Abstract
Mn2+ is an essential nutrient whose concentration is tightly controlled in bacteria. In Bacillus subtilis, the Mn2+-activated transcription factor MntR controls Mn2+ transporter genes. However, factors regulating intracellular Mn2+ concentration are incompletely understood. Here, we found that glucose addition induces an increase in intracellular Mn2+ concentration. We determined this upshift was mediated by glucose induction of the major Mn2+ importer gene mntH by the transcription factor AhrC, which is known to be involved in arginine metabolism and to be indirectly induced by glucose. In addition, we identified novel AhrC-regulated genes encoding the Mn2+ importer YcsG and the ABC-type exporter YknUV. We found the expression of these genes was also regulated by glucose and contributes to the glucose induction of Mn2+ concentrations. ycsG expression is regulated by MntR as well. Furthermore, we analyzed the interaction of AhrC and MntR with the promoter driving ycsG expression and examined the Mn2+-dependent induction of this promoter to identify the transcription factors responsible for the Mn2+ induction. RNA-Seq revealed that disruption of ahrC and mntR affected the expression of 502 and 478 genes, respectively (false discovery rate, <0.001, log2[fold change] ≥ |2|. The AhrC- and/or MntR-dependent expression of twenty promoters was confirmed by LacZ analysis, and AhrC or MntR binding to some of these promoters was observed via EMSA. The finding that glucose promotes an increase in intracellular Mn2+ levels without changes in extracellular Mn2+ concentrations is reasonable for the bacterium, as intracellular Mn2+ is required for enzymes and pathways mediating glucose metabolism.
Keywords: manganese homeostasis, manganese transporter genes, glucose induction, RNA-Seq, metabolic homeostasis
Glucose is the most favored carbon source for many bacteria; therefore, bacteria have developed several glucose-responsive systems (1, 2). In Gram-positive bacteria, including Bacillus subtilis, catabolite control protein A (CcpA) is the master transcription regulator for carbon catabolite regulation (1, 2). Incorporating glucose into bacterial cells increases the levels of metabolites such as fructose-1,6-bisphosphate in the glycolysis pathway, which triggers HPr phosphorylation at Ser46. HPr is a phosphocarrier protein in the sugar phosphotransferase system, and P-Ser-HPr activates CcpA, resulting in large transcriptome changes. Moreover, there are several additional glucose-responsive transcription factors, such as CcpC, CcpN, CggR, and GlcT (2). CcpN regulates the structural genes involved in gluconeogenesis (3). Our recent studies also revealed a glucose-responsive system that includes the nucleoid-associated protein YlxR (4, 5, 6) and ywlE encoding a phosphatase for protein Arg-phosphate, which is a regulatory factor for ylxR expression (7). Glucose induced ywlE expression through an unknown mechanism, leading to the induction of ylxR expression (7). YwlE counteracts arginine phosphorylation of proteins by McsB kinase, thus protecting the protein from degradation (8). Proteomic analyses have revealed that most glycolytic enzymes are targets of McsB (8), which suggests that YwlE protects glycolytic enzymes from degradation (7).
Mn2+ is an essential nutrient in organisms, including bacteria such as B. subtilis, because it plays roles in cell differentiation, biofilm formation, many metabolic pathways, and stress responses including oxidative stress (9, 10, 11, 12, 13, 14, 15, 16, 17). In Streptococci, the intracellular Mn2+ concentration affects the glycolytic enzymes expression and metabolome (10, 11). In Staphylococcus aureus, glycolysis increases the cellular demand for Mn2+ and this bacterium has a metal-independent and a metal (Mn2+ or Zn2+)-dependent fructose 1,6-bisphosphate aldolase, which enables S. aureus to resist against host-imposed Mn2+ limitation (12). In Bacillus, Mn2+ is a cofactor for two glycolytic enzymes, namely phosphoglycerate mutase and pyruvate kinase (18, 19), indicating a link between glycolytic enzymes and Mn2+. However, excess Mn2+ is harmful and causes intoxication, mainly through mismetallation of proteins (16, 17). Thus, intracellular Mn2+ concentrations are tightly regulated at transcription and post-transcription levels (20, 21). In B. subtilis, the Mn2+-activated transcription factor MntR plays a central role in Mn2+ homeostasis (21). B. subtilis is known to contain two Mn2+ importers, MntABCD (ABC-type transporter) and MntH (primary importer), and major and minor exporters MneP and MneS, respectively, both of which are cation diffusion facilitators (22, 23). TerC-type transporters YkoY and YceF have also been reported; however, their involvement in Mn2+ transport remains unclear (23). When intracellular Mn2+ concentrations are low, MntR does not repress importer genes or activate exporter genes. In contrast, MntR represses importer genes under high intracellular Mn2+ concentrations, and a further upshift in Mn2+ concentrations leads to MntR-mediated activation of exporter genes. This MntR-mediated Mn2+ homeostasis is dependent on the fact that MntR requires higher levels of Mn2+ to activate exporter genes (24). In other bacteria, for example Escherichia coli, Mn2+ homeostasis is maintained by MntR and additional transcription factors such as H-NS, OxyR, and ferric uptake regulator (17).
We started this study from the mechanistic analysis of the previous observation of glucose induction (GI) of ywlE. We found GI of Mn2+ concentrations, leading to GI of ywlE expression. The GI of translation of AhrC, an arginine metabolism-controlling transcription factor, is already known (25, 26, 27), and we found that AhrC regulates the four known MntR-regulated Mn2+ transporter loci. RNA-Seq analysis of ahrC and mntR disruptants resulted in the identification of two new Mn2+ transporter loci, contributing to the GI of Mn2+ concentrations.
Results
Cellular Mn2+ concentrations regulate ywlE expression
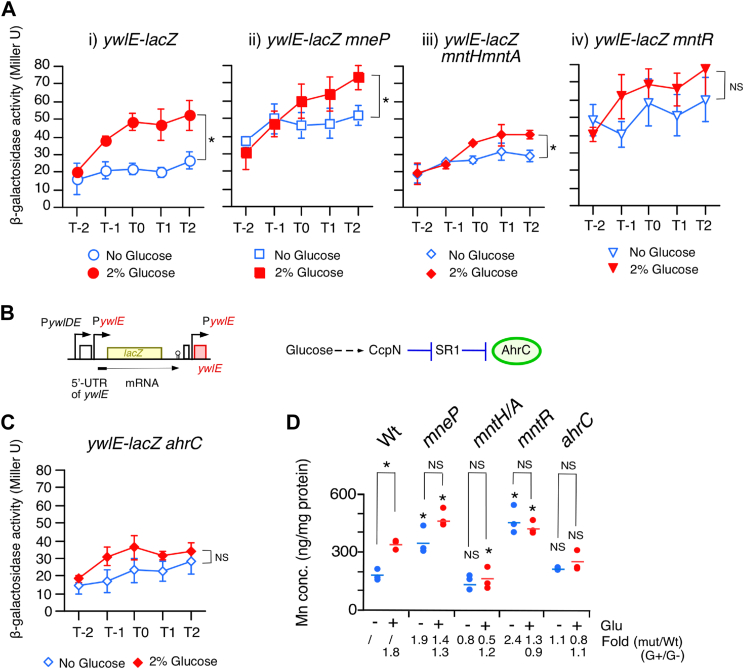
Previously, we observed that ywlE-lacZ expression (Fig. 1B) was induced by glucose in sporulation medium (i, Fig. 1A), which was confirmed at the protein level (7). The ywlD gene, whose product is similar to the Mn2+ exporter MntP in E. coli, is located upstream of ywlE (Fig. S1A) (28). We observed that glucose completely inhibited PywlDE-lacZ at the amyE locus (left, Fig. S1B), which seems to be incompatible with GI of ywlE. We therefore investigated the glucose effect on the overall expression of ywlE using (PywlDE plus PywlE)-lacZ (Fig. S1A). We confirmed GI of ywlE using this fusion (middle, Fig. S1B). Generally, genes whose function is related to each other in cellular physiology tend to form a cluster in the bacterial genome (29). Thus, we hypothesized that the putative Mn2+ exporter is downregulated by glucose, resulting in an increase in cellular Mn2+ concentration, somehow leading to the upregulation of ywlE-lacZ. Indeed, glucose addition elevated the Mn2+ concentration by 1.8-fold, which has not been previously reported, to the best of our knowledge (Fig. 1D). To investigate whether YwlD is involved in Mn2+ transport, we examined Mn2+ concentration in the ywlD disruptant and observed no changes of cellular Mn2+ concentration and GI of ywlE-lacZ (right, Fig. S1, B and C). We conclude that YwlD is not involved in Mn2+ homeostasis.
Figure 1.
Glucose induction of ywlE-lacZ and increase in cellular Mn concentrations. (A) and (C) β-Galactosidase activities were measured by using highly sensitive substrate CPRG shown in Miller units. Means from three independent experiments and the SDs are shown. Significant differences in the effects of glucose addition at T2 were determined using nonpaired t test. ∗p < 0.05; NS, no significant differences. The x-axis represents the growth time in hours relative to the end of vegetative growth (T0). Cells were grown in sporulation medium with (closed symbols) or without (open symbols) 2% glucose and sampled hourly. Strains: WT (OAM888), and derivatives of OAM888, mneP (OAM1004), mntH/mntA (OAM1003), mntR (OAM1007), and ahrC (OAM1006). (B) left: relevant structure of OAM888. Box, bent arrows, and stem–loop show ORF, promoter, and terminator, respectively. Right: flow of glucose-mediated regulation of ahrC. Dotted arrow and T-bar mean indirect activation and direct inhibition, respectively. SR1, small regulatory RNA, inhibits translation of ahrC. (D) cellular Mn concentrations. T2 cells grown in sporulation medium were harvested and processed. Strains; WT (168), mneP (OAM993), mntH/mntA (OAM992), mntR (OAM996), and ahrC (OAM995). “Glu” represents glucose. Three biologically independent samples were measured. Significant differences between Wt and mutants, with or without glucose (∗ and “NS” above each data point indicate p < 0.05 and no significant difference, respectively) and the effect of glucose addition to each strain were determined using nonpaired t test. ∗p < 0.05; NS, no significant differences. The short horizontal lines indicate the mean of the data points. CPRG, chlorophenolred β-D-galactopyranoside.
When the gene encoding the Mn2+ exporter MneP was disrupted, Mn2+ concentration was increased as expected (Fig. 1D), and ywlE-lacZ expression was also increased independent of glucose (ii, Fig. 1A). Conversely, in the mntA/mntH double disruptant of the importer genes, the Mn2+ concentration was decreased in the presence of glucose, compared to that in the WT (Fig. 1D). Concomitantly, ywlE-lacZ expression decreased in the presence of glucose (iii, Fig. 1A). Thus, changes in cellular Mn2+ concentration cause the changes of ywlE expression. This mntA/mntH strain showed slightly longer growth lag in the semisynthetic modified competence (MC) medium containing glucose, suggesting a role of the GI of Mn2+ (Fig. S2A) (30). Notably, in this double mutant, Mn2+ concentrations were observed to sustain growth, suggesting the presence of a third unknown importer; this has been reported previously (22). Based on these results, we concluded that ywlE-lacZ expression is an indicator of Mn2+ concentration. Furthermore, Mn2+ concentration is known to increase in the mntR disruptant (1.6-fold enhancement in LB medium, 31), which we also confirmed (Fig. 1D). Thus, the 1.8-fold change (FC) induced by glucose addition in the WT strain is significant. In addition, we confirmed that higher ywlE-lacZ expression was observed with and without glucose in the mntR disruptant (iv, Fig. 1A). This supports the idea that Mn2+ concentration regulates the expression of ywlE-lacZ. To elucidate the GI of Mn2+ concentrations, we examined Mn2+ concentrations in the ccpA disruptant; however, compared to the WT, no change in the GI pattern was observed (Fig. S1C). Next, we tested Mn2+ concentrations in the ahrC disruptant, which encodes a transcriptional regulator for arginine metabolism genes and is activated indirectly by glucose (Fig. 1B) (26, 27). In the ahrC disruptant, GI of neither Mn2+ concentration nor ywlE-lacZ expression was observed (Fig. 1, C and D). Hence, ahrC is involved in regulating Mn2+ concentrations.
Regulation of four known Mn2+ transporter genes by AhrC
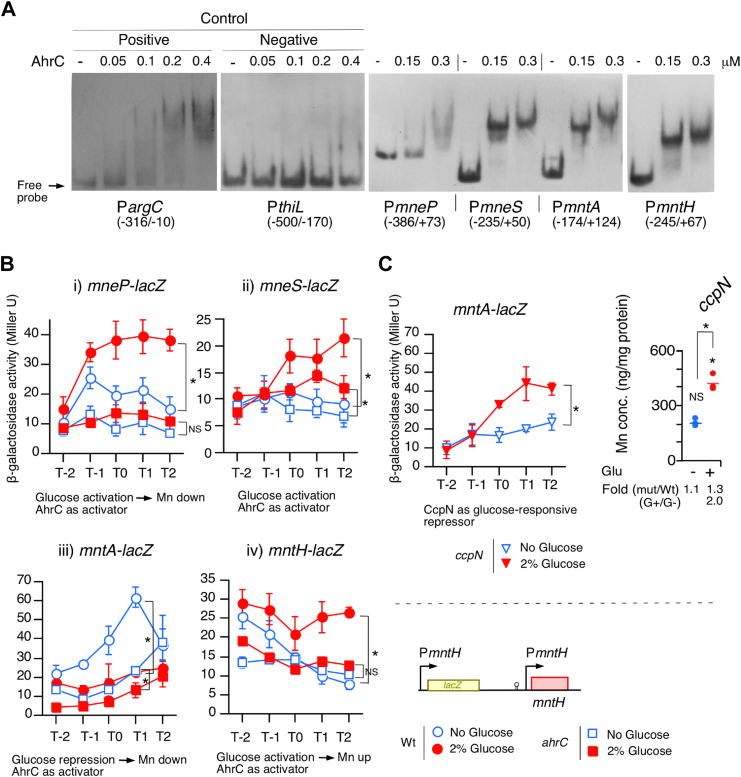
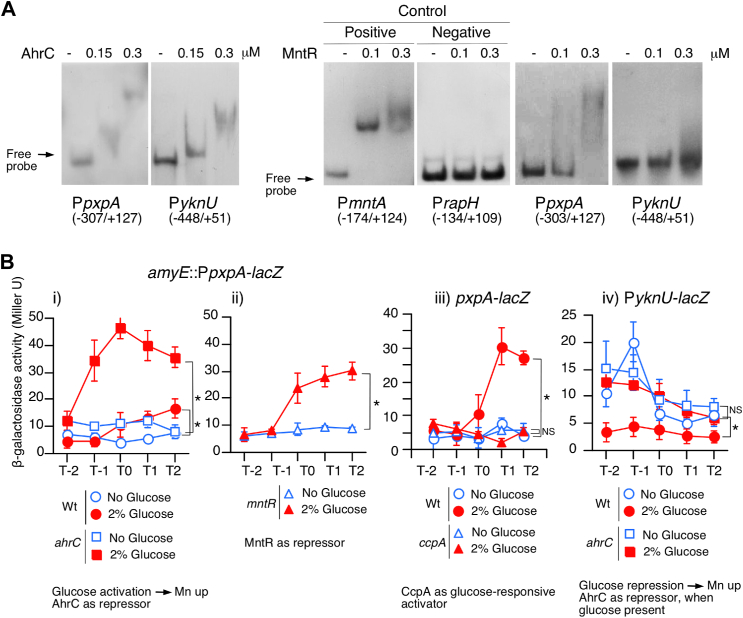
In the ahrC disruptant, GI of both Mn2+ concentration and ywlE-lacZ expression were abolished; therefore, we examined whether AhrC regulates Mn2+ transporter genes. AhrC was purified and used for the EMSA. Within the protein concentrations at which specific binding was observed (positive control, PargC; negative control, PthiL, Figure 2A), AhrC bound to the promoter regions of all known Mn2+ transporter genes (Fig. 2A). Next, we constructed transcriptional fusions in the original chromosomal context (Fig. 2B) and used them for the expression assay of WT and ahrC disruptant with or without glucose. Except for mntH, highly sensitive substrate chlorophenolred β-D-galactopyranoside were used for the β-galactosidase assay. We noted that the expression levels of mntH-lacZ were at least five times or higher than those of the other fusions when 2-Nitrophenyl-β-D-Galactopyranoside was used as the substrate for all assays (data not shown). For lacZ fusion with mneP (exporter), glucose activated its expression, leading to a decrease in Mn2+ concentration, and further ahrC disruption decreased the elevated expression (i, Fig. 2B). AhrC functions as an activator irrespective of the presence of glucose. Disruption of ahrC abolished the GI of the fusion, indicating that glucose induces fusion through AhrC activation. MneS did not play any role in regulating the Mn2+ concentration (Fig. S1C), which is consistent with a previous report (23). Glucose also activated mneS-lacZ, and ahrC functions as an activator, as in the case of mneP (ii, Fig. 2B). For lacZ fusion with mntA (importer), glucose repressed its expression, leading to a decrease in Mn2+ concentration (iii, Fig. 2B). ahrC disruption decreased its expression significantly in the absence of glucose, indicating that AhrC functions as an activator. Moreover, glucose-mediated repression in the ahrC disruptant was still observed, suggesting no involvement of ahrC in glucose repression. We found that ccpN disruption resulted in GI of mntA-lacZ, suggesting that CcpN is responsible for glucose repression (Fig. 2C). These results are consistent with the observation that in the ccpN disruptant GI of Mn2+ concentration was further enhanced. Since CcpN indirectly activates AhrC, the ccpN disruptant should also be considered as an ahrC-depleting strain. Thus, for mntA-lacZ, the results for the ccpN disruptant should be compared to those for the ahrC disruptant. For lacZ fusion with mntH (importer), glucose activated its expression, leading to an increase in Mn2+ concentrations, and further ahrC disruption decreased elevated expression (iv, Fig. 2B). AhrC functions as an activator irrespective of the presence of glucose. Disruption of ahrC abolished the GI of the fusion, indicating that glucose induces fusion through AhrC activation. These transporter genes are known to be regulated by MntR (23); thus, these results indicate that all four genes/operons are also regulated by AhrC.
Figure 2.
Involvement of AhrC in Mn2+transporter genes expression by EMSA. (A) EMSA. Protein concentrations and probe names are shown. Numbers in parentheses show nucleotides position to the relative to the translation start point for argC and thiL. For the others, numbers in parentheses show nucleotides position to the relative to the transcription start point. (B) and left panel in (C) representsβ-Galactosidase activities. Substrate used was 2-Nitrophenyl-β-D-Galactopyranoside for mntH-lacZ and CPRG for the others. Significant differences in the effects of glucose addition at T2 (T1 for mntA-lacZ) were determined using nonpaired t test. ∗p < 0.05; NS, no significant differences. The x-axis represents the growth time in hours relative to the end of vegetative growth (T0). Cells were grown in sporulation medium with (closed symbols) or without (open symbols) 2% glucose and sampled hourly. Strains: PmneP-lacZ (wt, OAM1016; ahrC, OAM1020), PmneS-lacZ (wt, OAM1017; ahrC, OAM1021), PmntA-lacZ (wt, OAM1014; ahrC, OAM1018; ccpN, OAM1024). Schematic representation of the structure of the PmntH-lacZ fusion is shown. Box, bent arrows, and stem–loop indicate ORF, promoter, and terminator, respectively. Right panel in (C) shows cellular Mn concentrations in the ccpN strain (OAM998). T2 cells grown in sporulation medium were harvested and processed. “Glu” represents glucose. Three biologically independent samples were measured. Significant differences between Wt and mutant, with or without glucose (∗ and “NS” above each data point indicate p < 0.05 and no significant difference, respectively) and the effect of glucose addition to the strain were determined using nonpaired t test. ∗p < 0.05; NS, no significant differences. The short horizontal lines indicate the mean of the data points. CPRG, chlorophenolred β-D-galactopyranoside.
These fusion analyses showed that the effects of glucose and ahrC disruption on Mn2+ concentrations can be either positive or negative. This may not be consistent with the observation that there was no GI for Mn2+ concentrations in the ahrC disruptant. Thus, these analyses suggest that there might be AhrC-regulated unknown Mn2+ transporter genes.
Transcriptomes of ahrC and mntR disruptants
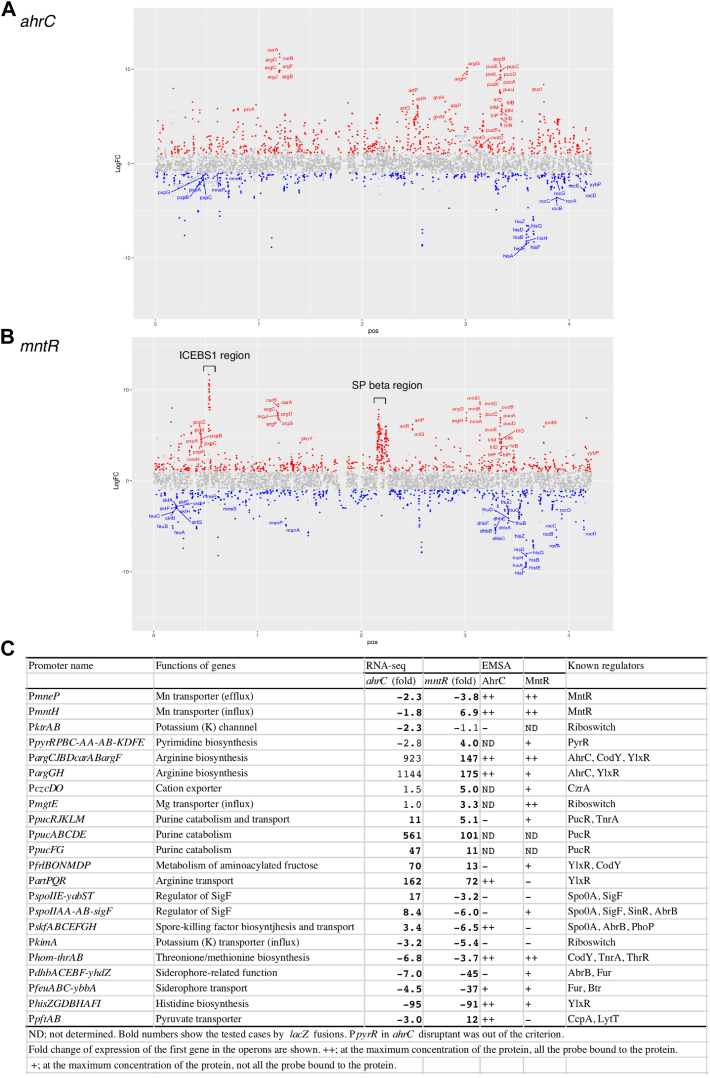
To identify unidentified Mn2+ transporter genes, we first determined the transcriptomic profile of the ahrC disruptant in the presence of glucose through comparative RNA-Seq analysis of the ahrC disruptant. The results using four biological replicates are shown in Figure 3A and Table S1. To determine whether new transporter genes are present in the MntR-regulated genes, we performed RNA-Seq analysis of the mntR disruptant in the presence of glucose using four biological replicates (Fig. 3B and Table S1). In both cases a very large number of AhrC- and/or MntR-regulated genes were observed. The ahrC and mntR disruptants showed the growth profiles similar to that in the WT strain, excluding secondary effects due to growth retardation (Fig. S2C).
Figure 3.
Comparative RNA-Seq analyses of the ahrC and mntR mutants. Values of fold change of transcripts between WT and ahrC (A) or mntR (B) mutant cells were calculated from four independent RNA-Seq analyses that were performed using cells at T2 in sporulation medium with 2% glucose. All genes (ordered clockwise from the +1 position of the chromosome) are plotted against fold-change values. Red and blue points represent upregulation and downregulation, respectively. (C) confirmation of the expression of the selected promoters by β-Gal analysis and EMSA. The data or images of both analyses are shown in this figure.
We chose 20 operons composed of metabolic and transporter genes, whose expression is affected by ahrC and/or mntR disruption (Fig. 3C), and investigated the reproducibility of expression changes, and confirmed the mntR- and/or ahrC-dependent regulation of all promoters (Fig. S3A). We examined protein binding to these promoter regions using EMSA and identified six new targets for AhrC and twelve new targets for MntR (Fig. S3B). These results also suggest that many of the differentially expressed genes (DEGs) in the ahrC/mntR disruptants are indirectly regulated by AhrC/MntR.
While AhrC was thought to bind to only eight Arg-box in the operons related to arginine metabolism (32), our analysis revealed 1176 candidate targets (false discovery rate [FDR] <0.05, log2[FC] ≥ |1|). Among the DEGs in the ahrC disruptant, we detected all eight known operons in addition to histidine and purine metabolism genes (Fig. 3).
We have identified 1316 candidate targets for the MntR regulated genes, including the known directly MntR-regulated loci (FDR <0.05, log2[FC] ≥ |1|). Among these, we found large fluctuations in the expression of genes belonging to the SPbeta phage and ICEBs1, which is an integrative and conjugative element (Fig. 3B) (33), although their physiological roles in MntR-dependent regulation are currently unknown. The members of the MntR-regulated genes would be affected by intracellular Mn2+ concentrations because MntR is activated by Mn2+ binding (22). Hence, the expression of more than 1300 genes could potentially be affected by Mn2+. Indeed, MntR binding to the newly identified target gene argC was affected by Mn2+ (Fig. S3C).
Interestingly, 472 genes were identified to have altered expression pattern in both ahrC and mntR disruptants. Enrichment analyses were performed for both transcriptomes (Fig. S4). These results revealed that many metabolic genes including several amino acid biosynthetic genes were observed in both DEGs.
Identification of candidate AhrC-regulated Mn2+ transporter genes
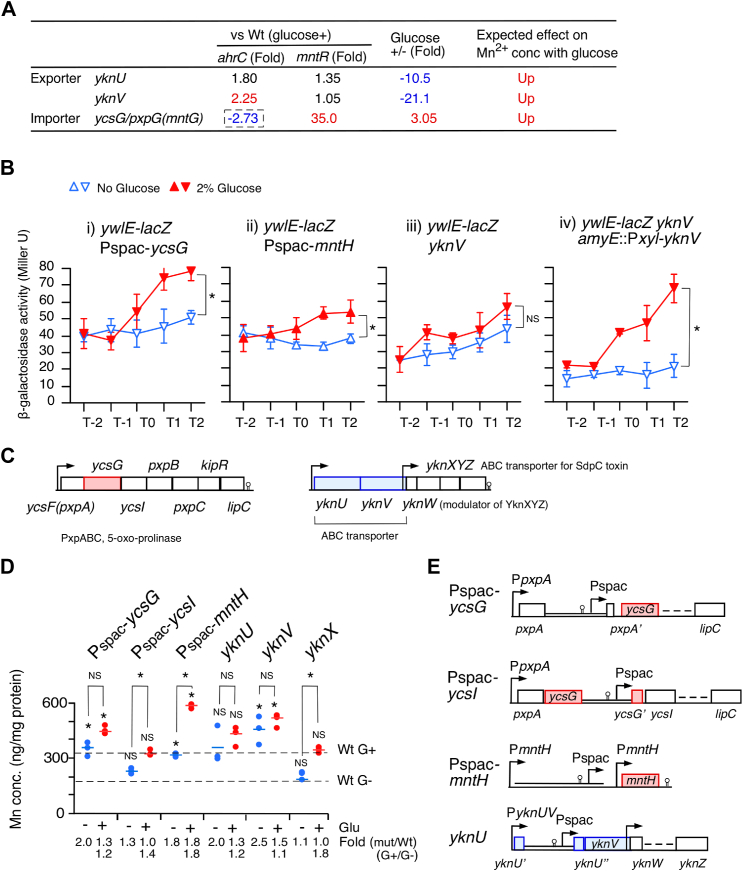
To identify candidate Mn2+ transporter genes, DEGs in the ahrC disruptant were screened. We searched for disruptants without GI of ywlE-lacZ among several AhrC-activated importers with unknown substrates that were under GI or AhrC-repressed exporters with unknown substrates that were under glucose repression (Fig. 4A). Next, we examined ywlE-lacZ expression in the disruptants. The ycsG gene encodes an importer with an unknown substrate, which has been annotated as a 5-oxoproline importer; however, the evidence for this annotation is limited (34), although the other members of this operon are involved in utilizing 5-oxoproline as a carbon source (Fig. 4C) (34). In addition to the GI observed in our RNA-Seq (35), the GI of this operon has been previously reported (36). Initial attempts to construct the ywlE-lacZ strain with ycsG disruption resulted in a highly unstable strain with respect to its no-GI phenotype (data not shown); thus, we adopted an overproduction strategy. Pspac is an IPTG-inducible promoter and Pspac-ycsG elevated ywlE-lacZ expression irrespective of the presence of glucose (i, Fig. 4B) (37). As a control, we also constructed the ywlE-lacZ strain with Pspac-mntH and observed similarly elevated ywlE-lacZ expression (ii, Fig. 4B). Moreover, construction of a triple mutant mntH mntA ycsG was failed in several trials, while the triple mutant was obtained when the strain carried amyE::Pxyl-ycsG. This triple mutant showed retarded growth in MC medium under the condition of leaky ycsG expression without xylose addition (Fig. S2B). These results supported the nature of ycsG, that is, the third Mn2+ importer in B. subtilis.
Figure 4.
Newly identified Mn2+transporters. (A) Fold changes of three candidate genes involved in Mn2+ transport in RNA-Seq. Fold changes in RNA-Seq when glucose was added to the WT were given (35). The dotted square indicates that the decreased expression in the ahrC strain using RNA-Seq, while in the β-Gal analysis of the fusion, the increased expression was observed (see Fig. 5B). B, β-Galactosidase activities. CPRG was used as substrate. Significant differences in the effects of glucose addition at T2 were determined using nonpaired t test. ∗p < 0.05; NS, no significant differences. The x-axis represents the growth time in hours relative to the end of vegetative growth (T0). Cells were grown in sporulation medium with (closed symbols) or without (open symbols) 2% glucose and sampled hourly. Strains: Pspac-mntH (OAM1009), Pspac-ycsG (OAM1010), yknV (OAM1011), and yknV amyE::Px-yknV (OAM1012). 0.2 mM and 0.1 mM IPTG was added, 0.2 mM for Pspac-mntH and 0.1 mM for Pspac-ycsG, respectively. For PyknU-lacZ no xylose was added. C and E, schematic representation of the structure of the ycsG-containing operon and yknUV operon, and Pspac-ycsG, Pspac-ycsI, Pspac-mntH, and yknU. Box, bent arrows, and stem–loop indicate ORF, promoter, and terminator, respectively. Double lines indicate the inserted plasmid sequences. D, cellular Mn concentrations. T2 cells grown in sporulation medium were harvested and processed. “Glu” represents glucose. Three biologically independent samples were measured. Significant differences between Wt and mutants, with or without glucose (∗ and “NS” above each data point indicate p < 0.05 and no significant difference, respectively) and the effect of glucose addition to each strain were determined using nonpaired t test. ∗p < 0.05; NS, no significant differences. The short horizontal lines indicate the mean of the data points. Strains: Pspac-ycsI (OAM1002), Pspac-mntH (OAM1000), yknU (YKNUd), yknV (OAM999), and yknX (YKNXd). 0.1 mM, 0.5 mM, 0.2 mM, and 1 mM IPTG was added for Pspac-ycsG, Pspac-ycsI, Pspac-mntH, and yknU, respectively. CPRG, chlorophenolred β-D-galactopyranoside.
We also tested the yknUV genes encoding an ABC transporter without substrate-binding protein, which has been annotated as an exporter with unknown substrates (38, 39). In the yknV disruptant, the GI of ywlE-lacZ was abolished (iii, Fig. 4B). To perform a complementation test, we constructed a ywlE-lacZ strain with the yknV disruption and ectopic yknV transcribed by the xylose-inducible promoter Pxyl (40). Ectopic and artificial expression of yknV resulted in the original GI phenotype (iv, Fig. 4B), indicating that yknV is responsible for the GI of ywlE-lacZ expression. It should be noted that GI was observed even in the absence of xylose, suggesting that trace activity of Pxyl is sufficient for YknV activity in the cell.
Functional characterization of ycsG encoding Mn2+ importer and yknUV encoding Mn2+ exporter
Next, the cellular Mn2+ concentrations in the mutants were measured to determine the nature of the putative transporters (Fig. 4D). In Pspac-ycsG, ycsG and downstream genes, including ycsI, are simultaneously upregulated by the Pspac promoter (Fig. 4E). In contrast, in Pspac-ycsI, only genes downstream of ycsG are upregulated by the Pspac promoter (Fig. 4E). Mn2+ concentrations were enhanced irrespective of glucose in Pspac-ycsG, whereas Mn2+ concentrations similar to those in the WT were observed with or without glucose in Pspac-ycsI(Fig. 4D). These show that the effect of Pspac-ycsG was not due to the enhancement of downstream genes such as ycsI. Hence, we concluded that ycsG encodes the Mn2+ importer. In the control strain with Pspac-mntH, Mn2+ concentrations were enhanced, and glucose addition further increased the Mn2+ concentrations. In this strain, mntH expression was driven by Pspac and its own promoters (Fig. 4E). Irrespective of glucose, in the yknU and yknV disruptants, Mn2+ concentrations were elevated, whereas they were not changed in the yknX disruptant, ruling out the possible polar effect of yknUV disruption on yknX. Thus, we concluded that YknUV was involved in Mn2+ export. To date, however, the ABC transporter for Mn2+ export is unidentified (17).
Expression of ycsG
To examine the direct binding of AhrC to PpxpA driving ycsG (importer), we performed EMSA using protein concentrations for specific binding. PpxpA was bound by AhrC, demonstrating direct regulation of PpxpA by AhrC (Fig. 5A). Next, we performed EMSA using MntR, as enhancement of ycsG transcripts was observed in RNA-Seq for MntR (Fig. 4A). MntR directly bound to PpxpA at concentrations of MntR within those permitting specific binding for PmntA (Fig. 5A). Next, we analyzed PpxpA expression. For the PpxpA-lacZ fusion, glucose activated its expression, leading to an increase in Mn2+ concentration, and further ahrC disruption increased the elevated expression, contrary to the RNA-Seq results due to unknown reason (i, Fig. 5B). PpxpA-lacZ contains the 5′-UTR of pxpA, which may work for post-transcription regulation. These results showed that AhrC functions as a repressor irrespective of the presence of glucose. The disruption of ahrC did not abolish the GI of the fusion, indicating that the GI of the fusion is not through AhrC. MntR functions as a repressor, as expected, and the GI of the fusion was still observed, indicating that the GI of the fusion is not through MntR (ii, Fig. 5B). Thus, we searched for several transcription factors related to this glucose effects and found that CcpA is responsible for the GI of the ycsG-containing operon, because GI was abolished in the ccpA disruptant (iii, Fig. 5B).
Figure 5.
AhrC/MntR-binding and expression of two newly identified Mn2+transporter loci. (A) EMSA. Concentrations of AhrC/MntR and probe names are shown. Numbers in parentheses show nucleotides position to the relative to the translation start point for yknU and rapH. For mntA and pxpA, position to the relative to the transcription start point. (B) β-Galactosidase activities. CPRG was used for PyknU-lacZ. 2-Nitrophenyl-β-D-Galactopyranoside was used for the others. Significant differences in the glucose effect in each strain at T2 were determined using nonpaired t test. ∗p < 0.05; NS, no significant differences. The x-axis represents the growth time in hours relative to the end of vegetative growth (T0). Cells were grown in sporulation medium with (closed symbols) or without (open symbols) 2% glucose and sampled hourly. Strains: PycsF-lacZ (wt, OAM1027; ahrC, OAM1028), ycsF-lacZ (wt, YCSFd; ccpA, OAM1031), and PyknU-lacZ (wt, OAM1025; ahrC, OAM1026). CPRG, chlorophenolred β-D-galactopyranoside.
Determination of cis-acting sequences of multiple transcription factors in PpxpA
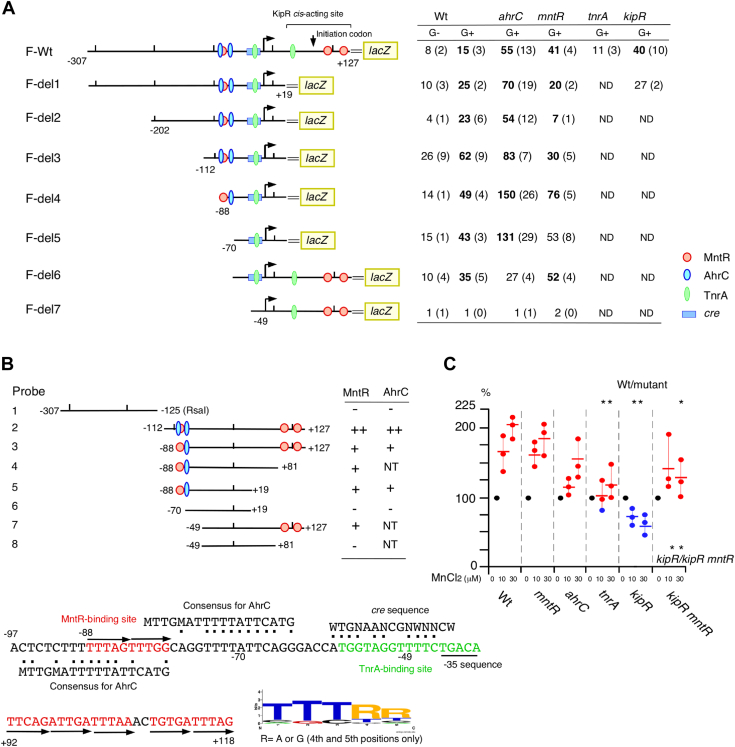
To obtain deeper understanding of regulation of PpxpA, we analyzed the expression of variously deleted promoter-lacZ fusions in disruptants of the gene encoding transcription factor. First, we examined CcpA-dependent GI of the constructed fusions, and GI was observed in all the fusions except for F-del7 (Fig. 6A). Thus, it is reasonable that CcpA-binding cre sequence was detected within the −70/−49 region (Fig. 6) (2). The decrease and increase of PpxpA activity in the tnrA and kipR disruptants, respectively, have been previously reported (36) and we confirmed the expected changes using F-Wt (Fig. 6A). Two TnrA-binding sequences were reported (41) and shown in Figure 6A, and the upstream site for TnrA is overlapped to the detected cre sequence. AhrC-binding sequences were conserved in many bacteria (32) and we detected two adjacent candidates in PpxpA. In EMSA, DNA probe 2 up to −112 position showed high affinity to AhrC, while DNA probe 3 up to −88 position showed low affinity to AhrC (see 0.15 μg of AhrC lanes and smear band in probe 5, Fig. S5A). This observation is consistent with the presence of two active AhrC-binding sites. Increases in fusions expression (Wt, del1, del2, del3, and del4) by the ahrC disruption supported the EMSA results. However, increased expression of del5 in the ahrC disruptant seems to be strange because this fusion does not carry any AhrC-binding site. Contrary to this, the del6 fusion expression up to −70 position did not change in the ahrC disruptant, which is consistent with the EMSA results. RNA-seq of the ahrC disruptant revealed significantly increased tnrA expression (Table S1), which may have promoted del5 expression. If so, introducing tnrA disruption in the del5 fusion strain with ahrC would suppress del5 expression. Our experiment confirmed that this was the case (data not shown). As introducing tnrA disruption to F-Wt with ahrC resulted in still 2.5-fold enhanced expression in OAM1123 (Table S2), del5 expression in the ahrC disruptant may be due to the artificial deletion of the fusion structure. Next, deletion of the downstream region from +1 resulted in the loss of mntR- and kipR-dependent promoter repression (F-del1). This suggested MntR binding to this region and indeed binding to the +81/+127 region was observed (Fig. 6B). The three fusions expression (del1, 2, and 3) was decreased in the mntR disruptant, suggesting the positive role of MntR in the upstream region from +1 and additional binding of MntR to the region. EMSA revealed the MntR binding to the −88/−70 region. The expression of del4 with this region increased in the mntR disruptant, showing in this fusion MntR plays a negative role contrary to the above three fusions. The apparent contradiction is resolved by considering that the upstream bound AhrC is absent in this fusion, so the anti-AhrC activity of the MntR is lost. Indeed, putative MntR cis-acting site (direct repeat of TTTRG) is within the upstream AhrC-binding site, thus MntR would function as an anti-repressor through competition for binding to this sequence. However, it should be noted that overall MntR-regulation is apparently repressive. In the ahrC mntR double disruptant slight additive enhanced expression of PpxpA was observed (Fig. S6). This is consistent with the downstream MntR-binding site being independent of the AhrC-binding sites. The direct and triple repeat of TTTRG is also within the downstream region required for the effect of MntR, suggesting this motif would be MntR-binding motif.
Figure 6.
Expression of PpxpA-lacZ. (A) Deletion analysis. Strains were grown in sporulation medium with or without 2% glucose and sampled hourly. Means of peak values (Miller units) from three independent experiments and the SDs are shown in parenthesis. Numbers in bold letter indicate statistically significant differences (Comparison between with or without glucose in Wt or between wild and disruptant; p < 0.05). Bent arrow and double line show promoter and vector sequence, respectively. Protein stoichiometry in the figure is not taken into account. AhrC consensus is from DBTBS (66). Numbers along the line indicate position relative to the transcription start site (SubtiWiki) (67). Strains: F-Wt, OAM1027 (wt); OAM1028 (ahrC); OAM1029 (mntR); OAM1089 (tnrA); OAM1090 (kipR); F-del1, OAM1091 (wt); OAM1092 (ahrC); OAM1093 (mntR); OAM1094 (kipR); F-del2, OAM1095 (wt); OAM1096 (ahrC); OAM1097 (mntR); F-del3, OAM1098 (Wt); OAM1099 (ahrC); OAM1100 (mntR); F-del4, OAM1101 (Wt); OAM1102 (ahrC); OAM1103 (mntR); F-del5, OAM1104 (wt); OAM1105 (ahrC); OAM1106 (mntR); F-del6, OAM1107 (Wt); OAM1108 (ahrC); OAM1109 (mntR); F-del7, OAM1110 (wt); OAM1111 (ahrC); OAM1112 (mntR). (B) EMSA results and sequence alignments. Numbers at the ends of the line indicate position relative to the transcription start site. ++ indicates the situation where free probe disappeared at low protein levels (0.1 μM for MntR and 0.15 μM for AhrC). EMSA images are shown in Fig. S5A. The consensus for MntR binding is generated from all motifs in pxpA, mntH, and mneP. (C) Mn2+ response of the PpxpA-lacZ. Strains were grown in sporulation medium with 2% glucose and without supplementation of MnCl2. Indicated MnCl2 (final concentrations) was added. For each experiment three independent trials were performed and asterisks show p < 0.05. The short horizontal lines show means of the shown data points. Strains: OAM1027 (wt); OAM1029 (mntR); OAM1028 (ahrC); OAM1089 (tnrA); OAM1090 (kipR); and OAM1121 (kipR mntR). ND, not determined; NT, not tested.
MntR-binding motif in regulatory region of mntH and mneP
For mntH and mneP, minimum MntR-binding regions were determined with multiple binding sites (22, 23). Thus, we examined whether the putative MntR-binding sequences detected in PpxpA are in the regulatory regions of mntH and mneP. The low expression of mntH-lacZ-Wt fusion in the WT was 3-fold enhanced by mntR disruption. MntR was bound to the Wt probe, generating two bands (Fig. S5B). Deleting 37 bases from the 3′-end of Wt resulted in 8-fold enhancement of the fusion expression, and further enhancement was observed in the mntR disruptant. In EMSA, using probe del1 MntR generated single band with lower mobility. Deleting further 24 bases resulted in MntR-independent enhanced expression and no MntR binding. These stepwise alterations of fusion expression and shift patterns in EMSA showed that the +67/+31 and +30/+6 regions contain independent MntR-responsive elements. Indeed, within the +6/+67 region three independent TTTRR repeats were detected. Next, the mneP regulatory region was analyzed using EMSA. The previous report showed the minimum MntR-binding region in mneP, that is, the −100/+133 region (23). We confirmed this in EMSA. The −386/+118 probe was completely shifted by the binding of MntR at 0.1 μM (Fig. S5C). Deletion of 41 bases resulted in scarce MntR binding, indicating that this region contains MntR cis-acting site(s) and the two direct repeat of the TTTRR motif were detected. These results supported the notion that the TTTRR motif is recognized by MntR. As the both promoters were regulated by AhrC, in addition to mneS and mntA, we scanned the four promoter sequences and detected putative AhrC-binding sites (Fig. S5). We introduced ahrC disruption into the PmntH-lacZ strain with the disruption of mntR and examined fusion expression. The elevated fusion expression in the mntR disruptant decreased in the double mutant, which is consistent with independent location of AhrC- and MntR-binding sites (Fig. S6).
Induction of PpxpA by Mn2+
The expression of the genes encoding two Mn2+ importers, mntH and mntABCD, was repressed by under high Mn2+ conditions through Mn2+-activated MntR (22, 42). We therefore investigated whether the PpxpA expression was altered by Mn2+ addition. Contrary to the expectation, PpxpA was induced by Mn2+ addition (Fig. 6C). Thus, we examined the PpxpA expression in the disruptants of the transcription factors. In the mntR and ahrC disruptants, PpxpA was still induced similarly to the WT, whereas in the tnrA and kipR disruptants weakened induction and strong repression of the fusion expression, respectively, were observed. We hypothesized that in the kipR disruptant, residual Mn2+-activated MntR may repress fusion expression. Thus, the mntR kipR double disruptant was constructed, and a modest induction perhaps by TnrA was observed. This indicated that in the absence of induction by KipR, repression by MntR is at work. Thus, the induction of PpxpA is mainly caused by KipR and to a lesser extent by TnrA. MntR-dependent repression appears to be hidden by the positive effects of KipR and TnrA, and therefore the effect of mntR disruption was not observed in the mntR disruptant with the normal kipR.
Expression of PyknU
Glucose repressed the expression of PyknU-lacZ, leading to an increase in Mn2+ concentration, and further disruption of ahrC increased the glucose-repressed expression (iv, Fig. 5B). As AhrC-dependent regulation was expected from the EMSA results, where AhrC, but not MntR, bound to PyknU (Fig. 5A), this is consistent with the increased expression in the ahrC disruptant. Moreover, in the ahrC disruptant, the fusion expression was not affected by glucose. Therefore, AhrC functions as a repressor only in the presence of glucose.
Conclusion
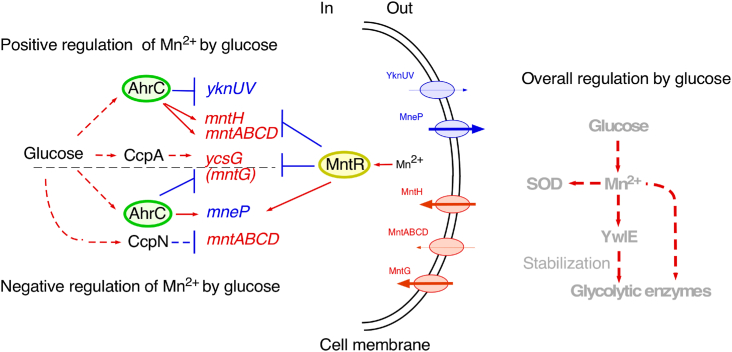
Based on these analyses, we concluded that two newly identified and known Mn2+ transporters contribute to the GI of Mn2+ concentrations (Fig. 7). Glucose, both negatively and positively, affects the expression of different genes encoding Mn2+ transporters through several transcription factors. However, overall, glucose induces an increase in Mn2+ concentrations.
Figure 7.
Schematic representation of glucose-mediated regulation of Mn2+transport.Left: T-bar and arrow indicate inhibition and activation of gene expression, respectively. Dotted arrow and T-bar indicate indirect effects. Proteins in red and blue represent Mn2+ importer and exporter, respectively. The arrow width in the transporter indicates putative overall effects of glucose on transporter genes expression. Direction of arrows indicates ion influx or efflux. Right: overall glucose-mediated effects of upshift of cellular Mn2+ equilibrium is shown. YwlE is a protein arginine phosphatase, which counteracts the arginine phosphorylation of proteins by McsB kinase, leading to protection of the protein from degradation including glycolytic enzymes. Glucose addition results in upshift of central carbon flow including glycolysis, tricarbonic acid cycle, and respiratory chain, leading to generation of toxic reactive oxygen species (ROS). Addition of glucose will increase the demand for superoxide dismutase (SOD).
Discussion
This study shows mechanism of the GI of Mn2+ concentrations. An increase in Mn2+ concentrations resulted in the induction of ywlE. YwlE counteracts arginine phosphorylation of glycolytic enzymes by McsB kinase, thereby protecting the proteins from degradation, which explains the role of GI of ywlE (Fig. 7). It was reported that in S. aureus cells using glucose as a sole carbon source, the cellular demand for Mn2+ and mntH expression were increased, compared to cells using amino acids as a sole carbon source, although the mechanisms were not explored (43). Cellular Mn2+ concentrations are tightly maintained at steady state levels corresponding to extracellular Mn2+ concentrations in the current model (17). Glucose upregulates Mn2+ concentrations by imposing AhrC regulation on MntR-regulated mntH. The reason for the AhrC-dependent regulation of Mn2+ transporter genes is currently unknown; however, we note that the arginase encoded by AhrC-regulated rocF requires Mn2+ for its enzymatic activity (44). Moreover, glucose addition results in activation of the respiratory chain, generating toxic reactive oxygen species. Superoxide dismutase, which is required for detoxification of reactive oxygen species, also requires Mn2+ as a cofactor (45). Since glucose addition increases the demand for superoxide dismutase, GI of Mn2+ concentration is advantageous for the cell (Fig. 7).
Mn2+ importer YcsG is a member of the natural resistance–associated macrophage protein family of metal ion transporters, which is highly conserved across three kingdoms and also contains MntH (46). YcsG was first identified as a member of the operon-containing kipI, which was annotated as a phosphorelay-controlling gene for sporulation initiation (36), and then the three genes in the operon (ycsF, kipI, and kipA) were reannotated for pxpABC encoding ATP-dependent 5-oxoprolinase (34) (Fig. 4C). 5-oxoproline is spontaneously generated from glutamine or glutamate and 5-oxoprolinase catalyzes the conversion of 5-oxoproline back into glutamate (34). It is therefore plausible that this operon is regulated by the global nitrogen metabolism regulator TnrA. The arrangement of pxpABC and ycsG in the same operon is found in Vibrio fischeri, Agrobacterium tumefaciens, and Micrococcus luteus (34). A similar operon structure, in which nitrogen metabolism genes (urea utilization) are associated with ycsG, has been reported in Acinetobacter baumannii (47). These facts may suggest a link between nitrogen metabolism and Mn2+. In previous studies, ycsG was reported to be involved in 5-oxoproline utilization; however, this effect did not fully rule out the possible polar effect on downstream pxpBC genes (34). This study provides evidence that YcsG is involved in Mn2+ import; thus, we renamed this gene as mntG. This study revealed that the operon expression is controlled by AhrC, MntR, and CcpA in addition to KipR, which is in this operon, and TnrA (36). Our analysis of their interaction with the promoter region revealed that CcpA and TnrA may compete for binding to the same sequence in PpxpA. This situation suggests that CcpA-mediated carbon regulation and TnrA-mediated nitrogen regulation intercrosses at PpxpA. The overlapping of the sites for TnrA and CcpA is not unprecedented (48). Characterization of MntR binding to PpxpA in addition to mntH and mneP led to the identification of MntR-recognized sequences and we present a consensus sequence for MntR binding. The likelihood of this consensus will be more certain when the newly identified MntR targets are studied experimentally. The identified sequences do not match the previously reported recognition sequences (23). The discrepancy may be due to the fact that the consensus sequence is partly based on sequences found in the mutational analysis using lacZ fusion in the mneP regulatory region, where MntR does not actually bind in our study.
PpxpA was induced by high Mn2+ concentrations. Under high Mn2+ conditions, two Mn2+ importers MntH and MntABCD were downregulated (22). Thus, at high Mn2+ concentrations, changes in the composition of three Mn2+ importers occur. The reason for this is still unknown. This Mn2+ response of PpxpA is mediated by MntR, TnrA, and KipR. The TnrA-dependent Mn2+ response has been reported and presented the possible mechanism for this as below (42). TnrA is bound by feedback-inhibited glutamine synthetase by glutamine, resulting in inhibition of TnrA (49). The Mn2+ form of glutamine synthetase is more resistant to inhibition by glutamine than the Mg2+ form, resulting in less inhibition of TnrA activity (42). B. subtilis KipR is poorly characterized; however, the crystal structure of the KipR homologue in Thermotoga maritima revealed zinc binding (50). Possible metal binding of B. subtilis KipR may be related to Mn2+-dependent operon regulation by KipR.
AhrC, which controls arginine metabolic genes expression, is known to be glucose-induced through CcpN via ncRNA, SR1 (26, 27). This study expanded the inventory of the genes involved in AhrC-mediated regulation. Thus, the CcpN/AhrC axis appears to be a global glucose regulatory system. In Streptomyces coelicolor and E. coli ArgR (AhrC analogue) has been shown to regulate numerous genes on a genome-wide scale (51, 52), and in Enterococcus faecalis, AhrC regulates genes with functions other than arginine metabolism (53), which corresponds with our RNA-Seq results. L-Arginine is a cofactor that binds to the C-terminal region of AhrC, and thus, arginine would be involved in the AhrC-mediated transcriptional regulation (54).
The MntR-regulated operons also contain many members. The comparative DNA microarray analyses of mntR at high Mn2+ concentration revealed many genes that belong to the ferric uptake regulator and SigB-regulons (42). A recent proteomic analysis of MntR-regulated proteins found limited numbers of MntR-regulated genes because of the technical limitations of the proteome analysis. They, however, reported the products of genes whose expression fluctuated highly during our RNA-Seq (31).
This study uncovered previously unknown aspects of Mn2+ homeostasis control and expansion of the glucose-mediated CcpN/AhrC regulatory axis in many genes. New aspects of Mn2+ homeostasis control are important because Mn2+ is required for many cellular processes and excess levels of Mn2+ can lead to intoxication by this metal.
Experimental procedures
Strains, media, plasmid, and β-galactosidase analysis
B. subtilis strains and plasmids used in this study are listed in Tables S2. The construction of plasmids is provided in Supplementary methods. A one-step modified competence medium (MC, 100 mM potassium phosphate [pH 7], 3 mM trisodium citrate, 3 mM MgSO4, 2% glucose, 22 mg/ml ferric ammonium citrate, 50 mg/ml tryptophan, 0.1% casein lysate, 0.2% potassium glutamate) (30), Schaeffer’s sporulation medium (55), antibiotic III medium (Difco), and lysogeny broth (LB-Lenox) medium (Difco) were used. Antibiotic concentrations were used as described previously (56). Synthetic oligonucleotides were commercially prepared by Tsukuba Oligo Service and are listed in Table S3. Growth conditions and methods for β-galactosidase analysis have been previously described (57). The use of the highly sensitive substrate chlorophenolred β-D-galactopyranoside for the β-galactosidase assay provides 5 to 10 times higher activities than those of 2-Nitrophenyl-β-D-Galactopyranoside, whereas the background activities were at the same level (around 1 Miller units).
Purification of AhrC and MntR
The E. coli strain BL21(DE3) bearing pGEX4T1-ahrC was grown in 600 ml of LB medium (100 μg/ml ampicillin) at 37 °C for 4 h after 1:100 inoculation of overnight culture in LB medium. After 0.5 mM IPTG was added, the cells were further incubated for 20 h at 23 °C. For BL21(DE3) bearing pGEX4T1-mntR, the similar conditions were used, except for the addition of 0.2 mM IPTG and further incubation for 4 h at 23 °C. The cells were harvested, resuspended in 3 ml of the thrombin buffer (20 mM Tris–HCl [pH 8.5], 150 mM NaCl, 2.5 mM CaCl2), and disrupted by French pressure cell. After centrifugation (25,000 rpm, 20 min, 4 °C), 2 ml of Glutathione-Sepharose 4B resin slurry (GE Healthcare) was added to the supernatant and gently stirred for 30 min. The mixture was then packed into a column and washed twice with a 10-column volume of the same buffer. After adding biotinylated-thrombin (Novagen) (2U/0.5 ml), the column was left for 20 h at 23 °C. Next, 2 ml of thrombin buffer containing 300 mM NaCl was added to the column. The resulting eluate was passed through a 0.5 ml of Streptavidin-agarose (Novagen) column. After SDS-PAGE analysis, the protein solution was dialyzed against a buffer containing 10% glycerol, 10 mM Tris–HCl [pH 7.5], 1 mM DTT, and 100 mM NaCl, and aliquots of the resultant supernatant were stored at −80 °C after centrifugation. The purified AhrC and MntR proteins that were produced in E. coli cells were almost intact but had with two additional amino acids derived from the BamHI restriction site of pGEX4T1 at the N terminus.
Electromobility shift assays
EMSA was performed using the essentially same methods as the previously published procedures (23). Appropriate amounts of purified AhrC or MntR were added to a final volume of 14 μl buffer containing 5% glycerol, 10 mM Tris–HCl [pH 7.5], 43 mM NaCl, 1 mM MnCl2, 1 mM DTT, 1 μg of poly[dI-dC (deoxyinosinic-deoxycytidylic acid)] (Sigma-Aldrich), and biotinylated DNA probe. After adding the protein, the reaction mixture was left for 15 min at 23 °C, following which 2 μl of loading buffer (10% glycerol, 40 mM Tris-acetate buffer [pH 7.5], and 2 mg/ml bromophenol blue) were added and applied to a 5% polyacrylamide gel, and electrophoresis was performed in 40 mM Tris-acetate buffer at 4 °C. The detection of biotin-labeled DNA has been previously described (56).
Measurement of Mn concentrations
Cells were grown in 50 ml of sporulation medium with or without 2% glucose. Aliquots of 4 ml of the cell culture were harvested at T2. The processes of washing, cell lysis, protein concentration assay, and pretreatment of samples with HNO3 were the same as the previously published procedures (22). The 800 μl cleared cell lysate solution was mixed with 3.2 ml of 0.08 M HNO3 solution. The Mn concentrations in these solutions were measured using quadrupole inductively coupled plasma mass spectrometry (Agilent 7800, Agilent, Santa Clara). H2 gas flow (10 L/min) into the collision cell was used for Mn measurements. 55Mn was used as the measurement isotopes and 115In was used as an internal standard. The uptake time for each isotope is 0.3 s.
RNA isolation and RNA-Seq analysis
B. subtilis WTe (168), ahrC (OAM995) mntR (OAM996), and mneP (OAM993) strains were newly prepared by transformation of the gene disruption into the WT strain so as to obtain a clean genome background. The cells were grown in 50 ml of sporulation medium with 2% glucose, and 4 ml of cell culture was sampled at T2 for RNA isolation. Four independent cultures were used for each experiment. RNA was isolated from the cells collected by centrifugation using an RNeasy mini kit (Qiagen) with DNase I (Takara) treatment, according to the manufacturer’s instructions. RNA quality was confirmed based on an RNA integrity number >7 using an Agilent RNA 6000 Nano Kit in an Agilent 2100 Bioanalyzer (Agilent Technologies). Ribosomal RNA elimination and complementary DNA library construction was performed using a NEBNext rRNA Depletion Kit (Bacteria) and NEBNext Ultra Ⅱ RNA Library Prep Kit (Illumina) for 1000 ng of total RNA, according to the manufacturer’s protocol. The library was sequenced on the Illumina sequencing platform (Illumina NextSeq 500), and 2 × 75-bp paired-end reads were generated. Adapter sequences in each read were removed using CLC Genomics Workbench 20× software (Qiagen) (https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-clc-genomics-workbench/). The cleaned read data were mapped to the reference genome (RefSeq assembly accession: GCF_000009045.1). Mapping parameters were as follows: mismatch cost, 2; insertion cost, 3; deletion cost, 3; length fraction, 0.8; and similarity fraction, 0.8. DEGs of each condition and control were identified with significant thresholds of a FC ≥ |2|, and FDR adjusted p-value (q-value) <0.05 was obtained by a generalized linear model approach using the CLC Genomics Workbench built-in tools differential expression for RNA-Seq.
Data availability
Original sequence reads were deposited in the DRA/SRA database (accession number: DRR445917-DRR445928).
Supporting information
This article contains supporting information 4, 7, 13, 22, 23, 30, 35, 37, 58, 59, 60, 61, 62, 63, 64, 65.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We are indebted to Shohei Sakuda (Teikyo University), Rieko Takeyama (The University of Tokyo), and Tsutomu Sato (Hosei University) for set-up of this study, technical assistances, and the gift of the strains, respectively.
Author contributions
M. O. conceptualization; M. O. validation; M. O., M. M., and M. S. investigation; M. O. writing-original draft; M. O. writing-review and editing; M. O. and K. A. project administration; M. O. and M. S. funding acquisition; M. M. formal analysis; M. M. data curation; K. A. supervision.
Funding and additional information
This work was supported by JSPS KAKENHI (21K05349 for M. O. and 19H05771 for M. S.).
Reviewed by members of the JBC Editorial Board. Edited by Chris Whitfield
Supporting information
References
- 1.Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2008;11:87–93. doi: 10.1016/j.mib.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Fujita Y. Carbon catabolite control of the metabolic network in Bacillus subtilis. Biosci. Biotechnol. Biochem. 2009;73:245–259. doi: 10.1271/bbb.80479. [DOI] [PubMed] [Google Scholar]
- 3.Servant P., Le Coq D., Aymerich S. CcpN (YqzB), a novel regulator for CcpA-independent catabolite repression of Bacillus subtilis gluconeogenic genes. Mol. Microbiol. 2005;55:1435–1451. doi: 10.1111/j.1365-2958.2005.04473.x. [DOI] [PubMed] [Google Scholar]
- 4.Ogura M., Kanesaki Y. Newly identified nucleoid-associated-like protein YlxR regulates metabolic gene expression in Bacillus subtilis. mSphere. 2018;3:e00501–e00518. doi: 10.1128/mSphere.00501-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogura M., Sato T., Abe K. Bacillus subtilis YlxR, which is involved in glucose-responsive metabolic changes, regulates expression of tsaD for protein quality control of pyruvate dehydrogenase. Front Microbiol. 2019;10:923. doi: 10.3389/fmicb.2019.00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogura M. Identification of transposon-inserted mutations including rnpB::Tn that abolished glucose induction of sigX encoding extracytoplasmic function-sigma factor in Bacillus subtilis. Biosci. Biotechnol. Biochem. 2022;86:282–285. doi: 10.1093/bbb/zbab211. [DOI] [PubMed] [Google Scholar]
- 7.Ogura M. Glucose-mediated protein arginine phosphorylation/dephosphorylation regulates ylxR encoding nucleoid-associated protein and cell growth in Bacillus subtilis. Front Microbiol. 2020;11:2382. doi: 10.3389/fmicb.2020.590828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt A., Trentini D.B., Spiess S., Fuhrmann J., Ammerer G., Mechtler K., et al. Quantitative phosphoproteomics reveals the role of protein arginine phosphorylation in the bacterial stress response. Mol. Cell Proteomics. 2014;13:537–550. doi: 10.1074/mcp.M113.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohle T.H., O'Brian M.R. Manganese is required for oxidative metabolism in unstressed Bradyrhizobium japonicum cells. Mol. Microbiol. 2012;84:766–777. doi: 10.1111/j.1365-2958.2012.08057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogunniyi A.D., Mahdi L.K., Jennings M.P., McEwan A.G., McDevitt C.A., et al. Central role of manganese in regulation of stress responses, physiology, and metabolism in Streptococcus pneumoniae. J. Bacteriol. 2020;192:4489–4497. doi: 10.1128/JB.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puccio T., Misra B.B., Kitten T. Time-course analysis of Streptococcus sanguinis after manganese depletion reveals changes in glycolytic and nucleic acid metabolites. Metabolomics. 2021;17:44. doi: 10.1007/s11306-021-01795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Párraga Solórzano P.,K., Bastillem T.S., Radin J.N., Kehl-Fie T.E. A manganese-independent aldolase enables Staphylococcus aureus to resist host-imposed metal starvation. mBio. 2023;14 doi: 10.1128/mbio.03223-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shemesh M., Chai Y. A combination of glycerol and manganese promotes biofilm formation in Bacillus subtilis via histidine kinase KinD signaling. J. Bacteriol. 2013;195:2747–2754. doi: 10.1128/JB.00028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaluskar Z.M., Garrison-Schillng K.L., McCarter K.S., Lambert B., Simer S.R., Pettis G.S. Manganese is an additional cation that enhances colonial phase variation of Vibrio vulnificus. Environ. Microbiol. 2015;7:789–794. doi: 10.1111/1758-2229.12318. [DOI] [PubMed] [Google Scholar]
- 15.Hood G., Ramachandran V., East A., Downie A., Poole P.S. Manganese transport is essential for N2-fixation by Rhizobium leguminosarum in bacteroids from galegoid but not phaseoloid nodules. Environ. Microbiol. 2017;19:2715–2726. doi: 10.1111/1462-2920.13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrangsu P., Rensing C., Helmann J.D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017;15:338–350. doi: 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosma E.F., Rau M.H., van Gijtenbeek L.A., Siedler S. Regulation and distinct physiological roles of manganese in bacteria. FEMS Microbiol. Rev. 2021;45:1–16. doi: 10.1093/femsre/fuab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuominen F.W., Bernlohr R.W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. II. Kinetic properties. J. Biol. Chem. 1971;246:1746–1755. [PubMed] [Google Scholar]
- 19.Watabe K., Freese E. Purification and properties of the manganese-dependent phosphoglycerate mutase of Bacillus subtilis. J. Bacteriol. 1979;137:773–778. doi: 10.1128/jb.137.2.773-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dambach M., Sandoval M., Updegrove T.B., Anantharaman V., Aravind L., Waters L.S., et al. The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Mol. Cell. 2015;57:1099–1109. doi: 10.1016/j.molcel.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters L.S. Bacterial manganese homeostasis. Curr. Opin. Chem. Biol. 2020;55:96–102. doi: 10.1016/j.cbpa.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Que Q., Helmann J.D. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 2000;35:1454–1568. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang X., Shin J.-H., Pinochet-Barros A., Su T., Helmann J.D. Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Mol. Microbiol. 2017;103:253–268. doi: 10.1111/mmi.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paruthiyil S., Pinochet-Barros A., Huang X., Helmann J.D. TerC family proteins help prevent manganese intoxication. J. Bacteriol. 2019;202 doi: 10.1128/JB.00624-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czaplewski L.G., North A.K., Smith M.C., Baumberg S., Stockley P.G. Purification and initial characterization of AhrC: the regulator of arginine metabolism genes in Bacillus subtilis. Mol. Microbiol. 1992;6:267–275. doi: 10.1111/j.1365-2958.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 26.Licht A., Preis S., Brantl S. Implication of CcpN in the regulation of a novel untranslated RNA (SR1) in Bacillus subtilis. Mol. Microbiol. 2005;58:189–206. doi: 10.1111/j.1365-2958.2005.04810.x. [DOI] [PubMed] [Google Scholar]
- 27.Heidrich N., Chinali A., Gerth U., Brantl S. The small untranslated RNA SR1 from the Bacillus subtilis genome is involved in the regulation of arginine catabolism. Mol. Microbiol. 2006;62:520–536. doi: 10.1111/j.1365-2958.2006.05384.x. [DOI] [PubMed] [Google Scholar]
- 28.Waters L.S., Sandoval M., Storz G. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J. Bacteriol. 2011;193:5887–5897. doi: 10.1128/JB.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf Y., Rogozin I., Kondrashov S., Koonin E. Genome alignment, evolution of prokaryotic genome organization, and prediction of gene function using genomic context. Genome Res. 2001;11:356–372. doi: 10.1101/gr.gr-1619r. [DOI] [PubMed] [Google Scholar]
- 30.Kunst F., Msadek T., Rapoport G. In: Regulation of Bacterial Differentiation. Piggot P.J., Moran C.P. Jr., Youngman P., editors. ASM Press; Washington, DC: 1994. Signal transduction network controlling degradative enzyme synthesis and competence in Bacillus subtilis. p 1-20. [Google Scholar]
- 31.Randazzo P., Anba-Mondoloni J., Aubert-Frambourg A., Guillot A., Pechoux C., Vidic J., et al. Bacillus subtilis regulators MntR and Zur participate in redox cycling, antibiotic sensitivity, and cell wall plasticity. J. Bacteriol. 2019;202 doi: 10.1128/JB.00547-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makalova K.S., Mironov A.A., Gelfand M.S. Conservation of the binding site for the arginine repressor in all bacterial lineages. Genome Biol. 2002;2 doi: 10.1186/gb-2001-2-4-research0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki S., Yoshikawa M., Imamura D., Abe K., Eichenberger P., Sato T. Compatibility of site-specific recombination units between mobile genetic elements. iScience. 2020;23 doi: 10.1016/j.isci.2019.100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niehaus T.D., Elbadawi-Sidhu M., de Crécy-Lagard V., Fiehn O., Hanson A.D. Discovery of a widespread prokaryotic 5-oxoprolinase that was hiding in plain sight. J. Biol. Chem. 2017;292:16360–16367. doi: 10.1074/jbc.M117.805028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanesaki Y., Ogura M. RNA-seq analysis identified glucose-responsive genes and YqfO as a global regulator in Bacillus subtilis. BMC Res. Notes. 2021;14:450. doi: 10.1186/s13104-021-05869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L., Grau R., Perego M., Hoch J.A. A novel histidine kinase inhibitor regulating development in Bacillus subtilis. Genes Dev. 1997;11:2569–2579. doi: 10.1101/gad.11.19.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vagner V., Dervyn E., Ehrlich S.D. A vector for systematic gene inactivation in Bacillus subtilis. Microbiol. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
- 38.Quentin Y., Fichant G., Denizot F. Inventory, assembly and analysis of Bacillus subtilis ABC transport systems. J. Mol. Biol. 1999;287:467–484. doi: 10.1006/jmbi.1999.2624. [DOI] [PubMed] [Google Scholar]
- 39.Davidson A.L., Dassa E., Orelle C., Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008;72:317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hori K., Kaneko M., Tanji Y., Xing X.H., Unno H. Construction of self-disruptive Bacillus megaterium in response to substrate exhaustion for polyhydroxybutyrate production. Appl. Microbiol. Biotechnol. 2002;59:211–216. doi: 10.1007/s00253-002-0986-8. [DOI] [PubMed] [Google Scholar]
- 41.Mirouze N., Bidnenko E., Noirot P., Auger S. Genome-wide mapping of TnrA-binding sites provides new insights into the TnrA regulon in Bacillus subtilis. Microbiologyopen. 2015;4:423–435. doi: 10.1002/mbo3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guedon E., Moore C.M., Que Q., Wang T., Ye R.W., Helmann J.D. The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sigmaB regulons. Mol. Microbiol. 2003;49:1477–1491. doi: 10.1046/j.1365-2958.2003.03648.x. [DOI] [PubMed] [Google Scholar]
- 43.Radin J.N., Kelliher J.L., Parraga Solorzano P.K., Kehl-Fie T.E. The two-component system ArlRS and alterations in metabolism enable Staphylococcus aureus to resist calprotectin-induced manganese starvation. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekowska A., Danchin A., Risler J.L. Phylogeny of related functions: the case of polyamine biosynthetic enzymes. Microbiol. 2000;146:1815–1828. doi: 10.1099/00221287-146-8-1815. [DOI] [PubMed] [Google Scholar]
- 45.Inaoka T., Matsumura Y., Tsuchido T. Molecular cloning and nucleotide sequence of the superoxide dismutase gene and characterization of its product from Bacillus subtilis. J. Bacteriol. 1998;180:3697–3703. doi: 10.1128/jb.180.14.3697-3703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nebo Y., Nelson N. The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta. 2006;1763:609–620. doi: 10.1016/j.bbamcr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Juttukonda L.J., Chazin W.J., Skaar E.P. Acinetobacter baumannii coordinates urea metabolism with metal import to resist host-mediated metal limitation. mBio. 2016;7 doi: 10.1128/mBio.01475-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishii H., Tanaka T., Ogura M. The Bacillus subtilis response regulator gene degU is positively regulated by CcpA and by catabolite-repressed synthesis of ClpC. J. Bacteriol. 2013;195:193–201. doi: 10.1128/JB.01881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wray L.V., Jr., Zalieckas J.M., Fisher S.H. Bacillus subtilis glutamine synthetase controls gene expression through a protein-protein interaction with transcription factor TnrA. Cell. 2001;107:427–435. doi: 10.1016/s0092-8674(01)00572-4. [DOI] [PubMed] [Google Scholar]
- 50.Zhang R.G., Kim Y., Skarina T., Beasley S., Laskowski R., Arrowsmith C., et al. Crystal structure of Thermotoga maritima 0065, a member of the IclR transcriptional factor family. J. Biol. Chem. 2002;277:19183–19190. doi: 10.1074/jbc.M112171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho B.K., Federowicz S., Park Y.S., Zengler K., Palsson B.Ø. Deciphering the transcriptional regulatory logic of amino acid metabolism. Nat. Chem. Biol. 2011;8:65–71. doi: 10.1038/nchembio.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Botas A., Pérez-Redondo R., Rodríguez-García A., Álvarez-Álvarez R., Yagüe P., Manteca A., et al. ArgR of Streptomyces coelicolor is a pleiotropic transcriptional regulator: effect on the transcriptome, antibiotic production, and differentiation in liquid cultures. Front Microbiol. 2018;9:361. doi: 10.3389/fmicb.2018.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manias D.A., Dunny G.M. Expression of adhesive pili and the collagen-binding adhesin ace is activated by ArgR family transcription factors in Enterococcus faecalis. J. Bacteriol. 2018;200 doi: 10.1128/JB.00269-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garnett J.A., Baumberg S., Stockley P.G., Phillips S.E. Structure of the C-terminal effector-binding domain of AhrC bound to its corepressor L-arginine. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2007;63:918–921. doi: 10.1107/S1744309107049391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaeffer P., Millet J., Aubert J. Catabolite repression of bacterial sporulation. Proc. Natl. Acad. Sci. U. S. A. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogura M., Ohshiro Y., Hirao S., Tanaka T. A new Bacillus subtilis gene, med, encodes a positive regulator of comK. J. Bacteriol. 1997;179:6244–6253. doi: 10.1128/jb.179.20.6244-6253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogura M., Asai K. Glucose induces ECF sigma factor genes, sigX and sigM, independent of cognate anti-sigma factors through acetylation of CshA in Bacillus subtilis. Front Microbiol. 2016;7:1918. doi: 10.3389/fmicb.2016.01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogura M., Tanaka T. Transcription of Bacillus subtilis degR is σD-dependent and suppressed by multicopy proB through σD. J. Bacteriol. 1996;178:216–222. doi: 10.1128/jb.178.1.216-222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsukahara K., Ogura M. Promoter selectivity of the Bacillus subtilis response regulator DegU, a positive regulator of the fla/che operon and sacB. BMC Microbiol. 2008;8:8. doi: 10.1186/1471-2180-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogura M., Shimane K., Asai K., Ogasawara N., Tanaka T. Binding of response regulator DegU to the aprE promoter is inhibited by RapG, which is counteracted by extracellular PhrG in Bacillus subtilis. Mol. Microbiol. 2003;49:1685–1697. doi: 10.1046/j.1365-2958.2003.03665.x. [DOI] [PubMed] [Google Scholar]
- 61.Murayama S., Ishikawa S., Chumsakul O., Ogasawara N., Oshima T. The role of α-CTD in the genome-wide transcriptional regulation of the Bacillus subtilis cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guérout-Fleury A.M., Frandsen N., Stragier P. Plasmids for ectopic integration in Bacillus subtilis. Gene. 1996;180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- 63.Hayashi K., Kensuke T., Kobayashi K., Ogasawara N., Ogura M. Bacillus subtilis RghR (YvaN) represses rapG and rapH, which encode inhibitors of expression of the srfA operon. Mol. Microbiol. 2006;59:1714–1729. doi: 10.1111/j.1365-2958.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi K., Ehrlich S.D., Albertini A., Amati G., Andersen K.K., et al. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinmetz M., Richter R. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene. 1994;142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 66.Sierro N., Makita Y., de Hoon M.J.L., Nakai K. DBTBS: a database of transcriptional regulation in Bacillus subtilis containing upstream intergenic conservation information. Nucleic Acids Res. 2008;36:D93–D96. doi: 10.1093/nar/gkm910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pedreira T., Elfmann C., Stülke J. The current state of SubtiWiki, the database for the model organism Bacillus subtilis. Nucleic Acids Res. 2022;50:D875–D882. doi: 10.1093/nar/gkab943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original sequence reads were deposited in the DRA/SRA database (accession number: DRR445917-DRR445928).