Abstract
Introduction: Although several meta-analyses support the positive effect of coenzyme Q10 (CoQ10) on biomarkers of oxidative stress and inflammation, the results of some other studies reject such effects.
Methods: Therefore, in this umbrella meta-analysis, we performed a comprehensive systematic search in such databases as Web of Science, PubMed, Scopus, Embase, and Google Scholar up to January 2023.
Results: Based on standardized mean difference analysis, CoQ10 supplementation significantly decreased serum C-reactive protein (CRP) (ESSMD = −0.39; 95% CI: 0.77, −0.01, p = 0.042) and malondialdehyde (MDA) (ESSMD = −1.17; 95% CI: 1.55, −0.79, p < 0.001), while it increased the total antioxidant capacity (TAC) (ESSMD = 1.21; 95% CI: 0.61, 1.81, p < 0.001) and serum superoxide dismutase (SOD) activity (ESSMD = 1.08; 95% CI: 0.37, 1.79, p = 0.003). However, CoQ10 supplementation had no significant reducing effect on tumor-necrosis factor-alpha (TNF- α) (ESSMD = −0.70; 95% CI: 2.09, 0.68, p = 0.320) and interleukin-6 (IL-6) levels (ESSMD = −0.85; 95% CI: 1.71, 0.01, p = 0.053). Based on weighted mean difference analysis, CoQ10 supplementation considerably decreased TNF-α (ESWMD = −0.46, 95% CI: 0.65, −0.27; p < 0.001), IL-6 (ESWMD = −0.92, 95% CI: 1.40, −0.45; p < 0.001), and CRP levels (effect sizes WMD = −0.28, 95% CI: 0.47, −0.09; p < 0.001).
Discussion: The results of our meta-analysis supported the alleviating effects of CoQ10 on markers of inflammation cautiously. However, CoQ10 had antioxidant effects regarding the improvement of all the studied antioxidant and oxidative stress biomarkers.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=323861, identifier CRD42022323861
Keywords: interleukin-6, C-reactive protein, antioxidant, biomarkers, coenzyme Q10, umbrella meta-analysis
1 Introduction
Inflammation is a biological and physiological response of the immune system against infection and tissue injury (Hotamisligil. 2017). However, increased levels of reactive oxygen species (ROS) following chronic inflammation and reduced antioxidant capacity, known as oxidative stress, can cause structural damage to cells (Zhang et al., 2017). A number of transcription factors can be activated by oxidative stress, leading to differential expression of several genes linked to inflammatory pathways. Therefore, oxidative stress is in relation with inflammation, and each can easily induce the other (Bessler et al., 2010; Tarry-Adkins et al., 2016; Mancini et al., 2021). Besides, both inflammation and oxidative stress can be associated with the development of chronic diseases such as diabetes mellitus (DM), obesity, metabolic syndrome, autoimmune diseases, and various types of cancers (Prasad et al., 2016; Ellulu et al., 2017).
Different types of markers are used to detect oxidative and inflammatory stress. Superoxide dismutase (SOD) and glutathione peroxidase (GPx) are the first line of antioxidant defense. SOD metabolizes superoxide radicals and GPx breaks down hydroperoxides into harmless molecules (Zarezadeh et al., 2022). Total antioxidant capacity (TAC) is the measure of the amount of ROS removed by a test solution, being used to assess the antioxidant capacity of biological samples (Rubio et al., 2016). Malondialdehyde (MDA) is used as a marker of free radical formation by lipid peroxidation (Musazadeh et al., 2021). The production of free radicals leads to the translocation of the nuclear-factor-Kappa-B (NF-kB) molecule into the nucleus and the production of pro-inflammatory cytokines such as tumor-necrosis factor-alpha (TNF- α) and interleukin-6 (IL-6).
The scientific community is persistently seeking to find nutrients or compounds that have anti-inflammatory, immunomodulatory, and antioxidant properties, particularly for diseases whose prevention is influenced by unhealthy diet. Coenzyme Q10 (CoQ10) has been discussed as a potential treatment option for chronic diseases in which oxidative stress plays a significant pathophysiological role (Arenas-Jal et al., 2020; Gutierrez-Mariscal et al., 2020; Mancini et al., 2021).
CoQ10 is one of the non-enzymatic antioxidants (Cicero et al., 2018; Samimi et al., 2019), which has both endogenous and exogenous sources (Fakhrolmobasheri et al., 2023). However, its endogenous biosynthesis is impaired in some conditions; therefore, CoQ10 supplementation can be useful in these conditions. In a study, endogenous CoQ10 biosynthesis decreased in patients with type 2 diabetes mellitus (T2DM) compared to subjects with normal glucose tolerance (Gholami et al., 2018). This could be due to impaired CoQ10 metabolism in T2DM or statin therapy in these patients, which may contribute to a decrease in the synthesis of CoQ10 substrates (Gholami et al., 2018). CoQ10 is involved in mitochondrial bioenergetics as well as ROS scavenging due to its participation in redox reactions (Abdeen et al., 2020; Hidalgo-Gutiérrez et al., 2021). Also, CoQ10 can inhibit inflammation through modulation of NF-kB-related pathways (Shukla and Dubey. 2018). Thus, CoQ10 has been proposed as a potential anti-inflammatory and antioxidant agent (Hernández-Camacho et al., 2018).
Several meta-analyses have reported controversial results related to the effect of CoQ10 on oxidative stress and inflammation biomarkers. In a study by Fan et al. (2017), CoQ10 significantly lowered inflammatory factors [TNF-α, IL-6, and C-reactive protein (CRP)], while its supplementation did not affect these factors in other studies (Farsi et al., 2019; Jorat et al., 2019; Dludla et al., 2020). In addition, supplementation with CoQ10 significantly increased TAC and serum SOD activity in several studies (Jorat et al., 2019; Akbari et al., 2020; Sangsefidi et al., 2020; Hajiluian et al., 2021). However, other studies reported that CoQ10 intake could not affect MDA (Dludla et al., 2020) and TAC (Hajiluian et al., 2021). Therefore, the present umbrella meta-analysis aimed to examine the effects of CoQ10 supplementation on serum TNF-α, IL-6, CRP, MDA, SOD, and TAC pooling from the selected meta-analyses. Given that different studies have used various statistical methods and reported diverging results, we used a single statistical method in this study to reach a definite conclusion regarding the anti-inflammatory and antioxidant effects of CoQ10.
2 Methods
This study was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Moher et al., 2015). Also, we registered the study protocol in PROSPERO (CRD42022323861).
2.1 Search strategy and study selection
To find the related literature, we systematically searched databases including Web of Science, PubMed, Scopus, Embase, and Google Scholar up to January 2023. The search strategy was developed using the following MeSH terms and keywords (Q10 [Mesh] OR “coenzyme Q10” [tiab] OR ubidecarenone [tiab] OR ubiquinone [Mesh] OR “Bio-Quinone Q10” [tiab] OR ubiquinol [tiab] OR “ubiquinol-10” [tiab]) AND (inflammation [Mesh] OR “C-Reactive Protein” [Mesh] OR “c-reactive protein” [tiab] OR crp [tiab] OR “hs-crp” [tiab] OR “high sensitivity-CRP” [tiab] OR “high sensitivity C-reactive protein” [tiab] OR “Tumor Necrosis Factor-alpha” [Mesh] OR “tumor necrosis factor-alpha” [tiab] OR “tumor necrosis factor-α” [tiab] OR “tnf-alpha” [tiab] OR “tnf-α” [tiab] OR “Interleukin-6” [Mesh] OR “interleukin-6” [tiab] OR IL-6 [tiab] OR “interleukin 6” [tiab] OR “Oxidative Stress” [MeSH] OR “Oxidative Stress” [tiab] OR “Total Antioxidant Capacity” [tiab] OR antioxidant [tiab] OR Oxidant [tiab] OR “reactive oxygen species” [tiab] OR Malondialdehyde [tiab] OR glutathione [tiab] OR TAC [tiab] OR GSH [tiab] OR MDA [tiab]) AND (“systematic review’’ [Publication Type] OR “meta-analysis” [tiab]). To increase the sensitivity of search strategy, the wild-card term “*’’ was used.
2.2 Inclusion and exclusion criteria
We included all meta-analyses of randomized controlled trials (RCTs) evaluating the effects of CoQ10 supplementation on biomarkers of inflammatory and stress oxidative, including TNF-α, IL-6, CRP, MDA, TAC, and SOD in adults (>18 years old). All the included studies reported the estimates of effect sizes (ES) and corresponding confidence intervals (CIs). We also excluded the following studies: in vitro, in vivo, and ex vivo studies; quasi-experimental studies; observational studies; case reports; editorials; and controlled clinical trials. In addition, studies on infants and juveniles were excluded. We also screened the reference lists of all studies manually to ensure the inclusion of all the related literature. Furthermore, we only included articles written in English language.
2.3 Study selection and data extraction
Two independent reviewers (ZK and VM) screened the articles according to the eligibility criteria. First, the title and abstract of the retrieved articles were reviewed. Next, we assessed the full-text of all the relevant articles to determine their eligibility for inclusion in the meta-analyses. Any disagreements were resolved by consensus with the third author (AHF).
We extracted the following information from the included meta-analyses: first author’s name, year of publication, sample size, study location, dose and duration range of supplementation, type of ESs [(weighted mean difference (WMD) and standardized mean difference (SMD)], as well as CIs for TNF- α, IL-6, CRP, MDA, TAC, and SOD.
2.4 Quality assessment and assessment of the meta-evidence
Methodological quality was assessed independently by two reviewers (MSH and VM) using the AMSTAR2 questionnaire. The AMSTAR2 questionnaire consists of 16 items that ask reviewers to choose one of the following options: “Yes” or “Partial Yes” or “No” or “No Meta-analysis”. The AMSTAR2 checklist was categorized into “critically low quality”, “low quality”, “moderate quality”, and “high quality” (Shea et al., 2017).
The GRADE (Grading of Recommendations, Assessment and Evaluation) approach was used to evaluate the credibility of the included meta-analyses. This approach includes five factors, including risk of bias, consistency of results, directness, precision, and potential for publication bias. The evidence is finally classified into four categories of high, moderate, low, or very low (Guyatt et al., 2008).
2.5 Data synthesis and statistical analysis
To estimate overall ES, we used the reported ESs and CIs. Analysis was performed separately for SMD and WMD due to their natural differences. Cochran’s Q test and I 2 statistics were used to determine the heterogeneity. In the current study, p-values less than 0.10 and I 2 values higher than 50% were considered as significant for between-study heterogeneity. A random-effects model was performed using the restricted maximum likelihood (REML) strategy when between-study heterogeneity was critical (I 2 > 50% or p < 0.1). To explore the sources of heterogeneity, we performed subgroup analysis by duration, mean age, sample size, and dose. The sensitivity analysis was conducted to determine whether removing a single study would affect the overall ES. Publication bias was assessed using funnel plots, and the Egger’s test was applied if the number of included datasets was ten or higher; otherwise, only the Begg’s test result was reported. To simulate a model without publication bias, the trim-and-fill method was used when publication bias was detected. All statistical analyses were carried out using STATA, version 16 (Stata Corporation, College Station, TX, US). p-values less than 0.05 were considered significant.
3 Results
3.1 Systematic review
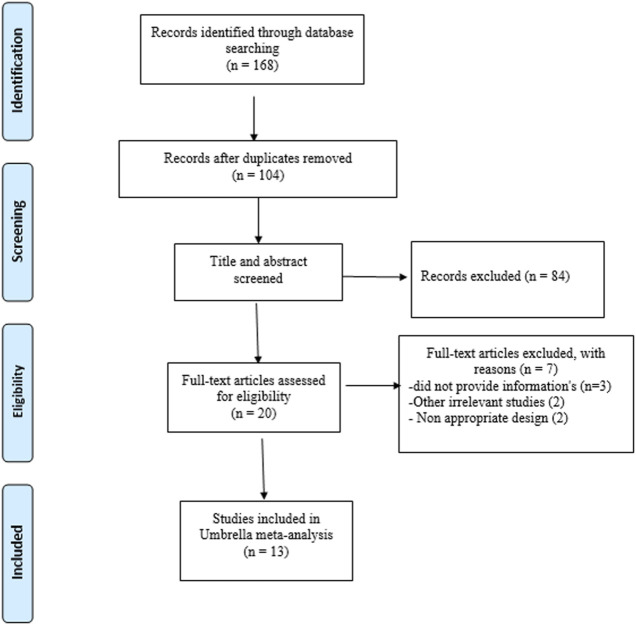
The flowchart of the search process is presented in Figure 1. After screening all the related data, a total of 13 meta-analyses published between 2017 and 2022 were included in our umbrella meta-analysis. The characteristics of the included studies are summarized in Table 1. The participants’ ages ranged from 43 to 69 years. Based on the country of the first author, the studies were as follows: seven in Iran (Farsi et al., 2019; Jorat et al., 2019; Akbari et al., 2020; Sangsefidi et al., 2020; Alimohammadi et al., 2021; Hajiluian et al., 2021; Sedaghat et al., 2022), five in China (Fan et al., 2017; Zhai et al., 2017; Mazidi et al., 2018; Zhang et al., 2019; Dai et al., 2022), and one in South Africa (Dludla et al., 2020). Interventions varied in duration, from 6 to 20 weeks, and supplement doses varied from 65 to 300 mg/day. There were also differences in the health status of the participants (Table 1). The quality of the trials was assessed using the “Jadad score” (Clark et al., 1999) system and the Cochrane risk of bias tool (Higgins et al., 2011). Overall, nearly 90% of the included meta-analyses contained high-quality RCTs (Table 1).
FIGURE 1.
PRISMA flow diagram of selection studies.
TABLE 1.
Characteristics of the included studies.
| Citation (first author et al., year) | Location | Study population | Sample size | Mean age | Q10 dosage (mg) | Duration (Week) | Main outcome |
|---|---|---|---|---|---|---|---|
| Zhai et al. (2017) | China | NAFLD, MS, CAD, MI | 180 | 46.9 | 275 | 12 | IL-6 ↔ |
| risk of CVD in CKD, obesity, CAD, NAFLD, MetS, HTN | 331 | 52.2 | 185.7 | 10 | CRP ↔ | ||
| NAFLD, MS, CAD | 128 | 43.5 | 300 | 12 | TNF-α↓ | ||
| Fan et al. (2017) | China | runners, NAFLD, CAD, mild hypertensive, RA, renal lithiasis, hyperlipidemic with MI, MS | 402 | 45.5 | 201.1 | 9 | IL-6↓ |
| runners, NAFLD, CAD, end stage heart failure, RA, MS | 217 | 43.6 | 194.8 | 9.5 | TNF-α ↓ | ||
| systolic dysfunction, runners, T2DM, NAFLD, obese, CAD, hypercholesterolemia, chronic renal impairment, mild hypertensive, heart failure, ESRD | 543 | 53.4 | 159 | 11 | CRP ↓ | ||
| Mazidi et al. (2018) | China | ischemic LVSD, DM with neuropathy, BMI>25, CAD | 385 | 65.9 | 188.75 | 11 | IL-6↓ |
| stenosis of one major, coronary artery, chronic renal impairment, mildly hypertensive | CRP↔ | ||||||
| Jorat et al. (2019) | Iran | CAD, AMI, MI | 295 | 54.4 | 114 | 13 | MDA↓ |
| CHF, end stage heart failure, CAD | 102 | 66 | 170 | 20 | TNF-α ↔ | ||
| CAD, T2DM with coronary heart disease | 155 | 68.3 | 152.5 | 11 | IL-6 ↔ | ||
| CAD, systolic dysfunction, CHF, ischemic heart disease | 313 | 63.6 | 218.3 | 10.5 | CRP↔ | ||
| CAD, ischemic heart disease, systolic dysfunction | 184 | 67.9 | 222 | 10 | SOD↑ | ||
| Zhang et al. (2019) | China | CKD | 115 | 67.2 | 65 | 12 | MDA↓ |
| Farsi et al. (2019) | Iran | migraine, HCC, NAFLD, RA, mild HTN, MS, CAD, end-stage heart failure | 348 | 48.7 | 232.5 | 11.5 | TNF-α ↔ |
| migraine, HCC, T2DM, NAFLD, RA, mild HTN, MS, HLP with MI, CAD | 454 | 55.9 | 210 | 11 | IL-6 ↓ | ||
| HCC, NAFLD, mild HTN, CAD | 208 | 65.4 | 168.3 | 12 | CRP ↔ | ||
| Dludla et al. (2020) | South Africa | mildly hypertensive, NAFLD | 101 | NR | 100 | 12 | TNF-α↔ |
| CRP ↓ | |||||||
| NAFLD, T2DM | 161 | 47.5 | 366.6 | 6.5 | MDA↔ | ||
| Akbari et al. (2020) | Iran | HD, T2DM, T1DM, bipolar disorders, acute coronary syndrome, RA, NAFLD | 540 | 48.8 | 114 | 11.5 | MDA ↓ |
| bipolar disorders, HD, T2DM, RA, NAFLD | 464 | 52.8 | 165 | 11 | TAC↑ | ||
| Hepatocellular carcinoma, renal injury, CAD, systolic dysfunction | 237 | 62.2 | 275 | 8 | SOD↑ | ||
| Sangsefidi et al. (2020) | Iran | T2D, ESRF, healthy, CRF, DN, HCC, MS, CAD, RA, NAFLD | 715 | NR | 182.1 | 11 | MDA↓ |
| NAFLD, RA, MS, dyslipidemia, T2DM | 481 | NR | 167.4 | 12 | TAC↑ | ||
| ischemic LVSD, CAD, MS, HCC, T2DM | 284 | NR | 244.2 | 11.5 | SOD↑ | ||
| Hajiluian et al. (2021) | Iran | RA, kidney disease, NAFLD, T2DM, CVD | 480 | 53.9 | 104.4 | 11 | MDA ↓ |
| T2DM | 236 | 57.2 | 100 | 10 | MDA ↓ | ||
| RA, kidney disease, NAFLD, CVD | 244 | 50.6 | 108 | 12 | MDA↓ | ||
| RA, T2DM, kidney disease, NAFLD | 406 | 54.8 | 160 | 11.5 | TAC↑ | ||
| RA, NAFLD, kidney disease | 148 | 51.3 | 106.6 | 8 | TAC↔ | ||
| T2DM | 258 | 57.5 | 200 | 14 | TAC↑ | ||
| kidney disease, CVD, cancer | 248 | 62.2 | 275 | 8.5 | SOD↑ | ||
| Alimohammadi et al. (2021) | Iran | breast cancer | 156 | 57 | 100 | 9.5 | SOD↑ |
| TNF-α↓ | |||||||
| IL-6↓ | |||||||
| Dai et al. (2022) | China | Healthy, dyslipidemia, NAFLD, T2DM, hepatocellular carcinoma, coronary artery disease, bipolar disorder, MS, hemodialysis | 1912 | 50 | 200 | 11.5 | MDA ↓ |
| TAC↑ | |||||||
| SOD↔ | |||||||
| Sedaghat et al. (2022) | Iran | Healthy, dyslipidemia, NAFLD, T2DM, hepatocellular carcinoma, coronary artery disease, bipolar disorder, MS, renal injury, ICU patients, autism, migraine | 1,267 | NR | 195 | 9 | MDA ↓ |
| TAC↑ | |||||||
| SOD↑ |
Abbreviations: NR, not reported; NAFLD, nonalcoholic fatty liver disease; MS, multiple sclerosis; CAD, coronary artery disease; MI, myocardial infarction; MetS, metabolic syndrome; HTN, hypertension; RA, rheumatoid arthritis; T2DM, Type 2 diabetes; CVD, cardiovascular disease; ESRD, End-Stage Renal Disease; LVSD, Left Ventricular Systolic Dysfunction; HCC, hepatocellular carcinoma; DN, diabetic nephropathy; AMI, acute myocardial infarction.
3.2 Risk of bias assessment and quality of evidence
Applying the AMSTAR 2 tool showed that the meta-analyses are of high quality (Table 2). Out of 13 meta-analyses, ten studies had high quality and three studies had moderate quality. Regarding SMD and WMD analyses, while biomarkers had high-quality evidence, oxidative stress had low-quality evidence (Table 3).
TABLE 2.
Results of assessing the methodological quality of all the meta-analyses included in the meta-analysis.
| Study | Q11 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhai et al. (2017) | No | Partial Yes | Yes | Partial Yes | Yes | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Moderate |
| Fan et al. (2017) | No | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Mazidi et al. (2018) | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Hajiluian et al. (2021) | No | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Jorat et al. (2019) | No | Partial Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Zhang et al. (2019) | No | Partial Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Moderate |
| Farsi et al. (2019) | No | Partial Yes | Yes | Partial Yes | No | Yes | No | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Moderate |
| Dludla et al. (2020) | No | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | High |
| Alimohammadi et al. (2021) | No | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Akbari et al. (2020) | No | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Sangsefidi et al. (2020) | No | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Dai et al. (2022) | No | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Sedaghat et al. (2022) | No | Partial Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | High |
Did the research questions and inclusion criteria for the review include the components of PICO? 2. Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? 3. Did the review authors explain their selection of the study designs for inclusion in the review? 4. Did the review authors use a comprehensive literature search strategy? 5. Did the review authors perform study selection in duplicate? 6. Did the review authors perform data extraction in duplicate? 7. Did the review authors provide a list of excluded studies and justify the exclusions? 8. Did the review authors describe the included studies in adequate detail? 9. Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review? 10. Did the review authors report on the sources of funding for the studies included in the review? 11. If meta-analysis was performed, did the review authors use appropriate methods for statistical combination of results? 12. If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? 13. Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? 14. Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? 15. If they performed quantitative synthesis, did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?
Each question was answered with “Yes”, “Partial Yes” or “No”. When no meta-analysis was done, question 11, 12 and 15 were answered with “No meta-analysis conducted.
TABLE 3.
Summary of findings and quality of evidence of the CoQ10 supplementation on inflammatory biomarkers.
| Outcome measures | Summary of findings | Quality of evidence assessment (GRADE) | ||||||
|---|---|---|---|---|---|---|---|---|
| No of patients (meta-analysis) | Effect size (95% CI) | Risk of bias a | Inconsistency b | Indirectness c | Imprecision d | Publication bias e | Quality of evidence f | |
| SMD analysis | ||||||||
| CRP | 622 (3) | −0.39 (−0.77, −0.01) | Not Serious | Not Serious | Serious | Not Serious | Not Serious | High |
| TNF-α | 885 (4) | −0.70 (−2.09, 0.68) | Not Serious | Not Serious | Serious | Not Serious | Not Serious | High |
| IL-6 | 1,076 (4) | −0.85 (−1.71, 0.01) | Not Serious | Not Serious | Serious | Not Serious | Not Serious | High |
| MDA | 4,550 (9) | −1.17 (−1.55, −0.79) | Not Serious | Serious | Serious | Serious | Not Serious | Low |
| TAC | 2,796 (6) | 1.21 (0.61, 1.81) | Not Serious | Serious | Serious | Serious | Not Serious | Low |
| SOD | 2,280 (7) | 1.08 (0.37, 1.79) | Not Serious | Serious | Serious | Serious | Not Serious | Low |
| WMD analysis | ||||||||
| CRP | 1,259 (3) | −0.28 (-0.47, −0.09) | Not Serious | Not Serious | Serious | Not Serious | Not Serious | High |
| TNF-α | 345 (2) | −0.46 (−0.65, −0.27) | Not Serious | Not Serious | Serious | Not Serious | Not Serious | High |
| IL-6 | 967 (3) | −0.92 (−1.40, −0.45) | Not Serious | Not Serious | Serious | Not Serious | Not Serious | High |
CRP, C-reactive protein; TNF, tumor necrosis factor; IL-6, interleukin-6; TAC, total antioxidant capacity; MDA, malondialdehyde; SOD, superoxide dismutase.
Risk of bias according to AMSTAR2 results.
Downgraded if there was a substantial unexplained heterogeneity (I 2 > 50%, p < 0.10) that was unexplained by meta-regression or subgroup analyses.
Downgraded if there were factors present relating to the participants, interventions, or outcomes that limited the generalizability of the results. Participants of the included studies were from different health conditions (subgroup analysis was not performed for each disease).
Downgraded if the 95% confidence interval (95% CI) crossed the minimally important difference (MID) for benefit or harm. MIDs, used for each outcome were: 3.16 mg/L for CRP, 7.9 pg/mL for TNF-α, and 2 pg/mL for IL-6, 0.59 mmol/mL for MDA, and 0.08 mmol/L for TAC.
Downgraded if there was an evidence of publication bias using funnel plot.
Since all the included studies were meta-analyses, the certainty of the evidence was graded as high for all outcomes by default and then downgraded based on prespecified criteria. Quality was graded as high, moderate, low, or very low.
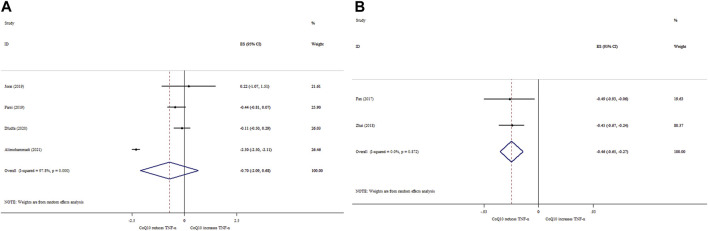
3.3 Effects of CoQ10 supplementation on TNF-α based on SMD analysis
Data from four meta-analyses indicated that CoQ10 supplementation did not significantly reduce TNF-α levels (ESSMD = −0.70; 95% CI: 2.09, 0.68, p = 0.320) (Figure 2A). Meanwhile, between-study heterogeneity was found to be quite high (I 2 = 97.8%, p < 0.001). In contrast to overall effect, subgroup analysis showed that CoQ10 supplementation significantly decreased TNF-α levels when intervention duration and dose were ≤10 weeks and >200 mg/day, respectively (Table 4). According to the sensitivity analysis, excluding any of the studies did not affect the estimate of the overall ES. No indication of publication bias was observed according to the Begg’s test (p = 0.707).
FIGURE 2.
Forest plot of impacts of CoQ10 supplementation on TNF-α based on SMD (A) and WMD (B) analysis.
TABLE 4.
Pooled estimates of the effect of coenzyme Q10 on inflammatory and stress oxidative biomarkers according to SMD analysis.
| Group | No. of comparisons | SMD (95% CI) | p-value | I 2 (%) | P-heterogeneity |
|---|---|---|---|---|---|
| Q10 supplementation on TNF-α levels | |||||
| Total | 4 | −0.70 (−2.09, 0.68) | 0.320 | 97.8 | <0.001 |
| Age (years) | |||||
| ≤55 | 1 | −0.44 (−0.88, - 0.00) | 0.050 | - | - |
| >55 | 2 | −1.12 (−3.59, 1.34) | 0.371 | 93.0 | <0.001 |
| NR | 1 | −0.11 (−0.50, 0.28) | 0.585 | - | - |
| Duration (week) | |||||
| ≤10 | 1 | −2.30 (−2.49, −2.11) | <0.001 | - | - |
| >10 | 3 | −0.23 (−0.52, 0.05) | 0.110 | 0.0 | 0.428 |
| Dose (mg) | |||||
| ≤200 | 3 | −0.78 (−2.58, 1.01) | 0.392 | 98.1 | <0.001 |
| >200 | 1 | −0.44 (−0.88, −0.00) | 0.050 | - | - |
| Q10 supplementation on IL-6 levels | |||||
| Total | 4 | −0.85 (−1.71, 0.01) | 0.053 | 95.9 | <0.001 |
| Age(years) | |||||
| ≤55 | 1 | −1.56 (−1.73, −1.39) | <0.001 | - | - |
| >55 | 2 | −0.67 (−1.73, 0.38) | 0.211 | 45.6 | 0.175 |
| NR | 1 | −0.24 (−0.63, 0.16) | 0.234 | - | - |
| Duration (weeks) | |||||
| ≤10 | 1 | −1.56 (−1.73, −1.39) | <0.001 | - | - |
| >10 | 3 | −0.35 (−0.61, −0.09) | 0.008 | 11.5 | 0.323 |
| Dose (mg) | |||||
| ≤200 | 3 | −1.06 (−2.17, 0.06) | 0.064 | 94.5 | <0.001 |
| >200 | 1 | −0.37 (−0.65, −0.09) | 0.010 | - | - |
| Q10 supplementation on CRP levels | |||||
| Total | 3 | −0.39 (−0.77, −0.01) | 0.042 | 38.5 | 0.197 |
| Q10 supplementation on MDA levels | |||||
| Total | 9 | −1.17 (−1.55, −0.79) | <0.001 | 62.4 | 0.007 |
| Sample size | |||||
| ≤300 | 5 | −1.36 (−2.17, −0.56) | <0.001 | 60.5 | 0.038 |
| >300 | 4 | −1.15 (−1.65, −0.66) | <0.001 | 73.0 | 0.011 |
| Age (year) | |||||
| ≤55 | 5 | −1.13 (−1.69, −0.57) | <0.001 | 56.4 | 0.057 |
| >55 | 2 | −0.79 (−1.28, −0.31) | <0.001 | 16.2 | 0.275 |
| NR | 2 | −1.84 (−3.42, −0.25) | 0.023 | 84.8 | 0.010 |
| Duration (weeks) | |||||
| ≤10 | 3 | −1.60 (−3.11, −0.09) | 0.038 | 82.3 | 0.004 |
| >10 | 6 | −1.07 (−1.45, −0.70) | <0.001 | 49.8 | 0.076 |
| Dose (mg/day) | |||||
| ≤200 | 8 | −1.16 (−1.55, −0.77) | <0.001 | 66.5 | 0.004 |
| >200 | 1 | −1.57 (−3.61, 047) | 0.131 | - | - |
| Q10 supplementation on TAC levels | |||||
| Total | 6 | 1.21 (0.61, 1.81) | <0.001 | 76.9 | <0.001 |
| Sample size | |||||
| ≤300 | 2 | 0.66 (0.16, 1.16) | 0.010 | 19.4 | 0.265 |
| >300 | 4 | 1.65 (0.67, 2.62) | <0.001 | 83.6 | <0.001 |
| Age (year) | |||||
| ≤55 | 3 | 0.92 (0.13, 1.70) | 0.022 | 77.7 | 0.011 |
| >55 | 1 | 0.84 (0.32, 1.36) | 0.002 | - | - |
| NR | 2 | 2.27 (0.21, 4.34) | 0.031 | 82.9 | 0.016 |
| Duration (weeks) | |||||
| ≤10 | 2 | 1.79 (−1.24, 4.83) | 0.247 | 92.8 | <0.001 |
| >10 | 4 | 1.07 (0.58, 1.56) | <0.001 | 61.2 | 0.052 |
| Dose (mg/day) | |||||
| ≤200 | 4 | 0.73 (0.45, 1.01) | <0.001 | 0.0 | 0.435 |
| >200 | 2 | 2.49 (0.97, 4.01) | <0.001 | 72.5 | 0.057 |
| Q10 supplementation on SOD levels | |||||
| Total | 7 | 1.08 (0.37, 1.79) | 0.003 | 95.7 | <0.001 |
| Sample size | |||||
| ≤200 | 2 | 2.43 (2.14, 2.72) | <0.001 | 0.0 | 0.782 |
| >200 | 5 | 0.48 (0.32, 0.64) | <0.001 | 0.0 | 0.420 |
| Age (year) | |||||
| ≤60 | 2 | 1.45 (−0.46, 3.36) | 0.136 | 97.9 | <0.001 |
| >60 | 3 | 0.62 (0.11, 1.12) | 0.017 | 77.3 | 0.012 |
| NR | 2 | 0.77 (0.26, 1.28) | 0.003 | 33.1 | 0.222 |
| Duration (weeks) | |||||
| ≤10 | 5 | 0.98 (0.06, 1.89) | 0.036 | 96.9 | <0.001 |
| >10 | 2 | 1.49 (−0.46, 3.45) | 0.134 | 85.5 | 0.009 |
| Dose (mg/day) | |||||
| ≤200 | 1 | 2.42 (2.13, 2.72) | <0.001 | - | - |
| >200 | 6 | 0.59 (0.32, 0.87) | <0.001 | 58.8 | 0.033 |
Abbreviation: N, number; NR, not reported.
3.4 Effects of CoQ10 supplementation on TNF-α based on WMD analysis
Our findings based on two meta-analyses revealed that CoQ10 supplementation considerably decreased TNF-α levels (ESWMD = −0.46, 95% CI: 0.65, −0.27; p < 0.001), with no considerable between-study heterogeneity (I 2 = 0.0%; p = 0.872) (Figure 2B).
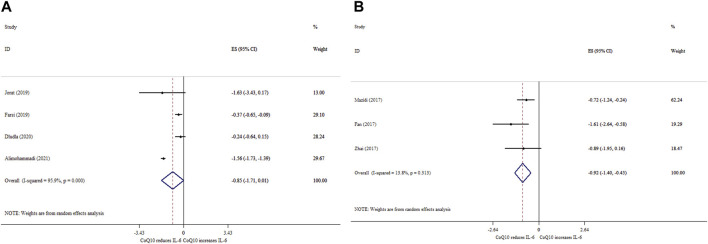
3.5 Effects of CoQ10 supplementation on IL-6 based on SMD analysis
CoQ10 supplementation did not significantly reduce IL-6 levels (ESSMD = −0.85; 95% CI: 1.71, 0.01, p = 0.053) (Figure 3A). Also, a high degree of heterogeneity was detected (I 2 = 95.9%, p < 0.001). Subgroup analysis revealed that the ameliorative effects of CoQ10 supplementation on the IL-6 levels were stronger when the treatment dose was >200 mg/day, the duration was ≤10 weeks, and age was ≤55 years (Table 4). Furthermore, the overall effects of CoQ10 on IL-6 changed to be statistically significant when the studies were removed using sensitivity analysis (Dludla et al., 2020; Alimohammadi et al., 2021). In addition, the Begg’s test did not reveal any evidence of publication bias (Begg’s, p = 0.548).
FIGURE 3.
Forest plot of impacts of CoQ10 supplementation on IL-6 based on SMD (A) and WMD (B) analysis.
3.6 Effects of CoQ10 supplementation on IL-6 based on WMD analysis
CoQ10 supplementation significantly decreased IL-6 levels (ESWMD = −0.92; 95% CI: 1.40, −0.45, p < 0.001; I 2 = 13.8%, p = 0.313) (Figure 3B).
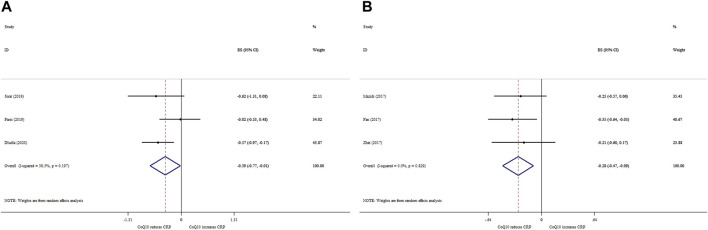
3.7 Effects of CoQ10 supplementation on CRP based on SMD analysis
CoQ10 supplementation significantly reduced CRP levels (ESSMD = −0.39; 95% CI: 0.77, −0.01, p = 0.042) (Figure 4A). Nevertheless, there was no significant between-study heterogeneity (I 2 = 38.5%, p = 0.197).
FIGURE 4.
Forest plot of impacts of CoQ10 supplementation on CRP based on SMD (A) and WMD (B) analysis.
3.8 Effects of CoQ10 supplementation on CRP based on WMD analysis
Pooling three meta-analyses revealed a significant reduction in CRP levels (ES WMD = −0.28, 95% CI: 0.47, −0.09; p < 0.001) (Figure 4B), with no considerable between-study heterogeneity (I 2 = 0.0%; p = 0.829).
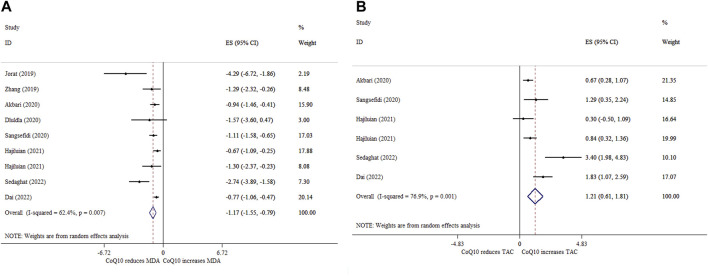
3.9 Effects of CoQ10 supplementation on MDA based on SMD analysis
Eight meta-analyses with nine ESs reported that CoQ10 supplementation significantly reduced MDA levels (ESSMD = −1.17; 95% CI: 1.55, −0.79, p < 0.001) (Figure 5A). Moreover, there was a significant between-study heterogeneity (I 2 = 62.4%, p = 0.007) (Table 4). Subgroup analysis demonstrated that CoQ10 supplementation had a greater reducing effect on MDA in people aged under 55 years, intervention duration ≤10 weeks, and sample size ≤300 (Table 4). Sensitivity analysis revealed no change in the direction of ESs when we removed any of the ESs in the overall analysis. No proof of publication bias was observed after performing the Begg’s test (p = 0.174).
FIGURE 5.
Forest plot of impacts of CoQ10 supplementation on MDA (A) and TAC (B) levels based on SMD analysis.
3.10 Effects of CoQ10 supplementation on TAC based on SMD analysis
Five meta-analyses with six ESs indicated that CoQ10 supplementation significantly increased TAC (ESSMD = 1.21; 95% CI: 0.61, 1.81, p < 0.001) (Figure 5B). Also, there was a high between-study heterogeneity (I 2 = 76.9%, p = 0.435) (Table 4). The effects of CoQ10 on TAC levels at doses >200 mg/day in studies with an intervention duration of >10 weeks and a sample size of >300 participants were stronger than other subgroups (Table 4). According to sensitivity analysis, the overall ES did not change significantly by excluding any of the studies. No evidence of publication bias was found following Begg’s test (p = 0.806).
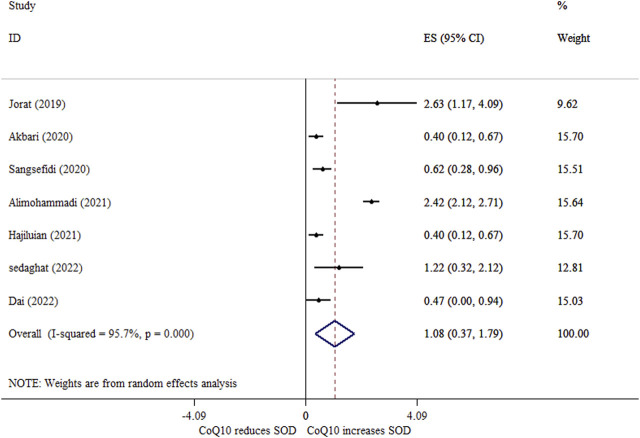
3.11 Effects of CoQ10 supplementation on SOD activity based on SMD analysis
Seven meta-analyses indicated that CoQ10 supplementation significantly increased serum SOD activity (ESSMD = 1.08; 95% CI: 0.37, 1.79, p = 0.003) (Figure 6). Moreover, we found a high degree of between-study heterogeneity (I 2 = 95.7%, p < 0.001) with sample size as its source (Table 4). Based on the sensitivity analysis results, the overall ES did not depend on a single study. There was no sign of publication bias based on Begg’s test (p = 0.327).
FIGURE 6.
Forest plot of impacts of CoQ10 supplementation on SOD levels based on SMD analysis.
4 Discussion
The current umbrella meta-analysis evaluated the therapeutic effects of CoQ10 on inflammation and oxidative stress. To this end, we included 13 meta-analyses containing 77 trials. Based on SMD analysis, our findings showed that CoQ10 supplementation enhanced serum activity of SOD and TAC, but it declined CRP and MDA levels. Meanwhile, CoQ10 supplementation did not affect TNF-α and IL-6 levels. However, according to the results of WMD analysis, CoQ10 reduced IL-6 and TNF-α levels. Since the WMD depends only on the weight of each study, it can be concluded that the standard deviation of the ESs related to IL-6 and TNF-α was effective in the final result (Andrade. 2020). Moreover, the limited number of analyzed studies with WMD could reduce the power of its related findings. On the other hand, the high heterogeneity of the analyzed studies with SMD can also question the validity of its related findings. Consequently, definitive conclusions regarding the effect of CoQ10 supplementation on IL-6 and TNF-α should be made with caution.
Due to the limited number of studies that reported WMD, subgroup analysis was performed only for studies that reported SMD. However, the small number of studies in some subgroups led to a low-powered ES. Nonetheless, this limited subgroup analysis also showed that the effect of CoQ10 on inflammatory and oxidative stress indices was not dependent on sample size. Regarding other subgroups, >200 mg/day for ≤10 weeks of CoQ10 supplementation showed more improving outcomes in patients with mean age of ≤55 years. However, there were some exceptions. For example, >10 weeks of CoQ10 supplementation and doses of ≤200 mg/day resulted in further increases in TAC and serum SOD activity, respectively. There are various oral doses for CoQ10 over the counter from 30 mg to 600 mg (Raizner. 2019). However, doses up to 1,200 mg/day have been reported to be tolerated (Garrido-Maraver et al., 2014).
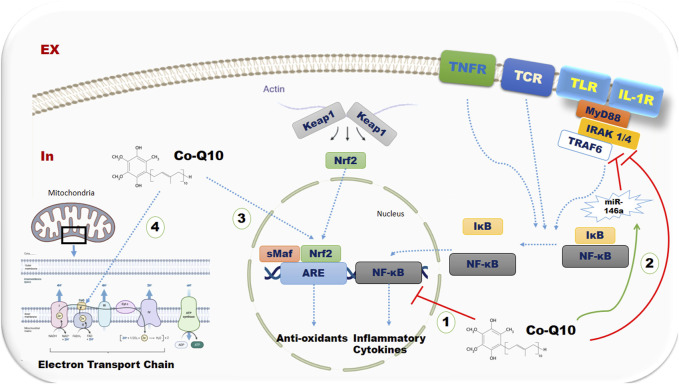
CoQ10, a well-known nutritional supplement with antioxidant properties, exerts protective roles in various metabolic and inflammatory processes (Zhai et al., 2017). Numerous potential mechanisms can explain these features (Figure 7). CoQ10 may play a role in declining the production of pro-inflammatory cytokines by inhibiting NF-κB gene expression, which is involved in the expression of pro-inflammatory cytokines, such as TNF-α and IL-6 (Schmelzer et al., 2008; Fan et al., 2017). Moreover, CoQ10 modulates the expression of microRNAs-146a (miR-146a), which is an NF-κB-dependent gene. However, through direct downregulation of IL-1 receptor-associated kinase 1 (IRAK-1) and TNF receptor-associated factor 6 (TRAF6), it exerts a negative feedback effect on Toll-like receptor and cytokine signaling, leading to the suppression of NF-κB-mediated inflammatory molecules (Taganov et al., 2006; Schmelzer et al., 2009; Xie et al., 2018; Long et al., 2019). CoQ10 has been reported to have a downregulating effect on the secretion of CRP (Schmelzer et al., 2008) and pro-inflammatory chemokines such as macrophage inflammatory protein-1 alpha (Schmelzer et al., 2007), possibly due to the inhibitory effect of CoQ10 on NF-kB. Moreover, CoQ10 has been found to have an increasing effect on nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and heme oxygenase 1 (HO-1) in the oxidative state (Pala et al., 2016). Nrf2 is a key transcription factor that targets the genes of antioxidant proteins (Gao et al., 2022). Also, it has been suggested that CoQ10 is a peroxisome proliferator-activated receptor gamma and alpha (PPAR-γ and α) ligand (Tiefenbach et al., 2018). Activated PPARs modulate the inflammatory responses through their regulatory effects on the expression of several genes involved in inflammation (Wu et al., 2020). In addition, CoQ10, as a redox carrier in the mitochondrial membrane, declines cellular oxidative stress and free radical production (Sangsefidi et al., 2020).
FIGURE 7.
The possible mechanisms of CoQ10 supplementation on inflammatory and oxidative stress biomarkers Abbreviations: TNFR, TNF receptor; ,TLRs, Toll-like receptors; TCR, T lymphocyte receptor; Keap 1, kelch-like ECH-associated protein 1; Nrf2, nuclear factor (erythroid-derived 2)-like 2; NF-kB, nuclear-factor-Kappa-B; IKB, I-kappa-B; miR-146a, microRNAs-146a; MYD88, Myeloid differentiation primary response 88; IRAK4, Interleukin-1 receptor–associated kinase 4; TRAF6, TNF receptor associated factor 6
Although most studies reported beneficial effects of CoQ10 on inflammation and oxidative stress, some other studies showed different findings. The possible reasons for such inconsistencies include differences in study design (Farsi et al., 2019; Alimohammadi et al., 2021), sample size (Zhai et al., 2017; Mazidi et al., 2018; Farsi et al., 2019; Alimohammadi et al., 2021), doses of CoQ10 (Fan et al., 2017; Zhai et al., 2017; Farsi et al., 2019; Jorat et al., 2019; Zhang et al., 2019; Akbari et al., 2020), duration of treatment (Fan et al., 2017; Jorat et al., 2019; Zhang et al., 2019; Akbari et al., 2020; Dludla et al., 2020; Hajiluian et al., 2021), formulation type of the supplement (Fan et al., 2017; Farsi et al., 2019; Sangsefidi et al., 2020), baseline characteristics such as gender, age (Farsi et al., 2019; Zhang et al., 2019; Hajiluian et al., 2021), body mass index (BMI), and lipoprotein concentration (Zhai et al., 2017), as well as low-grade inflammation and/or oxidative stress among healthy subjects (Mazidi et al., 2018; Sangsefidi et al., 2020).
The results of WMD analysis reflected a significant decline in IL-6 and TNF-α levels. Regarding CRP, a significant reduction was observed for both SMD and WMD analyses. In inflammatory pathways, CRP is in the downstream of pro-inflammatory cytokines such as IL-6 and TNF-α, and its hepatic biosynthesis is mainly dependent on IL-6 (Farsi et al., 2019). Accordingly, CRP is an independent risk factor for cardiovascular diseases and diabetes; it also appears to be less sensitive than IL-6 in response to the inflammatory state (Fan et al., 2017; Farsi et al., 2019). The contradictory reports regarding the effect of CoQ10 supplementation on inflammatory markers such as CRP and IL-6 might be attributed to the following two reasons: 1) some studies included healthy subjects with low baseline levels of inflammation that may not be affected by CoQ10, and 2) the dose of CoQ10 supplements may have been too low to observe anti-inflammatory effects. Zhai et al. (2017) mentioned that the association between CRP, IL-6, and CoQ10 serum concentration was influenced by age, sex, BMI, lipoprotein concentration, and health status. Accordingly, many studies have repeatedly shown that individuals with chronic inflammation may be more likely to benefit from CoQ10 intervention (Fan et al., 2017). In fact, differences in baseline levels of oxidative stress indices determine the effectiveness of CoQ10 supplementation on inflammation and oxidative stress (Sangsefidi et al., 2020).
Based on subgroup analyses, CoQ10 supplementation was more effective in reducing inflammation and oxidative stress in subjects younger than 55 years old. Aging increases the production of inflammatory mediators and can continuously diminish the rate of CoQ10 biosynthesis. This supports the hypothesis that exogenous supplementation can compensate for the low levels of CoQ10 (Farsi et al., 2019). As a result, it seems that elderly people should consume more than young people to compensate for this deficiency. Since the mean age of participants in the included studies was 43–69 years old, those under 55 years of age benefited the most from CoQ10 supplementation. However, due to the limited age range, generalizing the efficiency of CoQ10 supplementation to the general population should be done with caution. In this study, due to the limited number of studies with similar health status, subgroup analysis based on health status was not applicable.
Differences in subgroup analyses for dose and duration, regarding TAC and SOD, reflect that the effect of CoQ10 supplementation on inflammation and oxidative stress parameters was neither dose-dependent nor time-dependent. Different CoQ10 formulations with various bioavailability may account for the unclear direct dose-effect or duration-effect relationships observed (Fan et al., 2017). Few studies have shown a more beneficial effect of CoQ10 supplementation on oxidative stress factors over longer periods (>8 weeks) compared to shorter ones (<8 weeks) (Akbari et al., 2020; Hajiluian et al., 2021; Dai et al., 2022). Nevertheless, other studies claimed that the beneficial effects were observed in shorter durations (Farsi et al., 2019). The broad range of CoQ10 supplementation among studies (65–300 mg/day) can also explain the unclear dose-effect relationship between CoQ10 supplementation with TAC and SOD levels. Thus, the optimal dose of CoQ10 supplementation could not be determined precisely for the general population. These discrepancies are mainly related to the small number of studies and participants, as well as the inclusion of healthy population in some studies, which may not be affected by CoQ10 (Fan et al., 2017; Zhai et al., 2017; Mazidi et al., 2018). Although we witnessed a low heterogeneity and publication bias in the reported findings, the results of the present study should be interpreted with caution.
The most notable strength of this umbrella meta-analysis was that it included a considerable number of high-quality methodological studies. Subgroup analyses, controlling publication bias, and conducting a comprehensive systematic search were among the other strengths of our study. However, there were a few limitations that must be noted. First, we were unable to assess the effect of CoQ10 supplementation on other oxidative stress parameters since there were insufficient studies. Second, some studies had been repeated in several meta-analyses. Although this could affect the results, further assessments indicated that repeated studies did not affect the final results. Third, the included studies had been accomplished in certain geographic regions, which may enhance the possibility of selection bias. Fourth, due to the limited number of SMD studies on CRP, subgroup analysis for this biomarker was not possible. Hence, we could not reach a conclusive finding regarding the effect of CoQ10 supplementation on CRP level in different subgroups. Fifth, since most studies did not determine the serum level of CoQ10, they could not ensure that the patients were actually taking their CoQ10 supplements. However, to evaluate the degree of compliance, they used other ways such as assessing the remaining supplements returned by the patients.
5 Conclusion
The present umbrella meta-analysis confirmed the potential benefits of CoQ10 supplementation in reducing inflammatory and oxidative stress parameters. Moreover, acute CoQ10 supplementation (≤10 weeks) at doses of >200 mg/day contributed to lower MDA, TNF-α, and IL-6 levels. However, regarding TAC and SOD, over 10 weeks of CoQ10 supplementation with ≤200 mg/day doses resulted in greater increases in TAC and serum SOD activity, respectively. Hence, it appears that the effect of CoQ10 supplementation on inflammatory and oxidative stress can be found in both acute and chronic interventions at low doses and high doses. In this regard, CoQ10 supplementation can be advised as a complementary treatment in chronic inflammatory conditions.
Funding Statement
The research protocol was approved and supported by the Student Research Committee of Tabriz University of Medical Sciences, Iran (Registration code: 71811).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
VM, and AF designed research; ZK, VM, and FH conducted systematic search; SA, and SR screened articles; ZK, AM, and SS extracted data; VM and AF analyzed and interpreted data; SD and ZK drew tables; SD, VM, ZK wrote the paper. PD had primary responsibility for final content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CoQ10, Coenzyme Q10; WMD, weighted mean difference; SMD, standardized mean difference; CRP, C-reactive protein; MDA, malondialdehyde; TAC, total antioxidant capacity; SOD, superoxide dismutase; TNF- α, tumor-necrosis factor-alpha; IL-6, interleukin-6; ROS, reactive oxygen species; NF-kB, nuclear-factor-kappa-B; DM, diabetes mellitus; T2DM, 2 diabetes mellitus; ES, effect sizes; CIs, confidence intervals; GRADE, Grading of Recommendations, Assessment and Evaluation; miR-146a, microRNAs-146a; IRAK-1, IL-1 receptor-associated kinase-1; TRAF6, TNF receptor-associated factor 6; Nrf2, nuclear factor (erythroid-derived 2)-like 2; HO-1, heme oxygenase 1; PPAR-γ and α, peroxisome proliferator- activated receptor gamma and alpha; BMI, body mass index.
References
- Abdeen A., Abdelkader A., Elgazzar D., Aboubakr M., Abdulah O. A., Shoghy K., et al. (2020). Coenzyme Q10 supplementation mitigates piroxicam-induced oxidative injury and apoptotic pathways in the stomach, liver, and kidney. Biomed. Pharmacother. 130, 110627. 10.1016/j.biopha.2020.110627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari A., Mobini G. R., Agah S., Morvaridzadeh M., Omidi A., Potter E., et al. (2020). Coenzyme Q10 supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Eur. J. Clin. Pharmacol. 76 (11), 1483–1499. 10.1007/s00228-020-02919-8 [DOI] [PubMed] [Google Scholar]
- Alimohammadi M., Rahimi A., Faramarzi F., Golpour M., Jafari-Shakib R., Alizadeh-Navaei R., et al. (2021). Effects of coenzyme Q10 supplementation on inflammation, angiogenesis, and oxidative stress in breast cancer patients: A systematic review and meta-analysis of randomized controlled-trials. Inflammopharmacology 29 (3), 579–593. 10.1007/s10787-021-00817-8 [DOI] [PubMed] [Google Scholar]
- Andrade C. (2020). Mean difference, standardized mean difference (SMD), and their use in meta-analysis: As simple as it gets. J. Clin. Psychiatry 81 (5), 20f13681. 10.4088/JCP.20f13681 [DOI] [PubMed] [Google Scholar]
- Arenas‐Jal M., Suñé‐Negre J., García‐Montoya E. (2020). Coenzyme Q10 supplementation: Efficacy, safety, and formulation challenges. Compr. Rev. Food Sci. Food Saf. 19 (2), 574–594. 10.1111/1541-4337.12539 [DOI] [PubMed] [Google Scholar]
- Bessler H., Bergman M., Blumberger N., Djaldetti M., Salman H. (2010). Coenzyme Q10 decreases TNF-alpha and IL-2 secretion by human peripheral blood mononuclear cells. J. Nutr. Sci. Vitaminol. (Tokyo) 56 (1), 77–81. 10.3177/jnsv.56.77 [DOI] [PubMed] [Google Scholar]
- Cicero A. F., Fogacci F., Colletti A. (2018). Commentary to:“The effects of coenzyme Q10 supplementation on blood pressures among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials”. High. Blood Press Cardiovasc Prev. 25 (1), 51–52. 10.1007/s40292-018-0248-1 [DOI] [PubMed] [Google Scholar]
- Clark H. D., Wells G. A., Huët C., McAlister F. A., Salmi L. R., Fergusson D., et al. (1999). Assessing the quality of randomized trials: Reliability of the jadad scale. Control Clin. Trials 20 (5), 448–452. 10.1016/s0197-2456(99)00026-4 [DOI] [PubMed] [Google Scholar]
- Dai S., Tian Z., Zhao D., Liang Y., Liu M., Liu Z., et al. (2022). Effects of coenzyme Q10 supplementation on biomarkers of oxidative stress in adults: A GRADE-assessed systematic review and updated meta-analysis of randomized controlled trials. Antioxidants 11 (7), 1360. 10.3390/antiox11071360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dludla P. V., Orlando P., Silvestri S., Marcheggiani F., Cirilli I., Nyambuya T. M., et al. (2020). Coenzyme Q10 supplementation improves adipokine levels and alleviates inflammation and lipid peroxidation in conditions of metabolic syndrome: A meta-analysis of randomized controlled trials. Int. J. Mol. Sci. 21 (9), 3247. 10.3390/ijms21093247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellulu M. S., Patimah I., Khaza’ai H., Rahmat A., Abed Y. (2017). Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 13 (4), 851–863. 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhrolmobasheri M., Hosseini M.-S., Shahrokh S.-G., Mohammadi Z., Kahlani M.-J., Majidi S.-E., et al. (2023). Coenzyme Q10 and its therapeutic potencies against COVID-19 and other similar infections: A molecular review. Adv. Pharm. Bull. 13 (2), 233–243. 10.34172/apb.2023.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Feng Y., Chen G. C., Qin L. Q., Fu C. L., Chen L. H. (2017). Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 119, 128–136. 10.1016/j.phrs.2017.01.032 [DOI] [PubMed] [Google Scholar]
- Farsi F., Heshmati J., Keshtkar A., Irandoost P., Alamdari N. M., Akbari A., et al. (2019). Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-α and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 148, 104290. 10.1016/j.phrs.2019.104290 [DOI] [PubMed] [Google Scholar]
- Gao W., Guo L., Yang Y., Wang Y., Xia S., Gong H., et al. (2022). Dissecting the crosstalk between Nrf2 and NF-κB response pathways in drug-induced toxicity. Front. Cell. Dev. Biol. 9, 809952. 10.3389/fcell.2021.809952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Maraver J., Cordero M. D., Oropesa-Ávila M., Fernández Vega A., de la Mata M., Delgado Pavón A., et al. (2014). Coenzyme q10 therapy. Mol. Syndromol. 5 (3-4), 187–197. 10.1159/000360101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami M., Zarei P., Sadeghi Sedeh B., Rafiei F., Khosrowbeygi A. (2018). Effects of coenzyme Q10 supplementation on serum values of adiponectin, leptin, 8-isoprostane and malondialdehyde in women with type 2 diabetes. Gynecol. Endocrinol. 34 (12), 1059–1063. 10.1080/09513590.2018.1481944 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mariscal F. M., Arenas-de Larriva A. P., Limia-Perez L., Romero-Cabrera J. L., Yubero-Serrano E. M., López-Miranda J. (2020). Coenzyme Q10 supplementation for the reduction of oxidative stress: Clinical implications in the treatment of chronic diseases. Int. J. Mol. Sci. 21 (21), 7870. 10.3390/ijms21217870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G. H., Oxman A. D., Vist G. E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336 (7650), 924–926. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiluian G., Heshmati J., Jafari Karegar S., Sepidarkish M., Shokri A., Shidfar F. (2021). Diabetes, age, and duration of supplementation subgroup analysis for the effect of coenzyme Q10 on oxidative stress: A systematic review and meta-analysis. Complement. Med. Res. 28 (6), 557–570. 10.1159/000515249 [DOI] [PubMed] [Google Scholar]
- Hernández-Camacho J. D., Bernier M., López-Lluch G., Navas P. (2018). "Coenzyme Q10 supplementation in aging and disease." Front. Physiol. 9:44. 10.3389/fphys.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Gutiérrez A., González-García P., Díaz-Casado M. E., Barriocanal-Casado E., López-Herrador S., Quinzii C. M., López L. C. (2021). "Metabolic targets of coenzyme Q10 in mitochondria." Antioxidants . 10(4): 520. 10.3390/antiox10040520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., Altman D. G., Gøtzsche P. C., Jüni P., Moher D., Oxman A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343, d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S. (2017). Inflammation, metaflammation and immunometabolic disorders. Nature 542 (7640), 177–185. 10.1038/nature21363 [DOI] [PubMed] [Google Scholar]
- Jorat M. V., Tabrizi R., Kolahdooz F., Akbari M., Salami M., Heydari S. T., et al. (2019). The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 27 (2), 233–248. 10.1007/s10787-019-00572-x [DOI] [PubMed] [Google Scholar]
- Long J. P., Dong L. F., Chen F. F., Fan Y. F. (2019). miR-146a-5p targets interleukin-1 receptor-associated kinase 1 to inhibit the growth, migration, and invasion of breast cancer cells. Oncol. Lett. 17 (2), 1573–1580. 10.3892/ol.2018.9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini A., Bruno C., Vergani E., d’Abate C., Giacchi E., Silvestrini A. (2021). Oxidative stress and low-grade inflammation in polycystic ovary syndrome: Controversies and new insights. Int. J. Mol. Sci. 22 (4), 1667. 10.3390/ijms22041667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazidi M., Kengne A. P., Banach M., Lipid L., Group B. P. M.-a. C. (2018). Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 128, 130–136. 10.1016/j.phrs.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Sys Rev. 4 (1), 1–9. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazadeh V., Jafarzadeh J., Keramati M., Zarezadeh M., Ahmadi M., Farrokhian Z., et al. (2021). Flaxseed oil supplementation augments antioxidant capacity and alleviates oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2021, 4438613. 10.1155/2021/4438613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala R., Orhan C., Tuzcu M., Sahin N., Ali S., Cinar V., et al. (2016). Coenzyme Q10 supplementation modulates NFκB and Nrf2 pathways in exercise training. J. Sports Sci. Med. 15 (1), 196–203. [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Tyagi A. K., Aggarwal B. B. (2016). Detection of inflammatory biomarkers in saliva and urine: Potential in diagnosis, prevention, and treatment for chronic diseases. Exp. Biol. Med. 241 (8), 783–799. 10.1177/1535370216638770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizner A. E. (2019). Coenzyme Q(10). Methodist Debakey Cardiovasc J. 15 (3), 185–191. 10.14797/mdcj-15-3-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio C. P., Hernández-Ruiz J., Martinez-Subiela S., Tvarijonaviciute A., Ceron J. J. (2016). Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res . 12 (1), 166. 10.1186/s12917-016-0792-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimi F., Baazm M., Eftekhar E., Rajabi S., Goodarzi M. T., Mashayekhi F. J. (2019). Possible antioxidant mechanism of coenzyme Q10 in diabetes: Impact on sirt1/nrf2 signaling pathways. Res. Pharm. Sci. 14 (6), 524–533. 10.4103/1735-5362.272561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangsefidi Z. S., Yaghoubi F., Hajiahmadi S., Hosseinzadeh M. (2020). The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta‐analysis of randomized controlled clinical trials. Food Sci. Nutr. 8 (4), 1766–1776. 10.1002/fsn3.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer C., Lindner I., Rimbach G., Niklowitz P., Menke T., Döring F. (2008). Functions of coenzyme Q10 in inflammation and gene expression. Biofactors 32 (1-4), 179–183. 10.1002/biof.5520320121 [DOI] [PubMed] [Google Scholar]
- Schmelzer C., Lorenz G., Rimbach G., Döring F. (2007). Influence of Coenzyme Q_ {10} on release of pro-inflammatory chemokines in the human monocytic cell line THP-1. Biofactors 31 (3-4), 211–217. 10.1002/biof.5520310308 [DOI] [PubMed] [Google Scholar]
- Schmelzer C., Kitano M., Rimbach G., Niklowitz P., Menke T., Hosoe K., et al. (2009). "Effects of ubiquinol-10 on microRNA-146a expression in vitro and in vivo ." Mediat. Inflamm. 2009, 415437. 10.1155/2009/415437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat A., Samadi M., Shirvani H., Sepandi M., Tahmasebi W. (2022). Coenzyme Q10 supplementation and oxidative stress parameters: An updated systematic review and meta-analysis of randomized controlled clinical trials. Asian J. Sports Med. 13 (3). 10.5812/asjsm-131308 [DOI] [Google Scholar]
- Shea B. J., Reeves B. C., Wells G., Thuku M., Hamel C., Moran J., et al. (2017). Amstar 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. bmj 1, 358. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S., Dubey K. K. (2018). CoQ10 a super-vitamin: Review on application and biosynthesis" 3. Biotech 8 (5), 1–11. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taganov K. D., Boldin M. P., Chang K. J., Baltimore D. (2006). NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 103 (33), 12481–12486. 10.1073/pnas.0605298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarry-Adkins J. L., Fernandez-Twinn D. S., Hargreaves I. P., Neergheen V., Aiken C. E., Martin-Gronert M. S., et al. (2016). Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth. Am. J. Clin. Nutr. 103 (2), 579–588. 10.3945/ajcn.115.119834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenbach J., Magomedova L., Liu J., Reunov A. A., Tsai R., Eappen N. S., et al. (2018). Idebenone and coenzyme Q10 are novel PPARα/γ ligands, with potential for treatment of fatty liver diseases. Dis. Model. Mech. 11 (9), dmm034801. 10.1242/dmm.034801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Ni X. X., Xu Q. Y., Wang Q., Li X. Y., Hua J. (2020). Regulation of lipid‐induced macrophage polarization through modulating peroxisome proliferator‐activated receptor‐gamma activity affects hepatic lipid metabolism via a Toll‐like receptor 4/NF‐κB signaling pathway. J. Gastroenterol. Hepatol. 35 (11), 1998–2008. 10.1111/jgh.15025 [DOI] [PubMed] [Google Scholar]
- Xie Y., Chu A., Feng Y., Chen L., Shao Y., Luo Q., et al. (2018). MicroRNA-146a: A comprehensive indicator of inflammation and oxidative stress status induced in the brain of chronic T2DM rats. Front. Pharmacol. 9, 478. 10.3389/fphar.2018.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarezadeh M., Barzegari M., Aghapour B., Adeli S., Khademi F., Musazadeh V., et al. (2022). Melatonin effectiveness in amelioration of oxidative stress and strengthening of antioxidant defense system: Findings from a systematic review and dose-response meta-analysis of controlled clinical trials. Clin. Nutr. ESPEN 48, 109–120. 10.1016/j.clnesp.2022.01.038 [DOI] [PubMed] [Google Scholar]
- Zhai J., Bo Y., Lu Y., Liu C., Zhang L. (2017). Effects of coenzyme Q10 on markers of inflammation: A systematic review and meta-analysis. PloS one 12 (1), e0170172. 10.1371/journal.pone.0170172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhang F., Chen Y., Dou Y., Tao H., Zhang D., et al. (2017). Structure–property correlations of reactive oxygen species-responsive and hydrogen peroxide-eliminating materials with anti-oxidant and anti-inflammatory activities. Chem. Mater. 29 (19), 8221–8238. 10.1021/acs.chemmater.7b02412 [DOI] [Google Scholar]
- Zhang X., Shi Z., Liu Q., Quan H., Cheng X. (2019). Effects of coenzyme Q10 intervention on diabetic kidney disease: A systematic review and meta-analysis. Medicine 98 (24), e15850. 10.1097/MD.0000000000015850 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.