Abstract
BACKGROUND:
Cardiometabolic abnormalities are a leading cause of death among women, including women with cancer.
METHODS:
This study examined the association between prediagnosis cardiovascular health and total and cause-specific mortality among 12,076 postmenopausal women who developed local- or regional-stage invasive cancer in the Women’s Health Initiative (WHI). Cardiovascular risk factors included waist circumference, hypertension, high cholesterol, and type 2 diabetes. Obesity-related cancers included breast cancer, colorectal cancer, endometrial cancer, kidney cancer, pancreatic cancer, ovarian cancer, stomach cancer, liver cancer, and non-Hodgkin lymphoma. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) adjusted for important predictors of survival.
RESULTS:
After a median follow-up of 10.0 years from the date of the cancer diagnosis, there were 3607 total deaths, with 1546 (43%) due to cancer. Most participants (62.9%) had 1 or 2 cardiometabolic risk factors, and 8.1% had 3 or 4. In adjusted models, women with 3 to 4 risk factors (vs none) had a higher risk of all-cause mortality (HR, 1.99; 95% CI, 1.73–2.30), death due to cardiovascular disease (CVD) (HR, 4.01; 95% CI, 2.88–5.57), cancer-specific mortality (HR, 1.37; 95% CI, 1.1–1.72), and other-cause mortality (HR, 2.14; 95% CI, 1.70–2.69). A higher waist circumference was associated with greater all-cause mortality (HR, 1.17; 95% CI, 1.06–1.30) and cancer-specific mortality (HR, 1.22; 95% CI, 1.04–1.42).
CONCLUSIONS:
Among postmenopausal women diagnosed with cancer in the WHI, cardiometabolic risk factors before the cancer diagnosis were associated with greater all-cause, CVD, cancer-specific, and other-cause mortality. These results raise hypotheses regarding potential clinical intervention strategies targeting cardiometabolic abnormalities that require future prospective studies for confirmation.
Keywords: cancer, cardiometabolic risk factors, survival, Women’s Health Initiative
INTRODUCTION
Cardiometabolic abnormalities characterizing metabolic syndrome have long been linked to incident cardiovascular disease (CVD)1–3 and diabetes3 as well as deaths due to CVD and all causes.4 Specific clinical characteristics of compromised cardiometabolic health include an increased waist circumference, elevated triglycerides, hypertension, elevated fasting glucose levels, and low high-density lipoprotein cholesterol levels.5,6
In cohort studies including women, metabolic syndrome has been statistically significantly associated with a risk of endometrial, pancreatic, gastric, colorectal, ovarian, and postmenopausal breast cancers and non-Hodgkin lymphoma.7–10 To our knowledge, only 2 other large cohort studies have shown a significant relationship between indicators of cardiometabolic health and mortality across several cancer sites together11,12; however, no other group has comprehensively evaluated the relationship between cardiometabolic abnormalities determined before the cancer diagnosis and specific causes of death among postmenopausal women.
The continued improvement in outcomes after cancer due to advances in treatment and screening, which have resulted in an increase in the number of cancer survivors,13 coupled with the high prevalence of obesity and related comorbidities,14–16 highlights the need to better understand the relationship between cardiometabolic abnormalities and outcomes after cancer. We previously reported in the Women’s Health Initiative (WHI) on the relationship between preexisting cardiometabolic abnormalities and a higher risk of death due to CVD and other causes after the diagnosis of breast cancer.17 In this analysis, we evaluated associations between prediagnosis cardiometabolic abnormalities and mortality after cancer in an expanded cohort of WHI participants who were diagnosed with obesity-related cancers.
MATERIALS AND METHODS
Study Population
The WHI consists of an observational cohort (n = 93,676) and 3 overlapping clinical trials (n = 68,132), including trials of hormone therapy, dietary modification, and calcium plus vitamin D supplementation.18,19 Women were eligible to participate in the WHI if they were between the ages of 50 and 79 years, were postmenopausal, and had a predicted survival of at least 3 years at study entry. Participants were recruited from 40 US clinical centers between October 1, 1993, and December 31, 1998, and were initially followed through March 2005. WHI participants were subsequently followed through 2 extension studies, with follow-up now ongoing at least through 2020. The study design was approved by the institutional review boards at the participating centers.
Our study population included WHI participants who developed pathologically confirmed local- or regional-stage breast, colorectal, endometrial, kidney, pancreatic, ovarian, stomach, or liver cancer or non-Hodgkin lymphoma after enrollment in either the observational cohort or the clinical trial but before December 12, 2018 (n = 18,911). These cancers were chosen because of established associations between the risk of these cancers and either individual cardiometabolic features or metabolic syndrome. We excluded the following groups from the study cohort: those with cancer diagnosed at the time of death (n = 1114) or within 1 month of death (n = 339), those with a history of cancer at study entry or an unknown medical history (n = 1403), those with a distant (n = 1998) or unknown stage at diagnosis (n = 455), those with a second primary cancer (n = 1495), and those with missing information on waist circumference (n = 31). This resulted in an analytic cohort of 12,076 (Fig. 1). Women with distant-stage disease were excluded to include a more homogeneous study population of women with early-stage cancer.
Figure 1.
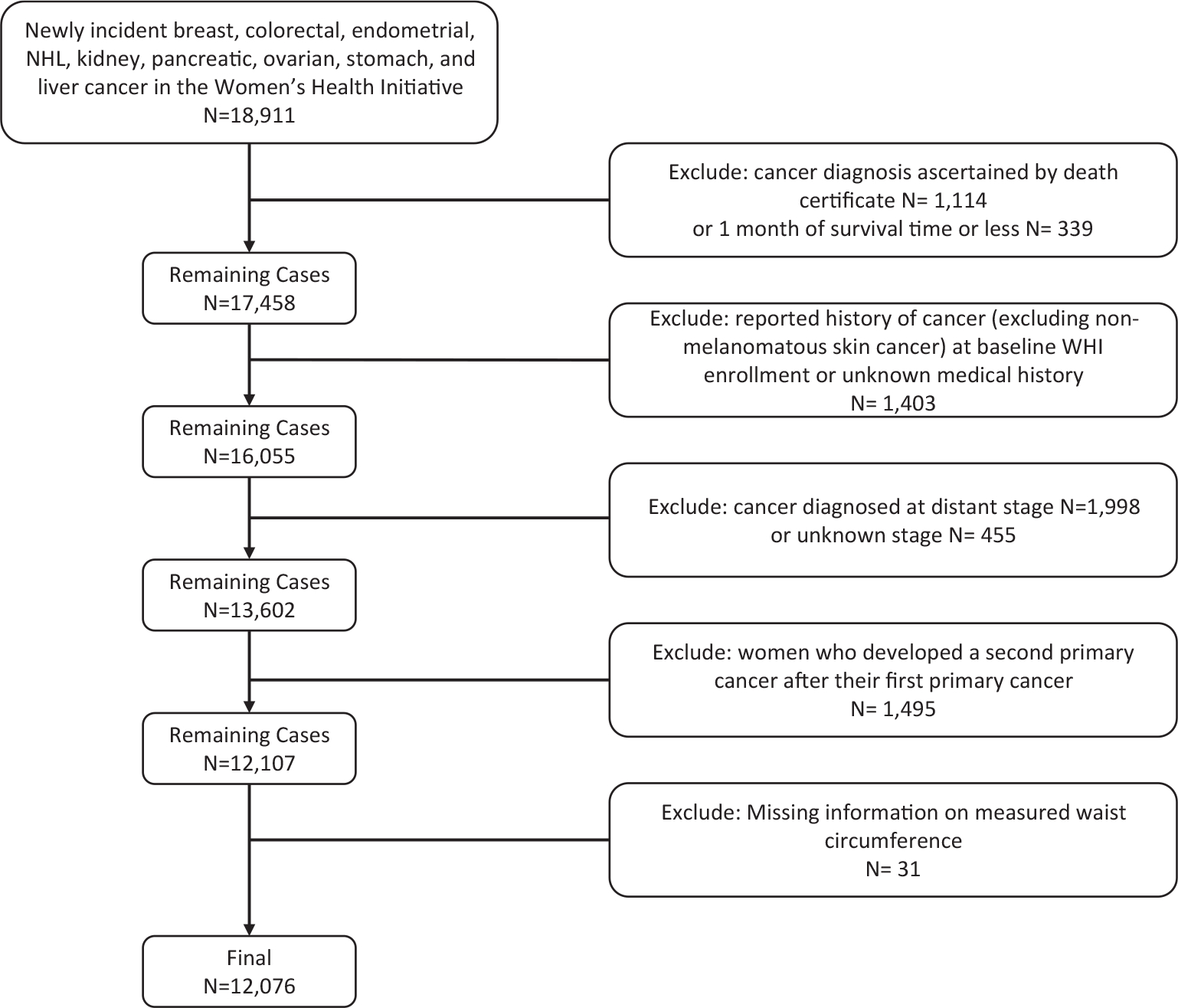
Study inclusion and exclusion criteria. NHL indicates non-Hodgkin lymphoma; WHI, Women’s Health Initiative.
Cardiometabolic Abnormalities
We used information on cardiometabolic abnormalities, including waist circumference and hypertension and self-reported histories of hypertension, diabetes, and high cholesterol. A high waist circumference was defined as ≥88 cm,20 and hypertension was defined as a systolic blood pressure > 130 mg Hg and/or a diastolic blood pressure > 85 mm Hg based on measurements taken by trained study staff at WHI clinical sites or as self-reported use of medications to treat hypertension at the baseline. Participants were considered to have diabetes or elevated cholesterol on the basis of self-reported histories or the use of medication used to treat each disease at baseline entry into the WHI.18,19 The use of information on self-reported diabetes has been previously validated in the WHI.21
Outcomes
Outcomes included all-cause mortality, mortality from cancer, mortality from CVD, and mortality from other causes. Participants were followed for outcomes from the date of their cancer diagnosis until the date of death or last contact before December 12, 2018. Deaths from CVD included deaths due to coronary heart disease, cerebrovascular accident, pulmonary embolism, possible coronary heart disease, other cardiovascular causes, and unknown CVD. The cause of death was determined by medical record or death certificate review and, in some cases, by a relative’s report. Mortality findings and information on cause of death were enhanced by serial linkage to the National Death Index.22
Covariates
Standardized questionnaires were used at the baseline to collect information on the following: age at cancer diagnosis, race/ethnicity, education, affiliation with a current health care provider, smoking history, alcohol consumption, recreational physical activity, postmenopausal hormone therapy, and history of cancer before study enrollment.18 Height, weight, and waist circumference were measured at the baseline according to a standardized protocol by trained study staff at WHI clinical sites. The body mass index (BMI) was computed as the weight (in kilograms) divided by the height (in meters squared). The summary stage was based on a review at the clinical coordinating center of the pathology report using the Surveillance, Epidemiology, and End Results coding system.22
Statistical Analysis
The distributions of demographics, exposures, selected health behaviors, and cardiometabolic abnormalities at the baseline were summarized overall and by the number of cardiometabolic abnormalities (0, 1 or 2, or 3 or 4). Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the association between each of the 4 cardiometabolic features identified at the baseline and death due to all causes as well as the competing risks of death due to cancer, CVD, and other causes. The survival time was calculated from the date of cancer diagnosis to death or last contact before December 12, 2018. The final models assessing individual abnormalities were mutually adjusted for the other 3 cardiovascular abnormalities as well as all variables listed in Table 1 (age group at cancer diagnosis, education, race/ethnicity, WHI study group, current health care provider, BMI, smoking status, alcohol intake, physical activity, and hormone therapy use). Sensitivity analyses were conducted that excluded BMI from the final model and were stratified by the time from WHI enrollment to cancer diagnosis (<5 and ≥5 years). In addition, we evaluated cancer site–specific mortality for the 3 most common cancers identified (stratified by the corresponding cancer site) and plotted the HRs and CIs associated with an increasing number of cardiometabolic abnormalities (ordinal variable: 0, 1 or 2, or 3 or 4). We also conducted a Kaplan-Meier analysis for overall survival by the number of abnormalities at the baseline.
TABLE 1.
Select Demographic and Clinical Characteristics Stratified by the Number of Cardiometabolic Abnormalities at Baseline Enrollment Into WHI
| No. of Cardiometabolic Abnormalities at Baseline |
||||||||
|---|---|---|---|---|---|---|---|---|
| All Women |
0 |
1 or 2 |
3 or 4 |
|||||
| Characteristic | No. | % | No. | % | No. | % | No. | % |
|
| ||||||||
| Total | 12,076 | 3508 | 7593 | 975 | ||||
| Age group at diagnosis | ||||||||
| <60 y | 747 | 6 | 316 | 9 | 395 | 5 | 36 | 4 |
| 60–64 y | 1445 | 12 | 536 | 15 | 797 | 10 | 112 | 11 |
| 65–69 y | 2277 | 19 | 708 | 20 | 1422 | 19 | 147 | 15 |
| 70–74 y | 2882 | 24 | 791 | 23 | 1823 | 24 | 268 | 27 |
| ≥75 y | 4725 | 39 | 1157 | 33 | 3156 | 42 | 412 | 42 |
| Education | ||||||||
| High school or less | 2343 | 20 | 484 | 14 | 1581 | 21 | 278 | 29 |
| More than high school | 9655 | 80 | 3000 | 86 | 5967 | 79 | 688 | 71 |
| Racea | ||||||||
| White (not of Hispanic origin) | 10,504 | 87 | 3184 | 91 | 6565 | 86 | 755 | 77 |
| Black or African American | 820 | 7 | 101 | 3 | 576 | 8 | 143 | 15 |
| Other | 752 | 6 | 223 | 6 | 452 | 6 | 77 | 8 |
| WHI study | ||||||||
| Observational | 6536 | 54 | 2105 | 60 | 3916 | 52 | 515 | 53 |
| DM: intervention arm | 1623 | 13 | 439 | 13 | 1072 | 14 | 112 | 11 |
| DM: control arm | 2548 | 21 | 639 | 18 | 1709 | 23 | 200 | 21 |
| CT not randomized to DM | 1369 | 11 | 325 | 9 | 896 | 12 | 148 | 15 |
| Current health care provider | ||||||||
| No | 630 | 5 | 221 | 6 | 394 | 5 | 15 | 2 |
| Yes | 11,353 | 95 | 3264 | 94 | 7137 | 95 | 952 | 98 |
| BMI | ||||||||
| <25.0 kg/m2 | 4004 | 33 | 2151 | 62 | 1823 | 24 | 30 | 3 |
| 25.0–29.9 kg/m2 | 4037 | 34 | 1181 | 34 | 2588 | 34 | 268 | 28 |
| 30.0–34.9 kg/m2 | 2357 | 20 | 134 | 4 | 1873 | 25 | 350 | 36 |
| >35.0 kg/m2 | 1584 | 13 | 15 | 0 | 1246 | 17 | 323 | 33 |
| Cardiometabolic abnormalities | ||||||||
| Waist circumference | ||||||||
| <88 cm | 6927 | 57 | 3508 | 100 | 3390 | 45 | 29 | 3 |
| ≥88 cm | 5149 | 43 | 0 | – | 4203 | 55 | 946 | 97 |
| High cholesterol | ||||||||
| No | 10,486 | 87 | 3508 | 100 | 6673 | 88 | 305 | 31 |
| Yes | 1590 | 13 | 0 | – | 920 | 12 | 670 | 69 |
| Diabetes | ||||||||
| No | 11,412 | 95 | 3508 | 100 | 7401 | 97 | 503 | 52 |
| Yes | 664 | 5 | 0 | – | 192 | 3 | 472 | 48 |
| Hypertension | ||||||||
| No | 5570 | 46 | 3508 | 100 | 2038 | 27 | 24 | 2 |
| Yes | 6506 | 54 | 0 | – | 5555 | 73 | 951 | 98 |
| Lifestyle factors | ||||||||
| Smoking status | ||||||||
| Never smoked | 5993 | 50 | 1754 | 51 | 3755 | 50 | 484 | 51 |
| Past smoker | 5247 | 44 | 1486 | 43 | 3333 | 44 | 428 | 45 |
| Current smoker | 684 | 6 | 221 | 6 | 418 | 6 | 45 | 5 |
| Alcohol intake | ||||||||
| Nondrinker | 1190 | 10 | 265 | 8 | 780 | 10 | 145 | 15 |
| Past drinker | 2002 | 17 | 410 | 12 | 1292 | 17 | 300 | 31 |
| <1 drink/mo | 1524 | 13 | 383 | 11 | 1008 | 13 | 133 | 14 |
| <1 drink/wk | 2508 | 21 | 704 | 20 | 1629 | 22 | 175 | 18 |
| 1 to <7 drinks/wk | 3145 | 26 | 1153 | 33 | 1854 | 25 | 138 | 14 |
| ≥7 drinks/wk | 1637 | 14 | 577 | 17 | 982 | 13 | 78 | 8 |
| Episodes of moderate to strenuous activity | ||||||||
| No activity | 1708 | 15 | 334 | 10 | 1180 | 16 | 194 | 21 |
| Some activity of limited duration | 4594 | 40 | 1178 | 36 | 2985 | 42 | 431 | 46 |
| 2 or 3 episodes/wk | 2147 | 19 | 692 | 21 | 1294 | 18 | 161 | 17 |
| ≥4 episodes/wk | 2982 | 26 | 1113 | 34 | 1727 | 24 | 142 | 15 |
| HT usage status | ||||||||
| Never used | 5054 | 42 | 1193 | 34 | 3352 | 44 | 509 | 52 |
| Past or current user | 7010 | 58 | 2310 | 66 | 4235 | 56 | 465 | 48 |
Abbreviations: BMI, body mass index; CT, clinical trial; DM, dietary modification; HT, hormone therapy; WHI, Women’s Health Initiative.
Other includes Hispanic/Latino, Asian or Pacific Islander, American Indian or Alaskan Native, and other.
All analyses were completed with SAS software (version 9.4; SAS, Carey, North Carolina). To test the proportionality assumption for the Cox proportional hazards regression analysis, an interaction term between the log of time and the ordinal exposure variable was added to each adjusted model, and we observed no statistically significant departures from proportionality. P values were 2-sided and were considered significant when they were <.005 (adjusted to take into consideration multiple comparisons).
RESULTS
Table 1 describes the demographic and clinical characteristics stratified by the number of cardiometabolic abnormalities. The median time between the baseline clinic visit and cancer diagnosis was 7.8 years (range, <1 to 22 years). Overall, the mean age at WHI study enrollment was 63.1 years (standard deviation [SD], 7.0 years), and the mean age at cancer diagnosis was 72.1 years (SD, 7.9 years); a majority of the participants were non-Hispanic White (87%) and were educated beyond high school (80%). The mean BMI at enrollment was 28.2 kg/m2 (SD, 6.0 kg/m2), and approximately one-third of the participants were in each of the established BMI categories (normal weight, overweight, and obese). The distribution of participants by the number of cardiometabolic abnormalities included 29% with no abnormalities, 63% with 1 or 2 abnormalities, and 8% with 3 or 4 abnormalities. The most common cardiometabolic abnormality was hypertension (54%), which was followed by an increased waist circumference (43%), a history of high cholesterol (13%), and a history of diabetes (5%). Older women, African American women, and women with an increased BMI, less education, and less participation in moderate to strenuous activity were more likely to have a higher number of cardiometabolic abnormalities.
After a median follow-up of 10 years starting at the date of the cancer diagnosis, there were a total of 3607 deaths, including 1546 deaths due to cancer (42.9%), 733 due to CVD (20.3%), and 1328 due to other causes (36.8%). The most prevalent known “other” causes of death were dementia or Alzheimer disease (n = 236), chronic obstructive pulmonary disease (n = 89), and pneumonia (n = 72; Table 2).
TABLE 2.
Numbers and Top 5 Causes of Death for Each Competing Risk Category
| Cancer Deaths | No. | CVD Deaths | No. | Other Causes | No. |
|---|---|---|---|---|---|
|
| |||||
| Total | 1546 | Total | 733 | Total | 1328 |
| Top 5 causes | Top 5 causes | Top 5 causes | |||
| Breast cancer | 597 | Possible CHD | 227 | Unknown cause | 398 |
| Colon cancer | 272 | Other cardiovascular | 221 | Known other cause | 268 |
| Pancreas cancer | 222 | Cerebrovascular | 177 | Dementia/Alzheimer disease | 236 |
| Endometrial cancer | 82 | Definite CHD | 94 | COPD | 89 |
| Lymphoma | 82 | Pulmonary embolism | 11 | Pneumonia | 72 |
Abbreviations: CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
Table 3 shows the unadjusted and adjusted HRs and 95% CIs for the association of cardiometabolic abnormalities and all-cause mortality and mortality due to cancer, CVD, and other causes. In the adjusted models, in comparison with women with no cardiometabolic abnormalities, the risk of death for women with 1 or 2 cardiometabolic abnormalities at WHI enrollment was 1.5 times greater (HR, 1.50; 95% CI, 1.36–1.65), and for women with 3 or 4 abnormalities, it was 2-fold greater (HR, 1.99; 95% CI, 1.73–2.30; P for trend <.001). For women with 3 or 4 abnormalities versus no abnormalities, the risk of death from CVD was 4 times greater (HR, 4.01; 95% CI, 2.88–5.57; P for trend <.001), the risk of death from other causes was 2.14-fold greater (HR, 2.14; 95% CI, 1.70–2.69; P for trend <.001), and the risk of death from cancer was 1.37 times greater (HR, 1.37; 95% CI, 1.1–1.72; P for trend = .001). An analysis for individual abnormalities showed that an elevated prediagnosis waist circumference at WHI enrollment was associated with a higher risk of death due to all causes (HR, 1.17; 95% CI, 1.06–1.30) and cancer (HR, 1.22; 95% CI, 1.04–1.42), whereas diabetes was associated with a higher risk of death due to all causes, CVD, and other causes, and hypertension was associated with higher mortality due to all causes, cancer, CVD, and other causes.
TABLE 3.
HRs and 95% Cis for Cancer, CVD, and Other-Cause Mortality by the Presence of Cardiometabolic Abnormalities at Baseline Enrollment Into WHI
| All-Cause Mortality |
Cancer Mortality |
CVD Mortality |
Other-Cause Mortality |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | HR | 95% CI | Deaths | HR | 95% CI | Deaths | HR | 95% CI | Deaths | HR | 95% CI | |
|
| ||||||||||||
| Unadjusted | 3607 | 1546 | 733 | 1328 | ||||||||
| Waist circumference | ||||||||||||
| <88 cm | 1876 | Reference | 817 | Reference | 368 | Reference | 691 | Reference | ||||
| >88 cm | 1731 | 1.33 | 1.24–1.42 | 729 | 1.25 | 1.14–1.39 | 365 | 1.44 | 1.25–1.67 | 637 | 1.35 | 1.21–1.51 |
| History of high cholesterol | ||||||||||||
| No | 3035 | Reference | 1333 | Reference | 597 | Reference | 1105 | Reference | ||||
| Yes | 572 | 1.29 | 1.18–1.41 | 213 | 1.08 | 0.93–1.24 | 136 | 1.57 | 1.30–1.89 | 223 | 1.40 | 1.21–1.62 |
| History of diabetes | ||||||||||||
| No | 3295 | Reference | 1444 | Reference | 660 | Reference | 1191 | Reference | ||||
| Yes | 312 | 1.83 | 1.63–2.05 | 102 | 1.32 | 1.08–1.61 | 73 | 2.18 | 1.72–2.78 | 137 | 2.29 | 1.92–2.73 |
| Hypertension | ||||||||||||
| No | 1235 | Reference | 630 | Reference | 161 | 444 | Reference | |||||
| Yes | 2372 | 1.74 | 1.62–1.86 | 916 | 1.28 | 1.16–1.42 | 572 | 3.25 | 2.73–3.87 | 884 | 1.84 | 1.64–2.06 |
| Cardiometabolic abnormalities | ||||||||||||
| 0 | 697 | Reference | 363 | Reference | 87 | Reference | 247 | Reference | ||||
| 1 or 2 | 2480 | 1.77 | 1.63–1.93 | 1037 | 1.39 | 1.23–1.56 | 534 | 3.10 | 2.48–3.89 | 909 | 1.88 | 1.63–2.16 |
| 3 or 4 | 430 | 2.56 | 2.27–2.88 | 146 | 1.58 | 1.30–1.91 | 112 | 5.49 | 4.15–7.26 | 172 | 3.02 | 2.49–3.67 |
| P for trend | <.001 | <.001 | <.001 | <.001 | ||||||||
| Adjusteda | ||||||||||||
| Waist circumference | ||||||||||||
| <88 cm | 1876 | Reference | 817 | Reference | 368 | Reference | 691 | Reference | ||||
| >88 cm | 1731 | 1.17 | 1.06–1.30 | 729 | 1.22 | 1.04–1.42 | 365 | 1.13 | 0.90–1.43 | 637 | 1.15 | 0.97–1.36 |
| History of high cholesterol | Reference | |||||||||||
| No | 3035 | Reference | 1333 | Reference | 597 | Reference | 1105 | |||||
| Yes | 572 | 1.06 | 0.96–1.16 | 213 | 0.99 | 0.85–1.16 | 136 | 1.14 | 0.93–1.39 | 223 | 1.09 | 0.93–1.27 |
| History of diabetes | ||||||||||||
| No | 3295 | Reference | 1444 | Reference | 660 | Reference | 1191 | Reference | ||||
| Yes | 312 | 1.45 | 1.27–1.64 | 102 | 1.04 | 0.83–1.29 | 73 | 1.53 | 1.18–2.00 | 137 | 1.93 | 1.59–2.35 |
| Hypertension | ||||||||||||
| No | 1235 | Reference | 630 | Reference | 161 | Reference | 444 | Reference | ||||
| Yes | 2372 | 1.36 | 1.26–1.47 | 916 | 1.17 | 1.04–1.31 | 572 | 2.33 | 1.92–2.83 | 884 | 1.25 | 1.11–1.42 |
| Cardiometabolic abnormalities | ||||||||||||
| 0 | 697 | Reference | 363 | Reference | 87 | Reference | 247 | Reference | ||||
| 1 or 2 | 2480 | 1.50 | 1.36–1.65 | 1037 | 1.29 | 1.12–1.48 | 534 | 2.52 | 1.95–3.26 | 909 | 1.45 | 1.23–1.70 |
| 3 or 4 | 430 | 1.99 | 1.73–2.30 | 146 | 1.37 | 1.10–1.72 | 112 | 4.01 | 2.88–5.57 | 172 | 2.14 | 1.70–2.69 |
| P for trend | <.001 | .001 | <.001 | <.001 | ||||||||
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; WHI, Women s Health Initiative.
Adjustments were made for the following: age group at cancer diagnosis, education, race, WHI study group, current health care provider, body mass index, smoking status, alcohol intake, physical activity, and hormone therapy use.
Excluding BMI from the adjusted models did not materially change the statistical significance of the results. Stratification by time from study entry to diagnosis had a significant impact on mortality due to cancer. Having 3 or 4 cardiometabolic abnormalities was associated with a 74% higher risk of death due to cancer for women diagnosed 5 or more years from study enrollment (HR, 1.74; 95% CI, 1.30–2.34) but not for women diagnosed less than 5 years from enrollment (HR, 0.91; 95% CI, 0.65–1.29).
Table 4 shows the adjusted HRs and 95% CIs for the association of cardiometabolic risk factors with mortality due to cancer for women diagnosed with 1 of the 3 most common cancer types evaluated in this analysis (breast, colorectal, and endometrial cancer). A higher number of cardiometabolic abnormalities was not associated with death due to breast, colorectal, or endometrial cancer individually.
TABLE 4.
Adjusted HRs and 95% CIs for Cancer Site-Specific Mortality by the Presence of Cardiometabolic Abnormalities at Baseline Enrollment Into WHI
| No. of Deaths | Site-Specific Cancer Mortality |
||
|---|---|---|---|
| HRa | 95% CI | ||
|
| |||
| Breast cancer | |||
| High waist circumference | 266 | 1.21 | 0.93–1.58 |
| History of high cholesterol | 75 | 0.95 | 0.73–1.22 |
| History of diabetes | 29 | 0.83 | 0.55–1.26 |
| Hypertension | 330 | 1.10 | 0.92–1.32 |
| Cardiometabolic abnormalities | |||
| 1 or 2 | 386 | 1.13 | 0.91–1.41 |
| 3 or 4 | 46 | 1.14 | 0.78–1.66 |
| P for trend | .333 | ||
| Colorectal cancer | |||
| High waist circumference | 139 | 1.27 | 0.88–1.85 |
| History of high cholesterol | 42 | 0.91 | 0.64–1.28 |
| History of diabetes | 25 | 1.10 | 0.70–1.73 |
| Hypertension | 193 | 1.43 | 1.09–1.86 |
| Cardiometabolic abnormalities | |||
| 1 or 2 | 195 | 1.28 | 0.93–1.78 |
| 3 or 4 | 34 | 1.48 | 0.90–2.42 |
| P for trend | .095 | ||
| Endometrial cancer | .095 | ||
| High waist circumference | 49 | 0.85 | 0.39–1.86 |
| History of high cholesterol | 9 | 1.20 | 0.59–2.45 |
| History of diabetes | 5 | 1.14 | 0.44–2.96 |
| Hypertension | 39 | 0.64 | 0.39–1.04 |
| Cardiometabolic abnormalities | |||
| 1 or 2 | 57 | 1.04 | 0.53–2.04 |
| 3 or 4 | 7 | 0.89 | 0.32–2.48 |
| P for trend | .867 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; WHI, Women’s Health Initiative
Adjustments were made for the following: age group at cancer diagnosis, education, race, WHI study group, current health care provider, body mass index, smoking status, alcohol intake, physical activity, and hormone therapy use.
Figure 2 shows the results of the Kaplan-Meier analysis for overall survival by the number of abnormalities at the baseline and demonstrates an increased risk of death for women with 1 or 2 cardiometabolic abnormalities or 3 or 4 cardiometabolic abnormalities versus none (log-rank P < .001).
Figure 2.
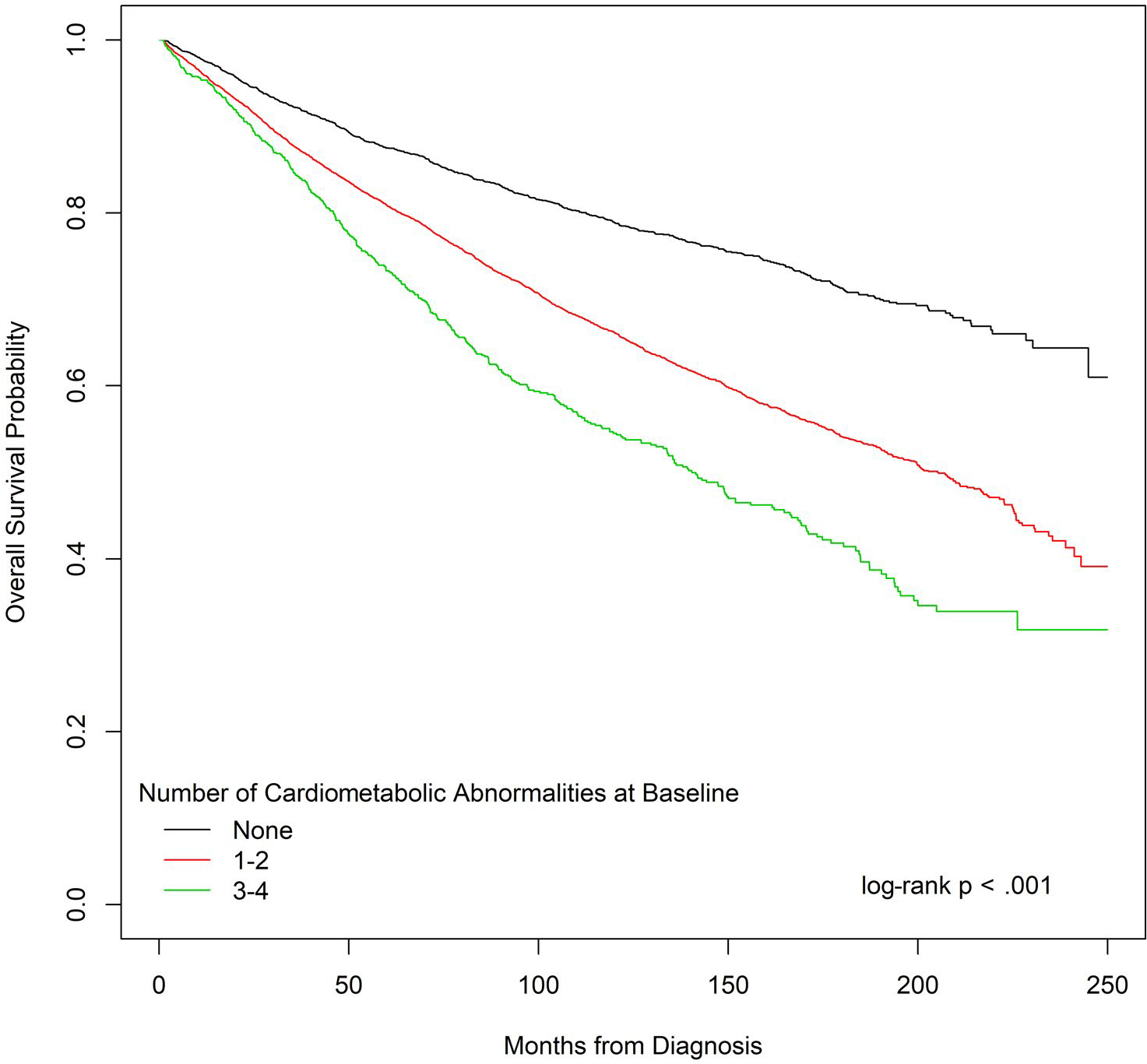
Overall survival probability by the number of cardiometabolic abnormalities at the baseline.
DISCUSSION
The results from this analysis suggest that prediagnosis cardiometabolic abnormalities are associated with death after diagnosis in a group of postmenopausal women diagnosed with cancers previously associated with metabolic syndrome. Importantly, HRs for death increased with an increased number of cardiometabolic abnormalities. The results shown in this analysis are consistent with a prior WHI report demonstrating a significant relationship between cardiometabolic abnormalities and death due to CVD and other causes among women diagnosed with early-stage breast cancer.17 The current study expands on our previous work with an expanded cohort of women diagnosed with 9 cancers previously associated with obesity.
The cardiometabolic abnormalities examined here have both unique mechanisms of action and shared mechanisms of action with cancer, and this may be in part explained through a common pathway of altered sensitivity to insulin, alterations in the hormonal profile, and chronic inflammation. The common soil hypothesis suggests that shared cellular and tissue mechanisms that are perturbed in the setting of obesity and physical inactivity may predispose individuals to cancer and CVD as well as diabetes.23 There is a large body of mechanistic evidence supporting the role of the cardiometabolic abnormalities examined in this analysis as drivers of a higher risk of mortality after cancer. In a mouse model of obesity-induced lipogenesis, Liu et al24 demonstrated the impact of dietary lipid loading on ovarian cancer cell adhesion and intraperitoneal tumor burden with histological analyses implicating changes in lipid regulatory factors, increased vascularity, and higher M1/M2 macrophage ratios in the increased tumor burden. Other studies have shown that the microenvironment provided by adipose tissue, particularly central or visceral adiposity, is rich in signaling molecules and acts as an endocrine organ promoting tumor progression and proliferation via various cellular mediators, adipokines, cytokines, and inflammatory markers.25,26 Others have shown that inflamed breast adipose tissue may influence cancers by way of white adipose tissue cell death, which leads to macrophage infiltration forming crownlike structures that stimulate aromatase activity, which subsequently leads to peripheral estrogen production.27 Hyperglycemia and insulin resistance could serve both as an energy reservoir and as drivers of a proinflammatory state as well as signals for various cell growth and proliferation pathways.1,28,29
Prior investigations have evaluated the relationship between metabolic syndrome or components of metabolic syndrome, a clinical condition characterized by the cardiometabolic exposures evaluated here, and cancer-specific outcomes. The literature suggests that a history of cardiometabolic abnormalities is associated with a poorer prognosis,20,30 local or distant relapse,1,31–34 cancer-specific mortality,31,33,35–37 and overall survival.31,33,35,36,38 Importantly, our analysis builds evidence for the relationship between cardiometabolic abnormalities and cancer outcomes among postmenopausal women, with 1 other study showing similar findings in men.39
To our knowledge, there have been only 2 other large published studies that have grouped together several relevant cancers and have also demonstrated a relationship between the exposures of interest and an increased risk of cancer mortality. In an analysis of the Third National Health and Nutrition Examination Survey, Gathirua-Mwangi et al11 evaluated the relationship between metabolic syndrome and overall cancer mortality (including lung cancer) in a population of 14,916 men and women followed longitudinally over a 12- to 18-year period of follow-up, of whom 687 died as a result of cancer. Their results demonstrated a significant relationship between having metabolic syndrome (vs not having metabolic syndrome) and higher total cancer mortality for men and women combined (HR, 1.33; 95% CI, 1.04–1.70) with an increasing risk associated with an increasing number of metabolic syndrome features (P for trend = .005). In contrast to our findings, elevated glucose was associated with an increased risk of dying from cancer, and as shown in our analysis, an elevated waist circumference was marginally associated with cancer death (HR, 1.32; 95% CI, 0.98–1.77). In another analysis of 25,038 participants in a community-based study of risk factors for stroke, REasons for Geographic and Racial Differences in Stroke (REGARDS) followed for 5 to 9 years, Akinyemiju et al12 demonstrated a higher risk of all cancer mortality associated with metabolic syndrome (HR, 1.22; 95% CI, 1.03–1.45), and consistently with the results from the WHI, they demonstrated a trend toward worsening all cancer mortality with an increased number of metabolic syndrome features.
Although the relevance of coexisting comorbidities, including obesity, hypertension, diabetes, and high cholesterol, to cancer risk and cancer outcomes has been well established, the current analyses raise the hypothesis that, in patients with cancer, interventions targeting metabolic abnormalities, which include modifiable lifestyle factors, may affect cancer outcomes. In this regard, earlier findings from the WHI dietary modification trial, which included 48,835 postmenopausal women without a history of breast cancer who were randomized to a low-fat dietary pattern that statistically significantly increased vegetable, fruit, and grain intake, showed an association with a reduction in metabolic syndrome components as well as a reduction in cholesterol-lowering and antihypertensive medication use in comparison with randomization to a comparison group.40 In addition, breast cancer incidence was a primary study endpoint, and with a long-term follow-up of 19.6 years, a statistically significant reduction in deaths from breast cancer (breast cancer followed by death directly attributed to the cancer) emerged (HR, 0.79; 95% CI 0.64–0.97; P = .02). This finding from a full-scale randomized trial represents a partial test of our study hypothesis, at least for the breast cancer outcome. Future studies should evaluate the potential impact of other interventions on outcomes in other cancers.
The strengths of our analysis include the large sample size, the long period of follow-up, physician-adjudicated cancer cases and outcomes, the inclusion of obesity-related cancers previously associated with metabolic syndrome, and a focus on women with early-stage disease who have the potential for long-term survival. This is especially important because of competing risks for morbidity and mortality among women with advanced-stage cancer. We acknowledge the limitations of residual confounding and the observational nature of our work. We could not comment on whether there was a differential impact of cardiometabolic risk factors by race or ethnicity because only 7% of the participants were Black and 6% belonged to other racial or ethnic groups in our study cohort. Other limitations include the lack of blood levels of glucose, triglycerides, and high-density lipoprotein and the reliance on participant questionnaires for information on the history of diabetes and high cholesterol, which could result in underestimations of those diagnoses, although prior analyses in the WHI have demonstrated that 92% of self-reported diabetes cases were confirmed by medical record review.41 Another limitation is the absence of information on cancer-directed therapy, which has been shown to have a direct impact on outcomes after cancer. Lastly, we evaluated the presence of cardiometabolic abnormalities that occurred at study entry, and we did not have data on these factors at the time of cancer diagnosis. The variability in the time between the assessment of cardiometabolic risk factors (median, 7.1 years; range, <1 to 20.6 years) could have potentially weakened the associations between cardiometabolic abnormalities and cancer-specific mortality in particular. In our sensitivity analysis, we showed that the time between study entry and diagnosis had no impact on the relationship between cardiovascular abnormalities and overall, CVD, or other-cause mortality; however, the relationship between cardiovascular abnormalities and death due to cancer was significant only for women who were diagnosed more than 5 years after study entry. These results might be explained by the possibility that the prevalence of the measured cardiometabolic risk factors could have changed over time, and this might have resulted in a stronger relationship with mortality for women diagnosed later; however, we were not able to assess this with our data set.
In conclusion, in a cohort of postmenopausal women with select early-stage cancers, a higher number of cardiometabolic abnormalities is associated with significantly higher overall mortality in addition to higher mortality due to cancer, CVD, and other causes. These findings suggest that interventions targeting these modifiable risk factors could potentially have a clinically meaningful impact on outcomes for cancer survivors. The results also point out a major gap in the survivorship care of patients with cancer and the need for improved efforts by public health systems to improve survival. This hypothesis requires future prospective studies for confirmation.
LAY SUMMARY:
This study uses information from the Women’s Health Initiative (WHI) to find out whether cardiac risk factors are related to a greater risk of dying among older women with cancer. The WHI is the largest study of medical problems faced by older women in this country.
The results show that women who have 3 or 4 risk factors are more likely to die of any cause, heart disease, or cancer in comparison with women with no risk factors.
It is concluded that interventions to help to lower the burden of cardiac risk factors can have an important impact on survivorship among women with cancer.
FUNDING SUPPORT
The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute (National Institutes of Health) through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32, and 44221; by the National Cancer Institute (National Institutes of Health) through Cancer Center Support Grant P30CA022453; and by the Blue Cross Blue Shield Foundation of Michigan. Randi Foraker also reports a grant from the National Cancer Institute during the conduct of the study.
This work was presented as a poster at the American Society of Clinical Oncology Meeting; June 3, 2019; Chicago, Illinois.
We acknowledge the dedicated efforts of the investigators and staff at the Women’s Health Initiative (WHI) clinical centers, the WHI Clinical Coordinating Center, the WHI Life and Longevity After Cancer Study (funded by National Cancer Institute grant 1UM1CA173642), and the National Heart, Lung, and Blood Institute program office; for a list of all the investigators who have contributed to WHI science, please visit http://www.whiscience.org/publications/WHI_investigators_longlist.pdf. We also recognize the WHI participants for their extraordinary commitment to the WHI program.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Michael S. Simon reports personal fees from the Women’s Health Initiative during the conduct of the study and that he is on a speakers’ bureau for AstraZeneca for non–treatment-related genetic counseling and testing. Theresa A. Hastert reports personal fees from the Women’s Health Initiative during the conduct of the study and grants from the American Cancer Society and the National Cancer Institute outside the submitted work. Ana Barac reports working on a data and safety monitoring board for CTI Biopharma and receiving honoraria from Bristol-Myers Squibb. Rowan T. Chlebowski reports personal fees from Novartis, Pfizer, AstraZeneca, Puma, Genentech, Immunomedics, and Merck during the conduct of the study. Aladdin H. Shadyab reports working as a consultant for Ranchio Biosciences, LLC. The other authors made no disclosures.
REFERENCES
- 1.Gurka MJ, Guo Y, Filipp SL, DeBoer MD. Metabolic syndrome severity is significantly associated with future coronary heart disease in type 2 diabetes. Cardiovasc Diabetol. 2018;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadi A, Leipsic J, Feuchtner G, et al. Is metabolic syndrome predictive of prevalence, extent, and risk of coronary artery disease beyond its components? Results from the multinational coronary CT angiography evaluation for clinical outcome: an international multicenter registry (CONFIRM). PLoS One. 2015;10:e0118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. [DOI] [PubMed] [Google Scholar]
- 4.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 6.Lin CC, Liu CS, Li CI, et al. The relation of metabolic syndrome according to five definitions to cardiovascular risk factors—a population-based study. BMC Public Health. 2009;9:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabat GC, Xue X, Kamensky V, et al. Risk of breast, endometrial, colorectal, and renal cancers in postmenopausal women in association with a body shape index and other anthropometric measures. Cancer Causes Control. 2015;26:219–229. [DOI] [PubMed] [Google Scholar]
- 9.Kabat GC, Kim MY, Lane DS, et al. Serum glucose and insulin and risk of cancers of the breast, endometrium, and ovary in postmenopausal women. Eur J Cancer Prev. 2018;27:261–268. [DOI] [PubMed] [Google Scholar]
- 10.Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care. 2008;31:2391–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gathirua-Mwangi WG, Monahan PO, Murage MJ, Zhang J. Metabolic syndrome and total cancer mortality in the Third National Health and Nutrition Examination Survey. Cancer Causes Control. 2017;28:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinyemiju T, Moore JX, Judd S, et al. Metabolic dysregulation and cancer mortality in a national cohort of Blacks and Whites. BMC Cancer. 2017;17:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 14.NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. [DOI] [PubMed] [Google Scholar]
- 16.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon MS, Beebe-Dimmer JL, Hastert TA, et al. Cardiometabolic risk factors and survival after breast cancer in the Women’s Health Initiative. Cancer. 2018;124:1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. [DOI] [PubMed] [Google Scholar]
- 19.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. [DOI] [PubMed] [Google Scholar]
- 20.Gathirua-Mwangi WG, Monahan P, Song Y, et al. Changes in adult BMI and waist circumference are associated with increased risk of advanced colorectal neoplasia. Dig Dis Sci. 2017;62:3177–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J, Hendryx M, Virnig B, et al. Pre-existing diabetes and breast cancer prognosis among elderly women. Br J Cancer. 2015;113:827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–S128. [DOI] [PubMed] [Google Scholar]
- 23.Bellastella G, Scappaticcio L, Esposito K, Giugliano D, Maiorino MI. Metabolic syndrome and cancer: “the common soil hypothesis”. Diabetes Res Clin Pract. 2018;143:389–397. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Metzinger MN, Lewellen KA, et al. Obesity contributes to ovarian cancer metastatic success through increased lipogenesis, enhanced vascularity, and decreased infiltration of M1 macrophages. Cancer Res. 2015;75:5046–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohmann AE, Goodwin PJ, Chlebowski RT, Pan K, Stambolic V, Dowling RJ. Association of obesity-related metabolic disruptions with cancer risk and outcome. J Clin Oncol. 2016;34:4249–4255. [DOI] [PubMed] [Google Scholar]
- 27.Argolo DF, Hudis CA, Iyengar NM. The impact of obesity on breast cancer. Curr Oncol Rep. 2018;20:47. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. [DOI] [PubMed] [Google Scholar]
- 29.Vrachnis N, Iavazzo C, Iliodromiti Z, et al. Diabetes mellitus and gynecologic cancer: molecular mechanisms, epidemiological, clinical and prognostic perspectives. Arch Gynecol Obstet. 2016;293:239–246. [DOI] [PubMed] [Google Scholar]
- 30.Ni J, Zhu T, Zhao L, et al. Metabolic syndrome is an independent prognostic factor for endometrial adenocarcinoma. Clin Transl Oncol. 2015;17:835–839. [DOI] [PubMed] [Google Scholar]
- 31.Fan Y, Ding X, Wang J, et al. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int J Biol Markers. 2015;30:e200–e207. [DOI] [PubMed] [Google Scholar]
- 32.Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147:159–165. [DOI] [PubMed] [Google Scholar]
- 33.Calip GS, Malone KE, Gralow JR, Stergachis A, Hubbard RA, Boudreau DM. Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res Treat. 2014;148:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muniz J, Kidwell KM, Henry NL. Associations between metabolic syndrome, breast cancer recurrence, and the 21-gene recurrence score assay. Breast Cancer Res Treat. 2016;157:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George SM, Bernstein L, Smith AW, et al. Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast Cancer Res Treat. 2014;146:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum. 2013;56:1304–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjorge T, Lukanova A, Jonsson H, et al. Metabolic syndrome and breast cancer in the Me-Can (Metabolic Syndrome and Cancer) project. Cancer Epidemiol Biomarkers Prev. 2010;19:1737–1745. [DOI] [PubMed] [Google Scholar]
- 38.Shen Z, Ye Y, Bin L, et al. Metabolic syndrome is an important factor for the evolution of prognosis of colorectal cancer: survival, recurrence, and liver metastasis. Am J Surg. 2010;200:59–63. [DOI] [PubMed] [Google Scholar]
- 39.Jaggers JR, Sui X, Hooker SP, et al. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer. 2009;45: 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuhouser ML, Howard B, Lu J, et al. A low-fat dietary pattern and risk of metabolic syndrome in postmenopausal women: the Women’s Health Initiative. Metabolism. 2012;61:1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson JM, Defor TA, Crain AL, et al. Self-reported diabetes is a valid outcome in pragmatic clinical trials and observational studies. J Clin Epidemiol. 2013;66:349–350. [DOI] [PubMed] [Google Scholar]


