Abstract
Erythropoietin (Epo) is a major regulator of erythropoiesis that alters the survival, proliferation, and differentiation of erythroid progenitor cells. The mechanism by which these events are regulated has not yet been determined. Using HB60, a newly established erythroblastic cell line, we show here that Epo-induced terminal erythroid differentiation is associated with a transient downregulation in the expression of the Ets-related transcription factor Fli-1. Constitutive expression of Fli-1 in HB60 cells, similar to retroviral insertional activation of Fli-1 observed in Friend murine leukemia virus (F-MuLV)-induced erythroleukemia, blocks Epo-induced differentiation while promoting Epo-induced proliferation. These results suggest that Fli-1 modulates the response of erythroid cells to Epo. To understand the mechanism by which Fli-1 regulates erythropoiesis, we searched for downstream target genes whose expression is regulated by this transcription factor. Here we show that the retinoblastoma (Rb) gene, which was previously shown to be involved in the development of mature erythrocytes, contains a Fli-1 consensus binding site within its promoter. Fli-1 binds to this cryptic Ets consensus site within the Rb promoter and transcriptionally represses Rb expression. Both the expression level and the phosphorylation status of Rb are consistent with the response of HB60 cells to Epo-induced terminal differentiation. We suggest that the negative regulation of Rb by Fli-1 could be one of the critical determinants in erythroid progenitor cell differentiation that is specifically deregulated during F-MuLV-induced erythroleukemia.
Genetic and biochemical studies of mature erythrocytes and their immediate progenitors have led to the identification of a number of important intracellular and extracellular factors that determine the fate of erythroid progenitor cells, namely, whether to proliferate (self-renew), differentiate, or die (apoptosis). Hematopoietic growth factors and transcription factors have been the more thoroughly characterized regulators of erythropoiesis. For example, erythropoietin (Epo), a low-molecular-weight glycoprotein hormone, is vital to adult definitive erythropoiesis (27, 37, 76). The intracellular signaling initiated by the binding of Epo to the Epo receptor (Epo-R) promotes either a mitogenic or a differentiation response (36, 77), although the mechanism by which these responses are mediated remains unknown.
Another important signaling pathway essential to proper erythroid development is defined by c-Kit and its ligand stem cell factor (SCF; also known as Steel factor). The importance of SCF/c-Kit to erythroid development is clearly evident from the study of W (White spotting) and Sl (Steel locus) mutant mice. These mice exhibit erythroid and other lineage-specific defects due to inherited mutations within the c-kit and SCF genes, respectively (56). In addition to these proximal signaling components, several nuclear factors (NFs), specifically DNA binding transcription factors that regulate erythroid cell-specific gene expression, have been intensively pursued. Both erythroid cell-specific factors and the widely expressed NFs have been shown to profoundly influence erythroid development (65). This has been aptly demonstrated in genetically engineered mouse strains where expected and unexpected determinants of erythroid differentiation have been identified. NFs that have been knocked out in mice and that generate discernible erythroid cell-specific defects include c-Myb (51), GATA-1 (58), EKLF (57), and Rb, the product of the retinoblastoma (Rb) tumor suppressor gene (7, 23, 35).
Targeted disruption of Rb delays erythroid maturation (7, 23, 35). Although a cell-autonomous role of Rb in erythroid differentiation was not observed in Rb−/−:Rb+/+ chimeric mice (39, 74), development of erythropoiesis in mice transplanted with Rb−/− fetal liver cells is impaired (22). The continuous presence of nucleated Rb−/− erythrocytes in the peripheral blood and extensive extramedullary erythropoiesis indicate that Rb is required for erythropoiesis.
Other lines of evidence in support of Rb’s involvement in erythropoiesis include studies with murine erythroleukemia cell lines derived from the spleens of mice infected with Friend virus (FV). These cell lines undergo an Epo-like differentiation program in response to polar compounds such as dimethyl sulfoxide and hexamethylene bisacetamide (HMBA) (13, 43). Treatment of erythroleukemia cells with HMBA induces a dramatic decrease in the expression of cdk4 and subsequent dephosphorylation of Rb (28). This response is blocked when these erythroleukemic cells ectopically overexpress cdk4, which subsequently blocks erythroid differentiation. Therefore, it appears that the ability of Rb to trigger erythroid differentiation is coupled to its negative effects on cell cycle progression (63). However, the ability of these polar compounds to induce erythroid differentiation is not a universal feature of erythroleukemia cell lines (64). Moreover, the activity of these cell cycle components, particularly Rb, has not been examined with respect to the primary oncogenic events responsible for FV-induced erythroleukemia.
Over the past decade, the role of various oncogenes and tumor suppressor genes during clonal transformation of erythroleukemia by FV has been studied in detail. Interestingly, the tumor suppressor gene p53 was shown to be inactivated in almost all erythroleukemia cell lines induced by various strains of FV (3, 18, 50, 52). In addition, retroviral insertional activation of genes for two members of the Ets family of transcription factors, Spi-1/PU.1 and Fli-1, has been identified in FV-induced erythroleukemia. The involvement of these two Ets-related transcription factors in Friend erythroleukemia is strictly dependent on the particular strain of FV used to induce the disease. Specifically, Fli-1 is activated during Friend murine leukemia virus (F-MuLV)-induced erythroleukemia, while Spi-1/PU.1 is activated during anemia (FV-A) or polychythemia (FV-P) FV-induced erythroleukemia (2, 15, 49). Recent studies have indicated that insertional activation of Fli-1 is the first detectable genetic alteration in F-MuLV-induced primary erythroleukemia and appears to alter the self-renewal properties of erythroid progenitor cells (21). Interestingly, Fli-1 is also activated in Ewing’s sarcoma as the result of a chromosomal translocation that creates a novel fusion protein in which the DNA-binding domain of Fli-1 is fused to a putative RNA-binding protein (EWS) from chromosome 22 (9).
In this study, we set out to determine how Fli-1 alters the self-renewal potential of erythroid progenitor cells. We utilized a novel erythroleukemic cell line, designated HB60-5, that has acquired an insertionally activated Spi-1 but contains a normal Fli-1 allele. Similar to erythroblasts, HB60-5 cells are capable of undergoing terminal differentiation in response to Epo. We show that the levels of endogenous Fli-1 expression modulate the response of HB60-5 cells to Epo. A dramatic but transient decrease in the expression of Fli-1 allows these cells to undergo cell cycle arrest and terminal differentiation. These results suggest that alterations in the levels of Fli-1 expression constitute an important molecular switch that commits HB60 cells to an irreversible program of Epo-induced terminal differentiation. We also demonstrate that Fli-1 binds to the Rb promoter and suppresses its transcription. In this respect, regulation of the Rb gene by the Fli-1 protein could constitute one of the pathways by which this transcription factor inhibits erythroid differentiation in transformed cells.
MATERIALS AND METHODS
Tumors and cell lines.
The erythroleukemic cell lines CB3 and CB7 were derived from methylcellulose colonies from the greatly enlarged spleens of BALB/c mice injected at birth with F-MuLV (64). The erythroleukemia cell lines DP16-1 and DP27-17 were derived from methylcellulose colonies of spleen cells from DBA/2J adult mice injected with FV-P (3, 50). Cells were maintained in alpha minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS).
Primary erythroleukemias were induced following injection of BALB/c mice at birth with clone 57 of F-MuLV helper virus as described previously (21). To establish cell lines from these tumors, erythroleukemic cells from tumor HB60-t were cultured in α-MEM supplemented with 15% FBS, 1 U of Epo (Boehringer) per ml, and 100 mg of SCF per ml. After several days of culture in the presence of Epo and SCF, a small population of the HB60 splenic tumor cells survived and proliferated in the presence of 20% FBS. While these cells grow slowly under this condition, a rare and fast-growing population of these cells had emerged after approximately 2 months in culture. The HB60 cells were cloned by limited dilution, and the clone HB60-5 was used in further studies. To induce differentiation, HB60-5 cells were washed twice with phosphate-buffered saline (PBS) and incubated in the presence of 15% FBS and 0.1 U of Epo per ml.
Tumor DNA and molecular hybridization.
High-molecular-weight DNA was isolated from tumor tissues by a modification of the proteinase K-phenol-chloroform method of Gross-Bellard et al. (15a) as described elsewhere (50). DNA was digested with restriction enzymes and electrophoresed on agarose gels. The DNA was acid depurinated before denaturation and transferred to nitrocellulose filters. The filters were hybridized with 2 × 106 cpm of random-primed probe as previously described (3).
DNA probes.
The NF-E2 p45 probe is an EcoRI cDNA fragment derived from Fli-2 locus (38). The F-MuLV envelope probe is a 830-bp BamHI fragment derived from plasmid pHC6 (6). The Rb probe, which corresponds to the C-terminal end of the Rb cDNA, is a 1.3-kbp PstI fragment derived from plasmid pECE-ΔBX-HA (17). The Spi-1 probe A is a 1-kbp PstI fragment, described elsewhere (49). The 750-bp PstI/XbaI fragment of mouse GAPDH cDNA was used to check the amount of RNA loaded. The Spi-1/PU.1 cDNA is a 1.2-kbp fragment of plasmid Spi-5. The GATA-1 probe (a gift of Hagop Youssoufian) was excised from plasmid pXM by XhoI digestion (71). All DNA probes were free of plasmid sequences, gel purified, and labeled with [α-32P]dCTP by random priming (12).
Expression vectors.
The SV40-Fli-1 vector was constructed by cloning a 1.7 kbp Fli-1 cDNA fragment into the EcoRI site of the pECE vector. The CMV-Fli-1 vectors were constructed by cloning the EcoRI 1.7-kbp Fli-1 cDNA in either orientation (sense or antisense) into the EcoRI site of the cytomegalovirus (CMV) expression vectors (Invitrogen). The pmRbmg vector was generated by cloning the 1.3-kbp mouse Rb promoter and part of exon 1 (78) upstream of the 2.7-kbp Rb cDNA plus simian virus 40 (SV40) poly(A) signal. The SV40-Fli-ΔEBD construct was generated by removing the 0.4-kbp NcoI fragment of Fli-1 from the SV40-Fli-1 vector.
RNA extraction and Northern blotting.
Total cellular RNA from cultured cells was isolated by using TRIzol reagent as described by the supplier (Gibco BRL) and used for poly(A)+ mRNA isolation (Pharmacia). Twenty micrograms of total RNA was dissolved in 2.2 M formaldehyde, denatured at 65°C for 5 min, and electrophoresed in a 1% agarose gel containing 0.66 M formaldehyde. After transfer to nylon membranes (Zetaprobe; Bio-Rad Laboratories), the filters were hybridized with 2 × 106 cpm of [α-32P]dCTP-labeled probes per ml.
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts from the erythroleukemic cell lines CB3, CB7, and DP16-1 (107 cells) were prepared as described previously (1). Protein concentration was determined by Bio-Rad protein assay. In some experiments, the bacterially expressed glutathione S-transferase (GST) GST–Fli-1, and GST–Spi-1 proteins were used as described previously (79). The sequences of the sense strands of synthetic oligonucleotides are 5′-AATAACCGGAAGTAACTC-3′ (E74), 5′-TGAGCGCGGGCGGAAGTGACGTTTTCCCGCGG-3′ (Rb), and 5′-TGAGCGCGGGCGGTTGTGACGTTTTCCCGCGG-3′ (Rb mutant). Fifty nanogram-aliquots of single-stranded oligonucleotides were labeled at their 5′ ends with T4 polynucleotide kinase (New England) and [γ-32P]ATP. The labeled single-stranded oligonucleotides were purified by passage through G-50 columns. They were then annealed with a twofold excess of unlabeled (cold) complementary oligonucleotides by boiling for 2 min and cooling slowly to room temperature.
Fli-1–DNA binding reactions were performed in a 10-μl volume containing 1 to 4 μl of nuclear extracts or 1 to 5 μl of bacterially expressed proteins in a mixture of binding buffer (20 mM HEPES [pH 7.9], 1 mM EDTA, 70 mM KCl, 6 mM MgCl2, 1 mM dithiothreitol, 10% glycerol), 1 mg of poly(dI-dC), and 0.1 to 0.5 ng of γ-32P-end-labeled oligonucleotide probes. Reaction mixtures were incubated at room temperature for 25 min. Samples were resolved by electrophoresis on 5% polyacrylamide gels in 0.25× Tris-borate-EDTA buffer at room temperature at 150 V; the gels were dried and exposed to film. Protein-DNA binding specificity was tested by adding antibodies to the nuclear extracts 1 h prior to addition of the radiolabeled probe or by using competition assays where an unlabeled specific or nonspecific competitor oligonucleotide probe was added to the nuclear extracts 5 min prior to addition of the radiolabeled probes.
Chromatin immunoprecipitation assay.
In vivo formaldehyde-mediated protein-DNA cross-linking was carried out as described previously (55, 66), with some modifications. Briefly, Friend erythroleukemic cells from the murine cell line DP27-17, which expresses Fli-1 at a moderate level, were grown at 37°C in α-MEM supplemented with 10% heat-inactivated FBS to a cell density of 2 × 106 to 5 × 106 cells/ml. Formaldehyde fixation was carried out by adding directly to the growth medium 11% formaldehyde to a final concentration of 1%. After 15 min at 37°C, glycine was added to a final concentration of 125 mM, and the cells were incubated for 1 h at 4°C. Fixed cells were pelleted and rinsed with PBS, and chromatin was isolated (55). Immunoprecipitations were performed in a 300-μl volume with 60 μg of chromatin preparation and 5 μl of anti-Fli-1 antibody. PCRs were performed in a 50-μl volume with an initial denaturation of 4 min at 94°C, followed by 29 cycles of 1-min denaturation at 94°C, 1-min annealing at 55°C, and 2-min extension at 72°C. PCR primers used were GTCCAGCGTTCTCCCAGAGG (forward) and CCGTCCTCACCCGACTCC (reverse).
Immunoblotting and antibodies.
Fifty-microgram aliquots of lysates from HB60-5 cells and derivative HB60-ED cells were lysed with radioimmunoprecipitation buffer (0.5% Nonidet P-40, 50 mM Tris HCl [pH 8.0], 120 mM NaCl, 50 mM NaF, 10 μg of aprotinin per ml, 100 μg of leupeptin per ml, 10 mM phenylmethylsulfonyl fluoride), resolved on sodium dodecyl sulfate (SDS)–6% polyacrylamide gels, and immunoblotted as described elsewhere (20). Antibody to pRb was obtained from Santa Cruz Biotechnology, Inc., and polyclonal antibody to Fli-1 was obtained from Alan Bernstein (79). The GABPα antibody, a gift from Steven McKnight, was previously described (34).
Transient and stable transfections.
C33A cervical carcinoma cells were maintained in α-MEM containing 10% FBS. Calcium phosphate transfections were performed in triplicates in 60-mm-diameter plates with 4 μg of one of the Rb-CAT (chloramphenicol acetyltransferase) constructs (pmRbP-198.CAT, pmRbP-198ΔFli-CAT, or pmRbP-1300.CAT), 5 μg of SV40 vector alone (pECE), or the reporter plasmid which consists of Fli-1 cDNA driven by the SV40 promoter (SV40-Fli-1) and 1 μg of pGKβGAL as internal control. Extracts from transfected cells were assayed for β-galactosidase (β-Gal) activity as described elsewhere (41). CAT analysis was performed with [14C]acetyl coenzyme A as a donor and chloramphenicol as an acceptor as described elsewhere (78). The luciferase assay was performed by transfecting the RBP0.69 Luc construct (14) with the indicated amount of SV40-Fli-1, SV40-Fli-ΔEBD, SV40 vector, and pGKβGAL into C33A cells. Luciferase activities were determined as described elsewhere (14).
For stable transfection, 5 × 106 HB60-5 cells were mixed with 30 μg of CMV-Fli-1 expression vector under either sense or antisense orientation in 0.8 ml of PBS and then subjected to electroporation (Bio-Rad) at 960 mF and 280 V. After 48 h of recovery in a medium containing Epo and SCF, the cells were selected for neomycin resistance by growth in medium containing G418 (0.8 mg/ml; Gibco BRL) for 2 weeks. 3T3 cells (2 × 105) were cotransfected with 5 μg of SV40-Fli-1 and 1 μg of Pgk-neo or 10 μg of pECE vector and 1 μg of Pgk-neo, using a Lipofectin transfection kit (Life Technologies), pooled (more than 50 colonies), and subjected to Northern blot analysis. Similarly, SAOS-2 cells were cotransfected with 1 μg of Rb minigene (pmRbmg) and with either 2 μg of CMV-Fli-1 sense or antisense expression vector, using Lipofectin. After selection with G418 (0.8 mg/ml) for about 2 weeks, the plates were stained with crystal violet and colonies with 50 to 500 cells were scored.
PCR amplification.
The fragment spanning the C-terminal region of Fli-1 and the transcription termination signal from bovine growth hormone of the pRc/CMV vector (Invitrogen) was amplified by PCR using the primers Fli-1 (TGCTGGGATCTATCCAAACC) and pRc/CMV (AGTCGAGGCTGATCAGCGAG), as described above.
RESULTS
Establishment of HB60, an erythroblastic cell line that undergoes cell cycle arrest and terminal differentiation in response to Epo.
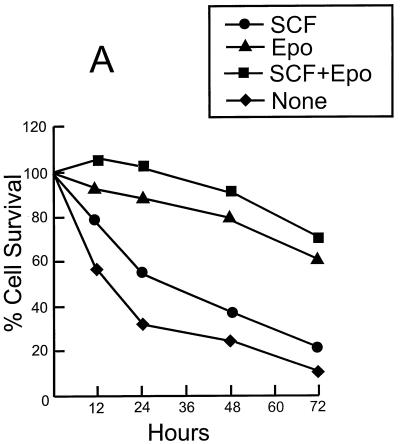
We have previously shown that F-MuLV-induced primary erythroleukemias undergo apoptosis when cultured in vitro but are capable of surviving when transplanted in vivo into syngeneic adult mice (21). Identifying factors present within the in vivo splenic microenvironment that are required for the survival of primary erythroleukemic cells in vitro has been an ongoing pursuit of our laboratory. Epo, a low-molecular-weight glycoprotein, plays an important role in erythroid progenitor cell survival via its antiapoptotic activity (30). The antiapoptotic activity of Epo also promotes the growth of immortalized erythroleukemic cell lines that have acquired, in addition to a constitutively activated Fli-1 gene, other genetic alterations, notably the inactivation of the p53 tumor suppressor gene (19, 21). In addition to Epo, SCF also promotes erythroid proliferation by inhibiting differentiation (53), as well as providing protection from apoptosis in a number of hematopoietic lineages (45, 47). Accordingly, the addition of both Epo and SCF to the growth medium of F-MuLV-induced primary erythroleukemic cells (HB60-t cells) extends their survival for several days (Fig. 1A). Although the majority of the HB60-t cell population died within the first week of culture in the presence of both Epo and SCF, a very small number of tumor cells remained viable. Extended culture (∼1 month) of these surviving tumor splenic cells resulted in the emergence of a rare cell population that possesses a short doubling time. Supplementation of the Epo-SCF culture medium with additional FBS (final FBS concentration of 20%) allowed us to establish an immortalized tumor cell line, termed HB60.
FIG. 1.
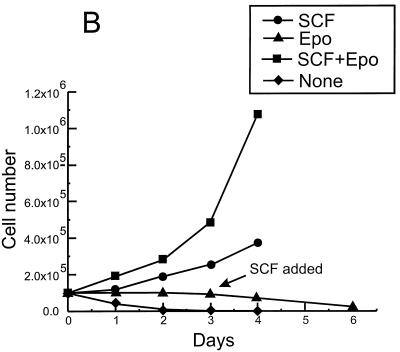
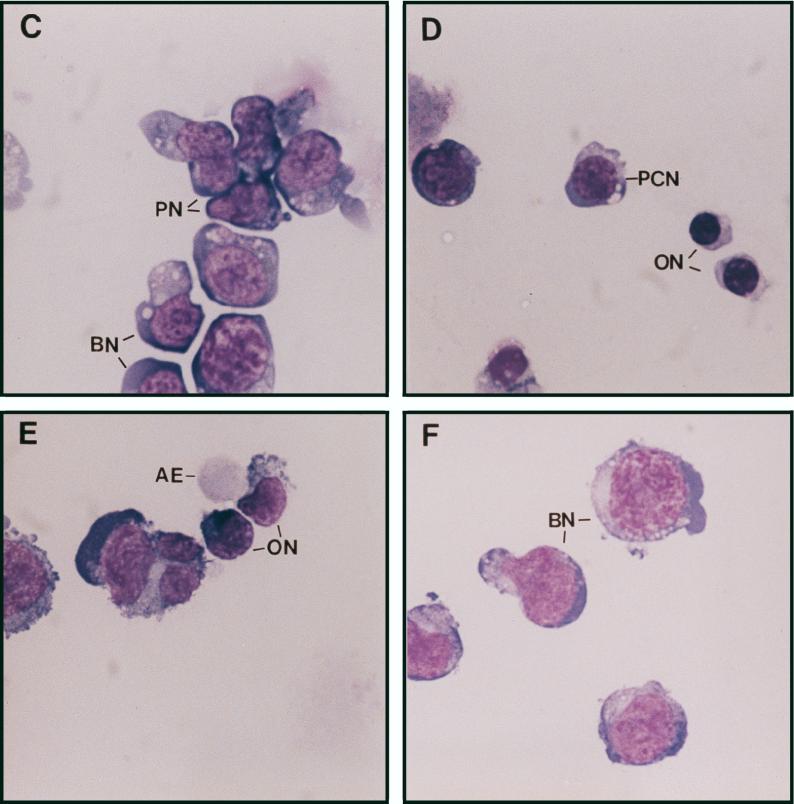
Establishment of the erythroblastic cell line HB60. Duplicate cultures (106) of the F-MuLV-induced primary erythroleukemia cell line HB60-t (A) or its derivative cell line HB60 (B) were incubated in the presence or absence of recombinant SCF (100 ng/ml) and/or Epo (0.1 U/ml) for the indicated times. The number of viable cells was determined by trypan blue dye exclusion. The arrow indicates the time at which SCF was added to the Epo-treated culture of HB60 cells, which did not lead to proliferation. (C to E) The clonal HB60-5 cells were grown in the presence of either SCF or Epo. At day 3 of incubation, the cells were harvested and stained with Wright’s stain. HB60-5 cells grown in the presence of SCF plus Epo exhibit the features of pronormoblast (PN) and basophilic normoblast (BN), which define the earliest recognizable stages of erythroid differentiation (C). HB60-5 cells grown in the presence of Epo (D and E) show a wider range of maturation stages, including polychromatophilic normoblast (PCN), orthochromatic normoblast (ON), normoblast (N), and anucleated erythrocyte (AE). HB60-ED cells expressing the exogenous Fli-1 (F) have morphological features of undifferentiated basophilic normoblast (BN) similar to the SCF-Epo-treated cells.
The established HB60 cell line grew slowly in the presence of SCF–20% FBS, and removal of SCF triggered rapid cell death (Fig. 1B). However, the growth rate of the HB60 cell line increased significantly in the presence of both recombinant Epo (0.1 U/ml) and SCF (100 ng/ml). Notably, Epo acts synergistically with SCF to stimulate the proliferation of HB60 cells in vitro, which is consistent with the effect described for normal erythroid progenitors (45, 47). In the presence of Epo alone, HB60 cells underwent terminal differentiation that was initiated by cell cycle arrest and maintained throughout the differentiation program. Addition of SCF to HB60 cells previously treated for 3 days with Epo did not induce proliferation, indicating commitment to terminal differentiation at this stage (Fig. 1B). The morphological characteristics of the various stages of HB60 differentiation are depicted in Fig. 1C to E. This includes the identification of distinct basophilic normoblasts and orthochromatic normoblasts with condensed nuclei and reduced cytoplasmic volume (Fig. 1D). In addition, there were a considerable number of mature anucleated erythrocytes detected after 3 days of exposure to Epo (Fig. 1E). In contrast, HB60 cells grown in the presence of SCF (data not shown) or SCF and Epo displayed an undifferentiated normoblast morphology, characterized by large nuclei and minimal cytoplasm (Fig. 1C). HB60 subclones, isolated by limited dilution, responded to SCF and Epo in a manner similar or identical to that of the parental HB60 cell line. One of these clones, designated HB60-5, was used for subsequent experimental analysis.
Epo-induced terminal differentiation of HB60 cells involves alterations in erythroid cell-specific gene expression.
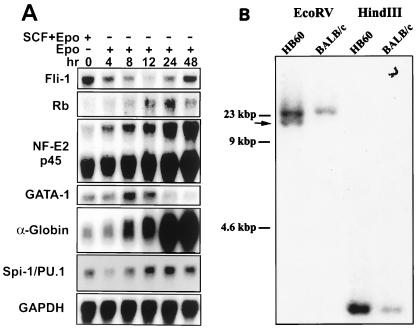
The normal program of Epo-induced erythroid differentiation is accompanied by distinct alterations in the expression of a number of erythroid cell-specific genes, most notably the induction of globin genes (36). Northern blot analysis of HB60-5 cells cultured in the presence of Epo showed a steady increase in the expression of α-globin and the p45 subunit of NF-E2, an erythroid/megakaryocytic cell-specific gene (Fig. 2A). In contrast, GATA-1 expression peaked by 8 h and then slowly declined thereafter. This transient increase in GATA-1 expression has also been reported for normal erythroblasts undergoing terminal differentiation (11). Based on the morphology of HB60-5 cells (Fig. 1C) and their lack of responsiveness to growth factors such as interleukin-3 and granulocyte-macrophage colony-stimulating factor (data not shown), they are likely derived from committed burst-forming-erythroid (BFU-E) CFU-erythroid (CFU-E)-like erythroid progenitor cells.
FIG. 2.
Fluctuation in the expression of Fli-1 during Epo-induced differentiation. (A) HB60-5 cells (5 × 106) were grown in the presence of Epo or Epo plus SCF for the indicated times. Total RNAs (20 μg) prepared from these cells were Northern blotted, transferred to nitrocellulose, and sequentially hybridized with cDNA probes for the following genes: Fli-1, GATA-1, NF-E2 p45, Rb, α-globin, Spi-1/PU.1, and GAPDH. (B) Ten-microgram aliquots of genomic DNA extracted from the HB60 cells and normal BALB/c spleen cells were digested with the indicated restriction enzymes, Southern blotted, and hybridized with Spi-1 probe A. The arrow shows the position of the rearranged band.
Erythroleukemia cell lines derived from mice infected with F-MuLV have an activated Fli-1 gene due to the proviral insertional activation of Fli-1 (21). We therefore examined the genomic organization of the Fli-1 locus in the HB60 clones. Surprisingly, although the primary tumor had acquired Fli-1 rearrangement, the derived HB60 cell line possessed no such rearrangement, as determined by Southern blot analysis. Independent HindIII and BamHI digests of HB60 genomic DNA revealed an intact Fli-1 (data not shown). Interestingly, rearrangement of Spi-1/PU.1 gene was detected in HB60 cells compared to the normal BALB/c DNA (Fig. 2B). Rearrangement of this locus resulted in induction of Spi-1 mRNA in HB60 cells (Fig. 2A). The expression of Spi-1 mRNA was slightly reduced 4 h following Epo-induced differentiation of HB60 cells and then after gradually increased (Fig. 2A). These observations suggest that the HB60 cell line may have been derived from a subpopulation of tumor cells that acquired proliferative ability in response to growth factors and the activation of Spi-1. The late emergence and expansion of the HB60 cell population from the cultured tumor population further suggests that these cells were selected for additional genetic alterations that conferred immortalization in culture. Indeed, immunoprecipitation with PAB 240, a monoclonal antibody that specifically recognizes mutant forms of p53, detected an abundant expression of p53 mutant protein (data not shown).
In summary, we have isolated a unique SCF-dependent erythroblastic cell line that is capable of Epo-induced terminal differentiation. J2E, a similar erythroblastic cell line previously isolated from murine fetal liver cells coinfected with v-Raf and v-Myc (29), is also capable of undergoing Epo-induced terminal differentiation. However, unlike the case for HB60 cells, Epo-induced differentiation of J2E cells is dependent on proliferation. Thus, the unique characteristics of HB60 cells allow us to dissect the molecular events involved in the transition of erythroblasts from proliferation to terminal differentiation.
Fli-1 expression levels modulate the response of HB60-5 cells to Epo.
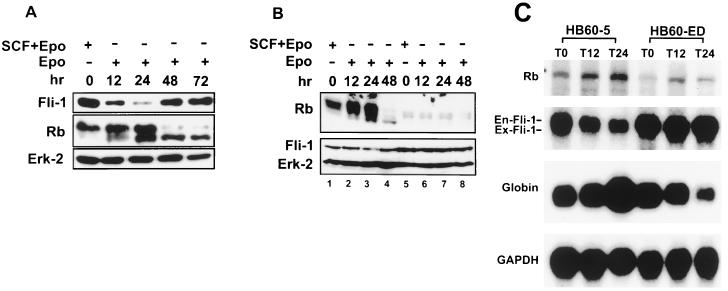
We have previously shown that activation of the Epo gene is a common but late event in vivo that confers enhanced tumorigenicity to F-MuLV-induced erythroleukemias (20). The ability of HB60 cells to undergo Epo-induced terminal differentiation raised the possibility that proviral insertional activation of Fli-1 may alter the responsiveness of erythroid progenitor cells to Epo, perhaps by promoting cellular proliferation at the expense of differentiation. Surprisingly, HB60-5 cells express a significant amount of Fli-1, despite having an apparently intact Fli-1 locus. Furthermore, the levels of Fli-1 expression changes dramatically during Epo-induced differentiation of HB60-5 cells, falling precipitously to almost undetectable levels by 12 h (Fig. 2A). At subsequent times, there was a slow but steady increase in Fli-1 expression levels. By 48 h, HB60-5 cells expressed Fli-1 at levels comparable to that of SCF-Epo-supplemented cultures of HB60-5 cells. A similar pattern of Fli-1 expression was also detected at the protein level (see Fig. 4A).
FIG. 4.
Negative correlation between the expression levels of Fli-1 and Rb proteins and mRNAs. (A) HB60-5 cells were cultured in the presence of SCF-Epo or Epo alone for the indicated times. Cells were lysed, separated on an SDS-acrylamide gel, and subjected to Western blotting using antibodies against the Rb and Fli-1 proteins. Equal loading was determined by blotting the same filter with an anti-mitogen-activated protein kinase (Erk-2) antibody. (B) HB60-5 (lanes 1 to 4) and HB60-ED (lanes 5 to 8) cells were treated with SCF-Epo or Epo alone for the indicated times and Western blotted with Rb, Fli-1, or Erk-2 antibodies as described above. (C) Two-microgram aliquots poly(A)+ mRNA isolated from HB60-5 or HB60-ED cells treated for the indicated times with Epo were Northern blotted and sequentially hybridized with Rb, Fli-1, α-globin, or GAPDH cDNA. The positions of endogenous (En-Fli-1) and exogenous (Ex-Fli-1) Fli-1 mRNAs are shown on the left.
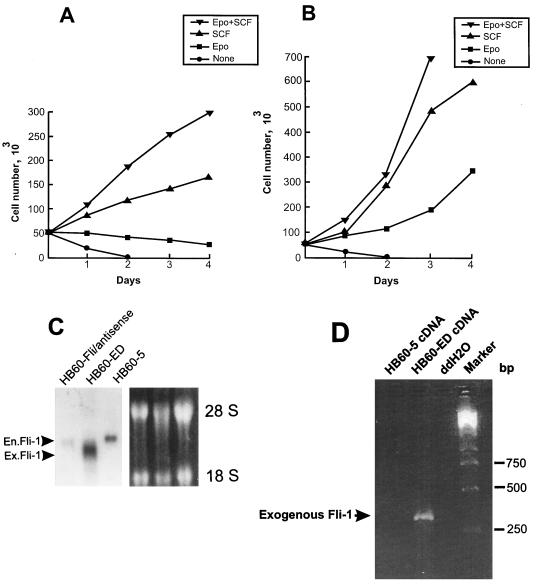
Together, these results suggest that the transient downregulation of Fli-1 is an important event in the commitment to terminal erythroid differentiation upon Epo induction. To test this hypothesis, we engineered HB60-5 cells to constitutively express either sense or antisense Fli-1, under the control of the CMV promoter. Mock and antisense Fli-1-transfected HB60-5 cells responded identically to Epo-SCF-supplemented culture (Fig. 3A). In contrast, a large population of pooled sense Fli-1-transfected HB60-5 cells grew in Epo-supplemented medium (Fig. 3B). We derived from these cells a polyclonal Epo-dependent cell line, designated HB60-ED, that expressed approximately two- to threefold more exogenous Fli-1 mRNA than the parental HB60-5 cells (Fig. 3C). Since the size of the band corresponding to the exogenous Fli-1 is very close to the endogenous species, these two bands were not completely resolved on the gel. PCR amplification of the Fli-1-transfected cDNA shows that this gene is expressed in HB60-ED cells (Fig. 3D). Interestingly, a negligible level of Fli-1 mRNA was detected in the mock-transfected antisense cells, while they still retained resistance to neomycin. This observation suggests that cells expressing exogenous Fli-1 antisense may not survive during the course of stable transfection. Indeed, very few cells survived after Fli-1 antisense transfection compared to the sense-transfected cells (data not shown). Moreover, the constitutive expression levels of Fli-1 protein and mRNA remained unchanged even after briefly supplementing, for several passages, and then removing SCF from Epo-cultured HB60-ED cells (Fig. 4B and C, respectively). Lastly, there was no morphological evidence of any terminal differentiation in the Epo cultures of HB60-ED (Fig. 2F). Furthermore, the induction of globin was not seen in these cells compared to Epo-treated cultures of HB60-5 cells (Fig. 4C). This result suggests that the downregulation of Fli-1 in Epo-induced HB60-5 cells is involved in the commitment to terminal differentiation.
FIG. 3.
Effect of overexpression of Fli-1 on proliferation of HB60-5 cells by Epo. HB60-5 cells (5 × 106) were transfected with either the sense or antisense CMV-Fli-1 expression vector (Fig. 8A), and the pools of transfected cells were incubated in the presence of growth factors as indicated. (A) Growth rate of the Fli-1 antisense-transfected HB60-5 cells. (B) Growth rate of the Epo-dependent HB60-ED cells, which are derived from the Fli-1 sense-transfected HB60-5 cells. (C) Analysis of expression of exogenous Fli-1 mRNA in transfected HB60-5 cells. mRNA extracted from HB60-5 cells, the pools of Fli-1 antisense-transfected HB60-5 cells, and HB60-ED cells were Northern blotted and hybridized with Fli-1 cDNA probe. The position of the exogenous Fli-1 (Ex.Fli-1) band, which is slightly smaller than endogenous Fli-1 (En.Fli-1) transcript, is shown by an arrowhead. The ethidium bromide-stained gel shows equal RNA loading. (D) Expression of the exogenous Fli-1 in HB60-ED cells was verified by PCR analysis using two primers corresponding to Fli-1 and transcription termination sequences from the pRc/CMV construct. The PCR products were separated on a 2% agarose gel and stained with ethidium bromide. The arrow shows the location of the 304-bp amplified fragment. A PCR using no cDNA(ddH2O) was used as a negative control.
Fli-1 binds an Ets site in the Rb promoter.
To understand the mechanism by which Fli-1 is involved in differentiation and proliferation of erythroid progenitor cells, we searched for potential target genes that might be regulated by this transcription factor. Fli-1 has been shown to bind to the consensus sequence CCGGAAGT (42, 61, 79). A nearly identical sequence (GCGGAAGT) is present within the Rb promoter (see Fig. 7A). This potential binding site for Fli-1 overlaps an SP1 binding motif which binds RBF-1, a heterodimer complex of E4TF1-60 and E4TF1-53 (62), which are homologous to the murine GABPα and GABPβ, respectively (34, 69). GABPα is an Ets-related transcription factor, whereas GABPβ belongs to a family of proteins that contain ankyrin-binding domains.
FIG. 7.
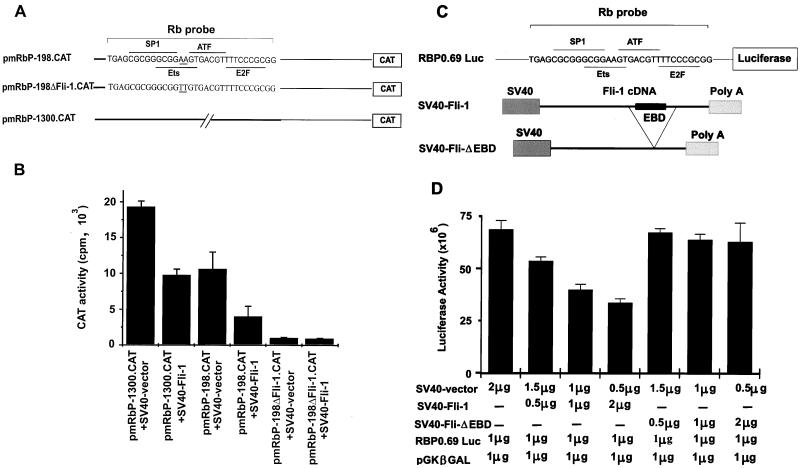
Suppression of the Rb promoter by Fli-1. (A) The pmRbP-1300.CAT and pmRbP-198.CAT constructs have been described elsewhere (78). The pmRbPΔFli-1.CAT construct was generated by converting neighboring AA nucleotides to TT in the core Ets binding site and are double underlined. The overlapping recognition sequences for transcription factors RBF-1, SP1, ATF, and E2F as well as the sequences used in the EMSA (Rb probe) are indicated. (B) The Rb promoter-CAT constructs were cotransfected into Rb-negative C33A cells with SV40-Fli-1 or SV40 vector alone, and the levels of CAT production were determined 3 days later. CAT levels were normalized for the levels of β-Gal. (C) The RBP0.69 Luc construct has been previously described. SV40-Fli-ΔEBD is a derivative of SV40-Fli-1 plasmid in which the EBD was deleted by removing the internal NcoI fragment from the Fli-1 cDNA. (D) The Rb promoter construct was cotransfected into C33A cells with the indicated amount of SV40-Fli-1, SV40-Fli-ΔEBD, SV40 vector, and pGKβGAL, and luciferase levels were measured 2 days later. Luciferase activity was normalized for the level of β-Gal.
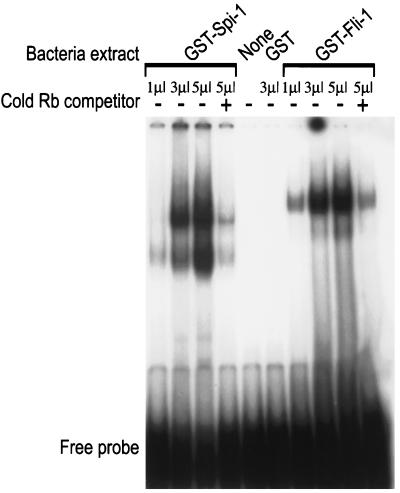
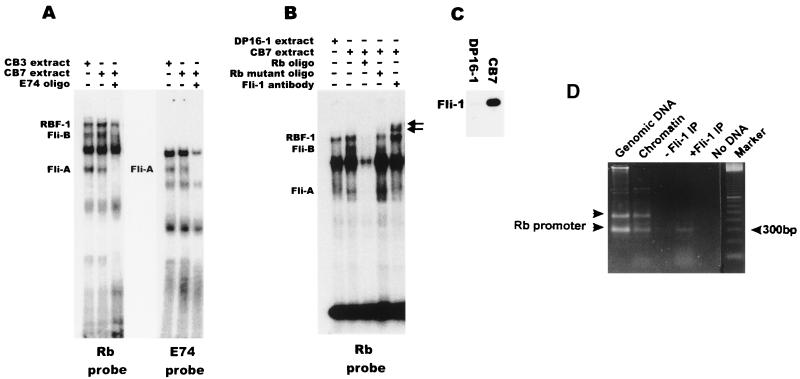
Although Rb was first identified as a tumor suppressor gene involved in retinoblastoma, genetic evidence in mice has shown that Rb is essential for normal erythroid differentiation (7, 23, 35). Thus, Fli-1 could be involved in blocking erythropoiesis by downregulating Rb expression at the transcriptional level. In an EMSA, incubation of oligonucleotides that contain the Ets site and surrounding sequences in the Rb promoter (see Fig. 7A) with bacterially expressed GST–Fli-1 protein leads to formation of a single bound complex (Fig. 5). The binding of GST–Fli-1 was specific, as it was competed by a 100-fold excess of cold Rb probe (Fig. 5). To verify the significance of this binding at the cellular level, nuclear extracts from CB3 and CB7 (F-MuLV-induced erythroleukemia cell lines that express high levels of Fli-1) were incubated with the Rb oligonucleotides and subjected to EMSA. As shown in Fig. 6A, three specific bands, RBF-1, Fli-A, and Fli-B, were identified. The identification of one of the shifted bands as corresponding to a RBF-1–DNA complex was verified by adding anti-GABPα or GABPβ antibodies to the EMSA, which resulted in the supershift of this band (data not shown). We concluded that the Fli-A and Fli-B shifted bands contain the Fli-1 protein, as suggested by (i) the elimination of the Fli-A and Fli-B shifted bands by competition with cold E74 probe, an oligonucleotide that specifically binds Fli-1 (Fig. 6A), as previously described (79); (ii) supershift of the Fli-A and Fli-B band with anti Fli-1 antibodies (Fig. 6B); (iii) competitive inhibition with cold Rb probe but not with cold Rb mutant probe that substitutes AA for TT within the Ets consensus binding site (Fig. 6B); and (iv) the substantial reduction in the levels of Fli-A and Fli-B but not RBF-1 shifted bands when the reaction was carried out with nuclear extracts from DP16-1 cells, an FV-P-induced erythroleukemia cell line that expresses low levels of Fli-1 (Fig. 6B and C). The Fli-A band was also observed with the E74 probe and likely corresponds to the monomeric form of Fli-1 (Fig. 6A), while the slower-migrating Fli-B band may represent a complex containing Fli-1 and an unknown protein partner that is perhaps specific to the Rb promoter (e.g., SP1, ATF, or E2F).
FIG. 5.
DNA binding activity of recombinant Fli-1 and Spi-1/PU.1 proteins to the Rb promoter. Lysates of bacterial cells (1, 3, or 5 μl) expressing GST–Fli-1, GST–Spi-1/PU.1, or GST were incubated with the 32P-labeled Rb oligonucleotides. Binding reactions were performed in the presence of the nonspecific competitor poly(dI-dC) and the presence (+) or absence (−) of cold specific Rb competitor DNA. Complexes were resolved on a 5% polyacrylamide gel. As a control, binding with no protein (None) was performed.
FIG. 6.
Binding of Fli-1 to the Ets site in the Rb promoter. Nuclear extracts (2 μg) prepared from the erythroleukemia cell lines CB3, CB7, and DP16-1 were incubated with 32P-labeled Rb probe (A and B) or E74 probe (A) in the absence or presence of 100-fold excess cold DNA competitor or Fli-1 antibody as indicated. The position of the supershifted Fli-1 complex is indicated by arrows. The nature of major bands in panels A and B is unknown. (C) Total cellular extracts (20 μg) prepared from the cell lines CB7 and DP16-1 were Western blotted and hybridized with anti-Fli-1 polyclonal antibodies. (D) In vivo association of Fli-1 with the Rb promoter by formaldehyde cross-linking. PCR was performed on chromatin fragments isolated after immunoprecipitation with or without Fli-1 antibody or as a control on total genomic or chromatin isolated from DP27-17 erythroleukemic cells. The lower arrowhead on the left marks the position of the 330-bp fragment corresponding to the Rb promoter, while the upper arrowhead marks the position of a 450-bp nonspecific fragment. No DNA, no DNA in the PCR; Marker, 100-bp DNA ladder.
To confirm the results of our in vitro studies and to verify whether Fli-1 associates with the Rb promoter in vivo, we used formaldehyde-mediated protein-DNA cross-linking of live proliferating DP27-27 cells which express Fli-1 at moderate levels. In vivo formaldehyde cross-linked chromatin fragments from DP27-27 cells were immunoprecipitated with antibodies directed against the Fli-1 protein. DNA from the resulting immunoprecipitates was then purified and subjected to PCR amplification of a 330-bp fragment corresponding to the Rb promoter. In agreement with our in vitro studies, the results of our in vivo studies show that Fli-1 is indeed associated with the Rb promoter in proliferating cells. As shown in Fig. 6D, anti-Fli-1 antibody can precipitate chromatin fragments containing the Rb promoter. The same 330-bp DNA fragment is also amplified from genomic DNA or chromatin fragments not subjected to immunoprecipitation.
Since HB60-5 cells also express the Spi-1 gene, its binding to the Ets site in the Rb promoter was examined. As shown in Fig. 5, bacterially expressed GST–Spi-1 also associates with the Rb probe in EMSA. However, the binding of Spi-1 to the Rb promoter is not evident with nuclear extract from DP16-1, a cell line that expresses Spi-1 due to insertional activation of this gene (Fig. 6B). In view of the pattern of Spi-1 RNA expression during differentiation which appears to parallel that of the Rb gene (Fig. 2A), it is unclear whether Spi-1 is involved in regulation of the Rb gene.
Fli-1 negatively regulates Rb expression.
The repressor effect of Fli-1 on the Rb promoter was also determined in a reporter assay of transiently transfected cells. For this purpose, we used either a pmRbP-1300.CAT or a pmRbP-198.CAT expression construct (Fig. 7A) that contains either a 1.3-kbp or a 198-bp sequence corresponding to the promoter region upstream of the Rb transcription start site (78). Cotransfection of C33A, an Rb- and Fli-1-nonproducing cervical carcinoma cell line, with either pmRbP-1300.CAT or pmRbP-198.CAT, together with the SV40-Fli-1 expression plasmid, resulted in CAT activity 50 to 60% lower than that for C33A cells transfected with either of the CAT expression reporter constructs alone. A variant of the pmRbP-198.CAT construct, containing a mutant Ets binding site (AA replaced by TT), termed pmRbP-198ΔFli-CAT, displayed ∼5% activity which was not further reduced by the addition of the SV40-Fli-1 expression construct (Fig. 7B).
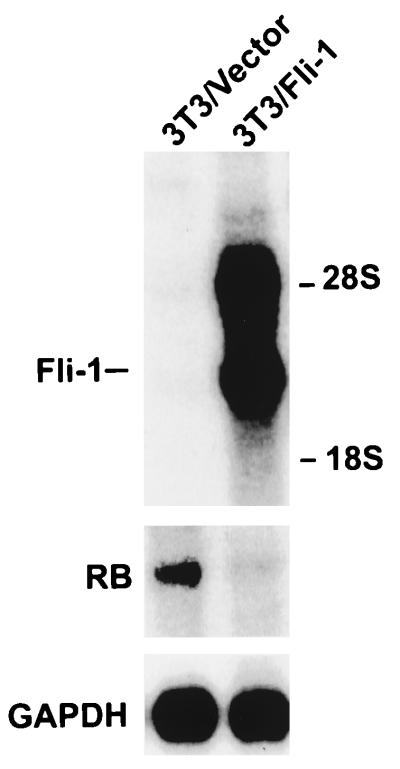
To confirm that suppression of the Rb promoter by Fli-1 is specific, the RBP0.69 Luc plasmid (14), in which the human Rb promoter (positions −677 to −56) is fused to the luciferase gene (Fig. 7C), was cotransfected into C33A cells with increasing amounts of SV40-Fli-1 vector. The human Rb promoter is identical in sequence to the murine promoter in the region which carries the consensus binding site for SP1, E2F, ATF, and Ets, as well as surrounding sequences (78). At the same time, C33A cells were cotransfected with RBP0.69 Luc and a Fli-1-defective construct (SV40-Fli-ΔEBD) in which the C-terminal end of Fli-1 containing the Ets binding domain (EBD) was deleted. As shown in Fig. 7D, the full-length Fli-1 suppresses the Rb-luciferase promoter in a dose-dependent manner. However, the SV40-Fli-ΔEBD had no effect on the promoter activity. The repression of the Rb promoter by Fli-1 peaked at 2 μg of SV40-Fli-1 DNA; higher DNA concentration resulted in only a slight increase in suppression (data not shown). These results suggest that Fli-1 competes with RBF-1 to repress Rb transcription. This hypothesis is also supported by the pattern of expression of both Fli-1 and Rb expression (RNA and protein) in HB60-5 cells undergoing Epo-induced differentiation. Figure 4A shows a negative correlation between the expression levels of Fli-1 and Rb. The drop in Fli-1 protein expression levels coincides with Rb’s transition from an inactive hyperphosphorylated form to an active hypophosphorylated form (Fig. 4A). In addition, the constitutive levels of Fli-1 expression in the HB60-ED cells dramatically suppressed Rb expression, which was mainly detected in its inactive hyperphosphorylated form (Fig. 4B). Suppression of Rb by Fli-1 occurs at the transcriptional level, as deduced from the negative correlation in the pattern of mRNA expression of these two genes in HB60-5 (Fig. 2A and 4C) and HB60-ED cells (Fig. 4C). Moreover, transcriptional repression of Rb by Fli-1 is also seen in fibroblast (Fig. 8). In this experiment, 3T3 cells were transfected with either vector alone or a Fli-1-expressing vector driven by the SV40 promoter. RNA from pooled cells was purified and subjected to Northern analysis. As shown in Fig. 8, Fli-1-transfected 3T3 cells showed a significant reduction in the level of endogenous Rb mRNA compared to vector-transfected cells.
FIG. 8.
Suppression of Rb by Fli-1 in fibroblasts. Two-microgram aliquots of poly(A)+ mRNA isolated from pooled 3T3 cells transfected with either SV40-Fli-1/Pgk-neo (3T3/Fli-1) or vector alone (pECE)/Pgk-neo (3T3/Vector) were Northern blotted and sequentially hybridized with Rb, Fli-1, or GAPDH probe.
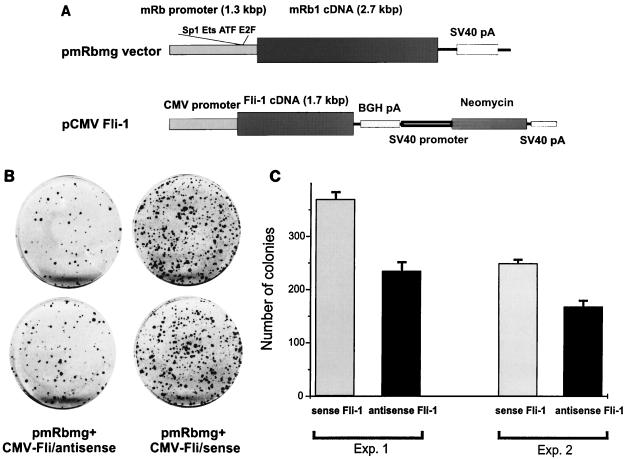
We further examined whether the transcriptional suppression of Rb by Fli-1 would confer a proliferative advantage to SAOS-2 cells transfected by an Rb minigene (pmRbmg). Previous studies have shown that SAOS-2 cells, an Rb (and Fli-1)-negative osteosarcoma cell line, transfected with wild-type Rb undergo cell cycle arrest (48, 68). Cotransfection of SAOS-2 cells with an Rb minigene driven by the 1.3-kbp Rb promoter and the CMV-Fli-1/sense expression vector generated a high number of colonies compared to cells cotransfected with either CMV-Fli-1 antisense and the Rb expression vector (Fig. 9A and B) or CMV vector and the Rb expression vector (data not shown). Similar results were obtained in at least two additional experiments using independent plasmid preparations (Fig. 9C). Together, these results strongly suggest that Fli-1 is a negative regulator of the Rb gene.
FIG. 9.
Suppression of the Rb promoter by Fli-1 in SAOS-2 cells. (A) The murine Rb and Fli-1 genes, driven by the Rb (pmRbmg) and CMV promoters, respectively. BGH, bovine growth hormone. (B) Duplicate cultures of SAOS-2 cells cotransfected with the pmRbmg construct and either CMV-Fli sense or CMV-Fli antisense. (C) Two additional cotransfection experiments using new plasmid preparations of the pmRbmg and CMV-Fli-1 constructs used for panel B.
DISCUSSION
In this study, we have analyzed the effect of Fli-1, a member of the Ets family of oncogenic transcription factors, on the Epo responsiveness of erythroblasts transformed by F-MuLV. We show that downregulation of Fli-1 is critical to Epo-induced terminal differentiation. Maintenance of high levels of Fli-1 by ectopic expression blocks differentiation and promotes the self-renewal of HB60 cells. We demonstrated that this inhibition of terminal differentiation may be partly mediated through direct transcriptional repression of Rb by Fli-1. These results establish a novel mechanism of transformation by retrovirus, where insertional activation of a proto-oncogene (Fli-1) negatively regulates the expression of a tumor suppressor gene (Rb).
Fli-1 induces erythroid transformation by switching Epo-induced differentiation to Epo-induced proliferation.
The Epo-induced differentiation of HB60 cells is accompanied by a transient but dramatic reduction in the expression of Fli-1. This Epo-induced fluctuation in the expression levels of Fli-1 is strikingly similar to the chemical changes (notably, dimethyl sulfoxide and HMBA) induced in c-myc and c-myb expression that have been observed in FV-A- and FV-P-induced erythroleukemia cell lines (8, 10, 32, 33, 59, 70, 72). Overexpression of these two nuclear proto-oncogenes inhibits chemically induced differentiation, suggesting that c-myc and c-myb are important transcriptional regulators of erythroid differentiation. Similarly, constitutive overexpression of Fli-1 in HB60-5 cells blocks Epo-induced terminal differentiation, resulting in enhanced self-renewal potential. However, unlike c-myc and c-myb, proviral insertional activation of Fli-1 is a primary transforming event associated with F-MuLV-induced erythroleukemias and therefore of more direct relevance to this murine model of leukemogenesis.
HB60-5 cells with an activated Spi-1/PU.1 gene resemble the FV-P-induced erythroleukemias with the exception of expressing the spleen focus-forming virus gp55 glycoprotein that is thought to mimic the effect of Epo. Since gp55 confers growth factor independence to the FV-P-induced erythroleukemic cells, induction of terminal differentiation by Epo suggests that stimulation of the Epo-R by these ligands may involve distinct signal transduction pathways. Since Epo induces downregulation of Fli-1 in HB60 cells, it will be intriguing to determine whether expression of gp55 confers growth factor independence through upregulation of Fli-1. This hypothesis is supported through a recent observation in which Spi-1/PU.1 has been shown to cooperate with an activated Epo-R in the inhibition of apoptosis and differentiation of erythroblasts (60).
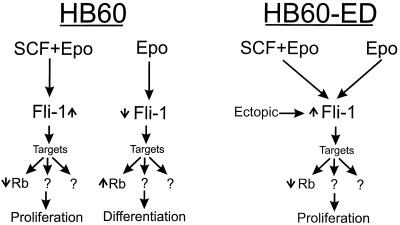
While the Epo responsiveness of the HB60 cell line is similar to that of normal erythroid progenitor cells, F-MuLV-induced erythroleukemic cells proliferate in response to Epo (20). This notable contrast in the behavior of these cell lines toward Epo is explained by the differences in the integrity of the Fli-1 locus within these two cell lines. The observation that constitutive Fli-1 expression can switch the Epo response of the HB60-5 cell line from a differentiation to self-renewal program is also consistent with the characteristic severe anemia and hyperproliferative proerythroblasts associated with F-MuLV-induced erythroleukemias. It is therefore possible to envisage Fli-1 as a master regulator of gene expression, capable of altering the responsiveness of erythroblasts to external signals (Epo) to either self-renewal or differentiation (Fig. 10).
FIG. 10.
Model depicting the role of Fli-1 during proliferation and differentiation of erythroblasts. Epo induces downregulation of Fli-1 (↓) in HB60 cells, which results in terminal differentiation (see text). Upregulation of Fli-1 (↑) by ectopic expression (HB60-ED cells) and the addition of SCF, with or without Epo, to the culture of HB60 cells inhibits differentiation and promotes proliferation. These observations suggest the Fli-1 replaces the proliferative effect of SCF signaling in erythroblasts. Moreover, they indicate that Fli-1 functions as a switch mechanism which alters the responsiveness of erythroblast to undergo differentiation or proliferation by ectopic expression. This response is mediated through the regulation of several target genes. Rb is one of these target genes that is negatively regulated by Fli-1. High expression of Rb as a consequence of low Fli-1 could be one of the events involved in erythroid differentiation.
SCF and c-Kit, proximal components of a signaling pathway critical to erythroid proliferation.
Although Epo signaling is crucial in vivo for definitive erythropoiesis, it is dispensable for BFU-E proliferation and differentiation to CFU-Es (76). SCF, however, is vital to this stage of erythroid progenitor cell development, since inbred strains of mice carrying germ line mutations in SCF (Sl locus) and c-Kit (W locus) suffer from severe anemia due to a CFU-E deficiency (5, 54). SCF (Sl) and c-Kit (w) mutant mice are also resistant to FV-induced erythroleukemias (40), which is consistent with the view that BFU-Es and CFU-Es are the targets of FV (26). However, leukemic clones isolated from wild-type mice infected with FV can proliferate when transplanted into Sl/Sld mutant mice, suggesting that during the evolution of Friend erythroleukemia the requirement for an intact SCF/c-Kit signaling pathway is lost (40).
Although SCF provides a modest proliferative signal, this response is strongly synergized by Epo, a response typical of normal erythroid progenitor cells. The basis for this synergism is unknown. However, recent evidence suggests a functional cross talk between c-Kit and the Epo-R (24, 76). Using Epo-R−/− fetal liver cells infected with a retrovirus expressing mutant forms of the Epo-R, these investigators have shown that CFU-E formation requires both Epo/Epo-R and SCF/c-Kit signal transduction pathways, presumably with c-Kit-mediated phosphorylation of specific Epo-R cytosolic tyrosine residues facilitating an important proliferative signal (75).
The ability to switch the growth factor dependence of these cells from SCF to that of Epo solely by overexpressing Fli-1 suggests that Fli-1 is an important nuclear effector of SCF/c-Kit signaling. Enforced expression of Fli-1 in HB60-5 cells could fulfill in whole, or in large part, the requirement for c-Kit signaling in promoting cellular proliferation. This would be consistent with the ability of FV-induced leukemic clones to be transplanted in Sl/Sld mice (40). This possibility is further supported by recent experiments showing the inhibitory effects of SCF and Epo on the differentiation of highly purified human erythroid colony-forming cells (53). These results suggest that modulating the physiological levels of SCF within hematopoietic microenvironments could produce conditions that are permissive to Epo-induced terminal differentiation.
Fli-1-blocked terminal erythroid differentiation may be mediated through transcriptional repression of the Rb gene.
The Fli-1 proto-oncogene is activated in murine erythroleukemia and Ewing’s sarcoma in humans. Identification of the downstream target genes for Fli-1 in normal cells and in other malignancies may provide an insight into the oncogenic processes. Our results suggest that the Rb tumor suppressor gene is one such downstream target of Fli-1 in murine erythroleukemias. This conclusion is supported by several lines of evidence. First, Fli-1 can bind the Ets consensus site in the Rb promoter (GCGGAAGT). This DNA-binding sequence is nearly identical to a Fli-1 consensus sequence (CCGGAAGT) (42, 79). Second, the inverse patterns of Rb and Fli-1 expression in HB60-5 cells is consistent with in vivo repression of Rb by Fli-1. Third, constitutive expression of Fli-1 reduces the level of Rb in these cells and also in fibroblasts. Fourth, Fli-1 can block the growth-suppressive effect of an Rb minigene driven by its own promoter in SAOS-2 cells. Together, these results provide strong evidence for the involvement of Fli-1 in the negative regulation of Rb transcription.
Although Spi-1 activation appears to play a major role in the establishment of HB60-5 cells, its involvement in the regulation of Rb has not been defined. Bacterially expressed Ets proteins Spi-1 and Fli-1 appear to bind the Rb promoter in vitro. However, bandshift analysis with unclear extracts from erythroleukemic cells expressing either or both proteins indicates that only Fli-1 binds to the Rb promoter. Moreover, Spi-1 expression increases during Epo-induced differentiation in a manner that is parallel to that of the Rb gene. Thus, our results suggest that during erythrocyte differentiation, Fli-1 but not Spi-1 is involved in negative regulation of the Rb gene. Interestingly, Spi-1 was previously shown to bind to the Rb protein in vivo (16). While the significance of this protein-protein interaction has not yet been determined, it is possible that Spi-1 regulates Rb at the posttranslational level.
Concerning the nature of Rb transcriptional repression, one possible mechanism would be that binding of Fli-1 to the Ets site may affect the binding of other factors (e.g., ATF or SP1) to the Rb promoter. We exclude this possibility since the results of EMSAs indicate that mutation of the Ets site within the Rb promoter abolishes the binding of RBF-1 and Fli-1 without affecting the binding of other proteins (data not shown). We propose that the Ets binding site is required for stabilizing a protein complex at the Rb promoter. RBF-1 stabilizes the complex and contributes to transcriptional activation of the Rb gene. Mutations in the Ets binding site inhibit binding of RBF-1 to the promoter, resulting in destabilization of the transcriptional complex and loss of Rb expression. The EMSA analysis revealed that RBF-1 and Fli-1 form two distinct complexes with the Ets binding site in the Rb promoter. Moreover, overexpression of Fli-1 in transient transfection experiments suppresses the Rb promoter. Thus, we suggest that Fli-1 may compete with RBF-1 but that it is capable of promoting assembly of other factors, as is evident from our EMSA analysis. This hypothesis is consistent with a weak transactivation potential of Fli-1 (44, 79) and a recent observation that RBF-1 is a critical positive regulator of Rb transcription (67). In this respect, the potential role of the EWS–Fli-1 fusion protein in the transcriptional repression of Rb would be interesting to evaluate. In this chimeric transcript, the weak transactivation domain of Fli-1 is replaced by the strong transactivation domain of EWS (44). Thus, it is possible that in contrast to Fli-1, the chimeric protein activates Rb transcription, but this remains to be determined.
Our findings are also consistent with the results obtained from Rb−/− mice that die by prenatal days 13 to 14, with failure in hepatic erythropoiesis and defective neuronal development (7, 23, 35). The defect in erythroid differentiation is an intrinsic property of the Rb−/− erythroblasts, as it is also observed in mice transplanted with Rb−/− fetal liver cells (22). This observation is further supported by the inhibition of hormone-induced differentiation of K562 cells by antisense-mediated downregulation of Rb (4).
The central role of Rb in controlling the cell cycle renders it an ideal target for Fli-1. Eliminating Rb function results in the constitutive activation of a number of nuclear proteins, most notably the E2Fs, freeing these factors to activate downstream proliferative pathways (73). Our results with HB60 cells are consistent with this hypothesis. The significant, albeit transient, Epo-induced increase in the expression of Rb coincides with a critical change in the phosphorylation status of this protein (Fig. 4A). Since Fli-1 expression in HB60-5 cells results in Rb phosphorylation, we do not exclude the possibility that Fli-1 is also involved in the posttranslational modifications of the Rb protein. Thus, both the expression level and progressive accumulation of the active hypophosphorylated form of Rb appear to be vital to the terminal differentiation of these cells. This notion is further supported by a recent study in which the level of Rb transcripts was found to be specifically and temporally regulated during embryogenesis, including hematopoiesis (25). Perhaps the time required to attain critical levels of hypophosphorylated Rb corresponds to a narrow time period during which cells can escape commitment to end-stage differentiation and clonal extinction (Fig. 10). Alternatively, it is also possible that the DNA binding activity of Fli-1 is altered as HB60-5 cells become committed to terminal differentiation. The affinity of Fli-1 for its target site may be reduced as the result of posttranslational modifications and/or lack of interaction with a partner protein. Indeed, results of EMSAs show the appearance of a Fli-1-containing complex (Fli-B) that binds the Rb promoter more readily than Fli-1 alone (Fli-A). Interestingly, the Ets-related protein Tel, which is rearranged in acute and chronic leukemias, has been shown to bind to Fli-1 and inactivate its transcriptional activity (31). Thus, it will be interesting to learn if Fli-B complex contains the Tel protein.
While Fli-1 deregulation affects both differentiation and growth of erythroid progenitor cells, our results strongly support the notion that the negative regulation of Rb affects mainly the differentiation phenotype of erythroid progenitor cells. Therefore, other downstream targets of Fli-1 likely contribute to the transformation process. This conclusion is supported by the fact that unlike Fli-1, which is activated by insertional mutagenesis, there is no evidence that the Rb gene is inactivated by proviral insertion in FV-induced erythroleukemias. Furthermore, transplantation of Rb−/− fetal liver cells into lethally irradiated syngeneic mice resulted in growth stimulation of erythrocytes, but erythroleukemia was not induced (22). These observations suggest that combined alteration in the expression of a number of Fli-1 target genes is required in order for erythroid progenitor cells to manifest malignant transformation by this transcription factor. Thus, it is possible that some of the Fli-1 target genes may also modify the phosphorylation state of the Rb protein.
The ability of Fli-1 to disrupt erythropoiesis suggests that mice lacking Fli-1 may have profound defects in hematopoiesis. Recently, Mélet and colleagues, using a gene targeting strategy, generated mice that express lower levels of truncated form of Fli-1 that appear to retain some Fli-1 functions (46). These mice exhibited thymus hypocellularity and demonstrated a delayed response to FV-induced erythroleukemia. The results presented here suggest that mice homozygous for null mutations in Fli-1 may exhibit defects in erythropoiesis.
In summary, we have shown that Fli-1 acts as a molecular switch capable of governing the fate of erythroblasts, namely, whether to self-renew or differentiate, in response to Epo. We have provided evidence that part of the transforming activity of Fli-1 is exerted by direct transcriptional repression of the Rb tumor suppressor gene. This constitutes the first transformation-relevant target function of Fli-1. Our results reinforce the notion that Rb is positioned at a critical point in a regulatory pathway that is disrupted during tumorigenesis. Thus, F-MuLV joins an extensive list of oncogenic viruses that have developed strategies to specifically target Rb, a tumor suppressor gene, for inactivation.
ACKNOWLEDGMENTS
Equal contributions were made by the first three authors.
We thank Alan Bernstein and Steven McKnight for generous gifts of recombinant Fli-1 and GABP antibodies and Stuart Berger for recombinant SCF. We also thank Alan Bernstein, Jorge Filmus, and Benoit Chabot for their comments on the manuscript, Barry Zochodone and Qi Li for excellent technical assistance, and Mina Viscardi for help in preparation of the manuscript.
This work was supported by grants from the National Cancer Institute of Canada to Y.B.-D. and the Medical Research Council of Canada to E.Z. Y.B.-D. is a Research Scholar of the National Cancer Institute of Canada. A.T. and U.-J.L. are supported by a fellowship from the Sunnybrook Trust for Medical Research, and R.R.H. is supported by the Leukemia Research Fund of Canada.
REFERENCES
- 1.Andrews N C, Faller D V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-David Y, Giddens E G, Letwin K, Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991;5:908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- 3.Ben-David Y, Prideaux V R, Chow V, Benchimol S, Bernstein A. Inactivation of the p53 oncogene by internal deletion or retroviral integration in erythroleukemic cell lines induced by Friend leukemia virus. Oncogene. 1988;3:179–185. [PubMed] [Google Scholar]
- 4.Bergh G, Ehinger M, Olofsson T, Baldetorp B, Johnsson E, Brycke H, Lindgren G, Olsson I, Gullberg U. Altered expression of the retinoblastoma tumor-suppressor gene in leukemic cell lines inhibits induction of differentiation but not G1-accumulation. Blood. 1997;89:2938–2950. [PubMed] [Google Scholar]
- 5.Chabot B, Stephenson D, Chapman V M, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 6.Chow V, Ben-David Y, Bernstein A, Benchimol S, Mowat M. Multistage Friend erythroleukemia: independent origin of tumor clones with normal or rearranged p53 cellular oncogenes. J Virol. 1987;61:2777–2781. doi: 10.1128/jvi.61.9.2777-2781.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke A R, Maandag E R, van Roon M, van de Lugt N M T, van der Valk M, Hooper M L, Berns A, te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359:328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- 8.Clarke M F, Kukowska-Latallo J F, Westin E, Smith M, Prochownik E V. Constitutive expression of a c-myb cDNA blocks Friend murine erythroleukemia cell differentiation. Mol Cell Biol. 1988;8:884–892. doi: 10.1128/mcb.8.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert K, de Jong P, Rouleau G, Aurias A, Thomas G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 10.Dmitrovsky E, Kuehl W M, Hollis G F, Kirsh I R, Bender T P, Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukemia cell line. Nature. 1986;322:748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- 11.Dolznig H, Bartunek P, Nasmyth K, Mullner E W, Beug H. Terminal differentiation of normal chicken erythroid progenitors: shortening of G1 correlates with loss of D-cyclin/cdk4 expression and altered cell size control. Cell Growth Differ. 1995;6:1341–1352. [PubMed] [Google Scholar]
- 12.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonucleases fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 13.Friend C, Scher W, Holland J G, Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci USA. 1971;68:378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill R M, Hamel P A, Zhe J, Zacksenhaus E, Gallie B L, Phillips R A. Characterization of the human RB1 promoter and of elements involved in transcriptional regulation. Cell Growth Differ. 1994;5:467–474. [PubMed] [Google Scholar]
- 15.Gobel M G, Moreau-Gachelin F, Ray D, Tambourin P, Tavitian A, Klemsz M J, McKercher S C, Van Beveren C, Maki R A. The PU.1 transcription factor is the product of the putative oncogene Spi-1. Cell. 1990;61:1165–1166. doi: 10.1016/0092-8674(90)90676-6. [DOI] [PubMed] [Google Scholar]
- 15a.Gross-Bellard M, Oudet P, Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973;36:32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 16.Hagemeier C, Bannister A J, Cook A, Kouzarides T. The activation domain of transcription factor PU.1 binds the retinoblastoma (RB) protein and the transcription factor TFIID in vitro: RB shows sequence similarity to TFIID and TFIIB. Proc Natl Acad Sci USA. 1993;90:1580–1584. doi: 10.1073/pnas.90.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamel P A, Gill R M, Phillips R A, Gallie B L. Transcriptional repression of the E2-containing promoter EIIaE, c-myc, and RB1 by the product of the RB1 gene. Mol Cell Biol. 1992;12:3431–3438. doi: 10.1128/mcb.12.8.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicks G G, Mowat M. Integration of Friend murine leukemia virus into both alleles of the p53 oncogene in an erythroleukemia cell line. J Virol. 1988;62:4752–4755. doi: 10.1128/jvi.62.12.4752-4755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard J C, Ung Y, Adachi D, Ben-David Y. p53-independent tumor growth and in vitro cell survival for F-MuLV-induced erythroleukemias. Cell Growth Differ. 1996;7:1651–1660. [PubMed] [Google Scholar]
- 20.Howard J C, Berger L, Bani M R, Hawley R, Ben-David Y. Activation of the erythropoietin gene in the majority of F-MuLV-induced erythroleukemias results in growth factor independence and enhanced tumorigenicity. Oncogene. 1996;12:1405–1415. [PubMed] [Google Scholar]
- 21.Howard J C, Yousefi S, Cheong G, Bernstein A, Ben-David Y. Temporal order and functional analysis of mutations within the Fli-1 and p53 genes during the erythroleukemias induced by F-MuLV. Oncogene. 1993;8:2721–2729. [PubMed] [Google Scholar]
- 22.Hu N, Gulley M L, Kung J T, Lee E Y H P. Retinoblastoma gene deficiency has mitogenic but not tumorigenic effects on erythropoiesis. Cancer Res. 1997;57:4123–4129. [PubMed] [Google Scholar]
- 23.Jacks T, Fazeli A, Schmitt E M, Bronson R T, Goodell M A, Weinberg R A. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs-Helber S M, Penta K, Sun Z, Lawson A, Sawyer S T. Distinct signaling from stem cell factor and erythropoietin in HCD57 cells. J Biol Chem. 1997;272:6850–6853. doi: 10.1074/jbc.272.11.6850. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Z, Zacksenhaus E, Gallie B L, Phillips R A. The retinoblastoma gene family is differentially expressed during embryogenesis. Oncogene. 1997;14:1789–1797. doi: 10.1038/sj.onc.1201014. [DOI] [PubMed] [Google Scholar]
- 26.Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1990;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- 27.Kieran M W, Perkins A C, Orkin S H, Zon L I. Thrombopoietin rescues in vitro erythroid colony formation from mouse embryos lacking the erythropoietin receptor. Proc Natl Acad Sci USA. 1996;93:9126–9131. doi: 10.1073/pnas.93.17.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiyokawa H, Richon V M, Rifkind R A, Marks P A. Suppression of cyclin-dependent kinase 4 during induced differentiation of erythroleukemia cells. Mol Cell Biol. 1994;14:7195–7203. doi: 10.1128/mcb.14.11.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klinken S P, Nicola N A, Johnson G R. In vitro-derived leukemic erythroid cell lines induced by a raf- and myc-containing retrovirus differentiate in response to erythropoietin. Proc Natl Acad Sci USA. 1988;85:8506–8510. doi: 10.1073/pnas.85.22.8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koury M J, Bondurant M. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- 31.Kwiatkowski B A, Bastian L S, Bauer T R, Tsai S, Zielinska-Kwiatkowska A G, Hickstein D D. The ets family member Tel binds to the Fli-1 oncoprotein and inhibits its transcriptional activity. J Biol Chem. 1998;273:17525–17530. doi: 10.1074/jbc.273.28.17525. [DOI] [PubMed] [Google Scholar]
- 32.Lachman H M, Hatton K S, Skoultchi A I, Schildkraut C L. c-myc mRNA levels in the cell cycle change in mouse erythroleukemia cells following inducer treatment. Proc Natl Acad Sci USA. 1985;82:5323–5327. doi: 10.1073/pnas.82.16.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lachman H M, Skoultchi A I. Expression of c-myc changes during differentiation of mouse erythroleukemia cells. Nature. 1984;310:592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- 34.LaMarco K, Thompson C C, Byers B P, Walton E M, McKnight S L. Identification of Ets- and notch-related subunits in GA binding protein. Science. 1991;253:789–792. doi: 10.1126/science.1876836. [DOI] [PubMed] [Google Scholar]
- 35.Lee E Y-H P, Chang C-Y, Hu N, Wang Y-C J, Lai C-C, Herrup K, Lee W-H, Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992;359:288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 36.Liboi E, Carroll M, D’Andrea A D, Mathey-Prevot B. Erythropoietin receptor signals both proliferation and erythroid-specific differentiation. Proc Natl Acad Sci USA. 1993;90:11351–11355. doi: 10.1073/pnas.90.23.11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin C S, Lim S K, D’Agati V, Costantini F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 38.Lu S J, Rowan S, Bani M R, Ben-David Y. Retroviral integration within the Fli-2 locus results in inactivation of the erythroid transcription factor NF-E2 in Friend erythroleukemias: evidence that NF-E2 is essential for globin expression. Proc Natl Acad Sci USA. 1994;91:8398–8402. doi: 10.1073/pnas.91.18.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maandag E C, van der Valk M, Vlaar M, Feltkamp C, O’Brien J, van Roon M, van der Lugt N, Berns A, te Riele H. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 1994;13:4260–4268. doi: 10.1002/j.1460-2075.1994.tb06746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mager D, Mak T W, Bernstein A. Friend leukemia virus-transformed cells, unlike normal stem cells, form spleen colonies in Sl/Sld mice. Nature. 1980;288:592–594. doi: 10.1038/288592a0. [DOI] [PubMed] [Google Scholar]
- 41.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 42.Mao X, Miesfeldt S, Yang H, Leiden J M, Thompson C B. The FLI-1 and chimeric ES-FLI-1 oncoproteins display similar DNA binding specificities. J Biol Chem. 1994;269:16216–16222. [PubMed] [Google Scholar]
- 43.Marks P A, Richon V M, Kiyokawa H, Rifkind R A. Inducing differentiation of transformed cells with hybrid polar compounds: a cell cycle-dependent process. Proc Natl Acad Sci USA. 1994;91:10251–10254. doi: 10.1073/pnas.91.22.10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.May W A, Gishizky M L, Lessnick S L, Lunsford L B, Lewis B C, Delattre O, Zucman J, Thomas G, Denny C T. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci USA. 1993;90:5752–5756. doi: 10.1073/pnas.90.12.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNiece I K, Langley K E, Zsebo K M. Recombinant human stem cell factor synergises with GM-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp Hematol. 1991;19:226–231. [PubMed] [Google Scholar]
- 46.Mélet F, Motro B, Rossi D J, Zhang L, Berinstein A. Generation of a novel Fli-1 protein by gene targeting leads to a defect in thymus development and a delay in Friend virus-induced erythroleukemia. Mol Cell Biol. 1996;16:2708–2715. doi: 10.1128/mcb.16.6.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Migliaccio G, Migliaccio A R, Druzin M L, Giardina P J, Zsebo K M, Adamson J W. Effects of recombinant human stem cell factor (SCF) on the growth of human progenitor cells in vitro. J Cell Physiol. 1991;148:503–509. doi: 10.1002/jcp.1041480324. [DOI] [PubMed] [Google Scholar]
- 48.Mittnacht S, Weinberg R A. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell. 1991;65:381–393. doi: 10.1016/0092-8674(91)90456-9. [DOI] [PubMed] [Google Scholar]
- 49.Moreau-Gachelin F, Tavitian A, Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukemias. Nature. 1988;331:277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- 50.Mowat M, Cheng A, Kimura N, Bernstein A, Benchimol S. Rearrangements of the cellular p53 gene in erythroleukemic cells transformed by Friend virus. Nature. 1985;314:633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- 51.Mucenski M L, McLain K, Kier A B, Swerdlow S H, Schreiner C M, Miller T A, Pietryga D W, Scott W J J, Potter S S. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 52.Munroe D G, Peacock J W, Benchimol S. Inactivation of the cellular p53 gene is a common feature of Friend erythroleukemia: relation to dominant transforming alleles. Mol Cell Biol. 1990;10:3307–3313. doi: 10.1128/mcb.10.7.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muta K. Stem cell factor retards differentiation of normal human erythroid progenitor cells while stimulating proliferation. Blood. 1995;86:572–580. [PubMed] [Google Scholar]
- 54.Nocka K, Majumder S, Chabot B, Ray P, Cervone M, Bernstein A, Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice—evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989;3:816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- 55.Orlando V, Paro R. Mapping Polycomb-repressed domains in the bithorax complex using in vivo formaldehyde cross-linked chromatin. Cell. 1993;75:1187–1198. doi: 10.1016/0092-8674(93)90328-n. [DOI] [PubMed] [Google Scholar]
- 56.Pawson T, Bernstein A. Receptor tyrosine kinases: genetic evidence for their role in Drosophila and mouse development. Trends Genet. 1990;6:350–356. doi: 10.1016/0168-9525(90)90276-c. [DOI] [PubMed] [Google Scholar]
- 57.Perkins A C, Sharpe A H, Orkin S H. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 58.Pevny L, Simon M C, Robertson E, Klein W H, Tsai S F, D’Agati V, Orkin S H, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 59.Prochownik E V, Kukowska J. Deregulation expression of c-myc by murine erythroleukaemia cells prevents differentiation. Nature. 1986;322:848–850. doi: 10.1038/322848a0. [DOI] [PubMed] [Google Scholar]
- 60.Quang C T, Wessely O, Pironin M, Beug H, Ghysdael J. Cooperation of Spi-1/PU.1 with an activated erythropoietin receptor inhibits apoptosis and Epo-dependent differentiation in primary erythroblasts and induces their Kit ligand-dependent proliferation. EMBO J. 1997;16:5639–5653. doi: 10.1093/emboj/16.18.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao V N, Ohno T, Prasad D D, Bhattacharya G, Reddy E S. Analysis of the DNA-binding and transcriptional activation functions of human Fli-1 protein. Oncogene. 1993;8:2167–2173. [PubMed] [Google Scholar]
- 62.Savoysky E, Mizuno T, Sowa Y, Watanabe H, Sawada J, Nomura H, Ohsugi Y, Handa H, Sakai T. The retinoblastoma binding factor 1 (RBF-1) site in RB gene promoter binds preferentially E4TF1, a member of the Ets transcription factors family. Oncogene. 1994;9:1839–1846. [PubMed] [Google Scholar]
- 63.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 64.Shibuya T, Mak T. Isolation and induction of erythroleukemic cell lines with properties of erythroid progenitor burst-forming cell (BFU-E) and erythroid precursor cell (CFU-E) Proc Natl Acad Sci USA. 1983;80:3721–3725. doi: 10.1073/pnas.80.12.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shivdasani R A, Orkin S H. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- 66.Solomon M J, Larsen P L, Varshavsky A. Mapping protein-DNA interactions in vivo with formaldehyde: evidence that histone H4 is retained on a highly transcribed gene. Cell. 1988;53:937–947. doi: 10.1016/s0092-8674(88)90469-2. [DOI] [PubMed] [Google Scholar]
- 67.Sowa Y, Shiio Y, Fujita T, Matsumoto T, Okuyama Y, Kato D, Inoue J, Sawada J, Goto M, Watanabe H, Handa H, Sakai T. Retinoblastoma binding factor 1 site in the core promoter region of the human RB gene is activated by hGABP/E4TF1. Cancer Res. 1997;57:3145–3148. [PubMed] [Google Scholar]
- 68.Templeton D J, Park S H, Lanier L, Weinberg R A. Nonfunctional mutants of the retinoblastoma protein are characterized by defects in phosphorylation, viral oncoprotein association, and nuclear tethering. Proc Natl Acad Sci USA. 1991;88:3033–3037. doi: 10.1073/pnas.88.8.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson C C, Brown T A, McKnight S L. Convergence of Ets- and notch-related structure motifs in a heteromeric DNA binding complex. Science. 1991;253:762–768. doi: 10.1126/science.1876833. [DOI] [PubMed] [Google Scholar]
- 70.Todokoro J, Watson R J, Higo H, Amanuma H, Kuramochi S, Yanagisawa H, Ikawa Y. Down-regulation of c-myb gene expression is a prerequisite for erythropoietin-induced erythroid differentiation. Proc Natl Acad Sci USA. 1988;85:8900–8904. doi: 10.1073/pnas.85.23.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai S F, Martin D I, Zon L I, D’Andrea A D, Wong G G, Orkin S H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989;339:446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- 72.Weber B I, Westin E H, Clarke M F. Differentiation of mouse erythroleukemia cells enhanced by alternatively spliced c-myb mRNA. Science. 1990;249:1291–1293. doi: 10.1126/science.2205003. [DOI] [PubMed] [Google Scholar]
- 73.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 74.Williams B O, Schmitt E M, Remington L, Bronson R T, Albert D M, Weinberg R A, Jacks T. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 1994;13:4251–4259. doi: 10.1002/j.1460-2075.1994.tb06745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu H, Klingmuller U, Acurio A, Hsiao J G, Lodish H F. Functional interaction of erythropoietin and stem cell factor receptors is essential for erythroid colony formation. Proc Natl Acad Sci USA. 1997;94:1806–1810. doi: 10.1073/pnas.94.5.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu H, Liu X, Jaenisch R, Lodish H F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 77.Youssoufian H, Longmore G, Neumann D, Yoshimura A, Lodish H F. Structure, function, and activation of the erythropoietin receptor. Blood. 1993;81:2223–2236. [PubMed] [Google Scholar]
- 78.Zacksenhaus E, Gill M, Phillips R A, Gallie B L. Molecular cloning and characterization of the mouse RB1 promoter. Oncogene. 1993;8:2343–2351. [PubMed] [Google Scholar]
- 79.Zhang L, Lemarchandel V, Romeo P, Ben-David Y, Bernstein A. The Fli-1 proto-oncogene involved in erythroleukemia and Ewing’s sarcoma, encodes a transcriptional activator with DNA-binding specificities distinct from other ets family members. Oncogene. 1993;8:1621–1630. [PubMed] [Google Scholar]