Abstract
Objectives
To evaluate the 1-year efficacy and safety of once-monthly erenumab 70 mg following a 24-week double-blind treatment period (DBTP) of a phase III randomised study of Japanese patients with episodic migraine (EM) or chronic migraine (CM).
Design
Multicentre open-label study.
Setting
A total of 41 centres in Japan.
Participants
Patients completing the DBTP continued into the 28-week open-label treatment period (OLTP). 254 of 261 (97.3%) randomised patients continued into the OLTP; 244 (93.5%) completed treatment.
Interventions
Once-monthly subcutaneous erenumab 70 mg.
Main outcome measures
Changes from baseline in monthly migraine days (MMD) and monthly acute migraine-specific medication treatment days (MSMD) reported via patient eDiary; proportion of ≥50% and ≥75% responders in MMD reduction from baseline; incidence and exposure-adjusted incidence of treatment-emergent adverse events (TEAEs).
Results
At week 24 of the DBTP, the mean (SE) change from baseline in MMD for the erenumab group was –3.8 (0.4) days (EM, –3.0 (0.4); CM, –5.2 (0.8)); in MSMD, –2.6 (0.4) days (EM, –2.1 (0.4); CM, –3.4 (0.7)). At the end of the OLTP (52 weeks postbaseline), the mean (SE) change from baseline in MMD was –4.7 (0.3) days (EM, –3.4 (0.3); CM, –6.9 (0.6)); in MSMD, –3.3 (0.3) days (EM, –2.4 (0.3); CM, –4.6 (0.5)). The proportion of ≥50% responders for MMD reduction in the erenumab group was 34.1% at week 24; 44.4% at week 52. The exposure-adjusted incidence of TEAEs was 219.7 per 100 patient-years during the OLTP (DBTP, 251.0 for the erenumab group). The most common TEAEs during the OLTP were nasopharyngitis, constipation and influenza. No new safety concerns were identified.
Conclusions
Erenumab treatment was associated with reduced migraine frequency in Japanese patients with EM or CM for up to 1 year. Overall safety results from the OLTP were consistent with DBTP results.
Trial registration number
Keywords: NEUROLOGY, Adult neurology, Migraine, Neurological pain, Clinical trials
STRENGTHS AND LIMITATIONS OF THIS STUDY.
While the 28-week open-label treatment period (OLTP) was short relative to other studies in non-Japanese patients, this study represents the longest follow-up time with erenumab in Japanese patients with chronic migraine.
Patients and study staff remained blinded to assignment (placebo or erenumab) in double-blind treatment period (DBTP) during OLTP.
Reporting exposure-adjusted rates normalises the rates of adverse events occurring during the DBTP and OLTP to equal exposure periods (ie, events per 100 patient-years), and thus allows for proper comparison between the DBTP and OLTP.
The OLTP of this study lacked a comparator arm.
Introduction
Migraine is a common neurological disease worldwide and a leading cause of disability associated with significant personal and societal effects.1–3 In Japan, 6%–8% of the population is affected by migraine, which places a substantial burden on patients and society related to quality of life, work productivity and costs.4–7 Because of concerns related to inadequate efficacy and poor tolerability, the use of standard of care oral preventive medications is low and is associated with high rates of discontinuation.6 8–11 Therefore, there is an unmet need for new migraine preventive medications.
Erenumab (erenumab-aooe in the USA), a fully human monoclonal antibody against the calcitonin gene-related peptide receptor, has been approved for the preventive treatment of adult migraine in over 70 countries worldwide, including the USA (2018), Europe (2018) and Japan (2021).12 13 The sustained efficacy and safety of erenumab in the preventive treatment of episodic migraine (EM) and chronic migraine (CM) have been demonstrated in several global clinical studies.14–18 In Japan, approval was based on two clinical studies in adult patients with EM or CM, which demonstrated erenumab to be safe and efficacious.19 20 Sustained efficacy and safety of erenumab for up to 2 years in Japanese patients with EM have also been demonstrated.21
Here, we report on the long-term (up to 1 year) efficacy, safety and tolerability of once-monthly erenumab 70 mg during a 28-week open-label treatment period (OLTP) after a 24-week double-blind treatment period (DBTP) of a phase III study, which demonstrated favourable efficacy and safety results for erenumab 70 mg in EM and CM.20
Methods
Study design
This multicentre (41 centres across Japan), 28-week OLTP followed a 24-week, randomised, double-blind, placebo-controlled, phase III study of once-monthly erenumab 70 mg in patients with EM or CM in Japan (ClinicalTrials.gov identifier NCT03812224) (online supplemental Figure S1; online supplemental table S1). Patients who completed the DBTP in each treatment group were eligible to participate in the OLTP and receive once-monthly erenumab 70 mg. The first patient entered the OLTP on 2 October 2019, and the last patient ended the OLTP on 20 November 2020. Randomisation was stratified by migraine status (EM or CM) and migraine preventive treatment status (ever used or never used) and was assigned by the sponsor using an interactive response technology system. During the DBTP, patients received once-monthly erenumab 70 mg or placebo in a 1:1 ratio; in the OLTP, all patients received once-monthly erenumab 70 mg. The study conforms to the guidelines set by the International Council for Harmonisation for Good Clinical Practice and by the Pharmaceuticals and Medical Devices Agency (PMDA). The study was designed according to the European Medicines Agency guideline on Clinical Investigation of Medicinal Products for the Treatment of Migraine, the International Headache Society (IHS) Guidelines for Controlled Trials of Drugs in Migraine, and advice given by the PMDA.22 23
bmjopen-2022-068616supp001.pdf (194.1KB, pdf)
Patient and public involvement statement
No patients or public representatives were involved in the design, conduct, reporting or dissemination efforts of the study results.
Patients
Patients who completed the DBTP (parent study) in each treatment group were eligible to participate in the OLTP and receive once-monthly erenumab 70 mg. Japanese patients aged 20 through 65 years with a history of migraine with or without aura (based on medical records or patient self-report) for at least 12 months before screening, as defined in the third edition of the International Classification of Headache Disorders of the IHS, and a diagnosis of EM (<15 headache days/month, ≥4 monthly migraine days (MMD)) or CM (≥15 headache days/month, ≥8 MMD) over the 3 months before screening, were included. Patients had to demonstrate at least 80% compliance with the eDiary during the baseline period prior to the DBTP. A detailed description of the eligibility criteria in the parent study has been described previously.20
Endpoints and assessments
Efficacy outcomes during the OLTP included changes from baseline in MMD and monthly acute migraine-specific medication treatment days (MSMD), and the proportion of patients who achieved at least a 50% or 75% reduction in MMD from baseline.
Patients used an eDiary to report clinical outcome assessments daily during weeks 33–36 and weeks 49–52. Clinical outcome assessments included the date and time of headache start and end; the worst pain severity of the headache; pain features (eg, one-sided, throbbing, worsens with exercise/physical activity); associated symptoms (eg, aura, nausea, vomiting, photophobia, phonophobia), and use of acute headache medications. A migraine day was defined as a migraine (with or without aura) that lasted for at least 4 hours and had at least two of the following pain features: unilateral, throbbing, moderate to severe or exacerbated with exercise or physical activity; or was associated with nausea, vomiting or photophobia and phonophobia. A migraine day also included a day in which a patient took a migraine-specific medication during aura or to treat a headache regardless of the duration and associated symptoms. A qualified headache day was a day characterised by onset, continuation or recurrence of a headache and met one of the following criteria: a migraine headache treated with acute migraine-specific medication, a non-migraine headache that lasted for at least 4 hours, or a headache for which acute headache treatment was used. An acute migraine-specific medication treatment day was defined as any day during which migraine-specific medication was used.
Safety endpoints included the incidence and exposure-adjusted incidence of treatment-emergent adverse events (TEAEs), clinical laboratory values and vital signs, and the incidence of anti-erenumab antibodies. Exposure-adjusted rates (per 100 patient-years) were calculated by dividing the number of patients with at least one reported occurrence of the TEAE of interest by the total time at risk for reporting the TEAE (patient-year) multiplied by 100. The time at risk was defined as the time from the first dose of erenumab to the onset of the TEAE or the end of study date. Reporting exposure-adjusted rates normalises the rates of adverse events occurring during the DBTP and OLTP to equal exposure periods (ie, events per 100 patient-years), and thus allows for a proper comparison between the DBTP and OLTP.
Statistical analysis
Analysis was performed after all patients had completed safety follow-up at the end of the study and included patients who received at least one dose of erenumab 70 mg in the OLTP. Efficacy and safety data were tabulated by the double-blind treatment group. Efficacy endpoints were analysed by using descriptive statistics based on observed data without imputation and were tabulated by visit. No formal testing was conducted. Patient incidence and exposure-adjusted incidence of TEAEs were tabulated by treatment group and by system organ class and preferred term. All analyses were performed using SAS System V.9.4 (SAS Institute).
Results
Patients
Of the 261 patients enrolled and randomised in the parent study (erenumab 70 mg, n=130; placebo, n=131), 254 (97.3%) entered the OLTP and received at least one dose of the investigational product (IP) and 244 (93.5%) completed the IP. Ten patients (3.8%) discontinued the IP for the following reasons: patient request (n=4; 1.5%), COVID-19 control measures (n=4; 1.5%), adverse event (n=1; 0.4%) and pregnancy (n=1; 0.4%) (online supplemental figure S2). In the OLTP population, the mean age of patients was 44.3 years, 86.6% were female, and the majority (77.6%) had used or were using migraine preventive treatment at baseline (table 1).
Table 1.
Baseline demographics and characteristics of the OLTP population
| Total (N=254) |
|
| Age, mean (SD), years | 44.3 (9.0) |
| Sex, female, n (%) | 220 (86.6) |
| Migraine type*, n (%) | |
| EM | 155 (61.0) |
| CM | 99 (39.0) |
| Migraine preventive treatment use, n (%) | |
| Ever used (including prior and/or current users) | 197 (77.6) |
| Never used | 57 (22.4) |
| MMD, mean (SE) | 12.2 (0.4) |
| MSMD, mean (SE) | 9.4 (0.4) |
| MHD, mean (SE) | 13.8 (0.4) |
N=number of patients in the analysis set. n=number of patients with observed data.
*Based on actual data collected instead of randomisation stratification.
CM, chronic migraine; EM, episodic migraine; MHD, monthly headache days; MMD, monthly migraine days; MSMD, monthly acute migraine-specific medication treatment days; OLTP, open-label treatment period; SE, standard error of the mean.
Efficacy
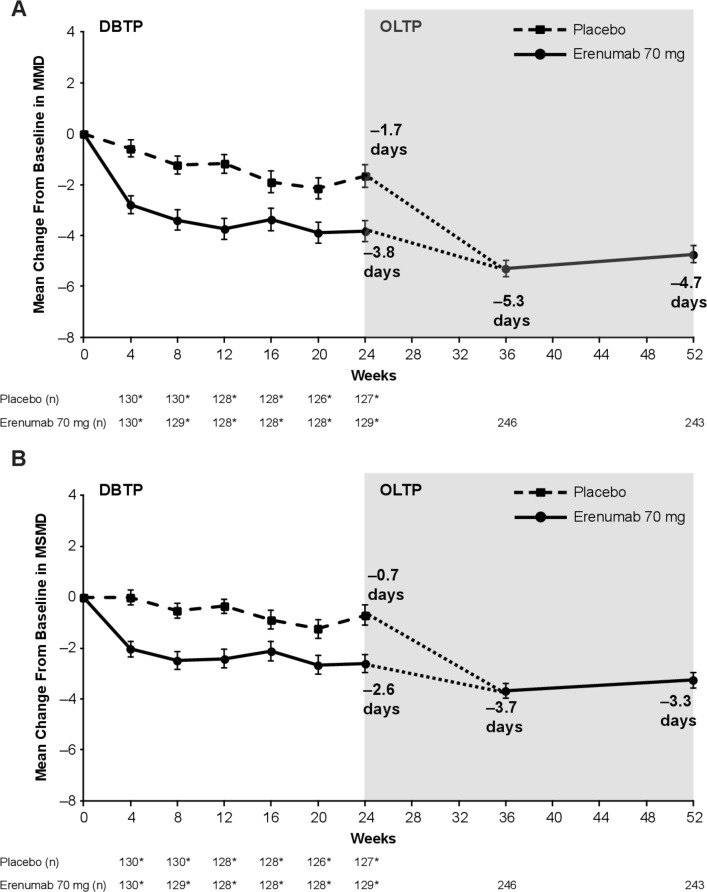
In the OLTP population (N=254; EM, n=155; CM, n=99), the mean (SE of the mean (SE)) MMD at baseline was 12.2 (0.4) days (EM, 8.3 (0.2) days; CM, 18.2 (0.4) days) and the mean (SE) MSMD was 9.4 (0.4) days (EM, 6.8 (0.3) days; CM, 13.6 (0.6) days) (table 1). At the end of the DBTP at week 24, the mean (SE) change from baseline in MMD for the erenumab 70 mg group was –3.8 (0.4) days (EM, –3.0 (0.4) days; CM, –5.2 (0.8) days)) and –1.7 (0.5) days for the placebo group; at the end of the OLTP at week 52, the mean (SE) change was –4.7 (0.3) days (EM, –3.4 (0.3); CM, –6.9 (0.6)) (figure 1, table 2).
Figure 1.
Change in (A) MMD and (B) MSMD from baseline. The mean (SE) change from baseline in MMD and MSMD during the DBTP and OLTP is shown for the treatment groups. For the OLTP, data are shown for the total population. The dotted line indicates that patients in the placebo group during the DBTP received erenumab 70 mg during the OLTP starting at week 24. *The number of patients in the efficacy analysis set during the DBTP. Error bars represent SE. n=number of patients with observed data. DBTP, double-blind treatment period; MMD, monthly migraine days; MSMD, monthly acute migraine-specific medication treatment days; OLTP, open-label treatment period.
Table 2.
Efficacy results during the OLTP
| EM (N=155) |
CM (N=99) |
Total (N=254) |
|
| Change from baseline in MMD, mean (SE) | |||
| Week 24* | n=78 –3.0 (0.4) |
n=51 –5.2 (0.8) |
n=129 –3.8 (0.4) |
| Week 36 | n=153 –3.7 (0.3) |
n=93 –8.0 (0.6) |
n=246 –5.3 (0.3) |
| Week 52 | n=147 –3.4 (0.3) |
n=96 –6.9 (0.6) |
n=243 –4.7 (0.3) |
| Change from baseline in MSMD, mean (SE) | |||
| Week 24* | n=78 –2.1 (0.4) |
n=51 –3.4 (0.7) |
n=129 –2.6 (0.4) |
| Week 36 | n=153 –2.8 (0.3) |
n=93 –5.2 (0.5) |
n=246 –3.7 (0.3) |
| Week 52 | n=147 –2.4 (0.3) |
n=96 –4.6 (0.5) |
n=243 –3.3 (0.3) |
| Achievement of ≥50% MMD response, n (%) | |||
| Week 24* | n=78 31 (39.7) |
n=51 13 (25.5) |
n=129 44 (34.1) |
| Week 36 | n=153 90 (58.8) |
n=93 40 (43.0) |
n=246 130 (52.8) |
| Week 52 | n=147 68 (46.3) |
n=96 40 (41.7) |
n=243 108 (44.4) |
Efficacy by EM and CM subgroups at week 24 of the DBTP and during the OLTP. N=number of patients in the open-label analysis set; n=number of patients with observed data.
*Data are shown for patients in the erenumab 70 mg group at week 24 of the DBTP in the efficacy analysis set.
CM, chronic migraine; DBTP, double-blind treatment period; EM, episodic migraine; MMD, monthly migraine days; MSMD, monthly acute migraine-specific medication treatment days; OLTP, open-label treatment period;SE, standard error of the mean;
At the end of the DBTP at week 24, the mean (SE) change from baseline in MSMD for the erenumab 70 mg group was –2.6 (0.4) days (EM, –2.1 (0.4); CM, –3.4 (0.7)) and –0.7 (0.4) days for the placebo group; at the end of the OLTP at week 52, the mean (SE) change was –3.3 (0.3) days (EM, –2.4 (0.3) days; CM, –4.6 (0.5) days) (figure 1, table 2). Throughout the 28-week OLTP, erenumab 70 mg demonstrated persistent efficacy in MMD and MSMD reduction in patients with EM or CM.
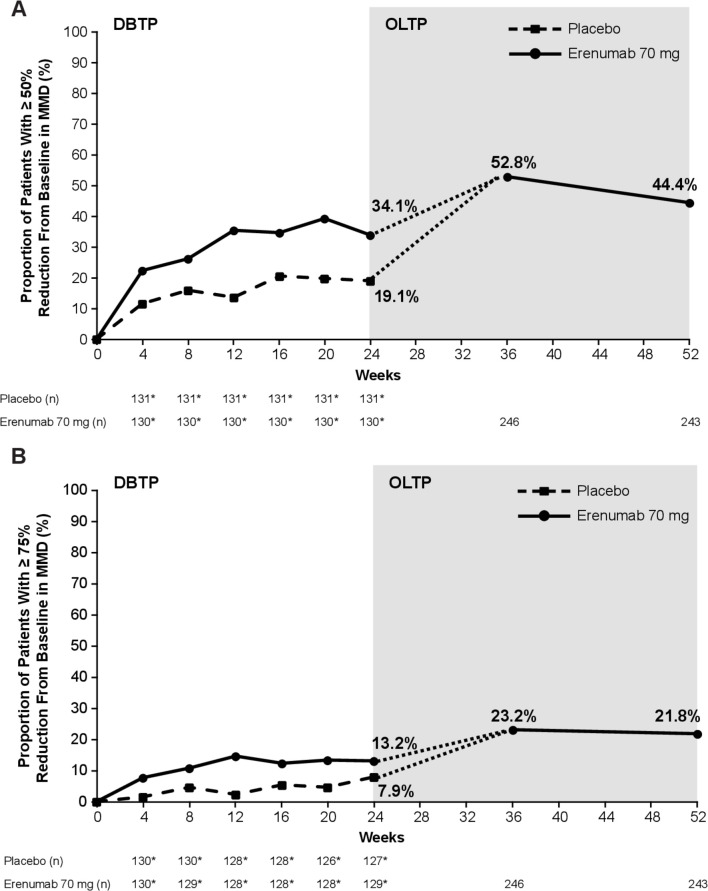
At week 24 of the DBTP, the proportion of patients who achieved at least a 50% reduction in MMD from baseline was 34.1% with erenumab 70 mg (EM, 39.7%; CM, 25.5%) and 19.1% with placebo (figure 2, table 2). The response was maintained and numerically higher throughout the OLTP than it was during the DBTP. At week 36 of the OLTP, 52.8% of the patients achieved the 50% threshold for MMD reduction (EM, 58.8%; CM, 43.0%); at week 52, it was 44.4% (EM, 46.3%; CM, 41.7%). The results were similar for patients responding at the 75% threshold for MMD reduction (figure 2).
Figure 2.
Patients achieving a (A) ≥50% and (B) ≥75% reduction in MMD from baseline. For the OLTP, data are shown for the total population. The dotted line indicates that patients in the placebo group during the DBTP received erenumab 70 mg during the OLTP starting at week 24. *The number of patients in the efficacy analysis set during the DBTP. n=number of patients with observed data. DBTP, double-blind treatment period; MMD, monthly migraine days; OLTP, open-label treatment period.
Safety
The mean (SD) exposure to erenumab 70 mg during the OLTP was 192.6 (20.0) days (total exposure to open-label treatment, 133.9 patient-years). The majority of patients (92.1%) received all seven doses of erenumab 70 mg during the OLTP.
During the OLTP, the incidence of TEAEs was 71.3% (181/254) (table 3). The exposure-adjusted incidence of TEAEs during the OLTP was 219.7 per 100 patient-years, which is similar to that in the erenumab group (251.0 per 100 patient-years) and in the placebo group (197.7 per 100 patient-years) during the DBTP. The majority of patients (62.2% (158/254)) experienced TEAEs of grade 2 or less. The most common (≥5 per 100 patient-years) TEAEs reported with erenumab (OLTP vs DBTP) were nasopharyngitis (32.8 vs 67.2 per 100 patient-years), constipation (7.8 vs 10.3 per 100 patient-years), influenza (6.6 vs 1.7 per 100 patient-years), gastroenteritis (6.5 vs 6.8 per 100 patient-years) and urticaria (5.9 vs 1.7 per 100 patient-years). Seven patients (2.8%) reported serious adverse events with erenumab during the OLTP, corresponding to an exposure-adjusted rate of 4.1 per 100 patient-years, which is similar to the rate reported during the DBTP in each treatment group (3.4 per 100 patient-years). During the OLTP, one patient with a serious adverse event discontinued treatment because of a grade 3 serious adverse event of drug eruption, which was considered by the investigator to be unrelated to erenumab treatment. No deaths were reported during the study. No clinically significant changes in laboratory values or vital signs were observed throughout the OLTP.
Table 3.
Safety results during the DBTP and OLTP
| DBTP | OLTP | ||
| Placebo (N=131) |
Erenumab 70 mg (N=130) |
Total (N=254) |
|
| All TEAEs, n (%) (r) | 78 (59.5) (197.7) | 86 (66.2) (251.0) | 181 (71.3) (219.7) |
| Grade ≥2 | 67 (51.1) (159.2) | 72 (55.4) (180.6) | 158 (62.2) (159.9) |
| Grade ≥3 | 2 (1.5) (3.4) | 4 (3.1) (6.8) | 12 (4.7) (7.1) |
| Serious AEs | 2 (1.5) (3.4) | 2 (1.5) (3.4) | 7 (2.8) (4.1) |
| Leading to IP discontinuation | 0 (0.0) (0.0) | 0 (0.0) (0.0) | 1 (0.4) (0.6) |
| Fatal AEs | 0 (0.0) (0.0) | 0 (0.0) (0.0) | 0 (0.0) (0.0) |
| Most common TEAEs, n (%) (r)* | |||
| Nasopharyngitis | 37 (28.2) (74.4) | 35 (26.9) (67.2) | 49 (19.3) (32.8) |
| Constipation | 1 (0.8) (1.7) | 6 (4.6) (10.3) | 13 (5.1) (7.8) |
| Influenza | 2 (1.5) (3.4) | 1 (0.8) (1.7) | 11 (4.3) (6.6) |
| Gastroenteritis | 4 (3.1) (6.7) | 4 (3.1) (6.8) | 11 (4.3) (6.5) |
| Urticaria | 0 (0.0) (0.0) | 1 (0.8) (1.7) | 10 (3.9) (5.9) |
| Developed antierenumab antibodies, n (%) | |||
| Developed binding anti-erenumab antibodies | NA | n’=129 7 (5.4) |
n’=254 9 (3.5) |
| Transient† | NA | 2 (28.6) | 4 (44.4) |
| Developed neutralising antierenumab antibodies | NA | NA | NA |
N=number of patients in the analysis set; n=number of patients with at least one occurrence of a TEAE or number of patients who developed anti-erenumab antibodies; n’=patients with a postbaseline result during the DBTP or OLTP; r=exposure-adjusted patient incidence rate per 100 patient-years.
*Exposure-adjusted rates of TEAEs of at least 5 per 100 patient-years during the OLTP.
†A negative result was reported at the patient’s last time point within the study period.
AE, adverse event; DBTP, double-blind treatment period; IP, investigational product; NA, not applicable; OLTP, open-label treatment period; TEAE, treatment-emergent adverse event.
Of the 254 patients in the OLTP, 9 (3.5%) developed antierenumab binding antibodies for the first time (negative or no result before the first OLTP dose), which is consistent with that observed during the DBTP (5.4%) (table 3). Of the nine patients who were positive for binding antibodies during the OLTP, six received placebo during the DBTP and three received erenumab during the DBTP and the OLTP. During the entire study, 16 patients (6.3%) developed antierenumab binding antibodies after erenumab treatment, of which 6 (37.5%) had transient antibodies (negative result at the last assessment). No patients developed antierenumab neutralising antibodies.
Discussion
The results of this 28-week OLTP study of erenumab 70 mg in Japanese patients with EM or CM demonstrated a persistence of efficacy for up to 1 year and a safety profile similar to that reported during the DBTP. From week 24 of the DBTP to the end of the OLTP at week 52, the reduction from baseline in MMD and MSMD, and the proportion of ≥50% and ≥75% responders in MMD reduction were maintained.
The incidence and exposure-adjusted incidence of TEAEs during the OLTP were consistent with those from the DBTP and previous studies,18 20 21 except for influenza and urticaria, which were numerically higher during the OLTP than they were during the DBTP. Furthermore, although the exposure-adjusted rates of constipation during the OLTP (7.8 per 100 patient-years) were consistent with those during the DBTP (10.0 per 100 patient-years), they were higher than those reported during the OLTP of the phase II study in Japanese patients with EM (2.6 per 100 patient-years).21 The development of antierenumab antibodies in 6.3% patients over the entire study was consistent with the 5.8% seen in the global CM OLE study and was lower than the 13.1% in the global EM OLE study.24 25 Neutralising antibodies were uncommon in the global studies and were not observed in this study. In addition, no new safety concerns regarding clinically relevant changes in laboratory assessments and vital signs were identified throughout the OLTP. Of the patients who entered the OLTP, 3.9% discontinued IP including 1 for an AE. The high proportion of patients completing erenumab treatment through both DBTP and OLTP (93.5%) reflects the excellent tolerability and sustained efficacy. In the pivotal topiramate trials, 28.7% of participants withdrew during the 8-month OLE, more than 40% of these due to AEs.26 The high retention rate also reduces the potential for bias that may be seen in open-label extension studies where patients may drop out for diminished efficacy, thus skewing the efficacy results over time.
The OLTP of this study was non-randomised and lacked a comparator arm, thus limiting the ability to distinguish study drug-specific effects on efficacy and safety from other factors. In addition, the duration of the 28-week OLTP was short relative to some other studies in non-Japanese patients.18 However, the study does represent the longest follow-up experience with erenumab in Japanese patients with CM and shows long-term efficacy and safety that are comparable to that seen in a global long-term study of erenumab in patients with CM.25 In the global CM study, the reduction in MMD and MSMD after 52 weeks for the erenumab 70 mg group was –7.8 days and –5.8 days, respectively; 47.4% of the patients achieved at least a 50% reduction from baseline in MMD. In the global EM study, the reduction in MMD and MSMD after the 52 week open-label period (study week 64) for the erenumab 70 mg group was –5.0 days and –2.4, respectively; 65% of the patients achieved at least a 50% reduction from baseline in MMD.24 This is comparable to the reductions in MMD and MSMD in this study at overall week 52 of –4.74 days and –3.26, respectively; 44.4% of the patients achieved at least a 50% reduction from baseline in MMD. These data support long-term treatment with erenumab in Japanese patients with EM and CM.
Conclusion
Treatment with erenumab was associated with a reduction in migraine frequency that was maintained for up to 1 year in Japanese patients with EM or CM. Erenumab had a safety profile similar to that observed in the DBTP; no new safety signals were identified during the OLTP.
Supplementary Material
Acknowledgments
Erenumab is codeveloped by Amgen and Novartis. The authors thank the patients and all the investigators who participated in this study. Medical writing support was provided by Qais Al-Hadid, PhD (Amgen). Editorial support was provided by Sangeeta P.C. (Cactus Communications).
Footnotes
Contributors: SC and GPdSL contributed to the conception and design of the study and acquired the data. KH, TT, FS, YN, RY, RK, MH, DY, GPdSL, and SC analyzed and interpreted the data, drafted the manuscript, critically reviewed and revised the manuscript for intellectual content, and provided final approval of the version to be published. KH, TT, FS, YN, RY, RK, MH, DY, GPdSL, and SC accept full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This study was funded by Amgen. No grants or awards were used for funding of this study.
Disclaimer: The Institutional review boards at each study centre (online supplemental table S1) approved the study protocol, informed consent forms, and any materials provided to the patients.
Competing interests: KH reports royalties from Amgen, Astellas, Daiichi Sankyo, Eisai, Merck Sharp & Dohme and Pfizer. TT has nothing to disclose. FS reports consulting fees from Amgen. RY, RK, MH, DY, GPdSL and SC are employees of and own stock in Amgen. YN owns stock in Amgen.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involved human patients and was approved by ethics committees (ECs) and institutional review boards (IRBs) listed in online supplemental table S1. Please note that the ECs and IRBs in Japan do not provide approval numbers. Participants gave informed consent to participate in the study before taking part.
References
- 1.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343–9. 10.1212/01.wnl.0000252808.97649.21 [DOI] [PubMed] [Google Scholar]
- 2.Stovner L, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27:193–210. 10.1111/j.1468-2982.2007.01288.x [DOI] [PubMed] [Google Scholar]
- 3.Agosti R. Migraine burden of disease: from the patient’s experience to a socio-economic view. Headache 2018;58 Suppl 1:17–32. 10.1111/head.13301 [DOI] [PubMed] [Google Scholar]
- 4.Sakai F, Igarashi H. Prevalence of migraine in Japan: a nationwide survey. Cephalalgia 1997;17:15–22. 10.1046/j.1468-2982.1997.1701015.x [DOI] [PubMed] [Google Scholar]
- 5.Takeshima T, Ishizaki K, Fukuhara Y, et al. Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache 2004;44:8–19. 10.1111/j.1526-4610.2004.04004.x [DOI] [PubMed] [Google Scholar]
- 6.Hirata K, Ueda K, Komori M, et al. Comprehensive population-based survey of migraine in Japan: results of the observational survey of the epidemiology, treatment, and care of migraine (overcome [Japan]) study. Curr Med Res Opin 2021;37:1945–55. 10.1080/03007995.2021.1971179 [DOI] [PubMed] [Google Scholar]
- 7.Kikui S, Chen Y, Todaka H, et al. Burden of migraine among Japanese patients: a cross-sectional national health and wellness survey. J Headache Pain 2020;21:110. 10.1186/s10194-020-01180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyers JL, Davis KL, Lenz RA, et al. Treatment patterns and characteristics of patients with migraine in Japan: a retrospective analysis of health insurance claims data. Cephalalgia 2019;39:1518–34. 10.1177/0333102419851855 [DOI] [PubMed] [Google Scholar]
- 9.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second International burden of migraine study (IBMS-II). Headache 2013;53:644–55. 10.1111/head.12055 [DOI] [PubMed] [Google Scholar]
- 10.Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm 2014;20:22–33. 10.18553/jmcp.2014.20.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 2015;35:478–88. 10.1177/0333102414547138 [DOI] [PubMed] [Google Scholar]
- 12.Aimovig (erenumab-aooe) . Full prescribing information. Thousand Oaks, CA: Amgen, Inc, 2020. [Google Scholar]
- 13.Aimovig (erenumab) . Summary of product characteristics. Dublin, Ireland: Novartis Europharm Limited, 2018. [Google Scholar]
- 14.Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016;15:382–90. 10.1016/S1474-4422(16)00019-3 [DOI] [PubMed] [Google Scholar]
- 15.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017;16:425–34. 10.1016/S1474-4422(17)30083-2 [DOI] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017;377:2123–32. 10.1056/NEJMoa1705848 [DOI] [PubMed] [Google Scholar]
- 17.Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018;38:1026–37. 10.1177/0333102418759786 [DOI] [PubMed] [Google Scholar]
- 18.Ashina M, Goadsby PJ, Reuter U, et al. Long-term efficacy and safety of erenumab in migraine prevention: results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol 2021;28:1716–25. 10.1111/ene.14715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai F, Takeshima T, Tatsuoka Y, et al. A randomized phase 2 study of erenumab for the prevention of episodic migraine in Japanese adults. Headache 2019;59:1731–42. 10.1111/head.13652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshima T, Sakai F, Hirata K, et al. Erenumab treatment for migraine prevention in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. Headache 2021;61:927–35. 10.1111/head.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakai F, Takeshima T, Tatsuoka Y, et al. Long-term efficacy and safety during open-label erenumab treatment in Japanese patients with episodic migraine. Headache 2021;61:653–61. 10.1111/head.14096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tassorelli C, Diener H-C, Dodick DW, et al. Guidelines of the international headache society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia 2018;38:815–32. 10.1177/0333102418758283 [DOI] [PubMed] [Google Scholar]
- 23.Tfelt-Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012;32:6–38. 10.1177/0333102411417901 [DOI] [PubMed] [Google Scholar]
- 24.Ashina M, Dodick D, Goadsby PJ, et al. Erenumab (AMG 334) in episodic migraine: interim analysis of an ongoing open-label study. Neurology 2017;89:1237–43. 10.1212/WNL.0000000000004391 [DOI] [PubMed] [Google Scholar]
- 25.Tepper SJ, Ashina M, Reuter U, et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: results from a 52-week, open-label extension study. Cephalalgia 2020;40:543–53. 10.1177/0333102420912726 [DOI] [PubMed] [Google Scholar]
- 26.Rapoport A, Mauskop A, Diener HC, et al. Long-term migraine prevention with topiramate: open-label extension of pivotal trials. Headache 2006;46:1151–60. 10.1111/j.1526-4610.2006.00506.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-068616supp001.pdf (194.1KB, pdf)
Data Availability Statement
Data are available on reasonable request. Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request.