Abstract
The first edition of Europace journal in 1999 came right around the time of the landmark publication of the electrophysiologists from Bordeaux, establishing how elimination of ectopic activity from the pulmonary veins (PVs) resulted in a marked reduction of atrial fibrillation (AF). The past 25 years have seen an incredible surge in scientific interest to develop new catheters and energy sources to optimize durability and safety of ablation, as well as study the mechanisms for AF and devise ablation strategies. While ablation in the beginning was performed with classic 4 mm tip catheters that emitted radiofrequency (RF) energy to create tissue lesions, this evolved to using irrigation and contact force (CF) measurement while increasing power. Also, so-called single-shot devices were developed with balloons and arrays to create larger contiguous lesions, and energy sources changed from RF current to cryogenic ablation and more recently pulsed field ablation with electrical current. Although PV ablation has remained the basis for every AF ablation, it was soon recognized that this was not enough to cure all patients, especially those with non-paroxysmal AF. Standardized approaches for additional ablation targets have been used but have not been satisfactory in all patients so far. This led to highly technical mapping systems that are meant to unravel the drivers for the maintenance of AF. In the following sections, the development of energies, strategies, and tools is described with a focus on the contribution of Europace to publish the outcomes of studies that were done during the past 25 years.
Keywords: Atrial fibrillation, Ablation, Mapping, Radiofrequency, Cryoablation, Pulsed field ablation
Graphical Abstract
Graphical abstract.
Introduction
The first edition of Europace journal in 1999 came right around the time of the landmark publication of the electrophysiologists from Bordeaux, establishing how elimination of ectopic activity from the pulmonary veins (PVs) resulted in a marked reduction of atrial fibrillation (AF).1 The past 25 years have seen an incredible surge in scientific interest to develop new catheters and energy sources to optimize durability and safety of ablation, as well as study the mechanisms for AF and devise ablation strategies.2
While ablation in the beginning was performed with classic 4 mm tip catheters that emitted radiofrequency (RF) energy to create tissue lesions, this evolved to using irrigation and contact force (CF) measurement while increasing power. Also, so-called single-shot devices were developed with balloons and arrays to create larger contiguous lesions, and energy sources changed from RF current to cryogenic ablation and more recently pulsed field ablation (PFA) with electrical current.
Although PV ablation has remained the basis for every AF ablation, it was soon recognized that this was not enough to cure all patients, especially those with non-paroxysmal AF.3 Standardized approaches for additional ablation targets have been used but have not been satisfactory in all patients so far. This led to highly technical mapping systems like TOPERA, CardioInsight, ACUTUS, VOLTA, and ABLACON that are meant to unravel the drivers for the maintenance of AF. In the following sections, the development of energies, strategies, and tools is described with a focus on the contribution of Europace to publish the outcomes of studies that were done during the past 25 years.
Radiofrequency-guided point-by-point pulmonary vein isolation: evolution to irrigation, contact force sensing, contiguous lesions, and high-power short-duration ablation
While different forms of energy delivery exist for pulmonary vein isolation (PVI), radiofrequency catheter ablation (RFCA) remains a relevant and commonly employed thermal-based technique.2 A significant arrhythmia-free survival benefit with RF ablation over medical therapy has been demonstrated in several randomized controlled trials (RCTs).4,5 Outcome after PVI for paroxysmal AF was previously quoted at 70%,6–8 with lower success rates seen in persistent AF (PeAF).9–11 Combining medical therapy and RF ablation (hybrid therapy) might further improve outcome after catheter ablation.12
Initial studies, more than 20 years ago, reported on PVI using solid tip, non-irrigated catheters with RF delivery in ‘temperature-controlled’ mode.13 Since the introduction of irrigated catheters, reducing thrombus formation and allowing more power delivery, RF is most often delivered in ‘power-controlled’ mode with conventional power settings between 20 and 40 W.
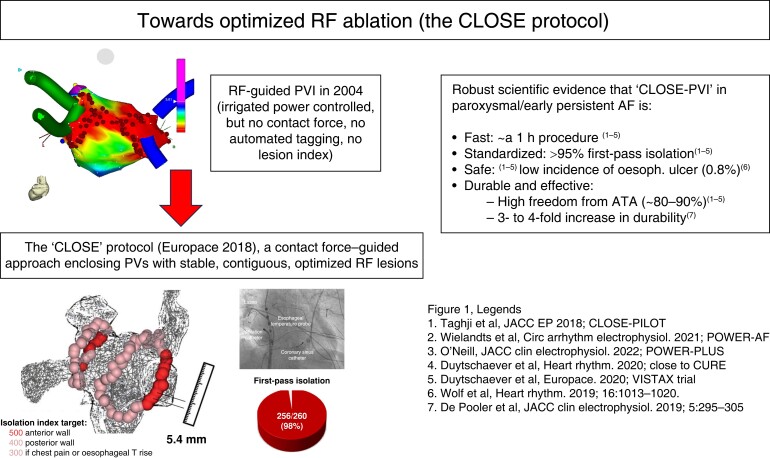
The positive impact of CF measurement on procedural time and recurrence rates7,14–16 has resulted in the adoption and widespread use of irrigated CF-sensing catheters. Contact force–sensing further facilitated the development of algorithms aimed at real-time assessment of lesion quality including the force time integral (FTI),17 lesion size index (LSI),18 and ablation index (AI).19 Together with automatic tagging and standardized workflows aiming for contiguous lesions,20 integration of lesion indices has resulted in improved outcomes for paroxysmal AF, with first-pass isolation rates of up to 98% and 1 year success rates in the range of 90% after CLOSE-PVI21–24 (Figure 1). The observation of more complete PV encirclement on cardiac MRI is in line with improved durability of isolation after CLOSE-PVI.25
Figure 1.
Evolution over 20 years towards a standardized and reproducible RF-PVI approach associated with an excellent efficacy/safety balance (the ‘CLOSE’ protocol; Europace 2018).
In the last 5 years, focus has centred on shortening the procedure time of RF-guided PVI by increasing power during RF delivery (up to 50 W).26 Several clinical studies, including two randomized trials, showed that contiguous, index-based encirclement with 40–50 W ablation in power-controlled mode does shorten procedure time (nearing a 1 h procedure) while preserving safety and effectiveness profile.27–33 With regard to oesophageal safety though, care should be taken using higher power at the posterior wall due to the potential issue of inadvertent overshoot.32 Newer stability algorithms, in combination with careful attention to CF and oesophageal temperature rise, may overcome this issue. One centre reported outcome after power-controlled ablation at 70 W over 7 s. Also, this strategy was associated with shorter procedure time and equivalent safety profile compared with conventional power ablation, although the absence of use of AI or LSI to standardize the lesion set may limit the reproducibility of the results.34
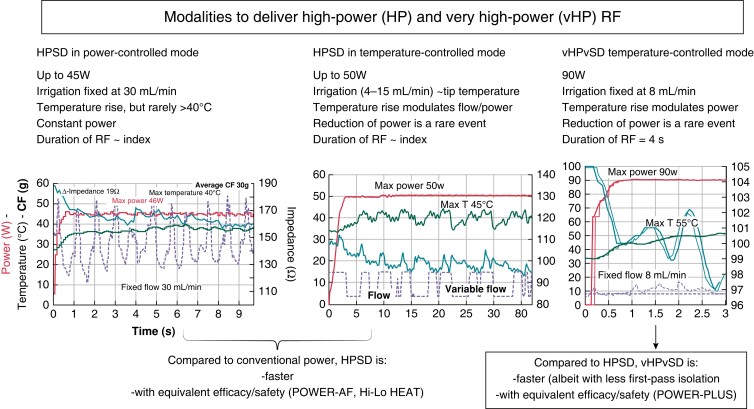
The limitation of reduced tissue temperature feedback accuracy during high-power irrigated ablation has led to the development of novel catheters equipped with multiple thermocouples (TCs) capable of more accurate, real-time tissue temperature monitoring, thus re-introducing the concept of ‘temperature-controlled’ RF delivery in PVI (DiamondTemp, Medtronic, QDot Micro, Biosense Webster). The randomized Diamond AF study demonstrated the non-inferiority of the DiamondTemp Ablation system with respect to standard CF-guided ablation with higher overall power delivery and reduced procedure times using temperature-controlled ablation.35 Similarly, a recent study showed that the CF-sensing QDOT catheter, together with temperature-controlled ablation up to 50 W during low flow irrigation, allowed AI-guided PVI with high first-pass isolation and preserved safety/effectiveness balance (Figure 2).36
Figure 2.
Different modalities to deliver high-power short-duration (HPSD) and very high-power very short-duration (vHPvSD) RF with re-introduction of temperature control in the era of irrigated RF ablation. The CLOSE protocol using HPSD and vHPvSD shortens the procedure time while preserving the excellent efficacy/safety balance.
The last chapter written in point-by-point RF ablation for PVI so far is the introduction of very high-power short-duration applications (vHPSD, 90 W for 4 s in temperature-controlled mode). Whereas some pre-clinical studies suggested a higher rate of contiguity and transmurality with 90 W/4 s ablations,37,38 a recent canine study described significantly smaller lesion sizes in 90 W/4 s ablation in line with a lower overall energy deposit.39 Initial studies demonstrated the feasibility and safety of 90 W/4 s ablation for PVI,40 with procedure times nearing 1 h and preserved safety and effectiveness.41 The safety profile of vHPSD was further supported in a two-centre study showing the lack of silent oesophageal injury.42 Finally, the POWER-PLUS study, a randomized study comparing 90 to 50 W PVI, confirmed that vHPSD shortens procedure time (albeit at the cost of a trend towards less first-pass isolation), with equivalent safety and effectiveness at 6 months.37,43
In summary, due to standardization and innovation, point-by-point RF ablation for PVI has evolved into a clinically safe and effective procedure over the last 25 years. With high- or very high-power ablation (in temperature- or power-controlled mode), procedural times of close to or under 1 h are achievable37,42 without compromising the excellent safety/efficacy profile. The decision to opt for a given power strategy may come down to operator preference or patient profile.
Cryoablation: balloon-based single-shot PVI
The concept of cooling to treat medical disorders dates back to the ancient Egyptians, who employed therapeutic hypothermic therapy 4000 years ago.44 The modern era of cryosurgery was in the 1960s45 with vacuum-insulated cryosurgical probes cooled by liquid nitrogen (−196°C).46 The transition to cooling via the Joule–Thomson effect (e.g. cooling from the expansion of a highly compressed non-ideal gas into a region of low pressure) enabled the development of the modern transvenous catheter cryoablation system in the late 1990s. In broad terms, the contemporary cryoablation system used for electrophysiology applications consists of a deflectable catheter with a hollow shaft, a cooling electrode tip (or balloon), and proximal TC. Ablation of the target tissue occurs through the delivery of pressurized cryorefrigerant from an external console to the catheter tip via an ultrafine injection tube. The cryorefrigerant absorbs heat from the myocardium and returns the vapour to the console via a central exhaust lumen maintained under vacuum.
Early attempts at cryothermal PV isolation were performed using a focal cryocatheter, in a fashion similar to point-by-point RF ablation. This strategy was associated with prolonged ablation time (e.g. mean cryoablation time 65 ± 39 min per vein) and procedure durations (mean procedural time 7.5 ± 2 h). Despite a reasonable acute success rate, the long-term results were disappointing (6–34% freedom from recurrent AF).47,48 Thereafter, efforts shifted to specialized PV isolation catheters, the first of which was a purpose-built self-expanding curvilinear 7-F Arctic Circler (Medtronic CryoCath LP). Again, while PV isolation could be acutely achieved (41/45 PVs; 4 PVs requiring focal RFCA for isolation), the procedure and ablation durations remained long (e.g. 63 min per patient) and only a minority of patients remained free of arrhythmia (4/18 after 14.8 ± 6.2 months of follow-up).49
Efforts to refine the cryothermal energy delivery system led to the creation of a specialized balloon catheter. The Arctic Front Cryoballoon (Medtronic CryoCath LP) entered clinical practice in the late 2000s.50,51 Early studies demonstrated a high acute success rate (>98% of patients achieving complete PVI) with a 1 year freedom from recurrent AF comparable to contemporary studies using RF ablation [1 year single procedure off anti-arrhythmic drugs (AAD) success of 60%; 73% if a 3 month blacking period was included].52 In 2012, the second-generation cryoballoon entered clinical practice. This catheter contained significant refinements, doubling and repositioning the number of refrigerant jets to increase the uniformity of cooling across the distal cryoballoon surface. These engineering changes resulted in a significantly improved efficacy [82% 1 year freedom from recurrent AF (11 studies; 1725 patients)] relative to the use of the first-generation cryoballoon [odds ratio (OR) of arrhythmia recurrence 0.34; 95% confidence interval (CI), 0.26–0.45; 10 studies, 2310 patients].53,54 Since then, the cryoballoon catheter design has undergone further minor refinements to the distal catheter tip (reducing the length from 13 to 8 mm) and catheter handle, without fundamentally altering refrigerant delivery.
To date, over 1 000 000 cryoballoon-based ablation procedures have been performed worldwide. Despite variability in operator skillset and experience, cryoballoon ablation has been noted to have a consistently high acute procedural success (>98% of patients achieving complete PVI) with excellent long-term freedom from recurrent AF.52,55,56 Compared to RF ablation, cryoballoon ablation has been associated with a significantly lower incidence of pericardial effusion (0.8% vs. 2.1% RF; OR 0.44; 95% CI 0.28–0.69) and tamponade (0.4% vs. 1.4% RF; OR 0.31; 95% CI 0.15–0.64) but a significantly greater incidence of cold-induced phrenic nerve injury (1.7% vs. 0.0% RF; OR 7.40; 95% CI 2.56–21.34).52,56,57 Despite the use of a large deflectable sheath, there does not appear to be a significant difference in the incidence of peripheral vascular complications (1.1% cryoballoon vs. 1.3% RF; OR 0.79; 95% CI 0.38–1.62). As such, the balance of safety, efficacy, and generalizability suggests that cryoballoon ablation may be a preferred first-ablation toolset, as it enables greater procedural standardization with more consistent clinical outcomes.58
More recently, a new cryoballoon technology has achieved regulatory approval. While the POLARx (Boston Scientific) cryoballoon is fundamentally similar to the Arctic Front system, there are noteworthy differences. Specifically, the POLARx sheath is more rigid and has an increased angle of deflection (155 degrees vs. 135 degrees with the Medtronic system). The POLARx cryoballoon has greater compliance and operates at a lower ablation pressure (∼3 PSI with Boston Scientific balloon vs. ∼20 PSI with Medtronic balloon). The second-generation POLARx FIT enables the operator to increase the balloon diameter from 28 to 31 mm duration the procedure, enabling more antral lesions with larger PV diameters. While differences in biophysical cryoablation parameters (e.g. rate of cooling, cryoballoon nadir temperatures, and thaw times) have been observed, it is unknown whether these impact procedural outcomes.59,60
Departing from balloon-based designs, the ultra-low temperature cryoablation (ULTC, Adagio Medical) system employs a single diagnostic and therapeutic flexible linear catheter.61 Pre-formed stylets enable the catheter to achieve multiple configurations (linear, circular, focal, or oval), facilitating pulmonary venous isolation as well as linear bi-atrial ablation lesions. In addition, while the cryoballoon systems rely on a liquid to gas phase change to realize isolation, the ULTC system maintains and circulates liquid nitrogen at its near-critical phase within a closed-loop system, achieving ultra-low temperatures (−185°C) and creating a more rapid and deeper myocardial lesion. Owing to the ultra-low cryoablation temperature, these ablation procedures must occur under general anaesthesia, as a specialized oesophageal warming balloon must be employed to reduce the risk of collateral damage to the oesophagus.
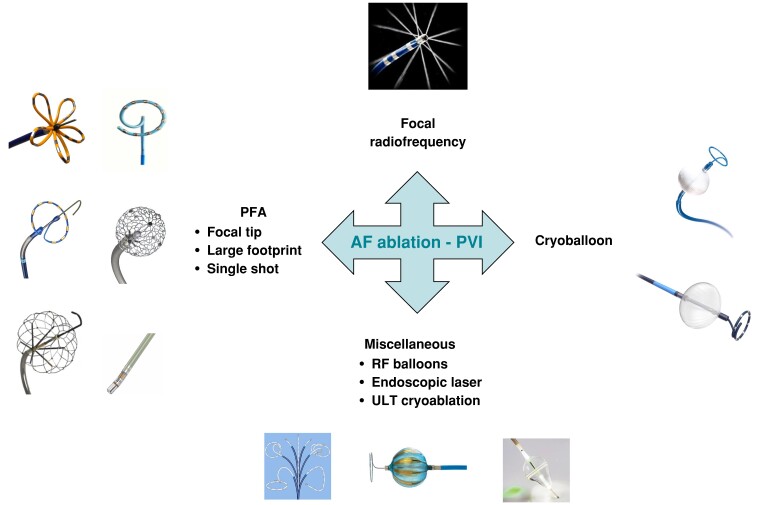
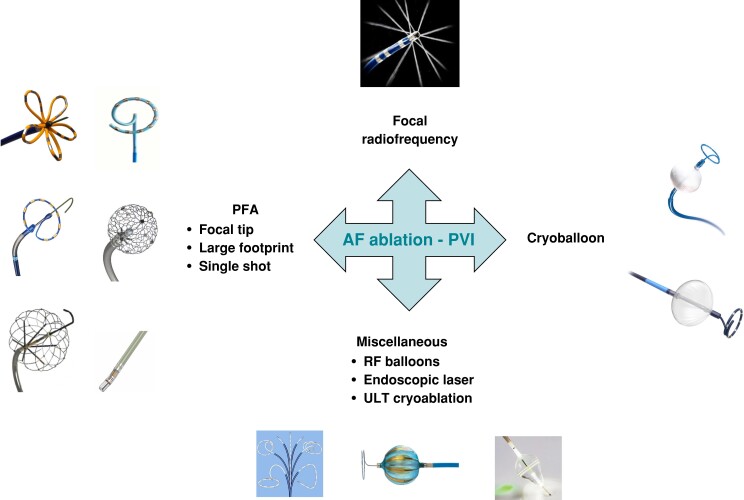
Single-shot ablation tools during 25 years of innovation
Several tools have been introduced to perform PV isolation with single-shot devices. Apart from cryoballoon, which has been described in previous section, several energy forms have been used including laser, high-intensity focus ultrasound, RF, and pulsed field activity (Figure 3).62
Figure 3.
New catheters to deliver ablation for AF.
Endoscopic laser balloon device
This ablation system is featured by a balloon-based ablation system that can deliver laser energy and an endoscope that enables an endoscopic view of the PV. Pulmonary vein isolation is performed under direct visual endoscopic control.63 No real-time electrograms (EGMs) of the PVs are provided; therefore, a separate circular mapping catheter is used to confirm successful PVI. Head-to-head comparison with RF showed no differences in terms of freedom from AF64 and good long-term results.65
The second-generation balloon system Heartlight (Excalibur, CardioFocus) includes real-time balloon sizing with different sizes of PV,66 and the third-generation balloon system Heartlight X3 (CardioFocus) provides an automated laser source rotation.67
High-intensity focused ultrasound (HIFU)
This system is mainly based on a fluid-filled balloon catheter with an ultrasound transducer and a dorsally attached parabolic CO2 balloon that can focus a 25 mm ring of ultrasound energy.68 This technology has several limitations, mainly based on difficulties in achieving PVI and periprocedural complication.69
Radiofrequency balloon
Radiofrequency energy has been added also in balloon systems. Currently, three systems are available. The RF hot balloon uses thermal energy conducted by the heated balloon to ablate the tissue, rather than direct RF ablation. This balloon achieves a target temperature of up to 70°C. Hot balloon PVI seems to create smaller antral lesions due to the more distal occlusion of the PV antrum. On the other side, efficacy in terms of AF freedom is similar to the one achieved with cryoballoon.70
Another recently produced RF balloon ‘Heliostar’ (Biosense Webster, Diamond Bar, California) is a spherical balloon catheter that uses irrigated RF energy. This catheter is a 28 mm balloon with ten gold-plated electrodes on the surface. Each electrode can deliver RF energy and can measure the temperature reached during ablation.
An inner lumen in the catheter can be used for the placement of a 3-F circular mapping catheter (Lassostar, Biosense Webster) that can record PV potentials and can be integrated into the electroanatomic mapping system (CARTO 3 Biosense Webster). Therefore, an anatomic map and voltage data pre- and post-ablation can be obtained. Observational registries showed good safety and efficacy profile of this device71. Impedance drop (>19.2 Ω) and temperature rise (>11.1°C) were associated with persistent single-shot isolation.72
The Luminize balloon catheter (Boston Scientific) is a multipolar RF system which has an endoscopic view of the PV through a camera. It is a 28 mm balloon with 18 electrodes providing RF energy; 12 electrodes are located along the equator of the device, and 6 electrodes are placed forward. Ablation electrodes can be together or independently selected. Preliminary data from a multicentre registry showed a good safety profile and 1 year survival freedom from any atrial tachycardia of 77.5%.73
Pulsed field ablation
Mechanism of action
Pulsed field ablation is a largely non-thermal energy modality which acts by exposing cardiac tissue to a short but intense electrical field, resulting in irreversible nanoscale pore formation on the lipid bilayer (electroporation) and subsequent cell death.74 The magnitude of mediated effect is dependent on catheter design, degree of electrode–tissue contact, and parameters of delivery protocol such as voltage, waveform shape, packet duration, and number of packets delivered.74 In this context, differences in proprietary delivery protocols of variant PFA systems result in diverse treatment effect. Numerous catheters with different designs (focal tip, larger footprint, and single-shot) are in different stages of development and clinical testing.75,76 Integration of PFA catheters with 3D electro-anatomical mapping systems and the possibility of switching between PF and RF energy enables the assessment of underlying substrate, versatility in energy modality, tagging of lesions, identification of gaps, and potentially reduction in fluoroscopy use.77
Safety profile
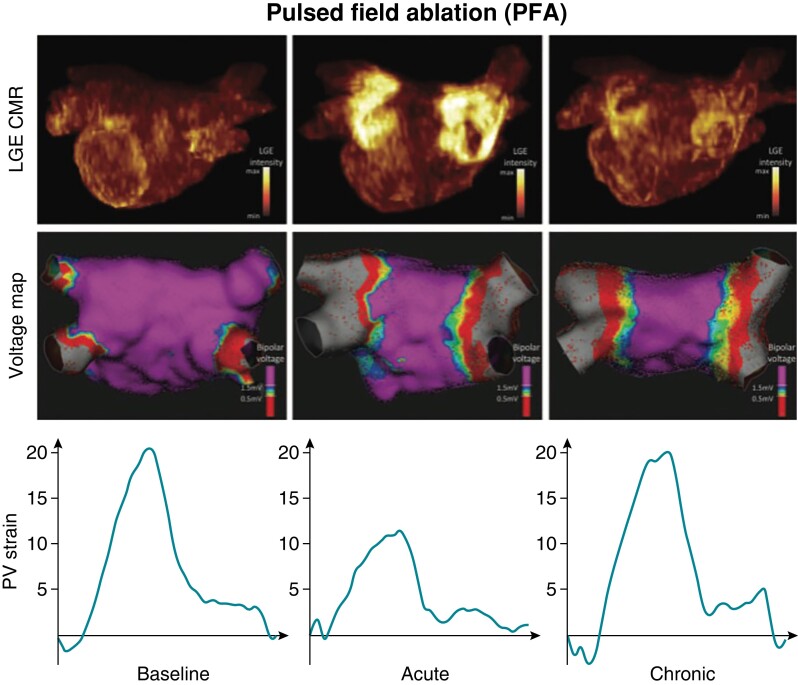
Pulsed field ablation lesions have specific characteristics with implications for clinical practice. The threshold for irreversible electroporation is tissue specific and relatively lower for cardiac tissue in relation to neighbouring structures.78 Pulsed field ablation–mediated myocardial cell death is associated with preservation of extracellular matrix architecture, reduced chronic atrial fibrosis, preserved atrial tissue compliance, and recovery of atrial mechanical function following ablation (Figure 4).79 These lesion characteristics enhance procedural safety by sparing collateral damage to neighbouring structures and by attenuating ablation-mediated restrictive physiology [e.g. stiff left atrial (LA) syndrome].80 Furthermore, PFA has minimal impact on the cardiac autonomic ganglionated plexi.81
Figure 4.
Top panels show MRI measurements at different stages before and after PFA. Middle panels show voltage maps before and acutely and chronically after PFA. Bottom panels show strain patterns before and acutely and chronically after PFA.
The first in-human trials and cohort studies have reported no oesophageal complications or phrenic nerve injury or PV stenosis in AF patients treated with PFA.82 More clinical data are needed to accurately assess procedural safety and the incidence of more rare complications such atrio-oesophageal fistula. Despite discrepant pre-clinical evidence, coronary arterial vasospasm has been reported when PFA is delivered with a pentaspline catheter immediately adjacent to coronary arteries, and nitroglycerin can attenuate or prevent this complication.83,84 It is not yet clarified whether this complication is also encountered with other PFA catheters or waveforms. Initial evidence of PFA procedural safety and lesion transmurality may reduce the threshold for delivery of adjunctive ablation beyond PVI in non-paroxysmal AF patients.
Procedural efficacy
Clinical evidence with different types of PFA catheters have demonstrated almost 100% acute PVI with enlarged LA isolation areas and increased durability documented by invasive remapping.85–88 A large, multinational European study enrolling 1233 consecutive paroxysmal and PeAF patients treated with a pentaspline PFA catheter has shown excellent acute performance in PVI achievement with procedural duration of about 1 h.89 Prospective, multicentre trials have validated the long-term clinical efficacy of PFA ablation, which was shown to be consistent with other energy modalities after indirect comparisons with RF or cryoballoon ablation studies with similar rigorous rhythm follow-up.90,91 However, the jury is still out in the wait of direct head-to-head, adequately powered randomized trials that will shed light on the relative efficacy and safety of PFA to thermal energy modalities.
Approach to persistent atrial fibrillation ablation
Catheter ablation of PeAF has proven very challenging over the years. The results in terms of freedom from recurrent arrhythmia are consistently worse after one or more procedures compared with paroxysmal AF.92 Part of the challenge is that the definition of PeAF encompasses a variable and complex group of patients for whom ablation may have greater or reduced efficacy. Persistent AF is defined arbitrarily as a duration of more than 7 days of continuous AF.92 Further, equally arbitrary sub-definitions include ‘early persistent’ which is AF continuing for up to 3 months; AF lasting more than 1 year continuously is ‘long-standing persistent’.92 However, when continuous monitoring is employed, patients with assumed PeAF may be found to have paroxysmal AF and vice versa.93,94 Late gadolinium-enhanced magnetic resonance imaging (MRI) has also shown that the degree of fibrotic remodelling of the atrium correlates little to whether the patient has paroxysmal, persistent, or long-standing PeAF.95 This ambiguity further complicates our ability to assess the success of various ablation techniques in PeAF.
According to current guidelines, any ablation procedure for PeAF should include PV antral isolation (PVAI).3 This is considered the ‘cornerstone’ of PeAF ablation and is based on early findings that reveal most triggers of AF originating in the PVs. Unfortunately, the outcome of PVAI alone in PeAF appears to be at least 15–40% worse compared with outcomes in paroxysmal AF.96 The gap appears to have narrowed more recently as we have developed better technologies to achieve durable PVAI. Cryoballoon technology, for example, may produce higher rates of durable PVAI compared with traditional RF.97 There have been several studies performed using a cryoballoon-based PVAI for PeAF without additional lesions which have reported single procedure success of about 60%.97,98 Newer open irrigation RF catheters coupled with CF sensing and, more recently, integration of highly sensitive tissue-tip temperature sensing may be improving PVAI outcomes in PeAF patients. In one trial, the 1 year freedom from AF was 71% (61% off drugs) for PeAF.99 One Norwegian study ultimately showed no difference between cryoballoon and CF RF with about 65% freedom from arrhythmia in both groups overall.100 There is still a significant proportion of patients, however, that will not be addressed by PVAI alone.
If PVAI is not enough for some patients, then could addition of further lesion sets on top of PVAI perform better? This was based on early work from the Cox Maze III surgical approach of isolating the posterior wall and PVs in addition to extensive additional linear scar creation in the left and right atria.3 This technique appeared to have a high success rate in PeAF patients. In fact, the 2012 Heart Rhythm Society Consensus Statement for Catheter Ablation of AF recommended that ‘operators should consider more extensive ablation based on linear lesions or complex fractionated electrograms’ in addition to PVAI for PeAF ablation (Figure 5).92 Some early pilot trials, like STAR AF 1, suggested there may be a clinical benefit when these techniques were applied using catheter ablation.101
Figure 5.
Examples of typical substrate ablation strategies beyond PVI. From: Eur Heart J, Volume 31, Issue 11, June 2010, Pages 1344–1356, https://doi.org/10.1093/eurheartj/ehq041.101
The hypothesis was more definitively tested in the STAR AF II study which compared PVAI alone, PVAI plus linear ablation (specifically a roof line and a mitral isthmus line), or PVAI plus ablation of complex fractionated EGMs for PeAF patients.102 Contrary to prevailing opinion at that time, the trial showed that the arms employing additional ablation showed no advantage in freedom from AF or atrial arrhythmias compared with PVAI alone. The overall success rate after one procedure was about 50%. Guidelines changed, and the addition of lesions beyond PVAI was given level IIb ratings, stating that further study and new targets may be required.92 Practice patterns also changed dramatically with European surveys suggesting that most operators had moved to a PVAI approach alone with very few adding linear or EGM-based ablation.96 Network and other meta-analyses of trials like STAR AF also failed to show improvement with strategies beyond PVAI.103,104 While the 50% success rate and lack of difference in the arms of STAR AF II were disappointing, a subsequent analysis of AF burden in these patients showed that 80% of patients experienced more than a 90% reduction in their AF burden which was correlated with improved quality of life.105 STAR AF II also subsequently started a search for new targets beyond linear and fractionated EGM ablation. In the large, Japanese EARNEST AF trial which came after STAR AF II, they showed a benefit to a ‘PVI-plus’ strategy and this helped propel a search for better targets.9
Scar-based mapping of the atria has also garnered a lot of interest since STAR AF II. Since fibrosis is the last common pathway of all electrical and physical remodelling of the atria, it is believed that characterizing fibrosis and homogenizing it with ablation could improve outcome in PeAF. Characterization of scar has been done with both electro-anatomical mapping, defining low-voltage regions as ‘scar’, or through late gadolinium MRI of the atrium.106 One of the key limitations to this approach is how scar should be defined. The low-voltage cut-off for mapping scar varies from 0.05 to 0.5 mV, and the thin atrial wall makes accurate MRI definition of scar thresholds challenging to perform reproducibly. Furthermore, there may be a mismatch between regions of scar localized by mapping and MRI.106 The study outcomes have been mixed. ERASE AF was the first RCT demonstrating a significant benefit [hazard ratio (HR) 0.62, 95% CI 0.43–0.88] adding low-voltage area (LVA) ablation to PVI alone, recently corroborated by Chen et al. showing a statistically significant reduction in atrial tachyarrhythmia recurrence (15% vs. 24%, HR 0.61, 95% CI 0.38–0.95).107 The large-scale DECAAF II study employed an MRI-based scar ablation strategy but did not show any benefit over PVAI alone and showed increased serious complications with the scar-based ablation.108 The ongoing COAST AF study will provide further answers to this important question.109
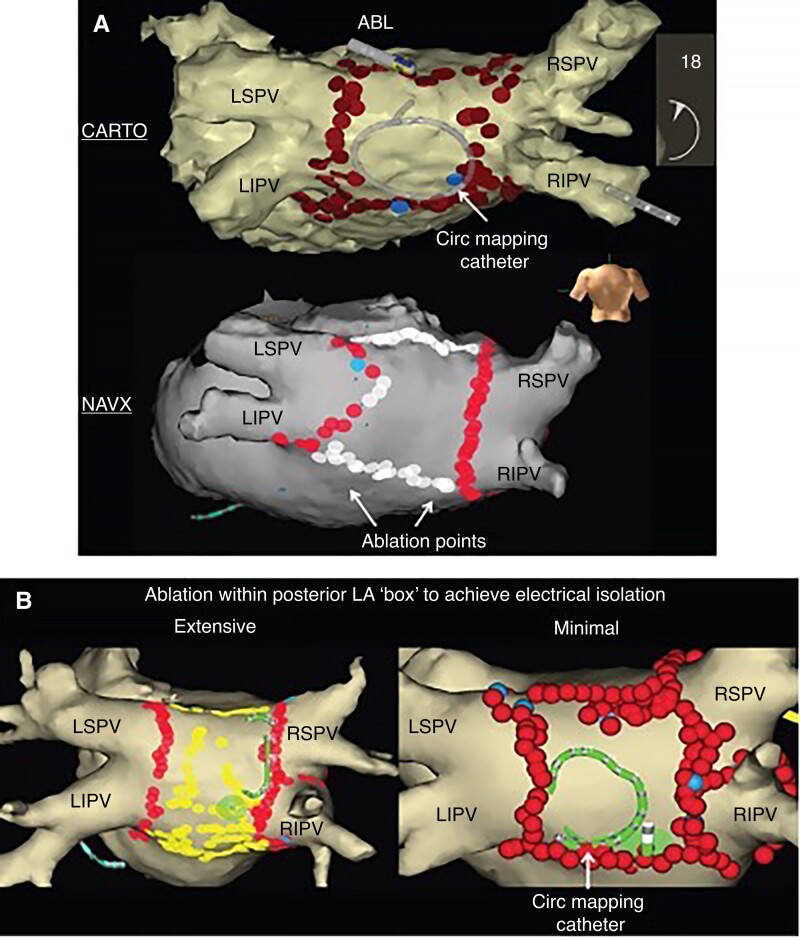
Persistent AF may also be treated with empiric isolation of atrial structures, namely the posterior wall and/or the LA appendage, in addition to PVAI. The posterior wall is a very common approach given the relative simplicity of ablating the region between two encircling PV lesions surrounding the left and right veins (Figure 6). The posterior wall is embryologically related to the PVs110 and is a common site for non-PV triggers and scar formation.95 It was also isolated during the Cox Maze III procedure. Ablation of the posterior wall could result in a higher risk of oesophageal damage, but with the advent of PFA, it is often being targeted because of the almost zero risk of oesophageal injury. A recent meta-analysis of these studies has suggested an overall relative reduction in atrial arrhythmia recurrence of 45% with additive ablation of the posterior wall.111 However, in a large, randomized trial comparing PVAI with PVAI plus posterior wall isolation, the CAPLA trial failed to show any difference.112 CAPLA was a very well-designed trial, but the population was more of an early persistent group. Whether posterior wall isolation can benefit a more long-standing persistent population is not well known.
Figure 6.
(A) Posterior wall ablation strategy. (B) Ablation within the margins of the posterior box (representative of extensive or limited ablation within box). LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein. From Europace, Volume 19, Issue 12, December 2017, Pages 1958–1966, https://doi.org/10.1093/europace/euw231.
Some groups have aggressively targeted the LA appendage for isolation with reporting better outcomes compared with PVAI alone.113 The thickness of the appendage can make it very challenging to isolate, and ablation within the appendage can cause perforation because of the thin tissue distally. The use of cryoballoon technology may help to make isolation of the appendage easier, but data are still limited.114 The frequency of triggers coming from the LA appendage has also been debated with some reporting that it contributes to only a minority of patients with long-standing PeAF. The AMAZE trial evaluated the use of a suture-based epicardial snare device to ligate the appendage as an adjuvant strategy to PVAI in PeAF patients.115 This trial reported no benefit of the approach in terms of arrhythmia recurrence, and it was associated with a high incidence of major complications. One of the key limitations of this approach is the risk of thrombus formation in an isolated appendage which requires either meticulous oral anticoagulation or percutaneous occlusion to avoid the risk of stroke.111
Finally, ablation of non-PV sources can also be performed, especially since they may be more common in the PeAF population.116 About 11% of patients may have non-vein triggers that are seen to initiate AF, but if frequent premature atrial beats are included (without documented initiation of AF), then more than 50% of patients may have potential non-vein triggers identified.117 Frequent sites of non-PV triggers include the superior vena cava, the crista terminalis, the inter-atrial septum, posterior LA wall, coronary sinus, ligament of Marshall, and the LA appendage. Limited studies have shown that PVAI followed by systematic provocation and ablation of all non-vein triggers can significantly improve freedom from recurrence in PeAF patients.117 Triggers are typically provoked by high-dose isoproterenol infusion (20–30 μg/min) for at least 10–15 min. The definition of a trigger remains a main limitation. Some will only ablate premature atrial beats demonstrated to initiate AF, while others ablate all premature beats seen. The former approach is time-consuming and may require multiple cardioversions, while the latter can result in extensive ablation. Localization of a premature beat is also challenging, often requiring multiple catheters inside the atria to help identify the origin, but even with this approach, exact localization can be difficult, and empiric ablation in a region of early activation may be employed. However, this approach does not guarantee that the specific trigger has been eliminated. Empiric ablation of the superior vena cava118 and the coronary sinus119 has been proposed as adjuvant strategies, but data are not compelling, and fewer than 15% of patient will demonstrate triggers from these regions.
In summary, we still do not know the optimal method of ablating PeAF. Strategies beyond PVAI have generally shown little benefit in larger trials. Even if a strategy works for selected operators, it cannot be recommended if larger application in multiple centres fails to show the same benefit. Even if a strategic target is found, how we ablate it (encircle, eliminate, and connect to an anatomical boundary); how to create durable, transmural lesions; and how to avoid pro-arrhythmia from these added lesions remain big challenges. Hopefully, a combination of technological development, creative thinking, and large-scale trials will eventually find the best approach.
Mapping of atrial fibrillation
Ever since the seminal publication by Haissaguerre,1 PVI has been the cornerstone of catheter ablation of AF and is mandated for every patient embarking on the ablation journey.3 Pulmonary vein isolation lends itself to an empiric, anatomy-based approach, and other than assessment of electrical isolation, no electrophysiological mapping is required (and even the necessity of PV electrical assessment has been questioned).120 However, PVI alone only achieves effective rhythm control in a proportion, and for patients with PeAF in particular, adjunctive ablation beyond the PVs (‘PVI-plus’) is often needed. Knowing what the ‘plus’ should be has been the focus of more than two decades of research.
Empiric approaches to PVI-plus, be it linear lesions, isolation of additional structures such as the SVC or LA appendage, and more recently, posterior wall isolation, have shown limited or no additional benefit when tested in large-scale randomized controlled trials.121 This has directed ablation for AF back to the roots of cardiac electrophysiology—mapping of the arrhythmia substrate—so that an individualized approach can be taken.
It could be argued that ablation of complex fractionated atrial EGMs (CFAEs), believed to be representative of underlying slow-velocity, rotational activation driving AF, was an early form of ablation guided by mapping; however, the technique was extremely subjective, often with no correlation between sites with CFAEs in AF and low-voltage or abnormal EGMs in sinus rhythm.122 When compared with PVI alone, adjunctive CFAE ablation offered no significant improvement in arrhythmia-free survival but was associated with increases in both procedural and fluoroscopy times.121 Other researchers have hypothesized that it is repetitive, regular activity that unmasks stable rotors and have proposed using conventional electro-anatomical mapping systems (Abbott EnSite Velocity) and bipolar EGMs collected from roving multipolar mapping catheters to perform a mean cycle length and cycle length standard deviation map.123 Repetitive–regular activity ablation more frequently resulted in acute arrhythmia termination and was associated with higher arrhythmia freedom during the follow-up.
An alternative strategy is to elucidate the underlying electrophysiological substrate through voltage (and by inference) scar mapping. In a randomized trial, an individually tailored substrate modification guided by voltage mapping was associated with a significantly higher arrhythmia-free survival rate compared with a conventional approach (68% vs. 42%).124 This is supported by a meta-analysis, with the caveat that there is a heterogeneous mix of patients and approaches and, perhaps, only a minority of patient have LVAs.125 Non-invasive detection of LVAs that can be targeted with adjunctive ablation using cardiac MRI has failed to demonstrate improved outcomes, which is unsurprising when investigators have shown a mismatch between delayed enhancement and low-voltage and arrhythmogenic areas defined by EGM criteria.106
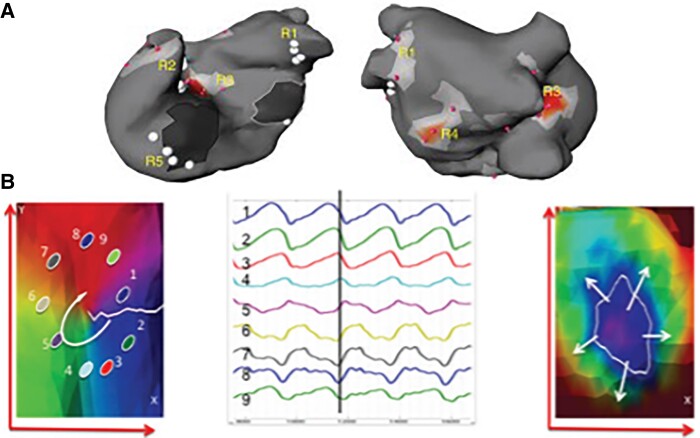
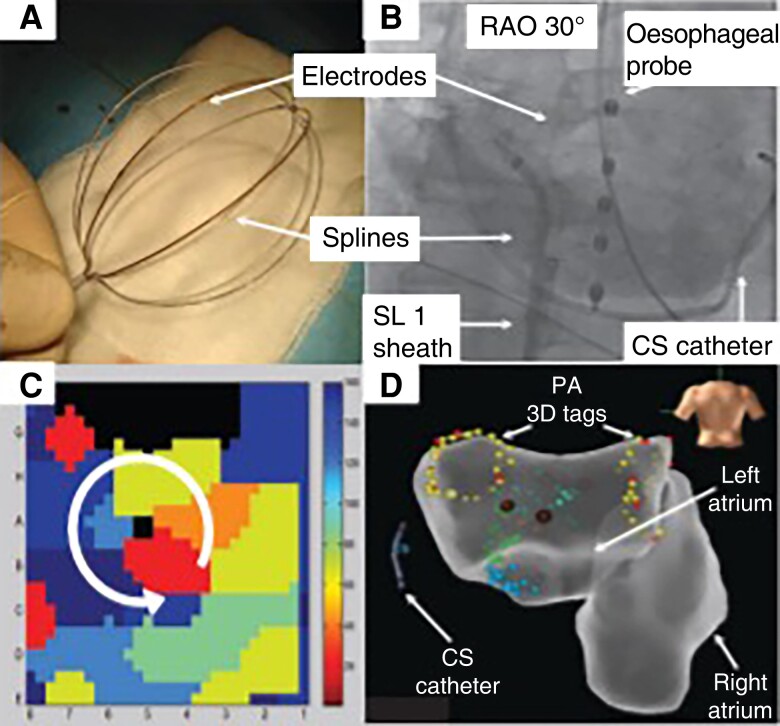
Focal impulse and rotor mapping (FIRM) utilizes a 64-pole basket catheter (Abbott Topera), aiming to cover >80% of the atrial surface, collecting monophasic action potential signals to undertake phase mapping of AF (Figure 7). Phase mapping is a mathematical technique to detect spatial and temporal periodicity and identify stable periodic rotations (i.e. rotors) and focal sources. Both the left and right atrium can be mapped. Analysis has shown that there are no characteristic EGM potentials that identify rotor activity.127 Multicentre studies have shown the technology to be safe, but efficacy outcomes have been disappointing and promising early results not replicated by other users, whether in de novo or re-do patients.128–130
Figure 7.
(A and B) See legend in the figure. From AFACART: Knecht, S., et al., Multicenter evaluation of non-invasive biatrial mapping for persistent atrial fibrillation ablation: the AFACART study. Europace, 2017. 19(8): p. 1302–1309.126
Non-invasive ECGi body surface potential mapping with a 256-electrode vest (CardioInsight) was examined in the multicentre AFACART trial, where AF drivers were initially targeted, followed by PV isolation, and then linear lesions if AF hadn’t terminated (Figure 8).126 Driver ablation alone resulted in acute termination of AF in 64% of patients. Although AF-free survival at 1 year was favourable at 78%, half of patients had AT recurrence requiring further management.
Figure 8.
See legend in the figure. From Lin, T., et al., Focal impulse and rotor modulation using the novel 64-electrode basket catheter: electrogram characteristics of human rotors. Europace, 2015. 17(12): p. 1791–7.127
An in-depth review of rotor mapping and ablation, highlighting the complex, three-dimensional spatiotemporal structure of spiral wavefront propagation and how that translated to relatively low-density contact endocardial or non-contact epicardial mapping, plus some of the limitations of phase mapping (i.e. identifying false positive rotors which are actually two wavefronts going in opposite directions on either side of a line of block), offers a number of explanations for these mapping systems’ fallibility.131
A more recent advance is non-contact charge density mapping (Acutus Medical). Using ultrasound to reconstruct atrial geometry, farfield unipolar EGMs from a 48-pole basket catheter calculate dipoles at >3000 vertices on the chamber surface and show propagation maps characterizing focal firing, rotational activation, and localized, irregular activation. Stable and repetitive phenomena are ablated using a core-to-boundary approach to minimize any pro-arrhythmic effect of lesion creation. In a challenging group of re-do AF ablation patients, a multicentre trial has shown a 74% freedom from AF at the 12 month follow-up (91% if only PVI performed at the de novo ablation).132 The mapping system also facilitates a more rapid understanding of organized sustained or non-sustained atrial tachycardias when compared with conventional electro-anatomical mapping.133
In the ultimate form of an individualized approach, computer modelling can be used to simulate electrophysiological mapping of AF mechanisms and the effect of ‘virtual’ ablation lesions. In a study comparing mapping-guided ablation to standard approaches, targeting high dominant frequency regions had a success of >98% despite only isolating 5–6% of the LA myocardium. In contrast, conventional ablation strategies targeting anatomical or structural substrate resulted in isolation of up to 20% of LA myocardium.134 Modelling studies support the need for minimizing the area of ablated tissue to prevent the formation of a pro-arrhythmic milieu while directing lesions to the key areas that drive the arrhythmia mechanism.
Although a recent consensus document advocates the pressing need to understand and identify AF mechanisms through electrophysiological mapping, it also acknowledges that currently available AF recording and processing technologies have technological limitations.135 Improvements in AF mapping by obtaining highest fidelity source signals for signal processing combined with novel acquisition instruments will enable enhanced and automated interpretation of EGM recordings.
Contributor Information
Lucas Boersma, Cardiology Department, St. Antonius Hospital Nieuwegein/Amsterdam University Medical Center, PO 2500, 3430 EM Nieuwegein, The Netherlands.
Jason G Andrade, Department of Medicine, University of British Columbia, Vancouver, Canada; Cardiology Department, Center for Cardiovascular Innovation, Vancouver, Canada; Montreal Heart Institute, Department of Medicine, Université de Montréal, Montreal, Canada.
Tim Betts, Department of Cardiology, Oxford University, Oxford, UK.
Mattias Duytschaever, Cardiology Department, AZ St. Jan, Brugge, Belgium.
Helmut Pürerfellner, Ordensklinikum Linz Elisabethinen, Academic Teaching Center, Linz, Austria.
Francesco Santoro, Department of Medical and Surgery Sciences, University of Foggia, Foggia, Italy.
Stylianos Tzeis, Cardiology Department, Mitera Hospital, Hygeia Group, Athens, Greece.
Atul Verma, Cardiology Department, McGill University Health Center, Montreal, Quebec, Canada.
Data availability
All relevant data are within the manuscript and can be derived from the papers that are reviewed.
References
- 1. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou Get al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 2. Boersma L. New energy sources and technologies for atrial fibrillation catheter ablation. Europace 2022;24:ii44–51. [DOI] [PubMed] [Google Scholar]
- 3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C; for the ESC Scientific Document Group . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 4. Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale Aet al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. Jama 2010;303:333–40. [DOI] [PubMed] [Google Scholar]
- 5. Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbia Ret al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498–505. [DOI] [PubMed] [Google Scholar]
- 6. Mont L, Bisbal F, Hernandez-Madrid A, Perez-Castellano N, Vinolas X, Arenal Aet al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Hear J 2014;35:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HTet al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014;64:647–56. [DOI] [PubMed] [Google Scholar]
- 8. Arbelo E, Brugada J, Hindricks G, Maggioni A, Tavazzi L, Vardas Pet al. The atrial fibrillation ablation pilot study: a European survey on methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J 2014;35:1466–78. [DOI] [PubMed] [Google Scholar]
- 9. Hikoso S, Masuda M, Furukawa Y, Hirata A, Egami Y, Watanabe Tet al. Pulmonary vein isolation alone vs. more extensive ablation with defragmentation and linear ablation of persistent atrial fibrillation: the EARNEST-PVI trial. Europace 2021;23:565–74. [DOI] [PubMed] [Google Scholar]
- 10. Dagres N, Bongiorni MG, Larsen TB, Hernandez-Madrid A, Pison L, Blomström-Lundqvist C. Current ablation techniques for persistent atrial fibrillation: results of the European Heart Rhythm Association Survey. Europace 2015;17:1596–600. [DOI] [PubMed] [Google Scholar]
- 11. Bertaglia E, Tondo C, De Simone A, Zoppo F, Mantica M, Turco Pet al. Does catheter ablation cure atrial fibrillation? Single-procedure outcome of drug-refractory atrial fibrillation ablation: a 6-year multicentre experience. Europace 2010;12:181–7. [DOI] [PubMed] [Google Scholar]
- 12. Duytschaever M, Demolder A, Phlips T, Sarkozy A, El Haddad M, Taghji Pet al. Pulmonary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J 2018;39:1429–37. [DOI] [PubMed] [Google Scholar]
- 13. Thomas S, Aggarwal G, Boyd A, Jin Y, Ross D. A comparison of open irrigated and non-irrigated tip catheter ablation for pulmonary vein isolation. Europace 2004;6:330–5. [DOI] [PubMed] [Google Scholar]
- 14. Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera Cet al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Hear Rhythm 2012;9:1789–95. [DOI] [PubMed] [Google Scholar]
- 15. Kautzner J, Neuzil P, Lambert H, Peichl P, Petru J, Cihak Ret al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace 2014;16:1459–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Itoh T, Kimura M, Tomita H, Sasaki S, Owada S, Horiuchi Det al. Reduced residual conduction gaps and favourable outcome in contact force-guided circumferential pulmonary vein isolation. Europace 2016;18:531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Squara F, Latcu DG, Massaad Y, Mahjoub M, Bun SS, Saoudi N. Contact force and force-time integral in atrial radiofrequency ablation predict transmurality of lesions. Europace 2014;16:660–7. [DOI] [PubMed] [Google Scholar]
- 18. Whitaker J, Fish J, Harrison J, Chubb H, Williams SE, Fastl Tet al. Lesion index-guided ablation facilitates continuous, transmural, and durable lesions in a porcine recovery model. Circ Arrhythmia Electrophysiol 2018;11. [DOI] [PubMed] [Google Scholar]
- 19. Das M, Loveday J, Wynn G, Gomes S, Saeed Y, Bonnett Jet al. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace 2017;19:775–83. [DOI] [PubMed] [Google Scholar]
- 20. Miller M, d'Avila A, Dukkipati S, Koruth J, Viles-Gonzalez J, Napolitano Cet al. Acute electrical isolation is a necessary but insufficient endpoint for achieving durable PV isolation: the importance of closing the visual gap. Europace 2012;14:653–60. [DOI] [PubMed] [Google Scholar]
- 21. Taghji P, El Haddad M, Phlips T, Wolf M, Knecht S, Vandekerckhove Yet al. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol 2018;4:99–108. [DOI] [PubMed] [Google Scholar]
- 22. Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Yet al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. Europace 2018;20:f419–27. [DOI] [PubMed] [Google Scholar]
- 23. Duytschaever M, Vijgen J, De Potter T, Scherr D, Van Herendael H, Knecht Set al. Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: the multicentre VISTAX trial. Europace 2020;22:1645–52. [DOI] [PubMed] [Google Scholar]
- 24. Teres C, Soto-Iglesias D, Penela D, Jáuregui B, Ordoñez A, Chauca Aet al. Personalized paroxysmal atrial fibrillation ablation by tailoring ablation index to the left atrial wall thickness: the ‘Ablate by-LAW’ single-centre study-a pilot study. Europace 2022;24:390–9. [DOI] [PubMed] [Google Scholar]
- 25. O'Neill L, Karim R, Mukherjee R, Whitaker J, Sim I, Harrison Jet al. Ablation index-guided point-by-point workflow: cardiovascular magnetic resonance assessment of left atrial scar formation. Europace 2019;21:1817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bourier F, Duchateau J, Vlachos K, Lam A, Martin CA, Takigawa Met al. High-power short-duration versus standard radiofrequency ablation: insights on lesion metrics. J Cardiovasc Electrophysiol 2018;29:1570–5. [DOI] [PubMed] [Google Scholar]
- 27. Chieng D, Segan L, Sugumar H, Al-Kaisey A, Hawson J, Moore Bet al. Higher power short duration vs. lower power longer duration posterior wall ablation for atrial fibrillation and oesophageal injury outcomes: a prospective multi-centre randomized controlled study (Hi-Lo HEAT trial). Europace 2023;25:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berte B, Hilfiker G, Russi I, Moccetti F, Cuculi F, Toggweiler Set al. Pulmonary vein isolation using a higher power shorter duration CLOSE protocol with a surround flow ablation catheter. J Cardiovasc Electrophysiol 2019;30:2199–204. [DOI] [PubMed] [Google Scholar]
- 29. Chen S, Schmidt B, Bordignon S, Urbanek L, Tohoku S, Bologna Fet al. Ablation index-guided 50W ablation for pulmonary vein isolation in patients with atrial fibrillation: procedural data, lesion analysis, and initial results from the FAFA AI High Power Study. J Cardiovasc Electrophysiol 2019;30:2724–31. [DOI] [PubMed] [Google Scholar]
- 30. O’Brien J, Obeidat M, Kozhuharov N, Yew Ding W, Tovmassian L, Bierme Cet al. Procedural efficiencies, lesion metrics, and 12-month clinical outcomes for ablation index-guided 50Wablation for atrial fibrillation. Europace 2021;23:878–86. [DOI] [PubMed] [Google Scholar]
- 31. Müller J, Nentwich K, Berkovitz A, Ene E, Sonne K, Zhuravlev Vet al. Acute oesophageal safety and long-term follow-up of AI-guided high-power short-duration with 50 W for atrial fibrillation ablation. Europace 2023;25:1379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wielandts JY, Kyriakopoulou M, Almorad A, Hilfiker G, Strisciuglio T, Phlips Tet al. Prospective randomized evaluation of high power during CLOSE-guided pulmonary vein isolation: the POWER-AF study. Circ Arrhythmia Electrophysiol 2021;14:49–55. [DOI] [PubMed] [Google Scholar]
- 33. Shin D G, Ahn J, Han S, Euy Lim H. Efficacy of high-power and short-duration ablation in patients with atrial fibrillation: a prospective randomized controlled trial. Europace 2020;22:1495–501. [DOI] [PubMed] [Google Scholar]
- 34. Popa M, Bourier F, Lengauer F, Krafft H, Bahlke F, Förschner Let al. Safety profile and long-term efficacy of very high-power short-duration (60–70 W) catheter ablation for atrial fibrillation: results of a large comparative analysis. Europace 2023;25:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kautzner J, Albenque JP, Natale A, Maddox W, Cuoco F, Neuzil Pet al. A novel temperature-controlled radiofrequency catheter ablation system used to treat patients with paroxysmal atrial fibrillation. JACC Clin Electrophysiol 2021;7:352–63. [DOI] [PubMed] [Google Scholar]
- 36. Almorad A, Wielandts JY, El Haddad M, Knecht S, Tavernier R, Kobza Ret al. Performance and safety of temperature- and flow-controlled radiofrequency ablation in ablation index–guided pulmonary vein isolation [internet]. JACC Clin. Electrophysiol 2021;7:408–9. [DOI] [PubMed] [Google Scholar]
- 37. Rozen G, Ptaszek L, Zilberman I, Douglas V, Heist K, Beeckler Cet al. Safety and efficacy of delivering high-power short-duration radiofrequency ablation lesions utilizing a novel temperature sensing technology. Europace 2018;20:f444–50. [DOI] [PubMed] [Google Scholar]
- 38. Bortone A, Albenque J-P, Ramirez FD, Haïssaguerre M, Combes S, Constantin Met al. 90 vs 50-watt radiofrequency applications for pulmonary vein isolation: experimental and clinical findings. Circ Arrhythmia Electrophysiol 2022:252–60. [DOI] [PubMed] [Google Scholar]
- 39. Nakagawa H, Ikeda A, Sharma T, Govari A, Ashton J, Maffre Jet al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with high power-short duration and moderate power-moderate duration effects of thermal latency and contact force on lesion formation. Circ Arrhythmia Electrophysiol 2021;14:605–17. [DOI] [PubMed] [Google Scholar]
- 40. Reddy VY, Grimaldi M, De Potter T, Vijgen JM, Bulava A, Duytschaever MFet al. Pulmonary vein isolation with very high power, short duration, temperature-controlled lesions: the QDOT-FAST trial. JACC Clin Electrophysiol 2019;5:778–86. [DOI] [PubMed] [Google Scholar]
- 41. Heeger C, Sano M, Popescu S, Subin B, Feher M, Phan Het al. Very high-power short-duration ablation for pulmonary vein isolation utilizing a very-close protocol-the FAST AND FURIOUS PVI study. Europace 2022:euac243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Halbfass P, Wielandts J-Y, Knecht S, Le Polain de Waroux J-B, Tavernier R, De Wilde Vet al. Safety of very high-power short-duration radiofrequency ablation for pulmonary vein isolation: a two-centre report with emphasis on silent oesophageal injury. Europace 2021;24:400–5. [DOI] [PubMed] [Google Scholar]
- 43. O'Neill L, El Haddad M, Berte B, Kobza R, Hilfiker G, Scherr Det al. Very high-power ablation for contiguous pulmonary vein isolation: results from the randomized POWER PLUS trial. JACC Clin Electrophysiol 2022. S2405-500X(22)01048-9. [DOI] [PubMed] [Google Scholar]
- 44. Khairy P, Dubuc M. Transcatheter cryoablation part I: preclinical experience. Pacing Clin Electrophysiol 2008;31:112–20. [DOI] [PubMed] [Google Scholar]
- 45. Cooper IS, Lee AS. Cryostatic congelation: a system for producing a limited, controlled region of cooling or freezing of biologic tissues. J Nerv Ment Dis 1961;133:259–63. [PubMed] [Google Scholar]
- 46. Gallagher JJ, Sealy WC, Anderson RW, Kasell J, Millar R, Campbell RWet al. Cryosurgical ablation of accessory atrioventricular connections: a method for correction of the pre-excitation syndrome. Circulation 1977;55:471–9. [DOI] [PubMed] [Google Scholar]
- 47. Tse HF, Reek S, Timmermans C, Lee KL, Geller JC, Rodriguez LMet al. Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J Am Coll Cardiol 2003;42:752–8. [DOI] [PubMed] [Google Scholar]
- 48. Wong T, Markides V, Peters NS, Davies DW. Percutaneous pulmonary vein cryoablation to treat atrial fibrillation. J Interv Card Electrophysiol 2004;11:117–26. [DOI] [PubMed] [Google Scholar]
- 49. Skanes AC, Jensen SM, Papp R, Li J, Yee R, Krahn ADet al. Isolation of pulmonary veins using a transvenous curvilinear cryoablation catheter: feasibility, initial experience, and analysis of recurrences. J Cardiovasc Electrophysiol 2005;16:1304–8. [DOI] [PubMed] [Google Scholar]
- 50. Van Belle Y, Janse P, Rivero-Ayerza MJ, Thornton AS, Jessurun ER, Theuns Det al. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur Heart J 2007;28:2231–7. [DOI] [PubMed] [Google Scholar]
- 51. Van Belle Y, Janse P, Theuns D, Szili-Torok T, Jordaens L. One year follow-up after cryoballoon isolation of the pulmonary veins in patients with paroxysmal atrial fibrillation. Europace 2008;10:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Andrade JG, Khairy P, Guerra PG, Deyell MW, Rivard L, Macle Let al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm 2011;8:1444–51. [DOI] [PubMed] [Google Scholar]
- 53. Pandya B, Sheikh A, Spagnola J, Bekheit S, Lafferty J, Kowalski M. Safety and efficacy of second-generation versus first-generation cryoballoons for treatment of atrial fibrillation: a meta-analysis of current evidence. J Interv Card Electrophysiol 2016;45:49–56. [DOI] [PubMed] [Google Scholar]
- 54. Aytemir K, Gurses KM, Yalcin MU, Kocyigit D, Dural M, Evranos Bet al. Safety and efficacy outcomes in patients undergoing pulmonary vein isolation with second-generation cryoballoondagger. Europace 2015;17:379–87. [DOI] [PubMed] [Google Scholar]
- 55. Cardoso R, Mendirichaga R, Fernandes G, Healy C, Lambrakos LK, Viles-Gonzalez JFet al. Cryoballoon versus radiofrequency catheter ablation in atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol 2016;27:1151–9. [DOI] [PubMed] [Google Scholar]
- 56. Jin ES, Wang PJ. Cryoballoon ablation for atrial fibrillation: a comprehensive review and practice guide. Korean Circ J 2018;48:114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Andrade JG, Dubuc M, Ferreira J, Guerra PG, Landry E, Coulombe Net al. Histopathology of cryoballoon ablation-induced phrenic nerve injury. J Cardiovasc Electrophysiol 2014;25:187–94. [DOI] [PubMed] [Google Scholar]
- 58. Providencia R, Defaye P, Lambiase PD, Pavin D, Cebron JP, Halimi Fet al. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace 2017;19:48–57. [DOI] [PubMed] [Google Scholar]
- 59. Creta A, Kanthasamy V, Schilling RJ, Rosengarten J, Khan F, Honarbakhsh Set al. First experience of POLARx versus arctic front advance: an early technology comparison. J Cardiovasc Electrophysiol 2021;32:925–30. [DOI] [PubMed] [Google Scholar]
- 60. Kochi AN, Moltrasio M, Tundo F, Riva S, Ascione C, Dessanai MAet al. Cryoballoon atrial fibrillation ablation: single-center safety and efficacy data using a novel cryoballoon technology compared to a historical balloon platform. J Cardiovasc Electrophysiol 2021;32:588–94. [DOI] [PubMed] [Google Scholar]
- 61. Tohoku S, Schmidt B, Bordignon S, Chen S, Bologna F, Chun JK. Initial clinical experience of pulmonary vein isolation using the ultra-low temperature cryoablation catheter for patients with atrial fibrillation. J Cardiovasc Electrophysiol 2022;33:1371–9. [DOI] [PubMed] [Google Scholar]
- 62. Hindricks G, Packer DL. Catheter ablation of atrial fibrillation: recent advances and future challenges. Europace 2022;24:ii1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Šedivá L, Petrů J, Škoda J, Janotka M, Chovanec M, Reddy Vet al. Visually guided laser ablation: a single-centre long-term experience. Europace 2014;16:1746–51. [DOI] [PubMed] [Google Scholar]
- 64. Schmidt B, Neuzil P, Luik A, Osca Asensi J, Schrickel JW, Deneke Tet al. Laser balloon or wide-area circumferential irrigated radiofrequency ablation for persistent atrial fibrillation: a multicenter prospective randomized study. Circ Arrhythm Electrophysiol 2017;10:e005767. [DOI] [PubMed] [Google Scholar]
- 65. Reissmann B, Budelmann T, Wissner E, Schlüter M, Heeger CH, Mathew Set al. Five-year clinical outcomes of visually guided laser balloon pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation. Clin Res Cardiol 2018;107:405–12. [DOI] [PubMed] [Google Scholar]
- 66. Rovaris G, Ciconte G, Schiavone M, Mitacchione G, Gasperetti A, Piazzi Eet al. Second-generation laser balloon ablation for the treatment of atrial fibrillation assessed by continuous rhythm monitoring: the LIGHT-AF study. Europace 2021;23:1380–90. [DOI] [PubMed] [Google Scholar]
- 67. Schmidt B, Petru J, Chun KRJ, Sediva L, Bordignon S, Chen Set al. Pivotal study of a novel motor-driven endoscopic ablation system. Circ Arrhythm Electrophysiol 2021;14:e009544. [DOI] [PubMed] [Google Scholar]
- 68. Schmidt B, Chun KR, Metzner A, Fuernkranz A, Ouyang F, Kuck KH. Pulmonary vein isolation with high-intensity focused ultrasound: results from the HIFU 12F study. Europace 2009;11:1281–8. [DOI] [PubMed] [Google Scholar]
- 69. Metzner A, Chun KR, Neven K, Fuernkranz A, Ouyang F, Antz Met al. Long-term clinical outcome following pulmonary vein isolation with high-intensity focused ultrasound balloon catheters in patients with paroxysmal atrial fibrillation. Europace 2010;12:188–93. [DOI] [PubMed] [Google Scholar]
- 70. Nagashima K, Okumura Y, Watanabe I, Nakahara S, Hori Y, Iso Ket al. Hot balloon versus cryoballoon ablation for atrial fibrillation: lesion characteristics and middle-term outcomes. Circ Arrhythm Electrophysiol 2018;11:e005861. [DOI] [PubMed] [Google Scholar]
- 71. Schilling R, Dhillon GS, Tondo C, Riva S, Grimaldi M, Quadrini Fet al. Safety, effectiveness, and quality of life following pulmonary vein isolation with a multi-electrode radiofrequency balloon catheter in paroxysmal atrial fibrillation: 1-year outcomes from SHINE. Europace 2021;23:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Del Monte A, Almorad A, Pannone L, Della Rocca DG, Bisignani A, Monaco Cet al. Pulmonary vein isolation with the radiofrequency balloon catheter: a single centre prospective study. Europace 2023;25:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reddy VY, Al-Ahmad A, Aidietis A, Daly M, Melton I, Hu Yet al. A novel visually guided radiofrequency balloon ablation catheter for pulmonary vein isolation: one-year outcomes of the multicenter AF-FICIENT I trial. Circ Arrhythm Electrophysiol 2021;14:e009308. [DOI] [PubMed] [Google Scholar]
- 74. Verma A, Asivatham SJ, Deneke T, Castellvi Q. Neal RE 2nd. Primer on pulsed electrical field ablation: understanding the benefits and limitations. Circ Arrhythm Electrophysiol 2021;14:e010086. [DOI] [PubMed] [Google Scholar]
- 75. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet Het al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 76. Koruth J, Verma A, Kawamura I, Reinders D, Andrade JG, Deyell MWet al. PV isolation using a spherical array PFA catheter: preclinical assessment and comparison to radiofrequency ablation. JACC Clin Electrophysiol 2023:S2405–500X. (23)00078-6. [DOI] [PubMed] [Google Scholar]
- 77. Duytschaever M, De Potter T, Grimaldi M, Anic A, Vijgen J, Neuzil Pet al. Paroxysmal atrial fibrillation ablation using a novel variable-loop biphasic pulsed field ablation catheter integrated with a 3-dimensional mapping system: 1-year outcomes of the multicenter inspIRE study. Circ Arrhythm Electrophysiol 2023;16:e011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Moshkovits Y, Grynberg D, Heller E, Maizels L, Maor E. Differential effect of high-frequency electroporation on myocardium vs. non-myocardial tissues. Europace 2023;25:748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Goujeau C, Cet al A. Pulsed field ablation prevents chronic atrial fibrotic changes and restrictive mechanics after catheter ablation for atrial fibrillation. Europace 2021;23:1767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cochet H, Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Nakashima Tet al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace 2021;23:1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Musikantow DR, Neuzil P, Petru J, Koruth JS, Kralovec S, Miller MAet al. Pulsed field ablation to treat atrial fibrillation: autonomic nervous system effects. JACC Clin Electrophysiol 2022;9:481–93. S2405-500X(22)00943-4. [DOI] [PubMed] [Google Scholar]
- 82. Füting A, Reinsch N, Höwel D, Brokkaar L, Rahe G, Neven K. First experience with pulsed field ablation as routine treatment for paroxysmal atrial fibrillation. Europace 2022;24:1084–92. [DOI] [PubMed] [Google Scholar]
- 83. Du Pré BC, van Driel VJ, van Wessel H, Loh P, Doevendans PA, Goldschmeding Ret al. Minimal coronary artery damage by myocardial electroporation ablation. Europace 2013;15:144–9. [DOI] [PubMed] [Google Scholar]
- 84. Reddy VY, Petru J, Funasako M, Kopriva K, Hala P, Chovanec Met al. Coronary arterial spasm during pulsed field ablation to treat atrial fibrillation. Circulation 2022;146:1808–19. [DOI] [PubMed] [Google Scholar]
- 85. Bohnen M, Weber R, Minners J, Jadidi A, Eichenlaub M, Neumann FJet al. Characterization of circumferential antral pulmonary vein isolation areas resulting from pulsed-field catheter ablation. Europace 2023;25:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tohoku S, Chun KRJ, Bordignon S, Chen S, Schaack D, Urbanek Let al. Findings from repeat ablation using high-density mapping after pulmonary vein isolation with pulsed field ablation. Europace 2023;25:433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami Ket al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068–80. [DOI] [PubMed] [Google Scholar]
- 88. Kawamura I, Neuzil P, Shivamurthy P, Kuroki K, Lam J, Musikantow Det al. How does the level of pulmonary venous isolation compare between pulsed field ablation and thermal energy ablation (radiofrequency, cryo, or laser)? Europace 2021;23:1757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schmidt B, Bordignon S, Neven K, Reichlin T, Blaauw Y, Hansen J. European real world outcomes with pulsed field ablation in patients with symptomatic atrial fibrillation—lessons from the multicenter EU-PORIA registry. Europace 2023;25:euad185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako Met al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614–27. [DOI] [PubMed] [Google Scholar]
- 91. Verma A, Haines DE, Boersma LV, Sood N, Natale A, Marchlinski FEet al. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation 2023;147:1422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Calkins H, Hindricks G, Cappato R, Kim Y, Saad E, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Charitos E, Purerfellner H, Glotzer TV, Ziegler PD. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J Am Coll Cardiol 2014;63:2840–8. [DOI] [PubMed] [Google Scholar]
- 94. Hammond-Haley M, Providência R, Lambiase PD. Temporal pattern/episode duration-based classification of atrial fibrillation as paroxysmal vs. persistent: is it time to develop a more integrated prognostic score to optimize management? Europace 2018;20:f288–98. [DOI] [PubMed] [Google Scholar]
- 95. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish ENet al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dagres N, Bongiorni MG, Larsen TB, Hernandez-Madrid A, Pison L, Blomström-Lundqvist Cet al. Current ablation techniques for persistent atrial fibrillation: results of the European Heart Rhythm Association Survey. Europace 2015;17:1596–600. [DOI] [PubMed] [Google Scholar]
- 97. Kuniss M, Greiß H, Pajitnev D, Akkaya E, Deubner N, Hain Aet al. Cryoballoon ablation of persistent atrial fibrillation: feasibility and safety of left atrial roof ablation with generation of conduction block in addition to antral pulmonary vein isolation. Europace 2017;19:1109–15. [DOI] [PubMed] [Google Scholar]
- 98. Sawhney V, Schilling RJ, Providencia R, Cadd M, Perera D, Chatha Set al. Cryoablation for persistent and longstanding persistent atrial fibrillation: results from a multicentre European registry. Europace 2020;22:375–81. [DOI] [PubMed] [Google Scholar]
- 99. Conti S, Weerasooriya R, Novak P, Champagne J, Lim HE, Macle Let al. Contact force sensing for ablation of persistent atrial fibrillation: a randomized, multicenter trial. Heart Rhythm 2018;15:201–8. [DOI] [PubMed] [Google Scholar]
- 100. Shi LB, Rossvoll O, Tande P, Schuster P, Solheim E, Chen J. Cryoballoon vs. radiofrequency catheter ablation: insights from Norwegian randomized study of persistent atrial fibrillation (NO-PERSAF study). Europace 2022;24:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Verma A, Mantovan R, Macle L, De Martino G, Chen J, Morillo CAet al. Substrate and trigger ablation for reduction of atrial fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J 2010;31:1344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan Ret al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 103. Clarnette JA, Brooks AG, Mahajan R, Elliott A, Twomey D, Pathak Ret al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace 2018;20:f366–76. [DOI] [PubMed] [Google Scholar]
- 104. Saglietto A, Ballatore A, Gaita F, Scaglione M, De Ponti R, De Ferrari GMet al. Comparative efficacy and safety of different catheter ablation strategies for persistent atrial fibrillation: a network meta-analysis of randomized clinical trials. Eur Heart J Qual Care Clin Outcomes 2022;8:619–29. [DOI] [PubMed] [Google Scholar]
- 105. Terricabras M, Mantovan R, Jiang CY, Betts T, Chen J, Deisenhofer Iet al. Association between quality of life and procedural outcome after catheter ablation for atrial fibrillation: a secondary analysis of a randomized clinical trial. JAMA Netw Open 2020;3:e2025473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chen J, Arentz T, Cochet H, Müller-Edenborn B, Kim S, Moreno-Weidmann Zet al. Extent and spatial distribution of left atrial arrhythmogenic sites, late gadolinium enhancement at magnetic resonance imaging, and low-voltage areas in patients with persistent atrial fibrillation: comparison of imaging vs. electrical parameters of fibrosis and arrhythmogenesis. Europace 2019;21:1484–93. [DOI] [PubMed] [Google Scholar]
- 107. Huo Y, Gaspar T, Schönbauer R, Wójcik M, Fiedler L, Roithinger Fet al. Low-voltage myocardium-guided ablation trial of persistent atrial fibrillation. NEJM Evid 2022;1. [DOI] [PubMed] [Google Scholar]
- 108. Marrouche NF, Wazni O, McGann C, Greene T, Dean M, Dagher Let al. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nery PB, Wells GA, Verma A, Joza J, Nair GM, Veenhuyzen Get al. Characterization of arrhythmia substrate to ablate persistent atrial fibrillation (COAST-AF): randomized controlled trial design and rationale. Am Heart J 2022;254:133–40. [DOI] [PubMed] [Google Scholar]
- 110. Ruaengsri C, Schill MR, Khiabani AJ, Schuessler RB, Melby SJ, Damiano RJ. The Cox-maze IV procedure in its second decade: still the gold standard? Eur J Cardiothorac Surg 2018;53:i19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. He X, Zhou Y, Chen Y, Wu L, Huang Y, He J. Left atrial posterior wall isolation reduces the recurrence of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol 2016;46:267–74. [DOI] [PubMed] [Google Scholar]
- 112. Kistler PM, Chieng D, Sugumar H, Ling LH, Segan L, Azzopardi Set al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the CAPLA randomized clinical trial. JAMA 2023;329:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Romero J, Michaud GF, Avendano R, Briceño DF, Kumar S, Carlos Diaz Jet al. Benefit of left atrial appendage electrical isolation for persistent and long-standing persistent atrial fibrillation: a systematic review and meta-analysis. Europace 2018;20:1268–78. [DOI] [PubMed] [Google Scholar]
- 114. Yorgun H, Şener YZ, Tanese N, Keresteci A, Sezenöz B, Çöteli Cet al. Long-term outcomes of left atrial appendage isolation using cryoballoon in persistent atrial fibrillation. Europace 2023;25:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lee RJ, Lakkireddy D, Mittal S, Ellis C, Connor JT, Saville BRet al. Percutaneous alternative to the Maze procedure for the treatment of persistent or long-standing persistent atrial fibrillation (aMAZE trial): rationale and design. Am Heart J 2015;170:1184–94. [DOI] [PubMed] [Google Scholar]
- 116. Hu X, Jiang W, Wu S, Xu K, Zhang D, Zhang Y, et al. Extra-pulmonary vein driver mapping and ablation for persistent atrial fibrillation in obese patients. Europace 2021;23:701–9. [DOI] [PubMed] [Google Scholar]
- 117. Della Rocca DG, Di Biase L, Mohanty S, Trivedi C, Gianni C, Romero J, et al. Targeting non-pulmonary vein triggers in persistent atrial fibrillation: results from a prospective, multicentre, observational registry. Europace 2021;23:1939–49. [DOI] [PubMed] [Google Scholar]
- 118. Xu K, Wang Y, Wu S, Zhou L, Zhao L, Jiang Wet al. The role of superior vena cava in catheter ablation of long-standing persistent atrial fibrillation. Europace 2017;19:1670–5. [DOI] [PubMed] [Google Scholar]
- 119. Mohanty S, Trivedi C, Gianni C, Della Rocca D, Hamilton Morris E, Burkhardt Det al. Procedural findings and ablation outcome in patients with atrial fibrillation referred after two or more failed catheter ablations. J Cardiovasc Electrophysiol 2017;28:1379–86. [DOI] [PubMed] [Google Scholar]
- 120. Kanagaratnam P, McCready J, Tayebjee M, Shepherd E, Sasikaran T, Todd Det al. Ablation versus anti-arrhythmic therapy for reducing all hospital episodes from recurrent atrial fibrillation: a prospective, randomized, multi-centre, open label trial. Europace 2023;25:863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Scott P, Silberbauer J, Murgatroyd F. The impact of adjunctive complex fractionated atrial electrogram ablation and linear lesions on outcomes in persistent atrial fibrillation: a meta-analysis. Europace 2016;18:359–67. [DOI] [PubMed] [Google Scholar]
- 122. The A, Kistler P, Lee G, Medi C, Heck P, Spence Set al. The relationship between complex fractionated electrograms and atrial low-voltage zones during atrial fibrillation and paced rhythm. Europace 2011;13:1709–16. [DOI] [PubMed] [Google Scholar]
- 123. Ciconte G, Vicedomini G, Wenwen Li W, Mangual J, McSpadden L, Ryu Ket al. Non-paroxysmal atrial fibrillation mapping: characterization of the electrophysiological substrate using a novel integrated mapping technique. Europace 2019;21:1193–202. [DOI] [PubMed] [Google Scholar]
- 124. Kircher S, Arya A, Altmann D, Rolf S, Bollmann A, Sommer Pet al. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace 2018;20:1766–75. [DOI] [PubMed] [Google Scholar]
- 125. Junarta J, Siddiqui M, Riley J, Dikdan S, Patel A, Frisch Det al. Low-voltage area substrate modification for atrial fibrillation ablation: a systematic review and meta-analysis of clinical trials. Europace 2022;24:1585–98. [DOI] [PubMed] [Google Scholar]
- 126. Knecht S, Sohal M, Deisenhofer I, Albenque JP, Arentz T, Neumann Tet al. Multicentre evaluation of non-invasive biatrial mapping for persistent atrial fibrillation ablation: the AFACART study. Europace 2017;19:1302–9. [DOI] [PubMed] [Google Scholar]
- 127. Lin T, Rillig A, Bucur T, Metzner A, Mathew S, Wissner Eet al. Focal impulse and rotor modulation using the novel 64-electrode basket catheter: electrogram characteristics of human rotors. Europace 2015;17:1791–7. [DOI] [PubMed] [Google Scholar]
- 128. Krummen D, Baykaner T, Schricker A, Kowalewski CA, Swarup V, Miller Ket al. Multicenter safety of adding focal impulse and rotor modulation (FIRM) to conventional ablation for atrial fibrillation. Europace 2017;19:769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Parameswaran R, Voskoboinik A, Gorelik A, Lee G, Kistler P, Sanders Pet al. Clinical impact of rotor ablation in atrial fibrillation: a systematic review. Europace 2018;20:1099–106. [DOI] [PubMed] [Google Scholar]
- 130. Spitzer S, Miller J, Sommer P, Szili-Torok T, Reddy V, Nölker Get al. Randomized evaluation of redo ablation procedures of atrial fibrillation with focal impulse and rotor modulation-guided procedures: the REDO-FIRM study. Europace 2023;25:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xu C, Xiong F, Jiang WF, Liu X, Liu T, Qin Met al. Rotor mechanism and its mapping in atrial fibrillation. Europace 2023;25:783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Betts T, Good W, Melki L, Metzner A, Grace A, Verma Aet al. Treatment of pathophysiologic propagation outside of the pulmonary veins in retreatment of atrial fibrillation patients: RECOVER AF study. Europace 2023;25:euad097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pope M, Leo M, Briosa E, Gala A, Betts T. Clinical utility of non-contact charge density ‘SuperMap’ algorithm for the mapping and ablation of organized atrial arrhythmias. Europace 2022;24:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Azzolin L, Eichenlaub M, Nagel C, Nairn D, Sanchez J, Unger Let al. Personalized ablation vs. conventional ablation strategies to terminate atrial fibrillation and prevent recurrence. Europace 2023;25:211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. de Groot N, Shah D, Boyle P, Anter E, Clifford G, Deisenhofer Iet al. Critical appraisal of technologies to assess electrical activity during atrial fibrillation: a position paper from the European Heart Rhythm Association and European Society of Cardiology Working Group on eCardiology in collaboration with the Heart Rhythm Society, Asia Pacific Heart Rhythm Society. Europace 2022;24:313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and can be derived from the papers that are reviewed.