Abstract
COVID-19 infection not only profoundly impacts the detection of tuberculosis infection (Tbc) but also affects modality in tuberculosis patient immune response. It is important to determine immune response alterations in latent tuberculosis infection as well as in SARS-CoV-2-infected tuberculosis patients. Such changes may have underlying effects on the development and course of further tuberculosis. Here, we aimed to review the characteristics of immune response in TB patients or convalescent COVID-19 patients with latent TB infection (LTBI). Materials and Methods. We analyzed the features of immune response in tuberculosis and COVID-19 patients. For this, we analyzed publications released from December 2019 to March 2023; those which were published in accessible international databases (“Medline”, “PubMed”, “Scopus”) and with keywords such as “COVID-19”, “SARS-CoV-2”, “tuberculosis”, “pulmonary tuberculosis”, “latent tuberculosis infection”, “Treg”, “follicular Treg”, and “Treg subsets”, we considered. Results. Through our analysis, we found that tuberculosis patients who had been infected with COVID-19 previously and elevated Th1 and Th2 cell levels. High levels of Th1 and Th2 cells may serve as a positive marker, characterizing activated immune response during TB infection. COVID-19 or post-COVID-19 subjects showed decreased Th17 levels, indicating a lack of tuberculosis development. Moreover, the typical course of tuberculosis is associated with an increase in Treg level, but COVID-19 contributes to a hyperinflammatory response. Conclusion. According to the data obtained, the course of tuberculosis proceeds in a dissimilar way due to the distinct immune response, elicited by SARS-CoV-2. Importantly, the development of active tuberculosis with a severe course is associated with a decline in Treg levels. Both pathogens lead to disturbed immune responses, increasing the risk of developing severe TB. The insights and findings of this paper may be used to improve the future management of individuals with latent and active tuberculosis.
Keywords: tuberculosis, pulmonary tuberculosis, latent tuberculosis infection, coronavirus infection, COVID-19, SARS-CoV-2, autoimmunity, Treg, follicular Treg, Treg subsets
1. Introduction
The rapid spread of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), which induced a novel coronavirus (COVID-19) caused a pandemic and global public health concerns [1,2]. Currently, we know that COVID-19 potentiates the development of diverse pathological events by altering the immune status of patients [3,4,5]. In many countries around the world, the COVID-19 pandemic has had an impact on establishing approaches for the diagnosis, surveillance, and treatment of tuberculosis (TB) patients [6]. The WHO estimated that, mortality attributed to increased TB infection up to 1.5 million people (1.4 million people died from TB in 2019)) in 2021. At the same time, there has been an 18% decline in TB prevalence as evidenced by a decrease from 7.1 million cases in 2019 to 5.8 million in 2020 [7,8].
However, our focus as researchers is not solely on the potential issues, related to the diagnosis of TB and how it is able to influence increased morbidity and mortality in TB patients, as we believe the specificity of altered immune response in COVID-19 patients, displaying characteristics similar to that of the cellular immune response of TB patients is deserving of study [9,10,11]. The immunological processes understanding may provide a basis for developing new approaches for early TB diagnostics and development. Strikingly, extensive organ damage in COVID-19 occurs due to ACE2 (angiotensin-converting enzyme 2) expression [12,13], allowing SARS-CoV-2 to enter host cells due to several receptors and accessory proteins, including CD147, NRP-1, CD26, AGTR2, Band3, KREMEN1, ASGR1, ANP, TMEM30A, CLEC4G, and LDLRAD3, which promote entry into human cells. It should be noted that the altered characteristics of SARS-CoV-2 (B.1.1.7 (Alpha)) and its different variants—B.1.617.1 (Kappa), B.1.617.2 (Delta), B.1.617.2+ (Delta+), and B.1.1.529 (Omicron)—reflect changes in its ability to enter host cells [14,15].
Currently, receptors (CD147, ASGR1, etc.) are known to facilitate the entry of various viruses (and bacteria) such as measles, HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), Ebola virus, etc. [16,17]. COVID-19, caused by SARS-CoV-2, has been shown to follow a seasonal trend, presented by varying symptoms and severity governed by the variant strain of SARS-CoV-2, as well as patient’s age, sex, underlying comorbidities, and individual immune status [18,19].
Most of the immune-mediated changes are associated with COVID-19 [19]. The suppression of proinflammatory mechanisms contributes to virus replication, resulting in potentiated pyroptosis, an inflammatory form of programmed cell death typical of cytopathic viral infections [20]. At the same time, there is an elevated release of necrosis products that enhance inflammation due to pyroptosis products and the development of an aberrant inflammatory response [21].
Moreover, some virus strains are responsible for the upregulated surface expression of MHC molecules on infected cells that are able to promote autoimmune response in virus-infected lung cells. COVID-19 augments MHC expression and promotes autoimmune responses; however, this remains poorly understood [21].
Immunity suppression is found in TB patients, which can be aggravated during or after COVID-19 [22] and deteriorate disease course, resulting in developing severe pulmonary fibrosis and antibacterial resistance. Obtaining new data on the pathogenesis of fibrosis, as well as immunologic and autoimmune alterations in individuals with LTBI and TB after COVID-19, may be a key in predicting TB course and adjusting therapeutic protocols [23].
At the same time, recent evidence suggests that SARS-CoV-2 may affect TB pathogenesis and its clinical course. Despite the recency effect of the COVID-19 pandemic, a comprehensive body of research on the innate and adaptive immune responses and molecular mechanisms underlying SARS-CoV-2 entry, as well as its spread in human cells, has accumulated. Here, we aimed to identify SARS-CoV-2-driven alterations in immune response in patients with LTBI and TB patients. Similarities and differences in immune cell phenotype, driven by both pathogens, have been uncovered. It should be of importance for the prognosis behind TB and its prophylaxis.
2. Methods
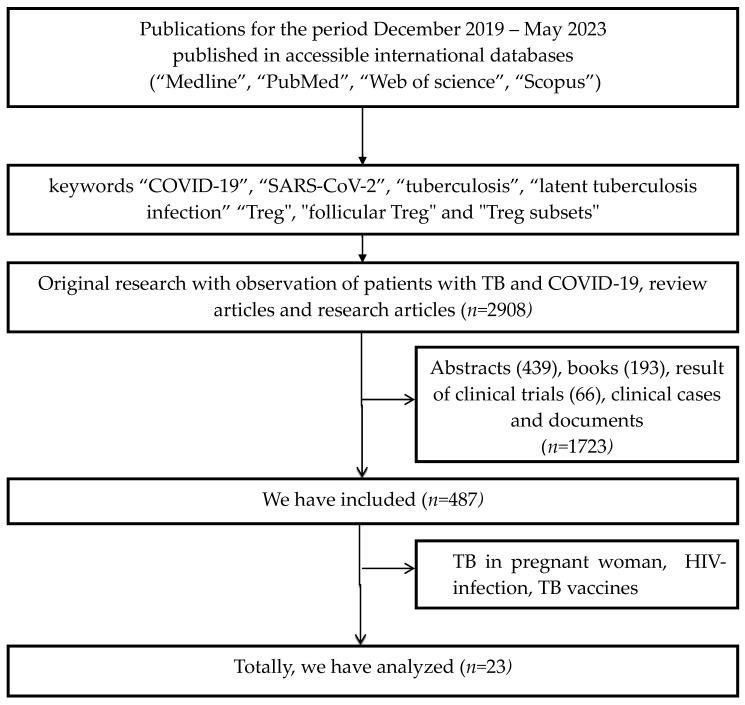
We analyzed publications, released from December 2019 to May 2023 after searching for works published in accessible international databases (“Medline”, “PubMed”, “Web of Science”, “Scopus”) with search parameters for keywords such as “COVID-19”, “SARS-CoV-2”, “tuberculosis”, “latent tuberculosis infection”, “pulmonary tuberculosis”, “autoimmune inflammation”, “Treg”, “follicular Treg”, and “Treg subsets”. Inclusion criteria were: Original studies with TB patients and COVID-19, reviews, and research articles. Exclusion criteria were: Clinical cases, abstracts, clinical trials, books, TB in pregnant women, HIV-infection, and TB vaccines. We analyzed 2908 publications excluding abstracts (439), books (193), results of clinical trials (66), and clinical cases and documents (n = 1723). We included 487 publications, analyzing a total of 23 publications (Figure 1).
Figure 1.
Flow chart depicting a systematic literature search.
The narrative review was carried out in accordance with the PRISMA protocol (http://www.prisma-statement.org, 6 March 2023 (registration ID 420213). The JBI checklist was used to evaluate the quality of the study.
3. Results
3.1. COVID-19 Affecting Immune Response in LTBI and Active Tuberculosis
According to the data analysis, a whole body of publications (n = 2908) related to COVID-19 affecting TB course were identified. However, while analyzing the publications in detail by taking into account the exclusion criteria, only 23 publications were selected, which contained the information on the published data and relevant results. The results of immunological studies underlying COVID-19-related effect on TB development and course are presented in Table 1.
Table 1.
COVID-19 affects immune response and development of tuberculosis infection.
| TB Infection and COVID-19 | Immune Response | First Author, Year of Publication |
|---|---|---|
| LTBI + COVID-19 (clinical case) | decline in CD4+ T cell count along with latent-to-active TB progression | Khayat M, 2021 [24] |
| LTBI + COVID-19 (study data) | LTBI with COVID-19 showed significantly higher plasma levels of IFN γ, IL-2, TNF α, IL- 1 α, IL-1 β, IL-6, IL-12, IL-15, IL-17, IL-3, GM-CSF, IL-10, IL-25, IL-33, CCL3 and CXCL10 | Rajamanickam A, 2021 [25] |
| LTBI + COVID-19 (review) | decreased cytokine levels shown for IL-2, IL-4, IL-5 and IL-13 | Shariq M, 2022 [26] |
| LTBI + COVID-19 (study data) |
LTBI in COVID subjects had lowered CD8+ T cell count and IFN-γ level | Palacios-Gutiérrez JJ, 2022 [27] |
| TB +COVID-19 (study data) |
low level of IFN-γ in COVID-19 and TB patients | Petrone L, 2021 [28] |
| TB +COVID-19 (study data) |
decreased SARS-CoV-2-specific CD4 T cell level in COVID-19 and TB patients | Flores-Lovon K, 2022 [29] |
According to the data presented in Table 1, four publications provided comparable data, regarding decreased CD4 T cell level in LTBI. At the onset of the COVID-19 pandemic, Rajamanickam A (2021) analyzed a wide range of immunological parameters in LTBI subjects [25], finding significantly different plasma levels for IFN γ, IL-2, TNF α, IL-1 α, IL-1 β, IL-6, IL-12, IL-15, IL-17, IL-3, GM-CSF, IL-10, IL-25, IL-33, CCL3, and CXCL10 in LTI along with COVID-19. A 2022 study by Petrone L et al. in LTBI during COVID-19 revealed a decline in CD8+ T cell count and IFN-γ level [28], accounting for a decreased immune response in LTBI. Such alterations may suggest a negative QFT Plus Gold test data in COVID-19 convalescents with LTBI. Hence, it necessitates a demand to develop new methods for LTBI investigation by taking into account altered immune response in SARS-CoV-2-infected subjects.
Single studies analyzed immunological parameters in tuberculosis patients, suffering from COVID-19. According to the published data, COVID-19 infection potentiates a decline in IFN-γ level, as well as magnitude of SARS-CoV-2-specific CD4 T cells [28,29]. Most researchers conclude that immune response in LTBI and tuberculosis is clearly impaired, which may presumably result in developing active tuberculosis or severe TB course in COVID-19 convalescents [29,30,31]. However, it was noted to continue performing further studies. We also attempted to analyze immune response in TB patients and compare alterations in immunological parameters due to the SARS-CoV-2 virus.
3.2. Immunological Features in Patients in Tuberculosis
TB infection is characterized by a great variability observed at diverse disease stages ranging from premalignant to manifest disease. It is accounted for by pathogen virulence and quantity upon body invasion, duration of contact, routes of infection, as well as host immune state [32,33].
Currently, it is acknowledged that Th1 cells in M. tuberculosis-infected humans are considered key effector T-helper cells. Upon recognition of the M. tuberculosis-specific antigen in peripheral tissues, Th1 cells begin to produce IFN-γ by activating a broad spectrum of immunocompetent cells, including cytotoxic CD8+ T cells, ILC1, macrophages, and B cells, which are involved in elimination of pathogens localized within host cells [34].
After M. tuberculosis infection, the first stage in developing tuberculosis is presented as a latent tuberculosis infection [35,36,37]. The major criteria for LTBI diagnostics include positive in vitro or in vivo immunological tests along with the lack of criteria for active tuberculosis infection [37]. The methodology of in vitro immunological tests is based on assessing activation of CD4+ or CD8+ T cells by specific peptides ESAT-6 and SFP-10, as well as IFN-γ release by antigen-specific activated CD4+ T cells [36,37,38]. Previously, it was shown that COVID-19 represents one of the causes eliciting a decline in CD4+ and CD8+ T cells, as well as IFN-γ levels, which may affect lowered diagnostic efficacy for LTBI [39,40].
As stated before the COVID-19 pandemic, about 10% of individuals with LTBI may develop active tuberculosis [36,41] under unfavorable conditions, such as stress, comorbidities, immunosuppression, etc., affecting macro-organisms and resulting in some immunologic disorders. At present, it is unclear how such a situation may change after the exposure of M. tuberculosis-infected subjects to SARS-CoV-2. Moreover, no follow-up data are available yet.
The identification of peptides that bind to MHC I and II molecules is crucial for the activation of CD8+ and CD4+ T-lymphocytes. Recent studies revealed that the engagement of both helper T-lymphocyte (HTL) epitopes binding to MHC II molecule and cytotoxic T-lymphocyte (CTL) epitopes interacting with MHC I molecule is required for mounting a strong immune response against M. tuberculosis (Mtb) [42].
Using TB human studies and experimental models enabled us to find out that lungs infected with Mtb contain organized ectopic lymphoid structures, bearing CXCR5+ T cells. In experimental models, the data uncovered that CXCR5 is expressed on T cells to ensure their proper localization within tuberculosis granulomas and promote effective macrophage activation to elicit a full defense against Mtb infection. Such data demonstrate that CD4+CXCR5+ T cells play a protective role in anti-tuberculosis immune response. According to the data obtained, the level of peripheral blood CXCR5-expressing Tfh cells in patients with active pulmonary tuberculosis is significantly lower than that in LTBI patients [41]. An imbalance in the Tfh subset profile is observed in tuberculosis, skewed towards a higher level of Tfh2 and Tfh17 cells, along with decreased Tfh1 cell level [43]. A similar pattern is also found in patients with autoimmune pathology [44]. Some studies have shown that Th17 cells play an important role in the production of cytokines, such as IL-23 and IL-17, that are necessary for the formation of tertiary lymphoid structures [45,46].
Increased IL-17 and IL-23, along with CXCL13 expression, has been verified in animal models, which provided CXCR5+ recruitment of Tfh and B cells into tertiary lymphoid structures. This event underlies an emergence of humoral immune response [47]. The role of Th17 cells in tuberculosis remains debated. It is known that some polymorphisms in the genes encoding signature Th17-associated cytokines, IL-17A and IL-17A, may be associated with a predisposition to tuberculosis [48]. It was noted that a decreased Th17 level is associated with a clinical cure for tuberculosis. At the same time, the suppression of Th17 cell pool due to lowered IL-6R expression may represent a crucial mechanism in the regulation of developing active TB [49]. It has been shown that the Th17.1 cell subset level is decreased in TB patients vs the control group, whereas peripheral blood “classic” and CCR6+DP Th17 cell levels are significantly elevated [50]. According to some data, an increased IFN-γ+IL-17+ T cell count correlated with disease severity. High levels of CD4+IFN-γ+IL-17+ T cells were most often detected in TB patients with low therapeutic efficacy and widespread lung damage, as well as prolonged disease course, which is related to impaired immune response [51,52]. Finally, animal models uncovered that IFN-γ-secreting Th17 lymphocytes negatively affect the development of long-term immune defense upon reinfection with M. tuberculosis [53,54].
According to studies, tuberculosis patients have a decreased level of memory B-cells and increased count of “naive” B-cells, compared to healthy subjects [55,56,57]. However, B-cell alterations have not been observed in other lung diseases, such as viral or bacterial pneumonia, bronchiectasis lung disease, bronchial asthma, or COPD [58]. Regulatory B-cells producing IL-10 and IL-35 were found in TB granulomas [57]. A subset of immature transient CD24++CD38+ B-cells performs diverse regulatory functions, the elevation of which was detected in tuberculosis patients compared to healthy subjects. CD5+ B-cells are also defined as IL-10-producing regulatory subset additionally involved in the synthesis of low-affinity autoreactive IgM antibodies [59].
The mouse tuberculosis model revealed an increased count of IL-35- and IL-10-co-producing regulatory B-cells. In turn, IL-35 can contribute to IL-35+ B-cell infiltration into lung tissue and suppress recruitment of tuberculosis-specific effector Th1/Th17 cells. An increase in the latter led to increased Foxp3+ Treg level in the site of lung tissue infiltration [57,60]. In addition, the level of Foxp3+ Treg cells differed significantly, in parallel with mycobacteria sputum microscopy data [53,54].
Peripheral blood Treg cells were more frequently found in patients with active vs. latent pulmonary tuberculosis and healthy subjects [61]. Mouse models demonstrated that the effect of Tregs in tuberculosis is related to the phase of inflammation so that, at a high level, they are observed in the acute phase of the disease, being associated with poor efficacy of disease treatment. A significantly higher percentage of Tregs was found in patients with drug-sensitive vs. multidrug-resistant TB patients. Studies showed that high Treg levels downmodulated PBMC-related microbiologic activity in Tbc patients [62]. Treg levels were significantly higher in BAL vs. peripheral blood [63].
CD127, an α-chain IL-7 receptor, is used to identify Tregs among CD4+CD25+ T cells. FoxP3, a signature marker of regulatory T lymphocytes interacts with the CD127 gene promoter and to suppress it [64]. CD39hi Tregs produce more IL-10 and exert stronger suppressor activity [65]. It has been shown that activation markers are expressed on Treg surface in patients with tuberculosis. The predominant Treg subset in both LNMC and PBMC from TB patients co-expresses HLA-DR and CD38 [66].
The presented data characterize the immune response mounted by Mycobacterium tuberculosis prior to the emergence of SARS-CoV-2 in global medical practice. Clearly, exposure to a single causative agent, resulting in a very specific bacterial infection, accompanied by an immunosuppressive response, along with autoimmune inflammation. It may aggravate immunocompromised condition after infection with a viral agent. The occurrence of such disorders can simultaneously alter the course of TB infection similar to co-infection with HIV [67]. The goal of our study in the context of the limited number of available studies and currently accumulated experience was to identify potential alterations, while comparing the data published on tuberculosis and COVID-19 co-infection.
3.3. Immune Response in COVID-19 Patients
SARS-CoV-2 directly affects airway tract cells, mediated by circulatory disorders along with profoundly altered immune response in both severe and mild COVID-19 [12,13,19].
SARS-CoV-2 causes the dysregulation of innate immunity with increased peripheral blood neutrophil levels that promote de novo formation of neutrophil extracellular traps (NETs) [68,69,70]. The emergence of tissue NET components, such as proteases, DNA degradation products, and neutrophil histones, as well as high levels of IL-6 and IL-17A, support a vicious circle of hyperinflammation, endothelial damage, thrombosis, and cardiovascular dysfunction [71,72].
Lymphopenia has been described in many patients with COVID-19, primarily characterized by lowered CD4+ and CD8+ T-cell counts also observed in several coronavirus infections [28]. SARS-CoV-2-specific CD4+ T cells express IFN-γ, TNF-ά, and IL-2, evidencing about developing Th1-type cell response [73]. An important role of CD4+ T-cells has been shown in murine models, wherein reduced T-lymphocyte count promoted the emergence of more prominent pulmonary inflammation. Transfer of SARS-CoV-2-specific CD4+ and CD8+ T cells to immunodeficient mice also resulted in mounting an effective immune response to the mouse-adapted SARS-CoV-2 strain [26]. CD8+ T cells were shown to be critical for controlling SARS-CoV-2 infection during the acute phase of COVID-19 and altered CD8+ T cell subset composition, dramatically influencing an efficient antiviral immune response [74,75].
A decrease in circulating follicular Treg cell count was noted in peripheral blood of acute COVID-19 patients compared to control group [76]. Moreover, Zahran et al. obtained similar data, showing that hospitalized severe patients had lower level of peripheral blood CD4+CXCR5+ICOS+Foxp3+ Tfr cells [77,78]. It should be noted that a consistently low level of circulating CD45RA-CD127-CD25+CXCR5hiPD-1h Tfr was also observed in COVID-19 patients [79].
As previously mentioned, the most important phenotypic characteristic of Th1 cells is that they express CXCR3 on the surface of the chemokine receptor, allowing it to enter the foci of inflammation along the gradient of relevant chemokines—CXCL9, CXCL10, and CXCL11 [80,81]. Patients with severe COVID-19 had increased serum levels of CXCR9 and CXCR10, which, along with the elevated count of both cellular (“unclassified” monocytes, CD38+HLA-DR+ T-cells and granzyme-B+/perforin+ T-cells) and serum (CXCL8, IL-6 and IL-10 levels) cues, allowed us to differentiate mild from severe disease [82]. In addition, COVID-19 convalescent patients showed a negative correlation between levels of circulating Tfr cells and serum virus-specific IgM, IgG, and IgA antibodies. Follicular Treg cells may potentially play an important role in controlling humoral memory response and antibody specificity, as well as preventing auto-antibody formation [83].
COVID-19 is also featured with decreased percentage of T-helper cells bearing key surface Th17 cell markers—CD161 and CCR6—compared to control group [84]. Th17 cells during COVID-19 are characterized by phenotype typical to tissue resident memory T cells also expressing the genes associated with cytolytic potential (SRGN, GZMB, and GNLY) and cytokine genes encoding IL-21, IL-17F, IL-17A, IFN-γ, and GM-CSF. Moreover, lung tissues collected from COVID-19 patients were enriched in CCR6 and IL-17A co-expressing cells [85]. Thus, patients with acute COVID-19 infection are characterized by decreased Tfr, along with elevated Tfh levels, that were able to contribute to mounting humoral autoimmune responses and the emergence of autoimmune pathologies in post-COVID-19 syndrome [86,87,88].
The aberrant organization of B-dependent zones, resulting in poor humoral immune response in patients with severe acute COVID-19, was noted in numerous studies [89]. The number of follicular dendritic cells, Bcl-6+ Tfh, and B-cells decreased in lymph nodes, whereas AID+ B-cell level was preserved most often [90]. The rise of the latter in cases of severe COVID-19 may suggest things about immunosuppression and the spread of virus-related pathological effects. CD25 expression was increased on Treg cells in patients with severe COVID-19, which was partially reduced after recovery that, however, remained elevated compared with healthy subjects. A unique type of hyperinflammatory response against SARS-CoV-2 may develop in some patients, eliciting autoimmune reactions. One of the plausible causes, resulting in development of autoimmune complications, may be due to molecular mimicry between SARS-CoV-2 S proteins and human proteins [28]. At the same time, the course of COVID-19 infection can be markedly affected, not only by host genetic predisposition, but also by underlying lung diseases. In this regard, the investigation of various types COVID-19 course in tuberculosis patients is of particular interest [23,80].
Thus, severe acute COVID-19 was found to have frequent compromised adaptive immune response due to the decline of the T-helper cell arm and reduced B-cell-dependent zones in secondary lymphoid organs. However, primarily enhanced activity of the above-mentioned cell types may develop allergic or autoimmune pathology during the recovery period.
3.4. Comparison of M. tuberculosis and SARS-CoV-2-Triggered Immune Response
Clearly, the interplay and impact of these two distinct pathogens on the development and course of tuberculosis infection have been discussed. Prior to the COVID-19 pandemic, more relevant insights into tuberculosis/HIV co-infection have been obtained. Now, it is clear that the effect on SARS-CoV-2-related immune response is not limited to a single COVID-19 episode. SARS-CoV-2 exerts long-lasting effects on the human immune system not always driven by COVID-19 severity. Tuberculosis infection is caused by M. tuberculosis that may proceed as a latent or active tuberculosis capable of resulting in generalization, low therapeutic efficiency, and development of drug resistance. Altogether, such events not only result from pathogen-related characteristics, but also due to features of host immune response. Not only the course of tuberculosis process per se, but also a crosstalk with a pathogen, such as SARS-CoV-2, are shaped by immune response parameters related to individualized genetic background. Circulating Tfh cells are distinguished from other Th cell subsets by CXCR5 chemokine receptor expression [59] also expressing chemokine receptors specific to polarized non-follicular Th cell subsets, such as Th1, Th2, and Th17 [60]. Regulatory T cells also play a crucial role in controlling chronicity of inflammatory reactions. An immune response triggered in COVID-19 and Tbc patients is characterized below (Table 2).
Table 2.
Th cell subset alterations in tuberculosis and COVID-19.
| Cells | COVID-19 | Tuberculosis |
|---|---|---|
| Th1 | ↑ [65,91,92]; ↓ [93,94] |
↑ [95,96] |
| Th2 | ↑ [97,98,99] | ↑ [100,101]; not significant [95] |
| Th17 | ↑ [99,102]; ↓ [93,96,101] |
↓ [100,103] |
| Tfh | ↑ [98,104]; ↓ [87,91,99,105] |
↑ [100]; ↓ [101] not significant [95] |
| Treg | ↑ [65,106,107]; ↓ [66,108,109] |
↑ [43,60,110,111,112] |
↑—high level; ↓—low level.
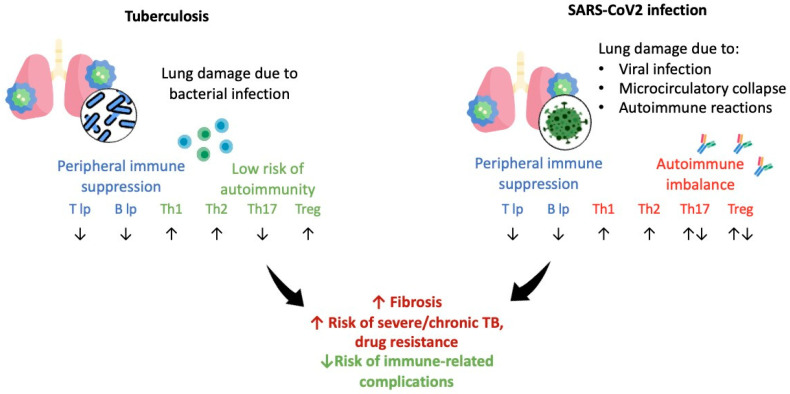
According to the current data, both tuberculosis and COVID-19 patients show elevated Th1 and Th2 cell levels typical to activated immune response. More often, tuberculosis is associated with a decline in Th17 cell level that may be further exacerbated by COVID-19. The typical course of tuberculosis is coupled to increased Treg cell level, whereas COVID-19, along with Tbc, results in a hyperinflammatory response. A shift to the latter may prevent virus spread while exacerbating the course of TB infection [108,111]. However, no unequivocal data on long-term data related to treatment and the course of tuberculosis in COVID-19 convalescent patients are available [112]. Currently, published study data suggest the need to further evaluate and monitor patients with high risk of active tuberculosis by taking into consideration development of post-COVID-19 immunosuppression.
4. Discussion
It is recognized that Th17 cells are actively involved in the pathogenesis of multiple pro-inflammatory events in both autoimmune and infectious disorders. The cytokines IL-1β, IL-6, and IL-23 play a crucial role in Th0-to-Th17 cell “polarization” [53,54].
COVID-19 is characterized by a decreased percentage of T-helper cells bearing the key Th17 surface markers, CD161 and CCR6 [91]. A similar pattern was found by using diverse biological research approaches. It was shown that the expression of Th17 cell-associated genes (RORC, IL17A, IL17F, and CCR6) was downmodulated in peripheral blood CD4+ T cells in COVID-19 patients [80]. Such cells migrated to the foci of inflammation that was confirmed by BAL studies. During COVID-19 infection, BAL Th17 cells had phenotype of tissue resident memory T cells and also expressed genes associated with cytolytic properties (SRGN, GZMB, and GNLY), and cytokine genes (IL-21, IL-17F, IL -17A, IFN-γ, and GM-CSF). Moreover, the lung tissues in COVID-19 patients were enriched in CCR6- and IL17A-co-expressing cells. The high concentration of IL-6, IL-17A, GM-CSF, and IFN-γ were found in the BAL liquid phase which may account for the volumetric inflammatory changes in severe patients with pneumonia [14,95]. IL-17A was shown to be required for granuloma maturation during mycobacterial infections [54].
Circulating human Tfh cells can be divided into four subsets based on the expression of two chemokine receptors, CXCR3 and CCR6. Thus, the Tfh1 population are phenotyped as CXCR3+CCR6−, Tfh2—CXCR3−CCR6−, Tfh17—CXCR3−CCR6+, as well as CXCR3+CCR6+ “double-positive” DP Tfh or Tfh17.1 cells, which differ in related functional properties. Hence, Tfh2 and Tfh17 cells (CXCR3–CCR6– and CXCR3–CCR6+, respectively) are able to promote antibody class switch in the “naive” B cells they synthesize antibodies and secrete (from IgM to IgG and IgE or IgG and IgA, respectively), whereas CXCR3+ Tfh1 memory cells are unable to exert such a stimulating effect, and can induce apoptosis in activated “naive” B-lymphocytes [94].
Peripheral blood CD8+ T cells can be subdivided into Tc1, Tc2, and Tc17 subsets based on surface chemokine receptor expression. Moreover, each type of such cells plays a distinct role in inflammation, both in vivo and in vitro. Few studies on this type of CD8+ T cell subset have been published. However, researchers who were engaged in this field obtained a rather complete picture for pathogenesis of certain pathologies. At the same time, the vast majority of Tc17 cells display a terminally differentiated phenotype (CD27-/CD45RO-) and profoundly suppress Th1 and Th17 cell functional activity evidencing their potent in relation to anti-inflammatory effects [95].
We also consider the importance of evaluating a pool of T-regulatory cells firstly by distinguishing based on surface expression of chemokine receptors, CCR4, CCR6, and CXCR3, enabling the indirect assessment of the migration of such cells into the lung tissue. In the context of the above pathologies, no studies have been conducted yet. According to the chemokine receptors noted above, regulatory T cells are subdivided into CCR4+CCR6–CXCR3+ Th1-like (Tr1), CCR4+CCR6–CXCR3- Th2-like (Tr2), CCR4+CCR6+CXCR3- Th17-like (Tr17), and CCR4+CCR6+CXCR3+ Th17.1-like (Tr17.1) cell subsets. The Th2-like Tregs display higher survival and migratory potential, as well as more prominent selectivity for T-effector cell suppression [96,113]. Th1-like T-regulatory cells and Th17-like T-regulatory cells exert increased pro-inflammatory activity by secreting IFN-γ and IL-17, respectively. However, they can also secrete IL-10, but at a lower level [97]. The former have the CD4+Foxp3+CD25+CD31+Helios+Nrp1+ phenotype, whereas the latter have the CD4+Foxp3+CD25+CD31-Helios-Nrp1 expression pattern [6]. Recent peripheral blood studies revealed that the prevalence of CD4+CD25+ T cells was higher in patients with active Tcb vs. LTBI. However, no differences in the number of CD4+CD25+CD127lo, CD4+CD25+FoxP3+, or CD4+CD25+FoxP3+CD127lo T cells were found [103].
Severe COVID-19, similarly to tuberculosis, was characterized by increased Th cell count, which may be related to immunosuppression. CD25 expression was upregulated on regulatory T cells in severe COVID-19 that was partially decreased after recovery but remained higher than in the control group. CD127 expression was evenly downmodulated in both mild and severe COVID-19 patients compared with healthy subjects. At the same time, after recovery, its expression returned back to normal level solely in patients with the mild disease, while continued to decrease in severe COVID-19 [14]. In addition, it was also shown that all COVID-19 convalescent vs. healthy subjects have low levels of CD25+Foxp3+CD127- regulatory T cells [26,59]. Kalfaoglu et al. found a decline in FOXP3+IL2RA+CD4+ T-cell levels during severe vs. moderate COVID-19 [91]. This immune response coupled to emerging post-COVID-19 immunosuppression in patients with tuberculosis may profoundly impact on further progression of the pathology [103,113].
The alterations in cell type profile during tuberculosis and COVID-19 are shown in Figure 2.
Figure 2.
The scheme depicting Th cell immune response altered in tuberculosis and COVID-19, ↑—high level; ↓—low level.
Antigen-specific peripheral tissue effector Th1 cells can produce IFN-γ to activate a broad range of immunocompetent cells, including CD8+ T cells, ILC1, macrophages, and B-cells, which are involved in intracellular pathogen elimination [80,114].
IFN-γ and TNF-α overproduced by Th1 cells during response to SARS-CoV-2 elicit a mass death of virus-infected cells resulting in lung tissue damage and triggering acute respiratory distress syndrome [113,114,115]. Hence, Th1 cell migration to inflamed tissues barely accounts for a decline in some fraction of such peripheral blood cells during acute TB infection that was noted in several independent studies [105,108,116,117].
5. Conclusions
Current data on the alterations in immune response during LTBI and tuberculosis after COVID-19 remain insufficient. Numerous conflicting clinical data suggest a decreased pulmonary function in TB patients and an increased risk of adverse COVID-19 outcomes. To some extent, immune responses in COVID-19 and TB infection overlap ranging from imbalanced Th cell subset composition, pro-inflammatory cytokine production to altered B-cell activation, and excessive infiltration of inflammatory sites by highly activated peripheral blood cells. It may result in excessive tissue damage. Both SARS-CoV-2 and Mtb lead to imbalanced and dysregulated immune response that elevates a risk of developing TB infection and its severe course. An introduction of such findings may improve the future management of individuals with LTBI and tuberculosis. Therefore, identification of novel immunological features for tuberculosis during or after COVID-19 will provide a deeper insights into diagnostics and treatment of relevant pathological conditions.
Acknowledgments
Government funding was obtained from Almazov National Medical Research Centre of the Ministry of Health of Russian Federation. We are thankful for the assistance provided by Min Zhuang, Chun-Lei Zhang, Hong Ling at the Center for Innovative Research in Infection and Immunity of Heilongjiang Province.
Author Contributions
A.S., I.K. and A.R.—performed data analysis, wrote the manuscript; I.D. and A.M.—performed data analysis, wrote the manuscript, coordinated the project; D.K., A.S., I.D., A.M. and I.K.—wrote the manuscript, coordinated the project. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was carried out with financial support from the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2022-301).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO . Coronavirus Disease (COVID-19) Pandemic. WHO; Geneva, Switzerland: 2020. [(accessed on 8 June 2023)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Google Scholar]
- 2.Sharma A., Balda S., Apreja M., Kataria K., Kapalas N., Sharma P. COVID-19 Diagnosis: Current and Future Techniques. Int. J. Biol. Macromol. 2021;193 (Pt B):1835–1844. doi: 10.1016/j.ijbiomac.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenollar F., Bouam A., Ballouche M., Fuster L., Prudent E., Colson P., Tissot-Dupont H., Million M., Drancourt M., Raoult D., et al. Evaluation of the Panbio COVID-19 Rapid Antigen Detection Test Device for the Screening of Patients with COVID-19. J. Clin. Microbiol. 2021;59:e02589-20. doi: 10.1128/JCM.02589-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., Ravelli R.B.G., Paul van Schayck J., Mykytyn A.Z., Duimel H.Q., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santa Cruz A., Mendes-Frias A., Oliveira A.I., Dias L., Matos A.R., Carvalho A., Capela C., Pedrosa J., Castro A.G., Silvestre R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021;12:613422. doi: 10.3389/fimmu.2021.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guglielmetti L., Veziris N., Aubry A., Brossier F., Bernard C., Sougakoff W., Jarlier V., Robert J. Risk factors for extensive drug resistance in multidrug-resistant tuberculosis cases: A case-case study. Int. J. Tuberc. Lung Dis. 2018;22:54–59. doi: 10.5588/ijtld.17.0387. [DOI] [PubMed] [Google Scholar]
- 7.Global Tuberculosis Report 2022. World Health Organization; Geneva, Switzerland: 2022. [Google Scholar]
- 8.Bigio J., Viscardi A., Gore G., Matteelli A., Sulis G. A scoping review on the risk of tuberculosis in specific population groups: Can we expand the World Health Organization recommendations? Eur. Respir. Rev. 2023;32:220127. doi: 10.1183/16000617.0127-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertz P., Jeannel J., Guffroy A., Lescuyer S., Korganow A.S., Rondeau-Lutz M., Weber J.C. Granulomatous manifestations associated with COVID19 infection: Is there a link between these two diseases? Autoimmun. Rev. 2021;20:102824. doi: 10.1016/j.autrev.2021.102824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starshinova A., Malkova A., Kudryavtsev I., Kudlay D., Zinchenko Y., Yablonskiy P. Tuberculosis and autoimmunity: Common features. Tuberculosis. 2022;134:102202. doi: 10.1016/j.tube.2022.102202. [DOI] [PubMed] [Google Scholar]
- 11.Stojanovic Z., Gonçalves-Carvalho F., Marín A., Capa J.A., Domínguez J., Latorre I., Lacoma A., Prat-Aymerich C. Advances in diagnostic tools for respiratory tract infections: From tuberculosis to COVID-19—Changing paradigms? ERJ Open Res. 2022;8:00113–2022. doi: 10.1183/23120541.00113-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trougakos I.P., Stamatelopoulos K., Terpos E., Tsitsilonis O.E., Aivalioti E., Paraskevis D., Kastritis E., Pavlakis G.N., Dimopoulos M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021;28:9. doi: 10.1186/s12929-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S., Zhang M., Chang T.L. ACE2-Independent Alternative Receptors for SARS-CoV-2. Viruses. 2022;14:2535. doi: 10.3390/v14112535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alipoor S.D., Mirsaeidi M. SARS-CoV-2 cell entry beyond the ACE2 receptor. Mol. Biol. Rep. 2022;49:10715–10727. doi: 10.1007/s11033-022-07700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe A., Yoneda M., Ikeda F., Terao-Muto Y., Sato H., Kai C. CD147/EMMPRIN Acts as a Functional Entry Receptor for Measles Virus on Epithelial Cells. J. Virol. 2010;84:4183–4193. doi: 10.1128/JVI.02168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 2016;159:481–490. doi: 10.1093/jb/mvv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Berger N.A., Kaelber D.C., Pamela BDavis P.B., Volkow N.D., Xu R. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. medRxiv. 2022 doi: 10.1101/2021.12.30.21268495. [DOI] [Google Scholar]
- 19.Chan K.W., Wong V.T., Tang S.C.W. COVID-19: An update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative chinese-western medicine for the management of 2019 novel coronavirus disease. Am. J. Chin. Med. 2020;48:737–762. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 20.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raucci F., Mansour A.A., Casillo G.M., Saviano A., Caso F., Scarpa R., Mascolo N., Iqbal A.J., Maione F. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmun. Rev. 2020;19:102572. doi: 10.1016/j.autrev.2020.102572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkington P., Tebruegge M., Mansour S. Tuberculosis: An Infection-Initiated Autoimmune Disease? Trends Immunol. 2016;37:815–818. doi: 10.1016/j.it.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starshinova A., Malkova A., Zinchenko Y., Kudryavtsev I., Serebriakova M., Akisheva T., Lapin S., Mazing A., Kudlay D., Glushkova A., et al. Identification of autoimmune markers in pulmonary tuberculosis. Front. Immunol. 2023;13:1059714. doi: 10.3389/fimmu.2022.1059714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khayat M., Fan H., Vali Y. COVID-19 promoting the development of active tuberculosis in a patient with latent tuberculosis infection: A case report. Respir. Med. Case Rep. 2021;32:101344. doi: 10.1016/j.rmcr.2021.101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajamanickam A., Kumar N.P., Padmapriyadarsini C., Nancy A., Selvaraj N., Karunanithi K., Munisankar S., Bm S., Renji R.M., Ambu T.C., et al. Latent tuberculosis co-infection is associated with heightened levels of humoral, cytokine and acute phase responses in seropositive SARS-CoV-2 infection. J. Infect. 2021;83:339–346. doi: 10.1016/j.jinf.2021.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shariq M., Sheikh J.A., Quadir N., Sharma N., Hasnain S.E., Ehtesham N.Z. COVID-19 and tuberculosis: The double whammy of respiratory pathogens. Eur. Respir. Rev. 2022;31:210264. doi: 10.1183/16000617.0264-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palacios-Gutiérrez J.J., Rodríguez-Guardado A., Arias-Guillén M., Alonso-Arias R., Palacios-Penedo S., García-García J.M., Balbín M., Pérez-Hernández D., Sandoval-Torrientes M., Torreblanca-Gil A., et al. Clinical and Epidemiological Correlates of Low IFN-Gamma Responses in Mitogen Tube of QuantiFERON Assay in Tuberculosis Infection Screening During the COVID-19 Pandemic: A Population-Based Marker of COVID-19 Mortality? Arch. Bronconeumol. 2022;58:649–659. doi: 10.1016/j.arbres.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrone L., Petruccioli E., Vanini V., Cuzzi G., Gualano G., Vittozzi P., Nicastri E., Maffongelli G., Grifoni A. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int. J. Infect. Dis. 2021;113((Suppl. 1)):S82–S87. doi: 10.1016/j.ijid.2021.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores-Lovon K., Ortiz-Saavedra B., Cueva-Chicaña L.A., Aperrigue-Lira S., Montes-Madariaga E.S., Soriano-Moreno D.R., Bell B., Macedo R. Immune responses in COVID-19 and tuberculosis coinfection: A scoping review. Front. Immunol. 2022;13:992743. doi: 10.3389/fimmu.2022.992743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bostanghadiri N., Jazi F.M., Razavi S., Fattorini L., Darban-Sarokhalil D. Mycobacterium tuberculosis and SARS-CoV-2 Coinfections: A Review. Front. Microbiol. 2022;12:747827. doi: 10.3389/fmicb.2021.747827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Maio F., Bianco D.M., Delogu G. The Dark Side of the COVID-19 Treatments on Mycobacterium Tuberculosis Infection. Mediterr. J. Hematol. Infect. Dis. 2022;14:e2022021. doi: 10.4084/MJHID.2022.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf A.J., Desvignes L., Linas B., Banaiee N., Tamura T., Takatsu K., Ernst J.D. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf A.J., Linas B., Trevejo-Nuñez G.J., Kincaid E., Tamura T., Takatsu K., Ernst J.D. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 34.Corleis B., Dorhoi A. Early dynamics of innate immunity during pulmonary tuberculosis. Immunol. Lett. 2020;221:56–60. doi: 10.1016/j.imlet.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Buonsenso D., Noguera-Julian A., Moroni R., Hernández-Bartolomé A., Fritschi N., Lancella L., Cursi L., Soler-Garcia A., Krüger R., Feiterna-Sperling C., et al. Performance of QuantiFERON-TB Gold Plus assays in paediatric tuberculosis: A multicentre PTBNET study. Thorax. 2022;78:288–296. doi: 10.1136/thorax-2022-218929. [DOI] [PubMed] [Google Scholar]
- 36.Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. World Health Organization; Geneva, Switzerland: 2018. [(accessed on 23 February 2022)]. Available online: https://apps.who.int/iris/handle/10665/260233. [PubMed] [Google Scholar]
- 37.Module 3: Diagnosis. Tests for Tuberculosis Infection. World Health Organization; Geneva, Switzerland: 2022. WHO Consolidated Guidelines on Tuberculosis. [PubMed] [Google Scholar]
- 38.Zellweger J.P., Sotgiu G., Corradi M., Durando P. The diagnosis of latent tuberculosis infection (LTBI): Currently 622 available tests, future developments, and perspectives to eliminate tuberculosis (TB) Med. Lav. 2020;111:170–183. doi: 10.23749/mdl.v111i3.9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starshinova A., Zhuravlev V., Dovgaluk I., Panteleev A., Manina V., Zinchenko U., Istomina E., Pavlova M., Yablonskiy P. A Comparison of Intradermal Test with Recombinant Tuberculosis Allergen (Diaskintest) with Other Immunologic Tests in the Diagnosis of Tuberculosis Infection. Int. J. Mycobacteriol. 2018;1:32–39. doi: 10.4103/ijmy.ijmy_17_18. [DOI] [PubMed] [Google Scholar]
- 40.Tang X.L., Zhou Y.X., Wu S.M., Pan Q., Xia B., Zhang X.L. CFP10 and ESAT6 aptamers as effective 628 Mycobacterial antigen diagnostic reagents. J. Infect. 2014;69:569–580. doi: 10.1016/j.jinf.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Slight S.R., Rangel-Moreno J., Gopal R., Lin Y., Fallert Junecko B.A., Mehra S., Selman M., Becerril-Villanueva E., Baquera-Heredia J., Pavon L., et al. CXCR5⁺ T helper cells mediate protective immunity against tuberculosis. J. Clin. Investig. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong W., Pan C., Cheng P., Wang J., Zhao G., Wu X. Peptide-Based Vaccines for Tuberculosis. Front. Immunol. 2022;13:830497. doi: 10.3389/fimmu.2022.830497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Zhang M., Liao M., Graner M.W., Wu C., Yang Q., Liu H., Zhou B. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am. J. Respir. Crit. Care Med. 2010;181:734–742. doi: 10.1164/rccm.200909-1463OC. [DOI] [PubMed] [Google Scholar]
- 44.Li X.Y., Wu Z.B., Ding J., Zheng Z.H., Li X.Y., Chen L.N., Zhu P. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjögren’s syndrome. Biochem. Biophys. Res. Commun. 2012;422:238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 45.Kumar N.P., Sridhar R., Hanna L.E., Banurekha V.V., Nutman T.B., Babu S. Decreased frequencies of circulating CD4⁺ T follicular helper cells associated with diminished plasma IL-21 in active pulmonary tuberculosis. PLoS ONE. 2014;9:e111098. doi: 10.1371/journal.pone.0111098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L., Jiang Y., Lao S., Yang B., Yu S., Zhang Y., Wu C. Mycobacterium tuberculosis-Specific IL-21+IFN-γ+CD4+ T Cells Are Regulated by IL-12. PLoS ONE. 2016;11:e0147356. doi: 10.1371/journal.pone.0147356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gopal R., Monin L., Slight S., Uche U., Blanchard E., Fallert Junecko B.A., Ramos-Payan R., Stallings C.L., Reinhart T.A., Kolls J.K., et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014;10:e1004099. doi: 10.1371/journal.ppat.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang M., Xu G., Lü L., Xu K., Chen Y., Pan H., Burstrom B., Burstrom K., Wang J. Genetic polymorphisms of IL-17A, IL-17F, TLR4 and miR-146a in association with the risk of pulmonary tuberculosis. Sci. Rep. 2016;6:28586. doi: 10.1038/srep28586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mourik B.C., Lubberts E., de Steenwinkel J.E.M., Ottenhoff T.H.M., Leenen P.J.M. Interactions between Type 1 Interferons and the Th17 Response in Tuberculosis: Lessons Learned from Autoimmune Diseases. Front. Immunol. 2017;8:294. doi: 10.3389/fimmu.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kudryavtsev I.V., Serebriakova M.K., Starshinova A.A., Zinchenko Y.S., Basantsova N.Y., Belyaeva E.N., Pavlova M.V., Yablonskiy P.K. Altered peripheral blood Th17 and follicular T-helper subsets in patients with pulmonary tuberculosis. Russ. J. Infect. Immun. 2019;9:304–314. doi: 10.15789/2220-7619-2019-2-304-314. [DOI] [Google Scholar]
- 51.Lyadova I., Nikitina I. Cell Differentiation Degree as a Factor Determining the Role for Different T-Helper Populations in Tuberculosis Protection. Front. Immunol. 2019;10:972. doi: 10.3389/fimmu.2019.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jurado J.O., Pasquinelli V., Alvarez I.B., Peña D., Rovetta A.I., Tateosian N.L., Romeo H.E., Musella R.M., Palmero D., Chuluyán H.E., et al. IL-17 and IFN-γ expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J. Leukoc. Biol. 2012;91:991–1002. doi: 10.1189/jlb.1211619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikitina I.Y., Panteleev A.V., Kosmiadi G.A., Serdyuk Y.V., Nenasheva T.A., Nikolaev A.A., Gorelova L.A., Radaeva T.V., Kiseleva Y.Y., Bozhenko V.K., et al. Th1, Th17, and Th1Th17 Lymphocytes during Tuberculosis: Th1 Lymphocytes Predominate and Appear as Low-Differentiated CXCR3+CCR6+ Cells in the Blood and Highly Differentiated CXCR3+/-CCR6- Cells in the Lungs. J. Immunol. 2018;200:2090–2103. doi: 10.4049/jimmunol.1701424. [DOI] [PubMed] [Google Scholar]
- 54.Monin L., Griffiths K.L., Slight S., Lin Y., Rangel-Moreno J., Khader S.A. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 2015;8:1099–1109. doi: 10.1038/mi.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linge I., Tsareva A., Kondratieva E., Dyatlov A., Hidalgo J., Zvartsev R., Apt A. Pleiotropic Effect of IL-6 Produced by B-Lymphocytes During Early Phases of Adaptive Immune Responses Against TB Infection. Front. Immunol. 2022;13:750068. doi: 10.3389/fimmu.2022.750068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malkova A., Starshinova A., Zinchenko Y., Gavrilova N., Kudryavtsev I., Lapin S., Mazing A., Surkova E., Pavlova M., Belaeva E., et al. New laboratory criteria of the autoimmune inflammation in pulmonary sarcoidosis and tuberculosis. Clin. Immunol. 2021;227:108724. doi: 10.1016/j.clim.2021.108724. [DOI] [PubMed] [Google Scholar]
- 57.Willem J., du Plessis W.J., Keyser A., Walzl G., Loxton A.G. Phenotypic analysis of peripheral B cell populations during Mycobacterium tuberculosis infection and disease. J. Inflamm. 2016;13:23. doi: 10.1186/s12950-016-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinze C.H., Colbert R.A. B-cell depletion in Wegener’s granulomatosis. Clin. Rev. Allergy Immunol. 2008;34:327–379. doi: 10.1007/s12016-007-8057-7. [DOI] [PubMed] [Google Scholar]
- 59.Soe P.T., Hanthamrongwit J., Saelee C., Kyaw S.P., Khaenam P., Warit S., Satproedprai N., Mahasirimongkol S., Yanai H., Chootong P., et al. Circulating IgA/IgG memory B cells against Mycobacterium tuberculosis dormancy-associated antigens Rv2659c and Rv3128c in active and latent tuberculosis. Int. J. Infect. Dis. 2021;110:75–82. doi: 10.1016/j.ijid.2021.07.033. [DOI] [PubMed] [Google Scholar]
- 60.Semple P.L., Binder A.B., Davids M., Maredza A., van Zyl-Smit R.N., Dheda K. Regulatory T cells attenuate mycobacterial stasisin alveolar and blood-derived macrophages from patients with tuberculosis. Am. J. Respir. Crit. Care Med. 2013;187:1249–1258. doi: 10.1164/rccm.201210-1934OC. [DOI] [PubMed] [Google Scholar]
- 61.Seddiki N., Santner-Nanan B., Martinson J., Zaunders J., Sasson S., Landay A., Solomon M., Selby W., Alexander S.I., Nanan R., et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai Y.C., Wang W.D., Zhang J.A., Chen C., Luo H.L., Xu H., Peng Y., Luo H., Yang X.R., Chen X., et al. MTB driven B cells producing IL-35 and secreting high level of IL-10 in the patients with active pulmonary tuberculosis. Mol. Immunol. 2019;112:175–181. doi: 10.1016/j.molimm.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Ahmed A., Adiga V., Nayak S., Kumar J.A.J.U., Dhar C., Sahoo P.A., Sundararaj B.K., Souza G.D., Vyakarnam A. Circulating HLA-DR+CD4+ effector memory T cells resistant to CCR5 and PD-L1 mediated suppression compromise regulatory T cell function in tuberculosis. PLOS Pathog. 2018;14:e1007289. doi: 10.1371/journal.ppat.1007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu W., Putnam A.L., Xu-Yu Z., Szot G.L., Lee M.R., Zhu S., Gottlieb P.A., Kapranov P., Gingeras T.R., Fazekas de St Groth B., et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schultheiß C., Paschold L., Simnica D., Mohme M., Willscher E., von Wenserski L., Scholz R., Wieters I., Dahlke C., Tolosa E., et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires from COVID-19 Patients Showed Signatures Associated with Severity of Disease. Immunity. 2020;53:442–455.e4. doi: 10.1016/j.immuni.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiacchio T., Casetti R., Butera O., Vanini V., Carrara S., Girardi E., Di Mitri D., Battistini L., Martini F., Borsellino G., et al. Characterization of regulatory T cells identified as CD4(+)CD25(high)CD39(+) in patients with active tuberculosis. Clin. Exp. Immunol. 2009;156:463–470. doi: 10.1111/j.1365-2249.2009.03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng X., Ouyang J., Isnard S., Mohme M., Willscher E., von Wenserski L., Scholz R., Wieters I., Dahlke C., Tolosa E., et al. Sharing CD4+ T Cell Loss: When COVID-19 and HIV Collide on Immune System. Front. Immunol. 2020;11:596631. doi: 10.3389/fimmu.2020.596631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A., Peccatori J., D’Angelo A., De Cobelli F., Rovere-Querini P., et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Kuraishy H.M., Al-Gareeb A.I., Al-Hussaniy H.A., Al-Harcan N.A.H., Alexiou A., Batiha G.E. Neutrophil Extracellular Traps (NETs) and COVID-19: A new frontiers for therapeutic modality. Int. Immunopharmacol. 2022;104:108516. doi: 10.1016/j.intimp.2021.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalinina O., Golovkin A., Zaikova E., Aquino A., Bezrukikh V., Melnik O., Vasilieva E., Karonova T., Kudryavtsev I., Shlyakhto E. Cytokine Storm Signature in Patients with Moderate and Severe COVID-19. Int. J. Mol. Sci. 2022;23:8879. doi: 10.3390/ijms23168879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan C.W., Low J.G.H., Wong W.H., Chua Y.Y., Goh S.L., Ng H.J. Critically ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. [(accessed on 22 June 2021)];Am. J. Hematol. 2021 95:E156–E158. doi: 10.1002/ajh.25822. Available online: https://pubmed.ncbi.nlm.nih.gov/32267008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jovanovic M., Sekulic S., Jocic M., Jurisevic M., Gajovic N., Jovanovic M., Arsenijevic N., Jovanovic M., Mijailovic M., Milosavljevic M., et al. Increased Pro Th1 and Th17 Transcriptional Activity in Patients with Severe COVID-19. Int. J. Med. Sci. 2023;20:530–541. doi: 10.7150/ijms.80498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kudryavtsev I.V., Arsentieva N.A., Korobova Z.R., Isakov D.V., Rubinstein A.A., Batsunov O.K., Khamitova I.V., Kuznetsova R.N., Savin T.V., Akisheva T.V., et al. Heterogenous CD8+ T Cell Maturation and ‘Polarization’ in Acute and Convalescent COVID-19 Patients. Viruses. 2022;14:1906. doi: 10.3390/v14091906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malkova A.M., Kudlay D.A., Kudryavtsev I.V., Starshinova A.A., Yablonsky P.K., Shoenfeld Y. Immunogenetic predictors of severe COVID-19. Vaccines. 2021;9:211. doi: 10.3390/vaccines9030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Søndergaard J.N., Tulyeu J., Edahiro R., Shirai Y., Yamaguchi Y., Murakami T., Morita T., Kato Y., Hirata H., Takeda Y., et al. A sex-biased imbalance between Tfr, Tph, and atypical B cells determines antibody responses in COVID-19 patients. Proc. Natl. Acad. Sci. USA. 2023;120:e2217902120. doi: 10.1073/pnas.2217902120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng H.Y., Zhang M., Yang C.X., Zhang N., Wang X.C., Yang X.P., Dong X.Q., Zheng Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zahran A.M., Abdel-Rahim M.H., Nasif K.A., Hussein S., Hafez R., Ahmad A.B., Saad K., Elhoufey A., Hussein H.A.M., Thabet A.A., et al. Association of follicular helper T and follicular regulatory T cells with severity and hyperglycemia in hospitalized COVID-19 patients. Virulence. 2022;13:569–577. doi: 10.1080/21505594.2022.2047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gong F., Dai Y., Zheng T., Cheng L., Zhao D., Wang H., Liu M., Pei H., Jin T., Yu D., et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J. Clin. Investig. 2020;130:6588–6599. doi: 10.1172/JCI141054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Starshinova A.A., Kudryavtsev I., Malkova A., Zinchenko U., Karev V., Kudlay D., Glushkova A., Starshinova A.Y., Dominguez J., Villar-Hernández R., et al. Molecular and Cellular Mechanisms of M. tuberculosis and SARS-CoV-2 Infections—Unexpected Similarities of Pathogenesis and What to Expect from Co-Infection. Int. J. Mol. Sci. 2022;23:2235. doi: 10.3390/ijms23042235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bonecchi R., Bianchi G., Bordignon P.P., D’Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P.A., Mantovani A., et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tincati C., Cannizzo E.S., Giacomelli M., Badolato R., d’Arminio Monforte A., Marchetti G. Heightened Circulating Interferon-Inducible Chemokines, and Activated Pro-Cytolytic Th1-Cell Phenotype Features COVID-19 Aggravation in the Second Week of Illness. Front. Immunol. 2020;11:580987. doi: 10.3389/fimmu.2020.580987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qi J., Liu C., Bai Z., Li X., Yao G. T follicular helper cells and T follicular regulatory cells in autoimmune diseases. Front. Immunol. 2023;14:1178792. doi: 10.3389/fimmu.2023.1178792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Biasi S., Lo Tartaro D., Meschiari M., Gibellini L., Bellinazzi C., Borella R., Fidanza L., Mattioli M., Paolini A., Gozzi L., et al. Expansion of plasmablasts and loss of memory B cells in peripheral blood from COVID-19 patients with pneumonia. Eur. J. Immunol. 2020;50:1283–1294. doi: 10.1002/eji.202048838. [DOI] [PubMed] [Google Scholar]
- 85.Zhao Y., Kilian C., Turner J.E., Bosurgi L., Roedl K., Bartsch P., Gnirck A.C., Cortesi F., Schultheiß C., Hellmig M., et al. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci. Immunol. 2021;6:eabf6692. doi: 10.1126/sciimmunol.abf6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caso F., Costa L., Ruscitti P., Navarini L., del Puente A., Giacomelli R., Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020;19:102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaneko N., Kuo H.H., Boucau J., Farmer J.R., Allard-Chamard H., Mahajan V.S., Piechocka-Trocha A., Lefteri K., Osborn M., Bals J., et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell. 2020;183:143–157.e13. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kudryavtsev I., Rubinstein A., Golovkin A., Kalinina O., Vasilyev K., Rudenko L., Isakova-Sivak I. Dysregulated Immune Responses in SARS-CoV-2-Infected Patients: A Comprehensive Overview. Viruses. 2022;14:1082. doi: 10.3390/v14051082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haslbauer J.D., Matter M.S., Stalder A.K., Tzankov A. Reaktionsmuster der lokoregionären Lymphknoten im Abflussgebiet von COVID-19-Lungen [Histomorphological patterns of regional lymph nodes in COVID-19 lungs] Pathologe. 2021;42:188–196. doi: 10.1007/s00292-021-00914-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duan Y.Q., Xia M.H., Ren L., Zhang Y.-F., Ao Q.-L., Xu S.-P., Kuang D., Liu Q., Yan B., Zhou Y.-W., et al. Deficiency of Tfh Cells and Germinal Center in Deceased COVID-19 Patients. Curr. Med. Sci. 2020;40:618–624. doi: 10.1007/s11596-020-2225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalfaoglu B., Almeida-Santos J., Tye C.A., Satou Y., Ono M. T-cell dysregulation in COVID-19. Biochem. Biophys. Res. Commun. 2021;538:204–210. doi: 10.1016/j.bbrc.2020.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu Q., Xu Y., Wang T., Xie F. Innate and adaptive immune response in SARS-CoV-2 infection-Current perspectives. Front Immunol. 2022;13:1053437. doi: 10.3389/fimmu.2022.1053437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sattler A., Angermair S., Stockmann H., Heim K.M., Khadzhynov D., Treskatsch S., Halleck F., Kreis M.E., Kotsch K. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J. Clin. Investig. 2020;130:6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.San Segundo D., Arnaiz de Las Revillas F., Lamadrid-Perojo P., Comins-Boo A., Gonzalez-Rico C., Alonso-Pena M., Irure-Ventura J., Olmos J.M., Farinas M.C., Lopez-Hoyos M. Innate and Adaptive Immune Assessment at Admission to Predict Clinical Outcome in COVID-19 Patients. Biomedicines. 2021;9:917. doi: 10.3390/biomedicines9080917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lo C.-Y., Huang Y.-C., Huang H.-Y., Chung F.-T., Lin C.-W., Chung K.F., Wang C.-H. Increased Th1 Cells with Disease Resolution of Active Pulmonary Tuberculosis in Non-Atopic Patients. Biomedicines. 2021;9:724. doi: 10.3390/biomedicines9070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shu C.C., Wu M.F., Wang J.Y., Lai H.C., Lee L.N., Chiang B.L., Yu C.J. Decreased T helper 17 cells in tuberculosis is associated with increased percentages of programmed death ligand 1, T helper 2 and regulatory T cells. Respir. Res. 2017;18:128. doi: 10.1186/s12931-017-0580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varnaitė R., García M., Glans H., Maleki K.T., Sandberg J.T., Tynell J., Christ W., Lagerqvist N., Asgeirsson H., Ljunggren H.G., et al. Expansion of SARS-CoV-2-Specific Antibody-Secreting Cells and Generation of Neutralizing Antibodies in Hospitalized COVID-19 Patients. J Immunol. 2020;205:2437–2446. doi: 10.4049/jimmunol.2000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen X., Huang J., Huang Y., Chen J., Jiang X., Shi Y. Characteristics of immune cells and cytokines in patients with coronavirus disease 2019 in Guangzhou, China. Hum Immunol. 2020;81:702–708. doi: 10.1016/j.humimm.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gutiérrez-Bautista J.F., Rodriguez-Nicolas A., Rosales-Castillo A., Jiménez P., Garrido F., Anderson P., Ruiz-Cabello F., López-Ruz M. Negative Clinical Evolution in COVID-19 Patients Is Frequently Accompanied with an Increased Proportion of Undifferentiated Th Cells and a Strong Underrepresentation of the Th1 Subset. Front. Immunol. 2020;11:596553. doi: 10.3389/fimmu.2020.596553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun H., Liu J., Feng R., Wang C., Li Y., Wang X. Follicular Helper T Cells in Pulmonary Tuberculosis: A Retrospective Study. Iran J Immunol. 2022;19:2. doi: 10.22034/IJI.2022.90588.2017. [DOI] [PubMed] [Google Scholar]
- 101.Kozlov V.A., Savchenko A.A., Kudryavtsev I.V., Kozlov I.G., Kudlay D.A., Prodeus A.P., Borisov A.G. Krasnoyarsk. Polycor; Krasnoyarsk, Russia: 2020. Clinical Immunology.386p. [Google Scholar]
- 102.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guyot-Revol V., Innes J.A., Hackforth S., Hinks T., Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 2006;173:803–810. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 104.Fathi F., Sami R., Mozafarpoor S., Hafezi H., Motedayyen H., Arefnezhad R., Eskandari N. Immune system changes during COVID-19 recovery play key role in determining disease severity. Int. J. Immunopathol. Pharmacol. 2020;34:2058738420966497. doi: 10.1177/2058738420966497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kudryavtsev I.V., Arsentieva N.A., Batsunov O.K., Korobova Z.R., Khamitova I.V., Isakov D.V., Kuznetsova R.N., Rubinstein A.A., Stanevich O.V., Lebedeva A.A., et al. Alterations in B Cell and Follicular T-Helper Cell Subsets in Patients with Acute COVID-19 and COVID-19 Convalescents. Curr. Issues Mol. Biol. 2022;44:14. doi: 10.3390/cimb44010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tan M., Liu Y., Zhou R., Deng X., Li F., Liang K., Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160:261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mohebbi S.R., Baghaei K., Rostami-Nejad M., Mojarad E.N., Mirjalali H., Yadegar A., Asri N., Abdoulahi S., Assadzadeh-Aghdaei H. Significant changes of CD4, FOXP3, CD25, and IL6 expression level in Iranian COVID-19 patients. Gastroenterol. Hepatol. Bed Bench. 2020;13:388. [PMC free article] [PubMed] [Google Scholar]
- 108.Almatrafi M.A., Awad K., Alsahaf N., Tayeb S., Alharthi A., Rabie N., Fadag R., Alwafi H., Salawati R., Alhindi A.K., et al. Disseminated Tuberculosis Post COVID-19 Infection: A Case Report. Cureus. 2022;14:e31489. doi: 10.7759/cureus.31489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dhawan M., Rabaan A.A., Alwarthan S., Alhajri M., Halwani M.A., Alshengeti A., Najim M.A., Alwashmi A.S.S., Alshehri A.A., Alshamrani S.A., et al. Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities with a Special Focus on Long COVID. Vaccines. 2023;11:699. doi: 10.3390/vaccines11030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kratzer B., Trapin D., Ettel P., Körmöczi U., Rottal A., Tuppy F., Feichter M., Gattinger P., Borochova K., Dorofeeva Y., et al. Immunological imprint of COVID-19 on human peripheral blood leukocyte populations. Allergy. 2021;76:751–765. doi: 10.1111/all.14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-kayali Rawaa S., Kashkash Mohamad F., Alhussein Alhajji Azzam H., Khouri A. Activation of tuberculosis in recovered COVID-19 patients: A case report. Ann. Med. Surg. 2023;85:280–283. doi: 10.1097/MS9.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y.C., Chin C.H., Liu S.F., Wu C.C., Tsen C.C., Wang Y.H., Chao T.Y., Lie C.H., Chen C.J., Wang C.C., et al. Prognostic values of serum IP-10 and IL-17 in patients with pulmonary tuberculosis. Dis. Markers. 2011;31:101–110. doi: 10.1155/2011/938794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alemu A., Bitew Z.W., Seid G., Diriba G., Gashu E., Berhe N., Mariam S.H., Gumi B. Tuberculosis in individuals who recovered from COVID-19: A systematic review of case reports. PLoS ONE. 2022;17:e0277807. doi: 10.1371/journal.pone.0277807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cardona P., Cardona P.-J. Regulatory T Cells in Mycobacterium tuberculosis Infection. Front. Immunol. 2019;10:2139. doi: 10.3389/fimmu.2019.02139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kozlov V.A., Tikhonova E.P., Savchenko A.A., Kudryavtsev I.V., Andronova N.V., Anisimova E.N., Golovkin A.S., Demina D.V., Zdzitovetsky D.E., Kalinina Y.S., et al. Clinical Immunology. A Practical Guide for Infectious Disease Specialists. Krasnoyarsk; Polikor, Russia: 2021. 563p. (In Russian) [DOI] [Google Scholar]
- 116.Lyadova I.V., Panteleev A.V. Th1 and Th17 Cells in Tuberculosis: Protection, Pathology, and Biomarkers. Mediat. Inflamm. 2015;2015:854507. doi: 10.1155/2015/854507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Renavikar P.S., Crawford M.P., Sinha S., Karandikar N.J. Human Tc17 cells harbor potent immune suppressive potential, whereas Tc1 cells lack suppressive ability. J. Immunol. 2019;202((Suppl. 1)):57.13. doi: 10.4049/jimmunol.202.Supp.57.13. [DOI] [Google Scholar]