Abstract
Phytoremediation has been explored as a cost‐effective method to remediate soil Pb contamination. A greenhouse study was conducted to evaluate the efficacy of Vigna unguiculata, Brassica pekinensis, Gomphrena globose, and Helianthus annuus for removing and immobilizing Pb in soil collected from the Westside Lead Superfund site in Atlanta. Plants were cultivated in sampled soil with a Pb concentration of 515 ± 10 mg/kg for 60 days. Soils growing H. annuus were additionally treated with ethylenediaminetetraacetic acid (EDTA) (0.1 g/kg) or compost (20% soil blend) to assess their capabilities for enhancing phytoremediation. Mean post‐phytoremediation Pb concentrations in the four plant species were 23.5, 25.7, 50.0, and 58.1 mg/kg dry weight (DW), respectively, and were substantially higher than 1.55 mg/kg DW in respective plant species grown in control soils with no Pb contamination. The highest Pb concentration, translocation factor, and biomass were found in V. unguiculate among four species without soil amendments. H. annuus treated with EDTA and compost resulted in a significant increase in the total Pb uptake and larger biomass compared to non‐treated plants, respectively. Although this study found that V. unguiculata was the best candidate for Pb accumulation and immobilization among four species, soil remediation was limited to 54 mg/kg in a growing season. We find that it is critically important to perform phytostabilization in a secure manner, since Pb bioavailability of edible plant parts implies the potential risk associated with their unintentional consumption. Efficiently and effectively remediating Pb‐contaminated soils in a low‐cost manner needs to be further studied.
Keywords: phytoremediation, soil, lead
Key Points
Phytoremediations using Helianthus annuus, Gomphrena globose, Brassica pekinensis, and Vigna unguiculata were evaluated
Selected plants were not able to reduce the soil Pb concentration to a regulatory standard (<400 mg/kg) in a single growing season
Phytoremediation can be enhanced with soil amendents, including EDTA and compost, which increased the lead uptake and the plant biomass
1. Introduction
Lead (Pb) contamination and subsequent human exposure is still a major public health concern, especially for children. The exposure routes of Pb include ingestion, inhalation, and dermal absorption. Common sources of Pb exposure are food, drinking water, and Pb‐contaminated soil (Lanphear & Roghmann, 1997). Children are more likely to be exposed to Pb in soil because of unintentional soil ingestion via hand‐to‐mouth behaviors, and they are particularly vulnerable to Pb poisoning, since the proportion of Pb absorbed in empty stomach is 4–5 times higher in infants and young children than in adults (U.S. EPA, 2011). As a neurodevelopmental toxicant, Pb affects the central nervous system, especially the developing brain. Because of the biologically immature blood‐brain barrier and liver detoxification systems, children are at greater risk of the neurotoxic effects of Pb, including cognitive and motor impairments (Sanders et al., 2009).
The conventional method to remove soil contaminants is soil replacement, which requires excavation of the contaminated soil, backfilling with clean soil, and landfill disposal (Salt et al., 1995). Chemical and thermal technologies, such as soil washing and vitrification, have been used as alternatives to soil replacement (Dermont et al., 2008). However, these methods are not only expensive and laborious but also they can negatively impact soil functions. The relocated soil contaminants can also become another potential exposure source.
Phytoremediation, an approach using living plants to clean up contaminants in soil, air, and water, has been developed to remediate contamination without ecosystem disturbance. Compared to other aforementioned remediation methods, phytoremediation is a much cheaper alternative. For example, according to Salt et al. (1995), to accomplish the same level of contaminant reduction in an acre of soil with a depth of 50 cm, the cost of phytoremediation is approximately $80,000, while the cost of soil excavation, backfilling with clean soil, and landfill disposal of waste is at least $400,000. In addition, contaminated plant biomass produced by phytoremediation can be disposed of through newer techniques, such as liquid extraction and biosynthesis of metal nanomaterials, to reduce possible secondary contamination (Liu & Tran, 2021). More importantly, phytoremediation has the potential to recover soil ecosystems by improving soil quality, reducing soil erosion, and adding organic matter (Salt et al., 1998).
There are two common processes of phytoremediation for soil contamination: phytoextraction and phytostabilization (Sharma et al., 2023). Phytoextraction refers to the process where plants remove heavy metals and metalloids (HMM) from the soil through the uptake and accumulation of HMM into the plant tissues (Garbisu & Alkorta, 2001). The efficacy of phytoextraction for contaminated soils is significantly influenced by the bioavailability of HMM for plant uptake (Lasat, 2002). Since plants can only absorb free metal ions and soluble metal complexes from the soil solution, a higher percentage of bioavailable metal in the soil means a higher accumulation of metals through phytoextraction. Pb bioavailability is typically less than 20% in soil (Giacalone et al., 2005; Sun et al., 2009). Phytostabilization involves a different mechanism, in which plants immobilize HMM by sequestering them in the rhizosphere to reduce resuspension and leaching (Vangronsveld et al., 1995). In phytostabilization, the mobility of HMM can be reduced through the accumulation in plant roots, adsorption on root surfaces, and precipitation within the rhizosphere (Hinsinger et al., 2005). Immobilization of metals can also be achieved by decreasing wind‐blown dust and reducing soil erosion. An effective phytostabilization can result in decreased HMM bioavailability in soil (Kumpiene et al., 2007; Rizzi et al., 2004).
The efficacy of phytoremediation is determined by not only the capability of plants to accumulate contaminants in soil but also HMM bioavailability and plant biomass. The usage of chemical and biomass amendments to enhance phytoremediation has been widely studied in the literature. As a chelating agent, ethylenediaminetetraacetic acid (EDTA) has been used to increase the bioavailability of contaminants in soil. EDTA promotes the dissolution of metal deposits associated with oxides and carbonates, increasing metal bioavailability and mobility (Zhang et al., 2010). Turgut et al. (2004) have reported that EDTA at a concentration of 0.1 g/kg of soil resulted in the largest increase in the total plant uptake of HMM including Pb, Hg, Cu, As, and Al. Biomass amendments, such as compost, can assist both phytoextraction and phytostabilization by enhancing plant growth. The increase in plant biomass is associated with a higher accumulation of HMM including Zn, Cu, Cr, Cd, and Pb (Sheoran et al., 2016). Compost is, however, preferable for phytostabilization compared to phytoextraction, because of its demonstrated ability to immobilize selected HMM and reduce their bioavailability in soil (Attanayake et al., 2015; Kumpiene et al., 2007; Rizzi et al., 2004). Attanayake et al. (2015), for example, demonstrated that the amendment of composted class‐A biosolids (44 kg/m2) decreased the Pb bioavailability in soil by 17% compared to the non‐treated soil.
In 2018, Emory University researchers, in partnership with community partner Historic Westside Gardens, discovered Pb contamination and slag, which are wastes from smelting, in residential and garden soil within the Westside of Atlanta neighborhoods. This finding has led to an United States Environmental Protection Agency (EPA) investigation, and this contamination site was listed on the National Priorities List on 16 March 2022 (Peters et al., 2023). There is no safe Pb level for children and so any soil Pb concentrations can be considered a health risk to residents, especially young children that may accidently ingest or inhale contaminated soil and dust. The EPA investigation has so far found that 508 out of the 2,097 lots in the Westside (U.S. EPA, 2023) have soil Pb levels above 400 ppm (U.S. EPA, 2020), which is the current regional screening level considered to pose a risk to residents. Moreover, urban farmers in the Westside neighborhoods can be exposed to Pb by consuming vegetables grown in the Pb‐contaminated soil.
To provide urban farmers and communities in the Westside with potentially cost‐effective and green approaches to reduce their exposure to Pb in soil, we investigated using the phytoremediation of these Pb‐contaminated soils. The goal of this study was to determine the intervention potential of cultivating common plants to reduce residents' exposure to Pb in soil via the removal and immobilization of Pb. The following non‐invasive plant species, which have been found in the literature to possess bioaccumulation capacity of Pb and Pb tolerance were selected for this study: Vigna unguiculata (cowpea), Brassica pekinensis (Chinese cabbage), Gomphrena globose (globe amaranth), and Helianthus annuus (sunflower) (Adejumo et al., 2019; Alaboudi et al., 2018; Odoh et al., 2017; Xiong et al., 2006).
Past studies had found H. annuus and B. pekinensis to be effective for phytoextraction of Pb in the medium with 200 mg/kg and 500 mg/kg of Pb added as a solution, respectively (Alaboudi et al., 2018; Xiong et al., 2006). It is noteworthy that flowering species are suitable phytoremediation candidates because they are preferred by urban farmers for decorating their gardens and yards. Although there is an increased risk through accidental ingestion, many vegetable species, especially those with a high potential for HMM bioaccumulation and large biomass, have been evaluated for their capacity to accumulate HMM (Sheoran et al., 2016). For instance, some Brassica species used as food plants have been reported to be HMM hyperaccumulators (Chaudhry et al., 2020). To evaluate the risk associated with the consumption of vegetables grown in Pb‐contaminated soil, this study investigated the bioavailability of Pb in edible parts of B. pekinensis and V. unguiculata cultivated in Pb‐contaminated soil. We further compared these values to the Interim Reference Level (IRL), a daily allowance for Pb intake established by the U.S. Food and Drug Administration (FDA), which is 2.2 μg/day for children and 8.8 μg/day for females of childbearing age (Flannery & Middleton, 2022).
2. Materials and Methods
2.1. Soil Sampling and Characterization
Pb‐contaminated soil was collected from an empty lot in the Westside of Atlanta (zip code 30318), where high Pb concentrations in soil and slag were detected (U.S. EPA, 2023). In this investigation, other HMM concentrations in soil were found to be below EPA regulation levels and thus were not taken into account. The soil was sampled at a depth of 0–15 cm via the incremental sampling method (ISM) to prevent soil heterogeneity from adversely influencing the environmental data (EPA Soil Sampling Operating Procedure, U.S. EPA, 2022). To ensure that all experimental pots contained soils with the same Pb concentration, pH, and organic content, soil samples were thoroughly homogenized following repeated quartering procedures (EPA Soil Sampling Operating Procedure, U.S. EPA, 2022). Three decision units (DUs) were positioned within the contamination site, and three homogeneous samples were generated for each DU, using the ISM. Equal amounts of soil from three samples were combined and homogenized to generate a single sample for each DU. Then, equal amounts of three DU samples were mixed thoroughly to produce a homogeneous soil mixture for pot experiment. Approximately 1.5 kg of soil mixture was transferred to each plastic pot. A portion of three soil samples from each DU was air‐dried, screened to pass through a 0.15 mm sieve, and analyzed for soil pH and Pb concentrations. Soil pH was measured using Hanna Instrument direct soil measurement pH portable meter, and Pb concentration in soil was measured using a field portable X‐ray fluorescence (XRF) analyzer (Niton® XL3t Series Multi‐element XRF Spectrum Analyzer) (EPA method 6200).
2.2. Plant Materials and Pot Experiment
Pot experiments were conducted in a greenhouse at Emory University. The treatments were comprised of four plant species: V. unguiculata (Cowpea), B. pekinensis (Chinese cabbage), G. globose (Globe Amaranth), and H. annuus (Sunflower) (Table 1). Each treatment of flowering plants was replicated three times for assessing phytoremediation potential, and each treatment of vegetables was replicated six times for evaluating the bioavailability of Pb in edible parts, in addition to the phytoremediation potential. For each plant species, three pots had normal potting soil as a control group and received the same treatment as experimental plants. Control soil was determined to have no Pb contaminant (Pb concentration was ∼8.3 mg/kg; limit of detection = 5 mg/kg) using an XRF analyzer (EPA method 6200). The sample size for spiked pots per each species with no soil amendment and corresponding control pots is summarized in Table 1. EDTA and compost amendments were applied to three additional sunflower pots because it has been shown that they can enhance Pb phytoremediation in H. annuus (Seth et al., 2011; Zhou et al., 2020). Each of the sunflower treatments was replicated three times.
Table 1.
Overview of Phytoremediation Experiments
| Species | Spiked pots | Control pots |
|---|---|---|
| Vigna unguiculate (Cowpea) | 6 (3 additional for bioavailability analysis) | 3 |
| Brassica pekinensis (Chinese cabbage) | 6 (3 additional for bioavailability analysis) | 3 |
| Gomphrena globose (Globe Amaranth) | 3 | 3 |
| Helianthus annuus (Sunflower) | No treatment: 3 | 3 |
| EDTA treatment: 3 | ||
| Compost treatment: 3 | ||
| Total | 24 | 12 |
2.3. Cultivation Practices
All plant seeds were germinated in the sampled soil within pots. Each pot contained 2–4 plants based on the expected size of mature plants. Only tap water was added to the soil during cultivation. Soil moisture was measured by using HydroSense II handheld soil moisture sensor, and the watering schedule was adjusted based on the weekly measurements to maintain consistent moisture content. Plants were harvested after 60 days of cultivation. For compost treatment, compost made from garden debris was mixed into the soil to create a 20% soil blend by weight before the cultivation. For the EDTA treatment, EDTA disodium salt solution was added to each pot 2 weeks before harvest to reach a final concentration of 0.1 g EDTA per kg of soil.
2.4. Post‐Phytoremediation Analyses
After plants were gently removed from pots, they were washed, separated into three to five plant parts (roots, stems, leaves, flowers, and fruits) based on species, and dried at 70°C for 24 hr in a drying oven. Dry biomass was measured for each plant part. Experimental soil was dried at 250°C until all moisture was removed and sieved into fine particles using 0.15 mm sieves for analysis. Pb concentrations in soil were measured using an XRF analyzer (EPA method 6200). Dried plants were ground into fine powders using SPEX 8000M Mixer Mill. To determine Pb concentrations in the plant tissues, 0.5 g of sieved plant compartment samples were digested separately by concentrated nitric acid (HNO3) and 30% hydrogen peroxide (H2O2) (EPA test methods 3050B). The acid‐digested samples were diluted and analyzed for Pb concentrations using inductively coupled plasma mass spectrometry (ICP‐MS).
The bioavailability of Pb in shoots of cabbages and fruits of cowpeas was assessed using in vitro gastrointestinal digestion followed by ICP‐MS (Minekus et al., 2014). In vitro digestion adjusts the ionic strength and pH as well as utilizes enzymes and bile salts to integrally simulate the in vivo conditions of oral, gastric, small intestinal, and large intestinal digestive processes. Six Chinese cabbage shoot samples and four cowpea fruit samples were digested separately. The digestion results were compared to the IRL for Pb.
2.5. Determination of Translocation and Bioconcentration Factor
HMM concentrations in plant tissues including roots, shoots, and in soil were used to calculate the translocation factor (TF) and bioconcentration factor (BCF) using the formula of Yadav et al. (2009).
TF = Metal concentration in the shoot of the plant (mg/kg DW)/Metal concentration in the root of the plant (mg/kg DW).
BCF = Metal concentration in the plant (mg/kg DW)/Metal concentration in soil (mg/kg).
2.6. Determination of Total Lead Uptake
The amount of Pb accumulated in roots and shoots of selected plant species was calculated separately using Pb concentrations found in the specific plant compartment and the dry weight of that compartment, using the following equation:
The total amount of Pb in plants (mg) = Dry biomass of roots (kg) x Pb concentration in roots (mg/kg DW) + Dry biomass of shoots (kg) x Pb concentration in shoots (mg/kg DW).
2.7. Statistical Analysis
Using Microsoft Excel, t‐test and one‐way Analysis of Variance were performed to detect statistically significant differences (p < 0.05) of the mean Pb concentrations, TFs, BCFs, and biomass of study species.
3. Results
3.1. Lead in Soil
Pre‐phytoremediation soil pH was 7.43 ± 0.2, and Pb concentration in sampled soil was 515 ± 10 mg/kg. The post‐phytoremediation mean Pb concentrations in soil were 461 mg/kg DW in cowpea, 456 mg/kg DW in Chinese cabbage, 444 mg/kg DW in amaranth, 474 mg/kg DW in sunflower, 454 mg/kg DW in sunflower with EDTA, and 421 mg/kg DW in sunflower with compost.
3.2. Biomass Production
The mean total dry biomass of control plants was 38.7 g in cowpea, 25.9 g in Chinese cabbage, 30.1 g in globe amaranth, and 33.5 g in sunflower (Table 2). The total dry biomass of cowpea was statistically significantly higher than that of other plants in control pots. Based on the mean dry biomass of four plant species without any treatment, the highest dry biomass yield of 32.8 g was also found in cowpea among the spiked concentration pots (Table 2). The root dry biomass of cowpea (5.43 g) in spiked pots was significantly larger than the other three species (p < 0.05). Total (15.5 g) and shoot (13.0 g) dry biomass of Chinese cabbage in spiked pots were, on the other hand, significantly smaller than those of the other three species (p < 0.05).
Table 2.
Mean Dry Biomass of Four Plant Species Studied
| Control | Spiked | |||
|---|---|---|---|---|
| Species | Total dry biomass (g) | Total dry biomass (g) | Root dry biomass (g) | Shoot dry biomass (g) |
| Vigna unguiculate (cowpea) | 38.7a | 32.8a | 5.43a | 27.4a |
| Brassica pekinensis (Chinese cabbage) | 25.9b | 15.5b | 2.50b | 13.0b |
| Gomphrena globose (Globe amaranth) | 30.1b | 27.6a | 2.54b | 25.1a |
| Helianthus annuus (Sunflower) | 33.5b | 29.4a | 2.38b | 27.0a |
Note. abWithin a column, means without a common superscript differ (p < 0.05).
In the spiked pots, plant growth and development were impacted by Pb toxicity. Pb contaminations impede germination and early plant growth, disturb plant water relations, reduce nutrient uptake, induce oxidative stress, and inhibit enzymatic activity (Zulfiqar et al., 2019). All four species used in this study that were grown in Pb‐contaminated soil showed delayed seed germination. Compared to the same species grown in control soil, the biomass production of all plants cultivated in Pb‐contaminated soil was decreased. The dry biomass was reduced by 15.2% in cowpea, 40.3% in Chinese cabbage, 8.3% in globe amaranth, and 12.2% in sunflower. Our results suggest that Chinese cabbage was less tolerant of Pb toxicity compared to the other three species.
3.3. Lead Accumulation in Plant Tissues
The baseline Pb concentration in plants was 1.55 mg/kg DW on average, according to Pb concentrations measured in control plants. For spiked pots, Pb accumulation varied in plant species and tissues. The mean total Pb concentrations in plant species were 58.1 mg/kg DW in cowpea, 50.0 mg/kg DW in Chinese cabbage, 25.7 mg/kg DW in amaranth, and 23.5 mg/kg DW in sunflower. There was no significant difference between the total Pb concentrations of cowpea and Chinese cabbage and between those of amaranth and sunflower (p < 0.05). The total Pb concentrations in cowpea and Chinese cabbage were significantly higher than those in amaranth and sunflower (p < 0.05).
The distribution of Pb concentrations in plant tissues shows that the main accumulation site for Pb was the root in all four plant species (Figure 1). Except for cowpea that had higher concentrations of Pb in leaves than in stems, the concentrations of Pb in different plant compartments were in the following order: roots > stems > leaves > flowers. In addition, root Pb concentrations in cowpea and Chinese cabbage were significantly higher than those in amaranth and sunflower (p < 0.05), while shoot Pb concentrations in cowpea and sunflower were significantly higher than those in Chinese cabbage and amaranth (p < 0.05).
Figure 1.
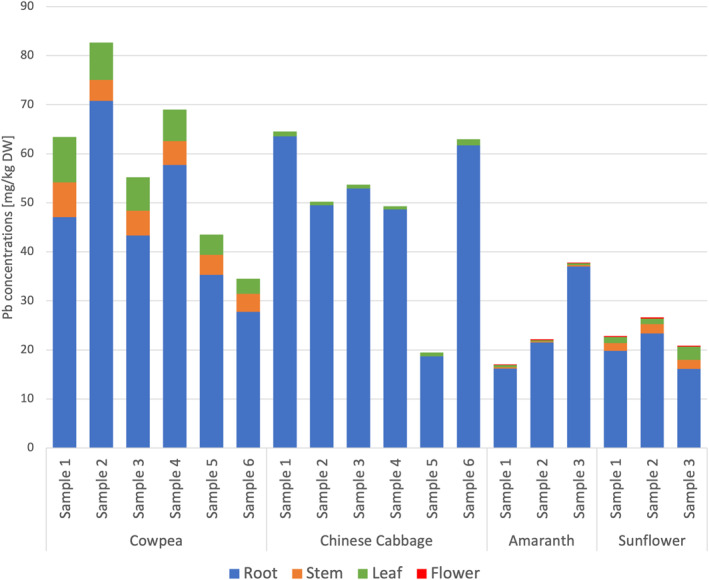
The concentration of Pb in plant compartments of the four plant species studied.
3.4. Translocation Factor and Bioconcentration Factor
The TF and BCF of four plant species for Pb concentrations are summarized in Table 3. TFs of cowpea and sunflower were significantly higher than those of Chinese cabbage and amaranth (p < 0.05). The highest TF was recorded in cowpea, which indicates that cowpea was most capable of translocating Pb from roots to shoots, compared to the other species studied. BCFs of cowpea and Chinese cabbage were significantly higher than those of amaranth and sunflower (p < 0.05). The highest BCF was also found in cowpea, indicating that cowpea had the highest efficiency of Pb accumulation among all plants studied.
Table 3.
Translocation Factor (TF) 1 and Bioconcentration Factor (BCF) 2 of Plant Species for Pb Concentrations
| Species | TF | BCF |
|---|---|---|
| Vigna unguiculate (Cowpea) | 0.24a | 0.13a |
| Brassica pekinensis (Chinese cabbage) | 0.020b | 0.11a |
| Gomphrena globose (Globe amaranth) | 0.034b | 0.060b |
| Helianthus annuus (Sunflower) | 0.19a | 0.049b |
Note. abWithin a column, means without a common superscript differ (p < 0.05).
TF = Metal concentration in the shoot of the plant (mg/kg DW)/Metal concentration in the root of the plant (mg/kg DW).
BCF = Metal concentration in the plant (mg/kg DW)/Metal concentration in soil (mg/kg).
3.5. Total Lead Uptake
The amount of Pb in the root and shoot of cowpea was significantly higher than that of other species (p < 0.05) (Figure 2). The amount of Pb in the root of Chinese cabbage was significantly higher than that of amaranth and sunflower (p < 0.05). In addition, the amount of Pb in the shoot of sunflower was significantly higher than that in Chinese cabbage and amaranth (p < 0.05).
Figure 2.
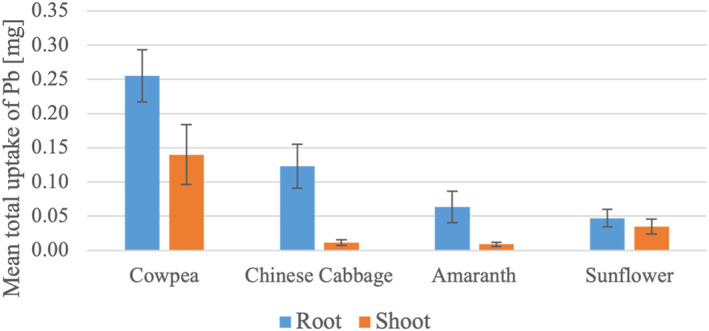
Mean total uptake of Pb of the four plant species studied with one standard deviation.
3.6. Bioavailability of Lead in Vegetables
In vitro gastrointestinal extraction of edible parts of selected plant species (fruits of cowpea and shoots of cabbage) provided the concentrations of Pb in vegetables that are bioavailable for human absorption. The results showed that the mean bioavailable Pb concentrations in the stomach and intestine environments were 1.28 and 2.2 μg/kg DW in cowpea and 3.43 and 8.66 μg/kg DW in Chinese cabbage, respectively.
3.7. Enhanced Phytoremediation With EDTA and Compost
The mean Pb concentrations in sunflowers without treatment and with EDTA or compost addition were 23.45, 52.27, and 16.01 mg/kg DW, respectively. The Pb concentrations in the roots and shoots of sunflowers treated with EDTA increased significantly compared to those without treatment (p < 0.05) (Figure 3). TF and BCF were also significantly increased by EDTA application and there was no significant reduction in plant biomass (p < 0.05) (Table 4). The results indicate that the EDTA enhanced the translocation of Pb from the root to the shoot and the total uptake of Pb, without influencing biomass production. In contrast, the Pb concentration in the tissues of sunflower with the compost treatment decreased significantly compared to those without treatment (p < 0.05) (Figure 3), suggesting that compost potentially reduced the bioavailability of Pb in soil via immobilization. Additionally, compost increased the root biomass of sunflowers by 61.8% (Table 4).
Figure 3.
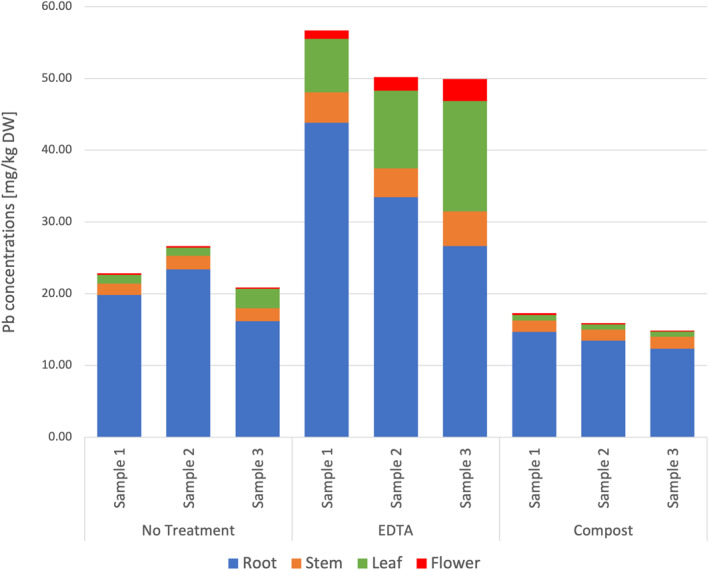
The concentration of Pb in sunflower compartments with and without treatments.
Table 4.
Translocation Factor (TF), 1 Bioconcentration Factor (BCF), 2 and Mean Dry Biomass of Sunflower With and Without Treatments for Pb Concentrations
| Species | Treatment | TF | BCF | Total dry biomass (g) | Root dry biomass (g) | Shoot dry biomass (g) |
|---|---|---|---|---|---|---|
| Helianthus annuus (Sunflower) | No treatment | 0.19a | 0.049a | 29.4a | 2.38a | 27.0a |
| EDTA | 0.56b | 0.12b | 27.6a | 2.21a | 25.4a | |
| Compost | 0.19a | 0.045a | 32.6a | 3.85b | 28.7a |
Note. abWithin a column, means without a common superscript differ (p < 0.05).
TF = Metal concentration in the shoot of the plant (mg/kg DW)/Metal concentration in the root of the plant (mg/kg DW).
BCF = Metal concentration in the plant (mg/kg DW)/Metal concentration in soil (mg/kg).
4. Discussion
The obtained results showed that the root is the major site of Pb accumulation for all selected plant species. This finding is consistent with the previous studies reporting that sunflower, cowpea, and Chinese cabbage accumulated Pb mainly in roots in the presence of 200–500 mg/kg Pb (Alaboudi et al., 2018; Fatnassi et al., 2014; Xiong et al., 2006). However, Pb concentrations in sunflower and Chinese cabbage grown in soil with Pb around 515 mg/kg in this study were lower compared to the values found in studies that investigated the same species in Pb‐enriched media with soluble Pb aqueous solution added at 200 mg/kg for sunflower (Alaboudi et al., 2018) and at 828.8 and 1,657.6 mg/kg for Chinese cabbage (Xiong et al., 2006). Alaboudi et al. (2018) showed that the highest Pb concentrations in shoots and roots were 40.1 and 107.7 mg/kg DW in sunflowers cultivated in soils amended with 200 mg/kg Pb. Pb concentrations of 202.3 and 418.2 mg/kg DW in the shoots were found in Chinese cabbage grown in soil with 828.8 and 1,657.6 mg/kg Pb treatments, respectively (Xiong et al., 2006).
The reason that Pb concentrations in the plants in this study were 27%–73% of what was observed in previous studies could potentially be explained by the difference in the amount of Pb that was bioavailable in the soil (Lasat, 2002). In previous studies, Pb ions in soluble forms were added to soil or culture medium to have high bioavailability for plant uptake. In contrast, according to chemical speciation, Pb usually presents in five fractions in the native soil (Tessier et al., 1979). In real‐life scenarios, only a small proportion of Pb is in exchangeable forms, which is bioavailable for plant uptake. Pb interacts with minerals, organic matter, and clays in soil, forming complexes that are less bioavailable for plant uptake (Giacalone et al., 2005; Sun et al., 2009). Therefore, even though sunflowers have been reported to be effective for phytoremediation in laboratory settings, this study showed that the low bioavailability of Pb in the actual field could be a major drawback of this remediation method. Soil pH is a critical factor that influences the concentration of bioavailable HMM for plant absorption. The solubility of Pb increases as soil pH decreases (Esbaugh et al., 2012). Precipitation of insoluble solids occurs in a soil environment with a pH range of 6–9, which reduces Pb ion concentration in a solution (Esbaugh et al., 2012). Soil pH was 7.43 in this study, indicating a condition that limits Pb solubility. The bioavailability of Pb was expected to be lower in the soil sampled from the urban site in this study, as was observed.
All four plant species resulted in BCFs < 1, indicating that they were excluders of Pb in soil sampled from the contaminated site. Plants classified as excluders can tolerate high soil HMM concentrations by maintaining a low metal concentration in the shoot (Baker, 1981). None of the four species are suitable for phytoextraction, since hyperaccumulator species that have BCFs > 10 are preferred by phytoextraction (Baker, 1981). The low bioavailability of Pb in sampled soil could be a factor limiting phytoextraction. It is important to choose the most appropriate phytoremediation strategy to overcome this limitation. The standards for identifying hyperaccumulators for phytoextraction are well established in the literature. However, there is no generally recognized standard for selecting plant species used for phytostabilization. Several plant parameters were considered to identify a suitable candidate for phytostabilization in previous studies. Ideal plants for phytostabilization are characterized by well‐developed root systems, large biomass, high accumulation of metals in roots, and restricted translocation of metals from roots to shoots when they thrive in HMM‐contaminated soil (Lee et al., 2014; Zou et al., 2011). Extensive root systems are important for the success of phytostabilization. Larger root biomass is more desirable because it provides a larger surface area for penetration into contaminated soil. It is also critical to choose plants with low translocation of metals from roots to shoots, which prevents metals from entering the food chain (Lee et al., 2014; Zou et al., 2011). In this study, all selected plant species had TFs < 1. The TFs of cowpea, Chinese cabbage, and sunflower are consistent with previous studies reporting that these species had TFs < 1 when they were cultivated in the medium with similar Pb concentrations (Alaboudi et al., 2018; Odoh et al., 2017; Xiong, 1998). Among the four species, cowpeas appeared as the best candidate for phytostabilization, because it had the highest accumulation of Pb in roots, highest root biomass and total biomass, and low translocation of Pb from roots to shoots.
Furthermore, the phytoremediation potential of G. globose (globe amaranth) was investigated in this study, which has not been done previously to the best of our knowledge. Globe amaranth had a similar capacity of Pb accumulation and biomass to sunflower in the presence of 515 mg/kg Pb concentration. The TF value of amaranth was much smaller than that of sunflower and cowpea. Root was the major site of Pb accumulation in amaranth, and this finding is in accordance with Adejumo et al. (2019), reporting that Gomphrena celosioides accumulated most of Pb in the root system.
Although plants used for phytostabilization should have a low accumulation of HMM in edible parts of leafy greens and fruit vegetables, it is important to investigate the food safety concerns. The FDA has used the IRL to evaluate the amount of Pb in a food product that is high enough to increase blood lead level (BLL) to a point that requires clinical monitoring. The IRL was determined by considering the amount of a specific food consumed daily and other factors that can lead to BLL of 3.5 μg/dL as the blood lead reference value established by the Centers for Disease Control and Prevention (CDC) (Flannery & Middleton, 2022). There is no safe level of Pb, and medical treatment is suggested for children with BLL higher than 5 μg/dL (Schmidt, 2017). The mean bioavailable Pb concentrations in gastrointestinal tract environments were 3.48 μg/kg in cowpea and 12.09 μg/kg in Chinese cabbage. Based on these values and the IRLs for Pb as mentioned earlier (2.2 μg/day for children and 8.8 μg/day for females of childbearing age), the bioavailable Pb concentrations in these vegetables found in this study would be higher than the IRL for children if more than 630 g of cowpea or 182 g of Chinese cabbage were consumed per day. According to the CDC, children aged 4–8 years should consume 128–192 g of vegetables daily (CDC, 2014), implying that consuming Chinese cabbage grown in 500 mg/kg Pb contaminated soil alone may pose a risk to children. Therefore, it is important to prevent the unintentional consumption of plants used for phytoremediation.
EDTA application was proven to enhance phytoextraction in this study. The total Pb accumulation, TF, and BCF of sunflowers were significantly increased by EDTA treatment. This finding is consistent with the study of Seth et al. (2011), which showed that the addition of EDTA (500 μM) led to an increase in the Pb accumulation in roots and shoots of sunflowers by 12% and 88%, respectively.
Our results also confirmed that the usage of chelating agents can increase the shoot‐to‐root ratio of Pb, which is consistent with the literature (Wu et al., 2004). Additionally, the result showed that compost amendment improved phytostabilization by increasing the root biomass of sunflowers. A similar finding has also been reported by Rizzi et al. (2004), which showed that the growth of Lolium italicum and Festuca arundinacea was improved by compost application when they were cultivated in Pb‐contaminated soil (Rizzi et al., 2004). The reduction of Pb concentration in the plant tissues agrees with a previous study reporting that the compost treatment reduced Pb accumulation in Phaseolus vulgaris from 10.7 to 6 mg/kg DW (Ruttens et al., 2006). Our finding suggests that the compost may suppress plant Pb uptake by reducing the mobility and bioavailability of Pb in soil. Furthermore, our results suggest that compost‐assisted phytostabilization, using cowpea could be a promising approach to reduce Pb exposure from contaminated soil. The addition of compost can potentially increase root accumulation of Pb by supporting the development of root systems, as well as decreasing the mobility and bioavailability of Pb in soil (Kumpiene et al., 2007; Rizzi et al., 2004). Compost amendment is also a safer strategy compared to the usage of EDTA to enhance phytoremediation, since EDTA application is associated with the risk of adversely impacting the food chain via animal exposures and leaching of metals that can potentially contaminate groundwater due to its long persistence and slow degradation in soil (Chen et al., 2004; Zhang et al., 2010).
This study found an inconsistency between the post‐phytoremediation Pb concentrations in soil measured by the XRF analyzer and Pb concentrations in the plant tissues evaluated by ICP‐MS. Theoretically, the mass of Pb taken up by plants should equal the mass of Pb removed from the soil. Our findings, however, showed that the total quantity of Pb accumulated in the plants was approximately one‐thirtieth of that reduced in the soil. A plausible explanation is that some of the soil adhering to the roots was washed away when we cleaned the plant roots for further analysis. As a result, the amount of Pb in soil attached to roots was excluded from the analysis, and the post‐phytoremediation soil Pb concentrations were most likely higher than our measurements. Consequently, the low reliability of post‐phytoremediation soil Pb concentration data measured by the XRF analyzer is a major limitation in this study. This finding suggests a modification in the method of sample preparation and the soil adhered to the plant roots should be carefully removed and saved for further analysis as an important proportion of the soil sample.
Because only one Pb concentration level in soil was investigated in this study, the potential change in plant phytoremediation capabilities under different Pb concentrations could not be evaluated. In this study, homogeneous soil samples were used, which represent a single set of soil physical properties. However, soil properties vary among different sites, and variances in parameters such as pH and organic matter content most likely impact phytoremediation efficacy. Therefore, the generalizability of this study is limited, suggesting that further research into plants' capacity for accumulating Pb in the presence of varying soil Pb concentrations and physical properties is needed. Moreover, the bioavailability of Pb in soil, which influences the efficacy of phytoremediation, was not determined. Additional studies of Pb speciation in soil are needed to investigate the effect of metal bioavailability on phytoremediation, as well as the ability of EDTA and compost amendments to increase and decrease Pb bioavailability in soil, respectively.
5. Conclusions
This study was performed to evaluate the potential of cultivating H. annuus, G. globose, B. pekinensis, and V. unguiculata in Pb‐contaminated soil found in the Westside of Atlanta to reduce residents' exposure to Pb in soil. The results showed that in the presence of approximately 515 mg/kg Pb concentration in soil, V. unguiculata (cowpea) was the most qualified for phytostabilization among the four species. However, even then it was unable to reduce the soil Pb concentration to be lower than 400 mg/kg in a single growing season. In addition, there is a risk of using edible plants for remediating soil contamination. Enhancing Pb uptake and the translocation of Pb from roots to shoots using EDTA amendment was found to be a viable approach for optimizing phytoextraction, but it is also important to emphasize that EDTA has an adverse impact on the environment. Adding compost to soil was shown to enhance phytostabilization by increasing root biomass and potentially decreasing the mobility and bioavailability of Pb in soil. Compost also improves soil fertility and is highly suggested for boosting phytostabilization. A combination of phytoextraction and soil amendments could be a feasible solution to the soil Pb contamination problem at the Westside of Atlanta, but further research is needed to find plants that can act as hyperaccumulator species in the contaminated field at this specific site and beyond.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Acknowledgments
This publication was developed under Assistance Agreement No. 84019801 awarded by the U.S. Environmental Protection Agency to Emory University. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
Yao, X. , Saikawa, E. , Warner, S. , D’Souza, P. E. , Ryan, P. B. , & Barr, D. B. (2023). Phytoremediation of lead‐contaminated soil in the Westside of Atlanta, GA. GeoHealth, 7, e2022GH000752. 10.1029/2022GH000752
Data Availability Statement
All the data are available in the open‐access public repository in Mendeley Data. Saikawa and Yao (2023), https://doi.org/10.17632/ysdtp3xkr9.2.
References
- Adejumo, S. A. , Tiwari, S. , Thul, S. , & Sarangi, B. K. (2019). Evaluation of lead and chromium tolerance and accumulation level in Gomphrena celosoides: A novel metal accumulator from lead acid battery waste contaminated site in Nigeria. International Journal of Phytoremediation, 21(13), 1341–1355. 10.1080/15226514.2019.1633258 [DOI] [PubMed] [Google Scholar]
- Alaboudi, K. A. , Ahmed, B. , & Brodie, G. (2018). Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Annals of Agricultural Science, 63(1), 123–127. 10.1016/j.aoas.2018.05.007 [DOI] [Google Scholar]
- Attanayake, C. P. , Hettiarachchi, G. M. , Martin, S. , & Pierzynski, G. M. (2015). Potential bioavailability of lead, arsenic, and polycyclic aromatic hydrocarbons in compost amended urban soils. Journal of Environmental Quality, 44(3), 930–944. 10.2134/jeq2014.09.0400 [DOI] [PubMed] [Google Scholar]
- Baker, A. J. M. (1981). Accumulators and excluders ‐trategies in the response of plants to heavy metals. Journal of Plant Nutrition, 3(1–4), 643–654. 10.1080/01904168109362867 [DOI] [Google Scholar]
- Chaudhry, H. , Nisar, N. , Mehmood, S. , Iqbal, M. , Nazir, A. , & Yasir, M. (2020). Indian Mustard Brassica juncea efficiency for the accumulation, tolerance and translocation of zinc from metal contaminated soil. Biocatalysis and Agricultural Biotechnology, 23, 101489. 10.1016/j.bcab.2019.101489 [DOI] [Google Scholar]
- Chen, Y. , Li, X. , & Shen, Z. (2004). Leaching and uptake of HMM by ten different species of plants during an EDTA‐assisted phytoextraction process. Chemosphere, 57(3), 187–196. 10.1016/j.chemosphere.2004.05.044 [DOI] [PubMed] [Google Scholar]
- Dermont, G. , Bergeron, M. , Mercier, G. , & Richer‐Laflèche, M. (2008). Soil washing for metal removal: A review of physical/chemical technologies and field applications. Journal of Hazardous Materials, 152(1), 1–31. 10.1016/j.jhazmat.2007.10.043 [DOI] [PubMed] [Google Scholar]
- Esbaugh, A. J. , Brix, K. V. , Mager, E. M. , De Schamphelaere, K. , & Grosell, M. (2012). Multi‐linear regression analysis, preliminary biotic ligand modeling, and cross species comparison of the effects of water chemistry on chronic lead toxicity in invertebrates. Comparative Biochemistry and Physiology—Part C: Toxicology & Pharmacology, 155(2), 423–431. 10.1016/j.cbpc.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Fatnassi, I. C. , Chiboub, M. , Jebara, M. , & Jebara, S. H. (2014). Bacteria associated with different legume species grown in heavy‐metal contaminated soils. International Journal of Agricultural Policy and Research, 2(12), 460–467. [Google Scholar]
- Flannery, B. M. , & Middleton, K. B. (2022). Updated interim reference levels for dietary lead to support FDA's closer to zero action plan. Regulatory Toxicology and Pharmacology, 133, 105202. 10.1016/j.yrtph.2022.105202 [DOI] [PubMed] [Google Scholar]
- Garbisu, C. , & Alkorta, I. (2001). Phytoextraction: A cost‐effective plant‐based technology for the removal of metals from the environment. Bioresource Technology, 77(3), 229–236. 10.1016/s0960-8524(00)00108-5 [DOI] [PubMed] [Google Scholar]
- Giacalone, A. , Gianguzza, A. , Orecchio, S. , Piazzese, D. , Dongarrà, G. , Sciarrino, S. , & Varrica, D. (2005). Metals distribution in the organic and inorganic fractions of soil: A case study on soils from Sicily. Chemical Speciation and Bioavailability, 17(3), 83–93. 10.3184/095422905782774892 [DOI] [Google Scholar]
- Hinsinger, P. , Gobran, G. R. , Gregory, P. J. , & Wenzel, W. W. (2005). Rhizospheregeometry and heterogeneity arising from root‐mediated physical and chemical processes. New Phytol.168,293–303. contaminated soil and their bioavailability in the human gastrointestinal tract. Food Additives & Contaminants, 23(1), 36–48. 10.1080/02652030500387554 [DOI] [PubMed] [Google Scholar]
- Kumpiene, J. , Lagerkvist, A. , & Maurice, C. (2007). Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Management, 28(1), 215–225. 10.1016/j.wasman.2006.12.012 [DOI] [PubMed] [Google Scholar]
- Lanphear, B. P. , & Roghmann, K. J. (1997). Pathways of lead exposure in urban children. Environmental Research, 74(1), 67–73. 10.1006/enrs.1997.3726 [DOI] [PubMed] [Google Scholar]
- Lasat, M. M. (2002). Phytoextraction of toxic metals. Journal of Environmental Quality, 31(1), 109–120. 10.2134/jeq2002.1090 [DOI] [PubMed] [Google Scholar]
- Lee, S.‐H. , Ji, W. H. , Lee, W.‐S. , Koo, N. , Koh, I. H. , Kim, M.‐S. , & Park, J.‐S. (2014). Influence of amendments and aided phytostabilization on metal availability and mobility in PB/zn mine tailings. Journal of Environmental Management, 139, 15–21. 10.1016/j.jenvman.2014.02.019 [DOI] [PubMed] [Google Scholar]
- Liu, Z. , & Tran, K.‐Q. (2021). A review on disposal and utilization of phytoremediation plants containing heavy metals. Ecotoxicology and Environmental Safety, 226, 112821. 10.1016/j.ecoenv.2021.112821 [DOI] [PubMed] [Google Scholar]
- Minekus, M. , Alminger, M. , Alvito, P. , Ballance, S. , Bohn, T. , Bourlieu, C. , et al. (2014). A standardised static in vitro digestion method suitable for food—An international consensus. Food & Function, 5(6), 1113–1124. 10.1039/c3fo60702j [DOI] [PubMed] [Google Scholar]
- Odoh, C. K. , Martins, P. E. , Okekeaji, U. , Akpi, U. K. , & Adobu, U. S. (2017). Phytoremediation potential of Vigna Unguiculata on lead polluted soil and its biotoxic effects on soil microbial activities. Global Journal of Science Frontier Research, 17(3). [Google Scholar]
- Peters, S. J. , Warner, S. M. , Saikawa, E. , Ryan, P. B. , Panuwet, P. , Barr, D. B. , et al. (2023). Community‐engaged assessment of soil lead contamination in Atlanta urban growing spaces. GeoHealth, 7(3). 10.1029/2022gh000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi, L. , Petruzzelli, G. , Poggio, G. , & Guidi, G. V. (2004). Soil physical changes and plant availability of Zn and Pb in a treatability test of phytostabilization. Chemosphere, 57(9), 1039–1046. 10.1016/j.chemosphere.2004.08.048 [DOI] [PubMed] [Google Scholar]
- Ruttens, A. , Mench, M. , Colpaert, J. V. , Boisson, J. , Carleer, R. , & Vangronsveld, J. (2006). Phytostabilization of a metal contaminated sandy soil. I: Influence of compostand/or inorganic metal immobilizing soil amendments on phytotoxicity and plantavailability of metals. Environmental Pollution, 144(2), 524–532. 10.1016/j.envpol.2006.01.038 [DOI] [PubMed] [Google Scholar]
- Saikawa, E. , & Yao, X. (2023). GeoHealth_Pb_Phytoremediation_dataset [Dataset]. Mendeley Data, V1. 10.17632/ysdtp3xkr9.2 [DOI]
- Salt, D. E. , Blaylock, M. , Kumar, N. P. , Dushenkov, V. , Ensley, B. D. , Chet, I. , & Raskin, I. (1995). Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Nature Biotechnology, 13(5), 468–474. 10.1038/nbt0595-468 [DOI] [PubMed] [Google Scholar]
- Salt, D. E. , Smith, R. D. , & Raskin, I. (1998). Phytoremediation. Annual Review of Plant Physiology and Plant Molecular Biology, 49(1), 643–668. 10.1146/annurev.arplant.49.1.643 [DOI] [PubMed] [Google Scholar]
- Sanders, T. , Liu, Y. , Buchner, V. , & Tchounwou, P. B. (2009). Neurotoxic effects and biomarkers of lead exposure: A review. Reviews on Environmental Health, 24(1). 10.1515/reveh.2009.24.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, C. W. (2017). After the screening: What happens next for children with elevated blood lead? Environmental Health Perspectives, 125(10), 102001. 10.1289/ehp2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth, C. S. , Misra, V. , Singh, R. R. , & Zolla, L. (2011). EDTA‐enhanced lead phytoremediation in sunflower (Helianthus annuus L.) hydroponic culture. Plant and Soil, 347(1–2), 231–242. 10.1007/s11104-011-0841-8 [DOI] [Google Scholar]
- Sharma, J. K. , Kumar, N. , Singh, N. P. , & Santal, A. R. (2023). Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Frontiers, 14. 10.3389/fpls.2023.1076876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheoran, V. , Sheoran, A. S. , & Poonia, P. (2016). Factors affecting phytoextraction: A re‐view. Pedosphere, 26(2), 148–166. 10.1016/s1002-0160(15)60032-7 [DOI] [Google Scholar]
- Sun, Y. B. , Zhou, Q. X. , An, J. , Liu, W. T. , & Liu, R. (2009). Chelator‐enhanced phytoextraction of HMM from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma, 150(1–2), 106–112. 10.1016/j.geoderma.2009.01.016 [DOI] [Google Scholar]
- Tessier, A. , Campbell, P. G. , & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851. 10.1021/ac50043a017 [DOI] [Google Scholar]
- Turgut, C. , Pepe, M. K. , & Cutright, T. J. (2004). The effect of EDTA and citric acid on phytoremediation of Cd, Cr, and Ni from soil using Helianthus annuus . Environmental Pollution, 131(1), 147–154. 10.1016/j.envpol.2004.01.017 [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (EPA) . (2011). Exposure factors handbook: 2011 edition. National Center for Environmental Assessment. EPA/600/R‐09/052F. Available from the National Technical Information Service. Retrieved from http://www.epa.gov/ncea/efh [Google Scholar]
- U.S. Environmental Protection Agency (EPA) . (2020). Soil sampling operating procedure. Retrieved from https://www.epa.gov/sites/default/files/2015-06/documents/Soil-Sampling.pdf
- U.S. Environmental Protection Agency (EPA) . (2023). Westside lead site profile. Retrieved from https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.Cleanup&%20id=0407160#bkground
- Vangronsveld, J. , Assche, F. V. , & Clijsters, H. (1995). Reclamation of a bare industrial area contaminated by non‐ferrous metals: In situ metal immobilization and revegetation. Environmental Pollution, 87(1), 51–59. 10.1016/s0269-7491(99)80007-4 [DOI] [PubMed] [Google Scholar]
- Wu, L. H. , Luo, Y. M. , Xing, X. R. , & Christie, P. (2004). EDTA‐enhanced phytoremediation of HMM contaminated soil with Indian mustard and associated potential leaching risk. Agriculture, Ecosystems & Environment, 102(3), 307–318. 10.1016/j.agee.2003.09.002 [DOI] [Google Scholar]
- Xiong, Z. (1998). Heavy metal contamination of urban soils and plants in relation to traffic in Wuhan city, China. Toxicological and Environmental Chemistry, 65(1–4), 31–39. 10.1080/02772249809358555 [DOI] [Google Scholar]
- Xiong, Z.‐T. , Zhao, F. , & Li, M. (2006). Lead toxicity in Brassica pekinensis Rupr.: Effect on nitrate assimilation and growth. Environmental Toxicology, 21(2), 147–153. 10.1002/tox.20167 [DOI] [PubMed] [Google Scholar]
- Yadav, S. K. , Juwarkar, A. A. , Kumar, G. P. , Thawale, P. R. , Singh, S. K. , & Chakrabarti, T. (2009). Bioaccumulation and phyto‐translocation of arsenic, chromium and zinc by Jatropha Curcas L.: Impact of dairy sludge and biofertilizer. Bioresource Technology, 100(20), 4616–4622. 10.1016/j.biortech.2009.04.062 [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Huang, H. , Tan, F. , Wang, H. , & Qiu, R. (2010). Influence of EDTA washing on the species and mobility of HMM residual in soils. Journal of Hazardous Materials, 173(1–3), 369–376. 10.1016/j.jhazmat.2009.08.087 [DOI] [PubMed] [Google Scholar]
- Zhou, J. , Chen, L. H. , Peng, L. , Luo, S. , & Zeng, Q. R. (2020). Phytoremediation of heavy metals under an oil crop rotation and treatment of biochar from contaminated biomass for safe use. Chemosphere, 247, 125856. 10.1016/j.chemosphere.2020.125856 [DOI] [PubMed] [Google Scholar]
- Zou, T. , Li, T. , Zhang, X. , Yu, H. , & Huang, H. (2011). Lead accumulation and phytostabilization potential of dominant plant species growing in a lead–zinc mine tailing. Environmental Earth Sciences, 65(3), 621–630. 10.1007/s12665-011-1109-6 [DOI] [Google Scholar]
- Zulfiqar, U. , Farooq, M. , Hussain, S. , Maqsood, M. , Hussain, M. , Ishfaq, M. , et al. (2019). Lead toxicity in plants: Impacts and remediation. Journal of Environmental Management, 250, 109557. 10.1016/j.jenvman.2019.109557 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Saikawa, E. , & Yao, X. (2023). GeoHealth_Pb_Phytoremediation_dataset [Dataset]. Mendeley Data, V1. 10.17632/ysdtp3xkr9.2 [DOI]
Data Availability Statement
All the data are available in the open‐access public repository in Mendeley Data. Saikawa and Yao (2023), https://doi.org/10.17632/ysdtp3xkr9.2.


