Abstract
Coxsackievirus and adenovirus receptor (CAR) from which the cytoplasmic domain had been deleted and glycosylphosphatidylinositol (GPI)-anchored CAR lacking both transmembrane and cytoplasmic domains were both capable of facilitating adenovirus 5-mediated gene delivery and infection by coxsackievirus B3. These results indicate that the CAR extracellular domain is sufficient to permit virus attachment and entry and that the presence of a GPI anchor does not prevent infection.
Coxsackie B viruses and adenoviruses 2 and 5 initiate infection by attaching to the coxsackievirus and adenovirus receptor (CAR) (2, 25). CAR is a 46-kDa protein composed of an extracellular domain containing two disulfide-linked loops, a typical hydrophobic transmembrane domain, and a 107-amino-acid-long cytoplasmic domain. The murine CAR homolog also functions as a receptor for both viruses (3, 25). Human and murine CAR proteins show 91% amino acid identity within the extracellular domain, 77% identity within the transmembrane domain, and 95% identity within the cytoplasmic domain. CAR’s cellular function has not been determined, but the high degree of sequence conservation suggests a significant role for the cytoplasmic domain. Whether the cytoplasmic domain is important for virus internalization and infection is not known.
All coxsackie B viruses tested so far use CAR as a cellular receptor (19a), but some coxsackie B viruses bind to an additional receptor, decay-accelerating factor (DAF) (4, 22). Although virus attachment to CAR on transfected rodent cells leads to productive infection, attachment to DAF does not, indicating that DAF-transfected cells are deficient in a postattachment function essential for virus replication. DAF is distinctive among identified virus receptor proteins in that it lacks typical transmembrane and cytoplasmic domains and is linked directly to the outer leaflet of the cell membrane by a glycolipid (glycosylphosphatidylinositol [GPI]) anchor (9, 19). GPI-anchored proteins are localized in distinct membrane microdomains (6), and several studies indicate that GPI-anchored and transmembrane proteins are internalized by different routes (1, 16). Whether the DAF glycolipid anchor is a barrier to coxsackievirus infection has not been determined.
Adenovirus infection involves the attachment of the viral fiber to a primary receptor, such as CAR; virus internalization is facilitated by a subsequent interaction between the viral penton base and cell surface integrins (αvβ3 and αvβ5) (29). On cells that lack fiber receptors, penton base-mediated attachment to other cell surface proteins may permit virus uptake (15). In addition, modifications of the adenovirus fiber that permit attachment to other proteins allow adenoviruses to enter cells by a CAR-independent route (30). The observation that virus attachment to other molecules may substitute for interaction with CAR suggests that CAR may not function in postattachment events in infection. If CAR functions primarily in virus attachment, CAR structures not directly involved in attachment may be dispensable.
In these studies we examined whether the highly conserved CAR cytoplasmic domain is essential for virus entry and infection. We also examined whether infection requires the CAR transmembrane domain or whether CAR expressed with the DAF glycolipid anchor can also promote infection by coxsackie B viruses and adenoviruses.
CAR mutants that lack the cytoplasmic and transmembrane domains.
To assess the roles of the transmembrane and cytoplasmic domains in virus infection, we engineered two truncated forms of human CAR (hCAR) (Fig. 1). CAR with a deletion of the cytoplasmic domain (tailless CAR) was obtained by the use of PCR mutagenesis to insert a stop codon within CAR cDNA at the position corresponding to amino acid 261 (2). To delete both the cytoplasmic and transmembrane domains, we used splice overlap extension PCR (13, 32) to fuse the CAR extracellular domain to the 37 carboxy-terminal amino acids of human DAF, which are known to contain signals that permit proteolytic cleavage and attachment to a glycolipid anchor (7). This construct is referred to as GPI-CAR.
FIG. 1.
Truncated CAR molecules lacking transmembrane and cytoplasmic domains. Wild-type CAR consists of an extracellular domain, a transmembrane (Tm) domain, and a cytoplasmic domain. Based on N-terminal sequencing of the mature protein (8), cleavage of the signal peptide occurs between amino acids 19 and 20 (numbered as in reference 2). Tailless CAR consists of amino acids 1 to 260, i.e., from MALL to IFCC. GPI-CAR consists of CAR amino acids 1 to 235, i.e., from MALL to PSNK, fused to the 37 C-terminal amino acids of DAF, i.e., from PNKG to GLLY. Ig, immunoglobulin.
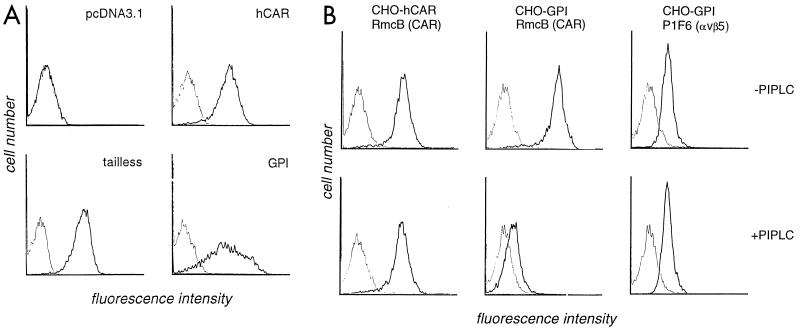
Wild-type and truncated CAR cDNAs were inserted into the EcoRI and XbaI sites of the expression vector pcDNA3.1 (Invitrogen, Carlsbad, Calif.). Each CAR cDNA, or empty pcDNA3.1 used as a negative control, was cotransfected into dihydrofolate reductase-deficient CHO cells with a plasmid encoding dihydrofolate reductase, and transfectants were selected in nucleoside-free medium as previously described (11). Cell populations with surface CAR expression were isolated by fluorescence-activated cell sorting with the anti-CAR monoclonal antibody (MAb) RmcB (14) (Fig. 2A).
FIG. 2.
Expression of CAR on transfected CHO cells. (A) Flow cytometry. CHO cells transfected with cDNA encoding wild-type hCAR or truncated CAR (tailless and GPI), and control cells transfected with the empty pcDNA3.1 vector, were incubated first with MAb RmcB (heavy line) or with a control antibody (MOPC 195 [thin line]) and then with fluorescein isothiocyanate-conjugated goat antibody to mouse immunoglobulin. All panels are shown on the same scale. (B) Flow cytometry after PIPLC treatment. Transfected CHO cells were incubated for 30 min at 37°C in RPMI 1640 supplemented with 0.2% bovine serum albumin, 50 μM 2-mercaptoethanol, 10 mM HEPES (pH 7.0), and 0.1% sodium azide, with or without the addition of PIPLC (0.4 U per million cells; Sigma). Cells were then washed and stained with MAb RmcB, MOPC 195, or MAb P1F6, which recognizes the integrin αvβ5. All panels are shown on the same scale. CAR expression on GPI-CHO cells was reduced 30-fold after PIPLC treatment.
GPI-anchored proteins are released from the cell membrane by the action of a specific enzyme, phosphatidylinositol-specific phospholipase C (PIPLC) (9, 19). To confirm that GPI-CAR was in fact linked to the membrane by a glycolipid anchor, transfected cells were incubated with PIPLC before flow cytometry analysis (Fig. 2B). As expected, PIPLC treatment significantly reduced the level of CAR expression on GPI-CAR transfectants but did not diminish CAR expression on CHO cells transfected with wild-type CAR. Control cells and CAR transfectants all expressed the integrin αvβ5, as determined by staining with MAb P1F6 (27) (Chemicon, Temecula, Calif.). As expected, the expression of this transmembrane protein was not affected by treatment with PIPLC.
Adenovirus and coxsackievirus infection of transfected CHO cells.
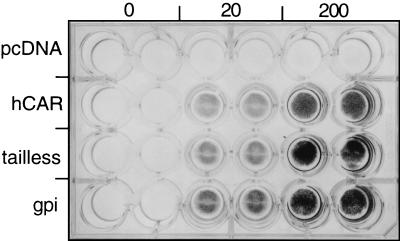
To determine their susceptibility to adenovirus entry, we exposed CHO cell monolayers to adenovirus 5 engineered to encode β-galactosidase (20 and 200 PFU/cell; 1 h at room temperature) and then washed and incubated the cells at 37°C for 48 h. β-Galactosidase expression was measured by in situ staining with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (Fig. 3) and by a quantitative β-galactosidase assay performed on cell lysates (data not shown). Adenovirus-mediated gene delivery was equally efficient in cells transfected with wild-type CAR, tailless CAR, and GPI-CAR, indicating that the cytoplasmic and transmembrane domains are not required for adenovirus entry.
FIG. 3.
Adenovirus-mediated gene transfer. Duplicate monolayers of CHO-hCAR, CHO-tailless CAR, CHO-GPI-CAR, or CHO-pcDNA3.1 were exposed to Ad.CMV.β-gal (0, 20, or 200 PFU per cell) for 1 h at room temperature and then washed. After incubation for 2 days at 37°C, β-galactosidase activity was detected by in situ staining with X-Gal.
Radiolabeled coxsackievirus B3 (Nancy) bound equally well to CHO cells expressing wild-type CAR, tailless CAR, or GPI-CAR (data not shown). To measure susceptibility to coxsackievirus infection, we exposed monolayers of transfected CHO cells to virus (1 PFU/cell) in 24-well plates for 1 h at room temperature. Monolayers were washed three times to remove unbound virus and then incubated at 37°C for 1, 24, or 48 h. Monolayers were frozen and thawed to release virus, and virus titers were determined by plaque assay as described previously (5). CHO cells expressing tailless CAR and GPI-CAR became infected, as demonstrated by viral cytopathic effect (data not shown) and by an increase in virus titer (Fig. 4A); no cytopathic effect or virus replication was observed in control transfectants. These results indicate that neither the CAR cytoplasmic domain nor the transmembrane domain is essential for coxsackievirus infection.
FIG. 4.
Coxsackievirus B3 replication. (A) Infection mediated by truncated CAR. CHO-hCAR, CHO-tailless CAR, CHO-GPI-CAR, or CHO-pcDNA3.1 monolayers were exposed to coxsackievirus B3 (Nancy) (obtained from Richard Crowell; 5 PFU per cell) for 1 h at room temperature, washed four times to remove unbound virus, and incubated at 37°C for 1 h (0 days), 1 day, or 2 days. Monolayers were frozen and thawed to release virus, and then plaque assays were performed. The mean virus titers for duplicate cultures are shown. (B) Infection mediated by transmembrane DAF. CHO-hCAR monolayers, CHO-pcDNA3.1 monolayers, or CHO-DAF-Tm cells were exposed to DAF-binding coxsackievirus B3 RD (4) (3 PFU per cell), and then incubations and plaque assays were performed as described above.
Infection of GPI-CAR cells by coxsackievirus B3 (Nancy) suggested that the GPI anchor does not account for the block to productive infection previously observed in DAF-transfected CHO (CHO-DAF) cells (4, 22). To confirm this, we tested whether expression of DAF as a transmembrane protein could render CHO cells susceptible to infection by coxsackievirus B3 RD, a virus isolate that binds efficiently to DAF (4). We used an available CHO cell line (CHO-DAF-Tm [18]) expressing the extracellular portion of DAF fused to the transmembrane and cytoplasmic domains of membrane cofactor protein. In a preliminary experiment we confirmed that radiolabeled coxsackievirus B3 RD bound to monolayers of CHO-DAF-Tm cells as efficiently as it bound to wild-type DAF transfectants (47,000 cpm was added; CHO-DAF-Tm, 24,804 cpm bound; CHO-DAF, 21,506 cpm bound; CHO mock transfectant, 88 cpm bound). When CHO-DAF-Tm cells were exposed to coxsackievirus B3 RD, no viral cytopathic effects and no increase in viral titer were seen (Fig. 4B). Thus cells transfected with transmembrane DAF, like those transfected with GPI-anchored DAF (4, 22), are deficient in some postattachment function required for virus replication.
Discussion.
These results show that the CAR extracellular domain is sufficient for attachment and internalization of both coxsackie B viruses and adenoviruses. Expression of CAR with a deletion of its cytoplasmic domain, or of GPI-anchored CAR with deletions of both the transmembrane and cytoplasmic domains, promoted virus infection of transfected CHO cells.
Although some coxsackie B virus strains bind to DAF-transfected rodent cells, productive infection does not occur (4, 22). The fact that DAF is a GPI-anchored (rather than a transmembrane) protein does not explain the postattachment block to infection. The expression of CAR with a GPI anchor did not abolish its capacity to mediate infection, and the expression of DAF with a transmembrane anchor did not permit infection to proceed beyond the attachment stage. Transmembrane and GPI-anchored proteins have been reported to enter cells by different routes (1, 16), but such differences do not explain the failure of coxsackieviruses to infect CHO-DAF cells. Rhinovirus, another human picornavirus, infects cells expressing an engineered GPI-anchored form of the rhinovirus receptor (24), and similar results have been obtained with human immunodeficiency virus (10), measles virus (26), and subgroup A avian leukosis virus (33). As has been proposed for echovirus 7 (21), coxsackievirus B3 infection of CHO-DAF cells may be blocked because interaction with DAF does not trigger viral uncoating.
The role of DAF in coxsackievirus infection is not clearly defined. Hsu and colleagues suggested that coxsackievirus B3 RD interaction with DAF on RD rhabdomyosarcoma cells is sufficient for productive infection (14). In contrast, other investigators reported that attachment of another virus strain to DAF on RD cells does not lead to infection in the absence of CAR (23). Even if it functions only in attachment, DAF may enhance susceptibility to infection by concentrating virus particles at the cell surface and facilitating interaction with CAR.
The mechanism by which picornaviruses enter cells (or release infectious RNA into the cytoplasm) is poorly understood, but adenovirus entry is somewhat better defined. After attachment to a primary receptor such as CAR, adenoviruses are internalized in clathrin-coated vesicles (20), and within an endosomal compartment, the virion is dismantled, resulting in the eventual release of the viral genome and its transport to the nucleus (12). Virus internalization (29) and endosomal disruption (28) are facilitated by interactions between the virus and αv integrins on the cell surface. Integrin-dependent signaling events—including the activation of phosphoinositide-3-OH kinase—appear to be important for virus entry (17).
Our results suggest that if CAR-dependent signals are involved in virus entry their transmission does not require the CAR cytoplasmic and transmembrane domains. The demonstration that these CAR domains are not essential is consistent with experiments in which adenovirus attachment to cell surface molecules other than CAR, accomplished by modification of the viral fiber (30) or the use of bispecific bridging antibodies (31), permits adenovirus entry and adenovirus-mediated gene delivery. Although these data do not exclude the possibility that CAR-mediated signals occur or are important in virus delivery, it is possible that CAR functions solely as an attachment molecule and that once adenovirus has attached to CAR or any other cell surface molecule, subsequent events in entry are mediated entirely by integrins or other secondary receptors.
Acknowledgments
We thank JenniElizabeth Petrella and Mariam Rahman for technical assistance, Richard Lublin for DAF cDNA and CHO-DAF-Tm cells, Scott Baldwin for Ad.CMV.β-gal, and Susan Coffin and Paul Bates for comments on the manuscript.
This work was supported by grants from the NIH (AI35667 and HL54734). J.M.B. is an Established Investigator of the American Heart Association.
ADDENDUM IN PROOF
Leon et al. (Proc. Natl. Acad. Sci. USA 95:13159–13164, 1998) have also reported that tailless CAR permits adenovirus-mediated gene delivery.
REFERENCES
- 1.Bamezi A, Goldmacher V, Rock K. Internalization of glycosyl-phosphatidylinositol (GPI)-anchored lymphocyte proteins. II. GPI-anchored and transmembrane molecules internalize through distinct pathways. Eur J Immunol. 1992;22:15–21. doi: 10.1002/eji.1830220104. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson J M, Mohanty J G, Crowell R L, St. John N F, Lublin D M, Finberg R W. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55) J Virol. 1995;69:1903–1906. doi: 10.1128/jvi.69.3.1903-1906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergelson J M, St. John N, Kawaguchi S, Chan M, Stubdal H, Modlin J, Finberg R W. Infection by echoviruses 1 and 8 depends on the α2 subunit of human VLA-2. J Virol. 1993;67:6847–6852. doi: 10.1128/jvi.67.11.6847-6852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown D A, Rose J K. Sorting of GPI-anchored glycoproteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 7.Caras I W, Weddell G N, Williams S R. Analysis of the signal for attachment of a glycophospholipid membrane anchor. J Cell Biol. 1989;108:1387–1396. doi: 10.1083/jcb.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson S D, Chapman N N, Tracy S M. Purification of the putative coxsackievirus B receptor from HeLa cells. Biochem Biophys Res Commun. 1997;233:325–328. doi: 10.1006/bbrc.1997.6449. [DOI] [PubMed] [Google Scholar]
- 9.Davitz M A, Low M G, Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement-regulatory protein. J Exp Med. 1986;163:1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond D C, Finberg R, Chaudhuri S, Sleckman B P, Burakoff S J. Human immunodeficiency virus infection is efficiently mediated by a glycolipid-anchored form of CD4. Proc Natl Acad Sci USA. 1990;87:5001–5005. doi: 10.1073/pnas.87.13.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giancotti F, Ruoslahti E. Elevated levels of the α5β1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 12.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 13.Horton R M, Cai Z, Ho S N, Pease L R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- 14.Hsu K-H L, Lonberg-Holm K, Alstein B, Crowell R L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller G-A, Siegel M, Caras I W. Endocytosis of glycophospholipid-anchored and transmembrane forms of CD4 by different endocytic pathways. EMBO J. 1992;11:863–874. doi: 10.1002/j.1460-2075.1992.tb05124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li E, Stupack D, Klemke R, Cheresh D A, Nemerow G R. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lublin D M, Coyne K E. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J Exp Med. 1991;174:35–44. doi: 10.1084/jem.174.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medof M E, Walter E I, Roberts W L, Haas R, Rosenberry T L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986;25:6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- 19a.Modlin, J., R. Finberg, and J. Bergelson. Unpublished data.
- 20.Pastan I, Seth P, FitzGerald D, Willingham M. Adenovirus entry into cells: some new observations on an old problem. In: Notkins A, Oldstone M, editors. Concepts in viral pathogenesis. New York, N.Y: Springer-Verlag; 1987. pp. 141–146. [Google Scholar]
- 21.Powell R M, Ward T, Evans D J, Almond J W. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J Virol. 1997;71:9306–9312. doi: 10.1128/jvi.71.12.9306-9312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafren D R, Bates R C, Agrez M V, Herd R L, Burns G F, Barry R D. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3877. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafren D R, Williams D T, Barry R D. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J Virol. 1997;71:9844–9848. doi: 10.1128/jvi.71.12.9844-9848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staunton D, Gaur A, Chan P-Y, Springer T. Internalization of a major group human rhinovirus does not require cytoplasmic or transmembrane domains of ICAM-1. J Immunol. 1992;148:3271–3274. [PubMed] [Google Scholar]
- 25.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varior-Krishnan G, Trescol-Biémont M-C, Naniche D, Rabourdin-Combe C, Gerlier D. Glycosyl-phosphatidylinositol-anchored and transmembrane forms of CD46 display similar measles virus receptor properties: virus binding, fusion, and replication; down-regulation by hemagglutinin; and virus uptake and endocytosis for antigen presentation by major histocompatibility complex class II molecules. J Virol. 1994;68:7891–7899. doi: 10.1128/jvi.68.12.7891-7899.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinacker A, Chen A, Agrez M, Cone R I, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin αvβ6 in cell attachment to fibronectin: heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- 28.Wickham T J, Filardo E J, Cheresh D A, Nemerow G R. Integrin αvβ5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 30.Wickham T J, Roelvink P W, Brough D E, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to different cell types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 31.Wickham T J, Segal D M, Roelvink P W, Carrion M E, Lizonova A, Lee G M, Kovesdi I. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yon J, Fried M. Precise gene fusion by PCR. Nucleic Acids Res. 1989;17:4895. doi: 10.1093/nar/17.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zingler K, Bélanger C, Peters R, Agard D, Young J A T. Identification and characterization of the viral interaction determinant of the subgroup A avian leukosis virus receptor. J Virol. 1995;69:4261–4266. doi: 10.1128/jvi.69.7.4261-4266.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]