Abstract
Conventional wisdom suggests that widely-utilized self-assembled alkylthiolate monolayers on gold are too unstable to last more than several days when exposed to complex fluids such as raw serum at body temperature. Demonstrated here is that these monolayers can not only last at least one week under such harsh conditions, but that significant applied value can be captured for continuous electrochemical aptamer biosensors. Electrochemical aptamer biosensors provide an ideal tool to investigate monolayer degradation, as aptamer sensors require a tightly-packed monolayer to preserve sensor signal versus background current and readily reveal fouling by albumin and other solutes when operating in biofluids. Week-long operation in serum at 37 °C is achieved by: (1) increasing van der Waals interactions between adjacent monolayer molecules to increase the activation energy required for desorption; (2) optimizing electrochemical measurement to decrease both alkylthiolate oxidation and electric-field induced desorption; (3) mitigating fouling by using protective zwitterionic membranes and zwitterion-based blocking layers with antifouling properties. This work further proposes origins and mechanisms of monolayer degradation in a logical stepwise manner that was previously unobservable over multi-day time scales. Several of the observed results are surprising, revealing that short term improvements to sensor longevity (i.e., hours) actually increase sensor degradation in the longer term (i.e., days). The results and underlying insights on mechanisms not only push forward fundamental understanding of stability for self-assembled monolayers, but demonstrate an important milestone for continuous electrochemical aptamer biosensors.
Keywords: Biosensors, self-assembled monolayers, biofouling, electrochemical aptamer sensors, desorption
Graphical Abstract

One to two weeks of wearable continuous glucose monitoring in dermal interstitial fluid for diabetes management is a historical achievement in clinical diagnostics. Unfortunately, glucose monitoring remains an isolated success despite acute needs for continuous measure of many other molecules across the field of human disease management such as in cardiac, fertility, and therapeutic drug monitoring applications.1,2 The major limitation is that electrochemical glucose sensors are enzymatic, thus limiting generalizability to other analytes due to a modest number of oxidoreductase enzymes that could oxidize/reduce analytes of interest. Unlike enzymatic sensors, electrochemical aptamer sensors are affinity-based and broadly generalizable by relying on large oligonucleotide libraries of 1012 to 1015 possible aptamer sequences,3 which can be further chemically modified to enhance binding capabilities during selection for a specific target.4 There are now over a dozen examples of continuous in-vivo aptamer sensor monitoring at nanomolar to micromolar concentration ranges.5–7 These early successes place aptamer sensors within a very limited collection of continuous chemical sensors that have been in-vivo demonstrated for clinically oriented applications, including ion-selective/ionophore sensors and enzymatic biosensors.1 Unfortunately, aptamer sensor longevity has remained a significant challenge for the clinical adoption of aptamer sensors, especially when tested in representative in-vivo conditions.7
Electrochemical aptamer sensors conventionally use a mixed monolayer of redox-tagged aptamers and alkylthiolate passivating molecules chemisorbed onto the surface of a gold electrode (Figure 1a). These self-assembled monolayers have been shown to rapidly degrade on gold electrodes in blood or serum at a body temperature of 37 °C.8 The mechanisms of monolayer degradation in complex biofluids have not been fully understood, which has limited the ability to pursue specific approaches to improve sensor longevity. Part of the challenge is that, if there are a plurality of mechanisms rapidly and simultaneously degrading a sensor’s monolayer and its biorecognition element, it becomes highly difficult to isolate and observe individual degradation mechanisms in a manner that is representative of the end application in whole biofluid. A recent review by Shaver and Arroyo-Currás9 concluded that experimental conditions such as elevated temperature, repeatedly applied voltages, and protein-rich biofluids can have a detrimental effect on monolayer integrity, limiting sensor operation to at most a few hours. This mirrors a generally accepted opinion that widely utilized self-assembled alkylthiolate monolayers on gold are too unstable to last more than several days when repeatedly electrochemically scanned and exposed to complex fluids such as raw serum or blood at body temperature.
Figure 1.

Proposed mechanisms of monolayer degradation and solutions for reaching multi-day stability for electrochemical aptamer sensors. (A) Thermal desorption and/or desorption mediated by serum solutes can be partially mitigated by increasing alkylthiol chain length while (B) Electrochemical scanning can be optimized to reduce both electric field induced desorption and spontaneous monolayer oxidation. (C) Enzymatic attack of aptamer probes is a concern that can largely be circumvented by utilizing non-native DNA chemistry. (D) While biofouling alters sensor signaling properties, membrane protection and surface chemistry modifications can mitigate these detrimental changes and retain sensor performance in multiday operation.
Herein, we demonstrate week-long operational stability of electrochemical aptamer sensors in bovine serum at 37 °C achieved by: (1) increasing the van der Waals interaction between adjacent monolayer molecules to increase the activation energy required for desorption, (2) optimizing electrochemical measurement to mitigate both alkylthiolate oxidation and electric-field induced desorption, and (3) protecting the surface from fouling by using either size-exclusion membrane protection or zwitterion antifouling chemistry. Electrochemical aptamer biosensors provide an ideal tool to investigate monolayer degradation mechanisms, as aptamer sensors require a tightly packed monolayer to preserve redox tag current versus background current, and because aptamer sensors readily reveal monolayer fouling by albumin and other solutes. As a result, this work further presents the origins and solutions for these degradation mechanisms in a logical stepwise manner that was previously unobservable over multiday operation in biofluid.
We first provide a brief overview of the leading mechanisms speculated to cause monolayer degradation along with proposed solutions to decrease the degradation rate, followed by experimental results obtained in buffer and raw undiluted serum at 4 °C and 37 °C. Several of the observed results are surprising, revealing that short term solutions to improving sensor longevity over several hours may actually increase sensor degradation during multi-day operation. The results and underlying insights on mechanisms not only push forward fundamental understanding of stability for self-assembled monolayer chemistry, but demonstrate an important milestone for continuous electrochemical aptamer biosensors and other biosensors that rely on molecular self-assembly on gold substrates, such as immunosensors, peptide-based sensors, and other electrochemical nucleic acid sensors.8
RESULTS AND DISCUSSION
Overview of proposed mechanisms of mixed monolayer degradation for electrochemical aptamer sensors.
As illustrated in Figure 1, electrochemical aptamer sensors rely on a redox-molecule tagged aptamer with specific affinity-based binding capability to an analyte. When affinity-based binding to the analyte occurs, the aptamer undergoes a binding-induced conformational change. As the aptamer geometry changes, the redox-tag’s availability for electron transfer with the electrode is altered and therefore is measurable as a change in electrochemical current.7,10,11 There have been numerous mechanisms proposed in recent literature regarding the degradation of electrochemical aptamer sensors, especially when deployed in complex media such a whole blood or raw undiluted serum. Part of the limitations of these previous studies is that they did not achieve multi-day results with fully functioning aptamer sensors and, if they did, there were a plurality of mechanisms rapidly degrading the sensor. Given a plurality of degradation mechanisms simultaneously occurring, it becomes highly difficult to isolate and observe individual degradation mechanisms and propose feasible solutions.
Before presenting the experimental results, a brief overview on degradation mechanisms is introduced along with potential solutions to mitigate specific degradation phenomena. The following terms will be used consistently throughout the paper: redox tag current is the amplitude of the faradaic redox tag peak current minus the background current amplitude outside the redox peak in a given voltammetric scan; normalized redox-tag current is the redox-tag current normalized to the first measurement taken; background current is the voltammetric current that would be measured if the aptamer molecules were not distally tagged with a redox reporter including, for example, capacitive currents and competing redox processes such as oxygen reduction; adjusted current is the combined redox tag current and background current of a square-wave voltammogram adjusted such that the minimum current is set to 0 A in the presentation of the voltammogram such that voltammograms can be plotted side by side and compared with greater ease; sensor response is the change in redox tag current due to binding of the target analyte to the aptamer, also known as signal gain, which can either increase or decrease based on the aptamer and the voltammetric time scale.12 We define these terms here because simpler terms used in the past such as ‘sensor signal’ can be misleading based on our findings because, as will be seen in the results, a strong redox tag current does not always result in a strong sensor response and therefore is not representative of a ‘strong sensor signal’. Lastly, the term scanning as used herein means performing a single complete square-wave voltammetric (SWV) measurement.13
The overview of degradation mechanisms begins with Figure 1a, thermally driven monolayer desorption. We speculate and observe at this time that there are two major factors that determine the degree of thermal desorption: monolayer defects and the degree of lateral van der Waals interactions between neighboring monolayer molecules. The molecules residing on the edges of large monolayer defects have reduced van der Waals interactions with adjacent molecules and therefore require less energy for desorption (Figure 1a1 energy plot).
Even using the best protocols for monolayer self-assembly, defects are unavoidable and found at mismatched gold crystallite domain boundaries and at large gold step edges. Recent literature has shown that the roughness of the gold is resultingly critical for monolayer stability, as smaller gold step edges force smaller-sized monolayer defects compared to those on highly smooth gold or even monocrystalline gold surfaces.14 However, even with an appropriate gold roughness for minimizing monolayer defect size (Figure 1a2), thermal desorption of the monolayer still remains a significant challenge. Moving to body temperature and/or complex biofluids further increases desorption through increased thermal energy and/or the presence of foulants that provide an ‘energetic bridge’ to reduce the single-step energy needed for desorption (Figure 1a3). As shown in Figure 1a4 and demonstrated later in our experimental results, even small increases in van der Waals interaction between adjacent monolayer molecules, achieved by increasing alkylthiolate chain length, can greatly decrease monolayer desorption by increasing the energy step required for thermal desorption and/or desorption mediated by monolayer solubilization via interaction with proteins and other amphipathic molecules present in biofluids (Figure 1a4).
Next, the effects of electrochemical scanning on the monolayer (Figure 1b1) are reviewed, which can both diminish and, as we show here for the first time for electrochemical aptamer sensors, also enhance monolayer stability. First, there have been multiple recent demonstrations that show that more gradual15 or reduced potential window scanning16,17 can reduce electrical stress on the monolayer. Particularly, when operating at negative potentials, the applied electric field can strain the negatively charged phosphate backbone of the aptamer away from the electrode surface.18,19 We additionally show here in the experimental results that, while operating at negative potentials can accelerate monolayer desorption, the same cathodic scanning also mitigates the oxidation of alkylthiolate molecules, setting the stage for optimized scanning as shown in Figure 1b2. While literature has demonstrated that attaching single molecules to gold with multiple thiol groups reduces their desorption due to thermal or electric-field effects,20,21 we will not test those methods herein because in most examples poorer packing-density of the resulting monolayer22,23 would increase electrochemical background current due to higher capacitance and increased oxygen reduction at the surface.
Another concern for biosensor longevity is chemical and enzymatic attack of the biorecognition element itself (Figure 1c). In particular for aptamers, processes such as nuclease cleavage, enzymatic methylation, and oxidation of the single-stranded DNA can all potentially degrade the sensor redox tag current and the sensor response over time. We will not spend time discussing enzymatic modification of aptamers in detail here because it is largely aptamer dependent and resolvable, if needed, by chemically modifying the aptamer24 or providing membrane protection to block nucleases and other enzymes.25
Lastly, one of the major remaining concerns that extends beyond just aptamer sensors is protein biofouling. Aptamer sensors employing alkylthiolate monolayers on mechanically polished gold typically exhibit a rapid initial 30–50% reduction of redox tag current due to biofouling when scanned in protein rich fluids such as serum.16 We speculate that this is due to hydrophobic protein domains fouling at defect sites where the inner hydrophobicity of the monolayer molecules is partially exposed (Figure 1d1, left plot). Even more problematic, but not previously discussed in the literature, is the reorganization of biofouled protein molecules and the resulting protein interactions with surface immobilized aptamer molecules over multiple days. While these interactions may not significantly impact redox tag and background currents over multiple days, they can constrain the aptamers’ ability to move freely and therefore prevent proper sensor response to changes in analyte concentration (Figure 1d1, middle and right plots).
Fouling is resolvable for at minimum multi-day operation if not multi-week operation using size-selective membranes that pass the analyte but not large foulants such as albumin (Figure 1d2). However, this precludes quantification of proteins that have similar size to large protein foulants since they will also be excluded by protective membranes. Fouling is also potentially resolvable using antifouling chemistry at the fluid-facing terminus of monolayer molecules,26 albeit less proven in practice for multi-day operation.
There are numerous other factors that can degrade self-assembled monolayers and aptamer sensors; however, they will not be discussed here as the sensor preparation methods and results described herein show that the aforementioned mechanisms are the most dominant in pursuit of week-long operation in a raw biofluid at body temperature. The experimental results are organized in subsections, each of which represent key insights produced in this work. The subsections are organized in the following order:
Extending alkylthiolate chain length increases monolayer intermolecular forces and therefore improves monolayer stability in buffer conditions (Figure 1a1–1a2)
Although short term experiments suggest that foulants in serum can further stabilize the monolayer, over multiple days these foulants locally promote desorption, which can also be resolved by increased alkylthiolate chain length (Figure 1a2–1a3)
While short term experiments suggest that minimizing electrochemical scanning improves monolayer stability, longer term data reveal that for a monolayer stable in terms of intermolecular forces, scanning actually further improves monolayer stability by suppressing oxidation of alkylthiolate molecules (Figure 1b)
Fouling affects electron transfer rates and has a significant impact on freedom of mobility of the aptamer. (Figure 1d)
Extending alkylthiolate chain length increases monolayer intermolecular forces and therefore improves monolayer stability in buffer conditions (Figure 1a1–1a2).
Self-assembled monolayers of alkylthiolates on gold substrates have been extensively studied due to their use in a variety of applications spanning from biosensing and drug delivery to nanotechnology and fundamental research on electrochemical properties of adsorbed/immobilized layers.8,27–29 Longer chain alkylthiolates can inherently offer more stability due to an increased number of points for van der Waals interactions with neighboring molecules, thus resulting in more orderly packed monolayers.30 However, longer chain monolayers pose a challenge for electrochemical aptamer sensors which, as previously mentioned, rely on electron transfer from a distally tagged redox reporter. Not only do longer alkylthiolate chains increase the electron-tunneling distance across the monolayer, but, given that longer monolayers also pack better,31,32 there are also less defects in the monolayer, which are necessary to some degree to support efficient electron transfer.14,33
Traditional electrochemical aptamer sensors predominantly employ the hydroxyl-terminated alkylthiolate 6-mercapto-1-hexanol (MCH) as the passivating blocking layer molecule to backfill unoccupied gold atoms after aptamer immobilization.34 MCH has previously been demonstrated to be somewhat sufficient for reducing nonspecific adsorption and mitigating unwanted redox processes (e.g., oxygen reduction) while still allowing for robust electron transfer and offering moderate stability for multi-hour deployment.7,9
Longer alkylthiolate monolayers (i.e., 11 methylene groups) have been attempted in the past for electrochemical aptamer sensors in efforts to improve stability.26,34 However, issues with impeded electron transfer have largely precluded practical application as slower electron transfer rates decrease redox tag current and can alter frequency response such that dual frequency approaches to drift-corrected5 and calibration-free35 operation may not be plausible. Additionally, in the context of desorption, the biocompatibility of these longer chain alkylthiolates must be considered for multiday operation in implantable applications, given that some longer chain alkylthiols are reported to cause acute toxicity upon ingestion according to safety data sheets. However, given that the surface coverage of typical electrodes is on the order of picomoles (Table S1), minute concentrations are likely to be experienced with gradual desorption of sensor lifetime.
Therefore, there is a tradeoff that must be considered between the stability that should theoretically be achieved by longer alkylthiolates and the resulting decrease in electrochemical aptamer biosensor performance (i.e., diminished redox tag current and altered response to target). Furthermore, to the best of our knowledge there are no literature reports that assess the multi-day stability of electrochemical aptamer sensors employing longer chain alkylthiolates as part of mixed monolayers with thiolated aptamers in undiluted biofluids at body temperature.
Empirically, we have observed that electrochemical aptamer sensors employing MCH passivation layers are quite stable when testing at refrigerated (~4 °C) or room temperatures (~20 °C) in buffer conditions (Figure 1a). This is also corroborated in the literature, where sensors stored at room temperature survived >7 days in solution.20,36 However, changing the testing environment to body temperature (~37 °C) leads to more rapid degradation, consistent with the literature on temperature-programmed desorption for pure alkylthiolate monolayers on gold.30,37 Table 1 illustrates how simply increasing alkylthiolate length by two methylene groups from MCH to 8-mercapto-1-octanol (MCO) can result in desorption rates at 37 °C similar to MCH desorption rates at room temperature based on the Arrhenius equation:
where k is the rate of desorption, A is a preexponential factor specific to the reaction, Ea is the activation energy required for desorption, R is the universal gas constant, and T is temperature in Kelvin.
Table 1.
Increasing alkylthiolate chain length by 2 methylene groups theoretically results in desorption rates at body temperature similar to those seen with MCH at room temperature.
| Increasing from room (20°C) to body temperature (37°C) | Increasing alkylthiolate chain length by 2 methylene groups from MCH (~288 kJ/mola) to MCO (~306 kJ/mola) |
|---|---|
Theoretical Ea values obtained from density functional theory calculations.38
Based on this analysis, we prepared electrochemical aptamer sensors for the antibiotic vancomycin39 with MCO blocking layers in efforts to compare their relative stability to those prepared with traditional MCH blocking layers. As anticipated, MCO-based sensors had reduced initial methylene blue currents compared to those with MCH due to the increased barrier for electron transfer (Figure S1). This is also reflected in the electron transfer rate constants for MCH- and MCO-based sensors (i.e., 33 s−1 and 18 s−1, respectively) as determined by the Laviron formalism (Table S1).40 Furthermore, these values, along with measures of capacitance and DNA surface density, are consistent with a previous report comparing MCH and MCO for electrochemical DNA sensor applications under high temperature hybridization.41 Of note in the present work, the thiolated linker on the DNA, which is typically matched to the length of MCH, was kept constant despite the mismatch between the number of methylene groups (i.e., eight vs. six) present in MCO and the linker moieties. While this could potentially introduce disruptions in the packing of MCO molecules around the aptamer probes due to steric hinderance from the first 5’ phosphate, reductive desorption of the mixed monolayer for both MCH and MCO sensors reveals a statistically insignificant difference in overall surface loading (Table S1).
Furthermore, these reductive desorption voltammograms (Figure S1) demonstrate the larger energetic barrier required for MCO desorption compared to MCH, with the difference in the overpotential of ~30 mV between the two groups consistent with prior literature.31 With regards to desorption in multiday operation, the experimental results directly support our hypothesis via the Arrhenius equation that the monolayer stability of MCO should be far superior to MCH at body temperature. Figure 2a–b shows the results for electrochemical aptamer sensors for vancomycin fabricated with either MCH or MCO passivation layers repeatedly scanned for over 1 week in target-free phosphate buffered saline (PBS) at body temperature (i.e. 37 °C). Here and in subsequently presented data, repeated scanning refers to consecutive SWV scans conducted approximately every 30 seconds with a square wave frequency of 300 Hz.
Figure 2.

Electrochemical aptamer sensors with MCO passivation layers are more stable than those with MCH when repeatedly scanned with a periodicity of every 30 seconds in PBS at 37°C. (a) MCH sensors suffered ~50% more signal loss over 7 days compared to MCO sensors and (b) accrued a significantly more drastic degradation in voltammogram quality. (c) Sensors repeatedly scanned at 4 °C for 7 days showed significantly less degradation for those with MCH while similar preservation of voltammogram quality was seen for those with MCO. All SWVs were recorded at 300 Hz. Shaded areas on all curves represent standard deviations (n=4).
Consistent with theoretical assumptions, MCO fabricated sensors exhibited superior stability during 7 days of operation (~40% redox tag current loss) compared to MCH sensors (~80% redox tag current loss) with the quality of the square-wave voltammograms well preserved after multiple days of scanning (Figure 2b). After ~3 days, MCH sensor voltammograms showed background current contributions due to oxygen reduction at −0.5 V that exceeded the redox tag current, indicating excessive desorption of the passivation layer. Beyond day 3, the quality of MCH sensor voltammograms became progressively worse as redox tag current decreased exponentially to approximately 20% of its initial value. Such excessive loss of redox tag current is likely due to aptamer desorption, which becomes even more energetically favorable after the desorption of surrounding MCH molecules. In comparison, the MCO sensors only exhibited a slight increase in background current due to oxygen reduction, suggesting that the MCO monolayer remained largely intact.
To further illustrate the strong temperature dependance of monolayer degradation, we repeated the same electrochemical aptamer sensor testing with MCH and MCO passivation layers in buffer conditions at a low temperature of 4 °C, which based on the Arrhenius equation should theoretically result in monolayer desorption rates ~106 times slower than those at 37 °C. Figure 2c shows the voltammogram results of MCH and MCO fabricated sensors repeatedly scanned for 7 days at 4 °C in PBS. Repeated scanning still resulted in significant loss of redox tag current as expected and previously reported, where excessive scanning is suspected to desorb the monolayer over time.16 However, MCH sensors lost significantly less current at 4 °C (Figure S2) compared to sensors tested at 37 °C (i.e., ~35% vs ~85% respectively), and voltammograms show that oxygen reduction increased only slightly over 7 days (Figure 2c), indicating that the passivating monolayer was well-preserved at this temperature. Surprisingly, at 4 °C MCH and MCO sensors lost similar amounts of redox tag current (~35 and ~40% respectively, Figure S2), suggesting that these losses were largely due to electric-field induced desorption of aptamer upon repeated scanning since thermal desorption of the monolayer at this temperature should be negligible.
Although Short Term Experiments Suggest That Foulants in Serum Can Further Stabilize the Monolayer, Over Multiple Days These Foulants Locally Promote Desorption, Which Can Also Be Resolved by Increased Alkylthiolate Chain Length (Figure 1a2–1a3).
While electrochemical aptamer sensors have been shown to operate in complex biofluid media, such as blood and serum, demonstrations have previously been limited to several hours and longer-term studies for more than 24 hours have been limited to more hydrophobic monolayers, such as those employing 1-hexanethiol, which are shown to exhibit superior electrochemical stability but foul much more severely.34 It is known that biofouling of the electrode surface by large solutes such as albumin can alter sensor performance and electron transfer kinetics.16,35 Typically, for aptamer sensors with hydroxyl-terminated alkylthiolates such as MCH or MCO, a rapid exponential loss of sensor redox tag current (~30 to 50%) is seen within the first 3 hours, followed by a period of slow and more linearly stable current loss for several hours thereafter.7,9,16 It has also been shown that the foulants in serum driving this loss in current are passivating unoccupied sites on the gold surface, thus flattening the baseline in the range of the potentials where otherwise oxygen reduction would be pronounced.42 The results here show that while foulants may initially passivate larger defects in the monolayer, over multiple days the biofouled layer actually caused desorption of the monolayer at a faster rate compared to experiments performed in PBS.
Figure 3a–b shows the results of electrochemical aptamer sensors for vancomycin with MCH and MCO passivation layers repeatedly scanned in target-free serum at 37 °C for over 1 week. The data in Figure 3 not only show the expected early stage foulant passivation of monolayer defects within the first few hours, but also show that after 24 hours, monolayer desorption for MCH sensors actually accelerated compared to those tested in PBS. This is evident by the excessive increase in sensor background currents such that redox tag currents became virtually indistinguishable by day 3 (Figure 3b). We speculate this is largely due to foulants creating an energetic bridge between the surface-bound and solubilized states for monolayer molecules (Figure 1a3). For example, adjacent foulant and alkylthiolate molecules could interact through attractive inter-molecular interactions that in turn may energetically favor desorption compared to desorption in buffer conditions.
Figure 3.
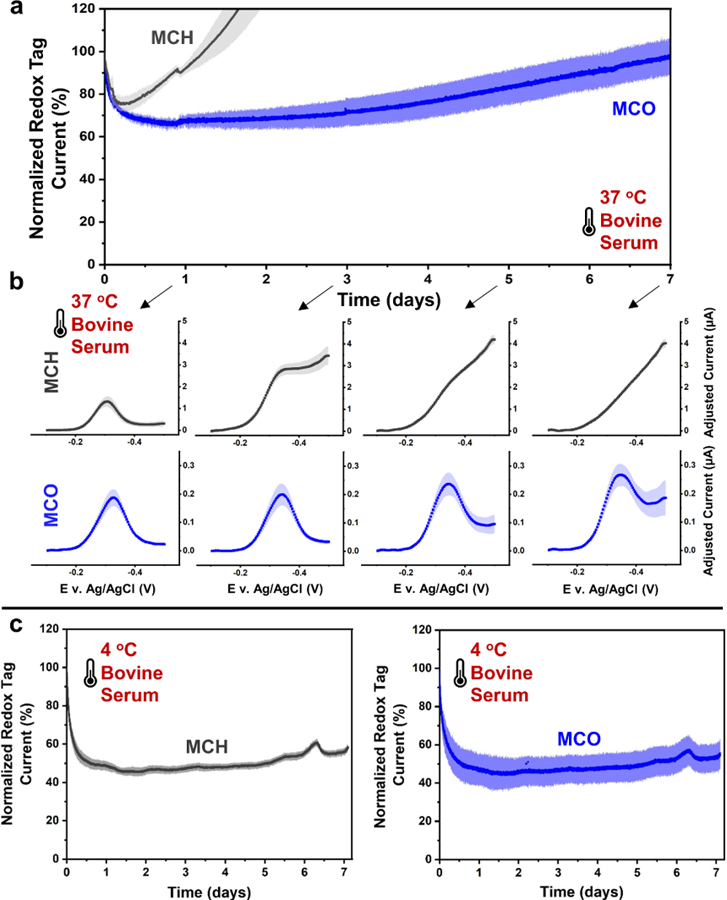
Biofouling in undiluted bovine serum accelerates degradation of both MCH and MCO fabricated sensors after multiple days of repeated scanning (i.e., ca. every 30s) at 37°C. (a) MCO sensor currents were more stable compared to MCH sensors and (b) maintained quality voltammograms with discernable redox peaks over 7 days. (c) Repeated scanning at 4 °C resulted in significantly more stable redox tag currents for both MCH and MCO sensors. All SWVs were recorded at 300 Hz. Shaded areas on all curves represent standard deviations (n=4).
MCO sensors on the other hand provided much more stable redox tag currents and voltammograms over 7 days with only modest increases in oxygen reduction currents (Figure 3b). Similar to how adding two methylene groups to passivation layer molecules (i.e., MCO vs. MCH) creates a larger energy barrier for thermal desorption in PBS (Table 1 and Figure 2), increased intermolecular forces between neighboring MCO molecules also makes it more difficult for foulants to act as an energetic bridge for MCO desorption in serum (Figure 1a4). However, once MCO sensor background currents began to increase around day 7, sensor degradation rapidly accelerated and soon resulted in voltammograms with indistinguishable redox tag currents.
An important difference between observations in PBS and serum at 37 °C is that the redox-tag currents eventually increased for both the MCH and MCO sensors at 37 °C in serum (Figure 3a), whereas in PBS at 37 °C (Figure 2a) the redox tag currents monotonically decreased with time. While MCH sensors showed a sharp rise in their measured redox tag currents after the first 12 hours (Figure 3a), this apparent increase was likely due to degraded monolayers better facilitating the parasitic oxygen reduction reaction, thus contributing to the total current measured near the potential of the methylene blue peak. However, large increases in oxygen reduction were also observed in buffer conditions as a result of sensors degradation but did not contribute to a similar increase in measured redox tag current over 7 days (Figure 2). The reason for this discrepancy has yet to be elucidated and will be the subject of future work.
Contrary to the results obtained at 37 °C, these degradative processes were largely impeded at 4 °C, resulting in much more stable redox-tag currents and lower oxygen reduction currents after multiday operation (Figure 3c).
Also of interest, the initial ~30 to 50% exponential loss in redox tag current typically seen at 37 °C over the first ~3 hours (Figure S3) occurs more slowly over ~24 hours at the lower temperature of 4 °C. Furthermore, the first 12 hours at 37 °C and the first several days at 4 °C both reveal the “exponential” and subsequent ‘linear’ phases of degradation previously described in the literature,16 as data from sensors tested at both temperatures closely mirror one other and differ only in the timescales over which the aforementioned phases occur (see Supporting Figure S3). This observation demonstrates the energetic dependence governing biofouling kinetics as dictated by the rate of protein unfolding and rearrangement. This is further illustrated by the finding that the rate of fouling is somewhat dependent on the periodicity of electrochemical scanning (Figure S4), a process that locally imparts energy and likely drives rearrangement of the biofouled layer.
Although Short-Term Experiments Suggest That Minimizing Electrochemical Scanning Improves Monolayer Stability, Longer-Term Data Reveals That for a Monolayer Stable in Terms of Intermolecular Forces, Scanning Actually Further Improves Monolayer Stability by Suppressing Oxidation of Alkylthiolate Molecules (Figure 1b).
Several papers have assessed the impact of electrochemical scanning on the degradation of aptamer sensors9 and have shown that loss of sensor redox tag current is dependent upon the number of scans performed and the width of the potential window used for interrogation.15–17 To assess the effects of electrochemical scanning in light of the thermal effects discussed in the previous sections, we additionally tested MCH and MCO sensors by performing scanning only once a day at either 4 °C or 37 °C. Tests were performed in both PBS (Figure 4) and in serum (Figure S5) with a surprising and similar conclusion for both media: longer-term data over multiple days show that more frequent scanning is preferred as it can actually help further stabilize the monolayer. To illustrate this improved stability, we compared the voltammograms obtained by repeated scanning in PBS at 37 °C (Figure 2b) with those recorded once-a-day (Figure 4a). MCH sensors scanned once-a-day underwent rapid degradation such that they were unreadable after just 2 days. While the MCO sensors were able to survive for 7 days (Figure 4a–b), background currents increased significantly more than those repeatedly scanned in the same conditions (Figure 2b).
Figure 4.

Elevated temperatures lead to excessive passivation layer desorption when sensors are only scanned once-a-day. (A) MCO sensors maintained fairly constant redox tag currents over 7 days, while MCH sensors were practically unreadable after Day 2. (B) MCH sensors underwent rapid degradation, evident by excessive background currents after only 2 days in buffer conditions at 37°C. MCO sensors suffered less overall degradation but showed background currents comparable to redox tag currents after 3 days in buffer at 37 °C. (C) Sensors interrogated at 4 °C in buffer conditions did not experience the same degradation seen at 37 °C and lost minimal current over 7 days. All SWVs were collected at 300 Hz. Shaded areas and error bars represent standard deviations (n=4).
We speculate that the applied negative potentials used during scanning suppress the oxidation of neighboring passivating alkylthiolates to disulfide dimers and therefore can reduce desorption of the monolayer by keeping surface-bonded passivation molecules in their reduced thiolate form. The dimerization of thiols has previously been observed in thermal desorption spectroscopy experiments with self-assembled monolayers on gold, where disulfide homodimers of alkylthiolate molecules were detected as the primary species desorbing from tested gold substrates (TDS and solution phase SAM desorption papers). Furthermore, gold has been shown to serve as a catalyst for the oxidation of thiols to disulfides in the presence of oxygen.45 At elevated temperatures, this dimerization process is accelerated and leads to a more rapid loss of passivating molecules. This results in the formation of unoccupied sites on the gold surface available for accessory redox processes, such as oxygen reduction, leading to excessive background currents.
With repeated scanning in reduction mode from −0.1 to − 0.5 V (vs. Ag/AgCl), excess electrons are made available to the monolayer during each scan, which could potentially suppress oxidation of neighboring alkylthiolates to disulfides and thus reduce the rate of monolayer desorption. Given that reductive desorption of alkylthiolates has been shown to begin beyond −0.7 V (vs. Ag/AgCl),31,38 it is unlikely that repeated scanning within this potential window can induce excessive desorption of passivating molecules. We conclude that under the test conditions presented here, and potentially in most other aptamer sensor experiments, thermal desorption of passivating moieties via oxidation to disulfides is a major driving factor of monolayer degradation and can be partially mitigated via repetitive operation in reduction mode and/or by increasing alkylthiolate length. Longer alkylthiolates (e.g., MCO vs. MCH) are less susceptible to oxidation to disulfides since the oxidation process first requires overcoming van der Waals interactions with neighboring molecules such that the disulfide can form and then diffuse from the electrode surface.
However, a balance in scanning periodicity is needed, because as previously discussed and shown in Figures 2 and 3, the electric field generated during scanning can also drive desorption of aptamer molecules due to straining of the negatively charged DNA backbone away from the electrode surface.18,19 When the same once-a-day scanning tests were performed at 4 °C in PBS, redox tag currents for both MCH and MCO sensors were fairly constant and only decreased by ~10% over 7 days, indicating that less frequent scanning resulted in less aptamer desorption. Additionally, the excessive increase in background current observed at 37 °C in PBS was not observed for either MCH or MCO sensors scanned once a day at 4 °C (Figure S5), further supporting the conclusion that thermally-induced alkylthiolate oxidation is largely driving monolayer desorption at elevated temperatures.
The tradeoff between increased scanning periodicity for alkylthiolate layer preservation in light of thermal effects and reduced scanning for aptamer preservation is summarized in Table 2. It has recently been shown by our group that partial scanning (Figure 1b2) about the redox peak can reduce the electrochemical stress on the sensor while still providing a robust redox-current for determining sensor response to analyte,17 which therefore may provide even greater longevity results than the unoptimized results demonstrated herein.
Table 2.
Summary of the tradeoffs between infrequent (once daily) and frequent (every 30 s) scanning at 4 °C and 37 °C in PBS (Figure 4) or serum (Supplemental Figure 6).
| 4 °C | 37 °C | |
|---|---|---|
| Scanning once-a-day | ✔ Low thermal desorption ✔ No electric-field desorption |
Х Excessive thermal desorption ✔ No electric-field desorption of aptamer |
| Repeated scans every 30s | ✔ Negligible thermal desorption - Mild electric-field desorption |
✔ Less oxidation = slow desorption - Mild electric-field desorption |
Fouling Affects Electron Transfer Rates and Has a Significant Impact on Freedom of Mobility of The Aptamer (Figure 1d).
While MCO passivated sensors are more resistant to monolayer desorption in multiday operation and maintain a more stable redox tag current relative to MCH sensors (Figure 3), both MCH and MCO based sensors suffer from a severe loss in sensor response (i.e., sensitivity) to vancomycin molecules after only 3 days of repeated scanning in serum at 37 °C (Figure 5a–b). Titration curves were obtained on day 0 and day 3 with the most sensitive “signal-on” and “signal-off” SWV frequencies12 based on the frequency response curves measured for each batch of sensors (Figure S7). These curves were used to construct the “kinetic differential measurement” (KDM) response, which is routinely used in aptamer sensors to enhance gain and correct for signal drift over time.5 The data were then fit to a Hill-Langmuir binding model for quantification of apparent binding affinity (KD,app).46
Figure 5:

Fouling in serum severely impacts signal gain in response to analyte and is solvable by antifouling strategies such as membrane protection. (A) MCH sensors repeatedly scanned for 3 days in bovine serum at 37 °C suffered drastic losses in sensor response to vancomycin. (B) MCO sensors responded similarly to MCH sensors on Day 0 and suffered less severe but significant losses of sensor responses after 3 days. (C) Hydrogel protection reduced sensor response for MCH sensors but preserved initial response over 3 days while (D) protected MCO sensors maintained their sensor response both initially and over 3 days. Error bars represent standard deviations (n=4).
Sensor response to vancomycin was similar for both MCH and MCO sensors on day 0 (Figure 5a–b), indicating that the additional methylene groups and mismatch between the aptamer linker and passivation layer length are largely inconsequential to initial sensor response. However, maximum sensor responses were reduced from ~200% to ~75% for MCH sensors after 3 days of repeated scanning, whereas those for MCO sensors decreased more moderately from ~180% for freshly prepared sensors to ~120% at day 3. Additionally, the apparent affinity drastically decreased for both sensors over this timeframe. This large reduction in sensor performance was not seen in sensors repeatedly scanned in PBS at 37 °C, as titration curves after 7 days showed virtually no loss in maximum sensor response for both sensor and only modest shifts in apparent affinity (Figure S6).
These results further suggests that over multiple days, rearrangement of the biofouled layer can hinder the physical mobility of aptamer molecules and prevent conformational change in the presence of analyte. With more aptamer molecules being incapable of bringing their redox reporter closer to the electrode surface, while still contributing to the redox tag current measured in analyte-free serum, a higher concentration is needed to drive a higher proportion of the unrestrained aptamer population toward the bound state. Of note, MCO sensors still showed appreciable sensor response after 7 days of continuous scanning in serum at 37 °C, albeit with further reductions in sensor response and affinity (see Supporting Figure S7).
Fouling also strongly affects the optimal frequency at which the highest response can be obtained. The maximally responsive signal-on frequency for both MCH- and MCO-based sensors determined at day 0 was 300 Hz. After 3 days, the maximally responsive signal on frequency shifted to 750 Hz for MCH while it remained at 300 Hz for the MCO sensors.
These shifts demonstrate that biofouling alters electron transfer kinetics on the electrode surface, either in the analyte-bound or in the unbound states for aptamer molecules, which is consistent with results seen in the literature where the frequency of maximum electron transfer for the unbound aptamer is shown to shift during the initial 2–3 hour exponential fouling phase.16 Over multiple days, desorption of passivation molecules allows for replacement with foulants, a process that occurs much more rapidly on MCH sensors than on MCO sensors, thus resulting in the shifts in maximally responsive frequency seen in Supporting Figure S7.
There is also both a strong temperature dependence and scanning periodicity dependence on both fouling-induced loss in sensor response and shifts in frequency response. Over 7 days of testing, MCH sensors repeatedly scanned at 4 °C only showed a decrease from a maximum response of ~200% to ~175%, whereas MCO based sensors showed virtually no loss in maximum sensor response (Figure S8). These results further support our previous conclusion that fouling is largely temperature dependent, which includes the subsequent rearrangement that can hinder aptamer mobility and sensor response to analyte. Regarding a dependence on the frequency of scanning, sensors scanned once a day in serum at 4 °C suffered greater reductions in their maximum sensor response compared to those repeatedly scanned at 4 °C (see Supporting Figure S8). These results further suggest a dependance for biofouling on how often scanning is performed, likely due to electric field perturbation and reorganization of the biofouled layer, consistent with our previous data (Figure S4) and discussion in this paper.
We further demonstrate here that fouling-induced loss of sensor response is preventable via robust membrane protection or via a monolayer with a terminal moiety that has stronger antifouling properties than the simple hydroxyl group at the end of MCH or MCO. First, the impact of hydrogel membrane protection was assessed on multi-day sensor response in serum at 37 °C. We note that for these results a roughened gold surface was used (see the Methods section in Supporting Information) to promote adhesion of a zwitterionic polybetaine hydrogel to the electrode surface given that the hydrogel was unable to adhere to smooth gold surfaces.
While application of the hydrogel to our sensors drastically reduced the initial maximum response for MCH sensors (Figure 5c), the response of the MCO based sensors was virtually unchanged (Figure 5d) compared to sensors without hydrogel (Figure 5b), although there was a considerable shift in apparent affinity. While the source of this large initial decrease in maximum response for MCH sensors is unknown, it is suspected that a higher degree of disorder for MCH monolayers makes them more susceptible to surface changes induced by the hydrogel. This is consistent with the larger reduction in sensor performance seen due to biofouling for MCH sensors compared to MCO sensors (Figure 5a–b). More importantly, both hydrogel-protected MCH and MCO sensors were able to largely maintain their initial maximum sensor responses from day 0 to day 3, although there was a notable reduction in apparent affinity seen in both cases (Figure 5c–d). These results indicate that the protection was largely successful in mitigating fouling that leads to the drastic reduction in sensor performance seen in unprotected sensors
Secondly, fouling can be partially reduced using antifouling chemistries, such as those previously demonstrated using a zwitterionic phosphatidylcholine-terminated passivating monolayer.26 We initially sought to test this phosphatidylcholine-terminated monolayer with the vancomycin aptamer used throughout this work but were unable to generate sensors with appreciable response to vancomycin. We therefore utilized previously reported aptamer sequences targeting cortisol47 and L-phenylalanine,48 with which the phosphatidylcholine-terminated passivation layer resulted in significant improvement in maintaining sensor response with repeated scanning in serum at 37 °C as compared to MCH (Figure S9). The hydrogel protection and anti-fouling monolayer strategies utilized here are unoptimized and are reported to simply demonstrate that fouling-induced loss in sensor response can be prevented over multi-day time scales, and therefore we expect can perform for at least one week or more under optimized conditions.
CONCLUSION
Collectively, these insights support the framework discussed for Figure 1 on degradation mechanisms for electrochemical aptamer sensors and for applications based on self-assembled alkylthiolate monolayers in general. By simply increasing passivating alkylthiolate chain length by two methylene groups, sensors demonstrated superior stability in multiday operation with further improvement in sensor performance realized by robust hydrogel protection. The results reported herein also challenge conventional wisdom regarding self-assembled monolayer stability on gold surfaces and shed important light on several previous conclusions that were only accurate over shorter term operation of just a few hours.
As a result, it appears promising that soon aptamer sensors will be able to operate in-vivo for at least one week at body temperature. Optimization of gold roughness, electrochemical interrogation, passivation layer length, surface chemistry, and aptamer linker length will serve beneficial to further improving sensor performance in such conditions. Additionally, future work will be needed to evaluate the overall biocompatibility of these systems complete with optimized surface chemistry and membrane coatings.
Based on these results and progress made in the field over the past few years, we expect that 2-week longevity mirroring commercial continuous glucose meters is a fully capturable reality for the field of electrochemical aptamer biosensors.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by National Institutes of Health NIBIB Award 1R21EB033874-01, National Institutes of Health NICHD Award F30HD107927, the Air Force Office of Scientific Research (FA9550-20-1-0117), the National Science Foundation (STTR Award #2212221, CBET Award #2125056, ECCS Award #2025720), the Office of Naval Research (#N00014-20-1-2764 via the University of California-Santa Barbara), and the University of Cincinnati Venture Lab.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Materials; methods for sensor preparation, sensor modification with antibiofouling coating, and electrochemical measurements; and data regarding the temperature and scan periodicity dependence on biofouling/degradation, sensor titrations in PBS, SWV frequency responses/titrations in serum, and sensor titrations with phosphatidylcholine passivation layer (PDF)
The authors declare the following competing financial interest(s): All the authors have intellectual property in terms of patents that could generate royalties with commercialization of electrochemical aptamer sensors, and co-author Heikenfeld is a co-founder of Kilele Health which is commercializing electrochemical aptamer sensors for chronic disease monitoring.
REFERENCES
- (1).Heikenfeld J; Jajack A; Rogers J; Gutruf P; Tian L; Pan T; Li R; Khine M; Kim J; Wang J; Kim J Wearable Sensors: Modalities, Challenges, and Prospects. Lab. Chip 2018, 18 (2), 217–248. 10.1039/C7LC00914C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Heikenfeld J; Jajack A; Feldman B; Granger SW; Gaitonde S; Begtrup G; Katchman BA Accessing Analytes in Biofluids for Peripheral Biochemical Monitoring. Nat. Biotechnol 2019, 37 (4), 407–419. 10.1038/s41587-019-0040-3. [DOI] [PubMed] [Google Scholar]
- (3).Song K-M; Lee S; Ban C Aptamers and Their Biological Applications. Sensors 2012, 12 (1), 612–631. 10.3390/s120100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Gold L; Ayers D; Bertino J; Bock C; Bock A; Brody EN; Carter J; Cunningham V; Dalby A; Fitzwater T; Flather D; Forbes A; Foreman T; Fowler C; Gawande B; Goss M; Gunn M; Gupta S; Halladay D; Heil J; Heilig J; Hicke B; Husar G; Janjic N; Jarvis T; Jennings S; Katilius E; Keeney TR; Kim N; Kaske T; Kraemer S; Kroiss L; Le N; Levine D; Lindsey W; Lollo B; Mayfield W; Mehan M; Mehler R; Nelson SK; Nelson M; Nieuwlandt D; Nikrad M; Ochsner U; Ostroff RM; Otis M; Parker T; Pietrasiewicz S; Resnicow D; Rohloff J; Sattin S; Schneider D; Singer B; Stanton M; Stewart A; Stratford S; Vaught JD; Vrkljan M; Walker JJ; Watrobka M; Waugh S; Weiss A; Wilcox SK; Wolfson A; Wolk S; Zhang C; Zichi D Aptamer-Based Multiplexed Proteomic Technology for Biomarker Discovery. Nat. Preced 2010, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ferguson BS; Hoggarth DA; Maliniak D; Ploense K; White RJ; Woodward N; Hsieh K; Bonham AJ; Eisenstein M; Kippin T; Plaxco KW; Soh HT Real-Time, Aptamer-Based Tracking of Circulating Therapeutic Agents in Living Animals. Sci. Transl. Med 2013, 5 (213), 213ra165. 10.1126/scitranslmed.3007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Arroyo-Currás N; Somerson J; Vieira PA; Ploense KL; Kippin TE; Plaxco KW Real-Time Measurement of Small Molecules Directly in Awake, Ambulatory Animals. Proc. Natl. Acad. Sci 2017, 114 (4), 645–650. 10.1073/pnas.1613458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Arroyo-Currás N; Dauphin-Ducharme P; Scida K; Chávez JL From the Beaker to the Body: Translational Challenges for Electrochemical, Aptamer-Based Sensors. Anal. Methods 2020, 12 (10), 1288–1310. 10.1039/D0AY00026D. [DOI] [Google Scholar]
- (8).Gooding JJ; Ciampi S The Molecular Level Modification of Surfaces: From Self-Assembled Monolayers to Complex Molecular Assemblies. Chem. Soc. Rev 2011, 40 (5), 2704–2718. 10.1039/C0CS00139B. [DOI] [PubMed] [Google Scholar]
- (9).Shaver A; Arroyo-Currás N The Challenge of Long-Term Stability for Nucleic Acid-Based Electrochemical Sensors. Curr. Opin. Electrochem 2022, 32, 100902. 10.1016/j.coelec.2021.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Xiao Y; Lubin AA; Heeger AJ; Plaxco KW Label-Free Electronic Detection of Thrombin in Blood Serum by Using an Aptamer-Based Sensor. Angew. Chem. Int. Ed 2005, 44 (34), 5456–5459. 10.1002/anie.200500989. [DOI] [PubMed] [Google Scholar]
- (11).Schoukroun-Barnes LR; Macazo FC; Gutierrez B; Lottermoser J; Liu J; White RJ Reagentless, Structure-Switching, Electrochemical Aptamer-Based Sensors. Annu. Rev. Anal. Chem 2016, 9 (1), 163–181. 10.1146/annurev-anchem-071015-041446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).White RJ; Plaxco KW Exploiting Binding-Induced Changes in Probe Flexibility for the Optimization of Electrochemical Biosensors. Anal. Chem 2010, 82 (1), 73–76. 10.1021/ac902595f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Dauphin-Ducharme P; Arroyo-Currás N; Kurnik M; Ortega G; Li H; Plaxco KW Simulation-Based Approach to Determining Electron Transfer Rates Using Square-Wave Voltammetry. Langmuir 2017, 33 (18), 4407–4413. 10.1021/acs.langmuir.7b00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Green J-BD; Clarke E; Porter MD; McDermott CA; McDermott MT; Zhong C-J; Bergren AJ On the Counter-Intuitive Heterogeneous Electron Transfer Barrier Properties of Alkanethiolate Monolayers on Gold: Smooth versus Rough Surfaces. Electroanalysis 2022, n/a (n/a). 10.1002/elan.202100704. [DOI] [Google Scholar]
- (15).Pellitero MA; Curtis SD; Arroyo-Currás N Interrogation of Electrochemical Aptamer-Based Sensors via Peak-to-Peak Separation in Cyclic Voltammetry Improves the Temporal Stability and Batch-to-Batch Variability in Biological Fluids. ACS Sens 2021, 6 (3), 1199–1207. 10.1021/acssensors.0c02455. [DOI] [PubMed] [Google Scholar]
- (16).Leung KK; Downs AM; Ortega G; Kurnik M; Plaxco KW Elucidating the Mechanisms Underlying the Signal Drift of Electrochemical Aptamer-Based Sensors in Whole Blood. ACS Sens 2021. 10.1021/acssensors.1c01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).McHenry A; Friedel M; Heikenfeld J Voltammetry Peak Tracking for Longer-Lasting and Reference-Electrode-Free Electrochemical Biosensors. Biosensors 2022, 12 (10), 782. 10.3390/bios12100782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kelley SO; Barton JK; Jackson NM; McPherson LD; Potter AB; Spain EM; Allen MJ; Hill MG Orienting DNA Helices on Gold Using Applied Electric Fields. Langmuir 1998, 14 (24), 6781–6784. 10.1021/la980874n. [DOI] [Google Scholar]
- (19).Kaiser W; Rant U Conformations of End-Tethered DNA Molecules on Gold Surfaces: Influences of Applied Electric Potential, Electrolyte Screening, and Temperature. J. Am. Chem. Soc 2010, 132 (23), 7935–7945. 10.1021/ja908727d. [DOI] [PubMed] [Google Scholar]
- (20).Phares N; White RJ; Plaxco KW Improving the Stability and Sensing of Electrochemical Biosensors by Employing Trithiol-Anchoring Groups in a Six-Carbon Self-Assembled Monolayer. Anal. Chem 2009, 81 (3), 1095–1100. 10.1021/ac8021983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Li F; Zhang H; Dever B; Li X-F; Le XC Thermal Stability of DNA Functionalized Gold Nanoparticles. Bioconjug. Chem 2013, 24 (11), 1790–1797. 10.1021/bc300687z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Shon Y-S; Lee TR Desorption and Exchange of Self-Assembled Monolayers (SAMs) on Gold Generated from Chelating Alkanedithiols. J. Phys. Chem. B 2000, 104 (34), 8192–8200. 10.1021/jp000651h. [DOI] [Google Scholar]
- (23).Park J-S; Vo AN; Barriet D; Shon Y-S; Lee TR Systematic Control of the Packing Density of Self-Assembled Monolayers Using Bidentate and Tridentate Chelating Alkanethiols. Langmuir 2005, 21 (7), 2902–2911. 10.1021/la0475573. [DOI] [PubMed] [Google Scholar]
- (24).Shaver A; Kundu N; Young BE; Vieira PA; Sczepanski JT; Arroyo-Currás N Nuclease Hydrolysis Does Not Drive the Rapid Signaling Decay of DNA Aptamer-Based Electrochemical Sensors in Biological Fluids. Langmuir 2021, 37 (17), 5213–5221. 10.1021/acs.langmuir.1c00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Santos-Cancel M; White RJ Collagen Membranes with Ribonuclease Inhibitors for Long-Term Stability of Electrochemical Aptamer-Based Sensors Employing RNA. Anal. Chem 2017, 89 (10), 5598–5604. 10.1021/acs.analchem.7b00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Li H; Dauphin-Ducharme P; Arroyo-Currás N; Tran CH; Vieira PA; Li S; Shin C; Somerson J; Kippin TE; Plaxco KW A Biomimetic Phosphatidylcholine-Terminated Monolayer Greatly Improves the In Vivo Performance of Electrochemical Aptamer-Based Sensors. Angew. Chem. Int. Ed 2017, 56 (26), 7492–7495. 10.1002/anie.201700748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gooding JJ; Darwish N The Rise of Self-Assembled Monolayers for Fabricating Electrochemical Biosensors—an Interfacial Perspective. Chem. Rec 2012, 12 (1), 92–105. 10.1002/tcr.201100013. [DOI] [PubMed] [Google Scholar]
- (28).Vericat C; E. Vela, M.; Benitez, G.; Carro, P.; C. Salvarezza, R. Self-Assembled Monolayers of Thiols and Dithiols on Gold : New Challenges for a Well-Known System. Chem. Soc. Rev 2010, 39 (5), 1805–1834. 10.1039/B907301A. [DOI] [PubMed] [Google Scholar]
- (29).Love JC; Estroff LA; Kriebel JK; Nuzzo RG; Whitesides GM Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev 2005, 105 (4), 1103–1170. 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- (30).Bain CD; Troughton EB; Tao YT; Evall J; Whitesides GM; Nuzzo RG Formation of Monolayer Films by the Spontaneous Assembly of Organic Thiols from Solution onto Gold. J. Am. Chem. Soc 1989, 111 (1), 321–335. 10.1021/ja00183a049. [DOI] [Google Scholar]
- (31).Widrig CA; Chung C; Porter MD The Electrochemical Desorption of N-Alkanethiol Monolayers from Polycrystalline Au and Ag Electrodes. J. Electroanal. Chem. Interfacial Electrochem 1991, 310 (1), 335–359. 10.1016/0022-0728(91)85271-P. [DOI] [Google Scholar]
- (32).Schessler HM; Karpovich DS; Blanchard GJ Quantitating the Balance between Enthalpic and Entropic Forces in Alkanethiol/Gold Monolayer Self Assembly. J. Am. Chem. Soc 1996, 118 (40), 9645–9651. 10.1021/ja961565r. [DOI] [Google Scholar]
- (33).Kiani A; Alpuche-Aviles MA; Eggers PK; Jones M; Gooding JJ; Paddon-Row MN; Bard AJ Scanning Electrochemical Microscopy. 59. Effect of Defects and Structure on Electron Transfer through Self-Assembled Monolayers. Langmuir 2008, 24 (6), 2841–2849. 10.1021/la702811t. [DOI] [PubMed] [Google Scholar]
- (34).Shaver A; Curtis SD; Arroyo-Currás N Alkanethiol Monolayer End Groups Affect the Long-Term Operational Stability and Signaling of Electrochemical, Aptamer-Based Sensors in Biological Fluids. ACS Appl. Mater. Interfaces 2020, 12 (9), 11214–11223. 10.1021/acsami.9b22385. [DOI] [PubMed] [Google Scholar]
- (35).Li H; Dauphin-Ducharme P; Ortega G; Plaxco KW Calibration-Free Electrochemical Biosensors Supporting Accurate Molecular Measurements Directly in Undiluted Whole Blood. J. Am. Chem. Soc 2017, 139 (32), 11207–11213. 10.1021/jacs.7b05412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lai RY; Seferos DS; Heeger AJ; Bazan GC; Plaxco KW Comparison of the Signaling and Stability of Electrochemical DNA Sensors Fabricated from 6- or 11-Carbon Self-Assembled Monolayers. Langmuir 2006, 22 (25), 10796–10800. 10.1021/la0611817. [DOI] [PubMed] [Google Scholar]
- (37).Lavrich DJ; Wetterer SM; Bernasek SL; Scoles G Physisorption and Chemisorption of Alkanethiols and Alkyl Sulfides on Au(111). J. Phys. Chem. B 1998, 102 (18), 3456–3465. 10.1021/jp980047v. [DOI] [Google Scholar]
- (38).Salvarezza RC; Carro P The Electrochemical Stability of Thiols on Gold Surfaces. J. Electroanal. Chem 2018, 819, 234–239. 10.1016/j.jelechem.2017.10.046. [DOI] [Google Scholar]
- (39).Dauphin-Ducharme P; Yang K; Arroyo-Currás N; Ploense KL; Zhang Y; Gerson J; Kurnik M; Kippin TE; Stojanovic MN; Plaxco KW Electrochemical Aptamer-Based Sensors for Improved Therapeutic Drug Monitoring and High-Precision, Feedback-Controlled Drug Delivery. ACS Sens 2019, 4 (10), 2832–2837. 10.1021/acssensors.9b01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Laviron E General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. Interfacial Electrochem 1979, 101 (1), 19–28. 10.1016/S0022-0728(79)80075-3. [DOI] [Google Scholar]
- (41).Yang W; Lai Y, R. Effect of Diluent Chain Length on the Performance of the Electrochemical DNA Sensor at Elevated Temperature. Analyst 2011, 136 (1), 134–139. 10.1039/C0AN00644K. [DOI] [PubMed] [Google Scholar]
- (42).Liu J; Wagan S; Dávila Morris M; Taylor J; White RJ Achieving Reproducible Performance of Electrochemical, Folding Aptamer-Based Sensors on Microelectrodes: Challenges and Prospects. Anal. Chem 2014, 86 (22), 11417–11424. 10.1021/ac503407e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Nishida N; Hara M; Sasabe HSH; Knoll WKW Thermal Desorption Spectroscopy of Alkanethiol Self-Assembled Monolayer on Au(111). Jpn. J. Appl. Phys 1996, 35 (11R), 5866. 10.1143/JJAP.35.5866. [DOI] [Google Scholar]
- (44).Yang G; Amro NA; Starkewolfe ZB; Liu G Molecular-Level Approach To Inhibit Degradations of Alkanethiol Self-Assembled Monolayers in Aqueous Media. Langmuir 2004, 20 (10), 3995–4003. 10.1021/la0499160. [DOI] [PubMed] [Google Scholar]
- (45).Corma A; Ródenas T; Sabater MJ Aerobic Oxidation of Thiols to Disulfides by Heterogeneous Gold Catalysts. Chem. Sci 2012, 3 (2), 398–404. 10.1039/C1SC00466B. [DOI] [Google Scholar]
- (46).Downs AM; Gerson J; Leung KK; Honeywell KM; Kippin T; Plaxco KW Improved Calibration of Electrochemical Aptamer-Based Sensors. Sci. Rep 2022, 12 (1), 5535. 10.1038/s41598-022-09070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Yuan Y; DeBrosse M; Brothers M; Kim S; Sereda A; Ivanov NV; Hussain S; Heikenfeld J Oil-Membrane Protection of Electrochemical Sensors for Fouling- and PH-Insensitive Detection of Lipophilic Analytes. ACS Appl. Mater. Interfaces 2021, 13 (45), 53553–53563. 10.1021/acsami.1c14175. [DOI] [PubMed] [Google Scholar]
- (48).Idili A; Gerson J; Kippin T; Plaxco KW Seconds-Resolved, In Situ Measurements of Plasma Phenylalanine Disposition Kinetics in Living Rats. Anal. Chem 2021, 93 (8), 4023–4032. 10.1021/acs.analchem.0c05024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


