Abstract
Introduction
Orthokeratology (OK) and low-concentration atropine are recommended approaches for controlling myopia. However, children with younger age and lower myopia are more likely to experience rapid axial progression during OK or atropine monotreatment. This study aimed to assess the efficacy of OK combined with low-concentration atropine for myopia control in children over 24 months and to determine whether the effect was sustainable.
Methods
In this retrospective study, we reviewed medical records of baseline and follow-up visits from children (7–14 years) applying OK for myopia control. Sixty-eight children receiving monoorthokeratology treatment (OK group) and 68 children who received 0.01% atropine in combination with orthokeratology simultaneously (AOK group) were included. A series of ophthalmic tests at baseline were conducted, and axial length (AL) was measured every 6 months. The comparison of AL change at different visits between the two groups was performed by repeated measures multivariate analyses of variance (RM–MANOVA).
Results
There were no significant differences in baseline characters between the two groups (p > 0.05). The AL significantly increased over time in both groups (all p < 0.05), and the 2-year change in AOK was 0.16 mm (36%) lower than in OK (0.28 ± 0.22 mm versus 0.44 ± 0.34 mm, p = 0.001). Compared with OK group, the significant suppression of AL elongation in the AOK group was observed in 0–6, 6–12, and 12–18 month periods (suppression rate: 62.5%, 33.3%, 38.5%, respectively, p < 0.05), while there was no significant difference in the 18–24 month period (p = 0.105). The multiple regression analysis showed an interaction between age and treatment effect (interaction coefficient = 0.06, p = 0.040), indicating one year age decrease approximately associated with 0.06 mm increased retardation in AL elongation in the AOK group.
Conclusion
The add-on effect of 0.01% atropine in OK wearers only occurred within 1.5 years, and younger children benefited more from the combination treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-023-00755-4.
Keywords: Orthokeratology, Low-concentration atropine, Axial length, Myopia
Key Summary Points
| Why carry out this study? |
| Children with younger age and lower degree of myopia are more likely to experience rapid axial progression during orthokeratology (OK) or atropine monotreatment. |
| We conducted this study to assess whether there is an add-on effect of 0.01% atropine in OK wearers for children’s myopia control and if the effect is sustainable. |
| What was learned from the study? |
| We confirmed that axial elongation over 2 years might be distinctly suppressed by the combination of OK and 0.01% atropine, but the add-on effect of 0.01% atropine in OK wearers only occurred within 1.5 years of treatment. |
| We found that children of a younger age would benefit more from the OK lenses and 0.01% atropine combination therapy. |
Introduction
Myopia, featured by images of distant objects coming into focus in front of the retina and blurred distance vision, has become a widely acknowledged global public health concern, especially in East Asia [1, 2]. A recent study estimated that 30% of the world population is currently myopic, and this number is expected to significantly increase by 2050, roughly affecting half of the worldwide population, 10% of whom will be affected by high myopia [3]. Furthermore, myopia, particularly high myopia, elevates the risk of sight-threatening ocular complications, such as retinal detachment and myopic maculopathy [4]. Therefore, various potential pharmaceutical and optical treatments with different effects are warranted to retard childhood myopia progression.
A network meta-analysis has compared the effectiveness of 16 optical and pharmaceutical myopia control methods. Certain specially designed contact lenses and pharmacologic muscarinic antagonists were reported to be the moderate and most effective approach [5]. For example, orthokeratology (OK), a rigid gas-permeable contact lens with a reverse geometry design worn overnight, can improve daytime uncorrected visual acuity by reshaping the corneal contour and slow eye enlargement by 36%–52% [6–8]. As for pharmaceutical interventions, a network meta-analysis of eight atropine concentrations demonstrated that higher dose atropine (1%, 0.5%) can effectively slow down myopia progression [9]. Nevertheless, atropine-related side effects, such as photophobia, blurred near vision, and rebound phenomenon after withdrawn atropine, were also dose-related [10] and may lead to poor tolerance and high dropout rate in long-term use of higher dose atropine. Hence, low concentration atropine (0.01%), seems tolerable and well accepted in clinical use, since it could suppress myopia progression with less rebound effect when medication is cessation [11].
However, the control effect of OK and low concentration atropine showed significant variation between individuals. Children with younger age [12], and lower initial refractive error [6] were more likely to experience rapid axial progression during OK treatment. Meanwhile, post hoc analysis of the Low-Concentration Atropine for Myopia Progression (LAMP) study found that poor atropine response is associated with younger age, suggesting altering concentration or combining other treatments in poor responders [13]. Since different mechanisms regulate OK and atropine, a combination of the two treatments might be more effective. Several studies have investigated the effectiveness of the combined therapy. Two randomized control trials (RCT) conducted by Kinoshita et al. [14] and Tan et al. [15], and a retrospective study authored by Chen et al. [16] indicated that the combination of OK and low concentration atropine was more effective for slowing axial elongation than OK alone. Meanwhile, attenuation of the effect was observed in these studies. Kinoahita and his team found that the AL change was not significantly smaller in the combination group over the 2nd year [14]. In their study, Tan et al. also reported that a significant between group difference was not found in the second half year [15]. Due to this attenuation phenomenon, it remains unclear that how long the add-on myopia suppression effect would last in AOK therapy.
This retrospective study used clinical data to investigate the short-term and long-term combination effect of low concentration atropine and OK in Chinese myopic children over a period of 24 months and to determine whether the combined effect is sustainable over time.
Methods
Participants
The baseline and 2-year follow-up medical records of children who visited Shanghai Eye Disease Prevention and Treatment Center for vision correction using OK lenses between September 2017 and March 2021 were reviewed. Children who met the following criteria were enrolled in this retrospective study: (1) age between 7–14 years, (2) without keratoconus, binocular vision problems and other ocular diseases, aside from refractive error, (3) intraocular pressure (IOP) < 21 mmHg, (4) astigmatism (axes 180 ± 30) no more than 1.50 D, (5) best-corrected visual acuity equal or less than 0.00 logMAR unit in both eyes, (6) no history of OK or contact lenses, and (7) discontinued lens wearing or atropine use less than a total of 30 days during the 2 years. A total of 136 children were included, 68 of whom had chosen to use 0.01% atropine ophthalmic solution while wearing OK lenses. Children using atropine simultaneously with OK lenses were included in the combination therapy group (AOK group), and those only wearing OK lenses were placed in the monotherapy group (OK group).
The study was approved by the Institutional Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University (No. [2021]089) and adhered to the tenets of the Declaration of Helsinki. In this study, only the clinical data of patients were retrospectively collected, and the requirement for informed consent was waived.
Orthokeratology Lenses
The orthokeratology lenses, Euclid overnight OK lenses (Euclid Systems Corporation, Herndon, USA), were made of Boston EQUALENS II (oprifocon A) with an oxygen permeability coefficient of 127 × 10−11 (cm2·mL02)/(s·mL·mmHg), a diameter of 10.6–11.0 mm, an optical center thickness of 0.21–0.23 mm, a wetting angle of 36° and a four anti-arc inner surface geometry. The subjects in both groups were provided with clear instructions regarding wearing and maintaining lenses and were instructed to wear their OK lenses on both eyes every night for at least 7 consecutive hours.
Atropine Ophthalmic Solution
The children’s parents purchased the 0.01% atropine ophthalmic solution at Fudan University Eye and Ear, Nose and Throat (ENT) Hospital. The 0.01% solution was obtained by diluting atropine sulfate injection of 0.05% (Hubei Xinghua Pharmaceutical, Wuhan, China) with sodium hyaluronate eye drops 0.3% (Santen Pharmaceutical) at a ratio of 1:4 in a sterile manner. Children in the combination group were instructed to instill the 0.01% atropine eye drop into both eyes once daily at night, at least 10 min before inserting the OK lenses.
Measurements
At the first visit, all participants underwent comprehensive ophthalmic testing, including cycloplegic refraction, axial length (AL) measurement, and corneal topography. The follow-up visits measuring AL were scheduled every 6 months. The AL was evaluated using an IOLMaster (Carl Zeiss Jena GmbH, Jena, Germany). All measurements were non-contacting. Three measurements were taken at each visit, and the average of the three was used as a representative value.
Return Visits and Compliance
Children were examined on days 1, 7, and 30 after beginning OK or AOK treatment, and then every 3 months thereafter. Corneal integrity, corneal topography, and visual acuity were examined at every follow-up visit. If corneal staining was observed, OK lenses were asked to be ceased for 2 days for low lever staining (grade 1 Efron Grading Scale), and 5–7 days for more intense staining (grade 2 and grade 3 Efron Grading Scale). Then subjects should return for examination following doctor’s suggestion and resume lens wearing until their corneal were completely recovered. Subjects were also examined whenever an abnormal symptom occurred. When visual disturbances associated with atropine use, for example, photophobia and blurred near vision, occurred in the AOK group, wearing sunglasses or using atropine early every night would be suggested to relieve these symptoms and ensure compliance. In addition, the reason for discontinuation of OK lenses or atropine, such as fever, conjunctivitis, photophobia, travel, and exams, would be inquired about and recorded in detail at each follow-up (including duration and reason for discontinuation).
Statistical Analyses
Only data from children who completed 5 visits were included in the data analysis. All statistical analyses were performed using software R (V4.1.3). The normality of numeric variables was confirmed by the Shapiro–Wilk test. The sex ratio was compared using the chi-squared test. Age, spherical equivalent refraction (SER), AL, flattest keratometry (FK), steepest keratometry (SK), average keratometry reading (AVE), central corneal thickness (CCT), and non-contact tonometer (NCT) at enrollment were compared between the two treatment groups using independent group t test. As the primary outcome, changes in AL over time in different groups were compared using repeated measures multivariate analyses of variance (RM–MANOVA), controlling for baseline factors with Bonferroni-adjusted pairwise comparisons (where necessary). A paired t test was used to compare AL changes in each group. The association between the changes in AL over 2 years and the baseline factors was analyzed by Pearson’s correlation and multiple linear regression analysis. A p value < 0.05 was considered statistically significant.
Results
A total of 136 right eyes were included in the data analysis since right and left eyes were highly correlated. As shown in Table 1, baseline characteristics, such as age (9.90 ± 1.59 versus 10.00 ± 1.57, p = 0.713), sex (girls 58.8% versus 58.8%, p = 1.000), spherical equivalent refraction (SER) (−3.68 ± 1.25 versus −3.25 ± 1.26, p = 0.052), AL (25.10 ± 0.87 versus 25.00 ± 0.85, p = 0.472), or AVE (42.59 ± 1.37 versus 42.60 ± 1.17, p = 0.953) did not significantly differ between the AOK and OK group.
Table 1.
Baseline characteristics of patients in the AOK group and the OK group
| AOK group (N = 68) | OK group (N = 68) | p value | |
|---|---|---|---|
| Age, year | 9.90 ± 1.59 | 10.00 ± 1.57 | 0.713* |
| Sex, no. (%) | 1.000a | ||
| Male | 28 (41.2) | 28 (41.2) | |
| Female | 40 (58.8) | 40 (58.8) | |
| SER, D | −3.68 ± 1.25 | −3.25 ± 1.26 | 0.052* |
| AL, mm | 25.10 ± 0.87 | 25.00 ± 0.85 | 0.472* |
| FK, D | 41.95 ± 1.29 | 42.06 ± 1.16 | 0.614* |
| SK, D | 43.25 ± 1.56 | 43.14 ± 1.23 | 0.649* |
| AVE, D | 42.59 ± 1.37 | 42.60 ± 1.17 | 0.953* |
| CCT, μm | 549.53 ± 25.64 | 549.47 ± 29.01 | 0.990* |
| NCT, mmHg | 16.06 ± 2.29 | 15.93 ± 2.28 | 0.725* |
Data are presented as mean ± standard deviation, unless otherwise indicated
AOK combination therapy group, OK monotherapy group, SER spherical equivalent refraction, D diopter, AL axial length, mm millimeter, FK flattest keratometry, SK steepest keratometry, AVE average kerotometry, CCT central corneal thickness, μm micrometer, NCT non-contact tonometer, mmHg millimeter of mercury
*Independent group t test
aChi-squared test
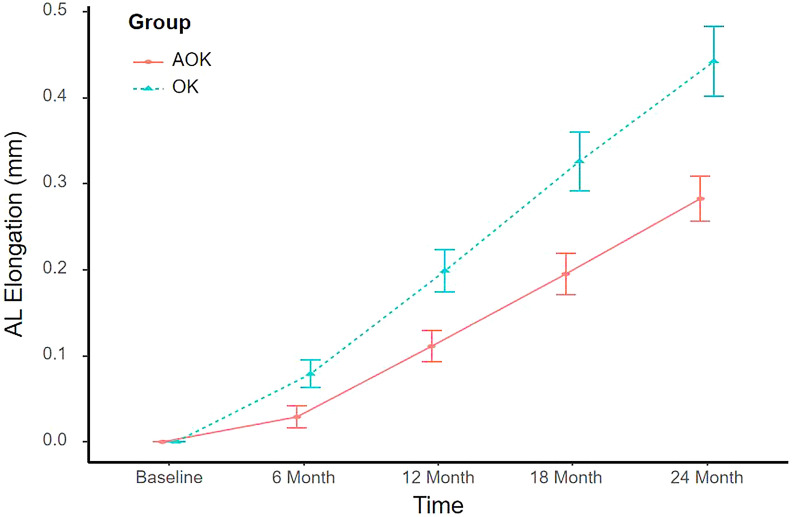
Figure 1 shows the time courses of changes in AL between the AOK and OK groups. AL changed over time (all p < 0.05), showing an increase in both study groups (Table 2). However, AL change was significantly different between the two study groups at 6-, 12-, 18- and 24-month visits (all p < 0.05), and there was a significant interaction between therapy and treatment time (p = 0.010) controlling for initial age and SER. During the study period, the cumulative axial elongation was significantly retarded by 0.05 mm, 0.09 mm, 0.14 mm, and 0.16 mm, at 6-, 12-, 18-, and 24-month visits, respectively, in the AOK group compared with the OK group, which indicated 63%, 45%, 42%, and 36% myopia control effectiveness at different visits, respectively (Table 2).
Fig. 1.
Time course of changes in AL in the AOK group and the OK group. Error bars represent the standard error. AL axial length, AOK combination therapy group, OK monotherapy group
Table 2.
AL elongation at different times in the AOK group and the OK group
| AOK group | OK group | p value | |
|---|---|---|---|
| 6 month | 0.03 (0.00, 0.06) | 0.08 (0.05, 0.11) | 0.020a |
| 12 month | 0.11 (0.07, 0.15) | 0.20 (0.15, 0.25) | 0.005a |
| 18 month | 0.19 (0.15, 0.24) | 0.33 (0.26, 0.40) | 0.002a |
| 24 month | 0.28 (0.23, 0.34) | 0.44 (0.36, 0.53) | 0.001a |
| p value | < 0.001* | < 0.001* |
AOK combination therapy group, OK monotherapy group
Data are presented as mean (95% confidence interval)
*Repeated measures multivariate analyses of variance (RM–MANOVA) controlling for initial age and spherical equivalent refraction (SER) with Bonferroni-adjusted pairwise comparisons
aPaired t test
Table 3 and Fig. 2 compared the increment in AL over a 6-monthly period. The change in AL was significantly smaller in the AOK group compared with the OK group at the first, second, and third 6-monthly period (all p < 0.05), with 0.05 mm, 0.04 mm, and 0.05 mm between group differences, respectively. However, there was no difference in axial elongation between the two groups over the fourth 6-monthly period (difference = 0.03 mm, p = 0.105).
Table 3.
Six-monthly change in AL at different times in the AOK group and the OK group
| AOK group | OK group | p value | |
|---|---|---|---|
| Difference between 6-month and baseline visits | 0.03 (0.00, 0.06) | 0.08 (0.05, 0.11) | 0.020a |
| Difference between 12-month and 6-month visits | 0.08 (0.06, 0.10) | 0.12 (0.10, 0.15) | 0.013a |
| Difference between 18-month and 12-month visits | 0.08 (0.06, 0.11) | 0.13 (0.09, 0.17) | 0.043a |
| Difference between 24-month and 18-month visits | 0.09 (0.07, 0.10) | 0.12 (0.09, 0.15) | 0.105a |
Data are presented as mean (95% confidence interval)
AL axial length, AOK combination therapy group, OK monotherapy group
aPaired t test
Fig. 2.
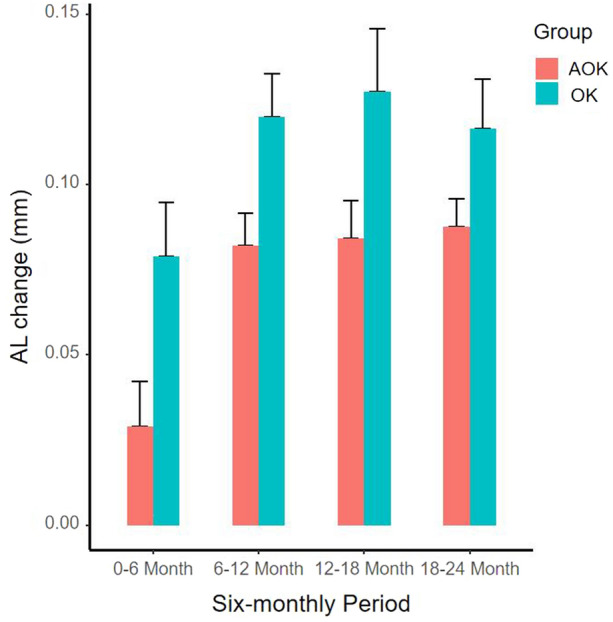
Bar plot of changes in AL over different 6-monthly period in the AOK group and the OK group. Error bars represent the standard error. AL axial length, AOK combination therapy group, OK monotherapy group
Pearson correlation analysis indicated that 2-year AL elongation was associated with SER at enrollment among the AOK group and was correlated with initial age, SER, and AL in the OK group (Table 4). As shown in Table 5, multiple regression analysis revealed that 2-year AL change had a negative relationship with initial age and AOK treatment, a positive relationship with SER at baseline, and the interaction between initial age and treatment when controlling for other factors. In addition, the AL increment of AOK group was modified by participants’ age (p = 0.040), which means that as age increased (no more than 14 years), the difference in 2-year AL elongation between AOK and OK decreased by approximately 0.06 mm. However, the interaction between initial SER and treatment was not significant in this regression analysis.
Table 4.
Correlation analysis of various variables associated with 2-year AL change in different treatment group
| Total | AOK group | OK group | ||||
|---|---|---|---|---|---|---|
| Correlation* | p value | Correlation* | p value | Correlation* | p value | |
| Age, year | −0.32 | < 0.001 | −0.21 | 0.092 | −0.44 | < 0.001 |
| SER, D | 0.32 | < 0.001 | 0.25 | 0.038 | 0.33 | 0.006 |
| AL, mm | −0.35 | < 0.001 | −0.16 | 0.188 | 0.49 | < 0.001 |
| NCT, mmHg | −0.09 | 0.302 | −0.13 | 0.309 | −0.06 | 0.628 |
| CCT, μm | 0.07 | 0.418 | 0.20 | 0.103 | 0.00 | 0.995 |
| FK, D | 0.18 | 0.038 | 0.12 | 0.330 | 0.22 | 0.069 |
| SK, D | 0.14 | 0.107 | 0.08 | 0.536 | 0.24 | 0.051 |
| AVE, D | 0.16 | 0.063 | 0.09 | 0.477 | 0.24 | 0.052 |
AOK combination therapy group, OK monotherapy group, SER spherical equivalent refraction, D diopter, AL axial length, mm millimeter, NCT non-contact tonometer, mmHg millimetre of mercury, CCT central corneal thickness, μm micrometer, FK flattest keratometry, SK steepest keratometry, AVE average kerotometry
*Pearson correlation coefficient
Table 5.
Multiple regression analysis of factors associated with 2-year axial elongation
| Variable | Coefficient | p value |
|---|---|---|
| Treatment | 0.004* | |
| OK | 0 | |
| AOK | −0.84 (−1.41, −0.27) | |
| Age, year | −0.08 (−0.12, −0.04) | < 0.001* |
| SER, D | 0.05 (0.01, 0.11) | 0.029* |
| Treatment* age | 0.040* | |
| OK* age | 0 | |
| AOK* age | 0.06 (0.00, 0.12) | |
| Treatment*.SER | 0.565 | |
| OK* SER | 0 | |
| AOK* SER | −0.02 (−0.10, 0.05) | |
AOK combination therapy group, OK monotherapy group, SER spherical equivalent refraction, D diopter
*Independent group t test
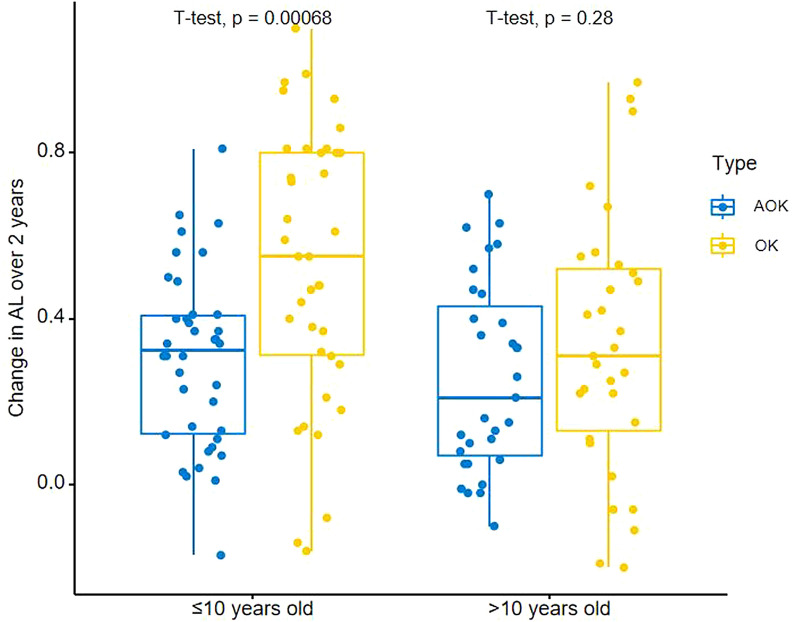
Next, we stratified the subjects according to the age at enrollment based on average (≤ 10 years old versus > 10 years old) and compared the change in AL between the combination and monotherapy groups. As shown in Fig. 3, children ≤ 10 years old (37 subjects in the AOK group and 37 subjects in the OK group) in the AOK group experienced a statistically significant reduction in axial elongation compared with the OK group (AOK: 0.30 mm versus OK: 0.54 mm), while there was no significant difference in 2-year AL change among children > 10 years old (31 subjects in AOK group and 31 subjects in OK group) between the two treatments (AOK: 0.26 mm versus OK: 0.33 mm). Baseline characteristics and 2-year AL elongation among children of different ages and SER were compared in the sensitivity analysis (see Supplementary Material Table S1).
Fig. 3.
Box plot of changes in AL over 2 years in the AOK group and the OK group in children with different ages. AL axial length, AOK combination therapy group, OK monotherapy group
Discussion
In this retrospective study, we found that a combination of OK and 0.01% atropine had a stronger suppression effect on axial elongation than OK alone; yet the significant suppression effect was observed only for the first 1.5 years. Besides, we also found that combination therapy applied in younger children could achieve more benefit.
In this study, a 2-year increase in AL was 0.44 mm in OK treatment, which is approximately 37% slower than the 0.70 mm enlargement of a spectacle control group in our previous study [17]. Yet, considering that part of the observation time (2019–2021) included a lockdown period caused by the coronavirus disease 2019 (COVID-19) epidemic in Shanghai, China, participants spent more time online and less time engaging in outdoor activities, which might have affected accelerated myopic progression in the present study [18]. Hence, the AL change observed in this study was larger than the value (approximately 0.31–0.39 mm) observed in other studies [6–8]. Meanwhile, in the combination therapy group, participants had an average of 0.28 mm axial elongation, indicating a 60% control effect compared with the spectacle group (0.70 mm) and an additional 23% retardation by add-on of nightly-use atropine. As shown in this study, the 1-year reduction rate in the AOK group was 45% (0.09 mm) compared with OK alone, which was similar to other 1-year adjunctive effects of atropine reported by Tan et al. (AOK 0.07 mm versus OK 0.16 mm) [15], Kinoshita et al. (AOK 0.09 mm versus OK 0.19 mm) [19], and Zhao et al. (AOK 0.14 mm versus OK 0.29 mm) [20]. The current result confirmed that adding atropine to OK wearers may distinctly suppress juvenile myopia progression compared with OK monotherapy.
The mechanism of orthokeratology and atropine in preventing myopia progression remains unclear. The possible explanation for OK lenses is the peripheral myopic defocus of the retina after redistributing corneal epithelium, which might reduce the rapid development of myopia [21, 22]. The antimuscarinic antagonist, atropine, reduces axial growth in myopia by regulating muscarinic receptors, which directly or indirectly stretches the sclera [23, 24]. OK lenses and low concentration atropine appear to have different mechanisms of myopia prevention, giving independent control effects of around 40% [6–8] and 20% [10, 11], respectively. Therefore, a combined effect of approximately 60% might be expected when used simultaneously. Our finding of a 60% total reduction compared with the spectacle was also consistent with the decrease rate. However, researchers also noticed that pupil diameter might participate in the combination mechanism. First, Chen et al. showed that large pupil diameters facilitated the effect of OK to slow axial growth [25]. Besides, studies of low concentration atropine had reported increased photopic and mesopic pupil size during treatment [11]. Tan et al. also observed pupil dilation in patients using AOK treatment [15]. Hence, we speculated that adding 0.01% atropine might increase the suppression of axial elongation by OK lenses through its mydriatic effect. Recently, new evidence has demonstrated that atropine could also alter peripheral refraction; furthermore, such change may also act on the control effect of the AOK treatment [26, 27]. Since pupil diameter and peripheral refraction were not measured in the current study, future investigation and discussion are required to determine whether the simple superposition of atropine and OK lenses or the synergistic effect through pupil dilation and peripheral refraction induced prominent myopia control.
Although the AL suppression effect of AOK therapy was obviously superior to OK monotherapy, when comparing axial elongation over different 6-monthly periods, unlike the other three periods, there was no difference between groups at 18–24 month, which means the combination effect was not sustainable. Other studies reported similar phenomena. For example, a 1-year RCT found that the additive effect of atropine only occurred in the first 6 months [15]. Meanwhile, the 2-year RCT in Japan also stated a significant difference between the two treatment groups in the first year, but not in the second year [14]. In their study, Chen et al. added nightly atropine to first year fast progressors and concluded that 3-year cumulative axial elongation was equal between the two groups, even though there was a significant reduction in the combined group during the second year in another similar study [16, 28]. One explanation was that the muscarinic receptors might be exhausted after a long time of binding with atropine, causing a reduction in efficacy [28]. From the regression model and stratified analysis of older children, we hypothesized that the combined effect of atropine and OK would decrease as age increased and might not be significant in children older than 10. Considering there may be differences in baseline age characteristics between this study and other studies mentioned above, this may be another reason for the differentiation in attenuate time among different studies. Thus, the application and duration of combination therapy in clinical practice should also consider children’s age. However, to find the exact attenuate time, more frequent follow-up and longer observation time research are warranted.
Previous studies have found that children of younger ages were more likely to experience more significant axial elongation in OK lenses and low concentration atropine treatment [6, 11–13, 29, 30]. In this study, correlation analysis and multiple regression showed that the efficacy of combination therapy was interacted with age, indicating younger children would benefit more from adding atropine to OK use in myopia control, which was consistent with a previous RCT [14]. Although the absolute value of axial increase in the AOK group among younger children is more extensive than the mean value of all participants, the add-on effect of atropine was 44% (AOK 0.30 mm versus OK 0.54 mm), which was higher than the reduction rate in the whole study population (control efficacy 36%). Meanwhile, the difference in AL elongation between the AOK and OK group was more significant in younger children with low myopia (AOK 0.26 mm versus OK 0.66 mm, control efficacy 61%, see Supplementary Material Table S1). However, OK monotherapy and AOK therapy could achieve nearly the same myopia retardation effect (AOK 0.26 mm versus OK 0.33 mm) in children older than 10. Children of different ages show various reactions to OK lenses combined with atropine, which might be due to different refractive development at different times. A longitudinal cohort reported that axial increment in children with persistent myopia slows from 0.44 mm at 8 years to 0.16 mm at 12 years [31], making it difficult to detect a significant reduction between AOK and OK in older juveniles. Therefore, we suggested that the combination of atropine and OK lenses be utilized in younger children to achieve better myopia control.
The present study has some limitations. First, although the two groups were well matched and comparable at baseline, the study design was retrospective in nature and was subject to bias. Only participants completing all follow-up visits were included in the data analysis, which might induce selection bias in this study. Second, although other researchers mentioned the hypothesis that pupil enlargement by atropine might facilitate the effect of OK lenses, we did not measure pupil diameters in follow-up visits in this study, which thus might not be able to clarify the potential mechanism of the combination of OK and low concentration atropine in slowing axial growth. We also did not measure the peripheral refraction in this study. In our future perspective research, we plan to measure theses values. Third, we did not measure and analyze the environmental factors, such as the amount of time spent on near-working and outdoor activities, as well as genetic parameters like parental myopia, which might affect the progression of myopia. Future studies should take into consideration the environmental factors.
Conclusions
During the 2-year study period, the add-on effect of 0.01% atropine in preventing axial elongation among OK wearers only occurred in the first 1.5 years. Younger children would gain more myopia suppression benefits from the atropine and OK combined therapy. However, a larger perspective study is warranted to determine whether the rebound effect exists after discontinuing atropine and the exact mechanism of the combined effect.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was supported by Shanghai Health Commission Research Project (no. 201840199), The Shanghai Science and Technology Commission Research Project (no. 18ZR1435700), Excellent Discipline Leader Cultivation Program of Shanghai (no. GWV-10.2-XD09), Three-year Action Program of Public Health (2020–2022) (no. GWV-9.1), and National Key R and D Program (no. 2021YFC2702100, China). The journal’s Rapid Service Fee and editorial assistance was funded by the author, Mengjun Zhu.
Medical Writing, Editorial, and Other Assistance
We would like to thank MedSci (www.medsci.cn) for English language editing.
Author Contributions
Linlin Du: data curation, formal analysis, and writing—original draft. Jun Chen: data curation, formal analysis, and writing—review and editing. Li Ding: resources, and writing—review and editing. Jingjing Wang: writing—review and editing. Jinliuxing Yang: writing—review and editing. Hui Xie: writing—review and editing. Xun Xu: supervision and funding acquisition. Xiangui He: conceptualization, investigation, resources, project administration, funding acquisition, and writing—review and editing. Mengjun Zhu: conceptualization, investigation, resources, project administration, funding acquisition, and writing—review and editing.
Disclosures
Linlin Du, Jun Chen, Li Ding, Jingjing Wang, Jinliuxing Yang, Hui Xie, Xun Xu, Xiangui He, and Mengjun Zhu all confirm that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Compliance with Ethics Guidelines
The study was approved by the Institutional Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University (no. [2021]089) and adhered to the tenets of the Declaration of Helsinki. Only the clinical data of patients were retrospectively collected, and the requirement for informed consent was waived.
Data Availability
The data that support the findings of this study are available from the corresponding author, Mengjun Zhu, upon reasonable request.
Footnotes
Xiangui He and Mengjun Zhu contributed equally as co-last authors.
Contributor Information
Xiangui He, Email: xianhezi@163.com.
Mengjun Zhu, Email: morning5012@163.com.
References
- 1.Morgan IG, French AN, Ashby RS, et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 3.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123(4):697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Kakita T, Hiraoka T, Oshika T. Influence of overnight orthokeratology on axial elongation in childhood myopia. Invest Ophthalmol Vis Sci. 2011;52(5):2170–2174. doi: 10.1167/iovs.10-5485. [DOI] [PubMed] [Google Scholar]
- 7.Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53(11):7077–7085. doi: 10.1167/iovs.12-10565. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Cheung SW, Cho P. Myopia control using toric orthokeratology (TO-SEE study) Invest Ophthalmol Vis Sci. 2013;54(10):6510–6517. doi: 10.1167/iovs.13-12527. [DOI] [PubMed] [Google Scholar]
- 9.Ha A, Kim SJ, Shim SR, Kim YK, Jung JH. Efficacy and safety of 8 atropine concentrations for myopia control in children: a network meta-analysis. Ophthalmology. 2022;129(3):322–333. doi: 10.1016/j.ophtha.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123(2):391–399. doi: 10.1016/j.ophtha.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Yam JC, Zhang XJ, Zhang Y, et al. Three-year clinical trial of low-concentration atropine for myopia progression (LAMP) study: continued versus washout: phase 3 report. Ophthalmology. 2022;129(3):308–321. doi: 10.1016/j.ophtha.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutiérrez-Ortega R. Factors preventing myopia progression with orthokeratology correction. Optom Vis Sci. 2013;90(11):1225–1236. doi: 10.1097/OPX.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 13.Li FF, Zhang Y, Zhang X, et al. Age effect on treatment responses to 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine for myopia progression study. Ophthalmology. 2021;128(8):1180–1187. doi: 10.1016/j.ophtha.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita N, Konno Y, Hamada N, et al. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep. 2020;10(1):12750. doi: 10.1038/s41598-020-69710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan Q, Ng AL, Choy BN, Cheng GP, Woo VC, Cho P. One-year results of 0.01% atropine with orthokeratology (AOK) study: a randomised clinical trial. Ophthalmic Physiol Opt. 2020;40(5):557–566. doi: 10.1111/opo.12722. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Huang S, Zhou J, Xiaomei Q, Zhou X, Xue F. Adjunctive effect of orthokeratology and low dose atropine on axial elongation in fast-progressing myopic children-a preliminary retrospective study. Cont Lens Anterior Eye. 2019;42(4):439–442. doi: 10.1016/j.clae.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Zhu MJ, Feng HY, He XG, Zou HD, Zhu JF. The control effect of orthokeratology on axial length elongation in Chinese children with myopia. BMC Ophthalmol. 2014;14:141. doi: 10.1186/1471-2415-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai T, Zhao L, Kong L, Du X. Complex Interplay Between COVID-19 Lockdown and Myopic Progression. Front Med (Lausanne) 2022;9:853293. doi: 10.3389/fmed.2022.853293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinoshita N, Konno Y, Hamada N, Kanda Y, Shimmura-Tomita M, Kakehashi A. Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: first year results. Jpn J Ophthalmol. 2018;62(5):544–553. doi: 10.1007/s10384-018-0608-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Q, Hao Q. Clinical efficacy of 0.01% atropine in retarding the progression of myopia in children. Int Ophthalmol. 2021;41(3):1011–1017. doi: 10.1007/s10792-020-01658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur A, Atchison DA. Effect of orthokeratology on peripheral aberrations of the eye. Optom Vis Sci. 2009;86(5):E476–E484. doi: 10.1097/OPX.0b013e31819fa5aa. [DOI] [PubMed] [Google Scholar]
- 22.Hiraoka T, Kakita T, Okamoto F, Oshika T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology. 2015;122(1):93–100. doi: 10.1016/j.ophtha.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 23.McBrien NA, Stell WK, Carr B. How does atropine exert its anti-myopia effects? Ophthalmic Physiol Opt. 2013;33(3):373–378. doi: 10.1111/opo.12052. [DOI] [PubMed] [Google Scholar]
- 24.Barathi VA, Weon SR, Beuerman RW. Expression of muscarinic receptors in human and mouse sclera and their role in the regulation of scleral fibroblasts proliferation. Mol Vis. 2009;15:1277–1293. [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Niu L, Xue F, et al. Impact of pupil diameter on axial growth in orthokeratology. Optom Vis Sci. 2012;89(11):1636–1640. doi: 10.1097/OPX.0b013e31826c1831. [DOI] [PubMed] [Google Scholar]
- 26.Sun HY, Lu WY, You JY, Kuo HY. Peripheral refraction in myopic children with and without atropine usage. J Ophthalmol. 2020;2020:4919154. doi: 10.1155/2020/4919154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian J, Wei S, Li S, et al. The effect of atropine 0.01% eyedrops on relative peripheral refraction in myopic children. Eye (Lond) 2023;37(2):356–361. doi: 10.1038/s41433-021-01923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Zhou J, Xue F, Qu X, Zhou X. Two-year add-on effect of using low concentration atropine in poor responders of orthokeratology in myopic children. Br J Ophthalmol. 2022;106(8):1069–1072. doi: 10.1136/bjophthalmol-2020-317980. [DOI] [PubMed] [Google Scholar]
- 29.Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci. 2012;53(7):3913–3919. doi: 10.1167/iovs.11-8453. [DOI] [PubMed] [Google Scholar]
- 30.Li SM, Kang MT, Wu SS, et al. Efficacy, safety and acceptability of orthokeratology on slowing axial elongation in myopic children by meta-analysis. Curr Eye Res. 2016;41(5):600–608. doi: 10.3109/02713683.2015.1050743. [DOI] [PubMed] [Google Scholar]
- 31.Rozema J, Dankert S, Iribarren R, Lanca C, Saw SM. Axial growth and lens power loss at myopia onset in singaporean children. Invest Ophthalmol Vis Sci. 2019;60(8):3091–3099. doi: 10.1167/iovs.18-26247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Mengjun Zhu, upon reasonable request.