Abstract
Spinal cord injury (SCI) destroys the sensorimotor pathway and blocks the information flow between the peripheral nerve and the brain, resulting in autonomic function loss. Numerous studies have explored the effects of obstructed information flow on brain structure and function and proved the extensive plasticity of the brain after SCI. Great progress has also been achieved in therapeutic strategies for SCI to restore the “re-innervation” of the cerebral cortex to the limbs to some extent. Although no thorough research has been conducted, the changes of brain structure and function caused by “re-domination” have been reported. This article is a review of the recent research progress on local structure, functional changes, and circuit reorganization of the cerebral cortex after SCI. Alterations of structure and electrical activity characteristics of brain neurons, features of brain functional reorganization, and regulation of brain functions by reconfigured information flow were also explored. The integration of brain function is the basis for the human body to exercise complex/fine movements and is intricately and widely regulated by information flow. Hence, its changes after SCI and treatments should be considered.
Keywords: Spinal cord injury, neural regeneration, information flow, brain functional network, functional reorganization
Introduction
Spinal cord injury (SCI) is a serious neural injury that usually causes hemorrhage and disrupts neural parenchyma, axonal network, and glial membrane, resulting in sensorimotor disorders, neuropathic pain, bladder/intestinal/sexual dysfunctions, and other complications. Its mechanisms mainly include primary injury due to traumatic impact and secondary injury induced by subsequent hemorrhagic and inflammation. 1 In the initial stage, traumatic impact leads to the irreversible destruction of the neural parenchyma, axon disruption, cellular electrolyte disturbances, and hemorrhage, thus triggering secondary injury. 2 Subsequent biochemical events in secondary injury include vascular damage, ischemia, hypoxia, increased cell permeability, ionic deregulation, excitotoxicity, lipid peroxidation, free radical formation, apoptosis, inflammation, edema, demyelination, fibroglial scar, and cyst formation.3,4 Great progress has been achieved in SCI treatments, including the transplantation of exogenous stem/progenitor cells,5–8 olfactory ensheathing cells,9–11 glial cell/Schwann cells,12–15 and peripheral nerve grafts16,17; the knockout of Chondroitin sulfate proteoglycans receptors18–20; and the implantation of biomaterials21–27 (Table 1). These experimental treatments promote the restoration of residual structure/function, the integration of replacement tissues, and the formation of new neurons/fibers in the damaged zone. These strategies exhibited good effects in rodents or non-human primate experiments28–32 and thus have potential application prospects. 33 A broad definition of neural regeneration includes multiple types of function-restoring axon growth. 34 The neural regenerative approach usually involves multiple forms of axon growth and synapse remodeling in the central nervous systems (CNS)21,32,35 to regain neural conduction mechanisms, 36 which could promote recovery as measured by BBB and BMS locomotor scales31,37 and restore gait 21 and upper limb function. 38
Table 1.
Comparison among different SCI therapy approaches.
| Approaches | Cell therapy | Nerve graft | Gene knockout | Neurotrophic factor | Biomaterials | |
|---|---|---|---|---|---|---|
| Examples | Neural stem cells, progenitor cells, olfactory ensheathing cells, glial cells, Schwann cells, etc. | Peripheral nerve grafts | LINGO-1, TLR9, LAR and PTPσ, etc. | BDNF, NT3, BFGF, NGF, and GDNF, etc. | Materials: neurogel, chitosan, PLGA, and 3D biomimetic scaffolds, etc. | Combinatorial approach: NT3-chitosan, Schwann cell channels, and collagen scaffold-collagen-NT3, etc. |
| Strategy | Differentiate into neurons or glia and incorporate into host neural networks | Bridge the site of injury with healthy neural tissues | Inhibit the expression of negative regulator | Provide a sufficient trophic support | Provide suitable environment | Blend growth factors/therapeutic molecules with scaffolds |
| Provide a physical substrate for neural tissue growth | ||||||
| Advantages | Introduce new cells instead of the injured tissues | Across long injury distances | Reduce cell apoptosis | Maintains residual neuronal survival | Direct and support axonal regrowth | Direct and support neural/axonal regrowth |
| Moderate inflammation/glial scar formation | ||||||
| Promote neuronal differentiation/regeneration | Improve microenvironment | Improve microenvironment | ||||
| Targeted drug delivery to the exact injury site | ||||||
| Maintain drug concentrations | ||||||
| Disadvantages | Ethical concerns regarding the formation of a cyst or tumor | Random projection pathway connectivity | Difficult to handle in clinical setting | Short half-life period | Require invasive surgery | Require invasive surgery |
| Difficulty of continuous administration | Poor efficiency | |||||
| The risks of immune rejection/microbial infection |
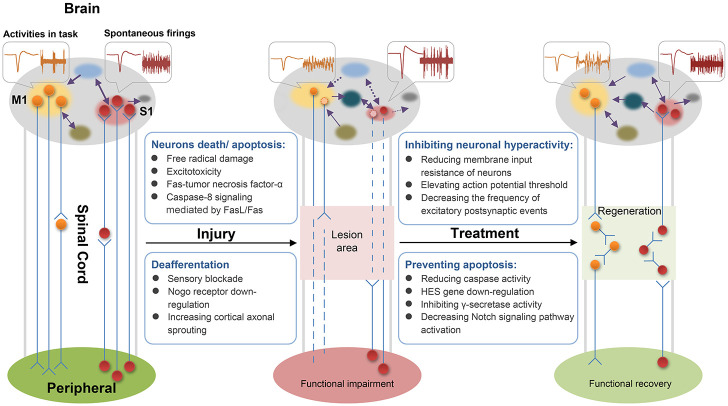
To date, SCI treatment still focuses on neural regeneration in the injured site accompanied by functional recovery. However, the spinal cord and brain make up the CNS and thus have a close structural and functional relationship. After SCI, the interruption and recovery of the information flow between the peripheral nerve and the brain affect the area below the damage level and change the current state of the brain. The brain is a complex system, and many interactions between neuronal populations occur in its different regions in broad spatiotemporal scales. 39 Each brain region is organized in a specific connection pattern to form a brain functional network, the physiological basis of brain function integration that completes information processing and cognitive expression. 40 In theory, neural tissue protection/reconstruction after SCI treatment can restore the brain neuronal structure and function, reshape the communication between the peripheral nerve and the brain, and widely affect multiple brain regions through complex interactions (Figure 1). The reorganization of structure and function in the brain also influences the peripheral nerve and eventually the limb function.
Figure 1.
Spinal cord injury (SCI) blocks the ascending and descending conduction pathways. Brain neurons loss/atrophy might be attributed to a series of biochemical processes leading to neurons death/apoptosis. Deafferentation produces altered electrophysiological properties (amplitude and firing rate) and disrupts the original pattern of brain functional connectivity. In a new potential manner, therapeutic interventions prevent apoptosis to improve neuron survival in the brain, inhibit neuron hyperexcitability to restore homeostatic neural electrical activity, and reconstruct spinal cord conduction pathways to modulate the distinctive patterns of brain functional connectivity and promote sensorimotor recovery.
Schematic of electrical activity: orange indicates neural electrical activities in the executing motor-tasks, and red indicates cortical spontaneous electrical activity. Circles represent neurons, and light circles represent “silent” ones. Purple arrows indicate the information flow in the brain, and dashed lines represent disappeared connections.
Although no in-depth research has been conducted in this area, the increasing application of brain-computer interface (BMI) in CNS injury provides evidence for the above theory. BMI does not involve the regeneration of damaged neural tissues but instead establishes a connection between the peripheral nerve and the brain for SCI subjects through visual feedback.41–44 After a long training, the patients showed increased functional connectivity across the whole brain, including the temporal, parietal, and occipital lobes and subcortical regions. The reorganization of brain functional network topology allows for the increased coordination between the multi-sensory, motor-related cortex, and the extrapyramidal system, thereby partially improving the limb functions. 45
Relevant research on BMI suggested the importance of limb function recovery and brain function changes in SCI and treatments due to the connection between brain and limb functions. This review summarizes the changes in brain structure and function after SCI from single cell (micro-level) to brain regions (macro-level) and discussed future research directions. Broadening the knowledge on the role of brain plasticity in functional restoration after SCI may support the development of effective repair strategies.
SCI changes the structure of brain neurons and the characteristics of neuronal discharge
SCI causes a series of complex biochemical processes in and around the injured area and thus affects neuronal survival and function. Apoptosis, a programmed and energy-dependent mode of cell death, is the most studied mechanism of neuronal death after SCI 46 and is mainly attributed to injury-induced ionic flow disturbances (e.g. Ca2+/Na+ influx and K+ efflux). These disruptions increase the mitochondrial permeability transition pores and consequently induce mitochondrial dysfunction,47,48 activate calpain-mediated protein degradation, 49 and trigger Fas-receptors that release caspases.50,51 Given that calcium from damaged cells in the lesion area cannot reach the remote areas, Dumont et al. 1 and Amemiya et al. 52 believed the death of neurons in the brain after SCI can be regulated by Fas-tumor necrosis factor-α, free radical damage, and excitotoxicity.
Keane et al. 53 first reported the appearance of caspase-8 immunoreactivity mediated by FasL/Fas in the neurons of the spinal gray matter and the consequent apoptosis in rats with a contused spinal cord. A recent study in SCI lampreys revealed that caspase-8 signaling is retrogradely transported by microtubules from the site of axotomy to the soma of neurons, indicating that the extrinsic pathway of apoptosis is involved in neuronal loss in the brain. 54 GABA/baclofen (a GABAB agonist) can reduce the caspase activity of brain neurons at 2 weeks after the complete transection of the lamprey’s spinal cord, thereby inhibiting the progression of apoptosis and promoting the survival/regeneration of descending projections.55,56 Further RNA-sequencing results revealed that GABA or baclofen treatment substantially down-regulated the HES gene and decreased the activation of the Notch signaling pathway after SCI, thus revealing a novel therapeutic target that can inhibit γ-secretase activity to promote axon regeneration. 57 These studies from lamprey SCI models revealed the effects of SCI on brain structure and proposed several (and are still expanding) drugs to alleviate the death/atrophy of descending projection neurons caused by degeneration. These pharmacological approaches focus on blocking apoptosis at different targets to improve the survival of neurons in the brain, thus providing a prerequisite for axonal regeneration and show potential as an SCI treatment strategy. 58
No report is available on the expression of Fas receptor or activated caspase-8 in descending neurons after SCI in mammalian animals. 50 Although many studies suggesting that inhibiting Fas signaling protects the descending projection pathways and promotes possible axonal regeneration, whether SCI causes neuronal loss or atrophy in the brain remains controversial. Hains et al. 59 transected corticospinal tracts (CSTs) in the dorsal funiculus at T9 level spinal cord of adult (150–175 g) male Sprague–Dawley rats and introduced the retrograde tracer Fluoro–Gold into the lesion site to label the pyramidal cells of the damaged axons in the motor cortex. The labeled cells showed morphological manifestations of apoptosis such as chromatin condensation and apoptotic body formation at 1 week after injury, and most of these cells died at 2 weeks after injury. These results documented the features of apoptotic cell death in a proportion of axotomized cortical motor neurons after SCI, suggesting that protection from apoptosis may be a prerequisite for regenerative approaches to SCI. Wannier et al. 60 quantified the surviving neurons in the hindlimb area of the motor cortex at 2 months after CST unilateral interruption in the C7/8 level of rhesus monkeys. They found that the vast majority of the corticospinal neurons did not degenerate. Compared with those on the ipsilateral side, the neurons shrunk after injury. These findings confirmed the effect of SCI on neuronal structure and proved that neuronal cell bodies with damaged axons remain alive but exhibit an altered structural morphology.
Endo et al. 61 completely transected the T9 segment of rat spinal cord. They found that at 1 day after SCI, the Nogo receptor and its co-receptor LINGO-1 in the deafferent cortical regions and adjacent areas were down-regulated, whereas BDNF was up-regulated. Gene transcriptional activities occur mainly in the superficial (II/III/IV) but not in deeper cortical layers (V and VI) where corticospinal pyramidal neurons reside, suggesting that the primary cause of Nogo receptor down-regulation is the sensory blockade, not long-distance axotomy. 61 Strengthening the synaptic connections among the hindlimb representative and adjacent areas in the S1 cortex may increase cortical axonal sprouting. 62 Structurally altered neural fiber networks with aberrant synaptic connections and information transmission/integration can trigger changes in the electrical activity of neurons/neuronal populations.
Aguilar et al. 63 studied the responses of primary somatosensory cortex (S1) neurons to forepaw stimuli in anesthetized rats within 10–30 min after a complete thoracic spinal transection. They found a remarkable increase in evoked local field potentials in the forepaw cortex, indicating that SCI can modulate cortical neuronal response amplitude. The cortical spontaneous activity of the reversible pharmacological block of spinal cord conduction was similar to that in SCI, suggesting that the immediate alteration of electrical activity in the S1 cortex is due to deafferentation, not descending projection axotomy or unspecific systemic response. 63 Xiong et al. 64 prepared a sensory input deprivation model rat by inducing thoracic spinal cord or peripheral nerve injury to the hind paw and recorded the changes in the spontaneous firings of S1 pyramidal neurons due to neural interruption by in vivo two-photon imaging and patch clamps. The number of active neurons decreased to ~40% of the baseline 6 h after injury and then increased to ~140% of the baseline within 48 h. Neurons in layers IV and V of the S1 cortex showed increased spontaneous firing rates after the loss of afferent connections post-SCI. These findings confirmed that SCI can change the number and spike frequency of active neurons in cortical representation and revealed that the alterations in the discharge characteristics of S1 pyramidal neurons are induced by SCI at the single-cell level.
Changes in the electrical activities of the neural population in the local brain area after SCI have been explored. Sawada et al. 65 examined the electrocorticography and the cortical local field potentials in macaque monkeys with lateral CST injury at the cervical cord performing a reach-and-grasp task that requires dexterous finger movements. Compared with those in normal organisms, a sustained γ high-frequency activity appeared in the sensorimotor cortex of injured animals during grasping. Meanwhile, the low-frequency burst in nucleus accumbens decreased substantially. Lucci et al. 66 compared the brain electroencephalogram (EEG) signals of paraplegic patients from acute to chronic phase with those of the normal control group, all of whom performed a visual–motor discrimination task with the right hand. The reaction time and omission percentage of patients with SCI were significantly higher than those of normal subjects due to the reduced activity in their supplementary motor areas. By contrast, the amplitude of Bereitschafts potential decreased during the pre-active motor preparation, regardless of lesion time. These studies suggested that SCI affects the electrical activity characteristics of the neural population in brain areas, changes the frequency and amplitude of sensorimotor neuronal bursts, and regulates the function of the frontal and parietal lobes.
A well-established mechanism by which SCI alters neuronal firing is that cortical neurons dynamically modulate their synaptic strength and intrinsic properties in response to an imposed increase or decrease in synaptic input.67,68 This homeostatic regulation compensates for input loss from the damaged pathway and may also cause and maintain cortical neuronal hyperexcitability, which is an underlying condition of neuropathic pain. 64 Abnormal S1 cortex activity after SCI has been associated with neuropathic pain. 69 A study explored the effects of optogenetic stimulation on the S1 cortex and found that this intervention reduces the membrane input resistance of layer V pyramidal neurons, elevates the action potential threshold, and decreases the frequency of excitatory postsynaptic events, thereby normalizing the pyramidal neuronal hyperexcitability. 64 This study also employed bicuculline-containing Elvax implants, a GABAA receptor antagonist, to achieve similar results. 64 Several other works adopted transcranial direct current stimulation 70 and exoskeleton training 71 to enhance sensorimotor cortex activity and repetitive transcranial magnetic stimulation72,73 to improve M1 cortical hypersensitivity. All these therapeutic strategies relieve SCI-induced neuropathic pain to some extent. These findings suggest that external interventions targeting the sensorimotor cortical activity are beneficial in restoring the normal state of neural electrical activity, which in turn modulates local brain function and alleviates pain syndromes after SCI.
Numerous studies on the property changes of brain neurons induced by SCI provide a basis in understanding the microscopic and local physiological structure and function changes in the cerebral cortex after SCI. The mechanisms underlying the initiation of brain functional reorganizations are also revealed. However, the brain is a complex machine that controls numerous conscious and subconscious functions attributed to the complex coordination of different brain regions. 40 On the surface, many of these functions appear to be controlled by specific anatomical structures. In reality, numerous dynamic networks within the brain contribute to its function through an interconnected web of neuronal and synaptic pathways. 74 Therefore, the effect of SCI on brain functions must be studied from the macroscopic and overall levels (i.e. brain network level) to further understand the normal and pathological state of the brain and its plasticity as manifested by neurons and glial cells.
Reorganization of brain functional network induced by SCI
The spinal cord and the brain have a close structural relationship and share many information transmission pathways. Task-functional magnetic resonance imaging (fMRI) revealed that the dorsal column lesions at a squirrel monkey’s cervical spinal cord inhibit the tactile stimulation-induced activation of Brodmann 3b and 1 areas. 75 Another study on rhesus monkeys 17 combining fMRI, cortical somatosensory-evoked potential, and motor-evoked potential reported that the hemisection of the thoracic spinal cord diminishes the response of the hindlimb representation in the S1 cortex, which is induced by innocuous temperature and electrical stimulations, and the response of hindlimbs to the primary motor cortex activations. In the clinical setting, SCI in humans is often different from that in the animal models established in the laboratory; regardless, similar phenomena have been observed. Chen et al. 76 performed electrical stimulation on the skin surface of the wrist and medial malleolus of right extremities in 11 patients with subacute incomplete cervical cord injury. Although these patients still retained some sensory functions, their cortical response amplitudes in the left postcentral gyrus, right brainstem, and cerebellar lobules IV–VI are remarkably weaker than those of healthy individuals. Therefore, SCI leads to afferent information loss in somatosensory cerebral regions and motor innervation in various body parts, thereby causing functional “silence” in the corresponding representative areas of the sensorimotor cortex. 77
Deafferentation increases the density of dendritic protrusions in layer IV but decreases it in layer VI 78 and up-regulates BDNF. 61 Owing to the plasticity of CNS, the functional “silence” after SCI triggers neuronal circuit remodeling, changes the functional map at the spinal cord34,79,80 and brain81,82 levels of the sensorimotor pathway, and further reorganizes brain functions. 83 To date, many studies have been conducted on the reorganization of brain functions after SCI. Wall and Egger 84 first observed the reorganization of cortical morphological maps caused by the blockage of corticospinal pathways in SCI rats. They suggested that the reorganization of the cerebral cortex in adult rats depends on the establishment of new projection pathways. Halder et al. 85 reported that lesions on the dorsal columns at cervical cord levels in monkeys can expand the intact chin inputs into the deafferented hand representation area in 3b, second somatosensory, and parietal ventral cortexes. Another longitudinal fMRI research in three squirrel monkeys with a unilateral dorsal column lesion at the mid-cervical level revealed the inactivation of tactile stimulation in the S1 cortex after injury and a considerable period of recovery. 86 Rao et al. 87 conducted temperature stimulation on the upper and lower limbs of rhesus monkeys with thoracic SCI. They found that the activation of the upper limb in S1 shifts to the region that generally dominates the lower limb, thus proving the occurrence of cortical reorganization. A study involving human patients also reported that SCI alters the function of the cerebral cortex. 88 Researchers examined the S1 cortex of patients with complete thoracic SCI through fMRI and found that the medial area of S1, which was originally the representative area of foot sensory projection, exhibits a significant response to chest tactile stimuli. The representative area of foot projection is involved in sensory information processing in the chest, thereby suggesting a segregated reorganization in the S1 cortex. This coactivation of non-adjacent cortical representations might be the underlying mechanism of perceptual and cortical reorganization after SCI and may be related to phantom sensations. 88 The above task-fMRI studies suggested a new functional integration between the “silent area” after SCI and the adjacent or distinct functional area, thus preliminarily reflecting the establishment of new functional synchronization. However, the overall change process of the brain from the spatial dimension is difficult to fully describe by task-fMRI for specific brain areas.
With the development of resting-state (rs) fMRI technology, recent studies have used this method to analyze brain function reorganization after SCI. Seminowicz et al. 89 produced a cervical SCI model with an electrolytic lesion (~5 mm2) in rats and found a significant decrease in the resting-state functional connectivity (rs-FC) between the ventroposterior lateral nucleus of the thalamus and the S1 cortex at 7 days after injury. Meanwhile, the functional synchronization within the thalamus and between S1 and retrosplenial cortex was abnormally increased at 14 days after injury. Rao et al. 90 conducted a longitudinal study of adult rhesus monkeys to evaluate functional network reorganization in 20 sensorimotor cortical regions of the brain after thoracic SCI. They found the opposite trend of rs-FC strength between the sensory and motor networks. The enhanced FC strength between the putamen and supplementary motor area in the contralateral hemisphere, which regulates the motor functions of the ipsilateral side, was negatively correlated with the gait performance of the ipsilateral hindlimb. Choe et al. 91 reported an increase in rs-FC between sensorimotor and visual cortex in patients with complete SCI. Kaushal et al. 92 tested the whole-brain rs-FC of patients with complete SCI and found a decrease in their connectivity in a subnetwork of the whole brain and an increase in rs-FC between the cerebellum and bilateral paracentral lobule compared with those of the control subjects. Hawasli et al. 93 examined the brain functional network in 10 patients with SCI and found that this condition reduces the connectivity strength of the neural circuits in the sensorimotor, salience, and default mode networks and disrupts the anti-correlated coupling between control and sensorimotor networks. Further observations at multiple time points revealed a continuously weakened connectivity between the left primary motor and sensory cortex over time. Pan et al. 94 explored the correlation between brain anatomical changes and functional reorganizations after SCI and reported a significant decrease in rs-FC in the neural networks within the left hemisphere and inter-hemispheres for patients with incomplete SCI, regardless of any significant alteration in gray matter volume. This finding indicated that the alterations of cortical anatomical structure and network functional connectivity are non-concomitant.
In addition to fMRI research, some studies explored the changes in brain functional network connections through electrophysiology. Fallani et al. 95 used task-EEG to record the electrical activity in patients with SCI who were attempting to move a paralyzed limb. Significant differences in the local efficiency of the brain network were found between patients and healthy subjects. These variations mainly occurred at θ, α, and β frequency bands, which are involved in the execution of motor acts. The high values in these three bands of patients engender a large level of internal organization and fault tolerance to hold an efficient communication in the M1 cortical areas for foot. Li et al. 96 realized phase synchronization (PS) in the motor cortex recorded by EEG in six patients with SCI and six normal controls performing two gait-like movements. Compared with that in normal subjects, the PS of the premotor cortical connective network in α and β bands significantly increased in patients with SCI during passive movement. In addition, the PS in δ and β bands in the posterior parietal cortex connective network increased during the attempted voluntary movement. Compared with normal subjects, patients with SCI require increased mental effort to engage in the task (in passive gait-like movement) or accomplish leg movement (in attempted/active movement). These results also implied that various neural networks with different neuronal oscillations frequencies exist in the brain and regulate different types of movements; these networks could be targeted to help patients with SCI recover from gait disturbance. Sawada et al. 65 conducted a retrieve food study in SCI macaque monkeys and found different changes in the functional connections between nucleus accumbens and sensorimotor cortex in the early and late recovery stages after injury. Their findings revealed that the nucleus accumbens is important in controlling the recovery of dexterous finger movements and regulating the sensorimotor cortex. A study by Chao et al. 97 on incomplete cervical SCI monkeys showed that interactions among brain regions, which are initiated from the premotor cortex contralateral to the lesion, are related to grasping onset in the γ band and to motor-preparation onset in α and β bands. Thus, two separate functional networks with the contralateral premotor cortex are formed as the core.
The above studies showed that with regard to temporal correlation, SCI induces the extensive reorganization of neuronal circuits in the cerebral cortex. Functional connections are also altered, implying that the reorganization of brain function is one of the key factors for spontaneous functional recovery after SCI. 98 However, the regulatory mechanism of SCI on brain functional reorganization remains unclear. Recent studies on other diseases also considered the mechanism of brain functional reorganization. Yan et al. 99 analyzed the changes of the functional network in patients with concussion. They found that the dependence on the right hippocampus and right medial frontal gyrus after concussion leads to the loss of network hubs in the left superior temporal gyrus. This finding suggested that the over-recruitment of the right prefrontal cortex is crucial for episodic memory dysfunction and disability. By recording the brain local field potentials of anesthetized rats, LeBlanc et al. 100 showed that the informational flow from ventral posterolateral thalamus to S1 cortex is decreased at 7 days after sciatic chronic constriction injury, and thalamus dysfunction induces thalamocortical dysconnectivity. These studies suggested that the combination of Granger Causality connectivity of the whole functional network and the structure and functional information of local brain regions can provide in-depth information on brain functional reorganization and thus is an important direction of future research.
Effect of reinnervation on brain functional reorganization
In addition to studying the effect of “denervation” on the brain functional reorganization caused by neural injury, the influence of “reinnervation” on the brain induced by SCI treatment, including neuronal protection/regeneration and the absence of reactive glia in the damaged cord, must be explored. Animal experiments suggested that the role of large-scale brain functional reorganization in regulating functional recovery is more important than that of new, indirect spinal pathways. 98 However, most studies still focused on achieving rapid and effective neural regeneration. Brain function changes after treatment are rarely analyzed. Researchers either adopted other types of models or only observed the functional recruitment in local brain regions.
Chen et al. 78 studied the effects of the loss and recovery of sensory afferents on the cerebral cortex structure of mice at various developmental periods and found that whisker regrowth protrusion densities are comparable with those of normal controls in layer VI but not in layer IV. This finding implied that reinnervation can restore the cortical structure to some extent but cannot completely return it to the normal state. By using fMRI, Perani et al. 101 observed patients with SCI and paraplegia who were treated with ulnar nerve grafting and suggested that the brain “silence” caused by long-term paraplegia could be reversed. The lower limbs were reinnervated after ulnar nerve grafting, and the corresponding sensorimotor cortex was “re-awakened.” Pelled et al. 102 explored the response of hindlimb cortical representative areas after the loss and regain of sensory afferent in sciatic nerve injury/regeneration models. With the regeneration of sciatic nerve at 9 days after injury, the cortical recruitment in the corresponding representative area gradually reappeared in response to stimuli, but the intensity was always lower than that in the intact group. After a peripheral nerve injury, the inherent plasticity of the brain remodels the synaptic contacts and integrates many cortical areas in the somatotopic postcentral gyrus, thereby allowing the takeover of deafferented representations by adjacent areas. However, re-innervation could still reactivate the correct cortical representative areas to process afferent information. The hindlimb representative cortex cannot be fully activated, implying that reinnervation could not completely reverse reorganizations.
The above studies analyzed the effects of reinnervation on the brain from the perspective of structure and function and suggested that local functional areas change accordingly after reinnervation. However, research focusing on the “self-recovery” of the corresponding cortical representative areas cannot sufficiently describe the overall change in brain functions. Clarifying the mechanism underlying functional recovery remains difficult. In addition to reinnervation, in-depth investigations aimed at pharmacological treatments or adequate transplants can also elaborate the rules of brain functional reorganization.
Conclusion
Although SCI effects on brain functions have been widely observed, the regulatory mechanism is still unclear. Change process, reorganization mode, and change rules of brain functional plasticity after SCI remain to be elucidated. Research on brain function regulation by SCI treatments is still in the initial stage. Longitudinal progress of brain functional reorganization in neural regeneration has never been reported, and the relationship between the change in brain functions and the recovery of limb functions has not been established.
Further in-depth exploration of brain function alterations induced by therapies after SCI is needed to reveal the underlying neural mechanisms of large-scale brain functional reorganization and its role in restoring physical functions. Identifying the relationship between brain functional reorganization and change progress for anatomy and limb function after SCI and interventions will contribute in developing precise therapeutic strategies.
Author biographies
Can Zhao works as a research assistant in the China Rehabilitation Science Institute, Beijing, China. She received her Ph.D. in Biomedical Engineering from Beihang University (2018), focusing on the accurate diagnosis of acute spinal cord injury, non-invasively identification of chronic spinal cord scar tissues, and clarification of the relationship between the changes of central nervous system and motor functions by multimodal magnetic resonance imaging and kinematic-based gait examination, and pursued postdoctoral research (2018-2020) at Beihang University. Her research interests are Neuroimaging and Preclinical research of spinal cord injury and treatment.
Shu-Sheng Bao is affiliated with Beijing Key Laboratory for Biomaterials and Neural Regeneration, Department of Biomedical Engineering, Beihang University, Beijing, China. He is currently pursuing his M.S. degree in Biomedical Engineering at Beihang University. He received the B.S. degree in Biomedical Engineering from Beihang University (2018). His main research includes developing the new technology in detecting the spinal cord injury and exploring the effects of regenerative therapy on brain structure in spinal cord injured rhesus monkeys.
Meng Xu is a chief pathologist at Department of Orthopedics, The First Medical Center of PLA General Hospital (Beijing, China). He received his PhD from H. Lee Moffitt Cancer Center and Research Institute (2013). He used to be a visiting scholar in Helios ENDO-Clinic, Germany (2008), Chonnam National University, Korea (2010) and Istituto Ortopedico Rizzoli, Italy (2014). His research interests include Resection of spinal tumor and reconstruction of spinal stability, Repair of spinal cord injury, Epidural spinal cord stimulation and Pelvic tumor resection and reconstruction.
Jia-Sheng Rao works as an assistant professor at Beihang University, Beijing, China. He is affiliated with Beijing Key Laboratory for Biomaterials and Neural Regeneration and Beijing Advanced Innovation Center for Biomedical Engineering. He received the Ph.D. degree in Biomedical Engineering from Beihang University (2016). His research interests include Central nervous system injury and treatment, Plasticity mechanism of spinal cord injury, Preclinical translational research on non-human primates, Development of rehabilitation technology, and Quantitative assessment of gait function.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grants 31970970, 31900980), Natural Science Foundation of Beijing Municipality (Grant 7194286), China Postdoctoral Science Foundation (Grant 2018M640046), Fundamental Research Funds for Central Public Welfare Research Institutes (Grant 2021CZ-10), Fundamental Research Funds for the Central Universities (Grant YWF-21-BJ-J-811).
ORCID iD: Jia-Sheng Rao  https://orcid.org/0000-0002-5196-0912
https://orcid.org/0000-0002-5196-0912
References
- 1.Dumont RJ, Okonkwo DO, Verma S, et al. Acute spinal cord injury, Part I: pathophysiologic mechanisms. Clin Neuropharmacol 2001; 24: 254–264. [DOI] [PubMed] [Google Scholar]
- 2.Tator CH. Biology of neurological recovery and functional restoration after spina cord injury. Neurosurgery 1998; 42: 696–708. [DOI] [PubMed] [Google Scholar]
- 3.Anjum A, Yazid MD, Fauzi Daud M, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 2020; 21: 7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimitrijevic MR, Danner SM, Mayr W. Neurocontrol of movement in humans with spinal cord injury. Artif Organs 2017; 39: 823–833. [DOI] [PubMed] [Google Scholar]
- 5.Khazaei M, Ahuja CS, Nakashima H, et al. GDNF rescues the fate of neural progenitor grafts by attenuating Notch signals in the injured spinal cord in rodents. Sci Transl Med 2020; 12: eaau3538. [DOI] [PubMed] [Google Scholar]
- 6.Zhong D, Cao Y, Li CJ, et al. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp Biol Med 2020; 245: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dugan EA, Jergova S, Sagen J. Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp Neurol 2020; 327: 113208. [DOI] [PubMed] [Google Scholar]
- 8.Zarei-Kheirabadi M, Sadrosadat H, Mohammadshirazi A, et al. Human embryonic stem cell-derived neural stem cells encapsulated in hyaluronic acid promotes regeneration in a contusion spinal cord injured rat. Int J Biol Macromol 2020; 148: 1118–1129. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zhuang X, Chen Y, et al. Intravenous transplantation of olfactory bulb ensheathing cells for a spinal cord hemisection injury rat model. Cell Transplant 2019; 28: 1585–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delarue Q, Mayeur A, Chalfouh C, et al. Inhibition of ADAMTS-4 expression in olfactory ensheathing cells enhances recovery after transplantation within spinal cord injury. J Neurotrauma 2020; 37(3): 507–516. [DOI] [PubMed] [Google Scholar]
- 11.Zhong W, Bian K, Hu Y, et al. Lysophosphatidic acid guides the homing of transplanted olfactory ensheathing cells to the lesion site after spinal cord injury in rats. Exp Cell Res 2019; 379: 65–72. [DOI] [PubMed] [Google Scholar]
- 12.Kobashi S, Terashima T, Katagi M, et al. Transplantation of M2-deviated microglia promotes recovery of motor function after spinal cord injury in mice. Mol Ther 2020; 28(1): 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerqueira SR, Lee YS, Cornelison RC, et al. Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials 2018; 177: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Liu Z, Zhang W, et al. Melatonin improves functional recovery in female rats after acute spinal cord injury by modulating polarization of spinal microglial/macrophages. J Neurosci Res 2019; 97(7): 733–743. [DOI] [PubMed] [Google Scholar]
- 15.Babaloo H, Ebrahimi-Barough S, Derakhshari MA, et al. PCL/gelatin nanofibrous scaffolds with human endometrial stem cells/Schwann cells facilitate axon regeneration in spinal cord injury. J Cell Physiol 2019; 234: 11060–11069. [DOI] [PubMed] [Google Scholar]
- 16.Dibble CF, Khalifeh JM, Van Voorhis A, et al. Novel nerve transfers for motor and sensory restoration in high cervical spinal cord injury. World Neurosurg 2019; 128: 611–615. [DOI] [PubMed] [Google Scholar]
- 17.Fox IK, Davidge KM, Novak CB, et al. Nerve transfers to restore upper extremity function in cervical spinal cord injury: update and preliminary outcomes. Plast Reconstr Surg 2015; 136: 780–792. [DOI] [PubMed] [Google Scholar]
- 18.Huang LJ, Li G, Ding Y, et al. LINGO-1 deficiency promotes nerve regeneration through reduction of cell apoptosis, inflammation, and glial scar after spinal cord injury in mice. Exp Neurol 2019; 320: 112965. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Ni L, Eugenin EA, et al. Toll-like receptor 9 antagonism modulates astrocyte function and preserves proximal axons following spinal cord injury. Brain Behav Immun 2019; 80: 328–343. [DOI] [PubMed] [Google Scholar]
- 20.Dyck S, Kataria H, Akbari-Kelachayeh K, et al. LAR and TPTσ receptors are negative regulators of oligodendrogenesis and oligodendrocyte integrity in spinal cord injury. Glia 2019; 67: 125–145. [DOI] [PubMed] [Google Scholar]
- 21.Rao JS, Zhao C, Zhang A, et al. NT3-chitosan enables de novo regeneration and functional recovery in monkeys after spinal cord injury. Proc Natl Acad Sci U S A 2018; 115: E5595–E5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koffler J, Zhu W, Qu X, et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med 2019; 25: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchini A, Raspa A, Pugliese R, et al. Multifunctionalized hydrogels foster hNSC maturation in 3D cultures and neural regeneration in spinal cord injuries. Proc Natl Acad Sci U S A 2018; 116: 7483–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D, Li X, Xiao Z, et al. Different functional bio-scaffolds share similar neurological mechanism to promote locomotor recovery of canines with complete spinal cord injury. Biomaterials 2019; 214: 119230. [DOI] [PubMed] [Google Scholar]
- 25.Kong W, Qi Z, Xia P, et al. Local delivery of FTY720 and NSCs on electrospun PLGA scaffolds improves functional recovery after spinal cord injury. RSC Adv 2019; 9: 17801–17811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Zhang F, Zhong W, et al. Transplantation of neural scaffolds consisting of dermal fibroblast-reprogrammed neurons and 3D silk fibrous materials promotes the repair of spinal cord injury. J Mater Chem B 2019; 7: 7525–7529. [DOI] [PubMed] [Google Scholar]
- 27.Kalotra S, Saini V, Singh H, et al. 5-Nonyloxytryptamine oxalate-embedded collagen-laminin scaffolds augment functional recovery after spinal cord injury in mice. Ann N Y Acad Sci 2020; 1465(1): 99–116. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MA, O’Shea TM, Burda JE, et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 2018; 561: 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang ZY, Zhang AF, Duan HM, et al. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci U S A 2015; 112: 13354–13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumamaru H, Lu P, Rosenzweig ES, et al. Activation of intrinsic growth state enhances host axonal regeneration into neural progenitor cell grafts. Stem Cell Rep 2018; 11: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oudega M, Hao P, Shang J, et al. Validation study of neurotrophin-3-releasing chitosan facilitation of neural tissue generation in the severely injured adult rat spinal cord. Exp Neurol 2019; 312: 51–62. [DOI] [PubMed] [Google Scholar]
- 32.Rosenzweig ES, Hrock JH, Lu P, et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med 2018; 24: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtis E, Martin JR, Gabel B, et al. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell 2018; 22: 941–950. [DOI] [PubMed] [Google Scholar]
- 34.Sofroniew M. Dissecting spinal cord regeneration. Nature 2018; 557: 343–350. [DOI] [PubMed] [Google Scholar]
- 35.Liu XY, Liang J, Wang Y, et al. Diffusion tensor imaging predicting neurological repair of spinal cord injury with transplanting collagen/chitosan scaffold binding bFGF. J Mater Sci Mater Med 2019; 30: 123–139. [DOI] [PubMed] [Google Scholar]
- 36.Abbas WA, Ibrahim ME, El-Naggar M, et al. Recent advances in the regenerative approaches for traumatic spinal cord injury: materials perspective. ACS Biomater Sci Eng 2020; 6: 6490–6509. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Yang C, Zhu X, et al. 3D printing collagen/chitosan scaffold ameliorated axon regeneration and neurological recovery after spinal cord injury. J Biomed Mater Res A 2019; 107: 1898–1908. [DOI] [PubMed] [Google Scholar]
- 38.Fox IK, Novak CB, Krauss EM, et al. The use of nerve transfers to restore upper extremity function in cervical spinal cord injury. PM R 2018; 10: 1173–1184.e2. [DOI] [PubMed] [Google Scholar]
- 39.Liao W, Ding J, Marinazzo D, et al. Small-world directed networks in the human brain: multivariate Granger causality analysis of resting-state fMRI. NeuroImage 2011; 54: 2683–2694. [DOI] [PubMed] [Google Scholar]
- 40.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science 2004; 304: 1926–1929. [DOI] [PubMed] [Google Scholar]
- 41.Bonizzato M, Pidpruzhnykova G, DiGiovanna J, et al. Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat Commun 2018; 9: 3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Larraz E, Escolano C, Montesano L, et al. Reactivating the dormant motor cortex after spinal cord injury with EEG neurofeedback: a case study with a chronic, complete C4 patient. Clin EEG Neurosci 2019; 50: 100–110. [DOI] [PubMed] [Google Scholar]
- 43.Keyl P, Schneiders M, Schuld C, et al. Differences in characteristics of error-related potentials between individuals with spinal cord injury and age- and sex-matched able-bodied controls. Front Neurol 2019; 9: 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capogrosso M, Milekovic T, Borton D, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016; 539: 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Q, Yue Z, Ge Y, et al. Brain functional networks study of subacute stroke patients with upper limb dysfunction after comprehensive rehabilitation including BCI training. Front Neurol 2020; 10: 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol 2019; 10: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pivovarova NB, Andrews SB. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J 2010; 277: 3622–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao Y, Lv G, Wang YS, et al. Mitochondrial fusion and fission after spinal sacord injury in rats. Brain Res 2013; 1522: 59–66. [DOI] [PubMed] [Google Scholar]
- 49.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp 2011; 71: 281–299. [DOI] [PubMed] [Google Scholar]
- 50.Sobrido-Cameán D, Barreiro-Iglesias A. Role of caspase-8 and Fas in cell death after spinal cord injury. Front Molec Neurosci 2018; 11: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S, Li Y, Choi HMC, et al. Lysosomal damage after spinal cord injury causes accumulation of RIPK1 and RIPK3 proteins and potentiation of necroptosis. Cell Death Dis 2018; 9: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amemiya S, Kamiya T, Nito C, et al. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur J Pharmacol 2005; 516: 125–130. [DOI] [PubMed] [Google Scholar]
- 53.Keane RW, Kraydieh S, Lotocki G, et al. Apoptotic and anti-apoptotic mechanisms following spinal cord injury. J Neuropathol Exp Neurol 2001; 60: 422–429. [DOI] [PubMed] [Google Scholar]
- 54.Barreiro-Iglesias A, Sobrido-Cameán D, Shifman MI. Retrograde activation of the extrinsic apoptotic pathway in spinal-projecting neurons after a complete spinal cord injury in lampreys. BioMed Res Int 2017; 2017: 5953674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romaus-Sanjurjo D, Rodicio MC, Barreiro-lglesias A. Gamma-aminobutyric acid (GABA) promotes recovery from spinal cord injury in lampreys: role of GABA receptors and perspective on the translation to mammals. Neural Regen Res 2019; 14: 1695–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romaus-Sanjurjo D, Ledo-García R, Fernández-López B, et al. GABA promotes survival and axonal regeneration in identifiable descending neurons after spinal cord injury in larval lampreys. Cell Death Dis 2018; 9: 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sobrido-Cameán D, Robledo D, Romaus-Sanjurjo D, et al. Inhibition of gamma-secretase promotes axon regeneration after a complete spinal cord injury. Front Cell Dev Biol 2020; 8: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodemer W, Selzer ME. Role of axon resealing in retrograde neuronal death and regeneration after spinal cord injury. Neural Regen Res 2019; 14: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hains BC, Black JA, Waxman SG. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J Comp Neurol 2003; 462: 328–341. [DOI] [PubMed] [Google Scholar]
- 60.Wannier T, Schmidlin E, Bloch J, et al. A unilateral section of the corticospinal tract at cervical level in primate does not lead to measurable cell loss in motor cortex. J Neurotrauma 2005; 22: 703–717. [DOI] [PubMed] [Google Scholar]
- 61.Endo T, Spenger C, Tominaga T, et al. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain 2007; 130: 2951–2961. [DOI] [PubMed] [Google Scholar]
- 62.Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature 1994; 368: 737–740. [DOI] [PubMed] [Google Scholar]
- 63.Aguilar J, Humanes-Valera D, Alonso-Calvino E, et al. Spinal cord injury immediately changes the state of the brain. J Neurosci 2010; 30: 7528–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong W, Ping X, Ripsch MS, et al. Enhancing excitatory activity of somatosensory cortex alleviates neuropathic pain through regulating homeostatic plasticity. Sci Rep 2017; 7(1): 12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawada M, Kato K, Kunieda T, et al. Function of the nucleus accumbens in motor control during recovery after spinal cord injury. Science 2015; 350: 98–101. [DOI] [PubMed] [Google Scholar]
- 66.Lucci G, Pisotta I, Berchicci M, et al. Proactive cortical control in spinal cord injury subjects with paraplegia. J Neurotrauma 2019; 36(24): 3347–3355. [DOI] [PubMed] [Google Scholar]
- 67.Turrigiano GG, Leslie KR, Desai NS, et al. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 1998; 391: 892–896. [DOI] [PubMed] [Google Scholar]
- 68.Keck T, Keller GB, Jacobsen I, et al. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron 2013; 80: 327–334. [DOI] [PubMed] [Google Scholar]
- 69.Endo T, Spenger C, Hao JX, et al. Functional MRI of the brain detects neuropathic pain in experimental spinal cord injury. Pain 2008; 138: 292–300. [DOI] [PubMed] [Google Scholar]
- 70.Fregni F, Boggio PS, Lima MC, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006; 122: 197–209. [DOI] [PubMed] [Google Scholar]
- 71.Sczesny-Kaiser M, Höffken O, Aach M, et al. HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J NeuroEng Rehabil 2015; 12: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun XL, Long H, Zhao CG, et al. Analgesia-enhancing effects of repetitive transcranial magnetic stimulation on neuropathic pain after spinal cord injury: an fNIRS study. Restor Neurol Neurosci 2019; 37: 497–507. [DOI] [PubMed] [Google Scholar]
- 73.Zhao CG, Sun W, Ju F, et al. Analgesic effects of directed repetitive transcranial magnetic stimulation in acute neuropathic pain after spinal cord injury. Pain Med 2019; 21: 1216–1223. [DOI] [PubMed] [Google Scholar]
- 74.Edelman BJ, Johnson N, Sohrabpour A, et al. Systems neuroengineering: understanding and interacting with the brain. Engineering 2015; 1: 292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qi HX, Liao CC, Reed JL, et al. Reorganization of higher-order somatosensory cortex after sensory loss from hand in squirrel monkeys. Cereb Cortex 2019; 29: 4347–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Q, Zheng W, Chen X, et al. Reorganization of the somatosensory pathway after subacute incomplete cervical cord injury. Neuroimage Clin 2019; 21: 101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist 2009; 15(2): 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen CC, Bajnath A, Brumberg JC. The impact of development and sensory deprivation on dendritic protrusions in the mouse barrel cortex. Cereb Cortex 2015; 25: 1638–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Endo T, Spenger C, Westman E, et al. Reorganization of sensory processing below the level of spinal cord injury as revealed by fMRI. Exp Neurol 2008; 209: 155–160. [DOI] [PubMed] [Google Scholar]
- 80.Zhong XP, Chen YX, Li ZY, et al. Cervical spinal functional magnetic resonance imaging of the spinal cord injured patient during electrical stimulation. Eur Spine J 2017; 26: 71–77. [DOI] [PubMed] [Google Scholar]
- 81.Ghosh A, Haiss F, Sydekum E, et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci 2010; 13: 97–104. [DOI] [PubMed] [Google Scholar]
- 82.Wrigley PJ, Siddall PJ, Gustin SM. New evidence for preserved somatosensory pathways in complete spinal cord injury: a fMRI study. Hum Brain Mapp 2018; 39: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Filipp ME, Travis BJ, Henry SS, et al. Differences in neuroplasticity after spinal cord injury in varying animal models and humans. Neural Regen Res 2019; 14: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wall PD, Egger MD. Formation of new connexions in adult rat brains after partial deafferentation. Nature 1971; 232: 542–545. [DOI] [PubMed] [Google Scholar]
- 85.Halder P, Kambi N, Chand P, et al. Altered expression of reorganized inputs as they ascend from the cuneate nucleus to cortical area 3b in monkeys with long-term spinal cord injuries. Cereb Cortex 2018; 28: 3922–3938. [DOI] [PubMed] [Google Scholar]
- 86.Qi HX, Wang F, Liao CC, et al. Spatiotemporal trajectories of reactivation of somatosensory cortex by direct and secondary pathways after dorsal column lesions in squirrel monkeys. Neuroimage 2016; 142: 421–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao JS, Ma M, Zhao C, et al. Atrophy and primary somatosensory cortical reorganization after unilateral thoracic spinal cord injury: a longitudinal functional magnetic resonance imaging study. Biomed Res Int 2013; 2013: 753061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moore CI, Stern CE, Dunbar C, et al. Referred phantom sensations and cortical reorganization after spinal cord injury in humans. Proc Natl Acad Sci U S A 2000; 97: 14703–14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seminowicz DA, Jiang L, Ji Y, et al. Thalamocortical asynchrony in conditions of spinal cord injury pain in rats. J Neurosci 2012; 22: 15843–15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rao JS, Liu Z, Zhao C, et al. Longitudinal evaluation of functional connectivity variation in the monkey sensorimotor network induced by spinal cord injury. Acta Physiol 2016; 217: 164–173. [DOI] [PubMed] [Google Scholar]
- 91.Choe AS, Belegu V, Yoshida S, et al. Extensive neurological recovery from a complete spinal cord injury: a case report and hypothesis on the role of cortical plasticity. Front Hum Neurosci 2013; 7: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaushal M, Oni-Orisan A, Chen G, et al. Evaluation of whole-brain resting-state functional connectivity in spinal cord injury: a large-scale network analysis using network-based statistic. J Neurotrauma 2017; 34: 1278–1282. [DOI] [PubMed] [Google Scholar]
- 93.Hawasli AH, Rutlin J, Roland JL, et al. Spinal cord injury disrupts resting-state networks in the human brain. J Neurotrauma 2018; 35(6): 864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pan Y, Dou WB, Wang YH, et al. Non-concomitant cortical structural and functional alterations in sensorimotor areas following incomplete spinal cord injury. Neural Regen Res 2017; 12: 2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fallani FDV, Astolfi L, Cincotti F, et al. Cortical functional connectivity networks in normal and spinal cord injured patients: evaluation by graph analysis. Hum Brain Mapp 2007; 28: 1334–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li W, Xu J, Chen X, et al. Phase synchronization between motor cortices during gait movement in patients with spinal cord injury. IEEE Trans Neural Syst Rehabil Eng 2016; 24: 151–157. [DOI] [PubMed] [Google Scholar]
- 97.Chao ZC, Sawada M, Isa T. Dynamic reorganization of motor networks during recovery from partial spinal cord injury in monkeys. Cereb Cortex 2019; 29: 3059–3073. [DOI] [PubMed] [Google Scholar]
- 98.Isa T. The brain is needed to cure spinal cord injury. Trends Neurosci 2017; 40: 625–636. [DOI] [PubMed] [Google Scholar]
- 99.Yan H, Feng Y, Wang Q. Altered effective connectivity of hippocampus-dependent episodic memory network in mTBI survivors. Neural Plast 2016; 2016: 6353845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LeBlanc BW, Lii TR, Huang JJ, et al. T-type calcium channel blocker Z944 restores cortical synchrony and thalamocortical connectivity in a rat model of neuropathic pain. Pain 2016; 157: 255–263. [DOI] [PubMed] [Google Scholar]
- 101.Perani D, Brunelli GA, Tettamanti M, et al. Remodelling of sensorimotor maps in paraplegia: a functional magnetic resonance imaging study after a surgical nerve transfer. Neurosci Lett 2001; 303: 62–66. [DOI] [PubMed] [Google Scholar]
- 102.Pelled G, Dodd SJ, Koretsky AP. Catheter confocal fluorescence imaging and functional magnetic resonance imaging of local and systems level recovery in the regenerating rodent sciatic nerve. Neuroimage 2006; 30: 847–856. [DOI] [PubMed] [Google Scholar]