Abstract
Aim
The risk for Herpes zoster (HZ) and its complications is higher in people with diabetes mellitus (DM). Our aim is to assess efficacy and effectiveness of the currently available live-attenuated zoster vaccine (LZV) and recombinant zoster vaccine (RZV) in adults with DM.
Methods
A Systematic Review and Meta-analysis of clinical trials and observational studies comparing incidence of HZ and its complications in vaccinated and unvaccinated people with DM was performed, on PubMed, Cochrane, Clinical Trials.gov and Embase databases, up to January 15th, 2023. Risk of bias was assessed through the Cochrane Collaboration tool and the Newcastle–Ottawa Scale. The protocol was registered on the PROSPERO website (CRD42022370705).
Results
Only three observational studies reported LZV efficacy and effectiveness in people with DM. A lower risk for HZ infection (MH-OH Ratio 95% CI = 0.52 [0.49, 0.56] was observed, for unadjusted analysis, and 0.51 [0.46, 0.56] for adjusted analysis, both with P < 0.00001 and no heterogeneity). No data on LZV safety were reported. A pooled analysis of two trials comparing RZV and placebo, showed a reduced risk for HZ incidence: (95% CI Odds Ratio: 0.09 [0.04–0.19]), with no difference in severe adverse events and mortality.
Conclusions
In our meta-analysis of three observational studies LZV showed a 48% effectiveness in reducing HZ incidence in adults with diabetes whereas in a pooled analysis of two RCTs, RZV showed a 91% efficacy. No data are available on the effects of vaccination on the incidence and severity of HZ-related complications among subjects with diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00592-023-02127-7.
Keywords: Diabetes, Herpes zoster-related severe outcome, Efficacy and effectiveness of Herpes zoster vaccination, Herpes zoster vaccine, Meta-analysis
Introduction
Herpes zoster (HZ), or shingles, is a neurocutaneous disease determined by the reactivation of a latent varicella zoster virus (VZV) in the dorsal root ganglion, characterized by unilateral radicular pain and a vesicular rash, both usually following a dermatomal pattern [1]. Potential complications of HZ include encephalitis, myelitis, nerve palsies, and, more frequently, postherpetic neuralgia (PHN), defined as pain lasting for more than 3 months after the onset of an HZ infection [2, 3]. PHN may last for years, greatly affecting quality of life, and its management is challenging [4].
The lifetime risk of developing HZ is 25%, but this risk increases sharply after 50 years of age, when two-thirds of HZ cases occur [5, 6]. VZV reactivation has been demonstrated to involve a defect in cell-mediated immunity [7] associated with aging and with diabetes mellitus (DM), leading to increased susceptibility to HZ [8]. Other risk factors include female gender, white race, and recent psychological stress [9].
Two recent observational studies [10, 11] suggested that the association of DM with risk for HZ may disappear after adjusting for age and sex; however, all available meta-analyses of observational studies confirm a significant increase of risk in diabetes mellitus, ranging from 24 to 60%, with an estimated yearly incidence of HZ in people with DM of 7.23–9.36/1.000 [12]–[15]. A further increase in risk of HZ has been observed in older people with diabetes, and in those with diabetes and cardiovascular disease [15]. Patients with diabetes are also at higher risk of complications of HZ, such as acute pain and PNH [16–18], leading to a more frequent use of medication (e.g., opioids) [19], outpatient visits, hospitalizations, sick leave, reduced quality of life and deterioration of glucose control [20].
Two vaccines for HZ are currently available. A live attenuated vaccine (LZV) was first licensed in 2006; it contains the Oka VZV strain (with high antigen content), which has been proved to be safe [21] and effective in a large randomized controlled trial (RCT), reducing the HZ incidence by 51.3%, and PHN by 66.5% [22]; on the other hand, its efficacy is lower in those aged more than 70 years, and it progressively declines over the time. More recently, in 2014, a recombinant subunit zoster vaccine (RZV), containing VZV glycoprotein E and the AS01B adjuvant system was introduced, showing a greater efficacy in two RCTs conducted in the general population: 97.2% reduction of HZ incidence in the older than 50 years, 91.3% in those older than 70 years, without any decrease in efficacy in those older than 80 years [23, 24], nor any decline over 10 years of follow-up [25]; furthermore, a 88.8% reduction in PHN incidence was shown [23, 24]. Based on these results, the Advisory Committee on Immunization Practices USA recommends RZV, rather than LZV, in patients with diabetes older than 50 years [26]. RZV is also being increasingly recommended in national vaccination guidelines across Europe and Canada [27–29].Nevertheless, HZ vaccine coverage is still suboptimal, likely due not only to logistic and economic difficulties [30], but also to the lack of physician recommendations [31], although some virtuous experiences have been reported [32].
A Cochrane review of RCTs performed to date in the general population, has shown that HZ vaccines are efficacious in reducing HZ incidence, and overall safe [33]; however, no systematic review or meta-analysis has explored, to our knowledge, their performance in adults with diabetes, a condition which may theoretically hamper vaccine efficacy [34]. The aim of this Systematic Review and Meta-analysis is therefore to collect the available evidence on efficacy and safety of available HZ vaccines in people with diabetes mellitus. The present work was performed to provide a reliable evidence base for the formulation of a position statement of the Scientific Societies involved.
Methods
This meta-analysis was performed in according to the criteria of Preferred Reporting Items for Systematic Reviews and Meta Analyses guidelines [35] (Table 1S). Review Protocol was submitted for registration to the PROSPERO website (CRD42022370705).
Search strategy and selection criteria
A systematic search on PubMed, Cochrane, Clinical Trials.gov and Embase databases was performed, collecting all randomized clinical trials and observational studies performed on humans up to January 15th, 2023. Search string included “Herpes Zoster”. The full search string is reported in Appendix, Table 2S. Further studies were manually searched in references from retrieved papers.
Inclusion criteria
Full-text publications and conference abstracts showing results of phase II, III and IV RCTs and observational studies were included, provided that:
Only adults with DM were enrolled, or separate analyses for patients with diabetes were available.
Efficacy, effectiveness and/or safety of any HZ vaccine, regardless of dose, schedule, preparation, or route of administration, were compared to other HZ vaccines, placebo, or no intervention.
Reports included at least one of the following outcomes: incidence or severity of HZ or PHN at any time point equal to or longer than 12 months, or for the entire duration of the study; incidence of serious adverse events (SAEs); overall mortality.
Other variables of interest retrieved from selected studies were year of publication, study duration, number, age and sex of participants.
Data collection
Titles and abstracts were screened independently by eight of the authors, and potentially relevant articles were retrieved in full text. For all published studies, results reported in published papers and supplements were used as the primary source of information; when the required information on protocol or outcomes was not available in the main publication secondary publications were used for retrieval of missing information; whenever needed an attempt at retrieval of missing information was performed consulting the clinicaltrials.gov registry. The identification of relevant abstracts, the selection of studies, and data extraction were performed independently by six of the authors, and conflicts were resolved by a distinct investigator. The risk of bias was assessed independently by two of the authors, and conflicts were resolved through discussion with a third investigator. The Cochrane Collaboration tool [36] was used for RCTs, whereas the Newcastle- Ottawa Scale, available at the https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp website, was adopted for nonrandomized studies; reporting bias was assessed for each main outcome. The GRADE methodology [37] was used to assess the quality of the body of retrieved evidence, using the GRADE pro-GDT software (GRADEpro Guideline Development Tool. McMaster University, 2015).
Statistical analyses
For each outcome, the number of events and patients enrolled in both arms were retrieved at any time-point for which they were available; when they were not available, or to meta-analyze adjusted analyses, Odds Ratios were retrieved; forest plot were then built collecting all data for each outcome at any given time-point. Between-group Mantel–Haenszel Odds ratio (MH-OR) with 95%, Confidence Intervals (CI) were calculated, on an intention-to-treat basis, for each outcome at any given time-point, using the Wald type confidence interval methods calculator. Heterogeneity was assessed by means of I2 statistics, through the Der Simonian and Laird variance estimator. We applied a random-effects model as the primary analysis, because it is more reliable than fixed-effect when the number of component studies is small. If at least six studies were included in a metanalysis for an outcome, a leave-one out analysis was conducted to assess robustness of the synthesized results. If a relevant heterogeneity was detected, subgroup-analyses or meta-regressions were performed taking year of publication, study duration, number, age and sex of participants into account, provided that a sufficient number of studies was available. Funnel plots and Egger regression were examined to estimate possible publication/disclosure bias, if a sufficient number of studies was detected (at least nine). All analyses were performed using Review Manager 5.3.5; The Cochrane Collaboration, 2014, and IBM SPSS Statistics 28.
Results
The flow research chart is reported in Fig. 1S in the supplementary appendix. The Systematic Search retrieved 12.076 titles, after removing duplicates; of those, 11.969 were excluded after reading titles and abstract. Of the 132 full-text selected, only 5 papers [38]–[42] reported analyses performed on people with diabetes, of which one [42], reported a pooled analysis from two RCTs on RZV, (see below). Therefore, 6 studies were included in this Systematic Review and Meta-analysis.
Recombinant Zoster Vaccine: Two randomized clinical trials compared RZV and placebo on people older than 50 [23] and 70 [24] years, respectively. The risk of bias was low (see Table 1 for general Characteristics). A pooled post-hoc analysis of subgroups of patients with diabetes (2,372 patients on active treatment and 2,350 on placebo) enrolled in these two trials has been published [42], showing a significant reduction of HZ (OR [95% CI] was 0.09 [0.04, 0.19]), with incidence of 0.8 and 9.1/1000 patients*years in the RZV and placebo arms, respectively. The quality of Evidence was rated as Moderate with the GRADE Methodology (Table 3S). The incidence of SAEs was similar in the two arms, as it was (15.2 [13.8–16.7]/1.000 patient*years with RZV and 15.4 [14.0–16.9] /1.000 patient*years with placebo. Reported all-cause mortality was 7.3 (6.3–8.4) /1000 patient*years in the RZV arm and 8.3 (7.2–9.4) /1000 patient*years in the placebo arm [42].
Table 1.
Characteristics of the included randomized controlled studies comparing HZ infection in people with diabetes mellitus with or without prior HZ recombinant subunit zoster vaccine (RZV)
| Study | Years of observation | Country | Design | Age Group | Vaccine | Comparator | Patient-years | Risk of Bias | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated | Unvaccinated | A | B | C | D | E | F | G | |||||||
| ZOE-70 | 2013–2015 | UK | RCT | ≥ 70 year | RZV | placebo | 8723.8 | 8652.7 | L | L | L | L | L | L | L |
| ZOE-50 | 2007–2009 | USA | RCT | ≥ 50 year | RZV | Placebo | L | L | L | L | L | L | L | ||
| Hata 2015 UMIN000004771) | 2007–2014 | JAPAN | RCT | 60–70 year | LZV | Placebo | 125,038 | 317,917 | L | L | L | L | L | L | L |
A Random sequence generation (Selection bias), B Allocation concealment (selection bias), C Blinding of participants and personnel (performance bias), D Blinding of outcome assessment (Detection bias), E Incomplete outcome data (attrition bias), F selective reporting for weight (reporting bias), G selective reporting for renal function (reporting bias), H other bias, L low risk, U unknown risk, H high risk
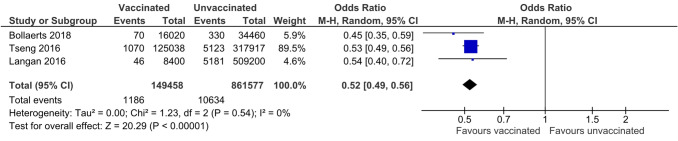
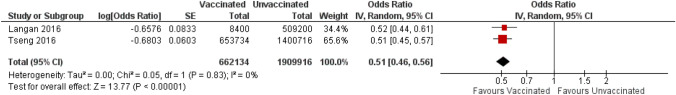
Live-attenuated Zoster vaccine: Only one small RCT performed with the LZV on people with diabetes was retrieved, with only 27 patients per treatment arm, detecting no cases of HZ in the 1-year follow-up [38] (Table 1). Three observational studies, performed on the LZV, provided separate data on people with diabetes mellitus [39]–[41], with a total observation of 149,458 and 861,577 patient*years for vaccinated and unvaccinated individuals, respectively; 1,186 and 10,634 cases of HZ were recorded in vaccinated and unvaccinated individuals. LZV was associated with a significant reduction in risk for HZ in unadjusted analysis (MH-OH Ratio [95% CI] 0.52 [0.49, 0.56], P < 0.00001, I2 = 0%; Fig. 1). When combining the results on patients with diabetes of the two studies reporting analyses adjusted for some confounding factors [39, 41] (Table 2), MH-OH Ratio [95% CI] was 0.51 [0.46, 0.56], with P < 0.00001 and I2 = 0% (Fig. 2). The quality of Evidence was rated as Low with the GRADE Methodology (Table 3S).
Fig. 1.
Differences in incidence of Herpes Zoster between vaccinated or unvaccinated (live attenuated vaccine, LZV) patients with diabetes mellitus, unadjusted odds ratio. M-H = Mantel Haenszel; CI = Confidence Intervals
Table 2.
Characteristics of the included observational studies comparing HZ infection in people with diabetes mellitus with or without prior HZ live attenuated vaccine (LZV)
| Study | Years of observation | Country | Vaccine | Design | Age group | Adjustments | Patient-years | NOS selection | NOS comparability | NOS exposure | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vaccinated | unvaccinated | 1 | 2 | 3 | 4 | 1 | 2 | ||||||||
| Bollaerts 2018 | 2013–2015 | UK | LZV | Retrospective | ≥ 70 year | None | 34,460 | 16,020 | + | − | + | + | − | + | + |
| Langan 2016 | 2007–2009 | USA | LZV | Retrospective | ≥ 65 year | Age, sex, race, comorbidities, immunosuppression, income | 8400 | 509,200 | + | + | + | + | + + | + | + |
| Tseng 2016 | 2007–2014 | USA | LZV | Retrospective | ≥ 60 year | age, sex, race, healthcare utilization, comorbidities | 125,038 | 317,917 | + | + | + | + | + + | + | + |
NOS Newcastle–Ottawa Scale
Fig. 2.
Differences in incidence of Herpes Zoster between vaccinated or unvaccinated (live attenuated vaccine, LZV) patients with diabetes mellitus, adjusted odds ratio. IV = Inverse Variance; SE = Standard Error CI = Confidence Intervals
Discussion
Both LZV and RZV appear to reduce the incidence of HZ in patients with diabetes. However, available data suggest possible differences in efficacy/effectiveness: the incidence of HZ in people with DM is reduced by 95% by RZV, with a number needed to treat (NNT) of 119 for avoiding one case of HZ in one year, whereas the reported reduction with LZV is 48%, with a NNT of 227. Such estimates, however, are derived from studies of different design: results with LZV were obtained meta-analysing three observational studies, whereas those with RZV were reported as a pooled analysis of patient-level data from two randomized controlled trials. The quality of evidence for efficacy of RZV is therefore higher than that for LZV. It is possible that apparent differences in efficacy (95 vs 48%) are at least partly determined by diversities in study design and/or characteristics of enrolled subjects, although the incidence of HZ in control groups of studies on LZV was similar to that of control arm of trials on RZV.
In particular, in observational studies with LZV, those receiving vaccination actively decided to undergo the procedure, whereas in randomized trials vaccination was a play of chance, thus excluding selection bias. It is possible that patients with previous episodes of HZ, or with relatives with a history of recurrent HZ, who could be at greater risk of HZ, were more prone to seek vaccination, thus producing an underestimation of effectiveness of vaccine in observational studies. On the other hand, the more controlled conditions of clinical trials could select subjects who are not fully representative of the general population, generating the possibility of an overestimation of efficacy.
Two network meta-analyses of trials conducted in the general population, showed that the adjuvant RZV is probably superior to LZV, with a greater risk of adverse events at injection sites, but no statistically significant differences for serious adverse events, or death were reported [44, 45]; however, no definitive conclusion can be drawn on this point, since there are no head to head comparisons between the two available vaccines in people with DM.
A previous meta-analysis including three observational studies, although limited to elderly subjects only, reported a reduction in the incidence of HZ associated with ZLV [43] similar to that observed in our meta-analysis. Our work is, to our knowledge, the first to systematically assess the efficacy/effectiveness of available HZ vaccines in people with DM [17], with no age limits and including recombinant vaccines.
One of the main goals of vaccination is the prevention of complications of HZ, such as PHN or the rare neurological complications. Diabetes mellitus is associated with an increased risk of both incidence and severity of HZ, including a higher risk for acute and chronic pain [16–18]. Unfortunately, neither studies on LZV or trials with RZV specifically reported the effects of vaccines on HZ complications in people with diabetes. Furthermore, available observational studies on LZV in people with diabetes do not include data mortality and adverse events with LZV, allowing a specific assessment of safety only for RZV. A further limitation is that the presently available data do not allow the assessment of efficacy and safety for subpopulations of diabetic patients stratified for age or comorbidities, preventing the collection of useful information for more targeted recommendations [46].
Overall, available data on people with DM are scarce, which is indeed disappointing given that DM is among the conditions for which a specific recommendation for vaccination has been provided [26, 27] [47]. Such scarcity is a major limitation of our work; on the other hand, the quality of the RCTs and observational studies retrieved is high, and no heterogeneity was detected in our meta-analysis of observational studies. On the other hand, the small number of included studies limits the reliability of I2 statistics and prevents the assessment of publication bias.
Recommendations on medical interventions should be based on a careful assessment of risk–benefit and cost-utility ratios. Such assessment requires an estimate of efficacy/effectiveness, such as the one provided by the present meta-analysis. Further data on safety and cost will allow the formulation of properly evidence-based recommendations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding and data transparency. This research was performed as a part of the institutional activity of the units, with no specific funding.
Authors' contribution
GAS and ID were involved in design, data collection, analysis and writing manuscript. AC, RF, GG, OC, OP, VS, ST were involved in data collection and manuscript revision. EM was involved as the external reviewer of the working Group in design, analysis, manuscript revision. The manuscript was drafted, revised and approved by all the authors in accordance with ICJME standards for authorship. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Declarations
Conflict of interest
GG declares fees from Sanofi Pasteur MSD, Pfizer, GSK Biologicals SA, Sanofi Pasteur, MSD Italy, Emergent BioSolutions, Moderna and Seqirus for taking part to advisory boards, expert meetings, for acting as speaker and/or organizer of meetings/congresses and as principal investigator and chief of O.U. in RCTs. All the others authors have no conflict of interest to disclose directly related to this manuscript.
Human and animal rights participations
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cohen JI. Herpes Zoster. N Engl J Med. 2013;369(3):255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galil K, Choo PW, Donahue JG, Platt R. The sequelae of herpes zoster. Arch Intern Med. 1997;157(11):1209–1213. doi: 10.1001/archinte.1997.00440320105010. [DOI] [PubMed] [Google Scholar]
- 3.Kawajiri S, Tani M, Noda K, Fujishima K, Hattori N, Okuma EY. (2007) Segmental Zoster Paresis of limbs: report of three cases and review of literature. Neurol. 2007;13(5):313–317. doi: 10.1097/NRL.0b013e31811e9d6d. [DOI] [PubMed] [Google Scholar]
- 4.Votrubec M, Thong EI. Neuropathic pain–a management update. Aust Fam Phys. 2013;42(3):92–97. [PubMed] [Google Scholar]
- 5.Hillebrand K, Bricout H, Schulze-Rath R, Schink T, Garbe E. Incidence of herpes zoster and its complications in Germany, 2005–2009. J Inf. 2015;70(2):178–186. doi: 10.1016/j.jinf.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81(10):928–930. doi: 10.1212/WNL.0b013e3182a3516e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin MJ, et al. Varicella-Zoster virus-specific immune responses in elderly recipients of a Herpes Zoster vaccine. J Infect Dis. 2008;197(6):825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto S, Hata A, Sadaoka K, Yamanishi K, Mori Y. Comparison of Varicella-Zoster Virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis. 2009;200(10):1606–1610. doi: 10.1086/644646. [DOI] [PubMed] [Google Scholar]
- 9.Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster». J Clin Virol. 2010;48:S2–S7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadogan SL, Mindell JS, Breuer J, Hayward A, Warren-Gash C. Prevalence of and factors associated with herpes zoster in England: a cross-sectional analysis of the Health Survey for England. BMC Infect Dis. 2022;22(1):513. doi: 10.1186/s12879-022-07479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes HJ, et al. Incidence of acute complications of herpes zoster among immunocompetent adults in England: a matched cohort study using routine health data*. Br J Dermatol. 2021;184(6):1077–1084. doi: 10.1111/bjd.19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mareque M, Oyagüez I, Morano R, Casado MA. Systematic review of the evidence on the epidemiology of herpes zoster: incidence in the general population and specific subpopulations in Spain. Public Health. 2019;167:136–146. doi: 10.1016/j.puhe.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Lai S-W, Liu C-S, Kuo Y-H, Lin C-L, Hwang B-F, Liao K-F. The incidence of herpes zoster in patients with diabetes mellitus: a meta-analysis of cohort studies». Medicine. 2021;100(16):e25292. doi: 10.1097/MD.0000000000025292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marra F, Parhar K, Huang B, Vadlamudi N. Risk factors for Herpes Zoster infection: a meta-analysis. Open Forum Infect Dis. 2020;7(1):ofaa005. doi: 10.1093/ofid/ofaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C-T, Lee C-Y, Sung H-Y, Liu S-J, Liang P-C, Tsai M-C. Association between diabetes mellitus and the risk of Herpes Zoster: a systematic review and meta-analysis. J Clin Endocrinol Metabol. 2022;107(2):586–597. doi: 10.1210/clinem/dgab675. [DOI] [PubMed] [Google Scholar]
- 16.Saadatian-Elahi M, Bauduceau B, Del-Signore C, Vanhems P. Diabetes as a risk factor for herpes zoster in adults: a synthetic literature review. Diabet Res Clin Pract. 2020;159:107983. doi: 10.1016/j.diabres.2019.107983. [DOI] [PubMed] [Google Scholar]
- 17.Papagianni M, Metallidis S, Tziomalos K. Herpes Zoster and diabetes mellitus: a review. Diabet Ther. 2018;9(2):545–550. doi: 10.1007/s13300-018-0394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes HJ, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157(1):30–54. doi: 10.1097/j.pain.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirrier J-E, et al. Burden of opioid use for pain management among adult herpes zoster patients in the US and the potential impact of vaccination. Hum Vaccin Immunother. 2022;18(5):2040328. doi: 10.1080/21645515.2022.2040328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz-Quiles C, López-Lacort M, Ampudia-Blasco FJ, Díez-Domingo J. Risk and impact of herpes zoster on patients with diabetes: a population-based study, 2009–2014. Human Vacc Immunother. 2017;13(11):2606–2611. doi: 10.1080/21645515.2017.1368600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohfuji S, et al. Safety of live attenuated varicella-zoster vaccine in patients with underlying illnesses compared with healthy adults: a prospective cohort study. BMC Infect Dis. 2019;19(1):95. doi: 10.1186/s12879-019-3719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oxman MN, et al. A vaccine to prevent Herpes Zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 23.Lal H, et al. Efficacy of an adjuvanted Herpes Zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham AL, et al. Efficacy of the Herpes Zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 25.Strezova A, et al. Long-term protection against Herpes Zoster by the adjuvanted recombinant zoster vaccine: interim efficacy, immunogenicity, and safety results up to 10 years after initial vaccination. Open Forum Infect Dis. 2022;9(10):ofac085. doi: 10.1093/ofid/ofac485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dooling KL, et al. Recommendations of the Advisory committee on immunization practices for use of Herpes Zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–108. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh R, Widenmaier R, Lecrenier N. A practitioner’s guide to the recombinant zoster vaccine: review of national vaccination recommendations. Exp Rev Vacc. 2021;20(9):1065–1075. doi: 10.1080/14760584.2021.1956906. [DOI] [PubMed] [Google Scholar]
- 28.“Calendario per la Vita” Position on the utilization of the herpes zoster vaccine (HZ). Available on: http://www.igienistionline.it/docs/2021/09zoster.pdf
- 29.German Standing Committee on Vaccination (STIKO) at the Robert Koch Institute (RKI) Epidimiological bulletin No. 50. 2018, Available on: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2018/Ausgaben/50_18.pdf?__blob =publicationFile
- 30.Ceccarelli A, et al. Adherence to Herpes Zoster (Shingles) catch-up campaign at the romagna local health authority (Italy), a multi-center retrospective observational study. Vaccines. 2022;10(10):1770. doi: 10.3390/vaccines10101770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabutti G, Valente N, Kuhdari P, Lupi S, Stefanati A. Prevention of herpes zoster and its complications: from the clinic to the real-life experience with the vaccine. J Med Microbiol. 2016;65(12):1363–1369. doi: 10.1099/jmm.0.000386. [DOI] [PubMed] [Google Scholar]
- 32.Cocchio S, Gallo T, Baldo V. Herpes zoster vaccination status and virtuous experiences. Minerva Med. 2020;111:1. doi: 10.23736/S0026-4806.19.06412-7. [DOI] [PubMed] [Google Scholar]
- 33.Gagliardi AM, et al. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst Rev. 2019;2019:11. doi: 10.1002/14651858.CD008858.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verstraeten T, et al. Diabetes mellitus as a vaccine-effect modifier: a review. Expert Rev Vaccines. 2020;19(5):445–453. doi: 10.1080/14760584.2020.1760098. [DOI] [PubMed] [Google Scholar]
- 35.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews». Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterne JAC, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clin Res ed) 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 37.Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hata A, et al. Efficacy and safety of live varicella zoster vaccine in diabetes: a randomized, double-blind, placebo-controlled trial. Diabet Med. 2016;33(8):1094–1101. doi: 10.1111/dme.13038. [DOI] [PubMed] [Google Scholar]
- 39.Langan SM, Thomas SL, Smeeth L, Margolis DJ, Nitsch D. Zoster vaccination is associated with a reduction of zoster in elderly patients with chronic kidney disease. Nephrol Dial Transpl. 2016;31(12):2095–2098. doi: 10.1093/ndt/gfv432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bollaerts K, Alexandridou M, Verstraeten T. Risk factors for modified vaccine effectiveness of the live attenuated zoster vaccine among the elderly in England. Vacc X. 2019;1:100007. doi: 10.1016/j.jvacx.2019.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng HF, et al. Declining effectiveness of Herpes Zoster vaccine in adults aged ≥60 years. J Infect Dis. 2016;213(12):1872–1875. doi: 10.1093/infdis/jiw047. [DOI] [PubMed] [Google Scholar]
- 42.Oostvogels L, et al. Medical conditions at enrollment do not impact efficacy and safety of the adjuvanted recombinant zoster vaccine: a pooled post-hoc analysis of two parallel randomized trials. Hum Vaccin Immunother. 2019;15(12):2865–2872. doi: 10.1080/21645515.2019.1627818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mbinta JF, Nguyen BP, Awuni PMA, Paynter J, Simpson CR. Post-licensure zoster vaccine effectiveness against Herpes Zoster and postherpetic neuralgia in older adults: a systematic review and meta-analysis. Lancet Healthy Longevity. 2022;3(4):e263–e275. doi: 10.1016/S2666-7568(22)00039-3. [DOI] [PubMed] [Google Scholar]
- 44.Tricco AC, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis». BMJ. 2018 doi: 10.1136/bmj.k4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGirr A, et al. The comparative efficacy and safety of herpes zoster vaccines: a network meta-analysis. Vaccine. 2019;37(22):2896–2909. doi: 10.1016/j.vaccine.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Tafuri S, Bianchi FP, Stefanizzi P. The public health and the question of the “best vaccine”. Vaccine. 2022;40(28):3813–3814. doi: 10.1016/j.vaccine.2022.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreoni M, Sticchi L, Nozza S, et al. Recommendations of the Italian society for infectious and tropical diseases (SIMIT) for adult vaccinations. Hum Vaccin Immunother. 2021;17(11):4265–4282. doi: 10.1080/21645515.2021.1971473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.