Abstract
Background
We recently demonstrated that more intensive blood pressure (BP) treatment lowered risk of orthostatic hypotension (OH) measured with a seated-to-standing protocol. However, seated-to-standing OH assessments are less sensitive than supine-to-standing and could miss clinically relevant OH.
Objectives
Using data from the Systolic Hypertension in Europe (Syst-Eur) trial, we examined the effect of hypertension treatment on incidence of OH based on the difference in BP from 3 body positions.
Methods
Syst-Eur was a multi-center, randomized trial that enrolled adults with isolated systolic hypertension to investigate whether active hypertension treatment could reduce cardiovascular events. Participants underwent BP measurement in supine, seated, and standing positions. Using differences in BP between the 3 body positions (seated minus supine, standing minus seated, and standing minus supine), we defined OH as a drop in systolic BP ≥20 mm Hg or diastolic BP ≥10 mm Hg. We included measurements from baseline and follow-up visits.
Results
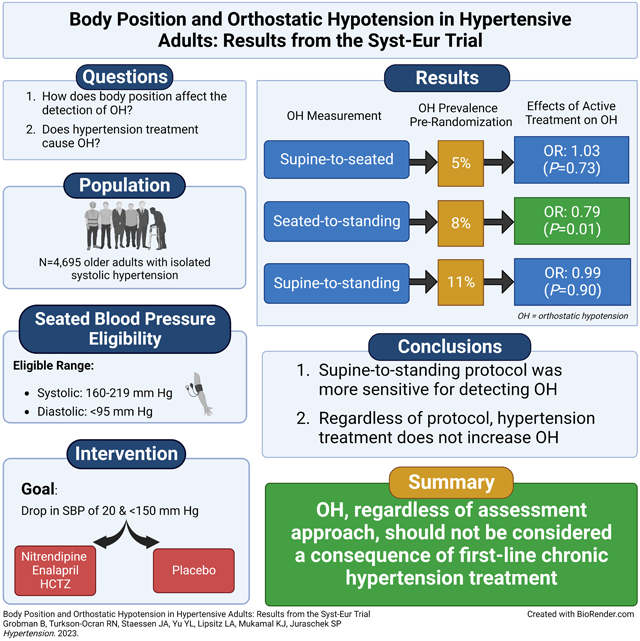
Among 4,695 participants (mean age 70.2 ± 6.7 years, 66.9% female) with 42,636 BP measurements, OH was present in 4.9% of measures with supine-to-seated, 7.9% with seated-to-standing, and 11.4% with supine-to-standing protocols, respectively. Compared with placebo, BP treatment did not increase OH with any set of maneuvers, OR 0.79 (95% CI: 0.65, 0.95) with seated-to standing, 1.03 (95% CI: 0.86, 1.24) with supine-to-seated, and 0.99 (95% CI: 0.86, 1.15) with supine-to-standing.
Conclusion
Regardless of protocol, active hypertension treatment did not increase the risk of OH, reinforcing evidence that OH should not be viewed as a complication of hypertension treatment.
Keywords: hypertension, orthostatic hypotension, cardiovascular outcomes, positional changes, measurement
Graphical Abstract
Introduction
Orthostatic hypotension (OH) is an important risk factor for stroke, cardiovascular disease, and premature death.1,2 While OH disproportionately affects older adults with uncontrolled hypertension,3 in a recent meta-analysis of 9 randomized trials we demonstrated that more intensive hypertension treatment lowered risk of OH among hypertensive adults, regardless of their age.4 However, the majority of these studies assessed OH using a seated-to-standing protocol, which underestimates the risk of OH.5 Whether more aggressive hypertension treatment increases the risk of OH among older adults assessed using a supine-to-standing protocol has not been determined.
The Systolic Hypertension in Europe (Syst-Eur) trial was a multi-center, randomized controlled trial that examined the effect of active hypertension treatment with nitrendipine, as well as enalapril and hydrochlorothiazide as necessary, for blood pressure (BP) reduction versus placebo on stroke outcomes among adults aged 60 years and older.6 Throughout the study, adults with isolated systolic hypertension (systolic BP 160–219 mm Hg, diastolic BP <95 mm Hg) underwent supine, seated, and standing BP readings.6,7 However, the effects of treatment on supine-to-standing OH has not been reported.
Our objectives in the present study were: (1) to characterize the effect of change in body position (supine-to-seated, seated-to-standing, supine-to-standing) on the prevalence of OH, (2) to determine the effect of treatment with nitrendipine (and enalapril and/or hydrochlorothiazide as necessary) versus placebo on risk for developing OH according to change in body position, and (3) to determine whether treatment modified the relationships between OH determined based on the three body positions with trial outcomes (coronary heart disease, stroke, congestive heart failure, death). We hypothesized that despite a higher prevalence of OH based on change from the supine-to-standing position (compared to supine-to-seated or seated-to-standing), hypertension treatment would not cause OH nor alter its relationships with trial outcomes.
Methods
Data Availability
The data that support the findings of this study are available from the original Syst-Eur investigators upon reasonable request.
Overview
The Syst-Eur Trial was an investigator-initiated, multi-center randomized controlled trial conducted by the European Working Party on High Blood Pressure in the Elderly between 1990 and 1997.6,8 The trial was performed at 198 sites in 23 countries across Europe.8 Funding for coordination was supplied by the European Union and the trial was sponsored by Bayer AG with additional funding from the Flemish Ministry of Public Health and the Belgian National Research Fund.6,8 The primary goal of the study was to investigate the effect of hypertension treatment on stroke morbidity and mortality among older adults.6,8 All participants provided consent prior to enrollment and the protocol was approved by the ethics committees at the University of Leuven and all participating centers.8,9
Study Population
Syst-Eur investigators enrolled 4,695 European adults aged 60 years and older with isolated systolic hypertension (systolic BP 160–219 mm Hg, diastolic BP <95 mm Hg).8 Participants with hypertension secondary to a specific condition requiring treatment (e.g., hyperthyroidism or renal artery stenosis), and those with severe hypertension were ineligible.6,8 Also ineligible were those participants with severe cardiac conditions such as congestive heart failure and aortic aneurysm, patients with renal failure, patients with a myocardial infarction within the prior year, and patients with other severe diseases.6,8
Trial Interventions
Prior to randomization, participants underwent a placebo run-in period during which all BP lowering medications were discontinued. They were then given a placebo tablet and their BP was measured at one-month intervals to ascertain their eligibility for the study (systolic BP 160–219 mm Hg, diastolic BP <95 mm Hg, standing systolic BP ≥ 140 mm Hg). Participants who met eligibility criteria for randomization were randomly assigned to the treatment or placebo group. Participants in the treatment group were given titrated doses of nitrendipine (10–40 mg/day) until their BP dropped at least 20 mm Hg and below 150 mm Hg.6,9 If participants on the maximum dose of nitrendipine did not reach appropriate BP control they were given enalapril (5–20 mg/day) and optionally hydrochlorothiazide (12.5–25 mg/day) to achieve treatment goals.6,8,9 In the case that the first medication caused side effects, the second and third medications were substituted sequentially.6
During the double-blind study period, participants were examined at least every 3 months during follow-up visits with more frequent visits as needed during medication adjustment (Supplement Figure S1).6 At each visit, participants’ BP was measured in the supine, seated, and standing positions. Randomization ended upon withdrawal from the trial or reaching an endpoint, whether fatal or nonfatal.6 The trial was stopped in February 1997 due to a significant benefit of treatment on incidence of stroke.9
Orthostatic Hypotension
The original Syst-Eur trial followed the British Hypertension Society guidelines for BP measurement: BP was measured twice after a 2-minute rest in the supine position, then measured twice after a 5-minute rest while sitting, and then twice after a 2-minute rest while standing.6,8 The supine, seated, and standing measurements were based on the average of the two measurements taken in each of these three positions.7 OH was defined using thresholds of the consensus definition as a drop of at least 20 mm Hg systolic and/or at least 10 mm Hg diastolic BP.1,10,11
Cardiovascular Disease Outcomes
Endpoints were determined by committee based on participants’ files and information from the investigators. Cardiovascular disease endpoints were based on review of ICD-9 codes.8 Stroke was defined as a neurological deficit which lasted for longer than 24 hours or led to death, and with no apparent cause other than vascular etiologies.6,8 Myocardial infarction was defined as at least 2 of 3 factors: retrosternal pain with or without radiations and not responding to nitroglycerine, abnormalities on electrocardiogram corresponding to myocardial infarction, and increased cardiac enzymes.6,8 Congestive heart failure was defined as a combination of symptoms such as dyspnea, clinical signs such as crackles or edema, and treatment with vasodilators, antihypertensives, or diuretics.8,9
Other Covariates
Age and sex were self-reported. Baseline GFR was calculated using the 2021 CKD-EPI equation based on creatinine measurements.12 Body mass index (kg/m2) was derived from height and weight measurements. History of diabetes, history of stroke, and history of cardiovascular disease were self-reported.
Statistical Analyses
Baseline characteristics were summarized overall and by treatment assignment, using means, standard deviations (SD), and proportions. Change in BP by study protocol was determined using generalized estimating equations (normal family, identity link, robust variance estimator with an exchangeable covariate matrix). The relationship between changes by protocol were visualized via scatter plots and Lowess curves.
In addition, we used generalized estimating equations (binomial family, logit link, robust variance estimator with an exchangeable covariate matrix) with a treatment-by-visit interaction term (visits rendered as pre-randomization: 0, post-randomization: 1) to determine the effect of treatment assignment on both the prevalence of OH as well as the odds of developing new or recurrent OH post-randomization with OH defined using each of the 3 protocols. Prevalence was plotted by study assignment and protocol based on each individual measure as well as by individual participants (by counting any OH case within pre-specified time intervals: 0 months, 0–6 months, 6–12 months, 12–24 months, 24–36 months, 36–48 months, and >48 months).
We also examined the associations between OH, measured via three different protocols, on endpoints: all-cause mortality, fatal CVD, myocardial infarction (fatal & nonfatal), stroke (fatal & nonfatal), and heart failure (fatal & nonfatal) in both independent and joint (i.e., mutually adjusted for all 3 protocols) Cox proportional hazards models. Model 1 was adjusted for age and sex. Model 2 was further adjusted for baseline GFR, body mass index, history of diabetes, history of stroke, history of cardiovascular disease, and treatment assignment. Model 3 was further adjusted for baseline seated systolic BP. Using these same models, we also examined the associations of change in systolic BP per 10 mm Hg and in diastolic BP per 5 mm Hg on study endpoints. Kaplan-Meier failure functions were used to visualize the cumulative incidence of outcomes by protocol.
A two-tailed P-value of <0.05 was considered significant. All analyses were conducted using Stata version 15.1 (Stata Corporation, College Station, TX, USA).13
Results
Baseline Characteristics
At baseline, the sample was 66.8% female with a mean age of 70.2±6.7 years (Table 1). The overall mean seated SBP was 173.8±10.0 mm Hg and the mean DBP was 85.5±5.9 mm Hg. Other baseline characteristics were evenly balanced between randomized assignments.
Table 1.
Baseline characteristics by overall and by randomized treatment assignment
| Overall, N=4695 | Placebo, N = 2297 | Active Therapy, N = 2398 | |
|---|---|---|---|
| Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | |
| Mean age, y | 70.2 (6.7) | 70.2 (6.7) | 70.3 (6.7) |
| Female, % | 67 | 66 | 68 |
| Mean supine systolic blood pressure, mm Hg | 173.6 (12.5) | 173.8 (12.6) | 173.4 (12.5) |
| Mean supine diastolic blood pressure, mm Hg | 85.4 (6.3) | 85.4 (6.4) | 85.4 (6.2) |
| Mean seated systolic blood pressure, mm Hg | 173.8 (10.0) | 173.9 (10.1) | 173.8 (9.9) |
| Mean seated diastolic blood pressure, mm Hg | 85.5 (5.9) | 85.5 (5.9) | 85.5 (5.8) |
| Mean standing systolic blood pressure, mm Hg | 169.0 (12.2) | 169.2 (12.1) | 168.8 (12.4) |
| Mean standing diastolic blood pressure, mm Hg | 87.4 (7.7) | 87.4 (7.7) | 87.3 (7.7) |
| Body mass index, kg/m2 | 27.0 (4.1) | 27.0 (4.0) | 27.0 (4.2) |
| Body mass index ≥30 kg/m2, % | 20 | 20 | 20 |
| Estimated glomerular filtration rate, ml/min per 1.73 m2 | 66.0 (15.1) | 66.0 (14.9) | 66.0 (15.4) |
| eGFR < 60 ml/min per 1.73 m2 | 37.6 | 37.4 | 37.9 |
| History of diabetes, % | 10 | 10 | 9 |
| History of stroke, % | 1 | 1 | 1 |
| History of cardiovascular disease, % | 6 | 6 | 6 |
Abbreviations: eGFR, estimated glomerular filtration rate; OH, orthostatic hypotension; SD, standard deviation. Mean supine systolic blood pressure N overall/placebo/active: 4691/2294/2397. Mean supine diastolic blood pressure N overall/placebo/active: 4691/2294/2397. Body mass index and body mass index ≥30 kg/m2 N overall: 4661/2280/2381. eGFR and eGFR < 60 ml/min per 1.73 m2 N overall/placebo/active: 4688/2296/2392.
Position and mean blood pressure
Prior to randomization, SBP and DBP did not differ significantly among participants after changing from the supine to seated positions (Table 2). However, we observed significant differences between the supine and standing position in SBP (−4.7 mm Hg; 95% CI: −4.9, −4.4) and DBP (2.0 mm Hg; 95% CI: 1.8, 2.1) and between the seated and standing position in SBP (−4.9 mm Hg; 95% CI: −5.1, −4.7) and DBP (1.9 mm Hg; 95% CI: 1.7, 2.0). These changes were virtually identical regardless of assignment to placebo or active therapy.
Table 2.
Position and mean blood pressure by study assignment
| Systolic blood pressure, mm Hg | |||
|---|---|---|---|
| Mean (SD) | Change (95% CI) | Change (95% CI) | |
| Pre-randomization, N=4,695 (42,636 measurements) | |||
| Supine | 173.6 (173.3, 174.0) | Ref | - |
| Seated | 173.8 (173.5, 174.1) | 0.2 (−0.0, 0.4) | Ref |
| Standing | 169.0 (168.6, 169.3) | -4.7 (−4.9, −4.4) | -4.9 (−5.1, −4.7) |
| Post-randomization Placebo, N=2,250 (55,240 measurements) | |||
| Supine | 164.1 (163.5, 164.7) | Ref | - |
| Seated | 162.8 (162.2, 163.4) | -1.3 (−1.6, −1.1) | Ref |
| Standing | 160.6 (159.9, 161.2) | -3.5 (−3.9, −3.2) | -2.2 (−2.5, −2.0) |
| Post-randomization Active, N=2,354 (62,914 measurements) | |||
| Supine | 154.2 (153.7, 154.7) | Ref | - |
| Seated | 152.3 (151.8, 152.8) | -1.9 (−2.2, −1.6) | Ref |
| Standing | 150.1 (149.6, 150.6) | -4.0 (−4.4, −3.7) | -2.2 (−2.4, −1.9) |
| Diastolic blood pressure, mm Hg | |||
| Mean (SD) | Change (95% CI) | Change (95% CI) | |
| Pre-randomization, N=4,695 (42,633 measurements) | |||
| Supine | 85.4 (85.2, 85.6) | Ref | - |
| Seated | 85.5 (85.3, 85.7) | 0.1 (−0.0, 0.2) | Ref |
| Standing | 87.4 (87.2, 87.6) | 2.0 ( 1.8, 2.1) | 1.9 ( 1.7, 2.0) |
| Post-randomization Placebo, N=2,248 (55,239 measurements) | |||
| Supine | 84.0 (83.7, 84.3) | Ref | - |
| Seated | 83.8 (83.6, 84.1) | -0.2 (−0.3, −0.0) | Ref |
| Standing | 86.2 (85.9, 86.5) | 2.2 ( 2.0, 2.5) | 2.4 ( 2.2, 2.6) |
| Post-randomization Active, N=2,352 (62,909 measurements) | |||
| Supine | 80.2 (80.0, 80.5) | Ref | - |
| Seated | 79.8 (79.5, 80.0) | -0.5 (−0.6, −0.3) | Ref |
| Standing | 82.1 (81.7, 82.4) | 1.8 ( 1.6, 2.1) | 2.3 ( 2.1, 2.5) |
Note: A few diastolic blood pressure measurements were missing.
Prior to randomization, changes in seated-to-standing and supine-to-seated BP tended to be inversely related (i.e., participants for whom blood pressure decreased in the seated-to-standing protocol generally had a blood pressure increase in the supine-to-standing protocol and vice versa). In comparisons of the supine-to-standing protocol with either seated-to-standing or supine-to-seated protocols, the slopes tended to be less than 1, suggesting that changes in supine-to-standing BP were generally smaller than corresponding changes in the supine-to-seated and seated-to-standing protocols (see Supplement Figures S2–S3). These relationships did not differ by treatment assignment.
Effects of Active Treatment on OH
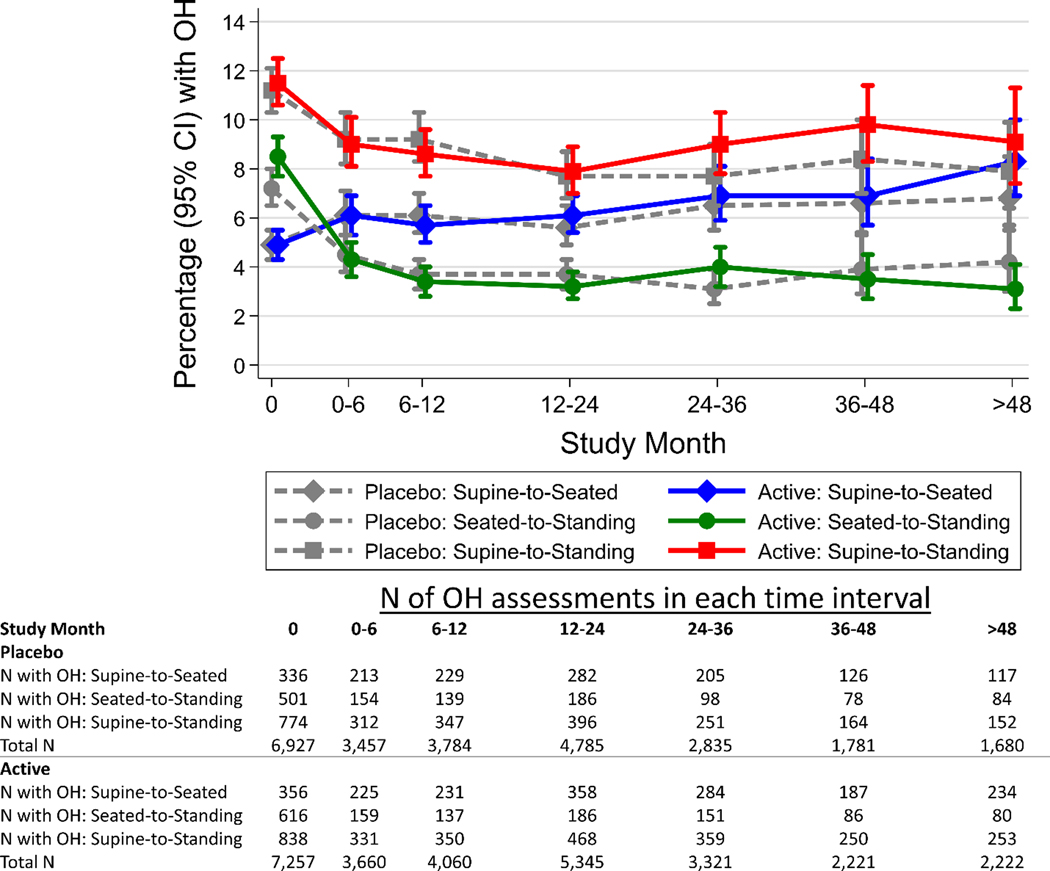
At baseline, OH was present in 4.9% of measurements with supine-to-seated, 7.9% with seated-to-standing, and 11.4% with supine-to-standing protocols (Supplement Table S1). Compared with placebo, active BP treatment was not associated with OH regardless of position, ORs being 1.03 (95% CI: 0.86, 1.24) with supine-to-seated, 0.79 (95% CI: 0.65, 0.95) with seated-to-standing, and 0.99 (95% CI: 0.86, 1.15) with supine-to-standing protocols (Table 3). Similarly, treatment for hypertension did not increase the prevalence of OH at any of the follow-up intervals, regardless of measurement protocol (Figure 1, Supplement Figure S1).
Table 3.
Effects of Active Treatment on OH, N = 4694 participants with 53,335 measures
| Placebo | Active | OR (95% CI) for new or recurrent OH | P | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-randomized OH, % | Follow-up OH, % | Pre-randomized OH, % | Follow-up OH, % | |||
| Supine-to-Seated | 4.9 | 6.1 | 4.9 | 6.4 | 1.03 (0.86, 1.24) | 0.73 |
| Seated-to-Standing | 7.2 | 3.8 | 8.5 | 3.6 | 0.79 (0.65, 0.95) | 0.013 |
| Supine-to-Standing | 11.2 | 8.5 | 11.5 | 8.7 | 0.99 (0.86, 1.15) | 0.90 |
Abbreviations: CI, confidence intervals; OH, orthostatic hypotension
Figure 1.
Percentage (95% CI) of measurements with OH over study follow-up months estimated using generalized estimating equations by protocol (supine-to-seated, seated-to-standing, supine-to-standing) and by active (solid lines) or placebo (dashed lines) assignments. Models included a Poisson family, log link with a robust variance estimator and exchangeable covariance matrix.
Association of OH prior to randomization with outcomes
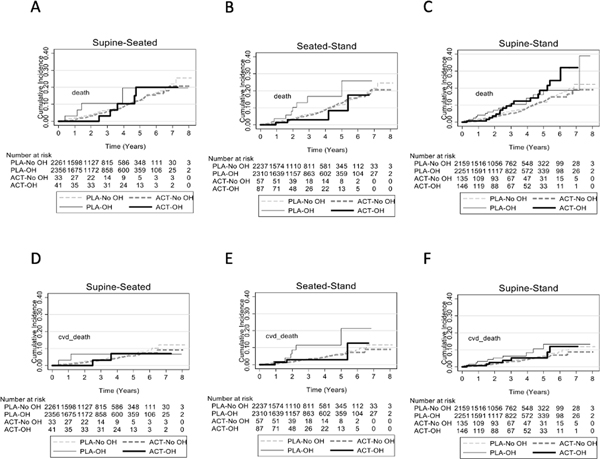
Prior to randomization, OH measured using the supine-to-standing protocol was associated with higher all-cause mortality after adjustment for age and sex, with an adjusted hazard ratio of 1.45 (95% CI: 1.01, 2.09) (Supplement Table S2; Figure 2). Otherwise, OH did not independently or jointly predict any fatal or nonfatal endpoint, regardless of how OH was measured. When examined as a continuous change, supine-to-seated change in SBP was inversely associated with mortality and heart failure in joint models (Supplement Table S3). In contrast, both supine-to-seated and seated-to-standing changes in DBP were inversely associated with all-cause mortality, while only seated-to-standing changes in DBP were inversely associated with fatal CVD in joint models (Supplement Table S4).
Figure 2.
Cumulative incidence plots according to orthostatic hypotension status and treatment group for all-cause mortality (A-C) and cardiovascular disease death (D-F) for the supine-to-seated protocol, the seated-to-standing protocol, and the supine-to-standing protocol. ACT represents active therapy, PLA represents placebo, and OH represents orthostatic hypotension.
Discussion
In this study of older adults with hypertension, we found that the proportion with OH differed based on the measurement protocol used with a higher number of OH cases detected via a supine-to-standing protocol versus a seated-to-standing protocol. Moreover, the supine-to-standing protocol was more strongly associated with all-cause mortality, although this association was weak and not independent of other positions in joint models. Nevertheless, regardless of measurement protocol used, hypertension treatment did not increase the risk of OH, reinforcing observations that hypertension treatment does not cause OH.
The finding that there are significant differences in the prevalence of OH when evaluated via the three different protocols aligns with our hypothesis. OH based on the consensus definition is defined as a drop of at least 20 mm Hg systolic and/or at least 10 mm Hg diastolic BP within 3 minutes of standing or sitting up at least 60 degrees on a tilt table.1,10,11 The present study shows that these definitions may not result in an equivalent prevalence of OH, and as such clinicians should be aware of possible discrepancies in the sensitivity of these measurement protocols. This finding is supported by our previous work using data from the STURDY trial, which found OH among 3.9% of participants with a seated-to-standing protocol versus 20.4% of participants with a supine-to-standing protocol.14 Similarly, a recent study found that BP drops were more pronounced among geriatric patients with higher levels of frailty when using a supine-to-standing as supposed to seated-to-standing measurement protocol. However, this relationship was not found among patients with lower or medium levels of frailty.15
Prior work suggests that seated-to-standing changes in BP are equivalent to greater magnitude changes in supine-to-standing BP based on observations in populations with neurogenic OH.16 Contrary to these observations, we found that the changes in SBP for supine-to-seated and seated-to-standing protocols were inversely related. Moreover, the overall change in BP between the supine and standing positions was often less than the individual changes between the supine-to-seated and seated-to-standing protocols. This questions the idea that different seated cut points can be used as a substitute for supine BP when assessing OH.17 This inverse relationship is supported by prior findings which show that both systolic and diastolic BP readings are on average lower in the supine position as compared to the seated position.18,19
Regardless of OH measurement protocol, intensive treatment for hypertension did not increase the risk of having OH. Some observational studies have shown a higher incidence of OH after hypertension treatment using alpha blockers,20 beta blockers,20–22 and centrally acting drugs.20 The impact of hypertension treatments targeting the renin-angiotensin-aldosterone system on OH are less consistent.20–22 Two of these trials used a seated-to-standing protocol21,22 while one used a supine-to-standing protocol.20 Nevertheless, in a recent meta-analysis of 9 randomized clinical trials, we showed that intensive BP lowering treatment decreased the risk of OH based on seated-to-standing protocols.4 However, the question remained whether these studies missed OH elicited from a supine-to-standing protocol. The present study addresses this concern, demonstrating that there was no increased risk of OH from treatment with a number of first-line antihypertensive agents, regardless of protocol used.8,23–30
OH detected by a supine-to-standing protocol was only mildly associated with all-cause mortality and this relationship was not independent from OH from the seated-to-standing protocol. While other studies have demonstrated OH to be associated with all-cause mortality,2,31 these relationships are inconsistent.32,33 Our study suggests that the protocol used to detect OH may not fully explain this heterogeneity in the literature. Further research is needed to understand both the optimal context, population, and protocol for predicting adverse events in relation to OH.
Our study has a few limitations. First, the measurement of OH via a supine-to-standing protocol was calculated as a composite measurement in which participants first sat up and were measured and then stood, compared to standing up in a single motion. This may limit the clinical implications of our findings regarding the supine-to-standing protocol. Moreover, temporal effects of the protocols cannot be isolated from the protocols, which may explain, in part, why joint models attenuated the relationship between supine-to-standing OH and all-cause mortality. Second, this study only included three classes of anti-hypertensive medications: a dihydropyridine calcium channel blocker that served as first-line treatment, an ACE inhibitor, and a thiazide diuretic. Thus, while our findings are applicable to first-line hypertension treatments, they may not be applicable to the range of antihypertension treatment regimens employed in clinical practice. Finally, our study sample was limited to a group of European adults aged 60 years and older. It is unclear whether the association between OH and outcomes varies by age. This may limit the generalizability of this study to younger populations.
Our study also has multiple strengths. Our study represents one of the largest comparisons of OH protocol in the literature among older adults with hypertension, a group with a higher prevalence of OH. Moreover, this is one of the only trials of hypertension treatment with supine-to-standing OH assessments, allowing us to examine OH using a more sensitive protocol. Finally, events were adjudicated and all assessments rigorously performed.
In conclusion, while more OH was detected via a supine-to-standing protocol, hypertension treatment did not increase the risk of having OH with any of the three protocols used. These findings reinforce evidence that the discovery of OH should not be considered a reason to discontinue or down-titrate hypertension therapy.
Perspectives
In a previous work, we demonstrated that hypertension treatment decreased the risk of orthostatic hypotension.4 However, these studies only assessed orthostatic hypotension when participants transferred from a seated-to-standing position. The present study, a post-hoc analysis of Syst-Eur, included supine, seated, and standing positions in its OH assessment. While OH was most prevalent when based on a supine-to-standing protocol, hypertension treatment did not increase the risk of orthostatic hypotension regardless of position. These findings reinforce that OH should not be considered a consequence of first-line hypertension treatments nor a reason to reduce hypertension therapy.
Supplementary Material
Pathophysiological Novelty and Relevance.
What is New?
Although more orthostatic hypotension was detected based on a supine-to-standing protocol, hypertension treatment did not increase risk of orthostatic hypotension regardless of protocol used for the assessment of OH.
What is Relevant?
Prior reports demonstrated no effect from hypertension treatment on OH based on seated-to-standing protocols, but it was unknown whether effects might differ with a more sensitive protocol, involving the supine position.
Clinical/pathophysiological relevance
Our findings suggest that hypertension treatment does not contribute to a higher risk of orthostatic hypotension regardless of measurement protocol
Acknowledgements
We thank participants of the Syst-Eur study who volunteered in support of this research.
Sources of Funding
SPJ is supported by NIH/ NHLBI 7K23HL135273. KJM is supported by NIH/NIA 5K24AG065525.
Nonstandard abbreviations:
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HR
hazard ratio
- OH
orthostatic hypotension
- SBP
systolic blood pressure
- Syst-Eur
Systolic Hypertension in Europe
- STURDY
The Study to Understand Fall Reduction and Vitamin D in You
Footnotes
Disclosure
The authors declare that there is no conflict of interest associated with this manuscript.
Syst-Eur is registered on clinicaltrials.gov under identifier NCT02088450
References
- 1.Freeman R, Abuzinadah AR, Gibbons C, Jones P, Miglis MG, Sinn DI. Orthostatic Hypotension. Journal of the American College of Cardiology. 2018;72(11):1294–1309. doi: 10.1016/j.jacc.2018.05.079 [DOI] [PubMed] [Google Scholar]
- 2.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31(1):85–91. doi: 10.1093/eurheartj/ehp329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gangavati A, Hajjar I, Quach L, et al. Hypertension, Orthostatic Hypotension, and the Risk of Falls in a Community-Dwelling Elderly Population: The Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study: HYPERTENSION, ORTHOSTATIC HYPOTENSION, AND FALLS. Journal of the American Geriatrics Society. 2011;59(3):383–389. doi: 10.1111/j.1532-5415.2011.03317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juraschek SP, Hu JR, Cluett JL, et al. Effects of Intensive Blood Pressure Treatment on Orthostatic Hypotension: A Systematic Review and Individual Participant–based Meta-analysis. Ann Intern Med. 2021;174(1):58–68. doi: 10.7326/M20-4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juraschek SP, Appel LJ, Mitchell C, et al. Comparison of supine and seated orthostatic hypotension assessments and their association with falls and orthostatic symptoms. J American Geriatrics Society. Published online April 22, 2022:jgs.17804. doi: 10.1111/jgs.17804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amery A, Birkenhäger W, Bulpitt CJ, et al. Syst-Eur. A multicentre trial on the treatment of isolated systolic hypertension in the elderly: Objectives, protocol, and organization. Aging Clin Exp Res. 1991;3(3):287–302. doi: 10.1007/BF03324024 [DOI] [PubMed] [Google Scholar]
- 7.Vanhanen H, Thijs L, Birkenhäger W, et al. Prevalence and persistency of orthostatic blood pressure fall in older patients with isolated systolic hypertension. Syst-Eur Investigators. J Hum Hypertens. 1996;10(9):607–612. [PubMed] [Google Scholar]
- 8.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Lancet. 1997;350(9080):757–764. doi: 10.1016/S0140-6736(97)05381-6 [DOI] [PubMed] [Google Scholar]
- 9.Hara A, Thijs L, Asayama K, Jacobs L, Wang JG, Staessen JA. Randomised Double-Blind Comparison of Placebo and Active Drugs for Effects on Risks Associated with Blood Pressure Variability in the Systolic Hypertension in Europe Trial. Fuchs FD, ed. PLoS ONE. 2014;9(8):e103169. doi: 10.1371/journal.pone.0103169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. doi: 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 11.Tzur I, Izhakian S, Gorelik O. Orthostatic hypotension: definition, classification and evaluation. Blood Pressure. 2019;28(3):146–156. doi: 10.1080/08037051.2019.1604067 [DOI] [PubMed] [Google Scholar]
- 12.Inker LA, Eneanya ND, Coresh J, et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.StataCorp. Stata Statistical Software: Release 17. Published online 2021. [Google Scholar]
- 14.Juraschek SP, Appel LJ, Mitchell C, et al. Comparison of supine and seated orthostatic hypotension assessments and their association with falls and orthostatic symptoms. J American Geriatrics Society. 2022;70(8):2310–2319. doi: 10.1111/jgs.17804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabriele S, Georgiopoulos I, Labat C, et al. Can sitting and lying blood pressure measurements be considered interchangeable in older frail adults? Eur Geriatr Med. Published online September 2, 2022. doi: 10.1007/s41999-022-00669-7 [DOI] [PubMed] [Google Scholar]
- 16.Shaw BH, Garland EM, Black BK, et al. Optimal diagnostic thresholds for diagnosis of orthostatic hypotension with a “sit-to-stand test.” J Hypertens. 2017;35(5):1019–1025. doi: 10.1097/HJH.0000000000001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casiglia E, Jordan J. Orthostatic hypotension: new views for an old problem. Journal of Hypertension. 2017;35(5):947–949. doi: 10.1097/HJH.0000000000001272 [DOI] [PubMed] [Google Scholar]
- 18.Privšek E, Hellgren M, Råstam L, Lindblad U, Daka B. Epidemiological and clinical implications of blood pressure measured in seated versus supine position. Medicine. 2018;97(31):e11603. doi: 10.1097/MD.0000000000011603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu L-C, Wei T-M, Li S, Ye X-L, Zeng C-L, Wang L-X. Differences in blood pressure readings between supine and sitting positions in hypertensive patients. Acta Cardiologica. 2008;(6):707–711. doi: 10.2143/AC.63.6.2033387 [DOI] [PubMed] [Google Scholar]
- 20.Di Stefano C, Milazzo V, Totaro S, et al. Orthostatic hypotension in a cohort of hypertensive patients referring to a hypertension clinic. J Hum Hypertens. 2015;29(10):599–603. doi: 10.1038/jhh.2014.130 [DOI] [PubMed] [Google Scholar]
- 21.Kamaruzzaman S, Watt H, Carson C, Ebrahim S. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age and Ageing. 2010;39(1):51–56. doi: 10.1093/ageing/afp192 [DOI] [PubMed] [Google Scholar]
- 22.Canney M, O’Connell MDL, Murphy CM, et al. Single Agent Antihypertensive Therapy and Orthostatic Blood Pressure Behaviour in Older Adults Using Beat-to-Beat Measurements: The Irish Longitudinal Study on Ageing. Rengo G, ed. PLoS ONE. 2016;11(1):e0146156. doi: 10.1371/journal.pone.0146156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright Jr JT, Bakris G, Greene T, et al. ; African American Study of Kidney Disease and Hypertension Study Group. Effect of Blood Pressure Lowering and Antihypertensive Drug Class on Progression of Hypertensive Kidney DiseaseResults From the AASK Trial. JAMA. 2002;288(19):2421. doi: 10.1001/jama.288.19.2421 [DOI] [PubMed] [Google Scholar]
- 24.Cushman W, Evans G, Byington R et al. ;, ACCORD Study Group. Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright JJ, Wiliamson J, Whelton P, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benavente O, Coffey C, Conwit R, SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. The Lancet. 2013;382(9891):507–515. doi: 10.1016/S0140-6736(13)60852–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 28.Beckett NS, Peters R, Fletcher AE, et al. Treatment of Hypertension in Patients 80 Years of Age or Older. N Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 29.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265(24):3255–3264. [PubMed] [Google Scholar]
- 30.Neaton JD, Grimm RH, Prineas RJ, et al. Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270(6):713–724. [PubMed] [Google Scholar]
- 31.Juraschek SP, Lipsitz LA, Beach JL, Mukamal KJ. Association of Orthostatic Hypotension Timing With Clinical Events in Adults With Diabetes and Hypertension: Results From the ACCORD Trial. Am J Hypertens. 2019;32(7):684–694. doi: 10.1093/ajh/hpz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss A, Beloosesky Y, Kornowski R, Yalov A, Grinblat J, Grossman E. Influence of orthostatic hypotension on mortality among patients discharged from an acute geriatric ward. J Gen Intern Med. 2006;21(6):602–606. doi: 10.1111/j.1525-1497.2006.00450.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss A, Beloosesky Y, Grossman A, Shlesinger A, Koren-Morag N, Grossman E. The association between orthostatic hypertension and all-cause mortality in hospitalized elderly persons. J Geriatr Cardiol. 2016;13(3):239–243. doi: 10.11909/j.issn.1671-5411.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the original Syst-Eur investigators upon reasonable request.