ABSTRACT
Phytobacter diazotrophicus is an Enterobacterales species that was originally identified as a plant growth-promoting, Gram-negative bacterium. Recently, this species has been recognized as relevant to opportunistic human and nosocomial infections in clinical settings. Its frequent misidentification as other Enterobacterales species from clinical examination occasionally causes a delay in the identification of nosocomial outbreaks. Here, we report the emergence of New Delhi metallo-β-lactamase (NDM)-producing P. diazotrophicus isolated from hospitalized pediatric patients and hospital environments in Tokyo, Japan. In our case, these isolates were found during an investigation of carbapenem-resistant Enterobacterales in relation to nosocomial infections. Whole-genome sequencing is useful for overcoming the difficulty of species identification. Furthermore, we found that blaNDM-1 was carried by an IncA/C2 plasmid (approximately 170 kbp), which was transferrable from the clinical isolates to the recipient strain Escherichia coli J53. Our study demonstrated that P. diazotrophicus behaves as a carrier of blaNDM-harboring plasmids, potentially disseminating resistance to carbapenems among Enterobacterales.
IMPORTANCE
Early detection of nosocomial outbreaks is important to minimize the spread of bacteria. When an outbreak is caused by multidrug-resistant bacteria such as carbapenem-resistant Enterobacterales, a delay in findings makes it difficult to control it because such bacteria often spread not only among human patients but also in hospital environments. Phytobacter diazotrophicus, an Enterobacterales species that has recently been found to be relevant to clinical settings, is often misidentified as other bacteria in clinical laboratories. Here, we found NDM-producing P. diazotrophicus in hospitalized pediatric patients and their environment in Tokyo, Japan. Given that the isolates carried blaNDM-1-harboring transferrable plasmids, the influence of such bacteria could be greater with the mediation of horizontal transfer of carbapenem resistance. Our findings suggest that P. diazotrophicus should be recognized as an NDM-carrier, for which more attention should be paid in clinical settings.
KEYWORDS: Enterobacteriaceae, antibiotic resistance, plasmid-mediated resistance, molecular epidemiology, genome analysis, genotypic identification
OBSERVATION
Carbapenem resistance in Enterobacterales is frequently acquired through horizontal transfer of carbapenemase genes mediated by plasmids, and this transfer has occurred among different Enterobacterales species (1). The spread of a plasmid carrying carbapenemase genes among several Enterobacterales species often causes large-scale (2, 3) or small-scale (4) outbreaks. One group of the globally disseminated carbapenemase genes is the blaNDM family, which was first identified in New Delhi, India, in 2008 (5). NDM-producing bacteria generally exhibit high levels of resistance to most β-lactams, including carbapenems, and the blaNDM family is mostly carried by plasmids (6).
Phytobacter diazotrophicus was originally isolated from wild rice and first noticed as a plant-associated bacteria (7). P. diazotrophicus promotes plant growth via nitrogen fixation, but this species often causes opportunistic human infections, occasionally causing nosocomial outbreaks (8). P. diazotrophicus has frequently been misidentified as other Enterobacterales species, such as Pantoea spp., because of the difficulty in identification using general methods (9). As a result, a certain proportion of P. diazotrophicus isolates may not be precisely identified as this species, even though they are present and significant in clinical settings (10). In fact, the genus Phytobacter has been recognized as a new member with clinical significance (11, 12).
Here, we report the emergence of P. diazotrophicus, which produces an NDM-type metallo-β-lactamase. In September 2020, a NDM-producing Klebsiella pneumoniae was isolated from a hospitalized patient at Nihon University Itabashi Hospital in Tokyo, Japan, and NDM-producing Enterobacterales were screened in other hospitalized patients and environments where NDM-producing K. pneumoniae was isolated during an outbreak investigation. Consequently, several NDM-producing Enterobacterales were found; however, identifying the bacterial species for four of these, isolated from three hospitalized pediatric patients in a pediatric ward and one environmental specimen around them (Table 1), was difficult. All three patients had suffered from severe congenital disorders at the time of admission. Thus, they had received many medical practices including antibiotic treatments, which may put them in a situation where they tended to carry antimicrobial-resistant organisms. Although fever had developed on all three patients during hospitalization, this was indistinguishable from the results of chronic diseases, and no signs and symptoms of infectious diseases had been detected. Finally, the patients were handled as carriers of these bacteria after detecting NDM-producing bacteria from their nonsterile sites (feces or biliary drain) (Table 1), and contact precautions were implemented. Minimum inhibitory concentrations (MICs) were determined using a RAISUS instrument (Nissui Pharmaceutical Co., Tokyo, Japan). NDM production was examined using NG-Test CARBA 5 (NG Biotech, Guipry, France). This study was approved by the Ethics Committee of the Nihon University Itabashi Hospital (RK-210608-1). Informed consent was obtained via an opt-out form, which clarified the current study on the website of the Nihon University Itabashi Hospital (https://www.itabashi.med.nihon-u.ac.jp/cr/open_information.html).
TABLE 1.
Isolation profiles and MICs of clinical isolates
| Isolate | TA9730 | TA9734 | TA9759 | TA9832 | ||||
|---|---|---|---|---|---|---|---|---|
| Patient/environment | Patient 1 | Patient 2 | Environment | Patient 3 | ||||
| Isolation date | 2020.09.02 | 2020.09.04 | 2020.09.08 | 2020.09.09 | ||||
| Gender | Female | Female | N/A c | Female | ||||
| Age (years) | 5 | 5 | N/A | 4 | ||||
| Origin | Feces | Feces | Waste channel | Biliary drain | ||||
| MIC (µg/mL) a | ||||||||
| Ampicillin-sulbactam | >16 | R | >16 | R | >16 | R | >16 | R |
| Piperacillin-tazobactam | >64 | R | >64 | R | >64 | R | >64 | R |
| Cefazolin | >16 | R | >16 | R | >16 | R | >16 | R |
| Cefotiam b | >4 | − | >4 | − | >4 | − | >4 | − |
| Cefotaxime | >32 | R | >32 | R | >32 | R | >32 | R |
| Ceftazidime | >16 | R | >16 | R | >16 | R | >16 | R |
| Cefepime | >16 | R | >16 | R | >16 | R | >16 | R |
| Cefmetazole | >32 | R | >32 | R | >32 | R | >32 | R |
| Moxalactam | >32 | R | >32 | R | >32 | R | >32 | R |
| Imipenem | >8 | R | 8 | R | >8 | R | 8 | R |
| Meropenem | >8 | R | >8 | R | >8 | R | >8 | R |
| Doripene | >8 | R | >8 | R | >8 | R | >8 | R |
| Aztreonam | ≤1 | S | ≤1 | S | ≤1 | S | ≤1 | S |
| Gentamicin | >8 | R | >8 | R | >8 | R | >8 | R |
| Tobramycin | >8 | R | >8 | R | >8 | R | >8 | R |
| Amikacin | >32 | R | >32 | R | >32 | R | >32 | R |
| Ciprofloxacin | ≤0.06 | S | 0.5 | I | ≤0.06 | S | 0.5 | I |
| Levofloxacin | ≤0.12 | S | 2 | R | ≤0.12 | S | 1 | I |
| Minocycline | ≤1 | S | 2 | S | ≤1 | S | 2 | S |
| Sulfamethoxazole/trimethoprim | >80 | R | >80 | R | >80 | R | >80 | R |
| Carbapenemase production | NDM (+) | NDM (+) | NDM (+) | NDM (+) | ||||
The initial identification using the MALDI Biotyper system (Bruker, Billerica, MA, USA) indicated that these four isolates were possibly Cronobacter sakazakii or Pluralibacter gergoviae with low identification scores (<2.0). The ID 32 E Api Kit (bioMérieux, Marcy-l’Etoile, France) indicated that one of these was Pantoea spp. Due to the necessity of another approach for identification, 16S rRNA sequencing using a MicroSEQ 500 16S rDNA Sequencing Kit (Thermo Fisher Scientific, Massachusetts, CA, USA) was performed, and a BLAST search for these sequences on the NCBI website using the megablast algorithm made another candidate, P. diazotrophicus (Table S1).
Finally, we employed whole-genome sequencing (WGS) as a definitive analysis. A next-generation sequencing (NGS) library was prepared from genomic DNA using the Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA, USA) to obtain 2 × 300 bp paired-end short reads on the MiSeq platform (Illumina). Quality trimming was performed using Trim Galore v.0.6.7 (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore) and assembled using Spades v.3.12.0 (14). The obtained contigs and reference sequences were applied to Prokka v.1.14.6 (15) for gene prediction. Core genes defined as having more than 95% identity were extracted and connected for core genome alignment using Roary v.3.13.0 (16). A distance matrix based on the average nucleotide identity (ANI) (17) was estimated using the Kostas Lab website (18). Phylogenetic trees for both methods were constructed using the MEGA7 software (19).
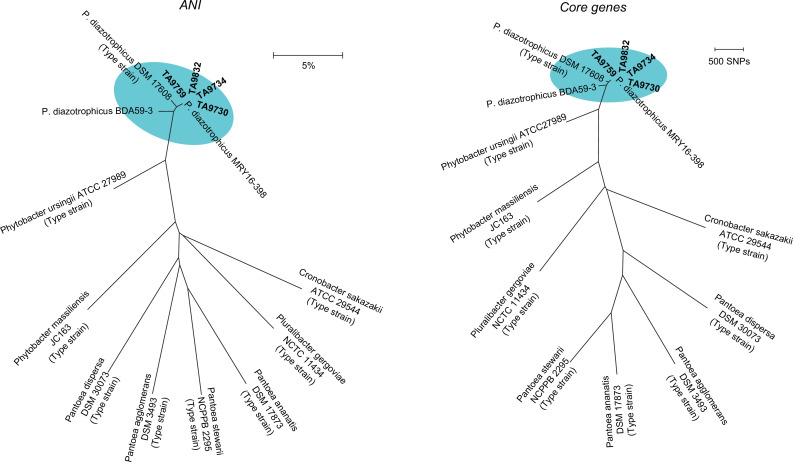
All four isolates were much closer to P. diazotrophicus than other Enterobacterales species in terms of ANI and core-gene similarity (Fig. 1). The ANI values among these isolates and P. diazotrophicus references were less than 5%, which is considered identical (17). Metakosakonia sp. MRY16-398 (20), which was later identified as P. diazotrophicus (10, 21), was closest to our four isolates. Besides the close epidemiological relationship such as their isolations within a week (Table 1), the clonality observed in the pulsed-field gel analysis demonstrated that P. diazotrophicus have disseminated through nosocomial infections in the current cases (Fig. S1). After coordinated, enhanced infection control measures by the hospital and the responsible public health authorities, no additional NDM-producing Enterobacterales have been identified in the hospital.
Fig 1.
Phylogenetic trees based on whole-genome data. Clinical isolates were compared to reference strains based on ANI (left) and core genes (right). These trees were constructed using the neighbor-joining method. Clinical isolates and P. diazotrophicus references are shown by the elliptical area highlighted in cyan. Metakosakonia sp. MRY16-398 and Citrobacter sp. BDA59-3 are regarded as P. diazotrophicus MRY16-398 and P. diazotrophicus BDA59-3, respectively, and the reclassification of Metakosakonia massiliensis to Phytobacter massiliensis is included, according to recent studies (10, 21). Scale bars = distance.
After the current study concluded, we conducted a more thorough investigation of the isolates in April 2023. First, we tested Type Strain Genome Server, another genome-based species identification tool (22). The contig data used for ANI and core-gene analyses with all four isolates were matched with Kluyvera intestini GT-16 and P. diazotrophicus DSM 17806, with more than 80% digital DNA-DNA hybridization scores, which is sufficient for species identification (22). Given that K. intestini GT-16 was recently reclassified as P. diazotrophicus (10, 21), these findings support the original identification of our isolates as P. diazotrophicus. Second, we reanalyzed our isolates after the database for the MALDI Biotyper system (the MBT Compass reference library, MBT-BDAL-10833, Bruker) was updated in our lab in April 2022. As a result, Phytobacter ursingii was hit with all four isolates with high scores: 2.22, 2.06, 2.11, and 1.99, for TA9730, TA9734, TA9759, and TA9832, respectively. The species identification is less accurate with the MALDI system than with WGS, but it is useful for the recognition of the P. diazotrophicus outbreak because all four isolates are determined to be identical species.
To investigate the carriage of plasmids, long-read sequencing was performed using a MinION sequencer (Oxford Nanopore Technologies, Oxford, UK) with a library prepared using the Native Barcoding Kit (Oxford Nanopore Technologies). After quality trimming using NanoFilt v.0.1.0 (23) and adaptor trimming using Porechop v.0.2.4 (https://github.com/rrwick/Porechop), these long reads were assembled with trimmed paired-end short reads using Unicycler v.0.4.8 (24). Genes were predicted and annotated using the DFAST pipeline (https://dfast.ddbj.nig.ac.jp). Gene markers for plasmid replicon type and antimicrobial resistance (AMR)-related genes were identified using PlasmidFinder (25) and ResFinder (26), respectively.
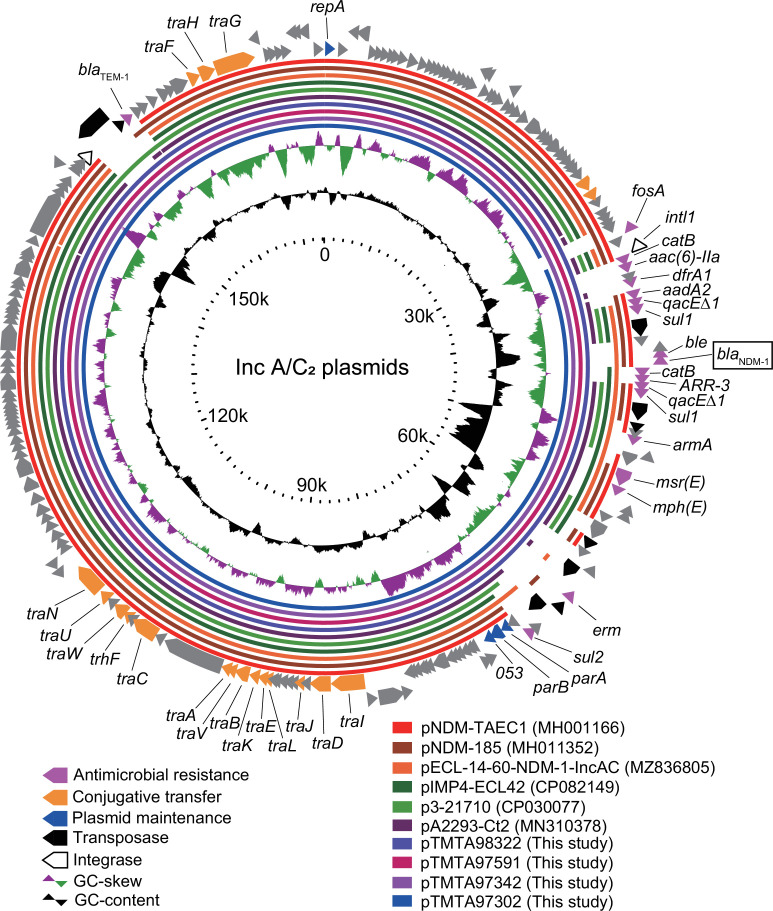
Four P. diazotrophicus isolates carried IncFIB(K) and IncA/C2 plasmids (Table 2), and multiple antimicrobial resistance genes, including blaNDM-1, were harbored by IncA/C2 plasmids. TA9734, TA9759, and TA9832 carried 174,343 bp IncA/C2 plasmids named pTMTA97342, pTMTA97591, and pTMTA98322, respectively. These three 174,343 bp plasmids were identical. The IncA/C2 plasmid from TA9730 (pTMTA97302) was identical to the other three plasmids, except for the lack of a fosfomycin-resistant gene (fosA) (Table 2; Fig. 2), where a slight modification of the plasmid occurred on pTMTA97302 during a series of nosocomial infections. pTMTA97342 shared nucleotide sequences of blaNDM-harboring IncA/C2 plasmids pECL-14–60-NDM-1-IncAC, pNDM-185, and pNDM-TAEC1 with 90%, 89%, and 89% identity, respectively. In addition, it was closely related to blaNDM-non-harboring IncA/C2 plasmids pA2293-Ct2, p3-20710, and pIMP4-ECL42 (with 92%, 90%, and 90% identities, respectively), suggesting that the common backbone of these plasmids contributes to the dissemination among Enterobacterales, even though the β-lactamase genes carried are diverse.
TABLE 2.
Genetic profile of P. diazotrophicus isolates
| Isolate | Chromosome/plasmids b | Accession no. | Length (bp) | Plasmid replicon type | AMR-related genes |
|---|---|---|---|---|---|
| TA9730 | Chromosome | AP028041 | 5,610,297 | N/A a | Not found |
| pTMTA97301 | AP028042 | 175,916 | IncFIB(K) | Not found | |
| pTMTA97302 | AP028043 | 172,011 | IncA/C 2 | ARR-3 , aac(6')-IIa , aadA2 , armA , bla NDM-1 , bla TEM-1B , catB4 , dfrA1 , mph(E) , m sr(E), sul1 , sul2 | |
| pTMTA97303 | AP028044 | 98,997 | Not identified | Not found | |
| pTMTA97304 | AP028045 | 3,530 | Not identified | Not found | |
| pTMTA97305 | AP028046 | 2,496 | Not identified | Not found | |
| TA9734 | Chromosome | AP025334 | 5,709,362 | N/A | Not found |
| pTMTA97341 | AP025335 | 174,856 | IncFIB(K) | Not found | |
| pTMTA97342 | AP025336 | 174,343 | IncA/C 2 | ARR-3 , aac(6')-IIa , aadA2 , armA , bla NDM-1 , bla TEM-1B , catB4 , dfrA1 , fosA3 , mph(E) , msr(E) , sul1 , sul2 | |
| pTMTA97343 | AP025337 | 3,530 | Not identified | Not found | |
| pTMTA97344 | AP025338 | 2,496 | Not identified | Not found | |
| TA9759 | Chromosome | AP028047 | 5,709,306 | N/A | Not found |
| pTMTA97591 | AP028048 | 174,343 | IncA/C 2 | ARR-3 , aac(6')-IIa , aadA2 , armA , bla NDM-1 , bla TEM-1B , catB4 , dfrA1 , fosA3 , mph(E) , msr(E) , sul1 , sul2 | |
| pTMTA97592 | AP028049 | 173,698 | IncFIB(K) | Not found | |
| TA9832 | Chromosome | AP028050 | 5,675,557 | N/A | Not found |
| pTMTA98321 | AP028051 | 174,856 | IncFIB(K) | Not found | |
| pTMTA98322 | AP028052 | 174,343 | IncA/C 2 | ARR-3 , aac(6')-IIa , aadA2 , armA , bla NDM-1 , bla TEM-1B , catB4 , dfrA1 , fosA3 , mph(E) , msr(E) , sul1 , sul2 | |
| pTMTA98323 | AP028053 | 3,530 | Not identified | Not found | |
| pTMTA98324 | AP028054 | 2,496 | Not identified | Not found |
N/A, not applicable.
IncA/C2 plasmids harboring blaNDM-1 are shown in bold characters.
Fig 2.
Circular map of the pTMTA97342 plasmid. IncA/C2 plasmids were compared and visualized using the GView Server (27). Alignment length and percentage of identity cutoff values for sequence-based BLASTn analysis revealed that the colored bars for these plasmids were 80% and 100%, respectively. The positions of open reading frames were derived from those in pTMTA98742, except for repA, which was derived from pNDM-TAEC1.
The conjugative transfer of pTMTA97302 and pTMTA97342 from clinical isolates to Escherichia coli J53 was tested using the mating method (28). The transfer frequency, calculated according to a previous report (29), was approximately 2.8 × 10−3 and 4.1 × 10−3 for pTMTA97302 and pTMTA97342, respectively.
In summary, our initial identification using general methods had failed to identify P. diazotrophicus as shown by the previous reports which mentioned frequent misidentification of this species (9, 10), and the difficulty in identification was overcome using whole-genome analysis. Even though P. diazotrophicus is an opportunistic pathogen (10), the correct identification is important to prevent delays in detecting nosocomial outbreaks. In fact, multi-state sepsis outbreaks caused by contaminated total parenteral nutrition had been reported in Brazil (9). Notably, such a delay can be more serious because this species often carries antimicrobial resistance genes including blaKPC and blaIMP-6 (10). Our study consolidates the importance of focusing on P. diazotrophicus because our isolates carried a blaNDM-1-harboring plasmid, which could spread carbapenem resistance via the horizontal transfer of plasmids in clinical settings. Considering the potential as a carrier of antimicrobial resistance genes, P. diazotrophicus should be more recognized as a clinically relevant pathogen.
ACKNOWLEDGMENTS
We thank the members of the Division of Laboratory Medicine, Department of Pathology and Microbiology, Nihon University School of Medicine, Tokyo, Japan, and the members of the Itabashi Healthcare Center, Tokyo, Japan, for their contributions.
This study was supported by JSPS KAKENHI (Grant No. 18K09593). T.N. and S.T. are endowed chairs at JEOL Ltd., Tokyo, Japan, and the study was partially supported by the funding received as part of these honorific positions.
The authors declare no conflicts of interest associated with this manuscript.
Contributor Information
Hiroaki Kubota, Email: Hiroaki_Kubota@member.metro.tokyo.jp.
Mariana Castanheira, JMI Laboratories, North Liberty, Iowa, USA .
DATA AVAILABILITY
The raw sequence reads and the complete genome sequences in this study were deposited in the DNA Data Bank of Japan, under the BioProject ID PRJDB12598.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00147-23.
BLAST hit results on partial 16S rRNA sequence and pulsed-field gel electrophoresis for four P. diazotrophicus isolates.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Bush K, Bradford PA. 2020. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev 33:e00047-19. doi: 10.1128/CMR.00047-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poirel L, Bonnin RA, Nordmann P. 2012. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother 56:559–562. doi: 10.1128/AAC.05289-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Y, Tong M-K, Chow K-H, Cheng V-C, Tse C-S, Wu A-L, Lai R-M, Luk W-K, Tsang D-C, Ho P-L. 2018. Occurrence of highly conjugative IncX3 epidemic plasmid parrying blaNDM in Enterobacteriaceae isolates in geographically widespread areas. Front. Microbiol 9:2272. doi: 10.3389/fmicb.2018.02272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamagishi T, Matsui M, Sekizuka T, Ito H, Fukusumi M, Uehira T, Tsubokura M, Ogawa Y, Miyamoto A, Nakamori S, Tawa A, Yoshimura T, Yoshida H, Hirokawa H, Suzuki S, Matsui T, Shibayama K, Kuroda M, Oishi K. 2020. A prolonged multispecies outbreak of IMP-6 carbapenemase-producing Enterobacterales due to horizontal transmission of the IncN plasmid. Sci Rep 10:4139. doi: 10.1038/s41598-020-60659-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054. doi: 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. 2019. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev 32:e00115-18. doi: 10.1128/CMR.00115-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang GX, Peng GX, Wang ET, Yan H, Yuan QH, Zhang W, Lou X, Wu H, Tan ZY. 2008. Diverse endophytic nitrogen-fixing bacteria isolated from wild rice Oryza rufipogon and description of Phytobacter diazotrophicus gen. nov. sp. nov. Arch Microbiol 189:431–439. doi: 10.1007/s00203-007-0333-7 [DOI] [PubMed] [Google Scholar]
- 8. Pillonetto M, Arend L, Gomes SMT, Oliveira MAA, Timm LN, Martins AF, Barth AL, Mazzetti A, Hersemann L, Smits THM, Mira MT, Rezzonico F. 2018. Molecular investigation of isolates from a multistate polymicrobial outbreak associated with contaminated total parenteral nutrition in Brazil. BMC Infect Dis 18:397. doi: 10.1186/s12879-018-3287-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pillonetto M, Arend LN, Faoro H, D’Espindula HRS, Blom J, Smits THM, Mira MT, Rezzonico F. 2018. Emended description of the genus Phytobacter, its type species Phytobacter diazotrophicus (Zhang 2008) and description of Phytobacter ursingii sp. nov. Int J Syst Evol Microbiol 68:176–184. doi: 10.1099/ijsem.0.002477 [DOI] [PubMed] [Google Scholar]
- 10. Smits THM, Arend L, Cardew S, Tång-Hallbäck E, Mira MT, Moore ERB, Sampaio JLM, Rezzonico F, Pillonetto M. 2022. Resolving taxonomic confusion: establishing the genus Phytobacter on the list of clinically relevant Enterobacteriaceae. Eur J Clin Microbiol Infect Dis 41:547–558. doi: 10.1007/s10096-022-04413-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janda JM, Abbott SL. 2021. The changing face of the family Enterobacteriaceae (order: "Enterobacterales"): new members, taxonomic issues, geographic expansion, and new diseases and disease syndromes. Clin Microbiol Rev 34:e00174-20. doi: 10.1128/CMR.00174-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munson E, Carroll KC. 2021. Summary of novel bacterial isolates derived from human clinical specimens and nomenclature revisions published in 2018 and 2019. J Clin Microbiol 59:e01309-20. doi: 10.1128/JCM.01309-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute . 2023. CLSI M100-Ed33:2023 performance standards for antimicrobial susceptibility testing, 33rd ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 16. Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. doi: 10.1093/bioinformatics/btv421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91. doi: 10.1099/ijs.0.64483-0 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-R LM, Konstantinidis KT. 2016. The enveomics collection: a toolbox for specialized analyses of microbial genomes and metagenomes. Peerj Preprints 4:e1900v1. doi: 10.7287/peerj.preprints.1900v1 [DOI] [Google Scholar]
- 19. Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger Ddtasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sekizuka T, Matsui M, Takahashi T, Hayashi M, Suzuki S, Tokaji A, Kuroda M. 2018. Complete genome sequence of blaIMP-6-positive Metakosakonia sp. MRY16-398 isolate from the ascites of a diverticulitis patient. Front Microbiol 9:2853. doi: 10.3389/fmicb.2018.02853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma Y, Yao R, Li Y, Wu X, Li S, An Q. 2020. Proposal for unification of the genus Metakosakonia and the genus Phytobacter to a single genus Phytobacter and reclassification of Metakosakonia massiliensis as Phytobacter massiliensis comb. nov. Curr Microbiol 77:1945–1954. doi: 10.1007/s00284-020-02004-4 [DOI] [PubMed] [Google Scholar]
- 22. Meier-Kolthoff JP, Göker M. 2019. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun 10:2182. doi: 10.1038/s41467-019-10210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Coster W, D’Hert S, Schultz DT, Cruts M, Van Broeckhoven C. 2018. Nanopack: visualizing and processing long-read sequencing data. Bioinformatics 34:2666–2669. doi: 10.1093/bioinformatics/bty149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLOS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carattoli A, Hasman H. 2020. PlasmidFinder and in silico pMLST: identification and typing of plasmid replicons in whole-genome sequencing (WGS). Methods Mol Biol 2075:285–294. doi: 10.1007/978-1-4939-9877-7_20 [DOI] [PubMed] [Google Scholar]
- 26. Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petkau A, Stuart-Edwards M, Stothard P, Van Domselaar G. 2010. Interactive microbial genome visualization with GView. Bioinformatics 26:3125–3126. doi: 10.1093/bioinformatics/btq588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kubota H, Suzuki Y, Okuno R, Uchitani Y, Ariyoshi T, Takemura N, Mihara F, Mezaki K, Ohmagari N, Matsui M, Suzuki S, Sekizuka T, Kuroda M, Yokoyama K, Sadamasu K. 2019. IMP-68, a novel IMP-type metallo-β-lactamase in imipenem-susceptible Klebsiella pneumoniae. mSphere 4:e00736-19. doi: 10.1128/mSphere.00736-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ariyoshi T, Aoki K, Kubota H, Sadamasu K, Ishii Y, Tateda K. 2022. Molecular characterization of blaNDM-carrying IncX3 plasmids: blaNDM-16b likely emerged from a mutation of blaNDM-5 on IncX3 plasmid. Microbiol Spectr 10:e0144922. doi: 10.1128/spectrum.01449-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BLAST hit results on partial 16S rRNA sequence and pulsed-field gel electrophoresis for four P. diazotrophicus isolates.
Data Availability Statement
The raw sequence reads and the complete genome sequences in this study were deposited in the DNA Data Bank of Japan, under the BioProject ID PRJDB12598.