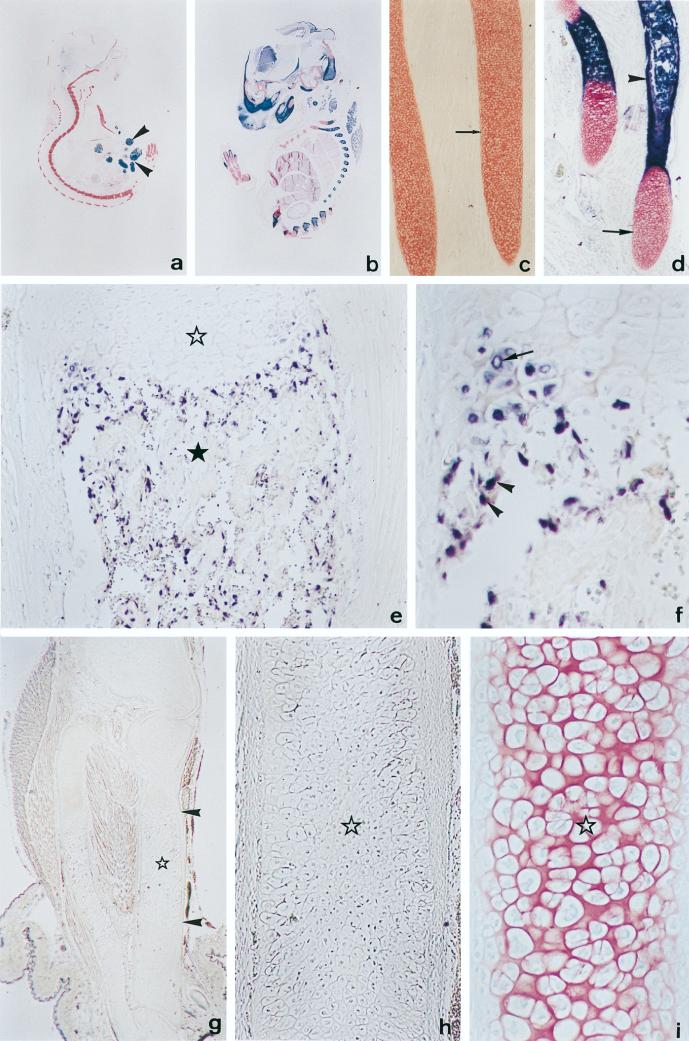
To examine the possibility that Cbfa1 influences the in vivo expression of collagenase 3, we analyzed the level of collagenase 3 transcripts by in situ hybridization on sections of late embryos (18.5pc) either from wild-type mice or from mice in which the Cbfa1 gene has been targeted (Fig. 9a to d). As previously reported (37, 53), wild-type embryos at this stage of development showed calcified bones in which the periosteal bud (blood vessels and perivascular mesenchyme) had entered at the middle of the cartilaginous template and formed the primary center of ossification (Fig. 9b and d). High levels of collagenase 3 transcripts were found in areas of endochondral and intramembranous bone formation. Labeling was restricted to osteoblastic cells localized along the newly formed trabeculae, hypertrophic chondrocytes found in the most distal portion of the epiphyses, and cells from the periosteal bud, likely of mesenchymal origin (Fig. 9e and f). Hybridization signal was not found in any other cell type. A similar expression pattern was found in 18.5-dpc heterozygous Cbfa1+/− embryos, although the intensity of signals was significantly lower (data not shown). By contrast, collagenase 3 transcripts were virtually absent in sections from homozygous embryos deficient in Cbfa1 (Fig. 9g and h), and only a very low number of scattered cells located near the periosteal bud showed weak specific signals. The virtual absence of collagenase 3 expression was coincident with a complete lack of ossification in these mutant mice (Fig. 9a and c). In addition, neither vascular nor mesenchymal cell invasion was observed in the calcified cartilage (Fig. 9g and h). Finally, Cbfa1-deficient mice exhibited hypertrophic chondrocytes (37) (Fig. 9i), which together with osteoblasts are the major cells producing collagenase 3 during fetal development (23, 30, 45, 70). Consequently, the absence of collagenase 3 production in these hypertrophic chondrocytes, which are cells with ability to produce Cbfa1 in normal mice (38), provides further in vivo support for the above results indicating that this gene is a transcriptional target of Cbfa1.
FIG. 9.
Collagenase 3 expression in Cbfa1-deficient mice. (a and b) Saggital sections of 18.5-dpc Cbfa1−/− (a) and wild-type (b) embryos showing alkaline phosphatase activity (dark blue) and stained with nuclear fast red. An intense alkaline phosphatase activity is observed in the skeletal tissues of the wild-type embryo. This activity is virtually absent in the skeletal tissues of the Cbfa1-deficient embryo, composed only of cartilage (stained in red), although it is present in epithelial cells from the small intestine (arrowheads). (c and d) Higher magnifications of ribs from embryos shown in panels a and b, respectively. In the wild-type embryo (d), cartilage templates are ossified at the central region, as revealed by an intense alkaline phosphatase activity (arrowhead), and show hyaline cartilage at the edges (arrow). By contrast, ribs from Cbfa1-deficient embryos (c) are devoid of alkaline phosphatase activity and appear mainly formed by hypertrophic chondrocytes. (e) In situ hybridization of a wild-type embryo with collagenase 3 antisense probe in a saggital section of the proximal half of the tibia. Labeling is found in both hypertrophic chondrocytes from the cartilage (white star) and osteoblastic cells localized along forming bone trabeculae present in the bone marrow cavity (black star). (f) Higher magnification of a region of the tibia from panel e, showing specific labeling in distal hypertrophic chondrocytes (arrow) and small cells from the periosteal collar (arrowheads). (g) In situ hybridization of Cbfa1-deficient embryos with collagenase 3 antisense probe. No specific signal is found, although long bones show a central part (arrowheads) occupied mainly by hypertrophic chondrocytes (star). (h) Higher magnification of the central region of the bone template shown in panel g. Hypertrophic chondrocytes are devoid of specific signal. (i) Parallel section of the bone presented in panel h stained with fast red and showing cells having morphological features of hypertrophic chondrocytes (star). Original magnifications: a and b, ×2.8; c and d, ×40; e, ×100; f, ×225; g, ×16; h and i, ×256.
DISCUSSION
In this work we have shown that collagenase 3, a metalloprotease overexpressed in malignant tumors and arthritic processes, is a target of Cbfa1, a transcriptional activator belonging to the runt domain gene family (32, 48, 69) that plays a major role in the process of bone formation (37, 53).
This study was originally aimed at analyzing the mechanisms controlling the expression of human collagenase 3 during fetal ossification, a physiological process in which this protease has been found to be produced at high levels (30, 70). The first indication that collagenase 3 expression could be induced by Cbfa1 was based on the finding of a Cbfa/NMP-2/OSE2 element, recognized and bound by this transcription factor, in the promoter region of this MMP gene (56). The functional relevance of the Cbfa element found in the collagenase 3 promoter was subsequently confirmed by several lines of evidence. Thus, cotransfection experiments with a Cbfa1 expression vector resulted in the transcriptional activation of all analyzed fragments of the collagenase 3 promoter containing the consensus Cbfa element. This transcriptional activity was completely abolished when point mutations were introduced in this Cbfa site of the collagenase 3 gene. In addition, introduction of multiple copies of this element upstream of the collagenase 3 promoter led to a high increase in the Cbfa1-induced transcriptional activity. Furthermore, gel mobility shift assay analysis with Cbfa oligonucleotides and nuclear extracts from Cbfa1-expressing cells revealed the formation of a specific protein-DNA complex, which was supershifted by antibodies against Cbfa1 and competed by an excess of oligonucleotides derived from the Cbfa element of the collagenase 3 promoter. Finally, overexpression of Cbfa1 in human osteoblastic cells without ability to produce collagenase 3, followed by TGF-β treatment, resulted in the expression of this metalloproteinase gene, thus suggesting that participation of other factors in addition to Cbfa1 may be necessary to achieve a full inducibility of this protease. In this regard, it is likely that the cooperative effect of these additional factors can be mediated through another promoter elements such as the AP-1 site, whose role in collagenase 3 inducibility in both human and murine tissues has been widely demonstrated (54, 56, 78). Nevertheless, it is also possible that some of these factors could act by increasing levels of Cbfa1 or by inducing posttranslational modifications of this transcriptional activator, which could result in an increased efficiency to induce collagenase 3 expression. Further studies will be necessary to clarify the precise mechanism by which other factors such as TGF-β contribute to enhance collagenase 3 expression in Cbfa1-producing cells. Also in relation to this question, recent studies have shown that PTH regulates the rat collagenase 3 promoter in osteoblastic cells through the cooperative interaction of an AP-1 site and the runt domain binding sequence present in this promoter (67). In our study on the human collagenase 3 promoter, we have shown that Cbfa and AP-1 sites can function independently since the activation of human collagenase 3 promoter constructs containing the Cbfa site by Cbfa1 was not diminished when the AP-1 site was mutated. However, we cannot exclude the possibility that a cooperative interaction is needed in vivo to achieve full collagenase 3 expression. Nevertheless, it is also possible that the minor structural differences between human and rat collagenase 3 promoters led to different properties in terms of regulatory mechanisms. In fact, there are numerous data indicating that the human and murine collagenase 3 genes are subjected to different regulatory controls, the human gene being more restricted in its expression in normal tissues (21, 30, 70). Finally, the possibility that the observed in vitro differences in activity of the two promoters were due to variations in the functional properties of the human and murine osteoblastic cell lines used in these studies cannot be ruled out.
In agreement with results of the cell culture experiments presented in this work, we have also provided evidence that mice deficient in Cbfa1 do not express significant amounts of collagenase 3. Recent studies have demonstrated that homozygous Cbfa1−/− mice show dwarfism and die soon after delivery due to respiratory failure, presumably caused by the inefficient functioning of the rib cage (37, 53). Analysis of the skeletal system of these mutant animals has revealed a complete lack of ossification in both membranous bones of the skull and endochondral bones of the rest of the body. They also exhibit retention of the partially calcified cartilaginous skeleton. Heterozygous Cbfa1+/− mice also show some skeletal abnormalities that recapitulate the phenotype of cleidocranial dysplasia, an autosomal-dominant skeletal disorder caused by mutations in Cbfa1 (40, 51). Detailed histochemical analysis of Cbfa1−/− mice has shown that both intramembranous and endochondral ossification processes are blocked as a consequence of the maturational arrest of osteoblastic cells. However, these mutant mice contain intact hypertrophic chondrocytes (37). Interestingly, mature osteoblasts and hypertrophic chondrocytes are the only cells expressing collagenase 3 during fetal development in both human and murine tissues (23, 30, 45, 70). In addition, both cell types have the ability to produce Cbfa1 (38). Therefore, and although the absence of this protease in Cbfa1-deficient mice could be explained in part by the fact that these animals do not contain mature osteoblasts, its absence in hypertrophic chondrocytes from Cbfa1−/− mice provides evidence for a role of this factor in the transcriptional activation of collagenase 3 in these cells. These results also support the idea that Cbfa1 may also mediate responses in cells distinct from osteoblasts, which have been demonstrated to be the major targets of this factor (15, 17, 37).
Previous studies have reported that Cbfa1−/− animals have a marked reduction of expression of different noncollagenous bone matrix proteins, such as osteocalcin and osteopontin, which also contain Cbfa1 binding elements in their gene promoter regions (17). These bone matrix proteins have been proposed to play different roles during osteogenesis. Thus, osteocalcin appears to control bone matrix deposition by slowing down the anabolic responses of osteoblasts (16), whereas osteopontin is thought to promote the attachment of these cells to the extracellular matrix (50, 60). However, our finding that Cbfa1 mutant embryos also lack a proteolytic enzyme such as collagenase 3 suggests that this protease may serve a distinct and specific role during skeletal development. It is well known that bone formation and remodeling are a highly coordinated process which involves a series of successive events of cell proliferation and differentiation, extracellular matrix destruction and turnover, angiogenesis, and apoptosis (18, 62, 71). Collagenase 3 may play important roles in several of these highly regulated events. A likely possibility in the context of osteogenesis is that collagenase 3 can degrade different matrix components of the bone anlage in order to initiate the formation of mature bone. Consistent with this possibility, we and others have provided evidence that collagenase 3 is a potent protease capable of degrading an exceptionally wide range of collagenous and noncollagenous components of the extracellular matrix (19, 21, 33–35, 49). In addition to this direct role in bone matrix degradation, collagenase 3 could regulate the availability and/or activity of bone growth factors, through releasing factors sequestered as inactive molecules in the matrix or by degrading their binding proteins, as demonstrated in the case of insulin-like growth factor binding proteins expressed by skeletal cells and susceptible to the proteolytic action of diverse metalloproteinases (31, 75). In this regard, it is of interest that collagenase 3 also has the ability to degrade perlecan, leading to the release of bFGF stored in the extracellular matrix through binding to the heparan sulfate chains of this proteoglycan (82). The down-regulation of collagenase 3 expression in Cbfa1-deficient embryos would hamper all of these proteolytic processes occurring during the cartilage bone transition and would explain at least in part the fact that these mutant animals retain a calcified cartilagenous skeleton without exhibiting any evidence of bone formation.
Another plausible role of collagenase 3 during bone formation could be related to the matrix-invasive process occurring after cartilage calcification. Thus, during the development of long bones in mammals, subperiosteal bone is formed around calcified cartilage before the formation of bone marrow. Osteogenic cells and blood capillaries then invade from the periosteal region into the calcified cartilage to form endochondral bone and the bone marrow cavity (18). This invasive process is somewhat reminiscent of those taking place during the invasion and metastasis of tumor cells in which diverse MMPs, including collagenase 3, appear to play essential roles (43, 72, 76). The absence of this proteolytic enzyme in Cbfa1−/− mice may explain the observation that neither vascular nor mesenchymal cell invasion was observed in the calcified cartilage of these mutant embryos. Finally, it must be taken into account that osteogenesis involves not only the deposition of newly formed bone but also the resorption of existing bone as embryonic bone matures into lamellar bone. This process first requires the degradation of the nonmineralized osteoid layer covering bone surfaces by the action of proteases secreted by osteoblasts. This proteolytic activity leads to exposure of the underlying mineralized matrix which is subsequently degraded by osteoclastic cells (22, 46). Since collagenase 3 is produced by osteoblastic cells but not by osteoclasts, Cbfa1-mediated induction of collagenase 3 expression in fully differentiated osteoblasts could be a critical step in the initiation of the resorptive process, acting in concert with the subsequent participation of an osteoclastic protease like gelatinase B or cathepsin K (58, 61). In this regard, of interest is the recent finding that collagenase-3 is an activator of progelatinase B, which should be consistent with the possibility that these enzymes can form a proteolytic cascade in vivo during bone remodeling processes (36). The participation of gelatinase B in these processes is underlined by recent findings showing an abnormal pattern of skeletal growth plate vascularization and ossification in animals deficient in this protease (80). In addition to a putative direct action of collagenase 3 on the removal of type I collagen of the osteoid layer, this protease could also indirectly participate in the process through the release of collagen fragments from the calcified cartilage which, after diffusion to the bone collar would act as chemoattractant for the preosteoclasts (10, 63). Consistent with the participation of collagenase 3 in the resorptive process, a number of studies have reported that this enzyme is strongly induced by bone-resorbing agents such as PTH and IL-6 in diverse in vitro systems, including osteoblastic cell lines and mouse calvarial osteoblasts (20, 39, 54). Further studies will be required to elucidate the participation of these agents in the context of factors such as Cbfa1, which according to data presented in this report are necessary for the transcriptional induction of collagenase 3 in bone-forming cells. Finally, ongoing work directed to create mutant animals in which the collagenase 3 gene has been inactivated by homologous recombination will be essential to determine the precise role of this enzyme during bone formation and remodeling.
ACKNOWLEDGMENTS
M. J. G. Jiménez and M. Balbín contributed equally to this work.
We thank J. Rey, I. Santamaría, A. M. Pendás, J. P. Freije, S. Cal, and G. Velasco for helpful comments; Y. Ito (Kyoto University, Kyoto, Japan), J. Kimura (Henry Ford Hospital, Detroit, Mich.), and G. Karsenty (University of Texas M. D. Anderson Cancer Center) for kindly providing antibodies, cells, and plasmids; and S. Alvarez for excellent technical support.
This work was supported by grants from Comisión Interministerial de Ciencia y Tecnología (SAF97-0258), EU-BIOMED II (BMH4-CT96-0017), and Glaxo-Wellcome, Spain.
REFERENCES
- 1.Airola K, Johansson N, Kariniemi A L, Kähäri V M, Saarialho-Kere U K. Human collagenase-3 is expressed in malignant squamous epithelium of the skin. J Investig Dermatol. 1997;109:225–231. doi: 10.1111/1523-1747.ep12319441. [DOI] [PubMed] [Google Scholar]
- 2.Angel P, Baumann I, Stein B, Delius H, Rahmsdorf H J, Herrlich P. 12-O-Tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5′-flanking region. Mol Cell Biol. 1987;7:2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anglard P, Melot T, Guérin E, Thomas G, Basset P. Structure and promoter characterization of the human stromelysin-3 gene. J Biol Chem. 1995;270:20337–20344. doi: 10.1074/jbc.270.35.20337. [DOI] [PubMed] [Google Scholar]
- 4.Aronson B D, Fisher A L, Blechman K, Caudy M, Gergen J P. Groucho-dependent and -independent repression activities of Runt domain proteins. Mol Cell Biol. 1997;17:5581–5587. doi: 10.1128/mcb.17.9.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balbín M, López-Otín C. Hormonal regulation of the human pepsinogen C gene in breast cancer cells: identification of a cis-acting element mediating its induction by androgens, glucocorticoids, and progesterone. J Biol Chem. 1996;271:15175–15181. doi: 10.1074/jbc.271.25.15175. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee C, Hiebert S W, Stein J L, Lian J B, Stein G S. An AML-1 consensus sequence binds an osteoblast-specific complex and transcriptionally activates the osteocalcin gene. Proc Natl Acad Sci USA. 1996;93:4968–4973. doi: 10.1073/pnas.93.10.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee C, McCabe L R, Choi J Y, Hiebert S W, Stein J L, Stein G S, Lian J B. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem. 1997;66:1–8. doi: 10.1002/(sici)1097-4644(19970701)66:1<1::aid-jcb1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Bidwell J P, van Wijnen A J, Fey E G, Dworetzky S, Penman S, Stein J L, Lian J B, Stein G S. Osteocalcin gene promoter-binding factors are tissue-specific nuclear matrix components. Proc Natl Acad Sci USA. 1993;90:3162–3166. doi: 10.1073/pnas.90.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkedal-Hansen H, Moore W G Y, Bodden M K, Windsor L J, Birkedal-Hansen B, DeCarlo A, Engler J A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 10.Blavier L, Delaissé J M. Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J Cell Sci. 1995;108:3649–3659. doi: 10.1242/jcs.108.12.3649. [DOI] [PubMed] [Google Scholar]
- 11.Borden P, Solymar D, Sucharczuk A, Lindman B, Cannon P, Heller R A. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J Biol Chem. 1996;271:23577–23581. doi: 10.1074/jbc.271.38.23577. [DOI] [PubMed] [Google Scholar]
- 12.Braissant O, Wahli W. A simplified in situ hybridization protocol using non-radioactively labelled probes to detect abundant and rare mRNAs on tissue sections. Biochemica (Boehringer Mannheim) 1998;1:10–16. [Google Scholar]
- 13.Cazorla M, Hernández L, Nadal A, Balbín M, López J M, Vizoso F, Fernández P L, Iwata K, Cardesa A, López-Otín C, Campo E. Collagenase-3 overexpression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. J Pathol. 1998;186:144–150. doi: 10.1002/(SICI)1096-9896(1998100)186:2<144::AID-PATH147>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol. 1995;15:1858–1869. doi: 10.1128/mcb.15.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 17.Ducy P, Zhang R, Geoffroy V, Ridall A L, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 18.Erlebacher A, Filvaroff E H, Gitelman S E, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995;80:371–378. doi: 10.1016/0092-8674(95)90487-5. [DOI] [PubMed] [Google Scholar]
- 19.Fosang A J, Last K, Knäuper V, Murphy G, Neame P J. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 20.Franchimont N, Rydziel S, Delany A M, Canalis E. Interleukin-6 and its soluble receptor cause a marked induction of collagenase-3 expression in rat osteoblast cultures. J Biol Chem. 1997;272:12144–12150. doi: 10.1074/jbc.272.18.12144. [DOI] [PubMed] [Google Scholar]
- 21.Freije J P, Diez-Itza I, Balbín M, Sánchez L M, Blasco R, Tolivia J, López-Otín C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269:16766–16773. [PubMed] [Google Scholar]
- 22.Fuller K, Chambers T J. Localisation of mRNA for collagenase in osteocytic, bone surface and chondrocytic cells but not osteoclasts. J Cell Sci. 1995;108:2221–2230. doi: 10.1242/jcs.108.6.2221. [DOI] [PubMed] [Google Scholar]
- 23.Gack S, Vallon R, Schmidt J, Grigoriadis A, Tuckermann J, Schenkel J, Weiher H, Wagner E F, Angel P. Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ. 1995;6:759–767. [PubMed] [Google Scholar]
- 24.Gutman A, Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henney A M, Wakeley P R, Davies M J, Foster K, Hembry R, Murphy G, Humphries S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci USA. 1991;88:8154–8158. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heppner K J, Matrisian L M, Jensen R A, Rodgers W H. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol. 1996;149:273–282. [PMC free article] [PubMed] [Google Scholar]
- 27.Huhtala P, Chow L T, Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990;265:11077–11082. [PubMed] [Google Scholar]
- 28.Huhtala P, Tuuttila A, Chow L T, Lohi J, Keski-Oja J, Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase: divergent regulation of expression for the 92- and 72-kDa enzyme genes in HT-1080 cells. J Biol Chem. 1991;266:16485–16490. [PubMed] [Google Scholar]
- 29.Johansson N, Airola K, Grénman R, Kariniemi A L, Saarialho-Kere U, Kähäri V K. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol. 1997;151:499–508. [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson N, Saarialho-Kere U, Airola K, Herva R, Nissinen L, Westermarck J, Vuorio E, Heino J, Kähäri V M. Collagenase-3 is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during fetal bone development. Dev Dyn. 1997;208:387–397. doi: 10.1002/(SICI)1097-0177(199703)208:3<387::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31.Jones J I, Clemmons D R. Insulin-like growth factors and their binding proteins: biological actions. Endocrine Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 32.Kagoshima H, Shigesada K, Satake M, Ito Y, Miyoshi H, Ohki M, Pepling M, Gergen P. The Runt domain identifies a new family of heteromeric transcriptional regulators. Trends Genet. 1993;9:338–341. doi: 10.1016/0168-9525(93)90026-e. [DOI] [PubMed] [Google Scholar]
- 33.Knäuper V, López-Otín C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 34.Knäuper V, Will H, López-Otín C, Smith B, Atkinson S J, Stanton H, Hembry R, Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation: evidence that MT1-MMP (MMP-14) and gelatinase A (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271:17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 35.Knäuper V, Cowell S, Smith B, López-Otín C, O’Shea M, Morris H, Zardi L, Murphy G. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem. 1997;272:17124–17131. doi: 10.1074/jbc.272.12.7608. [DOI] [PubMed] [Google Scholar]
- 36.Knäuper V, Smith B, López-Otín C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13) Eur J Biochem. 1997;248:369–373. doi: 10.1111/j.1432-1033.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- 37.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson R T, Gao Y H, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 38.Komori, T. Unpublished results.
- 39.Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, Kamoi K, Suda T. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology. 1998;139:1338–1345. doi: 10.1210/endo.139.3.5818. [DOI] [PubMed] [Google Scholar]
- 40.Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, Geoffroy V, Ducy P, Karsenty G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 41.Lindy O, Konttinen Y T, Sorsa T, Ding Y, Santavirta S, Ceponis A, López-Otín C. MMP-13 (collagenase-3) in human rheumatoid synovium. Arthritis Rheum. 1997;40:1391–1399. doi: 10.1002/art.1780400806. [DOI] [PubMed] [Google Scholar]
- 42.Lu J, Maruyama M, Satake M, Bae S C, Ogawa E, Kagoshima H, Shigesada K, Ito Y. Subcellular localization of the α and β subunits of the acute myeloid leukemia-linked transcription factor PEBP2/CBF. Mol Cell Biol. 1995;15:1651–1661. doi: 10.1128/mcb.15.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDougall J R, Matrisian L M. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995;14:351–362. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- 44.Matrisian L M. The matrix degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 45.Mattot V, Raes M B, Henriet P, Eeckhout Y, Stehelin D, Vandenbunder B, Desbiens X. Expression of interstitial collagenase is restricted to skeletal tissue during mouse embryogenesis. J Cell Sci. 1995;108:529–535. doi: 10.1242/jcs.108.2.529. [DOI] [PubMed] [Google Scholar]
- 46.Meikle M C, Bord S, Hembry R M, Reynolds J J. The synthesis of collagenase, gelatinase-A (72 kDa) and -B (92 kDa), and TIMP-1 and -2 by human osteoblast from normal and arthritic bone. Bone. 1995;17:255–260. doi: 10.1016/8756-3282(95)00219-4. [DOI] [PubMed] [Google Scholar]
- 47.Merriman H L, van Wijnen A J, Hiebert S, Bidwell J P, Fey E, Lian J, Stein J, Stein G S. The tissue-specific nuclear matrix protein, NMP-2, is a member of the AML/CBF/PEBP2/runt domain transcription factor family: interactions with the osteocalcin gene promoter. Biochemistry. 1995;34:13125–13132. doi: 10.1021/bi00040a025. [DOI] [PubMed] [Google Scholar]
- 48.Meyers S, Downing J R, Hiebert S W. Identification of AML-1 and the (8;21) translocation protein (AML-1/ETO) as sequence-specific DNA-binding proteins: the runt homology domain is required for DNA binding and protein-protein interactions. Mol Cell Biol. 1993;13:6336–6345. doi: 10.1128/mcb.13.10.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell P G, Magna H A, Reeves L M, Lopresti-Morrow L L, Yocum S A, Rosner P J, Geoghegan K F, Hambor J E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Investig. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyauchi A, Alvarez J, Greenfield E M, Teti A, Grano M, Colucci S, Zembonin-Zallone A, Ross F P, Teitelbaum S L, Cheresh D, Hruska K A. Recognition of osteopontin and related peptides by an αvβ3 integrin stimulates inmediate cell signals in osteoblasts. J Biol Chem. 1991;266:20369–20374. [PubMed] [Google Scholar]
- 51.Mundlos S, Otto F, Mundlos C, Mulliken J B, Aylsworth A S, Albright S, Lindhout D, Cole W G, Henn W, Knoll J H M, Owen M J, Mertelsmann R, Zabel B U, Olsen B R. Mutations involving the transcription factor Cbfa1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 52.Murphy G, Hembry R M. The matrix metalloproteinases and their inhibitors. J Rheumatol. 1992;19:61–64. [Google Scholar]
- 53.Otto F, Thornell A P, Crompton T, Denzel A, Gilmour K C, Rosewell I R, Stamp G W H, Beddington R S P, Mundlos S, Olsen B R, Selby P B, Owen M J. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 54.Partridge N, Walling H W, Bloch S R, Omura T H, Chan P T, Pearman A T, Chou W Y. The regulation and regulatory role of collagenase in bone. Crit Rev Eukaryot Gene Expr. 1996;6:15–27. doi: 10.1615/critreveukargeneexpr.v6.i1.20. [DOI] [PubMed] [Google Scholar]
- 55.Pendás A M, Matilla T, Estivill X, López-Otín C. The human collagenase-3 (CLG3) gene is located on chromosome 11q22.3 clustered to other members of the matrix metalloproteinase gene family. Genomics. 1995;26:615–618. doi: 10.1016/0888-7543(95)80186-p. [DOI] [PubMed] [Google Scholar]
- 56.Pendás A M, Balbín M, Llano E, Jiménez M G, López-Otín C. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP-13) Genomics. 1997;40:222–233. doi: 10.1006/geno.1996.4554. [DOI] [PubMed] [Google Scholar]
- 57.Rajakumar R A, Quinn C O. Parathyroid hormone induction of rat interstitial collagenase mRNA in osteosarcoma cells is mediated through an AP-1 binding site. Mol Endocrinol. 1996;10:867–878. doi: 10.1210/mend.10.7.8813727. [DOI] [PubMed] [Google Scholar]
- 58.Rantakokko J, Aro H T, Savontaus M, Vuorio E. Mouse cathepsin K: cDNA cloning and predominant expression of the gene in osteoclasts, and in some hypertrophying chondrocytes during mouse development. FEBS Lett. 1996;393:307–313. doi: 10.1016/0014-5793(96)00907-6. [DOI] [PubMed] [Google Scholar]
- 59.Reboul P, Pelletier J P, Tardif G, Cloutier J M, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. J Clin Investig. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reinholt F P, Hultenby K, Olberg A, Heinegard D. Osteopontin: a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reponen P, Sahlberg C, Munaut C, Thesleff I, Tryggvason K. High expression of 92 kDa type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol. 1994;124:1091–1102. doi: 10.1083/jcb.124.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roach H I, Erenpreisa J, Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 1995;131:483–494. doi: 10.1083/jcb.131.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakamoto S, Sakamoto M. Biochemical and immunohistochemical studies on collagenase in resorbing bone in tissue culture. J Periodontal Res. 1982;17:523–526. doi: 10.1111/j.1600-0765.1982.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 64.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 65.Schorpp M, Mattei M G, Herr I, Gack S, Schaper J, Angel P. Structural organization and chromosomal localization of the mouse collagenase type I gene. Biochem J. 1995;308:211–217. doi: 10.1042/bj3080211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schreiber E, Matthias P, Müller M M, Schaffner W. Rapid detection of octamer binding proteins with “mini extracts” prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selvamurugan N, Chou W Y, Pearman A T, Pulumati M R, Partridge N C. Parathyroid hormone regulates the rat collagenase-3 promoter in osteoblastic cells through the cooperative interaction of the activator protein-1 site and the runt domain binding sequence. J Biol Chem. 1998;273:10647–10657. doi: 10.1074/jbc.273.17.10647. [DOI] [PubMed] [Google Scholar]
- 68.Sirum K L, Brinckerhoff C E. Cloning of the genes for human stromelysin and stromelysin 2: differential expression in rheumatoid synovial fibroblasts. Biochemistry. 1989;28:8691–8698. doi: 10.1021/bi00448a004. [DOI] [PubMed] [Google Scholar]
- 69.Speck N A, Stacy T. A new transcription factor family associated with human leukemias. Crit Rev Eukaryot Gene Expr. 1995;5:337–364. doi: 10.1615/critreveukargeneexpr.v5.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 70.Stahle-Bäckdahl M, Sandsted B, Bruce K, Lindahl A, Jiménez M G, Vega J A, López-Otín C. Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab Investig. 1997;76:717–728. [PubMed] [Google Scholar]
- 71.Stein G S, Lian J B, Stein J L, van Wijnen A J, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol Rev. 1996;76:593–629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- 72.Stetler-Stevenson W G, Aznavoorian S, Liotta L A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 73.Stewart M, Terry A, Hu M, O’Hara M, Blyth K, Baxter E, Cameron E, Onions D E, Neil J C. Proviral insertions induce the expression of bone-specific isoforms of PEBP2αA (CBFA1): evidence for a new myc collaborating oncogene. Proc Natl Acad Sci USA. 1997;94:8646–8651. doi: 10.1073/pnas.94.16.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thirunavukkarasu K, Mahajan M, McLarren K W, Stifani S, Karsenty G. Two domains unique to osteoblast-specific transcription factor Osf2/Cbfa1 contribute to its transactivation function and its inability to heterodimerize with Cbfβ. Mol Cell Biol. 1998;18:4197–4208. doi: 10.1128/mcb.18.7.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thrailkill K M, Quarles L D, Nagase H, Suzuki K, Serra D M, Fowlkes J L. Characterization of insulin-like growth factor-binding protein 5-degrading proteases produced throughout murine osteoblast differentiation. Endocrinology. 1995;136:3527–3533. doi: 10.1210/endo.136.8.7543045. [DOI] [PubMed] [Google Scholar]
- 76.Uría J A, Stahle-Bäckdahl M, Seiki M, Fueyo A, López-Otín C. Regulation of collagenase-3 expression in human breast carcinomas is mediated by stromal-epithelial cell interactions. Cancer Res. 1997;57:4882–4888. [PubMed] [Google Scholar]
- 77.Uría J A, Balbín M, López J M, Álvarez J, Vizoso F, Takigawa M, López-Otín C. Collagenase-3 (MMP-13) expression in chondrosarcoma cells and its regulation by basic fibroblast growth factor. Am J Pathol. 1998;153:91–101. doi: 10.1016/S0002-9440(10)65549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uría J A, Jiménez M G, Balbín M, Freije J M P, López-Otín C. Differential effects of TGF-β on the expression of collagenase-1 and collagenase-3 in human fibroblasts. J Biol Chem. 1998;273:9769–9777. doi: 10.1074/jbc.273.16.9769. [DOI] [PubMed] [Google Scholar]
- 79.Vincenti M P, Coon C I, Mengshol J A, Yocum S, Mitchell P, Brinckerhoff C E. Cloning of the gene for interstitial collagenase-3 (matrix metalloproteinase-13) from rabbit synovial fibroblasts: differential expression with collagenase-1 (matrix metalloproteinase-1) Biochem J. 1998;331:341–346. doi: 10.1042/bj3310341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vu T H, Shipley J M, Bergers G, Beger J E, Helms J A, Hanahan D, Shapiro S D, Senior R M, Werb Z. Matrix metalloproteinase gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 82.Whitelock J M, Murdoch A D, Iozzo R V, Underwood P A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 83.Xiao Z S, Thomas R, Hinson T K, Quarles L D. Genomic structure and isoform expression of the mouse, rat, and human Cbfa1/Osf2 transcription factor. Gene. 1998;214:187–197. doi: 10.1016/s0378-1119(98)00227-3. [DOI] [PubMed] [Google Scholar]