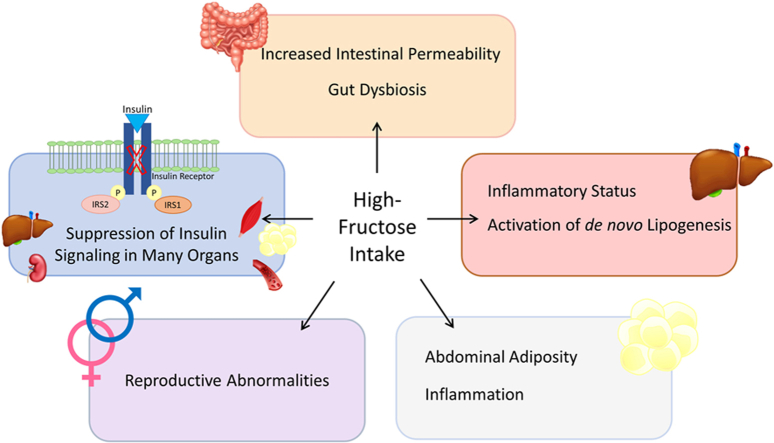
Abstract
The excessive intake of fructose in the regular human diet could be related to global increases in metabolic disorders. Sugar-sweetened soft drinks, mostly consumed by children, adolescents, and young adults, are the main source of added fructose. Dietary high-fructose can increase intestinal permeability and circulatory endotoxin by changing the gut barrier function and microbial composition. Excess fructose transports to the liver and then triggers inflammation as well as de novo lipogenesis leading to hepatic steatosis. Fructose also induces fat deposition in adipose tissue by stimulating the expression of lipogenic genes, thus causing abdominal adiposity. Activation of the inflammatory pathway by fructose in target tissues is thought to contribute to the suppression of the insulin signaling pathway producing systemic insulin resistance. Moreover, there is some evidence that high intake of fructose negatively affects both male and female reproductive systems and may lead to infertility. This review addresses dietary high-fructose-induced deteriorations that are obvious, especially in gut permeability, microbiota, abdominal fat accumulation, insulin signaling, and reproductive function. The recognition of the detrimental effects of fructose and the development of relevant new public health policies are necessary in order to prevent diet-related metabolic disorders.
Keywords: Dietary fructose, Gut permeability and dysbiosis, Abdominal adiposity, Inflammation, Insulin signaling, Reproductive function
Graphical abstract
1. Introduction
Global increases in metabolic disorders could be related to the excessive intake of fructose in the regular human diet. The current human diet contains high calorie mainly from the processed food rich in fat and sugar. Consistent evidence indicates an association between a high intake of processed food and increased cardiometabolic risks in people [1,2]. The experimental studies on high-fat diets have received more scientific attention than high-fructose diets; however, the destructive effect of excess calorie may not primarily depend on nutritional fat in the habitual human diet. In the last decades, the adverse health effect of fructose has been subjected to several scientific review articles [[3], [4], [5], [6]]. Sugar-sweetened soft drinks are the most commonly consumed source of fructose [[7], [8], [9]]. The added fructose in beverages is found as sucrose (glucose + fructose, disaccharide) or High-Fructose Corn Syrup (HFCS; free fructose, and glucose). Fructose consumption was determined to be higher especially in adolescents than in other age groups over the last decades [10,11]. The habitual daily fructose consumption was estimated as about 46 g/day in a study performed on 3817 children and adults from the Dutch National Food Consumption Survey between 2007 and 2010. The total fructose intake was the highest in boys aged 14–18 years as 61 g/day, whereas it was relatively low in the girls aged 9–13 years as 56 g/day. About one-third of the total fructose has been consumed as free fructose mainly from soft drinks [10]. In relation to diseases, epidemiological data indicated that diabetes prevalence is 20% higher in the countries utilizing HFCS as a sweetener than in the low-consuming countries [12]. The total fructose intake (approximately 61 g/day) especially from sweetened beverages was determined to be higher in children (5–9 years old) with Non-Alcoholic Fatty Liver Disease (NAFLD) than the overweight or normal weight participants. Additionally, the increased body weight and waist circumference were also considered in children who consumed a large amount of fructose [13]. In previous studies, higher consumption of soft drinks and fruit juices was also determined in both children and adults with NAFLD [14,15]. Recently, it was reported that the consumption of HFCS- or sucrose-sweetened beverages provided at 25% energy requirement for 16 days in healthy young adults increases hepatic lipid content and postprandial triglyceride level but decreases insulin sensitivity [16]. In a cross-sectional survey on adults (n = 283) living in Lebanon, the mean consumption of total fructose was estimated approximately as 51.4 g/day and a high intake of added fructose was correlated with a high incidence of metabolic syndrome [17]. These data pointed out that fructose is a significant component in the current human diet and its high intake is coincided with an increase in the prevalence of metabolic disorders including NAFLD, which is another worldwide disease although not completely included in this paper. The critical outcomes of high consumption of added sugar particularly fructose deserve special attention for public health. This review addresses crucial findings related to the dysregulatory impact of dietary fructose on gut permeability, microbiota, abdominal fat mass, insulin signaling, and reproductive function with a novel complimentary perspective.
2. Fructose absorption
Fructose is taken into the body as a monosaccharide or disaccharide from sucrose, HFCS, fruits, honey, and some vegetables [18]. When fructose is consumed as sucrose, it must be split into fructose and glucose before being absorbed. Fructose enters enterocytes and then transports to the portal bloodstream via the specific transporters. Absorption and transport of dietary fructose are performed by facilitative Glucose Transporters (GLUTs) located in the enterocyte membranes [19]. Of GLUTs GLUT2, 5, 8, and 12 are involved in fructose transport in the organ level [20]. As shown in Fig. 1, fructose is mainly taken up into the enterocyte by GLUT5 located on the apical side of the membrane and transported to the portal bloodstream by GLUT2 existed on the basolateral side of the enterocyte [21]. GLUT5 is mostly expressed in the small intestine, kidney, brain, fat, testes, muscle, and liver, whereas GLUT2 is presented in the intestine, liver, pancreas, and kidney [22]. GLUT5 transports only fructose, but GLUT2 both glucose and fructose as well as galactose. The intestinal fructose absorption is increased in the coingestion with glucose due to upregulation of GLUT5 [23]. This indicates that fructose can be more absorbed from enterocytes in the consumption of sucrose or HFCS, which contain both fructose and glucose.
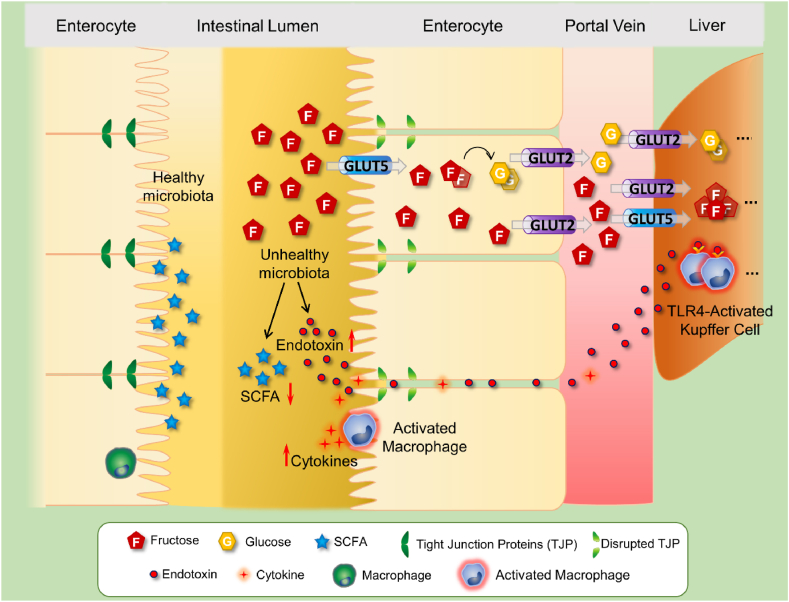
Fig. 1.
The outcomes of high-fructose intake in the intestine
Dietary fructose is transported into the enterocytes via GLUT5. Some part of the intestinal fructose is metabolized to other metabolites such as glucose and organic acids, by ketohexokinase, aldolase-B, and triokinase. Most of the intestinal fructose reaches the liver through the hepatic portal vein by GLUT2-mediated transport, thereby entering carbohydrate metabolism and participating in DNL. On the other hand, the unabsorbed fraction of fructose as well as its metabolites negatively affect the gut microbiota and thus lead to a decrease in SCFA levels and a change in intestinal microbial composition, mainly in the Firmicutes/Bacteriodetes ratio. Excessive fructose intake produces an elevation in the Gr (−) bacteria population, resulting in increased endotoxin levels. High levels of endotoxin cause increased intestinal permeability characterized by impaired gut barrier function and enhanced mucosal inflammation. The endotoxin triggers intestinal inflammation through macrophage activation, which leads to the release of inflammatory factors, and it also causes barrier dysfunction by disrupting tight junction proteins such as occludin, claudins, and zonula occludens. Also known as leaky gut, this condition contributes to the intestinal translocation of bacterial endotoxin via the portal vein and the subsequent activation of liver-resident macrophage (Kupffer) cells. The TLR-4-mediated activation of Kupffer cells induces the production of reactive oxygen species and inflammatory cytokines by stimulating the NF-κB pathway and thus contributes to hepatic inflammation and steatosis. Abbreviations: GLUT5: Facilitative Glucose Transporter-5, DNL: de novo lipogenesis, SCFA: Short Chain Fatty Acid, TLR-4: Toll-Like Receptor-4.
The uptake of fructose, but not glucose, into enterocytes is insulin and sodium-independent process without requiring ATP hydrolysis [18]. After taking fructose into the cytosol of enterocytes, most of the fructose is directly passed into the portal circulation where it participates in carbohydrate metabolism and de novo lipogenesis. Hepatic fructose is rapidly phosphorylated by Ketohexokinase (KHK) to Fructose-1-Phosphate (F1P), which is further metabolized to glyceraldehyde 3-phosphate and dihydroxyacetone phosphate by aldolase-B. Later, these products are converted to substrates for lipogenic and gluconeogenic pathways [4]. On the other hand, the remaining fructose in the enterocyte is also metabolized by KHK to F1P that is processed to further metabolites such as Short-Chain Fatty Acids (SCFAs), glucose, and organic acids by aldolase-B and triokinase sequentially [24]. It is generally accepted that the liver is the main site of fructose metabolism. However, a recent isotope tracing study by Jang et al. showed that some part of dietary fructose is metabolized in the small intestine [25]. In the intake of low-dose fructose (<0.5 g/kg), its approximately 90% is phosphorylated and then metabolized primarily to glucose and organic acids in the intestine, thus its unmetabolized small part passes into the portal circulation as fructose. Differently, in the high intake of fructose (1 g/kg), the capacity of the intestinal fructose metabolism surpasses and extra fructose carries to the liver [25]. The low doses of fructose is mainly cleared by the small intestine, while high-fructose exceeds the capacity of intestinal fructose metabolism and, the surplus fructose transports to the liver, thus may cause harmful metabolic effects. Also, an unabsorbed fraction of fructose is being an unfavorable substrate for gut microbiota (we mentioned this in the following section). On the other hand, fructose malabsorption which is an exceptional gastrointestinal dysfunction can occur in high-fructose ingestion. The absorption capacity of fructose has been proposed to extend to 5–50 g at one serving in a healthy adult [26]. Incomplete absorption of fructose was first considered in children with chronic diarrhea. Excessive intake of fruit juices containing high-fructose in children was determined to be associated with diarrhea and other gastrointestinal complications [27]. The fermentation of unabsorbed fructose by gut microbiota causes the accumulation of gaseous products that expand the intestinal wall. Accordingly, it has been stated that unabsorbed fructose results in abdominal pain, flatulence, diarrhea, and other gastrointestinal symptoms by drawing fluid into the intestinal lumen as well as forming gaseous compounds [28]. The prevalence of fructose malabsorption in toddlers was found to be higher compared to adults possibly due to the lower intestinal expression of GLUT5 [29].
Previous studies showed that high-fructose intake enhances the intestinal expression of GLUT2, GLUT5, GLUT8 and GLUT12 showing an adaptive mechanism for the overconsumption of monosaccharide [20,30]. This situation in the intestine appears to be a way of coping with excess fructose by enhancing its absorption. Therefore, in the malabsorption of fructose, it was proposed that activation of these transporters could be functional targets in the prevention and/or treatment of the disease [20]. However, the exertions to increase fructose absorption could enhance its circulatory fraction, which is a risky situation for many organs of the body against its harmful effects. On the other hand, intestine spesific inhibition of GLUT2 and GLUT5 would be a beneficial approach for fructose-dependent metabolic disorders [18]. However, a decrease in intestinal monosaccharide absorption would be expected to cause a pathology similar to fructose malabsorption. Therefore, the approaches to prevent the undesirable effects of high-fructose seem to be a double-edged sword. Further comprehensive studies are needed before the GLUTs can be used as therapeutic targets in the management of fructose-related disturbances. Alternatively, it can be proposed that the limitation of fructose intake would be a more efficient way rather than trying to modulate its absorption.
3. The impact of fructose on the intestinal permeability
The intestinal epithelium, being a single-cell lining, serves as a physical barrier to prevent the entry of microorganisms and certain microbial products into circulation. This selective permeable epithelial barrier is constructed by the tight junction, adherens junction, and desmosomes, which are connected with epithelial cells. Tight junctions play a critical role in regulating intestinal barrier function and coordinating the passage of small molecules and ions. The tight junction components are composed of occludin, claudins, zonula occludens (ZO-1, ZO-2, and ZO-3), and the junctional adhesion molecule [31]. Zonula occludens are essential proteins that link the transmembrane tight junction proteins to cytoskeleton. The junctional adhesion molecule has a regulatory role in the permeability; however, occludin and claudins are the main transmembrane proteins of the intestinal barrier construction [32].
The increased intestinal permeability reflects a deterioration in gut barrier function, which is especially associated with a decrease in the tight junction proteins. The barrier damage leads to the entry of microorganisms and their metabolites into the intestinal wall, also spreading inflammatory components to the whole body. Intestinal permeability may be changed in several situations, including gut microbiota modification, mucus layer alteration, and different eating habits such as a diet rich in fat and sugar [33,34]. Recent studies have shown that among dietary sugars, fructose has a specific function in the intestinal barrier destruction [[35], [36], [37], [38]]. As detailed above, in excessive fructose intake, the absorptive capacity of the ileum would be saturated; consequently, the unabsorbed fraction of fructose would be influential in the determination of the intestinal bacterial diversity, and inflammatory status and barrier function [35,37,39]. In addition, the increased inflammation in the intestine may enhance cell shedding and thus contributes to the impaired gut barrier function [32]. For the first time, Bergheim et al. reported that liver damage induced by 30% fructose administration in drinking water to mice was associated with increased intestinal translocation of endotoxin, which is also known as Lipopolysaccharide (LPS). In the same study, fructose, in comparison to sucrose, glucose, or artificial sweetener, was established to cause a more severe inflammatory condition that is evidenced by increased portal endotoxin levels and hepatic Tumor Necrosis Factor-α (TNF-α) expression. They also showed that sterilization of the gut with non-resorbable antibiotics remarkably reduced fructose-induced hepatic lipid accumulation [40]. The following study demonstrated that liver damage in high-fructose consumption is accompanied by hepatic Toll-Like Receptor-4 (TLR-4) activation induced by endotoxin derived from intestinal bacterial overgrowth [41]. TLRs can be activated by microbial pathogen-associated molecules such as endotoxin that occurs in the cell membrane of gram-negative bacteria and plays an influential role in fructose-induced endotoxemia [42]. In another investigation by the same research group, it has been reported that the intake of 30% fructose solution in mice leads to barrier dysfunction due to impairment of the tight junction proteins occludin and ZO-1, thus causing an increase in bacterial endotoxin level in the portal vein [37]. Consistent with previous studies, in our recent research, we found that 20% fructose intake in drinking water caused ileal inflammation evidenced by increased macrophage/leukocyte infiltration as well as expression of Inducible Nitric Oxide Synthase (iNOS) and Nuclear Factor kappa-B (NF-κB). Moreover, this dietary intervention resulted in increased intestinal permeability due to a decline in the ileal expression of occludin and claudin-1 and an alteration in gut microbial composition. All these intestinal changes are associated with increased plasma endotoxin levels [35]. In a very recent study, it was proposed that gut barrier dysfunction induced by high-fructose in rats may be related to nitration of intestinal tight junction and adherent junction proteins as well as apoptosis of intestinal enterocytes [43]. Moreover, a study by Volynets et al. showed that the excess fructose consumption in mice led to increased intestinal permeability, Firmicutes/Bacteriodetes ratio, and endotoxemia without increasing body weight, while a Western-style diet primarily caused weight gain but not endotoxin translocation [38]. It is clear that the overconsumption of fructose plays a specific role in the development of intestinal dysfunction by causing an increase in endotoxin levels due to the disruption of intestinal integrity and microbial community. The gastrointestinal changes caused by high-fructose intake are summarized in Fig. 1.
There is an anatomical link between the intestine and liver via the hepatic portal system, called as “gut-liver-axis”. The liver is directly exposed to gut microbiota and their products thereby acting as a first line of defense against harmful attacks [44]. As mention above, high-fructose intake can cause hepatic inflammatory damage by increasing intestinal translocation of endotoxin [40]. Also, gut-derived bacterial endotoxin due to high-fructose activates the resident macrophages Kupffer cells of the liver through a TLR-4-dependent mechanism [41]. As demonstrated in Fig. 2, the endotoxin translocates to the liver due to intestinal barrier disintegrity and microbial dysbiosis, thus binds to TLR-4 receptor on the surface of the macrophage cell. Endotoxin-induced TLR-4 stimulation activates NF-κB via the adaptor proteins Myeloid Differentiation Factor 88 (MyD88) and/or Interferon Regulatory Factor 3 (IRF3), subsequently leading to the production of inflammatory cytokines and reactive oxygen species [15,45]. Additionally, we recently show that the increased plasma level of endotoxin, TNF-α, and IL-1β in fructose-fed rats is associated with activation of Kupffer cells as well as hepatic expression of TNF-α, NF-κB and iNOS, implied that the liver is under attacked by an inflammatory infection [35]. A previous study demonstrated that TNF-α has a crucial function in the onset of fructose-induced liver damage as well as insulin resistance, through its receptor TNFR1 [46]. Moreover, it has been shown that an elevation in hepatic TNF-α level due to increased intestinal permeability, and endotoxemia stimulates lipogenic enzymes and lipid accumulation. Mechanistically, TNF-α activates caspase-2 by binding to TNFR1 on hepatocytes and then induces the expression of lipogenic genes including Sterol Regulatory Element-Binding Protein 1 (SREBP1), acetyl-CoA carboxylase, and Fatty Acid Synthase (FASN), thereby increasing hepatic steatosis [45]. In this line, high intake of fructose was reported to induced hepatic inflammatory status together with the increased expression of SREBP1 and FASN, thus contributed to the development of hepatic lipogenesis [33,35,47]. Taken together, it can be suggested that chronic fructose consumption both directly increases de novo lipogenesis, and indirectly enhances hepatic lipid accumulation through TNF-α activation due to the translocation of bacterial endotoxin. The hepatic alterations caused by high consumption of fructose are shown in Fig. 2.
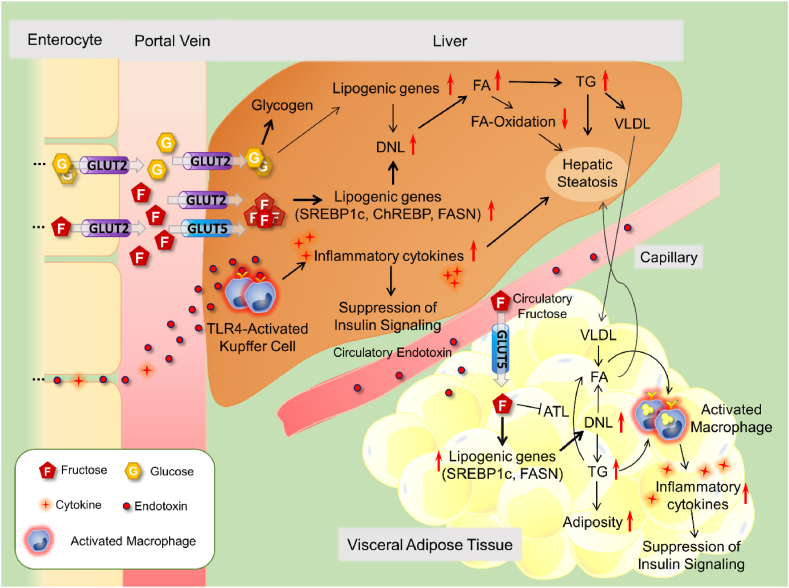
Fig. 2.
The effects of high-fructose intake in the liver and adipose tissue
Dietary monosaccharides are mainly metabolized in the liver. Fructose and glucose are taken up from the portal vein to the liver via GLUT2 and, then metabolized into pyruvate and acetyl-CoA that promote hepatic DNL. In this process, fructose as well as its metabolites stimulate the major transcription factors SREBP-1c and ChREBP, which increase the fatty acid synthesis by inducing gene expression of enzymes such as FASN. In addition to triggering DNL, fructose also contributes to hepatic steatosis by suppressing fatty acid oxidation. The increased hepatic fatty acids are converted to triglyceride and packaged as VLDL, which is released into circulation. High consumption of fructose causes a marked fat deposition, especially in the abdominal region. Fructose leads to fat accumulation in adipose tissue by both stimulating lipogenic gene expression and suppressing lipolytic enzyme adipose triglyceride lipase, thus triggering visceral adiposity. Enlarged visceral adipose tissue begins to produce inflammatory cytokines due to free fatty acids and/or endotoxin (derived from intestinal microbiota)-mediated TLR-4 activation, which then exacerbates macrophage infiltration into adipose tissue. In this way, the activation of inflammatory and lipogenic pathways caused by high-fructose intake contributes to both fat accumulation and suppression of insulin signaling in adipose tissue. Abbreviations: GLUT2: Facilitative Glucose Transporter-2, DNL: de novo lipogenesis, TLR-4: Toll-Like Receptor-4, SREBP-1c: Sterol Regulatory Element-Binding Protein-1c, ChREBP: Carbohydrate Response Element Binding Protein, FASN: Fatty Acid Synthase, VLDL: Very Low-Density Lipoprotein, ATL: Adipose Triglyceride Lipase, TG: Triglyceride, FA: Fatty Acid.
In parallel with preclinical studies, it has been reported that the intestinal barrier function and microbial community are changed in individuals with NAFLD. A clinical study has demonstrated that high-fructose consumption leads to an elevation in the translocation of bacterial endotoxin and the hepatic TLR-4 expression, thus contributing to the development of NAFLD [15]. In a subsequent study, patients with NAFLD were reported to have impaired gut permeability together with bacterial overgrowth and decreased ZO-1 expression in duodenal biopsy specimens [48]. Moreover, children with NAFLD were found to have excess fructose consumption in conjunction with elevated plasma inflammatory markers such as TNF-α, IL-6, and endotoxin, indicating increased intestinal permeability. Furthermore, chronic fructose intake for 2 weeks was shown to enhance circulating endotoxin levels in adolescents with NAFLD, and this elevation was found to be correlated with the increased markers of insulin resistance and inflammation [49]. In contrast to the above studies, a recent pilot study reported that a high intake of fructose did not affect intestinal permeability and gut microbiota as well as endotoxin level in obese humans who consume a certain amount of fructose known to develop NAFLD [50]. Thus, it can be suggested that the changes occurred in the gut-liver axis due to high-fructose consumption play a causative role in the development of dyslipidemia and hepatic steatosis. However, further clinical research is required to elucidate possible effects of fructose.
4. The impact of fructose on gut microbiota
In recent years, metagenomic approaches showed that the intestinal microbiota has a very complex structure and plays an influential role in health maintenance and disease status. Intestinal dysbiosis, which is described as an alteration in microbial abundance and/or diversity, was determined to be associated with several inflammatory metabolic disorders including obesity, NAFLD, type 1 and type 2 diabetes [51,52]. Intestinal microbiota comprising trillions of bacteria, which mainly belongs to Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia phyla, forms a heterogeneous and dynamic microbial ecosystem in the gastrointestinal tract [53]. In the first studies on the obesity-microbiota relationship in animals and humans, it was detected an increase in the abundance of Firmicutes, but a decrease in Bacteroidetes [54,55]. Following these initial findings, many studies demonstrated that the Firmicutes/Bacteroidetes ratio has increased in diseases such as obesity, type 2 diabetes, atherosclerosis, and NAFLD [35,56,57]. Contrary, this ratio was found to be decreased in clinical trials with obese, NAFLD, type 1 and 2 diabetes [[58], [59], [60], [61]].
From the studies conducted on animals, transplanting of the obese microbiota caused an increase in the body weight of lean mice [62]. In genetically obese ob/ob mice, it has been reported that two-week antibiotic treatment reduced the numbers of cecal aerobic and anaerobic bacteria, along with increased glucose tolerance as well as decreased liver triglyceride and plasma endotoxin levels [63]. However, the alteration of intestinal microbiota by probiotics bacteria such as Lactobacillus and Bifidobacterium species was shown to exert positive effects on health maintenance of rat [64]. Lactobacillus species from Firmicutes may produce improving effects on hyperglycemia, hyperinsulinemia, and dyslipidemia in metabolic disorders of rodents [[65], [66], [67], [68], [69]]. Similar to animal studies, a meta-analysis study evaluating 32 clinical trials has been demonstrated that probiotic treatment with especially Lactobacillus species improves metabolic parameters including total cholesterol, triglyceride, C reactive protein, HbA1c, fasting plasma glucose, fasting insulin levels, and both systolic and diastolic blood pressure in type 2 diabetic patients. As a result, probiotic supplementation is recommended to promote better metabolic control in patients with diabetes [70]. Another factor that directly affects the intestinal microbiota is prebiotics, known as indigestible carbohydrates. Prebiotic supplementation was shown to have a positive metabolic effect on obese and diabetic mice [71]. Also, gut microbiota affects host energy balance by regulating glucose and lipid metabolism through SCFAs like propionate, acetate, and butyrate production as a result of polysaccharides digestion induced by intestinal bacteria [72]. Most of the propionate and acetate reach the liver via the portal vein and metabolize in hepatocytes, while butyrate is utilized as a source of energy by intestinal epithelial cells [73]. Also, butyrate has been shown to have an antiinflammatory effect via the inhibition of NF-κB and proinflammatory cytokine production [74]. Moreover, SCFAs have a function in the suppression of appetite and attenuation of fat deposition by modulating relevant hormones and genes [75].
In the last decades, the changes in eating habits such as a Western-style diet containing high-sugar, fat, and salt may cause detrimental effects on host health by changing the microbial composition [76]. It has been shown that a diet containing 60% fat in mice causes an increase in the Firmicutes to Bacteriodetes ratio and also a decrease in Proteobacteria. This dietary intervention also increased the level of endotoxin in the feces and blood together with an induction of macrophage infiltration and inflammation in the adipose tissue in the presence of high circulatory cytokine level [77]. In a study investigating the effects of a diet rich in fat and sucrose on the gut microbiota of mice, two dietary interventions decreased the diversity and abundance of butyrate-producing bacteria as well as some other beneficial bacteria [78]. Another study reported that a high fat/high sucrose diet increases the abundance of Firmicutes but decreases Bacteroidetes, Actinobacteria, and Verrucomicrobia amounts in the fecal microbiota of mice [79]. Also, in high-fat-fed mice, there was an increase in the abundance of endotoxin-producing bacteria, but a decrease in SCFA-forming microorganisms [80]. Similar to high fat and sucrose diets, excess fructose intake has been shown to cause various changes in the composition of gut microbiota and its function [81]. In high-fructose intake, the intestinal monosaccharide transport capacity has saturated, and extra fructose reaches the colon and generates a partially selective medium of nutrients for the gut microbiota [25]. In progressing, colonic bacteria are able to metabolize the unabsorbed fructose into SCFAs and organic acids [81]. In a study performed in Sprague-Dawley rats, it was shown that fructose feeding increased Coprococcus and Ruminococcus bacterial strains which are significantly decreased after antibiotic treatment or fecal transplantation. The decrease in plasma endotoxin and TNF-α concentrations after antibiotic treatment indicates that the microbiota also contributes to the fructose-induced inflammatory condition [82]. In a study, dietary 30% liquid fructose in mice was shown to increase the Firmicutes to Bacteroidetes ratio by lowering the abundance of Bacteroidetes phyla, which is not altered by a Western-style diet. Furthermore, they observed that translocation of endotoxin in mice fed with a high-fructose or Western-style diet results in a reduction of mucus thickness in the colon [38]. In mice given high-fructose, the microbial alteration was accompanied by an elevation in serum endotoxin level, hepatic lipid accumulation, and inflammation [33]. In the other study, the high-fructose intake in mice caused a decrease in Bacteroidetes, but an enhancement in the pathogenic bacteria including Deferribacteraceae and Helicobacteraceae [79]. However, dietary fructose, which is given 40% in chow, did not change the abundance of Bacteroidetes but impaired Actinobacteria and Proteobacteria in the small intestine and colon in mice [83]. In a different study feeding protocol, we showed that dietary fructose, which is given 20% in drinking water, increased the Firmicutes/Bacteriodetes ratio as well as decreased the richness of Actinobacteria in rats [35]. In another experiment, it has been shown that a high-fructose diet which contains 60% fructose in chow increased the ratio of Firmicutes/Bacteroidetes and abundances of Deferribacteres, Verrucomicrobia, and Actinobacteria phyla in mice. At the genus level, high-fructose intake increased Bacteroides, Akkermansia, and Ruminococcus, which are mucin-degrading bacteria [84]. Akkermansia muciniphila is mostly thought to play a role in thinning of the mucus layer as a result of feeding on a high-fructose diet. However, the high-fructose diet did not produce a change in the fecal Akkermansia muciniphila abundance, but this bacterium has been found to be densely clustered at the colonic mucus layer [85]. It was also shown that dietary high-fructose reduces the richness of gut microbiota regarding both species and community, which are known as alpha and Shannon-Wiener diversity, respectively. The families of Bifidobacterceae, Enterococcaceae, and Erysipelotrichaceae were proposed as the prominent bacteria in the microbial changes induced by this dietary intervention using Linear discriminant analysis Effect Size (LEfSe) analysis. In addition, it was determined decreased concentration of most SCFAs, but increased inflammatory metabolites in feces [84]. The changes in gut microbiota and its metabolites were suggested to have a role in chronic intestinal inflammation caused by a high-fructose diet. In a recent study, fructose-rich chow was demonstrated to increase the Proteobacteria phylum in the cecal microbiota of mice. This phylum is known to be the main source of endotoxin in the gut microbiota, thus a rise of Proteobacteria has been claimed to be one of the causes of fructose-induced endotoxemia [86]. However, we and other researchers reported that there was no alteration in Proteobacteria abundance despite an elevation of plasma endotoxin level with the high-fructose diet [35,77,82].
In a human study, it was examined the influence of short-term two different formulations of high-fructose on the gut microbiota of healthy adult women [87]. The study subjects received fruit-rich and HFCS-supplemented diets with equal fructose amounts. In participants, the fruit-rich diet increased the abundance of Firmicutes, which contains butyrate-forming bacteria such as Anareostipes, Faecalibacterium, and Erysipelatoclostridium, while decreased the richness of Bacteroidetes with pathogenic genus Parabacteroides. On the contrary, the HFCS diet decreased Faecalibacterium and Erysipelatoclostridium, but increased the Bacteroidetes abundances [87]. Results of the above study show that different forms of fructose ingestion, such as fruit-rich diets and HFCS, may cause diverse effects on the gut microbiota. Therefore, the fructose intake from fruits appears to increase the favorable bacteria from Firmicutes phyla. This situation actually shows that the assessment of microbiota data by examining changes at the phylum level does not provide always precise information. Therefore, additional data analyzing family, genus, or species and also metabolites need to obtain accurate inferences from microbiota research.
In different perspective, the contribution of the microbiota to the fructose-induced metabolic disorder is evidenced that probiotic or prebiotic supplementation ameliorates the high-fructose-associated metabolic abnormalities. The probiotic supplementations containing L. acidophilus, L. casei and L. plantarum were shown to improve glucose intolerance, hyperinsulinemia, oxidative stress, and dyslipidemia in high-fructose-fed rats [67,88]. Other study results demonstrated that L. reuteri GMNL-263 treatment reduced serum lipid parameters including triglyceride and cholesterol levels, and also suppressed hepatic expression of lipogenic genes such as FASN and SREBP-1 in fructose-fed rats [89]. Previously, we have shown that supplementation of L. plantarum and L. helveticus decreased hepatic weight, triglyceride content, and FASN expression as well as improved insulin signaling pathway, endothelial Nitric Oxide Synthase (eNOS), and GLUT2 expression in liver of fructose-fed rats [68]. Moreover, our recent study results demonstrated that the treatment with kefir, which is a probiotic-prebiotic mixture, suppressed hepatic inflammation and lipogenesis, but promoted insulin signaling, in association with a change in the fecal microbiota, thus producing a more healthy status [35].
Evidence suggests that both the taxonomic and functional composition of the gut microbiota may have a role in the development of metabolic disorders. It is known that dietary fructose causes some changes in the intestinal microbial structure by both directly affecting the fecal content and also inducing metabolic disturbances. In fructose-induced microbial imbalance of the intestine, there is a decrease in beneficial commensal microorganisms that prevent the passage of pathogenic microbes and also maintain the integrity of the intestinal barrier through the production of mucus and lipid metabolites such as SCFAs. The fact that the treatments with probiotics and/or prebiotics targeting the intestinal microbiota have positive effects on metabolic syndrome reveals that microbiota involves in the pathogenesis of certain diseases [35,90,91]. Although there is no definitive treatment regimen for metabolic disorders, which are common health problem nowadays, consuming probiotic/prebiotic product for the regulation of intestinal microbiota will contribute to health maintenance, in addition to giving up or reducing unhealthy eating habits. In this regard, it is important to clarify the relationship between the pathological conditions and alterations in the gut microbiota induced by other diets rich in processed food.
5. The impact of fructose on abdominal adiposity
Abdominal adiposity as a regional excess fat accumulation could be a main driving force and potential health risk in metabolic syndrome and obesity, therefore has received increasing attention in the literature [92]. Regarding animal studies, Sprague-Dawley rats fed with a fructose-rich diet had more epididymal adipose tissue mass and high plasma insulin level than those of starch or dextrose-feeding rats [93]. The administration of 10% fructose solution to male rats for three weeks increased visceral adipose tissue mass and adipocyte size together with high blood levels of glucose, insulin, and triglyceride, but there was no change in body weight [94,95]. Similarly, it was found significant increases in plasma triglyceride level and visceral adipose tissues (epididymal or omental) of rats that consumed 10% fructose solution without having an effect on their body weight [[96], [97], [98]]. In rats fed with a diet containing 20% fructose for the 8-week study period, it was determined an increase in epididymal fat and mesenteric fat mass as well as adipocyte volume but no alteration in total body weight compared to control [82,99]. In compliance with the above studies, we have demonstrated that 10% fructose intake in drinking water for 6 months caused an expansion of omental adipose tissue of both male and female rats together with an increase in the plasma insulin and triglyceride levels. Metabolic disorder induced by long-term excess fructose intake was closely linked to abdominal fat accumulation, but independent of general obesity because there was a marked increase in body weight of males, but not that of females [100]. Recently in another study, 20% fructose in drinking water, relatively in a short feeding period of 15 weeks, also produced a remarkable increase in omental adipose tissue mass as well as plasma insulin and triglyceride levels in male rats without changing their body weight [35]. All these animal data disclosed common characteristics of metabolic disorder induced by dietary high-fructose, especially concerning the point of visceral obesity.
In humans, the first comprehensive analysis paper on the adverse health effects of dietary fructose by Elliot et al. elucidated the relation between excess consumption of fructose and body adiposity [101]. In a cross-sectional analysis study, total sugar intake was found to be positively correlated with body mass index and total fat accumulation in high-risk pediatric populations. The same investigation also reported that the modest reduction in sugar intake was effective in reducing obesity and the risk factors of type 2 diabetes [102]. A study performed on overweight and obese subjects, which are given either glucose- or fructose-sweetened drinks, providing 25% of energy requirements for 10 weeks, showed that fructose-consuming subjects had increased total abdominal fat, visceral adipose tissue volume, fasting plasma glucose, and insulin as well as postprandial triglyceride levels, but both groups displayed similar weight gain. Moreover, glucose consumption, but not fructose, specifically increased subcutaneous adipose tissue mass. Thus, the study results explored that fructose and glucose have different effect profiles on the distribution of regional adipose tissue independent of gaining weight [103]. The results of a cross-sectional study performed on 559 adolescents aged 14–18 y exhibited that total fructose intake (free fructose + sucrose) is positively associated with increases in visceral adiposity, systolic blood pressure, fasting glucose, HOMA-IR, and triglyceride, which are independent of age, sex, socioeconomic status, and energy intake [104]. In advance analyses, it was found that the connection between fructose intake and cardiometabolic risk markers may be related to visceral fat accumulation. Basically, it was known that hypertriglyceridemia and low HDL cholesterol were common abnormalities found in patients with abdominal obesity [105]. In subjects with abdominal obesity, the 75 g daily fructose intake, which is corresponding to 13% of the total energy for 12 weeks, significantly enhanced liver fat content, but produced a relatively low increase in their body weight and waist circumference. Also, visceral fat mass accretion was significantly correlated with liver fat accumulation. There was a considered individual difference in susceptibility to visceral adiposity as well as hepatic fat accumulation [106]. In a prospective study on middle-aged adults for 6 years, individuals who consumed regular sugar-sweetened beverages, at least 1 serving daily, were found to have a 29% greater increase in visceral adipose tissue mass compared to non-consumers [107]. The results from a double-blind study on 14 lean adolescents and 23 obese adolescents, who were matched for age, sex, and metabolic parameters, showed that ingestion of 75 g fructose, but not glucose, produced a hiperinsulinemia in obese participants than in leans [108]. In short-term studies, isocaloric fructose restriction for nine days in obese Latino and African American children noticeably reduced triglyceride and insulin levels, liver fat, and visceral fat accumulation determined by magnetic resonance spectroscopy [109,110]. All these human data demonstrated that excess fructose consumption in the diet was mostly associated with abdominal adiposity, hypertriglyceridemia, and hyperinsulinemia consequently causing a cardiometabolic risk.
Given studies on animals and humans, it is plausible to assume that excess intake of dietary fructose is a causative factor in the expansion of abdominal fat tissue in association with hypertriglyceridemia and hyperinsulinemia but independent of general obesity. In this assumption, some minor discrepancies can be ascribed to the methodological differences such as sugar concentration and feeding duration. Insulin may stimulate the uptake of fatty acids by adipocytes, which in turn activates lipogenic genes and lipogenesis, known as de novo fatty acid synthesis, but the mechanisms in adipose tissue are less understood than those of the liver [111]. Insulin causes lipid storage in adipocytes by both activating triacylglycerol synthesis and inhibiting its metabolism. In type 2 diabetes, insulin fails to inhibit hepatic gluconeogenesis but maintains to activate lipogenesis, thus producing a toxic combination of hyperglycemia and hypertriglyceridemia [112]. Likely, a high-fructose diet leads to the activation of hepatic lipogenesis and accumulation of ectopic fat in association with hyperinsulinemia [35,113,114]. Studies conducted on rodents demonstrated that a diet rich in fructose may stimulate lipogenesis by increasing gene expression of FASN, SREBP1, and Carbohydrate Response Element Binding Protein (ChREBP) in adipose tissue [35,97,115,116]. Dietary high-fructose also causes inhibition of adipose triglyceride lipase thus blocking lipid mobilization from adipocytes [97,117]. In a recent study, we showed that the gene expression of Angiopoietin-Like Protein 8 (ANGPTL8), which regulates plasma triglyceride levels by inhibiting lipoprotein lipase, is increased in adipose tissue in rats fed with a high-fructose diet [118]. Both its stimulatory effect on the adipose lipogenic genes and inhibitory effect on the lipolytic enzyme accounts for fat accumulation in the adipose tissue.
Fructose exposure also augments the accumulation of fatty acids in differentiated human adipocytes in culture and thus may lead to the initiation of adiposity accretion [119]. As presented in Fig. 2, the elevated fatty acids as well as triglyceride due to excessive fructose-induced hepatic de novo lipogenesis are transported to fat tissue for storage, thereby producing a dangerous metabolic link between liver and adipose tissue. Moreover, dietary fructose was proposed to activate intracellular cortisol, subsequently initiating fatty acid flux from subcutaneous adipocytes to visceral fat tissue for storage [120]. Fructose in the brain also stimulates Corticotropin-Releasing Hormone (CRH) and Adrenocorticotropic Hormone (ACTH), and thus causing an increase in cortisol secretion from the adrenal glands. The elevation in cortisol levels leads to hepatic gluconeogenesis, hyperglycemia, insulin resistance and fat accumulation in abdominal tissue [120]. It is well known that hypercortisolemia and chronic cortisol treatment cause an expansion of visceral adipose tissue [121]. This knowledge gives a support to the cortisol proposal to explain fructose-induced abdominal fat accumulation. Fructose has been also proposed to promote adipogenesis via ROS in the uric acid pathway [122]. However, why high-fructose specifically produces abdominal fat accumulation, but not subcutaneous, is not yet completely understood. Anyway, fructose appears as a unique nutritional component causing visceral fat deposition. Given, it is reasonable to propose that the reduction in dietary fructose intake will decrease abdominal fat mass and thereby could be imperative in health maintenance even without dramatic calorie restriction. Although the distribution of body fat is determined by many factors including age, sex, hormones, diet, physical activity, stress, and drugs, dietary fructose exclusively appears to be an effective factor in abdominal fat deposition. Based on the available findings on animals and humans with high-fructose intake, abdominal fat accumulation seems to be a critical outcome and a visible sign of adverse metabolic effects.
Functionally, adipose tissue regulates fat and glucose metabolism secreting several factors and showing specific adaptive changes in response to energy intake [123]. Adipose tissue has an ability to adjust rapid and long-term changes in energy balance leading to tissue expansion or reduction. In the over-calorie intake, triglycerides begin to accumulate in adipocytes causing a rise in the adipocyte size and the tissue mass [124]. In metabolic disorders, the enlargement of white adipose tissue is associated with insulin resistance as well as inflammatory status due to macrophage infiltration and cytokine generation [[125], [126], [127]]. The adipose tissue also generates many inflammatory adipokines such as leptin, adiponectin, resistin, and visfatin which can modify insulin sensitivity by affecting insulin signaling pathways and glucose transporters [128]. However, the relation between insulin resistance and inflammatory process in adipose tissue remains completely understood [124]. In vitro studies provide some insight into the subject, in which the long-term challenge of adipocytes with cytokines was found to suppress insulin signaling and lead to insulin resistance [129,130]. Several inflammatory and macrophage-specific genes were shown to upregulate in white adipose tissue in high-fat or high-fructose feeding of rodents together with an increase in plasma insulin level [131,132]. The depletion of macrophages from visceral adipose tissue prevents the development of insulin resistance and fat accumulation in mice on a high-fat diet [133]. Dietary fructose-induced insulin resistance in mice causes visceral fat accumulation, macrophage infiltration, production of proinflammatory cytokines, and activation of endoplasmic reticulum stress in the visceral adipose tissue [134]. Moreover, the number of macrophages and the level of inflammatory cytokine were found to be high in the adipose tissue of rodents fed with high-fructose diet [118,135]. On the other hand, lifestyle-based weight loss strategies such as caloric restriction diet may also have protective effects in patients with metabolic syndrome through attenuating visceral adipose tissue mass as well as improving peripheral lipid profile, reducing insulin levels and inflammatory factors [136]. In the clinical perspective, recent studies have proposed that Sodium-Dependent Glucose Cotransporter Proteins 2 (SGLT2) inhibitors and Glucagon Like Peptide-1 (GLP1) receptor agonists, which are among the novel anti-diabetic molecules, may have beneficial effects on metabolic disorders by inhibiting cytokine release from visceral adipose tissue as well as reducing body weight [137,138]. In this line, it is possible to propose that these new anti-diabetic drugs may counteract the pro-inflammatory state associated with the expansion of adipose tissue caused by a high-fructose diet. Hence, it is generally accepted that inflammation in adipose tissue plays a causative role in the development of insulin resistance in the metabolic disorders, but the mechanistic pathway is still unclear. Fig. 2 shows the alterations in adipose tissue due to the high intake of fructose.
6. The impact of fructose on insulin signaling
Insulin has essential metabolic and anabolic functions in the maintenance of health status. The action of insulin is disturbed in the setting of metabolic diseases including type-2 diabetes, obesity, and metabolic syndrome possibly due to suppression of the insulin signaling pathway. The binding of insulin to the specific membrane-bound receptors in target tissues is necessary to integrate its well-known physiologic effects in the body. For the establishment of metabolic homeostasis, insulin receptors are expressed on many somatic cells, especially in the liver, skeletal muscle, and white adipose tissue. Insulin produces tissue-specific physiologic effects in the insulin-responsive cells via almost the same signal transduction pathway that is diverged in the distal effectors [139]. In insulin resistance, the signal transduction system shows various changes in the different target tissues [112]. Also, the metabolic homeostatic effects are exerted to maintain with a high level of circulating insulin as observed in type-2 diabetes, obesity, overnutrition, and excess fructose intake [140]. In this section, rather than providing detailed knowledge of the insulin signaling system, we will try to delineate key molecules in insulin action and give special attention to modifying effect of dietary high-fructose.
In this pathway, insulin action begins with its binding to the Insulin Receptor (IR) on cell membranes, and the signal transmits to the downstream effectors. IR can connect to several phosphotyrosine-binding proteins including Insulin Receptor Substrates (IRS). Of IRS isoforms, IRS1 and IRS2 are known to mediate the metabolic effects of insulin. Phosphoinositide-3-kinase (PI3K), which is in a crucial position in insulin signaling, is activated after tyrosine phosphorylation of IRS proteins. The signal is maintained via AKT (protein kinase B) activation by phosphorylation. Then the activated AKT phosphorylates several downstream effectors in different functional pathways. IRS, PI3K, and AKT as proximal insulin signaling elements are essential for the initiation of insulin action [139]. The tyrosine phosphorylation of IRS1 protein involves the activation of the insulin signaling pathway; however, its serine phosphorylation has been implicated in insulin resistance [141]. Also, Protein Tyrosine Phosphatase Non-Receptor Type 1 b (PTP1b) negatively controls insulin signaling transduction through dephosphorylation of the IR and IRS1/2 [142]. Basically, insulin inhibits hepatic glucose output through the glucose transporter GLUT2 but enhances glucose uptake into muscle and adipose tissue via GLUT4. The main glucose uptake by GLUT4 into the tissues due to insulin stimulation is critically dependent on the activation of the IRS1/PI3K/AKT pathway. The IRS/PI3K/AKT-dependent mechanism controls glucose transport into tissues, gluconeogenesis, and de novo lipogenesis, but differently, this latest is also regulated by phosphorylation of lipogenic enzymes [140]. On the other hand, insulin also has a regulatory effect on vascular tone via activation of the IRS1/PI3K/AKT/eNOS pathway. Nitric oxide produced from endothelium is known to mediate the vasodilatory effect of insulin as well as regulate its delivery to target tissues such as the liver, skeletal muscle, and adipose tissue [143,144]. In a preceding study, IRS1 knockout mice were shown to have impaired endothelial vasodilation and insulin resistance [145]. Further studies explored that there was a functional link between insulin signaling and eNOS [146,147]. In the insulin signaling pathway, the mammalian Target of Rapamycin-1 (mTORC1) and Forkhead Box O1 (FOXO1) are well-characterized AKT targets with important physiological functions [140,148]. The insulin at high concentrations also stimulates the mitogenic pathway implying activation of the Mitogen-Activated Protein Kinase (MAPK) pathway [149].
In experimental insulin resistance, the disruption of insulin signaling in the tissues of animals may cause hyperinsulinemia and diabetes [150]. In insulin resistance, physiologic actions of insulin are exerted to preserve with a high level of insulin as a compensatory mechanism in which it can be observed some tissue-specific functional changes. This resistant circumstance could be the consequence of a defect in insulin activation at the levels of IR, IRS1, PI3K, and AKT which are key effectors of insulin signaling thus diminishing glucose entry into the tissues due to disruption of GLUT4 translocation [148]. The suppression in hepatic insulin signaling was proposed to lead to a reduction in hepatic glycogen synthesis without decreasing lipid synthesis, thereby causing the dangerous combination of hyperglycemia and hypertriglyceridemia [112,151]. The molecular mechanisms for insulin resistance may involve endoplasmic reticulum stress, reactive oxygen species, mitochondrial dysfunction, and branched-chain amino acids [139,[152], [153], [154]]. Moreover, the metabolic dysfunction in insulin resistance may be associated with inflammatory cytokines derived from activated macrophages of adipose tissue [155,156]. Expansion of adipose tissue may cause homeostatic stress and increase chemotactic signals in adipocytes macrophages [157]. The proposal of inflammatory cytokine in insulin resistance was initiated with studies on the neutralization of TNF-α and advanced with c-Jun N-terminal Kinase (JNK) activation in adipocytes [158,159]. However, the mechanistic link between inflammatory status and insulin resistance is still being elucidated [156,160]. In this section, insulin-resistant states in response to excess fructose intake have been presented by considering the insulin signaling pathway.
Animal and human studies indicate that similar to overnutrition, long-term excess fructose intake increases insulin secretion but decreases its metabolic actions in the target tissues thus leading to insulin resistance. Relatedly, accumulating evidence signified that high intake of fructose produces insulin resistance via suppressing proximal insulin signaling pathway, besides through the well-characterized mechanisms such as activation of de novo lipogenesis, reactive oxygen species, endoplasmic reticulum stress, mitochondrial dysfunction, and inflammatory cytokines [5]. An early study by Catena et al., in 2003 reported that fructose feeding (66% in diet) for two weeks causes a decline in the number of insulin receptors and IR gene expression as well as binding of radiolabeled insulin in the liver and skeletal muscle [161]. Moreover, hyperinsulinemia from fructose-fed rats was found to be associated with a decrease in nitric oxide-mediated vasodilation to insulin in the arteries, revealing vascular insulin resistance [162,163]. In a further step, a downregulation in IR, IRS1, pIRS1 (Tyr), pAKT, and PI3K as well as GLUT4 was established in the soleus muscle of male rats fed with a diet containing fructose [164]. Considering the negative control of insulin signaling, the intake of high-fructose led to an increase in PTP1b expression along with a decrease in hepatic IR, IRS1, and pAKT expression [151]. These preceding findings point to suppression in insulin action through signaling pathways in metabolic disturbance caused by dietary fructose. In this subject, we determined that dietary HFCS in male rats (20% in drinking water for 12 weeks) produced vascular insulin resistance by decreasing endothelial vasodilation to insulin as well as suppressing the expression of IRS1 and eNOS [165]. However, administration of pure fructose at 10% solution in drinking water for 24 weeks, reduced aortic expression of IRS2 and eNOS as well as insulin-induced vasodilation in female rats, but not males, showing a gender-dependent difference [100]. In relation to tissue division, we reported that HFCS-induced suppression in hepatic IRS1 and eNOS expression was associated with an increase in hepatic expression of FASN and SREBP-1c as well as triglyceride accumulation and microvesicular fat deposition in the liver [114]. In the development of hepatic insulin resistance after high-fat feeding, attenuation in liver eNOS content was proposed to play an important role in the suppression of insulin signaling at the level of IRS1 and p-AKT, besides the onset of hepatic inflammation [147,166]. Therefore, insulin-induced nitric oxide output in the liver sinusoidal endothelium via the stimulation of the AKT/eNOS signaling was proposed to participate in the maintenance of hepatic blood flow as well as liver function. In confirmation of this link between insulin signaling and eNOS in different tissue, fructose feeding in male rats was demonstrated to decrease IRS1/2, PI3K, AKT, and also eNOS protein expressions in vasculature, liver or skeletal muscle [167,168]. On the other hand, some studies suggested that suppression of insulin signaling due to dietary high-fructose depends on the downregulation of IRS2 in the liver. In this context, it has been shown that dietary fructose (10% solution for 14–60 days) reduced hepatic expression of IRS2, but not IRS1, in female rats [[169], [170], [171]]. Differently, in the liver of male rats, we displayed that administration of fructose (20% concentration in drinking water for 15 weeks) reduced both IRS1 and IRS2 gene and protein expressions besides IR mRNA, p-AKT, and p-eNOS protein expressions. These changes were associated with an increased hepatic triglyceride accumulation, fatty degeneration, and FASN expression indicating a reverse interaction between insulin signaling and lipogenesis [35,68]. The presence of insulin resistance is also evidenced by overt hyperinsulinemia, hypertriglyceridemia, and increased abdominal fat mass. Thus, it can be suggested that the attenuation of IR/IRS1-2/AKT/eNOS signaling may lead to lipogenic gene activation and liver fat accumulation in fructose-induced insulin resistance. However, the expressions of GLUT5 and GLUT2, which are the main fructose transporters, were augmented in the liver of rats upon fructose feeding, revealing excess fructose uptake by the liver evidenced by the increased amount of hepatic fructose content [35,68]. The insulin resistance due to suppression of hepatic insulin signaling may lead to a compensatory enrichment in fructose transporters, thereby providing substrate abundance for the overproduction of triglyceride by the liver. The impaired insulin signaling and increased fructose transporter expression in liver could be possible mechanisms underlying increased hepatic lipogenesis and fat accumulation in the high consumption of dietary fructose. In partial supporting this view, it was proposed that triglyceride synthesis in the liver is mainly dependent on substrate availability but generally independent of insulin action [172]. However, in liver insulin receptor knockout mice, overexpression of FASN and SREBP-1c induced by dietary fructose did not cause hepatic steatosis signifying a critical role of hepatic insulin signaling for the metabolism of triglycerides [173]. In aggregate, although hepatic insulin signaling and substrate availability as well as lipogenic genes have critical roles in de novo lipid synthesis, the mechanisms by which fructose led to fatty liver remain mostly unidentified [174].
With regard to the adipose tissue compartment, dietary high-fructose (20% in drinking water for 15 weeks) in rats was demonstrated to cause a downregulation in the gene expression of IRS1, IRS2, and eNOS, but upregulation in lipogenic genes including SREBP-1c and FASN, similar to the findings in the liver [35]. However, in a previous study investigating the impact of fructose feeding at long-term and relatively low concentrations (for 24 weeks, 10% solution in drinking water), it was not determined a suppression, but surprisingly an upregulation in the expression of insulin signaling genes in adipose tissues of both male and female rats together with a marked increase in omental adipose tissue mass [175]. These discrepancies point out possible compensatory and adaptive changes depending on fructose concentration and feeding duration. In another study, 10% fructose-sweetened water supplementation for 10 weeks did not change IR, IRS1, IRS2, PI3K, and AKT mRNA expressions but increased IRS1 (Ser) phosphorylation, accompanied by an increase in epididymal adipose tissue mass and adipocyte size [176]. However, in examining the sex-dependent effect of dietary fructose on the visceral adipose tissue insulin signaling pathway, it was found an increase in protein expressions of p-IRS1 (Ser) and PTP1b, whereas a decrease in p-AKT in female rats, but not in males, consuming 10% fructose solution for 9 weeks [177]. Considering with above section on abdominal adiposity, current findings signified that further investigation is required to elucidate the causal relationship between the excess fructose-induced expansion of adipose tissue and insulin signaling. On the other hand, as regards the kidney, it was found an increase in the expression level of p-IRS1 (Ser), but a decrease in p-IRS1 (Tyr) and p-AKT in renal tissues of rats fed 10% fructose solution for 8 or 12 weeks showing a suppression in the renal insulin signaling pathway [[178], [179], [180], [181]]. In partial agreement, dietary fructose in rats (20% solution for 15 weeks) led to an impairment in renal IRS1 and AKT protein expression together with an elevation in the expression of GLUT5 and sodium-glucose linked transporter-2 (SGLT2), which are specific fructose and glucose transporters respectively [67]. These results suggest an enhancement in tubular reabsorption of both monosaccharides in the blunted insulin signaling. Thus, excess fructose intake could be a risky nutritional component in the development of renal irregularities when considering its well-known uric acid-generating effect.
Inflammatory factors, especially iNOS, may have a role in the coupling of metabolic and vascular insulin resistance as well as impaired insulin signaling in the liver from diabetic obese mice [182,183]. In concert with this proposal, we reported that high vascular expression of iNOS was associated with suppression of aortic expression of IRS1 and eNOS as well dysregulation of metabolic parameters [165]. In the other studies, it was also reported an inverse association between the activation of inflammatory cytokines (iNOS, NF-κB, TNF-α, IL-1β, and IL-6) and suppression of insulin signaling pathway in the liver, adipose tissue, or kidney of rats fed with high-fructose [35,67]. In this line, high-fructose intake led to activation of Nod-like receptor pyrin domain-containing 3 (NLRP3) inflammasome in the kidney of rodents with decreased IRS1/AKT level [180,181,184]. Moreover, reduced expression of p-IR, p-IRS1 (Tyr), and/or p-IRS2 protein was associated with increased expression of inflammatory cytokines in the liver of fructose-fed animals [185,186]. The decreased expression of IRS1 in adipose tissue following high-fructose consumption of rats is accompanied by an increase in inflammatory factors such as NF-κB, IL-1β, IL-6, and TNF-α [177]. Also, fructose feeding-induced augmentation in p-IRS1 (Ser) expression in adipose tissue of rats was accompanied by an elevation in the inflammatory index with molecular and histopathological examinations [187]. In the soleus muscle of rats fed with fructose-rich diet, the elevated IRS1 (Ser) and reduced IRS1 (Tyr) expression were associated with an upregulation of Cyclooxygenase-2 (COX-2) and Inhibitor kappa-B alpha (IκB-α) expression [188]. As a result, excess fructose intake produces an activation of inflammatory status, but a suppression of insulin signaling, thereby suggesting a close reciprocal relationship between these two processes. Thus, we depicted a perspective on how fructose suppresses insulin signaling at the level of target organs including vasculature, liver, adipose tissue, skeletal muscle, and kidney. Suppression of insulin signaling in the main target tissues, possibly due to an elevation in inflammatory cytokines production, precedes impaired insulin action in the whole body characterized by systemic insulin resistance.
7. The impact of fructose on reproductive function
The low testosterone level might be an important characteristic of metabolic syndrome in men, like hyperglycemia, insulin resistance, dyslipidemia, abdominal obesity, and low-grade inflammation [189,190]. In men, testosterone deficiency is a constant indicator of infertility and is also interestingly involved in cardiovascular disease and mortality [191]. Studies have documented that at least 20–25% of men with metabolic syndrome or type 2 diabetes has low free testosterone levels and gonadal dysfunction [[191], [192], [193]]. Also, the increased body mass index in males is associated with a reduced plasma level of testosterone with a concomitant increase in plasma level of estrogen [192]. However, the meta-analysis results disclosed that the association between obesity and subfertility is somewhat controversial [194,195]. In relevance to fat distribution, the results of a retrospective study in men display a negative correlation between visceral adiposity and male fertility indicators such as sperm count, sperm motility, serum testosterone level, and testosterone/estradiol ratio [196]. Regulatory role of abdominal adiposity on testosterone levels appears to be important in the progress of complications of metabolic diseases in men. Notably, aromatase enzyme derived from white adipose tissue modulates the conversion of androgen to estrogen and thus affecting body fat distribution [197]. The aromatase activity is positively correlated with abdominal adiposity, pointing that there was an increased estrogen production, but a decreased testosterone, in visceral adipose tissue [198]. Moreover, in relation to regional adiposity, epididymal fat accumulation in the scrotum area may cause an increase in scrotal temperature thus disturbing the microenvironment of testes and maturation of spermatozoa [199]. On the other hand, systemic chronic inflammation in metabolic diseases may cover the male genital system and cause testicular dysfunction by disrupting hormone homeostasis and spermatogenesis [200]. The impact of dietary high-fructose, which is one of the contributory factors of metabolic syndrome, on male reproductive function is poorly understood.
Recently we showed that the intake of high-fructose in rats causes a decline in the testicular concentration of testosterone and testis weight [201,202]. Also, it was determined a decrease in sperm count and motility, but an increase in abnormal sperm morphology due to excess fructose consumption in rats [201,[203], [204], [205], [206], [207]]. Dietary high-fructose was also demonstrated to cause degeneration in the Sertoli cell and seminiferous tubule as well as a reduction in germ cell number [201,202,206,208,209]. It was also determined to produce architectural distortion with a high apoptosis index in seminiferous epithelial cells of rat testis [206]. The histochemical examination in the germ cells of high-fructose-fed rats disclosed the increased apoptotic activity evidenced by high expression intensity of Raf1, Extracellular Signal-Regulated Kinase 1/2 (ERK1/2), and caspase-3 as well as terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) positive staining [201]. In similarity, the damage in porcine epididymis due to exposure to cadmium, which is a major industrial and environmental toxicant, was associated with the increase in the apoptotic index, which is determined by the upregulation of Raf1 and caspase-3. TUNEL staining further verified the provocation of apoptosis [210]. Previously, cadmium was also shown to elevate the testicular Bax/Bcl-2 ratio, a main indicator of apoptosis, and cleaved caspase-3 activity together with an impairment in testis weight, sperm count, and motility in rats [211]. Another endocrine disrupter bisphenol-A activates the phosphorylation of ERK1/2 and caspase-3 in rat Sertoli cells [212,213]. ERK production depending on Raf cascade activation and caspase-3 plays an important role in the regulation of germ cell apoptosis and spermatogenesis [214,215]. The studies have ascertained that the imbalance between germ cell survival and apoptosis of testicular cells induced by environmental toxicants profoundly affects spermatogenesis [216]. The overexpression of Raf1 leads to disruption of intercellular tight junctions downregulating occludin transcription in the epithelial cells [217]. Activation of caspase-3, which is a constant marker of apoptosis, shows a negative correlation with sperm motility and viability [218]. These findings revealed that apoptotic factors have both physiologic and pathologic roles in the male reproductive system. Excess intake of dietary fructose like environmental harmful compounds provokes apoptosis in testicular tissues of animals, thus may lead to reproductive toxicity.
MAPK/ERK pathway consisting of p38 MAPK, ERK1/2, and JNK has a critical role in the modulation of apoptosis in the testis as well as some reproductive processes including the progression of germ cell and spermatogenesis [219,220]. An upregulation of p38 MAPK, ERK, and JNK expressions was determined in the testis of rodents with type-1 diabetes or high-fat diet-induced obesity [[221], [222], [223]]. The activation of p38 MAPK signaling and oxidative stress was shown to induce apoptotic cell death and testicular damage in rodents with type 1 diabetes [222,224,225]. Also, some toxic chemicals, such as dinitrobenzene, phthalates, and bisphenol A causes testicular injury by stimulating the MAPK pathway [[226], [227], [228]]. In the subject of dietary fructose, recently we demonstrated that excess fructose intake induces an elevation in the testicular expression of p38 MAPK, phosphorylation of p38 MAPK and ERK1/2 together with dysfunction and degeneration in the testis of rats [201]. Thus, it is plausible to propose that activation of apoptotic and mitogenic factors in the testis of rats following dietary high-fructose intake disturbs the development of germ cells and spermatogenesis.
MAPK/ERK pathway has also a modulatory role on the epididymal tight junction proteins thereby controlling permeability to water, ions, and solutes [219]. The blood-testis barrier produces a physical obstacle between the testes and the bloodstream. The barrier components such as claudins, occludins, and junctional adhesion molecules construct tight junctions inter-adjacent cells and adjust the selective permeability of the epithelium. The blood-testis barrier also protects the seminiferous tubules against detrimental insults and thereby playing a vital role in the process of spermatogenesis [229,230]. The barrier can be destroyed by certain chemicals and metabolic diseases. For example, exposure to cadmium was proposed to lead to male infertility damaging the blood-testis barrier [231,232]. The high-fat diet-induced obesity in male mice was shown to produce a decrease in the expression of tight junction proteins of the testis and fertility [178,233]. Of the conditions of metabolic disease, diabetes mellitus was suggested to change the permeability of blood-testis barrier and thus disturb spermatogenesis [234]. In compliance with this proposal, a downregulation in the expression of N-cadherin and claudin-11 was determined in the testicular tissue of type-1 diabetic mice [222,235]. Our study results for the first time demonstrated that dietary high-fructose causes a decrease in the expression of the major components of blood-testis barrier, including N-cadherin and claudin-11 in the testicular tissues of rats so rendering them defenseless against the damaging insults [201]. Besides, excess fructose intake activates the inflammatory process as evidenced by high expression levels of cytokines including TNF-α and iNOS as well as NF-κB in the testis of rats [202]. In the chemical and nutritional framework, testicular inflammation was also considered in exposure to reproductive toxicants such as manganese, cadmium, and bisphenol A [210,236,237] as well as in obesity induced by a high-fat diet [178]. The increase in seminal levels of inflammatory cytokines was determined to be associated with decreased total sperm count, motility, and vitality in patients with metabolic syndrome [238]. A study reported that the frequency of male accessory gland inflammation was around 43% among infertile type 2 diabetic patients [239]. The inflammation in the male accessory gland exhibits a high spreading rate in diabetic patients with testosterone deficiency displaying a regulatory role of inflammation on testosterone production [240]. These findings show the importance of the local inflammatory status of the male genital tract in metabolic diseases extending to reproductive dysfunction. In terms of a sugar viewpoint, high-fructose intake causes a hormonal dysfunction with low intra-testicular testosterone, activation of inflammatory cytokines, downregulation of the blood-testis barrier proteins, and upregulation of mitogenic and apoptotic pathways in the rats. In a further study, for the first time, we recently showed that excess consumption of fructose downregulates key molecules of the insulin signaling pathway including IR, IRS-1/2, PI3K-Akt-mTOR, and eNOS in rat testis, revealing testicular insulin resistance (unpublished yet). Taking all these molecular findings, in association with morphological changes including testicular degeneration, low sperm count, and sperm anomaly, we proposed that excess intake of fructose may generate male reproductive dysfunction. Although the relevance of these results to humans remains to be determined, the potential destructive effect of dietary high-fructose on the male system should be taken into consideration for the maintenance of public health. High-fructose-induced abnormalities and related potential mechanisms in the male reproductive system are summarized in Fig. 3.
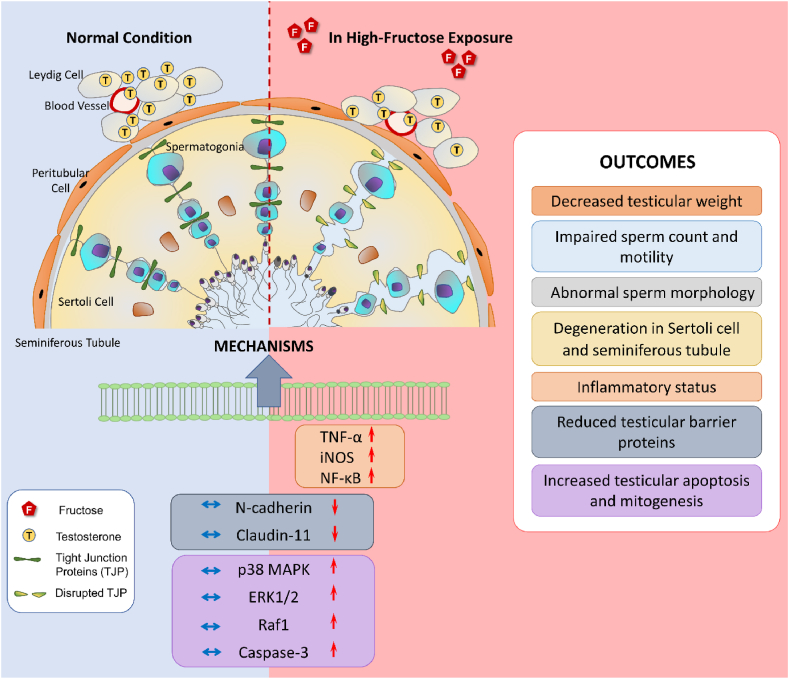
Fig. 3.
The impact of high-fructose intake on male reproductive system
The testes, which primarily contain Leydig, Sertoli, and germ cells, are responsible for male reproductive function and health. Leydig cells produce testosterone that plays an essential role in the maturation of spermatids and the maintenance of testicular homeostasis. Sertoli cells have a phagocytic function and support the proliferation and development of germ cells. The forming of spermatozoa involves the gradual differentiation of germ cells and their eventual transformation into mature sperm. The MAPK/ERK pathway, including p38 MAPK and ERK1/2, has an essential role in the regulation of spermatogenesis, progression of germ cells, and apoptosis as well as blood-testis barrier integrity. However, the overstimulation of Raf1, MAPK/ERK pathway, as well as caspase-3, negatively affects male reproductive function via disrupting sperm motility and viability as well as damaging sperm morphology. High intake of dietary fructose activates mitogenic and apoptotic factors in the testicular tissue thereby impairing blood-testis barrier integrity and spermatogenesis. The blood-testis barrier, which mainly consists of claudins, occludins, and junctional adhesion molecules, acts as a physical hindrance between the testes and the bloodstream. This obstacle protects the seminiferous tubules against harmful products, thereby supporting the maintenance of spermatogenesis. Overconsumption of fructose leads to a downregulation in the expression of tight junction-related proteins such as N-cadherin and claudin-11 and thus impairs barrier integrity and sperm production in testes. Additionally, high fructose causes testicular inflammation and degeneration through the upregulation of proinflammatory factors such as TNF-α, iNOS, and NF-κB. All these changes induced by high fructose could be responsible for the decreases in testicular weight and testosterone levels; the impairment in sperm count, motility, and morphology; the degeneration in Sertoli cells and seminiferous tubule, which are known to be closely related to male infertility.
The influence of dietary high-fructose on female gender function has been relatively less investigated than those of males in both animal and human studies. A study demonstrated that high-fructose corn syrup feeding for 28 days has adverse effects in adult female rats with changes in the length of the estrous cycle as well as ovarian and uterine histology [241]. Furthermore, high-fructose consumption caused an increase in ovarian weight, testosterone, and luteinising hormone level, and also a decrease in follicle-stimulating hormone in rats with the letrozole-induced polycystic ovarian syndrome [242]. Polycystic ovary syndrome is one of the most common endocrine disorders observed in premenopausal women leading to ovarian dysfunction. This syndrome was also associated with metabolic diseases including obesity and insulin resistance [243]. Currently, there is still little data to outline the adverse effect of a fructose-rich diet on the female reproductive system related to fertility and endocrine parameters. Further studies will be required to delineate the importance of the nutritional approach in female health. However, regarding offspring health, animal studies disclosed that excess maternal consumption of fructose resulted in fetal growth retardation, insulin resistance, hypertension, and sex-specific changes in offspring development as well as a reduction in fertility [[244], [245], [246], [247], [248]]. A large prospective cohort study showed that high maternal intake of sugar-sweetened beverages was associated with an increased risk of preterm birth [249]. Although the impact of perinatal fructose consumption during pregnancy in humans is largely undetermined, the above-mentioned experimental findings urge caution for potential adverse effects of high-fructose consumption on maternal and offspring health.
8. Conclusion
Although the presented documents are based somewhat on experimental data, the evidence indicates that a high intake of fructose, as a dietary sugar component, disturbs the metabolic physiologic balance. We are still unable to precisely define the mechanisms; however, dietary high inclusion of fructose causes serious health risks that are obvious, especially at the level of the intestine, microbiome, liver, adipose tissue and reproductive organs. Public health policy is necessary to encourage people by delineating a safe nutritional lifestyle to cope with the destructive consequences of diet-induced metabolic disorders. Giving up or reducing the consumption of fructose-sweetened beverages and foods, as a powerful lifestyle intervention, would produce a measurable effect in the prevention of metabolic diseases that could be a more cost-effective way than the other solutions such as drug therapy.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported in part by grants from the Gazi University Research Fund. BAP 02/2017–20, BAP 02/2018–14 and TDK-2022-7661.
Contributor Information
Ceren Guney, Email: cerennguney@gmail.com.
Nur Banu Bal, Email: nurbanubal@gazi.edu.tr.
Fatma Akar, Email: fakar@gazi.edu.tr.
References
- 1.Srour B., Fezeu L.K., Kesse-Guyot E., Alles B., Mejean C., Andrianasolo R.M., Chazelas E., Deschasaux M., Hercberg S., Galan P., Monteiro C.A., Julia C., Touvier M. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Sante) BMJ. 2019;365:l1451. doi: 10.1136/bmj.l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askari M., Heshmati J., Shahinfar H., Tripathi N., Daneshzad E. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int. J. Obes. 2020;44(10):2080–2091. doi: 10.1038/s41366-020-00650-z. [DOI] [PubMed] [Google Scholar]
- 3.Tran L.T., Yuen V.G., McNeill J.H. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009;332(1–2):145–159. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 4.Hannou S.A., Haslam D.E., McKeown N.M., Herman M.A. Fructose metabolism and metabolic disease. J. Clin. Invest. 2018;128(2):545–555. doi: 10.1172/JCI96702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Softic S., Stanhope K.L., Boucher J., Divanovic S., Lanaspa M.A., Johnson R.J., Kahn C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab Sci. 2020;57(5):308–322. doi: 10.1080/10408363.2019.1711360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taskinen M.R., Packard C.J., Boren J. Dietary fructose and the metabolic syndrome. Nutrients. 2019;11(9) doi: 10.3390/nu11091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popkin B.M. Patterns of beverage use across the lifecycle. Physiol. Behav. 2010;100(1):4–9. doi: 10.1016/j.physbeh.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosinger A., Herrick K., Gahche J., Park S. Sugar-sweetened beverage consumption among U.S. Youth, 2011-2014. NCHS Data Brief. 2017;271:1–8. [PubMed] [Google Scholar]
- 9.Rosinger A., Herrick K., Gahche J., Park S. vol. 270. NCHS Data Brief; 2017. pp. 1–8. (Sugar-sweetened Beverage Consumption Among U.S. Adults, 2011-2014). [PubMed] [Google Scholar]
- 10.Sluik D., Engelen A.I., Feskens E.J. Fructose consumption in The Netherlands: the Dutch national food consumption survey 2007-2010. Eur. J. Clin. Nutr. 2015;69(4):475–481. doi: 10.1038/ejcn.2014.267. [DOI] [PubMed] [Google Scholar]
- 11.Vos M.B., Kimmons J.E., Gillespie C., Welsh J., Blanck H.M. Dietary fructose consumption among US children and adults: the third national health and nutrition examination survey. Medscape J Med. 2008;10(7):160. [PMC free article] [PubMed] [Google Scholar]
- 12.Goran M.I., Ulijaszek S.J., Ventura E.E. High fructose corn syrup and diabetes prevalence: a global perspective. Global Publ. Health. 2013;8(1):55–64. doi: 10.1080/17441692.2012.736257. [DOI] [PubMed] [Google Scholar]
- 13.Nier A., Brandt A., Conzelmann I.B., Ozel Y., Bergheim I. Non-Alcoholic fatty liver disease in overweight children: role of fructose intake and dietary pattern. Nutrients. 2018;10(9) doi: 10.3390/nu10091329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelmalek M.F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R.J., Diehl A.M., Nonalcoholic Steatohepatitis Clinical Research N. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thuy S., Ladurner R., Volynets V., Wagner S., Strahl S., Konigsrainer A., Maier K.P., Bischoff S.C., Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J. Nutr. 2008;138(8):1452–1455. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 16.Sigala D.M., Hieronimus B., Medici V., Lee V., Nunez M.V., Bremer A.A., Cox C.L., Price C.A., Benyam Y., Chaudhari A.J., Abdelhafez Y., McGahan J.P., Goran M.I., Sirlin C.B., Pacini G., Tura A., Keim N.L., Havel P.J., Stanhope K.L. Consuming sucrose- or HFCS-sweetened beverages increases hepatic lipid and decreases insulin sensitivity in adults. J. Clin. Endocrinol. Metab. 2021;106(11):3248–3264. doi: 10.1210/clinem/dgab508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoun R., Chokor F.A.Z., Taktouk M., Nasrallah M., Ismaeel H., Tamim H., Nasreddine L. Dietary fructose and its association with the metabolic syndrome in Lebanese healthy adults: a cross-sectional study. Diabetol. Metab. Syndrome. 2022;14(1):29. doi: 10.1186/s13098-022-00800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraris R.P., Choe J.Y., Patel C.R. Intestinal absorption of fructose. Annu. Rev. Nutr. 2018;38:41–67. doi: 10.1146/annurev-nutr-082117-051707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueckler M. Facilitative glucose transporters. Eur. J. Biochem. 1994;219(3):713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 20.DeBosch B.J., Chi M., Moley K.H. Glucose transporter 8 (GLUT8) regulates enterocyte fructose transport and global mammalian fructose utilization. Endocrinology. 2012;153(9):4181–4191. doi: 10.1210/en.2012-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueckler M., Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol. Aspect. Med. 2013;34(2–3):121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holman G.D. Structure, function and regulation of mammalian glucose transporters of the SLC2 family. Pflügers Archiv. 2020;472(9):1155–1175. doi: 10.1007/s00424-020-02411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truswell A.S., Seach J.M., Thorburn A.W. Incomplete absorption of pure fructose in healthy subjects and the facilitating effect of glucose. Am. J. Clin. Nutr. 1988;48(6):1424–1430. doi: 10.1093/ajcn/48.6.1424. [DOI] [PubMed] [Google Scholar]
- 24.Diggle C.P., Shires M., Leitch D., Brooke D., Carr I.M., Markham A.F., Hayward B.E., Asipu A., Bonthron D.T. Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme. J. Histochem. Cytochem. 2009;57(8):763–774. doi: 10.1369/jhc.2009.953190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang C., Hui S., Lu W., Cowan A.J., Morscher R.J., Lee G., Liu W., Tesz G.J., Birnbaum M.J., Rabinowitz J.D. The small intestine converts dietary fructose into glucose and organic acids. Cell Metabol. 2018;27(2):351–361 e3. doi: 10.1016/j.cmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bidwell A.J. Chronic fructose ingestion as a major health concern: is a sedentary lifestyle making it worse? A Review, Nutrients. 2017;9(6) doi: 10.3390/nu9060549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duro D., Rising R., Cedillo M., Lifshitz F. Association between infantile colic and carbohydrate malabsorption from fruit juices in infancy. Pediatrics. 2002;109(5):797–805. doi: 10.1542/peds.109.5.797. [DOI] [PubMed] [Google Scholar]
- 28.Gaby A.R. Adverse effects of dietary fructose. Alternative Med. Rev. 2005;10(4):294–306. [PubMed] [Google Scholar]
- 29.Douard V., Ferraris R.P. The role of fructose transporters in diseases linked to excessive fructose intake. J. Physiol. 2013;591(2):401–414. doi: 10.1113/jphysiol.2011.215731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishi K., Tanaka T., Igawa M., Takase S., Goda T. Sucrase-isomaltase and hexose transporter gene expressions are coordinately enhanced by dietary fructose in rat jejunum. J. Nutr. 1999;129(5):953–956. doi: 10.1093/jn/129.5.953. [DOI] [PubMed] [Google Scholar]
- 31.Barbara G., Barbaro M.R., Fuschi D., Palombo M., Falangone F., Cremon C., Marasco G., Stanghellini V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.718356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bischoff S.C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.D., Serino M., Tilg H., Watson A., Wells J.M. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Do M.H., Lee E., Oh M.J., Kim Y., Park H.Y. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients. 2018;10(6) doi: 10.3390/nu10060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usuda H., Okamoto T., Wada K. Leaky gut: effect of dietary fiber and fats on microbiome and intestinal barrier. Int. J. Mol. Sci. 2021;22(14) doi: 10.3390/ijms22147613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akar F., Sumlu E., Alcigir M.E., Bostanci A., Sadi G. Potential mechanistic pathways underlying intestinal and hepatic effects of kefir in high-fructose-fed rats. Food Res. Int. 2021;143 doi: 10.1016/j.foodres.2021.110287. [DOI] [PubMed] [Google Scholar]
- 36.Nier A., Brandt A., Rajcic D., Bruns T., Bergheim I. Short-term isocaloric intake of a fructose- but not glucose-rich diet affects bacterial endotoxin concentrations and markers of metabolic health in normal weight healthy subjects. Mol. Nutr. Food Res. 2019;63(6) doi: 10.1002/mnfr.201800868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spruss A., Kanuri G., Stahl C., Bischoff S.C., Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab. Invest. 2012;92(7):1020–1032. doi: 10.1038/labinvest.2012.75. [DOI] [PubMed] [Google Scholar]
- 38.Volynets V., Louis S., Pretz D., Lang L., Ostaff M.J., Wehkamp J., Bischoff S.C. Intestinal barrier function and the gut microbiome are differentially affected in mice fed a western-style diet or drinking water supplemented with fructose. J. Nutr. 2017;147(5):770–780. doi: 10.3945/jn.116.242859. [DOI] [PubMed] [Google Scholar]
- 39.Yu S., Li C., Ji G., Zhang L. The contribution of dietary fructose to non-alcoholic fatty liver disease. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.783393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergheim I., Weber S., Vos M., Kramer S., Volynets V., Kaserouni S., McClain C.J., Bischoff S.C. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J. Hepatol. 2008;48(6):983–992. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 41.Spruss A., Kanuri G., Wagnerberger S., Haub S., Bischoff S.C., Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50(4):1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 42.Spruss A., Bergheim I. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2009;20(9):657–662. doi: 10.1016/j.jnutbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Cho Y.E., Kim D.K., Seo W., Gao B., Yoo S.H., Song B.J. Fructose promotes leaky gut, endotoxemia, and liver fibrosis through ethanol-inducible cytochrome P450-2E1-mediated oxidative and nitrative stress. Hepatology. 2021;73(6):2180–2195. doi: 10.1002/hep.30652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Z., Wang B. The gut-liver Axis in health and disease: the role of gut microbiota-derived signals in liver injury and regeneration. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.775526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Todoric J., Di Caro G., Reibe S., Henstridge D.C., Green C.R., Vrbanac A., Ceteci F., Conche C., McNulty R., Shalapour S., Taniguchi K., Meikle P.J., Watrous J.D., Moranchel R., Najhawan M., Jain M., Liu X., Kisseleva T., Diaz-Meco M.T., Moscat J., Knight R., Greten F.R., Lau L.F., Metallo C.M., Febbraio M.A., Karin M. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2020;2(10):1034–1045. doi: 10.1038/s42255-020-0261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanuri G., Spruss A., Wagnerberger S., Bischoff S.C., Bergheim I. Role of tumor necrosis factor alpha (TNFalpha) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J. Nutr. Biochem. 2011;22(6):527–534. doi: 10.1016/j.jnutbio.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Wagnerberger S., Spruss A., Kanuri G., Volynets V., Stahl C., Bischoff S.C., Bergheim I. Toll-like receptors 1-9 are elevated in livers with fructose-induced hepatic steatosis. Br. J. Nutr. 2012;107(12):1727–1738. doi: 10.1017/S0007114511004983. [DOI] [PubMed] [Google Scholar]
- 48.Miele L., Valenza V., La Torre G., Montalto M., Cammarota G., Ricci R., Masciana R., Forgione A., Gabrieli M.L., Perotti G., Vecchio F.M., Rapaccini G., Gasbarrini G., Day C.P., Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 49.Jin R., Willment A., Patel S.S., Sun X., Song M., Mannery Y.O., Kosters A., McClain C.J., Vos M.B. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int J Hepatol. 2014 doi: 10.1155/2014/560620. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aleman J.O., Henderson W.A., Walker J.M., Ronning A., Jones D.R., Walter P.J., Daniel S.G., Bittinger K., Vaughan R., MacArthur R., Chen K., Breslow J.L., Holt P.R. Excess dietary fructose does not alter gut microbiota or permeability in humans: a pilot randomized controlled study. J Clin Transl Sci. 2021;5(1):e143. doi: 10.1017/cts.2021.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015;26 doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Addio F., Fiorina P. Type 1 diabetes and dysfunctional intestinal homeostasis. Trends Endocrinol. Metabol. 2016;27(7):493–503. doi: 10.1016/j.tem.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012;9(10):577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 54.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U.S.A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 56.Fukuda S., Ohno H. Gut microbiome and metabolic diseases. Semin. Immunopathol. 2014;36(1):103–114. doi: 10.1007/s00281-013-0399-z. [DOI] [PubMed] [Google Scholar]
- 57.Saad M.J., Santos A., Prada P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology. 2016;31(4):283–293. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 58.Murri M., Leiva I., Gomez-Zumaquero J.M., Tinahones F.J., Cardona F., Soriguer F., Queipo-Ortuno M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. doi: 10.1186/1741-7015-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., Peng Y., Zhang D., Jie Z., Wu W., Qin Y., Xue W., Li J., Han L., Lu D., Wu P., Dai Y., Sun X., Li Z., Tang A., Zhong S., Li X., Chen W., Xu R., Wang M., Feng Q., Gong M., Yu J., Zhang Y., Zhang M., Hansen T., Sanchez G., Raes J., Falony G., Okuda S., Almeida M., LeChatelier E., Renault P., Pons N., Batto J.M., Zhang Z., Chen H., Yang R., Zheng W., Li S., Yang H., Wang J., Ehrlich S.D., Nielsen R., Pedersen O., Kristiansen K., Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 60.Schwiertz A., Taras D., Schafer K., Beijer S., Bos N.A., Donus C., Hardt P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 61.Wang B., Jiang X., Cao M., Ge J., Bao Q., Tang L., Chen Y., Li L. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci. Rep. 2016;6 doi: 10.1038/srep32002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., Egholm M., Henrissat B., Heath A.C., Knight R., Gordon J.I. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Membrez M., Blancher F., Jaquet M., Bibiloni R., Cani P.D., Burcelin R.G., Corthesy I., Mace K., Chou C.J. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. Faseb. J. 2008;22(7):2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 64.Liang Y., Lin C., Zhang Y., Deng Y., Liu C., Yang Q. Probiotic mixture of Lactobacillus and Bifidobacterium alleviates systemic adiposity and inflammation in non-alcoholic fatty liver disease rats through Gpr109a and the commensal metabolite butyrate. Inflammopharmacology. 2018;26(4):1051–1055. doi: 10.1007/s10787-018-0479-8. [DOI] [PubMed] [Google Scholar]
- 65.Choi I.D., Kim S.H., Jeong J.W., Lee D.E., Huh C.S., Hong S.S., Sim J.H., Ahn Y.T. Triglyceride-lowering effects of two probiotics, Lactobacillus plantarum KY1032 and Lactobacillus curvatus HY7601, in a rat model of high-fat diet-induced hypertriglyceridemia. J. Microbiol. Biotechnol. 2016;26(3):483–487. doi: 10.4014/jmb.1512.12018. [DOI] [PubMed] [Google Scholar]
- 66.Honda K., Moto M., Uchida N., He F., Hashizume N. Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J. Clin. Biochem. Nutr. 2012;51(2):96–101. doi: 10.3164/jcbn.11-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korkmaz O.A., Sumlu E., Koca H.B., Pektas M.B., Kocabas A., Sadi G., Akar F. Effects of Lactobacillus plantarum and Lactobacillus helveticus on renal insulin signaling, inflammatory markers, and glucose transporters in high-fructose-fed rats. Medicina. 2019;55(5) doi: 10.3390/medicina55050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sumlu E., Bostanci A., Sadi G., Alcigir M.E., Akar F. Lactobacillus plantarum improves lipogenesis and IRS-1/AKT/eNOS signalling pathway in the liver of high-fructose-fed rats. Arch. Physiol. Biochem. 2022;128(3):786–794. doi: 10.1080/13813455.2020.1727527. [DOI] [PubMed] [Google Scholar]
- 69.Wu C.C., Weng W.L., Lai W.L., Tsai H.P., Liu W.H., Lee M.H., Tsai Y.C. Effect of Lactobacillus plantarum strain K21 on high-fat diet-fed obese mice. Evid Based Complement Alternat Med. 2015 doi: 10.1155/2015/391767. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kocsis T., Molnar B., Nemeth D., Hegyi P., Szakacs Z., Balint A., Garami A., Soos A., Marta K., Solymar M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-68440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Everard A., Lazarevic V., Derrien M., Girard M., Muccioli G.G., Neyrinck A.M., Possemiers S., Van Holle A., Francois P., de Vos W.M., Delzenne N.M., Schrenzel J., Cani P.D. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donohoe D.R., Garge N., Zhang X., Sun W., O'Connell T.M., Bunger M.K., Bultman S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabol. 2011;13(5):517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segain J.P., Raingeard de la Bletiere D., Bourreille A., Leray V., Gervois N., Rosales C., Ferrier L., Bonnet C., Blottiere H.M., Galmiche J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47(3):397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiao A., Yu B., He J., Yu J., Zheng P., Luo Y., Luo J., Yan H., Wang Q., Wang H., Mao X., Chen D. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition. 2021;87–88 doi: 10.1016/j.nut.2021.111198. [DOI] [PubMed] [Google Scholar]
- 76.Di Rienzi S.C., Britton R.A. Adaptation of the gut microbiota to modern dietary sugars and sweeteners. Adv. Nutr. 2020;11(3):616–629. doi: 10.1093/advances/nmz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim K.A., Gu W., Lee I.A., Joh E.H., Kim D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kong C., Gao R., Yan X., Huang L., Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2019;60:175–184. doi: 10.1016/j.nut.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Li J.M., Yu R., Zhang L.P., Wen S.Y., Wang S.J., Zhang X.Y., Xu Q., Kong L.D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: a benefit of short-chain fatty acids. Microbiome. 2019;7(1):98. doi: 10.1186/s40168-019-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X., Zhao Y., Zhang M., Pang X., Xu J., Kang C., Li M., Zhang C., Zhang Z., Zhang Y., Li X., Ning G., Zhao L. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lambertz J., Weiskirchen S., Landert S., Weiskirchen R. Fructose: a dietary sugar in crosstalk with microbiota contributing to the development and progression of non-alcoholic liver disease. Front. Immunol. 2017;8:1159. doi: 10.3389/fimmu.2017.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di Luccia B., Crescenzo R., Mazzoli A., Cigliano L., Venditti P., Walser J.C., Widmer A., Baccigalupi L., Ricca E., Iossa S. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0134893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brutting C., Lara Bisch M., Brandsch C., Hirche F., Stangl G.I. Impact of dietary propionate on fructose-induced changes in lipid metabolism, gut microbiota and short-chain fatty acids in mice. Int. J. Food Sci. Nutr. 2021;72(2):160–173. doi: 10.1080/09637486.2020.1773415. [DOI] [PubMed] [Google Scholar]
- 84.Tan R., Dong H., Chen Z., Jin M., Yin J., Li H., Shi D., Shao Y., Wang H., Chen T., Yang D., Li J. Intestinal microbiota mediates high-fructose and high-fat diets to induce chronic intestinal inflammation. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.654074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montrose D.C., Nishiguchi R., Basu S., Staab H.A., Zhou X.K., Wang H., Meng L., Johncilla M., Cubillos-Ruiz J.R., Morales D.K., Wells M.T., Simpson K.W., Zhang S., Dogan B., Jiao C., Fei Z., Oka A., Herzog J.W., Sartor R.B., Dannenberg A.J. Dietary fructose alters the composition, localization, and metabolism of gut microbiota in association with worsening colitis. Cell Mol Gastroenterol Hepatol. 2021;11(2):525–550. doi: 10.1016/j.jcmgh.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vasques-Monteiro I.M.L., Silva-Veiga F.M., Miranda C.S., de Andrade Goncalves E.C.B., Daleprane J.B., Souza-Mello V. A rise in Proteobacteria is an indicator of gut-liver axis-mediated nonalcoholic fatty liver disease in high-fructose-fed adult mice. Nutr. Res. 2021;91:26–35. doi: 10.1016/j.nutres.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Beisner J., Gonzalez-Granda A., Basrai M., Damms-Machado A., Bischoff S.C. Fructose-induced intestinal microbiota shift following two types of short-term high-fructose dietary phases. Nutrients. 2020;12(11) doi: 10.3390/nu12113444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yadav H., Jain S., Sinha P.R. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23(1):62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh F.C., Lee C.L., Chai C.Y., Chen W.T., Lu Y.C., Wu C.S. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr. Metab. 2013;10(1):35. doi: 10.1186/1743-7075-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beserra B.T., Fernandes R., do Rosario V.A., Mocellin M.C., Kuntz M.G., Trindade E.B. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin. Nutr. 2015;34(5):845–858. doi: 10.1016/j.clnu.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019;16(10):605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 92.Tchernof A., Despres J.P. Pathophysiology of human visceral obesity: an update. Physiol. Rev. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 93.Rizkalla S.W., Boillot J., Tricottet V., Fontvieille A.M., Luo J., Salzman J.L., Camilleri J.P., Slama G. Effects of chronic dietary fructose with and without copper supplementation on glycaemic control, adiposity, insulin binding to adipocytes and glomerular basement membrane thickness in normal rats. Br. J. Nutr. 1993;70(1):199–209. doi: 10.1079/bjn19930117. [DOI] [PubMed] [Google Scholar]
- 94.Alzamendi A., Giovambattista A., Raschia A., Madrid V., Gaillard R.C., Rebolledo O., Gagliardino J.J., Spinedi E. Fructose-rich diet-induced abdominal adipose tissue endocrine dysfunction in normal male rats. Endocrine. 2009;35(2):227–232. doi: 10.1007/s12020-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 95.Zubiria M.G., Farina J.P., Moreno G., Gagliardino J.J., Spinedi E., Giovambattista A. Excess fructose intake-induced hypertrophic visceral adipose tissue results from unbalanced precursor cell adipogenic signals. FEBS J. 2013;280(22):5864–5874. doi: 10.1111/febs.12511. [DOI] [PubMed] [Google Scholar]
- 96.Kovacevic S., Nestorov J., Matic G., Elakovic I. Dietary fructose-related adiposity and glucocorticoid receptor function in visceral adipose tissue of female rats. Eur. J. Nutr. 2014;53(6):1409–1420. doi: 10.1007/s00394-013-0644-1. [DOI] [PubMed] [Google Scholar]
- 97.Li J.X., Ke D.Z., Yao L., Wang S., Ma P., Liu L., Zuo G.W., Jiang L.R., Wang J.W. Response of genes involved in lipid metabolism in rat epididymal white adipose tissue to different fasting conditions after long-term fructose consumption. Biochem. Biophys. Res. Commun. 2017;484(2):336–341. doi: 10.1016/j.bbrc.2017.01.119. [DOI] [PubMed] [Google Scholar]
- 98.Prince P.D., Santander Y.A., Gerez E.M., Hocht C., Polizio A.H., Mayer M.A., Taira C.A., Fraga C.G., Galleano M., Carranza A. Fructose increases corticosterone production in association with NADPH metabolism alterations in rat epididymal white adipose tissue. J. Nutr. Biochem. 2017;46:109–116. doi: 10.1016/j.jnutbio.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 99.Crescenzo R., Bianco F., Falcone I., Coppola P., Liverini G., Iossa S. Increased hepatic de novo lipogenesis and mitochondrial efficiency in a model of obesity induced by diets rich in fructose. Eur. J. Nutr. 2013;52(2):537–545. doi: 10.1007/s00394-012-0356-y. [DOI] [PubMed] [Google Scholar]
- 100.Pektas M.B., Sadi G., Akar F. Long-term dietary fructose causes gender-different metabolic and vascular dysfunction in rats: modulatory effects of resveratrol. Cell. Physiol. Biochem. 2015;37(4):1407–1420. doi: 10.1159/000430405. [DOI] [PubMed] [Google Scholar]
- 101.Elliott S.S., Keim N.L., Stern J.S., Teff K., Havel P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002;76(5):911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 102.Davis J.N., Alexander K.E., Ventura E.E., Kelly L.A., Lane C.J., Byrd-Williams C.E., Toledo-Corral C.M., Roberts C.K., Spruijt-Metz D., Weigensberg M.J., Goran M.I. Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight Latino youth. Am. J. Clin. Nutr. 2007;86(5):1331–1338. doi: 10.1093/ajcn/86.5.1331. [DOI] [PubMed] [Google Scholar]
- 103.Stanhope K.L., Schwarz J.M., Keim N.L., Griffen S.C., Bremer A.A., Graham J.L., Hatcher B., Cox C.L., Dyachenko A., Zhang W., McGahan J.P., Seibert A., Krauss R.M., Chiu S., Schaefer E.J., Ai M., Otokozawa S., Nakajima K., Nakano T., Beysen C., Hellerstein M.K., Berglund L., Havel P.J. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 2009;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pollock N.K., Bundy V., Kanto W., Davis C.L., Bernard P.J., Zhu H., Gutin B., Dong Y. Greater fructose consumption is associated with cardiometabolic risk markers and visceral adiposity in adolescents. J. Nutr. 2012;142(2):251–257. doi: 10.3945/jn.111.150219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Despres J.P., Lemieux I., Prud'homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ. 2001;322(7288):716–720. doi: 10.1136/bmj.322.7288.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taskinen M.R., Soderlund S., Bogl L.H., Hakkarainen A., Matikainen N., Pietilainen K.H., Rasanen S., Lundbom N., Bjornson E., Eliasson B., Mancina R.M., Romeo S., Almeras N., Pepa G.D., Vetrani C., Prinster A., Annuzzi G., Rivellese A., Despres J.P., Boren J. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J. Intern. Med. 2017;282(2):187–201. doi: 10.1111/joim.12632. [DOI] [PubMed] [Google Scholar]
- 107.Ma J., McKeown N.M., Hwang S.J., Hoffmann U., Jacques P.F., Fox C.S. Sugar-sweetened beverage consumption is associated with change of visceral adipose tissue over 6 Years of follow-up. Circulation. 2016;133(4):370–377. doi: 10.1161/CIRCULATIONAHA.115.018704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galderisi A., Giannini C., Van Name M., Caprio S. Fructose consumption contributes to hyperinsulinemia in adolescents with obesity through a GLP-1-mediated mechanism. J. Clin. Endocrinol. Metab. 2019;104(8):3481–3490. doi: 10.1210/jc.2019-00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lustig R.H., Mulligan K., Noworolski S.M., Tai V.W., Wen M.J., Erkin-Cakmak A., Gugliucci A., Schwarz J.M. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity. 2016;24(2):453–460. doi: 10.1002/oby.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwarz J.M., Noworolski S.M., Erkin-Cakmak A., Korn N.J., Wen M.J., Tai V.W., Jones G.M., Palii S.P., Velasco-Alin M., Pan K., Patterson B.W., Gugliucci A., Lustig R.H., Mulligan K. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology. 2017;153(3):743–752. doi: 10.1053/j.gastro.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Czech M.P., Tencerova M., Pedersen D.J., Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia. 2013;56(5):949–964. doi: 10.1007/s00125-013-2869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown M.S., Goldstein J.L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metabol. 2008;7(2):95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 113.Dekker M.J., Su Q., Baker C., Rutledge A.C., Adeli K. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2010;299(5):E685–E694. doi: 10.1152/ajpendo.00283.2010. [DOI] [PubMed] [Google Scholar]
- 114.Sadi G., Ergin V., Yilmaz G., Pektas M.B., Yildirim O.G., Menevse A., Akar F. High-fructose corn syrup-induced hepatic dysfunction in rats: improving effect of resveratrol. Eur. J. Nutr. 2015;54(6):895–904. doi: 10.1007/s00394-014-0765-1. [DOI] [PubMed] [Google Scholar]
- 115.Bursac B.N., Vasiljevic A.D., Nestorovic N.M., Velickovic N.A., Vojnovic Milutinovic D.D., Matic G.M., Djordjevic A.D. High-fructose diet leads to visceral adiposity and hypothalamic leptin resistance in male rats--do glucocorticoids play a role? J. Nutr. Biochem. 2014;25(4):446–455. doi: 10.1016/j.jnutbio.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 116.Khitan Z., Harsh M., Sodhi K., Shapiro J.I., Abraham N.G. HO-1 upregulation attenuates adipocyte dysfunction, obesity, and isoprostane levels in mice fed high fructose diets. J Nutr Metab. 2014 doi: 10.1155/2014/980547. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang D.W., Chang W.C., Yang H.J., Wu J.S., Shen S.C. Gallic acid alleviates hypertriglyceridemia and fat accumulation via modulating glycolysis and lipolysis pathways in perirenal adipose tissues of rats fed a high-fructose diet. Int. J. Mol. Sci. 2018;19(1) doi: 10.3390/ijms19010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Akar F., Guney C., Ozer H.B., Pektaş M.B., Koca H.B., Kocabaş A., Sadi G. The inverse association between ANGPTL8 and PI3K-mTOR-PPARγ expressions in adipose tissue of high-fructose-fed rats: the modulatory effect of kefir. Istanbul J Pharm. 2021;51(3):7. doi: 10.26650/IstanbulJPharm.2021.933139. [DOI] [Google Scholar]
- 119.Varma V., Boros L.G., Nolen G.T., Chang C.W., Wabitsch M., Beger R.D., Kaput J. Metabolic fate of fructose in human adipocytes: a targeted (13)C tracer fate association study. Metabolomics. 2015;11(3):529–544. doi: 10.1007/s11306-014-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.DiNicolantonio J.J., Mehta V., Onkaramurthy N., O'Keefe J.H. Fructose-induced inflammation and increased cortisol: a new mechanism for how sugar induces visceral adiposity. Prog. Cardiovasc. Dis. 2018;61(1):3–9. doi: 10.1016/j.pcad.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 121.Lee M.J., Pramyothin P., Karastergiou K., Fried S.K. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim. Biophys. Acta. 2014;1842(3):473–481. doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hernandez-Diazcouder A., Romero-Nava R., Carbo R., Sanchez-Lozada L.G., Sanchez-Munoz F. High fructose intake and adipogenesis. Int. J. Mol. Sci. 2019;20(11) doi: 10.3390/ijms20112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scherer P.E. The many secret lives of adipocytes: implications for diabetes. Diabetologia. 2019;62(2):223–232. doi: 10.1007/s00125-018-4777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klein S., Gastaldelli A., Yki-Jarvinen H., Scherer P.E. Why does obesity cause diabetes? Cell Metabol. 2022;34(1):11–20. doi: 10.1016/j.cmet.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ballak D.B., Stienstra R., Tack C.J., Dinarello C.A., van Diepen J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine. 2015;75(2):280–290. doi: 10.1016/j.cyto.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bastard J.P., Maachi M., Lagathu C., Kim M.J., Caron M., Vidal H., Capeau J., Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 127.Torres-Leal F.L., Fonseca-Alaniz M.H., Rogero M.M., Tirapegui J. The role of inflamed adipose tissue in the insulin resistance. Cell Biochem. Funct. 2010;28(8):623–631. doi: 10.1002/cbf.1706. [DOI] [PubMed] [Google Scholar]
- 128.Nicholson T., Church C., Baker D.J., Jones S.W. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J. Inflamm. 2018;15:9. doi: 10.1186/s12950-018-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jager J., Gremeaux T., Cormont M., Le Marchand-Brustel Y., Tanti J.F. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148(1):241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rotter V., Nagaev I., Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003;278(46):45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 131.Bargut T.C.L., Santos L.P., Machado D.G.L., Aguila M.B., Mandarim-de-Lacerda C.A. Eicosapentaenoic acid (EPA) vs. Docosahexaenoic acid (DHA): effects in epididymal white adipose tissue of mice fed a high-fructose diet. Prostaglandins Leukot. Essent. Fatty Acids. 2017;123:14–24. doi: 10.1016/j.plefa.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 132.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A., Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bu L., Gao M., Qu S., Liu D. Intraperitoneal injection of clodronate liposomes eliminates visceral adipose macrophages and blocks high-fat diet-induced weight gain and development of insulin resistance. AAPS J. 2013;15(4):1001–1011. doi: 10.1208/s12248-013-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marek G., Pannu V., Shanmugham P., Pancione B., Mascia D., Crosson S., Ishimoto T., Sautin Y.Y. Adiponectin resistance and proinflammatory changes in the visceral adipose tissue induced by fructose consumption via ketohexokinase-dependent pathway. Diabetes. 2015;64(2):508–518. doi: 10.2337/db14-0411. [DOI] [PubMed] [Google Scholar]
- 135.Choi Y., Abdelmegeed M.A., Song B.J. Diet high in fructose promotes liver steatosis and hepatocyte apoptosis in C57BL/6J female mice: role of disturbed lipid homeostasis and increased oxidative stress. Food Chem. Toxicol. 2017;103:111–121. doi: 10.1016/j.fct.2017.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Montefusco L., D'Addio F., Loretelli C., Ben Nasr M., Garziano M., Rossi A., Pastore I., Plebani L., Lunati M.E., Bolla A.M., Porta M.D., Piuri G., Rocchio F., Abdelsalam A., Assi E., Barichella M., Maestroni A., Usuelli V., Loreggian L., Muzio F., Zuccotti G.V., Cazzola R., Fiorina P. Anti-inflammatory effects of diet and caloric restriction in metabolic syndrome. J. Endocrinol. Invest. 2021;44(11):2407–2415. doi: 10.1007/s40618-021-01547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bendotti G., Montefusco L., Lunati M.E., Usuelli V., Pastore I., Lazzaroni E., Assi E., Seelam A.J., El Essawy B., Jang J., Loretelli C., D'Addio F., Berra C., Ben Nasr M., Zuccotti G., Fiorina P. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022;182 doi: 10.1016/j.phrs.2022.106320. [DOI] [PubMed] [Google Scholar]
- 138.Lazzaroni E., Ben Nasr M., Loretelli C., Pastore I., Plebani L., Lunati M.E., Vallone L., Bolla A.M., Rossi A., Montefusco L., Ippolito E., Berra C., D'Addio F., Zuccotti G.V., Fiorina P. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol. Res. 2021;171 doi: 10.1016/j.phrs.2021.105782. [DOI] [PubMed] [Google Scholar]
- 139.Petersen M.C., Shulman G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Czech M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017;23(7):804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aguirre V., Werner E.D., Giraud J., Lee Y.H., Shoelson S.E., White M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002;277(2):1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 142.Tiganis T. PTP1B and TCPTP--nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J. 2013;280(2):445–458. doi: 10.1111/j.1742-4658.2012.08563.x. [DOI] [PubMed] [Google Scholar]
- 143.Muniyappa R., Montagnani M., Koh K.K., Quon M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007;28(5):463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 144.Zeng G., Nystrom F.H., Ravichandran L.V., Cong L.N., Kirby M., Mostowski H., Quon M.J. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101(13):1539–1545. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
- 145.Abe H., Yamada N., Kamata K., Kuwaki T., Shimada M., Osuga J., Shionoiri F., Yahagi N., Kadowaki T., Tamemoto H., Ishibashi S., Yazaki Y., Makuuchi M. Hypertension, hypertriglyceridemia, and impaired endothelium-dependent vascular relaxation in mice lacking insulin receptor substrate-1. J. Clin. Invest. 1998;101(8):1784–1788. doi: 10.1172/JCI1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Montagnani M., Chen H., Barr V.A., Quon M.J. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179) J. Biol. Chem. 2001;276(32):30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 147.Tateya S., Rizzo N.O., Handa P., Cheng A.M., Morgan-Stevenson V., Daum G., Clowes A.W., Morton G.J., Schwartz M.W., Kim F. Endothelial NO/cGMP/VASP signaling attenuates Kupffer cell activation and hepatic insulin resistance induced by high-fat feeding. Diabetes. 2011;60(11):2792–2801. doi: 10.2337/db11-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang X., Liu G., Guo J., Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018;14(11):1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bedinger D.H., Adams S.H. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol. Cell. Endocrinol. 2015;415:143–156. doi: 10.1016/j.mce.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 150.Boucher J., Kleinridders A., Kahn C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harbor Perspect. Biol. 2014;6(1) doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li J.M., Li Y.C., Kong L.D., Hu Q.H. Curcumin inhibits hepatic protein-tyrosine phosphatase 1B and prevents hypertriglyceridemia and hepatic steatosis in fructose-fed rats. Hepatology. 2010;51(5):1555–1566. doi: 10.1002/hep.23524. [DOI] [PubMed] [Google Scholar]
- 152.Bashan N., Kovsan J., Kachko I., Ovadia H., Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev. 2009;89(1):27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 153.Lynch C.J., Adams S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014;10(12):723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 155.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 156.Shimobayashi M., Albert V., Woelnerhanssen B., Frei I.C., Weissenberger D., Meyer-Gerspach A.C., Clement N., Moes S., Colombi M., Meier J.A., Swierczynska M.M., Jeno P., Beglinger C., Peterli R., Hall M.N. Insulin resistance causes inflammation in adipose tissue. J. Clin. Invest. 2018;128(4):1538–1550. doi: 10.1172/JCI96139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murano I., Barbatelli G., Parisani V., Latini C., Muzzonigro G., Castellucci M., Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008;49(7):1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 158.Hirosumi J., Tuncman G., Chang L., Gorgun C.Z., Uysal K.T., Maeda K., Karin M., Hotamisligil G.S. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 159.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 160.McLaughlin T., Ackerman S.E., Shen L., Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Invest. 2017;127(1):5–13. doi: 10.1172/JCI88876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Catena C., Giacchetti G., Novello M., Colussi G., Cavarape A., Sechi L.A. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am. J. Hypertens. 2003;16(11 Pt 1):973–978. doi: 10.1016/s0895-7061(03)01002-1. [DOI] [PubMed] [Google Scholar]
- 162.Miller A.W., Tulbert C., Puskar M., Busija D.W. Enhanced endothelin activity prevents vasodilation to insulin in insulin resistance. Hypertension. 2002;40(1):78–82. doi: 10.1161/01.hyp.0000022806.87281.62. [DOI] [PubMed] [Google Scholar]
- 163.Verma S., Bhanot S., Yao L., McNeill J.H. Vascular insulin resistance in fructose-hypertensive rats. Eur. J. Pharmacol. 1997;322(2–3):R1–R2. doi: 10.1016/s0014-2999(97)00104-0. [DOI] [PubMed] [Google Scholar]
- 164.Su C.F., Chang Y.Y., Pai H.H., Liu I.M., Lo C.Y., Cheng J.T. Infusion of beta-endorphin improves insulin resistance in fructose-fed rats. Horm. Metab. Res. 2004;36(8):571–577. doi: 10.1055/s-2004-825763. [DOI] [PubMed] [Google Scholar]
- 165.Babacanoglu C., Yildirim N., Sadi G., Pektas M.B., Akar F. Resveratrol prevents high-fructose corn syrup-induced vascular insulin resistance and dysfunction in rats. Food Chem. Toxicol. 2013;60:160–167. doi: 10.1016/j.fct.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 166.Eccleston H.B., Andringa K.K., Betancourt A.M., King A.L., Mantena S.K., Swain T.M., Tinsley H.N., Nolte R.N., Nagy T.R., Abrams G.A., Bailey S.M. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxidants Redox Signal. 2011;15(2):447–459. doi: 10.1089/ars.2010.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu X., Zhao C.X., Wang L., Tu L., Fang X., Zheng C., Edin M.L., Zeldin D.C., Wang D.W. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes. 2010;59(4):997–1005. doi: 10.2337/db09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhao C.X., Xu X., Cui Y., Wang P., Wei X., Yang S., Edin M.L., Zeldin D.C., Wang D.W. Increased endothelial nitric-oxide synthase expression reduces hypertension and hyperinsulinemia in fructose-treated rats. J. Pharmacol. Exp. Therapeut. 2009;328(2):610–620. doi: 10.1124/jpet.108.143396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Baena M., Sanguesa G., Davalos A., Latasa M.J., Sala-Vila A., Sanchez R.M., Roglans N., Laguna J.C., Alegret M. Fructose, but not glucose, impairs insulin signaling in the three major insulin-sensitive tissues. Sci. Rep. 2016;6 doi: 10.1038/srep26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rebollo A., Roglans N., Baena M., Padrosa A., Sanchez R.M., Merlos M., Alegret M., Laguna J.C. Liquid fructose down-regulates liver insulin receptor substrate 2 and gluconeogenic enzymes by modifying nutrient sensing factors in rats. J. Nutr. Biochem. 2014;25(2):250–258. doi: 10.1016/j.jnutbio.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 171.Vila L., Roglans N., Perna V., Sanchez R.M., Vazquez-Carrera M., Alegret M., Laguna J.C. Liver AMP/ATP ratio and fructokinase expression are related to gender differences in AMPK activity and glucose intolerance in rats ingesting liquid fructose. J. Nutr. Biochem. 2011;22(8):741–751. doi: 10.1016/j.jnutbio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 172.Vatner D.F., Majumdar S.K., Kumashiro N., Petersen M.C., Rahimi Y., Gattu A.K., Bears M., Camporez J.P., Cline G.W., Jurczak M.J., Samuel V.T., Shulman G.I. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc. Natl. Acad. Sci. U.S.A. 2015;112(4):1143–1148. doi: 10.1073/pnas.1423952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Haas J.T., Miao J., Chanda D., Wang Y., Zhao E., Haas M.E., Hirschey M., Vaitheesvaran B., Farese R.V., Jr., Kurland I.J., Graham M., Crooke R., Foufelle F., Biddinger S.B. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metabol. 2012;15(6):873–884. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang D., Tong X., VanDommelen K., Gupta N., Stamper K., Brady G.F., Meng Z., Lin J., Rui L., Omary M.B., Yin L. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. J. Clin. Invest. 2017;127(7):2855–2867. doi: 10.1172/JCI89934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pektas M.B., Koca H.B., Sadi G., Akar F. Dietary fructose activates insulin signaling and inflammation in adipose tissue: modulatory role of resveratrol. BioMed Res. Int. 2016 doi: 10.1155/2016/8014252. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li Y., Wang J., Gu T., Yamahara J., Li Y. Oleanolic acid supplement attenuates liquid fructose-induced adipose tissue insulin resistance through the insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt signaling pathway in rats. Toxicol. Appl. Pharmacol. 2014;277(2):155–163. doi: 10.1016/j.taap.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 177.Kovacevic S., Brkljacic J., Vojnovic Milutinovic D., Gligorovska L., Bursac B., Elakovic I., Djordjevic A. Fructose induces visceral adipose tissue inflammation and insulin resistance even without development of obesity in adult female but not in male rats. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.749328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fan Y., Liu Y., Xue K., Gu G., Fan W., Xu Y., Ding Z. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gu T.T., Chen T.Y., Yang Y.Z., Zhao X.J., Sun Y., Li T.S., Zhang D.M., Kong L.D. Pterostilbene alleviates fructose-induced renal fibrosis by suppressing TGF-beta1/TGF-beta type I receptor/Smads signaling in proximal tubular epithelial cells. Eur. J. Pharmacol. 2019;842:70–78. doi: 10.1016/j.ejphar.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 180.Gu T.T., Song L., Chen T.Y., Wang X., Zhao X.J., Ding X.Q., Yang Y.Z., Pan Y., Zhang D.M., Kong L.D. Fructose downregulates miR-330 to induce renal inflammatory response and insulin signaling impairment: attenuation by morin. Mol. Nutr. Food Res. 2017;61(8) doi: 10.1002/mnfr.201600760. [DOI] [PubMed] [Google Scholar]
- 181.Hu Q.H., Zhang X., Pan Y., Li Y.C., Kong L.D. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem. Pharmacol. 2012;84(1):113–125. doi: 10.1016/j.bcp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 182.Fujimoto M., Shimizu N., Kunii K., Martyn J.A., Ueki K., Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes. 2005;54(5):1340–1348. doi: 10.2337/diabetes.54.5.1340. [DOI] [PubMed] [Google Scholar]
- 183.Koh K.K., Han S.H., Quon M.J. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J. Am. Coll. Cardiol. 2005;46(11):1978–1985. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 184.Fan C.Y., Wang M.X., Ge C.X., Wang X., Li J.M., Kong L.D. Betaine supplementation protects against high-fructose-induced renal injury in rats. J. Nutr. Biochem. 2014;25(3):353–362. doi: 10.1016/j.jnutbio.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 185.Castro M.C., Massa M.L., Arbelaez L.G., Schinella G., Gagliardino J.J., Francini F. Fructose-induced inflammation, insulin resistance and oxidative stress: a liver pathological triad effectively disrupted by lipoic acid. Life Sci. 2015;137:1–6. doi: 10.1016/j.lfs.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 186.Tamer F., Ulug E., Akyol A., Nergiz-Unal R. The potential efficacy of dietary fatty acids and fructose induced inflammation and oxidative stress on the insulin signaling and fat accumulation in mice. Food Chem. Toxicol. 2020;135 doi: 10.1016/j.fct.2019.110914. [DOI] [PubMed] [Google Scholar]
- 187.Kubacka M., Mogilski S., Zadrozna M., Nowak B., Szafarz M., Pomierny B., Marona H., Waszkielewicz A., Jawien W., Sapa J., Bednarski M., Knutelska J., Kotanska M. MH-76, a novel non-quinazoline alpha(1)-adrenoceptor antagonist, but not prazosin reduces inflammation and improves insulin signaling in adipose tissue of fructose-fed rats. Pharmaceuticals. 2021;14(5) doi: 10.3390/ph14050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Maithilikarpagaselvi N., Sridhar M.G., Swaminathan R.P., Zachariah B. Curcumin prevents inflammatory response, oxidative stress and insulin resistance in high fructose fed male Wistar rats: potential role of serine kinases. Chem. Biol. Interact. 2016;244:187–194. doi: 10.1016/j.cbi.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 189.Traish A.M., Johansen V. Impact of testosterone deficiency and testosterone therapy on lower urinary tract symptoms in men with metabolic syndrome. World J Mens Health. 2018;36(3):199–222. doi: 10.5534/wjmh.180032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wickramatilake C.M., Mohideen M.R., Pathirana C. Association of metabolic syndrome with testosterone and inflammation in men. Ann. Endocrinol. 2015;76(3):260–263. doi: 10.1016/j.ando.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 191.Bianchi V.E., Locatelli V. Testosterone a key factor in gender related metabolic syndrome. Obes. Rev. 2018;19(4):557–575. doi: 10.1111/obr.12633. [DOI] [PubMed] [Google Scholar]
- 192.Dandona P., Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J. Clin. Endocrinol. Metab. 2011;96(9):2643–2651. doi: 10.1210/jc.2010-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rovira-Llopis S., Banuls C., de Maranon A.M., Diaz-Morales N., Jover A., Garzon S., Rocha M., Victor V.M., Hernandez-Mijares A. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic. Biol. Med. 2017;108:155–162. doi: 10.1016/j.freeradbiomed.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 194.MacDonald A.A., Herbison G.P., Showell M., Farquhar C.M. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum. Reprod. Update. 2010;16(3):293–311. doi: 10.1093/humupd/dmp047. [DOI] [PubMed] [Google Scholar]
- 195.Sermondade N., Faure C., Fezeu L., Shayeb A.G., Bonde J.P., Jensen T.K., Van Wely M., Cao J., Martini A.C., Eskandar M., Chavarro J.E., Koloszar S., Twigt J.M., Ramlau-Hansen C.H., Borges E., Jr., Lotti F., Steegers-Theunissen R.P., Zorn B., Polotsky A.J., La Vignera S., Eskenazi B., Tremellen K., Magnusdottir E.V., Fejes I., Hercberg S., Levy R., Czernichow S. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum. Reprod. Update. 2013;19(3):221–231. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Turan E., Oztekin U. Relationship between visceral adiposity index and male infertility. Andrologia. 2020;52(4) doi: 10.1111/and.13548. [DOI] [PubMed] [Google Scholar]
- 197.Wake D.J., Strand M., Rask E., Westerbacka J., Livingstone D.E., Soderberg S., Andrew R., Yki-Jarvinen H., Olsson T., Walker B.R. Intra-adipose sex steroid metabolism and body fat distribution in idiopathic human obesity. Clin. Endocrinol. 2007;66(3):440–446. doi: 10.1111/j.1365-2265.2007.02755.x. [DOI] [PubMed] [Google Scholar]
- 198.Meseguer A., Puche C., Cabero A. Sex steroid biosynthesis in white adipose tissue. Horm. Metab. Res. 2002;34(11–12):731–736. doi: 10.1055/s-2002-38249. [DOI] [PubMed] [Google Scholar]
- 199.Katib A. Mechanisms linking obesity to male infertility. Cent European J Urol. 2015;68(1):79–85. doi: 10.5173/ceju.2015.01.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Azenabor A., Ekun A.O., Akinloye O. Impact of inflammation on male reproductive tract. J. Reproduction Infertil. 2015;16(3):123–129. [PMC free article] [PubMed] [Google Scholar]
- 201.Akar F., Yildirim O.G., Yucel Tenekeci G., Tunc A.S., Demirel M.A., Sadi G. Dietary high-fructose reduces barrier proteins and activates mitogenic signalling in the testis of a rat model: regulatory effects of kefir supplementation. Andrologia. 2022;54(3) doi: 10.1111/and.14342. [DOI] [PubMed] [Google Scholar]
- 202.Yildirim O.G., Sumlu E., Aslan E., Koca H.B., Pektas M.B., Sadi G., Akar F. High-fructose in drinking water initiates activation of inflammatory cytokines and testicular degeneration in rat. Toxicol. Mech. Methods. 2019;29(3):224–232. doi: 10.1080/15376516.2018.1543745. [DOI] [PubMed] [Google Scholar]
- 203.Akarca-Dizakar S.O., Erdogan D., Peker T., Coskun Akcay N., Turkoglu I., Esmekaya M.A., Omeroglu S. Effects of co-administered melatonin, fructose and bisphenol A (BPA) on rat epididymis and sperm characteristics. Biotech. Histochem. 2020;95(1):18–26. doi: 10.1080/10520295.2019.1627418. [DOI] [PubMed] [Google Scholar]
- 204.Aslankoc R., Ozmen O. The effects of high-fructose corn syrup consumption on testis physiopathology-The ameliorative role of melatonin. Andrologia. 2019;51(8) doi: 10.1111/and.13327. [DOI] [PubMed] [Google Scholar]
- 205.Dokumacioglu E., Iskender H., Yenice G., Kapakin K.A.T., Sevim C., Hayirli A., Saral S., Comakli S. Effects of astaxanthin on biochemical and histopathological parameters related to oxidative stress on testes of rats on high fructose regime. Andrologia. 2018;50(7) doi: 10.1111/and.13042. [DOI] [PubMed] [Google Scholar]
- 206.Meydanli E.G., Gumusel A., Ozkan S., Tanriverdi G., Balci M.B.C., Develi Is S., Hazar A.I., Uysal M., Bekpinar S. Effects of resveratrol on high-fructose-induced testis injury in rats. Ultrastruct. Pathol. 2018;42(1):65–73. doi: 10.1080/01913123.2017.1397075. [DOI] [PubMed] [Google Scholar]
- 207.Ramirez N.D., Luque E.M., Jones X.M., Torres P.J., Moreira Espinoza M.J., Cantarelli V., Ponzio M.F., Arja A., Rabaglino M.B., Martini A.C. Modulatory effects of ghrelin on sperm quality alterations induced by a fructose-enriched diet. Heliyon. 2019;5(11) doi: 10.1016/j.heliyon.2019.e02886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.El-Mehi A.E., Faried M.A. Effect of high-fructose diet-induced metabolic syndrome on the pituitary-gonadal axis from adolescence through adulthood in male albino rats and the possible protective role of ginger extract. A biochemical, histological and immunohistochemical study. Folia Morphol. 2020;79(4):690–708. doi: 10.5603/FM.a2019.0139. [DOI] [PubMed] [Google Scholar]
- 209.Medaglia D.S.A., Vieira H.R., Silveira S.D.S., Siervo G., Marcon M., Mathias P.C.F., Fernandes G.S.A. High-fructose diet during puberty alters the sperm parameters, testosterone concentration, and histopathology of testes and epididymis in adult Wistar rats. J Dev Orig Health Dis. 2022;13(1):20–27. doi: 10.1017/S2040174420001385. [DOI] [PubMed] [Google Scholar]
- 210.Zhang Y., Li Y., Zhang J., Qi X., Cui Y., Yin K., Lin H. Cadmium induced inflammation and apoptosis of porcine epididymis via activating RAF1/MEK/ERK and NF-kappaB pathways. Toxicol. Appl. Pharmacol. 2021;415 doi: 10.1016/j.taap.2021.115449. [DOI] [PubMed] [Google Scholar]
- 211.Nna V.U., Ujah G.A., Mohamed M., Etim K.B., Igba B.O., Augustine E.R., Osim E.E. Cadmium chloride-induced testicular toxicity in male wistar rats; prophylactic effect of quercetin, and assessment of testicular recovery following cadmium chloride withdrawal. Biomed. Pharmacother. 2017;94:109–123. doi: 10.1016/j.biopha.2017.07.087. [DOI] [PubMed] [Google Scholar]
- 212.Izumi Y., Yamaguchi K., Ishikawa T., Ando M., Chiba K., Hashimoto H., Shiotani M., Fujisawa M. Molecular changes induced by bisphenol-A in rat Sertoli cell culture. Syst. Biol. Reprod. Med. 2011;57(5):228–232. doi: 10.3109/19396368.2011.574248. [DOI] [PubMed] [Google Scholar]
- 213.Qi S., Fu W., Wang C., Liu C., Quan C., Kourouma A., Yan M., Yu T., Duan P., Yang K. BPA-induced apoptosis of rat Sertoli cells through Fas/FasL and JNKs/p38 MAPK pathways. Reprod. Toxicol. 2014;50:108–116. doi: 10.1016/j.reprotox.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 214.Aziz N., Said T., Paasch U., Agarwal A. The relationship between human sperm apoptosis, morphology and the sperm deformity index. Hum. Reprod. 2007;22(5):1413–1419. doi: 10.1093/humrep/dem016. [DOI] [PubMed] [Google Scholar]
- 215.Wong C.H., Cheng C.Y. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data. Dev. Biol. 2005;286(1):1–15. doi: 10.1016/j.ydbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 216.Wang M., Su P. The role of the Fas/FasL signaling pathway in environmental toxicant-induced testicular cell apoptosis: an update. Syst. Biol. Reprod. Med. 2018;64(2):93–102. doi: 10.1080/19396368.2017.1422046. [DOI] [PubMed] [Google Scholar]
- 217.Wang Z., Wade P., Mandell K.J., Akyildiz A., Parkos C.A., Mrsny R.J., Nusrat A. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 2007;26(8):1222–1230. doi: 10.1038/sj.onc.1209902. [DOI] [PubMed] [Google Scholar]
- 218.Said T.M., Grunewald S., Paasch U., Glander H.J., Baumann T., Kriegel C., Li L., Agarwal A. Advantage of combining magnetic cell separation with sperm preparation techniques. Reprod. Biomed. Online. 2005;10(6):740–746. doi: 10.1016/s1472-6483(10)61118-2. [DOI] [PubMed] [Google Scholar]
- 219.Kim B., Breton S. The MAPK/ERK-Signaling pathway regulates the expression and distribution of tight junction proteins in the mouse proximal epididymis. Biol. Reprod. 2016;94(1):22. doi: 10.1095/biolreprod.115.134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li M.W., Mruk D.D., Cheng C.Y. Mitogen-activated protein kinases in male reproductive function. Trends Mol. Med. 2009;15(4):159–168. doi: 10.1016/j.molmed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fan W., Xu Y., Liu Y., Zhang Z., Lu L., Ding Z. Obesity or overweight, a chronic inflammatory status in male reproductive system, leads to mice and human subfertility. Front. Physiol. 2017;8:1117. doi: 10.3389/fphys.2017.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jiang Y.P., Yang J.M., Ye R.J., Liu N., Zhang W.J., Ma L., Zheng P., Niu J.G., Liu P., Yu J.Q. Protective effects of betaine on diabetic induced disruption of the male mice blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Biomed. Pharmacother. 2019;120 doi: 10.1016/j.biopha.2019.109474. [DOI] [PubMed] [Google Scholar]
- 223.Rashid K., Sil P.C. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim. Biophys. Acta. 2015;1852(1):70–82. doi: 10.1016/j.bbadis.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 224.Zha W., Bai Y., Xu L., Liu Y., Yang Z., Gao H., Li J. Curcumin attenuates testicular injury in rats with streptozotocin-induced diabetes. BioMed Res. Int. 2018 doi: 10.1155/2018/7468019. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhao Y., Tan Y., Dai J., Li B., Guo L., Cui J., Wang G., Shi X., Zhang X., Mellen N., Li W., Cai L. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol. Lett. 2011;200(1–2):100–106. doi: 10.1016/j.toxlet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 226.Lee Y.S., Yoon H.J., Oh J.H., Park H.J., Lee E.H., Song C.W., Yoon S. 1,3-Dinitrobenzene induces apoptosis in TM4 mouse Sertoli cells: involvement of the c-Jun N-terminal kinase (JNK) MAPK pathway. Toxicol. Lett. 2009;189(2):145–151. doi: 10.1016/j.toxlet.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 227.Peretz J., Vrooman L., Ricke W.A., Hunt P.A., Ehrlich S., Hauser R., Padmanabhan V., Taylor H.S., Swan S.H., VandeVoort C.A., Flaws J.A. Bisphenol a and reproductive health: update of experimental and human evidence, 2007-2013. Environ. Health Perspect. 2014;122(8):775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang H., Zhou W., Zhang J., Li H. Role of JNK and ERK1/2 MAPK signaling pathway in testicular injury of rats induced by di-N-butyl-phthalate (DBP) Biol. Res. 2019;52(1):41. doi: 10.1186/s40659-019-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cheng C.Y., Mruk D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012;64(1):16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cyr D.G., Gregory M., Dube E., Dufresne J., Chan P.T., Hermo L. Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood-epididymal barrier in animals and humans. Asian J. Androl. 2007;9(4):463–475. doi: 10.1111/j.1745-7262.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 231.Chung N.P., Cheng C.Y. Is cadmium chloride-induced inter-sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142(5):1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- 232.Li Y., Wu J., Zhou W., Gao E. Association between environmental exposure to cadmium and human semen quality. Int. J. Environ. Health Res. 2016;26(2):175–186. doi: 10.1080/09603123.2015.1061115. [DOI] [PubMed] [Google Scholar]
- 233.Ye J., Luo D., Xu X., Sun M., Su X., Tian Z., Zhang M., Yu C., Guan Q. Metformin improves fertility in obese males by alleviating oxidative stress-induced blood-testis barrier damage. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/9151067. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Alves M.G., Martins A.D., Cavaco J.E., Socorro S., Oliveira P.F. Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers. 2013;1(2) doi: 10.4161/tisb.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jiang Y.P., Ye R.J., Yang J.M., Liu N., Zhang W.J., Ma L., Sun T., Niu J.G., Zheng P., Yu J.Q. Protective effects of Salidroside on spermatogenesis in streptozotocin induced type-1 diabetic male mice by inhibiting oxidative stress mediated blood-testis barrier damage. Chem. Biol. Interact. 2020;315 doi: 10.1016/j.cbi.2019.108869. [DOI] [PubMed] [Google Scholar]
- 236.Du Y., Zhu Y., Teng X., Zhang K., Teng X., Li S. Toxicological effect of manganese on NF-kappaB/iNOS-COX-2 signaling pathway in chicken testes. Biol. Trace Elem. Res. 2015;168(1):227–234. doi: 10.1007/s12011-015-0340-5. [DOI] [PubMed] [Google Scholar]
- 237.Ogo F.M., de Lion Siervo G.E.M., Staurengo-Ferrari L., de Oliveira Mendes L., Luchetta N.R., Vieira H.R., Fattori V., Verri W.A., Jr., Scarano W.R., Fernandes G.S.A. Bisphenol A exposure impairs epididymal development during the peripubertal period of rats: inflammatory profile and tissue changes. Basic Clin. Pharmacol. Toxicol. 2018;122(2):262–270. doi: 10.1111/bcpt.12894. [DOI] [PubMed] [Google Scholar]
- 238.Leisegang K., Bouic P.J., Henkel R.R. Metabolic syndrome is associated with increased seminal inflammatory cytokines and reproductive dysfunction in a case-controlled male cohort. Am. J. Reprod. Immunol. 2016;76(2):155–163. doi: 10.1111/aji.12529. [DOI] [PubMed] [Google Scholar]
- 239.Condorelli R.A., Calogero A.E., Vicari E., Duca Y., Favilla V., Morgia G., Cimino S., Di Mauro M., La Vignera S. Prevalence of male accessory gland inflammations/infections in patients with Type 2 diabetes mellitus. J. Endocrinol. Invest. 2013;36(9):770–774. doi: 10.3275/8950. [DOI] [PubMed] [Google Scholar]
- 240.Calogero A.E., Duca Y., Condorelli R.A., La Vignera S. Male accessory gland inflammation, infertility, and sexual dysfunctions: a practical approach to diagnosis and therapy. Andrology. 2017;5(6):1064–1072. doi: 10.1111/andr.12427. [DOI] [PubMed] [Google Scholar]
- 241.Ko E.A., Kim H.R., Kim Y.B., Kim H.S., Lee S.H. Effect of high fructose corn syrup (HFCS) intake on the female reproductive organs and lipid accumulation in adult rats. Dev Reprod. 2017;21(2):151–156. doi: 10.12717/DR.2017.21.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Akintayo C.O., Johnson A.D., Badejogbin O.C., Olaniyi K.S., Oniyide A.A., Ajadi I.O., Ojewale A.O., Adeyomoye O.I., Kayode A.B. High fructose-enriched diet synergistically exacerbates endocrine but not metabolic changes in letrozole-induced polycystic ovarian syndrome in Wistar rats. Heliyon. 2021;7(1) doi: 10.1016/j.heliyon.2020.e05890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Murri M., Insenser M., Fernandez-Duran E., San-Millan J.L., Luque-Ramirez M., Escobar-Morreale H.F. Non-targeted profiling of circulating microRNAs in women with polycystic ovary syndrome (PCOS): effects of obesity and sex hormones. Metabolism. 2018;86:49–60. doi: 10.1016/j.metabol.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 244.Asghar Z.A., Thompson A., Chi M., Cusumano A., Scheaffer S., Al-Hammadi N., Saben J.L., Moley K.H. Maternal fructose drives placental uric acid production leading to adverse fetal outcomes. Sci. Rep. 2016;6 doi: 10.1038/srep25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gray C., Long S., Green C., Gardiner S.M., Craigon J., Gardner D.S. Maternal fructose and/or salt intake and reproductive outcome in the rat: effects on growth, fertility, sex ratio, and birth order. Biol. Reprod. 2013;89(3):51. doi: 10.1095/biolreprod.113.109595. [DOI] [PubMed] [Google Scholar]
- 246.Rodriguez L., Otero P., Panadero M.I., Rodrigo S., Alvarez-Millan J.J., Bocos C. Maternal fructose intake induces insulin resistance and oxidative stress in male, but not female, offspring. J Nutr Metab. 2015 doi: 10.1155/2015/158091. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Seong H.Y., Cho H.M., Kim M., Kim I. Maternal high-fructose intake induces multigenerational activation of the renin-angiotensin-aldosterone system. Hypertension. 2019;74(3):518–525. doi: 10.1161/HYPERTENSIONAHA.119.12941. [DOI] [PubMed] [Google Scholar]
- 248.Vickers M.H., Clayton Z.E., Yap C., Sloboda D.M. Maternal fructose intake during pregnancy and lactation alters placental growth and leads to sex-specific changes in fetal and neonatal endocrine function. Endocrinology. 2011;152(4):1378–1387. doi: 10.1210/en.2010-1093. [DOI] [PubMed] [Google Scholar]
- 249.Englund-Ogge L., Brantsaeter A.L., Haugen M., Sengpiel V., Khatibi A., Myhre R., Myking S., Meltzer H.M., Kacerovsky M., Nilsen R.M., Jacobsson B. Association between intake of artificially sweetened and sugar-sweetened beverages and preterm delivery: a large prospective cohort study. Am. J. Clin. Nutr. 2012;96(3):552–559. doi: 10.3945/ajcn.111.031567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.