Abstract
Objective
A new formulation of benzoyl peroxide (E-BPO cream, 5%) entraps benzoyl peroxide (BPO) in silica microcapsules. This study assesses the efficacy, safety, and tolerability of E-BPO cream, 5%, in rosacea in two Phase III clinical trials.
Methods
In two 12-week, randomized, double-blind, vehicle cream-controlled Phase III trials, 733 subjects at least 18 years old with moderate to severe rosacea were randomized (2:1) to once-daily E-BPO cream, 5%, or vehicle.
Results
In Study 1, the proportion of subjects achieving IGA clear/almost clear at Week 12 was 43.5 percent for E-BPO cream, 5%, and 16.1 percent for vehicle. In Study 2, the respective values were 50.1 percent and 25.9 percent. In Study 1, the decrease in lesion count from baseline to Week 12 was –17.4 for E-BPO cream, 5%, versus –9.5 for vehicle. In Study 2, the respective values were –20.3 and –13.3 (all P<0.001). The difference was also significant at Week 2. There were no treatment-related serious adverse events; 1.4 percent of subjects (1.8% E-BPO cream, 5%, 0.4% vehicle) discontinued due to adverse events. Assessed local tolerability was found to be similar among subjects in both E-BPO and vehicle.
E-BPO was not compared with unencapsulated BPO.
Conclusion
E-BPO is an effective and well tolerated treatment for rosacea. Clinicaltrials.gov Identifiers: NCT03564119, NCT03448939.
Keywords: Benzoyl peroxide, microencapsulation, rosacea, papulopustular, topical, BPO
Rosacea is a chronic, relapsing, inflammatory skin disease with a high prevalence and significant patient burden.1–11 Numerous topical and systemic therapies have been employed to treat rosacea, but current available options have less than optimal efficacy in many patients, resulting in significant dissatisfaction with therapy.11–14
Benzoyl peroxide (BPO) is a powerful oxidizing agent15 that is used extensively in acne treatment, more often than in rosacea.16 While a mechanism of action in rosacea remains hypothetical, in the small number of studies in which BPO has been employed in rosacea, it has demonstrated efficacy,17,18 but its use is associated with local skin irritation, including stinging, burning, and itching.15,16,19–21 Various methods have been employed in an attempt to improve tolerability while maintaining or improving efficacy.22–25
Encapsulated BPO (E-BPO) cream, 5%, is a new formulation of BPO developed using the sol-gel process, in which the drug is enclosed in a silica microcapsule. The capsule provides gradual release of BPO, thereby allowing potential for improved efficacy and tolerability compared with conventional delivery. Results from a Phase II study that compared the safety and efficacy of 1% and 5% silica-encapsulated BPO indicated significant superiority versus vehicle cream for decreasing papulopustular lesions and improving the Investigator Global Assessment (IGA) in subjects with rosacea. Both strengths of E-BPO were well tolerated.26
The objective of these two trials was to compare efficacy, safety, and tolerability of E-BPO cream, 5%, monotherapy versus vehicle in subjects with moderate to severe rosacea. This paper will discuss the results of two double-blind, randomized, vehicle-controlled studies of E-BPO cream, 5%.
METHODS
Study subjects. Subjects were men and women, aged 18 years or older, with moderate to severe rosacea and a baseline IGA score of 3 (moderate) or 4 (severe) on a severity scale of 0 to 4. Each had a minimum of 15 and a maximum of 70 papules and/or pustules and two or less nodules (a papule or pustule >5mm in diameter). Subjects were healthy and willing and able to understand and comply with the study requirements, apply the medication as instructed, and minimize factors that could trigger rosacea flare-ups throughout the study. They were also instructed to refrain from using prohibited medications during the study. Sexually active women of childbearing potential were required to use an accepted method of birth control.
Potential subjects were excluded if they had any history or evidence of ocular rosacea, other facial skin dermatoses, other uncontrolled diseases, excessive facial hair, a history of unresponsiveness or sensitivities to BPO, or were concurrently using drugs known to cause acneiform eruptions. Subjects who consumed excessive alcohol or abused drugs were also excluded.
Study design and treatment. The two trials (NCT03448939, NCT03564119) were randomized (2:1), double-blind, multicenter, parallel-group, vehicle-controlled studies that included a screening visit and 12 weeks of treatment. They were conducted between June 18, 2018, and June 10, 2019, at 54 sites total in the United States. In the two trials, a total of 493 subjects were randomized to E-BPO cream, 5%, and 240 to vehicle (Supplementary Figure 1).
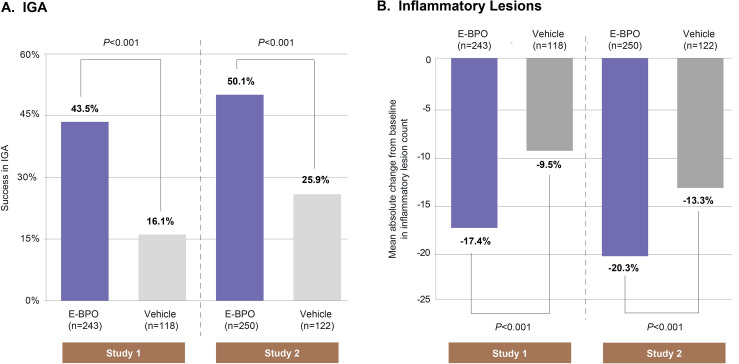
FIGURE 1.
The percentage of subjects who achieved (A) Investigator Global Assessment (IGA) success at Week 12; and (B) the mean reduction in inflammatory lesion count at Week 12 in subjects with rosacea.
E-BPO: Encapsulated benzoyl peroxide
Randomization was performed according to a computer-generated schedule by an unblinded statistician. A pea-sized amount of the cream or vehicle was applied as a thin layer to each area of the face (forehead, chin, nose, and each cheek) once daily at approximately the same time of day. After screening, randomization, and a baseline evaluation (Day 1), subjects returned to the study sites for four evaluation visits (Weeks 2, 4, 8, and 12) during the double-blind period. Safety assessments were performed throughout the study.
This study was conducted in compliance with United States Food and Drug Administration regulations, the ethical principles of the Declaration of Helsinki, and the current International Council for Harmonisation Good Clinical Practice guidelines. The protocol, informed consent documents, any information provided to subjects, recruitment advertisements, and any amendments to these items had Institutional Review Board approval prior to their use in the study. Voluntary informed consent was given by every patient prior to the initiation of any study-related procedures.
Efficacy. Each study had two coprimary endpoints: (1) treatment success as determined by the IGA, which was completed at screening/baseline and Weeks 2, 4, 8, and 12; success was defined as achieving an IGA rating of “clear” (0) or “almost clear” (1) on the five-point IGA scale at Week 12 (0=clear, 1=almost clear, 2=mild disease, 3=moderate disease, and 4=severe disease; Supplementary Table 1); and (2) the absolute change in inflammatory lesion count from baseline to Week 12. Erythema was included as a component in the IGA scale. Secondary endpoints included percentage change in inflammatory lesion count from baseline to Week 12; absolute changes in inflammatory lesion count from baseline to Weeks 2, 4, and 8; and the proportion of subjects achieving IGA success at Weeks 2, 4, and 8. The Week 2 endpoints were analyzed as a post-hoc analysis.
TABLE 1.
Pooled adverse events (Safety population)
| CHARACTERISTIC | E-BPO CREAM, 5% N=488 | VEHICLE N=234 |
|---|---|---|
| TEAEs, n (%) | ||
| Any TEAE | 99 (20.3) | 39 (16.7) |
| Serious TEAE | 1 (0.2)1 | 1 (0.4)2 |
| Severe TEAE | 4 (0.8)3 | 0 |
| Discontinuation | 9 (1.8) | 2 (0.9)4 |
| Treatment related | 23 (4.7) | 3 (1.3) |
| AEs reported for ≥1% of subjects in any treatment arm (%) related to study drug | ||
| Application site erythema | 11 (2.3) | 2 (0.9) |
| Application site pain | 8 (1.6) | 2 (0.9) |
| Application site pruritus | 6 (1.2) | 1 (0.4) |
1Spinal compression fracture.
2Femur fracture.
3One severe TEAE deemed not related to study drug by the investigator.
4Urinary tract infection (discontinuation classified as “other reason”).
AE: adverse event; E-BPO: encapsulated benzoyl peroxide; TEAE: treatment-emergent adverse event
Standardized photography of facial rosacea at baseline and Weeks 2, 4, 8, and 12 was performed at selected sites, but the photographs were not used for efficacy assessment.
Safety. Safety was assessed by recording vital signs, monitoring any adverse events (AEs), and recording results of cutaneous safety and local tolerability assessments at baseline and all postbaseline study visits. Cutaneous safety was assessed by evaluating dryness and scaling (each measured on a four-point scale: 0=none, 1=mild, 2=moderate, 3=severe), and tolerability was assessed by evaluating itching and stinging/burning using a similar scale.
Statistical analysis. Based on results from the Phase II trial of microencapsulated BPO for rosacea,28 a sample size of 86 in the E-BPO cream, 5%, group and 43 in the vehicle group had 95-percent power to detect a statistically significant difference in the proportion of subjects who had at least a two-grade reduction at Week 12 from baseline in IGA and were clear or almost clear. A sample size of 200 in the E-BPO cream, 5%, group and 100 in the vehicle group had 95-percent power to detect a statistically significant difference in inflammatory lesions. The estimated absolute change from baseline in treatment mean was –14.1 for E-BPO cream, 5%, and –7.4 for vehicle, with a standard deviation (SD) of 6.70 and 17.24, respectively. The sample sizes above were increased to give a planned enrollment of 234 in the E-BPO cream, 5%, group and 117 in the vehicle group in each study since the Phase III trials were expected to enroll subjects with more severe rosacea than those in the Phase II trial based on differences in the inclusion criteria.
The primary population for efficacy analysis was the intent-to-treat population, which consisted of all subjects who were randomized and received study treatment. The safety population consisted of subjects in the randomized population who were presumed to have used the study product at least once and provided at least one post-baseline safety evaluation.
The analysis of the dichotomized IGA was based on a logistic regression test with factors for treatment group and analysis center. Tests of superiority for the absolute change from baseline in inflammatory lesions were based on an analysis of covariance with factors of treatment, center, and baseline lesion count as covariates. The primary method of handling missing efficacy data was based on estimation using Markov chain Monte Carlo imputation.
RESULTS
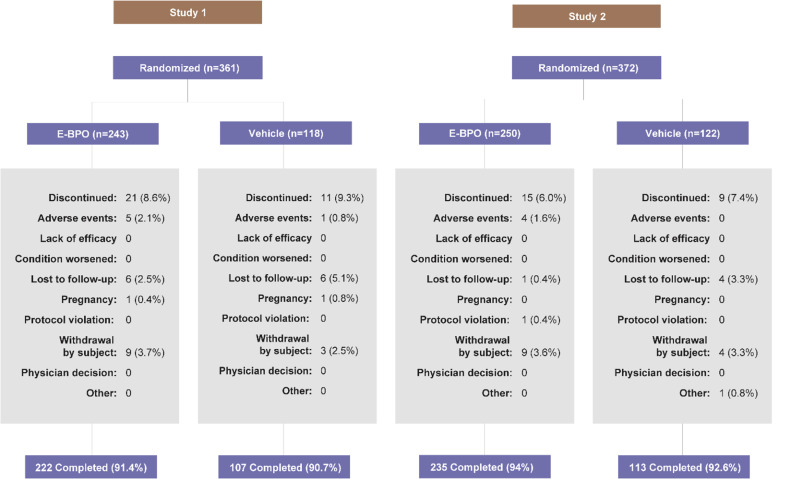
Subject disposition and demographics. In Study 1, a total of 243 subjects were randomized to E-BPO cream, 5%, and 222 (91.4%) completed the study. The respective values for vehicle were 118 and 107 (90.7%). In Study 2, a total of 250 subjects were randomized to E-BPO cream, 5%, and 235 (94.0%) completed the study. The respective values for vehicle were 122 and 113 (92.6%) (Supplementary Figure 1). Baseline demographic and clinical characteristics were well balanced between treatment groups in both studies (Supplementary Table 1).
SUPPLEMENTAL FIGURE 1.
CONSORT Diagram
SUPPLEMENTAL TABLE 1.
Investigator Global Assessment scale
| GRADE | DESCRIPTION |
|---|---|
| 0–Clear | Skin is clear of inflammatory papules or pustules |
| 1–Almost Clear | Very few small papules or pustules and very mild dull erythema are present |
| 2–Mild | Few small papules or pustules and mild dull or light pink erythema are present |
| 3–Moderate | Several to many small or larger papules or pustules and moderate light to bright red erythema are present |
| 4–Severe | Numerous small and/or larger papules or pustules and severe erythema that is bright red to deep red are present |
Efficacy. E-BPO cream, 5%, was significantly superior to placebo for both coprimary endpoints. At Week 12 in Study 1, 43.5 percent of subjects randomized to E-BPO cream, 5%, and 16.1 percent of those randomized to vehicle achieved IGA success (P<0.001). The respective values for Study 2 were 50.1 percent and 25.9 percent (P<0.001) (Figure 1A). In Study 1, 47.4 percent of subjects treated with E-BPO cream, 5%, achieved IGA success versus 20.7 percent for vehicle based on multiple imputation (MI) analysis (95% CI: 16.7%, 36.8%). The respective values in Study 2 were 49.2 percent and 28.2 percent based on MI analysis (95% CI: 10.7%, 31.3%). At Week 12 in Study 1, the mean (SD) reductions in lesion count were –17.4 (9.3) for subjects randomized to E-BPO cream, 5%, and –9.5 (9.4) for those randomized to vehicle (P<0.001). The respective values for Study 2 were –20.3 (9.6) and –13.3 (9.6; P<0.001) (Figure 1B). Baseline lesion counts are presented in Supplementary Table 2.
SUPPLEMENTAL TABLE 2.
Baseline demographic and clinical characteristics
| CHARACTERSITICS | STUDY 1 | STUDY 2 | ||
|---|---|---|---|---|
| E-BPO CREAM, 5% (N=243) |
VEHICLE (N=118) |
E-BPO CREAM, 5% (N=250) |
VEHICLE (N=122) |
|
| Age, years | ||||
| Mean (SD) | 52.8 (13.21) | 52.4 (13.26) | 49.5 (14.04) | 51.5 (12.55) |
| Median (range) | 54.0 (19–81) | 52.5 (24–85) | 50.0 (18–79) | 53 (22–84) |
| Sex, n (%) | ||||
| Male | 60 (24.7) | 35 (29.7) | 69 (27.6) | 35 (28.7) |
| Female | 183 (75.3) | 83 (70.3) | 181 (72.4) | 87 (71.3) |
| Race, n (%) | ||||
| American Indian/Alaska Nat. | 0 | 0 | 0 | 2 (1.6) |
| Asian | 9 (3.7) | 2 (1.7) | 20 (8.0) | 8 (6.6) |
| Black/African American | 0 | 0 | 2 (0.8) | 0 |
| Nat. Hawaiian/Pac. Islander | 0 | 0 | 3 (1.2) | 2 (1.6) |
| White | 233 (95.9) | 116 (98.3) | 220 (88.0) | 110 (90.2) |
| Multiple/Other | 1 (0.4) | 0 | 5 (2.0) | 0 |
| Ethnicity, n (%) | ||||
| Hispanic/Latino | 86 (35.4) | 39 (33.1) | 55 (22.0) | 30 (24.6) |
| Not Hispanic or Latino | 156 (64.2) | 77 (65.3) | 195 (78.0) | 92 (75.4) |
| Unknown | 1 (0.4) | 2 (1.7) | 0 | 0 |
| IGA severity (%) | ||||
| Moderate | 210 (86.4) | 104 (88.1) | 227 (90.8) | 112 (91.8) |
| Severe | 33 (13.6) | 14 (11.9) | 23 (9.2) | 10 (8.2) |
| Lesion count | ||||
| Mean (SD) | 25.7 (11.07) | 26.3 (12.45) | 29.8 (14.00) | 27.5 (13.04) |
| Median (range) | 22.0 (15–69) | 21.0 (15–70) | 25.0 (15–70) | 22.5 (15–70) |
E-BPO: encapsulated benzoyl peroxide; IGA: Investigator Global Assessment; Nat.: native; Pac.: Pacific; SD: standard deviation
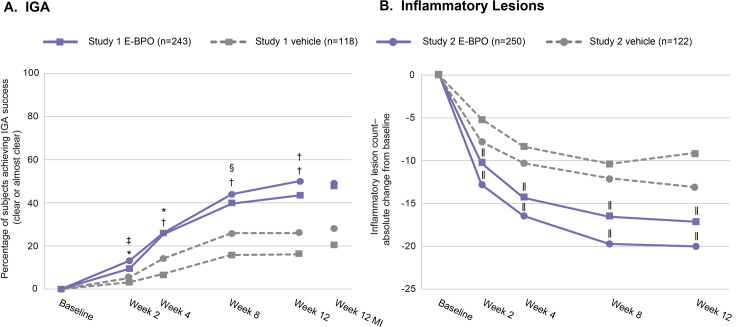
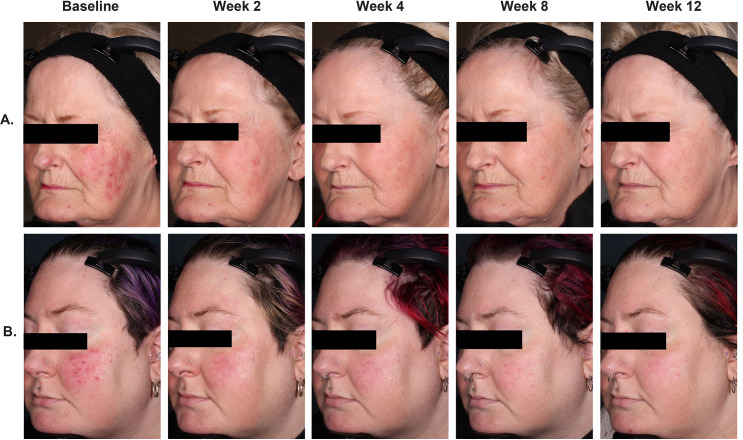
Assessment of efficacy at each evaluation indicated that E-BPO cream, 5%, achieved significant superiority over vehicle by two weeks (the first post-baseline visit) and that differences remained significant at all subsequent assessments (Figure 2). At Week 2 in Study 1, 9.5 percent of subjects randomized to E-BPO cream, 5%, and 3.1 percent of those randomized to vehicle achieved IGA success (P=0.009). The respective values for Study 2 were 13.2 percent and 5.5 percent (P=0.017) (Figure 2A). At Week 2 in Study 1, the mean (SD) reduction in lesion count was –10.5 (7.8) for subjects randomized to E-BPO cream, 5%, and–5.5 (7.9) for those randomized to vehicle (P<0.001). The respective values for Study 2 were –13.0 (9.8) and –8.0 (9.8; P<0.001) (Figure 2B). Rapid IGA improvement and lesion reduction achieved in two subjects with E-BPO cream, 5%, are illustrated in the photographs in Figure 3.
FIGURE 2.
The percentage of subjects who achieved (A) Investigator Global Assessment (IGA) success; and (B) the mean reduction in inflammatory lesion count in subjects with rosacea over the course of the study. *P=0.009; †P<0.001; ‡P=0.017; §P=0.006; ||P<0.001
E-BPO: encapsulated benzoyl peroxide; MI: multiple imputation
FIGURE 3.
The results from two subjects with rosacea who achieved Investigator Global Assessment (IGA) success demonstrate the rapid improvement in IGA and reduction in lesions achieved with E-BPO cream, 5%. (A) 69-year-old woman, lesion counts at each time point were 33, 15, 1, 0, 0. (B) 41-year-old woman, they were 31, 0, 0, 0, 1.
E-BPO: encapsulated benzoyl peroxide
The percentage reductions in lesion counts also indicate significant superiority of E-BPO cream, 5%, versus vehicle at Week 12. In Study 1, the percentage reduction in lesion count for E-BPO cream, 5%, was –68.2 percent versus –38.3 percent for vehicle (P<0.001). The respective values in Study 2 were –69.4 percent and –46.0 percent (P<0.001).
Safety and tolerability. Most treatment-emergent adverse events (TEAEs) related to E-BPO cream, 5%, were cutaneous and occurred primarily at the application site (Table 1). There were two serious AEs reported in the study, which were not considered by the investigator to be related to the study drug (a spinal compression fracture and a femur fracture). Severe AEs deemed related to E-BPO were reported in three subjects (two with severe application site erythema and one with severe application site pruritus and application site pain). There were no severe AEs in the vehicle group. The most commonly reported TEAEs were at the application site and included pain, erythema, pruritus, and edema. AEs led to discontinuation in 1.4 percent of total study participants: nine (1.8%) treated with E-BPO cream, 5%, and one (0.4%) in the vehicle group. There were no significant clinically relevant changes in vital signs or physical examination findings.
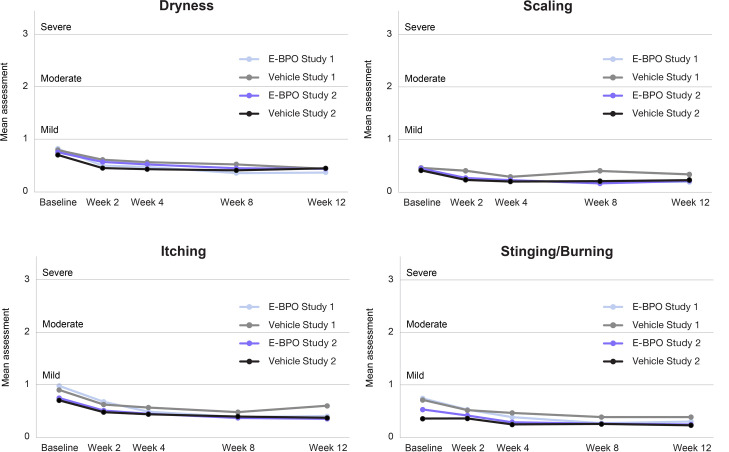
Local tolerability was assessed by the investigator. Subjects in both arms reported improved skin tolerability (scaling, itching, dryness, and burning/stinging) after 12 weeks of treatment compared with baseline (Figure 4). The mean tolerability parameters of E-BPO and vehicle stayed below one on a scale of 0–3 and were not statistically different.
FIGURE 4.
Mean assessment for local tolerability was assessed by the investigators are all visits in subjects with rosacea. The mean tolerability parameters of encapsulated benzoyl peroxide (E-BPO) and vehicle stayed below 1 on a scale of 0 to 3 and were not statistically different.
DISCUSSION
Results from the two Phase III trials indicate that a 12-week regimen of once-daily E-BPO cream, 5%, monotherapy is efficacious, safe, and well tolerated. E-BPO cream, 5%, was significantly superior to vehicle for the coprimary endpoints of IGA success and reduction in the number of lesions. Significant superiority over vehicle was achieved at two weeks (the first postbaseline evaluation) and was maintained for the remainder of the study. Nine of 493 subjects (1.8%) treated with E-BPO cream, 5%, discontinued treatment because of TEAEs. Treatment with E-BPO cream, 5%, also reduced the percentage of subjects reporting skin dryness, scaling, itching, or stinging/burning compared with vehicle.
The efficacy, safety, and tolerability demonstrated for E-BPO cream, 5%, contrast with prior results reported in studies of unencapsulated BPO for rosacea. Montes et al17 reported a study in which subjects with rosacea were treated with 5% BPO acetone gel for four weeks followed by 10% BPO acetone gel for four more weeks. The control group was treated with acetone gel vehicle. At the end of the first four weeks of treatment, the dropout rate due to lack of improvement was 63 percent for vehicle and 23 percent for BPO acetone gel.17 Leyden et al18 reported a photographic analysis of a randomized, double-blind, vehicle-controlled, 12-week study that assessed the efficacy of topical BPO 5%/clindamycin 1% versus vehicle in the treatment of rosacea. Their analysis indicated that 7.7 percent of 26 subjects treated with the combination were rated as “clear/nearly clear” from baseline compared with 0 percent for vehicle.18 In another small-scale study of 26 subjects with rosacea who were treated with combined BPO and clindamycin, 15.4 percent reported application site burning with active treatment versus 0 percent for vehicle.27 Results from a retrospective cohort study using medical and pharmacy claims also indicated poor tolerability of BPO in subjects with rosacea. Overall, 22.5 percent of 1,084 subjects treated with BPO had AEs versus 12.9 percent of 10,721 subjects treated with azelaic acid and 12.3 percent of the 35,868 who received metronidazole.20 While there are many well-known limitations to comparing data collected in different types of studies, the historically observed efficacy and tolerability of unencapsulated BPO appear to differ substantially from the efficacy and tolerability of microencapsulated BPO for rosacea.
BPO is rapidly metabolized in the skin to benzoic acid28 and may result in very limited exposure to the drug. Application of conventional BPO to the skin results in high transient exposure that may be linked to increased AEs and poor tolerability. This limitation of the free drug is also addressed by microencapsulation. The controlled, gradual release of BPO from the silica capsules is believed to result in slower, more sustained BPO exposure, which may lead to improved tolerability.
Limitations. This study has two limitations. First, unencapsulated BPO was not used as a control. While historical data indicated that BPO had the potential to treat rosacea, this study did not seek to compare unencapsulated with microencapsulated BPO. Poor tolerability with unencapsulated BPO has already been reported.20,29 In this regard, it is worth noting that most of the Phase III studies of treatments for rosacea did not include active control groups.30-33 A second limitation is the short (12-week) duration of the trial, which limits understanding of the durability of the clinical efficacy of E-BPO cream, 5%. Rosacea is a chronic and relapsing condition, and a 12-week treatment course is unlikely to reflect real-world practice. Nevertheless, the study design reported here is in line with other efficacy and safety studies,30–33 and longer-term treatment has been addressed in a 40-week safety extension study.34
The results from the two Phase III studies summarized in this report indicate that E-BPO cream, 5%, is a safe, well-tolerated, and effective treatment option for papulopustular rosacea. This new formulation of BPO should be a useful addition to the armamentarium of agents used to treat this common, yet difficult to manage, disease.
ACKNOWLEDGEMENTS
The authors would like to dedicate this manuscript in memory of Thomas Prunty.Additionally, the authors wish to recognize the contributions of Ofra Levy-Hacham, PhD, Ori Nov, PhD, and Vered Ram, MSc, of Sol-Gel Technologies for design and supervision of these clinical trials. The authors also wish to thank Dr. Robert Rhoades and Mr. Thomas Prunty of AraMed Strategies, LLC, and Alyssa Theodore, PhD, Heather Anderson, MD, and Jesse Watts, MPA-C, of Simpson Healthcare for their assistance in medical writing and editing.
REFERENCES
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9(1):e1361574. doi: 10.1080/19381980.2017.1361574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexis AF, Callender VD, Baldwin HE et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80(6):1722–1729.e7. doi: 10.1016/j.jaad.2018.08.049. [DOI] [PubMed] [Google Scholar]
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000. doi: 10.12688/f1000research.16537.1. Faculty Rev-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Steinhoff M, Bewley A et al. Characterizing high-burden rosacea subjects: a multivariate risk factor analysis from a global survey [published correction appears in J Dermatolog Treat. 2020;31:[2210]. J Dermatolog Treat. 2020;31(2):168–174. doi: 10.1080/09546634.2019.1623368. [DOI] [PubMed] [Google Scholar]
- Zeichner JA, Eichenfield LF, Feldman SR et al. Quality of life in individuals with erythematotelangiectatic and papulopustular rosacea: findings from a web-based survey. J Clin Aesthet Dermatol. 2018;11(2):47–52. [PMC free article] [PubMed] [Google Scholar]
- Oussedik E, Bourcier M, Tan J. Psychosocial burden and other impacts of rosacea on patients’ quality of life. Dermatol Clin. 2018;36(2):103–113. doi: 10.1016/j.det.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Harper J, Del Rosso JQ, Ferrusi IL. Cross-sectional survey of the burden of illness of rosacea by erythema severity. J Drugs Dermatol. 2018;17(2):150–158. [PubMed] [Google Scholar]
- Abokwidir M, Feldman SR. Rosacea management. Skin Appendage Disord. 2016;2(1-2):26–34. doi: 10.1159/000446215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Schöfer H, Araviiskaia E et al. Prevalence of rosacea in the general population of Germany and Russia—the RISE study. J Eur Acad Dermatol Venereol. 2016;30(3):428–434. doi: 10.1111/jdv.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient's quality of life. Am Health Drug Benefits. 2013;6(6):348–354. [PMC free article] [PubMed] [Google Scholar]
- Anzengruber F, Czernielewski J, Conrad C et al. Swiss S1 guideline for the treatment of rosacea. J Eur Acad Dermatol Venereol. 2017;31(11):1775–1791. doi: 10.1111/jdv.14349. [DOI] [PubMed] [Google Scholar]
- Schaller M, Almeida LMC, Bewley A et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182(5):1269–1276. doi: 10.1111/bjd.18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuuren EJ, Fedorowicz Z, Tan J et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol. 2019;181(1):65–79. doi: 10.1111/bjd.17590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson T, Cheng WY, McCormick N et al. Patient preferences and therapeutic satisfaction with topical agents for rosacea: a survey-based study. Am Health Drug Benefits. 2018;11(2):97–106. [PMC free article] [PubMed] [Google Scholar]
- Russell JJ. Topical therapy for acne. Am Fam Physician. 2000;61(2):357–366. [PubMed] [Google Scholar]
- Matin T, Goodman MB. Benzoyl peroxide. In: StatPearls [Internet]. 2022. Treasure Island (FL): StatPearls Publishing; October 20. [PubMed]
- Montes LF, Cordero AA, Kriner J et al. Topical treatment of acne rosacea with benzoyl peroxide acetone gel. Cutis. 1983;32(2):185–190. [PubMed] [Google Scholar]
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11–17. [PubMed] [Google Scholar]
- Bandyopadhyay D. Topical antibacterials in dermatology. Indian J Dermatol. 2021;66(2):117–125. doi: 10.4103/ijd.IJD_99_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson T, Kamalakar R, Ogbonnaya A et al. Rate of adverse events and healthcare costs associated with the topical treatment of rosacea. Am Health Drug Benefits. 2017;10(3):113–119. [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Barankin B, Lam JM et al. Dermatology: how to manage acne vulgaris. Drugs Context. 2021;10:2021–2086. doi: 10.7573/dic.2021-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosso JQ, Tanghetti E. The clinical impact of vehicle technology using a patented formulation of benzoyl peroxide 5%/clindamycin 1% gel: comparative assessments of skin tolerability and evaluation of combination use with a topical retinoid. J Drugs Dermatol. 2006;5(2):160–164. [PubMed] [Google Scholar]
- Fyrand O, Jakobsen HB. Water-based versus alcohol-based benzoyl peroxide preparations in the treatment of acne vulgaris. Dermatologica. 1986;172(5):263–267. doi: 10.1159/000249352. [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Phan J, Schairer DO et al. Antimicrobial and anti-inflammatory activity of chitosan-alginate nanoparticles: a targeted therapy for cutaneous pathogens. J Invest Dermatol. 2013;133(5):1231–1239. doi: 10.1038/jid.2012.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11(7):830–833. [PubMed] [Google Scholar]
- Leyden JJ. Randomized, phase 2, dose-ranging study in the treatment of rosacea with encapsulated benzoyl peroxide gel. J Drugs Dermatol. 2014;13(6):685–688. [PubMed] [Google Scholar]
- Breneman D, Savin R, VandePol C et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43(5):381–387. doi: 10.1111/j.1365-4632.2004.02283.x. [DOI] [PubMed] [Google Scholar]
- Kern D. What is benzoic acid and can it help clear acne? https://www.acne.org/what-is-benzoic-acid-and-can-it-help-clear-acne.html Accessed June 7, 2022.
- Shields CW IV, White JP, Osta EG et al. Encapsulation and controlled release of retinol from silicone particles for topical delivery. J Control Release. 2018;278:37–48. doi: 10.1016/j.jconrel.2018.03.023. [DOI] [PubMed] [Google Scholar]
- Del Rosso JQ, Webster GF, Jackson M et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791–802. doi: 10.1016/j.jaad.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Thibutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48(6):836–845. doi: 10.1067/mjd.2003.308. [DOI] [PubMed] [Google Scholar]
- Stein L, Kircik L, Fowler J et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13(3):316–323. [PubMed] [Google Scholar]
- Draelos ZD, Elewski B, Staedtler G et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306–317. [PubMed] [Google Scholar]
- Werschler WP, Sugarman J, Bhatia N et al. Long-term efficacy and safety of microencapsulated benzoyl peroxide cream, 5%, in rosacea: Results from an extension of two Phase III, vehicle-controlled trials. J Clin Aesthet Dermatol. 2023;16(8):25–31. [PMC free article] [PubMed] [Google Scholar]