Abstract
Rationale
Chronic obstructive pulmonary disease (COPD) is a prevalent and burdensome condition in low- and middle-income countries (LMICs). Challenges to better care include more effective diagnosis and access to affordable interventions. There are no previous reports describing therapeutic needs of populations with COPD in LMICs who were identified through screening.
Objectives
To describe unmet therapeutic need in screening-detected COPD in LMIC settings.
Methods
We compared interventions recommended by the international Global Initiative for Chronic Obstructive Lung Disease COPD strategy document, with that received in 1,000 people with COPD identified by population screening at three LMIC sites in Nepal, Peru, and Uganda. We calculated costs using data on the availability and affordability of medicines.
Measurement and Main Results
The greatest unmet need for nonpharmacological interventions was for education and vaccinations (applicable to all), pulmonary rehabilitation (49%), smoking cessation (30%), and advice on biomass smoke exposure (26%). Ninety-five percent of the cases were previously undiagnosed, and few were receiving therapy (4.5% had short-acting β-agonists). Only three of 47 people (6%) with a previous COPD diagnosis had access to drugs consistent with recommendations. None of those with more severe COPD were accessing appropriate maintenance inhalers. Even when available, maintenance treatments were unaffordable, with 30 days of treatment costing more than a low-skilled worker’s daily average wage.
Conclusions
We found a significant missed opportunity to reduce the burden of COPD in LMIC settings, with most cases undiagnosed. Although there is unmet need in developing novel therapies, in LMICs where the burden is greatest, better diagnosis combined with access to affordable interventions could translate to immediate benefit.
Keywords: COPD, LMIC, pulmonary rehabilitation, bronchodilator, guidelines
At a Glance Commentary
Scientific Knowledge on the Subject
There are no previous reports describing therapeutic needs in people with chronic obstructive pulmonary disease who were identified through population screening in low- and middle-income countries.
What This Study Adds to the Field
There is great unmet need in managing chronic obstructive pulmonary disease in low- and middle-income countries. This includes underdiagnosis; provision of nonpharmacological interventions, such as education and pulmonary rehabilitation; and access to affordable preventative inhaled medicines, notably, long-acting bronchodilators.
Chronic obstructive pulmonary disease (COPD) is the most prevalent of the chronic respiratory diseases that, combined, are the third most common cause of death worldwide (1). COPD is associated with systematic disadvantage through the life course and respiratory exposures (2). The greatest burden of COPD, therefore, falls in low- and middle-income countries (LMICs), which account for 75% of the cases; 80% of the deaths; and considerable morbidity, premature mortality, and lost productivity (3).
There are many challenges to mitigating COPD in LMICs; notably, underdiagnosis, absence of LMIC-relevant evidence and guidelines (4), access to affordable interventions (5, 6), and heterogeneous implementation of evidence-based care (7). Changing this requires an investment in healthcare staff, services, and facilities. Better care for COPD in LMICs begins with improved access to diagnostics, and we have recently reported on the discriminative accuracy of simple screening tools to identify people at high risk for COPD in three diverse LMIC sites in Nepal, Peru, and Uganda (8). The overall prevalence of COPD was 9.4%, and this varied by site (9). Forty-nine percent of the cases had clinically significant disease, and more than 95% were previously undiagnosed (9). Our findings contrast with recommendations from the U.S. Preventative Services Task Force, which recently reiterated their statement that screening-detected cases of COPD are generally mild, for which intervention other than smoking cessation is not indicated (10). This is not true in LMIC settings.
After a diagnosis of COPD, the next consideration is implementation of evidence-based interventions, and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy provides a framework to do that for both pharmacological and nonpharmacological treatments (11). Interventions such as respiratory exposure reduction, pulmonary rehabilitation, and drugs have proven efficacy in reducing symptoms and exacerbations, improving functional status and quality of life, and reducing premature mortality in clinically significant COPD (11). Not using these in people with newly diagnosed COPD represents a missed therapeutic opportunity, which we define as guideline-based care that has not been received.
There are no previous studies reporting the missed therapeutic opportunity in a sample of people with COPD identified through population-based screening in LMICs. In this paper, we report on the scale of that missed opportunity by applying the GOLD 2022 (12) and 2023 strategy recommendations (11) to a population-based sample of people with COPD detected by screening at three diverse LMIC sites in Nepal, Peru, and Uganda (9).
Methods
Study Setting
We have previously reported on the methodology (8) and results (9) of the parent Global Excellence in COPD Outcomes study which was prospectively registered at clinicaltrials.gov (NCT03365713). In short, the present study uses data from 10,664 individuals screened for COPD with quality-assured postbronchodilator spirometry at three LMIC sites (8)—urban Bhaktapur, Nepal; Lima, Peru; and the rural Nakaseke district in Uganda—between January 2018 and March 2020. COPD was defined as a ratio of postbronchodilator FEV1 to FVC less than the fifth percentile of the Global Lung Function Initiative’s mixed ethnic population reference lower limit of normal. Participants were randomly selected at those sites from a census of people over 40 years of age and so are representative of the communities from which they were drawn. A total of 9.4% of subjects (1,000/10,664) had COPD: 642 (18.2% prevalence) in Nepal, 261 (7.3%) in Uganda, and 97 (2.7%) in Peru (9). Results were prepared in line with Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Study Design
Collection of data on prior diagnosis and medication use
Each participant was asked about a previous diagnosis of COPD. Those who answered in the affirmative were classified as being previously diagnosed with COPD. Each participant was also asked about a previous diagnosis of asthma. The use of medications was self-reported to field workers using a questionnaire. The use of regular maintenance medication was defined as using the medication at least twice per week in the past 12 months. Combinations of inhaled therapies did not need to be delivered in the same inhaler device. The use of a short-acting β-agonist (SABA) reliever inhaler was defined as either using this regularly, or using this for a short period of time for respiratory symptoms in the past 12 months.
Defining optimal COPD care
The 2022 GOLD strategy document was used to assess recommended interventions for each participant (12). GOLD has separate algorithms for people with new and preexisting diagnoses of COPD and for pharmacological and nonpharmacological interventions. Cases were classified using the GOLD ABCD assessment using self-reported exacerbation history in the previous year and the higher of the scores on the modified Medical Research Council Questionnaire (mMRC) and COPD Assessment Test (CAT) (8). Since collecting these data, the 2022 GOLD system has been revised to A/B/E (11), and we also report therapy by these 2023 categories.
Recommended interventions are summarized in Table 1, with those in group D divided by the presence or absence of self-reported asthma and CAT score (13). As would be typical in community settings, we did not have blood eosinophil counts to guide inhaled corticosteroid (ICS) use, and we, therefore, used a self-reported diagnosis of asthma to guide the need for ICS. The recommended interventions for each subject were then compared with the actual treatment currently received, assessing the scale of missed therapeutic opportunity.
Table 1.
| GOLD 2022 Grade | GOLD 2023 Grade | Nonpharmacological | Pharmacological |
|---|---|---|---|
| A | A | Vaccinations Advising on physical activity Smoking cessation, if smoker Biomass exposure reduction |
SABA reliever |
| B | B | Vaccinations Advising on physical activity Pulmonary rehabilitation Smoking cessation, if smoker Biomass exposure reduction |
SABA reliever LAMA* or LABA-LAMA† |
| C | not applicable | Vaccinations Advising on physical activity Pulmonary rehabilitation Smoking cessation, if smoker Biomass exposure reduction |
SABA reliever LAMA* |
| D | E | Vaccinations Advising on physical activity Pulmonary rehabilitation Smoking cessation, if smoker Biomass exposure reduction |
SABA reliever No asthma and CAT score ⩽20: LAMA* or LABA-LAMA† No asthma and CAT score >20: LABA-LAMA Asthma and CAT score ⩽20: ICS-LABA* or ICS-LABA-LAMA† Asthma and CAT score >20: ICS-LABA* or ICS-LABA-LAMA† |
Definition of abbreviations: CAT = COPD Assessment Test; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting β agonist.
2022 guidance.
2023 guidance.
Where individuals had an existing diagnosis and were on maintenance therapy, which was unusual, therapeutic escalation per GOLD (12) was considered, with the caveat that we do not have information on adherence to therapy. GOLD recommends that, if the exacerbation frequency in the previous year was two or more events, or one or more respiratory hospitalizations, and/or the mMRC dyspnea scale score in the previous year was ⩾2, escalation of treatment should be considered. Previous hospitalization data were not available—only the number of exacerbations in the past year—so the mMRC dyspnea scale and frequency of exacerbations were used. If an mMRC score was ⩾2, pulmonary rehabilitation (PR) and a specific breathlessness action plan should be recommended, and if the frequency of exacerbations was two or more events in the past year, a specific exacerbation plan would be recommended.
Access, availability, and affordability of medicines
Regarding access to and availability of medications, we used data from our previous surveys of local pharmacies at our three LMIC sites (6). In brief, we surveyed 63 pharmacies in Nepal, 104 pharmacies in Peru, and 53 pharmacies in Uganda that served the communities in which we worked, and we assessed the cost and availability of medications for the management of COPD.
We determined affordability by using standardized metrics including the total cost of a medication for a standard course of one month’s treatment in U.S. dollars. We also compared costs with the daily average wage of a low-skilled worker at each location. This metric was used as a proxy for the World Health Organization/Health Action International–recommended measure of number of days of wages of the lowest paid unskilled government worker (14) because of a lack of reliable data on government salaries. We used the 2017 estimate of the daily wage of a low-skilled worker from the International Labor Organization’s Statistics on Wages (15). Paying over one day’s wage for 30 days of treatment is considered unaffordable (16, 17). Treatment costs for both maintenance and reliever therapy were calculated. For GOLD A disease, a treatment schedule of one SABA inhaler per month was used, but for reliever therapy in GOLD B-D disease, a treatment schedule of one SABA inhaler per year was used, reflecting treatment of 200 doses per year (three to four doses per week).
We do not have contemporaneous information on the availability of local PR, biomass mitigation, or smoking cessation services. We did not consider the use of medicines outside of the GOLD guidelines, including complementary (nonallopathic) medicines.
Statistics
Descriptive statistics (number and percent) are reported. For comparisons between groups, we used chi-square analysis. Analysis was completed in RStudio, Version 4.1.2 or above (18). P values were two-tailed, and P < 0.05 was taken as statistically significant.
Ethical Considerations
All participants provided written informed consent. Ethics permissions were obtained from the University College London Research Ethics Committee, the Johns Hopkins School of Medicine, the Nepal Health Research Council, A.B. PRISMA in Peru, the Makerere University School of Medicine in Uganda, and the Uganda National Council for Science and Technology.
Results
We have previously reported on the baseline characteristics of the population by site (9), and the original CONSORT (Consolidated Standards of Reporting Trials) diagram and relevant participant characteristics are reported separately (see Figure E1 and Table E1 in the online supplement). In Nepal, the mean age was 56.2 years (SD = 11.7), and 50.1% were female. In Peru, the participants had a mean age of 56.6 years (SD = 11.3), and 49.8% were female; in Uganda, the mean age was 56.1 years (SD = 12.1), and 51.0% were female. For the purposes of this analysis, there were 1,000 COPD cases among the 10,664 people randomly screened and who had spirometry data available that met quality assurance standards (8): A total of 952 (95.3%) were previously undiagnosed, 47 (4.7%) were previously diagnosed, and one had missing diagnostic status. Unmet therapeutic opportunity is, therefore, principally driven by limited access to diagnosis. GOLD grouping (A–D) was available for 999 of the 1,000 cases. A past history of asthma was missing for one person, and the prevalence of a previous diagnosis of asthma was 14.7% (147/999).
Unmet Therapeutic Opportunity: Nonpharmacological Interventions
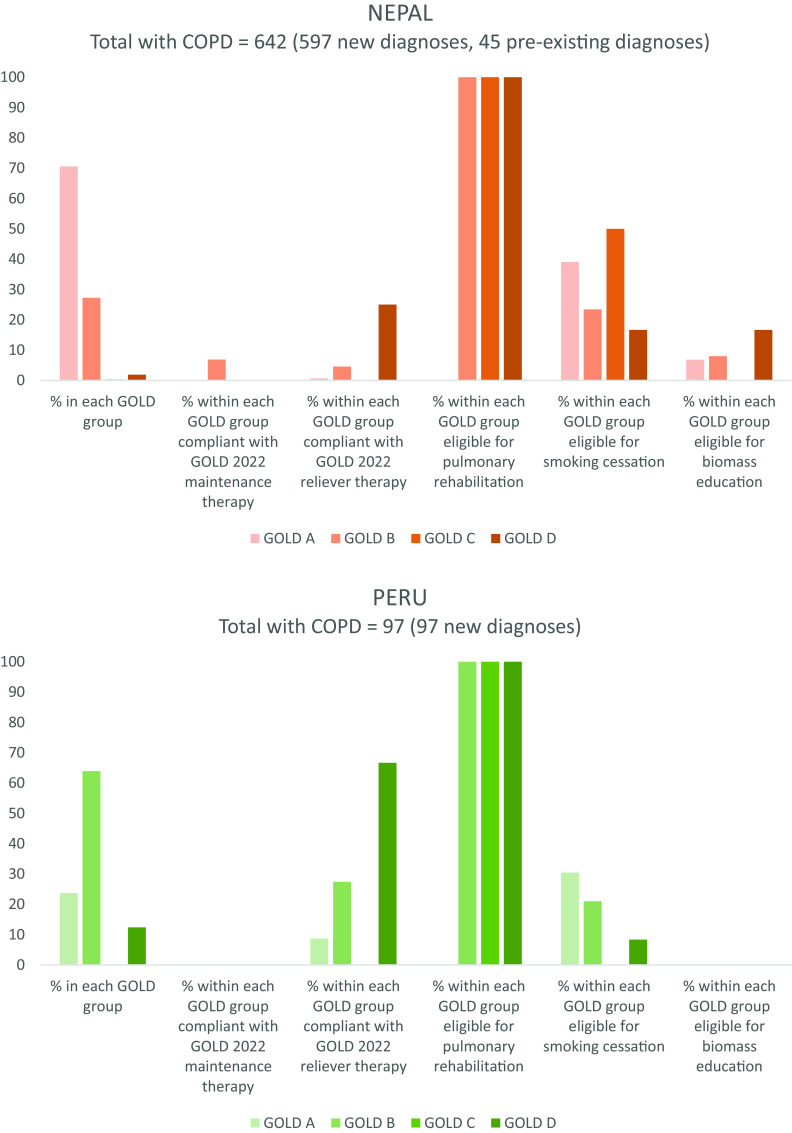
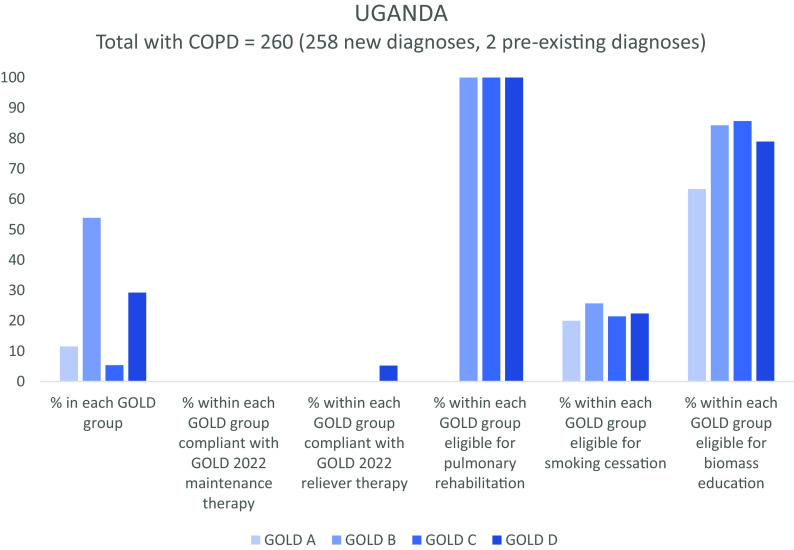
Regarding nonpharmacological interventions, all cases of COPD would be eligible for annual vaccinations, such as influenza, and self-management advice. A total of 493/999 (49.3%) people with GOLD B, C, or D disease would be eligible for pulmonary rehabilitation. Three hundred four (30.4%) current smokers would be eligible for smoking cessation support, and the 256 (25.6%) people currently exposed to biomass would be eligible for education and/or intervention to reduce environmental respiratory exposures. Figure 1 illustrates eligibility for nonpharmacological interventions classified by site and GOLD group for 999 people with COPD.
Figure 1.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2022 group, concordance with pharmacotherapy recommendations, and eligibility for nonpharmacological interventions for 999 people with COPD. A random sample of 10,664 people over 40 years of age was collected at three sites in Nepal, Peru, and Uganda. In the 2023 update to GOLD guidance, groups C and D have been combined as group E. Percentages are indicated on the y-axis. COPD = chronic obstructive pulmonary disease.
Unmet Therapeutic Opportunity: Pharmacological Interventions
Figure 1 also illustrates concordance (or lack of concordance) with GOLD 2022 pharmacological guidance, as classified by GOLD group and site, emphasizing the unmet pharmacotherapeutic need (see Table E2). A total of 50.6% of people with GOLD A disease would not meet criteria for regular maintenance pharmacotherapy.
Unmet Therapeutic Opportunity: Pharmacological Interventions in People with No Prior Diagnosis
For people with newly diagnosed COPD, the results of the 2022 ABCD classification by site and need for pharmacotherapy are reported in Table 2. Only 38 (4.0%) of 952 people were already using a SABA (eight in Nepal, 27 in Peru, and three in Uganda). Just nine people (0.9%), all in Nepal and all in group B, had been previously prescribed a long-acting muscarinic antagonist (LAMA). Two of these were also on a long-acting β-agonist (LABA), which would satisfy GOLD 2023 guidance. Six more people in group B had (non-GOLD compliant) single-agent LABA. Notably, not a single person with GOLD C or D disease had access to appropriate preventative medication (although, in group D, three were using LABA; one LAMA and two ICS monotherapy).
Table 2.
Unmet Therapeutic Need in Screen-Detected, Previously Undiagnosed COPD at Three Sites in LMICs (n = 952)
| Grade and Subtype | Nepal (n) | Peru (n) | Uganda (n) | Total | Reliever | No. (%) of Subjects Using Reliever | Maintenance* | No. (%) of Subjects Using Maintenance |
|---|---|---|---|---|---|---|---|---|
| GOLD A | 436 | 23 | 30 | 489 | SABA | 3 (0.6) | — | — |
| GOLD B | 152 | 62 | 139 | 353 | SABA | 23 (6.5) | LAMA† | 9 (2.5)‡ |
| GOLD C | 2 | 0 | 14 | 16 | SABA | 0 (0) | LAMA† | 0 (0) |
| GOLD D | ||||||||
| Asthma history, CAT score ⩽20 | 3 | 5 | 4 | 12 | SABA | 5 (41.7) | ICS-LABA§ | 0 (0) |
| No asthma, CAT score ⩽20 | 3 | 3 | 34 | 40 | SABA | 1 (2.5) | LAMA† | 0 (0) |
| Asthma history and CAT score >20 | 0 | 4 | 6 | 10 | SABA | 5 (50.0) | ICS-LABA§ | 0 (0) |
| No asthma, CAT score >20 | 1 | 0 | 31 | 32 | SABA | 1 (3.1) | LABA-LAMA | 0 (0) |
Definition of abbreviations: CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; LMICs = low- and middle-income countries; SABA = short-acting β agonist.
2022 guidance.
2023 guidance would be LABA-LAMA.
Two were also on LABA.
2023 guidance would be ICS-LABA-LAMA. History of asthma was used instead of blood eosinophils to guide the need for ICS, see text for details.
Unmet Therapeutic Opportunity: Pharmacological Interventions in People with a Prior Diagnosis of COPD
Of 1,000 people with COPD, 47 (4.7%) were previously aware of that diagnosis: 45 in Nepal and two in Uganda. Seventeen had GOLD A disease, 24 had GOLD B disease; none had GOLD C disease, and six had GOLD D disease. Seven (14.9%) were on reliever SABA, and five (10.6%) others had reliever short-acting muscarinic antagonist (SAMA). Of 47 people, 18 (38.3%) were on some form of maintenance treatment, but eight of those had GOLD A disease.
Three of these 47 people (6.4%), all in Nepal, were using GOLD-recommended pharmacotherapy (two with GOLD A disease on SABA and one with GOLD B disease on maintenance LAMA and reliever SABA). Six further subjects (12.8% of those with an existing diagnosis) could be considered partially compliant with GOLD 2022– recommended pharmacotherapy. Two people in Nepal with GOLD B disease were using maintenance LAMA (one with xanthines) but did not have rescue SABA. One person in Nepal with GOLD B disease was using LABA and SABA rather than LAMA and SABA combination. Three others, all with GOLD D disease, had access to reliever SABA (two in Nepal, one in Uganda) but were on maintenance treatment that was not compliant with the GOLD 2022 strategy: One person in Nepal was on LABA instead of LAMA, and the other was on LAMA instead of LABA-LAMA. The subject in Uganda with a history of asthma was on ICS and oral steroids instead of ICS-LABA.
Of the remaining 38 subjects with a previous diagnosis of COPD, 15 with GOLD A disease did not have reliever SABA, although nine were on an alternative treatment (one on SAMA, four on LABA, two on LAMA, one on LAMA and SAMA and one on LABA and LAMA). One subject with GOLD D disease and a CAT score >20 was not on recommended LABA-LAMA, SABA, or any other treatment. The remaining 22 (20 with GOLD B and two with GOLD D disease) were not on recommended LAMA maintenance or SABA reliever (2022 guidance). Two of those 22 with GOLD B disease and one with GOLD D disease were on LABA, but the remaining 19 were not on any maintenance treatment.
Unmet Therapeutic Opportunity: Pharmacological Escalation in People Receiving Guideline-Compliant Maintenance Therapy
The need for treatment escalation was determined for people who were fully or partially GOLD compliant (although without knowledge of adherence). The two people with GOLD A disease on reliever SABA had an mMRC score of 1 and no exacerbations in the previous year and would, therefore, not be considered for escalation. The person on LAMA and SABA with GOLD B disease had an mMRC score of 2, one exacerbation in the past year, and a history of asthma, so recommended therapy escalation would be to ICS-LABA-LAMA. Of the two people with GOLD B disease compliant with maintenance therapy (LAMA) but without access to SABA, both of whom had a preexisting diagnosis of asthma, one had an mMRC score of 2 with no exacerbations and so would be escalated to ICS-LABA-LAMA with SABA, and one had an mMRC score of 1 with one exacerbation and would not require escalation but would require access to SABA.
Factors Associated with Use of SABA
Use of SABA varied by site and was highest in Peru (27.8%) compared with those in Nepal and Uganda (2.2% and 1.5% respectively; P < 0.001). Use of SABA was also more common in those with a previous history of asthma (22.4% vs. 1.4%; P < 0.001).
Availability and Affordability of Medicines
We have previously reported on medicine availability and costs (6). Treatment costs of both reliever and maintenance treatments for all those with COPD (both new and preexisting diagnoses) are reported in Table 3. Most maintenance medications were not available at pharmacies in Peru and Uganda.
Table 3.
Cost of Meeting Unmet Therapeutic Need for Both New and Preexisting Diagnoses of COPD per Month in Each Population
| GOLD Group | Maintenance Treatment | Treatment Schedule | Reliever Treatment | Treatment Schedule | Nepal |
Peru |
Uganda |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maintenance Mean Monthly Cost (USD) | Reliever Mean Monthly Cost (USD) | COPD (n) | No. of Days’ Wages* Needed to Fund 30 d of Treatment | Maintenance Mean Monthly Cost (USD) | Reliever Mean Monthly Cost (USD) | COPD (n) | No. of Days’ Wages* Needed to Fund 30 d of Treatment | Maintenance Mean Monthly Cost (USD) | Reliever Mean Monthly Cost (USD) | COPD (n) | No. of Days’ Wages* Needed to Fund 30 d of Treatment | |||||
| A | — | — | SABA (100 μg salbutamol) | One inhaler per month | — | 1.90 | 453 | 0.44 | — | 3.94 | 23 | 0.43 | — | 3.08 | 30 | 1.78 |
| B | LAMA (9 μg tiotropium) | One inhaler per month | SABA (100 μg salbutamol) | One inhaler per year | 7.05 | 0.16 | 175 | 1.66 | NA | 0.33 | 62 | — | NA | 0.26 | 140 | — |
| C | LAMA (9 μg tiotropium) | One inhaler per month | SABA (100 μg salbutamol) | One inhaler per year | 7.05 | 0.16 | 2 | 1.66 | NA | 0.33 | 0 | — | NA | 0.26 | 14 | — |
| D (asthma history, CAT score ⩽20) | ICS + LABA (25 + 250 μg fluticasone + salmeterol) | One inhaler per month | SABA (100 μg salbutamol) | One inhaler per year | 7.09 | 0.16 | 3 | 1.67 | 17.88 | 0.33 | 5 | 1.98 | NA | 0.26 | 5 | — |
| D (no asthma, CAT score ⩽20) | LAMA (9 μg tiotropium) | One inhaler per month | SABA (100 μg salbutamol) | One inhaler per year | 7.05 | 0.16 | 6 | 1.66 | NA | 0.33 | 3 | — | NA | 0.26 | 34 | — |
| D (asthma history, CAT score >20) | ICS + LABA (25 + 250 μg fluticasone + salmeterol) | One inhaler per month | SABA (100 μg salbutamol) | One inhaler per year | 7.09 | 0.16 | 0 | 1.67 | 17.88 | 0.33 | 4 | 1.98 | NA | 0.26 | 6 | — |
| D (no asthma, CAT score >20) | LAMA + LABA (9 μg tiotropium inhaler, 25 μg salmeterol) | Two inhalers per month | SABA (100 μg salbutamol) | One inhaler per year | 9.63 | 0.16 | 3 | 2.25 | NA | 0.33 | 0 | — | NA | 0.26 | 31 | — |
| Total | — | — | — | — | — | — | 642 | — | — | — | 97 | — | — | — | 260† | — |
Definition of abbreviations: CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; NA = not available; SABA = short-acting β agonist; USD = U.S. dollars.
Of a low-skilled worker.
One person in Uganda had GOLD group data missing; therefore, n = 260.
When we compare these costs to the daily wage of a low-skilled worker, the cost of one SABA inhaler was the most expensive in Uganda (1.78 day’s wages, compared with 0.44 day’s wages in Nepal and 0.43 day’s wages in Peru). Further, even where medicines were stocked by pharmacies, maintenance drugs for COPD were unaffordable (requiring more than a day’s wage of a low-skilled worker for 30 days treatment).
Discussion
This is the first description of “missed therapeutic opportunity” from three populations with screening-detected COPD in LMIC settings, randomly selected across diverse sites in Nepal, Peru, and Uganda. We defined missed therapeutic opportunity as guideline-based care that has not been received. The vast majority of cases were previously undiagnosed. In addition to the provision of basic education and vaccines, recommended for everyone with COPD, the major unmet needs for nonpharmacological interventions across this population of 999 people with COPD were PR (493; 49.3%), biomass exposure reduction (256; 25.6%), and smoking cessation support for current smokers (304; 30.4%). With regard to pharmacologic interventions, the major needs were access to SABA (954; 95.5%) and LAMA (424; 42.4%) or, with the 2023 updates to GOLD COPD guidance (11), LABA-LAMA dual bronchodilators. Both access and affordability differed by site. We note that few of those with a new diagnosis of COPD had been previously treated, suggesting that patients were not accessing care for symptoms or receiving treatment without a diagnosis. A past history of asthma was associated with increased use of SABA. Even where there was a previous diagnosis of COPD, few patients were receiving guideline-recommended care. Even when available, maintenance therapy was unaffordable.
Our previous survey on access and affordability of medicines found that pharmacies in all countries stocked SABA reliever inhalers (Nepal, 87%; Peru, 69%; and Uganda, 32%) (6). ICS-LABA maintenance treatment (for patients with GOLD D disease with asthma and a CAT score ⩽20, in the 2022 guidance) was available in Nepal and Peru. LAMA (for patients with GOLD B, C, or D disease with no asthma and a CAT score <20) and LAMA-LABA maintenance (for patients with GOLD D disease with no asthma and a CATscore >20) were only available in Nepal. ICS-LABA-LAMA triple-combination inhalers were not available at this time but could be used as “open triple” by combining ICS-LABA with LAMA. No one was prescribed open triple. It is important to note that there are challenges with access and affordability within countries, including important differences between rural and urban locations (5, 6).
The scale of the missed therapeutic opportunity is large, given that interventions in COPD have proven efficacy in reducing symptoms and exacerbations, improving functional status and quality of life, and reducing premature mortality (11). They are also cost-effective when used for clinically significant COPD in high-income settings (2). Although the evidence for such interventions in LMIC settings is much less robust, extrapolating the benefit would suggest real potential to significantly mitigate the morbidity, premature mortality, and costs associated with COPD in LMICs.
PR is a combined exercise and education class for people living with respiratory disease, typically delivered face-to-face for groups in an outpatient setting, and it has shown clinically significant improvements in symptoms, health status, and exercise capacity (19). Despite these benefits and evidence of successful pilot implementation projects in LMICs (20), significant barriers to wider introduction remain (21), such as awareness, facilities, and staff training.
Smoking cessation remains a key intervention for people living with COPD who continue to smoke and the only intervention definitively shown to reduce the subsequent rate of lung function decline (22). Access to treatment for tobacco addiction in LMICs is variable (23). Opportunities to mitigate COPD in LMICs also require addressing occupational and domestic air pollution, although the evidence of benefit from clean-cookstove interventions remains limited by the absence of longer term studies (24). The benefits of smoking cessation and clean air initiatives at a population level, in addition to improving outcomes in those with COPD, will reduce the burden of future COPD (and other noncommunicable diseases).
SABAs remain the treatment of choice for short-term relief of breathlessness in COPD (11), whereas the most frequently indicated maintenance therapy would be LAMA, or LABA-LAMA in the updated GOLD document. LAMA and LABA-LAMA improve symptoms and lung function and reduce the risk of exacerbations (25). LAMA has now been added to the World Health Organization Model Lists of Essential Medicines (26). However, as we have previously reported, this does not translate to an accessible and affordable supply (6).
Missed therapeutic opportunities might best be considered an evidence-practice gap, which varies across settings and is associated with the systemic inequities that drive COPD. This emphasizes the need for further implementation science research in this area and for multilevel interventions that address the social and structural determinants of health that underlie gaps in care. Even where LMIC-relevant guidelines exist, and they often do not (4), there remain significant challenges to effective implementation (7). For example, considering those with the most impactful (GOLD D and E) COPD, even when people were accessing inhaled maintenance therapy, in no case was this aligned with GOLD recommendations.
Given that the majority of people with COPD were undiagnosed and that only 6.4% of those previously diagnosed were receiving recommended pharmacotherapy, we are not powered to examine differences by participant characteristics. However, we note that use of SABA was significantly more common in those with a previous diagnosis of asthma. A key finding of our analysis is the high prevalence of undiagnosed COPD, with this acting as a major barrier to the introduction of effective interventions. Underdiagnosis of COPD is not a problem unique to LMIC settings and is well described (27).
The strengths of our study are the random (age- and sex-stratified) population samples across three diverse sites and comparison with both contemporaneous and the latest GOLD guidance. There are also limitations to our study. We did not have all the available information that GOLD considers necessary to judge the need for ICS (e.g., blood eosinophil counts, although these would generally be absent in clinical services in community settings). We did not have information on local PR or smoking cessation services, including the costs of these services or the costs of vaccines, but our data suggest considerable need for these interventions. Education costs could be estimated from the hourly wage of local healthcare workers, although there is no standard COPD education program in LMICs. Assessing cost-effectiveness is not possible without knowledge of the effectiveness of interventions, especially long-acting bronchodilators in the context of LMICs. Cost data in GOLD subgroups should be interpreted with caution and have high uncertainty because of the small number of cases. It is not known whether use of the mMRC dyspnea scale and CAT score in people not previously known to have COPD in LMICs have similar performance characteristics to those with known respiratory disease. Understanding how and from whom the medicines used were obtained among the small numbers who received them would be important future work.
In conclusion, we report significant missed therapeutic opportunities to reduce the burden of population screening–detected COPD in three LMIC settings. The majority of cases were undiagnosed. Although there remains unmet need in developing novel therapies for COPD, in LMIC settings where the burden of COPD is greatest, better diagnosis together with better access to affordable nonpharmacological (e.g., smoking cessation, clean air initiatives, PR, and vaccinations) and pharmacological interventions (e.g., short- and long-acting bronchodilators) could translate to immediate benefit for individuals and society. Addressing this will require a system-level approach with coordinated health-system strengthening between policy makers, clinicians, transnational organizations, and the pharmaceutical industry.
Acknowledgments
Acknowledgment
The authors acknowledge the contributions of the field research teams and participants in the trial, the Trial Steering Committee, the funder, and the Global Alliance for Chronic Disease research network. This research was conducted while S.L.P. was employed at Johns Hopkins University.
Additional GECo Study Investigators: Maria Kathia Cárdenas, Santa Kumar Das, Shakir Hossen, Robert Kalyesubula, Denis Mawanda, Faith Nassali, Shumonta A. Quaderi, Nicole Robertson, Laxman Shrestha, and Karbir N. Yogi.
A complete list of the GECo Study Investigators may be found before the beginning of the References.
Footnotes
Supported by the Medical Research Council (grant MR/P008984/1) under a Global Alliance for Chronic Disease call and by NIH grant K23HL126946 (T.S.). The opinions expressed in this article are the authors’ own and do not reflect the views of the NIH, the Department of Health and Human Services, or the U.S. government. Neither the Medical Research Council nor the NIH had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author Contributions: All authors meet the authorship criteria of the International Committee of Medical Journal Editors. The first draft was written by K.E.H.F. and J.R.H. Data analysis was led by K.E.H.F., with support from S.M. and J.R.H. The data were available to all authors. All authors reviewed the paper for important intellectual content and approved the final version for submission.
This article has an online data supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202302-0289OC on June 27, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Additional GECo Study Investigators:
Maria Kathia Cárdenas, Santa Kumar Das, Shakir Hossen, Robert Kalyesubula, Denis Mawanda, Faith Nassali, Shumonta A. Quaderi, Nicole Robertson, Laxman Shrestha, and Karbir N. Yogi
References
- 1. GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med . 2020;8:585–596. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stolz D, Mkorombindo T, Schumann DM, Agusti A, Ash SY, Bafadhel M, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet . 2022;400:921–972. doi: 10.1016/S0140-6736(22)01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I, NIHR RESPIRE Global Respiratory Health Unit Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med . 2022;10:447–458. doi: 10.1016/S2213-2600(21)00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tabyshova A, Hurst JR, Soriano JB, Checkley W, Wan-Chun Huang E, Trofor AC, et al. Gaps in COPD guidelines of low- and middle-income countries: a systematic scoping review. Chest . 2021;159:575–584. doi: 10.1016/j.chest.2020.09.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stolbrink M, Thomson H, Hadfield RM, Ozoh OB, Nantanda R, Jayasooriya S, et al. The availability, cost, and affordability of essential medicines for asthma and COPD in low-income and middle-income countries: a systematic review. Lancet Glob Health . 2022;10:e1423–e1442. doi: 10.1016/S2214-109X(22)00330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siddharthan T, Robertson NM, Rykiel NA, Underhill L, Rahman N, Kafle S, et al. Availability, affordability and access to essential medications for asthma and chronic obstructive pulmonary disease in three low- and middle-income country settings. PLOS Glob Public Health . 2022;2:e0001309. doi: 10.1371/journal.pgph.0001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hurst JR, Buist AS, Gaga M, Gianella GE, Kirenga B, Khoo EM, et al. Challenges in the implementation of chronic obstructive pulmonary disease guidelines in low- and middle-income countries: an official American Thoracic Society workshop report. Ann Am Thorac Soc . 2021;18:1269–1277. doi: 10.1513/AnnalsATS.202103-284ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siddharthan T, Pollard SL, Quaderi SA, Mirelman AJ, Cárdenas MK, Kirenga B, et al. GECo Study Investigators Effectiveness-implementation of COPD case finding and self-management action plans in low- and middle-income countries: Global Excellence in COPD Outcomes (GECo) study protocol. Trials . 2018;19:571. doi: 10.1186/s13063-018-2909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siddharthan T, Pollard SL, Quaderi SA, Rykiel NA, Wosu AC, Alupo P, et al. GECo Study Investigators Discriminative accuracy of chronic obstructive pulmonary disease screening instruments in 3 low- and middle-income country settings. JAMA . 2022;327:151–160. doi: 10.1001/jama.2021.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mangione CM, Barry MJ, Nicholson WK, Cabana M, Caughey AB, Chelmow D, et al. U.S. Preventive Services Task Force Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force reaffirmation recommendation statement. JAMA . 2022;327:1806–1811. doi: 10.1001/jama.2022.5692. [DOI] [PubMed] [Google Scholar]
- 11.2022. https://goldcopd.org/2023-gold-report-2/
- 12.2021. https://goldcopd.org/archived-reports/
- 13. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J . 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organisation WHO/HAI. Measuring medicine prices, availability, affordability and price components. WHO Tech Rep Ser . 2007;20:763–765. [Google Scholar]
- 15.2017. https://ilostat.ilo.org/topics/wages/#
- 16. Saeed A, Saeed H, Saleem Z, Fang Y, Babar ZUD. Evaluation of prices, availability and affordability of essential medicines in Lahore Division, Pakistan: a cross-sectional survey using WHO/HAI methodology. PLoS One . 2019;14:e0216122. doi: 10.1371/journal.pone.0216122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zarei L, Karimzadeh I, Moradi N, Peymani P, Asadi S, Babar ZUD. Affordability assessment from a static to dynamic concept: a scenario‐based assessment of cardiovascular medicines. Int J Environ Res Public Health . 2020;17:1710. doi: 10.3390/ijerph17051710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2020. https://www.rstudio.com/
- 19. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2015:CD003793. doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sooriyakanthan M, Orme MW, Sivapalan K, Selvaratnam G, Singh SJ, Wimalasekera S. A feasibility trial of pulmonary rehabilitation for patients with COPD in a low resource setting: Jaffna, Sri Lanka. BMC Pulm Med . 2022;22:302. doi: 10.1186/s12890-022-02092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bickton FM, Shannon H. Barriers and enablers to pulmonary rehabilitation in low- and middle-income countries: a qualitative study of healthcare professionals. Int J Chron Obstruct Pulmon Dis . 2022;17:141–153. doi: 10.2147/COPD.S348663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA . 1994;272:1497–1505. [PubMed] [Google Scholar]
- 23. Hipple Walters B, Petrea I, Lando H. Tobacco control in low- and middle-income countries: changing the present to help the future. J Smok Cessat . 2018;13:187–188. [Google Scholar]
- 24. Zhou Y, Zou Y, Li X, Chen S, Zhao Z, He F, et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med . 2014;11:e1001621. doi: 10.1371/journal.pmed.1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang C, Zhang M, Wang Y, Xiong H, Huang Q, Shuai T, et al. Efficacy and cardiovascular safety of LAMA in patients with COPD: a systematic review and meta-analysis. J Investig Med . 2021;69:1391–1398. doi: 10.1136/jim-2021-001931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.2021. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
- 27. Lamprecht B, Soriano JB, Studnicka M, Kaiser B, Vanfleteren LE, Gnatiuc L, et al. BOLD Collaborative Research Group, the EPI-SCAN Team, the PLATINO Team, and the PREPOCOL Study Group Determinants of underdiagnosis of COPD in national and international surveys. Chest . 2015;148:971–985. doi: 10.1378/chest.14-2535. [DOI] [PubMed] [Google Scholar]