Abstract
Polycystic ovary syndrome (PCOS) is a complex endocrine disease that can cause female infertility and bring economic burden to families and to society. The clinical and/or biochemical manifestations include hyperandrogenism, persistent anovulation, and polycystic ovarian changes, often accompanied by insulin resistance and obesity. Although its pathogenesis is unclear, PCOS involves the abnormal regulation of the hypothalamic-pituitary-ovarian axis and the abnormal activation of GnRH neurons. Neuropeptide Y (NPY) is widely distributed in the arcuate nucleus of the hypothalamus and functions as the physiological integrator of two neuroendocrine systems, one governing feeding and the other controlling reproduction. In recent years, an increasing number of studies have focused on the improvement of the reproductive and metabolic status of PCOS through the therapeutic application of NPY and its receptors. In this review, we summarize the central and peripheral regulation of NPY and its receptors in the development of PCOS and discuss the potential for NPY receptor–related therapies for PCOS.
Keywords: Polycystic ovary syndrome, Neuropeptide Y, Gonadotropin-releasing hormone, Dysgenesis, Metabolic disturbance, Leptin
Introduction
Polycystic ovary syndrome (PCOS)—also known as the Stein-Leventhal syndrome which was first reported by Stein and Leventhal in 1935 [1, 2]—is the most common endocrine disease among women of childbearing age. According to the current diagnostic criteria, the global prevalence of PCOS is 4–21% [3, 4]. Patients with PCOS have various clinical sequelae that are serious in nature, including severe mental health problems (e.g., reduced quality of life, poor self-esteem, depression, and anxiety), reproductive complications (infertility and pregnancy issues), and metabolic implications (insulin resistance (IR), metabolic syndrome, and diabetes). Due to the heterogeneity and clinical characteristics of PCOS, its course may vary throughout a person’s lifetime [5]. At present, the most common treatment options for PCOS include lifestyle changes (especially diet and strengthening exercises) and drugs, which help regulate the menstrual cycle, reduce androgen levels, improve IR, and promote ovulation. Treatment for PCOS may also require surgery. These therapeutic options primarily focus on the treatment of symptoms; however, in some cases, they do not produce satisfactory results [6].
Although PCOS has been known for a long time, its pathophysiological mechanism remains unclear. Imbalance of the hypothalamic-pituitary-ovarian (HPO) axis is considered an important pathophysiological mechanism of PCOS [7]. Gonadotropin-releasing hormone (GnRH) neurons project to the median eminence and release pulses of GnRH peptide directly into the pituitary portal vasculature that drive the pulsatile release of the gonadotropin-luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland [1, 8]. Abnormal GNRH pulse release can lead to an abnormal LH/FSH ratio. Hormone tests in women with PCOS show elevated LH levels. This has also been observed in letrozole-induced PCOS mouse models [9]. Studies have shown that NPY acts directly on GnRH neurons [10, 11] and affects metabolism and reproduction.
Neuropeptide Y (NPY) is a 36–amino acid neuropeptide that is highly conserved among species and is one of the most abundant neuropeptides in the central nervous system of mammals [12–15]. Five mammalian NPY receptors (Y1, Y2, Y4, Y5, and Y6) have been cloned in mammals. The Y1, Y2, Y4, and Y5 receptors are all G-protein coupled. The Y6 receptor is truncated in most mammals (including humans) but is functional in mice [16]. Neuronal NPY participates in the regulation of feeding behavior [17], reproductive behavior [18], energy homeostasis [19], and memory storage [20]. Hypothalamic NPY is an important central regulator of sexual behavior and reproductive functions [21]. In addition, NPY is the strongest appetite-promoting factor in the hypothalamus and controls eating behavior [22]. NPY stimulates appetite, causes overeating, increases body fat, lowers body temperature, and inhibits sympathetic nerve activity [22].
Pathophysiology of PCOS
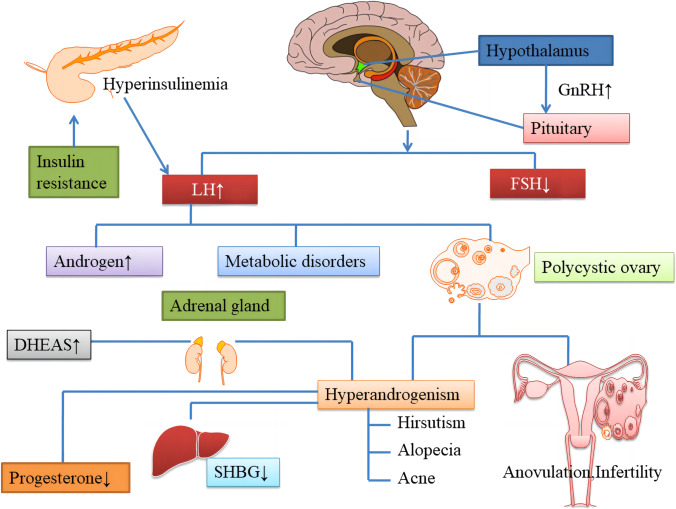
Globally, PCOS is the most common endocrine disease and one which causes female infertility [23]. Despite decades of research, the etiology and pathophysiological mechanisms of PCOS are as yet poorly understood [24–26]. Abnormal ovarian steroid production [27, 28], IR and hyperinsulinemia [29], and abnormalities in neuroendocrine control [30] are considered to be the main causes of PCOS. In most patients with PCOS, the pulse frequency of LH release increases and that of FSH release decreases, suggesting that GnRH pulse frequency is faster [31]. Conversely, high LH levels contribute to an increase in androgen secretion from the ovarian follicular membrane cells, whereas a decrease in FSH levels can disrupt follicular maturation and ovulation. PCOS results in increased secretion of GnRH/LH and a weaker response to exogenous estrogen and P4 [32], indicating that the negative feedback effect of steroid hormones on GnRH neurons is impaired [33]. Some neurotransmitter and neuropeptide receptors expressed in GnRH neurons directly regulate the release of GnRH, LH, and FSH [34]. Moreover, IR and compensatory hyperinsulinemia play a major role in the pathophysiology of PCOS. Excessive levels of insulin act synergistically with LH to stimulate the production of excessive levels of androgens. This inhibits the production of sex hormone-binding globulin (SHBG) by the liver [35] and increases the concentration of free testosterone. The pathophysiology of PCOS is depicted in Fig. 1.
Fig. 1.
The pathophysiology of PCOS. Several theories have been proposed to explain the etiology of PCOS. Abnormal GnRH pulsation leads to an increase in the pulse frequency and amplitude of LH release and a relatively low release of FSH. This causes excessive androgen production, metabolic disorders, and other related performances. IR with hyperinsulinemia further increases ovarian androgen production directly and indirectly by inhibiting the production of SHBG by the liver. PCOS, polycystic ovary syndrome; GnRH, gonadotropin releasing hormone; LH, luteinizing hormone; FSH, follicle stimulating hormone
Effect of NPY on the reproductive system
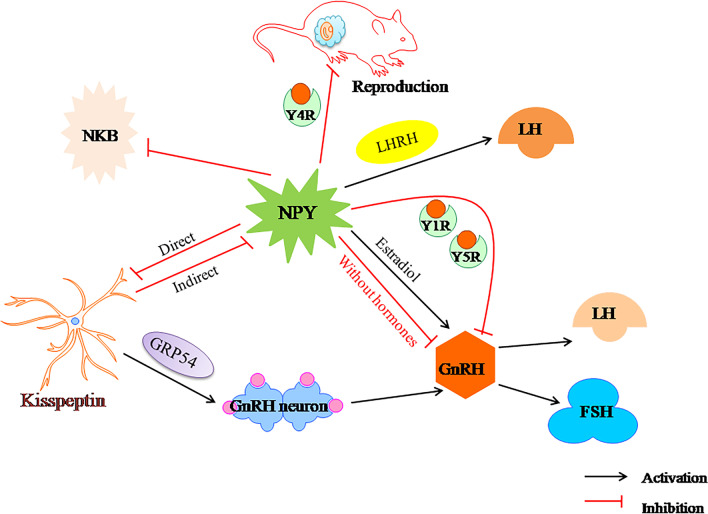
Infertility is a common manifestation of PCOS, and approximately 90–95% of anovulatory infertility is caused by PCOS [5]. The reproductive system is controlled by the hypothalamic–pituitary–gonadal (HPG) axis [36], and depends on the proper functioning of the GnRH neuron network [23]. GnRH neurons secrete GnRH peptide into the pituitary portal system in timed pulses to promote the pulsating release of LH and FSH [37, 38]. Evidence suggests that NPY neurons have a negative effect on the HPO axis in female castrated animals [39, 40]. Results from prenatal androgen-induced sheep and mouse PCOS models suggest that an altered GABAergic input to GnRH neurons may play a role in the elevated GnRH/LH secretion [33, 41, 42]. Therefore, dysfunction of the GnRH neuronal network can lead to infertility [23]. NPY plays an important role in regulating the pulsatile release of GnRH [43], and regulates female reproductive function through the central nervous system [44]. The following section provides key evidence supporting the generally accepted effects of NPY on reproduction (Fig. 2).
Fig. 2.
Relationship between NPY and fertility in polycystic ovary syndrome. NPY, neuropeptide Y; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; LHRH, luteinizing hormone–releasing hormone; FSH, follicle stimulating hormone
NPY stimulates LH release by regulating GnRH
NPY can act as a physiological stimulus to promote GnRH release before ovulation [13] affects the binding of GnRH and its receptors in the anterior pituitary of rats, and increases the response of anterior pituitary cells to GnRH release [43]. Notably, the fact that NPY enhances GnRH activity suggests that it interacts with GnRH to regulate LH secretion [45]. Evans et al. reported that NPY can regulate both the basal and GnRH-stimulated release of LH [45]. Moreover, NPY can also enhance GnRH-stimulated FSH secretion [46]. In ovary-intact ewes, the injection of NPY stimulates the release of GnRH in the follicular phase, but not in the luteal phase [47]. Additionally, Francis et al. found that the NPY-stimulated release of GnRH from the hypothalamus and of LH from the anterior pituitary requires normal ovarian function [48].
Interestingly, NPY can exert both stimulatory and inhibitory effects on GnRH neurons, and several studies have reported this to be steroid-dependent [49–51]. For example, NPY stimulates the release of GnRH in the presence of estradiol, but inhibits the release of GnRH in rats without sex hormones [52]. Coutinho et.al showed that arcuate nucleus neurons (ARN) NPY neurons inhibit GnRH/LH pulse frequency and decreased LH secretion in PCOS-like mice models [53].These inhibitory effects of NPY on reproductive function may lead to a decline in fertility in conditions of negative energy balance, such as food restriction or strenuous exercise, that are related to an increase in hypothalamic NPY expression. However, in a prospective study, women with PCOS had lower NPY levels than weight-matched healthy women [54]. Another study demonstrated that circulating NPY levels in obese and non-obese PCOS adolescents are significantly higher than those in healthy adolescents [55]. The reasons for the difference in results are unknown and may require more research.
NPY receptors and GnRH
The NPY released from the hypothalamus regulates the activity of the GnRH neuronal system through the NPY receptor and the secretion of LH through the pituitary gland [56–60]. NPY stimulates LH-releasing hormone (LHRH) secretion by directly acting on LHRH neurons, which process is mediated by Y1-like receptors in vivo [29]. Moreover, NPY neurons participate in the LH surge by increasing the production of NPY and subsequently promoting the release of LHRH and/or enhancing its effects [29]. Sainsbury et al. found that when NPY expression in the hypothalamus increases under normal physiological conditions, the Y4 receptor causes a decline in reproductive capacity. Knocking out the Y4 receptor restored the reproductive capacity of ob/ob mice [21]. One study showed that NPY inhibits the excitability of GnRH neurons through the Y1 receptor and stimulates their excitability through the Y4 receptor [61]. The affinity of NPY for the Y4 receptor is 1000 × lower than that for Y1 receptor [62], indicating that endogenous NPY affects GnRH neurons via inhibitory events mediated by the Y1 receptor [61].
In addition, a study in lactating rats demonstrated that NPY exerts a direct inhibitory effect on GnRH neurons via postsynaptic Y5R [11]. Direct effects of NPY on proliferation and apoptosis of porcine ovarian cells have been reported [63]. Urata and colleagues found that the NPY5R in granulosa cells isolated from early antral follicles was significantly higher than that in late antral follicles [12]. Moreover, Pinilla et al. observed that Y2 receptors play a complex dual role in controlling gonadotropin secretion at various levels of the hypothalamic-pituitary unit in rats. PYY [13–36] (a selective Y2 receptor agonist) inhibits GnRH release. In contrast, the GnRH-stimulated response of gonadotropins is enhanced in the presence of PYY13-36 [59]. Table 1 provides a snapshot of the functions in which the NPY receptor is critical and the studies which support these findings. The above results may point to new strategies for using NPY receptors to improve neuroendocrine and ovarian function in PCOS patients.
Table 1.
The major physiological roles of NPY receptors in humans, their agonists, and their antagonists
| NPY receptors | Expression | Function | Agonist | Antagonist | References |
|---|---|---|---|---|---|
| Y1R |
Periphery, hypothalamus, hippocampus, neocortex, thalamus |
Food intake, energy homeostasis, body weight, angiogenesis, anti-anxiety, ethanol consumption, pain signaling, bone homeostasis, regulation blood pressure, sedation |
[Leu31,Pro34]NPY, [Phe7,Pro34]pNPY, [D-Arg25]NPY, [D-His26]-NPY |
1229U91,J-104870, J-115814,BMS-193885, SAR-135966,BVD-10, compound 3,BIBP3226, compound 4,SR120819A, BIBO3304 |
[14, 86, 124, 125] |
| Y2R |
Brain, hippocampus, thalamus, hypothalamus |
Food intake, energy homeostasis, colonic transit, pain signaling, cardiovascular regulation, anxiety, neuronal excitability, angiogenesis, ethanol consumption, bone formation |
PYY(3–36),TM-30335, NPY13-36,obinepitide |
BII20246,JNJ-31020028, JNJ-5207787(compound 7), T4-[NPY(33–36)]4 |
[14, 59, 86, 124, 125] |
| Y4R |
Gastrointestinal tract, hippocampus, pancreas, hypothalamus, prostate, human epidermis |
Food intake, energy homeostasis, regulates bone volume, body weight, affects fertility, muscle contraction |
BVD-74D,1229U91, Sub[-T yr-Arg-Leu-Arg-T yr-NH2]2, TM-30339,obinepitide |
[14, 21, 86, 124, 125] | |
| Y5R | Hypothalamus |
Food intake, energy homeostasis, anticonvulsant, anxiety, mood control |
[D-Trp32]NPY, [D-Trp34]NPY, [cPP1-7, N P Y19-23, A l a31, Aib32, G l n34]hPP |
CGP71683A,S-25585, GW438014A,MK-0557, FMS-586,L-152,804 velneperit (S-2367), |
[14, 86, 124, 125] |
NPY inhibits kisspeptin
Kisspeptin is known to be a potent regulator of GnRH neuronal activity [1]. Several studies have confirmed that kisspeptin levels are increased in women with PCOS [64, 65] as well as in rodent models of PCOS [9]. However, kisspeptin levels were decreased in rats with dihydrotestosterone-induced PCOS, which conflicting results may be caused by different modeling methods [66]. Kisspeptin is a hypothalamic neuropeptide that drives fertility via the stimulation of GnRH neurons [67, 68] and induces the secretion of LH and FSH by directly activating GnRH neurons [67, 69, 70]. In addition, kisspeptin activates hypothalamic GnRH secretion through G-protein-coupled receptor GRP54 [71]. Abbara and coworkers demonstrated that kisspeptin receptor agonist (MVT-602) increases the firing duration of GnRH neurons and regulates LH levels, thus improving fertility outcomes in PCOS [72].
Sabine et al. reported that NPY had a direct inhibitory effect on a subpopulation of arcuate kisspeptin neurons in mice and suppressed neurokinin B-evoked firing. Fu and colleagues showed that kisspeptin inhibits NPY neurons through an indirect mechanism involving enhancement gamma-aminobutyric acid (GABA)-mediated inhibitory synaptic tone [73]. This indicates that NPY is negatively correlated with kisspeptin. However, the effect of NPY on kisspeptin neurons is still unclear [74], and, given the insufficient evidence in this regard, the subject requires further exploration.
Considering all the above, it is evident that NPY plays a significant role in the anovulatory infertility caused by PCOS, data which, on the other hand, potentially point to new strategies for PCOS infertility treatment. As present-day research is, however, primarily focused on animal models providing only limited clinical data, more studies are certainly needed.
NPY affects metabolic disorders
The hypothalamus plays an essential role in the regulation of reproduction and energy balance [75]. Arcuate nucleus neurons coexpress NPY and agouti-related peptide (AgRP), which are key regulators of central energy homeostasis [76].
NPY and obesity
Obesity is a growing global epidemic that creates both health and economic challenges [77, 78]. Weight gain and central obesity are the common features of PCOS, and usually occur before the start of the anovulatory cycle. Visceral obesity in PCOS patients is associated with elevated IR, which leads to an increase in reproductive disorders [79]. Obesity increases the risk for adverse metabolic and reproductive outcomes in patients with PCOS. Obesity also increases inflammatory adipokines, thereby promoting hyperinsulinemia, and amplifies functional ovarian hyperandrogenism by upregulating ovarian androgen production: this causes further weight gain, thereby forming a vicious feedback loop. Obesity increases IR and compensatory hyperinsulinemia, glucose intolerance, dyslipidemia, and the risk for pregnancy complications [80, 81]. The NPY system is hypothesized to play a key role in regulating energy balance and the pathophysiology of obesity [19].
Several studies have demonstrated that NPY regulates feeding behavior, body composition, and energy homeostasis and improves food efficiency, while it also induces food cravings and hormonal and metabolic changes and promotes fat gain [44]. In the arcuate nucleus, two types of neurons have opposite effects on food intake, namely, (1) neurons that coexpress NPY and AgRP and stimulate food intake (orexigenic), or those that can express insulin receptors [82, 83]; and (2) neurons that coexpress pro-opiomelanocortin (POMC) and cocaine and amphetamine-regulated transcript (CART), which restrain food intake (anorexigenic) [19, 44]. Consistent with its role as an orexigenic peptide, NPY is increased during fasting or calorie restriction, and is inhibited by feeding and the presence of leptin and insulin [84]. Sadeghian et al. found elevated NPY levels in obese women during a fasting mimicking diet [85]. In many cases of obesity, the elevated NPY-ergic tone may be due to central resistance to peripheral signals of energy excess such as leptin, which increases with long-term exposure to positive energy balance [86]. Similarly, Hansen et al. found that NPY synthesis is reduced in animals fed a high-fat diet [87]. Moreover, Baranowska et al. investigated the relationship between NPY levels and body weight and observed that plasma NPY levels increased significantly in both obese and non-obese patients with PCOS [29].
In addition, a number of studies have also demonstrated the importance of NPY receptors in mediating feeding responses. For example, specific ablation of Y2 receptors on NPY neurons led to a marked increase in obesity in female mice [88]. This was also the case in mice with conditional Y1 receptor knockout [89]. Moreover, the NPY Y5 receptor is also known to mediate NPY-induced feeding [90]. Fukasaka et al. demonstrated that NPY Y5 receptor antagonists significantly reduced weight gain and food intake [91]. Accumulating knowledge on the effect of NPY and its receptor on obesity may provide new insights into the treatment of obese women with PCOS.
NPY and its effects on insulin resistance and hyperinsulinemia
IR and hyperinsulinemia are metabolic features characteristic of patients with PCOS and are considered an important component of the pathogenesis of this endocrine disease [92]. The prevalence of IR in PCOS patients is estimated to be 53–76% [24, 93]. IR is defined as decreased sensitivity of peripheral tissues to insulin. Therefore, higher insulin levels are needed to achieve its metabolic function, which leads to pancreatic β cells producing and releasing more insulin. This explains why IR is often associated with compensatory hyperinsulinemia [92, 94, 95]. Many studies have shown that the decrease of glucose transporter type 4 (GLUT-4) expression is one of the mechanisms underlying IR and PCOS [96–98]. Feng et al. found that insulin resistance reduced the expression of sex hormone-binding protein (SHBG) in human villous trophoblast cells, thereby inhibiting the expression of GLUT-4 and phosphatidylinositol 3-kinase (PI3K) p85α mRNA. This suggests that SHBG may be involved in PI3K/protein kinase B (Akt) pathway-mediated systemic insulin resistance [99]. In addition, IR and related hyperinsulinemia promote pituitary LH release, and increase testosterone production and SHBG synthesis, resulting in high levels of free testosterone (FT) [5, 100, 101]. On the other hand, IR stimulates GnRH gene transcription through the mitogen- activated protein kinase (MAPK) pathway in PCOS and increases LH secretion, thereby significantly increasing ovarian androgen synthesis [102].
Hyperinsulinemia and hyperandrogenism may promote the occurrence of acne. An observational study reported that the severity of acne in women with PCOS was associated with increased concentrations of FT and dehydroepiandrosterone sulfate [103]. High levels of insulin can lead to an increase in the concentration of insulin-like growth factor 1 (IGF-1). IGF-1 may stimulate the secretion of facial average sebum, increase the level of dehydroepiandrosterone sulfate, and induce the proliferation of sebocytes. In addition, hyperinsulinemia promotes the production of epidermal growth factor and transforming growth factor β, thereby increasing the level of non-esterified fatty acids in plasma, causing inflammation; it may thus lead to the colonization of epidermal bacteria in the follicles and the development of acne vulgaris [104]. Moreover, Kim et al. reported that insulin directly affects GnRH neurons, especially by stimulating GnRH gene expression to regulate reproductive function [105]. In summary, given that IR and hyperinsulinemia play a key role in PCOS and associated metabolic complications, targeting these disorders may prove to be beneficial in the treatment of this syndrome.
Sato et al. demonstrated that insulin played a role in reducing NPY gene expression in the hypothalamus. Insulin functions via neurotransmission, and the GABAergic system may also be involved in its effects on NPY neurons [106]. Singhal et al. reported that central resistin induced hepatic IR in mice through NPY [107]. Moreover, Hoek et al. reported that in rodents and humans fed a high-fat diet, increased levels of NPY in the hypothalamus may enhance glucose production and lead to sympathetic hyperactivity and hepatic IR [108]. Previous studies have shown that the activation of AgRP neurons induces IR partly through the acute suppression of sympathetic activation (SNA) in brown adipose tissue (BAT) [109]. However, the ability of AgRP neurons to induce IR depends on NPY expression. Consistent with this, intravenous cephalic injection of NPY rapidly and profoundly reduces BAT SNA [110, 111] and improves systemic insulin sensitivity [112]. In addition, prenatal exposure to androgens also reduces the colocalization of AgRP and insulin receptors. This may affect hepatic insulin sensitivity, as insulin in these neurons plays a prominent role in the regulation of hepatic glucose production [92]. Cernea et al. found that the decreased colocalization of IRβ in AgRP neurons may be a contributing factor to hyperinsulinemia and IR in adult ewes exposed to prenatal testosterone [113]. Interestingly, in patients with anorexia, leptin and insulin enter the brain, inhibit the activity of NPY/AgRP neurons, simultaneously stimulate the activity of POMC/CART neurons, and inhibit food intake [19]. The effects of leptin are discussed below.
NPY and leptin
Leptin, a 167–amino acid polypeptide that is primarily synthesized and expressed in adipocytes [79, 114], is known to play an important role in energy homeostasis and reproduction. Leptin can induce anorexia and regulates energy requirements, fat reserves, and food intake. Insufficient energy intake (for instance, during fasting) leads to a decrease in leptin levels: this in turn stimulates intense hunger, causing an increase in food intake, which may subsequently lead to obesity [13]. Low leptin concentrations are an important signal of an energy deficit in the HPG axis, while high leptin concentrations in obese patients are usually associated with leptin resistance [115]. In patients with PCOS, plasma leptin levels are positively correlated with BMI [29]. NPY mRNA levels are increased in ob/ob mice but decrease after treatment with leptin. The knockout of NPY can attenuate obesity and other related symptoms in ob/ob mice, suggesting that NPY plays a role in the response to leptin deficiency [116]. In the brain, leptin regulates energy expenditure and other physiological functions through the leptin receptor (LepRb) [117, 118]. Zhang et al. showed that under high-fat diet conditions, NPY neurons’ lack of LepRb signaling leads to a significant increase in food intake accompanied by a decrease in energy expenditure, resulting in accelerated cellulite accumulation [119]. Leptin acts indirectly on kisspeptin neurons through POMC/CART and AgRP/NPY neurons to affect energy metabolism and GnRH release [75].
Leptin may also promote high androgen production by promoting steroid production and inhibiting NPY, leading to high levels of GnRH and LH [120]. The administration of leptin increases the levels of LH, FSH, and testosterone in fasting and ob/ob mice [114]. Barash et al. found that leptin specifically stimulates gonadal function in male and female ob/ob mice. Leptin treatment increased the weight of the ovaries and testes, and promoted follicular development in the ovary, which was consistent with the activation of ovarian function. Low levels of leptin stimulate the secretion of gonadotropins, whereas high levels of leptin have an inhibitory effect on the gonads. High levels of leptin have been shown to inhibit E2 synthesis and interfere with the formation of follicles, the production of steroid hormones, and the maturation of oocytes [121]. This suggests a potential for leptin therapy in patients with PCOS. However, the mechanism of leptin is still unclear and further research is needed.
Conclusion and prospective directions
PCOS is a complex endocrine disease affecting reproduction, and metabolism [122]. It is a chronic lifelong disease that is a major health concern and poses an economic burden on patients and society [5]. Due to the complex etiology of the disease, the mechanism of its phenotypic development has not been fully elucidated. The classical theory suggests that the abnormal activation of hypothalamic GnRH neurons and ovarian androgen synthesis are involved in the core pathogenesis of PCOS [7]. In patients with PCOS, NPY not only regulates fertility by regulating GnRH/LH release and affecting the HPO axis, but also plays an important role in maintaining energy balance and regulating body weight and circulating glucose and lipid levels. In recent years, increasing numbers of studies have focused on the NPY receptors; however, the safety of this neuropeptide remains unclear, which precludes large-scale treatment of PCOS through its usage [123]. Therefore, further research should focus on exploring the safety of NPY application, determining what is the biological the mechanism of PCOS and identifying NPY receptors with high affinity and specificity as potential therapeutic agents.
Acknowledgements
We are thankful to The Second Affiliated Hospital of Fujian Medical University for providing infrastructure facilities.
Abbreviations
- AgRP
Agouti-related peptide
- Akt
Protein kinase B
- BAT
Brown adipose tissue
- CART
Cocaine and amphetamine-regulated transcript
- FSH
Follicle stimulating hormone
- FT
Free testosterone
- GABA
Gamma-aminobutyric acid
- GLUT-4
Glucose transporter type 4
- GnRH
Gonadotropin releasing hormone
- HPG
Hypothalamic pituitary gonadal
- HPO
Hypothalamic-pituitary-ovarian
- IR
Insulin resistance
- LH
Luteinizing hormone
- LEpRb
Leptin receptor
- LHRH
Luteinizing hormone releasing hormone
- MAPK
Mitogen-activated protein kinase
- NPY
Neuropeptide Y
- PCOS
Polycystic ovary syndrome
- P13K
Phosphatidylinositol 3–kinase
- POMC
Pro-opiomelanocortin
- PYY13-36
A selective Y2 receptor agonist
- SHBG
Sex hormone-binding globulin
- SNA
Sympathetic nerve activation
Author contribution
The role of all authors in the writing of the work is as follows: Shu Lin:25%, Qiyang Shi:25%, Weihong Chen:25%, Yan-chuan Shi:10%, Qiaoyi Huang:5%, Jiaming Chen:5%, Zhiyi Wang:5%. Shu Lin and Qiyang Shi: Funding acquisition, project administration, supervision, validation, writing, reviewing and editing. Weihong Chen: Writing-original draft, writing, reviewing and editing. Yan-chuan Shi: Supervision, validation, writing, reviewing and editing. Qiaoyi Huang, Jiaming Chen and Zhiyi Wang: Writing, reviewing and editing.
Funding
This work was supported by the Science and Technology Bureau of Quanzhou (grant number 2020CT003) and the Science and Technology Project of Fujian Provincial Health Commission (grant number 2020CXB027).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shu Lin, Email: shulin1956@126.com.
Qi-yang Shi, Email: wsqy214@163.com.
References
- 1.Ruddenklau A, Campbell RE. Neuroendocrine impairments of polycystic ovary syndrome. Endocrinology. 2019;160:2230–2242. doi: 10.1210/en.2019-00428. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Adashi EY. Stein and Leventhal: 80 years on. Am J Obstet Gynecol. 2016;214:247.e241–247.e211. doi: 10.1016/j.ajog.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Lizneva D, Suturina L, Walker W, et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106:6–15. doi: 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 5.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wawrzkiewicz-Jalowiecka A, Kowalczyk K, Trybek P, et al. In search of new therapeutics-molecular aspects of the PCOS pathophysiology: genetics, hormones, metabolism and beyond. Int J Mol Sci. 2020;21(19):7054. doi: 10.3390/ijms21197054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao B, Qiao J, Pang Y. Central regulation of PCOS: abnormal neuronal-reproductive-metabolic circuits in PCOS pathophysiology. Front Endocrinol (Lausanne) 2021;12:667422. doi: 10.3389/fendo.2021.667422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore AM, Campbell RE. Polycystic ovary syndrome: understanding the role of the brain. Front Neuroendocrinol. 2017;46:1–14. doi: 10.1016/j.yfrne.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Esparza LA, Schafer D, Ho BS et al (2020) Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology 16(4):bqaa018. 10.1210/endocr/bqaa018 [DOI] [PMC free article] [PubMed]
- 10.Marshall CJ, Prescott M, Campbell RE. Investigating the NPY/AgRP/GABA to GnRH neuron circuit in prenatally androgenized PCOS-like mice. J Endocr Soc. 2020;4:bvaa129. doi: 10.1210/jendso/bvaa129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Kirigiti MA, Cowley MA, et al. Suppression of basal spontaneous gonadotropin-releasing hormone neuronal activity during lactation: role of inhibitory effects of neuropeptide. Y Endocrinology. 2009;150:333–340. doi: 10.1210/en.2008-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urata Y, Salehi R, Lima PDA, et al. Neuropeptide Y regulates proliferation and apoptosis in granulosa cells in a follicular stage-dependent manner. J Ovarian Res. 2020;13:5. doi: 10.1186/s13048-019-0608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manfredi-Lozano M, Roa J, Tena-Sempere M. Connecting metabolism and gonadal function: novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front Neuroendocrinol. 2018;48:37–49. doi: 10.1016/j.yfrne.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Yi M, Li H, Wu Z, et al. A promising therapeutic target for metabolic diseases: neuropeptide Y receptors in humans. Cell Physiol Biochem. 2018;45:88–107. doi: 10.1159/000486225. [DOI] [PubMed] [Google Scholar]
- 15.Lee NJ, Herzog H. NPY regulation of bone remodelling. Neuropeptides. 2009;43:457–463. doi: 10.1016/j.npep.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Gehlert DR. Introduction to the reviews on neuropeptide. Y Neuropeptides. 2004;38:135–140. doi: 10.1016/j.npep.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muroi Y, Ishii T. A novel neuropeptide Y neuronal pathway linking energy state and reproductive behavior. Neuropeptides. 2016;59:1–8. doi: 10.1016/j.npep.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Loh K, Herzog H, Shi YC. Regulation of energy homeostasis by the NPY system. Trends Endocrinol Metab. 2015;26:125–135. doi: 10.1016/j.tem.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Gotzsche CR, Woldbye DP. The role of NPY in learning and memory. Neuropeptides. 2016;55:79–89. doi: 10.1016/j.npep.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Sainsbury A, Schwarzer C, Couzens M, et al. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mircea CN, Lujan ME, Pierson RA. Metabolic fuel and clinical implications for female reproduction. J Obstet Gynaecol Can. 2007;29:887–902. doi: 10.1016/S1701-2163(16)32661-5. [DOI] [PubMed] [Google Scholar]
- 23.Silva MSB, Desroziers E, Hessler S, et al. Activation of arcuate nucleus GABA neurons promotes luteinizing hormone secretion and reproductive dysfunction: implications for polycystic ovary syndrome. EBioMedicine. 2019;44:582–596. doi: 10.1016/j.ebiom.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12:351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 25.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: what's new? Adv Clin Exp Med. 2017;26:359–367. doi: 10.17219/acem/59380. [DOI] [PubMed] [Google Scholar]
- 27.al-Shoumer KA, Anyaoku V, Richmond W, et al. Elevated leptin concentrations in growth hormone-deficient hypopituitary adults. Clin Endocrinol (Oxf) 1997;47:153–159. doi: 10.1046/j.1365-2265.1997.2131054.x. [DOI] [PubMed] [Google Scholar]
- 28.Kahsar-Miller MD, Conway-Myers BA, Boots LR, et al. Steroidogenic acute regulatory protein (StAR) in the ovaries of healthy women and those with polycystic ovary syndrome. Am J Obstet Gynecol. 2001;185:1381–1387. doi: 10.1067/mob.2001.118656. [DOI] [PubMed] [Google Scholar]
- 29.Baranowska B, Radzikowska M, Wasilewska-Dziubinska E, et al. Neuropeptide Y, leptin, galanin and insulin in women with polycystic ovary syndrome. Gynecol Endocrinol. 1999;13:344–351. doi: 10.3109/09513599909167578. [DOI] [PubMed] [Google Scholar]
- 30.Marshall JC, Eagleson CA. Neuroendocrine aspects of polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:295–324. doi: 10.1016/s0889-8529(05)70071-2. [DOI] [PubMed] [Google Scholar]
- 31.McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20:317–326. doi: 10.1055/s-2002-36706. [DOI] [PubMed] [Google Scholar]
- 32.Burt Solorzano CM, Beller JP, Abshire MY, et al. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77:332–337. doi: 10.1016/j.steroids.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore AM, Prescott M, Marshall CJ, et al. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci U S A. 2015;112:596–601. doi: 10.1073/pnas.1415038112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilie IR. Neurotransmitter, neuropeptide and gut peptide profile in PCOS-pathways contributing to the pathophysiology, food intake and psychiatric manifestations of PCOS. Adv Clin Chem. 2020;96:85–135. doi: 10.1016/bs.acc.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Nestler JE, Jakubowicz DJ, de Vargas AF, et al. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83:2001–2005. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhari N, Dawalbhakta M, Nampoothiri L. GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod Biol Endocrinol. 2018;16:37. doi: 10.1186/s12958-018-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159:3723–3736. doi: 10.1210/en.2018-00653. [DOI] [PubMed] [Google Scholar]
- 38.Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: signaling and gene expression. Mol Cell Endocrinol. 2018;463:131–141. doi: 10.1016/j.mce.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunes M, Bukan N. Examination of angiopoietin-like protein 4, neuropeptide Y, omentin-1 levels of obese and non-obese patients with polycystic ovary syndrome. Gynecol Endocrinol. 2015;31:903–906. doi: 10.3109/09513590.2015.1068285. [DOI] [PubMed] [Google Scholar]
- 40.Sun J, Shen X, Liu H, et al. Caloric restriction in female reproduction: is it beneficial or detrimental? Reprod Biol Endocrinol. 2021;19:1. doi: 10.1186/s12958-020-00681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter DT, Moore AM, Cobern JA, et al. Prenatal testosterone exposure alters GABAergic synaptic inputs to GnRH and KNDy neurons in a sheep model of polycystic ovarian syndrome. Endocrinology. 2019;160:2529–2542. doi: 10.1210/en.2019-00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva MS, Prescott M, Campbell RE (2018) Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI Insight 3(7):e99405. 10.1172/jci.insight.99405 [DOI] [PMC free article] [PubMed]
- 43.Mikolajczyk A, Zlotkowska D. Subclinical lipopolysaccharide from Salmonella enteritidis induces dysregulation of bioactive substances from selected brain sections and glands of neuroendocrine axes. Toxins (Basel) 2019;11(2):91. doi: 10.3390/toxins11020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen AD, Herzog H, Sainsbury A. Neuropeptide Y and peptide YY: important regulators of energy metabolism. Curr Opin Endocrinol Diabetes Obes. 2011;18:56–60. doi: 10.1097/MED.0b013e3283422f0a. [DOI] [PubMed] [Google Scholar]
- 45.Evans JJ, Pragg FL, Mason DR. Release of luteinizing hormone from the anterior pituitary gland in vitro can be concurrently regulated by at least three peptides: gonadotropin-releasing hormone, oxytocin and neuropeptide Y. Neuroendocrinology. 2001;73:408–416. doi: 10.1159/000054659. [DOI] [PubMed] [Google Scholar]
- 46.O'Conner JL, Wade MF, Brann DW, et al. Direct anterior pituitary modulation of gonadotropin secretion by neuropeptide Y: role of gonadal steroids. Neuroendocrinology. 1993;58:129–135. doi: 10.1159/000126521. [DOI] [PubMed] [Google Scholar]
- 47.Advis JP, Klein J, Kuljis RO, et al. Regulation of gonadotropin releasing hormone release by neuropeptide Y at the median eminence during the preovulatory period in ewes. Neuroendocrinology. 2003;77:246–257. doi: 10.1159/000070280. [DOI] [PubMed] [Google Scholar]
- 48.Pau KY, Kaynard AH, Hess DL, et al. Effects of neuropeptide Y on the in vitro release of gonadotropin-releasing hormone, luteinizing hormone, and beta-endorphin and pituitary responsiveness to gonadotropin-releasing hormone in female macaques. Neuroendocrinology. 1991;53:396–403. doi: 10.1159/000125747. [DOI] [PubMed] [Google Scholar]
- 49.Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology. 2010;151:2736–2746. doi: 10.1210/en.2009-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Yorio MP, Bilbao MG, Faletti AG. Neuropeptide Y regulates the leptin receptors in rat hypothalamic and pituitary explant cultures. Regul Pept. 2014;188:13–20. doi: 10.1016/j.regpep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Hessler S, Liu X, Herbison AE. Direct inhibition of arcuate kisspeptin neurones by neuropeptide Y in the male and female mouse. J Neuroendocrinol. 2020;32:e12849. doi: 10.1111/jne.12849. [DOI] [PubMed] [Google Scholar]
- 52.Kalra SP, Crowley WR. Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol. 1992;13:1–46. [PubMed] [Google Scholar]
- 53.Coutinho EA, Prescott M, Hessler S, et al. Activation of a classic hunger circuit slows luteinizing hormone pulsatility. Neuroendocrinology. 2020;110:671–687. doi: 10.1159/000504225. [DOI] [PubMed] [Google Scholar]
- 54.Romualdi D, De Marinis L, Campagna G, et al. Alteration of ghrelin-neuropeptide Y network in obese patients with polycystic ovary syndrome: role of hyperinsulinism. Clin Endocrinol (Oxf) 2008;69:562–567. doi: 10.1111/j.1365-2265.2008.03204.x. [DOI] [PubMed] [Google Scholar]
- 55.Guzelkas I, Orbak Z, Doneray H, et al. Serum kisspeptin, leptin, neuropeptide Y, and neurokinin B levels in adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2022;35:481–487. doi: 10.1515/jpem-2021-0487. [DOI] [PubMed] [Google Scholar]
- 56.Barb CR, Kraeling RR, Rampacek GB, et al. The role of neuropeptide Y and interaction with leptin in regulating feed intake and luteinizing hormone and growth hormone secretion in the pig. Reproduction. 2006;131:1127–1135. doi: 10.1530/rep.1.01108. [DOI] [PubMed] [Google Scholar]
- 57.Foradori CD, Whitlock BK, Daniel JA, et al. Kisspeptin stimulates growth hormone release by utilizing neuropeptide Y pathways and is dependent on the presence of ghrelin in the ewe. Endocrinology. 2017;158:3526–3539. doi: 10.1210/en.2017-00303. [DOI] [PubMed] [Google Scholar]
- 58.Wojcik-Gladysz A, Polkowska J. Neuropeptide Y–a neuromodulatory link between nutrition and reproduction at the central nervous system level. Reprod Biol. 2006;6(Suppl 2):21–28. [PubMed] [Google Scholar]
- 59.Pinilla L, Fernandez-Fernandez R, Roa J, et al. Selective role of neuropeptide Y receptor subtype Y2 in the control of gonadotropin secretion in the rat. Am J Physiol Endocrinol Metab. 2007;293:E1385–1392. doi: 10.1152/ajpendo.00274.2007. [DOI] [PubMed] [Google Scholar]
- 60.Siawrys G, Buchowski H (2018) Modulation of anterior pituitary cell luteinizing hormone secretory activity by neuropeptide Y in early pregnant pigs. J Physiol Pharmacol 69(5). 10.26402/jpp.2018.5.06 [DOI] [PubMed]
- 61.Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012;153:5587–5599. doi: 10.1210/en.2012-1470. [DOI] [PubMed] [Google Scholar]
- 62.Yan H, Yang J, Marasco J, et al. Cloning and functional expression of cDNAs encoding human and rat pancreatic polypeptide receptors. Proc Natl Acad Sci U S A. 1996;93:4661–4665. doi: 10.1073/pnas.93.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sirotkin AV, Kardošová D, Alwasel SH, et al. Neuropeptide Y directly affects ovarian cell proliferation and apoptosis. Reprod Biol. 2015;15:257–260. doi: 10.1016/j.repbio.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Umayal B, Jayakody SN, Chandrasekharan NV, et al. Polycystic ovary syndrome (PCOS) and kisspeptin - a Sri Lankan study. J Postgrad Med. 2019;65:18–23. doi: 10.4103/jpgm.JPGM_683_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorkem U, Togrul C, Arslan E, et al. Is there a role for kisspeptin in pathogenesis of polycystic ovary syndrome? Gynecol Endocrinol. 2018;34:157–160. doi: 10.1080/09513590.2017.1379499. [DOI] [PubMed] [Google Scholar]
- 66.Yuan C, Huang WQ, Guo JH, et al. Involvement of kisspeptin in androgen-induced hypothalamic endoplasmic reticulum stress and its rescuing effect in PCOS rats. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166242. doi: 10.1016/j.bbadis.2021.166242. [DOI] [PubMed] [Google Scholar]
- 67.Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 69.Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 70.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abbara A, Eng PC, Phylactou M, et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J Clin Invest. 2020;130:6739–6753. doi: 10.1172/jci139681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30:10205–10219. doi: 10.1523/jneurosci.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Bond JA, Smith JT. Kisspeptin and energy balance in reproduction. Reproduction. 2014;147:R53–63. doi: 10.1530/REP-13-0509. [DOI] [PubMed] [Google Scholar]
- 75.Chen X, Xiao Z, Cai Y, et al. Hypothalamic mechanisms of obesity-associated disturbance of hypothalamic-pituitary-ovarian axis. Trends Endocrinol Metab. 2022;33:206–217. doi: 10.1016/j.tem.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Parker JA, Bloom SR. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology. 2012;63:18–30. doi: 10.1016/j.neuropharm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2011;12:131–141. doi: 10.1111/j.1467-789X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 79.Orio F, Muscogiuri G, Nese C, et al. Obesity, type 2 diabetes mellitus and cardiovascular disease risk: an uptodate in the management of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;207:214–219. doi: 10.1016/j.ejogrb.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 80.Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med. 2012;30:496–506. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benoit SC, Air EL, Coolen LM, et al. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S56–62. doi: 10.1038/sj.ijo.0801915. [DOI] [PubMed] [Google Scholar]
- 84.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 85.Sadeghian M, Hosseini SA, Zare Javid A, et al. Effect of fasting-mimicking diet or continuous energy restriction on weight loss, body composition, and appetite-regulating hormones among metabolically healthy women with obesity: a randomized controlled, parallel trial. Obes Surg. 2021;31:2030–2039. doi: 10.1007/s11695-020-05202-y. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Bijker MS, Herzog H. The neuropeptide Y system: pathophysiological and therapeutic implications in obesity and cancer. Pharmacol Ther. 2011;131:91–113. doi: 10.1016/j.pharmthera.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 87.Hansen MJ, Jovanovska V, Morris MJ. Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. J Neurochem. 2004;88:909–916. doi: 10.1046/j.1471-4159.2003.02217.x. [DOI] [PubMed] [Google Scholar]
- 88.Shi YC, Lin S, Wong IP, et al. NPY neuron-specific Y2 receptors regulate adipose tissue and trabecular bone but not cortical bone homeostasis in mice. PLoS One. 2010;5:e11361. doi: 10.1371/journal.pone.0011361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oberto A, Bertocchi I, Longo A, et al. Hypothalamic NPY-Y1R interacts with gonadal hormones in protecting female mice against obesity and neuroinflammation. Int J Mol Sci. 2022;23(11):6351. doi: 10.3390/ijms23116351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vohra MS, Benchoula K, Serpell CJ, et al. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur J Pharmacol. 2022;915:174611. doi: 10.1016/j.ejphar.2021.174611. [DOI] [PubMed] [Google Scholar]
- 91.Fukasaka Y, Nambu H, Tanioka H, et al. An insurmountable NPY Y5 receptor antagonist exhibits superior anti-obesity effects in high-fat diet-induced obese mice. Neuropeptides. 2018;70:55–63. doi: 10.1016/j.npep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 92.Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937. doi: 10.1016/j.molmet.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ullah A, Jahan S, Razak S, et al. Protective effects of GABA against metabolic and reproductive disturbances in letrozole induced polycystic ovarian syndrome in rats. J Ovarian Res. 2017;10:62. doi: 10.1186/s13048-017-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marshall JC, Dunaif A. Should all women with PCOS be treated for insulin resistance? Fertil Steril. 2012;97:18–22. doi: 10.1016/j.fertnstert.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 96.Ezeh U, Chen IY, Chen YH, et al. Adipocyte expression of glucose transporter 1 and 4 in PCOS: relationship to insulin-mediated and non-insulin-mediated whole-body glucose uptake. Clin Endocrinol (Oxf) 2019;90:542–552. doi: 10.1111/cen.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen YH, Heneidi S, Lee JM, et al. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes. 2013;62:2278–2286. doi: 10.2337/db12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ezeh U, Chen IY, Chen YH, et al. Adipocyte insulin resistance in PCOS: relationship with GLUT-4 expression and whole-body glucose disposal and β-cell function. J Clin Endocrinol Metab. 2020;105:e2408–2420. doi: 10.1210/clinem/dgaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng C, Jin Z, Chi X, et al. SHBG expression is correlated with PI3K/AKT pathway activity in a cellular model of human insulin resistance. Gynecol Endocrinol. 2018;34:567–573. doi: 10.1080/09513590.2017.1411474. [DOI] [PubMed] [Google Scholar]
- 100.Homburg R. Androgen circle of polycystic ovary syndrome. Hum Reprod. 2009;24:1548–1555. doi: 10.1093/humrep/dep049. [DOI] [PubMed] [Google Scholar]
- 101.Zeng X, Xie YJ, Liu YT, et al. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–221. doi: 10.1016/j.cca.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Li M, Chi X, Wang Y, et al. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7:216. doi: 10.1038/s41392-022-01073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Franik G, Bizoń A, Włoch S, et al. Hormonal and metabolic aspects of acne vulgaris in women with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2018;22:4411–4418. doi: 10.26355/eurrev_201807_15491. [DOI] [PubMed] [Google Scholar]
- 104.Andreadi A, Muscoli S, Tajmir R, et al. Insulin resistance and acne: the role of metformin as alternative therapy in men. Pharmaceuticals (Basel) 2022;16(1):27. doi: 10.3390/ph16010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim HH, DiVall SA, Deneau RM, et al. Insulin regulation of GnRH gene expression through MAP kinase signaling pathways. Mol Cell Endocrinol. 2005;242:42–49. doi: 10.1016/j.mce.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 106.Sato I, Arima H, Ozaki N, et al. Insulin inhibits neuropeptide Y gene expression in the arcuate nucleus through GABAergic systems. J Neurosci. 2005;25:8657–8664. doi: 10.1523/JNEUROSCI.2739-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singhal NS, Lazar MA, Ahima RS. Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci. 2007;27:12924–12932. doi: 10.1523/JNEUROSCI.2443-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van den Hoek AM, van Heijningen C, Schroder-van der Elst JP, et al. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes. 2008;57:2304–2310. doi: 10.2337/db07-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Engstrom Ruud L, Pereira MMA, de Solis AJ, et al. NPY mediates the rapid feeding and glucose metabolism regulatory functions of AgRP neurons. Nat Commun. 2020;11:442. doi: 10.1038/s41467-020-14291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tanida M, Shen J, Nagai K. Possible role of the histaminergic system in autonomic and cardiovascular responses to neuropeptide Y. Neuropeptides. 2009;43:21–29. doi: 10.1016/j.npep.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 111.Shi YC, Lau J, Lin Z, et al. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013;17:236–248. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 112.Marks JL, Waite K. Intracerebroventricular neuropeptide Y acutely influences glucose metabolism and insulin sensitivity in the rat. J Neuroendocrinol. 1997;9:99–103. doi: 10.1046/j.1365-2826.1997.00554.x. [DOI] [PubMed] [Google Scholar]
- 113.Cernea M, Phillips R, Padmanabhan V, et al. Prenatal testosterone exposure decreases colocalization of insulin receptors in kisspeptin/neurokinin B/dynorphin and agouti-related peptide neurons of the adult ewe. Eur J Neurosci. 2016;44:2557–2568. doi: 10.1111/ejn.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mathew H, Castracane VD, Mantzoros C. Adipose tissue and reproductive health. Metabolism. 2018;86:18–32. doi: 10.1016/j.metabol.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 115.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366:74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- 116.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev. 2002;60:S1–14. doi: 10.1301/002966402320634878. [DOI] [PubMed] [Google Scholar]
- 117.Chu SC, Chen PN, Chen JR, et al. Role of hypothalamic leptin-LepRb signaling in NPY-CART-mediated appetite suppression in amphetamine-treated rats. Horm Behav. 2018;98:173–182. doi: 10.1016/j.yhbeh.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 118.Myers MG, Jr, Leibel RL, Seeley RJ, et al. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang L, Reed F, Herzog H. Leptin signalling on arcuate NPY neurones controls adiposity independent of energy balance or diet composition. J Neuroendocrinol. 2020;32:e12898. doi: 10.1111/jne.12898. [DOI] [PubMed] [Google Scholar]
- 120.Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic ovarian syndrome (PCOS): long-term metabolic consequences. Metabolism. 2018;86:33–43. doi: 10.1016/j.metabol.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 121.de Medeiros SF, Rodgers RJ, Norman RJ. Adipocyte and steroidogenic cell cross-talk in polycystic ovary syndrome. Hum Reprod Update. 2021;27:771–796. doi: 10.1093/humupd/dmab004. [DOI] [PubMed] [Google Scholar]
- 122.Dumesic DA, Oberfield SE, Stener-Victorin E, et al. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lepine S, Jo J, Metwally M, et al. Ovarian surgery for symptom relief in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2017;11:CD009526. doi: 10.1002/14651858.CD009526.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 125.Brothers SP, Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol Med. 2010;2:429–439. doi: 10.1002/emmm.201000100. [DOI] [PMC free article] [PubMed] [Google Scholar]