Abstract
In Hong Kong in 1997, a highly lethal H5N1 avian influenza virus was apparently transmitted directly from chickens to humans with no intermediate mammalian host and caused 18 confirmed infections and six deaths. Strategies must be developed to deal with this virus if it should reappear, and prospective vaccines must be developed to anticipate a future pandemic. We have determined that unadapted H5N1 viruses are pathogenic in mice, which provides a well-defined mammalian system for immunological studies of lethal avian influenza virus infection. We report that a DNA vaccine encoding hemagglutinin from the index human influenza isolate A/HK/156/97 provides immunity against H5N1 infection of mice. This immunity was induced against both the homologous A/HK/156/97 (H5N1) virus, which has no glycosylation site at residue 154, and chicken isolate A/Ck/HK/258/97 (H5N1), which does have a glycosylation site at residue 154. The mouse model system should allow rapid evaluation of the vaccine’s protective efficacy in a mammalian host. In our previous study using an avian model, DNA encoding hemagglutinin conferred protection against challenge with antigenic variants that differed from the primary antigen by 11 to 13% in the HA1 region. However, in our current study we found that a DNA vaccine encoding the hemagglutinin from A/Ty/Ir/1/83 (H5N8), which differs from A/HK/156/97 (H5N1) by 12% in HA1, prevented death but not H5N1 infection in mice. Therefore, a DNA vaccine made with a heterologous H5 strain did not prevent infection by H5N1 avian influenza viruses in mice but was useful in preventing death.
Prior to 1997, the avian influenza viruses were considered unable to be transmitted directly to humans because of the absence of appropriate human cellular receptors (1). However, in Hong Kong in the summer of 1997, a strain of avian influenza A (H5N1) virus was transmitted directly from birds to humans and caused 18 confirmed infections and six deaths (3).
Antigenic and genetic analyses of viral isolates from seven of the patients identified two closely related but distinguishable groups of influenza A (H5N1) viruses (3). The most notable difference was the presence or absence of a potential glycosylation site at residue 154 of the hemagglutinin (HA) in their HA1 regions. In all seven of the influenza A (H5N1) virus isolates from these patients, the eight RNA gene segments were those of avian viruses, indicating that the isolates had not undergone genetic reassortment with human viruses (3, 4, 26). Serologic data from the initial case suggests that this virus was not efficiently transmitted among humans (3).
One-third of the humans infected with the H5N1 virus in the Hong Kong outbreak died. Thus, this virus could have devastating consequences if it acquired efficient human-to-human transmissibility. Antiviral agents and vaccines offer the most promise for controlling a potential avian influenza pandemic, but supply and logistical constraints would preclude the widespread use of antivirals in such an event. Current inactivated vaccines require large numbers of embryonated chicken eggs and take 6 months to produce. Although the mass killing of poultry in Hong Kong apparently eliminated the immediate source of infection, a human pandemic caused by a novel avian influenza virus remains a real possibility. Thus, it is urgent that an appropriate strategy for dealing with such an eventuality be developed (5, 6).
Immunization with purified DNA is a powerful means of inducing immune responses. This approach has been applied to many infectious diseases, including influenza, malaria, and tuberculosis (15, 25, 27, 30). Importantly, the candidate vaccine can be recovered from infected tissue, thereby eliminating the time required to culture the virus. We had found that H5N1 viruses are directly pathogenic in mice, providing a useful immunologic model of avian virus infection in mammals. We assessed whether vaccination with DNA encoding the HA of influenza virus A/HK/156/97 (H5N1) (HK97), inoculated via a gene gun, could induce protective immunity against H5N1 in mice. To assess the feasibility of using a related H5 virus as the basis of a human vaccine, we also evaluated the ability of DNA encoding HA from an antigenically related H5N8 virus to protect against H5N1 infection. The DNA vaccine encoding HA from influenza virus HK97 was protective against influenza viruses HK97 and A/Ck/HK/258/97 (CkHK97). Mice vaccinated with DNA encoding HA from the related avian influenza virus A/Ty/Ir/1/83 (H5N8) (TyIr83) were not protected against H5N1 infection, but they survived the infection. This is contrary to our previous findings and those of others in which chickens immunized with HA DNA are protected against infection by antigenic variant strains in which the HA1 regions differ from the primary immunizing antigen by 11 and 13% (7, 18, 30). These results have serious implications regarding the use of related strains of H5 viruses in the development of vaccines for humans.
MATERIALS AND METHODS
Viruses and cells.
The influenza viruses CkHK97 and HK97 have been described (4). These viruses were cultivated in the allantoic cavities of embryonated eggs (31) and handled in the hospital’s U.S. Department of Agriculture-approved biosafety level 3 containment facility.
Replication of H5N1 viruses in mice.
To determine the infectivity of HK97, mice were inoculated intranasally with 0.1 ml of ∼103 to 104 egg infectious doses (EID50) of allantoic fluid. Mice were monitored daily for clinical signs, weight loss, and mortality. On day 5 postinfection, three mice from each group were sacrificed to collect lung and brain tissues for virus titrations. Virus titrations were done in embryonated chicken eggs, and the titers were expressed as log10 EID50 per organ.
Influenza virus genes and expression vectors.
A full-length cDNA copy of the HA gene of influenza virus TyIr83 was cloned into the pJW4303 vector under the control of the cytomegalovirus (CMV) immediate-early promoter as previously described (18) and designated pTyIrHA. A full-length cDNA copy of the HA gene of HK97 was cloned into the EcoRI and BglII sites of pCAGGS/MCS (18), a vector that contains a chicken β-actin promoter. This construct was designated pHKHA. Plasmids were grown in HB101 bacteria and purified on purification columns (Qiagen, Inc., Valencia, Calif.).
Hemadsorption.
The expression and biological activity of the influenza virus HAs were examined by hemadsorption to chicken erythrocytes (RBCs). Cos-1 cells were transfected with pHKHA as described previously (18). Forty-eight hours after transfection, cells were washed with phosphate-buffered saline (PBS) and treated with bacterial neuraminidase for 1 h at 37°C to remove host cell sialic acid. Cells were washed again with PBS and overlaid at 4°C with 1% chicken RBCs in isotonic PBS. After 30 min, unbound RBCs were removed by washing with PBS, and cells were fixed with 10% buffered formalin phosphate. Bound RBCs were visualized by Giemsa staining (17).
Gene gun delivery of DNA.
We used the gene gun for DNA delivery because of the efficiency of transfection by this method demonstrated in previous studies (8, 10). Plasmid DNA was affixed to 2.6-μm gold beads (Degussa, South Plainfield, N.J.), and the complexes were inoculated into shaved abdominal areas of mice by use of a helium pulse gene gun (Accell; Auragen, Inc., Middleton, Wis.) as previously described (18, 20).
Immunization and challenge infection.
Vaccine trials were conducted in U.S. Department of Agriculture-approved biosafety level 3 facility. Six- to seven-week-old BALB/c mice (n = 50) were inoculated via gene gun with 1 μg of either pTyIrHA or pHKHA affixed to gold particles and were given boosters of the same dose 4 weeks later. Sixty-four control mice were left untreated. Ten days after receiving the boosters, the mice were challenged with 10 50% lethal doses (LD50) of either CkHK97 or HK97 in 100-μl volumes, intranasally. The mice were monitored daily for weight loss, clinical signs, and mortality, and samples were taken from three or four from each group on day 5 postinfection for virus replication. In all of the above-described experiments, serum samples were collected prebooster, prechallenge, and 10 days postchallenge for antibody analyses.
Serology.
Serum samples were collected pre- and postchallenge for serum antibody analyses. HA and HA inhibition (HI) assays were performed with 0.5% chicken RBCs as previously described (31). Sera from mice were tested individually after treatment with receptor-destroying enzyme (32). HI titers were determined as the reciprocal of the highest serum dilution that gave complete inhibition of hemagglutination.
RESULTS
Experimental infection of mice with influenza virus HK97 and CkHK97.
Mice are not a natural host for influenza viruses, and usually human viruses must be adapted to grow in mice. Since the H5N1 influenza viruses in Hong Kong caused severe infection in humans, studies were done to establish the properties of these viruses in mammalian systems. Groups of mice were infected with either HK97 or CkHK97 viruses as described in Materials and Methods. The mice became sick by day 5 postinfection, showing clinical signs of infection including lethargy, huddling, and ruffled fur. Three mice from each group were sacrificed on day 5, and the concentrations of virus in their lungs and brains were quantitated by titration in embryonated eggs. Both HK97 and CkHK97 grew to high titers in the lungs (∼106 EID50/lung) and to moderate titers in the brain (∼102.5 EID50/brain). The virus replication in the brain tissue without adaptation suggests that these viruses are neurotropic.
Infected mice lost up to 25% of their body weight by day 5 postinfection, and the progressive weight loss continued until the mice died. There was 100% mortality in the mice infected with CkHK97 by day 7, and 100% mortality was observed by day 12 in mice infected with HK97. We determined the LD50 of these two viruses in mice. We also determined the infectious-virus units in 1 LD50 of both HK97 and CkHK97 viruses and found that 1 LD50 of HK97 virus contains 101.0 PFU, compared to 103.3 PFU in 1 LD50 of CkHK97 virus. This suggests that HK97 is more virulent than CkHK97 virus.
Expression of HA protein in vitro.
Before testing HA DNA vaccines for induction of immunity in the mouse model, we determined the expression and biological activity of the cloned HA in vitro. Cos-1 cells were transfected with pHKHA or pTyIrHA, and transfected cells were assayed for hemadsorption. The expressed HA adsorbed to chicken RBCs, confirming that HA is transported to the cell surface and is biologically active.
Protection induced by immunization with pHKHA.
We sought to determine the extent of protection conferred by the DNA encoding HA of HK97 against challenge with homologous H5 virus (HK97) or CkHK97 (Table 1). Gene gun immunization of 12 mice with 1 μg of pHKHA provided 100% protection against death from homologous challenge with HK97 virus. This protection was provided with the complete absence of virus replication in the brain tissue. However, only one of four mice tested had virus present at 104.5/ml in its lungs, which is 100-fold lower than that of controls. Four of four control mice had large amounts of virus in both lung (>106 EID50/lung) and brain (>102 EID50/brain) tissues. The immunized mice showed no signs of infection, whereas the control mice showed severe signs of infection (huddling, shivering, and ruffled fur). There was progressive weight loss observed in control mice after day 5 postinfection, while the immunized mice gained weight over the period of 12 days.
TABLE 1.
DNA encoding HA from HK97 virus protects mice against lethal influenza H5N1 virus challenge
| Vaccine | Challenge virusa | Virus replication on day 5 postinfection (log10 EID50/organ)b
|
Protection on day 12 postinfection (no. sick/no. dead/total) | Survival (%) | |
|---|---|---|---|---|---|
| Lung | Brain | ||||
| pHKHA | HK97 | 1.1c | <1d | 0/0/12 | 100 |
| Control | HK97 | 6.3 | 2.6 | 12/12/12 | 0 |
| pHKHA | CkHK97 | <1d | <1d | 0/0/12 | 100 |
| Control | CkHK97 | 6.9 | 2.5 | 12/11/12 | 8 |
Challenge by intranasal inoculation with 10 LD50.
The findings were recorded as mean titers in the tissues of four mice per group. Whole brain and lung tissues were homogenized and resuspended in 1 ml of PBS.
One of four had virus in the lung. Data shown are averaged from four lungs.
No virus at a 10−1 dilution.
Immunization with 1 μg of pHKHA-DNA administered via a gene gun also provided 100% protection against death from challenge with the CkHK97 virus in the absence of detectable virus in the lung and brain tissues (Table 1). All the control mice except one lost a significant amount of body weight and eventually died of infection. These results suggest that pHKHA induced protective immunity against homologous HK97 and antigenic variant CkHK97 viruses.
Protection induced by immunization with pTyIrHA.
An emerging pandemic may not allow time for making a DNA vaccine that encodes the genetically matched HA. Our previous studies with plasmid DNA encoding HA from TyIr83 have shown that HA DNA vaccines can protect chickens against challenge infection with antigenic variants (18). Here, we sought to determine whether immunization with pTyIrHA could effectively protect against infection with the HK97 virus. Groups of mice (n = 14) were gene gun immunized with pTyIrHA and then exposed to HK97 as described in Materials and Methods.
Although pTyIrHA vaccine prevented death from HK97 virus challenge in nine of nine immunized mice (Table 2), the mice showed transient signs of infection after challenge and had significant amounts of virus (log105.5) in their lungs, similar to amounts in untreated control mice (log106.5). However, in immunized mice there was no replication of virus in the brain, in contrast to control mice. The mice had lost 10% of their body weight by day 5 postchallenge, which was not significant when compared to the controls. The control mice also showed signs of severe infection, with marked weight loss (25% on day 5 postinfection) and 100% mortality by day 12. There was a difference of only 12% in the amino acid sequences of the HA1 regions of TyIr83 HA and HK97 HA, but the DNA encoding HA in TyIr83 did not prevent the challenge infection of immunized mice with HK97.
TABLE 2.
DNA encoding HA from TyIr83 virus protects mice against lethal influenza H5N1 virus challengea
| Vaccine | Virus replication on day 5 postinfection (log EID50/lung or brain)b
|
Body weight loss on day 5 postinfection (%) | Protection on day 12 postinfection (no. sick/no. dead/total) | Survival (%) | |
|---|---|---|---|---|---|
| Lung | Brain | ||||
| pTyIrHA | 5.5c | <1d | 10 | 9/0/9 | 100 |
| Control | 6.5 | 3.6 | 25 | 12/12/12 | 0 |
Challenge by intranasal inoculation with 10 LD50 of HK97.
The findings were recorded as mean titers in the tissues of four mice per group. Whole brain and lung tissues were homogenized and resuspended in 1 ml of PBS.
Four had virus in the lungs. The data is the average of four lungs.
No virus at a 10−1 dilution.
Antibody responses of mice immunized with HA DNA vaccines.
The ability of HA DNA vaccines (pTyIrHA and pHKHA) to induce serum antibodies was examined. Within the first 4 weeks of immunization there was no detectable antibody response to any of the antigens tested in mice immunized with either of the plasmids. After the booster vaccination, 40% of the mice immunized with pHKHA developed low levels of HI antibodies (Fig. 1A). There was no detectable antibody response after booster vaccination in mice immunized with pTyIrHA plasmid. The postchallenge serum antibody response was measured against both HK97 and CkHK97 viruses after challenge infection.
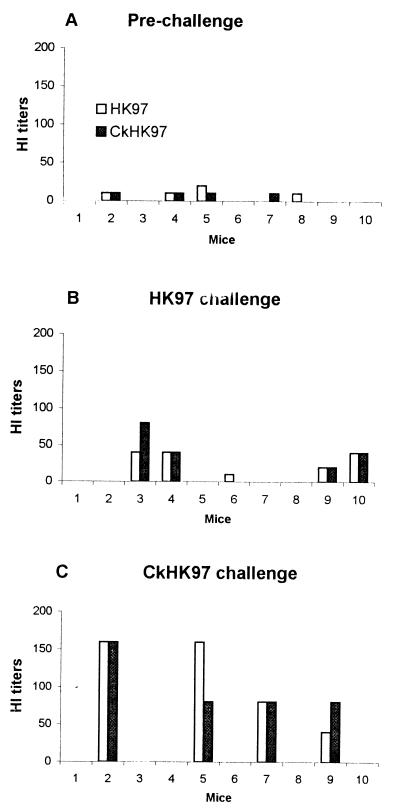
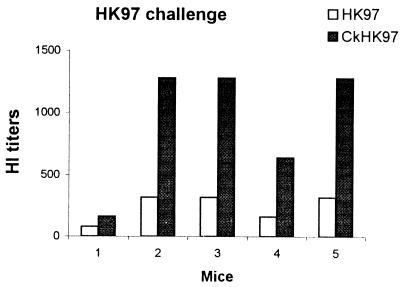
FIG. 1.
HI antibody titers of mice immunized with pHKHA and challenged with either HK97 or CkHK97. Serum samples were collected at 10 days postbooster (prechallenge) and at 10 days postchallenge. HI determinations were done with either HK97 or CkHK97 virus antigens. Each bar represents a titer from an individual mouse.
(i) pHKHA immunization and HK97 challenge.
We determined the postchallenge antibody response in the serum samples of mice immunized with pHKHA (Fig. 1B). The HK97 challenge of mice immunized with pHKHA induced very low levels of HI antibodies in 40 and 50% of the protected mice reacting to CkHK97 and HK97 viruses, respectively. There was virus shedding in 25% of the animals, suggesting that virus replication occurred in a limited number of animals. The small number of animals with an antibody response suggests that this response was due to virus replication.
(ii) pHKHA immunization and CkHK97 challenge.
The profile of antibody response to CkHK97 was very similar to the antibody response in mice challenged with HK97 virus. HI antibodies were detected in four of ten mice challenged with CkHK97, again probably due to limited replication of the virus (Fig. 1C). The HI titer in mice challenged with CkHK97 was slightly higher than that in HK97-challenged mice. This could be due to the larger number of infectious units in the challenge dose of CkHK97 virus since 1 LD50 of CkHK97 virus contains 100 times more infectious units than does 1 LD50 of the HK97 virus (Fig. 1C).
(iii) pTyIrHA immunization and HK97 challenge.
The HI assay showed high levels of postchallenge antibodies specific to both HK97 and CkHK97 in mice immunized with pTyIrHA (Fig. 2). Interestingly, these levels were two- to fourfold higher in their reactivity with CkHK97 than in their reactivity with HK97 antigen. It was also interesting that five of five immunized mice tested produced antibodies reacting with HK97 or CkHK97, unlike pHKHA-immunized mice. This antibody response could be due to the replication of large amounts of the challenge virus in the lungs.
FIG. 2.
HI antibody titers of mice immunized with pTyIrHA and challenged with HK97. Serum samples were collected at 10 days postbooster (prechallenge) and at 10 days postchallenge. There were no detectable antibodies in the serum samples collected from mice after booster. HI determinations were done with either HK97 or CkHK97 virus antigens. Each bar represents a titer from an individual mouse.
DISCUSSION
Highly lethal avian H5N1 viruses are extremely virulent in mice, causing disease that mimics that seen in chickens. The marked pathogenicity of these viruses in a well-defined mammalian system offers a model useful for evaluating vaccine efficacy. Despite the low or undetectable levels of antibodies in mice immunized with DNA encoding HA from HK97, the DNA vaccine provided immunity against H5N1 infection. This immunity was induced against both the homologous human isolate HK97, which lacks a glycosylation site at 154, and against the chicken isolate A/Ck/HK/258/97 virus, which has a glycosylation site at 154. Only one of eight immunized mice had virus present in the lungs at levels 100-fold times lower than that of controls. Previous studies (7, 18, 30) have shown that HA-based DNA vaccines can prevent infections in chickens and ferrets by antigenic variants of the primary immunizing HA. However, the DNA vaccine encoding the HA from TyIr83, which differs from HK97 by 12% in the antigenic region, did not prevent H5N1 infection in mice. It did protect mice from death, thereby suggesting that in an avian influenza pandemic, a DNA vaccine encoding an antigenically related HA might offer adequate protection until a genetically matched HA DNA vaccine could be made—a process that should require less than a month.
The HK97 and CkHK97 viruses replicated without adaptation in the brain tissues of mice after intranasal inoculation. Studies done by Scholtissek et al. suggest that a cleavable HA derived from influenza virus A/FPV/Rostock/34 is essential for the neurovirulence of several reassortants in mice (24). H5N1 HA is highly cleavable due to the basic amino acids (4, 26) at its cleavage site, and the viral HA is probably enzymatically cleaved in brain tissue. Internal genes may also be involved in the neurovirulence of these strains in mice, as described for mouse-adapted A/WSN/33 (H1N1) strains of influenza virus (23, 28). Although there was no difference between the HA cleavage sites of chicken isolate CkHK97 and human isolate HK97, these viruses had different virulences in mice. The LD50 of HK97 (101.0 PFU) was 100-fold lower than that of CkHK97 (103.3 PFU), when HK97 was inoculated intranasally. This difference may be explained by the presence of a glycosylation site at 154 in CkHK97 HA or by differences in any of the internal genes (2).
After immunization, antibody production is usually the major mechanism of protection against influenza infection, neutralizing the virus by specific immunoglobulin G or immunoglobulin A (12) at the surface of the lung mucosa. Antibodies to the HA molecule are necessary if the influenza virus is to be neutralized and the infection is to be prevented (10, 11, 30). DNA vaccination can induce the production of neutralizing antibodies in titers comparable to those induced by natural infection (21). Although the absence of virus replication suggested effective virus neutralization in mice immunized with DNA encoding HA of HK97, HI assays showed low to undetectable antibody responses. This observation suggests that B-cell memory plays a large role in mediating the immune response to influenza virus (12, 16). Consistent with this conclusion, in our previous studies DNA vaccine encoding H5 HA induced protection in chickens in the absence of HI antibodies (9, 10, 18, 22). Thus, H5-specific memory B cells activated after challenge infection may have prevented the development of lethal pneumonia following respiratory challenge. The levels of HI antibodies reacting with the CkHK97 virus are high compared to the levels of HI antibodies reacting with the HK97 virus. This is probably due to the presence of a glycosylation site in the CkHK97 virus.
We have shown previously that DNA encoding HA from TyIr83 effectively protects chickens against antigenically related viruses like A/Ck/Queretaro/19/95 (H5N2) and A/Ck/Pennsylvania/13073/83 (H5N2). This protection is remarkable, since the challenge viruses differed from the immunizing antigen by 11 and 13%, respectively, in amino acid sequence homology within the antigenic region (18). However, in the present study, DNA encoding the HA of TyIr83 generated limited immunity to subsequent challenge with HK97 virus in mice. This failure to prevent infection may be attributable to the species difference. It is very well established in the mouse model that susceptibility to viral challenge differs among mouse strains. This difference is attributed to the immune responsiveness (13) of the various strains. Thus, it is not surprising that the protective ability of pTyIrHA differs in avian and mammalian species. The second possibility is that the difference could be caused by the two different vector promoter systems. The plasmid DNA encoding TyIr83 HA is under the control of the CMV promoter, and the plasmid vector encoding HK97 HA is under the control of the chicken β-actin promoter. The rate of seroconversion to the DNA-encoded antigen is highly dependent on the strength of the promoter. However, it is unlikely that the reduced protection induced by pTyIrHA was caused by the vector containing the CMV promoter, since the CMV immediate-early promoter has been shown to be superior for gene expression in the mouse model (19). Whatever the reason for the reduced protection induced by pTyIrHA, it appears that available avian viruses may not be useful sources of vaccines against avian viruses introduced into the human population. The related HA may protect against death, as shown here with HK97 challenge infection, but may not prevent the spread of infection.
Postchallenge antibodies were induced in only 40 to 50% of the mice immunized with pHKHA, as opposed to the induction of antibodies in all of the mice immunized with pTyIrHA. The high levels of HI antibodies in the group immunized with pTyIrHA were probably induced by the replication of the challenge virus in the lungs of the mice, since infection was not prevented. However, in the mice immunized with pHKHA (homologous HA), the challenge virus was probably effectively neutralized by specific antibodies, since there was virus replication in only one of eight animals tested.
Currently circulating mammalian influenza viruses are not lethal to the host in the absence of complications. However, virulent strains of avian influenza viruses (H5 and H7 subtypes) could appear or reappear in humans. Although the mass killing of poultry in Hong Kong eliminated a source of infection, reservoirs of avian influenza viruses are expected to still exist, and other avian pathogenic strains may infect and become established in humans (29). The antivirals amantadine and rimantadine are potentially useful, but a number of factors, including their limited supply, their side effects, and the emergence of drug resistance, are likely to preclude their widespread use in a pandemic (14). Although available vaccines may not prevent infection, they may be useful in preventing deaths until a specific vaccine against the pandemic strain can be prepared. DNA immunization offers a unique advantage in that the candidate vaccine can be recovered from infected tissue and rapidly cloned into an expression vector, thereby eliminating the time required to culture the virus during the emerging pandemic. A DNA vaccine encoding HA from the pandemic strain could be rapidly prepared and evaluated in a mammalian host.
ACKNOWLEDGMENTS
This research is supported by Public Health Service grants AI-08831, AI-144388, and AI-33898 from the National Institute of Allergy and Infectious Diseases, Cancer Center Support (CORE) grant CA-21765, and by the American Lebanese Syrian Associated Charities (ALSAC) to R.G.W.
REFERENCES
- 1.Beare A S, Webster R G. Replication of avian viruses in humans. Arch Virol. 1990;119:37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- 2.Brown E G. Increased virulence of a mouse-adapted variant of influenza A/FM/1/47 virus is controlled by mutations in genome segments 4, 5, 7, and 8. J Virol. 1990;64:4523–4533. doi: 10.1128/jvi.64.9.4523-4533.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Update: isolation of avian influenza A(H5N1) viruses for humans—Hong Kong, 1997–1998. Morbid Mortal Weekly Rep. 1998;46:1245–1247. [PubMed] [Google Scholar]
- 4.Claas E C, Osterhaus A D M E, van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A(H5N1) virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. The flu pandemic that might have been. Science. 1997;277:1600–1601. doi: 10.1126/science.277.5332.1600. [DOI] [PubMed] [Google Scholar]
- 6.De Jong J C, Class E C, Osterhaus A D M E, Webster R G, Lim W L. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. . (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly J J, Friedman A, Martinez D, Montgomery D L, Shiver J W, Motzel S H, Ulmer J B, Liu M A. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995;6:583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 8.Eisenbraun M D, Fuller D H, Haynes J R. Examination of parameters affecting the elicitation of humoral immune responses by particle bombardment-mediated genetic immunization. DNA Cell Biol. 1993;12:791–797. doi: 10.1089/dna.1993.12.791. [DOI] [PubMed] [Google Scholar]
- 9.Fynan E F, Robinson H L, Webster R G. Use of DNA encoding influenza hemagglutinin as an avian influenza vaccine. DNA Cell Biol. 1993;12:785–789. doi: 10.1089/dna.1993.12.785. [DOI] [PubMed] [Google Scholar]
- 10.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerhard W. The analysis of the monoclonal immune response to influenza virus. III. The relationship between stimulation of virus primed precursor B-cells by heterologous virus and reactivity of selected antibodies. J Immunol. 1978;120:1164–1168. [PubMed] [Google Scholar]
- 12.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 13.Hartley J W, Fredrickson T N, Yetter R A, Makino M, Morse H C., III Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989;63:1223–1231. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden, F. G. 1997. Antivirals for pandemic influenza. J. Infect. Dis. 176(Suppl. 1):s56–s61. [DOI] [PubMed]
- 15.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souze C, Dowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 16.Justewicz D M, Doherty P C, Webster R G. The B-cell response in lymphoid tissue of mice immunized with various antigenic forms of the influenza virus hemagglutinin. J Virol. 1995;69:5414–5421. doi: 10.1128/jvi.69.9.5414-5421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaoka Y, Webster R G. Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci USA. 1988;85:324–328. doi: 10.1073/pnas.85.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodihalli S, Haynes J R, Robinson H L, Webster R G. Cross-protection among lethal H5N2 influenza viruses induced by DNA vaccine to the hemagglutinin. J Virol. 1997;71:3391–3396. doi: 10.1128/jvi.71.5.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A H, Suh Y S, Sung J H, Yang S H, Sung Y C. Comparison of various expression plasmids for the induction of immune response by DNA immunization. Mol Cells. 1997;7(4):495–501. [PubMed] [Google Scholar]
- 20.Pertmer T M, Eisenbraun M D, McCabe D, Prayaga S K, Fuller D H, Haynes J R. Gene-gun based nucleic acid immunization: elicitation of humoral and cytotoxic responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13:1427–1430. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 21.Robinson H L, Feltquate D M, Morin M J, Haynes J R, Webster R G. DNA vaccines: a new approach to immunizations. In: Brown F, Channock R M, Ginsberg H S, Norrby E, editors. Vaccines 95: molecular approaches to the control of infectious diseases. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 69–75. [Google Scholar]
- 22.Robinson H L, Hunt L A, Webster R G. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine. 1993;11:957. doi: 10.1016/0264-410x(93)90385-b. [DOI] [PubMed] [Google Scholar]
- 23.Schlesinger R W, Bradshaw G L, Barbone F, Reinacher M, Rott R, Husak P. Role of hemagglutinin cleavage and expression of M1 protein in replication of A/WS/33, A/PR/8/34, and WSN influenza viruses in mouse brain. J Virol. 1989;63:1695–1703. doi: 10.1128/jvi.63.4.1695-1703.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholtissek C, Vallbracht A, Flehmig B, Rott R. Correlation of pathogenicity and gene constellation of influenza A viruses. II. Highly neurovirulent recombinants derived from non-neurovirulent or weakly neurovirulent parent virus strains. Virology. 1979;95:492–500. doi: 10.1016/0042-6822(79)90503-8. [DOI] [PubMed] [Google Scholar]
- 25.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N J. Characterization of an avian influenza A(H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 27.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 28.Ward A C. Specific changes in the M1 protein during adaptation of influenza virus to mouse. Arch Virol. 1995;140:383–389. doi: 10.1007/BF01309872. [DOI] [PubMed] [Google Scholar]
- 29.Webster R G. Predictions for future human influenza pandemics. J Infect Dis. 1997;176:S14–S19. doi: 10.1086/514168. [DOI] [PubMed] [Google Scholar]
- 30.Webster R G, Fynan E F, Santro J C, Robinson H L. Protection of ferrets against influenza challenge with a DNA vaccine to haemagglutinin. Vaccine. 1994;12:1495–1498. doi: 10.1016/0264-410x(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 31.WHO Collaborating Center for Reference and Research on Influenza. Concepts and procedures for laboratory based influenza surveillance, B-19. Atlanta, Ga: Centers for Disease Control; 1982. [Google Scholar]
- 32.Wood J M, Kawaoka Y, Newberry L A, Bordwell E, Webster R G. Standardization of inactivated H5N2 influenza vaccine and efficacy against lethal A/Chicken/Pennsylvania/1370/83 infection. Avian Dis. 1985;29:867–872. [PubMed] [Google Scholar]