Abstract
Ketamine, including esketamine, is an effective treatment for patients with treatment-resistant depression (TRD); however, its long-term efficacy in real-world populations remains poorly characterized. This is a retrospective cohort study using TriNetX US Collaborative Network, a platform aggregating electronic health records (EHRs) data from 93 million patients from 56 health care organizations in the US, and the study population includes 321,367 patients with a diagnosis of TRD who were prescribed relevant treatment in their EHRs. The prescription of ketamine (including esketamine) was associated with significant decreased risk of suicidal ideation compared to prescription of other common antidepressants: HR = 0.65 (95% CI: 0.53 – 0.81) at 1 day – 7 days, 0.78 (95% CI: 0.66 – 0.92) at 1 day – 30 days, 0.81 (95% CI: 0.70 – 0.92) at 1 day – 90 days, 0.82 (95% CI: 0.72 – 0.92) at 1 day – 180 days, and 0.83 (95% CI: 0.74 – 0.93) at 1 day – 270 days. This trend was especially robust among adults over 24 years of age, males, and White patients with TRD. No significant difference was observed for suicide attempts, except significantly increased risk for adolescents (aged 10-24) at 1 day – 30 days with HR = 2.22 (95% CI: 1.01-4.87). This study provides real-world evidence that ketamine has long-term benefits in mitigating suicidal ideation in patients with treatment-resistant depression. Future work should focus on optimizing dosage regimens for ketamine, understanding the mechanism, and the difference in various demographic subpopulations.
Introduction
Ketamine, a Food and Drug Administration (FDA)-approved anesthetic, and its S enantiomer S-ketamine (esketamine), provide well-characterized antidepressant benefits, rapidly and transiently alleviating manifestations of treatment-resistant depression (TRD), including suicidal ideation1-7. Although ketamine and esketamine are not identical, they share a mechanism of action in that both antagonize N-methyl D-aspartate receptors4,8. The benefits of both drugs for TRD and suicidal ideation have been demonstrated in clinical trials for up to one month after drug administration, but evidence of longer-term benefits remains limited9-16. Additionally, there is evidence that both drugs are associated with reduction of suicidal ideation, but the risk of actual suicidal behavior remains unknown6.
Suicide is a leading cause of death in the United States (and is the second leading cause of death among 10-34 year-olds and fifth leading cause of death among 35-54 year-olds)17, and was associated with over 45,000 deaths in 2021 alone18. In the same year, an estimated 12.3 million American adults had suicidal thoughts, and 1.7 million attempted suicide18. Thirty percent of patients with TRD attempt suicide at least once during their lifetime19-21. In 2019, the FDA approved esketamine nasal spray for the treatment of TRD24; however, ketamine is not FDA-approved for the treatment of any psychiatric disorder8.
In this large-scale retrospective cohort study, we assessed the risk of suicidal ideation and attempted suicide among TRD patients prescribed ketamine (including esketamine) compared to those prescribed other antidepressants and how the risk evolves over time from 7 days to 270 days. Since risk of suicide ideations and behaviors varies by age, gender and race22,23, we also conducted stratified analyses to examine these effects in different demographic subgroups.
Methods
(1). Database Description
The TriNetX Analytics Network platform is a large-scale, deidentified database. Data were obtained from the US Collaborative Network, which consists of 93 million unique patients from 56 healthcare organizations, covering diverse geographic locations, age groups, race and ethnic groups, income levels, and insurance types25. Built-in statistical functions within the TriNetX Analytics Platform perform statistical analyses on patient-level data and report population-level results without including protected health information. The Institutional Review Board of the MetroHealth System, Cleveland, Ohio has determined that any research using TriNetX in this manner is not Human Subject Research and is therefore exempt from IRB review. We have previously used TriNetX to conduct retrospective cohort studies26-39. This study follows the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines40.
(2). Study population
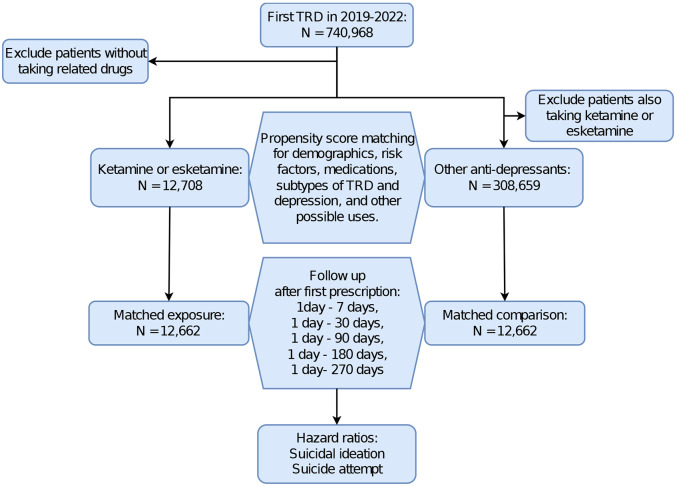
The study population consisted of patients who had their first encounter diagnosis of TRD and were followed by the prescription of related treatments between January 2019 and January 2022. To compare the prescription of ketamine (including esketamine) with that of other common antidepressants, the study population was divided into two cohorts: (1) an exposure cohort consisting of TRD patients who were prescribed ketamine (or esketamine), we will use “ketamine” through this paper to refer ketamine or esketamine, and (2) a comparison cohort consisting of TRD patients who were not prescribed ketamine but rather at least one of the following antidepressants: fluoxetine, paroxetine, sertraline, citalopram, escitalopram, vortioxetine, venlafaxine, duloxetine, doxepin, amitriptyline, trazodone, mirtazapine, or bupropion. The study population excluded patients not taking antidepressants as they may display relatively mild symptoms of depression or be in remission from TRD (Figure 1). Importantly, patients in the exposure cohort could be prescribed other antidepressants in addition to ketamine, as they are often prescribed in conjunction with other treatments41. Prescription of the non-ketamine antidepressants listed above was therefore balanced between the two cohorts using propensity-score matching.
Figure 1.
Flow diagram of patient selection and analysis in TriNetX. (TRD: treatment resistant depression)
(3). Statistical analysis
The outcomes of interest were one or more encounter diagnoses of suicidal ideation (ICD-10: R45.851 Suicidal ideation) and suicide attempt (ICD-10: T14.91 Suicide attempt).
Covariates that were matched between the exposure and comparison cohorts include demographics (age, gender, race, and ethnicity), and potential confounders5,42 including pre-existing medical conditions, medications, procedures, family history, and socioeconomical factors (Table 1). The full list of outcomes and covariates, as well as their standardized names, data types, and corresponding codes, are included in eTables 1 and 2.
Table 1.
The characteristics of patients prescribed ketamine (“Ketamine cohort”) and other antidepressants (“Comparison cohort”) before and after propensity-score matching (SMD: standardized mean differences, *SMD greater than 0.1, a threshold being recommended for declaring imbalance.)
| Characteristics | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Cohort, No. (%) | Cohort, No. (%) | |||||
| Ketamine cohort |
Comparison cohort |
SMD | Ketamine cohort |
Comparison cohort |
SMD | |
| Cohort size | 12,708 | 308,659 | 12,662 | 12,662 | ||
| Age at Index | 49.4 ± 16.7 | 43.2 ± 20.1 | 0.33* | 49.4 ± 16.7 | 50.2 ± 19.1 | 0.03 |
| Female | 65.8 | 69.0 | 0.07 | 65.8 | 65.8 | <.001 |
| Race and ethnicity | ||||||
| White | 74.5 | 72.8 | 0.04 | 74.4 | 74.4 | <.001 |
| Unknown Race | 12.2 | 13.7 | 0.05 | 12.2 | 12.5 | 0.01 |
| Black or African American | 11.8 | 11.2 | 0.02 | 11.8 | 11.4 | 0.01 |
| Hispanic or Latino | 6.2 | 7.5 | 0.05 | 6.2 | 6.1 | 0.01 |
| Asian | 0.8 | 1.6 | 0.08 | 0.8 | 0.8 | 0.01 |
| Risk factors and complications (based on encounter diagnosis International Classification of Diseases (ICD) codes) | ||||||
| Potential health hazards related to family and personal history | 85.6 | 57.3 | 0.66* | 85.5 | 86.7 | 0.03 |
| Anxiety disorder, unspecified | 59.9 | 44.9 | 0.31* | 59.8 | 60.7 | 0.02 |
| Hypertensive diseases | 56.7 | 33.3 | 0.48* | 56.6 | 57.1 | 0.01 |
| Sleep disorders | 49.9 | 28.3 | 0.45* | 49.8 | 50.1 | 0.01 |
| Chronic pain, not elsewhere classified | 47.3 | 19.9 | 0.61* | 47.2 | 47.6 | 0.01 |
| Overweight and obesity | 46.1 | 23.2 | 0.49* | 46.0 | 46.5 | 0.01 |
| Mental and behavioral disorders due to psychoactive substance use | 46.0 | 29.0 | 0.36* | 45.9 | 46.4 | 0.01 |
| Diabetes mellitus | 27.7 | 14.6 | 0.32* | 27.7 | 28.1 | 0.01 |
| Chronic ischemic heart disease | 17.7 | 7.5 | 0.31* | 17.6 | 18.3 | 0.02 |
| Post-traumatic stress disorder (PTSD) | 15.3 | 11.6 | 0.11* | 15.2 | 15.1 | <.001 |
| Heart failure | 14.5 | 4.8 | 0.33* | 14.5 | 14.5 | <.001 |
| Persons with potential health hazards related to socioeconomic and psychosocial circumstances | 14.3 | 11.3 | 0.09 | 14.3 | 14.2 | <.001 |
| Pre-existing suicidal ideations | 13.9 | 14.8 | 0.03 | 13.8 | 13.8 | <.001 |
| Epilepsy and recurrent seizures | 6.4 | 3.2 | 0.15* | 6.3 | 6.7 | 0.02 |
| Schizophrenia, schizotypal, delusional, and other non-mood psychotic disorders | 5.8 | 4.0 | 0.08 | 5.7 | 5.6 | 0.01 |
| Cerebral infarction | 5.7 | 2.8 | 0.14* | 5.7 | 6.0 | 0.01 |
| Personal history of self-harm | 5.7 | 6.0 | 0.01 | 5.6 | 5.4 | 0.01 |
| Intentional self-harm | 2.4 | 1.3 | 0.08 | 2.3 | 2.5 | 0.01 |
| Pre-existing suicide attempt | 2.1 | 1.5 | 0.04 | 2.0 | 1.9 | 0.01 |
Statistical analyses were conducted in the TriNetX Advanced Analytics platform. Cohorts were propensity-score matched (1:1 matching using the nearest neighbor greedy matching algorithm) for the above covariates. The index event for the exposure and comparison cohorts was the first prescription of either ketamine or other antidepressant after TRD diagnosis. Hazard ratios of the outcomes of interest at 1 day – 7 days, 1 day −30 days, 1 day – 90 days, 1 day – 180 days, and 1 day – 270 days after drug prescription were compared between matched cohorts using hazard ratios (HRs) and 95% confidence intervals (CIs), and p-values were calculated at a significance of P < 0.05 (2-sided t-test)43 (Figure 1).
As suicidal thoughts and behaviors vary by age, gender, and race18, stratified analyses were performed in subgroups distinguished by age (adolescents [10-24 years old]44,45, adults [>=24 years old])46, gender (female, male)22 and race (White, Black)47. Due to the relatively small sample size, stratified analyses were not performed for other races. Figure 1 presents the flow diagram of patient selection and analysis in TriNetX.
Results
(1). Patient characteristics
Table 1 and eTable 3 present the characteristics of the patients in the exposure cohort (those prescribed ketamine) and the comparison cohort (those prescribed other antidepressants) before and after propensity-score matching. Before matching, the exposure cohort was older (average 49.4 vs. 43.2 years) and had significantly higher prevalence of comorbidities and adverse socioeconomic determinants of health. The two cohorts were balanced after propensity-score matching, yielding 12,662 patients each in the exposure and comparison cohorts (Table 1).
(2). Ketamine prescription is associated with decreased suicidal ideation compared to other antidepressant prescription.
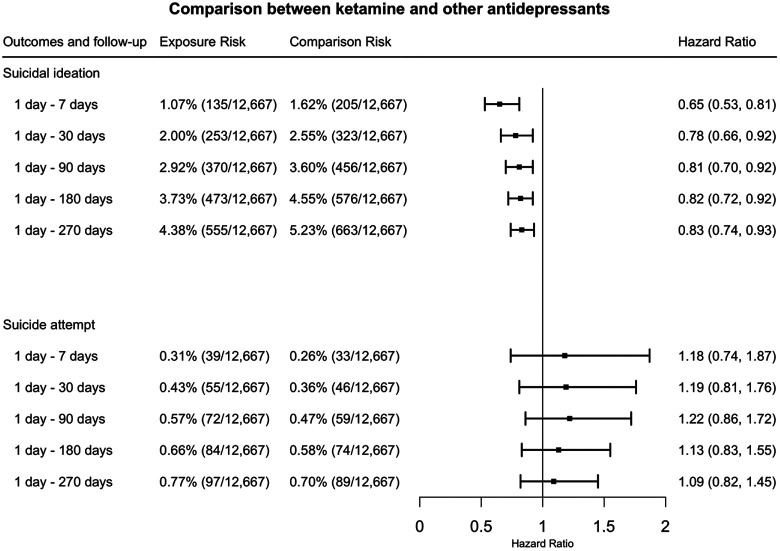
As shown in Figure 2, the prescription of ketamine was associated with significant decrease in suicidal ideation: HR = 0.65 (95% CI: 0.53 – 0.81) at 1 day – 7 days, 0.78 (95% CI: 0.66 – 0.92) at 1 day – 30 days, 0.81 (95% CI: 0.70 – 0.92) at 1 day – 90 days, 0.82 (95% CI: 0.72 – 0.92) at 1 day – 180 days, 0.83 (95% CI: 0.74 – 0.93) at 1 day – 270 days. No significant difference was observed for suicide attempt.
Figure 2.
Comparison of hazard of suicidal ideation and suicide attempt among patients with TRD between propensity-score matched ketamine cohort and the comparison cohort (other anti-depressants) at 1day – 7 days, 1 day −30 days, 1 day – 90 days, 1 day – 180 days, 1 day – 270 days after initial prescription. (TRD: treatment resistant depression)
(3). The association between ketamine prescription and suicidal ideation or suicide attempt varies by age, gender, and race.
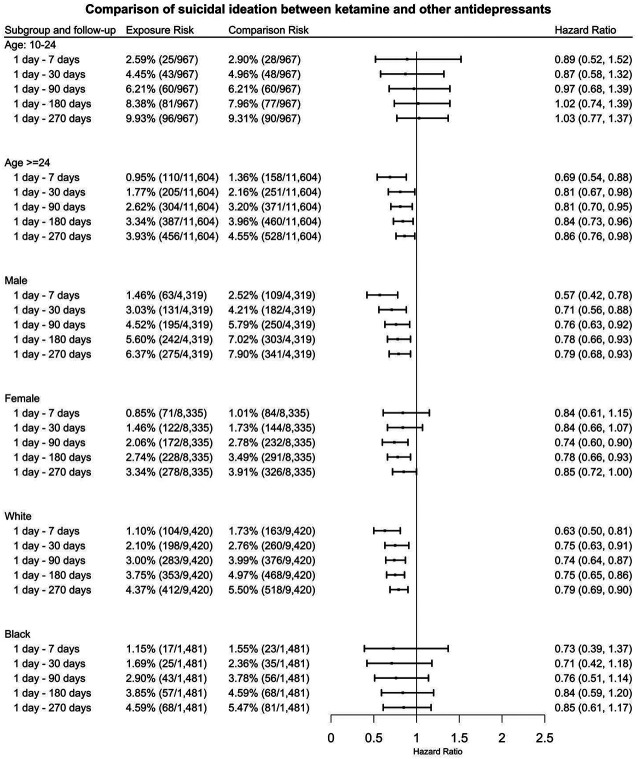
In patients ages 24 and older, ketamine prescription is associated with significant decrease in suicidal ideation compared to prescription of other antidepressants at 1 day – 7 days, 1 day −30 days, 1 day – 90 days, 1 day – 180 days, 1 day – 270 days after initial prescription: HR = 0.69 (95% CI: 0.54 – 0.88), 0.81 (95% CI: 0.67 – 0.98), 0.81 (95% CI: 0.70 – 0.95), 0.84 (95% CI: 0.73 - 0.96), 0.86 (95% CI: 0.76-0.98) (Figure 3). In male patients, ketamine prescription is associated with significant decrease in suicidal ideation compared to prescription of other antidepressants at 1 day – 7 days, 1 day −30 days, 1 day – 90 days, 1 day – 180 days, 1 day – 270 days after initial prescription: HR = 0.57 (95% CI: 0.42 – 0.78), 0.71 (95% CI: 0.56 – 0.88), 0.76 (95% CI: 0.63 – 0.92), 0.78 (95% CI: 0.66 - 0.93), 0.79 (95% CI: 0.68-0.93) (Figure 3). In White patients, the prescription of ketamine is associated with significant decrease in suicidal ideation compared to prescription of other common antidepressants at 1 day – 7 days, 1 day −30 days, 1 day – 90 days, 1 day – 180 days, 1 day – 270 days after initial prescription: HR = 0.63 (95% CI: 0.50 – 0.81), 0.75 (95% CI: 0.63 – 0.91), 0.74 (95% CI: 0.64 – 0.87), 0.75 (95% CI: 0.65 - 0.86), 0.79 (95% CI: 0.69-0.90) (Figure 3). In female patients, the prescription of ketamine is associated with significant decrease in suicidal ideation compared to prescription of other common antidepressants at 1 day – 90 days, 1 day – 180 days, 1 day – 270 days after initial prescription: HR = 0.74 (95% CI: 0.60 – 0.90), 0.78 (95% CI: 0.66 – 0.93), 0.85 (95% CI: 0.72 – 1.00). However, no significant differences were observed at 1 day – 7 days or 1 day – 30 days (Figure 3). No significant differences were observed for risk of suicidal ideation between the exposure and comparison cohorts in adolescents (aged 10-24 years) or Black patients.
Figure 3.
Comparison of hazard of suicidal ideation among patients with TRD (matched ketamine cohort vs. other anti-depressants cohort) stratified by age, gender, race groups at 1day – 7 days, 1 day −30 days, 1 day – 90 days, 1 day – 180 days, 1 day – 270 days after initial prescription. (TRD: treatment resistant depression)
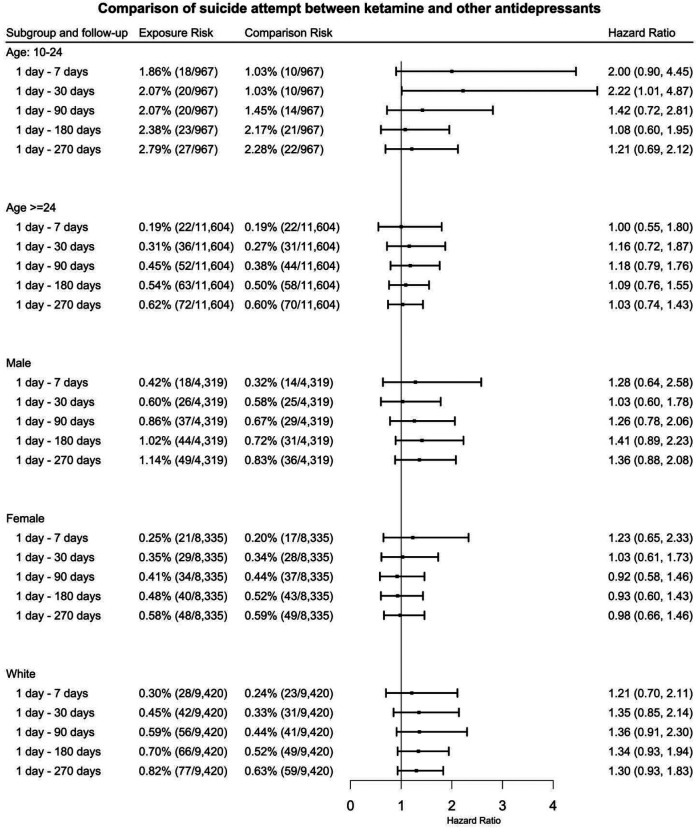
In patients aged 10-24, ketamine prescription is associated with increased risk of suicide attempt at 1 day – 30 days: HR = 2.22 (95% CI: 1.01-4.87). No other significant differences were observed for suicide attempt among the demographic-stratified subgroups (Figure 4), and the results for Black patients are not presented due to insufficient sample size.
Figure 4.
Comparison of hazard of suicide attempt among patients with TRD (matched ketamine cohort vs. other anti-depressants cohort) stratified by age, gender, race groups at 1day – 7 days, 1 day −30 days, 1 day – 90 days, 1 day – 180 days, 1 day – 270 days after initial prescription. (TRD: treatment resistant depression)
Discussion
Using a large-scale platform of aggregated patient electronic health record data, our study reveals that prescription of ketamine is associated with significant decrease in suicidal ideation in both the short- (1-30 days) and long-term (1-270 days) compared to other common antidepressants, and this trend was especially robust in those who were >=24 years of age, male, and White; however, no difference was observed in suicide attempt at any time point, except increased risk for adolescents (aged 10-24) at 1 day – 30 days. Randomized controlled trials have demonstrated that ketamine and esketamine can mitigate suicidal ideation in the short-term (one week, one month)2,3,48-50, consistent with our findings. This study also provides evidence of a long-term association between ketamine prescription and decrease in suicidal ideation in patients with TRD. This is also the first observational study to our knowledge that examines the risk over time of attempted suicide in patients with TRD who were prescribed ketamine versus other antidepressants.
In our results, decreased suicidal ideation does not translate into decreased suicide attempts. It has been recognized that suicidal ideation and suicide attempt have different mechanisms according to the ideation-to-action framework51,52, and there is no linear relationship between them53. Findings in stratified analyses suggest the decreased risk of suicidal ideation observed in those prescribed ketamine is especially strong among White, men, and over 24 years of age with TRD, which is possibly contributed by factors such as hormonal differences54, brain development44 and placebo effect55.
This study has several limitations. First, the results only represent individuals who had medical encounters with health care systems that provide data to TriNetX. Therefore, their generalizability to other populations needs to be further tested. Second, TriNetX only identifies the event of drug prescription, not the length of prescription or adherence to medication regimens. Since ketamine is potentially neurotoxic56, particularly with longer-term administration, clinicians typically prescribe ketamine for only a short period of time, after which patients are transitioned to maintenance treatment with antidepressants and/or psychotherapy57. Though our retrospective cohort study could not incorporate the duration of drug use or the specific antidepressants the exposure cohort switched to after the initial therapy of ketamine, the reduced effect of suicidal ideation persisted at 270 days post-prescription, indicating potential long-term benefits after pharmacotherapy transition. Third, we balanced the exposure and comparison groups using extensive propensity-score matching for demographics, risk factors, complications, and other treatments; however, there could be unmeasured confounders that have skewed the results. Fourth, due to the nature of retrospective cohort studies, our results cannot establish causality between ketamine prescription and reduction in suicidal ideation and cannot be used to impute the mechanism. Fifth, we utilized diagnosis of suicide attempt as a proxy for suicidal behavior, and TriNetX only includes the information that is entered during patients’ encounters with health organizations. Consequently, other suicidal behaviors that occurred outside of patient medical encounters may not be captured, resulting in missing outcome data and potential bias. Sixth, sufficient dosage data are not available on TriNetX, so it could not be determined if the relationship between ketamine prescription and suicidal ideation is dose dependent.
Future work should focus on optimizing dosage regimens for ketamine when it is used to treat TRD. The benefit and risk profile for esketamine has been established, as described in FDA-approved labelling58 and the approved Risk Evaluation and Mitigation Strategy program24. However, there is no FDA-approved dosing regimen for ketamine. Among the 740 968 patients from TriNetX platform who had first diagnosis of TRD during 2019-2022, there were 12,404 patients taking ketamine and 728 patients taking esketamine after their diagnosis. Considering the different dosing regimens, increasing popularity of ketamine for TRD, and potential adverse events related to ketamine prescription59, it is crucial to determine safe and effective dosing for ketamine when indicated for TRD8. In this study, we combined ketamine and esketamine together because of their similar mechanism of action and insufficient sample size in esketamine. A head-to-head comparison between ketamine and esketamine is necessary to examine the difference of these two drugs in the future. It is also important to further understand the underlying mechanism of ketamine effect among patients with TRD. One possible explanation is that ketamine can cause dissociative effects linked to an antidepressant response60. However, a recent clinical trial indicated that administration of ketamine under general anesthesia is no better than placebo at alleviating depression in the short term, and had similar effect to the previous ketamine trials in awake patients, suggesting that the drug may work through a patient’s interactions with medical professionals and a belief in improvement, rather than the biochemical effect of ketamine per se61. Further work is also needed to understand why the association between ketamine prescription with suicidal ideation and suicide attempt varies between different demographic subpopulations. It is worrisome that ketamine prescription did not mitigate suicidal ideation in adolescents and even was associated with increased trend of suicide attempt, which suggests the cautious prescription in this subpopulation. Future work is necessary to be conducted on larger sample size of adolescents.
Conclusion
Our study provides real-world evidence that patients with TRD who were prescribed ketamine experienced significant long-term decrease in suicidal ideation compared with patients who were prescribed other antidepressants, within 270 days following the prescription. Findings from this study provide data to balance the benefits of ketamine with its reported adverse effects, such as dissociation, psychosis, hypertension, tachycardia, tolerance, and addiction56,59,62. Future work should focus on longer follow-up time, optimized dosage regimens for ketamine, its mechanism of action with respect to TRD and suicidal ideation, and disparities in efficacy between various demographic subgroups.
Funding/Support:
We acknowledge support from National Institute on Aging (Grants nos. AG057557, AG061388, AG062272, AG076649), National Institute on Alcohol Abuse and Alcoholism (Grant nos. AA029831).
Role of the Funder/Sponsor:
The funders have no roles in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Disclosures: The authors have no conflict of interest.
Availability of data and materials
This study used population-level aggregate and de-identified data generated by the TriNetX Platform. Due to data privacy, patient-level data was not used and cannot be shared.
References
- 1.Andrade C. Ketamine for Depression, 3: Does Chirality Matter? J Clin Psychiatry. 2017;78(6):10104. doi: 10.4088/JCP.17f11681 [DOI] [PubMed] [Google Scholar]
- 2.Witt K, Potts J, Hubers A, et al. Ketamine for suicidal ideation in adults with psychiatric disorders: a systematic review and meta-analysis of treatment trials. Australian & New Zealand Journal of Psychiatry. 2020;54(1):29–45. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson ST, Ballard ED, Bloch MH, et al. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am J Psychiatry. 2018;175(2):150–158. doi: 10.1176/appi.ajp.2017.17040472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. American Journal of Psychiatry. 2015;172(10):950–966. [DOI] [PubMed] [Google Scholar]
- 5.Ketamine and esketamine for treating unipolar depression in adults: Administration, efficacy, and adverse effects - UpToDate. Accessed October 24, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects#H963778187
- 6.Trivedi MH. Antisuicidal Effects of Ketamine: A Promising First Step. Am J Psychiatry. 2018;175(2):97–98. doi: 10.1176/appi.ajp.2017.17111261 [DOI] [PubMed] [Google Scholar]
- 7.Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for Nonpsychotic Treatment-Resistant Major Depression. New England Journal of Medicine. 2023;0(0):null. doi: 10.1056/NEJMoa2302399 [DOI] [PubMed] [Google Scholar]
- 8.Research C for DE and. FDA alerts health care professionals of potential risks associated with compounded ketamine nasal spray. FDA. Published online February 16, 2022. Accessed March 1, 2023. https://www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-professionals-potential-risks-associated-compounded-ketamine-nasal-spray [Google Scholar]
- 9.Han Y, Chen J, Zou D, et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr Dis Treat. 2016;12:2859–2867. doi: 10.2147/NDT.S117146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Hackett M, Carter G, et al. Effects of Low-Dose and Very Low-Dose Ketamine among Patients with Major Depression: a Systematic Review and Meta-Analysis. Int J Neuropsychopharmacol. 2016;19(4):pyv124. doi: 10.1093/ijnp/pyv124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612. doi: 10.1002/14651858.CD011612.pub2 [DOI] [PubMed] [Google Scholar]
- 12.McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693–704. doi: 10.1017/S0033291714001603 [DOI] [PubMed] [Google Scholar]
- 13.Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl). 2014;231(18):3663–3676. doi: 10.1007/s00213-014-3664-5 [DOI] [PubMed] [Google Scholar]
- 14.Singh JB, Fedgchin M, Daly E, et al. Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study. Biol Psychiatry. 2016;80(6):424–431. doi: 10.1016/j.biopsych.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson ST, Katz RB, Toprak M, Webler R, Ostroff RB, Sanacora G. Acute and Longer-Term Outcomes Using Ketamine as a Clinical Treatment at the Yale Psychiatric Hospital. J Clin Psychiatry. 2018;79(4):17m11731. doi: 10.4088/JCP.17m11731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szymkowicz SM, Finnegan N, Dale RM. A 12-month naturalistic observation of three patients receiving repeat intravenous ketamine infusions for their treatment-resistant depression. J Affect Disord. 2013;147(1-3):416–420. doi: 10.1016/j.jad.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPhillips D. Suicide rises to 11th leading cause of death in the US in 2021, reversing two years of decline. CNN. Published April 13, 2023. Accessed May 30, 2023. https://www.cnn.com/2023/04/13/health/suicide-rates-2021-cdc/index.html [Google Scholar]
- 18.Facts About Suicide ∣ Suicide ∣ CDC. Accessed January 9, 2023. https://www.cdc.gov/suicide/facts/ [Google Scholar]
- 19.Cartwright C, Gibson K, Read J, Cowan O, Dehar T. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401–1407. doi: 10.2147/PPA.S110632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergfeld IO, Mantione M, Figee M, Schuurman PR, Lok A, Denys D. Treatment-resistant depression and suicidality. Journal of affective disorders. 2018;235:362–367. [DOI] [PubMed] [Google Scholar]
- 21.Nischal A, Tripathi A, Nischal A, Trivedi JK. Suicide and Antidepressants: What Current Evidence Indicates. Mens Sana Monogr. 2012;10(1):33–44. doi: 10.4103/0973-1229.87287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges G, Nock MK, Haro Abad JM, et al. Twelve-month prevalence of and risk factors for suicide attempts in the World Health Organization World Mental Health Surveys. J Clin Psychiatry. 2010;71(12):1617–1628. doi: 10.4088/JCP.08m04967blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nock MK, Borges G, Bromet EJ, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192(2):98–105. doi: 10.1192/bjp.bp.107.040113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic ∣ FDA. Accessed January 9, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified [Google Scholar]
- 25.TriNetX. TriNetX. Accessed June 1, 2022. https://trinetx.com/
- 26.Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R. Incidence rates and clinical outcomes of SARS-CoV-2 infection with the Omicron and Delta variants in children younger than 5 years in the US. JAMA pediatrics. Published online 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Davis PB, Kaelber DC, Xu R. COVID-19 breakthrough infections and hospitalizations among vaccinated patients with dementia in the United States between December 2020 and August 2021. Alzheimer’s & Dementia. Published online 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Davis PB, Kaelber DC, Volkow ND, Xu R. Comparison of mRNA-1273 and BNT162b2 vaccines on breakthrough SARS-CoV-2 infections, hospitalizations, and death during the delta-predominant period. Jama. 2022;327(7):678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Wang Q, Davis PB, Volkow ND, Xu R. Increased risk for COVID-19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry. 2022;21(1):124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Kaelber DC, Xu R, Berger NA. Breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with cancer in the US between December 2020 and November 2021. JAMA oncology. Published online 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Berger NA, Xu R. Risks of SARS-CoV-2 breakthrough infection and hospitalization in fully vaccinated patients with multiple myeloma. JAMA network open. 2021;4(11):e2137575–e2137575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Davis PB, Volkow ND, Berger NA, Kaelber DC, Xu R. Association of COVID-19 with New-Onset Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2022;89(2):411–414. doi: 10.3233/JAD-220717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-CoV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Network Open. 2022;5(9):e2233014–e2233014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Y, Davis PB, Kaebler DC, Blankfield RP, Xu R. Cardiovascular risk of gabapentin and pregabalin in patients with diabetic neuropathy. Cardiovascular Diabetology. 2022;21(1):170. doi: 10.1186/s12933-022-01610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Kaelber DC, Xu R, Berger NA. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: A clarion call for maintaining mitigation and ramping-up research. Blood reviews. Published online 2022:100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Volkow ND, Berger NA, Davis PB, Kaelber DC, Xu R. Association of COVID-19 with endocarditis in patients with cocaine or opioid use disorders in the US. Molecular Psychiatry. Published online 2022:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olaker VR, Kendall EK, Wang CX, et al. Association of Recent SARS-CoV-2 Infection With New-Onset Alcohol Use Disorder, January 2020 Through January 2022. JAMA Network Open. 2023;6(2):e2255496. doi: 10.1001/jamanetworkopen.2022.55496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorenflo MP, Davis PB, Kendall EK, Olaker VR, Kaelber DC, Xu R. Association of Aspirin Use with Reduced Risk of Developing Alzheimer’s Disease in Elderly Ischemic Stroke Patients: A Retrospective Cohort Study. Journal of Alzheimer’s Disease. 2023;91(2):697–704. doi: 10.3233/JAD-220901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding P, Pan Y, Wang Q, Xu R. Prediction and evaluation of combination pharmacotherapy using natural language processing, machine learning and patient electronic health records. Journal of Biomedical Informatics. 2022;133:104164. doi: 10.1016/j.jbi.2022.104164 [DOI] [PubMed] [Google Scholar]
- 40.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Annals of internal medicine. 2007;147(8):573–577. [DOI] [PubMed] [Google Scholar]
- 41.Kishimoto T, Chawla J, Hagi K, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychological medicine. 2016;46(7):1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unipolar treatment-resistant depression in adults: Epidemiology, risk factors, assessment, and prognosis - UpToDate. Accessed April 17, 2023. https://www.uptodate.com/contents/unipolar-treatment-resistant-depression-in-adults-epidemiology-risk-factors-assessment-and-prognosis?topicRef=119992&source=see_link#H6078918
- 43.Advanced Analytic. TriNetX. Accessed February 6, 2023. https://trinetx.com/real-world-data/advanced-analytics/ [Google Scholar]
- 44.Arain M, Haque M, Johal L, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449–461. doi: 10.2147/NDT.S39776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. The Lancet Child & Adolescent Health. 2018;2(3):223–228. doi: 10.1016/S2352-4642(18)30022-1 [DOI] [PubMed] [Google Scholar]
- 46.Disparities in Suicide ∣ CDC. Published January 12, 2023. Accessed March 21, 2023. https://www.cdc.gov/suicide/facts/disparities-in-suicide.html [Google Scholar]
- 47.Curtin SC, Warner M, Hedegaard H. Increase in Suicide in the United States, 1999-2014. NCHS Data Brief. 2016;(241):1–8. [PubMed] [Google Scholar]
- 48.Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. American Journal of Psychiatry. 2018;175(4):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study. Am J Psychiatry. 2018;175(7):620–630. doi: 10.1176/appi.ajp.2018.17060720 [DOI] [PubMed] [Google Scholar]
- 50.Fu DJ, Ionescu DF, Li X, et al. Esketamine Nasal Spray for Rapid Reduction of Major Depressive Disorder Symptoms in Patients Who Have Active Suicidal Ideation With Intent: Double-Blind, Randomized Study (ASPIRE I). J Clin Psychiatry. 2020;81(3):6605. doi: 10.4088/JCP.19m13191 [DOI] [PubMed] [Google Scholar]
- 51.Klonsky ED, May AM, Saffer BY. Suicide, Suicide Attempts, and Suicidal Ideation. Annual Review of Clinical Psychology. 2016;12(1):307–330. doi: 10.1146/annurev-clinpsy-021815-093204 [DOI] [PubMed] [Google Scholar]
- 52.Klonsky ED, May AM. The Three-Step Theory (3ST): A New Theory of Suicide Rooted in the “Ideation-to-Action” Framework. International Journal of Cognitive Therapy. 2015;8(2):114–129. doi: 10.1521/ijct.2015.8.2.114 [DOI] [Google Scholar]
- 53.De Luca S, Yan Y, O’Donnell K. Is anybody there? A longitudinal examination of help-seeking and suicidal risk among Latino, Black, and non-Hispanic white adolescents. Suicide and Life-Threatening Behavior. 2023;53(3):385–398. doi: 10.1111/sltb.12951 [DOI] [PubMed] [Google Scholar]
- 54.Jacklin CN. Female and male: Issues of gender. American Psychologist. 1989;44(2):127. [DOI] [PubMed] [Google Scholar]
- 55.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. The Lancet. 2010;375(9715):686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. The Lancet Psychiatry. 2018;5(1):65–78. doi: 10.1016/S2215-0366(17)30272-9 [DOI] [PubMed] [Google Scholar]
- 57.Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA psychiatry. 2017;74(4):399–405. [DOI] [PubMed] [Google Scholar]
- 58.Drug Approval Package: Spravato. Accessed March 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000TOC.cfm
- 59.Malhi GS, Byrow Y, Cassidy F, et al. Ketamine: stimulating antidepressant treatment? BJPsych Open. 2016;2(3):e5–e9. doi: 10.1192/bjpo.bp.116.002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pennybaker SJ, Niciu MJ, Luckenbaugh DA, Zarate CA. Symptomatology and predictors of antidepressant efficacy in extended responders to a single ketamine infusion. J Affect Disord. 2017;208:560–566. doi: 10.1016/j.jad.2016.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lii TR, Smith AE, Flohr JR, et al. Trial of Ketamine Masked by Surgical Anesthesia in Depressed Patients. Published online May 1, 2023:2023.04.28.23289210. doi: 10.1101/2023.04.28.23289210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Domany Y, Bleich-Cohen M, Tarrasch R, et al. Repeated oral ketamine for out-patient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. The British Journal of Psychiatry. 2019;214(1):20–26. [DOI] [PubMed] [Google Scholar]