Abstract
Interleukin-1β (IL-1β) concentrations are frequently elevated in central nervous system (CNS) viral infections, but the pathophysiologic significance of such elevations is not known. To examine the role of IL-1β in CNS viral pathogenesis, we compared the natural histories of IL-1β-deficient and wild-type 129 SV(ev) mice infected with a neurovirulent viral strain, neuroadapted Sindbis virus (NSV). We found that the incidence of severe paralysis and death was markedly decreased in NSV-infected IL-1β−/− mice compared to NSV-infected wild-type mice (4 versus 88%, P < 0.001). Despite this marked difference in clinical outcome, no differences in numbers of apoptotic cells or presence of histopathologic lesions in the brains of moribund wild-type mice and those of clinically healthy IL-1β−/− mice could be detected. These results suggest that IL-1β deficiency is protective against fatal Sindbis virus infection by a mechanism that does not involve resistance to CNS virus-induced apoptosis or histopathology.
The proinflammatory cytokine interleukin-1β(IL-1β) is a critical modulator of the host response to microbial infections. It is induced by a wide range of microbes and other inflammatory mediators and has pleiotropic effects on numerous cell types, including fever and the induction of other cytokines mediating the acute-phase response. The protective role of IL-1β in microbial host defense has been best characterized in bacterial infections, but at least two lines of evidence suggest that IL-1β also plays an important role in host defense against certain viral diseases. First, mice deficient in IL-1β demonstrate increased susceptibility to challenge with influenza virus (9). Second, deletions of poxvirus genes whose products directly interfere with the activation of IL-1β (e.g., poxvirus genes encoding serpins that inhibit IL-1β-converting enzyme) or the function of IL-1β (e.g., soluble IL-1β receptors that bind IL-1β) lead to decreased viral virulence and host pathology (27, 30). Thus, in influenza virus and poxvirus animal models, viral induction of IL-1β is beneficial for the host.
However, IL-1β may have adverse pathophysiologic consequences when levels are elevated in response to certain noninfectious stimuli, especially those involving the central nervous system (CNS). Mature IL-1β, secreted either by intrinsic brain cells or by infiltrating inflammatory cells, can result in neuronal dysfunction (reviewed in reference 25) by affecting neurotransmitter synthesis, ion influxes, or nitric oxide production. Mature IL-1β can also play a direct role in mediating irreversible brain damage. Ischemic and excitotoxic brain damage in rodents is significantly inhibited by treatment with IL-1 receptor antagonist (21, 34), and transgenic mice that are deficient in IL-1β-converting enzyme (23) or express a dominant negative mutant of IL-1β-converting enzyme (4) are protected against ischemic brain injury. Furthermore, IL-1β may be involved in mediating apoptotic death of neurons in response to trophic factor deprivation or oxidative stress (5, 32).
In several different viral infections of the nervous system, elevations of IL-1β levels have been described. These include human immunodeficiency virus (HIV) (6, 18), simian immunodeficiency virus (10), lymphocytic choriomeningitis virus (3), the nerotropic JHMV strain of mouse hepatitis virus (28), rabies virus (14), canine distemper virus (1), Semliki Forest virus (15), and Sindbis virus (33) infections. Despite the protective effect of IL-1β in influenza and poxvirus infections (which are outside the CNS), the deleterious role of IL-1β in nonviral CNS disorders raises the possibility that IL-1β also contributes to the pathogenesis of CNS viral diseases. A correlation between the level of IL-1β elevation and the severity of viral encephalitis has been previously noted (6, 33), but no studies have directly examined whether IL-1β plays a protective or deleterious role in CNS viral infections.
To examine the role of IL-1β in CNS viral pathogenesis, we used an IL-1β-deficient mouse model of Sindbis virus encephalitis. We used the strain neuroadapted Sindbis virus (NSV), which was derived from the prototype alphavirus, wild-type Sindbis virus, by serial intracerebral passage in mouse brain (7, 8). NSV, unlike wild-type Sindbis virus, produces fatal disease in both suckling and weanling mice. Both immunocompetent and severe combined immunodeficient (scid) suckling mice are susceptible to lethal wild-type Sindbis virus infection. In contrast, the fatal disease produced by NSV in weanling mice requires the contribution of an immunopathological response since weanling scid mice are resistant to NSV-induced paralysis and death (33). Previously, it has been shown that levels of IL-1β are higher in the brains of NSV-infected immunocompetent mice than in the brains of NSV-infected scid mice (33). In the present study, we examined the significance of IL-1β in the pathogenesis of fatal NSV encephalitis by comparing the natural histories of disease in IL-1β−/− and wild-type 129 SV(ev) mice.
IL-1β-deficient mice are resistant to fatal NSV infection.
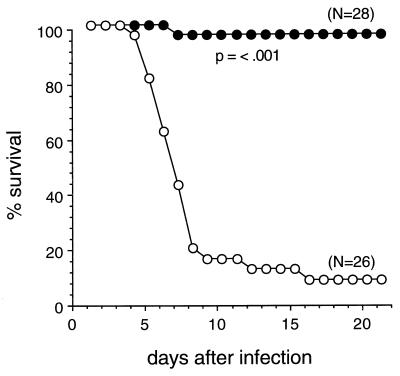
To evaluate the role of IL-1β in the pathogenesis of NSV encephalitis, we compared the natural histories of 5-week-old IL-1β-deficient 129 SV(ev) mice (36) (provided by Hui Zheng, Merck Research Laboratories) and wild-type 129 SV(ev) mice (Taconic Farms), both of which had been inoculated intracerebrally with NSV. Mice were observed for a 21-day period for mortality and maximal amount of paralysis. After NSV infection, 92% of 129 SV(ev) mice developed severe paralysis (Table 1) and 88% died (Fig. 1). (The one mouse that developed severe paralysis but did not die recovered completely by day 21.) In contrast, only 4% of the IL-1β-deficient mice developed severe paralysis (Table 1), and only 4% of the mice died (P < 0.001, life table analysis) (Fig. 1). These results show that IL-1β-deficient mice are resistant to paralysis and death induced by NSV infection.
TABLE 1.
Clinical assessment of paralysis in IL-1β-deficient and wild-type 129 SV(ev) mice after NSV infection
| 129 SV(ev) mouse strain | Total no. of mice | No. of mice with degree of paralysis
|
|||
|---|---|---|---|---|---|
| None | Milda | Mediumb | Severec | ||
| IL-1β−/− | 28 | 20 | 2 | 5 | 1 |
| Wild type | 26 | 0 | 2 | 0 | 24 |
Limp tail.
Hind limb paresis.
Hind limb paralysis.
FIG. 1.
Survival curves of IL-1β−/− (closed circles) and wild-type (open circles) 129 SV(ev) mice infected with NSV. Five-week-old mice were inoculated intracerebrally with 1,000 PFU of NSV in 0.03 ml of Hanks balanced salt solution. Five to ten mice of each strain were infected per experiment; data shown represent combined survival probabilities for four independent infections. Significance of survival differences determined by life table analysis.
Kinetics of viral spread and clearance in IL-1β-deficient mice.
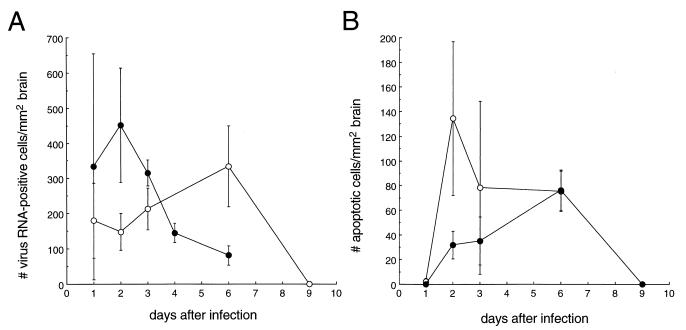
To assess whether IL-1β deficiency affects the CNS replication of NSV, we performed in situ hybridization to detect message-sense viral RNA in the brains of IL-1β-deficient and wild-type 129 SV(ev) mice. Computerized quantitative image analysis was used to calculate the number of viral RNA-positive cells in individual mouse brain sections at serial time points after infection (13). At day 2 after infection, there were more RNA-positive cells in the brains of wild-type mice than in the brains of IL-1β-deficient mice (Fig. 2A), but this difference did not reach statistical significance (P = 0.096, t test). The number of RNA-positive cells in wild-type-infected mice peaked at day 2 after infection, whereas the number of RNA-positive cells in IL-1β−/− mice did not peak until day 6 after infection. At day 6 after infection, when the peak number of RNA-positive cells was observed in IL-1β-deficient mice, the number of viral RNA-positive cells in the brains of wild-type mice had already declined markedly. This difference is illustrated in the representative photomicrographs of hippocampal sections shown in Fig. 3. By day 9 after infection, viral RNA was no longer detected in the brains of IL-1β−/− mice.
FIG. 2.
Viral growth and apoptosis in the brains of IL-1β−/− (closed circles) and wild-type (open circles) 129 SV(ev) mice infected with NSV. At serial time points after NSV infection, mice were sacrificed, and brains were fixed in 4% paraformaldehyde and embedded in paraffin. Parasagittal brain sections (4 μm) were cut at the level of the olfactory bulb, extending caudally from the bulb to the cerebellum and medulla. In situ hybridization to detect message-sense viral RNA (A) and ISEL to detect apoptotic nucleic (B) were performed as described previously (11, 22). The numbers of virus RNA and ISEL-positive cells were quantitated with Image-ProPlus software, exactly as previously described for Sindbis virus-infected brains (11). Each data point represents the mean ± the standard error of the mean of RNA- or ISEL-positive cells per square millimeter of brain for four to eight mice.
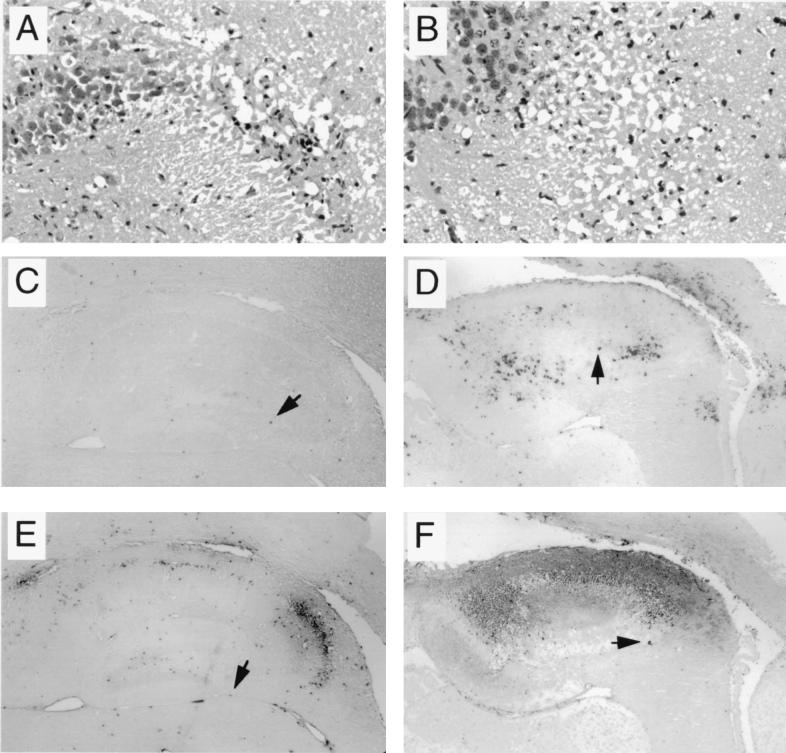
FIG. 3.
Representative photomicrographs of hippocampal sections of a moribund wild-type 129 SV(ev) mouse (A, C, and E) and a clinically healthy IL-1β−/− 129 SV(ev) mouse (B, D, and F) 6 days after infection with NSV. (A and B) H&E-stained sections showing necrosis and numerous pyknotic nuclei in both the wild-type and IL-1β−/− mice. (C and D) In situ hybridization to detect message-sense viral RNA. The images show fewer positive cells in the wild-type mouse than in the IL-1β−/− mouse. (E and F) ISEL staining to detect apoptotic nuclei. The images show numerous positive cells in both the wild-type and the IL-1β−/− mouse. Panels C to F illustrate representative fields used for computerized quantitative image analyses of viral RNA-positive and ISEL-positive cells (Fig. 2) and correspond to images observed with a 10× objective lens; arrowheads denote cells representative of those that were scored as positive by the Image-ProPlus software program. Panels A and B show images of the same hippocampal brain region observed with a 40× objective lens.
Apoptosis is not prevented in the brains of IL-1β-deficient mice.
To determine whether IL-1β deficiency protects against NSV-induced apoptosis, we compared the number of apoptotic nuclei in the brains of NSV-infected IL-1β−/− and wild-type mice. Computerized quantitative image analysis was used to calculate the number of apoptotic, in situ end labeling (ISEL)-positive cells in individual mouse brain sections at serial time points after infection (13). At day 2 after infection, parallel to the decrease in viral RNA-positive cells, the number of apoptotic cells was decreased in the brains of IL-1β-deficient mice (Fig. 2B), but this difference also did not reach statistical significance. Furthermore, within defined virus-infected brain regions, the number of apoptotic versus RNA-positive cells appeared to be at least as high in the IL-1β-deficient mice as in the wild-type 129 SV(ev) mice (data not shown). Thus, the decreased total number of apoptotic nuclei in the brains of IL-1β-deficient mice at day 2 after infection probably reflects the decreased number of virus-infected cells rather than any specific protective effects of IL-1β deficiency on virus-induced apoptosis. At day 6 after infection, when the number of viral RNA-positive cells was highest in NSV-infected IL-1β-deficient mouse brains, the number of apoptotic cells was also highest. At this time point, the number of apoptotic cells observed in the brains of IL-1β−/− mice was identical to that found in the brains of wild-type 129 SV(ev) mice.
CNS histopathology and apoptosis do not correlate with clinical status of NSV-infected mice.
To determine whether the clinical resistance of IL-1β-deficient mice to NSV-induced disease was associated with reduced CNS histopathology, hematoxylin-and-eosin (H&E)-stained brain sections were examined by a neuropathologist blinded to mouse genotype. At day 6 after infection, the majority of NSV-infected wild-type mice were lethargic, severely paralyzed, and moribund, whereas the majority of NSV-infected IL-1β-deficient mice appeared clinically healthy. Despite these pronounced differences in clinical status between the two groups of mice, no significant histopathologic differences could be detected between the brain tissues of the IL-1β−/− and the wild-type 129 SV(ev) mice. At day 6 after infection, both groups of mice demonstrated histopathologic evidence of severe encephalitis, including extensive perivascular cuffing, microglial activation, and pyknotic cell nuclei (see representative photomicrographs in Fig. 3A, 3B, and 4). The frequency, severity, and distribution of such lesions did not differ between the two strains of mice. In addition, as stated above, numbers of apoptotic brain cells in the brains of mice in both groups were identical. Thus, the resistance of IL-1β-deficient mice to fatal NSV encephalitis cannot be attributed to a decrease in CNS cell death, CNS inflammation, or other apparent histopathologic changes.
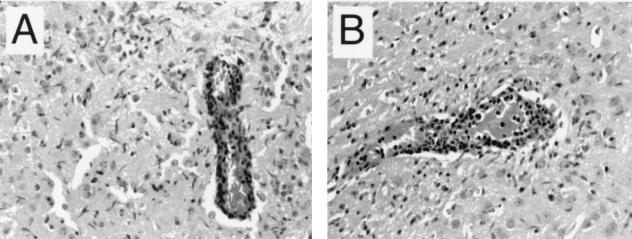
FIG. 4.
Perivascular inflammation in the anterior thalamus of a wild-type 129 SV(ev) mouse (A) and a IL-1β−/− 129 SV(ev) mouse (B) 6 days after NSV infection.
In summary, we found that wild-type 129 SV(ev) mice developed severe paralysis and death, whereas the majority of IL-1β−/− 129 SV(ev) mice remained clinically healthy, following intracerebral NSV infection. At day 2 after infection, there were decreases in the numbers of virus RNA-positive cells and apoptotic cells in IL-1β-deficient mice compared to wild-type 129 SV(ev) mice; these values did not reach statistical significance. Moreover, at 6 days after infection, when IL-1β-deficient mice were clinically healthy and wild-type mice were moribund, the brains of both groups of mice demonstrated extensive apoptosis and severe histopathology. These findings suggest that IL-1β deficiency protects against fatal NSV encephalitis by a mechanism that does not involve the blockade of virus-induced CNS apoptosis or histopathology. To our knowledge, these findings provide the first direct demonstration of a deleterious role of IL-1β in a CNS viral infection.
The mechanism by which IL-1β exerts deleterious effects in NSV encephalitis appears to differ somewhat from that postulated for nonviral CNS disorders. In ischemic and excitotoxic models of CNS diseases, IL-1β is thought to contribute directly to neuronal death (reviewed in reference 20). In contrast, in the NSV encephalitis model, IL-1β deficiency did not protect animals from virus-induced neuronal death. At a time after intracerebral NSV inoculation when infected wild-type animals were terminally ill and infected IL-1β−/− animals were clinically healthy, the number of pyknotic neuronal nuclei observed by H&E staining and the number of apoptotic nuclei observed by ISEL staining were similar in IL-1β-deficient and wild-type mice.
In addition to the lack of a role for IL-1β in NSV-induced neuronal death, our results also suggest the lack of a role for IL-1β-dependent tissue inflammation in the pathogenesis of fatal NSV encephalitis. Histopathologic hallmark lesions of viral encephalitis (e.g., perivascular cuffs and glial nodules) were present with equal frequencies and severities in the brains of NSV-infected, terminally ill wild-type mice and NSV-infected, clinically healthy IL-1β-deficient mice. This finding suggests that the occurrence of these lesions in NSV encephalitis is not mechanically related to the clinical manifestations of the disease and that IL-1β is not an essential cytokine for mediating virally induced CNS-inflammatory responses. Zheng et al. also found no difference in the histologic nature or the severity of inflammatory lesions between wild-type and IL-1β−/− mice in the turpentine model of inflammation, despite the failure of IL-1β−/− mice to produce elevations in levels of acute-phase reaction proteins (36).
We cannot exclude the possibility that the resistance of IL-1β mice to fatal NSV encephalitis is due to slower CNS replication of NSV in these animals. There was an approximately twofold decrease in the number of viral RNA-positive cells in IL-1β−/− mice compared to wild-type 129 SV(ev) mice at day 2 after infection; this difference did not reach statistical significance. The effects of IL-1β on alphavirus replication have not been studied, but neurons (the primary target of Sindbis virus infection in the CNS) have surface IL-1 receptors, and IL-1β is known to stimulate the replication of other, unrelated viruses. IL-1β can potentiate HIV replication in T cells (19) through a signalling pathway that likely involves p38 mitogen-activated protein kinase (26) and NF-κB transcriptional stimulation of the HIV long terminal repeat (17). IL-1β can also increase the susceptibility of respiratory epithelial cells to human rhinovirus infection by upregulating levels of the rhinovirus receptor, ICAM-1 (29). In addition, soluble IL-1 receptors that antagonize IL-1β reduce viral replication in BALB/c mice infected with murine cytomegalovirus (35). Further studies to examine the effects of IL-1β on NSV replication in mouse brain are warranted.
IL-1β may play a pathophysiologic role in fatal NSV encephalitis through mechanisms other than those affecting neuronal death, CNS inflammation, or viral replication. Given that neuronal death and CNS histopathology lack a relationship to animal mortality in NSV encephalitis, one possibility is that animal mortality in these cases results from IL-1β-dependent neuronal dysfunction and/or alterations in neurohormonal function. IL-1β stimulates the release of several neurotransmitters, including norepinephrine, dopamine, 5-hydroxytryptophan, and nitric oxide; induces alterations in the electrophysiologic properties of neurons; stimulates the hypothalamic-pituitary-thyroid axis, resulting in a systemic stress response; and modulates autonomic function, including body temperature and cardiovascular regulation (reviewed in reference 24). The reversibility of severe NSV-induced paralysis in mice that do not die supports the concept that at least some of the disease manifestations are due to potentially reversible neuronal dysfunction, which could be mediated by effects of IL-1β on neurotransmission or neuroelectrophysiology. In addition, it has been postulated that the systemic stress responses resulting from intracerebral production of IL-1β and other cytokines are important in the pathogenesis of encephalitis caused by neurovirulent strains of Sindbis virus (31) and herpes simplex virus (2).
The lack of a correlation between clinical outcome and amount of CNS apoptosis in NSV-infected IL-1β−/− and wild-type 129 SV(ev) mice may have important implications for understanding the role of apoptosis in CNS viral pathogenesis. Previously, we and others hypothesized that apoptosis plays a direct role in the pathogenesis of fatal Sindbis virus encephalitis. This hypothesis was based upon observations that the overexpression of antiapoptotic genes (e.g., bcl-2, beclin, and crm-A) in virally infected neurons protects 1- to 10-day-old mice from fatal Sindbis virus infection (11, 13, 16). In addition, Lewis et al. reported a close relationship between CNS apoptosis and fatal disease in older mice infected with different strains of Sindbis virus (12). However, the findings of the present study suggest that, at least in the NSV weanling-mouse model of alphavirus encephalitis, extensive CNS apoptosis may be present without adversely affecting the clinical status of the animal. The nature of the pathogenetically relevant IL-1β-dependent factors that are responsible for NSV-induced paralysis and death remains to be elucidated.
Acknowledgments
We thank H. Zheng and L. H. T. Van der Ploeg (Merck Research Laboratories) for providing IL-1β−/− 129 SV(ev) mice and Milton Packer for helpful comments.
This work was supported by a James S. McDonnell Foundation Scholar Award (B.L.) and NIH grants AI01217 (B.L.) and AI40246 (B.L.). B.L. was supported by an Irma T. Hirschl Trust Career Scientist Award.
REFERENCES
- 1.Bencsik A, Malcus C, Akaoka H, Giraudon P, Belin M F, Bernard A. Selective induction of cytokines in mouse brain infected with canine distemper virus: structural, cellular and temporal expression. J Neuroimmunol. 1996;65:1–9. doi: 10.1016/0165-5728(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Hur T, Rosenthal J, Itzik A, Weidenfeld J. Rescue of HSV-1 neurovirulence is associated with induction of brain interleukin-1 expression, prostaglandin synthesis and neuroendocrine responses. J Neurovirol. 1996;2:279–288. doi: 10.3109/13550289609146891. [DOI] [PubMed] [Google Scholar]
- 3.Campbell I L, Hobbs M V, Kemper P, Oldstone M B. Cerebral expression of multiple cytokine genes in mice with lymphocytic choriomeningitis. J Immunol. 1994;152:716–723. [PubMed] [Google Scholar]
- 4.Friedlander R M, Gagliardini V, Hara H, Fink K B, Li W, MacDonald G, Fishman M C, Greenberg A H, Moskovitz M A, Yuan J. Expression of a dominant negative mutant of interleukin-1B converting enzyme in transgenic mice prevents neuronal cell death induced by trophic factor withdrawal and ischemic brain injury. J Exp Med. 1997;185:933–940. doi: 10.1084/jem.185.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedlander R M, Gagliardini V, Rotello R J, Yuan J. Functional role of interleukin 1B (IL-1B) in IL-1B-converting enzyme-mediated apoptosis. J Exp Med. 1996;184:717–724. doi: 10.1084/jem.184.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin D E. Cytokines in the brain during viral infection: clues to HIV-associated dementia. J Clin Investig. 1997;100:2948–2951. doi: 10.1172/JCI119847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson A C, Moench T R, Griffin D E, Johnson R T. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Investig. 1987;56:418–423. [PubMed] [Google Scholar]
- 8.Jackson A C, Moench T R, Trapp B D, Griffin D E. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab Investig. 1988;58:503–509. [PubMed] [Google Scholar]
- 9.Kozak W. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1B-deficient mice. Am J Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- 10.Lane T E, Bucmeier M J, Watry D D, Fox H S. Expression of inflammatory cytokines and inducible nitric oxide synthase in brains of SIV-infected rhesus monkeys: applications to HIV-induced central nervous system disease. Mol Med. 1996;1:27–37. [PMC free article] [PubMed] [Google Scholar]
- 11.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang X H, Kleeman L K, Jiang H H, Gordon G, Goldman J E, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by Beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marquette C, Dam A M V, Ceccaldi P E, Weber P, Haour F, Tsiang H. Induction of immunoreactive interleukin-1 beta and tumor necrosis factor-alpha in the brains of rabies virus infected rats. J Neuroimmunol. 1996;68:45–51. doi: 10.1016/0165-5728(96)00056-2. [DOI] [PubMed] [Google Scholar]
- 15.Mokhtarian F, Wesselingh S L, Choi S, Maeda A, Griffin D E, Sobel R A, Grob D. Production and role of cytokines in the CNS of mice with acute viral encephalomyelitis. J Neuroimmunol. 1996;66:11–22. doi: 10.1016/0165-5728(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 16.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborn L, Kunkel S, Nabel G J. Tumor necrosis factor a and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kB. Proc Natl Acad Sci USA. 1989;87:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman H E. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- 19.Poli G, Kinter A L, Fauci A S. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc Natl Acad Sci USA. 1994;91:108–112. doi: 10.1073/pnas.91.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Investig. 1997;100:2648–2652. doi: 10.1172/JCI119808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothwell N J, Relton J K. Involvement of interleukin-1 and lipocortin-1 in ischaemic brain damage. Cerebrovasc Brain Metab Rev. 1993;5:178–198. [PubMed] [Google Scholar]
- 22.Santarosa R, Columbel M C, Kaplan S, Monson F, Levin R M, Buttyan R. Hyperplasia and apoptosis. Opposing cellular processes that regulate the response of the rabbit bladder to transient outlet obstruction. Lab Investig. 1994;70:503–510. [PubMed] [Google Scholar]
- 23.Schielke G P, Yang G Y, Shivers B D, Betz A L. Reduced ischemic brain injury in interleukin-1 beta converting enzyme-deficient mice. J Cereb Blood Flow Metab. 1998;18:180–185. doi: 10.1097/00004647-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Schobitz B, de Kloet E R, Holsboer F. Gene expression and function of interleukin 1, interleukin 6 and tumor necrosis factor in the brain. Prog Neurobiol. 1994;44:397–432. doi: 10.1016/0301-0082(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 25.Sei Y, Vitkovic L, Yokoyama M M. Cytokines in the central nervous system: regulatory roles in neuronal function, cell death and repair. Neuroimmunomodulation. 1995;2:121–133. doi: 10.1159/000096881. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro L, Heidenreich K A, Meintzer M K, Dinarello C A. Role of p38 mitogen-activated protein kinase in HIV type 1 production in vitro. Proc Natl Acad Sci USA. 1998;95:7422–7426. doi: 10.1073/pnas.95.13.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spriggs M K, Hruby D E, Maliszewski C R, Pickup D J, Sims J E, Buller R M, VanSlyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992;71:145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- 28.Stohlman S A, Yao Q, Bergmann C C, Tahara S M, Kyuwa S, Hinton D R. Transcription and translation of proinflammatory cytokines following JHMV infection. Adv Exp Med Biol. 1995;380:173–178. doi: 10.1007/978-1-4615-1899-0_28. [DOI] [PubMed] [Google Scholar]
- 29.Terajima M, Yamaya M, Sekizawa K, Okinaga S, Suzuki T, Yamada N, Nakayam K, Ohrui T, Oshima T, Numazaki Y, Sasaki H. Rhinovirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1beta. Am J Physiol. 1997;273:L749–L759. doi: 10.1152/ajplung.1997.273.4.L749. [DOI] [PubMed] [Google Scholar]
- 30.Thompson J P, Turner P C, Ali A N, Crenshaw B C, Moyer R W. The effects of serpin gene mutations on the distinctive pathobiology of cowpox and rabbitpox virus following intranasal inoculation of Balb/c mice. Virology. 1993;197:328–338. doi: 10.1006/viro.1993.1594. [DOI] [PubMed] [Google Scholar]
- 31.Trgovicich J, Ryman K, Extrom P, Eldridge J C, Aronson J F, Johnston R E. Sindbis virus infection of neonatal mice results in a severe stress response. Virology. 1997;227:234–238. doi: 10.1006/viro.1996.8289. [DOI] [PubMed] [Google Scholar]
- 32.Troy C M, Stefanis L, Prochiantz A, Greene L A, Shelanski M L. The contrasting roles of ICE family proteases and interleukin-1b in apoptosis induced by trophic factor withdrawal and by copper/zinc superoxide dismutase down-regulation. Proc Natl Acad Sci USA. 1996;93:5635–5640. doi: 10.1073/pnas.93.11.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wesselingh S L, Levine B, Fox R J, Choi S, Griffin D E. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J Immunol. 1994;152:1289–1297. [PubMed] [Google Scholar]
- 34.Yang G Y, Zhao Y J, Davidson B L, Betz A L. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 1997;751:181–188. doi: 10.1016/s0006-8993(96)01277-2. [DOI] [PubMed] [Google Scholar]
- 35.Yerkovich S T, Olver S D, Lenzo J C, Peacock C D, Price P. The roles of tumour necrosis factor-alpha, interleukin-1, and interleukin-12 in murine cytomegalovirus infection. Immunology. 1997;91:45–52. doi: 10.1046/j.1365-2567.1997.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann K, Soszynski C A, Grabiec C, Trumbauer M E, Shaw A, Kostura M J, Stevens K, Rosen H, North R J, Chen H Y, Tocci M J, Kluger M J, van der Ploeg L H T. Resistance to fever induction and impaired acute-phase response in interleukin-1β-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]