Abstract
Previous data have linked omega-3 fatty acids with risk of dementia. We aimed to assess the longitudinal relationships of omega-3 polyunsaturated fatty acid intake as well as blood biomarkers with risk of Alzheimer’s disease (AD), dementia, or cognitive decline. Longitudinal data were derived from 1135 participants without dementia (mean age = 73 y) in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort to evaluate the associations of omega-3 fatty acid supplementation and blood biomarkers with incident AD during the 6-y follow-up. A meta-analysis of published cohort studies was further conducted to test the longitudinal relationships of dietary intake of omega-3 and its peripheral markers with all-cause dementia or cognitive decline. Causal dose–response analyses were conducted using the robust error meta-regression model. In the ADNI cohort, long-term users of omega-3 fatty acid supplements exhibited a 64% reduced risk of AD (hazard ratio: 0.36, 95% confidence interval: 0.18, 0.72; P = 0.004). After incorporating 48 longitudinal studies involving 103,651 participants, a moderate-to-high level of evidence suggested that dietary intake of omega-3 fatty acids could lower risk of all-cause dementia or cognitive decline by ∼20%, especially for docosahexaenoic acid (DHA) intake (relative risk [RR]: 0.82, I2 = 63.6%, P = 0.001) and for studies that were adjusted for apolipoprotein APOE ε4 status (RR: 0.83, I2 = 65%, P = 0.006). Each increment of 0.1 g/d of DHA or eicosapentaenoic acid (EPA) intake was associated with an 8% ∼ 9.9% (Plinear < 0.0005) lower risk of cognitive decline. Moderate-to-high levels of evidence indicated that elevated levels of plasma EPA (RR: 0.88, I2 = 38.1%) and erythrocyte membrane DHA (RR: 0.94, I2 = 0.4%) were associated with a lower risk of cognitive decline. Dietary intake or long-term supplementation of omega-3 fatty acids may help reduce risk of AD or cognitive decline.
Keywords: omega-3 fatty acid, dementia, AD, cognitive decline, dietary, biomarker
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder with a high prevalence among aging populations [1]. Given the lack of effective therapeutic strategies and the high disease burden, it is imperative to identify modifiable risk factors to prevent or postpone the onset of AD. Interest has raised recently in the role of omega-3 fatty acid dietary intake and blood concentrations in the prevention of dementia. Omega-3 fatty acids are a heterogeneous group of fatty acids with a double bond at the ω−3 carbon atom, mainly including DHA (22:6n-3), EPA (20:5n-3), and ALA (18:3n-3). Omega-3 fatty acids are primarily obtained through dietary intake, and fish is the primary dietary source of EPA and DHA in humans [2]. Plant-derived ALA is the most abundant omega-3 fatty acid in the diets of people who do not regularly consume fish [3].
Omega-3 fatty acids are nootropic agents that are beneficial for brain development, anti-inflammation, and cognitive preservation [4]. DHA is an essential fatty acid that maintains brain function and integrity, and its derivatives can modulate glial cell activity and improve cognition in the early stages of AD [5]. However, evidence from observational studies and clinical trials has produced unclear conclusions regarding the efficacy of omega-3 fatty acid supplementation for prevention of cognitive decline, dementia, or AD. Prospective studies have suggested that individuals who consume higher amounts of omega-3 fatty acids are less likely to develop AD [6], and erythrocyte DHA levels are inversely associated with risk of AD and all-cause dementia [7]. Compared with cognitively healthy individuals, patients with AD have been found to have lower concentrations of omega-3 fatty acids, especially DHA, in the serum, plasma phospholipids, and erythrocyte membranes [8,9]. In contrast, randomized clinical trials have shown limited efficacy of omega-3 fatty acid supplementation in reducing cognitive decline and probable AD [10]. Further, apolipoprotein ε4 (APOE ε4) genotype might modify the association between omega-3 fatty acid supplementation and cognitive decline [11], dementia, or AD [12]. The APOE ε4 allele, the major genetic risk factor for AD, could mediate AD-associated pathology, including inducing abnormal cholesterol metabolism [13]. It remains unclear how APOE ε4 interacts with omega-3 fatty acids to affect risk of dementia and cognitive decline. A study has found an association between a higher intake of omega-3 fatty acids and slower rates of cognitive decline among APOE ε4 carriers but not among APOE ε4 noncarriers [11]. Other studies have reported opposite results, showing that omega-3 fatty acids are only beneficial for APOE ε4 noncarriers [9,14]. The underlying mechanism may involve the reduced delivery of DHA and EPA into the brain caused by APOE ε4 [15].
To assess the longitudinal relationships between the intake of omega-3 polyunsaturated fatty acids and blood biomarkers and risk of AD, dementia, or cognitive decline, we analyzed data from Alzheimer’s Disease Neuroimaging Initiative (ADNI) and conducted a systematic review and meta-analysis of newly published studies.
Methods
ADNI cohort study
Participants
Data were derived from the ADNI cohort (http://adni.loni.usc.edu/). As a multicenter study, the ADNI is designed to develop clinical, imaging, genetic, and biochemical biomarkers for the early detection and tracking of AD. The studied population are nondemented adults aged 55 y to 90 y at baseline. Participants underwent standardized neuropsychological assessments, in-person interviews for detailed medical history, and cognitive evaluation at study entry and follow-up (Figure 1A). The ADNI was approved by the institutional review boards of all participating institutions, and written informed consent was obtained from all participants according to the Declaration of Helsinki.
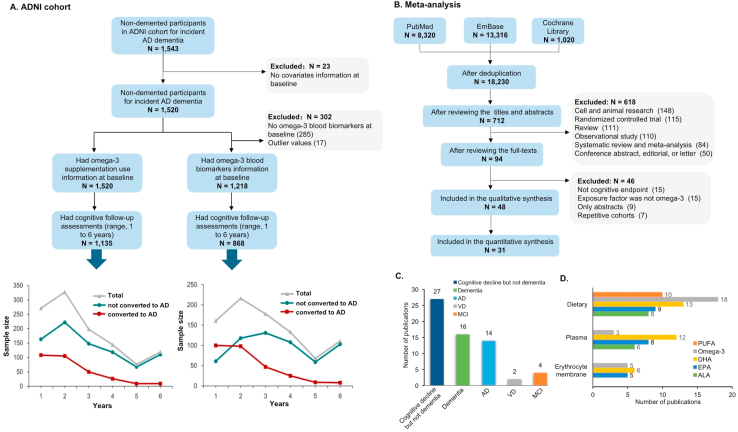
FIGURE 1.
Flowchart for the overall selection process and summary characteristics of included studies. (A) Flowchart of the participants included in the ADNI cohort study. (B) Overall selection process in the meta-analysis of published longitudinal studies. (C) Most studies reported cognitive decline but not dementia (n = 27) followed by dementia (n = 16) or AD (n = 14), and only a few reported MCI (n = 4) or VD (n = 2). Of all 48 studies included in systematic review, most studies reported omega-3 fatty acid dietary intake (n = 31), followed by plasma (n = 14) and erythrocyte membrane (n = 6). (D) All 31 studies included in the meta-analysis; 18 reported dietary omega-3 including DHA (n = 13), EPA (n = 9), and ALA (n = 8). AD, Alzheimer’s disease; MCI, mild cognitive impairment; VD, vascular dementia.
Supplementation and blood measurement of omega-3 fatty acids
Dietary supplement use information was collected at the initial screening visit as a part of the medication-taking questionnaire. The question was open-ended, and participants provided the information about all prescribed and over-the-counter medications as well as any oral supplement they are taking, including the name and the time from initiation of use to discontinuation. Omega-3 fatty acid supplements are defined as fish oil, omega-3 fatty acid, PUFA, DHA, EPA, or ALA. Self-reported omega-3 fatty acid supplementation information was recorded at the initial screening visit with participants and their partners. Duration of omega-3 fatty acid supplementation was calculated as the time from initiation of use to discontinuation. Participants who used omega-3 fatty acid supplements for over 1 y were defined as the “exposed group” and others as “nonexposed group.” The “exposed” subjects were further divided into “medium-term users (1 ∼ 9 y)” group and “long-term users (≥10 y).”
Plasma samples for each subject were obtained in the morning following an overnight fast. The time from collection to freezing was mostly within 2 h. Lab technicians were blinded to the clinical information of samples. Fatty acid composition was quantified using Nightingale Health’s nuclear magnetic resonance (NMR) metabolomics platform. The samples were processed following the automated standard protocol provided by Nightingale’s technology, and blood metabolites were quantified in absolute concentrations and percentages using NMR spectroscopy [16].
Ascertainment of AD
AD was diagnosed by neurologists according to brain structure scans, cognitive score, and independent living ability, based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA) for definite, probable, or possible AD [17].
Covariate measurements
The covariates included age (continuous variable), sex (female =1, male = 0), education (continuous variable), cognitive status (mild cognitive impairment =1, normal cognition = 0), and APOE ε4 status (“44/34/24” = 1, “33/22/23” = 0). rs7412 and rs429358 were used to define the APOE ε2/ε3/ε4 isoforms [18]. Other potential covariates were obtained from baseline medical history, including dichotomous variables (hyperlipidemia, hypertension, diabetes, stroke history, insomnia, depression, anxiety, current smoking status, coronary artery disease, alcohol intake, multivitamin, vitamin B12, and folate supplementation, anti-hypertensive drugs, and anti-diabetic drugs) and continuous variable (BMI).
Evidence evaluation via meta-analysis
Search strategy and selection criteria
We registered the protocol on PROSPERO in advance (CRD42021238393). Meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines (PRISMA) statement [19]. PubMed, EMBASE, and Cochrane Library were searched using the strategy: ((fish) OR (fish oil) OR (omega-3) OR (omega) OR (polyunsaturated fatty acid) OR (PUFA) OR (docosahexaenoic acid) OR (DHA) OR (eicosapentaenoic acid) or (EPA) OR (Alpha linolenic acid) OR (ALA)) AND ((cognitive) OR (dementia) OR (Alzheimer)) until March 17, 2022. Reference lists of articles identified through database searches were manually screened to locate additional relevant studies. The inclusion criteria were as follows: 1) the relationships of omega-3 fatty acid intake and its peripheral biomarkers with risk of AD, all-cause dementia, or cognitive decline were investigated; 2) study designs were cohort studies or nested case-control studies; 3) risk estimates or the raw data that can be used to calculate these numbers were available; 4) publication type was original article. No restriction was imposed on language. Studies were excluded if they did not meet the inclusion criteria mentioned above.
Data extraction
A pre-designed template was used to extract the data (Supplementary Table 1). For data unavailable in the article, we attempted to obtain them by contacting the corresponding authors. Literature selection and data extraction were performed by 2 experienced investigators (B-ZW and C-WD), and a third reviewer (WX) occasionally participated in the negotiation to solve any discrepancies.
Identification of AD, dementia, and cognitive decline
Cognitive decline is a common outcome of aging and may lead to dementia, and AD is the primary kind of dementia. Participants underwent cognitive assessments at baseline and during follow-up, and a decline in scores on the Mini-Mental State Examination scale was considered as cognitive decline. AD and dementia were diagnosed by the consensus of physicians according to the NINCDS-ADRDA and the Diagnostic and Statistical Manual of Mental Disorders 4th/5th Edition (DSM-IV/DSM-V), respectively.
Assessment of study quality and credibility of meta-analyses
An evolving Newcastle- Ottawa Quality Assessment Scale for observational cohort studies [20] was employed to evaluate the quality of eligible studies (Supplementary Table 2). The evidence robustness of the meta-analysis was assessed by summing scores from 5 domains: risk of bias, heterogeneity, publication bias, effect size, and imprecision. Scores were ranked in descending order; the top third was categorized into high level (H), the middle third was moderate level (M), and the bottom third was low level (L) (Supplementary Table 3). To better utilize the whole body of evidence and avoid missing important research unsuitable for meta-analysis, we developed a semiquantitative index named “index S” [21] (Supplementary Table 4).
Statistical analyses
In the ADNI, the statistical differences on baseline variables (see “Covariate measurements”) between participants who developed AD and those who were free of dementia during follow-up was compared by Pearson chi-squared test or independent samples t-test. Cox proportional hazards models with age as the time metric were used to assess the influence of omega-3 fatty acid at baseline on AD incidence. Risk estimate was expressed as hazard ratio (HR) and 95% CI. The proportional hazards assumption was checked using Schoenfeld’s global test. No variable was statistically significant, suggesting that the proportional hazards assumption was not violated (P > 0.05). To examine the potential stratification or interaction effect, stratified analyses were performed according to sex, age, APOE ε4 status, and baseline cognitive diagnosis. Sensitivity analyses were conducted by excluding those who progressed to dementia within 1 y follow-up.
As for meta-analyses, data were analyzed using the ‘metagen,’ ‘metabias,’ and ‘trimfill’ packages in R V.3.4.3 software (https://www.r-project.org). All statistical tests were 2-sided and used a significance level of P < 0.05. The pooled risk estimates were first calculated based on the comparison of the highest versus the lowest category of exposure. For studies wherein the reference group was not the lowest category, we recalculated the effect size using the method of Orsini [22]. For studies reporting odd ratios (ORs), we transformed ORs to RRs using the following algorithm: RRadjusted = ORadjusted/[(1 − P0) + (P0× ORadjusted)] where P0 indicates the incidence of the endpoint (AD, dementia, or cognitive decline) in the nonexposed group of the cohort. When P0 was not available, the incidence rate of total participants was used as a proxy [23]. The multivariable-adjusted risk estimates and 95% CIs were log-transformed and pooled using random models (DerSimonian–Laird method) [24]. Heterogeneity was examined using the I2 statistic, and the source of heterogeneity was explored via sensitivity analyses, meta-regression (if n ≥ 10), and subgroup analyses according to multiple variables, including study design, region, sex, sample size, cognitive status at baseline, age stage (late life [mean age ≥ 65 y] or midlife), follow-up duration, whether APOE ε4 status was adjusted, type of outcome, effect estimate, and quality score. Publication bias was assessed by determining the symmetry of the funnel plot by Egger’s test and enhanced-contour funnel plots after the trim-and-fill method. Dose–response meta-analyses were conducted using the inverse variance weighted least squares regression with cluster robust error variances (random effects meta-regression model) [25,26]. The midpoint of the lower and upper bounds was regarded as the dose of each category if the study only reported the range. For studies with an open-ended boundary, we multiplied or divided the reported boundary by 1.25.
Results
ADNI cohort study
Baseline characteristics of the study population
In the ADNI cohort for incident AD, a total of 1135 participants (46.2% females, aged 73.36 ± 7.22 y) were included, with a mean follow-up time of 2.81 ± 1.60 y (range, 1–6 y) (Figure 1A). Compared with participants free of dementia, those who developed AD tended to be APOE ε4 carriers, have a lower BMI, were more likely to have a history of depression, and less likely to have a history of insomnia (Table 1).
TABLE 1.
Population characteristics at baseline in ADNI cohort
| Variable | Total | Participants free of AD | Participants who developed AD | P |
|---|---|---|---|---|
| n | 1,135 | 828 | 307 | |
| Age, y, mean ± SD | 73.36 ± 7.22 | 73.11 ± 7.26 | 72.02 ± 7.05 | 0.057 |
| Female, % | 524 (46.2%) | 400 (48.3%) | 124 (40.4%) | 0.017 |
| Education, y | 16.16 ± 2.69 | 16.24 ± 2.66 | 15.94 ± 2.78 | 0.101 |
| MCI, % | 711 (62.6%) | 417 (50.4%) | 294 (95.8%) | <0.001 |
| APOE ε4 carriers, % | 513 (45.2%) | 314 (37.9%) | 199 (64.8%) | <0.001 |
| Omega-3 supplementation | ||||
| Exposure (yes or no) | 272 (24.0%) | 213 (25.7%) | 59 (19.2%) | 0.023 |
| Medium-term exposure (1∼9 y) | 204 (18.0%) | 153 (18.5%) | 51 (16.6%) | 0.397 |
| long-term exposure (≥10 y) | 68 (6.0%) | 60 (7.2%) | 8 (2.6%) | 0.030 |
| Blood markers (N-867) | ||||
| Omega-3 | 0.43 ± 0.12 | 0.43 ± 0.12 | 0.42 ± 0.12 | 0.420 |
| DHA | 0.13 ± 0.04 | 0.13 ± 0.04 | 0.13 ± 0.04 | 0.692 |
| ALA | 0.41 ± 0.06 | 0.41 ± 0.06 | 0.41 ± 0.06 | 0.786 |
| Insomnia, % | 82 (7.2%) | 71 (8.6%) | 11 (3.9%) | 0.004 |
| Depression, % | 247 (21.8%) | 163 (19.7%) | 84 (27.4%) | 0.005 |
| Anxiety, % | 76 (6.7%) | 52 (6.3%) | 24 (7.8%) | 0.357 |
| Hypertension, % | 528 (46.5%) | 383 (46.3%) | 145 (47.2%) | 0.770 |
| BMI (kg/m2) | 27.04 ± 4.86 | 27.31 ± 4.95 | 26.33 ± 4.54 | 0.002 |
| Diabetes, % | 102 (9.0%) | 75 (9.1%) | 27 (8.8%) | 0.891 |
| Hyperlipidemia, % | 225 (19.8%) | 162 (19.6%) | 63 (20.5%) | 0.720 |
| Stroke, % | 41 (3.6%) | 22 (2.7%) | 19 (6.2%) | 0.005 |
| CAD, % | 92 (8.1%) | 65 (7.9%) | 27 (8.8%) | 0.605 |
| Current smoker, % | 166 (14.6%) | 122 (14.7%) | 44 (14.3%) | 0.865 |
Abbreviations: AD, Alzheimer’s disease; ADNI, Alzheimer’s Disease Neuroimaging Initiative; MCI, mild cognitive impairment.
Association of omega-3 fatty acid supplementation use with AD risk
In the unadjusted analysis (Model 1), omega-3 fatty acid supplementation was significantly associated with a lower risk of AD (HR: 0.73, 95% CI: 0.55, 0.97; P = 0.029) compared with that of omega-3 fatty acid nonusers. Moreover, long-term users had a 64% lower AD risk (HR: 0.36, 95% CI: 0.18, 0.72; P = 0.004) compared with nonusers. The results were similar in Models 2 and 3, which included more confounders (Table 2). Separate analyses by subgroup indicated that compared with no consumption, long-term use of omega-3 dietary supplements was significantly associated with risk of incident AD for males, those of advanced age, APOE ε4 carriers, and patients with mild cognitive impairment (MCI) (Table 3). Similar results were obtained in the sensitivity analyses after removing cases diagnosed in the first year (Supplementary Table 5).
TABLE 2.
Cox proportional hazards regression analysis of omega-3 supplementation use and its blood biomarkers for AD risk in ADNI cohort
| AD cases/total | Model 1 |
P |
Model 2 |
P |
Model 3 |
P |
|
|---|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |||||
| Omega-3 supplementation | |||||||
| non-exposure | 248/863 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| exposure | 59/272 | 0.73 (0.55-0.97) | 0.029 | 0.71 (0.53-0.94) | 0.018 | 0.72 (0.54-0.97) | 0.031 |
| medium-term exposure | 51/204 | 0.87 (0.64-1.18) | 0.370 | 0.84 (0.62-1.14) | 0.262 | 0.85 (0.62-1.16) | 0.304 |
| long-term exposure | 8/68 | 0.36 (0.18-0.72) | 0.004 | 0.35 (0.17-0.71) | 0.004 | 0.37 (0.18-0.75) | 0.006 |
| Blood markers | |||||||
| omega-3 | 287/867 | 0.91 (0.52-1.61) | 0.752 | 1.15 (0.65-2.03) | 0.640 | 1.28 (0.71-2.30) | 0.407 |
| DHA | 287/867 | 0.86 (0.57-1.30) | 0.485 | 1.05 (0.70-1.57) | 0.832 | 1.13 (0.74-1.71) | 0.571 |
| ALA | 287/867 | 1.08 (0.64-1.83) | 0.781 | 1.23 (0.74-2.05) | 0.428 | 1.27 (0.75-2.17) | 0.374 |
Model 1: crude HR with no covariates adjusted;
Model 2: HR adjusted for age, sex, education, clinical diagnosis, and APOE ε4;
Model 3: HR adjusted for Model 2 plus insomnia, depression, anxiety, hypertension, diabetes mellitus, hyperlipidemia, smoking, BMI, stroke, and coronary artery disease, alcohol intake, multivitamins, vitamin B12, folate, anti-hypertensive drugs and antidiabetic drugs.
TABLE 3.
Hazard ratios with corresponding 95% CIs of the association of omega-3 supplementation use and its blood biomarkers with risk of AD according to strata of age, sex, APOE ε4 status and cognitive status in the ADNI cohort
| Omega-3 supplementation |
Blood markers |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-exposure | Exposure | Medium-term exposure | Long-term exposure | Omega-3 | DHA | ALA | ||
| Sex | Male | 1 (reference) | 0.80 (0.56-1.15) | 0.10 (0.68-1.46) | 0.33 (0.13-0.82) | 1.04 (0.50-2.18) | 1.02 (0.61-1.72) | 1.11 (0.57-2.17) |
| Female | 1 (reference) | 0.62 (0.38-1.02) | 0.65 (0.38-1.11) | 0.50 (0.16-1.61) | 1.84 (0.67-5.04) | 1.40 (0.67-2.92) | 1.85 (0.75-4.61) | |
| Age | Midlife <65 y | 1 (reference) | 0.87 (0.33-2.35) | 0.94 (0.35-2.53) | 0.78 (0.00-∞) | 0.91 (0.12-6.70) | 0.79 (0.18-3.44) | 4.09(0.76-22.00) |
| Late life ≥65 y | 1 (reference) | 0.69 (0.47-0.88) | 0.75 (0.54-1.05) | 0.35 (0.17-0.73) | 1.29 (0.71-2.37) | 1.15 (0.75-1.76) | 1.18 (0.68-2.06) | |
| APOE ε4 | APOE ε4 (+) | 1 (reference) | 0.75 (0.52-1.08) | 0.90 (0.61-1.32) | 0.29 (0.11-0.83) | 2.03 (0.98-4.23) | 1.60 (0.94-2.71) | 1.11 (0.58-2.14) |
| APOE ε4 (-) | 1 (reference) | 0.71 (0.44-1.14) | 0.81 (0.48-1.35) | 0.45 (0.16-1.25) | 0.49 (0.17-1.38) | 0.57 (0.28-1.18) | 1.72 (0.65-4.57) | |
| Cognitive status | CN | 1 (reference) | 0.27 (0.03-2.36) | 0.44 (0.05-3.84) | 0.45 (0.00-∞) | 0.10 (0.00-2.42) | 0.20 (0.02-2.13) | 0.44 (0.03-6.94) |
| MCI | 1 (reference) | 0.75 (0.56-1.00) | 0.87 (0.63-1.18) | 0.40 (0.20-0.82) | 1.36 (0.75-2.48) | 1.17 (0.77-1.80) | 1.41 (0.82-2.43) | |
A separate Cox regression model was conducted for each stratum of the covariate
Abbreviations: CN, cognitively normal; MCI, mild cognitive impairment.
Association of blood omega-3 fatty acid and its components with AD risk
Plasma omega-3 fatty acid and its components were not significantly associated with AD risk (Table 2). Similar results were obtained when performing sensitivity analyses after removing cases diagnosed in the first year. (Supplementary Table 5).
Meta-analysis
Searching results and characteristics of studies
We initially identified 18,230 articles after de-duplication. After scanning the titles and abstracts, 709 articles were considered potentially eligible. Following detailed assessments, 48 longitudinal studies met the inclusion criteria, of which 31 were included in the meta-analysis, with a total of 103,651 participants (Figure 1B). The detailed characteristics of the studies included in this meta-analysis are presented in Table 4. Most studies reported omega-3 fatty acid dietary intake (n = 31), followed by plasma (n = 14), and erythrocyte membrane (n = 6).
TABLE 4.
Characteristics of 48 studies included in the systematic review and meta-analysis
| N | First author, y | Design, Cohort name, Country | Follow-up duration (mean) | Age at baseline (mean) | Female (%) | Participants | Cases | Outcome | Categories | Exposure measurement | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Li 2021 | RC; ADNI; Canada, Unites States | 2.8y | 73y | 46% | 11351/867 2 | 3071/2872 | AD | 1 omega-3 2 omega-3, DHA, ALA | FFQ | 6 |
| 2 | Melo van Lent 2021 [27] | PC; AgeCoDe; Germany | 7y | 84y | 64% | 1264 | 233 | AD | 2 omega-3; DHA; EPA; ALA | Blood sample | 7 |
| 3 | Koch 2021 [28] | PNCC; GEMS; Unites States | 4.8y | 78y | 47% | 1252 | 498/334 | Dementia; AD | 2 omega-3; DHA; EPA; ALA | Blood sample | 6.5 |
| 4 | Nozaki 2021 [29] | PC; JPHC; Japan | 15y | 73y | 69% | 1127 | 380/54 | Dementia; MCI | 1 PUFA; omega-3; EPA; DHA | FFQ | 5.5 |
| 5 | Thomas 2020 [30] | PC; 3C; France | 174y | 74y | NA | 1279 | 271 | Dementia | 2 EPA; DHA | Blood sample | 7.5 |
| 6 | Jiang et al. [31] 2020 | PC; SCHS; Singapore | 20y | 45-74y | NA | 16736 | 2397 | CD | 1 PUFA; omega-3; ALA | FFQ | 8 |
| 7 | Gustafson et al. [32] 2020 | PC; WHICAP; Unites States | 4.9y | 76y | 67% | 2612 | 380 | AD | 1 PUFA; omega-3; DHA; EPA | FFQ | 7 |
| 8 | Mao 2019 [33] | PC; CARDIA; Unites States | 25y | 25y | 56% | 3231 | NA | CD | 1 omega-3; DHA; EPA | CARDI questionnaire | 7.5 |
| 9 | Bigornia 2018 [34] | PC; BPRHS; Unites States | 2y | 45-75y | 73% | 1032 | 151 | CD | 1 and 3 ALA; EPA; DHA | FFQ and blood sample | 6.5 |
| 10 | Haution-bitker 2018 [35] | PNCC; GERIOX; France | 11.55m | 80y | 65% | 1402/703 | 44 | CD | 2 and 3 omega-3; EPA; DHA; ALA | Blood sample | 3 |
| 11 | Nooyens 2018 [36] | PC; DS; Netherlands | 5y | 43-70y | 51% | 2612 | NA | CD | 1 PUFA; omega-3; DHA; ALA; EPA | FFQ | 7 |
| 12 | Ammann 2017 [37] | PC; WHIMS-ECHO; Unites States | 9.85y | 70y | 100% | 6706 | 587/671 | Dementia; MCI | 3 omega-3; DHA; EPA | Blood sample | 7.5 |
| 13 | Yamagishi 2017 [38] | PNCC; CIRCS; Japan | 12.5y | 64y | 66% | 7586 | 315 | Dementia | 2 DHA; EPA; ALA | Blood sample (serum) | 7.5 |
| 14 | Vanderest 2016 [39] | PC; MAP; Unites States | 4.9y | 81y | 75% | 915 | NA | CD | 1 omega3; ALA | FFQ | 7 |
| 15 | Otsuka 2014 [40] | PC; NILS-LSA; Japan | 10.2y | 67y | 46% | 430 | 36 | CD | 2 DHA; EPA | Blood sample | 7 |
| 16 | Bowman 2013 [41] | PC; OBAS; Unites States | 3.9y | 86y | 62% | 86 | NA | CD | 2 omega-3 | Blood sample | 6.5 |
| 17 | Titova 2013 [42] | PC; PIVUS; Sweden | 5y | 70y | 48% | 252 | NA | CD | 1 omega-3 | 7-d food protocol | 6.5 |
| 18 | Ammann 2013 [43] | PC; WHISCA; Unites States | 5.95y | 73y | 100% | 2157 | NA | CD | 3 omega-3 | Blood sample | 6 |
| 19 | Okereke 2012 [44] | PC; WHS; Unites States | 44y | 66y | 100% | 6183 | NA | CD | 1 PUFA | FFQ | 5 |
| 20 | Ronnemaa 2012 [45] | PC; ULSAM; Sweden | 354y | 50y | 0% | 2009 | 213/91 | Dementia; AD | 2 DHA; EPA; ALA | Blood sample | 6.5 |
| 21 | Lopez 2011 [46] | PC; RBS; Unites States | 3y | 80y | 44% | 2421/2662 | 42/30 | Dementia; AD | 1 and 2 DHA | FFQ | 6 |
| 22 | Kesse-guyot 2011 [47] | PC; SUVIMAX 2; France | 13y | 51y | 46% | 3294 | NA | CD | 1 omega-3; DHA; EPA | Repeated 24-h dietary records | 6 |
| 23 | Gao 2011 [48] | PC; SLAS; Singapore | 1.55y | 66y | 66% | 1475 | NA | CD | 1 omega-3 | A self-reported single question | 5.5 |
| 24 | Samieri 2011 [49] | PC; 3C; France | 74y | 74y | 61% | 1228 | NA | CD | 2 DHA; EPA | Blood sample | 7.5 |
| 25 | Vercambre 2010 [50] | PC; WACS; Unites States | 5.44y | 72y | 100% | 2551 | NA | CD | 1 PUFA | Willett FFQ | 5 |
| 26 | Vercambre 2009 [51] | PC; E3N; France | 134y | 78y | 100% | 4809 | 598 | CD | 1 PUFA; omega-3; ALA | FFQ | 5.5 |
| 27 | Devore 2009 [52] | PC; RS; Netherlands | 9.6y | 68y | 59% | 5395 | 465 | Dementia; | 1 omega-3; DHA; EPA | SFFQ | 7.5 |
| 28 | Devore 2009(2) [53] | PC; NHS; Unites States | 4.2y | 74y | 100% | 1486 | NA | CD | 1 PUFA | Willett FFQ | 6 |
| 29 | Kroger 2009 [54] | PC; CSHA; Canada | 4.95y | 81y | 66% | 663 | 149/105 | Dementia; AD | 3 omega-3; DHA; EPA | Blood sample | 7 |
| 30 | Vanderest 2009 [55] | PC; NAS; Unites States | 6y | 68y | 0% | 1025 | NA | CD | 1 omega-3; | Willett FFQ | 4 |
| 31 | Samieri 2008 [56] | PC; 3C; France | 4y4 | 74y | 62% | 1214 | 65 | Dementia | 2 PUFA; omega-3; DHA; EPA;ALA | Blood sample | 6.5 |
| 32 | Eskelinen 2008 [57] | PC; CAIDE; Finland | 21y | 50y | 62% | 1449 | 82 | MCI | 1 PUFA | FFQ | 6.5 |
| 33 | Velho 2008 [58] | PC; NA; Portugal | 8.5m | 70y | 71% | 187 | NA | CD | 1 omega-3 | 3- d food dietary record | 5 |
| 34 | Whalley 2008 [59] | PC; SCRE; Scotland, United Kingdom | 44y | 64y | 60% | 120 | NA | CD | 3 omega-3; DHA; EPA | Blood sample | 5 |
| 35 | Barberger-Gateau 2007 [60] | PC; 3C; France | 3.5y | ≥65y | NA | 8085 | 281/183 | Dementia; AD | 1 omega-3 | FFQ | 7 |
| 36 | Beydoun et al. [14] 2007 | PC; ARIC; Unites States | 6y | 50-65y | 55% | 78141/22512 | 486/140 | CD | 1,2 omega-3; ALA | Blood sample | 5.5 |
| 37 | Vangelder 2007 [61] | PC; ZES ;Netherlands | 5y | 70-89y | 51% | 210 | NA | CD | 1 omega-3 | Cross-check dietary history method | 6 |
| 38 | Dullemeijer 2007 [62] | PC;FACIT; Netherlands | 3y | 50-70y | 0% | 404 | NA | CD | 2 PUFA; omega-3; DHA; EPA; ALA | Blood sample | 6.5 |
| 39 | Solfrizzi 2006(2) [63] | PC; ILSA; Italy | 2.65y | 65-84y | 28% | 278 | 18 | MCI | 1 PUFA | FFQ | 5.5 |
| 40 | Schaefer 2006 [64] | PC; FHS; Unites States | 9.1y | 76y | 45% | 4881/8992 | 79 | AD | 1 and 2 DHA | FFQ | 7 |
| 41 | Laitinen 2006 [65] | PC; CAIDE; Finland | 21y | 50y | 64% | 1341 | 117/76 | Dementia; AD | 1 PUFA | FFQ | 8.5 |
| 42 | Solfrizzi 2006 [66] | PC; ILSA; Italy | 8.55y | 73y | 62% | 278 | NA | CD | 1PUFA | FFQ | 7 |
| 43 | Morris 2005 [67] | PC; CHAP; Unites States | 64y | 74y | 45% | 3718 | NA | CD | 1 omega-3; DHA; EPA; ALA | FFQ | 6.5 |
| 44 | Morris 2003 [68] | PC; CHAP; Unites States | 3.9y | 65-94y | 62% | 815 | 131 | AD | 1 omega-3; DHA; EPA; ALA | FFQ | 6 |
| 45 | Heude 2003 [69] | PC; EVA; France | 4y | 63-74y | 61% | 246 | 27 | CD | 3 PUFA; omega-3; DHA; EPA | Blood sample | 5 |
| 46 | Laurin 2003 [70] | PC; CSHA; Canada | 5y | 77y | 58% | 174 | 11 | Dementia | 2 PUFA; omega-3; DHA; EPA | Blood sample | 7.5 |
| 47 | Engelhart 2002 [71] | PC; RS;Netherlands | 6.0y | 68y | 66% | 5395 | 197/146 /29 | Dementia; AD; VD | 1 PUFA; omega-3 | FFQ | 6.5 |
| 48 | Kalmijn 1997 [72] | PC; ZES; Netherlands | 3y | 69-89y | 59% | 342 | 51 | CD | 1PUFA; omega-3; DHA; EPA | Cross-check dietary history method | 4.5 |
Abbreviations: AD, Alzheimer’s disease; ADNI, Alzheimer’s Disease Neuroimaging Initiative; AgeCoDe, German Study on Aging, Cognition, and Dementia; BPRHS, Boston Puerto Rican Health Study; CAIDE, Cardiovascular risk factors, Aging and Dementia study; CARDIA, Coronary Artery Risk Development in Young Adults; CD, cognitive decline; CIRCS, Circulatory Risk in Communities Study; CHAP, Chicago Health and Aging Project; CHCS, Cardiovascular Health Cognition Study; CHNS, China Health and Nutrition Survey; CLHLS, Chinese Longitudinal Health Longevity Study; CSHA, Canadian Study of Health and Aging; DS, Doetinchem Study; EVA, Etude du Vieillissement Artériel Study; E3N, Etude Epideámiologique de Femmes de la Mutuelle Geáneárale de l’Education Nationale study; FACIT, Folic Acid and Carotid Intima-media Thickness Trial; FHS, Framingham Heart Study; GEMS, Ginkgo Evaluation of Memory Study; ILSA, Italian Longitudinal Study on Aging; JPHC, Japan Public Health Center-based Prospective Study; MAP, Rush Memory and Aging Project; MCI, mild cognitive impairment; NA, not available; NAS, Normative Aging Study; NILS-LSA, National Institute for Longevity Sciences-Longitudinal Study for Aging; NHS, Nurses’ Health Study; OBAS, Oregon Brain Aging Study; Omega-3, omega-3 polyunsaturated fatty acid; PC, prospective cohort; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors Cohort; PNCC, Prospective nested case-control study; PUFA, polyunsaturated fatty acid; RBS, Rancho Bernardo Study; RC, retrospective cohort; RS, Rotterdam Study; SCHS, Singapore Chinese Health Study; SCRE, Scottish Council for Research in Education; SLAS, Singapore Longitudinal Aging Study; SUVIMAX, Supplementation with Antioxidant Vitamins and Minerals; ULSAM, Uppsala Longitudinal Study of Adult Men cohort; VD, vascular dementia; WACS, Women’s Antioxidant Cardiovascular Study; WHICAP, Washington Heights-Hamilton Heights-Inwood Columbia Aging Project; WHISCA, Women's Health Initiative Study of Cognitive Aging; WHIMS-ECHO, Woman's Health Initiative Memory Study - Epidemiology of Cognitive Health Outcomes; WHS, Women's Health Study; ZES, Zutphen Elderly Study; 3C, Three-City Study.
dietary
plasma/serum
erythrocyte
max age
median age
Association of dietary intake of omega-3 fatty acid and its components with cognitive decline
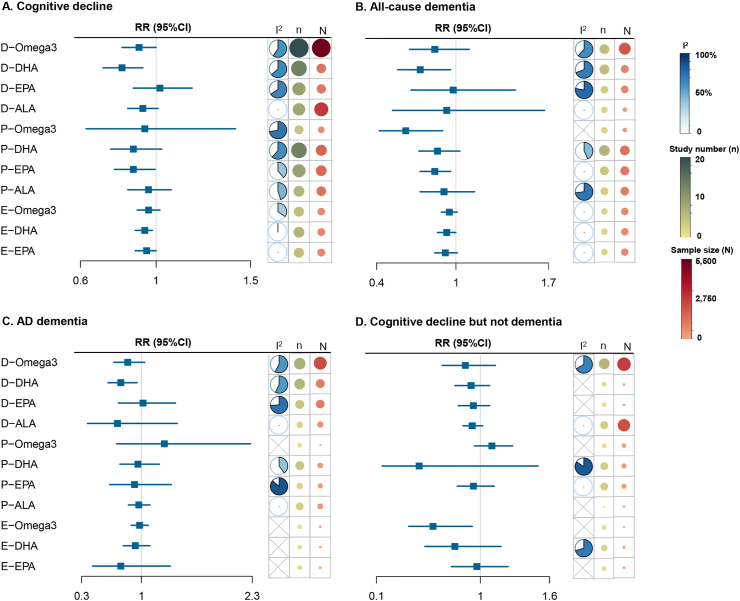
A meta-analysis of 18 studies with 46,548 participants revealed a significant protective effect of dietary omega-3 fatty acid intake on lower risk of cognitive decline (RR: 0.91, 95% CI: 0.82, 1.00; I2 = 60.0%, Level M) (Figure 2). Meta-regression analysis revealed that no factor could explain the source of heterogeneity. The significantly protective effect was observed in the subgroup adjusting for APOE ε4 status (n = 8, RR: 0.83, 95% CI: 0.71, 0.97; I2 = 65.0%) (Supplementary Table 6). In addition, the dose–response analysis (27,161 participants and 3797 cases) revealed that risk of cognitive decline was reduced when the intake of omega-3 fatty acids exceeded 1.0 g/d. However, the decrease in risk was not significantly linear with the increase in dietary intake (Figure 3A).
FIGURE 2.
Association of omega-3 and its peripheral biomarkers with risk of cognitive decline. Squares represent overall estimate effects and solid lines represent 95% CIs (Supplementary Table 6). The blue fan represents proportion of heterogeneity among studies. Green/red dots represent the number of studies/participants included, respectively. D-omega-3, dietary intake of omega-3 fatty acid; E-omega-3, erythrocyte omega-3 fatty acid; P-omega-3, plasma omega-3 fatty acid.
FIGURE 3.
Dose–response relationships between dietary omega-3 and cognitive decline. The dose–response analyses revealed significantly linear associations between dietary intake of DHA (B) or EPA (C) and risk of cognitive decline. An increment of 0.1 g/d of DHA or EPA intake was associated with an 8.0% (Plinear = 0.0005) or 9.9% (Plinear = 0.0004) lower risk of cognitive decline, respectively. The dose–response analyses revealed a nonsignificant relationship between dietary intake of omega-3 (A), ALA (D) and risk of cognitive decline. AD, Alzheimer’s disease; RR, relative risk.
Regarding its components, the pooled estimate for dietary DHA from 13 studies was 0.82 (95% CI: 0.72, 0.93; I2 = 63.6%, Level H) (Figure 2). Dietary intake of DHA was associated with a 27% decreased risk of dementia and a 24% decreased risk of AD, whereas dietary intake of ALA (Level H) and EPA (Level L) did not have a significant protective effect on cognitive decline (Supplementary Figures 1–3). The dose–response analysis revealed that an increment of 0.1 g/d of DHA or EPA intake was associated with an 8.0% (Plinear = 0.0005) or 9.9% (Plinear = 0.0004) lower risk of cognitive decline, respectively (Figure 3B, C). No publication bias was identified.
Association of plasma omega-3 fatty acid and its components with cognitive decline
No significant association was found between higher levels of plasma DHA and a lower risk of cognitive decline (RR: 0.88, 95% CI: 0.76, 1.03; I2 = 63.6%, Level L), with publication bias (Egger’s P = 0.007, corrected RR: 0.99, 95% CI: 0.85, 1.14; I2 = 69%) (Figure 2). Meta-regression analysis indicated that the mean age could partly account for the heterogeneity (P = 0.02, tau2 = 0.002). Risk of cognitive decline in older participants (with an average baseline age ≥ 65 y) was significantly reduced by 23%, whereas in younger participants, no significant association was indicated for plasma levels of DHA (Supplementary Table 6). Higher levels of plasma EPA were significantly associated with lower risk of cognitive decline (RR: 0.88, 95% CI: 0.78, 0.995; I2 = 38.1%, Level M), and dementia (RR: 0.84, 95% CI: 0.73, 0.96; I2 = 0.0%, Level M). Plasma levels of omega-3 fatty acid (Level L) and ALA (Level L) did not have a significant protective effect on cognitive decline (Figure 2 and Supplementary Figures 4 and 5).
Association of erythrocyte membrane omega-3 fatty acid and its components with cognitive decline
Five longitudinal studies involving 14,940 participants explored the association between omega-3 fatty acid levels in erythrocyte membranes and risk of cognitive decline. No significant protective effect was revealed (RR: 0.96, 95% CI: 0.90, 1.02; I2 = 34.5%, Level M). However, higher levels of erythrocyte membrane DHA (RR: 0.94, 95% CI: 0.89, 0.98; I2 = 0.4%, Level H) and EPA (RR: 0.95, 95% CI: 0.89, 1.00; I2 = 0%, Level H) were associated with a lower risk of cognitive decline (Figure 2 and Supplementary Figures 6 and 7).
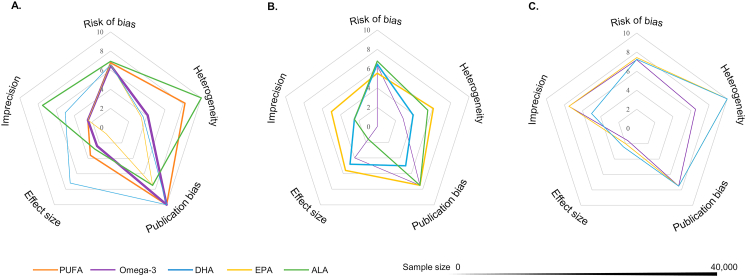
Summary of evidence credibility
The larger area of the pentagonal graph represents better evidence credibility, whereas Figures 4A–C represent omega-3 fatty acids in 3 states: dietary, plasma, and erythrocytes. The evidence credibility for dietary intake and erythrocytes was higher than that of plasma (Figure 4). A higher Index Sdifference indicates a stronger degree of consistency between the results of meta-analyses and the results of each individual cohort study included. Thus, the consistency of the results related to erythrocytes was greater than that of dietary intake and plasma. Overall, the evidence credibility, ranked from highest to lowest, was as follows: erythrocytes, diet, and plasma (Supplementary Table 4).
FIGURE 4.
Evidence rating results. The credibility of each meta-analysis result was categorized into 3 levels: ‘High (H)’, ‘Moderate (M)’, and ‘Low (L)’ by summing the scores of 5 domains: risk of bias, heterogeneity, publication bias, effect size, and imprecision. Scores in each domain ranged from 0 to 10, and a score of 50 represents the highest level of evidence. (A) dietary; (B) plasma; (C) erythrocyte.
Discussion
In the present study, we found that supplemental omega-3 fatty acid use was significantly associated with a lower risk of AD, with a potential moderating effect by APOE ε4. Our meta-analysis findings strengthened the possible association between dietary omega-3 fatty acid intake and its peripheral biomarkers with AD, dementia, or cognitive decline. Compared with previous studies [[73], [74], [75]] (Supplementary Table 7), we included 27 new cohorts and provided the most comprehensive evidence of the relationship of omega-3 fatty acids and dementia.
APOE ε4 status may influence the association between omega-3 fatty acids and AD. APOE ε4 is the strongest genetic risk factor for AD [76]. The ADNI cohort revealed an inverse association between omega-3 fatty acid dietary supplements and AD only among APOE ε4 carriers, which is consistent with our previous finding [12]. Consistently, several studies concluded that omega-3 fatty acid supplementation could attenuate the effect of APOE ε4 on AD pathologic changes and that such protective effects existed in APOE ε4 carriers only [11,77]. However, plasma DHA levels showed few changes after omega-3 fatty acid supplementation among APOE ε4 carriers [78,79]. It has also been proposed that APOE ε4 carriers are more likely to exhibit blood–brain barrier dysfunction, which can impair DHA delivery to the brain. Thus, it is questionable whether APOE ε4 carriers can gain benefits from supplementing with omega-3 fatty acids. A compensatory mechanism hypothesis might help explain the dispute, such that DHA utilization and metabolic demands are increased in APOE ε4 carriers. Consistent with the hypothesis, a positron emission tomography imaging study discovered a higher increase in DHA incorporation in several brain regions in APOE ε4 carriers as compared to noncarriers [80]. Future studies should focus on populations with high genetic susceptibility (e.g., APOE ε4 genotype) to test the efficacy of omega-3 fatty acid supplementation.
Our findings support the hypothesis that the efficacy of omega-3 fatty acid supplementation is dose-dependent. We found that omega-3 fatty acid supplementation was significantly associated with a decreased risk of AD, particularly among long-term users. Regular intake of omega-3 fatty acids may help maintain stable blood concentrations, which can benefit the prevention of dementia. Although we did not observe a significant linear relationship between dietary omega-3 fatty acid intake and risk of cognitive decline, risk of cognitive decline decreased when the intake of omega-3 fatty acids exceeded 1.0 g/d. For this reason, we propose that 1.0 g/d may be the threshold dosage of omega-3 fatty acids for the prevention of cognitive decline. Appropriate dosing of omega-3 fatty acids is beneficial not only for preventative purposes in individuals with healthy cognition but also for the treatment of patients with cognitive impairment. Previous randomized controlled trials discussed the appropriate dosage of omega-3 fatty acids and found that high doses (0.9–1.8 g/d) of DHA/EPA intake improved cognitive function [[81], [82], [83]], whereas low doses (0.3–0.7 g/d) failed to exert benefits [84,85]. Since the optimal doses for the prevention and treatment are not well established, future studies should explore the efficacious dose range. Furthermore, the recommended dose may differ for specific populations, such as APOE ε4 carriers.
This evidence-based summary revealed the significant role of erythrocyte DHA in predicting risk of cognitive decline and a suggestive role of plasma and erythrocyte EPA (high-level evidence). Several mechanisms may explain the importance of omega-3 fatty acid biomarkers in predicting dementia. First, DHA is commonly considered beneficial for maintaining the integrity of brain neurons and expressing neuroprotection by inhibiting tau phosphorylation. Second, DHA could target AD-specific pathology pathways adversely affected in APOE ε4 carriers, including microglia and inflammatory pathways, astrocytes and lipid metabolism, and pericytes and blood–brain barrier integrity [86]. Third, although EPA is rarely found in the brain, it is important to balance inflammation and immune function associated with AD pathogenesis. Based on our moderate-to-high evidence levels, it is reasonable to conclude that blood tests for EPA and DHA concentration may be useful for AD risk evaluation. Specifically, erythrocyte membrane concentration is a relatively more stable indicator for clinical research. Erythrocyte concentrations may reflect with higher precision dietary intake over a longer term (estimated range of the past 60 to 90 d) than plasma concentrations (estimated range of the past 7 to 14 d). Future studies should further investigate the different biological effects of EPA and DHA so that the assessment of their concentrations via regular blood tests in high-risk populations may be useful for early risk detection and reduction of AD.
Future cohort studies should be refined as follows: 1) baseline measures for omega-3 fatty acids should include the dosage of daily intake and more detailed supplement information, 2) baseline evaluation of population characteristics should include certain genetic factors (e.g., APOE ε4 genotype), and cognitive level (e.g., cognitively intact/nondemented/MCI and preclinical dementia) should be thoroughly screened using objective rating scales rather than self-reported questionnaire; 3) special attention should be paid to the interaction of omega-3 fatty acids with other kinds of fatty acids because balanced blood levels of omega-3 fatty acids and other fatty acids are necessary for favorable cognitive outcomes. Some implications for practical use also apply as follows: 1) maintaining an adequate intake of omega-3 fatty acids provides a tool for the potential prevention of dementia, especially in APOE ε4 carriers; 2) particular attention should be paid to the regular examination of erythrocyte DHA in populations who are at increased risk of dementia, either based on the APOE ε4 genotype or other risk factors.
However, potential limitations of this study must be considered. First, although we adjusted for several relevant potential confounders, residual confounding from unmeasured confounders (such as dietary intake and physical activity) remains an issue. Second, the total duration of supplementation but not the accurate dose was used in the ADNI analyses, which might introduce certain risk of measurement bias. According to previous publications, the omega-3 supplement dose varied slightly from 0.6 to 1.3 g/d for the general population [31,32]. Third, all cohorts assessed omega-3 fatty acid exposure only at baseline, which may have resulted in misclassifications as the actual intake amount may change over time. Fourth, most cohort studies included in the meta-analysis assessed omega-3 fatty acid intake using FFQ, which are designed to measure the frequency of intake rather than exposure time, which was not verified in the meta-analysis. Fifth, due to too little data in the included studies, the associations between dementia subtypes and the role of omega-3 fatty acids in different APOE ε4 groups have not been thoroughly explored.
In conclusion, our findings suggest that 1) long-term omega-3 fatty acid supplementation may reduce risk of AD; 2) dietary omega-3 fatty acid intake, especially DHA, may lower risk of dementia or cognitive decline; and 3) peripheral biomarkers of omega-3 fatty acids may serve as predictors of cognitive decline. However, further investigation is needed to understand the gene–environment interactions involved in the intake of omega-3 fatty acids.
Funding
This study was supported by grants from the National Natural Science Foundation of China (82001136) and the Tai-Shan Scholar Project.
Acknowledgments
The authors’ responsibilities were as follows – WX: conceptualization and design of the study, revision of the manuscript; B-ZW: analysis of published longitudinal data, drafting and revision of the manuscript, and figure preparation; LL: analysis of ADNI cohort data, drafting and revision of the manuscript, and figure preparation; C-WD and C-CT: revision of the manuscript; and all authors: read and approved the final manuscript. The authors thank contributors, including the staff at Alzheimer’s Disease Centers who collected samples used in this study, patients, and their families whose help and participation made this work possible. Data collection and sharing for this project were funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research provides funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author disclosures
The authors report no conflicts of interest.
Data availability
All data are available upon reasonable request or can be obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu).
Footnotes
The data used in preparation for this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-ontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.04.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. doi: 10.1016/s2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cholewski M., Tomczykowa M., Tomczyk M. A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients. 2018;10(11):1662. doi: 10.3390/nu10111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geleijnse J.M., de Goede J., Brouwer I.A. Alpha-linolenic acid: is it essential to cardiovascular health? Curr. Atheroscler. Rep. 2010;12(6):359–367. doi: 10.1007/s11883-010-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boudrault C., Bazinet R.P., Ma D.W. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer’s disease. J. Nutr. Biochem. 2009;20(1):1–10. doi: 10.1016/j.jnutbio.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Heras-Sandoval D., Pedraza-Chaverri J., Pérez-Rojas J.M. Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J Neuroinflammation. 2016;13(1):61. doi: 10.1186/s12974-016-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood A.H.R., Chappell H.F., Zulyniak M.A. Dietary and supplemental long-chain omega-3 fatty acids as moderators of cognitive impairment and Alzheimer’s disease. Eur. J. Nutr. 2022;61(2):589–604. doi: 10.1007/s00394-021-02655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sala-Vila A., Satizabal C.L., Tintle N., Melo van Lent D., Vasan R.S., Beiser A.S., et al. Red blood cell DHA is inversely associated with risk of incident Alzheimer’s disease and all-cause dementia: Framingham offspring study. Nutrients. 2022;14(12):2408. doi: 10.3390/nu14122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tully A.M., Roche H.M., Doyle R., Fallon C., Bruce I., Lawlor B., et al. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer’s disease: a case-control study. Br. J. Nutr. 2003;89(4):483–489. doi: 10.1079/bjn2002804. [DOI] [PubMed] [Google Scholar]
- 9.Whalley L.J., Deary I.J., Starr J.M., Wahle K.W., Rance K.A., Bourne V.J., et al. n-3 fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. Am. J. Clin. Nutr. 2008;87(2):449–454. doi: 10.1093/ajcn/87.2.449. [DOI] [PubMed] [Google Scholar]
- 10.Phillips M.A., Childs C.E., Calder P.C., Rogers P.J. No effect of omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable Alzheimer’s disease: a randomised controlled trial. Int. J. Mol. Sci. 2015;16(10):24600–24613. doi: 10.3390/ijms161024600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Rest O., Wang Y., Barnes L.L., Tangney C., Bennett D.A., Morris M.C. APOE ε4 and the associations of seafood and long-chain omega-3 fatty acids with cognitive decline. Neurology. 2016;86(22):2063–2070. doi: 10.1212/wnl.0000000000002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L., Xu W., Tan C.C., Cao X.P., Wei B.Z., Dong C.W., Tan L. A gene-environment interplay between omega-3 supplementation and APOE ε4 provides insights for Alzheimer’s disease precise prevention amongst high-genetic-risk population. Eur. J. Neurol. 2022;29(2):422–431. doi: 10.1111/ene.15160. [DOI] [PubMed] [Google Scholar]
- 13.Jeong W., Lee H., Cho S., Seo J. ApoE4-induced cholesterol dysregulation and its brain cell type-specific implications in the pathogenesis of Alzheimer’s disease. Mol. Cells. 2019;42(11):739–746. doi: 10.14348/molcells.2019.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beydoun M.A., Kaufman J.S., Satia J.A., Rosamond W., Folsom A.R. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr. 2007;85(4):1103–1111. doi: 10.1093/ajcn/85.4.1103. [DOI] [PubMed] [Google Scholar]
- 15.Barberger-Gateau P., Samieri C., Féart C., Plourde M. Dietary omega 3 polyunsaturated fatty acids and Alzheimer's disease: interaction with apolipoprotein E genotype. Curr. Alzheimer Res. 2011;8(5):479–491. doi: 10.2174/156720511796391926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu X., Jaeger M., Beumer J., Bakker O.B., Aguirre-Gamboa R., Oosting M., et al. Integration of metabolomics, genomics, and immune phenotypes reveals the causal roles of metabolites in disease. Genome Biol. 2021;22(1):198. doi: 10.1186/s13059-021-02413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Kim S., Swaminathan S., Shen L., Risacher S.L., Nho K., Foroud T., et al. Genome-wide association study of CSF biomarkers Abeta1-42, t-tau, and p-tau181p in the ADNI cohort. Neurology. 2011;76(1):69–79. doi: 10.1212/WNL.0b013e318204a397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J.T., Xu W., Tan C.C., Andrieu S., Suckling J., Evangelou E., et al. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry. 2020;91(11):1201–1209. doi: 10.1136/jnnp-2019-321913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H., Tan C.C., Tan L., Xu W. Predictors of cognitive deterioration in subjective cognitive decline: evidence from longitudinal studies and implications for SCD-plus criteria. J Neurol Neurosurg Psychiatry. 2023 doi: 10.1136/jnnp-2022-330246. Published online March 3. [DOI] [PubMed] [Google Scholar]
- 22.Orsini N. From floated to conventional confidence intervals for the relative risks based on published dose-response data. Comput Methods Programs Biomed. 2010;98(1):90–93. doi: 10.1016/j.cmpb.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Grant R.L. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014;348:f7450. doi: 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C., Doi S.A.R. The robust error meta-regression method for dose-response meta-analysis. Int J. Evid. Based Healthc. 2018;16(3):138–144. doi: 10.1097/xeb.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 26.Hedges L.V., Tipton E., Johnson M.C. Robust variance estimation in meta-regression with dependent effect size estimates. Res. Synth. Methods. 2010;1(1):39–65. doi: 10.1002/jrsm.5. [DOI] [PubMed] [Google Scholar]
- 27.Melo van Lent D., Egert S., Wolfsgruber S., Kleineidam L., Weinhold L., Wagner-Thelen H., et al. Eicosapentaenoic acid is associated with decreased incidence of alzheimer’s dementia in the oldest old. Nutrients. 2021;13(2) doi: 10.3390/nu13020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch M., Furtado J.D., DeKosky S.T., Fitzpatrick A.L., Lopez O.L., Kuller L.H., Mukamal K.J., Jensen M.K. Case-cohort study of plasma phospholipid fatty acid profiles, cognitive function, and risk of dementia: a secondary analysis in the Ginkgo Evaluation of Memory Study. Am J Clin Nutr. 2021;114(1):154–162. doi: 10.1093/ajcn/nqab087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nozaki S., Sawada N., Matsuoka Y.J., Shikimoto R., Mimura M., Tsugane S. Association between dietary fish and PUFA intake in midlife and dementia in later life: the JPHC saku mental health study. J Alzheimers Dis. 2021;79(3):1091–1104. doi: 10.3233/jad-191313. [DOI] [PubMed] [Google Scholar]
- 30.Thomas A., Baillet M., Proust-Lima C., Féart C., Foubert-Samier A., Helmer C., Catheline G., Samieri C. Blood polyunsaturated omega-3 fatty acids, brain atrophy, cognitive decline, and dementia risk. Alzheimers Dement. 2020 doi: 10.1002/alz.12195. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y.W., Sheng L.T., Pan X.F., Feng L., Yuan J.M., Pan A., et al. Midlife dietary intakes of monounsaturated acids, n-6 polyunsaturated acids, and plant-based fat are inversely associated with risk of cognitive impairment in older Singapore Chinese adults. J. Nutr. 2020;150(4):901–909. doi: 10.1093/jn/nxz325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustafson D.R., Bäckman K., Scarmeas N., Stern Y., Manly J.J., Mayeux R., et al. Dietary fatty acids and risk of Alzheimer’s disease and related dementias: observations from the Washington Heights-Hamilton Heights-Inwood Columbia Aging Project (WHICAP) Alzheimers Dement. 2020;16(12):1638–1649. doi: 10.1002/alz.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao X., Chen C., Xun P., Daviglus M., Steffen L.M., Jacobs D.R., Jr., Van Horn L., Sidney S., Zhu N., He K. Effects of seafood consumption and toenail mercury and selenium levels on cognitive function among American adults: 25 y of follow up. Nutrition. 2019;61:77–83. doi: 10.1016/j.nut.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bigornia S.J., Scott T.M., Harris W.S., Tucker K.L. Prospective associations of erythrocyte composition and dietary intake of n-3 and n-6 PUFA with measures of cognitive function. Nutrients. 2018;10(9) doi: 10.3390/nu10091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haution-Bitker M., Gilbert T., Vignoles A., Lecardonnel C., Watelet S., Blond E., Drai J., Bonnefoy M. Associations between plasmatic polyunsaturated fatty acids concentrations and cognitive status and decline in neurocognitive disorders. J. Nutr. Health Aging. 2018;22(6):718–725. doi: 10.1007/s12603-018-1010-z. [DOI] [PubMed] [Google Scholar]
- 36.Nooyens A.C.J., van Gelder B.M., Bueno-de-Mesquita H.B., van Boxtel M.P.J., Verschuren W.M.M. Fish consumption, intake of fats and cognitive decline at middle and older age: the Doetinchem Cohort Study. Eur. J. Nutr. 2018;57(4):1667–1675. doi: 10.1007/s00394-017-1453-8. [DOI] [PubMed] [Google Scholar]
- 37.Ammann E.M., Pottala J.V., Robinson J.G., Espeland M.A., Harris W.S. Erythrocyte omega-3 fatty acids are inversely associated with incident dementia: secondary analyses of longitudinal data from the Women’s Health Initiative Memory Study (WHIMS) Prostaglandins Leukot Essent Fatty Acids. 2017;121:68–75. doi: 10.1016/j.plefa.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamagishi K., Ikeda A., Chei C.L., Noda H., Umesawa M., Cui R., et al. Serum α-linolenic and other ω-3 fatty acids, and risk of disabling dementia: community-based nested case-control study. Clin. Nutr. 2017;36(3):793–797. doi: 10.1016/j.clnu.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 39.van de Rest O., Wang Y., Barnes L.L., Tangney C., Bennett D.A., Morris M.C. APOE ε4 and the associations of seafood and long-chain omega-3 fatty acids with cognitive decline. Neurology. 2016;86(22):2063–2070. doi: 10.1212/wnl.0000000000002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otsuka R., Tange C., Nishita Y., Kato Y., Imai T., Ando F., Shimokata H. Serum docosahexaenoic and eicosapentaenoic acid and risk of cognitive decline over 10 years among elderly Japanese. Eur. J. Clin. Nutr. 2014;68(4):503–509. doi: 10.1038/ejcn.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowman G.L., Dodge H.H., Mattek N., Barbey A.K., Silbert L.C., Shinto L., Howieson D.B., Kaye J.A., Quinn J.F. Plasma omega-3 PUFA and white matter mediated executive decline in older adults. Front. Aging Neurosci. 2013;5:92. doi: 10.3389/fnagi.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titova O.E., Sjögren P., Brooks S.J., Kullberg J., Ax E., Kilander L., et al. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordrecht, Netherlands) 2013;35(4):1495–1505. doi: 10.1007/s11357-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ammann E.M., Pottala J.V., Harris W.S., Espeland M.A., Wallace R., Denburg N.L., Carnahan R.M., Robinson J.G. ω-3 fatty acids and domain-specific cognitive aging: secondary analyses of data from WHISCA. Neurology. 2013;81(17):1484–1491. doi: 10.1212/WNL.0b013e3182a9584c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okereke O.I., Rosner B.A., Kim D.H., Kang J.H., Cook N.R., Manson J.E., Buring J.E., Willett W.C., Grodstein F. Dietary fat types and 4-year cognitive change in community-dwelling older women. Ann Neurol. 2012;72(1):124–134. doi: 10.1002/ana.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rönnemaa E., Zethelius B., Vessby B., Lannfelt L., Byberg L., Kilander L. Serum fatty-acid composition and the risk of Alzheimer’s disease: a longitudinal population-based study. Eur. J. Clin. Nutr. 2012;66(8):885–890. doi: 10.1038/ejcn.2012.63. [DOI] [PubMed] [Google Scholar]
- 46.Lopez L.B., Kritz-Silverstein D., Barrett Connor E. High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: the Rancho Bernardo study. J. Nutr. Health Aging. 2011;15(1):25–31. doi: 10.1007/s12603-011-0009-5. [DOI] [PubMed] [Google Scholar]
- 47.Kesse-Guyot E., Péneau S., Ferry M., Jeandel C., Hercberg S., Galan P. Thirteen-year prospective study between fish consumption, long-chain n-3 fatty acids intakes and cognitive function. J. Nutr. Health Aging. 2011;15(2):115–120. doi: 10.1007/s12603-011-0023-7. [DOI] [PubMed] [Google Scholar]
- 48.Gao Q., Niti M., Feng L., Yap K.B., Ng T.P. Omega-3 polyunsaturated fatty acid supplements and cognitive decline: Singapore Longitudinal Aging Studies. J. Nutr. Health Aging. 2011;15(1):32–35. doi: 10.1007/s12603-011-0010-z. [DOI] [PubMed] [Google Scholar]
- 49.Samieri C., Féart C., Proust-Lima C., Peuchant E., Dartigues J.F., Amieva H., Barberger-Gateau P. ω-3 fatty acids and cognitive decline: modulation by ApoEε4 allele and depression. Neurobiol. Aging. 2011;32(12):2317.e13–2317.e22. doi: 10.1016/j.neurobiolaging.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Vercambre M.N., Grodstein F., Kang J.H. Dietary fat intake in relation to cognitive change in high-risk women with cardiovascular disease or vascular factors. Eur. J. Clin. Nutr. 2010;64(10):1134–1140. doi: 10.1038/ejcn.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vercambre M.N., Boutron-Ruault M.C., Ritchie K., Clavel-Chapelon F., Berr C. Long-term association of food and nutrient intakes with cognitive and functional decline: a 13-year follow-up study of elderly French women. Br. J. Nutr. 2009;102(3):419–427. doi: 10.1017/s0007114508201959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devore E.E., Grodstein F., van Rooij F.J., Hofman A., Rosner B., Stampfer M.J., Witteman J.C., Breteler M.M. Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. Am. J. Clin. Nutr. 2009;90(1):170–176. doi: 10.3945/ajcn.2008.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devore E.E., Stampfer M.J., Breteler M.M., Rosner B., Kang J.H., Okereke O., Hu F.B., Grodstein F. Dietary fat intake and cognitive decline in women with type 2 diabetes. Diabetes Care. 2009;32(4):635–640. doi: 10.2337/dc08-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kröger E., Verreault R., Carmichael P.H., Lindsay J., Julien P., Dewailly E., Ayotte P, Laurin D. Omega-3 fatty acids and risk of dementia: the Canadian Study of Health and Aging. Am. J. Clin. Nutr. 2009;90(1):184–192. doi: 10.3945/ajcn.2008.26987. [DOI] [PubMed] [Google Scholar]
- 55.van de Rest O., Spiro A., 3rd, Krall-Kaye E., Geleijnse J.M., de Groot L.C., Tucker K.L. Intakes of (n-3) fatty acids and fatty fish are not associated with cognitive performance and 6-year cognitive change in men participating in the Veterans Affairs Normative Aging Study. J. Nutr. 2009;139(12):2329–2336. doi: 10.3945/jn.109.113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samieri C., Féart C., Letenneur L., Dartigues J.F., Pérès K., Auriacombe S., Peuchant E., Delcourt C., Barberger-Gateau P. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am. J. Clin. Nutr. 2008;88(3):714–721. doi: 10.1093/ajcn/88.3.714. [DOI] [PubMed] [Google Scholar]
- 57.Eskelinen M.H., Ngandu T., Helkala E.L., Tuomilehto J., Nissinen A., Soininen H., Kivipelto M. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int. J. Geriatr. Psychiatry. 2008;23(7):741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 58.Velho S., Marques-Vidal P., Baptista F., Camilo M.E. Dietary intake adequacy and cognitive function in free-living active elderly: a cross-sectional and short-term prospective study. Clin. Nutr. 2008;27(1):77–86. doi: 10.1016/j.clnu.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Whalley L.J., Deary I.J., Starr J.M., Wahle K.W., Rance K.A., Bourne V.J., Fox H.C. n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. Am. J. Clin. Nutr. 2008;87(2):449–454. doi: 10.1093/ajcn/87.2.449. [DOI] [PubMed] [Google Scholar]
- 60.Barberger-Gateau P., Raffaitin C., Letenneur L., Berr C., Tzourio C., Dartigues J.F., Alpérovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69(20):1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 61.van Gelder B.M., Tijhuis M., Kalmijn S., Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am. J. Clin. Nutr. 2007;85(4):1142–1147. doi: 10.1093/ajcn/85.4.1142. [DOI] [PubMed] [Google Scholar]
- 62.Dullemeijer C., Durga J., Brouwer I.A., van de Rest O., Kok F.J., Brummer R.J., van Boxtel M.P., Verhoef P. n 3 fatty acid proportions in plasma and cognitive performance in older adults. Am. J. Clin. Nutr. 2007;86(5):1479–1485. doi: 10.1093/ajcn/86.5.1479. [DOI] [PubMed] [Google Scholar]
- 63.Solfrizzi V., Colacicco A.M., D’Introno A., Capurso C., Del Parigi A., Capurso S.A., Argentieri G., Capurso A., Panza F. Dietary fatty acids intakes and rate of mild cognitive impairment. The Italian Longitudinal Study on Aging. Exp. Gerontol. 2006;41(6):619–627. doi: 10.1016/j.exger.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Schaefer E.J., Bongard V., Beiser A.S., Lamon-Fava S., Robins S.J., Au R., Tucker K.L., Kyle D.J., Wilson P.W., Wolf P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch. Neurol. 2006;63(11):1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 65.Laitinen M.H., Ngandu T., Rovio S, Helkala E.L., Uusitalo U., Viitanen M., Nissinen A., Tuomilehto J., Soininen H., Kivipelto M. Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study. Dement. Geriatr. Cogn. Disord. 2006;22(1):99–107. doi: 10.1159/000093478. [DOI] [PubMed] [Google Scholar]
- 66.Solfrizzi V., Colacicco A.M., D’Introno A., Capurso C., Torres F., Rizzo C., Capurso A., Panza F. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol. Aging. 2006;27(11):1694–1704. doi: 10.1016/j.neurobiolaging.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 67.Morris M.C., Evans D.A., Tangney C.C., Bienias J.L., Wilson R.S. Fish consumption and cognitive decline with age in a large community study. Arch. Neurol. 2005;62(12):1849–1853. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- 68.Morris M.C., Evans D.A., Bienias J.L., Tangney C.C., Bennett D.A., Wilson R.S., Aggarwal N., Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003;60(7):940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 69.Heude B., Ducimetière P., Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes–The EVA Study. Am. J. Clin. Nutr. 2003;77(4):803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 70.Laurin D., Verreault R., Lindsay J., Dewailly E., Holub B.J. Omega-3 fatty acids and risk of cognitive impairment and dementia. J. Alzheimers Dis. 2003;5(4):315–322. doi: 10.3233/jad-2003-5407. [DOI] [PubMed] [Google Scholar]
- 71.Engelhart M.J., Geerlings M.I., Ruitenberg A., Van Swieten J.C., Hofman A., Witteman J.C., Breteler M.M. Diet and risk of dementia: does fat matter?: The Rotterdam Study. Neurology. 2002;59(12):1915–1921. doi: 10.1212/01.wnl.0000038345.77753.46. [DOI] [PubMed] [Google Scholar]
- 72.Kalmijn S., Feskens E.J., Launer L.J., Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am. J. Epidemiol. 1997;145(1):33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- 73.Wu S., Ding Y., Wu F., Li R., Hou J., Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: a meta-analysis. Neurosci. Biobehav. Rev. 2015;48:1–9. doi: 10.1016/j.neubiorev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y., Chen J., Qiu J., Li Y., Wang J., Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016;103(2):330–340. doi: 10.3945/ajcn.115.124081. [DOI] [PubMed] [Google Scholar]
- 75.Kosti R.I., Kasdagli M.I., Kyrozis A., Orsini N., Lagiou P., Taiganidou F., et al. Fish intake, n-3 fatty acid body status, and risk of cognitive decline: a systematic review and a dose-response meta-analysis of observational and experimental studies. Nutr. Rev. 2022;80(6):1445–1458. doi: 10.1093/nutrit/nuab078. [DOI] [PubMed] [Google Scholar]
- 76.Barbash S., Garfinkel B.P., Maoz R., Simchovitz A., Nadorp B., Guffanti A., et al. Alzheimer’s brains show inter-related changes in RNA and lipid metabolism. Neurobiol. Dis. 2017;106:1–13. doi: 10.1016/j.nbd.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yassine H.N., Braskie M.N., Mack W.J., Castor K.J., Fonteh A.N., Schneider L.S., et al. Association of docosahexaenoic acid supplementation with Alzheimer disease stage in apolipoprotein E epsilon4 carriers: a review. JAMA Neurol. 2017;74(3):339–347. doi: 10.1001/jamaneurol.2016.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daiello L.A., Gongvatana A., Dunsiger S., Cohen R.A., Ott B.R. Alzheimer’s Disease Neuroimaging Initiative, Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimers Dement. 2015;11(2):226–235. doi: 10.1016/j.jalz.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quinn J.F., Raman R., Thomas R.G., Yurko-Mauro K., Nelson E.B., Van Dyck C., et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304(17):1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yassine H.N., Croteau E., Rawat V., Hibbeln J.R., Rapoport S.I., Cunnane S.C., et al. DHA brain uptake and APOE4 status: a PET study with [1-11C]-DHA. Alzheimers Res. Ther. 2017;9(1):23. doi: 10.1186/s13195-017-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiu C.C., Su K.P., Cheng T.C., Liu H.C., Chang C.J., Dewey M.E., et al. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(6):1538–1544. doi: 10.1016/j.pnpbp.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 82.Shinto L., Quinn J., Montine T., Dodge H.H., Woodward W., Baldauf-Wagner S., et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J. Alzheimers Dis. 2014;38(1):111–120. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yurko-Mauro K., McCarthy D., Rom D., Nelson E.B., Ryan A.S., Blackwell A., et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6(6):456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 84.Mahmoudi M.J., Hedayat M., Sharifi F., Mirarefin M., Nazari N., Mehrdad N., et al. Effect of low dose ω-3 poly unsaturated fatty acids on cognitive status among older people: a double-blind randomized placebo-controlled study. J. Diabetes Metab. Disord. 2014;13(1):34. doi: 10.1186/2251-6581-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dangour A.D., Allen E., Elbourne D., Fasey N., Fletcher A.E., Hardy P., et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am. J. Clin. Nutr. 2010;91(6):1725–1732. doi: 10.3945/ajcn.2009.29121. [DOI] [PubMed] [Google Scholar]
- 86.Norwitz N.G., Saif N., Ariza I.E., Isaacson R.S. Precision nutrition for Alzheimer’s prevention in ApoE4 carriers. Nutrients. 2021;13(4):1362. doi: 10.3390/nu13041362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon reasonable request or can be obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu).