Abstract
The emergence of immunotherapy has revolutionized the traditional treatment paradigm of colorectal cancer (CRC). Among them, immune checkpoint blockade has become the first-line treatment for metastatic colorectal cancer (mCRC) and has made significant progress in the treatment of locally advanced colorectal cancer (LACRC). We reviewed a series of clinical trials that have made breakthrough progress. We will emphasize the breakthrough progress in achieving organ preservation in patients with high microsatellite instability or DNA mismatch repair deficiency (MSI-H/dMMR), and based on this, we propose the concept of selective surgery, which includes selectively removing or preserving lymph nodes, with the aim of proving our idea through more research in the future.
Keywords: Immunotherapy, Colorectal cancer, Locally advanced rectal cancer, Organ preservation, Lymph nodes tracing, Selective surgery
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related deaths globally and the second leading cause of cancer-related deaths worldwide. It is considered a global health problem that requires new treatment strategies to meet urgent unmet needs [1,2]. Although screening has become more widespread and significantly reduced incidence and mortality, 20 % of newly diagnosed CRC patients present with metastatic disease, and an additional 25 % present with localized disease that subsequently progresses to distant metastasis.
The current standard treatment for CRC includes surgery, adjuvant chemotherapy and radiotherapy, as well as systemic therapy using chemotherapy and targeted drugs. Surgical treatment is the cornerstone of early-stage colorectal cancer treatment. Among them, targeted therapy refers to the treatment method that targets specific proteins or pathways that are overexpressed or abnormally expressed in colorectal cancer cells. Studies have shown that in patients with KRAS/NRAS/BRAF wild-type metastatic colorectal cancer (mCRC), the use of cetuximab and panitumumab in combination with chemotherapy can prolong median survival time (MST) [[3], [4], [5]]. For patients with BRAF V600E sequence mutations [[6], [7], [8]], targeted combination therapy using BRAF and EGFR inhibitors can prolong overall survival (OS) [7,8].
Despite reaching a plateau in the benefits of chemotherapy and targeted therapy, there is an urgent need to develop new effective treatment strategies to improve survival outcomes. Immunotherapy has become one of the most popular treatments in recent years. By utilizing the patient's own immune system to attack cancer cells, immunotherapy includes checkpoint inhibitors, monoclonal antibodies, chimeric antigen receptor-modified T (CAR-T) cells, and cancer vaccines. Immune checkpoint inhibitors (ICIs) can inhibit the activation and suppression signals of immune cells, thereby enhancing the immune cells' attack on cancer cells [9]. Pembrolizumab and nivolumab (used alone or in combination with ipilimumab) have been approved by the US Food and Drug Administration (FDA) as first-line treatment for mCRC patients due to their high efficacy, stability, and durability [[10], [11], [12], [13], [14]]. In this review, we summarize the existing evidence supporting the use of ICIs in CRC, with a focus on the latest clinical trials expanding the use of ICIs in mCRC and locally advanced CRC (LACRC). Immunotherapy has made a significant breakthrough in achieving organ preservation for some patients with locally advanced rectal cancer (LARC) to reach complete clinical response (cCR) status.
Based on these, we propose a promising vision for future CRC surgery with selective lymph node clearance or preservation, which is technically and theoretically supported. We look forward to more breakthroughs in the treatment of CRC in the future, making treatment more personalized and tailored to individual needs.
The impact of immunotherapy on the treatment paradigm of CRC
Studies have shown that mCRC is the main cause of cancer-related death, with a five-year survival rate of less than 15% [15]. Although conventional surgery, chemotherapy, and radiotherapy have improved patient survival to some extent, there are still many cases of recurrence and metastasis, indicating that current treatment strategies still have limitations. In recent years, the development of immunotherapy, particularly ICIs, has brought new hope for the treatment of CRC.
Application of ICIs
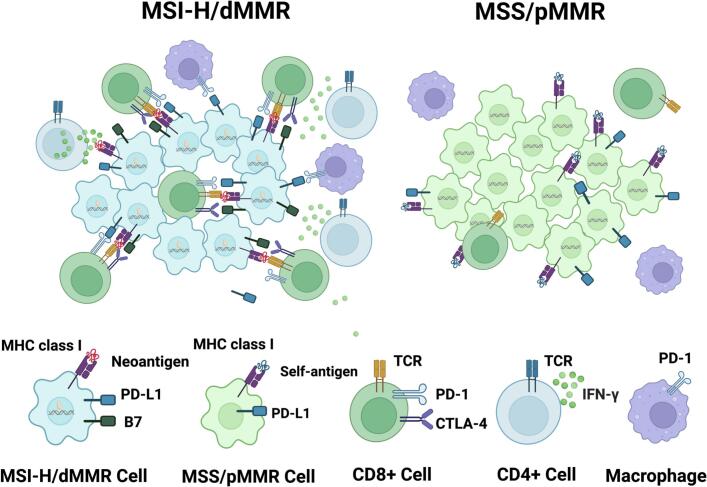
ICIs are a new cancer treatment modality that can effectively attack cancer cells by releasing the immune system's brakes. Numerous studies have shown that ICIs have better therapeutic effects on microsatellite instability-high (MSI-H)/deficient DNA mismatch repair (dMMR) mCRC. However, 95 % of mCRC patients belong to the microsatellite-stable (MSS)/proficient DNA mismatch repair (pMMR) subtype, which is insensitive to immunotherapy [16,17]. These patients are prone to DNA replication errors, frameshift mutations, and have reduced immune escape mechanisms, resulting in an increase in tumor mutation burden (TMB) and the production of abnormal proteins or neoantigens that can be recognized and activated by the immune system [32,33] (Fig. 1). ICIs have made significant breakthroughs in this patient population (Table 1), gradually shifting from second-line to first-line treatment.
Fig. 1.
Comparison between MSI-H/dMMR and MSS/pMMR CRC. MSI-H/dMMR CRC is characterized by a high mutational burden and continuous generation of neoantigens, which are favorable for immune surveillance. Neoantigens are presented by MHC class I molecules and recruit CD8+ T cells to the tumor microenvironment via interaction with T cell receptor (TCR). However, immune checkpoint proteins expressed on the surface of T cells, such as PD-1/PD-L1 and CTLA-4/B7, can attenuate antitumor immune responses by interacting with their ligands on antigen-presenting cells. In contrast, MSS/pMMR CRC has a lower mutational burden and lacks immune surveillance.
Table 1.
Representative clinical trials supporting checkpoint inhibitor use in MSI-H mCRC.
| Trials | Phase | Therapy | Outcome | Significance |
|---|---|---|---|---|
| KETNOTE016 | II | Pembrolizumab (PD-1) | ORR: 40 % | A new era of immunotherapy |
| KEYNOTE177 | III | Pembrolizumab (PD-1) | PFS: 16.5 m ORR: 45.1 % |
First-line: PD-1 monotherapy |
| CheckMate142 | II | Nivolumab±ipilimumab (PD-1 + CTLA-4) | 12mORR: 60 % 12 m PFS: 77 % OS: 83 % |
First-line: PD-1 + CTLA-4 |
PD-1 = programmed cell death 1; CTLA-4 = cytotoxic T-lymphocyte-associated protein 4; mCRC = metastatic CRC; MSI-H = microsatellite instability high; ORR = overall response rate; PFS = progression-free survival; OS = overall survival; m = month.
Two ICIs (pembrolizumab and nivolumab) have been approved by the FDA as the new standard of care for first-line treatment of MSI-H mCRC and have been designated as the standard of care for MSI-H/dMMR CRC by the FDA [18].
In 2015, a clinical phase II study named KEYNOTE-016 was presented at the American Society of Clinical Oncology (ASCO) annual meeting [19]. The study found that the objective response rate (ORR) was 40 % in MSI-H/dMMR CRC patients, compared to 0 % in non-MSI-H/pMMR CRC patients. This study marked the beginning of immune therapy for MSI-H/dMMR CRC, providing a promising new treatment option for this challenging disease.
In 2020, pembrolizumab was approved by the FDA as the first monotherapy PD-1 inhibitor for first-line treatment of MSI-H/dMMR CRC patients with unresectable or metastatic disease [20], marking significant progress in the treatment of this disease. Based on the KEYNOTE-177 trial, this is the first drug to be approved by the FDA for first-line treatment of MSI-H/dMMR CRC, providing a promising new treatment option for mCRC patients. The 2023 CSCO guidelines update recommends dual immunotherapy for MSI-H/dMMR patients based on the available evidence from ipilimumab and the CheckMate-142 study in China [21]. The study recommends dual immunotherapy as first-line treatment for advanced MSI-H/dMMR patients, but with a low recommendation level (expert consensus III).
Meanwhile, for MSS (microsatellite-stable)/pMMR (typical mismatch repair) CRC, single immunotherapy (such as monoclonal antibodies, cancer vaccines, etc.) cannot significantly improve patient survival and treatment outcomes (Fig. 1). Insufficient mutation neoantigens, immune evasion, and neovascularization are the reasons for the poor efficacy [22,23]. However, some studies have shown that combining immunotherapy with other treatments, such as chemotherapy or radiotherapy, may enhance the efficacy of immunotherapy in this patient population [24,25].
Adopting novel immunotherapies
CAR-T cell therapy (chimeric antigen receptor T cell therapy) is a treatment that modifies a patient's T cells into “biological weapons” that can recognize and attack cancer cells. Recent studies have shown that CAR-T cell therapy targeting CRC-related antigens such as CEA (carcinoembryonic antigen) and GUCY2C (guanylate cyclase 2C) has demonstrated good safety and efficacy in clinical trials [26].
Study of the immune microenvironment
The immune microenvironment, including tumor cells, immune cells, vascular cells, and extracellular matrix, plays an important role in the occurrence, development, and treatment response of tumors [27,28]. In recent years, researchers have found that tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), regulatory T cells (Tregs), and other immune cells play a promoting role in the development of CRC, while the activity of anti-tumor immune cells such as cytotoxic T cells and natural killer cells (NK cells) is often inhibited [[29], [30], [31]]. By intervening in the proportion and function of these immune cells, the efficacy of immunotherapy can be improved.
Study of gene vaccines
Gene vaccines are a new type of immunotherapy that activates or enhances the body's immune response to tumors by injecting genes containing tumor antigens or enhancing immune response. Some studies have shown that gene vaccines targeting CRC-related antigens such as CEA and MUC1 can improve the efficacy of immunotherapy [32]. Some studies have also explored different delivery methods for gene vaccines, such as viral vectors and liposomes, to improve the immunogenicity and biological stability of gene vaccines [33].
Immunotherapy targeting TdLNs
In recent years, more and more studies have shown that tumor-associated lymph nodes (TdLNs) in the tumor microenvironment have a strong therapeutic response to ICIs [34]. Therefore, some new clinical trials have begun to explore the possibility of selectively removing or preserving TdLNs to improve the efficacy of immunotherapy.
In summary, immunotherapy has made significant progress in the treatment of CRC, but further research is still needed. Although the MSS subtype of colorectal cancer has a lower response to single immunotherapy, combination immunotherapy may have some therapeutic effect. Future studies are needed to explore the application of combination immunotherapy in MSS colorectal cancer to improve treatment outcomes and survival in patients.
Exploration of ICIs in LACRC
LACRC refers to cancer that has spread to adjacent tissues or lymph nodes before surgical treatment. Immunotherapy has opened up new treatment options for these patients. Among them, neoadjuvant immunotherapy (nIT) [35], which involves administration of ICIs before surgery, has shown promising results, including reducing tumor staging, increasing R0 resection rates, reducing local recurrence rates, improving prognosis, and even achieving complete remission or pathological complete remission (pCR) in some patients [21,[36], [37], [38], [39], [40], [41]]. This provides more opportunities for subsequent surgical treatment and injects hope into LACRC patients. In addition, studies have shown that nIT is well-tolerated in LACRC patients, with only mild immune-related adverse events (irAEs) such as fever, fatigue, nausea, vomiting, and rash. Although further research is needed to determine its efficacy and safety in larger patient populations, nIT has the potential to become a more effective and less toxic treatment option for LACRC patients.
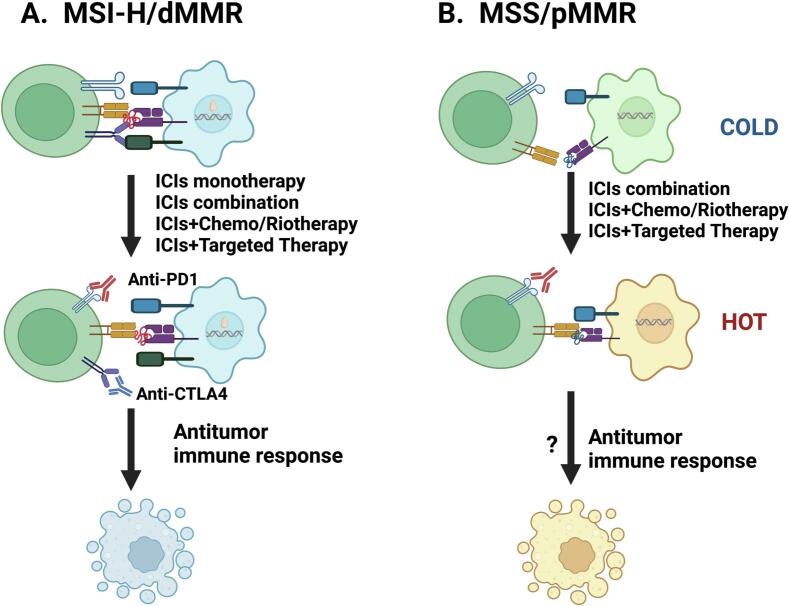
Overall, ICIs have made significant breakthroughs in the treatment of LACRC patients, with representative clinical trials shown in Table 2. To further improve treatment outcomes, various strategies based on ICIs have been developed, such as combining ICIs with radiotherapy, chemotherapy, and targeted therapy (Fig. 2A).
Table 2.
Representative clinical trials supporting checkpoint inhibitor use in CRC.
| Trials | Phase | Patients | Therapy | Outcome | Significance |
|---|---|---|---|---|---|
| NICHE | II | 32 | Nivolimab+ipilimumab | pCR: 69 % | The first nIT in early stage CRC |
| PICC (NCT03926338) | II | 17vs17 | Toripalimab vs Toripalimab+celecoxib | pCR: 65% vs 88% | Filled the gap in the field of nIT for MSI-H/dMMR LACRC |
| NCT04165772 | II | 14 | Dostarlimab | cCR: 100 % | Unprecedented 100 % cCR |
| NCT04304209 | II | 17 | Sintilimab | CR: 75 % | Achieving organ preservation |
| REGONIVO (NCT03406871) | Ib | 24 MSS/pMMR | Nivolimab+regorafenib | ORR: 33.3 % 1yPFS: 41.8 % 1yOS: 68 % |
The first phase Ib study exploring in MSS CRC |
| REGOTORI (NCT03946917) | Ib/II | 42MSS/pMMR/MSI-L Advanced CRC |
Regorafenib+teriparatide | ORR: 2.36 % DFS: 4.80 % mPFS: 2.1 m mOS: 15.5 m |
The first domestically conducted study of ICIs combined with anti-VEGF therapy. |
cCR = clinical complete remission; pCR = pathological complete remission; CR = complete response; ORR = overall response rate; PFS = progression-free survival; OS = overall survival; y = year; m = month; mOS: median overall survival; mPFS: median progression-free survival.
Fig. 2.
In CRC tumors, the combination of ICIs with other treatment modalities can enhance the anti-tumor treatment efficacy.
A. MSI-H/dMMR CRC patients have an immune system that is more capable of recognizing and attacking tumor cells than MSS/pMMR CRC patients, due to their higher mutational burden and expression of more neoantigens. Monotherapy with ICIs has become one of the first-line treatment options for MSI-H/dMMR CRC patients, but combination therapy with other treatment modalities such as chemotherapy, radiation therapy, and targeted therapy is also being studied and developed to further enhance its therapeutic efficacy.
B. On the other hand, the efficacy of monotherapy with ICIs is limited for MSS/pMMR CRC patients. Clinical trials are underway to develop combination therapy of ICIs with other treatment modalities. Combination therapy of ICIs with chemotherapy (e.g. FOLFOX, CAPEOX) has been shown to have some efficacy. In addition, combination therapy of ICIs with radiation therapy and targeted therapy is also widely studied. Combination therapy may enhance tumor immunogenicity by boosting the immune system response and promoting tumor cell apoptosis, thereby improving treatment efficacy.
ICIs in CRC patients with MSI-H/dMMR
Single-agent immunotherapy with ICIs
In a study conducted by Zhang et al. [42], two LARC patients with dMMR/MSI achieved complete remission with nivolumab alone in nIT. One of the patients underwent surgery after 6 cycles of nivolumab and achieved pCR, while the other patient achieved cCR. This is the first report of the use of anti-PD-1 antibody monotherapy as a neoadjuvant treatment for dMMR LARC.
Ding et al. [43] conducted a retrospective analysis of eight patients with MSI-H/dMMR LACRC who received treatment with pembrolizumab or nivolumab in China. All eight patients enrolled had a significant response on imaging and/or pathological assessment, with five of the seven patients who underwent surgery being assessed as pCR and one non-operated patient achieving cCR and then adopting a “w&w” treatment strategy was adopted for the one non-operated patient who achieved a cCR. More recently, Ding's latest study [44] showed that in 58 patients treated with PD-1 blockade monotherapy, final response rates and pCR/cCR were 87.9 % and 60.3 % respectively, with no difference from combination therapy. These results suggest that neoadjuvant therapy with PD-1 blockade alone is sufficient to achieve high response rates in localized MSI-H/dMMR CRC and may be as effective as combination therapy.
The results of the PICC study [45] suggest that patients in the toripalimab combined with celecoxib group had a pCR rate of 88 %, compared to 65 % in the toripalimab monotherapy group. This study is the first prospective clinical trial in the world to report preoperative nIT using anti-PD-1 monoclonal antibody-based, monotherapy or in combination with COX-2 inhibitors for MSI-H/dMMR in patients with locally progressive CRC.
A neoadjuvant monotherapy trial [38] targeting MSI-H LACRC was conducted in China. Eighteen MSI-H/dMMR LACRC patients received pembrolizumab treatment, and all patients (100 %) achieved significant tumor shrinkage. Five LARC patients underwent surgery, and all were diagnosed with pCR. LARC patients received “w&w” treatment after achieving cCR.
A groundbreaking study showed that monotherapy with PD-1 blockade for MSI-H/dMMR LARC patients achieved cCR in 100 % of patients after 6 months of treatment [35]. Their unprecedented results and 100 % clinical complete remission suggest that for some rectal cancers (such as low-grade rectal cancer), it may surpass standard treatment and require organ preservation.
A recent study [46] investigated the use of sintilimab as neoadjuvant therapy for LARC with MSI-H/dMMR. Of the 16 evaluable patients, 15 had tumor shrinkage following treatment, and nine achieved clinical complete response and opted for follow-up observation rather than more invasive treatments. The study achieved an impressive 75 % complete response rate, offering compelling evidence for the potential of immunotherapy in treating MSI-H/dMMR LARC. These promising results underscore the importance of continuing to explore the use of immunotherapy in CRC treatment.
ICIs combination
The NICHE study [47] was a pioneering investigation into the use of immunotherapy in neoadjuvant treatment for CRC. Specifically, it aimed to evaluate the safety and efficacy of nivolumab and ipilimumab in the context of early-stage CRC. Preliminary results from the study were highly promising, with all patients achieving objective remission and 67 % of patients achieving pCR. These findings suggest that the use of dual immune neoadjuvant therapy represents a significant opportunity for CRC patients, and the study marks a major milestone in the application of nIT in the treatment of this disease.
A recent study [48] reported that a female patient with MSI-H/dMMR LARC who refused radiotherapy received nIT consisting of ipilimumab + nivolumab for 3 cycles, underwent TME with pathologically confirmed pCR and showed no recurrence at the first evaluation 6 months after surgery. And another study [49] reported a young patient with MSI-H/dMMR LACRC in Lynch syndrome who refused nCRT to preserve fertility and eventually opted to receive nIT with ipilimumab in combination with nivolumab and subsequently underwent TME, with postoperative pathology confirming pCR.
ICIs in combination with radiotherapy and chemotherapy
In the VOLTAGE-A (NCT02948348) phase I/II trial, the addition of 3–5 courses of nivolumab consolidation therapy between nCRT and radical surgery in LARC patients with MSS or MSI-H status showed that pCR was achieved in 30 % (11/37) and 60 % (3/5) of LARC patients with MSS and MSI-H status, respectively [50],suggesting that the combination of nivolumab and radical surgery after nCRT is effective in treating patients with MSS/pMMR in LARC.
The ANAVA study [51], reported at the 2021 ASCO Annual Meeting, recruited 101 patients with LARC who were treated with 6 courses of avelumab following nCRT. Of the 96 patients whose pathology was finally assessed, 22 (23 %) achieved pCR and 59 (61.5 %) achieved major pathological remission (mPR).
The AVERECTAL study investigated the effectiveness of neoadjuvant short-course radiotherapy (SCRT) in combination with mFOLFOX6 plus avelumab for LARC [52]. Of 40 patients who completed at least 1 cycle of ICIs combined with radiotherapy followed by TME, 67.5 % achieved mPR and 37.5 % achieved pCR, with further follow-up studies to follow [53,54].
A R-IMMUNE phase Ib/II prospective trial study [55] included 26 LARC patients treated with atezolizumab for 4 cycles after the end of nCRT followed by surgery, of which 25 completed surgery and 6 had postoperative pathology showing pCR.
ICIs in combination with targeted therapy
The NSABP C-08 study included II/III stage CRC patients who received adjuvant FOLFOX and bevacizumab [56]. In the entire study population, bevacizumab did not significantly improve disease-free survival (HR, 0.89). However, post-hoc analysis of patients with dMMR or pMMR showed improved survival with bevacizumab compared to FOLFOX alone in the dMMR subgroup [57]. In contrast, no survival benefit was observed in the pMMR subgroup. This result suggests that at least in some CRC patients with pre-existing anti-cancer immunity, the inhibition of VEGF alone is sufficient to provide immune-stimulatory effects, enhance anti-cancer immune responses, and provide rationale for combining bevacizumab with ICIs to enhance immunity.
A retrospective case study from Zhang et al. [58] evaluated the reported efficacy and safety of nIT combined with targeted drug therapy in patients with MSI-H/dMMR gastrointestinal malignancies, in which two MSI-H LARC patients received nIT alone or in combination with bevacizumab, both patients were diagnosed with pCR postoperatively, and no severe irAEs occurred during treatment.
ICIs in CRC patients with MSS/pMMR
MSS/pMMR CRC is more common than MSI-H/dMMR CRC. In recent years, numerous studies have focused on overcoming the inherent resistance to ICIs observed in the majority (approximately 95 %) of MSS/pMMR tumor patients [59]. Efforts are being made to develop effective strategies and convert their immunogenicity “cold” microenvironment into a “hot” microenvironment (Fig. 2B) to enhance their anti-tumor immunity. The following are some of the latest and most promising clinical trials in this field.
Combination of ICIs
In the NICHE exploratory study (NCT03026140) mentioned earlier [47], 4 out of 15 (27 %) pMMR tumor patients who received a combination of ipilimumab and nivolumab with or without celecoxib achieved pCR levels. This suggests that combining ICIs with other drugs such as celecoxib may be a promising treatment modality for pMMR tumor patients.
ICIs in combination with radiotherapy and chemotherapy
Li et al. [60] included 24 patients with LARC at low and high risk in MSS/pMMR, treated with the CAPEOX regimen in combination with sintilimab followed by a full course of neoadjuvant therapy, followed by continuation of the CAPEOX regimen for consolidation of therapy; the results showed a pCR rate of 30 % in the 20 patients who underwent surgery; of the four patients who did not undergo surgery, three achieved a cCR and one declined surgery because the tumor remained stable.
The ongoing TORCH trial [61] reported an initial cCR or pCR rate of 81.3 % in 16 patients with MSS LARC treated with toripalimab in addition to SCRT or CAPEOX-based combination therapy.
Lin et al. [62] used a short course of radiotherapy sequential immunotherapy combined with chemotherapy to treat LARC patients for 2 cycles and showed a high pCR rate of 48 % in the total population of 27 subjects who underwent the procedure, 26 of whom were of the pMMR type with a pCR rate of 46 %. The programme is also currently in a clinical phase III study and it is expected that it will enable more patients to benefit from immunotherapy. So far, the safety appears to be manageable, with no level 4–5 irAEs.
ICIs in combination with targeted therapy
The REGONIVO study [63] enrolled 50 advanced gastrointestinal cancer patients who failed standard treatment, of which 24 were MSS CRC. The salvage treatment with regorafenib plus nivolumab achieved a good ORR of 40 % overall, with an ORR of 36 % in the CRC group. The median PFS was 7.9 months, and the 12-month PFS was 41.8 %. The median OS was not reached, and the 1-year OS rate was 68.0 %. REGONIVO achieved a “qualitative leap” in salvage treatment for CRC, opening up a new era of regorafenib plus PD-1 inhibitor therapy for refractory MSS CRC. This is currently the most effective third-line treatment for colorectal cancer.
The REGOTORI study [64] is the first clinical trial of PD-1 monoclonal antibody combined with regorafenib for refractory pMMR/MSS/MSI-L mCRC conducted in Chinese patients. In the evaluable patients at the recommended phase II dose (15 mg regorafenib plus toripalimab), the objective response rate (ORR) was 2.36 %, and the disease control rate was 4.80 %. The median PFS and median OS were 2.1 months and 15.5 months, respectively.
Overall, these advances highlight the growing importance of combination therapy in LACRC, especially for patients with lower tumor location, difficult anal preservation, or the need for organ and tissue preservation. Some patients may even have the opportunity to be cured through immunotherapy alone, providing a promising new treatment option for this challenging disease. Further research will optimize treatment strategies and outcomes for MSS CRC patients in the future.
The implementation of selective surgery
For patients with LACRC, the preferred surgical approach is complete mesocolic excision (CME)/total mesorectal excision (TME) surgery. The goal of this surgery is to completely remove the tumor and surrounding tissues and clear lymph nodes, including the mesentery (as shown in Fig. 3A). TME surgery can be performed through either the anus or abdominal incision, depending on the patient's specific situation. However, it should be noted that CME/TME surgery carries potential risks and complications, including bleeding, infection, urinary incontinence, sexual dysfunction, and pelvic pain.
Fig. 3.
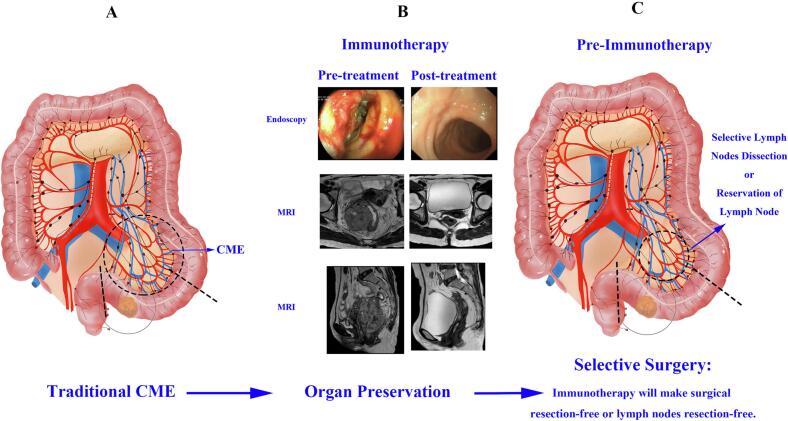
With the continuous development of surgical techniques, tumor treatment has evolved from traditional CME/TME surgery to the era of organ preservation and selective surgery, and will continue to develop towards a more individualized, precise, and efficient direction.
A. The traditional CME/TME surgery is the main curative approach for tumor treatment, aimed at controlling tumor progression by removing the tumor, surrounding tissue, and lymph nodes. The figure shows curative CME surgery for sigmoid colon cancer, which involves removing the colon within 10 cm above the tumor and at least 5 cm below it. Lymph nodes to be cleared include mesenteric lymph nodes, pericolonic lymph nodes, and 253 lymph nodes. Vessels to be dissected include the sigmoid artery.
B. Comparison of pre-nIT and post-nIT in MSI-H/dMMR LARC patients. Through follow-up with a combined approach of endoscopy, rectal MRI for at least 6 months, all patients achieved cCR.
C. Currently, organ preservation has been achieved, and we propose the concept of selective surgery. A promising idea for future surgical procedures is to selectively remove lymph nodes or preserve them after immunotherapy. The purpose of selective surgery is to ensure complete tumor removal while selectively clearing lymph nodes, or even preserving them, to improve patient prognosis and quality of life.
The “w&w” non-surgical organ preservation strategy was first reported by Harba-Gamal et al. in 2004. This approach is applied to LARC patients who have achieved cCR after receiving immunotherapy and who do not undergo conventional surgery but go directly to a close follow-up and observation period, preserving organ function without compromising patient survival and avoiding surgical complications or permanent colostomy. Patients with preserved organs have similar DFS and OS compared to those who undergo surgery, and LARC patients have a significantly improved quality of life.
As mentioned earlier, immunotherapy has shown promising results in LARC patients. Preliminary data suggest that immunotherapy may achieve high pCR or cCR rates as well as organ preservation [35]. This finding represents a major breakthrough in the field of CRC treatment, as it provides patients with an alternative treatment option while improving their overall prognosis. The “watch-and-wait” non-surgical organ preservation strategy was first reported by Habr-Gama et al. in 2004 [65]. This approach can be applied to LARC patients who achieve cCR through immunotherapy and do not undergo traditional surgery, but instead enter a close follow-up and observation period, preserving organ function without compromising patient survival and avoiding surgical complications or permanent colostomies [[66], [67], [68], [69], [70], [71], [72], [73]]. Patients with organ preservation have similar DFS and OS compared to those who undergo surgery, but with significantly improved quality of life [65,[74], [75], [76]].
As mentioned earlier, the breakthrough study [35] showed that LARC patients with MSI-H/dMMR achieved cCR in 100 % of patients after 6 months of PD-1 monotherapy, as assessed by a comprehensive evaluation of rectal MRI, endoscopy, and digital rectal examination. This allows patients to avoid radiotherapy and surgery and continue with observation only. The study provides revolutionary evidence of a paradigm shift from nCRT to organ preservation through immunotherapy for this patient population.
In 2023, Xu et al. [77] demonstrated that 9 out of 17 primary MSI-H/dMMR LARC patients achieved cCR after receiving Sintilimab neoadjuvant immunotherapy and were selected for follow-up observation. The overall study complete remission rate was as high as 75 %. These patients were followed up until November 2022 and did not experience tumor recurrence. In this study, most patients with MSI-H/dMMR LARC were able to achieve cCR after PD-1 antibody treatment, avoiding the need for radical surgery.
These findings provide compelling evidence supporting the use of immunotherapy as a potentially effective treatment strategy for locally advanced MSI-H/dMMR rectal cancer patients. Using a comprehensive approach of endoscopy, rectal MRI, or CT/PET-CT for at least 6 months, all patients achieved cCR (Fig. 3B), offering hope for improving LARC management.
Overall, the evidence so far indicates that the “watch-and-wait” strategy is a feasible breakthrough in selectively treating colorectal cancer with surgery, opening up a new era of anal preservation. Future research and discoveries will bring exciting prospects, providing patients with better treatment options and improving their quality of life.
Preserving lymph nodes or selective resection of metastatic lymph nodes
Theoretical feasibility
Currently, based on the breakthrough progress in research related to lymph nodes, we propose a beautiful vision of protecting lymph nodes or implementing a selective lymph node dissection strategy during surgery, to achieve the selective surgery we have discussed.
A study published in Cell by Maha et al. [78] has made us rethink the significance of preserving lymph nodes during treatment. This study shows that after immunotherapy of tumor patients, progenitor exhausted CD8+ T cells (Tpex) differentiate into transitional intermediate exhausted cells (Tex-int) in lymph nodes not invaded by tumor cells; subsequently, Tex-int then enter the blood system, expand and infiltrate the tumor. That is, the T cells responding to immunotherapy originate from the lymph nodes, suggesting that keeping the lymph nodes intact prior to immunotherapy may enhance the effectiveness of immunotherapy, and this solidifies the position of neoadjuvant immunotherapy. This study was conducted in head and neck squamous cell carcinoma, and we look forward to similar breakthroughs in the field of CRC in the future.
A new study by Huang et al. in the journal Cell identifies the presence of tumor-specific memory CD8+ T cells, or Tumor-draining lymph nodes (TdLNs)-TTSM cells, in TdLNs, considered to be true responders to ICIs blocking agents. Their findings suggest that we can make patients benefit more from immunotherapy by keeping tumor-free lymph nodes intact during surgery [79]. It has also been shown that the tumor-specific T-cell response of TdLNs is strongly correlated with the response to ICIs therapy [34].
The groundbreaking research led by Spitzer et al. [80] has shed new light on the mechanisms behind the effectiveness of immunotherapy in fighting against cancer. Contrary to the common belief that immunotherapy activates T-cells directly within the tumor, their study indicates that the immune response is initiated by activating T-cells within the lymph nodes, which in turn release circulating T-cells into the bloodstream. These T-cells can then infiltrate the tumor and attack cancer cells, ultimately leading to tumor regression and improved patient outcomes.
However, the removal of nearby lymph nodes prior to immunotherapy treatment can have a detrimental effect on the activation of T-cells and their subsequent infiltration into the tumor. This highlights the importance of preserving lymph nodes, especially those not affected by the tumor, as key sites for T-cell activation and immune response initiation.
In the field of cancer treatment, lymph nodes are an important component in identifying metastatic tumor cells. Removing nearby lymph nodes before immunotherapy may have adverse effects on T cell activation and subsequent infiltration into the tumor. This highlights the importance of protecting lymph nodes, especially those that are not affected by the tumor, as critical sites for T cell activation and immune response initiation. Therefore, we propose that protecting lymph nodes or implementing a selective lymph node dissection strategy during surgery may help reduce these risks and potentially improve patient outcomes (Fig. 3C).
Essentially, preserving some lymph nodes that are not invaded by the tumor may be a key factor in improving the effectiveness of immunotherapy. The emerging trend of selective lymph node dissection or lymph node preservation may be a crucial factor in improving the effectiveness of immunotherapy and improving patient outcomes. By preserving the critical sites where T cells can be activated, we can maximize the potential of immunotherapy and fundamentally change the way we treat cancer. Lymph node preservation in the treatment of malignant tumors is still in the exploratory stage and requires more clinical research to confirm its safety and efficacy.
Technological feasibility
Lymphatic mapping can visualize lymphatic drainage pathways and the distribution of regional lymph nodes. It is widely used in curative surgery for gastrointestinal malignancies to help determine the extent of lymph node dissection, detect lymph nodes, and provide intraoperative flexibility when dealing with lymph nodes outside the standard clearance area [[81], [82], [83], [84], [85]]. Tracer lymph node dissection commonly uses fluorescent tracers, with carbon nanoparticles (CNPs) and indocyanine green (ICG) being the most commonly used tracers.
CNPs
The principle behind the ability of CNPs to track lymph nodes lies in their high lymphatic tropism. When injected into local tumor tissue, they are phagocytosed by macrophages. Due to the difference between capillary lymphatic vessels and capillary endothelial cells, CNPs cannot enter blood vessels, but can quickly enter lymphatic vessels and stay and aggregate in lymph nodes. This causes the nodes to turn black, achieving the goal of tracking lymph nodes [[86], [87], [88]]. CNPs are widely used in CRC surgery to detect black areas of positive lymph nodes (Fig. 4A). Since CNPs are injected around the tumor, they mainly deposit in the lymph nodes and do not enter the cardiovascular or respiratory systems, thus avoiding potential toxic effects on these systems to some extent [89].
Fig. 4.
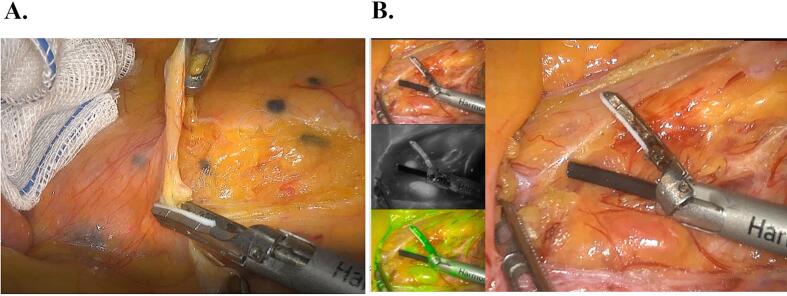
Lymphatic mapping refers to the injection of a tracer into the patient's body, and tracking its flow path during the surgery to determine the location and number of lymph nodes. This approach can help surgeons more accurately remove lymph nodes, thereby reducing the risk of tumor recurrence and metastasis.
A. CNPs are applied in laparoscopic surgery for colorectal cancer, where the black area indicates the detected positive lymph nodes.
B. ICG is applied in laparoscopic surgery for colorectal cancer, with the bottom left corner showing ICG-enhanced near-infrared fluorescence-guided imaging.
However, the use of CNPs also faces some challenges [90]. One difficulty is the difficulty of injecting CNPs into the submucosal layer around the tumor through an endoscope. Injection leakage may also interfere with tumor resection and cause adverse effects. In addition, CNPs are a non-specific lymph node tracer and cannot distinguish between metastatic and negative lymph nodes.
ICG
ICG fluorescence lymphography has been reported for assessing lymphatic pathways in CRC surgery. After submucosal, subserosal, or subcutaneous injection, ICG disperses in lymphatic fluid, binds to lipoproteins, and is excreted through lymphatic pathways and lymph nodes. Especially in laparoscopic right hemicolectomy, it can highlight watershed areas around major vascular branches to more accurately fix the mesentery and visualize lymphatic and blood flow [91,92] (Fig. 4B). ICG fluorescence has the advantages of low toxicity, high sensitivity, rapid feedback, and no radiation [93]. ICG can accurately assess the blood supply at the anastomotic site in real time and can detect organ ischemia before reconstruction, thus reducing the incidence of anastomotic fistula and reoperation rate [92,94]. The use of ICG in robot-assisted colorectal surgery is also feasible [95,96].
Choosing the appropriate tracer can help achieve selective lymph node dissection, and selecting the correct tracer requires consideration of factors such as lymph node uptake, imaging quality, dosage, and safety. This can assist in implementing the concept of selective surgery. Some tracers have a longer residence time in lymph nodes and can provide more accurate lymph node localization information, such as CNPs, which can migrate to lymph nodes through the lymphatic system and have a high lymph node accumulation rate. Other tracers, such as ICG, can provide real-time lymph node imaging information through ultraviolet or near-infrared light excitation. Therefore, choosing the appropriate tracer requires consideration of multiple factors to help achieve the concept of selective surgery.
Conclusions
Undoubtedly, in recent years, immunotherapy has had a significant impact on the treatment of CRC, including immune checkpoint inhibitors, CAR-T cell therapy, cancer vaccines, and all of these have made significant progress. Among them, based on the strong data from the KEYNOTE-177 trial, Pembrolizumab was approved by the FDA in 2020 as a first-line treatment for mCRC. In recent years, the application of immunotherapy in LACRC has also made breakthroughs. Currently, multiple clinical trials are underway to explore the potential benefits of ICIs as a treatment for LACRC patients, which is highly anticipated.
Moreover, it is encouraging that ICIs treatment is no longer limited to MSI-H/dMMR patients who respond to immunotherapy. Some recent clinical trials have overcome the initial resistance of MSS/pMMR mCRC patients to immunotherapy and achieved good results. A series of clinical trials of combination therapy have shown promising data, but further research is needed to validate their safety and effectiveness.
In addition, based on the achievement of complete pathological remission through immunotherapy in MSI-H/dMMR LARC, patients who choose the “watch and wait” strategy have made breakthroughs in organ preservation, avoiding the harm caused by surgery, and taking a big step forward in the concept of selective surgery proposed by us. In addition, because TdLNs have a strong therapeutic response to ICIs, we propose the concept of future selective surgery, including selective clearance or preservation of lymph nodes. Lymph node preservation in the treatment of malignant tumors is still in the exploratory stage and requires more clinical studies to confirm its safety and effectiveness.
In conclusion, it is reasonable to say that immunotherapy has already changed the treatment paradigm of CRC. However, there are still a series of difficulties and challenges to be faced in the future, and the development of immunotherapy for CRC requires further research and exploration, including determining the optimal treatment regimen, prognostic factors, and biomarkers of patient response. With the development of new research and technologies, the vision of achieving selective surgery can be realized, providing individualized treatment plans for patients and improving their quality of life in advanced stages.
CRediT authorship contribution statement
Shiya Yao contributed to the design of the work. Huanrong Lan, Yuejun Han, and Chunsen Mao were responsible for literature review. Xuan Zhang and Mengxiang Yang created the figures. Shiya Yao and Huanrong Lan wrote the manuscript and conducted data analysis. Ketao Jin reviewed/edited the manuscript. Shiya Yao, Huanrong Lan, and Yuejun Han participated in discussions and reviewed/edited the manuscript.
Funding source
This work was supported by National Natural Science Foundation of China [grant no. 82104445 to HRL], Zhejiang Provincial Science and Technology Projects (grant no. LGF22H160046 to HRL), Jinhua Municipal Science and Technology Projects (grants no. 2021-3-040 to KTJ, and 2021-3-046 to HRL).
Ethics statement
This article is a review and does not involve ethics.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by National Natural Science Foundation of China [grant no. 82104445 to HRL], Zhejiang Provincial Science and Technology Projects (grant no. LGF22H160046 to HRL), Jinhua Municipal Science and Technology Projects (grants no. 2021-3-040 to KTJ, and 2021-3-046 to HRL).
Contributor Information
Xuan Zhang, Email: zhangxuan66@kmmu.edu.cn.
Ketao Jin, Email: jinketao2001@zju.edu.cn.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Goding Sauer A., Fedewa S.A., Butterly L.F., Anderson J.C., et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 3.Di Nicolantonio F., Martini M., Molinari F., Sartore-Bianchi A., Arena S., Saletti P., et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 4.Zhu G., Pei L., Xia H., Tang Q., Bi F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer. 2021;20:143. doi: 10.1186/s12943-021-01441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douillard J.Y., Siena S., Cassidy J., Tabernero J., Burkes R., Barugel M., et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 6.Tabernero J., Grothey A., Van Cutsem E., Yaeger R., Wasan H., Yoshino T., et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39:273–284. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopetz S., Grothey A., Yaeger R., Van Cutsem E., Desai J., Yoshino T., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 8.Sharma V., Vanidassane I. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2020;382:876. doi: 10.1056/NEJMc1915676. [DOI] [PubMed] [Google Scholar]
- 9.Jin Z., Sinicrope F.A. Mismatch repair-deficient colorectal cancer: building on checkpoint blockade. J Clin Oncol. 2022;40:2735–2750. doi: 10.1200/JCO.21.02691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le D.T., Kim T.W., Van Cutsem E., Geva R., Jäger D., Hara H., et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanani A., Veen T., Søreide K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br J Surg. 2021;108:1417–1425. doi: 10.1093/bjs/znab342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan A., Wang B., Wang X., Nie Y., Fan D., Zhao X., et al. Immunotherapy in colorectal cancer: current achievements and future perspective. Int J Biol Sci. 2021;17:3837–3849. doi: 10.7150/ijbs.64077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao W., Jin L., Chen P., Li D., Gao W., Dong G. Colorectal cancer immunotherapy-recent progress and future directions. Cancer Lett. 2022;545 doi: 10.1016/j.canlet.2022.215816. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A., et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 16.Kalyan A., Kircher S., Shah H., Mulcahy M., Benson A. Updates on immunotherapy for colorectal cancer. J Gastrointest Oncol. 2018;9:160–169. doi: 10.21037/jgo.2018.01.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.André T., Shiu K.-K., Kim T.W., Jensen B.V., Jensen L.H., Punt C., et al. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 19.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.André T., Shiu K.K., Kim T.W., Jensen B.V., Jensen L.H., Punt C., et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 21.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.J., Gelsomino F., Aglietta M., et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 22.Kloor M., von Knebel Doeberitz M. The immune biology of microsatellite-unstable cancer. Trends Cancer. 2016;2:121–133. doi: 10.1016/j.trecan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Schwitalle Y., Linnebacher M., Ripberger E., Gebert J., MvK Doeberitz. Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. Cancer Immun. 2004:4. [PubMed] [Google Scholar]
- 24.Mettu N.B., Twohy E., Ou F.S., Halfdanarson T.R., Lenz H.J., Breakstone R., et al. 533PD - BACCI: a phase II randomized, double-blind, multicenter, placebo-controlled study of capecitabine (C) bevacizumab (B) plus atezolizumab (A) or placebo (P) in refractory metastatic colorectal cancer (mCRC): an ACCRU network study. Ann Oncol. 2019;30 [Google Scholar]
- 25.Fang X., Zhong C., Zhu N., Weng S., Hu H., Wang J., et al. A phase 2 trial of sintilimab (IBI 308) in combination with CAPEOX and bevacizumab (BBCAPX) as first-line treatment in patients with RAS-mutant, microsatellite stable, unresectable metastatic colorectal cancer. J Clin Oncol. 2022;40:3563. [Google Scholar]
- 26.Golubovskaya V. CAR-T cell therapy: from the bench to the bedside. Cancers (Basel) 2017:9. doi: 10.3390/cancers9110150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotwals P., Cameron S., Cipolletta D., Cremasco V., Crystal A., Hewes B., et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y., Goel S., Duda D.G., Fukumura D., Jain R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motz G.T., Santoro S.P., Wang L.-P., Garrabrant T., Lastra R.R., Hagemann I.S., et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 32.Gao T., Cen Q., Lei H. A review on development of MUC1-based cancer vaccine. Biomed Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110888. [DOI] [PubMed] [Google Scholar]
- 33.Gupta R., Arora K., Roy S.S., Joseph A., Rastogi R., Arora N.M., et al. Platforms, advances, and technical challenges in virus-like particles-based vaccines. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1123805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma S., Chee J., Fear V.S., Forbes C.A., Boon L., Dick I.M., et al. Pre-treatment tumor neo-antigen responses in draining lymph nodes are infrequent but predict checkpoint blockade therapy outcome. Oncoimmunology. 2020;9 doi: 10.1080/2162402X.2019.1684714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cercek A., Lumish M., Sinopoli J., Weiss J., Shia J., Lamendola-Essel M., et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386:2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz L.A., Jr., Shiu K.K., Kim T.W., Jensen B.V., Jensen L.H., Punt C., et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659–670. doi: 10.1016/S1470-2045(22)00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amin M.B., Greene F.L., Edge S.B., Compton C.C., Gershenwald J.E., Brookland R.K., et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X., Wu T., Cai X., Dong J., Xia C., Zhou Y., et al. Neoadjuvant immunotherapy for MSI-H/dMMR locally advanced colorectal cancer: new strategies and unveiled opportunities. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.795972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauer R., Liersch T., Merkel S., Fietkau R., Hohenberger W., Hess C., et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 40.Petrelli F., Trevisan F., Cabiddu M., Sgroi G., Bruschieri L., Rausa E., et al. Total neoadjuvant therapy in rectal cancer a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271:440–448. doi: 10.1097/SLA.0000000000003471. [DOI] [PubMed] [Google Scholar]
- 41.De Felice F., Benevento I., Magnante A.L., Musio D., Bulzonetti N., Caiazzo R., et al. Clinical benefit of adding oxaliplatin to standard neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a meta-analysis oxaliplatin in neoadjuvant treatment for rectal cancer. BMC Cancer. 2017:17. doi: 10.1186/s12885-017-3323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J., Cai J., Deng Y., Wang H. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2019.1663108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu D.X., Li D.D., He W., Ke C.F., Jiang W., Tang J.H., et al. PD-1 blockade in neoadjuvant setting of DNA mismatch repair-deficient/microsatellite instability-high colorectal cancer. Oncoimmunology. 2020;9 doi: 10.1080/2162402X.2020.1711650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao B.Y., Zhang X., Cao T.Y., Li D.D., Jiang W., Kong L.H., et al. Neoadjuvant immunotherapy leads to major response and low recurrence in localized mismatch repair-deficient colorectal cancer. J Natl Compr Canc Netw. 2023;21:60–66.e5. doi: 10.6004/jnccn.2022.7060. [DOI] [PubMed] [Google Scholar]
- 45.Hu H., Kang L., Zhang J., Wu Z., Wang H., Huang M., et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:38–48. doi: 10.1016/S2468-1253(21)00348-4. [DOI] [PubMed] [Google Scholar]
- 46.Chen G., Jin Y., Guan W.L., Zhang R.X., Xiao W.W., Cai P.Q., et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol. 2023;8:422–431. doi: 10.1016/S2468-1253(22)00439-3. [DOI] [PubMed] [Google Scholar]
- 47.Chalabi M., Fanchi L.F., Dijkstra K.K., Van den Berg J.G., Aalbers A.G., Sikorska K., et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 48.Mans L., Pezzullo M., D’Haene N., Van de Stadt J., Van Laethem J.L. Pathological complete response after neoadjuvant immunotherapy for a patient with microsatellite instability locally advanced rectal cancer: should we adapt our standard management for these patients? Eur J Cancer. 2020;135:75–77. doi: 10.1016/j.ejca.2020.04.046. [DOI] [PubMed] [Google Scholar]
- 49.Trojan J., Stintzing S., Haase O., Koch C., Ziegler P., Demes M., et al. Complete pathological response after neoadjuvant short-course immunotherapy with ipilimumab and nivolumab in locally advanced MSI-H/dMMR rectal cancer. Oncologist. 2021;26:e2110–e2114. doi: 10.1002/onco.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bando H., Tsukada Y., Inamori K., Togashi Y., Koyama S., Kotani D., et al. Preoperative chemoradiotherapy plus nivolumab before surgery in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Clin Cancer Res. 2022;28:1136–1146. doi: 10.1158/1078-0432.CCR-21-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salvatore L., Bensi M., Corallo S., Bergamo F., Pellegrini I., Rasola C., et al. Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus avelumab (AVE) in patients (PTS) with locally advanced rectal cancer (LARC): the AVANA study. J Clin Oncol. 2021;39:3511. [Google Scholar]
- 52.Shamseddine A., Zeidan Y., Bouferraa Y., Turfa R., Kattan J., Mukherji D., et al. SO-30 efficacy and safety of neoadjuvant short-course radiation followed by mFOLFOX-6 plus avelumab for locally-advanced rectal adenocarcinoma: Averectal study. Ann Oncol. 2021;32:S215. [Google Scholar]
- 53.Yuki S., Bando H., Tsukada Y., Inamori K., Komatsu Y., Homma S., et al. Short-term results of VOLTAGE-A: nivolumab monotherapy and subsequent radical surgery following preoperative chemoradiotherapy in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. J Clin Oncol. 2020;38:4100. [Google Scholar]
- 54.Shamseddine A., Zeidan Y.H., El Husseini Z., Kreidieh M., Al Darazi M., Turfa R., et al. Efficacy and safety-in analysis of short-course radiation followed by mFOLFOX-6 plus avelumab for locally advanced rectal adenocarcinoma. Radiat Oncol. 2020;15:233. doi: 10.1186/s13014-020-01673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carrasco J., Schröder D., Sinapi I., De Cuyper A., Beniuga G., Delmarcelle S., et al. 397P R-IMMUNE interim analysis: a phase Ib/II study to evaluate safety and efficacy of atezolizumab combined with radio-chemotherapy in a preoperative setting for patients with localized rectal cancer. Ann Oncol. 2021;32:S537. [Google Scholar]
- 56.Allegra C.J., Yothers G., O’Connell M.J., Sharif S., Petrelli N.J., Colangelo L.H., et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pogue-Geile K., Yothers G., Taniyama Y., Tanaka N., Gavin P., Colangelo L., et al. Defective mismatch repair and benefit from bevacizumab for colon cancer: findings from NSABP C-08. J Natl Cancer Inst. 2013;105:989–992. doi: 10.1093/jnci/djt140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z., Cheng S., Gong J., Lu M., Zhou J., Zhang X., et al. Efficacy and safety of neoadjuvant immunotherapy in patients with microsatellite instability-high gastrointestinal malignancies: a case series. Eur J Surg Oncol. 2020;46:e33–e39. doi: 10.1016/j.ejso.2020.06.034. [DOI] [PubMed] [Google Scholar]
- 59.Akin Telli T., Bregni G., Vanhooren M., Saude Conde R., Hendlisz A., Sclafani F. Regorafenib in combination with immune checkpoint inhibitors for mismatch repair proficient (pMMR)/microsatellite stable (MSS) colorectal cancer. Cancer Treat Rev. 2022;110 doi: 10.1016/j.ctrv.2022.102460. [DOI] [PubMed] [Google Scholar]
- 60.Li Y.J., Zhang L., Dong Q.S., Cai Y., Zhang Y.Z., Wang L., et al. Short-term outcome of programmed cell death protein1 (PD-1) antibody combined with total neoadjuvant chemoradiotherapy in the treatment of locally advanced middle-low rectal cancer with high risk factors. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:998–1007. doi: 10.3760/cma.j.cn441530-20210927-00386. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Xia F., Shen L., Wan J., Zhang H., Wu R., et al. Short-course radiotherapy based total neoadjuvant therapy combined with toripalimab for locally advanced rectal cancer: preliminary findings from a randomized, prospective, multicenter, double-arm, phase II trial (TORCH) Int J Radiat Oncol*Biol*Phys. 2022;114 doi: 10.1186/s12885-022-09348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin Z., Cai M., Zhang P., Li X., Cai K., Nie X., et al. Short-course radiotherapy and subsequent CAPOX plus camrelizumab followed by delayed surgery for locally advanced rectal cancer: short-term results of a phase II trial. J Clin Oncol. 2021;39:63. [Google Scholar]
- 63.Fukuoka S., Hara H., Takahashi N., Kojima T., Kawazoe A., Asayama M., et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603) J Clin Oncol. 2020;38:2053–2061. doi: 10.1200/JCO.19.03296. [DOI] [PubMed] [Google Scholar]
- 64.Wang F., He M.M., Yao Y.C., Zhao X., Wang Z.Q., Jin Y., et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Habr-Gama A., Perez R.O., Nadalin W., Sabbaga J., Ribeiro U., Jr., Silva e Sousa A.H., Jr., et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–717. doi: 10.1097/01.sla.0000141194.27992.32. [discussion 7-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iv A.A., Koprowski M.A., Nabavizadeh N., Tsikitis V.L. The evolution of rectal cancer treatment: the journey to total neoadjuvant therapy and organ preservation. Ann Gastroenterol. 2022;35:226–233. doi: 10.20524/aog.2022.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Sluis F.J., Couwenberg A.M., de Bock G.H., Intven M.P.W., Reerink O., van Leeuwen B.L., et al. Population-based study of morbidity risk associated with pathological complete response after chemoradiotherapy for rectal cancer. Br J Surg. 2020;107:131–139. doi: 10.1002/bjs.11324. [DOI] [PubMed] [Google Scholar]
- 68.Kim J.Y., Kim N.-K., Lee K.Y., Hur H., Min B.S., Kim J.H. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol. 2012;19:2485–2493. doi: 10.1245/s10434-012-2262-1. [DOI] [PubMed] [Google Scholar]
- 69.Formijne Jonkers H.A., Draaisma W.A., Roskott A.M., van Overbeeke A.J., Broeders I.A.M.J., Consten E.C.J. Early complications after stoma formation: a prospective cohort study in 100 patients with 1-year follow-up. Int J Colorectal Dis. 2012;27:1095–1099. doi: 10.1007/s00384-012-1413-y. [DOI] [PubMed] [Google Scholar]
- 70.Reese J.B., Finan P.H., Haythornthwaite J.A., Kadan M., Regan K.R., Herman J.M., et al. Gastrointestinal ostomies and sexual outcomes: a comparison of colorectal cancer patients by ostomy status. Support Care Cancer. 2014;22:461–468. doi: 10.1007/s00520-013-1998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peeters K.C.M.J., Velde CjHvd, Leer J.W.H., Martijn H., Junggeburt J.M.C., Kranenbarg E.K., et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients—a Dutch Colorectal Cancer Group Study. J Clin Oncol. 2005;23:6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 72.Maas M., Nelemans P.J., Valentini V., Das P., Rödel C., Kuo L.J., et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 73.van der Valk M.J.M., Hilling D.E., Bastiaannet E., Meershoek-Klein Kranenbarg E., Beets G.L., Figueiredo N.L., et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–2545. doi: 10.1016/S0140-6736(18)31078-X. [DOI] [PubMed] [Google Scholar]
- 74.Smith J.J., Strombom P., Chow O.S., Roxburgh C.S., Lynn P., Eaton A., et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol. 2019;5 doi: 10.1001/jamaoncol.2018.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maas M., Beets-Tan R.G., Lambregts D.M., Lammering G., Nelemans P.J., Engelen S.M., et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 76.Park I.J., You Y.N., Agarwal A., Skibber J.M., Rodriguez-Bigas M.A., Eng C., et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen G., Jin Y., Guan W.-L., Zhang R.-X., Xiao W.-W., Cai P.-Q., et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol. 2023;8:422–431. doi: 10.1016/S2468-1253(22)00439-3. [DOI] [PubMed] [Google Scholar]
- 78.Rahim M.K., Okholm T.L.H., Jones K.B., McCarthy E.E., Liu C.C., Yee J.L., et al. Dynamic CD8(+) T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell. 2023;186:1127–1143.e18. doi: 10.1016/j.cell.2023.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yin E., Sun N., He J. Tumor-draining lymph node-derived tumor-specific memory CD8(+) T cells: a key player in PD-1/PD-L1 immunotherapy. Signal Transduct Target Ther. 2023;8:111. doi: 10.1038/s41392-023-01356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reticker-Flynn N.E., Zhang W., Belk J.A., Basto P.A., Escalante N.K., Pilarowski G.O.W., et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell. 2022;185:1924–1942.e23. doi: 10.1016/j.cell.2022.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noura S., Ohue M., Seki Y., Tanaka K., Motoori M., Kishi K., et al. Feasibility of a lateral region sentinel node biopsy of lower rectal cancer guided by indocyanine green using a near-infrared camera system. Ann Surg Oncol. 2010;17:144–151. doi: 10.1245/s10434-009-0711-2. [DOI] [PubMed] [Google Scholar]
- 82.Watanabe J., Ota M., Suwa Y., Ishibe A., Masui H., Nagahori K. Evaluation of lymph flow patterns in splenic flexural colon cancers using laparoscopic real-time indocyanine green fluorescence imaging. Int J Colorectal Dis. 2017;32:201–207. doi: 10.1007/s00384-016-2669-4. [DOI] [PubMed] [Google Scholar]
- 83.Chen Q.Y., Xie J.W., Zhong Q., Wang J.B., Lin J.X., Lu J., et al. Safety and efficacy of indocyanine green tracer-guided lymph node dissection during laparoscopic radical gastrectomy in patients with gastric cancer: a randomized clinical trial. JAMA Surg. 2020;155:300–311. doi: 10.1001/jamasurg.2019.6033. [DOI] [PubMed] [Google Scholar]
- 84.Ho M.F., Futaba K., Mak T.W.C., Ng S.S.M. Personalized laparoscopic resection of colon cancer with the use of indocyanine green lymph node mapping: technical and clinical outcomes. Asian J Endosc Surg. 2022;15:563–568. doi: 10.1111/ases.13050. [DOI] [PubMed] [Google Scholar]
- 85.Liberale G., Bohlok A., Bormans A., Bouazza F., Galdon M.G., El Nakadi I., et al. Indocyanine green fluorescence imaging for sentinel lymph node detection in colorectal cancer: a systematic review. Eur J Surg Oncol. 2018;44:1301–1306. doi: 10.1016/j.ejso.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 86.Liu C.L., Yang T.L., Chen B.F. Sentinel lymph node mapping with emulsion of activated carbon particles in patients with pre-mastectomy diagnosis of intraductal carcinoma of the breast. J Chin Med Assoc. 2003;66:406–410. [PubMed] [Google Scholar]
- 87.Hao R.T., Chen J., Zhao L.H., Liu C., Wang O.C., Huang G.L., et al. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroid microcarcinoma. Eur J Surg Oncol. 2012;38:718–724. doi: 10.1016/j.ejso.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 88.Cousins A., Thompson S.K., Wedding A.B., Thierry B. Clinical relevance of novel imaging technologies for sentinel lymph node identification and staging. Biotechnol Adv. 2014;32:269–279. doi: 10.1016/j.biotechadv.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Madannejad R., Shoaie N., Jahanpeyma F., Darvishi M.H., Azimzadeh M., Javadi H. Toxicity of carbon-based nanomaterials: reviewing recent reports in medical and biological systems. Chem Biol Interact. 2019;307:206–222. doi: 10.1016/j.cbi.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 90.Liu P., Tan J., Tan Q., Xu L., He T., Lv Q. Application of carbon nanoparticles in tracing lymph nodes and locating tumors in colorectal cancer: a concise review. Int J Nanomedicine. 2020;15:9671–9681. doi: 10.2147/IJN.S281914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keller D.S., Joshi H.M., Rodriguez-Justo M., Walsh D., Coffey J.C., Chand M. Using fluorescence lymphangiography to define the ileocolic mesentery: proof of concept for the watershed area using real-time imaging. Tech Coloproctol. 2017;21:757–760. doi: 10.1007/s10151-017-1677-x. [DOI] [PubMed] [Google Scholar]
- 92.Nishigori N., Koyama F., Nakagawa T., Nakamura S., Ueda T., Inoue T., et al. Visualization of lymph/blood flow in laparoscopic colorectal cancer surgery by ICG fluorescence imaging (Lap-IGFI) Ann Surg Oncol. 2016;23(Suppl. 2):S266–S274. doi: 10.1245/s10434-015-4509-0. [DOI] [PubMed] [Google Scholar]
- 93.Marano A., Priora F., Lenti L.M., Ravazzoni F., Quarati R., Spinoglio G. Application of fluorescence in robotic general surgery: review of the literature and state of the art. World J Surg. 2013;37:2800–2811. doi: 10.1007/s00268-013-2066-x. [DOI] [PubMed] [Google Scholar]
- 94.Sujatha-Bhaskar S., Jafari M.D., Stamos M.J. The role of fluorescent angiography in anastomotic leaks. Surg Technol Int. 2017;30:83–88. [PubMed] [Google Scholar]
- 95.Watanabe M., Tsunoda A., Narita K., Kusano M., Miwa M. Colonic tattooing using fluorescence imaging with light-emitting diode-activated indocyanine green: a feasibility study. Surg Today. 2009;39:214–218. doi: 10.1007/s00595-008-3849-9. [DOI] [PubMed] [Google Scholar]
- 96.Cahill R.A., Ris F., Mortensen N.J. Near-infrared laparoscopy for real-time intra-operative arterial and lymphatic perfusion imaging. Colorectal Dis. 2011;13(Suppl. 7):12–17. doi: 10.1111/j.1463-1318.2011.02772.x. [DOI] [PubMed] [Google Scholar]